Abstract
Intra-articular (IA) injection is an attractive route of administration for the treatment of osteoarthritis (OA). However, free drugs injected into the joint space are rapidly cleared and many of them can induce adverse off-target effects on different IA tissues. To overcome these limitations, we designed nano-composite 4-arm-poly(ethylene glycol)-maleimide (PEG-4MAL) microgels, presenting cartilage- or synoviocyte-binding peptides, containing poly(lactic-co-glycolic) acid (PLGA) nanoparticles (NPs) as an IA small molecule drug delivery system. Microgels containing rhodamine B (model drug)-loaded PLGA NPs were synthesized using microfluidics technology and exhibited a sustained, near zero-order release of the fluorophore over 16 days in vitro. PEG-4MAL microgels presenting synoviocyte- or cartilage-targeting peptides specifically bound to rabbit and human synoviocytes or to bovine articular cartilage in vitro, respectively. Finally, using a rat model of post-traumatic knee OA, PEG-4MAL microgels were shown to be retained in the joint space for at least 3 weeks without inducing any joint degenerative changes as measured by EPIC-μCT and histology. Additionally, all microgel formulations were found trapped in the synovial membrane and significantly increased the IA retention time of a model small molecule near-infrared (NIR) dye compared to the free dye. These results suggest that peptide-functionalized nano-composite PEG-4MAL microgels represent a promising intra-articular vehicle for tissue-localized drug delivery and prolonged IA drug retention for the treatment of OA.
Keywords: Microgels, tissue-binding, intra-articular, drug delivery, osteoarthritis
Graphical Abstract
1. Introduction
Osteoarthritis (OA) is a progressive joint degenerative disease that affects approximately 303 million people in the world1 and 30.8 million adults in the U.S.2 There are no FDA-approved disease modifying OA drugs (DMOADs).3 OA treatment is limited to weight management and physical therapy;4 systemic pain management using non-steroidal anti-inflammatory drugs (NSAIDs), cyclooxygenase inhibitors and weak opioids, whose long-term use has been associated with adverse effects on the gastrointestinal, renal and circulatory system;5,6 and viscosupplementation injections of hyaluronic acid.7 Nevertheless, these management approaches do not address the underlying joint degeneration and have limited long-term benefits.4,8,9
Intra-articular (IA) injection offers an attractive route of drug administration, which can reduce systemic off-target effects and allow for a higher local drug concentration while using lower doses compared to systemic delivery.3 However, free drugs injected in the IA space are rapidly cleared, which leads to insufficient retention times and effective concentrations for drugs to elicit a therapeutic effect.10 For example, the IA half-life time of small molecule drugs (<1 kDa) is less than 5 h11 and that of larger molecules such as proteins does not exceed 24 h.12,13 Although recently discovered DMOADs have demonstrated promising results in preclinical models of OA, their translation into the clinic has been hindered, in part due to the lack of IA drug delivery vehicles able to support long-term treatment.3
Extensive research has led to the development of novel DMOADs that act on different joint tissues.3 However, non-tissue specific drug delivery may result in unwanted off-target effects. For example, drugs targeting processes occurring in the synovial membrane such as NSAIDs have been related to reduced proteoglycan secretion and increased cartilage degradation.14 Similarly, nerve growth factor (NGF) blockade for pain relief resulted in rapid OA progression and osteonecrosis in a phase III clinical trial.15 Likewise, drugs that induce chondrogenesis such as TGF-β and kartogenin increase synovium hyperplasia and formation of cartilage-like tissues in ligaments and synovial membrane.16,17
To overcome these limitations, targeting strategies have been used to confer the delivery vehicle the ability to bind articular cartilage18-22 or synovial membrane.23-29 Targeting peptides that bind the inflamed synovial vasculature28,29 or fibroblast-like synoviocytes have been identified.25 In particular, the HAP-1 peptide (SFHQFARATLAS), which targets fibroblast-like synoviocytes, has been conjugated to drug-loaded liposomes, demonstrating encouraging results in rat models of adjuvant- and zymosan-induced arthritis.26,27 On the other hand, active cartilage targeting has been achieved via anti-type II collagen (Coll-2) monoclonal antibodies18-20 and Coll-2-targeting peptides.22 Rothenfluh et al. reported the peptide WYRGRL (WYR), which specifically binds to Coll-2 α1 chain22 and has shown efficient cartilage-binding in a variety of diagnostic and drug delivery applications.5,30-33
Despite advances on tissue targeting, only nano-sized carriers have been used for IA tissue-localized drug delivery.19-22,26-28 Even though the use of NPs may be advantageous for intra-cartilage and intra-cellular drug delivery, recent studies have shown that the use of targeting moieties does not improve IA drug carrier retention. Brown et al. demonstrated that cartilage-targeting PLGA-based NPs effectively penetrate the articular cartilage when conjugated with the targeting peptide WYR or to positively charged moieties. However, the use of these cartilage targeting strategies did not improve NPs IA retention compared to non-targeting control NPs.30 In general, particles larger than a few microns in diameter present better IA retention than NPs.3 Nevertheless, the use of larger particles may not always be beneficial. Polymeric microparticles resulting from joint arthroplasty implant wear are associated with articular cartilage and meniscus degradation and synovitis.34 Liggins et al. demonstrated that PLGA particles between 1 and 20 μm in size induced higher proteoglycan depletion and synovial cellular infiltration than larger particles (30-100 μm) in a rabbit model of antigen-induced arthritis.35 These observations raise concerns regarding the use of solid polymeric microparticles for long-term IA delivery. Therefore, there is an unmet need to develop safe intra-articular drug delivery systems with tissue-localized delivery capabilities and prolonged IA drug retention times.
Synthetic microgels, micron-sized hydrogel particles, offer attractive chemical and mechanical properties for the development of intra-articular drug delivery vehicles. In particular PEG-4MAL microgels allow for efficient incorporation of biological ligands and have been used for cell encapsulation and protein delivery applications.36-38 However, their use as small molecule intra-articular drug carriers has not been explored. Here, we developed nano-composite PEG-4MAL microgels containing poly(lactic-co-glycolic) acid (PLGA) NPs and functionalized with HAP-1 and WYR targeting peptides as synoviocyte-binding and articular cartilage surface-binding intra-articular small molecule delivery vehicles. We evaluated these systems for their ability to support the sustained release of model small molecule drugs and bind to synoviocytes and articular cartilage in vitro. Additionally, the IA retention of PEG-4MAL microgels and a loaded model small molecule were evaluated in a rat model of OA. Finally, the IA localization of peptide-functionalized microgels was evaluated and their effect on articular cartilage and synovial membrane structure were investigated.
2. Materials and Methods
2.1. Materials
The reagents and peptides used in this work are described in Supplementary Material.
2.2. PLGA nanoparticles synthesis and characterization
PLGA NPs containing model small molecules were synthesized by oil-in-water single emulsion method. PLGA (210 mg) was dissolved in 6 mL of dichloromethane (DCM) and mixed with 1 mL of a solution of either rhodamine B or cyanine 7 (Cy7) in DCM (1 mg/mL). The mixture was added to 50 mL of 1% polyvinyl alcohol (PVA) in distilled water and sonicated for 3 min (A=100%, VibraCell™ Ultrasonic Processor, VCX 130 PB). The emulsion was added to 50 mL 1% PVA and magnetically stirred for 4 h. PLGA NPs were washed with distilled water 3 times via centrifugation at 16,000g for 10 min, lyophilized and stored at −20°C. Nanoparticles size was determined using dynamic light scattering (Brookhaven 90 Plus Particle Size Analyzer, Brookhaven Instruments, Holtsville, NY, USA).
2.3. Preparation PEG-4MAL/PLGA NPs composite microgels and characterization
PEG microgels were synthesized using microfluidics technology as described elsewhere with minor modifications.36 Aqueous phase was composed of PBS with Ca2+ and Mg2+ (PBS+/+) and Optiprep (2:1) with 20 mM HEPES and oil phase contained mineral oil with 2% SPAN 80. Prior to microgel formation, PEG-4MAL macromer was reacted with cartilage- (WYR), synoviocyte- (HAP-1) or integrin- (RGD) binding peptides or their respective scrambled controls (WYRsc, HAP-1sc, RDG) at room temperature and pH 7.0 for 10 min, and mixed with an aqueous suspension of PLGA NPs. The final PEG-4MAL concentration was 6% w/v, and contained 1.0 mM peptide and either 0, 0.25, 0.50 or 1.00% w/v PLGA NPs. Cross-linker emulsion was prepared using a solution of dithiothreitol (DTT) in aqueous phase (30 mg/mL) mixed with oil phase at a 1:14 ratio. Microgels were synthesized using PDMS flow-focusing devices with a nozzle size of 46.5 ± 0.5 μm (Fig. 1A) and washed 5 times with a 1% bovine serum albumin (BSA) solution in PBS+/+ via centrifugation (1,000g for 5 min). Size distribution of PEG-4MAL microgels was determined via microscopy image analysis using ImageJ. Further microgels characterization methods are described in Supplementary Material.
Figure 1.
Nano-composite PEG-4MAL/PLGA NP microgels synthesis and characterization. A) Schematic of microgel fabrication. PEG-4MAL was functionalized with targeting peptides (1.0 mM) and mixed with PLGA NPs prior to microgels synthesis via microfluidics technology. B) Rhodamine B-loaded PLGA NP size distribution. C) Encapsulation of PLGA NPs into PEG-4MAL microgels did not affect microgel size (p>0.99). D) Rhodamine B release profile from empty microgels, nano-composite microgels and PLGA NPs. Rhodamine B rapidly diffuses out of empty PEG-4MAL microgels, whereas microgels containing PLGA NPs and PLGA NPs alone exhibited a zero-order release. E) Nano-composite PEG-4MAL microgels presented a rhodamine B release rate comparable to PLGA NPs alone (p=0.107).
2.4. Rhodamine B in vitro release
PEG-4MAL microgels containing different rhodamine B-loaded PLGA NPs concentrations (0, 0.25, 0.50, and 1.00% w/v) were prepared as described before (n = 4 independent runs, 27 μL of PEG-4MAL macromer precursor solution were used to prepare each batch). Empty microgels were loaded with a rhodamine B solution (5 μg/mL) by incubation and physical entrapment. PLGA NPs alone (1.35 mg/mL) were used as a control. Samples were then incubated at 4°C with constant stirring in a total volume of 200 μL of 1% BSA. At each time point, microgel samples were centrifuged at 1000g for 5 min, 150 μL of supernatant were taken for rhodamine B release measurements via spectrophotometry (λex/λem: 553/627 nm) and replaced with fresh 1% BSA solution. For PLGA NPs only, a centrifugation speed of 16,000g was used to ensure appropriate separation. Encapsulated rhodamine content in PLGA NPs was measured after extraction in methanol (1 mg/mL) at 4°C. Relative cumulative release profiles were analyzed using a zero-order curve fit.
2.5. HAP-1-functionalized PEG-4MAL microgel binding assay
To evaluate the ability of HAP-1 peptide-functionalized PEG-4MAL microgels to specifically bind to synovial cells, rabbit and human synoviocytes (HIG-82 and SW982), mouse myoblasts (C2C12) and mouse pre-osteoblasts (MC3T3-E1) were cultured in a 24-well plate at an initial seeding density of 100,000 cells/well for one day to achieve over 95% confluency. C2C12 and MC3T3-E1 cell lines were used as negative controls to assess microgel binding specificity. Cells were stained using CellTracker Green™ according to the manufacturer's instructions. PEG-4MAL microgels (19,000 microgels/mL, 500 μL/well) containing rhodamine B-loaded PLGA NPs and functionalized with either 1.0 mM HAP-1, HAP-1sc, RGD or RDG (n = 4 wells per group) were incubated for 30 min with the different cell lines in growth media (Ham’s F12 media supplemented with 10% fetal bovine serum (FBS)). The integrin-binding peptide RGD was used as positive control. Unbound microgels were washed 3 times using growth media, then 10 images per well were taken at random positions using confocal microscopy and bound microgels were manually quantified.
2.6. WYR-functionalized PEG-4MAL microgel binding assay
The ability of WYR-conjugated PEG-4MAL microgels to bind to articular cartilage was evaluated using 10 μm thick fresh bovine cartilage frozen sections. Sections (~1 cm2, n=3) were stained with DAPI and incubated for 30 min with 100 μL of WYR or WYRsc-functionalized microgels suspended in growth media (19,000 microgels/mL). Samples were washed twice using growth media and a minimum of 7 randomly located images per sample were taken using confocal microscopy to quantify bound microgels. To assess for the specificity of the cartilage-targeting peptide and confirm that it does not bind to synoviocytes, a microgels binding assay was conducted using the HIG-82 cells monolayer platform as described in section 2.4.
2.7. Surgical induction of OA in Lewis rats
All animal experiments were performed following Georgia Tech's Institutional Animal Care and Use Committee (IACUC) approval. Male Lewis rats (250-300g) were subjected to unilateral medial meniscus transection (MMT) as previously described.39
2.8. Peptide-functionalized PEG-4MAL/PLGA NPs microgel in vivo intra-articular retention
HAP-1 and WYR peptides were conjugated to Cy7-NHS ester prior to microgel formation following manufacturer’s instructions. Three weeks after MMT procedure, rats received 50 μL bilateral injections of the following formulations in sterile saline: (1) free sulfo-Cy7 dye (negative control), (2) Cy7-WYR-functionalized microgels or (3) Cy7-HAP-1-functionalized microgels (530,000 microgels/mL, n=9). Microgel formulations contained 0.5% w/v PLGA NPs. Rats were scanned before and immediately after injection and on days 1, 3, 5, 7, 10, 13, 16, 19 and 26 post-injection using an in vivo imaging system (Perkin Elmer IVIS Spectrum CT). At day 26, rats were euthanized and legs harvested to assess knee joint health and OA progression.
2.9. Assessment of the medial tibial articular cartilage and synovial membrane thickness
Harvested tissues were fixed in 10% neutral buffered formalin for 3 days and then stored in PBS without Ca++ and Mg++ (PBS−/−). Cartilage degradation and osteophyte formation were measured via equilibrium partitioning of ionic contrasting agent microcomputed tomography (EPIC μ-CT)40. Dissected tibias (n=6) were incubated in 37.5% Conray® contrast agent for 40 min at room temperature. Samples were scanned in a Scanco μCT40 (45 kVp, 177 μA, 8 W, 16 μm resolution) and images contoured and analyzed for cartilage attenuation, lesion volume, roughness and calcified osteophyte volume 40,41. Three samples per group were decalcified for 10 days, then knee joints were dissected leaving the knee cap intact, embedded in paraffin and sectioned through the coronal plane (5 μm thick sections). Samples were then stained using Toluidine Blue and synovial membrane thickness was measured using ImageJ.
2.10. In vivo localization of nano-composite microgels
Peptide-functionalized microgels (HAP-1, HAP-1sc, WYR, and WYRsc) were injected into rat knees 2 weeks after surgical induction of OA (50 μL/injection, 530,000 microgels/mL, n=4). Animals were euthanized 2 weeks after IA injections and knees were collected. Tissues were fixed in 10% neutral buffered formalin for 3 days, decalcified for 10 days and embedded in optimal cutting temperature compound (OCT) and sectioned through the mid-coronal plane. For each sample, 3 sections (15 μm thick) were collected and stained with hematoxylin and eosin (H&E) for microgels localization.
2.11. Small molecule cargo in vivo intra-articular retention
The near infrared dye Cy7, used as model small molecule drug, was loaded into PLGA NPs as described in section 2.1. Microgels functionalized with WYR, WYRsc, HAP-1 and HAP-1sc peptides, containing 0.5% w/v Cy7-loaded PLGA NPs, were injected into the knee joints of male Lewis rats (50 μL/injection, 530,000 microgels/mL) at 3 weeks after surgical induction of OA (n=7). Sulfo-Cy7 free dye and Cy7-loaded PLGA NPs (no microgels) were used as controls. Rats were scanned before and immediately after injection and on days 1, 3, 5, 7, 10, 13, 16, 19 and 26 post-injection using an in vivo imaging system (Perkin Elmer IVIS Spectrum CT).
3. Statistical Methods
All data are reported as mean ± standard deviation. Data obtained from the rhodamine B release assay was fitted using either zero- or first-order model, and the corresponding curve fits were compared for their goodness of fit using the Akaike’s Information Criterion (AIC). Data acquired from the in vivo microgels and Cy7 tracking studies were fitted to a one-phase exponential decay and curve fit parameters were then compared among groups. Outlier analysis was performed using a maximum false discovery rate of 1% prior to statistical analysis. Normally distributed data that presented equal variances was analyzed via one-way ANOVA. All other data that did not present a Gaussian distribution or homoscedasticity was subjected to a logarithmic transformation. If the transformation resulted in normally distributed data with equal variances, a one-way ANOVA was applied. If the logarithmic transformation only corrected for data non-normality, a Welch’s ANOVA was used on the transformed data. A p-value<0.05 was considered significant. All analyses were performed using Prism (GraphPad Software, San Diego, CA).
4. Results
4.1. Microfluidic polymerization produces PEG-4MAL/PLGA NP microgels
Small molecule-loaded PLGA NPs exhibited a mean size of 338 ± 91 nm (Fig. 1B) and contained 3 μg of rhodamine B per mg of PLGA (encapsulation efficiency 63%). The microfluidic technology generates PLGA NP-loaded PEG-4MAL microgels with tight size distribution (Fig. 1C). All formulations presented a mean size between 50.4 and 51.4 μm with a coefficient of variation (CV) below 6.5% (Table 1). PLGA NP encapsulation did not significantly affect microgels mean size (p>0.99). PLGA NP content per microgel followed a Poisson distribution for formulations containing 0.25% w/v PLGA NPs, whereas higher PLGA NP concentrations (0.50 and 1.00% w/v) led to a bimodal distribution with most microgels containing 10% or 90% of their cross-sectional area filled with PLGA NPs (Fig. S2). This phenomenon is more pronounced in the 1.00% w/v PLGA NP group and can be attributed to the NP hydrophobic nature, which leads to nanoparticle aggregation as its concentration increases in the precursor PEG-4MAL solution.
Table 1.
PLGA NPs-loaded microgels size and coefficient of variance (CV%).
| PLGA NPs (%w/v) | Diameter mean ± SD (μm) |
CV (%) |
|---|---|---|
| 0.00 | 51.0 ± 2.7 | 5.20 |
| 0.25 | 50.4 ± 2.2 | 4.38 |
| 0.50 | 50.9 ± 3.3 | 6.44 |
| 1.00 | 51.4 ± 3.3 | 6.48 |
4.2. Rhodamine B in vitro release is controlled by PLGA NPs
Rhodamine B was used as a model small molecule (M.W = 479.02 Da). When encapsulated into empty PEG-4MAL microgels by simple physical entrapment, over 80% rhodamine B was released within the first hour, consistent to our previous studies using other small molecules loaded into PEG-4MAL microgels.36 In contrast, PLGA alone or microgels containing rhodamine B-loaded PLGA NPs exhibited a sustained release over 16 days (Fig. 1D), which followed a zero-order release kinetics model (AIC test, >99% compared to first-order model). PLGA NPs alone presented an initial burst release (10.0 ± 2.8% by day 1) followed by a zero-order profile, characteristic of PLGA particulate systems.42,43 In contrast, nano-composite microgels did not exhibit an initial burst release (p<0.0001 compared to PLGA NPs only at day 1), which typically occurs due to rapid diffusion of molecules loaded at the surface of these systems.43 We attribute this difference to the multiple washing steps involved in microgels synthesis, which can remove the rhodamine B molecules at the surface of PLGA NPs. The release rate of rhodamine B from PLGA NPs alone was comparable to the rates observed for microgels containing different concentrations of PLGA NPs (p=0.107) (Fig. 1E). This result indicates that the release rate of small molecules, like rhodamine B, is mainly controlled by the encapsulated PLGA NPs and not by the hydrogel itself.
4.3. HAP-1-functionalized PEG-4MAL microgels bind synoviocytes
Microgels functionalized with synoviocyte-targeting peptide were evaluated for their ability to specifically bind to rabbit (HIG-82) and human (SW982) synovial cell lines (Fig. 2A). Positive control microgels functionalized with RGD bound to all evaluated cell types at a higher level compared to its scrambled control RDG (p<0.0025) (Fig. 2B). Moreover, HAP-1-functionalized PEG-4MAL microgels bound to rabbit and human synoviocytes to the same level as RGD microgels (p>0.59), but in a specific manner as evidenced by their lower binding capacity to C2C12 and MC3T3-E1 cells (p<0.0008 compared to RGD). Also, HAP-1sc presented a binding level comparable to RDG negative control microgels in all cell types (p>0.49). These results demonstrate that HAP-1 peptide confers nano-composite PEG-4MAL/PLGA NPs microgels the ability to bind specifically to rabbit and human synoviocytes.
Figure 2.
HAP-1-functionalized PEG-4MAL/PLGA NP microgels bind synoviocytes. A) Confocal images of peptide-functionalized microgels (magenta) bound to rabbit (HIG-82) and human (SW982) synoviocytes, mouse myoblasts (C2C12) and mouse pre-osteoblasts (MC3T3-E1) stained with Cell Tracker Green™. Scale bar 200 μm. B) RGD-functionalized microgels bound to HIG-82, SW982, C2C12 and MC3T3-E1 cell lines at a higher level than RDG microgels. HAP-1-functionalized PEG microgels bound to rabbit and human synoviocytes to the same extent as RGD-functionalized microgels and is specific for synovial cells lines as demonstrated by its low binding to C2C12 and MC3T3-E1 cells. Mean ± SD, *p<0.05 compared to RGD, #p<0.05 compared to HAP-1.
4.4. WYR-functionalized PEG-4MAL microgels bind articular cartilage
WYR peptide binds specifically to the collagen type II α1 chain.22 Therefore, a collagen type II-rich system such as bovine articular cartilage was used to assess WYR-functionalized PEG-4MAL microgels binding (Fig. 3A). WYR-presenting microgels bound to bovine cartilage fresh frozen sections to a greater extent than scrambled peptide control microgels (p=0.0015) (Fig. 3B). To investigate whether this formulation also binds to synoviocytes, a HIG-82 cell monolayer platform was used and WYR-presenting microgel binding was compared to positive control peptide (RGD, HAP-1) microgel formulations (Fig. 3C). WYR-presenting microgels exhibited significantly lower binding levels to rabbit synoviocytes compared to HAP-1- and RGD-functionalized microgels (p<0.025). Furthermore, their binding levels were comparable to those of negative control peptide-presenting microgels, including RDG, HAP-1sc and WYRsc (p>0.93) (Fig. 3D). These results demonstrate that WYR-functionalized PEG-4MAL microgels specifically bind to articular cartilage but not to synoviocytes.
Figure 3.
WYR-functionalized PEG microgels bind articular cartilage. A) Confocal images of PEG-4MAL microgels (red) bound to bovine articular cartilage sections stained with DAPI (blue). Scale bar 200 μm. B) WYR-functionalized microgels bind to bovine cartilage sections to a higher level than the scrambled control WYRsc-presenting microgels. Mean ± SD, n=3, *p<0.05. C) Confocal images of PEG-4MAL microgels (magenta) bound to rabbit synoviocytes stained with Cell Tracker Green™. Scale bar 200 μm. D) Binding of WYR-functionalized PEG-4MAL microgels to synoviocytes is significantly lower than RGD- and HAP-1-functionalized microgels. Mean ± SD, n=4, 8 images per well, *p<0.05 compared to HAP-1, #p<0.05 compared to RGD.
4.5. PEG-4MAL/PLGA NP microgels have increased in vivo intra-articular retention
An in vivo tracking study was conducted to determine the IA retention of HAP-1- and WYR-functionalized microgels compared to a free small molecule near-infrared dye (Cy7) in healthy and OA rat joints. Radiant efficiency data resulting from IVIS imaging (Fig. 4B) demonstrate that Cy7 free dye is cleared faster than the microgel formulations (Fig. 4C) in healthy and OA joints, exhibiting a half-life time of 0.14 ± 0.04 and 0.21 ± 0.06 days, respectively (Fig. 4D). In contrast, nano-composite microgels exhibited a significant increase in the intra-articular half-life time compared to free dye control in MMT knees (HAP-1: 6.03 ± 4.76 days, p<0.0005; WYR: 2.48 ± 0.70 days, p<0.006) and healthy joints (HAP-1: 3.37 ± 2.82 days, p<0.0056; WYR: 4.70 ± 1.78 days, p<0.0003). These findings demonstrate that peptide-functionalized PEG-4MAL nano-composite microgels are retained in the knee space for a significantly longer period of time compared to free small molecules such as Cy7. WYR-functionalized microgels in both healthy and diseased joints exhibited an increase in radiant efficiency at day 1 compared to day 0 (Fig. 4C). This increase in fluorescence can be attributed to a self-quenching effect characteristic of cyanine dyes.44 This phenomenon was more pronounced in WYR- than HAP-1-presenting microgels, probably due to a higher degree of Cy7 aggregation in WYR peptide molecules compared to HAP-1 (Fig. S3). Because of this self-quenching increase in radiant efficiency, one-phase exponential decay curve fit of WYR-conjugated PEG-4MAL microgels did not include values from the day 0 time point.
Figure 4.
In vivo retention of peptide-functionalized PEG-4MAL nano-composite microgels. A) Rats received MMT surgery in the left joint, and 21 days after OA induction, were injected bilaterally with Cy7, WYR- or HAP-1-functionalized PEG-4MAL microgels. B) IVIS images of healthy and OA rat knees show that free Cy7 dye is cleared from the joints faster that nano-composite microgels. C) Radiant efficiency of the different formulations as a function on time (Mean ± SD, n=9). D) Half-life time obtained from one-phase exponential decay curve fit shows that nano-composite PEG-4MAL microgels present a significantly higher retention time compared to free dye (Mean ± SD, n=9, *p<0.05 compared to dye in MMT joints, #p<0.05 compared to dye in healthy joints).
We next examined the size stability of WYR-functionalized microgels in the IA space to assess any microgel degradation due to joint motion and other in vivo effects. Microgel size was reduced by day 14 as evidenced by the presence of smaller, irregular hydrogel fragments (Fig. S4). We attribute this reduction in size to mechanical forces in the joint breaking up microgels. Nevertheless, 40% of the retrieved microgels were found intact at day 21, demonstrating that PEG-4MAL nano-composite microgels are retained in the rat knee joint for at least 3 weeks.
4.6. Microgels do not negatively impact cartilage structure
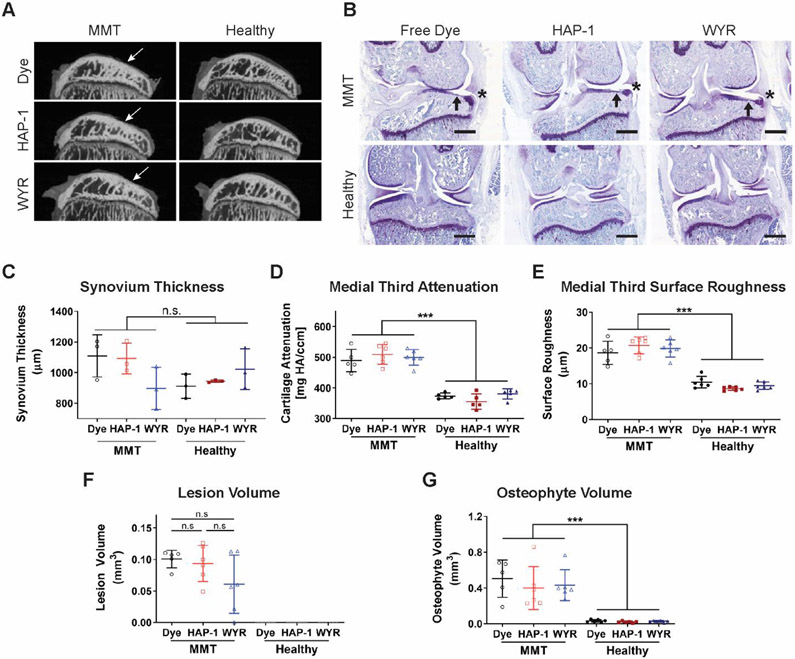
EPIC-μCT was used to evaluate whether peptide-functionalized nano-composite PEG-4MAL microgels affected articular cartilage integrity (Fig. 5A). We have previously shown that the MMT model induces a higher degree of articular cartilage damage in the medial third of the medial tibial plateau,39 therefore, parameters such as cartilage attenuation and surface roughness were evaluated in this region. As expected, animals subjected to surgical induction of OA presented significant cartilage damage compared to naïve joints as evidenced by a significant increase in tissue attenuation (p<0.0001) (Fig. 5D), an indication of proteoglycan depletion, an increase in surface roughness (p<0.0001) (Fig. 5E), and the presence of calcified osteophytes (p<0.0001) (Fig. 5G). Additionally, animals in the MMT groups presented full thickness articular cartilage lesions (Fig. 5F). WYR- and HAP-1-functionalized PEG-4MAL microgels did not induce any cartilage degenerative changes compared to free Cy7 dye in healthy joints and did not worsen OA progression in MMT animals. In this experiment, the free dye group was considered the negative control, given that free Cy7 dye is cleared from the joint in less than 5 h (Fig. 4D) and to the best of our knowledge, does not negatively affect the knee joint. Although injection of WYR-functionalized PEG-4MAL microgels did not induce a significant reduction in lesion volume compared to free dye and HAP-1-functionalized microgels in MMT rats (Fig. 5F), one of the animals did not exhibit any cartilage lesions and three of them presented smaller lesions than HAP-1 and free dye treated rats. This is an interesting observation because cartilage damage seen in the rat MMT model at 6 weeks post-surgery is usually characterized by the development of full-thickness lesions.45 This observation provides a basis for future evaluation of WYR-functionalized PEG-4MAL nano-composite microgels and their effect on OA progression. Additionally, synovial membrane thickness as a marker of inflammation was measured via histology image analysis (Fig. 5B). Synovium thickness was not affected either by the progression of the disease or the presence of microgels in the intra-articular space (Fig. 5C). Overall, these results demonstrate that IA injection of peptide-functionalized PEG-4MAL microgels containing PLGA NPs does not result in detectable cartilage damage or synovial membrane thickening in healthy joints and does not accelerate the progression of OA following meniscal injury.
Figure 5.
Effects of peptide-functionalized PEG-4MAL nano-composite microgels on cartilage degradation, osteophyte formation and synovial membrane thickness. A) EPIC-μCT images of the medial tibial plateau (sagittal view) show full-thickness cartilage lesions in MMT animals (white arrows) and intact articular cartilage in all naïve groups. B) Coronal rat knee sections stained with toluidine blue indicate presence of cartilage lesions (black arrows) and osteophytes (asterisk). Scale bar 2 mm. C) Synovial membrane thickness (Mean ± SD, n=3, p>0.13). D-G) Cartilage degradation and osteophyte formation metrics obtained via EPIC-μCT demonstrate that WYR- and HAP-1-functionalized PEG-4MAL nano-composite microgels do not induce damage in naïve joints and do not worsen OA progression compared to the free dye negative control (Mean ± SD, n=6). D) Medial third attenuation significantly increased in all MMT groups indicating a higher degree of proteoglycan depletion in diseased joints (***p<0.0001). E) Cartilage fibrillations were present in all MMT groups as evidenced by an increase in articular cartilage surface roughness (***p<0.0001). F) Animals that received surgical induction of OA presented full-thickness cartilage lesions whose volume was not different among injected formulations (p>0.13). G) MMT surgery induces calcified osteophyte formation compared to healthy joints (*** p<0.0001).
4.7. Microgels accumulate in the synovial membrane
Histological analysis of H&E-stained samples revealed that 2 weeks after injection all PEG-4MAL microgel formulations accumulate in the synovial membrane (Fig. 6). This result is consistent with prior work showing that micron-sized materials injected in the IA space are trapped in the synovial membrane and induce local hypercellularity.46,47 Therefore, it is not surprising that no gross differences in microgel accumulation into the synovial membrane were observed amongst formulations. Analysis of microgel localization in other areas of the joint such as the synovial fluid or the articular cartilage surface was not technically feasible. Hence, it was not possible to determine the ability of WYR-functionalized microgels to bind to the articular cartilage in vivo. The development of quantitative, three-dimensional techniques would be necessary to further evaluate the fate of peptide-functionalized microgels within the joint.
Figure 6.
In vivo localization of peptide-functionalized PEG-4MAL nano-composite microgels. Representative images of the synovial membrane 2 weeks after IA administration of PEG-4MAL microgels. Black arrows denote the accumulation of peptide-functionalized microgels within the synovial membrane, that appear as circular white pockets (scale bar 200 μm).
4.8. Small molecule cargo in vivo intra-articular retention
We investigated the IA retention of a model small molecule encapsulated in the nano-composite PEG-4MAL microgels, which are retained in the IA space for at least 3 weeks (Fig. 4 and Fig. S4). Comparable to our previous studies, IA injection of the NIR dye Cy7, used as a model small molecule, resulted in a short IA half-life time (0.25 ± 0.07 days) (Fig. 7A,B). The steady-state value obtained from a one-phase exponential decay demonstrate that free Cy7 dye is eliminated from the joint space (0.5 ± 0.2%), whereas PLGA NPs alone or encapsulated into any PEG-4MAL microgels formulation exhibited higher steady state values compared to free dye (p<0.0001) (Fig. 7C). PLGA NPs alone and WYR-functionalized PEG-4MAL microgels did not improve Cy7 IA half-life time over free dye (p=0.0652 and 0.0583, respectively) (Fig. 7B). Additionally, area under the curve (AUC) values, used as an overall metric of Cy7 IA retention, demonstrated that all PLGA NPs alone and nano-composite PEG-4MAL microgel formulations presented a significantly larger AUC compared to free dye (p<0.022) (Fig. 7D). All together, these results suggest that nano-composite PEG-4MAL microgels increase Cy7 retention in the joint.
Figure 7.
Intra-articular Cy7 retention time of Cy7-loaded peptide-functionalized PEG-4MAL microgels. A) Cy7 retention profile for free dye control, PLGA NPs alone and peptide-functionalized nano-composite PEG-4MAL microgels (Mean ± SD, n=7, data normalized to radiant efficiency measured immediately after injection). B) IA half-life of Cy7 dye was increased using HAP-1, HAP-1sc and WYRsc microgels (n=7, *p<0.05, **p<0.002 compared to free dye). C) Steady state radiant efficiency values demonstrate that free dye is eliminated from the rat knee joints whereas PLGA NPs and nano-composite PEG-4MAL microgels increased the IA retention of Cy7 (n=7, ***p<0.0001). D) Area under the curve (AUC) indicates that nanocomposite microgels can significantly increase model small molecule IA retention compared to free dye control (n=7, **p<0.002).
5. Discussion
Osteoarthritis has become one of the leading causes of disability in the world,53 and still there is no FDA-approved treatment to effectively slow down the progression of the disease.3 Multiple DMOAD candidates have shown promising results in pre-clinical studies, but their translation into the clinic has been limited in part due to the lack of appropriate IA drug delivery systems.54 In particular, small molecule drugs (<1 kDa) that are rapidly cleared from the joint space could greatly benefit from appropriate IA drug delivery vehicles.
Although microparticles composed of several materials have been used as IA drug delivery vehicles, to the best of our knowledge, this is the first report of nano-composite microgels as intra-articular drug delivery vehicles. Compared to free nano-size carriers, the nano-composite microgels presented in this study offer significant improvements in terms of IA retention. Nano-sized vehicles, such as polymeric NPs, are subjected to cell-mediated clearance and lymphatics drainage48 and are retained in the IA space for around a week,54 which can explain why PLGA NPs alone failed to improve the IA half-life time of the model small molecule Cy7 (Fig. 7B). In contrast, our nano-composite PEG-4MAL microgel system acts as a nanoparticle depot, which could extend their IA retention for at least 3 weeks. Additionally, we demonstrated that peptide-functionalized PEG-4MAL microgel formulations accumulate in the synovial membrane. Even though the incorporation of synoviocyte-binding peptides into PEG-4MAL microgels did not seem to increase their accumulation in the synovial lining compared to scrambled peptide controls, microgels accumulation into this tissue may help improve the IA retention of loaded small molecules as observed in Fig. 7. We hypothesize that microgels accumulation into the synovial membrane may help protect these structures from the IA compression and shear stresses and therefore improve their cargo IA retention time. Similar to our microgels, solid polymeric microparticles exhibit higher IA retention times compared to NPs.3,48 However, solid polymeric microparticles resulting from IA implant wear, as well as PLGA NPs bellow 20 μm in diameter have been shown to promote synovial cell infiltration and proteoglycan loss,34,35 raising concerns regarding the use of solid microparticles as intra-articular drug delivery vehicles. The use of a softer, water-swollen material such as PEG-4MAL microgels may be advantageous in terms of joint health after long term exposure to these carriers. In fact, Holyoak, et al. recently demonstrated that bulk PEG-4MAL hydrogels containing drug-loaded PLGA NPs act as “mechanical pillows” can protect the joint from cartilage degradation and osteophyte formation after IA injection in a mouse model of knee OA.55 These findings align with the results presented in this study, where no microgels-induced articular cartilage damage or significant synovial membrane thickening were observed. Importantly, in contrast to the bulk hydrogels proposed by Holyoak, et al., our approach supports the possibility of developing tissue-specific treatment strategies for the treatment of OA, an important feature that could minimize drugs’ adverse off-target effects.
An advantage of the proposed PEG-4MAL nano-composite microgels is the modular design which permits independent control over the hydrogel and the encapsulated NPs properties. This could allow for better control of microgel IA retention and stability. Our results demonstrate that WYR-functionalized microgels are subjected to mechanical degradation (Fig. S4), which may have a negative impact on the loaded small molecules’ IA retention time. This was observed in Fig. 7B, where WYR-presenting microgels were the only formulation that did not improve IA half-life time of the model small molecule Cy7 compared to the free dye control. This limitation could be addressed by changing the macromer molecular weight and/or concentration to optimize the microgels mechanical properties, while independently enhancing the small molecule release rate by tuning the encapsulated NPs properties. Moreover, the modular design of our nano-composite microgels allows for the incorporation of other functionalities. In the present study, we conjugated tissue-targeting peptides to promote microgel binding to articular cartilage and synoviocytes. Intra-articular tissue targeting has been explored to detect cartilage lesions, enhance NPs IA retention, and increase drug bioavailability into tissues of interest such as the articular cartilage.18,19,22,25,26,32 Additionally, a better understanding of the off-target effects of some promising drug candidates3,14-16,56-61 has evidenced the necessity of developing IA tissue-targeting drug delivery vehicles. Here, we showed that cartilage- (WYR) and synoviocyte-targeting (HAP-1) peptides can be conjugated to nano-composite PEG-4MAL microgels to promote specific binding to articular cartilage and synovial cell lines in vitro and in vivo binding to synovial membrane and may support microgels binding to the articular cartilage.
One limitation of the microgel drug delivery vehicles is that their size may not be ideal for direct intra-cartilage drug delivery applications. In particular, cartilage extracellular matrix presents a small mesh size (60-200 nm) and high negative charge, which impair the penetration of molecules and particles into this tissue.30,62 However, IA pharmacokinetics modeling suggests that cartilage-binding, non-penetrating particles, such as those presented in this study, may increase intra-cartilage drug concentrations compared to non-penetrating particles that do not present any cartilage-binding mechanism.62 Therefore, we hypothesize that WYR-functionalized PEG-4MAL microgels could improve the effectiveness of conventional non-targeting drug delivery vehicles for OA treatment by localizing them to the articular cartilage surface. Furthermore, we expect these microgels will enhance the therapeutic efficacy of cartilage targeting drugs by preferentially binding and delivering their cargo into damaged areas of the articular cartilage. In this regard, the proposed WYR-functionalized PEG-4MAL microgels are expected to exhibit different binding patterns as the disease progresses, probably exhibiting an optimal binding in moderate cases of OA where Coll-2 is exposed in cartilage fibrillations and lesions, but not fully degraded to subchondral bone as occurs in severe OA cases.63
Finally, we used PLGA NPs to fabricate nano-composite PEG-4MAL microgels. This specific polymeric system may not be ideal to achieve efficient loading and delivery of many DMOAD candidates, which may be more compatible with liposomes, lipid NPs or other polymeric NPs formulations.3 Microfluidic synthesis of PEG-4MAL nano-composite microgels could allow the encapsulation of multiple types of particulate drug delivery vehicles. In fact, preliminary studies conducted in our laboratory suggest that solid lipid NPs and gold NPs could be effectively loaded into PEG-4MAL microgels. Additionally, our research group has previously demonstrated that PEG-based microgels are effective methods for protein and cell delivery.36-38 The modular design of the proposed microgels offers a range of possibilities for the IA delivery of multiple types of therapeutics including synthetic small molecules, macromolecules, cells and their combinations. Therefore, we expect our tissue-binding nano-composite PEG-4MAL microgels to facilitate the clinical translation of DMOAD candidates as well as support the development of multi-target, combinatorial therapeutic strategies for OA treatment.
6. Conclusions
We synthesized cartilage- and synoviocyte-binging nano-composite PEG-4MAL microgels for improved IA small molecule drug delivery. Microgels containing PLGA NPs exhibited sustained delivery of a model small molecule. Additionally, tethering of tissue-targeting peptides to PEG-4MAL microgels provided specific binding to articular cartilage and synoviocytes in vitro. Moreover, nano-composite PEG-4MAL microgels were retained in the IA space for at least 3 weeks without inducing degenerative changes in the articular cartilage or promoting synovial membrane thickening. Synoviocyte-targeting PEG-4MAL microgels localized to the synovial membrane in the joint and improved the intra-articular retention time of a model small molecule. These results support the application of tissue-binding, nano-composite PEG-4MAL microgels as promising IA drug delivery systems for OA treatment.
Supplementary Material
Supplementary materials and methods. This includes a comprehensive list of reagents and methods for the determination of PLGA NP distribution within PEG-4MAL microgels and WYR-functionalized PEG-4MAL/PLGA NPs microgel in vivo stability.
Figure S1. Chemical structure of A) poly(lactic-co-glycolic) acid and B) 4-arm poly(ethylene glycol) maleimide
Figure S2. PLGA NP encapsulation into PEG-4MAL microgels
Figure S3. Self-quenching effect of peptide-functionalized PEG-4MAL microgels was evaluated in vitro.
Figure S4. Stability of WYR peptide-functionalized PEG-4MAL nano-composite microgels in vivo.
7. Acknowledgements
The authors acknowledge Casey Vantucci, Gilad Doron, Fabrice Bernard, Marissa Ruehle and Brett Klosterhoff for their assistance with surgeries and Dr. Nick Willett for his guidance throughout the development of this work.
9. Funding Sources
This research was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award numbers R01AR062920 and S10OD016264 and the Department of Defense PRMRP Grant (PR171379).
Footnotes
Supporting Information
The following files are available free of charge on the ACS publication website:
References
- (1).Kloppenburg M; Berenbaum F Osteoarthritis Year in Review 2019: Epidemiology and Therapy. Osteoarthr. Cart 2020, 28, 242–248. 10.1016/j.joca.2020.01.002. [DOI] [PubMed] [Google Scholar]
- (2).Lawrence RC; Felson DT; Helmick CG; Arnold LM; Choi H; Deyo RA; Gabriel S; Hirsch R; Hochberg MC; Hunder GG; Jordan JM; Katz JN; Kremers HM; Wolfe F Estimates of the Prevalence of Arthritis and Other Rheumatic Conditions in the United States, Part II. Arthritis Rheum 2008, 58 (1), 26–35. 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Maudens P; Jordan O; Allémann E Recent Advances in Intra-Articular Drug Delivery Systems for Osteoarthritis Therapy. Drug Discov. Today 2018, 23 (10), 1761–1775. 10.1016/j.drudis.2018.05.023. [DOI] [PubMed] [Google Scholar]
- (4).Mora JC; Przkora R; Cruz-Almeida Y Knee Osteoarthritis: Pathophysiology and Current Treatment Modalities. J. Pain Res 2018, 11, 2189–2196. 10.2147/JPR.S154002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Formica FA; Barreto G; Zenobi-Wong M Cartilage-Targeting Dexamethasone Prodrugs Increase the Efficacy of Dexamethasone. J. Control. Release 2019, 295, 118–129. 10.1016/j.jconrel.2018.12.025. [DOI] [PubMed] [Google Scholar]
- (6).Janssen M; Timur UT; Woike N; Welting TJM; Draaisma G; Gijbels M; van Rhijn LW; Mihov G; Thies J; Emans PJ Celecoxib-Loaded PEA Microspheres as an Auto Regulatory Drug-Delivery System after Intra-Articular Injection. J. Control. Release 2016, 244, 30–40. 10.1016/j.jconrel.2016.11.003. [DOI] [PubMed] [Google Scholar]
- (7).Johal H; Devji T; Schemitsch EH; Bhandari M Viscosupplementation in Knee Osteoarthritis: Evidence Revisited. JBJS Rev. 2016, 4 (4), 1–11. 10.2106/JBJS.RVW.15.00098. [DOI] [PubMed] [Google Scholar]
- (8).David Jevsevar, MD, MBA, Patrick Donnelly, MA, Brown Gregory A., MD, PhD, and Cummins Deborah S., P. Viscosupplementation for Osteoarthritis of the Knee: A Systematic Review of the Evidence. J. Bone Jt. Surg 2015, 97, 2047–2060. 10.1056/NEJMc1505801. [DOI] [PubMed] [Google Scholar]
- (9).Rutjes Antie W.S., Jiini Peter, da Costa Bruno R., Trelle Sven, Niiesch Eveline, S. R.; Background: Viscosupplementation for Osteoarthritis of the Knee. N. Engl. J. Med 2015, 372 (26), 2569–2570. 10.1056/NEJMc1505801. [DOI] [PubMed] [Google Scholar]
- (10).Brown T; Laurent U; Fraser. Turnover of Hyaluronan in Synovial Joints: Elimination of Labelled Hyaluronan from the Knee Joint of the Rabbit. Exp. Physiol 1991, 76 (1), 125–134. 10.1113/expphysiol.1991.sp003474. [DOI] [PubMed] [Google Scholar]
- (11).Larsen C, Ostergaard J, Larsen SW, Jensen H, Jacobsen S, Lindegaard C, Intra-Articular PHA Depot Formulation Principles: Role in the Management of Postoperative Pain and Arthritic Disorders. J. Pharm. Sci 2008, 97 (11), 4622–4654. 10.1002/jps. [DOI] [PubMed] [Google Scholar]
- (12).Whitmire RE; Wilson DS; Singh A; Levenston ME; Murthy N; García AJ Self-Assembling Nanoparticles for Intra-Articular Delivery of Anti-Inflammatory Proteins. Biomaterials 2012, 33 (30), 7665–7675. 10.1016/j.biomaterials.2012.06.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Singh A; Agarwal R; Diaz-Ruiz CA; Willett NJ; Wang P; Andrew Lee L; Wang Q; Guldberg RE; García AJ Nano-Engineered Particles for Enhanced Intra-Articular Retention and Delivery of Proteins. Adv Heal. Mater 2014, 3 (10), 1562–1567. 10.1002/adhm.201400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Suh J-K; Muzzonigro TS; Fu FH Injury and Repair of Articular Cartilage: Related Scientific Issues. Oper. Tech. Orthop 1997, 7 (4), 270–278. 10.1016/S1048-6666(97)80029-8. [DOI] [Google Scholar]
- (15).Hochberg MC; Tive LA; Abramson SB; Vignon E; Verburg KM; West CR; Smith MD; Hungerford DS When Is Osteonecrosis Not Osteonecrosis?: Adjudication of Reported Serious Adverse Joint Events in the Tanezumab Clinical Development Program. Arthritis Rheumatol. 2016, 68 (2), 382–391. 10.1002/art.39492. [DOI] [PubMed] [Google Scholar]
- (16).Bakker AC; Van De Loo FAJ; Van Beuningen HM; Sime P; Van Lent PLEM; Van Der Kraan PM; Richards CD; Van Den Berg WB Overexpression of Active TGF-Beta-1 in the Murine Knee Joint: Evidence for Synovial-Layer-Dependent Chondro-Osteophyte Formation. Osteoarthr. Cartil 2001, 9, 128–136. 10.1053/joca.2000.0368. [DOI] [PubMed] [Google Scholar]
- (17).Zhang J; Wang JH-C Kartogenin Induces Cartilage-like Tissue Formation in Tendon– Bone Junction. Bone Res. 2014, 28 (2), 1–10. 10.1038/boneres.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Hughes C; Faurholm B; Dell’Accio F; Manzo A; Seed M; Eltawil N; Marrelli A; Gould D; Subang C; Al-Kashi A; De Bari C; Winyard P; Chernajovsky Y; Nissim A Human Single-Chain Variable Fragment That Specifically Targets Arthritic Cartilage. Arthritis Rheum. 2010, 62 (4), 1007–1016. 10.1002/art.27346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Cho H; Pinkhassik E; David V; Stuart JM; Hasty KA Detection of Early Cartilage Damage Using Targeted Nanosomes in a Post-Traumatic Osteoarthritis Mouse Model. Nanomedicine Nanotechnology, Biol. Med 2015, 11, 939–946. 10.1016/j.nano.2015.01.011. [DOI] [PubMed] [Google Scholar]
- (20).Hongsik Cho, Byoung Ju Kim, Sang-Hyug Park, Hasty Karen A, M. B-H Noninvasive Visualization of Early Osteoarthritic Cartilage Using Targeted Nanosomes in a Destabilization of the Medial Meniscus Mouse Model. Int. J. Nanomedicine 2018, 13, 1215–1224. 10.2147/IJN.S149375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pi Y; Zhang X; Shi J; Zhu J; Chen W; Zhang C; Gao W; Zhou C; Ao Y Targeted Delivery of Non-Viral Vectors to Cartilage in Vivo Using a Chondrocyte-Homing Peptide Identified by Phage Display. Biomaterials 2011, 32 (26), 6324–6332. 10.1016/j.biomaterials.2011.05.017. [DOI] [PubMed] [Google Scholar]
- (22).Rothenfluh DA; Bermudez H; O ’neil CP; Hubbell JA Biofunctional Polymer Nanoparticles for Intra-Articular Targeting and Retention in Cartilage. Nat. Mater 2008, 7, 248–254. 10.1038/nmat2116. [DOI] [PubMed] [Google Scholar]
- (23).Lee L; Buckley C; Blades MC; Panayi G; George AJT Identification of Synovium-Specific Homing Peptides by In Vivo Phage Display Selection. 2002, 46 (8), 2109–2120. 10.1002/art.10464. [DOI] [PubMed] [Google Scholar]
- (24).Yang Y-H; Rajaiah R; Ruoslahti E; Moudgil KD Peptides Targeting Inflamed Synovial Vasculature Attenuate Autoimmune Arthritis. Proc. Natl. Acad. Sci. U. S. A 2011, 108 (31), 12857–12862. 10.1073/pnas.1103569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Mi Z; Lu X; Mai JC; Ng BG; Wang G; Lechman ER; Watkins SC; Rabinowich H; Robbins PD Identification of a Synovial Fibroblast-Specific Protein Transduction Domain for Delivery of Apoptotic Agents to Hyperplastic Synovium. Mol. Ther 2003, 8 (2), 295–305. 10.1016/S1525-0016(03)00181-3. [DOI] [PubMed] [Google Scholar]
- (26).You C; Zu J; Liu X; Kong P; Song C; Wei R; Zhou C; Wang Y; Yan J Synovial Fibroblast-Targeting Liposomes Encapsulating an NF-ΚB-Blocking Peptide Ameliorates Zymosan-Induced Synovial Inflammation. J. Cell. Mol. Med 2018, 22 (4), 2449–2457. 10.1111/jcmm.13549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Vanniasinghe AS; Manolios N; Schibeci S; Lakhiani C; Kamali-Sarvestani E; Sharma R; Kumar V; Moghaddam M; Ali M; Bender V Targeting Fibroblast-like Synovial Cells at Sites of Inflammation with Peptide Targeted Liposomes Results in Inhibition of Experimental Arthritis. Clin. Immunol 2014, 151 (1), 43–54. 10.1016/j.clim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- (28).Wythe SE; Dicara D; Taher TEI; Finucane CM; Jones R; Bombardieri M; Man YKS; Nissim A; Mather SJ; Chernajovsky Y; Pitzalis C Targeted Delivery of Cytokine Therapy to Rheumatoid Tissue by a Synovial Targeting Peptide. Ann Rheum Dis 2013, 72, 129–135. 10.1136/annrheumdis-2012-201457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Meka RR; Venkatesha SH; Moudgil KD Peptide-Directed Liposomal Delivery Improves the Therapeutic Index of an Immunomodulatory Cytokine in Controlling Autoimmune Arthritis. J. Control. Release 2018, 286, 279–288. 10.1016/j.jconrel.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Brown SB; Wang L; Jungels RR; Sharma B Effects of Cartilage-Targeting Moieties on Nanoparticle Biodistribution in Healthy and Osteoarthritic Joints. Acta Biomater. 2020, 101, 469–483. 10.1016/j.actbio.2019.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Hu H-Y; Lim N-H; Juretschke H-P; Ding-Pfennigdorff D; Florian P; Kohlmann M; Kandira A; Peter Von Kries J; Saas J; Rudolphi KA; Wendt KU; Nagase H; Plettenburg O; Nazare M; Schultz C In Vivo Visualization of Osteoarthritic Hypertrophic Lesions. Chem. Sci 2015, 6, 6256–6261. 10.1039/c5sc01301a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Chen H; Qin Z; Zhao J; He Y; Ren E; Zhu Y; Liu G; Mao C; Zheng L Cartilage-Targeting and Dual MMP-13/PH Responsive Theranostic Nanoprobes for Osteoarthritis Imaging and Precision Therapy. Biomaterials 2019, 225. 10.1016/j.biomaterials.2019.119520. [DOI] [PubMed] [Google Scholar]
- (33).Yi W; Zhou H; Li A; Yuan Y; Guo Y; Li P; Qi B; Xiao Y; Yu A; Hu X A NIR-II Fluorescent Probe for Articular Cartilage Degeneration Imaging and Osteoarthritis Detection. Biomater. Sci 2019, 7, 1043–1051. 10.1039/c8bm01440j. [DOI] [PubMed] [Google Scholar]
- (34).Park DY; Min BH; Kim DW; Song BR; Kim M; Kim YJ Polyethylene Wear Particles Play a Role in Development of Osteoarthritis via Detrimental Effects on Cartilage, Meniscus, and Synovium. Osteoarthr. Cartil 2013, 21 (12), 2021–2029. 10.1016/j.joca.2013.09.013. [DOI] [PubMed] [Google Scholar]
- (35).Liggins RT; , Cruz T, Min W, Liang L, H. WL and B. HM Intra-Articular Treatment of Arthritis with Microsphere Formulations of Paclitaxel: Biocompatibility and Efficacy Determinations in Rabbits. Inflamm. Res 2004, 53, 365–372. 10.1007/s00011-004-1273-1. [DOI] [PubMed] [Google Scholar]
- (36).Headen DM; Aubry G; Lu H; García AJ Microfluidic-Based Generation of Size-Controlled, Biofunctionalized Synthetic Polymer Microgels for Cell Encapsulation. Adv. Mater 2014, 26 (19), 3003–3008. 10.1002/adma.201304880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Foster GA; Headen DM; González-García C; Salmerón-Sánchez M; Shirwan H; García AJ Protease-Degradable Microgels for Protein Delivery for Vascularization. Biomaterials 2017, 113, 170–175. 10.1016/j.biomaterials.2016.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Headen DM; García JR; García AJ Parallel Droplet Microfluidics for High Throughput Cell Encapsulation and Synthetic Microgel Generation. Microsystems Nanoeng. 2018, 4 (17076), 1–9. 10.1038/micronano.2017.76. [DOI] [Google Scholar]
- (39).Thote T; Lin ASP; Raji Y; Moran S; Stevens HY; Hart M; Kamath RV; Guldberg RE; Willett Z Y NJ; Coulter WH Localized 3D Analysis of Cartilage Composition and Morphology in Small Animal Models of Joint Degeneration. Osteoarthr. Cartil 2013, 21, 1132–1141. 10.1016/j.joca.2013.05.018. [DOI] [PubMed] [Google Scholar]
- (40).Xie L; Lin ASP; Levenston ME; Guldberg RE Quantitative Assessment of Articular Cartilage Morphology via EPIC-MCT. Osteoarthr. Cartil 2009, 17 (3), 313–320. 10.1016/j.joca.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Reece DS; Thote T; Lin ASP; Willett NJ; Guldberg RE Contrast Enhanced MCT Imaging of Early Articular Changes in a Pre-Clinical Model of Osteoarthritis. Osteoarthr. Cartil 2018, 26 (1), 118–127. 10.1016/j.joca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- (42).Fredenberg S; Wahlgren M; Reslow M; Axelsson A The Mechanisms of Drug Release in Poly(Lactic-Co-Glycolic Acid)-Based Drug Delivery Systems - A Review. Int. J. Pharm Elsevier; August 30, 2011, pp 34–52. 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- (43).Fu Y; Kao WJ Drug Release Kinetics and Transport Mechanisms of Non-Degradable and Degradable Polymeric Delivery Systems. Expert Opin Drug Devil 2010, 7 (4), 429–444. 10.1517/17425241003602259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhegalova NG; He S; Zhou H; Kim DM; Berezin MY Minimization of Self-Quenching Fluorescence on Dyes Conjugated to Biomolecules with Multiple Labeling Sites via Asymmetrically Charged NIR Fluorophores. Contrast Media Mol. Imaging 2014, 9 (5), 355–362. 10.1002/cmmi.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Tsai LC; Cooper ES; Hetzendorfer KM; Warren GL; Chang YH; Willett NJ Effects of Treadmill Running and Limb Immobilization on Knee Cartilage Degeneration and Locomotor Joint Kinematics in Rats Following Knee Meniscal Transection. Osteoarthr. Cartil 2019, 27 (12), 1851–1859. 10.1016/j.joca.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bédouet L; Pascale F; Moine L; Wassef M; Ghegediban SH; Nguyen V-N; Bonneau M; Labarre D; Laurent A Intra-Articular Fate of Degradable Poly(Ethyleneglycol)-Hydrogel Microspheres as Carriers for Sustained Drug Delivery. Int. J. Pharm 2013, 456, 536–544. 10.1016/j.ijpharm.2013.08.016. [DOI] [PubMed] [Google Scholar]
- (47).Willett NJ; Thote T; Lin AS; Moran S; Raji Y; Sridaran S; Stevens HY; Guldberg RE Intra-Articular Injection of Micronized Dehydrated Human Amnion/Chorion Membrane Attenuates Osteoarthritis Development. Arthritis Res. Ther 2014, 16 (1), R47. 10.1186/ar4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Pradal J; Maudens P; Gabay C; Seemayer CA; Jordan O; Allémann E Effect of Particle Size on the Biodistribution of Nano- and Microparticles Following Intra-Articular Injection in Mice. Int. J. Pharm 2016, 498, 119–129. 10.1016/j.ijpharm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- (49).Singh A; Agarwal R; Diaz-Ruiz CA; Willett NJ; Wang P; Andrew Lee L; Wang Q; Guldberg RE; García AJ Nano-Engineered Particles for Enhanced Intra-Articular Retention and Delivery of Proteins. Adv Heal. Mater 2014, 3 (10), 1562–1567. 10.1002/adhm.201400051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Morgen M; Tung D; Boras B; Miller W; Malfait A-M; Tortorella M; Morgen M; Miller W; Tung D; Boras B; Malfait A-M; Tortorella M Nanoparticles for Improved Local Retention after Intra-Articular Injection into the Knee Joint. Pharm Res 2013, 30, 257–268. 10.1007/s11095-012-0870-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Arunkumar P; Indulekha S; Vijayalakshmi S; Srivastava R Synthesis, Characterizations, in Vitro and in Vivo Evaluation of Etoricoxib-Loaded Poly (Caprolactone) Microparticles-a Potential Intra-Articular Drug Delivery System for the Treatment of Osteoarthritis. J. Biomater. Sci 2016, 27 (4), 303–316. 10.1080/09205063.2015.1125564. [DOI] [PubMed] [Google Scholar]
- (52).Maarten Janssen, Ufuk Tan Timur, Nina Woike, Welting Tim J.M., Draaisma Guy, Gijbels Marion, van Rhijn Lodewijk W., Mihov George, Thies Jens, E. PJ Celecoxib-Loaded PEA Microspheres as an Auto Regulatory Drug-Delivery System after Intra-Articular Injection. J. Control. Release 2016, 244, 30–40. [DOI] [PubMed] [Google Scholar]
- (53).Chen D; Shen J; Zhao W; Wang T; Han L; Hamilton JL; Im H-J Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5 (16044), 1–13. 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Brown S; Kumar S; Sharma B Intra-Articular Targeting of Nanomaterials for the Treatment of Osteoarthritis. Acta Biomaterialia. July 15, 2019, pp 239–257. 10.1016/j.actbio.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Holyoak DT; Wheeler TA; van der Meulen MC; Singh A Injectable Mechanical Pillows for Attenuation of Load-Induced Post-Traumatic Osteoarthritis. Regen. Biomater 2019, 211–219. 10.1093/rb/rbz013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Geiger Brett C., Grodzinsky Alan J., H. PT Designing Drug Delivery Systems for Articular Jointst. Chem. Eng. Prog 2018, 114 (5), 46–51. [Google Scholar]
- (57).Kang ML; Ko J-Y; Kim JE; Im G-I Intra-Articular Delivery of Kartogenin-Conjugated Chitosan Nano/ Microparticles for Cartilage Regeneration. Biomaterials 2014, 35, 9984–9994. 10.1016/j.biomaterials.2014.08.042. [DOI] [PubMed] [Google Scholar]
- (58).Zhang J; Wang JHC Kartogenin Induces Cartilage-like Tissue Formation in Tendon-Bone Junction. Bone Res. 2014, 2 (January), 12–17. 10.1038/boneres.2014.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Shi D; Xu X; Ye Y; Song K; Cheng Y; Di J; Hu Q; Li J; Ju H; Jiang Q; Gu Z Photo-Cross-Linked Scaffold with Kartogenin- Encapsulated Nanoparticles for Cartilage Regeneration. ACS Nano 2016, 10, 1292–1299. 10.1021/acsnano.5b06663. [DOI] [PubMed] [Google Scholar]
- (60).Li X; Ding J; Zhang Z; Yang M; Yu J; Wang J; Chang F; Chen X Kartogenin-Incorporated Thermogel Supports Stem Cells for Significant Cartilage Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5148–5159. 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- (61).Mohan G; Magnitsky S; Melkus G; Subburaj K; Kazakia G; Burghardt AJ; Dang A; Lane NE; Majumdar S Kartogenin Treatment Prevented Joint Degeneration in a Rodent Model of Osteoarthritis: A Pilot Study. J. Orthop. Res 2016, 34 (10), 1780–1789. 10.1002/jor.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Bajpayee AG; Grodzinsky AJ Cartilage-Targeting Drug Delivery: Can Electrostatic Interactions Help? Nat. Publ. Gr 2017, 13 (3), 183–193. 10.1038/nrrheum.2016.210. [DOI] [PubMed] [Google Scholar]
- (63).Loeser RF; Goldring SR; Scanzello CR; Goldring MB Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64 (6), 1697–1707. 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials and methods. This includes a comprehensive list of reagents and methods for the determination of PLGA NP distribution within PEG-4MAL microgels and WYR-functionalized PEG-4MAL/PLGA NPs microgel in vivo stability.
Figure S1. Chemical structure of A) poly(lactic-co-glycolic) acid and B) 4-arm poly(ethylene glycol) maleimide
Figure S2. PLGA NP encapsulation into PEG-4MAL microgels
Figure S3. Self-quenching effect of peptide-functionalized PEG-4MAL microgels was evaluated in vitro.
Figure S4. Stability of WYR peptide-functionalized PEG-4MAL nano-composite microgels in vivo.