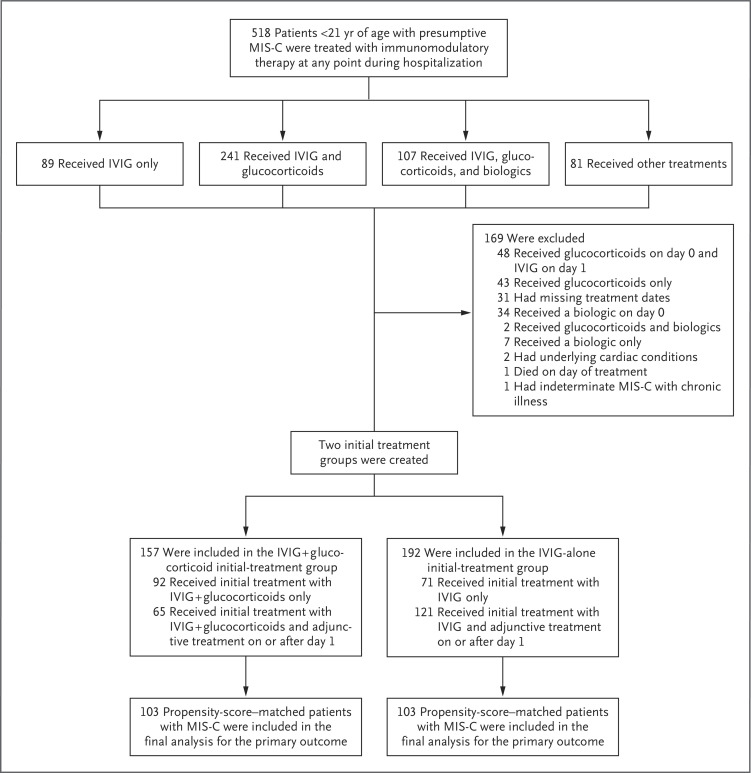
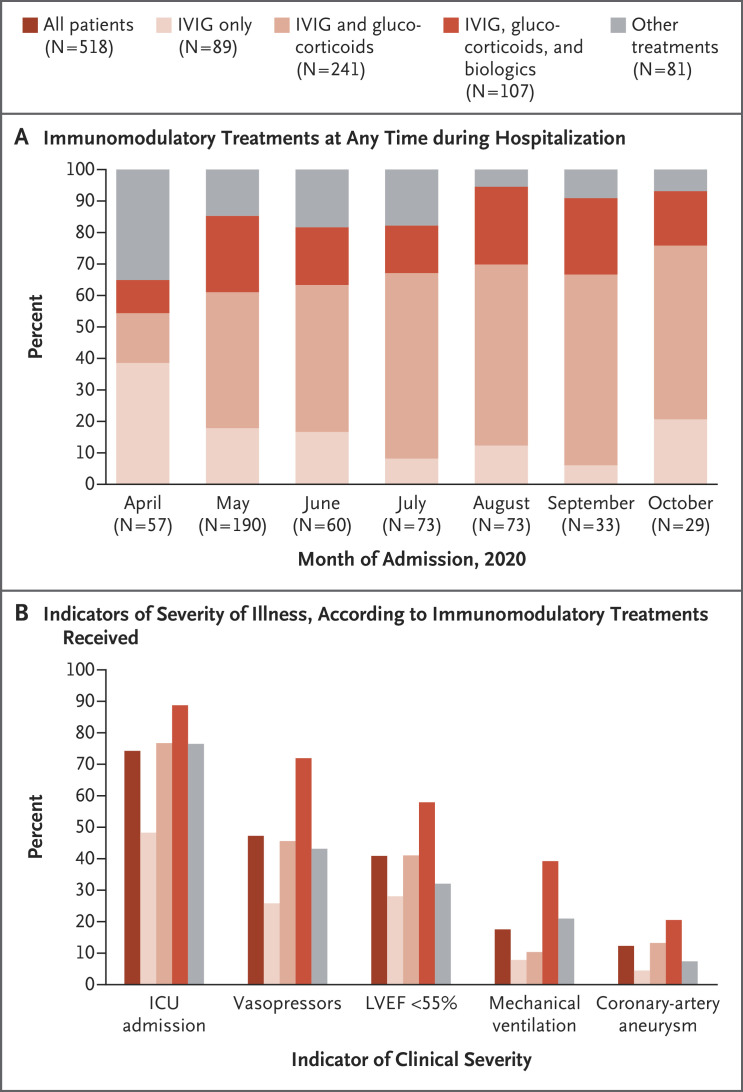
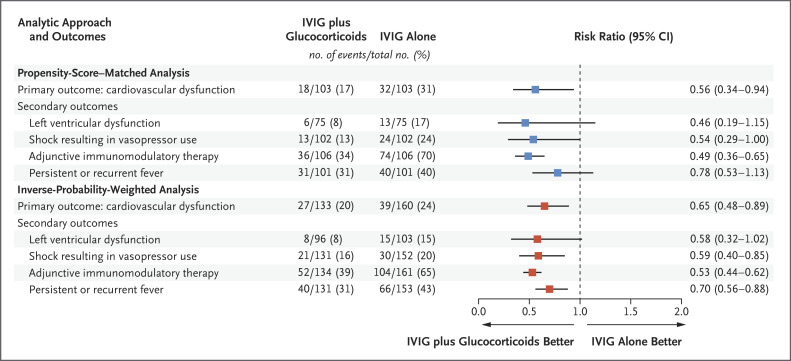
Mary Beth F Son
Mary Beth F Son, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Nancy Murray
Nancy Murray, M.Sc.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Kevin Friedman
Kevin Friedman, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Cameron C Young
Cameron C Young
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Margaret M Newhams
Margaret M Newhams, M.P.H.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Leora R Feldstein
Leora R Feldstein, Ph.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Laura L Loftis
Laura L Loftis, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Keiko M Tarquinio
Keiko M Tarquinio, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Aalok R Singh
Aalok R Singh, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Sabrina M Heidemann
Sabrina M Heidemann, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Vijaya L Soma
Vijaya L Soma, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Becky J Riggs
Becky J Riggs, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Julie C Fitzgerald
Julie C Fitzgerald, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Michele Kong
Michele Kong, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Sule Doymaz
Sule Doymaz, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
John S Giuliano Jr
John S Giuliano Jr, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Michael A Keenaghan
Michael A Keenaghan, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Janet R Hume
Janet R Hume, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Charlotte V Hobbs
Charlotte V Hobbs, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Jennifer E Schuster
Jennifer E Schuster, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Katharine N Clouser
Katharine N Clouser, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Mark W Hall
Mark W Hall, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Lincoln S Smith
Lincoln S Smith, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Steven M Horwitz
Steven M Horwitz, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Stephanie P Schwartz
Stephanie P Schwartz, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Katherine Irby
Katherine Irby, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Tamara T Bradford
Tamara T Bradford, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Aline B Maddux
Aline B Maddux, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Christopher J Babbitt
Christopher J Babbitt, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Courtney M Rowan
Courtney M Rowan, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Gwenn E McLaughlin
Gwenn E McLaughlin, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Phoebe H Yager
Phoebe H Yager, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Mia Maamari
Mia Maamari, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Elizabeth H Mack
Elizabeth H Mack, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Christopher L Carroll
Christopher L Carroll, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Vicki L Montgomery
Vicki L Montgomery, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Natasha B Halasa
Natasha B Halasa, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Natalie Z Cvijanovich
Natalie Z Cvijanovich, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Bria M Coates
Bria M Coates, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Charles E Rose
Charles E Rose, Ph.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Jane W Newburger
Jane W Newburger, M.D., M.P.H.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Manish M Patel
Manish M Patel, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,
Adrienne G Randolph
Adrienne G Randolph, M.D.
1From the Division of Immunology (M.B.F.S.), and the Departments of Cardiology (K.F., J.W.N.) and Anesthesiology, Critical Care, and Pain Medicine (C.C.Y., M.M.N., A.G.R.), Boston Children’s Hospital, the Division of Pediatric Critical Care Medicine, MassGeneral Hospital for Children (P.H.Y.), and the Departments of Anesthesia (A.G.R.) and Pediatrics (M.B.F.S., K.F., P.H.Y., J.W.N., A.G.R.), Harvard Medical School — all in Boston; the COVID-19 Response Team, Centers for Disease Control and Prevention (N.M., L.R.F., C.E.R., M.M.P.), and the Division of Critical Care Medicine, Department of Pediatrics, Emory University School of Medicine, Children’s Healthcare of Atlanta (K.M.T.) — both in Atlanta; the Commissioned Corps of the U.S. Public Health Service, Rockville (L.R.F., M.M.P.), and the Department of Anesthesiology and Critical Care Medicine, Division of Pediatric Anesthesiology and Critical Care Medicine, Johns Hopkins School of Medicine, Baltimore (B.J.R.) — both in Maryland; the Section of Critical Care Medicine, Department of Pediatrics, Baylor College of Medicine, Houston (L.L.L.); the Department of Pediatrics, Division of Critical Care Medicine, University of Texas Southwestern, Children’s Medical Center of Dallas, Dallas (M.M.); the Pediatric Critical Care Division, Maria Fareri Children’s Hospital at Westchester Medical Center and New York Medical College, Valhalla (A.R.S.), the Division of Pediatric Infectious Diseases, Department of Pediatrics, New York University Grossman School of Medicine, New York (V.L.S.), and the Division of Pediatric Critical Care, Department of Pediatrics, State University of New York Downstate Health Sciences University (S.D.), and Pediatric Critical Care, New York City Health and Hospitals, Kings County Hospital (M.A.K.), Brooklyn — all in New York; the Department of Pediatrics, Division of Pediatric Critical Care Medicine, Central Michigan University, Detroit (S.M. Heidemann); the Division of Critical Care, Department of Anesthesiology and Critical Care, University of Pennsylvania Perelman School of Medicine, Philadelphia (J.C.F.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Alabama at Birmingham, Birmingham (M.K.); the Department of Pediatrics, Division of Critical Care, Yale University School of Medicine, New Haven (J.S.G.), and the Division of Critical Care, Connecticut Children’s, Hartford (C.L.C.) — both in Connecticut; the Division of Pediatric Critical Care, M Health Fairview University of Minnesota Masonic Children’s Hospital, Minneapolis (J.R.H.); the Department of Pediatrics, Department of Microbiology, Division of Infectious Diseases, University of Mississippi Medical Center, Jackson (C.V.H.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Kansas City, Kansas City, MO (J.E.S.); the Department of Pediatrics, Joseph M. Sanzari Children’s Hospital at Hackensack University Medical Center, Hackensack (K.N.C.), and the Department of Pediatrics, Division of Pediatric Critical Care, Bristol-Myers Squibb Children’s Hospital at Robert Wood Johnson Medical School, Rutger’s University, New Brunswick (S.M. Horwitz) — both in New Jersey; the Division of Critical Care Medicine, Department of Pediatrics, Nationwide Children’s Hospital, Columbus, OH (M.W.H.); the Department of Pediatrics, Division of Pediatric Critical Care Medicine, University of Washington, Seattle (L.S.S.); the Department of Pediatrics, University of North Carolina Children’s Hospital, Chapel Hill (S.P.S.); the Section of Pediatric Critical Care, Department of Pediatrics, Arkansas Children’s Hospital, Little Rock (K.I.); the Department of Pediatrics, Division of Cardiology, Louisiana State University Health Sciences Center and Children’s Hospital of New Orleans, New Orleans (T.T.B.); the Department of Pediatrics, Section of Critical Care Medicine, University of Colorado School of Medicine and Children’s Hospital Colorado, Aurora (A.B.M.); the Division of Pediatric Critical Care, Miller Children’s and Women’s Hospital of Long Beach, Long Beach (C.J.B.), and the Division of Critical Care Medicine, University of California San Francisco Benioff Children’s Hospital Oakland, Oakland (N.Z.C.) — both in California; the Division of Pediatric Critical Care Medicine, Department of Pediatrics, Indiana University School of Medicine, Riley Hospital for Children, Indianapolis (C.M.R.); the Division of Pediatric Critical Care Medicine, Department of Pediatrics, University of Miami Miller School of Medicine, Miami (G.E.M.); the Division of Pediatric Critical Care Medicine, Medical University of South Carolina, Charleston (E.H.M.); the Department of Pediatrics, University of Louisville and Norton Children’s Hospital, Louisville, KY (V.L.M.); the Division of Pediatric Infectious Diseases, Department of Pediatrics, Vanderbilt University Medical Center, Nashville (N.B.H.); and the Division of Critical Care Medicine, Department of Pediatrics, Northwestern University Feinberg School of Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Chicago (B.M.C.).
1,✉,
the Overcoming COVID-19 Investigators