Abstract
Background
Fungal spores are ubiquitous allergens. Severe forms of asthma are particularly highly associated with fungal sensitization. National and international asthma guidelines recommend the implementation of allergen immunotherapy if indicated. Thus, detection and treatment of relevant allergies are key components of primary care of these patients.
Objectives
The aims of the study were (i) to investigate trends in the prevalence of sensitization to twelve fungi in central Germany over the last 20 years and (ii) to dissect specific sensitization patterns among the 3 most important fungi: Aspergillus, Alternaria, and Cladosporium.
Methods
This single-center study evaluated skin prick test (SPT) results of 3,358 patients with suspected airway allergies over a period of 20 years (1998–2017).
Results
While 19.2% of all study patients had positive test results to at least 1 of the 3 fungi (Alternaria, Aspergillus, or Cladosporium) in the first study decade, this rate increased to 22.5% in the second decade. Slight increases in sensitization rates to almost all fungi were observed over the 20-year period. In the last decade, polysensitization to Alternaria, Aspergillus, and Cladosporium increased significantly. Sensitization to fungi is age-dependent and peaks in the age-group of 21–40 years during the second decade.
Conclusion
Fungi are relevant allergens for perennial and seasonal allergy symptoms. We currently recommend including Aspergillus, Alternaria, and Cladosporium in the standard series of SPTs for airway allergies.
Keywords: Alternaria, Cladosporium, Penicillium, Aspergillus, Molds, Fungi
Introduction
Fungi are ubiquitously occurring eukaryotic organisms, and virtually all people are exposed to fungi. Fungal spores are found as indoor and outdoor allergens, mainly depending on humidity and climate conditions, and they can cause both seasonal and perennial allergy symptoms. The importance of fungi as relevant allergens in allergic airway diseases has been underappreciated for many years [1].
Genomic analyses have led to a reorganization of fungal taxonomy in recent years [2]. The new classification resulted in the renaming of several species and also in a modified cladogram [1, 2] (see online suppl. Fig. 1; seewww.karger.com/doi/10.1159/000512230 for all online suppl. material). Currently, 8 phyla of fungi are being differentiated, among which 3 are specifically relevant to allergic reactions: Zygomycota, Ascomycota, and Basidiomycota [2]. Although at least 112 genera are suspected allergen sources, the 4 genera most commonly associated with the development of allergy are Alternaria, Cladosporium, Penicillium, and Aspergillus [1]. The revised cladogram also seems to better reflect the close relationship between phylogeny and IgE against fungi [3]. Common medical terminology often uses the term “mold sensitization” or “mold allergy.” However, the term “mold” is taxonomically imprecise, as it phenotypically describes a large group of different genera across the fungal phylum mainly growing in the form of hyphae [4]. It also does not include fungal genera that are most frequently (albeit not exclusively) found as yeasts but still harbors species eliciting allergies such as Candida and Aureobasidium. Therefore, we use the more global term “fungi” throughout this article, even though most of the studied fungi indeed form hyphae. The prevalence of sensitization to inhalation allergens in Germany was examined in the population-based “German Health Interview and Examination Survey for Adults” (DEGS1) from 2008 to 2011 (n = 7,025 participants). Based on IgE analyses, 33.6% of the general population was sensitized to one or more inhalant allergens. Detailed prevalence analysis of fungal sensitization (Aspergillus fumigatus, Alternaria alternata, and Cladosporium herbarum) revealed 4.6% to be sensitized among the full cohort (3.2% of women and 6.1% of men). The sensitization peaked in adults aged 18–29, with a prevalence of 7.2%. The most common serological finding was sensitization to A. alternata at 3.0%, followed by A. fumigatus at 2.3% and C. herbarum at 1.3% [5]. In a large Italian analysis of patients with suspected allergic diseases (n = 4,962), skin prick tests (SPTs) were performed with A. alternata, A. fumigatus, Candida albicans, C. herbarum, Penicillium notatum, Trichophyton mentagrophytes, and Saccharomyces cerevisiae [6]. Two-thirds of this cohort revealed a positive SPT for at least one inhalation allergen, and 19.1% of those were sensitized to at least 1 fungal allergen. Among the fungal SPT-positive individuals, 77.6% were monosensitized to 1 fungus only, whereas 12.4% displayed sensitization to more than 2 fungi. Most patients sensitized to only 1 fungal species reacted to A. alternata, C. albicans, or T. mentagrophytes [6]. Fungal sensitization is also strongly associated with the severity of asthma. Patients with severe asthma demonstrate high sensitization rates to fungal allergens, between 35 and 75% [7]. In a subgroup analysis of patients with life-threatening asthma, sensitization rates ranged from 54 to 91%. Fatal asthma was previously found to be specifically associated with Alternaria sensitization and others [8]. Various studies have confirmed a connection between high exposure to many outdoor fungal spores and asthma hospitalization in children and adolescents (e.g., [9, 10]).
A novel and potentially promising therapeutic concept in allergen immunotherapy for airway allergies is treatment with purified major allergens from fungal extracts. A multicenter, randomized, double-blind, placebo-controlled clinical trial of 2 different dosing options of Alt a 1, the predominant allergen of A. alternata, was conducted between 2012 and 2016 in Spain [11]. A total of 113 adolescents (>12 years of age) and adults with a history of allergic rhinitis associated with fungal exposure, with or without mild or moderate asthma, were included. Patients who received the higher dose of Alt a 1 (0.37 μg) achieved a significant reduction in the combined symptom and medication score compared to those who received placebo after 12 months of treatment.
The German guidelines on indoor mold exposure recommend early immunotherapy for allergic patients [4]. However, fungal allergens are not regulated in the German “Therapy Allergen Ordinance,” in contrast to, for example, insect venom allergens or grass allergens; thus, authorities possess only limited data on the quality and allergen composition of these individually prepared fungal allergen immunotherapies. This heterogeneity of SPT extracts is a well-known issue. Detailed analysis of different fungal SPT extracts revealed substantial diversity in protein patterns and antigen and allergen contents [12]. Numerous factors may influence the composition of the final test extract. The fungal strain, its growth conditions, and the extraction procedure are all relevant determinants [12, 13]. In order to circumvent this problem, blood tests with purified or recombinant allergens have been increasingly suggested in recent years. However, SPTs remain much more affordable and sensitive than serological tests [14]. Thus, SPTs with fungal allergen extracts continue to be the cornerstone of initial diagnostic procedures in testing for airway allergies to fungi.
Larger studies to dissect sensitization rates to fungi in greater detail are completely missing in Germany. Thus, the primary aims of this study were to investigate prevalence rates of allergic sensitization by SPT to various fungi and to analyze changes in sensitization patterns over a 20-year period. These results should also help current guidelines in which fungal allergens should be included in screening test series. In addition, we analyzed cosensitization rates among different genera of fungi.
Materials and Methods
Study Population
We compiled retrospective data on 3,358 patients who underwent SPTs with inhalant allergens in our tertiary referral center at the University Medical Center Göttingen. Göttingen, a city of 135,000 inhabitants and a catchment area of approximately 1.5 million people, is located close to the geographical center of Germany.
As the study period spanned the years 1998–2017, a subgroup analysis was performed for the 2 decades: 1998–2007 (n = 2,018 patients) and 2008–2017 (n = 1,368 patients). Twenty-eight patients were tested in both decades. Additional subgroup analyses were performed on patients who received SPT for Alternaria, Aspergillus, and Cladosporium (1998–2007, n = 1,251; 2008–2017, n = 1,002). SPTs with fungal extracts were performed as part of an extensive standard diagnostic panel. Hence, we can largely exclude the possibility of selection bias due to changes in the specific indications for SPTs with fungi over the years. Briefly, test results of all patients were recorded in local databases and, after anonymization, transmitted to the IVDK central office at the University of Göttingen. This retrospective study has been reviewed and approved by the local ethics committee at the University Medical Center Göttingen.
Methods
SPTs were performed according to the national guidelines [15], with sensitization being defined as a positive SPT. Commercially available allergen extracts were used for all patients. Over the 20 years covered in this study, to our knowledge, fungal extracts were purchased exclusively from Allergopharma (Reinbek, Germany). The commercial SPT extracts were A. alternata (formerly Alternaria tenuis), Aspergillus sp., Aureobasidium pullulans (formerly Pullularia pullulans), Botrytis cinerea, Cladosporium sp., Curvularia sp., Fusarium sp., Helminthosporium sp., Mucor sp., Penicillium sp., Rhizopus nigricans, and Serpula lacrymans. SPTs were performed on the back or the forearm with standardized allergen dilutions. Histamine hydrochloride (10 mg/mL) was used as the positive control and saline solution as the negative control (both from Allergopharma). A reaction with an average wheal diameter ≥2 mm was considered positive, according to standards in the late 1990s [16, 17]. Especially within epidemiological studies, the interpretation of wheal diameters of 2–3 mm as positive was explicitly recommended at this time [18]. This parameter was changed to ≥3 mm in 2018 in our department; therefore, this convention of reading average wheal diameters ≥2 mm as positive was consistent over the 20 years covered in this study [15]. Use of the historical reading parameter may lead to an overestimation of positive SPTs in our study. Of note, only 3 different nurses performed the SPTs over these 20 years, ruling out significant investigator bias.
Statistical Analysis
Data were analyzed using the statistical analysis software SAS©, version 9.4 (SAS Institute, Cary, NC, USA), and the R statistical software package (version 3.4.4, https://www.r-project.org/, RRID: SCR_001905). Differences in proportions between patient subgroups were tested for significance using the χ2 test. Each figure or table contains information on the significance levels in the legend: *p < 0.05, **p < 0.01, and ***p < 0.001 were considered significant. Venn diagrams displaying overlapping sensitization patterns were generated according to the SPT data. To create area-proportional Venn diagrams, the software eulerAPE was used [19] (version 3.0.0, http://www.eulerdiagrams.org/eulerAPE).
Results
Sensitization Rates to Different Fungi Moderately Increased from 1998–2007 to 2008–2017
SPT results of 3,358 patients with suspected allergic rhinitis or bronchial asthma were retrospectively investigated for fungal sensitization. To determine trends, the study cohort was divided into 2 decades: 1998–2007 (2,018 patients) and 2008–2017 (1,368 patients). Age and gender distributions remained largely unchanged over time (online suppl. Fig. 2), with roughly a 2:1 female:male ratio in both decades. Over the 2 decades, we found a moderate increase in almost all sensitization rates to fungi (Fig. 1a). Significant increases in sensitization rates were seen for Alternaria, Aspergillus, Aureobasidium, Curvularia, Penicillium, and Rhizopus. In both decades, the highest rates were detected for Penicillium sensitization, while Fusarium sensitization ranked lowest. During 1998–2007, sensitization rates to all fungi ranged from 6.3 (Fusarium) to 12.6% (Penicillium). In the second decade, sensitization ranged from 7.2 (Fusarium) to 28.6% (Penicillium). No substantial change was observed for Mucor over time (7.5 vs. 9.9%). In-depth analysis of 5-year intervals of our study period revealed the most prominent change in sensitization rates of Curvularia and Penicillium, which doubled from the first 5-year interval (1998–2003) to the last (2013–2017) (Fig. 1b; extended data in online suppl. Table 1).
Fig. 1.
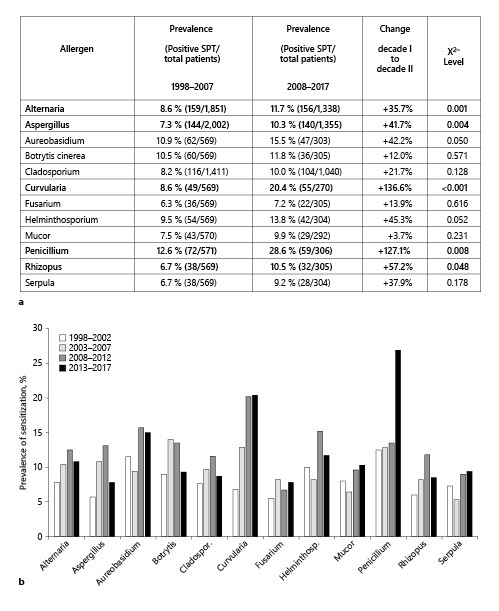
Prevalence of sensitization rates to the investigated fungi, as diagnosed by positive SPTs over 20 years. a Results are shown split into 2 decades, 1998–2007 (n = 2,018 patients) and 2008–2017 (n = 1,368 patients), showing the total number of positive SPTs and the total number of tested patients to the respective allergen. The increase in prevalence from 1998–2007 to 2008–2017 is depicted in the category “change.” b More detailed temporal sensitization trends to fungi among all patients (n = 3,358 patients), depicted here in the 5-year intervals. Raw data can be found in online suppl. Table 1. SPT, skin prick test.
Sensitization Rates to Fungi Are Highest in Middle-Aged Patients
The 2011 German guidelines for skin tests of allergic immediate-type reactions from [15] and the 2013 European baseline prick test series [20] suggest that all adult patients undergo testing with the 3 fungal genera Alternaria, Aspergillus, and Cladosporium. For this reason, further subanalyses were carried out with these 3 genera. Individuals sensitized to Alternaria, Aspergillus, and Cladosporium demonstrated a peak sensitization in 21- to 40-year-olds for all 3 genera in the second decade (Fig. 2). In contrast, sensitization rates to Aspergillus were evenly distributed over all age-groups in the first decade, ranging from 6.6 to 7.6%. In the second decade, the sensitization rates to Aspergillus nearly doubled, ranging from 7.6 to 13.6%, with the only substantial increase being in the age-group of 21–40 years (p < 0.001). Alternaria sensitization was most prevalent in the child/adolescent group during the first decade, decreasing with increasing age. In the second decade, the distribution shifted to a maximum in the 21- to 40-year-olds. Interestingly, the sensitization rate to Cladosporium decreased over time among children and adolescents (11.4% in the first decade vs. 6.3% in the second decade) and increased significantly (p < 0.01) in the age-group of 41–60 years by the second decade.
Fig. 2.
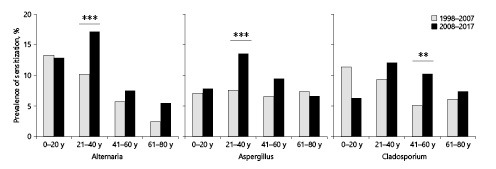
Prevalence of positive SPTs to Alternaria, Aspergillus, and Cladosporium according to age distribution (0–20, 21–40, 41–60, and 61–80 years) during the 2 decades investigated. Total patient numbers for the 2 decades 1998–2007 versus 2008–2017: Alternaria, n = 1,845 patients tested in 1998–2007 and n = 1,335 in 2008–2017; Aspergillus, n = 1,995 and n = 1,352 patients; Cladosporium, n = 1,408 patients and n = 1,037 patients. Significance levels reflect comparisons within each age-group between the first and second decades for each allergen. Significance levels: **p < 0.01, ***p < 0.001. SPT, skin prick test.
A Trend toward Fungal Polysensitization over Time
In further subanalyses, we investigated the prevalence of cosensitization to the most important fungi: Alternaria, Aspergillus, and Cladosporium. These genera belong to different orders of the phylum Ascomycota (online suppl. Fig. 1). While 19.2% of all patients in the first decade had positive test results for at least 1 of these 3 fungi, 22.5% tested positive in the second decade. Our results indicate that the majority of allergic patients were monosensitized to one fungal genus (13.0% in the first decade vs. 13.7% in the second decade) (Fig. 3). No relevant change was observed in the distribution of sensitization to different fungal genera among the monosensitized individuals. Most of these patients were monosensitized to Alternaria at a rate of 5.3% in the first decade. In the second decade, this rate remained almost unchanged (5.7%). In both decades, a minority was sensitized to all 3 fungal genera (1.7% in the first decade vs. 3.7% in the second decade of all patients tested). Of note, the increased sensitization rates were significant for all 3 genera. The data were compiled in Venn diagrams to visualize overlapping sensitizations and their changes (Fig. 4). Cosensitization to Alternaria and Aspergillus was the most common sensitization in the first decade. In the second decade, sensitization to all 3 genera almost doubled (8.8% in the first decade vs. 16.4% in the second decade of all positively tested patients to at least 1 of the 3 fungi). Sensitization to Alternaria and Aspergillus continued to be the most frequent pairing among patients cosensitized to 2 different fungi. Unfortunately, evaluation of cosensitizations between the other fungi was not feasible due to the limited number of positive patients in the subgroups. However, the preliminary analysis did not reveal a pattern that allowed the grouping of positive SPTs according to allergic cross-reactivity.
Fig. 3.
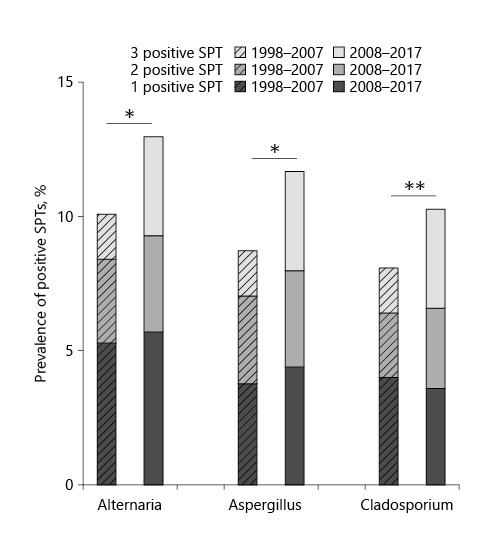
Prevalence of positive SPTs to the 3 fungal genera Alternaria, Aspergillus, and Cladosporium in the patient subgroup who received SPTs to all 3 fungi in the years 1998–2007 (n = 1,251) and 2008–2017 (n = 1,002). In each column, the proportion of patients with a positive SPT to 1, 2, or all 3 fungal genera is marked. SPT, skin prick test.
Fig. 4.
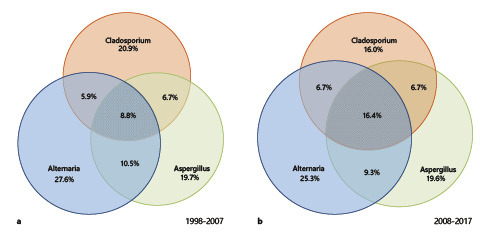
Venn diagrams displaying the overlapping profiles of sensitization to Alternaria, Aspergillus, and Cladosporium in the 2 decades investigated. Data were extracted from subgroup analyses of patients who underwent SPTs to all 3 fungal extracts and showed a positive reaction to at least one of them. a Sensitization profile for the decade 1998–2007 (n = 240 patients). b Sensitization profile for the decade 2008–2017 (n = 226 patients).
Discussion
Three of the 8 phyla from the kingdom Fungi produce relevant allergens: Ascomycota, Basidiomycota, and Zygomycota. We retrospectively analyzed SPT data from 1998 to 2017 for 12 different genera from these 3 phyla. A slight increase in the rate of sensitization for almost all analyzed fungi was detected from 1998–2007 to 2008–2017. The most prominent increases in sensitization were observed for Curvularia and Penicillium. In the first decade, 19.2% of the patients with suspected airway allergies were sensitized to at least one of the fungi Alternaria, Aspergillus, and Cladosporium, compared to 22.5% in the second decade (2008–2017). These slow increases in sensitization rates to different fungi are in agreement with the observed increasing rates of allergic sensitization for various other airborne allergens [5, 21, 22, 23]. We primarily focused on the latter 3 fungal allergens that are recommended for routine testing by the European GA2LEN consortium. However, our data show that for Penicillium and Curvularia, sensitization rates increased significantly, and probably also for Rhizopus. Due to the significantly lower number of tested patients for the rarer allergens, we opted for a cautious interpretation of these results. Furthermore, the production of SPT preparations for most of the rarer fungi has been terminated due to insufficient reimbursement in Germany. Thus, sensitization of especially rarer fungal allergens warrants further investigation. Of note, Curvularia spores are described as relevant allergens, with high prevalence in humid regions such as India [24]. Studies on the cross-reactivity of Curvularia to various other fungi have been published, including Cladosporium, Alternaria, and Aspergillus [25]. However, we currently lack studies that provide evidence for clinical relevance. In contrast, Penicillium belongs to the fungi with proven clinical relevance and should be considered for routine testing [1, 26]. With apparently increasing sensitization rates to Penicillium, a possible Penicillium allergy should also be taken into consideration, if a fungal allergy is suspected [1, 26].
During the first decade, sensitization to Aspergillus, the primary outdoor allergen, was equally distributed among the age-groups (0–20, 21–40, 41–60, and 61–80 years). Detailed analyses of age-dependent sensitization to Alternaria, Aspergillus, and Cladosporium demonstrated a peak in the 21- to 40-year age-group in the second study. This peak in sensitization in early adulthood is consistent with previously published data on weed allergy [22]. Allergen-specific differences in age were observed, and a general trend toward sensitization rates peaking in 20- to 40-year-old patients has been described previously [22, 27]. Among previous studies comparable to ours, a German population-based study randomly collected data and blood samples from 17,641 children aged 3–17 years in 2003–2006 [28], in which sensitization rates to C. herbarum and A. fumigatus were specifically determined by IgE analysis. In these children and adolescents, a steady age-dependent increase was found in fungal sensitization. The lowest sensitization rate was detected in children aged between 3 and 6 years (1.3%), while the highest rate was found in adolescents aged between 14 and 17 years at 4.2% [28]. In another study from the 1990s, the prevalence of inhalant allergen sensitization peaked between ages 16 and 25 years, independent of the type of allergen [29]. Moreover, a Danish study found no significant difference in sensitization to fungi (Cladosporium and/or Alternaria), as measured by specific IgE, according to children's upbringing in a city, town, or rural area, or on a farm. In contrast, highly significant differences were observed in sensitization rates to grass and birch pollen for children who lived in a city of >250,000 inhabitants.
In our cohort, most patients sensitized to Alternaria were monosensitized. This result is in line with previously published data [6, 30]. The serological analysis demonstrated that patients monosensitized to Alternaria predominantly reacted to the major allergen Alt a 1 [6]. Alt a 1 is localized within the cell wall of Alternaria spores, and specific IgE to Alt a 1 was found in 80–90% of the Alternaria-sensitized patients [31, 32]. In our subgroup analysis of the 3 most important fungal genera (Alternaria, Aspergillus, and Cladosporium), the majority of patients were sensitized to 1 fungus only. Similar findings were seen in a Mexican cohort [33]. Regarding these 3 fungi, monosensitization was always the most prevalent finding in our cohorts. We found a positive SPT to all 3 major fungal genera only in a minority of patients (1.7% in the first decade vs. 3.7% in the second decade). It is assumed that fungal sensitization is frequently observed in patients with polysensitization to other inhalant allergens [34].
A limiting factor in fungal allergy diagnostics is the lack of SPT extract standardization [35]. Nonetheless, SPT remains a sensitive method for allergy diagnostics and is a cost-effective and available diagnostic tool. Unfortunately, Germany is experiencing a rapid decline in the availability of commercial test extracts for SPT due to limited reimbursements and regulatory issues. Although our study carries some limitations due to its retrospective setting, the lack of patient serological samples, and the referral situation for patients with suspected airway allergies, our data reveal increasing sensitization rates and broadening sensitization patterns for the most relevant fungal allergens in Germany among symptomatic patients over the last 20 years. In addition, our data quality is very good because test substances were purchased from the same manufacturer over a very long period of time and the test procedure was carried out by the same personnel in a standardized manner. Frequent confounding factors were thus minimized.
From these data, we conclude that no single fungus should be used in limited screening series as a proxy for fungal allergies in general. The pan-European inhalant allergen baseline series proposed by the GA2LEN consortium recommends SPTs with Alternaria, Aspergillus, and Cladosporium [20], as these 3 genera are highly associated with asthma [14]. Additional testing with Penicillium should also be considered [14]. Our data are in line with these recommendations, and we describe a trend toward higher sensitization rates to multiple fungi in our study cohort. In order to correctly assess the clinical relevance of sensitization, a detailed patient history is required, along with challenge tests if necessary. In addition, the diagnosis of fungal airway allergy should be supplemented with serological analysis of recombinant allergens.
Statement of Ethics
This retrospective study has been reviewed and approved by the local ethics committee at the University Medical Center Göttingen.
Conflict of Interest Statement
None of the authors have a conflict of interest in relation to this work. The authors have no ethical conflicts to disclose.
Funding Sources
The authors did not receive any funding.
Author Contributions
S.F., J.G., and T.B. designed the study; S.F., T.F., C.B., S.S.S., and T.B. collected data; S.G. and J.G. extracted and compiled the data; all authors discussed the data; S.F. and T.B. drafted the manuscript; all authors jointly discussed, reviewed, and amended the manuscript. All authors reviewed the final manuscript version and consented to its submission.
Supplementary Material
Supplementary data
References
- 1.Twaroch TE, Curin M, Valenta R, Swoboda I. Mold allergens in respiratory allergy: from structure to therapy. Allergy Asthma Immunol Res. 2015;7((3)):205–20. doi: 10.4168/aair.2015.7.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levetin E, Horner WE, Scott JA, Environmental Allergens Workgroup Taxonomy of allergenic fungi. J Allergy Clin Immunol Pract. 2016;4:375–85.e1. doi: 10.1016/j.jaip.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Soeria-Atmadja D, Onell A, Borgå A. IgE sensitization to fungi mirrors fungal phylogenetic systematics. J Allergy Clin Immunol. 2010;125((6)):1379–86.e1. doi: 10.1016/j.jaci.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Wiesmuller GA, Heinzow B, Aurbach U, Bergmann KC, Bufe A, Buzina W, et al. Abridged version of the AWMF guideline for the medical clinical diagnostics of indoor mould exposure: S2K Guideline of the German Society of Hygiene, Environmental Medicine and Preventive Medicine (GHUP) in collaboration with the German Association of Allergists (AeDA), the German Society of Dermatology (DDG), the German Society for Allergology and Clinical Immunology (DGAKI), the German Society for Occupational and Environmental Medicine (DGAUM), the German Society for Hospital Hygiene (DGKH), the German Society for Pneumology and Respiratory Medicine (DGP), the German Mycological Society (DMykG), the Society for Pediatric Allergology and Environmental Medicine (GPA), the German Federal Association of Pediatric Pneumology (BAPP), and the Austrian Society for Medical Mycology (OGMM) Allergo J Int. 2017;26:168–93. doi: 10.1007/s40629-017-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haftenberger M, Laußmann D, Ellert U, Kalcklösch M, Langen U, Schlaud M, et al. [Prevalence of sensitisation to aeraoallergens and food allergens: results of the German Health Interview and Examination Survey for Adults (DEGS1)] Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2013;56((5–6)):687–97. doi: 10.1007/s00103-012-1658-1. [DOI] [PubMed] [Google Scholar]
- 6.Mari A, Schneider P, Wally V, Breitenbach M, Simon-Nobbe B. Sensitization to fungi: epidemiology, comparative skin tests, and IgE reactivity of fungal extracts. Clin Exp Allergy. 2003;33((10)):1429–38. doi: 10.1046/j.1365-2222.2003.01783.x. [DOI] [PubMed] [Google Scholar]
- 7.Del Giacco SR, Bakirtas A, Bel E, Custovic A, Diamant Z, Hamelmann E, et al. Allergy in severe asthma. Allergy. 2017;72((2)):207–20. doi: 10.1111/all.13072. [DOI] [PubMed] [Google Scholar]
- 8.Barnes C. Fungi and atopy. Clin Rev Allergy Immunol. 2019;57((3)):439–48. doi: 10.1007/s12016-019-08750-z. [DOI] [PubMed] [Google Scholar]
- 9.Pulimood TB, Corden JM, Bryden C, Sharples L, Nasser SM. Epidemic asthma and the role of the fungal mold Alternaria alternata. J Allergy Clin Immunol. 2007;120((3)):610–7. doi: 10.1016/j.jaci.2007.04.045. [DOI] [PubMed] [Google Scholar]
- 10.Tham R, Vicendese D, Dharmage SC, Hyndman RJ, Newbigin E, Lewis E, et al. Associations between outdoor fungal spores and childhood and adolescent asthma hospitalizations. J Allergy Clin Immunol. 2017;139((4)):1140–e4. doi: 10.1016/j.jaci.2016.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Tabar AI, Prieto L, Alba P, Nieto A, Rodríguez M, Torrecillas M, et al. Double-blind, randomized, placebo-controlled trial of allergen-specific immunotherapy with the major allergen Alt a 1. J Allergy Clin Immunol. 2019;144((1)):216–e3. doi: 10.1016/j.jaci.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Kespohl S, Maryska S, Zahradnik E, Sander I, Brüning T, Raulf-Heimsoth M. Biochemical and immunological analysis of mould skin prick test solution: current status of standardization. Clin Exp Allergy. 2013;43((11)):1286–96. doi: 10.1111/cea.12186. [DOI] [PubMed] [Google Scholar]
- 13.Esch RE, Codina R. Fungal raw materials used to produce allergen extracts. Ann Allergy Asthma Immunol. 2017;118((4)):399–405. doi: 10.1016/j.anai.2016.05.012. [DOI] [PubMed] [Google Scholar]
- 14.Kespohl S, Raulf M. Mold sensitization in asthmatic and non-asthmatic subjects diagnosed with extract-based versus component-based allergens. Adv Exp Med Biol. 2019;1153:79–89. doi: 10.1007/5584_2019_342. [DOI] [PubMed] [Google Scholar]
- 15.Rueff F, Bergmann KC, Brockow K, Fuchs T, Grubl A, Jung K, et al. [Skin tests for diagnostics of allergic immediate-type reactions. Guideline of the German Society for Allergology and Clinical Immunology] Pneumologie. 2011;65:484–95. doi: 10.1055/s-0030-1256476. [DOI] [PubMed] [Google Scholar]
- 16.Pepys J. “Atopy”: a study in definition. Allergy. 1994;49:397–9. doi: 10.1111/j.1398-9995.1994.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 17.Proceedings of the task force on guidelines for standardizing old and new technologies used for the diagnosis and treatment of allergic diseases. Washington, DC. 1987 June;:18–19. doi: 10.1016/0091-6749(88)90958-x. [DOI] [PubMed] [Google Scholar]
- 18.Bousquet J, Heinzerling L, Bachert C, Papadopoulos NG, Bousquet PJ, Burney PG, et al. Practical guide to skin prick tests in allergy to aeroallergens. Allergy. 2012;67((1)):18–24. doi: 10.1111/j.1398-9995.2011.02728.x. [DOI] [PubMed] [Google Scholar]
- 19.Micallef L, Rodgers P. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One. 2014;9((7)):e101717. doi: 10.1371/journal.pone.0101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heinzerling L, Mari A, Bergmann KC, Bresciani M, Burbach G, Darsow U, et al. The skin prick test: European standards. Clin Transl Allergy. 2013;3((1)):3. doi: 10.1186/2045-7022-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leth-Moller KB, Skaaby T, Linneberg A. Allergic rhinitis and allergic sensitisation are still increasing among Danish adults. Allergy. 2020;75:660–8. doi: 10.1111/all.14046. [DOI] [PubMed] [Google Scholar]
- 22.Forkel S, Beutner C, Heetfeld A, Fuchs T, Schön MP, Geier J, et al. Allergic rhinitis to weed pollen in Germany: dominance by plantain, rising prevalence, and polysensitization rates over 20 years. Int Arch Allergy Immunol. 2020;181((2)):128–35. doi: 10.1159/000504297. [DOI] [PubMed] [Google Scholar]
- 23.Beutner C, Werchan B, Forkel S, Gupta S, Fuchs T, Schön MP, et al. Sensitization rates to common inhalant allergens increase and change patterns over the last 20 years in Germany J Dtsch Dermatol Ges. Forthcoming. 2020 doi: 10.1111/ddg.14312. [DOI] [PubMed] [Google Scholar]
- 24.Bhattacharya K, Sircar G, Dasgupta A, Gupta Bhattacharya S. Spectrum of allergens and allergen biology in India. Int Arch Allergy Immunol. 2018;177((3)):219–37. doi: 10.1159/000490805. [DOI] [PubMed] [Google Scholar]
- 25.Sharma V, Gupta R, Jhingran A, Singh BP, Sridhara S, Gaur SN, et al. Cloning, recombinant expression and activity studies of a major allergen “enolase” from the fungus Curvularia lunata. J Clin Immunol. 2006;26((4)):360–9. doi: 10.1007/s10875-006-9032-4. [DOI] [PubMed] [Google Scholar]
- 26.Licorish K, Novey HS, Kozak P, Fairshter RD, Wilson AF. Role of Alternaria and Penicillium spores in the pathogenesis of asthma. J Allergy Clin Immunol. 1985;76((6)):819–25. doi: 10.1016/0091-6749(85)90755-9. [DOI] [PubMed] [Google Scholar]
- 27.Arbes SJ, Jr, Gergen PJ, Elliott L, Zeldin DC. Prevalences of positive skin test responses to 10 common allergens in the US population: results from the third National Health and Nutrition Examination Survey. J Allergy Clin Immunol. 2005;116:377–83. doi: 10.1016/j.jaci.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 28.Schmitz R, Ellert U, Kalcklösch M, Dahm S, Thamm M. Patterns of sensitization to inhalant and food allergens: findings from the German Health Interview and Examination Survey for Children and Adolescents. Int Arch Allergy Immunol. 2013;162((3)):263–70. doi: 10.1159/000353344. [DOI] [PubMed] [Google Scholar]
- 29.Boulet LP, Turcotte H, Laprise C, Lavertu C, Bédard PM, Lavoie A, et al. Comparative degree and type of sensitization to common indoor and outdoor allergens in subjects with allergic rhinitis and/or asthma. Clin Exp Allergy. 1997;27((1)):52–9. [PubMed] [Google Scholar]
- 30.Rick EM, Woolnough K, Pashley CH, Wardlaw AJ. Allergic fungal airway disease. J Investig Allergol Clin Immunol. 2016;26((6)):344–54. doi: 10.18176/jiaci.0122. [DOI] [PubMed] [Google Scholar]
- 31.Chruszcz M, Chapman MD, Osinski T, Solberg R, Demas M, Porebski PJ, et al. Alternaria alternata allergen Alt a 1: a unique β-barrel protein dimer found exclusively in fungi. J Allergy Clin Immunol. 2012;130:241–7.e9. doi: 10.1016/j.jaci.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Vouge MW, Thaker AJ, Curran IH, Zhang L, Muradia G, Rode H, et al. Isolation and expression of a cDNA clone encoding an Alternaria alternata Alt a 1 subunit. Int Arch Allergy Immunol. 1996;111((4)):385–95. doi: 10.1159/000237397. [DOI] [PubMed] [Google Scholar]
- 33.Fernandez-Soto R, Navarrete-Rodriguez EM, Del-Rio-Navarro BE, Sienra-Monge JJL, Meneses-Sanchez NA, Saucedo-Ramirez OJ. Fungal allergy: pattern of sensitization over the past 11 years. Allergol Immunopathol. 2018;46:557–64. doi: 10.1016/j.aller.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Reijula K, Leino M, Mussalo-Rauhamaa H, Nikulin M, Alenius H, Mikkola J, et al. IgE-mediated allergy to fungal allergens in Finland with special reference to Alternaria alternata and Cladosporium herbarum. Ann Allergy Asthma Immunol. 2003;91((3)):280–7. doi: 10.1016/S1081-1206(10)63531-4. [DOI] [PubMed] [Google Scholar]
- 35.Kespohl S, Maryska S, Bünger J, Hagemeyer O, Jakob T, Joest M, et al. How to diagnose mould allergy? Comparison of skin prick tests with specific IgE results. Clin Exp Allergy. 2016;46((7)):981–91. doi: 10.1111/cea.12733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


