Abstract
Background:
In utero exposure to heavy metals lead (Pb), mercury (Hg), and cadmium (Cd) may be associated with higher childhood blood pressure (BP), whereas trace elements selenium (Se) and manganese (Mn) may have protective antioxidant effects that modify metal-BP associations.
Objectives:
We examined the individual and joint effects of in utero exposure to Pb, Hg, Cd, Se, and Mn on childhood BP.
Methods:
We used data from the Boston Birth Cohort (enrolled 2002–2013). We measured heavy metals and trace elements in maternal red blood cells collected 24–72 h after delivery. We calculated child BP percentile per the 2017 American Academy of Pediatrics Clinical Practice Guideline. We used linear regression models to estimate the association of each metal, and Bayesian kernel machine regression (BKMR) to examine metal coexposures, with child BP between 3 to 15 years of age.
Results:
Our analytic sample comprised 1,194 mother-infant pairs (61% non-Hispanic Black, 20% Hispanic). Hg and Pb were not associated with child systolic BP (SBP). Se and Mn were inversely associated with child SBP percentiles, which, on average, were 6.23 points lower with a doubling of Se (95% CI: , ) and 2.62 points lower with a doubling of Mn (95% CI: , ). BKMR models showed similar results. Although Cd was not associated with child SBP overall, the inverse association between Mn and child SBP was stronger at higher levels of Cd (). Consistent with this finding, in utero exposure to cigarette smoke modified the Mn–child SBP association. Among children whose mothers smoked during pregnancy, a doubling of Mn was associated with a 10.09-point reduction in SBP percentile (95% CI: , ), compared with a 1.49-point reduction (95% CI: , 1.24) in children whose mothers did not smoke during pregnancy ().
Conclusion:
Se and Mn concentrations in maternal red blood cells collected 24–72 h after delivery were associated with lower child SBP at 3 to 15 years of age. There was an interaction between Mn and Cd on child SBP, whereby the protective association of Mn on child SBP was stronger among mothers who had higher Cd. The association of Mn and child SBP was also modified by maternal cigarette smoking—a source of Cd—during pregnancy. Optimizing in utero Se levels, as well as Mn levels in women who had high Cd or smoked during pregnancy, may protect offspring from developing high BP during childhood. https://doi.org/10.1289/EHP8325
Introduction
High blood pressure (BP) is the leading modifiable risk factor for cardiovascular diseases (CVD) (Virani et al. 2020) and is responsible for 7.8 million deaths per year worldwide (Forouzanfar et al. 2017). Despite efforts to control BP, the global burden of high BP is still on the rise (Danaei et al. 2011; Forouzanfar et al. 2017). BP tracks from childhood to adulthood (Chen and Wang 2008). Higher child BP is associated with hypertension and CVD in adulthood (Theodore et al. 2015; Yang et al. 2020). Identifying modifiable risk factors for high BP in childhood may thus pave the way for primordial prevention of CVD.
Lead (Pb), mercury (Hg), and cadmium (Cd) are highly toxic metals that have no known physiological role but can cause adverse health effects even at trace levels. Exposure to these metals is associated with higher risk of hypertension and CVD in adults (Chowdhury et al. 2018; Hu et al. 2018; Lanphear et al. 2018; Navas-Acien et al. 2007; Nawrot et al. 2002) and in pregnant women (Kahn and Trasande 2018; Liu et al. 2019). The prevalence of exposure to these metals among pregnant women is high. In the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2016 data, 83%–98% of pregnant women had detectable levels of Pb, Hg, and/or Cd, with a high frequency of coexposure (Watson et al. 2020). Pb, Hg, and Cd in maternal blood can cross the placenta, accumulate in fetal tissues (Gundacker and Hengstschläger 2012), and may affect offspring cardiovascular health; however, research is limited. Few studies have examined the association of in utero exposure to Pb (Farzan et al. 2018; Gump et al. 2005; Skröder et al. 2016; Zhang et al. 2012), Hg (Gregory et al. 2016; Kalish et al. 2014; Sørensen et al. 1999; Thurston et al. 2007), or Cd (Chatzi et al. 2019; Hawkesworth et al. 2013) with child BP, with mixed findings. Most of these studies, however, only measured child BP in early childhood and did not examine differences by child sex, despite literature showing sexual dimorphism in BP across life span (Ji et al. 2020; Xia et al. 2020). Moreover, Black and Hispanic populations have been underrepresented in these studies, despite their high exposure to metals (Shim et al. 2017) and high prevalence of hypertension and CVD (Fryar et al. 2017; Rosner et al. 2013).
Selenium (Se) and manganese (Mn) are essential trace elements for human health. In adults, some studies suggested that Se and Mn may have protective effects against hypertension and CVD, although there is also evidence of possible adverse effects, nonlinear relationships, or null effects of these elements on BP and CVD (Bulka et al. 2019; Laclaustra et al. 2009; Lee and Kim 2011; Mordukhovich et al. 2012; Nawrot et al. 2007; Wu et al. 2017). In pregnant women, we reported in the Boston Birth Cohort (Liu et al. 2019) and in Project Viva (Liu et al. 2020) that higher maternal Mn levels were prospectively associated with lower risk of preeclampsia, a pregnancy complication characterized by high BP and a known risk factor for child high BP (Zhang et al. 2020b). It is unclear how in utero exposure to Se and Mn is associated with BP in children.
Additionally, metals may interact with each other and have synergistic and/or antagonistic effects on health. For example, animal studies have found that Cd inhibits Mn uptake (Gruden and Matausić 1989), Mn reduces Cd-induced lethality and hepatotoxicity (Goering and Klaassen 1985), and Se reduces the toxicity of metals such as Cd, Hg, and Pb (ATSDR 2003; Frost 1972); these interactions were dependent on exposure dose, route, and target organ, and the mechanisms are still not fully understood. Epidemiological studies in children have also provided evidence of metal interactions on child neurodevelopmental outcomes (Henn et al. 2012; Sanders et al. 2015; Valeri et al. 2017; Wasserman et al. 2006). However, it remains unclear whether in utero coexposure to a mixture of metals and trace elements affects child BP and whether metal interactions exist. To our knowledge, no animal studies have examined interactions of prenatal metal exposures on offspring BP or CVD. One cohort study in Mexico City, Mexico, by Kupsco et al. reported a decrease in child systolic BP (SBP), with higher concentrations of a mixture of 11 metals in maternal blood collected during the second trimester, although Mn and Se were not individually associated with child SBP in the multivariable analyses that included one metal at a time (Kupsco et al. 2019). The authors also did not observe evidence of interactions of these metals on BP. More recently, Howe et al. found in a Greek cohort a potential interaction of Pb and molybdenum on child BP at 11 years of age (Howe et al. 2021).
In this study, we aimed to assess the impact of in utero coexposure to metals Pb, Hg, and Cd and trace elements Se and Mn on child BP from 3 to 15 years of age. We used data from the Boston Birth Cohort, one of the largest and longest running birth cohorts in the United States, comprising a predominantly urban, racially diverse, and low-income population from Boston, Massachusetts. We hypothesized that in utero metal exposures affect child BP, and the metals and trace elements interact with each other to affect child BP. Given the inconclusive prior evidence on how these metals and trace elements may affect child BP (e.g., adverse, protective, or null effects, nonlinear relationships, and potential interactions), we used Bayesian kernel machine regression (BKMR), a flexible mixture modeling approach that enabled us to examine the independent and joint effects of these metals and trace elements on child BP, while simultaneously allowing for evaluation of nonlinear effects and interactions of the metals and trace elements (Bobb et al. 2015).
Methods
Study Design and Population
The Boston Birth Cohort is an ongoing prospective birth cohort that follows up mother–child dyads at the Boston Medical Center, Boston, Massachusetts. Briefly, women admitted to the labor and delivery floor at the Boston Medical Center who delivered a singleton live infant without major birth defects were eligible for participation in this cohort. Research staff approached and recruited mothers 24 to 72 h after delivery.
Mother–child dyads in this analysis were enrolled between November 2002 to October 2013 (followed up until May 2018). Of the 1,501 mothers who had their blood samples collected and metal concentrations measured, 1,272 had their child’s BP measured between 3 to 15 years of age. We excluded 78 dyads with missing data on key maternal covariates, including maternal educational level, prepregnancy body mass index (BMI), or cigarette smoking history. A total of 1,194 mother–child dyads were included in the final analytic data set. A comparison of the characteristics of dyads included in vs. excluded from this analysis is provided in Table S1.
The Boston Medical Center and the Johns Hopkins Bloomberg School of Public Health institutional review boards approved this study. We obtained written informed consent from each child’s biological mother and assent from the child.
Metals and Trace Elements Analysis
We used heavy metal (Pb, Hg, Cd) and trace element (Se, Mn) concentrations measured in maternal red blood cells (RBCs) collected 24 to 72 h after delivery as a proxy for third trimester in utero exposure. We collected maternal blood samples, separated plasma and red blood cells by centrifugation, and kept these samples at in metal-free vials. Methods for the measurements of metals and trace elements were published previously (Chen et al. 2014). Aliquots () were transported on dry ice to the Public Health and Environmental Laboratories in Trenton, New Jersey, and were measured using inductively coupled plasma mass spectrometry (ICP-MS). We measured all five elements in the same run. Intra-assay coefficients of variation were . Table 1 provides the summary data, the limits of detection, and the number (%) of samples below the limits of detection for each metal and trace element. We assigned samples below the limits of detection a value as detection limit divided by the square root of 2. Histograms of metal and trace element concentrations are provided in Figure S1.
Table 1.
Distributions, limits of detection, and number (%) of samples below limits of detection for lead, mercury, cadmium, selenium, and manganese ().
| Metals and trace elements | Mean (SD) | 25th percentile | 50th percentile | 75th percentile | Limit of detection (LOD) | Number (%) of samples below LOD |
|---|---|---|---|---|---|---|
| Lead () | 3.29 (3.03) | 1.65 | 2.42 | 3.68 | 0.07 | 0 (0) |
| Mercury () | 3.15 (3.60) | 1.06 | 2.15 | 3.70 | 0.280 | 134 (11.2%) |
| Cadmium () | 0.86 (0.68) | 0.46 | 0.69 | 1.04 | 0.100 | 104 (8.7%) |
| Selenium () | 289.50 (60.49) | 248.00 | 278.00 | 316.00 | 24.5 | 0 (0) |
| Manganese () | 39.57 (15.28) | 28.80 | 37.30 | 48.00 | 0.99 | 2 (0.2%) |
Note: Mother–child dyads in the Boston Birth Cohort are from Boston, Massachusetts. Dyads included in this analysis were enrolled between November 2002 to October 2013 (followed up until May 2018). Metal and trace element concentrations were measured in maternal red blood cells collected 24–72 h after delivery. We assigned samples below the LOD a value as LOD divided by square root of 2. Distributions were based on data after values were imputed for samples below the LODs. LOD, limit of detection; SD, standard deviation.
Child BP
We used child BP measured at the most recent annual well-child visit, which fell between 3 to 15 years of age. Clinical staff measured child BP using a validated automatic sphygmomanometer Masimo SET with an appropriately sized cuff at the right brachial artery in a quiet room with the child in a sitting position. We calculated child age-, sex-, and height-specific BP percentile based on the 2017 American Academy of Pediatrics Clinical Practice Guideline (Flynn et al. 2017). This Guideline updated the 2004 Pediatric Hypertension Guideline to include revised definitions of BP categories that align with the 2017 American College of Cardiology/American Heart Association (ACC/AHA) Hypertension Guidelines for adults (Whelton et al. 2018). In this Guideline, BP percentiles were calculated using new normative BP tables based on SBP and diastolic BP (DBP) readings arranged by age, sex, and height from normal-weight children and adolescents (Flynn et al. 2017). We used SBP as the primary outcome and DBP as the secondary outcome, because SBP is more strongly associated with hypertension and CVD in adulthood (Chen and Wang 2008; Sun et al. 2007); this approach is consistent with our previous studies in the Boston Birth Cohort (Zhang et al. 2018, 2020b).
Covariates
We extracted information on maternal age at delivery, preeclampsia, chronic diabetes mellitus, gestational diabetes mellitus, child sex, birth weight, and gestational age at birth from the electronic medical records. We collected data on maternal prepregnancy weight, height, race/ethnicity, educational level, and cigarette smoking history from a standardized Maternal Postpartum Questionnaire administered 24 to 72 h after delivery. Mothers were also asked in the questionnaire how often they ate fish per week on average during pregnancy, and choices included “None,” “,” “1–2 d/week,” “3–5 d/week,” “6–7 d/week,” and “do not know.” We calculated maternal prepregnancy BMI as weight (kilograms) divided by height (meters) squared and classified mothers as underweight (), normal weight (), overweight (), and obese (). We defined low birth weight as birth weight and preterm birth as gestational age at birth . Child weight and height were measured by trained medical staff at the same clinical visit as child BP. We calculated child BMI as weight (kilograms) divided by height (meters) squared.
Statistical Analysis
We used linear regression models to examine the associations between in utero exposure to each metal and trace element with child BP. We included product terms of each pair of metals and trace elements (log2-transformed) in the regression models to examine their interactions. We modeled metal and trace element concentrations as continuous variables (log2-transformed to account for the right-skewness of the concentrations; Figure S1) and as categorical variables (quartiles).
We also used BKMR to assess potential independent effects of each metal and trace element, joint effects of each pair of metals and trace elements, and mixture effects of all metals and trace elements on child BP (Bobb et al. 2015; Valeri et al. 2017). Specifically, we used BKMR to estimate: a) the association of each metal and trace element with child BP, when all four other metals and trace elements are fixed at their 50th percentile; b) the association of each pair of metals and trace elements with child BP, when all three other metals and trace elements are fixed at their 50th percentile; and c) the overall effect of metal mixtures by estimating differences in BP, when all five metals and trace elements are held at their 10th to 90th percentile (with 10 percentile point increments) as compared to when they are held at their 50th percentile. We applied the default parameters in the kmbayes function in R package bkmr (version 0.2.0) (Bobb et al. 2018) and fitted four parallel Markov chain Monte Carlo (MCMC) chains (100,000 iterations for each chain that included 50,000 burn-in iterations) using the kmbayes_parallel function in R package bkmrhat (version 1.0.2). We investigated model convergence by inspecting the trace plots, autocorrelation plots, density plots, and the Gelman-Rubin convergence statistics. In an additional analysis, we grouped the five elements into heavy metals (Pb, Cd, Hg) and trace elements (Mn, Se) and conducted hierarchical variable selection that allowed us to estimate the posterior inclusion probability (PIP) for each group as well as conditional PIPs for each metal/trace element within the group. We used log2-transformed metals and trace elements in all BKMR analyses.
We defined confounders as covariates expected to be associated with in utero metal and trace element exposure and child BP but not on the potential causal pathway based on a priori information from the literature. In both the linear regression and the BKMR models, we adjusted for maternal age at delivery (continuous), self-reported race/ethnicity (non-Hispanic Black; non-Hispanic White; Hispanic; non-Hispanic others [including Asian, Pacific Islander, and mixed-race]), educational level (middle school or below; high school graduate or some college; college graduate and above), cigarette smoking history (never smoked; quit smoking before pregnancy; smoked during pregnancy), and maternal prepregnancy BMI (underweight; normal weight; overweight; obese). A Directed Acyclic Graph (DAG) that outlines the hypothesized relationships between all variables in this analysis is provided in Figure S2 (Textor et al. 2016).
A priori, we considered as potential effect modifiers child sex (male vs. female), age group (3–5 y, 6–9 y, 10–15 y), and maternal race/ethnicity (non-Hispanic Black vs. Hispanic). We estimated the stratum-specific associations for each subgroup, and we included a product term of the potential effect modifier and the log2-transformed metal/trace element concentration in the multivariable-adjusted linear models and used the -values of the product terms as interaction p-values. We did not conduct subgroup analysis in the White () or the “Others” () race/ethnicity groups due to the small sample size. We considered a two-sided as evidence for effect modification.
During the analysis, we observed an interaction of Mn and Cd with child SBP. Because cigarette smoking is a common major source of Cd exposure (Ashraf 2012), we conducted a post hoc analysis to examine whether maternal Cd levels differed by cigarette smoking during pregnancy (dichotomized as smoked vs. did not smoke, with mothers who never smoked and those who quit smoking before pregnancy combined into a single category) using a Wilcoxon rank-sum test. We estimated stratum-specific associations of Pb, Hg, Cd, Mn, or Se with child SBP by maternal smoking status. We included a product term of maternal smoking status and the log2-transformed metal/trace element concentration in the multivariable-adjusted linear models and used the -values of the product terms as interaction p-values. We considered a two-sided as evidence for effect modification.
We performed sensitivity analyses to assess the robustness of our findings. The sensitivity analyses were limited to SBP outcomes, the primary outcomes in this analysis. First, we additionally adjusted for the following covariates: a) maternal fish intake during pregnancy (none; ; 1–2 d/wk; 3–5 d/wk; 6–7 d/wk; do not know); b) maternal preeclampsia (yes; no) and diabetes mellitus (no; chronic; gestational); and c) child BMI (continuous) measured at the same clinical visit as child BP. Second, because percentiles are not on a scale with equal intervals (i.e., the difference between two BP readings may be different from other two BP readings whose percentile difference is the same), we used child SBP z-score as the outcome. Third, we estimated the association of each metal and trace element with child elevated SBP (defined as SBP percentile) using logistic regression. Fourth, we used the stabilized inverse probability weighting method to account for the potential selection bias due to missing child SBP and key covariates. We estimated the probability of being included in () vs. excluded from () the final analytic data set based on covariates including maternal age at delivery; race/ethnicity; educational level; cigarette smoking history; prepregnancy BMI; maternal RBC Pb, Hg, Cd, Se, Mn concentrations; and child birth weight and gestational age at birth and then applied the weights to the fully adjusted linear regression models.
We conducted analyses using Stata (version 15.1; StataCorp) and R (version 3.6.3; R Development Core Team).
Results
Descriptive Results
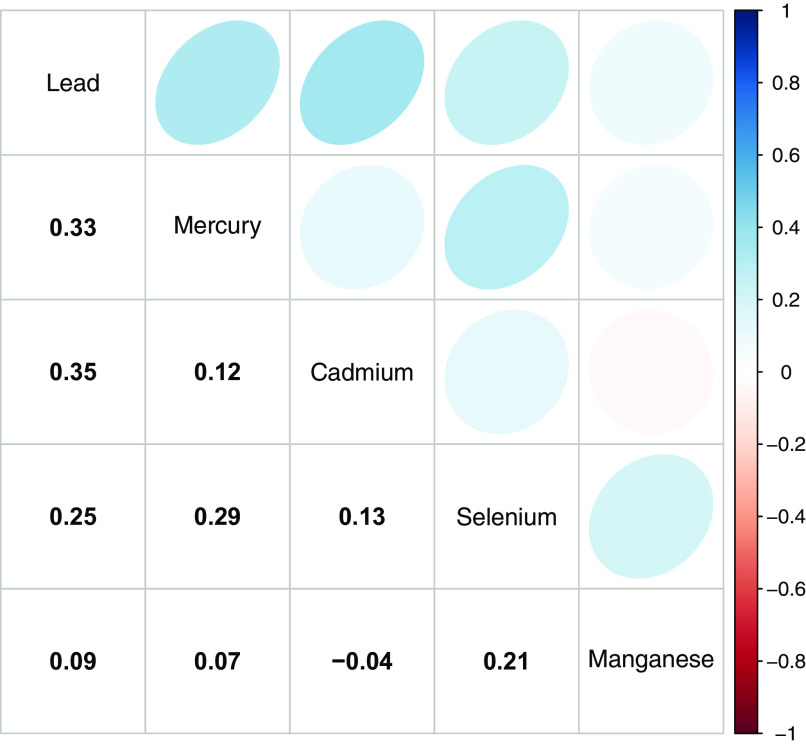
Of the 1,194 mothers in the analysis sample, the mean age at delivery was 28.7 [standard deviation (SD): 6.5 y], 722 (60.5%) were non-Hispanic Black, 302 (25.3%) did not finish high school, and 632 (52.9%) were overweight or obese prior to pregnancy. Of children, 603 (50.5%) were female, 291 (24.4%) were born preterm (Table 2), and 188 (15.7%) had elevated SBP. The median [interquartile range (IQR)] age at BP measurement was 8.4 (6.2–10.6) y (range: 3.0–15.4 y) (Table S2; Figure S3). Spearman correlations of each pair of metals ranged from to 0.35 (Figure 1). MCMC chains showed good convergence across all estimated parameters (Figure S4; Table S3).
Table 2.
Characteristics of the mothers and children included in this analysis ().
| Maternal characteristics | |
|---|---|
| Age at delivery (y, mean ± SD) | 28.7 ± 6.5 |
| Race/ethnicity [ (%)] | |
| Non-Hispanic Black | 722 (60.5) |
| Non-Hispanic White | 57 (4.8) |
| Hispanic | 238 (19.9) |
| Others | 177 (14.8) |
| Educational level [ (%)] | |
| Middle school or below | 302 (25.3) |
| High school graduate or some college | 731 (61.2) |
| College graduate and above | 161 (13.5) |
| Prepregnancy BMI categories [ (%)] | |
| Underweight | 44 (3.7) |
| Normal weight | 518 (43.4) |
| Overweight | 332 (27.8) |
| Obese | 300 (25.1) |
| Cigarette smoking history [ (%)] | |
| Never smoked | 983 (82.3) |
| Quit smoking before pregnancy | 103 (8.6) |
| Smoked during pregnancy | 108 (9.0) |
| Preeclampsiaa [ (%)] | |
| No | 1,065 (89.4) |
| Mild | 48 (4.0) |
| Severe | 78 (6.5) |
| Missing | 3 |
| Diabetes mellitus [ (%)] | |
| No | 1,039 (87.0) |
| Gestational diabetes mellitus | 100 (8.4) |
| Chronic diabetes mellitus | 55 (4.6) |
| Frequency of fish consumption during pregnancya [ (%)] | |
| None | 270 (22.9) |
| 333 (28.2) | |
| 1–2 d/wk | 422 (35.7) |
| 3–5 d/wk | 131 (11.1) |
| 6–7 d/wk | 25 (2.1) |
| Don’t know | 2 (0.2) |
| Missing | 11 |
| Child characteristics | |
|---|---|
| Child sex [ (%)] | |
| Female | 603 (50.5) |
| Male | 591 (49.5) |
| Preterm birth [ (%)] | |
| No | 903 (75.6) |
| Yes | 291 (24.4) |
| Low birth weight [ (%)] | |
| No | 925 (77.5) |
| Yes | 269 (22.5) |
| Child BMI at blood pressure measurement (kg/m2, mean ± SD) | 19.5 ± 5.0 |
Note: Mother–child pairs with missing data on maternal education level, prepregnancy BMI, or cigarette smoking history were excluded from this analysis. There were no missing data for age at delivery, race/ethnicity, diabetes mellitus, child sex, preterm birth, low birth weight, or child BMI. BMI, body mass index; SD, standard deviation.
For covariates with missing observations, missing observations were not included in the denominator when deriving percentages for the categories with known values.
Figure 1.
Spearman correlation matrix for lead, mercury, cadmium, selenium, and manganese measured in maternal red blood cells ().
In comparison with mothers excluded from the analysis (), mothers included were more likely to be non-Hispanic Black, were less likely to be Hispanic or to have smoked during pregnancy, and had different levels of education. Children included were less likely to have been born preterm and had higher BMI. Other maternal and child characteristics, including RBC metal and trace element concentrations, were not statistically significantly different between the two groups (Table S1).
Individual Metal and Trace Element with Child BP
Estimates from multivariable-adjusted linear regression models indicated inverse associations between SBP percentiles and a doubling of maternal Se (; 95% CI: , ) and Mn (; 95% CI: , ) (Table 3). When Se and Mn were modeled as quartiles, estimates were generally consistent with inverse associations, though support for an inverse trend was stronger for Se (successively stronger associations for the third and fourth quartiles) than for Mn (inverse for all exposures above the first quartile, but weaker for the highest quartile than the second and third relative to the first). Estimated associations for a doubling of Pb, Hg, and Cd were essentially null, and quartile-specific estimates did not suggest clear positive or negative trends. Figure 2 illustrates the multivariable adjusted exposure–response functions for each metal and trace element with child SBP percentile with all the other four metals and trace elements fixed at the 50th percentile, as estimated by the BKMR models; the results were generally consistent with the linear regression models. Results did not suggest associations of metal or trace element concentrations with DBP percentiles, with the possible exception of an inverse association with Se (; 95% CI: , 1.40 for a doubling of exposure, and a negative slope based on BKMR) (Table S4; Figure S5).
Table 3.
Associations of maternal lead, mercury, cadmium, selenium, and manganese concentrations with child systolic blood pressure percentile estimated using linear regression models ().
| Metals and trace elements | Levels of concentration | Difference (95% confidence interval) in child systolic blood pressure percentile | |
|---|---|---|---|
| Unadjusted models | Adjusted modelsa | ||
| Lead () | Quartile 1 (0.58 to 1.65) | Reference (0) | Reference (0) |
| Quartile 2 (1.65 to 2.42) | 0.76 (, 4.74) | 0.92 (, 4.97) | |
| Quartile 3 (2.44 to 3.68) | 1.61 (, 5.63) | 1.39 (, 5.64) | |
| Quartile 4 (3.70 to 24.8) | (, 3.66) | (, 3.74) | |
| A doubling of lead levels | 0.35 (, 1.92) | 0.30 (, 2.02) | |
| Mercury () | Quartile 1 () | Reference (0) | Reference (0) |
| Quartile 2 (1.07 to 2.14) | (, 2.76) | (, 2.70) | |
| Quartile 3 (2.16 to 3.70) | (, 1.24) | (, 1.41) | |
| Quartile 4 (3.72 to 27.80) | 0.58 (, 4.58) | 1.37 (, 5.59) | |
| A doubling of mercury levels | (, 0.84) | (, 1.05) | |
| Cadmium () | Quartile 1 () | Reference (0) | Reference (0) |
| Quartile 2 (0.46 to 0.69) | (, 3.57) | (, 3.62) | |
| Quartile 3 (0.69 to 1.04) | (, 2.70) | (, 2.61) | |
| Quartile 4 (1.04 to 4.76) | 1.76 (, 5.74) | 1.05 (, 5.55) | |
| A doubling of cadmium levels | 0.54 (, 2.00) | 0.14 (, 1.81) | |
| Selenium () | Quartile 1 (152.4 to 248.0) | Reference (0) | Reference (0) |
| Quartile 2 (250.0 to 278.0) | 0.15 (, 4.11) | 0.41 (, 4.42) | |
| Quartile 3 (280.0 to 316.0) | (, 2.15) | (, 2.65) | |
| Quartile 4 (318.0 to 624.0) | (, 0.66) | (, 0.89) | |
| A doubling of selenium levels | (, ) | (, ) | |
| Manganese () | Quartile 1 () | Reference (0) | Reference (0) |
| Quartile 2 (29.0 to 37.2) | (, ) | (, ) | |
| Quartile 3 (37.4 to 48.0) | (, ) | (, ) | |
| Quartile 4 (48.2 to 109.8) | (, ) | (, 0.24) | |
| A doubling of manganese levels | (, ) | (, ) | |
Note: Mother–child dyads in the Boston Birth Cohort are from Boston, Massachusetts. Mother–child dyads included in this analysis were enrolled between November 2002 to October 2013 (followed up until May 2018). LOD, limit of detection.
Models adjusted for maternal age at delivery, race/ethnicity, educational level, and maternal prepregnancy body mass index, and maternal cigarette smoking history.
Figure 2.
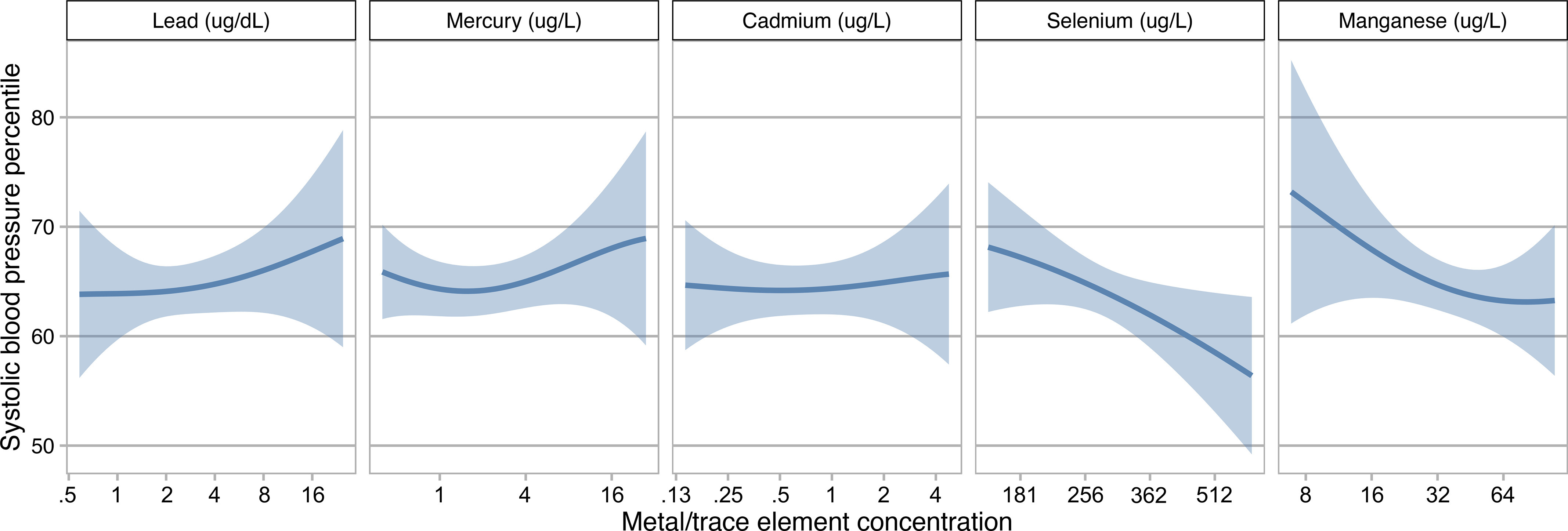
Associations (estimates and 95% credible intervals) of lead, mercury, cadmium, selenium, and manganese concentrations with child systolic blood pressure percentile estimated using Bayesian kernel machine regression (). This figure shows the association of each metal/trace element with child systolic blood pressure percentile when all other metals/trace elements are fixed at their 50th percentile. Models adjusted for maternal age at delivery, race/ethnicity, educational level, prepregnancy body mass index, and cigarette smoking history. Metals and trace element concentrations were log2-transformed for analyses and were back-transformed to their original scales in the figure.
Pairs of Metals and Trace Elements with Child BP
Figure 3 shows the multivariable adjusted exposure–response functions for each pair of metals and trace elements and child SBP with all other metals and trace elements fixed at the 50th percentile, as estimated by the BKMR models. There was evidence of an interaction between Mn and Cd, such that Cd was positively associated with child SBP when Mn levels were low (Figure 3, Panel 5.3), and the inverse association between Mn and child SBP was stronger at higher levels of Cd (Figure 3, Panel 3.5). Linear regression indicated a significant interaction between Mn and Cd on child SBP () resulting in a smaller estimated increase in SBP percentiles with a doubling of both Mn and Cd (7.02; 95% CI: , 16.31) than estimated for a doubling of Cd alone (13.52; 95% CI: 1.01, 26.03) (Table S5). BKMR and linear regression models did not provide evidence of interactions between pairs of metal/trace element exposures on child DBP percentiles (Table S6; Figure S6).
Figure 3.
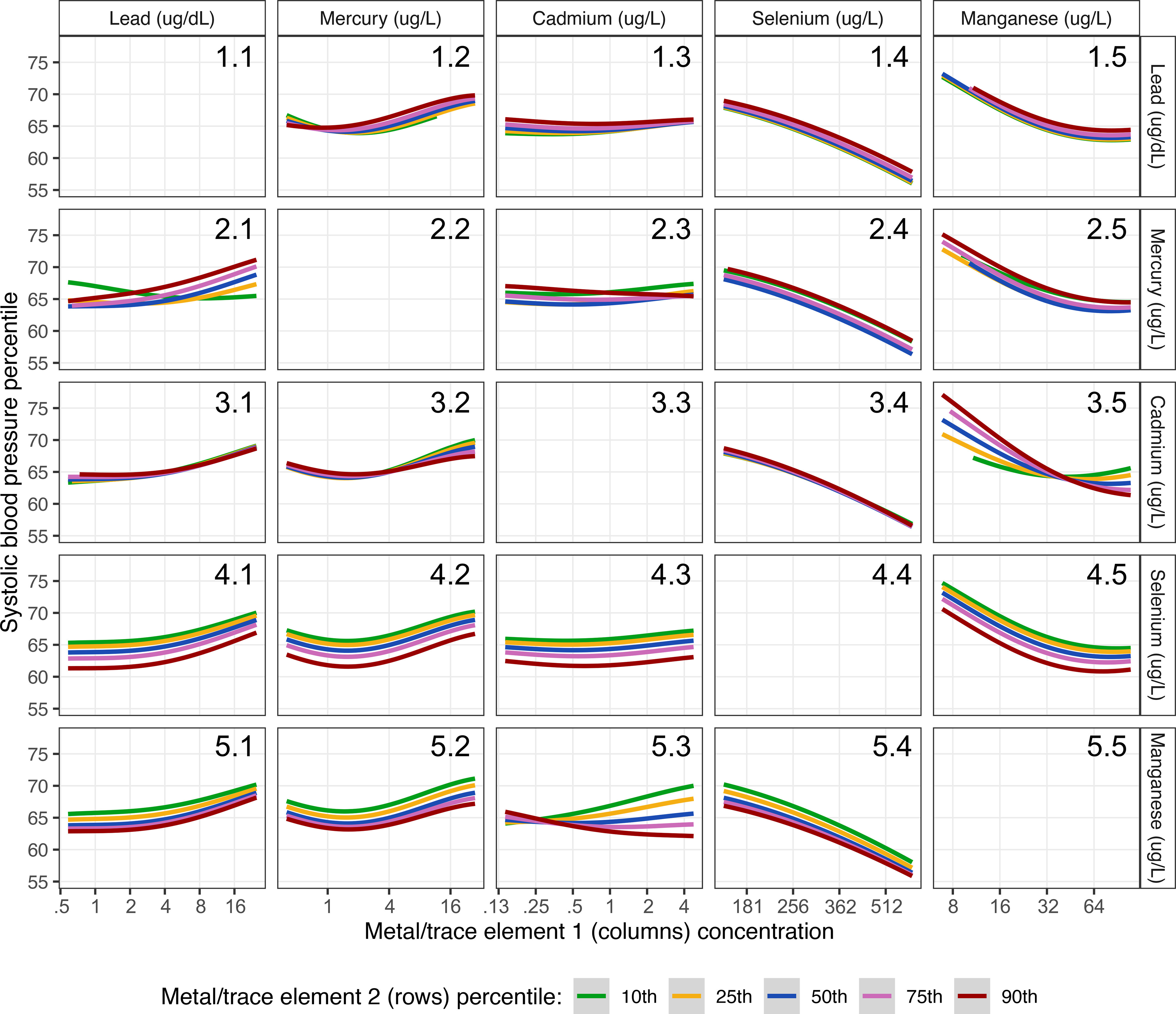
Associations of maternal metal/trace element one concentration (columns) and child systolic blood pressure percentile by levels (10th, 25th, 50th, 75th, and 90th percentile) of metal/trace element two concentration (rows) estimated using Bayesian kernel machine regression (). This figure shows the association of each pair of metals/trace elements with child systolic blood pressure percentile when all other metals/trace elements are fixed at their 50th percentile. Models adjusted for maternal age at delivery, race/ethnicity, educational level, prepregnancy body mass index, and cigarette smoking history. Metals and trace element concentrations were log2-transformed for analyses and were back-transformed to their original scales in the figure.
Group and Overall Effect of Metal Mixtures on Child BP
The mixture as a whole was not associated with child SBP (Figure S7) or DBP (Figure S8) percentiles. PIPs from a BKMR model with hierarchical variable selection indicate that trace elements as a group were more strongly associated with SBP than heavy metals as a group (PIPs of 0.852 and 0.042, respectively) (Table S7). Pb had the largest and Hg had the smallest conditional PIP of the three heavy metals, and for the trace elements, Se had a larger conditional PIP than Mn (Table S7).
Subgroup Analyses
The association between a doubling of Mn and SBP was similar by child sex and maternal race/ethnicity, but was strongest at 3–5 years of age (; 95% CI: , ) and null at 10–15 y () (Table S8). Associations between Mn and DBP were close to the null when stratified by child sex and age ( and 0.68, respectively), but differed between children of Hispanic mothers (; 95% CI: , 0.75) compared with non-Hispanic Black mothers (0.81; 95% CI: , 3.45) (). Associations between Cd and SBP and DBP were variable but did not show clear evidence of differences by child sex, age, or maternal race/ethnicity (p-interactions are 0.28–0.76 for SBP and 0.30–0.75 for DBP). Associations between Hg and child SBP and DBP differed by maternal race/ethnicity, with estimated differences for children born to non-Hispanic Black and Hispanic mothers of (95% CI: , 0.17) and 2.07 (95% CI: , 4.80), respectively, for SBP (), and (95% CI: , ) and 2.61 (95% CI: 0.44, 4.78), respectively, for DBP (). A doubling of Se was inversely associated with DBP in female children (; 95% CI: , ) but not in male children (1.71; 95% CI: , 7.88) (), but there was no clear difference in the association between Se and SBP by sex () or in associations between Se and SBP or DBP by age or maternal race/ethnicity. Associations between Pb and SBP and DBP also did not show clear differences by child sex, age, or maternal race/ethnicity ( for SBP, 0.63 to 0.99 for DBP). Child sex-, age-, and maternal race/ethnicity-stratified estimates for associations with SBP and DBP from BKMR models were consistent with corresponding estimates from linear regression (Figure S9; Figure S10).
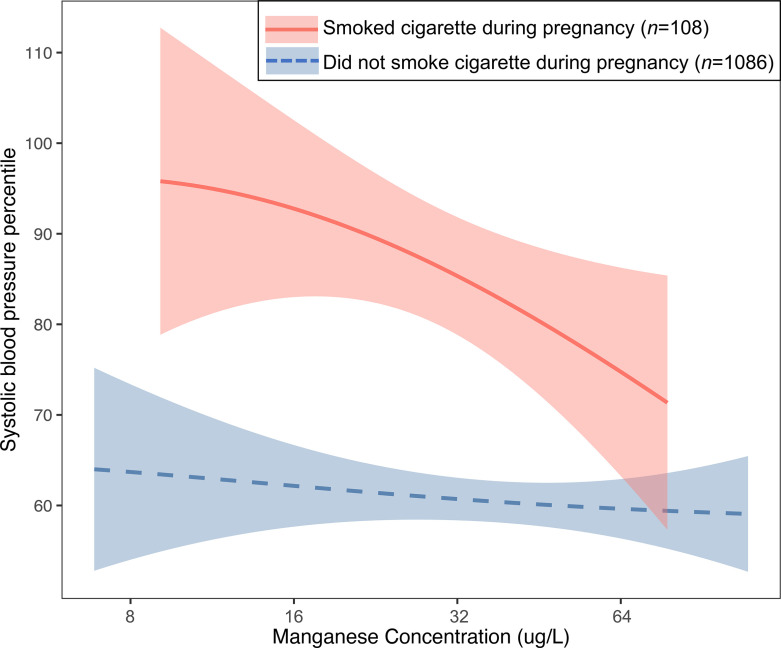
Maternal Cigarette Smoking during Pregnancy and the Mn–Cd Interaction on Child SBP
The median maternal Cd concentration in mothers who smoked during pregnancy [median (IQR): 1.41 (0.93–2.37) ; ] was more than twice the median concentration in mothers who never smoked or who quit smoking before pregnancy [median (IQR): 0.65 (0.43–0.96) ; ] (). The association of Mn and child SBP percentiles differed by maternal smoking status during pregnancy () (Table 4). In the multivariable linear regression models, a doubling of Mn levels was associated with a 10.09-point reduction in SBP percentile (95% CI: −18.03, −2.15) among mothers who smoked cigarettes during pregnancy; there was no association of Mn with child SBP percentiles among mothers who did not smoke cigarettes during pregnancy (, 95% CI: , 1.24 for a doubling of exposure). BKMR confirmed an inverse association of Mn and child SBP percentiles in mothers who smoked during pregnancy and a lack of association in mothers who did not smoke (Figure 4). Despite the inverse association of Mn and SBP percentiles, children whose mothers smoked cigarettes during pregnancy still had higher SBP percentiles at all Mn concentrations compared with children whose mothers did not smoke. The associations of Pb, Hg, Cd, and Se with child SBP percentiles did not significantly differ by maternal smoking status during pregnancy () (Table 4). Results from the BKMR models were consistent with corresponding estimates from the linear regression models (Figure S11).
Table 4.
Associations of maternal lead, mercury, cadmium, selenium, and manganese concentrations with child systolic blood pressure percentile by maternal cigarette smoking during pregnancy estimated using linear regression models ().
| Metals and trace elements | Groupsa | Difference (95% CI) in child systolic blood pressure percentile per doubling of metal or trace element levelsb | p-interactions | |
|---|---|---|---|---|
| Lead () | Did not smoke | 1,086 | 0.61 (, 2.37) | 0.11 |
| Smoked | 108 | (, 4.26) | ||
| Mercury () | Did not smoke | 1,086 | 0.07 (, 1.22) | 0.18 |
| Smoked | 108 | (, 4.34) | ||
| Cadmium () | Did not smoke | 1,086 | (, 1.31) | 0.33 |
| Smoked | 108 | 2.98 (, 9.00) | ||
| Selenium () | Did not smoke | 1,086 | (, ) | 0.13 |
| Smoked | 108 | (, 7.26) | ||
| Manganese () | Did not smoke | 1,086 | (, 1.24) | 0.08 |
| Smoked | 108 | (, ) |
Note: CI, confidence interval.
Maternal cigarette smoking during pregnancy was dichotomized as smoked vs. did not smoke, with mothers who never smoked and those who quit smoking before pregnancy combined into a single category.
Models adjusted for maternal age at delivery, race/ethnicity, educational level, and prepregnancy BMI.
Figure 4.
Associations (estimates and 95% credible intervals) of maternal manganese and child systolic blood pressure percentile for children born to mothers who smoked cigarette during pregnancy () vs. who did not smoke cigarette during pregnancy () estimated using Bayesian kernel machine regression. Models adjusted for maternal age at delivery, race/ethnicity, educational level, and maternal prepregnancy body mass index. Manganese concentrations were log2-transformed for analyses and were back-transformed to their original scales in the figure.
Sensitivity Analysis
Associations of metals and trace elements with child SBP percentiles did not markedly change when we further adjusted for maternal fish intake during pregnancy, preeclampsia, diabetes, or for child BMI (Table S9). Results showed consistent trends when we used SBP z-score (Table S10) or elevated SBP defined as SBP percentile (Table S11) as the outcome. Estimated differences in SBP percentiles with a doubling of each metal or trace element were similar to those from adjusted linear regression models when we applied stabilized inverse probability weights to account for potential selection bias due to exclusions (Table S12).
Discussion
Main Findings
In the Boston Birth Cohort, a prospective birth cohort with a predominately urban, low-income minority population from Boston, we found that a) in utero exposure to Mn and Se were associated with lower child SBP; b) in utero exposure to Pb and Hg were not associated with higher child SBP; and c) in utero exposure to Mn and Cd interacted with each other when affecting child SBP, in such a way that the inverse association of maternal Mn and child SBP was stronger when maternal Cd levels were higher. Median maternal Cd levels were more than doubled in mothers who smoked during pregnancy compared with mothers who did not. Consistent with the observed Mn–Cd interaction, there was an inverse association between Mn and child SBP among those born to mothers who smoked during pregnancy, and we found little evidence of an association between Mn and child SBP among those born to mothers who did not smoke during pregnancy. Despite the inverse association with Mn, SBP percentiles were higher at all Mn concentrations in the children of mothers who smoked compared with children whose mothers did not smoke. Our findings do not support associations (overall) of in utero metals or trace elements and child DBP. Subgroup analyses showed that: a) the associations of Hg with SBP and DBP were inverse for children whose mothers were non-Hispanic Black but positive for children whose mothers were Hispanic; b) the associations of Mn and SBP were strongest at 3–5 y and null at 10–15 y; and c) the associations of Se with DBP were inverse for female children but close to null for male children. Other associations did not show clear differences by child sex or age group, or by whether the child was born to non-Hispanic Black or Hispanic mothers.
Mn and Se
Our study is the first mixture analysis that shows an inverse association of in utero Mn and Se exposure with child SBP. Two studies also examined how prenatal metal mixtures affect child BP. Kupsco et al. found in 544 Mexico City children that a higher overall mixture of 11 metals in maternal blood collected in the second trimester was associated with lower child SBP (but not DBP) at 4 to 6 y (Kupsco et al. 2019); the mixture included Mn and Se, yet these two elements were not individually associated with SBP or DBP in either linear regression models or mixture models. In another mixture analysis in 176 mother–child pairs from Greece, Howe et al. also did not find an association of Se (measured in maternal urine samples in pregnancy) with child BP at 4, 6, or 11 y (Howe et al. 2021). Different findings between these two studies with ours could possibly be due to the differences in the biomarkers used, time points at biomarker collection and outcome assessment, metal mixtures that the children were exposed to, and unmeasured susceptibility factors and confounders. Compared with these two studies, we also had a larger sample size () to identify associations.
As oxidative stress is involved in the pathogenesis of hypertension and CVD (Ceriello 2008; Cervantes Gracia et al. 2017), the inverse associations of Mn and Se with child SBP may be due to the antioxidant effects of these two elements (Li and Yang 2018; Tinggi 2008). Mn is an essential component of the Mn superoxide dismutase located in the mitochondria that fights against superoxide and free radicals (Li and Yang 2018; Miriyala et al. 2012). Se may affect cardiovascular health through its effects on glutathione peroxidases and other selenoenzymes that can neutralize reactive oxygen and nitrogen species, regulate redox processes, and reduce oxidized methionine (Benstoem et al. 2015).
However, excess levels of Mn and Se could also result in toxic effects, including oxidative damage (ATSDR 2003; Williams et al. 2012). In adults, findings on the effects of Mn and Se on hypertension and CVD are inconclusive. For Se, a meta-analysis of 25 observational studies found that higher Se levels were associated with a lower risk of coronary heart disease in adults; in six clinical trials, however, Se supplementation was not associated with a lower risk of coronary heart disease (Flores-Mateo et al. 2006). A few studies reported an inverse association of Se and BP (Nawrot et al. 2007; Salonen et al. 1988), but an analysis of the NHANES 2003–2004 data found that higher serum Se levels were associated with a higher prevalence of hypertension in U.S. adults (Laclaustra et al. 2009). For Mn, an analysis of the U.S. NHANES 2011–2014 data found that higher urinary Mn levels were associated with lower BP in adults (Wu et al. 2017), whereas another study in Swiss adults found a positive association of plasma and urine Mn with BP (Zhang et al. 2020a). It should also be noted that many previous studies did not examine (and thus may have missed) the possible nonlinear associations of these two elements with BP. Two recent studies, one in U.S. children (i.e., the Boston Birth Cohort) (Wang et al. 2021) and one in Bangladeshi adults (Bulka et al. 2019), reported nonlinear associations of Mn or Se with BP.
Cd and the Mn–Cd Interaction
The overall null association of in utero Cd exposure and child BP is consistent with the literature. In a study of 1,887 children in rural Bangladesh (Hawkesworth et al. 2013) and a study of 515 children in Greece (Chatzi et al. 2019), maternal urinary Cd was not associated with early childhood SBP or DBP, and the association did not differ by child sex. Both studies accounted for maternal smoking as a major contributor to higher Cd levels. Unlike our study, neither of these two studies examined the coexposure of Mn and Cd. In our study, the overall null association of Cd and child SBP was modified by Mn, such that at low Mn levels there was a positive association of Cd with child SBP. Related to this finding, we found that cigarette smoking during pregnancy modified the Mn–child SBP association (inverse association among children whose mothers smoked during pregnancy, and no association among children whose mothers did not smoke during pregnancy). In contrast, Kupsco et al. did not observe a Mn–Cd interaction on child SBP using BKMR (Kupsco et al. 2019), which could reflect differences in metal mixtures, population characteristics (e.g., ethnicity and age), or a smaller sample size compared with our study. To our knowledge, no animal studies have examined how in utero exposure to metal mixture affects offspring BP.
Potential mechanisms for an interaction between Mn–Cd on child SBP are unclear. An antioxidative effect of Mn could play a role, but we did not observe an interaction between Cd and the antioxidant Se. Of the animal studies that examined Mn–Cd interactions, one study found in adult rats that Cd inhibited Mn uptake (Gruden and Matausić 1989), whereas a more recent study in mice found that offspring born to Cd-exposed mothers had increased levels of blood and liver Mn at birth (Hudson et al. 2019). Other possible mechanisms are that Mn increases the synthesis of the metal-binding protein metallothionein (Waalkes and Klaassen 1985), and the amount of Cd bound to metallothionein (Goering and Klaassen 1985) thus decreases unbound Cd and reduces Cd toxicity. However, whether evidence from these animal studies is applicable to humans is not clear. Future studies are needed to examine the possible mechanisms.
Pb
In adults, there is sufficient evidence linking Pb exposure and higher BP and higher risk of CVD (Chowdhury et al. 2018; Navas-Acien et al. 2007). Our findings on the lack of association between in utero Pb and child BP are consistent with a few prior studies. In 457 Hispanic children from Mexico City, maternal patella or cord blood Pb was not overall associated with child SBP or DBP from 7 to 15 y, whereas there was a positive association between maternal tibia Pb and SBP and DBP in girls (Zhang et al. 2012). In 1,574 children from rural Bangladesh, maternal blood Pb was not associated with child SBP or DBP at , and the association did not differ by child sex (Skröder et al. 2016). Some studies, in contrast, did observe a positive association. In 122 New York children, higher cord blood Pb levels were associated with higher SBP and DBP at 9.5 y; the authors did not examine whether the associations differed by child sex (Gump et al. 2005). In a prospective birth cohort of 323 children from New York, New York, higher maternal toenail Pb levels were associated with higher child SBP at 5.5 y, with a stronger association among boys than girls (Farzan et al. 2018).
Several reasons may have contributed to the differences in findings. First, we measured Pb in maternal RBCs, which represent long-term in utero exposure (life span ) and are not influenced by hemodilution during pregnancy (Chen et al. 2014). Second, children in our analysis were older and represented a wider age range (median age at BP measurement: 8.4 y; range: 3.0–15.4 y). Third, the Pb levels in our sample [median (IQR) RBC Pb concentration: 2.4 (1.6–3.7) ] were also lower in comparison with previous studies. Future studies are needed to examine whether the association between in utero Pb exposure and child high BP risk is more pronounced at higher Pb levels.
Hg
Our null finding on the association of in utero Hg exposure and child BP is also consistent with most prior studies in children. In 1,103 Boston children, there was no association of maternal RBC Hg and early- or mid-childhood SBP (Kalish et al. 2014). In 1,754 children from England, no association was observed for maternal whole blood Hg and child SBP or DBP from 7 to 17 years of age (Gregory et al. 2016). In both studies, the association did not differ by child sex. In 779 children from the Republic of Seychelles, there was a positive association of maternal hair Hg and child DBP (but not SBP) among boys (but not girls) at 15 y (but not 12 y); however, this finding may be a chance finding, according to the authors (Thurston et al. 2007). To our knowledge, the only study that showed a positive association of in utero Hg and child BP was from a cohort of 917 children from the Faroe Islands, where higher cord blood Hg was associated with higher SBP and DBP at 7 y (Sørensen et al. 1999). However, children in this study were recruited in 1986–1987 and were highly exposed to Hg (average cord blood Hg concentration was as high as ). The discrepancies in the associations by Hg levels is consistent with findings in adults. In a meta-analysis of 29 studies, Hu et al. concluded that there was a positive association of Hg and BP only in adults with higher Hg levels (Hu et al. 2018). Interestingly, we found that the associations of Hg with both SBP and DBP differed by maternal race/ethnicity, which merits further investigation.
It has been shown in adults that the association of Hg and BP may depend on Hg forms [i.e., elemental, inorganic, or organic such as methylmercury (MeHg)] (Hu et al. 2018). Because RBCs represent a mixture of organic and inorganic Hg (ATSDR 1999), the null results in our study could be due to the mixture of Hg forms that may have opposing effects on BP. Yet, the proportion of RBC inorganic Hg are expected to be very low in our study population because: a) MeHg is the predominant form of Hg in RBCs [ of MeHg is found in the RBCs (Hong et al. 2012)]; b) our cohort is not a cohort with high occupational inorganic Hg exposure; and c) mothers in our cohort are from the Boston area (on the Atlantic Ocean coast), and their primary source of Hg exposure is likely through eating contaminated fish or seafood that contains MeHg (Sagiv et al. 2012).
Validity of Exposure Assessment
We used maternal metal levels measured in RBCs collected 24 to 72 h after delivery as a biomarker for third trimester in utero metal and trace element exposure. Advantages of using this proxy to reflect in utero exposure include that RBCs are a major reservoir of blood metals and trace elements. For example, 99% of Pb, 90% of MeHg, and 66% of Mn in the blood are within RBCs (Abadin et al. 2007; Hong et al. 2012; Milne et al. 1990). RBCs have also been shown to better (vs. plasma) reflect the transplacental transfer of metals and trace elements from the mother to the fetus (Chen et al. 2014). Also, RBC metal and trace element levels are not influenced by hemodilution during pregnancy and are thus free from confounding by hemodilution. Admittedly, one limitation is that these RBCs were collected within 24 to 72 h post delivery. Yet, the average life span of RBCs is , and the biological half-lives for Pb, Hg, and Cd range from 2 to 4 months in blood (CDC 2017; Järup et al. 1983; Smith and Farris 1996). For Mn, its biological half-life in blood is , and its blood levels could reflect the accumulation of Mn in the body (Crossgrove and Zheng 2004; Nelson et al. 1993). For Se, although it has a much shorter half-life, our previous pilot study in a subset of children () in the Boston Birth Cohort showed a strong correlation of maternal RBC Se levels (collected 24 to 72 h after delivery) with cord blood RBC Se levels (collected at birth) () (Chen et al. 2014). As such, RBC metals and trace elements measured in this analysis could be a good proxy in reflecting third trimester in utero exposure. Nevertheless, without repeated measures or trimester-specific data, we cannot assess the impact of cumulative metal and trace element exposure during the entire in utero period. If the critical window of susceptibility for these metals on child BP is in early pregnancy, metal and trace element levels measured in this analysis may not reflect exposure at the relevant time period.
Limitations
Our study has limitations. First, although we controlled for a set of potential confounders (including maternal smoking and fish consumption), we cannot rule out unmeasured confounding by factors such as dietary patterns or residual confounding by imprecisely measured fish intake or other factors. For example, the inverse association of Se and Mn with child SBP may be due to confounding by nutrients in healthy diets. Second, measurement error of child BP may exist because we had only one BP measurement at each visit. This may have led to higher BP measures on average (Flynn et al. 2017; Pickering et al. 2005). Yet, such errors were unlikely to be differential by in utero metal exposure status and, if any, would have biased the estimates toward the null. Third, we did not have data on child metal levels measured in early childhood, which could have mediated the association of in utero metal exposure with child BP. We plan to examine the impact of prenatal metal mixture on child BP in other cohorts as well as in the Environmental influences on Child Health Outcomes (ECHO) program, which pooled data from multiple U.S. cohorts that have data on maternal metal and trace elements and child BP (Buckley et al. 2020).
Strengths and Innovations
Our study has several strengths and innovations. First, we were able to examine prospective exposure-response relationships between coexposures to metals and trace elements and child BP. Second, we used BKMR to examine whether metals and trace elements interact to affect child BP. This approach allowed us to assess in a large group of children () the nonlinear effects and interactions between the metals and trace elements on child BP while accounting for metal coexposures. Had we not used these methods, we may have missed the important interaction between Mn and Cd. Similar findings from both the linear regression and the BKMR strengthened our findings. Third, we measured metal and trace element concentrations in maternal RBCs, which reflect maternal–fetal transfer of the metals and trace elements better than plasma concentrations, represent in utero exposure, and are not influenced by hemodilution during pregnancy (Chen et al. 2014). Fourth, our study population was racially diverse with 61% non-Hispanic Black and 20% Hispanic, which made our findings particularly relevant to those disproportionally exposed to environmental toxic metals yet who are underrepresented in environmental health research.
Summary of Key Findings
In this U.S. urban, low-income, minority birth cohort, in utero exposure to trace elements Se and Mn was prospectively associated with lower child SBP between age 3 to 15 y, whereas Pb, Hg, and Cd exposures did not appear to be associated with child SBP. None of the metals or trace elements were clearly associated with child DBP. There was an interaction between Mn and Cd on child SBP, whereby the protective association of Mn was stronger among mothers with higher Cd. Maternal cigarette smoking during pregnancy, a primary source of Cd, also modified the association of Mn and child SBP. Our findings suggest that for children exposed to cigarette smoke during the prenatal period, those simultaneously exposed to lower Mn may be more susceptible to developing higher BP in childhood compared with those exposed to higher Mn. Although children would fare best if their mothers did not smoke, for mothers who smoked during pregnancy, optimizing Mn level may protect their children from developing high BP and future CVD.
Findings from our analysis provided evidence that analyzing one metal at a time, as has been done in most previous studies, may obscure the true effects of metals on human health outcomes. This evidence emphasizes the need for future epidemiological studies to focus on how metal and trace element coexposures affect health. This focus is in line with the National Institute of Environmental Health Sciences (NIEHS)’s priority to study the impact of cumulative exposure to multiple agents on human health as outlined in its 2018–2023 Strategic Plan (Braun et al. 2016; NIEHS 2018). Future studies should confirm our findings on the inverse association between in utero Mn and Se exposure with child BP and the interaction of Mn and Cd on child SBP. There is also need to understand the molecular and metabolic pathways underlying these associations and to understand how these findings could be translated to clinical and public health practice to prevent child high BP.
Supplementary Material
Acknowledgments
The authors extend acknowledgments to all study participants in the Boston Birth Cohort. The authors also acknowledge the nursing staff at the Boston University Medical Center and the Boston Birth Cohort field team for their support and help with the study.
The Boston Birth Cohort (the parent study) is funded by Maternal and Child Health Bureau (UJ2MC31074) and the National Institutes of Health (NIH; R01HD086013, R01HD041702, R01HD098232, R01ES031272, and R01ES031521). M.Z. is supported by the American Heart Association (Award Number 827990). N.T.M. is supported by the NIH (K01HL141589). J.P.B. is supported by the NIH (R01ES029531). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
References
- Abadin H, Ashizawa A, Stevens YW, Llados F, Diamond G, Sage G, et al. 2007. Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profiles. In: Toxicological Profile for Lead. Atlanta, GA: Agency for Toxic Substances and Disease Registry (US). [PubMed] [Google Scholar]
- Ashraf MW. 2012. Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. ScientificWorldJournal 2012:729430, PMID: 22489199, 10.1100/2012/729430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 1999. Toxicological Profile for Mercury. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- ATSDR. 2003. Toxicological Profile for Selenium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- Benstoem C, Goetzenich A, Kraemer S, Borosch S, Manzanares W, Hardy G, et al. 2015. Selenium and its supplementation in cardiovascular disease–what do we know? Nutrients 7(5):3094–3118, PMID: 25923656, 10.3390/nu7053094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Claus Henn B, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via Bayesian kernel machine regression. Environ Health 17(1):67, PMID: 30126431, 10.1186/s12940-018-0413-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16(3):493–508, PMID: 25532525, 10.1093/biostatistics/kxu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Gennings C, Hauser R, Webster TF. 2016. What can epidemiological studies tell us about the impact of chemical mixtures on human health? Environ Health Perspect 124(1):A6–A9, PMID: 26720830, 10.1289/ehp.1510569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Barrett ES, Beamer PI, Bennett DH, Bloom MS, Fennell TR, et al. 2020. Opportunities for evaluating chemical exposures and child health in the United States: the Environmental influences on Child Health Outcomes (ECHO) program. J Expo Sci Environ Epidemiol 30(3):397–419, PMID: 32066883, 10.1038/s41370-020-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulka CM, Scannell Bryan M, Persky VW, Daviglus ML, Durazo-Arvizu RA, Parvez F, et al. 2019. Changes in blood pressure associated with lead, manganese, and selenium in a Bangladeshi cohort. Environ Pollut 248:28–35, PMID: 30771745, 10.1016/j.envpol.2019.01.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. Biomonitoring Summary—Lead. https://www.cdc.gov/biomonitoring/Lead_BiomonitoringSummary.html [accessed 16 February 2021].
- Ceriello A. 2008. Possible role of oxidative stress in the pathogenesis of hypertension. Diabetes Care 31(suppl 2):S181–S184, PMID: 18227482, 10.2337/dc08-s245. [DOI] [PubMed] [Google Scholar]
- Cervantes Gracia K, Llanas-Cornejo D, Husi H. 2017. CVD and oxidative stress. J Clin Med 6(2):22, 10.3390/jcm6020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzi L, Ierodiakonou D, Margetaki K, Vafeiadi M, Chalkiadaki G, Roumeliotaki T, et al. 2019. Associations of prenatal exposure to cadmium with child growth, obesity, and cardiometabolic traits. Am J Epidemiol 188(1):141–150, PMID: 30252047, 10.1093/aje/kwy216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. 2014. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol 24(5):537–544, PMID: 24756102, 10.1038/jes.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang Y. 2008. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation 117(25):3171–3180, PMID: 18559702, 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. 2018. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362:k3310, PMID: 30158148, 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossgrove J, Zheng W. 2004. Manganese toxicity upon overexposure. NMR Biomed 17(8):544–553, PMID: 15617053, 10.1002/nbm.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. 2011. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5.4 million participants. Lancet 377(9765):568–577, PMID: 21295844, 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- Farzan SF, Howe CG, Chen Y, Gilbert-Diamond D, Cottingham KL, Jackson BP, et al. 2018. Prenatal lead exposure and elevated blood pressure in children. Environ Int 121(pt 2):1289–1296, PMID: 30389381, 10.1016/j.envint.2018.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. 2006. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr 84(4):762–773, PMID: 17023702, 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. 2017. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics 140(3):e20171904, PMID: 28827377, 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, et al. 2017. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm hg, 1990–2015. JAMA 317(2):165–182, PMID: 28097354, 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- Frost DV. 1972. The two faces of selenium–can selenophobia be cured? CRC Crit Rev Toxicol 1(4):467–514, PMID: 4564866, 10.3109/10408447209103467. [DOI] [PubMed] [Google Scholar]
- Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. 2017. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief: 1–8, PMID: 29155682. [PubMed] [Google Scholar]
- Goering PL, Klaassen CD. 1985. Mechanism of manganese-induced tolerance to cadmium lethality and hepatotoxicity. Biochem Pharmacol 34(9):1371–1379, PMID: 3994753, 10.1016/0006-2952(85)90673-2. [DOI] [PubMed] [Google Scholar]
- Gregory S, Iles-Caven Y, Hibbeln JR, Taylor CM, Golding J. 2016. Are prenatal mercury levels associated with subsequent blood pressure in childhood and adolescence? The Avon prebirth cohort study. BMJ Open 6(10):e012425, PMID: 27742626, 10.1136/bmjopen-2016-012425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruden N, Matausić S. 1989. Some factors influencing cadmium-manganese interaction in adult rats. Bull Environ Contam Toxicol 43(1):101–106, PMID: 2758124, 10.1007/BF01702244. [DOI] [PubMed] [Google Scholar]
- Gump BB, Stewart P, Reihman J, Lonky E, Darvill T, Matthews KA, et al. 2005. Prenatal and early childhood blood lead levels and cardiovascular functioning in 9½ year old children. Neurotoxicol Teratol 27(4):655–665, PMID: 15919179, 10.1016/j.ntt.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Gundacker C, Hengstschläger M. 2012. The role of the placenta in fetal exposure to heavy metals. Wien Med Wochenschr 162(9–10):201–206, PMID: 22717874, 10.1007/s10354-012-0074-3. [DOI] [PubMed] [Google Scholar]
- Hawkesworth S, Wagatsuma Y, Kippler M, Fulford AJ, Arifeen SE, Persson L-A, et al. 2013. Early exposure to toxic metals has a limited effect on blood pressure or kidney function in later childhood, rural Bangladesh. Int J Epidemiol 42(1):176–185, PMID: 23243118, 10.1093/ije/dys215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henn BC, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, Hernández-Avila M, et al. 2012. Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect 120(1):126–131, PMID: 21885384, 10.1289/ehp.1003300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YS, Kim YM, Lee KE. 2012. Methylmercury exposure and health effects. J Prev Med Public Health 45(6):353–363, PMID: 23230465, 10.3961/jpmph.2012.45.6.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe CG, Margetaki K, Vafeiadi M, Roumeliotaki T, Karachaliou M, Kogevinas M, et al. 2021. Prenatal metal mixtures and child blood pressure in the rhea mother-child cohort in Greece. Environ Health 20(1):1, PMID: 33407552, 10.1186/s12940-020-00685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu XF, Singh K, Chan HM. 2018. Mercury exposure, blood pressure, and hypertension: a systematic review and dose–response meta-analysis. Environ Health Perspect 126(7):076002, PMID: 30073953, 10.1289/EHP2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson KM, Belcher SM, Cowley M. 2019. Maternal cadmium exposure in the mouse leads to increased heart weight at birth and programs susceptibility to hypertension in adulthood. Sci Rep 9(1):13553, PMID: 31537853, 10.1038/s41598-019-49807-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järup L, Rogenfelt A, Elinder CG, Nogawa K, Kjellström T. 1983. Biological half-time of cadmium in the blood of workers after cessation of exposure. Scand J Work Environ Health 9(4):327–331, PMID: 6635611, 10.5271/sjweh.2404. [DOI] [PubMed] [Google Scholar]
- Ji H, Kim A, Ebinger JE, Niiranen TJ, Claggett BL, Bairey Merz CN, et al. 2020. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol 5(3):255–226, PMID: 31940010, 10.1001/jamacardio.2019.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LG, Trasande L. 2018. Environmental toxicant exposure and hypertensive disorders of pregnancy: recent findings. Curr Hypertens Rep 20(10):87, PMID: 30090982, 10.1007/s11906-018-0888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalish BT, Rifas-Shiman SL, Wright RO, Amarasiriwardena CJ, Jayawardene I, Gillman MW, et al. 2014. Associations of prenatal maternal blood mercury concentrations with early and mid-childhood blood pressure: a prospective study. Environ Res 133:327–333, PMID: 25019468, 10.1016/j.envres.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupsco A, Kioumourtzoglou M-A, Just AC, Amarasiriwardena C, Estrada-Gutierrez G, Cantoral A, et al. 2019. Prenatal metal concentrations and childhood cardiometabolic risk using Bayesian kernel machine regression to assess mixture and interaction effects. Epidemiology 30(2):263–273, PMID: 30720588, 10.1097/EDE.0000000000000962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laclaustra M, Navas-Acien A, Stranges S, Ordovas JM, Guallar E. 2009. Serum selenium concentrations and hypertension in the US population. Circ Cardiovasc Qual Outcomes 2(4):369–376, PMID: 20031863, 10.1161/CIRCOUTCOMES.108.831552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanphear BP, Rauch S, Auinger P, Allen RW, Hornung RW. 2018. Low-level lead exposure and mortality in US adults: a population-based cohort study. Lancet Public Health 3(4):e177–e184, PMID: 29544878, 10.1016/S2468-2667(18)30025-2. [DOI] [PubMed] [Google Scholar]
- Lee B-K, Kim Y. 2011. Relationship between blood manganese and blood pressure in the Korean general population according to KNHANES 2008. Environ Res 111(6):797–803, PMID: 21601843, 10.1016/j.envres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Li L, Yang X. 2018. The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev 2018:7580707, PMID: 29849912, 10.1155/2018/7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Hivert M-F, Rifas-Shiman SL, Rahman ML, Oken E, Cardenas A, et al. 2020. Prospective association between manganese in early pregnancy and the risk of preeclampsia. Epidemiology 31(5):677–680, PMID: 32618710, 10.1097/EDE.0000000000001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Zhang M, Guallar E, Wang G, Hong X, Wang X, et al. 2019. Trace minerals, heavy metals, and preeclampsia: findings from the Boston Birth Cohort. J Am Heart Assoc 8(16):e012436, PMID: 31426704, 10.1161/JAHA.119.012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne DB, Sims RL, Ralston NV. 1990. Manganese content of the cellular components of blood. Clin Chem 36(3):450–452, PMID: 2311212, 10.1093/clinchem/36.3.450. [DOI] [PubMed] [Google Scholar]
- Miriyala S, Spasojevic I, Tovmasyan A, Salvemini D, Vujaskovic Z, St. Clair D, et al. 2012. Manganese superoxide dismutase, MnSOD and its mimics. Biochim Biophys Acta 1822(5):794–814, PMID: 22198225, 10.1016/j.bbadis.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordukhovich I, Wright RO, Hu H, Amarasiriwardena C, Baccarelli A, Litonjua A, et al. 2012. Associations of toenail arsenic, cadmium, mercury, manganese, and lead with blood pressure in the normative aging study. Environ Health Perspect 120(1):98–104, PMID: 21878420, 10.1289/ehp.1002805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEHS (National Institute of Environmental Health Sciences). 2018. NIEHS Strategic Plan 2018–2023 - Advancing Environmental Health Sciences, Improving Health.https://www.niehs.nih.gov/about/strategicplan/strategicplan20182023_508.pdf [accessed 20 September 2020].
- Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. 2007. Lead exposure and cardiovascular disease–a systematic review. Environ Health Perspect 115(3):472–482, PMID: 17431501, 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Roels HA, Den Hond E, Thijs L, Fagard RH, et al. 2007. Blood pressure and blood selenium: a cross-sectional and longitudinal population study. Eur Heart J 28(5):628–633, PMID: 17242009, 10.1093/eurheartj/ehl479. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Thijs L, Den Hond EM, Roels HA, Staessen JA. 2002. An epidemiological re-appraisal of the association between blood pressure and blood lead: a meta-analysis. J Hum Hypertens 16(2):123–131, PMID: 11850770, 10.1038/sj.jhh.1001300. [DOI] [PubMed] [Google Scholar]
- Nelson K, Golnick J, Korn T, Angle C. 1993. Manganese encephalopathy: utility of early magnetic resonance imaging. Br J Ind Med 50(6):510–513, PMID: 8329316, 10.1136/oem.50.6.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, et al. 2005. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the subcommittee of professional and public education of the American Heart Association Council on High Blood Pressure Research. Circulation 111(5):697–716, PMID: 15699287, 10.1161/01.CIR.0000154900.76284.F6. [DOI] [PubMed] [Google Scholar]
- Rosner B, Cook NR, Daniels S, Falkner B. 2013. Childhood blood pressure trends and risk factors for high blood pressure. Hypertension 62(2):247–254, PMID: 23856492, 10.1161/HYPERTENSIONAHA.111.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Amarasiriwardena C, Korrick SA. 2012. Prenatal exposure to mercury and fish consumption during pregnancy and attention-deficit/hyperactivity disorder-related behavior in children. Arch Pediatr Adolesc Med 166(12):1123–1129, PMID: 23044994, 10.1001/archpediatrics.2012.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen JT, Salonen R, Ihanainen M, Parviainen M, Seppänen R, Kantola M, et al. 1988. Blood pressure, dietary fats, and antioxidants. Am J Clin Nutr 48(5):1226–1232, PMID: 3189209, 10.1093/ajcn/48.5.1226. [DOI] [PubMed] [Google Scholar]
- Sanders AP, Claus Henn B, Wright RO. 2015. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature. Curr Environ Health Rep 2(3):284–294, PMID: 26231505, 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim YK, Lewin MD, Ruiz P, Eichner JE, Mumtaz MM. 2017. Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: the United States NHANES, 2007–2012. J Toxicol Environ Health A 80(9):502–512, PMID: 28703686, 10.1080/15287394.2017.1330581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skröder H, Hawkesworth S, Moore SE, Wagatsuma Y, Kippler M, Vahter M. 2016. Prenatal lead exposure and childhood blood pressure and kidney function. Environ Res 151:628–634, PMID: 27611993, 10.1016/j.envres.2016.08.028. [DOI] [PubMed] [Google Scholar]
- Smith JC, Farris FF. 1996. Methyl mercury pharmacokinetics in man: a reevaluation. Toxicol Appl Pharmacol 137(2):245–252, PMID: 8661350, 10.1006/taap.1996.0078. [DOI] [PubMed] [Google Scholar]
- Sørensen N, Murata K, Budtz-Jørgensen E, Weihe P, Grandjean P. 1999. Prenatal methylmercury exposure as a cardiovascular risk factor at seven years of age. Epidemiology 10(4):370–375, PMID: 10401870, 10.1097/00001648-199907000-00006. [DOI] [PubMed] [Google Scholar]
- Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. 2007. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics 119(2):237–246, PMID: 17272612, 10.1542/peds.2006-2543. [DOI] [PubMed] [Google Scholar]
- Textor J, van der Zander B, Gilthorpe MS, Liskiewicz M, Ellison GT. 2016. Robust causal inference using directed acyclic graphs: the R package ‘dagitty’. Int J Epidemiol 45:1887–1894, PMID: 28089956, 10.1093/ije/dyw341. [DOI] [PubMed] [Google Scholar]
- Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, et al. 2015. Childhood to early-midlife systolic blood pressure trajectories. Hypertension 66(6):1108–1115, PMID: 26558818, 10.1161/HYPERTENSIONAHA.115.05831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurston SW, Bovet P, Myers GJ, Davidson PW, Georger LA, Shamlaye C, et al. 2007. Does prenatal methylmercury exposure from fish consumption affect blood pressure in childhood? Neurotoxicology 28(5):924–930, PMID: 17659343, 10.1016/j.neuro.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinggi U. 2008. Selenium: its role as antioxidant in human health. Environ Health Prev Med 13(2):102–108, PMID: 19568888, 10.1007/s12199-007-0019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA, et al. 2017. The joint effect of prenatal exposure to metal mixtures on neurodevelopmental outcomes at 20-40 months of age: evidence from rural Bangladesh. Environ Health Perspect 125(6):067015, PMID: 28669934, 10.1289/EHP614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. 2020. Heart Disease and Stroke Statistics-2020 Update: a report from the American Heart Association. Circulation 141(9):e139–e596, PMID: 31992061, 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Klaassen CD. 1985. Concentration of metallothionein in major organs of rats after administration of various metals. Fundam Appl Toxicol 5(3):473–477, PMID: 4007305, 10.1093/toxsci/5.3.473. [DOI] [PubMed] [Google Scholar]
- Wang G, Tang W-Y, Wills-Karp M, Ji H, Bartell TR, Ji Y, et al. 2021. A nonlinear relation between maternal red blood cell manganese concentrations and child blood pressure at age 6–12 y: a prospective birth cohort study. J Nutr 151(3):570–578, PMID: 33438012, 10.1093/jn/nxaa368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, et al. 2006. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect 114(1):124–129, PMID: 16393669, 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CV, Lewin M, Ragin-Wilson A, Jones R, Jarrett JM, Wallon K, et al. 2020. Characterization of trace elements exposure in pregnant women in the United States, NHANES 1999–2016. Environ Res 183:109208, PMID: 32058143, 10.1016/j.envres.2020.109208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2018. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APHA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138:e484–e594, PMID: 30354654, 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
- Williams M, Todd GD, Roney N, Crawford J, Coles C, McClure PR, et al. 2012. Agency for Toxic Substances and Disease Registry (ATSDR) toxicological profiles. In: Toxicological Profile for Manganese. Atlanta, GA: Agency for Toxic Substances and Disease Registry (US). [PubMed] [Google Scholar]
- Wu C, Woo JG, Zhang N. 2017. Association between urinary manganese and blood pressure: results from National Health and Nutrition Examination Survey (NHANES), 2011–2014. PLoS One 12(11):e0188145, PMID: 29141052, 10.1371/journal.pone.0188145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Du X, Guo L, Du J, Arnott C, Lam Carolyn SP, et al. 2020. Sex differences in primary and secondary prevention of cardiovascular disease in China. Circulation 141(7):530–539, PMID: 32065775, 10.1161/CIRCULATIONAHA.119.043731. [DOI] [PubMed] [Google Scholar]
- Yang L, Magnussen Costan G, Yang L, Bovet P, Xi B. 2020. Elevated blood pressure in childhood or adolescence and cardiovascular outcomes in adulthood. Hypertension 75(4):948–955, PMID: 32114851, 10.1161/HYPERTENSIONAHA.119.14168. [DOI] [PubMed] [Google Scholar]
- Zhang ZY, Carmeli C, Ponte B, Pruijm M, Ackermann D, Ehret G, et al. 2020a. Ambulatory blood pressure in relation to plasma and urinary manganese. Hypertension 75(4):1133–1139, PMID: 32114854, 10.1161/HYPERTENSIONAHA.119.13649. [DOI] [PubMed] [Google Scholar]
- Zhang M, Michos E, Wang G, Wang X, Mueller NT. 2020b. Association of cord blood vitamin D and preeclampsia with offspring blood pressure in childhood and adolescence. JAMA Netw Open 3(10):e2019046, PMID: 33017029, 10.1001/jamanetworkopen.2020.19046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Hu H, Sanchez BN, Ettinger AS, Park SK, Cantonwine D, et al. 2012. Association between prenatal lead exposure and blood pressure in children. Environ Health Perspect 120(3):445–450, PMID: 21947582, 10.1289/ehp.1103736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mueller NT, Wang H, Hong X, Appel LJ, Wang X. 2018. Maternal exposure to ambient particulate matter ≤2.5 µm during pregnancy and the risk for high blood pressure in childhood. Hypertension 72(1):194–201, PMID: 29760154, 10.1161/HYPERTENSIONAHA.117.10944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.