Abstract
The objective of this paper is to explain how to apply, interpret, and present the results of a new instrument to assess the risk of bias (RoB) in non-randomized studies (NRS) dealing with effects of environmental exposures on health outcomes. This instrument is modeled on the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) instrument. The RoB instrument for NRS of exposures assesses RoB along a standardized comparison to a randomized target experiment, instead of the study-design directed RoB approach. We provide specific guidance for the integral steps of developing a research question and target experiment, distinguishing issues of indirectness from RoB, making individual-study judgments, and performing and interpreting sensitivity analyses for RoB judgments across a body of evidence. Also, we present an approach for integrating the RoB assessments within the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework to assess the certainty of the evidence in the systematic review. Finally, we guide the reader through an overall assessment to support the rating of all domains that determine the certainty of a body of evidence using the GRADE approach.
Keywords: Risk of bias, Environmental health, GRADE, Non-randomized studies, Study limitations, ROBINS
1. Introduction
The evidence on the impact of environmental or occupational exposures on human health outcomes typically comes from non-randomized studies (NRS). Objective and transparent evaluation of evidence of exposures requires the use of systematic reviews (Woodruff and Sutton, 2014). A highly credible systematic review should include a standardized, rigorous, and transparent assessment of the risk of bias (RoB) in each included study and across the body of evidence (Balshem et al., 2011; Liberati et al., 2009). This is applicable when referring to studies evaluating the impact of an environmental, occupational or other type of exposure.
A recent study evaluated five RoB methods used in environmental health hazard assessments (Rooney et al., 2016). While all five methods considered similar issues (or domains) in RoB assessment, their relative emphasis on these issues varied. The study suggested a need for the harmonization and improvement of these methods. We developed the RoB instrument for NRS of exposures based on the feedback from developers of existing instruments and methods to address limitations such as outlining the ideal study, labelling of study designs, and the use of signaling questions (Rooney et al., 2016; Morgan et al., 2018a). The objective of this paper is to explain how to apply, interpret, and present the results of a new instrument to assess the RoB in NRS dealing with effects of environmental exposures on health outcomes.
2. Overview of the instrument
The RoB instrument for NRS of exposures is modeled after the Risk Of Bias In Non-randomized Studies of interventions (ROBINS-I) instrument (Sterne et al., 2016). In 1965, Cochran proposed evaluating NRS using the criteria for RCTs (Cochran and Chambers, 1965). Hernan et al. recently suggested that causal inference from NRS represents an attempt to emulate the ideal randomized trial (the target trial) that would answer the question of interest (Hernán and Robins, 2016). In fact, ROBINS-I uses a hypothetical ideal target trial that would be free of bias as a reference point. By using the target trial as the reference point, ROBINS-I moves away from a study-design directed approach. That is, the specific design of the NRS, e.g. a case-control design, does not a priori determine absence or presence of RoB (Schünemann et al., 2018). RoB instrument for NRS of exposures emulates these features of ROBINS-I.
In brief, the application of the RoB instrument for NRS of exposures consists of three steps:
Step I: presents the review question, potential confounders, co-interventions, and exposure and outcome measurement accuracy information;
Step II: describes each eligible study as a hypothetical target experiment, including specific confounders and co-interventions from that study that will require consideration; and
Step III: assesses RoB across seven items about the strengths and limitations of studies of environmental exposure.
The seven RoB items are: 1) Bias due to confounding, 2) Bias in selection of participants into the study, 3) Bias in classification of exposures, 4) Bias due to departures from intended exposures, 5) Bias due to missing data, 6) Bias in measurement of outcomes, and 7) Bias in selection of reported results. Judgments for each RoB item can be: ‘Low RoB’, ‘Moderate RoB’, ‘Serious RoB’, or ‘Critical RoB’. Similarly, an overall judgment about the bias at the study level is either ‘Low RoB’, ‘Moderate RoB’, ‘Serious RoB’, or ‘Critical RoB’. In order to reach a judgment for each RoB item, the rater first answers one or more signaling questions with ‘Yes’, ‘Probably yes’, ‘Probably no’, ‘or No’. The answer should be based on the information available in the publications/reports of the individual study and be justified in an accompanying free-text field.
Previously published guidance for the ROBINS-I instrument proposes that the study-level RoB should be the most concerning level among the RoB items for that study, unless raters determine the study-level RoB to be more severe because of compounded risks of more than one individual RoB item (Sterne et al., 2016). Identifying RoB per item and across items per study allows systematic-review authors to explore the possible influence of studies at less compared to more severe RoB on the pooled estimates of effect (Guyatt et al., 2011a). As in the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for the body of evidence, risk of bias is assessed by outcome in a study and study RoB could vary by outcome (e.g. subjective outcomes may have different levels of bias than objective outcomes) or group of outcomes, if pragmatic rationale supports the grouping of outcomes.
Systematic-review authors can then use the RoB instrument as part of the assessment of the certainty of the body of evidence using the GRADE framework. Within the GRADE framework, RoB is one domain for assessing the certainty of evidence (CoE), the others being inconsistency, indirectness, imprecision, publication bias, magnitude of effect, dose-response gradient, and plausible opposing residual confounding (Balshem et al., 2011). As per the current GRADE guidance, evidence from NRS, appraised using existing design-specific RoB instruments, starts with a default initial certainty of “Low” due to concerns of confounding and selection bias when randomization is lacking. Raters then downgrade or upgrade the body of evidence according to specific GRADE domain assessments, including a more detailed evaluation for RoB other than confounding. However, since the RoB instrument for NRS of exposures takes into account lack of randomization, evidence will not be automatically rated down because judgments of risk of bias would have been made with reference to a hypothetical target experiment (ideal target trial). Bodies of evidence of any study design will undergo the same RoB evaluation without specific reference to the study design. In the context of using ROBINS-like instruments, all studies within the bodies of evidence will start at the same ‘High’ initial certainty within GRADE regardless of study design. However, in general, NRS, due to potential for confounding and selection bias when compared with RCTs will receive a rating of low or very low depending on the degree of RoB. Raters must justify not rating down only in the presence of specific study design and execution or result features (Schünemann et al., 2018).
When conducting a systematic review, results from the study-level RoB instrument for NRS of health effects of exposures inform judgments about overall RoB for the body of evidence across studies. So far, no guidance on the use of the RoB instrument for NRS of effects of exposures for this purpose exists. This article provides guidance for the application of the RoB instrument for NRS of exposures at the study level and as part of a RoB judgment within the GRADE framework to determine the certainty across a body of evidence (Morgan et al., 2018a). Although the RoB instrument for NRS of exposures is still being refined in consultation with a diverse group of subject matter experts, we highlight a number of important procedural questions. Thus, describing our experience in implementing the RoB instrument for NRS of effects of exposures will facilitate future testing and clarification of the use of the instrument by systematic review authors and guideline developers.
3. Approach when conducting systematic reviews for studies of exposure
We previously described the development of the RoB instrument for NRS of exposures (Morgan et al., 2018a). In addition to this effort, we have solicited broader input on this instrument at workshops held at GRADE Working Group meetings in March 2015, October 2015, and April 2016; during a meeting to develop ROBINS of Exposures (ROBINS-E; an instrument based on the RoB instrument for NRS of exposures and ROBINS-I) in January 2017; and at the Global Evidence Summit in September 2017. Findings from these workshops, through this iterative process, have led to further refinement and pilot-testing of the RoB instrument for NRS of exposures.
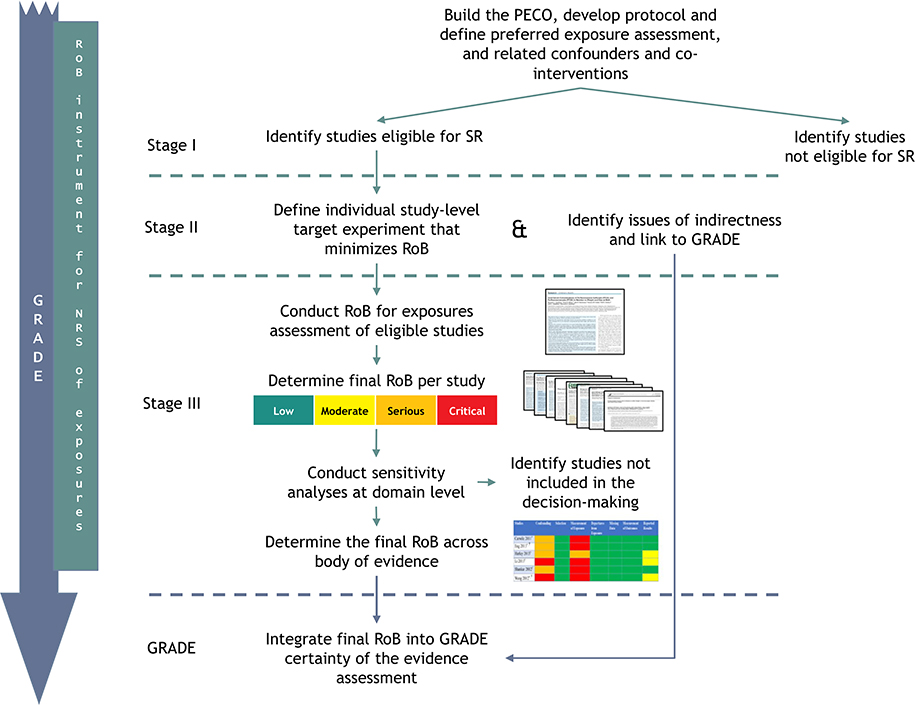
Fig. 1 presents a schematic of how the RoB instrument for NRS of exposures fits into the systematic review process. It illustrates steps for evaluating the RoB of individual studies in a systematic review and integrating the results across a body of evidence into the GRADE evidence-assessment framework. For each outcome in the review, authors of systematic reviews would go through Steps II and III, and GRADE.
Fig. 1.
Approach for conducting an assessment using the RoB instrument for NRS of exposures and the integration into GRADE when conducting systematic reviews of exposure.
GRADE: Grading of Recommendations Assessment, Development and Evaluation; PECO: population, exposure, comparator, outcome; RoB: risk of bias; SR: systematic review.
3.1. Complete step I of the RoB instrument for NRS of exposures
3.1.1. Define the research question
This process begins with the definition of the research question. For questions about exposures (i.e. unintentional interventions), namely the environmental and occupational type, the research question is formatted as a PECO (population, exposure, comparator(s), and outcomes) question (Morgan et al., 2018a; Morgan et al., 2016). For example, we may ask the following research question “In production workers exposed to steady state noise for ten years (population), what is the effect of exposure to a noise level of 80 dB(A) measured as LAeq,8h or greater (exposure) compared to less than 80 dB(A) also measured as LAeq,8h (comparison) in the same population on hearing level?” To understand the relation between noise and hearing loss, we may also ask the following PECO: “In production workers exposed to steady state noise louder than 80 dB(A) during ten years measured as L Aeq,8h, what is the effect of an increase of 5 dB(A) on hearing level compared to the level from where the increase started, over the whole range of exposure, assuming an exponential relationship between exposure and hearing level?”
Since the RoB instrument for NRS of exposures is set up as a comparison between groups that can be exposed or not, or exposed to different levels, it is necessary to clearly identify what is the exposure level of interest and what is the comparison. In some situations, little or nothing may be known about the relationship between an exposure and outcome to inform the PECO. There are at least five approaches to facilitate formulating and defining the levels of exposure within the PECO (Table 1) (Morgan et al., 2018b). Researchers should be transparent about which of these approaches they are using for definition of their PECO and ensure that the exposure and comparator(s) are explicitly defined.
Table 1.
Five paradigmatic approaches and examples for identifying the exposure and comparator in systematic review and decision-making questions (from Morgan RL, Whaley P, Thayer KA, Schünemann HJ: Identifying the PECO: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environment International 2018. (Morgan et al., 2018b))
| Potential systematic-review or research context | Approach | PECO example |
|---|---|---|
| 1. Calculate the health effect from an exposure; describing the dose-effect relationship between an exposure and an outcome for risk characterisation. | Explore the shape and distribution of the relationship between the exposure and the outcome in the systematic review. | Among newborns, what is the incremental effect of 10dB increase during gestation on postnatal hearing impairment? |
| 2. Evaluate the effect of an exposure cut-off on health outcomes, when the cut-off can be informed iteratively by the results of the systematic review. | Use cut-offs defined based on distribution in the studies identified in the systematic review. | Among newborns, what is the effect of the highest dB exposure compared to the lowest dB exposure (e.g. identified tertiles, quartiles, or quintiles) during pregnancy on postnatal hearing impairment? |
| 3. Evaluate the association between an exposure cut-off and a comparison cut-off, when the cut-offs can be identified or are known from other populations. | Use mean cut-offs from external or other populations (may come from other research). | Among commercial pilots, what is the effect of noise corresponding to occupational exposure compared to noise exposure experienced in other occupations on hearing impairment? |
| 4. Identify an exposure cut-off that ameliorates the effects on health outcomes. | Use existing exposure cut-offs associated with known health outcomes of interest. | Among industrial workers, what is the effect of exposure to <80 dB compared to ≥80 dB on hearing impairment? |
| 5. Evaluate the potential effect of a cut-off* that can be achieved through an intervention to ameliorate the effects of exposure on health outcomes. | Select the comparator based on what exposure cut-offs can be achieved through an intervention. | Among the general population, what is the effect of an intervention that reduces noise levels by 20 dB compared to no intervention on hearing impairment? |
3.1.2. Identify confounders, co-interventions, and measures of exposures and outcomes
In Step I, systematic-review authors list confounders and co-interventions that are associated with both the exposure and outcome. In addition, review authors assess the accuracy of the exposure and outcome measurements. These sections must be populated by knowledgeable members of the review team. While working through these sections, raters respond to signaling questions in the confounding, participant selection, and exposure measurement RoB items. Consideration of these issues may lead to the identification of different sources of indirectness (Morgan et al., 2018a). For example, the review team may identify obesity as one of their important outcomes; however, studies may measure waist circumference (and measure it accurately within the study) to inform the outcome of obesity. The review team may label waist circumference as an indirect measure of obesity.
We present the text used in the review-level protocol for an example on bisphenol A (BPA), comparing the highest exposure stratum and lowest exposure stratum of BPA in each eligible study (Appendix A). The PECO being: “What is the effect of highest levels compared with lowest levels of BPA exposure on body weight?” We reviewed published literature, as well as consulted with topic-specific experts, to determine the final set of responses to the Step I fields. For some exposures, a public database of confounders for measures of environmental exposures and health outcomes (i.e., PhenX Toolkit; https://www.phenxtoolkit.org/) may provide additional information.
3.2. Complete Step II of the RoB instrument for NRS of exposures for eligible studies
3.2.1. Construct the target experiment
At this point, the studies that meet the eligibility criteria of the review should have been identified. The reviewers should complete separate forms for each relevant outcome (group) within each study. At the start of Step II, reviewers construct a study-specific target experiment informed by the PECO question, the exposure and comparator exposure thresholds, outcome specific confounders, and health outcome measurements. As explained in previous GRADE guidance for the use of ROBINS-I, the target experiment provides a structured comparison with a reference experiment that is considered to be at low RoB (Schünemann et al., 2018). The target experiment need not be realistic, as it should reflect a study design that reduces known and unknown imbalance in prognostic factors and confounding (Morgan et al., 2018a). It then allows RoB assessment of individual studies and across studies at a later stage against the lowest possible bias that research could yield for the question at hand. Also, in Step II, the reviewer records how the individual studies measured the exposure and health outcome. The information recorded in Step II informs the RoB judgments made in Step III.
For example, let’s consider our review on BPA and weight. The PECO of the review is comparing the highest to the lowest level of BPA exposure. In Step II, we determine the target experiment for the included study (Appendix B). Based on the quantities identified in the study by Carwile & Michels (Carwile and Michels, 2011), the target hypothetical experiment would be framed as an experiment in which the general adult population is randomly allocated to a high level of BPA exposure (≥4.7 ng/mL) or a low level of BPA exposure (≤1.1 ng/mL) and body weight measured. In this situation, we compared two exposure cut-offs to determine the effect on obesity.
Confounders must be explored in each eligible study, as studies and outcomes may be affected by different confounders. For example, the review question may be about the general population, but the study includes only industrial workers, which may introduce additional confounders, such as exposure to other chemicals. Note that it may have impact on judging indirectness or selection bias, too. Also, in Step II, the reviewer makes a judgment of the potential magnitude and direction of the impact of the confounding factor on the effect estimate. For example, when examining the effect of BPA on body weight, consumption of processed foods is considered a confounder as it both increases the participants’ exposure to BPA through food packaging and increases overall caloric intake (Ranciere et al., 2015). We present the completed Step II sections for two studies from our BPA and obesity example: Carwile and Michels (2011); Harley et al. (2013) (Appendices B & C).
3.2.2. Identifying sources of indirectness to integrate within GRADE and their relation to risk of bias
While establishing the target experiment in Step II, individuals may identify studies that present evidence different from the PECO question (i.e., a restricted version of any concept such as only part of the population of interest or a section of the range of interest for high exposure) (Guyatt et al., 2011b). For example, consider again the review of hearing loss due to noise exposure. Studies with only shift workers may be considered indirect evidence for effects in the general population. Studies reporting on waist circumference may be considered indirect evidence for the measure of the outcome of obesity. Sources of indirectness may also come from studies that do not have a direct comparison (and therefore results would be compared to results from an external control or comparator group) or when using surrogate measures. While the review team may decide to include this study in the review, when evaluating the evidence within GRADE, differentiation between the domain of risk of bias and indirectness may be rather nuanced. Consider the following: the target experiment serves as the anchor point. If the study at hand tries to emulate the exposure specified in the target experiment but does not achieve what it sets out to do, it is subject to bias. If it acknowledges difficulty in mimicking and defines a proxy experiment, which the study appropriately implements, then it could be considered indirectness in relation to the question of interest.
Subsequent considerations for RoB when using indirect evidence in a review require critical evaluation to identify potential for misclassification of the exposure. While it is important to recognize the potential for more serious bias in classification of exposures when using an indirect comparison, there are situations in which they may present less risk because of clearly delineated exposure and comparison groups (e.g. there is little to no concern that the exposure groups are overlapping).
Similarly, studies identified for the review may use exposure measures that are indirect to those identified in the PECO, i.e., proxy or intermediate markers of measures. Within the BPA example, the measurement of exposure level based on a participant’s job title (e.g. cashier) would be indirect (Thayer et al., 2013). Extrapolating BPA exposure levels based on a participant’s job title may also introduce a risk to bias based on specific prognostic factors or the ability to differentiate between the levels of exposure.
3.3. Complete Step III of the RoB instrument for NRS of exposures assessment for eligible studies
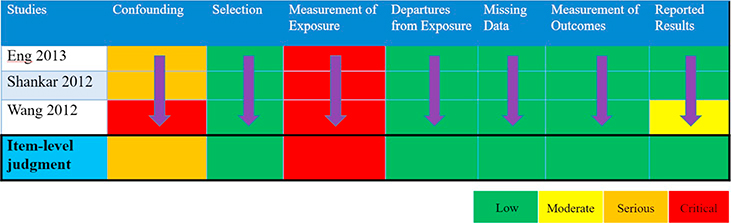
Raters evaluate eligible studies and determine RoB by responding to signaling questions for each of the seven RoB items listed previously. Appendices D & E present summaries from two studies addressing BPA and body weight (as measured by prevalent overweight and prevalent obesity). We present judgments across assessments of the RoB instrument items for NRS of exposures in a RoB matrix for all eligible studies in Table 2.
Table 2.
Risk of bias matrix presenting judgments for highest BPA exposure vs. lowest BPA exposure on the outcome of body weight, for the 7 RoB items, for 6 included studies.
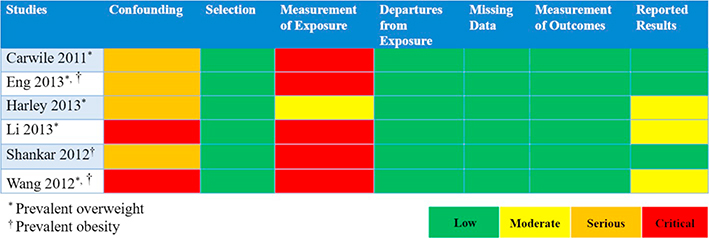 |
Due to the lack of randomization and allocation concealment, studies will typically be judged as ‘Serious’ RoB within the item of bias due to confounding and, also, may be judged as ‘Serious’ due to selection of participants. While RoB items 4-to-7 are similar to those used to evaluate RCTs (Sterne et al., 2016; Higgins et al., 2016), bias due to confounding, selection of participants, and classification of the exposure present considerations unique to studies of exposures (Morgan et al., 2018a). Below, we highlight some of these nuances and how raters can address them in their item- and study-level RoB judgments.
3.3.1.1. Bias due to confounding.
Three situations require particular attention when evaluating bias due to confounding for exposures: 1) the evaluation of cross-sectional studies; 2) considerations of large effects; and 3) opposing residual plausible confounding.
Cross-sectional studies can impact the judgment on the item-level RoB due to confounding (e.g. time-varying confounding). This is because we might be unable to evaluate time-varying confounding and it makes the measurement of the effect of known confounders more challenging. We present two examples from the BPA and body weight review. While Carwile & Michels adjusted for all critical confounders, the measurement of exposure and outcome at one time point lowers our certainty that temporal confounders (e.g. dietary preference for canned food) are not responsible for any observed long-term association (Appendix D) (Carwile and Michels, 2011). In this specific study, the data collection point is part of the National Health and Nutrition Examination Survey (NHANES), a nationally-representative dataset with years of prior data collection, therefore providing supplemental information about the adjustment of confounders. In contrast, within that review, neither Li nor Wang provide that same level of information about the data collection, therefore presenting “Critical” bias due to confounding (Table 1) (Li et al., 2013; Wang et al., 2012).
Studies judged as biased due to confounding with evidence of a large effect or opposing residual confounding (i.e. when residual confounders would result in the underestimating of an apparent exposure effect) may not require severe RoB item-level judgment (Guyatt et al., 2011c). This is due to the magnitude of the effect outweighing the size of the bias that might exist in the study or that all plausible biases go in a direction that would have reduced the observed effect or increased the observed lack of effect. These latter two domains contribute to increasing the CoE in a body of evidence of NRS in GRADE; however, within the RoB instrument for NRS of exposures they may also influence the study-level judgments (Guyatt et al., 2011c). To demonstrate this situation, we present an example on smoking and lung cancer-related mortality (Doll and Hill, 1950; Doll and Hill, 1964). A prospective cohort study compared lung cancer-related mortality rates among smokers and non-smokers (Doll and Hill, 1964). Although there are some concerns due to residual and unmeasured confounders, such as occupational or air pollution exposures, the large magnitude of effect (30 times greater mortality rate due to lung cancer among persons smoking 25 or more cigarettes vs. non-smokers) warrants a less severe RoB item-level judgment of ‘Low’ or ‘Moderate’, instead of ‘Serious’ for the RoB item of confounding (Doll and Hill, 1964). In this example, the large magnitude of effect reduces our concern that bias alone creates a spurious effect (Bross, 1966).
In addition, exploratory research conducted has suggested there is no relation between the 10 most common occupational exposures (i.e., sulfur dioxide, welding fumes, engine emissions, gasoline, lubricating oil, solvents, paints/varnishes, adhesives, excavation dust, and wood dust) and smoking history (Blair et al., 2007). This exploration into the relationship between exposures and the outcome of interest reduces our concern for potential residual plausible confounding due to other occupational or air pollution exposures even more.
3.3.1.2. Bias due to misclassification of exposure.
In NRS of exposure, there is a particular concern with distinguishing between the exposed and reference groups, as measuring exposure is difficult and the reference groups are often assumed to be non-exposed. Bias relating to exposure assessment is a major source of systematic error in studies of environmental exposures (Steenland and Savitz, 1997). This is dealt with explicitly in a separate paper (Kogevinas, 2011). It is crucial to identify the source and type of exposure misclassification. If non-differential, the exposure misclassification will usually bias associations to the null, although the final impact on the observed relative risk is also dependent on other factors (Jurek et al., 2005).
Systematic reviewers may be faced with different approaches to exposure assessment. In the example of noise exposure, this may be assessed by (in order of most severe to least severe exposure misclassification bias) (Nieuwenhuijsen, 2015):
Self-report questionnaire: Do you have to raise your voice to carry out a normal conversation with a colleague when approximately two metres apart for at least part of the working day (may indicate noise levels>80 dB);
Modelling: in the occupational setting, a job-exposure matrix would be an example, whereby an occupational hygienist classifies likely exposure ranges based on job title;
Environmental monitoring: using a noise monitor to measure noise in the workplace environment will give a continuous measurement but sensor measurement error likely to be optimised for certain exposure ranges;
Personal monitoring: using a personal noise monitor to measure exposure but sensor measurement error likely to be optimised for certain exposure ranges;
Individual dose: personal monitoring, additionally taking account of use of ear defenders, hearing acuity, etc.
In our example of BPA and body weight, the review team and topic-specific experts note the accuracy of the measurement of exposure requires multiple measurements (cited here from five-to-13 repeated measurements) at different time points, due to the non-persistent nature of BPA in the body (Cox et al., 2016). If an individual study uses fewer than the recommended number of samples, or since diagnostic accuracy of BPA with the collection of between five and 13 samples only yields ≥0.80 sensitivity and specificity depending on level of exposure (small, moderate, high), there are concerns for non-differential misclassification (i.e. random error) potentially conflating participants in the exposure and comparator groups, likely leading to little difference in the outcomes (i.e. bias toward the null). When the exposure is non-persistent, we have more confidence when studies use multiple timepoints to measure the exposure level. The number of collected samples increases our certainty in the correct classification of the higher exposed and lower exposed groups. In this situation we may consider the exposure domain for Harley to be of less potential risk of bias for misclassification of the exposure. Although repeated measures in urine are acceptable, there is still some scientific uncertainty about the most direct measure of BPA exposure (i.e. urine vs blood) (Vandenberg et al., 2013; Thayer et al., 2015). In Carwile & Michels, participants provided only one sample; therefore we may have critical concerns about bias due to misclassification of the exposure (Appendix D) (Carwile and Michels, 2011).
The single sampling method used in Carwile & Michels decreases our certainty that the higher exposed and lower exposed participants can be accurately distinguished. Returning to Fig. 1, in their protocol, review authors could have specified to exclude such studies a priori or identified this risk of bias item as a reason to conduct a sensitivity analysis (see below).
3.4. RoB judgments for an individual study for an outcome
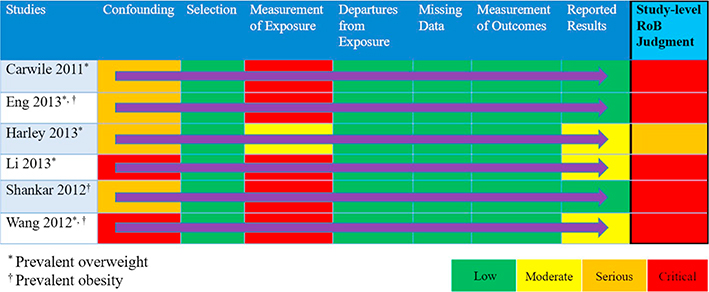
According to ROBINS-I guidance, raters should assign the study-level RoB according to the most severe of the RoB item-level judgments unless they determine the study to have more severe RoB based on a combination of RoB judgments across items (Sterne et al., 2016). We demonstrate this in our example of BPA and weight in Table 3. This approach relies on individuals critically evaluating the rationale and direction of the bias. For example, if more than one RoB item within a study were rated as serious RoB but no RoB items were of critical RoB, then the study-level RoB could either be serious or could be critical if the consideration of all serious ratings leads to greater concern than would be expressed by a rating of serious on the study level.
Table 3.
Study-level judgments for prevalent overweight and prevalent obesity.
 |
3.5. Sensitivity analyses and overall RoB across studies
Sensitivity analyses allow for exploration across a body of evidence to determine whether the pooled results are robust with including, versus excluding, studies with certain RoB (Higgins and Green, 2011). The variability in RoB judgments across individual studies may inform whether a selection of studies, rather than the whole body of evidence, best informs the research question. The approach to conducting sensitivity analyses (not to be confused with the sensitivity of a study) should be specified at the protocol step of the systematic review; however, may be identified after the preliminary analysis. For example, studies may be deemed critical in the domain of bias due to confounding resulting from unadjusted analyses of covariates. If a body of evidence includes studies with adjusted and unadjusted analyses, a sensitivity analysis could compare the estimates of effect for the adjusted (removing those studies not adjusting for covariates) and the total pooled estimate. If the effect estimates are not robust and differ between analyses (i.e. confounding may have an influence on the results), then review authors might consider whether to exclude the studies with unadjusted analyses; however, if the effect estimates do not differ (e.g. confounding apparently has no influence on the results), then the review authors may keep the unadjusted studies in the analysis because the suspicion of confounding apparently does not have a big impact. In these instances when the effect estimate is similar across studies then authors could consider updating the individual study level ratings to indicate a less severe RoB for the item and include the rationale that the sensitivity analysis showed no effect of RoB on the results.
Using BPA as an example, we compared studies for the body weight outcomes of prevalent overweight and prevalent obesity at higher and lower RoB in sensitivity analyses specifically across the domain of confounding (Tables 4 & 5; Appendices F & G). We conducted these sensitivity analyses to explore the potential for bias introduced by studies that did not adjust for all critical confounders. The sensitivity analysis for the outcome of prevalent overweight resulted in a difference between the effect estimates, demonstrating that bias due to confounding impacted the pooled estimate; therefore, the judgment would be reflective of the more severe RoB (Table 4). An additional option would be to only show results from Harley, Eng, and Carwile in the GRADE evidence assessment. In contrast, the sensitivity analysis of studies reporting on prevalent obesity demonstrated similar effect estimates (Appendix G). In this situation, all studies reflect the less serious RoB judgment (Table 5).
Table 4.
Item-level judgments for prevalent overweight.
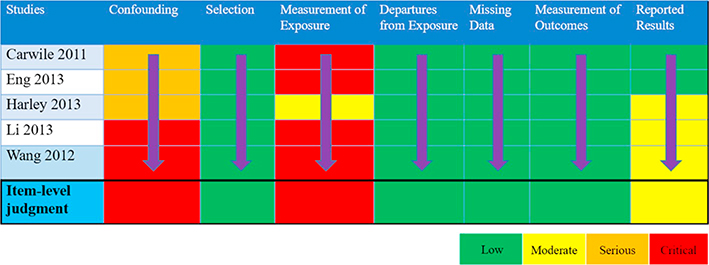 |
Table 5.
Item-level judgments for prevalent obesity.
 |
3.6. Integration of RoB judgments across a body of evidence into GRADE assessment
The overall rating of RoB across the body of evidence for an outcome is integrated into the GRADE assessment similar to what has been previously described in the literature for the result of RCTs and observational studies (Guyatt et al., 2011a). It is also during this process where indirectness, if identified during Steps I or II within the RoB instrument for NRS of exposures, would be integrated in the overall assessment of the evidence. When evaluating RoB using ROBINS-I and the RoB instrument for NRS of exposures, the body of evidence starts at ‘High’ initial CoE within GRADE. For the example of BPA and its effect on body weight, we present the outcomes of prevalent overweight (i.e., BMI≥85th percentile for age/sex in children; 25≤BMI<30 kg/m2 in adults) and prevalent obesity (BMI≥95th percentile for age/sex in children; BMI≥30 kg/m2 in adults) in a GRADE evidence profile (Table 6). It is across this body of evidence that we look for evidence of the three factors (magnitude of effect, dose-response gradient, and opposing residual confounding) considered in the past as mechanisms to upgrade the quality of the evidence for NRS within GRADE (Guyatt et al., 2011c). The BPA example does not demonstrate any situation, based on these three factors, which may lead to a less severe RoB judgment. Across the body of evidence for prevalent overweight, our RoB based on the RoB instrument for NRS of exposures and sensitivity analysis of the item of confounding is ‘Critical’, resulting in a rating down of three levels for RoB. In addition, we rate down for imprecision because the effect estimate crosses the null. Our final CoE would be ‘Very low’. Across the body of evidence for prevalent obesity, our RoB is ‘Serious’; therefore, we rate down two levels for RoB. There are no other GRADE domains that we would rate down for. Our final CoE would be ‘Low’.
Table 6.
Exposure to BPA on the outcome of birthweight GRADE evidence assessment.
| Question: Exposure to highest levels of BPA (CAS# 80-05-7) compared to exposure to lowest levels of BPA in general population | ||||||||||||
| Setting: Community | ||||||||||||
| Bibliography: Rancière, F., Lyons, J. G., Loh, V. H., Botton, J., Galloway, T., Wang, T., … & Magliano, D. J. (2015). Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environmental Health, 14(1), 46 (Ranciere et al., 2015). | ||||||||||||
| Quality assessment | No. of patients | Effect | Quality | Importance | ||||||||
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Exposure to highest BPA levels | Exposure to lowest BPA levels | Relative (95% CI) | Absolute (95% CI) | ||
| Prevalent overweight (assessed with: BMI≥85th percentile for age/gender in children; BMI 18.5–25/30 kg/m2) | ||||||||||||
| 5 | Studies | Very, very serious a | Not seriousb | Not seriousc | Seriousd | None | 1774/5403 (32.8%) | 1584/5657 (28.0%) | OR 1.21 (0.98 to 1.56) | 40 more per 1000 (from 4 fewer to 98 more) | ⊕◯◯◯ VERY LOW |
Critical |
| Prevalent obesity (assessed with: BMI≥95th percentile for age/gender in children; BMI≥25–30 kg/m2) | ||||||||||||
| 3 | Studies | Very seriousa | Not serious | Not seriousc | Not serious | None | 1425/5178 (27.5%) | 1204/5342 (22.5%) | OR 1.67 (1.32 to 1.93) | 102 more per 1000 (from 52 more to 134 more) | ⊕⊕◯◯ LOW |
Critical |
CI: Confidence interval; OR: Odds ratio.
Explanations
Most studies adjusted for known confounders of weight (age and gender) and diet; however, two studies did not account for caloric intake or diet which is relevant for evaluating weight-related outcomes, there is some risk of unmeasured confounding; BPA measurement present potential for bias as the chemical is non-persistent with a short half-life and exposure measurements were not repeated (except in one study), one study measures BPA three months post-BMI measurement, remaining studies measure BPA and BMI at the same time; however, the effect estimates may underestimate the true effect reducing our concern of non-differential misclassification; potential risk of reporting bias because three studies did not report prior publication of a protocol; however, all studies present outcome measures and analyses consistent with a priori plan outlined in the manuscript.
The I2 value=45% and exploration of the forest plot suggests some inconsistency introduced by one outlying study contributing 4.3% of the weight to the analysis of children.
Studies measured BPA concentration through urinary output. uBPA (BPA in urine) is considered a reliable and direct measure of BPA consumption and was not downgraded for indirectness.
Imprecision is present because the width of the confidence interval is consistent with no association.
4. Discussion
The RoB instrument for NRS of exposures presents a novel instrument for conducting the RoB assessment of individual studies included in a systematic review of the health effects of exposure. In this users’ guide, we suggest that the RoB instrument for NRS of exposures provides a standardized instrument for the transparent evaluation of RoB for NRS of exposures. We present an overview of the process, using examples to demonstrate specific issues encountered when formulating the PECO for the review, outlining a target experiment for an individual study, evaluating bias in individual studies, and summarizing judgments across the body of evidence. We highlight the need for critical consideration of the RoB judgments, including situations within individual studies and across a body of evidence when the judgments may be less severe. In addition, we present sources of indirectness identified in eligible studies that would inform the GRADE evidence assessment. We also present the steps for integrating the RoB across a body of evidence into a GRADE evidence profile.
4.1. Advantages and disadvantages of using the RoB instrument for NRS of exposures approach
Some challenges remain, specifically when defining the target experiment and making judgments at the study and review level. The major challenge when identifying a hypothetical target randomized experiment is that much of the research on environmental health exposures focuses on a potential link with a human health hazard. Defining a specific comparison to an exposure presents a challenge, as there may be a paucity of evidence to support the distinct exposure and comparator; however, in this paper we present five scenarios to facilitate the identification of an exposure and comparator (Morgan et al., 2018b). In addition, the best available studies to inform a review may only present data on one exposure category. In this situation, we recommend other sources of comparative exposure data, such as historical controls (i.e. source of data presents levels of exposure before and after introduction to a known source of exposure).
Inter-rater reliability of the RoB instrument for NRS of exposures has not yet been measured; however, the purpose of the RoB instrument for NRS of exposures is not necessarily to have different experts reach the same judgment per study and across studies, but instead to justify the judgements and make the judgements transparent. We present several examples when using the RoB instrument for NRS of exposures. More examples are needed to highlight nuances of this instrument when applied on an individual-study and across-study basis.
Based on concerns from systematic-review authors and guideline developers in the environmental health field, the RoB instrument for NRS of exposures evaluates bias using a standardized comparison to a hypothetical target experiment. This allows the body of evidence to start at ‘High’ initial CoE within the GRADE framework, potentially improving acceptability of this instrument and the use of GRADE for environmental decision-making assessments. Of note is that randomized controlled exposure trials in animals would be evaluated with the framework for randomized trials and not the herein described instrument.
4.2. Relation to other studies
This is the first article describing examples from systematic reviews using the RoB instrument for NRS of exposures to evaluate the RoB across a body of evidence for a specific outcome. We present one option of a RoB matrix displaying the RoB study- and item-level judgments. In addition, we present examples of when an individual and a body of evidence RoB judgment may be improved (determined to be a less severe RoB) based on further exploration of residual and unmeasured confounding. We highlight the value added by performing sensitivity analyses with the body of evidence to explore sources of bias.
The application of ROBINS-I for RoB assessment across a body of evidence is undergoing further development, as are the procedures for interpreting RoB within the GRADE approach when NRS are compared to RCTs as in the RoB instrument for NRS of exposures or ROBINS-I (Schünemann et al., 2018). Collaboration between the developers of the RoB instrument for NRS of exposures and these projects allows for an iterative approach to methods advancements. We expect that this approach would be applicable to broader research of exposures conducted in the fields of public health and nutrition, not limited to environmental exposures.
4.3. Implications for stakeholders using the RoB instrument for NRS of exposures
Evaluating the RoB across the body of evidence for an outcome informs one domain within the GRADE framework’s evidence assessment contributing to the understanding about the overall CoE. Using this instrument should not result in a final certainty distinct from the prior approach of starting NRS at ‘Low’ initial CoE within GRADE because the conceptual underpinnings are the same. However, the approach is fairer and more transparent. Indeed, users may prefer investigating the relationship between rating down for imbalances due to confounders, selection bias, or misclassification of the exposure instead of starting at ‘Low’ initial CoE as a general judgment about these items. The process and examples outlined in this manuscript provide guidance for researchers and guideline developers using evidence about exposures to inform their systematic reviews and decision making.
4.4. Unanswered questions and future research
This research provides many opportunities for further application and assessment of the RoB instrument for NRS of exposures and integration into GRADE. Specific areas of interest based on our research may include 1) how to apply the RoB instrument for NRS of exposures to primary studies that use different exposure measurement strategies; 2) the process for making a judgment about the body of evidence when using different techniques to synthesize evidence of the effects; and 3) the role of dose-response within RoB and GRADE assessments.
We present several measurement strategies that may be used when direct measures of the exposure are unfeasible or not available, such as modelling, or environmental or personal monitoring. Each method may be associated with greater or lesser specificity and/or potential for exposure misclassification. Application of the RoB instrument for NRS of exposures to topics using these measures is needed.
In addition, we present the process for when the RoB across a body of evidence can be further explored and assessed by using meta-analytic approaches; however, systematic reviews of exposures may use other approaches to summarize evidence, such as a qualitative analysis or narrative summary. Further exploration of how these methods may translate to different summary approaches is needed.
Lastly, while we present situations of where magnitude of effect and opposing residual confounding may decrease our concerns about bias within both individual assessments and across the body of evidence, more exploration of the role of dose-response is needed. Future research should provide examples of how to incorporate dose response into an assessment using the RoB instrument for NRS of exposures.
5. Conclusions
The RoB instrument for NRS of exposures provides a novel approach for evaluating RoB of exposures. Determining the RoB across a body of evidence is critical to inform decision making about health exposures. We present guidance and examples for systematic-review authors and guideline developers to follow when using this instrument.
Acknowledgments
We are grateful to all systematic review and GRADE members who have collaborated with their feedback and suggestions for this work. We thank Jani Ruotsalainen, Finnish Institute of Occupational Health, Cochrane Work Review Group, for his help in formulating the PECO questions on noise exposure.
Funding sources
This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences and the MacGRADE Centre at the McMaster University.
Appendix A. Step I of the RoB instrument for NRS of exposures for the PECO: “What is the effect of highest levels vs. lowest levels of BPA exposure on weight?”
| Step I Items | Response |
|---|---|
| Confounding for BPA and obesity | •Body composition (age, ethnicity, gender, height, race); •Weight (age, gender); •Waist circumference (age, gender); •Body mass index (age, ethnicity, gender, race); •In addition, consumption of canned or packaged food and drink (“processed” food) that is also energy dense and low-nutrient (e.g., soda) is a significant confounder because food packaging is a main source of exposure to BPA. •Co-exposures: There may be some concern for co-exposure to certain phthalates used in food packaging that have also been linked to obesity. However, phthalates are used in different types of food packaging than BPA (plastic wraps versus canned lining and polycarbonate materials). No other a priori co-exposures of particular concern are identified for general population studies. There may be some co-exposures that need to be considered in occupational studies and these should be assessed on a case by case basis if discovered. |
| Co-interventions | •None identified |
| Accuracy of the measurement of exposure to BPA (CAS# 80–05-7) | •BPA is a non-persistent compound (near 100% elimination within 24 h after oral exposure, possible longer elimination time from non-oral exposure but on order of days), so blood and urine measures only assess recent exposure. This means current exposure levels may NOT be indicative of past exposures. This is problematic for assessment of BPA as a risk factor for health outcomes that are not acute and take time to develop like obesity. •BPA measures are variable over time in the same person (even during the same day) so methods that utilize repeated measures of exposure are preferred. Some experts on BPA exposure assessment express less concern for lack of repeated measures for NHANES data because it is a large sample survey of the general population. •Standard analytical measures: Measurement of urine or blood by quantitative techniques such as liquid chromatography-triple quadrupole mass spectrometry (LC-MS/MS) and high-pressure liquid chromatography with tandem mass spectrometry (HPLC/MS) are preferred. Measurements made at CDC are considered high-quality. •Measures to minimize sample contamination with BPA should be taken (e.g., glass pipettes, polypropylene plastic lab ware and sample collection materials, water blanks). •Measures of unconjugated BPA in blood need to be very carefully considered based on extent to which investigators controlled for background exposures. •Questionnaire or self-reported measures of BPA exposure are more problematic due to the ubiquity of exposure and lack of knowledge on all possible routes of exposure, e.g., thermal paper, certain pharmaceuticals. However, there is some support for an association between higher urine/blood levels of BPA and higher reported use of BPA-containing food packaging (e.g., canned food consumption) or handling of BPA-containing thermal paper (cashiers) so questionnaire data that assess these types of exposure sources may have some utility in assessing longer-term time trends in exposure. |
| Accuracy of the measurement of outcome of obesity | •Body Composition: Dual-energy X-Ray absorptiometry, triceps skinfold thickness, subscapular skinfold thickness, suprailiac skinfold thickness •Measured waist circumference •Body mass index •Measured weight *Obesity typically develops relatively slowly over time so preferred follow-up times after start of exposure would be on the order of several months to years. |
Appendix B. Step II of the RoB instrument for NRS of exposures for Carwile and Michels, 2011
B.1. Specify a target randomized trial specific to the study
| Design | Individual randomized controlled trial |
|---|---|
| Participants | Adults of all ages, predominantly 18–35 years (8.2% < 18years and 7.9% > 35 years). Civilian, non-institutionalized, United States population. Analyses restricted to participants 18–74 years of age, who were included in the random subsample of participants, who supplied a spot urine sample analyzed for BPA. |
| Experimental intervention | BPA highest levels (quartile 4: ≥4.7 ng/mL) |
| Comparator | BPA lowest levels (quartile 1: ≤1.1 ng/mL) |
B.2. Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the Summary of Findings table). Specify whether this is a proposed benefit or harm of intervention.
| Prevalent overweight (Overweight: 25≤BMI<30 kg/m2 [reference: BMI<25 kg/m2]) |
B.3. Specify the numerical result being assessed
In case of multiple alternative analyses being presented, specify the numeric result (e.g. RR=1.52 (95% CI 0.83 to 2.77) and/or a reference (e.g., to a table, figure or paragraph) that uniquely defines the result being assessed.
| Participants in the upper BPA quartile 4 vs. participants in the lowest BPA quartile 1: OR: 1.76, 95% CI: 1.06–2.94) | ||||
| (i) Confounding domains listed in Step I | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is failure to adjust for this variable (alone) expected to favor the experimental intervention or the comparator? |
| Yes/No/No information | Favor experimental/Favor comparator/No information | |||
| Age, gender | Weight | No | Yes | Favor experimental |
| Consumption of canned or packaged food and drink (“processed” food) that is also energy dense and low-nutrient (- e.g., soda) | Daily caloric intake | No | No | Favor experimental because obese individuals (potentially caused by higher consumption of canned foods and drinks) have higher urinary BPA levels relative to those with normal weight. |
| (ii) Additional confounding domains relevant to the setting of this particular study, or which the study authors identified as important | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is failure to adjust for this variable (alone) expected to favor the experimental intervention or the comparator? |
| Yes/No/No information | Favor experimental/Favor comparator/No information | |||
| Alcohol drinking, fish intake, protein, fat, carbohydrate, and energy intake | None | No | No | |
Carwile JL, Michels KB: Urinary bisphenol A and obesity: NHANES 2003–2006. Environmental research 2011, 111(6):825–830 (Carwile and Michels, 2011).
Appendix C. Step II of the RoB instrument for NRS of exposures for Harley et al., 2013
C.1. Specify a target randomized trial specific to the study
| Design | Individual randomized controlled trial |
|---|---|
| Participants | Children at 5 and 9 years of age born to eligible pregnant women were at least 18 years of age, spoke English or Spanish, qualified for low-income health insurance, were at <20 weeks gestation, and were planning to deliver at the county hospital. Must have had a singleton, live birth. |
| Experimental intervention | BPA highest levels (tertile 3: 4.6–349.8 μg/g) |
| Comparator | BPA lowest levels (tertile 1: <LOD-2.4 μg/g) |
C.2. Specify the outcome
Specify which outcome is being assessed for risk of bias (typically from among those earmarked for the Summary of Findings table). Specify whether this is a proposed benefit or harm of intervention.
| Prevalent overweight (Overweight: BMI≥85th percentile at 5 and 9 years of age) |
C.3. Specify the numerical result being assessed
In case of multiple alternative analyses being presented, specify the numeric result (e.g. RR=1.52 (95% CI 0.83 to 2.77) and/or a reference (e.g. to a table, figure or paragraph) that uniquely defines the result being assessed.
| Participants in the upper BPA tertile 3 vs. participants in the lowest BPA tertile 1: OR=1.36 (0.75–2.47) | ||||
| (i) Confounding domains listed in Step I | ||||
| Confounding domain | Measured variable(s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is failure to adjust for this variable (alone) expected to favor the experimental intervention or the comparator? |
| Yes/No/No information | Favor experimental/Favor comparator/No information | |||
| Age, gender | Weight | No | Yes | Favor experimental |
| Consumption of canned or packaged food and drink (“processed” food) that is also energy dense and low-nutrient (e.g., soda) | Child consumption of soda, fast food, and sweets | No | Yes | Favor experimental because obese individuals (potentially caused by higher consumption of canned foods and drinks) have higher urinary BPA levels relative to those with normal weight. |
| (ii) Additional confounding domains relevant to the setting of this particular study, or which the study authors identified as important | ||||
| Confounding domain | Measured variable (s) | Is there evidence that controlling for this variable was unnecessary? | Is the confounding domain measured validly and reliably by this variable (or these variables)? | OPTIONAL: Is failure to adjust for this variable (alone) expected to favor the experimental intervention or the comparator? |
| Yes/No/No information | Favor experimental/Favor comparator/No information | |||
| Television watching | Average daily TV time | No | Yes | Favor experimental |
| Environmental tobacco smoke exposure | Self-reported mother’s smoking status | No | Yes | No information |
| Time spent playing outdoors | Unknown | No | No information | No information |
Harley KG, Schall RA, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B: Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environmental health perspectives 2013, 121(4):514 (Harley et al., 2013).
Appendix D. Summary of Step III of the RoB instrument for NRS of exposures and the direction of bias and reaching the overall bias judgement for Carwile and Michels (2011)
| Bias items | Risk of bias | Direction of bias | Rationale |
|---|---|---|---|
| Bias due to confounding | Serious | Unknown | NHANES data were used. Specific details were not provided in the study report, but NHANES co-variate data were obtained from either a standardized questionnaire or laboratory methods (e.g., creatinine). The reliability/validity of the questionnaire was not reported, but it is not expected to appreciably bias the results. Most of the critical confounders were considered statistically, but there is possibility of residual unmeasured (and unidentified) confounding. For the most part, although certain post-exposure variables are relevant to evaluating obesity (e.g., caloric intake), there is little information on the association of these variables to BPA exposure. No indication that time-varying confounding is a major concern given the cross-sectional nature of the study. Critical confounders (age, gender, and ethnicity) were accounted for in the analysis. Model 1 was adjusted for age, sex, and urinary creatinine. Model 2 was adjusted for race, education, and smoking in addition to Model 1 covariates. |
| Bias in selection of participants into the study | Low | N/A | Study is cross-sectional. Subjects were randomly selected from NHANES subjects with urinary BPA data available using the same criteria. Selection of subjects was unrelated to either exposure or outcome. While there is no information on start of exposure, everyone is exposed to BPA throughout their life, but the levels will change over time. Although BPA is ubiquitous, start of exposure and how exposure changes over time are not known. Timing of recruitment was similar (2003–2006) but given that the age ranged from 18 to 74 years, exposure could range by more than a decade. |
| Bias in classification of exposures | Critical | Concerns of bias toward the null due to non-differential misclassification of the exposure. | Urinary BPA concentration was measured in 1 spot sample from each participant. The lower limit of detection (LLOD) was 0.36 ng/mL in 2003/04 and 0.4 ng/mL in2005/06. For BPA concentrations below the LLOD (2003/04: n=110/1373 [8%]; 2005/06: n=114/1374 [8%]) NHANES assigned a value of the LLOD divided by the square root of two. BPA is a non-persistent compound and exposure measures were not repeated. Therefore, there is no confidence that the current exposure reflects exposure over the subject’s life time or even over any duration of time. Because this population is obtained from NHANES some experts consider the lack of repeated measures to be less of a concern because it is a large survey of the general population (this cross-sectional study had a population of 2747 adults). Exposure was measured at same time as outcome, but participants were likely exposed throughout life due to BPA being a ubiquitous exposure. Therefore, it is unlikely that entry into the cohort started with the exposure. Cross-sectional analyses with both BPA exposure and weight, height, and waist circumference used to define obesity assessed simultaneously. Urine samples were obtained at the time that obesity measurements were obtained and analyzed later in a laboratory separate from where the data were collected. In addition, NHANES collected data on a variety of compounds and health effects without knowledge of the intent for this current study indicating that exposure status is not likely to be biased by knowledge of the outcome. The range/variability in exposure was likely sufficient with a 25th to 75th percentile range of 1.18 to 3.33 ng/mL urinary BPA ng/mL and quartiles ranging from <1.1 ng/mL to >4.7 ng/mL. However, we are not confident that the subjects were exposed to this concentration for a long period of time. Lacking information on the duration that subjects were exposed to these levels, the single BPA measurement obtained at the same time as outcome is not of sufficient to detect an effect of exposure. Urinary BPA samples were collected at the same time that height, weight, and waist circumference were measured. Because BPA is not persistent, and obesity is not an acute effect, there is not adequate follow-up period to allow for the development of the outcome of interest. Total (free and conjugated) urinary BPA concentrations were measured at the Division of Environmental Health Laboratory Sciences (National Center for Environmental Health, CDC) using online solid-phase extraction coupled to isotope dilution high-performance liquid chromatography-tandem mass spectrometry. Quality control (QC) procedures included analysis of reagent blanks and samples of pooled human urine spiked with BPA at low-and high-concentrations. Coefficients of variation calculated for low-and high-concentration QC samples were 19% and 12% in 2003–2004 and 13% and 11% in 2005–2006. Additional information on laboratory methods is available online (CDC, 2004, 2006). |
| Bias due to deviations from intended exposures | Low | N/A | There is little concern that changes in exposure status occurred among participants. Although BPA levels may change overtime, the cross-sectional nature of the study and the intention-to-treat analyses this is of little concern because participants are analyzed based on the exposure group they are assigned from the single measurement. No critical co-exposures were identified and nothing about the subject characteristics suggests likelihood of differential exposure to other environmental contaminants at lower versus higher concentrations of BPA. |
| Bias due to missing data | Low | N/A | There is no information on the missing data by exposure level, but it is unlikely to be related to exposure level. The missing indicator method was used for covariates with missing data for ≥10% of observations, otherwise observations with missing covariate data were excluded. Data excluded from analysis did not exceed 4% and is considered relatively complete. 32 or 87 observations were stated excluded from analysis due to missing BMI data depending on the analysis conducted. 47 participants were excluded based on missing urinary BPA measurements. There were observations excluded based on missing covariate data. The number varied with the analysis but was only excluded if it was <10%. |
| Bias in measurement of the outcome | Low | N/A | It is unlikely that the outcome could be affected by knowledge of exposure. Height, weight, and waist circumference were measured using standard NHANES protocols (not described in the publication, but available on NHANES website). Body mass index was calculated (weight (kg)/height (m)2). The specific measurements would not be affected by knowledge of exposure, and it is unlikely that the calculation or assignment into obesity category would be affected by knowledge of exposure. Specific methods were not reported in the study report but are provided on NHANES website. Height and weight are likely sensitive measurements with waist circumference likely slightly less sensitive. Height, weight, and waist circumference were measured by trained technicians using a standardized protocol. Method details, including QA/QC procedures, are available on the NHANES website. BMI was calculated as weight in kilograms divided by height in meters squared and used to define overweight [25.0<BMI<29.9] and obesity [BMI >30.0]. It is unlikely that any systematic error in measuring height, weight, or waist circumference (or in calculating the BMI or assigning obesity category) would have been related to exposure. NHANES has a standard protocol for measuring height, weight, and waist circumference that would have been used for all subjects. Outcome was assessed at the time of sample collection for exposure. Therefore, exposure was unknown at time of outcome assessment. |
| Bias in selection of the reported result | Low | N/A | Reporting of the results is consistent with an a priori plan and data were readily available from NHANES that provides all protocols for obtaining the data online. Results were provided for two measurements of obesity, which were reported in the methods making it unlikely that there is selective reporting based on outcome. Statistical methods reported in the methods section were used and presented in the results. Associations between urinary BPA and obesity were assessed for effect modification by gender, which were provided in the supplemental material. |
| Overall bias | Serious | Possibly toward the null | Overall bias was judged as Serious due to concerns of potential unknown confounders, unmeasured confounding due to the single time-point data collection, and concerns of non-differential misclassification of the exposure. |
Carwile JL, Michels KB: Urinary bisphenol A and obesity: NHANES 2003–2006. Environmental research 2011, 111(6):825–830 (Carwile and Michels, 2011).
Appendix E. Summary of Step III of the RoB instrument for NRS of exposures and the direction of bias and reaching the overall bias judgement for Harley et al., 2013
| Bias items | Risk of bias | Direction of bias | Rationale |
|---|---|---|---|
| Bias due to confounding | Serious | Unknown | Most of the critical confounders were considered statistically, but there is possibility of residual unmeasured (e.g., diet, pesticide exposure) confounding. The study evaluated the child's BPA exposure throughout several points in their life. And used each one separately in the evaluation. Changes in BPA exposure could be related to changes in food consumption over time as BPA exposure is mainly through canned or processed food including soda, which could also be related to obesity. Since Harley follows participants over time, there is some concern for time-varying confounding as they may have changed their diet while pregnant. Potential confounders were identified a priori using directed acyclic graphs. Potential confounders included maternal pre-pregnancy BMI, age, education, years of residence in the United States, smoking during pregnancy, soda consumption during pregnancy, and family income. Time-varying covariates considered were child consumption of soda, fast food, and sweets, television watching, environmental tobacco smoke exposure, and time spent playing outdoors, assessed at multiple times during childhood. Covariates were included in the final models if they were associated with both exposure and any of the growth outcomes at p-value <0.2 or if removing them changed the coefficient for the main BPA exposure variable by >10%. Maternal age and pre-pregnancy BMI were analyzed as continuous variables. Other variables were categorical. Mothers were interviewed twice during pregnancy, after delivery, and when their children were 2, 3.5, 5, 7, and 9 years of age to obtain information about demographic characteristics, diet, and behaviors. All interviews were conducted in English or Spanish using structured questionnaires, but no information was provided on reliability/validity. At the baseline interview, we asked mothers about their race/ethnicity, education, income, marital status, and number of years they had lived in the United States, as well as information about soda consumption, smoking, and alcohol and drug use during pregnancy. We calculated pre-pregnancy BMI from self-reported pre-pregnancy weight and measured height. If self-reported pre-pregnancy weight was unavailable or invalid, we used measured weight at first prenatal visit (n = 23) if the first prenatal visit occurred at or before 13 weeks gestation or used regression models to impute pre-pregnancy weight based on weight at all prenatal visits if the first prenatal visit occurred after 13 weeks (n = 16). |
| Bias in selection of participants into the study | Low | N/A | Selection of subjects was unrelated to either exposure or outcome. The study sample consisted of participants in the Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS), a longitudinal cohort study of environmental factors and children's growth and development. Pregnant mothers were enrolled Selection of subjects was unrelated to either exposure or outcome in 1999 and 2000 from prenatal clinics serving the farmworker population in the Salinas Valley, California. Eligible women were at least 18 years of age, spoke English or Spanish, qualified for low-income health insurance, were at <20 weeks gestation, and were planning to deliver at the county hospital. Mothers provided written informed consent for themselves and their children to participate in the study. Start of exposure occurred in the first trimester and all subjects were followed through 9 years of age. |
| Bias in classification of exposures | Moderate | Some concern of bias toward the null due to non-differential misclassification of the exposure. | Urinary BPA concentration was measured in 4 spot samples, 2 during pregnancy and 2 from the child. LOD was 0.4 ng/mL. Concentrations< LOD for which a signal was detected were reported as measured. Concentrations < LOD with no signal detected were randomly imputed based on a log-normal probability distribution using maximum likelihood estimation. The number of collected samples increases our certainty in the correct classification of the higher exposed and lower exposed groups. Initial exposure was measured during the first trimester of pregnancy. While this may not be the exact date of start of exposure it would be very close for the children. Prenatal and five-year-old exposure measurements were taken prior to the assessment of BMI at 9 years. Exposure was assessed prior to the outcome at three different time points. Only one exposure measurement was obtained at the same time as the outcome; thus, it was not possible for classification of exposure to have been affected by the knowledge of the outcome. The range/variability in exposure was sufficient (range during pregnancy 0.5 to 4.6 ng/mL and during childhood 0.9 to 16.3 ng/mL). Although BPA levels change over time and we are not confident that the subjects were exposed to this concentration for a long period of time, the fact that there were 4 measurements per subject make us more confident in the exposure being represented of changes over time. In addition, since the child's exposure was first measured based on mother's levels when pregnant, then again when the children were 5 (4 years prior to measuring outcome) the duration of exposure would have been sufficient even if the level of this exposure was not consistent. BPA levels were also measured in the child at 9 years. However, data were not provided for the individual subjects to know how the BPA levels may have varied per subject. Children were followed up for 9 years, which would have been sufficient time for the outcome to develop. Spot urine samples were collected from mothers at two time points during pregnancy: near the end of the first (mean±SD, 13.8±5.0 weeks gestation) and second (mean±SD, 26.4±2.4 weeks gestation) trimester of pregnancy and from the children when they were 5 (mean±SD, 5.1±0.2 years) and 9 (mean±SD, 9.4±0.4 years) years of age. Urine samples were collected in polypropylene urine cups, aliquoted into glass vials, and frozen at −80°C until shipment to the CDC for analysis. Analysis of field blanks showed no detectable contamination by BPA using this collection protocol. Solid-phase extraction coupled to high performance liquid chromatography-isotope dilution tandem mass spectrometry to measure total urinary BPA concentration (conjugated plus unconjugated). Concentrations < LOD for which a signal was detected were reported as measured. Concentrations < LOD with no signal detected were randomly imputed based on a log-normal probability distribution using maximum likelihood estimation. Specific gravity was measured with a refractometer (National Instrument Company Inc., Baltimore, MD) for the maternal urine samples, but was unavailable for the children's samples. Thus, maternal concentrations were normalized for urinary dilution using urine specific gravity, and child BPA concentrations were normalized by dividing by urinary creatinine concentration. |
| Bias due to deviations from intended exposures | Low | N/A | There is little concern that changes in exposure status occurred among participants. Although BPA levels may change overtime, several measurements were obtained and evaluate separately by exposure they were assigned. Because each exposure was evaluated as an intent to treat, there is little concern about the potential changes in exposure. The study authors reanalyzed the models controlling separately for three important prenatal exposures in this population: organochlorine pesticides [using prenatal serum concentrations of dichlorodiphenyldichloroethylene (DDE)], organophosphate pesticides (using prenatal urinary metabolites of organophosphate pesticides), and brominated flame retardants [using prenatal serum concentrations of polybrominated diphenyl ethers (PBDEs)]. |
| Bias due to missing data | Low | N/A | Reasons for exclusion were documented and unlikely to differ across exposures threshold. Although some subjects were lost to follow-up and the missing data were not described by exposure status, the study authors conducted analyses that addressed loss to follow-up and are likely to have removed any risk of bias thus judged low risk of bias. There is no statement that participants with missing covariate data were excluded from analyses. There is no information on the missing data by exposure level. Although it is unlikely to be related to exposure level, they had the data in order to compare those lost to follow-up with those included in the analysis, but no information was provided. Of the 527 mothers meeting the inclusion criteria, 402 had at least one urine measurement available. There were 325 measurements in children at 5 years and 304 available at 9 years. Of the 402 children included in the analysis, anthropometric measurements were available for 319 children at 5 years and 311 children at 9 years. |
| Bias in measurement of the outcome | Low | N/A | It is unlikely that the outcome could be affected by knowledge of exposure. It was not noted that outcome assessors were blind to the exposure level, but it was likely given that separate individuals were used to measure the outcome parameters than conducted the exposure analysis (i.e., CDC). The same methods were used for all participants at all times measured. It is unlikely that any systematic error in anthropometric measurements (or calculating the BMI or assigning obesity category) would have been related to exposure. Children were weighed and measured without jackets or shoes by trained study staff. Weight was measured using a digital scale and rounded to the nearest 0.1 kg. Height was measured using a stadiometer and rounded to the nearest 0.1 cm. Starting at 5 years of age, waist circumference was measured at each visit by placing a measuring tape around the abdomen at the level of the iliac crest, parallel to the floor. Height and waist circumference measurements were conducted in triplicate and averaged for analysis. When the children were 9 years of age, fat percentage was measured using “foot-to-foot” bio-impedance technology with a Tanita TBF-300A body composition analyzer (Tanita Corp.). BMI was calculated as weight (kilograms) divided by height squared (square meters) and compared with the sex-specific BMI-for-age percentile data issued by CDC in 2000 (National Center for Health Statistics 2005). Children who were≥85th but <95th percentile for their age and sex were classified as overweight. Age- and sex-standardized BMI z-scores were also generated using the CDC norms. These methods are considered sensitive. |
| Bias in selection of the reported result | Moderate | Potential for bias away from the null. | Reported results are consistent with an a priori plan; however, as no protocol was published prior to the study there is potential for reporting bias to inflate results for publication success. Several measurements of obesity were evaluated and reported. These were also assessed at several different time periods in the children. Although the publication only shows a few of the results (both positive and negative), the BMI-z-scores for all ages are presented in the supplemental data indicating that it is unlikely that there was bias from selective reporting of outcome. Gender and age were evaluated as separate subgroups as described in the report. Statistical methods reported in the methods section were used and presented in the results or discussion. BPA was analyzed as categorical and continuous variable. |
| Overall bias | Moderate | Unknown | Overall bias was judged as Moderate due to concerns of potential unknown confounders, some concerns of non-differential misclassification of the exposure, and some concerns with bias in reported results. |
Harley KG, Schall RA, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B: Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environmental health perspectives 2013, 121(4):514 (Harley et al., 2013).
Appendix F. Sensitivity analysis for the outcome of prevalent overweight
Fig. F.1.
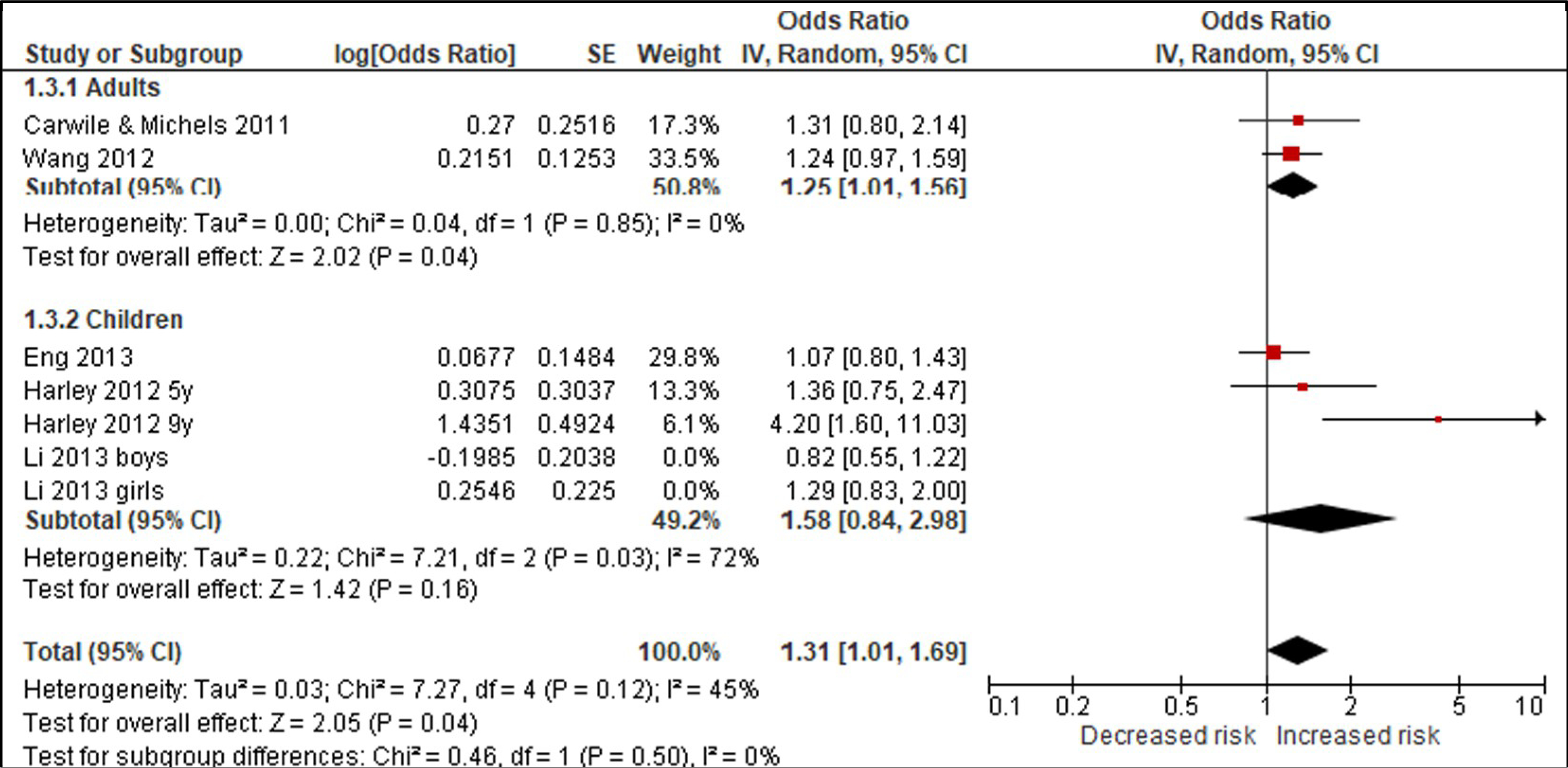
Sensitivity analysis of studies with ‘Serious’ bias due to confounding.
Fig. F.2.
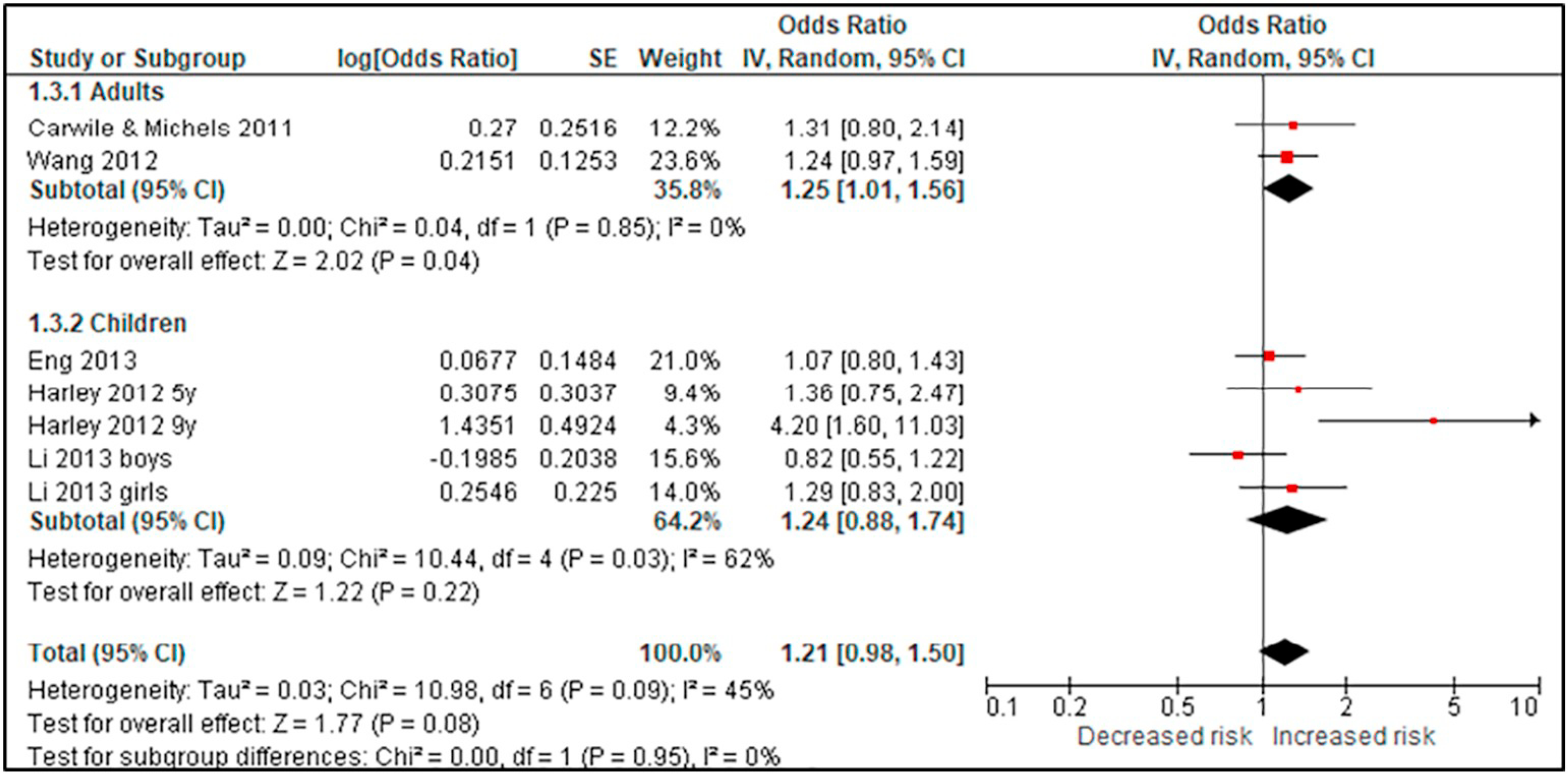
Sensitivity analysis of all studies.
Appendix G. Sensitivity analysis for the outcome of prevalent obesity
Fig. G.1.
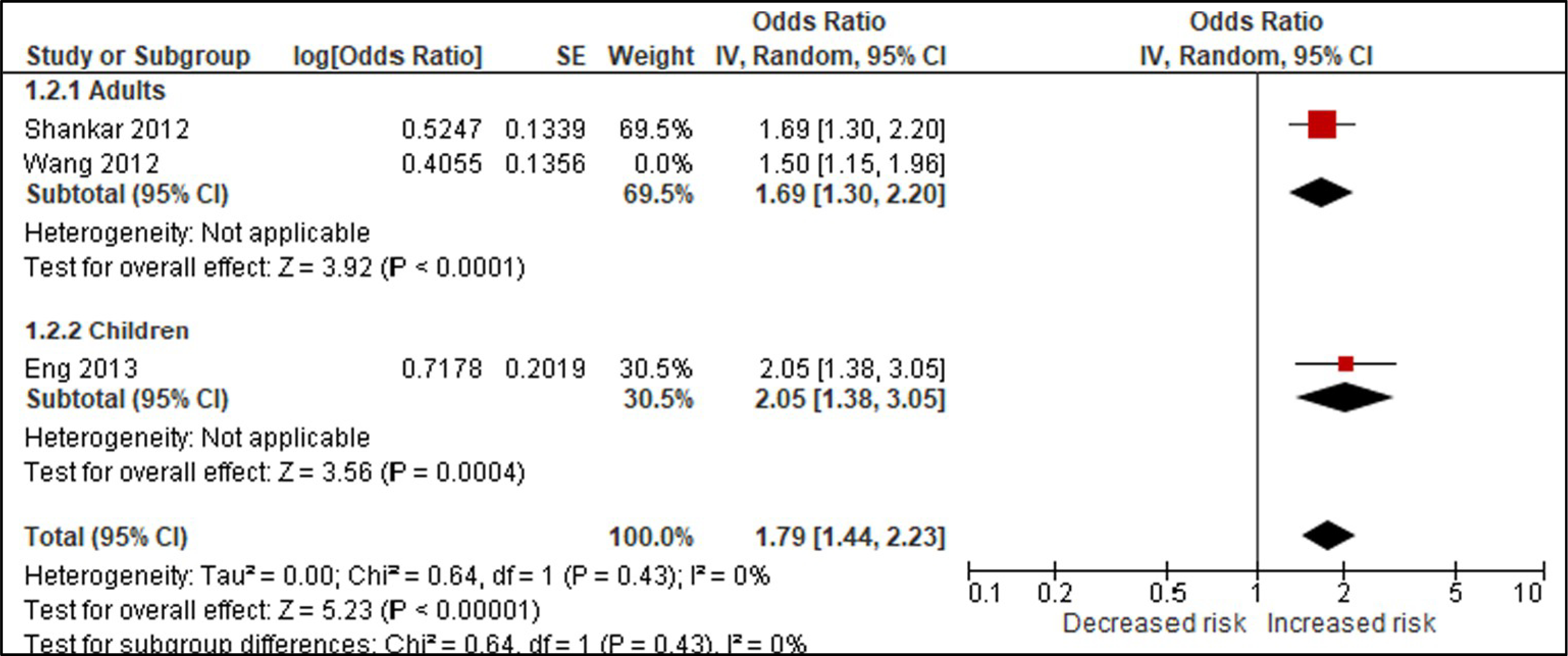
Sensitivity analysis of studies with ‘Serious’ bias due to confounding.
Fig. G.2.
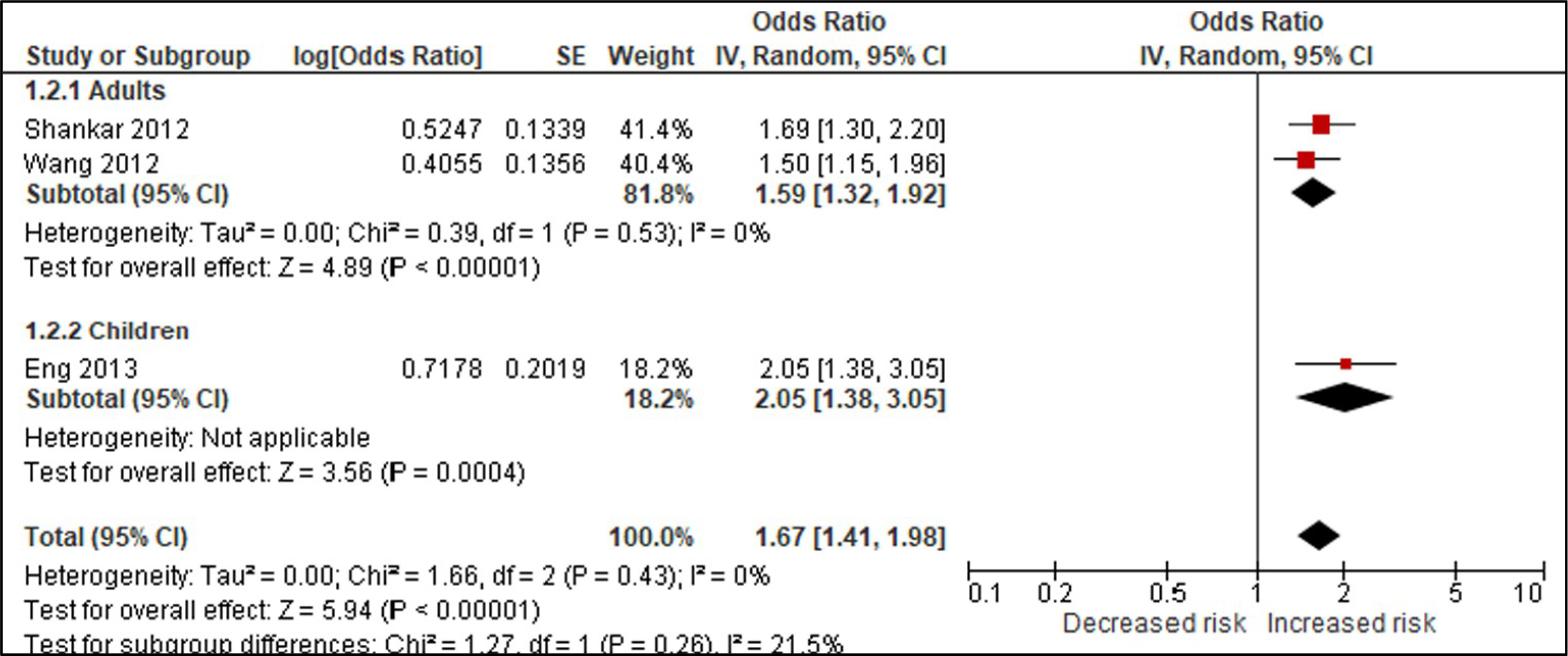
Sensitivity analysis of all studies.
References
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. , 2011. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol 64 (4), 401–406. [DOI] [PubMed] [Google Scholar]
- Blair A, Stewart P, Lubin JH, Forastiere F, 2007. Methodological issues regarding confounding and exposure misclassification in epidemiological studies of occupational exposures. Am. J. Ind. Med 50 (3), 199–207. [DOI] [PubMed] [Google Scholar]
- Bross ID, 1966. Spurious effects from an extraneous variable. J. Chronic Dis 19 (6), 637–647. [DOI] [PubMed] [Google Scholar]
- Carwile JL, Michels KB, 2011. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res 111 (6), 825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2004. NHANES 2003–2004. Laboratory Procedure Manual. Available: (http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/l24eph_c_met_phenols.pdf), Accessed date: 11 November 2018. [Google Scholar]
- Centers for Disease Control and Prevention (CDC), 2006. NHANES 2005–2006. Laboratory Procedure Manual. Available: 〈>http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/eph_d_met_phenols_parabens.pdf〉, Accessed date: 11 November 2018. [Google Scholar]
- Cochran WG, Chambers SP, 1965. The planning of observational studies of human populations. J. R. Stat. Soc. Ser. A (General) 128 (2), 234–266. [Google Scholar]
- Cox KJ, Porucznik CA, Anderson DJ, Brozek EM, Szczotka KM, Bailey NM, Wilkins DG, Stanford JB, 2016. Exposure classification and temporal variability in urinary bisphenol A concentrations among couples in Utah—the HOPE study. Environ. Health Perspect. 124 (4), 498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Hill AB, 1950. Smoking and carcinoma of the lung. Br. Med. J 2 (4682), 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll R, Hill AB, 1964. Mortality in relation to smoking: ten years’ observations of British doctors. Br. Med. J 1 (5395), 1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, et al. , 2011b. GRADE guidelines: 8. Rating the quality of evidence–indirectness. J. Clin. Epidemiol 64 (12), 1303–1310. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori V, et al. , 2011c. GRADE guidelines: 9. Rating up the quality of evidence. J. Clin. Epidemiol 64 (12), 1311–1316. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, et al. , 2011a. GRADE guidelines: 4. Rating the quality of evidence–study limitations (risk of bias). J. Clin. Epidemiol 64 (4), 407–415. [DOI] [PubMed] [Google Scholar]
- Harley KG, Schall RA, Chevrier J, Tyler K, Aguirre H, Bradman A, Holland NT, Lustig RH, Calafat AM, Eskenazi B, 2013. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ. Health Perspect 121 (4), 514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Robins JM, 2016. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol 183 (8), 758–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Green S, 2011. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). http://handbook.cochrane.org/ (Accessed 3 February 2013).
- Higgins J, Sterne J, Savovic J, Page M, Hrobjartsson A, Boutron I, Reeves B, Eldridge S, 2016. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V (Eds.), Cochrane Methods,. http://www.cochranelibrary.com/dotAsset/ecafc5c7-0b9b-4cd1-a4c1-8b0013aea046.pdf. [Google Scholar]
- Jurek AM, Greenland S, Maldonado G, Church TR, 2005. Proper interpretation of non-differential misclassification effects: expectations vs observations. Int. J. Epidemiol 34 (3), 680–687. [DOI] [PubMed] [Google Scholar]
- Kogevinas M, 2011. Epidemiological approaches in the investigation of environmental causes of cancer: the case of dioxins and water disinfection by-products. In: Environmental Health: 2011: BioMed Central, pp. S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D-K, Miao M, Zhou Z, Wu C, Shi H, Liu X, Wang S, Yuan W, 2013. Urine bisphenol-A level in relation to obesity and overweight in school-age children. PLoS One 8 (6), e65399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D, 2009. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 6 (7), e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RL, Thayer KA, Bero L, Bruce N, Falck-Ytter Y, Ghersi D, Guyatt G, Hooijmans C, Langendam M, Mandrioli D, et al. , 2016. GRADE: assessing the quality of evidence in environmental and occupational health. Environ. Int 92–93, 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RL, Thayer KA, Santesso N, Holloway AC, Blain R, Eftim SE, Goldstone AE, Ross P, Guyatt G, Schünemann HJ, 2018a. Evaluation of the risk of bias in non-randomized studies of interventions (ROBINS-I) and the ‘target experiment’concept in studies of exposures: rationale and preliminary instrument development. Environ. Int 120, 382–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RL, Whaley P, Thayer KA, Schunemann HJ, 2018b. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ. Int 121 (Part 1), 1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuijsen MJ, 2015. Exposure Assessment in Environmental Epidemiology. Oxford University Press, USA. [Google Scholar]
- Ranciere F, Lyons JG, Loh VH, Botton J, Galloway T, Wang T, Shaw JE, Magliano DJ, 2015. Bisphenol A and the risk of cardiometabolic disorders: a systematic review with meta-analysis of the epidemiological evidence. Environ. Health 14 (1), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Cooper GS, Jahnke GD, Lam J, Morgan RL, Boyles AL, Ratcliffe JM, Kraft AD, Schünemann HJ, Schwingl P, 2016. How credible are the study results? Evaluating and applying internal validity tools to literature-based assessments of environmental health hazards. Environ. Int 92, 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, Morgan RL, Gartlehner G, Kunz R, Katikireddi SV, 2018. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in non-randomized studies should be used to rate the certainty of a body of evidence. J. Clin. Epidemiol (February 9. pii: S0895–4356(17)31031–4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K, Savitz DA, 1997. Topics in Environmental Epidemiology. Oxford University Press, USA. [Google Scholar]
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. , 2016. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, 14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer K, Rooney A, Boyles A, Holmgren S, Walker V, Kissling G, National Toxicology Program, US Department of Health and Human Services, 2013. Draft protocol for systematic review to evaluate the evidence for an association between bisphenol A (BPA) exposure and obesity. Natl. Toxicol. Program. [Google Scholar]
- Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, 2015. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ. Int 83, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS, 2013. Human exposures to bisphenol A: mismatches between data and assumptions. Rev. Environ. Health 28 (1), 37–58. [DOI] [PubMed] [Google Scholar]
- Wang H-X, Zhou Y, Tang C-X, Wu J-G, Chen Y, Jiang Q-W, 2012. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ. Health 11 (1), 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Sutton P, 2014. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ. Health Perspect 122 (10), 1007–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]