Abstract
Background
Evidence is urgently needed to support treatment decisions for children with multisystem inflammatory syndrome (MIS-C) associated with severe acute respiratory syndrome coronavirus 2.
Methods
We performed an international observational cohort study of clinical and outcome data regarding suspected MIS-C that had been uploaded by physicians onto a Web-based database. We used inverse-probability weighting and generalized linear models to evaluate intravenous immune globulin (IVIG) as a reference, as compared with IVIG plus glucocorticoids and glucocorticoids alone. There were two primary outcomes: the first was a composite of inotropic support or mechanical ventilation by day 2 or later or death; the second was a reduction in disease severity on an ordinal scale by day 2. Secondary outcomes included treatment escalation and the time until a reduction in organ failure and inflammation.
Results
Data were available regarding the course of treatment for 614 children from 32 countries from June 2020 through February 2021; 490 met the World Health Organization criteria for MIS-C. Of the 614 children with suspected MIS-C, 246 received primary treatment with IVIG alone, 208 with IVIG plus glucocorticoids, and 99 with glucocorticoids alone; 22 children received other treatment combinations, including biologic agents, and 39 received no immunomodulatory therapy. Receipt of inotropic or ventilatory support or death occurred in 56 patients who received IVIG plus glucocorticoids (adjusted odds ratio for the comparison with IVIG alone, 0.77; 95% confidence interval [CI], 0.33 to 1.82) and in 17 patients who received glucocorticoids alone (adjusted odds ratio, 0.54; 95% CI, 0.22 to 1.33). The adjusted odds ratios for a reduction in disease severity were similar in the two groups, as compared with IVIG alone (0.90 for IVIG plus glucocorticoids and 0.93 for glucocorticoids alone). The time until a reduction in disease severity was similar in the three groups.
Conclusions
We found no evidence that recovery from MIS-C differed after primary treatment with IVIG alone, IVIG plus glucocorticoids, or glucocorticoids alone, although significant differences may emerge as more data accrue. (Funded by the European Union’s Horizon 2020 Program and others; BATS ISRCTN number, ISRCTN69546370.)
In April 2020, practitioners first noted a temporal association between infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)1-4 and multisystem inflammatory syndrome in children (MIS-C) as a rare but serious complication.5-8 Since that time, MIS-C has been reported to occur 2 to 6 weeks after SARS-CoV-2 infection and is characterized by persistent fever and nonspecific symptoms that include abdominal pain, vomiting, headache, and fatigue. Conjunctival injection (hyperemia) and rash resembling Kawasaki’s disease have occurred in a high percentage of children with this condition.4,6,9-11 Severely affected children have been reported with multiorgan failure and shock that requires inotropic support. Laboratory studies have shown intense inflammation with elevated levels of C-reactive protein, ferritin, troponin, and N-terminal pro–B-type natriuretic peptide and reduced levels of hemoglobin, platelets, and lymphocytes.
Faced with a new disease with no proven therapy, pediatricians have treated patients using their “best guess” as to what might be beneficial. On the basis of the resemblance of MIS-C to Kawasaki’s disease,2,4,9,11 macrophage activation syndrome,12 and staphylococcal toxic shock syndrome,13,14 practitioners have preferentially chosen immunomodulatory agents that have shown benefit in these diseases, with such drugs often used in combination or sequentially when initial treatments have failed.15-17
Since coronary-artery aneurysm is an important overlapping feature of both MIS-C and Kawasaki’s disease, intravenous immune globulin (IVIG), the proven treatment for Kawasaki’s disease,18 has been widely adopted as an initial therapy, and withholding IVIG is considered unacceptable by some clinicians. However, some children with MIS-C recover with supportive treatment alone,3,15 so aggressive attempts to suppress the inflammatory response may not necessarily translate into clinical benefit.
Randomized trials are needed to establish the most effective treatment of MIS-C. However, given the rapid emergence of MIS-C during the coronavirus disease 2019 (Covid-19) pandemic — and concern that it may lead to death, multiorgan failure, or long-term cardiac damage — practitioners have adopted the use of a range of unproven immunomodulators, which are now being recommended in local and national treatment guidelines.19,20 We performed the Best Available Treatment Study (BATS) to provide evidence for recommendations regarding the treatment of MIS-C by collecting and analyzing outcomes of the treatments chosen by individual pediatricians responsible for patient care.
Methods
Study Design
We invited pediatricians worldwide to join our study by uploading data from their patients with suspected MIS-C onto a Web-based Research Electronic Data Capture database.21 Since the accuracy of current MIS-C definitions is unknown and emerging experience suggests a wide spectrum of inflammatory illnesses after SARS-CoV-2 infection,22,23 our study invited pediatricians to enroll not only children who met the published criteria for MIS-C24-26 but also those with any suspected inflammatory illness after SARS-CoV-2 infection. We collected deidentified longitudinal data regarding presenting features, demographic characteristics, laboratory findings, immunomodulatory treatments (IVIG, glucocorticoids, or biologic agents), and supportive therapies. Treatments and daily data were collected according to the calendar day. We also recorded the duration of hospital admission, organ support required, and health status at the time of discharge. Details regarding the study methods are provided in the Supplementary Appendix, available with the full text of this article at NEJM.org.
Comparison of Treatments
Three primary treatment groups were large enough to be considered for comparison: IVIG alone, IVIG plus glucocorticoids, and glucocorticoids alone. We chose IVIG alone as the reference treatment since it is the accepted treatment for Kawasaki’s disease and has been widely adopted in the treatment of MIS-C. Thus, we compared IVIG plus glucocorticoids and glucocorticoids alone with IVIG.
The first calendar day of each of the three treatments was defined as day 0, and the subsequent treatment and outcomes were defined relative to this initiation. Additional immunomodulators that were administered on the same calendar day as the primary treatment were defined as coprimary treatments, whereas immunomodulators that were administered on subsequent days were considered to be secondary treatments.
Primary and Secondary Outcomes
There were two primary outcomes. The first was a composite of inotropic support or mechanical ventilation (invasive or noninvasive) by day 2 or later or death. The second was a reduction in disease severity on a seven-point ordinal scale between day 0 and day 2; the levels of disease severity from worst to least were as follows: receipt of mechanical ventilation and inotropic support, receipt of mechanical ventilation alone, receipt of inotropic support alone, receipt of oxygen alone, no supportive therapy with a C-reactive protein level of 50 mg per liter or more, no supportive therapy with a C-reactive protein level of less than 50 mg per liter, and hospital discharge.
Key secondary outcomes were temporal dynamics of blood markers of inflammation and organ damage; an escalation in the administration of immunomodulators, which was defined as the addition of a separate immunomodulator or (if the initial treatment included glucocorticoids) an incremental increase of 5 mg per kilogram of body weight or its equivalent in the daily dose of prednisolone; the time until a reduction of 1 point in disease severity on the ordinal scale; left ventricular dysfunction on echocardiography; coronary-artery aneurysm after treatment (coronary-artery z score of ≥2.5 or documented aneurysm)27; any increase in cardiorespiratory supportive therapy after day 0; and other therapeutic complications or death.
For the primary outcomes, we performed a subgroup analysis that included only the patients who met the World Health Organization (WHO) criteria for MIS-C26 and a sensitivity analysis in which we defined the primary treatments as those received during the first two calendar days of immunomodulatory treatment.
Oversight
The study was designed by the study team at Imperial College London. Data regarding individual patients were collected by local investigators. The statistical analysis plan was developed by the statistical group, who also performed the analyses. An international advisory board oversaw the progress of the study and the publication strategy. (Details regarding members of the study team, the investigator consortium, and international advisory board are provided in the Supplementary Appendix.)
The study was approved by the United Kingdom research ethics committee. Participating centers obtained ethical approval according to the requirements in each country. The initial draft of the manuscript was written by the last author and was developed by all the authors, who constitute the writing group. The last author and members of the data-management and analysis groups had access to all the data and vouch for the completeness and accuracy of the data and for the fidelity of the study to the protocol and the statistical analysis plan.
Statistical Analysis
We used weighted logistic-regression methods to analyze dichotomous outcomes and performed time-to-event analyses using weighted Cox regression methods, with weights determined by inverse probability according to covariate-balancing propensity scores to account for baseline differences among the three major treatment groups (IVIG alone, IVIG plus glucocorticoids, and glucocorticoids alone). These analyses included the same covariates as were used for the propensity scores to produce doubly robust estimates. The average treatment effect was estimated except as described otherwise. We reported outcomes as odds ratios or average hazard ratios with 95% confidence intervals, using IVIG alone as the reference category. (Details are provided in the Methods section in the Supplementary Appendix and in the statistical analysis plan in the protocol.)
Inflammatory markers were plotted as percentages of each patient’s peak value. Weighted generalized additive models were fitted to produce smoothed curves with confidence intervals. Weights were produced as outlined above; however, in comparing the patients who were treated with immunomodulatory drugs with those who did not receive such treatment, we estimated the average treatment effect in the untreated group to preserve the sample size of this smaller and more distinct subgroup.
Results
Patients
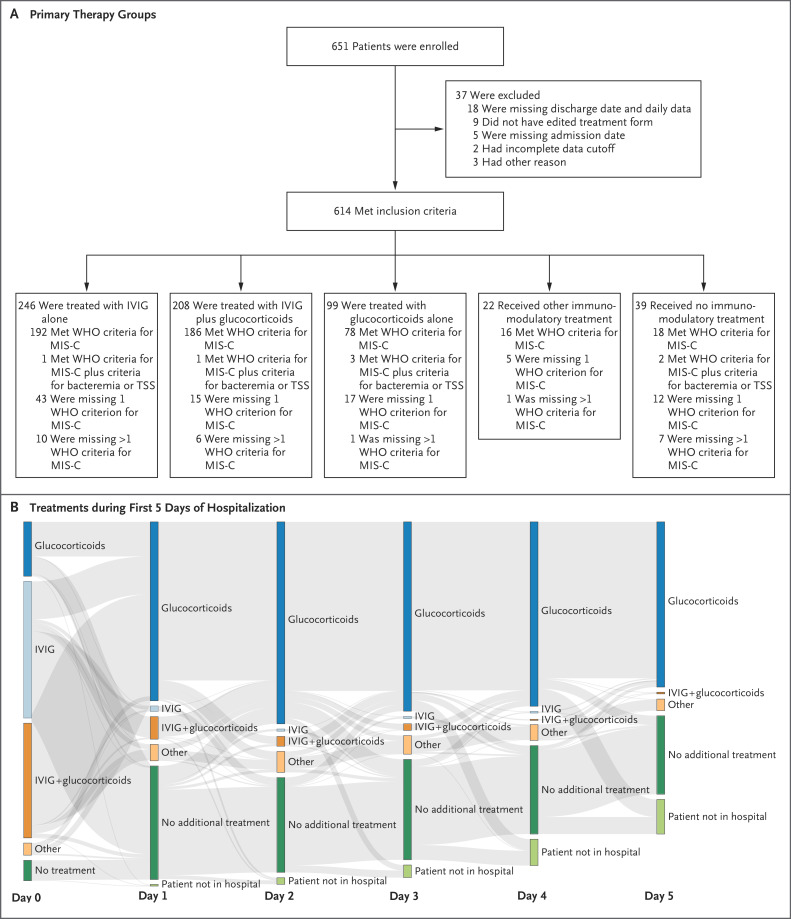
From June 20, 2020, to February 24, 2021, practitioners at 81 hospitals in 34 countries uploaded data for 651 patients with suspected MIS-C to the study database (Figs. S1, S2, and S3 in the Supplementary Appendix). Data for 37 patients were excluded owing to incomplete information or duplicate entries. Of the remaining 614 patients, 246 received primary treatment with IVIG alone, 208 with IVIG plus glucocorticoids, and 99 with glucocorticoids alone; another 22 patients received other immunomodulators, and 39 received no immunomodulatory therapy (Figure 1A). In the three primary treatment groups, 136 of 552 patients (25%) had received additional immunomodulators by day 2, and 238 patients (43%) received secondary agents at any time. The complex changes in treatments are shown in Figure 1B.
Figure 1. Study Enrollment and Treatments after Hospital Admission.
Panel A shows an overview of the total number of children with suspected multisystem inflammatory syndrome (MIS-C) associated with severe acute respiratory syndrome coronavirus 2 who were enrolled in the study, according to treatment received. Patients who met the inclusion criteria were categorized according to enrollment in the three main treatment groups — intravenous immune globulin (IVIG) alone, IVIG plus glucocorticoids, and glucocorticoids alone — along with other immunomodulatory treatments (including anti–tumor necrosis factor, anti–interleukin-1, and anti–interleukin-6). Patients were further categorized according to whether they met the clinical criteria of the World Health Organization (WHO) for MIS-C. TSS denotes toxic shock syndrome.
Panel B shows a Sankey diagram of treatments received by patients after hospital admission. Each vertical stack represents day 0 to 5 in the patient’s hospital admission. The arrows (gray bands) represent the movement of patients between treatment groups on subsequent days; the width of the arrows is proportional to the flow rate between days. Patients in the group that received glucocorticoids alone could have received either intravenous or oral formulations, and the continuation of glucocorticoid treatments on subsequent days at the same or lower dose did not constitute additional treatment. Other treatments — which included one or more other immunomodulatory therapies given alone or in combination with glucocorticoids, IVIG, or both — were anti–tumor necrosis factor, anti–interleukin-1, anti–interleukin-6, extracorporeal cytokine adsorber (CytoSorb), granulocyte colony-stimulating factor, colchicine, mesenchymal stem cells, and convalescent plasma.
Clinical and Laboratory Measures
Clinical and laboratory findings were similar among the treatment groups (Table 1 and Table S2). However, troponin levels and the percentage of patients who received inotropes on day 0 were higher in the group that received IVIG plus glucocorticoids (Figs. S4, S5, and S6). Of the 614 patients, 490 (80%) met the WHO criteria for MIS-C (Table S3). The most common criterion that was missing among the patients who did not meet the WHO criteria was evidence of SARS-CoV-2 exposure (Fig. S7). SARS-CoV-2 antibody measurements were not tested in 14% of the patients, and results were negative in 14%. Bacteria were cultured in blood samples obtained from 6 patients. The percentage of patients who met the American Heart Association (AHA) definition for Kawasaki’s disease18 was 37% in the overall population and 39% among those who met the WHO criteria for MIS-C (Table S4 and Fig. S8).
Table 1. Demographic and Clinical Characteristics of the Patients on Admission.*.
| Characteristic | IVIG Alone (N=246) |
IVIG plus Glucocorticoids (N=208) |
Glucocorticoids Alone (N=99) |
Other Immunomodulator (N=22) |
No Immunomodulator (N=39) |
|---|---|---|---|---|---|
| Median age (IQR) — yr | 7.0 (3.7–11.0) | 8.8 (4.6–12.0) | 8.8 (5.0–12.0) | 13 (9.5–15.0) | 9.6 (4.4–13.0) |
| Male sex — no. (%) | 157 (64) | 127 (61) | 59 (60) | 15 (68) | 18 (46) |
| Age-adjusted z score of ≥2 for weight — no. (%) | 28 (11) | 45 (22) | 10 (10) | 4 (18) | 3 (8) |
| Coexisting illness — no. (%) | 5 (2) | 5 (2) | 7 (7) | 1 (5) | 3 (8) |
| SARS-CoV-2 positive on PCR — no./total no. (%)† | 36/244 (15) | 53/204 (26) | 26/98 (27) | 8/22 (36) | 10/38 (26) |
| SARS-CoV-2 antibody positive — no./total no. (%) | 163/241 (68) | 163/203 (80) | 68/97 (70) | 16/22 (73) | 14/39 (36) |
| Organ support — no. (%)‡ | 38 (15) | 66 (32) | 20 (20) | 8 (36) | 6 (15) |
| Clinical features on admission — no./total no. (%) | |||||
| Fever | 237/246 (96) | 196/208 (94) | 92/99 (93) | 20/22 (91) | 35/39 (90) |
| Sore throat | 62/206 (30) | 50/191 (26) | 23/90 (26) | 3/17 (18) | 11/31 (35) |
| Cough | 49/229 (21) | 38/194 (20) | 24/95 (25) | 7/20 (35) | 6/31 (19) |
| Respiratory distress | 29/226 (13) | 36/198 (18) | 12/96 (12) | 6/21 (29) | 5/35 (14) |
| Abdominal pain | 142/223 (64) | 138/200 (69) | 48/93 (52) | 16/22 (73) | 21/35 (60) |
| Diarrhea | 100/231 (43) | 120/204 (59) | 36/94 (38) | 7/22 (32) | 18/35 (51) |
| Vomiting | 118/227 (52) | 135/203 (67) | 43/94 (46) | 13/20 (65) | 15/34 (44) |
| Headache | 66/198 (33) | 61/184 (33) | 22/86 (26) | 7/20 (35) | 8/31 (26) |
| Irritability | 39/215 (18) | 47/197 (24) | 22/92 (24) | 1/18 (6) | 7/32 (22) |
| Lethargy | 89/218 (41) | 64/198 (32) | 46/96 (48) | 11/20 (55) | 12/32 (38) |
| Met criteria for Kawasaki’s disease — no. (%) | 106 (43) | 82 (39) | 30 (30) | 5 (23) | 2 (5) |
| Laboratory values | |||||
| Median lymphocytes (IQR) — cells/mm3 | 1400 (800–2200) | 1100 (700–1700) | 1100 (760–1700) | 810 (480–1500) | 1100 (780–2300) |
| Median troponin (IQR) — ng/liter | 18 (8–55) | 50 (30–260) | 50 (16–150) | 200 (13–2900) | 11 (7–120) |
| Median C-reactive protein (IQR) — mg/liter | 150 (82–210) | 150 (90–250) | 130 (50–250) | 160 (120–260) | 160 (67–200) |
| Median ferritin (IQR) — μg/liter | 410 (200–620) | 560 (300–920) | 530 (230–1100) | 640 (310–1300) | 230 (140–330) |
| Median albumin (IQR) — g/liter | 34 (29–40) | 32 (28–38) | 31 (27–34) | 32 (29–37) | 34 (30–39) |
Other immunomodulatory therapies include biologic agents. A complete list of patients’ data is provided in Table S2 in the Supplementary Appendix. IQR denotes interquartile range, and IVIG intravenous immune globulin.
Data regarding the results on polymerase-chain-reaction (PCR) assay for severe acute respiratory syndrome coronavirus 2 were obtained during hospital admission.
Organ support refers to receipt of mechanical ventilation, inotropic support, or extracorporeal membrane oxygenation on admission.
Primary Outcomes
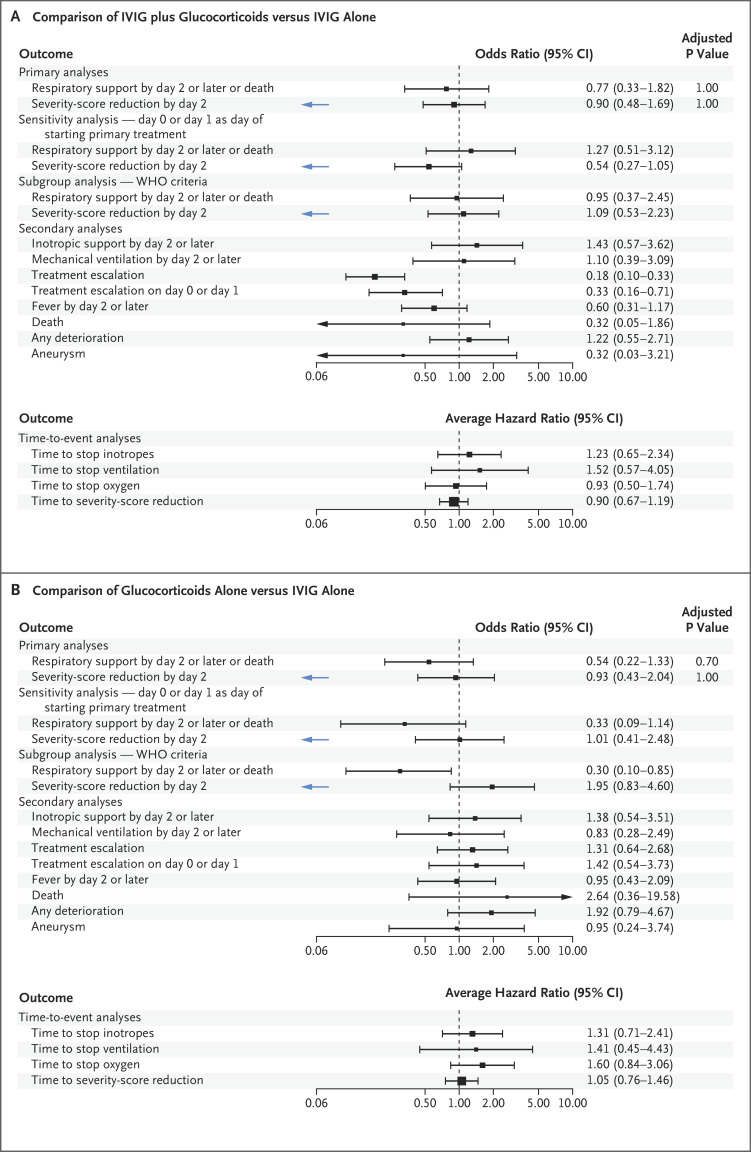
A total of 50 of 553 patients (9%) received immunomodulators before transfer to the reporting hospital and were excluded from the weighted analyses. The receipt of inotropic support or mechanical ventilation on day 2 or later or death (the first primary outcome) occurred in 56 patients who received initial treatment with IVIG plus glucocorticoids (adjusted odds ratio for the comparison with IVIG alone, 0.77; 95% confidence interval [CI], 0.33 to 1.82) and in 17 patients who received glucocorticoids alone (adjusted odds ratio, 0.54; 95% CI, 0.22 to 1.33) (Figure 2). Unadjusted values are shown in Table S5.
Figure 2. Forest Plots for Primary, Secondary, and Subgroup Analyses.
Shown are outcomes for patients with suspected MIS-C who received IVIG plus glucocorticoids (Panel A) or glucocorticoids alone (Panel B) as compared with those who received IVIG alone (reference group, indicated by an odds ratio or average hazard ratio of 1.00). Odds ratios are shown for all comparisons except time-to-event analyses, for which average hazard ratios were calculated. Values to the right of the dashed vertical line indicate the superiority of IVIG alone, except for the second primary outcome (a reduction in disease severity on the ordinal scale by day 2, indicated by blue arrows), for which values to the left indicate the superiority of IVIG alone.
In a subgroup analysis that included only the patients who met the WHO criteria for MIS-C, a first-primary-outcome event occurred in 40 patients who received initial treatment with IVIG plus glucocorticoids (adjusted odds ratio for the comparison with IVIG alone, 0.95; 95% CI, 0.37 to 2.45) and in 12 patients who received initial treatment with glucocorticoids alone (adjusted odds ratio, 0.30; 95% CI, 0.10 to 0.85). The results for the individual components of the composite outcome are shown in Figure 2 and Table S5.
A reduction in the score for disease severity on the ordinal scale by day 2 (the second primary outcome) occurred in 54 patients who received IVIG plus glucocorticoids (adjusted odds ratio for the comparison with IVIG alone, 0.90; 95% CI, 0.48 to 1.69) and in 20 patients who received glucocorticoids alone (adjusted odds ratio, 0.93; 95% CI, 0.43 to 2.04). When WHO criteria for MIS-C were considered in a subgroup analysis, a second-primary-outcome event occurred in 52 patients who received IVIG plus glucocorticoids (adjusted odds ratio for the comparison with IVIG alone, 1.09; 95% CI, 0.53 to 2.23) and in 16 patients who received glucocorticoids alone (adjusted odds ratio, 1.95; 95% CI, 0.83 to 4.60).
The results for the two primary outcomes showed an acceptable degree of balance with respect to the covariates (Fig. S9). Analyses that were performed with the use of standardized weights did not change the interpretation of the primary outcomes.
Secondary Outcomes
Escalation of immunomodulatory treatment was less common among the patients who received IVIG plus glucocorticoids than among those who received IVIG alone (odds ratio, 0.18; 95% CI, 0.10 to 0.33). The comparison was inconclusive between the patients who received glucocorticoids alone and those who received IVIG alone (odds ratio, 1.31; 95% CI, 0.64 to 2.68) (Table S5; Table S1 shows additional details regarding treatment escalation according to group). No clear between-group differences were seen in blood markers, inotropic support, or mechanical ventilation between patients who had an escalation to other treatments by day 2 and those who continued to receive the initial treatment (Figs. S5 and S6B).
Left ventricular dysfunction was reported in 12% of the 538 patients who had undergone echocardiography starting on day 2, with no substantial differences among the treatment groups. Coronary-artery aneurysm was present on the latest echocardiogram at 2 days after the initiation of treatment or later in 6% of the 326 patients for whom data were available. The low numbers of coronary-artery aneurysms that were detected preclude statistical comparisons among the treatment groups, although among the patients with data, the incidence of coronary-artery aneurysm was not greater among those who did not receive any IVIG as part of primary treatment than among those who did receive IVIG (Table S6). Death was reported in 3 of 238 patients (1%) who received IVIG alone, in 5 of 192 patients (3%) who received IVIG plus glucocorticoids, and in 4 of 91 patients (4%) who received glucocorticoids alone; status with respect to death was not reported for 32 patients (Table S5).
In the analysis of the time until an improvement in disease severity on the ordinal scale, the average hazard ratio for the comparison with IVIG alone was 0.89 (95% CI, 0.67 to 1.19) for IVIG plus glucocorticoids and 1.03 (95% CI, 0.72 to 1.46) for glucocorticoids alone (Fig. S9 and Table S5C).
Drug complications were reported by clinicians in 16 of 411 patients (4%) who received glucocorticoids in any combination and in 9 of 408 (2%) who received IVIG in any combination. Glucocorticoid-related complications were predominantly hypertension and hyperglycemia (Table S7).
Effect of Immunomodulation on Blood Markers
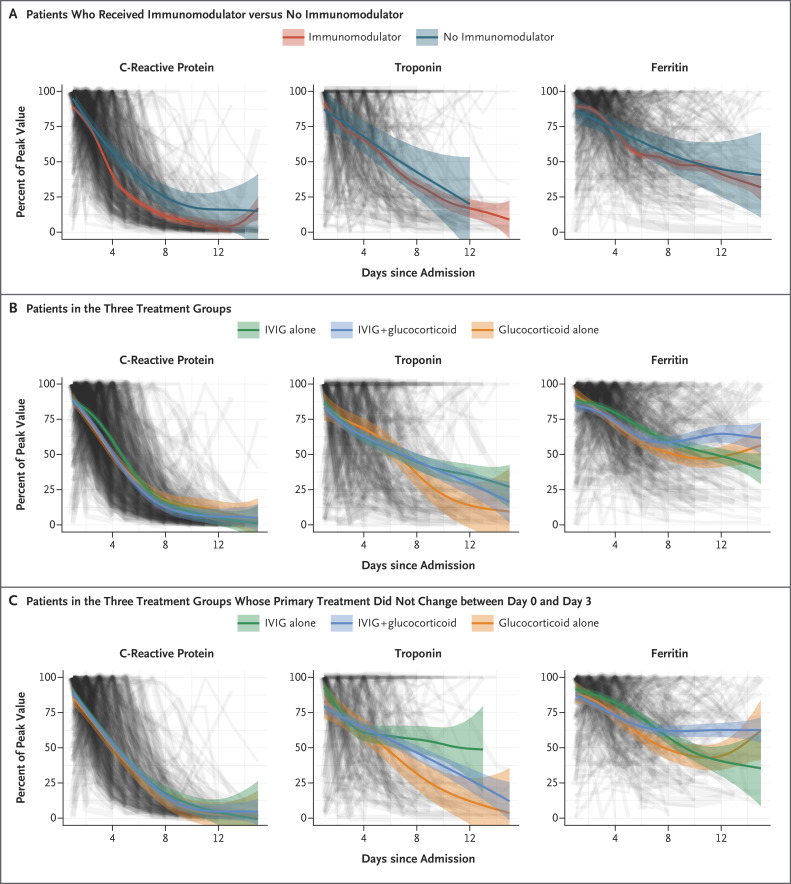
Levels of C-reactive protein decreased more rapidly in patients who received immunomodulators than in those who did not receive such treatment (Figure 3A). Changes in levels of C-reactive protein, troponin, and ferritin followed a similar temporal decrease in the three groups (Figure 3B), although there was some variation in the rate of decline, which was most obvious in the patients who did not change treatment before day 3 (Figure 3C).
Figure 3. Changes in Levels of C-Reactive Protein, Troponin, and Ferritin, According to Type of Treatment and Timing.
Each of three key markers of inflammation (C-reactive protein, troponin, and ferritin) is plotted as a line and weighted by the covariate-balancing propensity score. The levels are shown as a percentage of each patient’s peak value. A generalized additive model was used to fit the curves. Panel A shows the fitted curves for the three measures in patients who received any immunomodulators, as compared with those who did not receive immunomodulators. Panel B shows the fitted curves for patients who received IVIG alone, IVIG plus glucocorticoids, and glucocorticoids alone as their primary treatment. Panel C shows the fitted curves for the three treatments combined in the patients whose primary treatment did not change between the day of admission (0) and day 3.
To investigate whether the inclusion of children with Kawasaki’s disease in the present study might have influenced treatment responses, we explored changes in blood markers separately in children with a likely diagnosis of Kawasaki’s disease and in those without such a diagnosis. Since Kawasaki’s disease generally is more frequent in children before the age of 6 years and MIS-C is generally reported in older children, we compared the patients who met the AHA criteria for Kawasaki’s disease and all those under the age of 6 years (whose illness may be described as Kawasaki’s disease–like) with the remaining patients with MIS-C. Among the children who received IVIG alone, the smoothed curves showed rates of decline in C-reactive protein levels among those younger than 6 years of age who met the AHA criteria for Kawasaki’s disease that were similar to the rates among the remaining children. However, among the children who received glucocorticoids with or without IVIG, there was a more rapid decline in the C-reactive protein level in the group of children who did not meet the AHA criteria for Kawasaki’s disease or were over 6 years of age (Fig. S10).
Discussion
In a pragmatically defined international cohort of patients with suspected MIS-C, we found no evidence of substantial differences in the two primary outcomes among children who received the three most common treatments for this disorder (IVIG alone, IVIG plus glucocorticoids, and glucocorticoids alone). However, when we restricted the analyses to patients who met the WHO criteria for MIS-C, we found modest evidence of benefit with glucocorticoids alone over IVIG alone for both primary outcomes.
Analyses of secondary outcomes showed a lower frequency of escalation in immunomodulatory treatment (i.e., the addition of secondary agents) in patients receiving IVIG plus glucocorticoids than in those receiving either IVIG alone or glucocorticoids alone. We did not find clear evidence for an association between initial treatment with any of the three treatments and changes in organ failure, inflammation, or discharge from the hospital. The frequency of coronary-artery aneurysm was also similar in the three groups, but the percentage of patients with data regarding coronary-artery aneurysm was too low for firm conclusions to be made.
In a French national surveillance study involving patients with MIS-C,28 investigators found that patients who received IVIG plus glucocorticoids had a lower frequency of treatment escalation than those who received IVIG alone, along with a reduced need for hemodynamic support and a shorter stay in the intensive care unit. However, this study did not include a glucocorticoid-only group and had a smaller enrollment of patients than our study.
The high rates of escalation to additional treatments in patients receiving single agents may be explained by a failure of the initial treatment or by the severity of illness, as well as by a greater readiness to escalate therapy when only one treatment was given. An increased percentage of patients who received IVIG plus glucocorticoids were also receiving inotropes at day 0. These patients had higher levels of troponin than those in the other two groups, which suggests that patients who were more severely ill may have received IVIG plus glucocorticoids. However, patients who were receiving additional treatment by day 2 did not receive more inotropic or ventilatory support or have higher troponin levels than those who did not undergo treatment escalation.
We measured levels of C-reactive protein and ferritin to explore how the different agents may have affected inflammation as surrogates for a determination of overall inflammation, and we measured troponin as a marker of cardiac injury. After adjustment for baseline differences in illness severity, the rate of reduction in the C-reactive protein level appeared to be more rapid in patients who received immunomodulators.
Since the clinical features of MIS-C overlap with those of Kawasaki’s disease, a major dilemma in treatment decisions for MIS-C has been whether treatment regimens that do not include IVIG (the proven treatment for Kawasaki’s disease) may delay recovery and increase the risk of coronary-artery aneurysm. We found no evidence of delayed recovery from organ failure in patients who received glucocorticoids alone as their initial treatment. When we restricted the analysis to patients who met the WHO criteria for MIS-C, we found a possible benefit for glucocorticoids alone in reducing the frequency of organ failure and a reduction in the score on the ordinal scale, although this comparison was confounded by the high percentage of patients with treatment escalation to IVIG plus glucocorticoids. The frequency of coronary-artery aneurysm across all three treatment groups was low (6%), so firm conclusions could not be drawn with respect to that complication.
We explored whether the inadvertent inclusion of patients with Kawasaki’s disease in the MIS-C cohort may have prevented the detection of benefit from non-IVIG treatments. We found suggestive evidence that the rate of decline in the C-reactive protein level in response to immunomodulators may have differed between younger children who met the AHA criteria for Kawasaki’s disease and other children with suspected MIS-C. This finding supports our concern that the challenge of making a clinical distinction between these two diseases may increase the difficulty in identifying differences among the treatments for MIS-C.
Our study included patients who met the WHO definition of MIS-C as well as patients lacking one or more criteria for this diagnosis. The current criteria are imperfect, since antibody testing is not always available and a history of exposure to SARS-CoV-2 lacks specificity because asymptomatic infection is common. Since MIS-C includes a broad spectrum of illnesses, until a diagnostic test is developed, clinicians will face difficult treatment decisions when they encounter a wider group of inflammatory diseases that occur after SARS-CoV-2 infection than those identified by WHO criteria.
MIS-C has emerged as an important complication of SARS-CoV-2 infection among children in low- and middle-income countries.29,30 IVIG and biologic agents are costly and have limited availability in many countries, so evidence to support their use in preference to cheaper antiinflammatory agents such as glucocorticoids is needed. Since our study does not provide conclusive evidence for either equivalence or superiority of any of the three treatments that were evaluated, ongoing recruitment and analysis of a larger number of patients are needed to provide definitive evidence.
Our study has some limitations. Since our data did not come from randomized studies, a major concern is whether treatment selection was biased by the severity of illness. Our use of propensity-score weighting reduced bias. However, bias may also have been introduced by the fact that some patients received additional treatments after their primary treatment. Higher rates of treatment escalation in single-agent groups may have masked differences in efficacy. In addition, the status with respect to aneurysm on day 2 and beyond was unavailable for 35% of the study patients, and we did not specifically collect follow-up data on coronary-artery aneurysm. Thus, the current findings are insufficient to suggest equivalence of glucocorticoids with IVIG in preventing coronary-artery aneurysm.
Overall, we found no evidence of differences in outcomes between treatment with glucocorticoids or IVIG as single agents or between the single-agent and dual-agent primary treatments. The confidence intervals for inferences about treatment effect admit the possibility of actual benefit from one or more of the treatments relative to the others.
Protocol
Supplementary Appendix
Disclosure Forms
Data Sharing Statement
This article was published on June 16, 2021, at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
Footnotes
Supported by the European Union’s Horizon 2020 Program under grants (848196 DIAMONDS and 668303 PERFORM, to Dr. Levin), by the Imperial Biomedical Research Centre (BRC) of the National Institute for Health Research (NIHR) for the enrollment of patients in London, by a grant (to Dr. McArdle) from the Lee Family Foundation, a grant (203928/Z/16/Z, to Dr. Wilson) from the Wellcome Trust, a grant (RDA02, to Dr. Broderick) from the Wellcome Trust and the BRC, grants (206508/Z/17/Z and MRF-160-0008-ELP-KAFO-C0801, to Dr. Kaforou) from the Wellcome Trust and the Medical Research Foundation, a grant (ACL-2018-021-007, to Dr. Nijman) from the NIHR, and a grant (GA5R01AI128765, to Dr. Levin) from the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Riphagen S, Gomez X, Gonzalez-Martinez C, Wilkinson N, Theocharis P. Hyperinflammatory shock in children during COVID-19 pandemic. Lancet 2020;395:1607-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet 2020;395:1741-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whittaker E, Bamford A, Kenny J, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA 2020;324:259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdoni L, Mazza A, Gervasoni A, et al. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 2020;395:1771-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feldstein LR, Rose EB, Horwitz SM, et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med 2020;383:334-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldstein LR, Tenforde MW, Friedman KG, et al. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA 2021;325:1074-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levin M. Childhood multisystem inflammatory syndrome — a new challenge in the pandemic. N Engl J Med 2020;383:393-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfred-Cato S, Bryant B, Leung J, et al. COVID-19-associated multisystem inflammatory syndrome in children — United States, March–July 2020. MMWR Morb Mortal Wkly Rep 2020;69:1074-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toubiana J, Poirault C, Corsia A, et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ 2020;369:m2094-m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capone CA, Subramony A, Sweberg T, et al. Characteristics, cardiac involvement, and outcomes of multisystem inflammatory syndrome of childhood associated with severe acute respiratory syndrome coronavirus 2 infection. J Pediatr 2020;224:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pouletty M, Borocco C, Ouldali N, et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis 2020;79:999-1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crayne C, Cron RQ. Pediatric macrophage activation syndrome, recognizing the tip of the Iceberg. Eur J Rheumatol 2019;7:Suppl 1:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gottlieb M, Long B, Koyfman A. The evaluation and management of toxic shock syndrome in the emergency department: a review of the literature. J Emerg Med 2018;54:807-814. [DOI] [PubMed] [Google Scholar]
- 14.Wilkins AL, Steer AC, Smeesters PR, Curtis N. Toxic shock syndrome — the seven Rs of management and treatment. J Infect 2017;74:Suppl 1:S147-S152. [DOI] [PubMed] [Google Scholar]
- 15.Davies P, Lillie J, Prayle A, et al. Association between treatments and short-term biochemical improvements and clinical outcomes in post-severe acute respiratory syndrome coronavirus-2 inflammatory syndrome. Pediatr Crit Care Med 2021;22(5):e285-e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies P, Evans C, Kanthimathinathan HK, et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health 2020;4:669-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belhadjer Z, Auriau J, Méot M, et al. Addition of corticosteroids to immunoglobulins is associated with recovery of cardiac function in multi-inflammatory syndrome in children. Circulation 2020;142:2282-2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCrindle BW, Rowley AH, Newburger JW, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a scientific statement for health professionals from the American Heart Association. Circulation 2017;135(17):e927-e999. [DOI] [PubMed] [Google Scholar]
- 19.Harwood R, Allin B, Jones CE, et al. A national consensus management pathway for paediatric inflammatory multisystem syndrome temporally associated with COVID-19 (PIMS-TS): results of a national Delphi process. Lancet Child Adolesc Health 2021;5:133-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henderson LA, Canna SW, Friedman KG, et al. American College of Rheumatology clinical guidance for multisystem inflammatory syndrome in children associated with SARS-CoV-2 and hyperinflammation in pediatric COVID-19: version 1. Arthritis Rheumatol 2020;72:1791-1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) — a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowles L, Platton S, Yartey N, et al. Lupus anticoagulant and abnormal coagulation tests in patients with Covid-19. N Engl J Med 2020;383:288-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med 2020;382:2574-2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royal College of Paediatrics and Child Health. Paediatric multisystem inflammatory syndrome temporally associated with COVID-19 (PIMS) — guidance for clinicians. 2020. (https://www.rcpch.ac.uk/resources/paediatric-multisystem-inflammatory-syndrome-temporally-associated-covid-19-pims-guidance).
- 25.Centers for Disease Control and Prevention. Information for healthcare providers about multisystem inflammatory syndrome in children (MIS-C) (https://www.cdc.gov/mis-c/hcp/).
- 26.World Health Organization. Multisystem inflammatory syndrome in children and adolescents with COVID-19. Scientific brief. May 2020. (https://www.who.int/publications/i/item/multisystem-inflammatory-syndrome-in-children-and-adolescents-with-covid-19).
- 27.Lopez L, Colan SD, Stylianou M, et al. Relationship of echocardiographic Z scores adjusted for body surface area to age, sex, race, and ethnicity: the Pediatric Heart Network Normal Echocardiogram Database. Circ Cardiovasc Imaging 2017;10(11):e006979-e006979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ouldali N, Toubiana J, Antona D, et al. Association of intravenous immunoglobulins plus methylprednisolone vs immunoglobulins alone with course of fever in multisystem inflammatory syndrome in children. JAMA 2021;325:855-864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Irfan O, Muttalib F, Tang K, Jiang L, Lassi ZS, Bhutta Z. Clinical characteristics, treatment and outcomes of paediatric COVID-19: a systematic review and meta-analysis. Arch Dis Child 2021;106:440-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sood M, Sharma S, Sood I, Sharma K, Kaushik A. Emerging evidence on multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection: a systematic review with meta-analysis. SN Compr Clin Med 2021. January 7 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.