Abstract
Background:
Disparities in survival by race/ethnicity, socioeconomic status (SES), and geography in adolescent and young adult (AYA) patients with central nervous system (CNS) tumors have not been well studied.
Procedure:
A retrospective cohort study utilizing the Surveillance, Epidemiology, and End Results (SEER) database was conducted for AYA patients diagnosed with primary CNS tumors. Adjusted hazard ratios (aHR) were calculated using a multivariate Cox proportional hazard model to evaluate the association between race/ethnicity, SES, rurality, and hazard of death.
Results:
All minority groups showed an increased hazard of death with greatest disparities in the high-grade glioma cohort. Lower SES was associated with an increased hazard of death in non-Hispanic White (NHW) patients (aHR 1.12; 95% confidence interval [CI] 1.01–1.24), non-Hispanic Black (NHB) patients (aHR 1.34; 95% CI 1.00–1.80), and patients aged 25–29 years (aHR 1.29; 95% CI 1.07–1.55). Mediation analysis showed an indirect effect of SES on the effect of race/ethnicity on the hazard of death only among NHB patients, with SES accounting for 33.7% of the association between NHB and hazard of death. Rurality was associated with an increased hazard of death for patients in the lowest SES tertile (aHR 1.31; 95% CI 1.08–1.59) and NHW patients (aHR 1.20; 95% CI 1.08–1.34).
Conclusions:
Patients identified as a racial/ethnic minority, patients with a lower SES, and patients residing in rural areas had an increased hazard of death. Further studies are needed to understand and address the biological, psychosocial, societal, and economic factors that impact AYA neuro-oncology patients at highest risk of experiencing poorer outcomes.
Keywords: adolescent and young adults, central nervous system tumors, racial/ethnic disparities, socioeconomic disparities, survival disparities
1 |. BACKGROUND
Adolescent and young adult (AYA) patients, defined as ages 15 to 39 years, account for 77,000 of new cancer diagnoses each year in the United States.1,2 AYA patients have unique needs given their transition from dependent childhood to independent adulthood and have distinct social, psychological, biological, and physiological challenges that affect adherence to therapies and reduce clinical trial enrollment.3–5 Current methods to improve AYA outcomes have focused on biology, trial enrollment, and medical adherence.6,7 Race/ethnicity and socioeconomic status (SES) have also been shown to contribute to AYA disparities.6,8
Programs such as Healthy People 2020 highlight the impact of race/ethnicity and socioeconomic status (SES) on health equity.9 Liu et al. conducted a study in Los Angeles County and found that racial/ethnic and socioeconomic factors contribute to disparities in survival in the AYA oncology population.8 Another study looking at the AYA population within the California Cancer Registry noted that while overall survival in the AYA cancer population has improved in recent decades, there have also been growing survival disparities among AYA patients with cancer from racial/ethnic minorities and lower SES.6 AYA patients living in rural areas, older AYA patients, and those from lower SES have been found to have decreased access to National Cancer Institute (NCI) centers—a factor that has been shown to contribute to inferior outcomes amongst AYA oncology patients.10–13 As AYA patients face challenges in receiving appropriate care, it becomes imperative to understand the impact of disparities on survival within the AYA neuro-oncology population.
While there exists pediatric and adult literature evaluating the impact of racial, ethnic and SES disparities on outcomes in neuro-oncology patients, few studies focus on disparities in AYA patients with primary central nervous system (CNS) tumors, and even fewer focus on stratifying CNS tumors by histology.14–16 Evaluating tumors based on these factors is important, as histologically similar tumors receive similar therapies with similar outcomes.
Using the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database, we investigated the impact of race/ethnicity, SES, and rurality on the risk of death in AYA patients with primary CNS tumors. SEER is a population-based national cancer registry that was initially established in 1973 by the NCI to standardize the large-scale collection of cancer incidence, therapy, and survival data to improve the development of cancer diagnostics and therapeutics.17,18 The SEER database has expanded significantly over the last 50 years and now represents approximately 35% of the US population and includes registries from 19 major geographic areas.17
2 |. METHODS
2.1 |. Study population
The study population was selected from the specialized SEER Census Tract-level Socioeconomic Status and Rurality Database using SEER*Stat software (Version 8.3.6, Surveillance Research Program, National Cancer Institute, seer.cancer.gov/seerstat).19 A complete list of variables extracted can be found in Table S1. Patients aged 15 to 39 years, inclusive, who were diagnosed with select CNS tumors from January 1, 2000 to December 31, 2016 were included in the analysis (Table S2). Patients diagnosed prior to the year 2000, of unknown race and/or ethnicity, or missing SES data were excluded. Given the small sample size, patients designated as Native Americans and Alaska Natives were excluded from the analysis (Figure S1).
2.2 |. Socioeconomic status and race/ethnicity variables
Data provided by the specialized SEER database allows for analysis using census tracts, which were established by the Census Bureau and represent the smallest population unit for which economic data are available.20 The census tract-level SES index within SEER is based upon an algorithm delineated by Yost et al. using seven variables from the American Community Survey that measure SES at the census tract level. This algorithm has been validated by the NCI for applicability to SEER data.21,22 Parameters include median household income, median house value, median rent, percent of population below 150% of the poverty line, education level, percent of population identifying as working class, and percent of population unemployed.21 Within each of the registries that report to SEER, these index values were divided into tertiles, with the highest tertile as the reference group in analyses.
2.3 |. Outcome variables
Extracted outcome variables included patient vital status and months to death from date of diagnosis as of December 31, 2016. SEER utilized censoring to account for loss to follow up.23
2.4 |. Demographic and clinical variables
The following demographic and clinical variables were extracted for each case: age at diagnosis, year of diagnosis, race/ethnicity, and tumor histology. Age at diagnosis was divided into five categories: 15–19 years (reference), 20–24 years, 25–29 years, 30–34 years, and 35–39 years. Year of diagnosis was categorized by 4-year intervals: 2000–2004 (reference), 2005–2008, 2009–2012, and 2013–2016. Race/ethnicity was defined as non-Hispanic White (NHW) (reference group), non-Hispanic Black (NHB), non-Hispanic Asian/Pacific Islander (NHAPI), and Hispanic (all races). The tumors were divided into five groups based on histology: low-grade glioma (reference group), high-grade glioma, primitive neuroectodermal tumors (PNET)/medulloblastoma, malignant ependymoma, and benign ependymoma.
Rural classification was based on the Census Bureau measure derived from percentage of census-tract population living in an urban area. This variable was recorded to be dichotomous: ≥50% rural (rural) and <50% rural (urban) (reference group).
2.5 |. Statistical analysis
A multivariable Cox proportional hazard model was computed and the unadjusted and adjusted Cox proportional hazard ratios (HR and aHR) with 95% confidence intervals were calculated (Table S3). Joint hypothesis testing was done for each of the categorical variables to test for significant differences between the categories within each variable. Interaction terms were not included in the final model since they were not statistically significant. The proportional hazards assumption for each of the predictor variables was tested using Schoenfeld residuals. Given that race/ethnicity, histology type, age group, and Yost tertile violated the proportional hazard assumption, stratified analyses are reported. The complete stratified analysis by SES is included in Tables S4, with the stratified analyses by race/ethnicity, age group, and histology shown below.
Mediation analysis was conducted to determine the indirect effect of SES on the association between race/ethnicity and the hazard of death. The methodology used was inverse odds weighting (IOW) using the NHW cohort as the reference group.24,25 The weight for race/ethnicity was obtained from a multivariate logistic regression model. For each racial/ethnic group, two multivariate Cox proportional hazards were then calculated, one without the IOWs and one with IOWs.26 The model without the IOWs yielded the total effect (βtotal) and the model with the IOWs yielded the direct effect βdirect).26 The indirect effect (effect of race/ethnicity on hazard of death mediated by SES) was calculated by subtracting the direct effect from the total effect.26 If the p-value calculated for βindirect was statistically significant, mediation effect of SES on race/ethnicity associated with hazard of death was concluded to exist. Note that 95% confidence intervals (CIs) for βtotal, βdirect, and βindirect were estimated using bootstrapping.26
Kaplan–Meier curves for each of the predictor variables were constructed. The log-rank test was used to test for differences between groups. The threshold used for Type I errors for all analyses was p < 0.05. The SEER*Stat program with the Ederer II method and the 1992–2016 SES/geography/race annual life tables constructed by Mariotto et al. were used to analyze the differences in 5-year relative survival.27 Relative survival represents the ratio between observed survival within a cohort of cancer patients that survived and observed survival in a comparable cancer-free cohort.18 All data analysis and figure creation were conducted using StataSE Version 16.0 (College Station, TX).
3 |. RESULTS
3.1 |. Demographics
Total sample size was 11,386 with 7095 (62.3%) patients alive and 4291 (37.7%) deceased as of December 31, 2016 (Table 1). Within the cohort, 6485 patients (57.0%) were male and 4901 (43.0%) were female. The majority of patients were NHW (65.9%), had a tumor histology classification of high-grade glioma (53.6%), and lived in primarily urban areas (89.6%) (Table 1).
TABLE 1.
Characteristics of Adolescents and Young Adults with Primary Central Nervous System Tumors
| Characteristic | N (%) |
|---|---|
| Vital Status | |
| Alive | 7095 (62.3) |
| Dead | 4291 (37.7) |
| Age Group | |
| 15 – 19 years | 1734 (15.2) |
| 20 – 24 years | 1819 (16.0) |
| 25 – 29 years | 2275 (20.0) |
| 30 – 34 years | 2605 (22.9) |
| 35 – 39 years | 2953 (25.9) |
| Gender | |
| Female | 4901 (43.0) |
| Male | 6485 (57.0) |
| Year of Diagnosis | |
| 2000 – 2004 | 3072 (27.0) |
| 2005 – 2008 | 2679 (23.5) |
| 2009 – 2012 | 2841 (25.0) |
| 2013 – 2016 | 2794 (24.5) |
| Race/Ethnicity | |
| Non-Hispanic White | 7498 (65.9) |
| Non-Hispanic Black | 903 (7.9) |
| Non-Hispanic Asian or Pacific Islander | 797 (7.0) |
| Hispanic (All Races) | 2188 (19.2) |
| Histology | |
| Low Grade Glioma | 3173 (27.9) |
| High Grade Glioma | 6098 (53.6) |
| PNET/Medulloblastoma | 757 (6.7) |
| Malignant Ependymoma | 943 (8.3) |
| Benign Ependymoma | 415 (3.6) |
| Census Proportion Living in Urban Areas | |
| ≥ 50% Urban | 10200 (89.6) |
| ≥ 50% Rural | 1186 (10.4) |
| Yost SES Tertilesa | |
| Tertile 1 | 3328 (29.2) |
| Tertile 2 | 3867 (34.0) |
| Tertile 3 | 4191 (36.8) |
Abbreviations: HR, hazard ratio; PNET, primitive neuroectodermal tumor; SES, socioeconomic status
Tertile 1 corresponds to the lowest SES and Tertile 3 corresponds to the highest SES
3.2 |. Relative and overall survival
The 5-year relative survival for the entire cohort was 65.4% (95% CI 64.4–66.4%). The youngest age group was noted to have the highest 5-year relative survival of 75.3% (95% CI 73.1–77.3%) with the lowest 5-year relative survival of 54.8% (95% CI 52.8–56.9%) within the oldest age group. NHW patients were noted to have a statistically significant higher 5-year relative survival over NHB patients (66.9% vs. 62.0%, p = 0.001), NHAPI patients (66.9% vs. 60.0%, p < 0.001), and Hispanic patients (66.9% vs. 62.6%, p < 0.001). When evaluating by SES level, those from the lowest Yost tertile was found to have a statistically significant lower relative 5-year survival compared to the middle tertile (63.8% vs. 65.6%, p = 0.01) and highest tertile (63.8% vs. 67.1%, p = 0.003). Patients from racial/ethnic minorities had lower survival probability compared to NHW patients (Figure 1A). Patients from the lowest SES level or living in a rural area were found to have lower survival probability (Figure 1B and C). A decrease in survival probability as age increases was also noted (Figure 1D). The results of the log-rank test are included in Figure 1.
FIGURE 1.
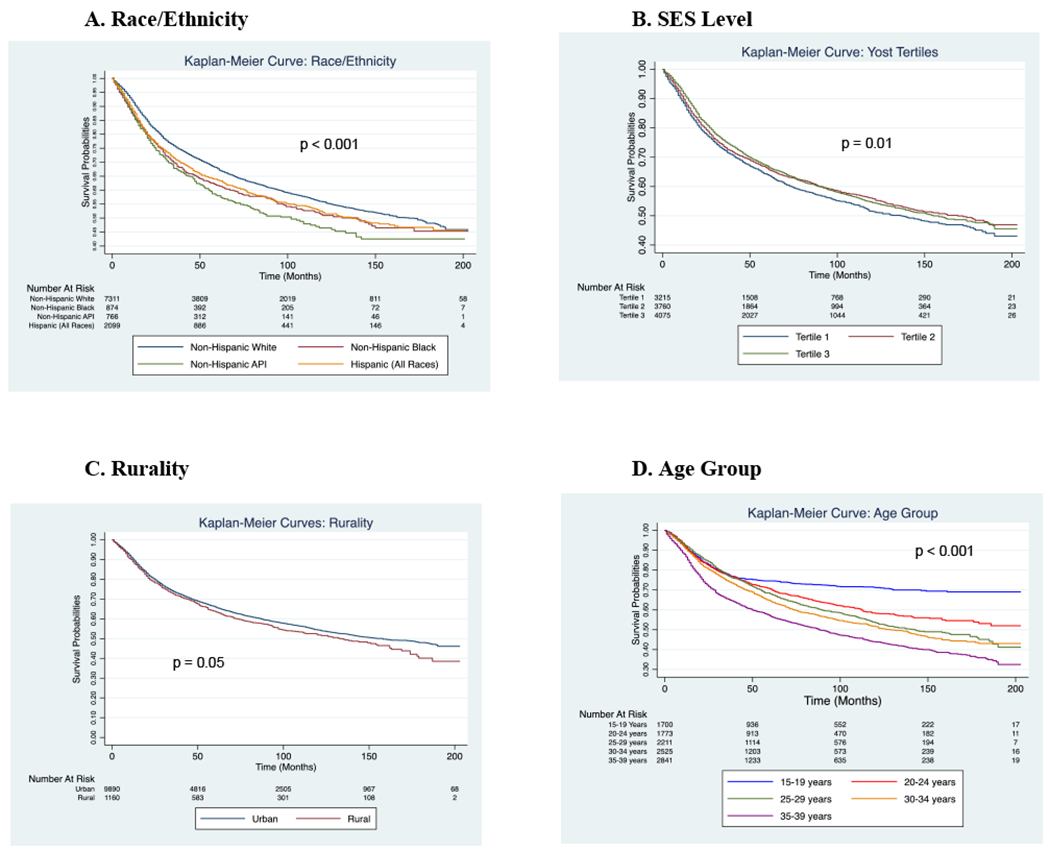
Survival probabilities stratified by race/ethnicity (A), SES level (B), rurality (C), and age group (D)
3.3 |. Stratified analysis by race/ethnicity
Lower SES was associated with an increased hazard of death in NHW (aHR 1.12; 95% CI 1.01–1.24) and NHB patients (aHR 1.34, 95% CI 1.00–1.80) but not Hispanic or NHAPI patients (Table 2). The highest hazard of death was noted among NHB with high-grade gliomas (Table 2). Among the patients of racial/ethnic minorities, NHB patients were noted to have the largest hazard of death from SES status (Table 2). Except for within the NHAPI cohort, patients aged 35–39 years were found to have the highest risk of death and females were noted to have a lower risk of death (Table 2). Amongst NHW patients, those from rural areas (aHR 1.20; 95% CI 1.08–1.34) were found to have an increased hazard of death (Table 2).
TABLE 2.
Hazard of Death for Adolescent and Young Adult Primary Central Nervous System Tumor Patients by Race/Ethnicity
| Non-Hispanic White | Non-Hispanic Black | Non Hispanic Asian/Pacific Islander | Hispanic (All Races) | |||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | aHRa (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p |
| Age Group | ||||||||
| 15 – 19 years | [reference] | [reference] | [reference] | [reference] | ||||
| 20 – 24 years | 1.30 (1.10 – 1.53) | 0.002 | 1.39 (0.95 – 2.03) | 0.11 | 0.88 (0.55 – 1.38) | 0.57 | 1.29 (0.87 – 1.52) | 0.07 |
| 25 – 29 years | 1.35 (1.15 – 1.58) | <0.001 | 1.20 (0.75 – 1.63) | 0.37 | 1.02 (0.69 – 1.49) | 0.93 | 1.21 (0.85 – 1.44) | 0.16 |
| 30 – 34 years | 1.49 (1.28 – 1.73) | <0.001 | 1.26 (0.79 – 1.65) | 0.22 | 0.76 (0.52 – 1.12) | 0.17 | 1.22 (0.91 – 1.54) | 0.14 |
| 35 – 39 years | 1.86 (1.61 – 2.15) | <0.001 | 1.98 (1.35 – 2.68) | <0.001 | 1.01 (0.70 – 1.47) | 0.94 | 1.42 (1.03 – 1.70) | 0.01 |
| Gender | ||||||||
| Male | [reference] | [reference] | [reference] | [reference] | ||||
| Female | 0.76 (0.71 – 0.83) | <0.001 | 0.74 (0.60 – 0.92) | 0.01 | 0.84 (0.67 – 1.06) | 0.14 | 0.83 (0.72 – 0.97) | 0.01 |
| Census Classification | ||||||||
| Urban | [reference] | [reference] | [reference] | [reference] | ||||
| Rural | 1.20 (1.08 – 1.34) | 0.001 | 1.15 (0.77 – 1.81) | 0.53 | 1.07 (0.26 – 4.38) | 0.93 | 1.47 (0.99 – 2.18) | 0.05 |
| Yost SES Tertilec | ||||||||
| Tertile 1 | 1.12 (1.01 – 1.24) | 0.03 | 1.34 (1.00 – 1.80) | 0.048 | 1.07 (0.79 – 1.43) | 0.67 | 1.12 (0.91 – 1.36) | 0.28 |
| Tertile 2 | 1.00 (0.91 – 1.09) | 0.96 | 1.39 (1.01 – 1.92) | 0.04 | 1.06 (0.82 – 1.36) | 0.70 | 1.07 (0.87 – 1.33) | 0.52 |
| Tertile 3 | [reference] | [reference] | [reference] | [reference] | ||||
Abbreviations: aHR, adjusted hazard ratio; PNET, primitive neuroectodermal tumor; SES, socioeconomic status
Adjusted hazard ratio adjusted for age group, year of diagnosis, gender, histology, census classification, and Yost SES tertile
Sample size too small to compute
Tertile 1 corresponds to the lowest SES and Tertile 3 corresponds to the highest SES
3.4 |. Mediation analysis of socioeconomic status on race/ethnicity
When evaluating the direct and total effects with mediation analysis, all racial/ethnic minorities were noted to have an increased hazard of death compared to NHW patients (Table 3). As noted in Table S4, the increased hazard of death for patients identified as racial/ethnic minorities and patients from rural areas was more significant in those from the lowest SES. When adjusting for the other co-variates, a mediating effect of SES on the association between race/ethnicity and hazard of death was observed in NHB patients (indirect aHR 1.11; 95% CI 1.01–1.22), but not in NHAPI or Hispanic patients (Table 3). SES accounted for 33.7% of the association between NHB and hazard of death and was found to be statistically significant.
TABLE 3.
Mediation Analysis of Socioeconomic Status on Race/Ethnicity
| Indirect Effect | % Accounted by Indirect Effectb | Direct Effect | Total Effect | ||||
|---|---|---|---|---|---|---|---|
| aHRa (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p | ||
| NHW vs. NHB | 1.11 (1.01 – 1.22) | 0.03 | 33.7 | 1.23 (1.05 – 1.43) | 0.01 | 1.36 (1.21 – 1.54) | <0.001 |
| NHW vs. NHAPI | 1.02 (0.94 – 1.10) | 0.69 | 5.3 | 1.35 (1.17 – 1.55) | 0.01 | 1.21 (1.06 – 1.37) | <0.001 |
| NHW vs. Hispanic | 1.05 (0.95 – 1.16) | 0.33 | 20.7 | 1.21 (1.06 – 1.37) | <0.001 | 1.27 (1.17 – 1.37) | <0.001 |
Abbreviations: aHR, adjusted hazard ratio; NHW, non-Hispanic White; NHB, non-Hispanic Black, NHAPI, non-Hispanic Asian/Pacific Islander
All hazard ratios adjusted for age group, year of diagnosis, gender, histology, and rural classification
% accounted for by indirect effect is calculated by [βindirect / βtotal] x 100
3.5 |. Stratified analysis by age group
Among patients aged 15–19 years, NHAPI and Hispanic patients were noted to have a statistically significant increased hazard of death, with all three race/ethnic minority groups having a statistically significant increased hazard of death within the 20–24 year age group (Table 4). Among patients aged 35–39 years of age, only NHB patients had a statistically significant increased hazard of death (aHR 1.43; 95% CI 1.15–1.77) (Table 4). Residence in a rural area was noted to be associated with a statistically significant increased hazard of death amongst patients aged 35–39 years (aHR 1.33; 95% CI 1.13–1.57) (Table 4). Lower SES was associated with a significantly increased hazard of death in patients aged 25–29 years and aged 35–39 years (Table 4). In all age groups, males were noted to have an increased risk of death as compared to females (Table 4).
TABLE 4.
Hazard of Death for Adolescent and Young Adult Primary Central Nervous System Tumor Patients by Age Group
| Ages 15 – 19 | Ages 20 – 24 | Ages 25 – 29 | Ages 30 – 34 | Ages 35 – 39 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | aHRa (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p | aHR (95% CI) | p |
| Gender | ||||||||||
| Male | [reference] | [reference] | [reference] | [reference] | [reference] | |||||
| Female | 0.76 (0.63 – 0.93) | 0.006 | 0.69 (0.59 – 0.82) | <0.001 | 0.76 (0.66 – 0.88) | <0.001 | 0.74 (0.65 – 0.85) | <0.001 | 0.85 (0.76 – 0.95) | 0.01 |
| Race/Ethnicity | ||||||||||
| Non-Hispanic White | [reference] | [reference] | [reference] | [reference] | [reference] | |||||
| Non-Hispanic Black | 1.31 (0.95 – 1.79) | 0.10 | 1.44 (1.09 – 1.90) | 0.01 | 1.15 (0.87 – 1.53) | 0.33 | 1.16 (0.91 – 1.48) | 0.24 | 1.43 (1.16 – 1.77) | 0.001 |
| Non-Hispanic API | 1.92 (1.39 – 2.66) | <0.001 | 1.44 (1.01 – 2.08) | 0.046 | 1.68 (1.31 – 2.16) | <0.001 | 1.12 (0.87 – 1.43) | 0.38 | 1.20 (0.97 – 1.49) | 0.10 |
| Hispanic [All Races] | 1.38 (1.06 – 1.80) | 0.02 | 1.46 (1.17 – 1.82) | 0.001 | 1.26 (1.04 – 1.52) | 0.02 | 1.26 (1.05 – 1.50) | 0.01 | 1.10 (0.95 – 1.29) | 0.20 |
| Histology | ||||||||||
| Low Grade Glioma | [reference] | [reference] | [reference] | [reference] | [reference] | |||||
| High Grade Glioma | 8.36 (6.31 – 11.07) | <0.001 | 3.11 (2.54 – 3.80) | <0.001 | 1.90 (1.59 – 2.26) | <0.001 | 2.15 (1.82 – 2.53) | <0.001 | 2.03 (1.75 – 2.35) | <0.001 |
| PNET/Medulloblastoma | 5.29 (3.77 – 7.42) | <0.001 | 1.60 (1.56 – 2.21) | 0.01 | 1.52 (1.12 – 2.06) | 0.01 | 0.90 (0.61 – 1.32) | 0.59 | 1.09 (0.76 – 1.56) | 0.66 |
| Malignant Ependymoma | 1.45 (0.83 – 2.51) | 0.19 | 0.49 (0.30 – 0.82) | 0.01 | 0.48 (0.33 – 0.71) | <0.001 | 0.34 (0.22 – 0.53) | <0.001 | 0.27 (0.18 – 0.40) | <0.001 |
| Benign Ependymoma | n/a | 1.00 | 0.21 (0.07 – 0.66) | 0.007 | 0.14 (0.05 – 0.38) | <0.001 | 0.08 (0.02 – 0.30) | <0.001 | 0.07 (0.02 – 0.27) | <0.001 |
| Census classification | ||||||||||
| Urban | [reference] | [reference] | [reference] | [reference] | [reference] | |||||
| Rural | 1.24 (0.91 – 1.70) | 0.17 | 1.09 (0.82 – 1.44) | 0.56 | 1.08 (0.84 – 1.38) | 0.56 | 1.21 (0.99 – 1.48) | 0.06 | 1.33 (1.13 – 1.57) | 0.001 |
| Yost SES Tertilesb | ||||||||||
| Tertile 1 | 1.00 (0.76 – 1.28) | 0.98 | 1.00 (0.81 – 1.23) | 0.96 | 1.29 (1.07 – 1.55) | 0.01 | 1.10 (0.94 – 1.29) | 0.24 | 1.16 (1.01 – 1.34) | 0.04 |
| Tertile 2 | 0.89 (0.71 – 1.12) | 0.30 | 1.00 (0.82 – 1.23) | 0.97 | 1.09 (0.92 – 1.30) | 0.31 | 1.02 (0.87 – 1.19) | 0.82 | 1.09 (0.95 – 1.24) | 0.21 |
| Tertile 3 | [reference] | [reference] | [reference] | [reference] | [reference] | |||||
Abbreviations: aHR, adjusted hazard ratio; API, Asian Pacific Islander; PNET, primitive neuroectodermal tumor; SES, socioeconomic status
Adjusted hazard ratio adjusted for race/ethnicity, year of diagnosis, gender, histology, census classification, and Yost SES tertile
Tertile 1 corresponds to the lowest SES and Tertile 3 corresponds to the highest SES
3.6 |. Stratified analysis by histology
Among patients with low-grade gliomas, the hazard of death increased significantly with increased age (Table 5). Similarly, among patients with high-grade gliomas, those from the oldest age group had the highest hazard of death (aHR 1.20; 95% CI 1.05–1.37) (Table 5). Survival disparities associated with race/ethnicity were more notable in the high-grade glioma cohort, with all racial/ethnic minorities having a statistically significant increased hazard of death compared to NHW patients (Table 5). Within the low-grade glioma cohort, NHAPI was the only racial/ethnic minority group with a statistically significant increased hazard of death (aHR 1.61; 95% CI 1.21–2.14). Rurality caused a statistically significant increased hazard of death in both the low-grade and high-grade glioma cohorts. Being from a low SES was statistically significantly associated with an increased hazard of death only among high-grade glioma patients (Table 5).
TABLE 5.
Hazard of Death for Adolescent and Young Adult Primary Central Nervous System Tumor Patients by Histology
| LGGs | HGGs | |||
|---|---|---|---|---|
| Characteristic | aHRa (95% CI) | p | aHR (95% CI) | p |
| Age Group | ||||
| 15 – 19 years | [reference] | [reference] | ||
| 20 – 24 years | 2.95 (2.17 – 4.01) | <0.001 | 0.97 (0.83 – 1.14) | 0.72 |
| 25 – 29 years | 4.26 (3.17 – 5.72) | <0.001 | 0.84 (0.73 – 0.97) | 0.02 |
| 30 – 34 years | 4.27 (3.18 – 5.73) | <0.001 | 0.96 (0.83 – 1.10) | 0.54 |
| 35 – 39 years | 5.53 (4.15 – 7.36) | <0.001 | 1.20 (1.05 – 1.37) | 0.01 |
| Year of Diagnosis | ||||
| 2000 – 2004 | [reference] | [reference] | ||
| 2005 – 2008 | 0.90 (0.76 – 1.07) | 0.25 | 0.86 (0.79 – 0.95) | 0.002 |
| 2009 – 2012 | 0.85 (0.69 – 1.05) | 0.13 | 0.81 (0.73 – 0.89) | <0.001 |
| 2013 – 2016 | 0.72 (0.52 – 1.01) | 0.06 | 0.78 (0.69 – 0.89) | <0.001 |
| Gender | ||||
| Male | [reference] | [reference] | ||
| Female | 0.71 (0.61 – 0.82) | <0.001 | 0.79 (0.73 – 0.85) | <0.001 |
| Race/Ethnicity | ||||
| Non-Hispanic White | [reference] | [reference] | ||
| Non-Hispanic Black | 1.12 (0.85 – 1.46) | 0.43 | 1.33 (1.16 – 1.52) | <0.001 |
| Non-Hispanic Asian or Pacific Islander | 1.61 (1.21 – 2.14) | 0.001 | 1.34 (1.17 – 1.54) | <0.001 |
| Hispanic [All Races] | 1.15 (0.93 – 1.41) | 0.20 | 1.26 (1.14 – 1.39) | <0.001 |
| Census Classification | ||||
| Urban | [reference] | [reference] | ||
| Rural | 1.26 (1.02 – 1.56) | 0.03 | 1.20 (1.06 – 1.35) | <0.001 |
| Yost SES Tertiles | ||||
| Tertile 1 | 1.15 (0.96 – 1.39) | 0.13 | 1.11 (1.01 – 1.22) | 0.03 |
| Tertile 2 | 1.10 (0.93 – 1.31) | 0.28 | 1.04 (0.96 – 1.14) | 0.33 |
| Tertile 3 | [reference] | [reference] | ||
Abbreviations: aHR, adjusted hazard ratio; LGG, low-grade gliomas; HGG, high-grade gliomas; PNET, primitive neuroectodermal tumor
Hazard ratios adjusted for age group, gender, year of diagnosis, race/ethnicity, census classification, and Yost SES tertiles
4 |. DISCUSSION
This is an in-depth population-based study using a national database to assess the impact of racial/ethnic, socioeconomic, and geographic factors on survival disparities in the AYA neuro-oncology population. In the largest sample of AYA patients with primary CNS tumors to date, this manuscript shows, through mediation analysis, that there was a mediating effect of SES on the effect of race/ethnicity on the hazard of death among NHB patients, but not NHAPI or Hispanic patients. There are numerous plausible hypotheses to explain the survival disparities amongst racial/ethnic minority patients, including biological differences, differences in stage at diagnosis, treatment received, and societal factors such as potential language barriers, systemic racism, and the ability to access and adhere to efficacious therapies including clinical trials and appropriate supportive care.28–31 The significant indirect effect in the NHB cohort indicates that SES does indeed have a more significant role in this population’s increased hazard of death.
The results provide evidence that patients being identified as a racial/ethnic minority, having a lower SES, and living in a rural area negatively impact survival in AYA patients with primary CNS tumors. This is in contrast to the adult literature, which found superior survival in Blacks with glioblastoma multiforme compared to White patients.32 These papers, however, were focused on the adult neuro-oncology population and have not emphasized the interplay between race/ethnicity, SES, and survival. Our differing findings are notable and could potentially be explained by unique AYA tumor biology or by more pronounced effects of previously described AYA challenges in the NHB population (i.e., social, psychological, adherence, as well as decreased clinical trial enrollment).3–6,33,34
The findings above highlight the need for further research to assess and address the role of other covariates in these survival disparities. Social determinants of health such as food insecurity, housing instability, ability to pay for utilities, and personal safety issues have been shown to negatively impact both therapy adherence and outcomes and need to be addressed at the time of diagnosis.35–37 Having a low SES translates to having less resources to pay for appropriate therapies and supportive care that can significantly affect outcomes. Decreased access to clinical trials as well as the complicated multidisciplinary management of these patients may also help explain survival disparities.30,38,39 Racial/ethnic minority patients and patients with low SES may also be more prone to loss to follow up due to a range of social barriers, including inherent distrust of the healthcare system fostered by experiences of discrimination.40,41 Additionally, there remains a disparity in insurance coverage amongst patients from racial/ethnic minorities despite the Affordable Care Act.42
Similarly, geography affects the ability to expediently access necessary care. Treatment at NCI centers has been shown to lessen the inferior outcomes noted in the AYA population.10 Most large cancer centers capable of managing CNS tumors with experienced multidisciplinary teams are located in urban areas. As such, those living in rural areas may have more difficulty accessing these centers, along with more sophisticated therapies.11
When evaluating different age groups, the youngest age group from the lowest SES level was not found to have a statistically significant increased mortality hazard. However, within the older age groups, those with the lowest SES did have an association with an increased hazard of death. In most US healthcare centers, the majority of patients aged 15–19 years receive their care at pediatric oncology centers, however, less frequently than their pediatric counterparts.10,12 These patients benefit from having significant multidisciplinary care with more resources and support, including financial support from parents or guardians.38 The frequency of referrals to these centers decrease with age, with older AYA patients with low SES or living further from NCI centers experiencing inferior outcomes.10,13
Of interest, patients with low-grade gliomas were found to have decreased overall survival compared to those with malignant ependymomas (Figure S2). While unexpected in the pediatric population, this correlates with data that shows that older patients with low-grade gliomas experience a substantial decrease in overall survival, compared to pediatric patients.43,44 This trend in low-grade gliomas also helps explain decreasing hazard ratios in high-grade gliomas and medulloblastomas/PNETs with increase in age.43–45 The decreased survival in older patients with low-grade gliomas is due to more aggressive tumor behavior in the adult population.46 Next-generation sequencing has revealed underlying molecular differences in pediatric and adult low-grade gliomas, including increased prevalence of v-Raf murine sarcoma viral oncogene homolog B (BRAF) mutations among pediatric low-grade gliomas and a higher incidence of isocitrate dehydrogenase 1/2 mutations in adult low-grade gliomas.47,48 This molecular variability establishes the need for a brain tumor database that incorporates molecular, clinical, demographic, and socioeconomic data to help truly identify biological, racial/ethnic, socioeconomic, and geographic sources of survival disparities.
4.1 |. Limitations
While SEER provides a robust sample size for analysis, the population included within SEER represents only 34% of the US population, with higher representation from the Northeast and the West and significant underrepresentation of the Native American and Alaska Native populations. There is also underrepresentation of individuals living in rural areas. Another limitation of the SEER database is that patient migration is not captured, and so those moving from within to outside SEER’s catchment areas are lost to follow up.49 Additionally, the insurance variable within SEER has substantial limitations, including the lack of availability of insurance information for patients diagnosed prior to 2007 and unknown insurance status for some patients diagnosed after 2007. The lack of detailed treatment data within SEER such as specific chemotherapy agents or type of radiation given is also a limitation, as therapy has a significant effect on outcomes.
The effect of rural residence can also greatly differ depending on the overall population density of each region. While there is some adjustment for the Yost tertiles based on the different state/regional registries within SEER, there is no such adjustment for rural classification. Lack of information regarding patients’ state and county of residence results in further limitations in assessing these regional differences. Additionally, the high density of racial/ethnic minorities in urban areas could affect the extrapolation of our results to the US population as a whole (Table S5). Lastly, while census tract-level SES data is the most granular data available, the SES status of each individual patient could vary drastically from that of his/her census tract.20
5 |. CONCLUSION
While there have been efforts to improve healthcare disparities for minority patients, those with low SES, and those living in rural areas, there data support the premise that the adolescent and young adult neuro-oncology population continues to experience disparities in outcomes from these factors. Further studies are warranted to explore and address the source of these disparities with a focus on developing and implementing specific interventions to bridge the gap in survival within this population.
Supplementary Material
Abbreviations:
- aHR
adjusted hazard ratio
- AYA
adolescent and young adult
- BRAF
v-Raf murine sarcoma viral oncogene homolog B
- CNS
central nervous system
- HR
hazard ratio
- IOW
inverse odds weighting
- NCI
National Cancer Institute
- NHAPI
non-Hispanic Asian and Pacific Islander
- NHB
non-Hispanic Black
- NHW
non-Hispanic White
- PNET
primitive neuroectodermal tumor
- SEER
Surveillance, Epidemiology, and End Results
- SES
socioeconomic status
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the Surveillance Epidemiology and End Results (SEER) Program. Restrictions apply to the availability of these data. Data are available with permission and approval of the SEER Program.
REFERENCES
- 1.United States Cancer Statistics. US cancer statistics data visualizations tool. US Centers for Disease Control and Prevention. [Google Scholar]
- 2.Coccia PF. Overview of adolescent and young adult oncology. J Oncol Pract. 2019;15(5):235–237. [DOI] [PubMed] [Google Scholar]
- 3.Smith AW, Seibel NL, Lewis DR, et al. Next steps for adolescent and young adult oncology workshop: an update on progress and recommendations for the future. Cancer. 2016;122(7):988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richter D, Koehler M, Friedrich M, Hilgendorf I, Mehnert A, Weissflog G. Psychosocial interventions for adolescents and young adult cancer patients: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2015;95(3):370–386. [DOI] [PubMed] [Google Scholar]
- 5.Jacob SA, Shaw PH. No improvement in clinical trial enrollment for adolescents and young adults with cancer at a children’s hospital. Pediatr Blood Cancer. 2017;64(12):e26638. [DOI] [PubMed] [Google Scholar]
- 6.Moke DJ, Tsai K, Hamilton AS, et al. Emerging cancer survival trends, disparities, and priorities in adolescents and young adults: a California Cancer Registry-Based Study. JNCI Cancer Spectr. 2019;3(2):pkz031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isenalumhe LL, Fridgen O, Beaupin LK, Quinn GP, Reed DR. Disparities in adolescents and young adults with cancer. Cancer Control. 2016;23(4):424–433. [DOI] [PubMed] [Google Scholar]
- 8.Liu LHA, Moke D, Tsai KY, Wojcik KY, Cockburn M, Deapen D, eds. Cancer in Los Angeles County: survival among adolescents and young adults 1988–2014. Los Angeles Cancer Surveillance Program. Los Angeles, CA: University of Southern California; 2017. [Google Scholar]
- 9.Braveman PA, Kumanyika S, Fielding J, et al. Health disparities and health equity: the issue is justice. Am J Public Health. 2011;101(Suppl 1):S149–S155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfson J, Sun CL, Kang T, Wyatt L, D’Appuzzo M, Bhatia S. Impact of treatment site in adolescents and young adults with central nervous system tumors. J Natl Cancer Inst. 2014;106(8):dju166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delavar A,Al Jammal OM, Maguire KR,Wali AR, Pham MH. The impact of rural residence on adult brain cancer survival in the United States. J Neurooncol. 2019;144(3):535–543. [DOI] [PubMed] [Google Scholar]
- 12.Yeager ND, Hoshaw-Woodard S, Ruymann FB, Termuhlen A. Patterns of care among adolescents with malignancy in Ohio. J Pediatr Hematol Oncol. 2006;28(1):17–22. [PubMed] [Google Scholar]
- 13.Albritton KH, Wiggins CH, Nelson HE, Weeks JC. Site of oncologic specialty care for older adolescents in Utah. J Clin Oncol. 2007;25(29):4616–4621. [DOI] [PubMed] [Google Scholar]
- 14.Cooney T, Fisher PG, Tao L, Clarke CA, Partap S. Pediatric neuro-oncology survival disparities in California. J Neurooncol. 2018; 138(1):83–97. [DOI] [PubMed] [Google Scholar]
- 15.Siegel DA, Li J, Ding H, Singh SD, King JB, Pollack LA. Racial and ethnic differences in survival of pediatric patients with brain and central nervous system cancer in the United States. Pediatr Blood Cancer. 2019;66(2):e27501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mukherjee D, Zaidi HA, Kosztowski T, et al. Disparities in access to neuro-oncologic care in the United States. Arch Surg. 2010;145(3):247–253. [DOI] [PubMed] [Google Scholar]
- 17.Surveillance E, and End Results (SEER) Program. Surveillance, Epidemiology, and End Results (SEER): SEER Overview. Bethesda, MD: National Cancer Institute; 2018. [Google Scholar]
- 18.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8(6):541–552. [DOI] [PubMed] [Google Scholar]
- 19.Surveillance E, and End Results (SEER) Program. SEER*Stat Database: Incidence-SEER 18 Regs (Excl AK) Custom Data (with additional treatment fields), Nov 2018 Sub (2000-2016) <Vintage 2016 Pops by Tract 2000/2010 Mixed Geographies>- Linked To Census Tract Attributes-Time Dependent (2000-2016)-SEER 18 (excl AK) Census 2000/2010 Geographies with Index Field Quantiles. National Cancer Institute, DCCPS, Surveillance Research Program, released January 2020, based on the November 2018 submission. [Google Scholar]
- 20.Kong AY, Zhang X. The use of small area estimates in place-based health research. Am J Public Health. 2020:e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12(8):703–711. [DOI] [PubMed] [Google Scholar]
- 22.Yu M, Tatalovich Z, Gibson JT, Cronin KA. Using a composite index of socioeconomic status to investigate health disparities while protecting the confidentiality of cancer registry data. Cancer Causes Control. 2014;25(1):81–92. [DOI] [PubMed] [Google Scholar]
- 23.Pinheiro PS, Morris CR, Liu L, Bungum TJ, Altekruse SF. The impact of follow-up type and missed deaths on population-based cancer survival studies for Hispanics and Asians. J Natl Cancer Inst Monogr. 2014;2014(49):210–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tchetgen Tchetgen EJ. Inverse odds ratio-weighted estimation for causal mediation analysis. Stat Med. 2013;32(26):4567–4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen QC, Osypuk TL, Schmidt NM, Glymour MM, Tchetgen Tchetgen EJ. Practical guidance for conducting mediation analysis with multiple mediators using inverse odds ratio weighting. Am J Epidemiol. 2015;181(5):349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williams LA, Frazier AL, Poynter JN. Survival differences by race/ethnicity among children and adolescents diagnosed with germ cell tumors. Int J Cancer. 2020;146(9):2433–2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mariotto AB, Zou Z, Johnson CJ, Scoppa S, Weir HK, Huang B. Geographical, racial and socio-economic variation in life expectancy in the US and their impact on cancer relative survival. PLoS One. 2018;13(7):e0201034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhatia S, Sather HN, Heerema NA, Trigg ME, Gaynon PS, Robison LL. Racial and ethnic differences in survival of children with acute lymphoblastic leukemia. Blood. 2002;100(6):1957–1964. [DOI] [PubMed] [Google Scholar]
- 29.Kahn JM, Keegan TH, Tao L, Abrahao R, Bleyer A, Viny AD. Racial disparities in the survival of American children, adolescents, and young adults with acute lymphoblastic leukemia, acute myelogenous leukemia, and Hodgkin lymphoma. Cancer. 2016;122(17):2723–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Rojas T, Neven A, Terada M, et al. Access to clinical trials for adolescents and young adults with cancer: a meta-research analysis. JNCI Cancer Spectr. 2019;3(4):pkz057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–3334. [DOI] [PubMed] [Google Scholar]
- 32.Ostrom QT, Cote DJ, Ascha M, Kruchko C, Barnholtz-Sloan JS. Adult glioma incidence and survival by race or ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alken SP, D’Urso P, Saran FH. Managing teenage/young adult (TYA) brain tumors: a UK perspective. CNS Oncol. 2015;4(4):235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tricoli JV, Blair DG, Anders CK, et al. Biologic and clinical characteristics of adolescent and young adult cancers: acute lymphoblastic leukemia, colorectal cancer, breast cancer, melanoma, and sarcoma. Cancer. 2016;122(7):1017–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bona K, London WB, Guo D, Frank DA, Wolfe J. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatr Blood Cancer. 2016;63(1):105–111. [DOI] [PubMed] [Google Scholar]
- 36.Bona K, Dussel V, Orellana L, et al. Economic impact of advanced pediatric cancer on families. J Pain Symptom Manage. 2014;47(3):594–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia S, Landier W, Hageman L, et al. 6MP adherence in a multiracial cohort of children with acute lymphoblastic leukemia: a Children’s Oncology Group study. Blood. 2014;124(15):2345–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parsons SK, Kumar AJ. Adolescent and young adult cancer care: financial hardship and continued uncertainty. Pediatr Blood Cancer. 2019;66(4):e27587. [DOI] [PubMed] [Google Scholar]
- 39.Colon-Otero G, Smallridge RC. Disparities in participation in cancer clinical trials in the United States : a symptom of a healthcare system in crisis. Cancer. 2008;112(3):447–454. [DOI] [PubMed] [Google Scholar]
- 40.Berkman JM, Dallas J, Lim J, et al. Social determinants of health affecting treatment of pediatric brain tumors. J Neurosurg Pediatr. 2019:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Penner LA, Harper FWK, Dovidio JF, et al. The impact of Black cancer patients’ race-related beliefs and attitudes on racially-discordant oncology interactions: a field study. Soc Sci Med. 2017;191:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buchmueller TC, Levinson ZM, Levy HG, Wolfe BL. Effect of the affordable care act on racial and ethnic disparities in health insurance coverage. Am J Public Health. 2016;106(8):1416–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ostrom QT, Gittleman H, de Blank PM, et al. American brain tumor association adolescent and young adult primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2016;18(Suppl 1):i1–i50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ostrom QT, Gittleman H, Truitt G, Boscia A, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Corell A, Carstam L, Smits A, Henriksson R, Jakola AS. Age and surgical outcome of low-grade glioma in Sweden. Acta Neurol Scand. 2018;138(4):359–368. [DOI] [PubMed] [Google Scholar]
- 46.Schomas DA, Laack NN, Brown PD. Low-grade gliomas in older patients: long-term follow-up from Mayo Clinic. Cancer. 2009;115(17):3969–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venneti S, Huse JT. The evolving molecular genetics of low-grade glioma. Adv Anat Pathol. 2015;22(2):94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blionas A, Giakoumettis D, Klonou A, Neromyliotis E, Karydakis P, Themistocleous MS. Paediatric gliomas: diagnosis, molecular biology and management. Ann Transl Med. 2018;6(12):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu JB, Gross CP, Wilson LD, Smith BD. NCI SEER public-use data: applications and limitations in oncology research. Oncology. 2009;23(3):288–295. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the Surveillance Epidemiology and End Results (SEER) Program. Restrictions apply to the availability of these data. Data are available with permission and approval of the SEER Program.


