Abstract
Background and aims
COVID-19 infection in paediatric patients with cancer is severe or critical in 20% of the patients. It can therefore directly affect paediatric patients with cancer and/or their care. We aimed at evaluating the safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine in adolescents and young adults (AYA) with solid tumour.
Methods
This study includes a retrospective analysis of safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine administered to patients, ≥16 years old, under treatment for a solid tumour or within 6 months after treatment from 15th February 2021 to 15th April 2021. Two administrations of the vaccine 3 weeks apart were given. Sera were tested for anti-SARS-Cov-2 immunoglobulin G (IgG) antibodies directed against the S1 domain of the spike protein. In case of positive serology, neutralisation of SARS-Cov-2 was tested.
Results
Twenty-three patients with solid tumours were identified and proposed to get vaccinated. Nine patients refused, and 1 previously developed COVID-19 infection with positive serology. At the time of writing, 13 patients (10 M/2 F; median age: 17) started vaccination. All patients received 2 injections except 2 patients who stopped vaccination because of tumour progression. Ten patients were under treatment (alone or in combination: chemotherapy: 7 patients [pts], immunotherapy: 2 pts, targeted therapy: 3 pts, follow-up: 3 patients). Overall, vaccines were well tolerated. Five patients did not report any side-effects after the first injection and 4 after the second injection. The main local reactivity symptom was mild pain at the site of injection (6 and 2 pts). Fatigue (2 pts and 5 pts) was the most frequent systemic symptom. One patient refused serology testing. All patients but 1 had pre-vaccination negative serology; 7 of 10 patients tested had positive serology before second vaccine injection, and 9 of 10 patients had positive serology one month after the second injection. All patients with seroconversion had positive COVID-19 neutralisation test. No patient developed COVID infections.
Conclusions
We report the good safety profile and good efficacy of the BNT162B2 vaccine in AYA with solid tumours. Larger series and monitoring of the kinetics of anti-Sars-Cov-2 IgG antibodies for several months are mandatory to confirm these preliminary results and to determine long-term vaccination.
Keywords: AYA, Oncology, Covid-19, Vaccine, Paediatric oncology, Serology
1. Introduction
Since its beginning, the COVID-19 pandemic has gradually directly and indirectly affected patients with chronic disease worldwide [1]. Although children are at lower risk of developing severe form of COVID-19 [2], children treated for malignancies may be at higher risk of developing severe forms than their healthy counterparts [[2], [3], [4], [5]]. The global registry of COVID-19 in childhood cancer [6] reports 20% of severe and critical forms among 1500 patients. Direct and indirect consequences of COVID-19 such as delays in diagnosis or treatment can impact outcomes of these patients [7]. For children receiving experimental therapies, data are even rarer, and physicians have been very cautious. The Innovative Therapies for Children with Cancer (ITCC) have thus reported a 60% decrease of enrolment of patients in early phase clinical trials in Europe [8].
Despite initial hope in drug repurposing, medical treatments such as chloroquine have largely failed to help clearing the virus, although the use of heparin, steroids and oxygen has greatly helped to improve outcomes of patients [[9], [10], [11]]. As for today, beyond social distancing, vaccines are the main tools to prevent further spread of the pandemic and protect people from the infection [12].
We report here a retrospective monocentric experience with the RNAm vaccine in adolescents and young adults (AYA) with solid malignancies under treatment or 6 months after completion of treatment treated in our department.
2. Patients and methods
2.1. Population
AYA older than 16 years old, under treatment for their malignancies or who had completed their treatment within 6 months and followed in the department of paediatric immunology, haematology and oncology, of the children hospital of La Timone, AP-HM, were proposed to receive the BNT162b2 mRNA COVID-19 vaccine.
2.2. Treatment
Patients were planned to receive two doses, 21 days apart, of the BNT162b2 vaccine (30 μg per dose). BNT162b2 is a lipid nanoparticle–formulated, nucleoside-modified RNA vaccine that encodes a prefusion-stabilised, membrane-anchored SARS-CoV-2 full-length spike protein [12].
2.3. Methods
Sera samples were tested for anti-SARS-Cov-2 immunoglobulin G (IgG) antibodies directed against the S1 domain of the spike protein of the virus using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Euroimmun®, Luebeck, Germany) as recommended by the manufacturer. All samples with an ELISA ratio ≥0.7, neutralising antibodies against SARS-Cov-2, were detected using a virus neutralisation test (VNT100), as previously described [13]. Serial dilutions of sera 1/10 to 1/160 were tested, and specimens with a VNT100 titre ≥40 were considered positive, those with a titre at 20 indeterminate and <20, negative. Samples were taken before first administration (T0), before second administration (T1) and one month after the second administration of the vaccine (T2).
2.4. Ethic
All data have been generated as part of the routine care at Assistance Publique-Hôpitaux de Marseille, and this study results from routine clinical management. The study was approved by the AP-HM, and access to the patients' biological and registry data issued from the hospital information system was approved by the data protection committee of Assistance Publique-Hôpitaux de Marseille (APHM PADS21-136). All patients signed informed consent.
3. Results
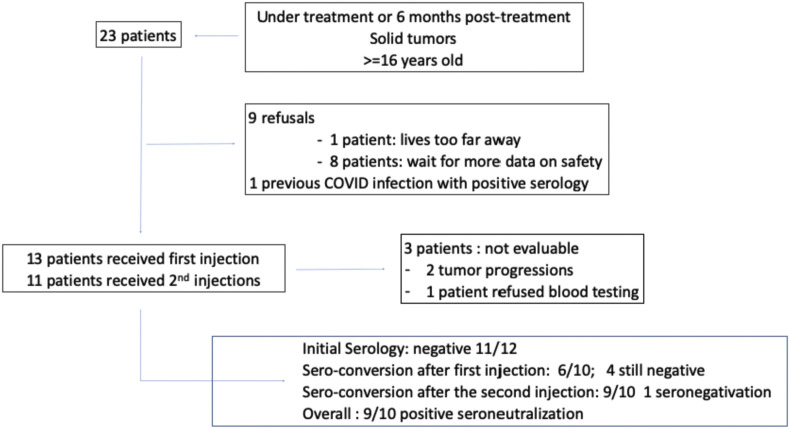
Twenty-three patients were identified and proposed the BNT162b2 mRNA COVID-19 vaccine. Of these 23 patients, 9 refused to receive the vaccine. The reasons for refusal were the fear of developing side-effect, the lack of long-term knowledge about tolerance for 8 patients and living too far from the hospital for 1 patient. One patient did not receive the vaccine as he already had developed COVID-19 [14] and still displayed positive serology and positive virus neutralisation test. Therefore, 13 patients started vaccination (see Fig. 1 ). The characteristics of these 13 patients are given in Table 1 . Patients were receiving chemotherapy (7 patients), targeted therapies (2 patients) or immunotherapies (2 patients) alone or in combination. Three patients were in follow-up.
Fig. 1.
Flowchart of patients.
Table 1.
Patient characteristics.
| Patient | Age (years) (at first injection) | Gender | Diagnosis | Ongoing treatment | No of previous lines of treatment |
|---|---|---|---|---|---|
| 1 | 21 | M | B cell lymphoma | Follow-up | 2 |
| 2 | 16 | M | Low-grade glioma | Trametinib | 0 |
| 3 | 19 | F | Ewing sarcoma | Metronomic cyclophosphamide | 0 |
| 4 | 18 | M | MGT | Follow-up | 2 |
| 5 | 17 | M | Ewing sarcoma | Metronomic navelbine-cyclophosphamide | 1 |
| 6 | 18 | M | ALCL | Vinblastine | 4 |
| 7 | 17 | M | Ewing sarcoma | Metronomic cyclophosphamide | 1 |
| 8 | 16 | F | Rhabdomyosarcoma | Chemotherapy | 0 |
| 9 | 17 | M | Osteosarcoma | Durvalumab-tremelimumab | 2 |
| 10 | 17 | M | Ewing Sarcoma | Nivolumab-lirilumab | 4 |
| 11 | 16 | M | MGT | Follow-up | 0 |
| 12 | 16 | M | Desmoid tumour | Pazopanib-vinblastine | 0 |
| 13 | 16 | M | Rhabdoid tumour | Metronomic chemotherapy + bevacizumab | 0 |
ALCL: anaplastic large-cell lymphoma, MGT: malignant germinal tumours.
Two patients did not receive the second injection because of tumour progression and also lived far away from our institution. They received palliative care only.
Overall, vaccines were well tolerated. Five of 13 patients did not report any side-effects after the first injection and 4 of 11 after the second injection. The most frequent side-effect was a mild-to-moderate pain at the injection site (6 patients) that resolved within 1–2 days. The second administration seemed to be slightly less well tolerated with more frequent systemic reactions. Details of side-effects are reported in Table 2 . Systemic events including fever and chills were observed within the first 1–2 days after vaccination and resolved shortly thereafter. Aside from transient local and systemic reactions, no safety concerns were identified (see Table 3).
Table 2.
Immunisation of patients.
| Patient | T0 | T1 | T2 | Seroneutralisation |
|---|---|---|---|---|
| 1 | Neg | Neg | Pos | Pos |
| 2 | Neg | Pos | Pos | Pos |
| 3 | Neg | Pos | Pos | Pos |
| 4 | Neg | Pos | Pos | Pos |
| 5 | Neg | Neg | Pos | ND |
| 6 | Neg | Neg | Pos | Pos |
| 7 | ND | Pos | Pos | Pos |
| 8 | Pos | Neg | Pos | Pos |
| 9 | Neg | ND | ND | ND |
| 10 | Neg | ND | ND | ND |
| 11 | ND | ND | ND | ND |
| 12 | Neg | Pos | Pos | Pos |
| 13 | Neg | Pos | Neg | Pos |
ND: not done.
One patient refused serology to be performed.
One patient stopped the vaccination program after tumour progression.
Table 3.
Toxicity of vaccines.
| Toxicity | First administration |
Second Administration |
|---|---|---|
| Number of patients (13 patients) | Number of patients (11 patients) | |
| No toxicity | 5 | 4 |
| Systemic reactivity | 3 | 5 |
|
2 | 4 |
|
2 | 5 |
|
0 | 1 |
|
1 | 0 |
| Local reactivity | 6 | 2 |
|
6 | 2 |
All patients but one were negative for COVID-19 serology before immunisation. This patient did not previously develop COVID-19 symptoms, and the second serology was negative. Overall, 3 weeks after the first injection, 8 of 10 tested patients developed seroconversion, and one month after the second administration, the vaccine led to a seroconversion in 9 of 10 tested patients with positive COVID neutralisation test in all. The only patient with negative serology 1 month after the second serology had a positive serology at the time of the second injection and positive seroneutralisation. No patients developed COVID after immunisation.
4. Discussion
We report here our experience with the immunisation with a COVID-19 mRNA vaccine of AYA under treatment or shortly after completion of treatment for a solid tumour. Our results show a high rate of refusal, a good tolerance and a high immunogenicity in a real-life setting of immunocompromised paediatric patients. To the best of our knowledge, our results are the first reported in AYA with cancer; it is therefore impossible to compare it with other experiences. Adverse events are consistent with those previously reported in healthy AYA [12].
Preliminary studies reported that healthy children and adolescents under the age of 15 vaccinated against sars-Cov-2 have a very high rate of protection compared with a healthy adult. In patients with cancer, only adult data are reported. Monin et al. [15] recently reported that in 56 adult patients with solid tumours, one dose of the BNT162b2 vaccine yielded only poor efficacy at day 21 with 38% of the patients with positive anti-S IgG titres. However, in our study, almost all children with solid tumours seroconverted after the second dose of the vaccine.
The series we report here has some limitations. We studied a small number of patients in a single centre. Patients were limited to AYA with solid tumour and therefore do not reflect most of the paediatric oncology patients. In addition, the study was conducted over a short period up to one month after the second dose of the vaccine; it would be necessary to continue monitoring the kinetics of anti–sars-Cov-2 IgG antibodies for several months to assess vaccine protection over time.
If our preliminary results are confirmed, such a vaccine could be proposed to AYA before the initiation of their treatment and during treatment to prevent severe forms of COVID-19 or additional complications related to the disease that may compromise their oncologic treatment and in turn their long-term outcomes.
Close follow-up remains mandatory to detect rare or unknown side-effects of the vaccine in this very specific population or interactions with oncologic treatment or procedures such as radiation recall [16] or hypermetabolic lymphadenopathy by [(18)F]fluorodeoxyglucose positron-emission tomography–computed tomography [17].
Importantly, although both the American Society of Clinical Oncology and the European Society of Medical Oncology have advocated for patients with cancer a high priority status to get access to COVID-19 vaccines [18], we have observed a high rate of refusal, much higher than recently reported by Di Noia et al. [19] in a large Italian adult series (904 patients), in which an 11% refusal rate was observed. Elsewhere, Barriere et al. [20] performed a survey among adult patients with cancer in France. Among the respondents, 536 (54%) reported their intent to be vaccinated, whereas 297 (30%) considered they were not ready yet but could change their mind, and 166 (17%) patients reported to definitely refuse vaccination.
It is therefore crucial that adequate information and education is brought to paediatric patients with cancer and their family to increase enrolment in vaccination programs and in turn the success of the campaign. Education strategies must be developed with respect to AYA-specific needs and habits, for example trough social media [21] or webinars [22] to make it happen as urged by Curigliano G and Eggermont [23].
5. Conclusion
We report here a preliminary experience with RNA vaccines in AYA with solid tumours and report a good safety profile and excellent immunogenicity. Larger series and monitoring of the kinetics of anti-Sars-Cov-2 IgG antibodies for several months are mandatory to confirm these preliminary results and to determine long-term vaccination effect.
Authors' contributions
Gabriel Revon-Riviere, Formal analysis, Investigation, Validation, Writing – original draft, Writing – review & editing; Laetitia Ninove, Methodology, Project administration, Writing – review & editing, Investigation, Validation; Victoria Min, Investigation, Validation, Writing – review & editing; Angélique Rome, Investigation, Validation, Writing – review & editing; Carole Coze, Investigation, Validation, Writing – review & editing; Arnauld Verschuur, Investigation, Validation, Writing – review & editing; Xavier de Lamballerie, Resources, Supervision, Validation, Writing – review & editing, Investigation; Nicolas André, Conceptualisation, Investigation, Writing – review & editing, Formal analysis, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
References
- 1.Zhu N., Zhang D., Wang W., et al. China novel coronavirus investigating and research team . A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.André N., Rouger-Gaudichon J., Brethon B., et al. COVID-19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Canc. 2020;67 doi: 10.1002/pbc.28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulad F., Kamboj M., Bouvier N., et al. COVID-19 in children with cancer in New York City. JAMA Oncol. 2020;6:1459–1460. doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Rojas T., Pérez-Martínez A., Cela E., et al. COVID-19 infection in children and adolescents with cancer in Madrid. Pediatr Blood Canc. 2020;67 doi: 10.1002/pbc.28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hrusak O., Kalina T., Wolf J., et al. Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment. Eur J Canc. 2020;132:11–16. doi: 10.1016/j.ejca.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global registry of COVID-19 in childhood cancer available at: https://app.powerbi.com/view?r=eyJrIjoiNGQ3NDAwZDItYjRjNi00MjNhLWE2NTMtNmFjNmU1YTgzZDMwIiwidCI6IjIyMzQwZmE4LTkyMjYtNDg3MS1iNjc3LWQzYjNlMzc3YWY3MiIsImMiOjN9.
- 7.Ding Y.Y., Ramakrishna S., Long A.H., et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr Blood Canc. 2020;67 doi: 10.1002/pbc.28427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubio-San-Simón A., André N., Giuseppina Cefalo M., et al. Impact of COVID-19 in paediatric early-phase cancer clinical trials in Europe: a report from the Innovative Therapies for Children with Cancer (ITCC) consortium. Eur J Canc. 2020;141:82–91. doi: 10.1016/j.ejca.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajjar L.A., Costa I.B.S.D.S., Rizk S.I., et al. Intensive care management of patients with COVID-19: a practical approach. Ann Intensive Care. 2021;11:36. doi: 10.1186/s13613-021-00820-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group, et al. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hippensteel J.A., LaRiviere W.B., Colbert J.F., et al. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol. 2020;319:L211–L217. doi: 10.1152/ajplung.00199.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack F.P., Thomas S.J., Kitchin N., et al. C4591001 Clinical Trial Group Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallian P., Pastorino B., Morel P., et al. Lower prevalence of antibodies neutralizing SARS-CoV-2 in group O French blood donors. Antivir Res. 2020;181:104880. doi: 10.1016/j.antiviral.2020.104880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Revon-Riviere G., Soler C., Andrianarivony T., et al. Favorable outcome of COVID-19 infection in a pediatric cancer patient receiving an anti-PD-L1/anti-CTLA-4 combination. J Pediatr Hematol Oncol. 2021 Mar 31 doi: 10.1097/MPH.0000000000002099. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Monin L., Laing A.G., Muñoz-Ruiz M., et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study. Lancet Oncol. 2021;S1470-2045(21):213–218. doi: 10.1016/S1470-2045(21)00213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soyfer V., Gutfeld O., Shamai S., Schlocker A. Merimsky O OVID-19 vaccine-induced radiation recall phenomenon. Int J Radiat Oncol Biol Phys. 2021;S0360-3016(21):233–239. doi: 10.1016/j.ijrobp.2021.02.048. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen D., Krauthammer S.H., Wolf I., Even-Sapir E. Hypermetabolic lymphadenopathy following administration of BNT162b2 mRNA Covid-19 vaccine: incidence assessed by [(18)F]FDG PET-CT and relevance to study interpretation. Eur J Nucl Med Mol Imag. 2021;27:1–10. doi: 10.1007/s00259-021-05314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corti C., Crimini E., Tarantino P., et al. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Canc. 2021;148:316–327. doi: 10.1016/j.ejca.2021.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Noia V., Renna D., Barberi V., et al. The first report on Covid-19 vaccine refusal by cancer patients in Italy: early data from a single-institute survey. Eur J Canc. 2021 May 26 doi: 10.1016/j.ejca.2021.05.006. S0959-8049(21)00310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barrière J., Gal J., Hoch B., et al. Acceptance of SARS-CoV-2 vaccination among French patients with cancer: a cross-sectional survey. Ann Oncol. 2021;32 doi: 10.1016/j.annonc.2021.01.066. 773-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelkar A.H., Blake J.A., Cherabuddi K., Cornett H., McKee B.L. Vaccine enthusiasm and hesitancy in cancer patients and the impact of a webinar. Healthcare (Basel) 2021;9:351. doi: 10.3390/healthcare9030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raza F., Lantos J.D. Promoting COVID-19 vaccination on social media. Pediatrics. 2021 Apr 14 doi: 10.1542/peds.2021-050049. [DOI] [PubMed] [Google Scholar]
- 23.Curigliano G., Eggermont A.M.M. Adherence to COVID-19 vaccines in cancer patients: promote it and make it happen! Eur J Canc. 2021 May 24 doi: 10.1016/j.ejca.2021.05.007. S0959-8049(21)00311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]