Abstract
Background
Melatonin has been known as an anti-inflammatory agent and immune modulator that may address progressive pathophysiology of coronavirus disease 2019 (COVID-19).Aim of the study. To evaluate the clinical efficacy of adjuvant, use of melatonin in patients with COVID-19.
Methods
This single-center, double-blind, randomized clinical trial included 74 hospitalized patients with confirmed mild to moderate COVID-19 at Baqiyatallah Hospital in Tehran, Iran, from April 25, 2020–June 5, 2020. Patients were randomly assigned in a 1:1 ratio to receive standard of care and standard of care plus melatonin at a dose of 3 mg three times daily for 14 d. Clinical characteristics, laboratory, and radiological findings were assessed and compared between two study groups at baseline and post-intervention. Safety and clinical outcomes were followed up for four weeks.
Results
A total of 24 patients in the intervention group and 20 patients in the control group completed the treatment. Compared with the control group, the clinical symptoms such as cough, dyspnea, and fatigue, as well as the level of CRP and the pulmonary involvement in the intervention group had significantly improved (p <0.05). The mean time of hospital discharge of patients and return to baseline health was significantly shorter in the intervention group compared to the control group (p <0.05). No deaths and adverse events were observed in both groups.
Conclusions
Adjuvant use of melatonin has a potential to improve clinical symptoms of COVID-19 patients and contribute to a faster return of patients to baseline health.
Keywords: COVID-19, Melatonin, Clinical trial, Adjunctive therapy
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is responsible for the outbreak of an intense respiratory illness named coronavirus disease 2019 (COVID-19). The COVID-19 was first reported in Wuhan, China in late December 2019 and soon spread around the world (1,2). As of May 4, 2021, the COVID-19 pandemic had infected nearly 152 million people and killed more than 3 million worldwide (3). A range of severity of COVID-19 has been reported from mild, self-limiting respiratory tract disease to severe progressive pneumonia, multiple organ dysfunction, and death. Most patients with COVID-19 have a self-limiting infection and have recover, however, about 15% of cases suffer from severe pneumonia and in 5% of cases a critical illness occurs which requires the intensive care unit (ICU) admission and some of them die (4,5).
Whereas COVID-19 infection continues to spread around the world, multiple therapeutic agents have been tested or are under clinical trials. However, these medications have not yet been proven effective in treating patients with COVID-19. Many papers have reported compassionate use of drugs with inconclusive therapeutic effects and many side effects (6., 7., 8.). Accordingly, there is currently no definitive treatment for this novel coronavirus. Due to the high contagiousness and rapid spread of SARS‐CoV‐2 infection, the need for safe and effective drugs for the treatment of COVID‐19 is becoming increasingly urgent.
The pathophysiology of COVD‐19 is triggered by the coronavirus cell entry via binding spike protein to angiotensin converting the enzyme 2 (ACE2) host receptor, which expresses in different human tissues and cell types (9,10). The COVID-19 infection launches a cytokine storm and an inflammatory cascade that can significantly contribute to disease progression and even leads to death (11). Therefore, modulation of the excessive inflammatory responses is thought to be important in improving the treatment outcomes for COVID-19 patients.
Melatonin is a multifunctional molecule that is well-known as an anti-inflammatory, antioxidant, and immune modulator. It has also been proven that melatonin is involved in regulating sleep, blood pressure, and vascular function and alleviates viral respiratory disorders. Due to these properties, recent publications have suggested the possible beneficial effects of melatonin on improving clinical outcomes of COVID-19 patients (12., 13., 14., 15.). However, there are few clinical and laboratory data on the use of melatonin as an adjunctive therapeutic agent in COVID-19 infection. Hence, in this study a randomized clinical trial was designed and performed to evaluate the efficacy and safety of oral melatonin in combination with standard treatment for improving clinical status in adult patients hospitalized with mild to moderate COVID-19.
Methods
Study Design and Patients
This study was a single-center, double-blind, randomized clinical trial to assess the efficacy and safety of melatonin in adults with COVID-19 admitted to Baqiyatallah Hospital, Iran, from April 25, 2020–June 5, 2020. The diagnosis of COVID-19 was confirmed by Reverse Transcription-Polymerase Chain Reaction (RT-PCR), as well as chest radiography or Computed Tomography (CT) findings consistent with the COVID-19 pneumonia. A total of 100 patients were screened, of whom 26 patients were excluded, due to the lack of inclusion criteria and non-acceptance to participate in the study. Thus, 74 patients were selected to participate in the trial.
Eligible patients fulfilled inclusion and exclusion criteria. The inclusion criteria were hospitalized patients with confirmed mild to moderate COVID-19, inclusion within the first 48 hours of hospital admission, males and non-pregnant females aged 18 years or older, and willingness to participate in this clinical trial. Mild cases were defined as mild clinical symptoms without CT manifestation of pneumonia. Moderate cases were defined as fever and respiratory symptoms with CT manifestation of pneumonia. The exclusion criteria were as follows: pregnancy or breastfeeding, neurological diseases, chronic hepatitis, kidney failure, use of antioxidants, anti-inflammatory and immunosuppressant drugs, known allergy to melatonin, and participation in another clinical trial at least 30 d prior to enrollment.
The study protocol was approved by the ethical committee of the Baqiyatallah University of Medical Sciences, Iran (IR.BMSU.REC.1399.039), and the trial was registered with ClinicalTrials.gov (Identifier: NCT04409522). Before enrollment, the study protocol and objectives were explained for all patients or/and their legal representatives, and a written informed consent was obtained from them.
Randomization and Procedures
Eligible patients were randomly divided into two groups of intervention and control using a random permuted block design (1∶1 ratio) based on the combined analysis. The control group received standard of care, and the intervention group received standard of care plus melatonin at a dose of 3 mg three times daily for 14 d.
The standard of care for Covid-19 was at the discretion of treating physicians and according to the Iranian national COVID-19 treatment protocol. Patients were evaluated once daily by trained nurses using a checklist, which recorded clinical outcomes and complications. Safety assessments included monitoring for adverse events, vital signs, laboratory parameters (days 1, and 14), and 12-lead electrocardiograms (days 1, and 14). If a specific complication was observed in the melatonin group, the use of melatonin was broken, and the patient was excluded from the group. All recorded data were entered into an electronic database after being reviewed again. Both researchers and patients were blinded to the details of therapeutic intervention. To ensure correct use of the tablets by patients, they were reminded via a phone call or text message. The patients who did not take more than 10% of the tablets were excluded from the study. The follow-up period was 4 weeks for all patients.
Outcomes
The primary clinical outcomes included evaluation of clinical improvement of symptoms and laboratory parameters including neutrophil-lymphocyte ratio (NLR), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP). Secondary clinical outcomes were chest radiographic improvement, time to discharge, transfer to ICU, and 28 d mortality. Safety outcomes included the occurrence of adverse events during treatment, serious infections, laboratory abnormalities, and premature discontinuation of melatonin.
Statistical Analysis
Assuming a 5% dropout rate, at least a sample size of 20 patients was estimated for each treatment group to make a difference of 3 d in clinical recovery time, with 80% power and a two-sided α level of 0.05. Normality distribution of variables was assessed by the Kolmogorov-Smirnov test. Continuous variables were reported as mean ± standard deviation (SD) or median with interquartile range (IQR) and were compared with the independent t-test or Mann-Whitney U test. Categorical ones were expressed as frequencies and percentages (%) and were compared by the Fisher exact test or χ2 test. Statistical analyses were performed using SPSS software (version 22.0; IBM), and p-value <0.05 was considered significant.
Results
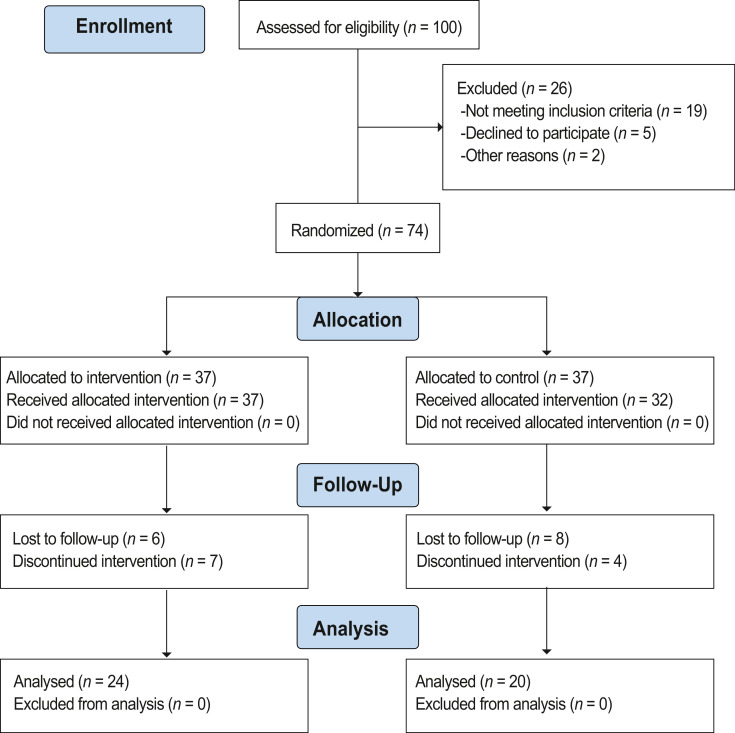
Among the 100 COVID–19 patients assessed for eligibility, 26 were excluded as they did not meet the eligibility criteria or declined to participate in the study. The remaining 74 patients with mild to moderate COVID-19 were randomized. Due to the dropouts, a total of 44 patients (24 [3 mild cases and 21 moderate cases] in the intervention and 20 [1 mild case and 19 moderate cases] in the control group) completed the scheduled treatment and were analyzed (Figure 1 ).
Figure 1.
CONSORT flow diagram of patient enrolled through the clinical trial.
The baseline demographic and clinical characteristics of the studied patients in both groups are shown in Table 1 . The mean ± SD age in the intervention and control group was 50.75 ± 14.43 and 52.95 ± 14.07 years, respectively. From among the patients, 26 of them were males (59.1%). There was no significant difference in terms of demographic data, underlying comorbidities, clinical signs/symptoms, laboratory findings, and pulmonary involvement between two groups at baseline (p ≥0.05). Hypertension (25%), diabetes (22.7%), rheumatic disease (9.1%), cardiovascular diseases (6.8%), and cancer (6.8%) were common underlying comorbidities. Also, no significant difference was noted in the median interval time from symptom onset to randomization between both groups (7 [IQR, 6–9] day in the intervention group versus 7 [IQR, 5–8] day in the control group, p = 0.435).
Table 1.
Demographics and clinical characteristics of COVID-19 patients at baseline
| Patients, No. (%) |
|||
| Characteristics | Intervention group (n = 24) | Control group (n = 20) | pa |
| Age, mean ± SD, years | 50.75 ± 14.43 | 52.95 ± 14.07 | 0.613 |
| Sex | |||
| Male | 14 (58.3) | 12 (60) | 0.911 |
| Female | 10 (41.7) | 8 (40) | |
| BMI, kg/m2 | 28.44 ± 4.69 | 27.37 ± 3.76 | 0.415 |
| Current smoker | 1 (4.2) | 1 (5) | 0.895 |
| Comorbidities | |||
| Hypertension | 8 (33.3) | 3 (15) | 0.162 |
| Diabetes | 6 (25) | 4 (20) | 0.694 |
| Rheumatic disease | 1 (4.2) | 3 (15) | 0.213 |
| Cardiovascular disease | 3 (12.5) | 0 (0) | 0.101 |
| Cancer | 1 (4.2) | 2 (10) | 0.445 |
| Signs and symptoms | |||
| Fever (temperature ≥37.3°C) | 7 (29.2) | 4 (20) | 0.484 |
| Cough | 11 (45.8) | 10 (50) | 0.783 |
| Dyspnea | 17 (70.8) | 13 (65) | 0.679 |
| Myalgia | 16 (66.7) | 8 (40) | 0.077 |
| Fatigue | 19 (79.2) | 14 (70) | 0.484 |
| Chill | 11 (45.8) | 9 (45) | 0.956 |
| Headache | 13 (54.2) | 7 (35) | 0.204 |
| GI symptoms | 9 (37.5) | 7 (35) | 0.864 |
| Chest pain | 11 (45.8) | 10 (50) | 0.783 |
| Sputum production | 8 (33.3) | 6 (30) | 0.813 |
| Rhinorrhea | 3 (12.5) | 4 (20) | 0.498 |
| Sore throat | 5 (20.8) | 6 (30) | 0.484 |
| Laboratory findings | |||
| NLR, median (IQR) | 3.3 (2.1–5.7) | 3.4 (2.3–5.6) | 0.822 |
| ESR, median (IQR), mm/h | 25 (14–35) | 27 (17–38) | 0.516 |
| Positive CRP ≥10 mg/L | 21 (87.5) | 18 (90) | 0.795 |
| Pulmonary involvement | 21 (87.5) | 19 (95) | 0.389 |
| Time from symptom onset to randomization, median (IQR), days | 7 (6–9) | 7 (5–8) | 0.435 |
COVID-19, coronavirus disease 2019; BMI, body mass index; NLR, neutrophil-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; No., number; SD, standard deviations; IQR, interquartile range.
p values indicate differences between patients in the intervention group and the control group. p <0.05 was considered statistically significant.
Table 2 shows the post-treatment clinical characteristics and outcomes of patients in the intervention and control group. Patients receiving melatonin compared with control patients showed a significant improvement of respiratory symptoms including cough (4.2 vs. 25%; p = 0.045) and dyspnea (0% vs. 15%; p = 0.049). Similar results were observed for fatigue (8.3 vs. 30%; p = 0.020), while there was no significant difference in other symptoms between the two groups. Comparing the laboratory and radiographic findings showed an improvement in the level of CRP (4.2 vs. 25%; p = 0.045) and the pulmonary involvement (4.2 vs. 25%; p = 0.045) in the intervention group compared to the control group. Figure 2 shows the chest CT results of the COVID-19 patients in the intervention and control group.
Table 2.
Clinical characteristics and outcomes of COVID-19 patients at post-intervention
| Patients, No. (%) |
|||
| Characteristics | Intervention group(n = 24) | Control group(n = 20) | pa |
| Signs and symptoms | |||
| Fever (temperature ≥37.3°C) | 1 (4.2) | 0 (0) | 0.356 |
| Cough | 1 (4.2) | 5 (25) | 0.045 |
| Dyspnea | 0 (0) | 3 (15) | 0.049 |
| Myalgia | 1 (4.2) | 2 (10) | 0.445 |
| Fatigue | 2 (8.3) | 6 (30) | 0.020 |
| Chill | 1 (4.2) | 1 (5) | 0.895 |
| Headache | 1 (4.2) | 2 (10) | 0.445 |
| GI symptoms | 1 (4.2) | 0 (0) | 0.356 |
| Sputum production | 0 (0) | 1 (5) | 0.268 |
| Sore throat | 0 (0) | 2 (10) | 0.113 |
| Chest pain | 0 (0) | 0 (0) | 1 |
| Rhinorrhea | 0 (0) | 0 (0) | 1 |
| Laboratory findings | |||
| NLR, median (IQR) | 1.7 (1–2.5) | 1.9 (1.2–2.8) | 0.464 |
| ESR, median (IQR), mm/h | 8 (5–13) | 9 (7–16) | 0.146 |
| Positive CRP ≥10 mg/L | 1 (4.2) | 5 (25) | 0.045 |
| Pulmonary involvement | 1 (4.2) | 5 (25) | 0.045 |
| Clinical outcome | |||
| Hospital discharge | 22 (91.7) | 17 (85) | 0.488 |
| Time to discharge, mean ± SD, day | 4.65 ± 3.37 | 8.15 ± 5.97 | 0.021 |
| Return to baseline health, mean ± SD, day | 15.09 ± 8.69 | 29.60 ± 21.12 | 0.004 |
| Hospital stays | 2 (8.3) | 3 (15) | 0.488 |
| ICU admission | 0 (0) | 2 (10) | 0.113 |
| Death | 0 (0) | 0 (0) | 1 |
COVID-19, coronavirus disease 2019; NLR, neutrophil-lymphocyte ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ICU, intensive care unit No., number; SD, standard deviations; IQR, interquartile range.
p values indicate differences between patients in the intervention group and the control group. p <0.05 was considered statistically significant. Values in bold indicate significant difference.
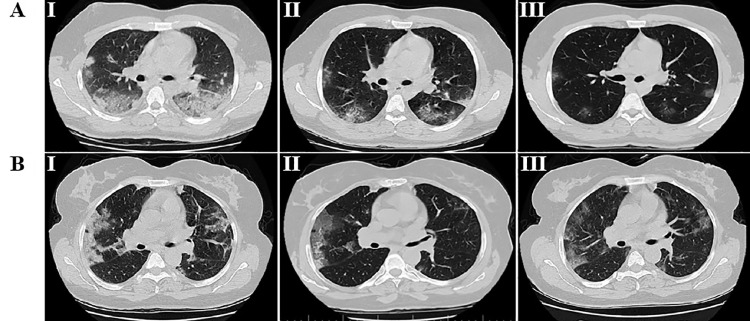
Figure 2.
Chest CT images of the representative hospitalized patients with COVID-19. A. chest CT scans of a 39 year old male patient with COVID-19 pneumonia who received melatonin as an adjuvant treatment; B. chest CT scans of a 48 year-old male patient with COVID-19 pneumonia who only received standard of care. CT was performed in three stages, including I) the day of admission before treatment onset, II) 5 d after treatment and, III) 12 d after treatment. COVID‐19, coronavirus disease 2019; CT, computed tomography. AI: CT image show multifocal ground-glass opacities and consolidation lesions. AII: CT image show bilateral multiple ground-glass opacity. AIII: CT image show very faint and smaller ground-glass opacities in both lungs. BI: CT image show consolidation lesions and ground-glass opacities with Interlobular septal thickening called crazy paving pattern. BII: CT image show bilateral ground-glass opacities and atelectasis in the segment of the left upper lobe. BIII: CT image show bilateral ground-glass opacities.
On day 14 of therapy, 91.7% of patients in the intervention group and 85% of patients in the control group were discharged. The mean time of hospital discharge of patients (4.65 ± 3.37 vs. 8.15 ± 5.97; p = 0.021) was significantly shorter in the intervention group compared to the control group. Moreover, during follow-up, patients treated with melatonin showed a return to baseline health sooner than control patients (15.09 ± 8.69 vs. 29.60 ± 21.12; p = 0.004).
There was no significant difference in the rate of ICU admissions and 28 d mortality between the two groups. This is while no patients died in both groups. No adverse events were also observed in patients receiving melatonin from the beginning of the treatment to the end of the follow-up period.
Discussion
With the growing spread of the COVID-19 outbreak globally, seeking an effective treatment strategy is essential. As of now, COVID-19 treatment is still empirically based, and drugs are prescribed in a compassionate manner. The efficacy of melatonin as an adjunctive therapy has been demonstrated in several diseases (13,16., 17., 18.). However, there are few trials regarding the use of melatonin in COVID-19 patients, and to our knowledge, this is the first randomized trial to evaluate the efficacy and safety of a low dose of oral melatonin as an adjunctive therapy in patients hospitalized with mild to moderate COVID-19.
The results of this clinical trial showed that two weeks consumption of oral melatonin 3 mg three times a day combined with standard of care could significantly relieve the clinical symptoms such as cough, dyspnea and fatigue in patients hospitalized with mild to moderate COVID-19. These are the major symptoms of lower respiratory tract infections during COVID-19, which are associated with high prevalence and mortality rates. It has been well found that melatonin administration could mitigate viral infection-induced oxidative stress through reducing the level of malondialdehyde, 8-isoprostane, as well as increasing the antioxidant enzymes activity, and improving respiratory symptoms (19).
The CRP is an acute-phase protein and an indicator of inflammation, infection, and tissue damage. Hepatic synthesis of CRP is mainly induced by IL-6 with a half-life of 19 hours. The measurement of CRP is a reliable test for early screening and rapid isolation of suspected COVID-19 patients, and elevated levels of CRP correlate with poor prognosis (20,21). In this study, 87.5 and 90% of patients were positive for the CRP test in the intervention and control group, respectively, at baseline. At post-intervention, improvement in CRP was observed in most patients with a significant difference between the two groups, indicating the effective impact of melatonin in inhibiting inflammation under the COVID-19 infection.
The chest radiographic results demonstrated a significant difference between the two groups. In this clinical trial, an improvement in the respiratory symptoms in COVID-19 patients who received melatonin, reflected a decline in the severity of pulmonary involvement through reducing airway oxidative stress and immunomodulatory in these patients.
During the follow-up, although no significant difference was observed in the rate of hospital discharge, hospital stay, ICU admissions and 28 d mortality, interestingly, the mean time of discharge and return to baseline health were significantly shorter in the patients receiving melatonin supplementation. Moreover, no adverse events were also observed until the end of the follow-up period, indicating that short-term use of melatonin to be safe. Cumulatively, these observations suggest that adjuvant use of melatonin may not only reduce the disease burden and healthcare utilization caused by COVID-19 but may also increase the effectiveness of antiviral drugs as a bridge to recovery, with less side effects and dose-limiting toxicity.
This study has a randomized double-blind structure to better clarify the results, and assuming that interventions may be more effective in the early course of the disease, early treatment was given. However, the main limitations of this trial included the small sample size and short-term follow-up, which may lead to bias. Therefore, further randomized clinical trials with larger sample sizes need to be undertaken to provide sufficient power for detecting the differences in the clinical outcomes and to also support the adjuvant use of a low dose of melatonin in COVID-19 patients.
Conclusion
To concluded, the results from this study revealed the efficacy of oral melatonin as an adjuvant therapy added to the standard of care compared with standard of care alone in patients hospitalized with mild to moderate COVID-19. Improving respiratory symptoms, time of patient discharge and return to baseline health are in favor of the efficacy of this adjuvant medication in mitigating this infectious disease. Considering the high performance of melatonin as an inexpensive, affordable, highly safe to human and readily available medication, it is suggested that it alone or in combination would be further investigated in COVID-19 prophylaxis in future studies.
Funding
None
Competing Interests
All authors have no conflict of interest to declare.
Acknowledgments
We would like to thank all the participants for their contribution in the current trial. Also, we thank the guidance and advice of the “Clinical Research Development Unit of Baqiyatallah Hospital”.
References
- 1.Wang C, Horby PW, Hayden FG, et al. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikpouraghdam M, Farahani AJ, Alishiri G, et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Weekly epidemiological update on COVID-19. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19-4-may-2021. (Accessed May 4, 2021).
- 4.Akbariqomi M, Hosseini MS, Rashidiani J, et al. Clinical characteristics and outcome of hospitalized COVID-19 patients with diabetes: A single-center, retrospective study in Iran. Diabetes Res Clin Pract. 2020;169 doi: 10.1016/j.diabres.2020.108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abuo-Rahma GE-DA, Mohamed MFA, Ibrahim TS, et al. Potential repurposed SARS-CoV-2 (COVID-19) infection drugs. RSC Adv. 2020;10:26895–26916. doi: 10.1039/d0ra05821a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davoodi L, Abedi SM, Salehifar E, et al. Febuxostat therapy in outpatients with suspected COVID-19: A clinical trial. Int J Clin Pract. 2020;74:e13600. doi: 10.1111/ijcp.13600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu R, Wang L, Kuo H-CD, et al. An update on current therapeutic drugs treating COVID-19. Curr Pharmacol Reports. 2020;6:56–70. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2020;54:159–163. doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li MY, Li L, Zhang Y, et al. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ragab D, Salah Eldin H, Taeimah M, et al. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleszczyński K, Slominski AT, Steinbrink K, et al. Clinical Trials for Use of Melatonin to Fight against COVID-19 Are Urgently Needed. Nutrients. 2020;12:2561. doi: 10.3390/nu12092561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang R, Wang X, Ni L, et al. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020 doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shneider A, Kudriavtsev A, Vakhrusheva A. Can melatonin reduce the severity of COVID-19 pandemic? Int Rev Immunol. 2020;39:153–162. doi: 10.1080/08830185.2020.1756284. [DOI] [PubMed] [Google Scholar]
- 15.Tan DX, Hardeland R. Potential utility of melatonin in deadly infectious diseases related to the overreaction of innate immune response and destructive inflammation: focus on COVID-19. Melatonin Res. 2020;3:120–143. [Google Scholar]
- 16.Li Y, Li S, Zhou Y, et al. Melatonin for the prevention and treatment of cancer. Oncotarget. 2017;8:39896. doi: 10.18632/oncotarget.16379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S-H, Liao C-L, Chen S-J, et al. Melatonin possesses an anti-influenza potential through its immune modulatory effect. J Funct Foods. 2019;58:189–198. [Google Scholar]
- 18.Biancatelli RMLC, Berrill M, Mohammed YH, et al. Melatonin for the treatment of sepsis: the scientific rationale. J Thorac Dis. 2020;12:S54. doi: 10.21037/jtd.2019.12.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habtemariam S, Daglia M, Sureda A, et al. Melatonin and respiratory diseases: a review. Curr Top Med Chem. 2017;17:467–488. doi: 10.2174/1568026616666160824120338. [DOI] [PubMed] [Google Scholar]
- 20.Liu F, Li L, Xu M, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potempa LA, Rajab IM, Hart PC, et al. Insights into the use of C-Reactive protein as a diagnostic index of disease severity in COVID-19 infections. Am J Trop Med Hyg. 2020;103:561–563. doi: 10.4269/ajtmh.20-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]