Abstract
Introduction
Syringe services programs (SSPs) are evidence-based HIV prevention programs for people who inject drugs. However, not all SSPs operate evidence-based syringe distribution models, such as needs-based distribution. This study aims to provide preliminary evidence from the IDEA SSP on changes in injection risk behaviors over time, and to examine factors, including syringe coverage, associated with injection risk behavior trajectories over time under a one-for-one syringe distribution model.
Methods
We used a prospective observational study design to generate a cohort of SSP clients who completed three behavioral assessments at SSP service visits between December 2016 and January 2020 (N=115). The study used generalized estimating equations (GEE) to examine the relationship between covariate measures and the primary outcomes. The primary outcomes were 1) sharing of any injection equipment (e.g. syringes, needles, cookers, cottons) in the previous 30 days (yes/no) and 2) reusing of needles/syringes in the previous 30 days (yes/no).
Results
Men were more likely to report reusing syringes (aRR=1.15, 95% CI: 1.01–1.37) and those who reported injecting in public were less likely to report reusing syringes (aRR=0.90, 95% CI: 0.82–0.99). HCV-positive clients had a 62% reduction in sharing injection equipment and those who reported public injection had a 62% increase in sharing injection equipment over time. Most importantly, increasing syringe coverage was associated with a decrease in both sharing injection equipment (aRR=0.42, 95% CI: 0.25–0.72) and reusing syringes (aRR=0.79, 95% CI: 0.66–0.95).
Conclusion
This study provides preliminary evidence of reductions in injection-related risk behaviors from the IDEA SSP and highlights potential high priority groups, such as people experiencing homelessness, that may need additional intervention. In addition, improving syringe coverage among SSP clients may be an important factor in reducing behaviors that place individuals at risk for contracting HIV and HCV.
1. Introduction
Globally, there are an estimated 15 million people who inject drugs (PWID) (Degenhardt et al., 2017). Of those, about 1.6 million PWID (13%) are living with HIV and nearly 6 million PWID (50%) are living with hepatitis C virus (HCV; UN World Drug Report, 2017). PWID are at increased risk for acquiring these viral blood borne infections as well as bacterial infections, such as infective endocarditis, due to a variety of risk factors, including injection and sexual risk behaviors (Broz et al., 2014; Edeza et al., 2020; Neaigus et al., 2013). High-risk injection behaviors include reusing syringes, sharing needles/syringes and drug preparation equipment, and using nonsterile injection supplies; and high risk sexual behavior includes multiple sexual partners, transactional sex, condomless sex, and condomless anal sex (Bogart et al., 2005). Due to lack of comprehensive implementation of harm reduction services in the United States, PWID often have limited access to life-saving resources, including sterile injection equipment.
In 2018, approximately 10% of the 37,968 new HIV cases diagnosed in the United States were attributed to PWID (CDC, 2019a). In addition, injection drug use remains the leading risk factor for HCV transmission in the United States, with a four-fold increase in acute HCV infection between 2010 and 2016 (Zibbell et al., 2018). Moreover, PWID living with HIV are six times more likely to be co-infected with HCV than their HIV negative counterparts (WHO, 2017). In total, 60% of PWID are living with at least one type of blood borne infection (Nelson et al., 2011). The Centers for Disease Control and Prevention’s National HIV Behavioral Surveillance (NHBS) system reports that of 9,425 PWID, 70% had vaginal sex without a condom and 30% shared syringes (Spiller, Broz, Wejnert, Nerlander, & Paz-Bailey, 2015), suggesting both sexual and injection risk for HIV and HCV among this population. In addition, data from the Medical Monitoring Project reports that about 10% of PWID living with HIV report sharing injection supplies (Dasgupta et al., 2019).
Within injection risk behaviors, sharing syringes has been shown to be a significant risk factor for both HIV and HCV infection (Des Jarlais, Kerr, Carrieri, Feelemyer, & Arasteh, 2016; Zibbell et al., 2018). However, syringes are only part of the equipment needed for injection. Additional materials, such as cookers and cottons, are essential supplies during the drug preparation process. Research has shown that sharing injection drug preparation equipment is a strong risk factor for HIV and HCV infection (Ball et al., 2019; Holly Hagan et al., 2001), suggesting that most equipment used for drug preparation and injection holds potential risk of HIV/HCV transmission. In addition to sharing of injection equipment, research has shown reusing syringes to be a risk factor for injection-related infections (IRI) among PWID (Colledge et al., 2020; Larney, Peacock, Mathers, Hickman, & Degenhardt, 2017), commonly referred to as bacterial infections at injecting sites, such as skin and soft tissue infections (SSTIs). With high prevalence and economic burden of these infections (Coye et al., 2020; Larney et al., 2017), reducing syringe reuse among this population remains imperative for public health.
Syringe services programs (SSPs) are part of a well-established, evidence-based global public health initiative to reduce the spread of blood borne pathogens like HIV and HCV by providing PWID with sterile syringes and injection supplies. SSPs often also provide HIV/HCV testing (Spielberg et al., 2003), HIV treatment (Altice, Springer, Buitrago, Hunt, & Friedland, 2003), HCV treatment (Schulkind et al., 2019), condoms (Bogart et al., 2005), linkage to substance use treatment (Strathdee et al., 1999; Strathdee et al., 2006), onsite medications for opioid use disorder (MOUD) (Bachhuber et al., 2018), overdose prevention through naloxone distribution (Ashford, Curtis, & Brown, 2018), and medical services (e.g. wound care, primary care) (Wang, 2017). Research has proven SSPs to be effective in reducing the spread of HIV among PWID (R. N. Bluthenthal, Kral, Erringer, & Edlin, 1998; Palmateer et al., 2014), based on their effectiveness in reducing injection risk behaviors, such as syringe sharing and reuse (Dezheng Huo & Ouellet, 2007; Kåberg et al., 2020). Previous studies of PWID recruited in diverse settings have examined injection and sexual risk behaviors and suggest that (Abdul-Quader et al., 2013; Dickson-Gomez et al., 2020; Gibson, Flynn, & Perales, 2001; H. Hagan & Thiede, 2000; D. Huo & Ouellet, 2009; Mateu-Gelabert et al., 2020; Mir, Akhtar, Zhang, Thomas, & Shao, 2018; Wejnert et al., 2016) additional intervention beyond the provision of new injection equipment might be needed (Strathdee et al., 2013). In addition, few studies have examined the impact of syringe coverage (i.e. provision of one new unused syringe per injection) on injection-related risk, and have utilized cross-sectional designs that do not capture changes over time (Ricky N Bluthenthal, Anderson, Flynn, & Kral, 2007; Noroozi et al., 2015).
While SSPs are not new, implementation of these programs has lagged in the southern United States, which has a disproportionately high burden of HIV infection (S. Reif, Safley, McAllaster, Wilson, & Whetten, 2017; S. S. Reif et al., 2014). In 2016, the Florida Legislature passed the Infectious Disease Elimination Act (IDEA) that approved the implementation of a pilot SSP at the University of Miami in Miami-Dade County—a jurisdiction with the highest incidence of HIV infection in the United States (CDC, 2019b). This study aims to provide preliminary evidence from the IDEA SSP on injection risk behaviors over time, specifically sharing injection equipment and reusing syringes, and to examine factors associated with injection risk behavior trajectories over time.
2. Methods
2.1. Ethics statement
The Institutional Review Board of the University of Miami determined this study was not human subject research (IRB #20200799), due to the use of anonymous program data as part of routine pilot program evaluation.
2.2. Study setting
The State of Florida established the IDEA SSP in December 2016 as the first legal SSP in the state, and authorized it as a five-year pilot program. It operates primarily from a fixed site but also has a mobile outreach unit. Participants are provided with new syringes and injection equipment through a statutorily restricted one-for-one exchange, brief risk reduction and educational material on proper injection practices, as well as naloxone and an array of wraparound services. The SSP primarily serves clients in Miami-Dade County.
2.3. Study design
We used a retrospective observational study design to generate a cohort of SSP clients who completed three behavioral assessments at SSP service visits between December 2016 and January 2020 (N=115). At initial enrollment into the program, participants received rapid HIV and HCV testing, along with a 15-minute face-to-face behavioral interview that SSP staff performed in a private location. This baseline assessment included collecting sociodemographics, and information on injection-related risk behaviors, drug use, and sexual risk. After baseline enrollment, the study collected minimal data at each exchange visit, including number of syringes disposed and number of syringes distributed. In addition, the study asked participants to complete follow-up behavioral assessments, in addition to HIV and HCV testing, on a quarterly basis. HIV/HCV screening took place in an opt-out testing framework per SSP protocol (Bartholomew, Tookes, Serota, et al., 2020). However, these assessments were not mandatory for receiving services at the SSP. All biological testing and behavioral assessments are collected as routine care (i.e. programmatic data). The study collected outcome and covariate measures, including HIV and HCV status, at all three timepoints. The study collected and managed all data using REDCap electronic data capture tools hosted at the University of Miami Department of Public Health Sciences (Harris et al., 2009). Participants at the IDEA SSP have a unique identifier that does not contain any protected health information, and the study used this identifier to track patients longitudinally for evaluation of the pilot program.
2.4. Primary outcome(s)
The primary outcomes of this current analysis were 1) sharing of any injection equipment (e.g. syringes, needles, cookers, cottons) in the previous 30 days (yes/no) and 2) reusing of needles/syringes in the previous 30 days (yes/no).
2.5. Measures
Sociodemographic measures included age (categorized as 18–35, 36–49, 50 and older), biological sex (male vs. female), race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Hispanic), and housing status (currently experiencing homelessness/in stable housing). Participants reported which substances they had injected in the previous 30 days (heroin, prescription opioids, cocaine, methamphetamine, crack-cocaine, heroin/cocaine co-injection, and fentanyl); frequency of injection per day in the previous 30 days (dichotomized to >=5/<5); and public (i.e. street, park, sidewalk) injection in the previous 30 days. In addition, the study calculated a syringe coverage estimate for each participant, with 100% representing one new syringe for every injection between assessment timepoints; this calculation served as a proxy for sterile injection equipment beyond syringes (e.g., cookers, cottons, sterile water) due to the concurrent administration of these supplies in harm reduction packs with syringes. The methodology on calculating syringe coverage has been previously published (Bartholomew, Tookes, Bullock, et al., 2020).
2.6. Statistical analysis
We described characteristics of the cohort, stratified by assessment timepoint, using frequencies and percentages for categorical variables and medians and interquartile range (IQR) for continuous variables. We compared our longitudinal cohort to the overall SSP cohort using Pearson’s chi-squared tests for categorical values and Mann-Whitney tests to compare medians. We used generalized estimating equations (GEE) models to account for the individual-level correlation created by repeated measures on individuals. The GEE method is a robust and commonly used methodology that can assess the time trend in longitudinal repeated measurements, addressing random missing, and misspecification of the true correlation structure (SAS & Procedures, 2019). We used multivariable Poisson regression models, adjusted for demographics and substance use, to assess the relationship between syringe coverage and our outcome measures to get more accurate estimates of relative risk than was possible with logistic regression (Knol, Le Cessie, Algra, Vandenbroucke, & Groenwold, 2012). We calculated robust standard errors using an unstructured working correlation matrix. Following a growth curve specification, we included time as a continuous variable to examine linear time trajectories, and we estimated both outcomes in separate models. In addition, final models presented included time by HCV status and time by public injection terms to examine differences in the trajectories of sharing injection equipment and reusing syringes among these two groups. The study reported adjusted relative risk (aRR) and 95% confidence intervals. Study staff conducted all regression analyses using PROC GENMOD in SAS 9.4 (SAS Institute Inc.) and significance was set at an alpha of 0.05.
3. Results
The overall sample included in this analysis were 115 SSP participants that provided baseline and two follow-up timepoint assessments at the program between December 2016 and January 2020. We provide a comparison between this cohort and those who only provided baseline data in Appendix A. The average time between each study assessments was 6 months. Descriptive statistics of the sample across timepoints can be found in Table 1. The median age of the sample was 38 (IQR=32–47). The majority of the participants were male (76.5%), non-Hispanic White (50.4%) and reported experiencing homelessness (58.3%) at baseline. The most common drug injected across timepoints was heroin (84.4%, 84.8%, 66.4%); however, participants reported increases in fentanyl injection across timepoints (14.8%, 25.0%, 41.4%). In addition, syringe coverage increased over time with a median syringe coverage of 39% (IQR=9%-83%) at last follow-up assessment. Baseline prevalence of HIV and HCV infection were 9.7% and 45.1%, respectively.
Table 1.
Descriptive characteristics of IDEA SSP clients at baseline and follow-up timepoints (N=115).
| Characteristic | Baseline (n,%) | 1st Follow-up (n,%) | 2nd Follow-up (n,%) |
|---|---|---|---|
| Age (median, IQR) | 38 (32–47) | --- | --- |
| Biological Sex | |||
| Male | 88 (76.5) | --- | --- |
| Female | 27 (23.5) | --- | --- |
| Race/Ethnicity | |||
| Non-Hispanic White | 57 (50.4) | --- | --- |
| Non-Hispanic Black | 5 (4.4) | --- | --- |
| Hispanic | 51 (45.1) | --- | --- |
| Housing Status | |||
| Currently experiencing homelessness | 56 (58.3) | 63 (56.3) | 59 (57.3) |
| Drugs Injected | |||
| Heroin | 97 (84.4) | 95 (84.8) | 69 (66.4) |
| Cocaine | 30 (26.1) | 31 (27.7) | 41 (39.4) |
| Methamphetamine | 10 (8.7) | 8 (7.1) | 10 (9.6) |
| Crack-cocaine | 11 (9.6) | 19 (17.0) | 20 (19.2) |
| Speedball | 25 (21.7) | 34 (30.4) | 34 (32.7) |
| Fentanyl | 17 (14.8) | 28 (25.0) | 43 (41.4) |
| HIV-positive | 11 (9.7) | 18 (17.1) | 18 (17.1) |
| HCV-positive | 50 (45.1) | 60 (58.2) | 64 (62.1) |
| Average number of injections per day | |||
| <5 | 64 (57.7) | 58 (53.2) | 47 (48.0) |
| ≥5 | 47 (42.3) | 51 (46.8) | 51 (52.0) |
| Injection in public places (e.g. streets) | |||
| Yes | 64 (55.7) | 71 (63.4) | 57 (54.8) |
| No | 51 (44.3) | 41 (36.6) | 47 (45.2) |
| Syringe coverage (median, IQR) | 0 (0–0) | 0.35 (0.09–0.67) | 0.39 (0.09–0.83) |
| Share injection equipment | |||
| Yes | 51 (47.2) | 27 (24.8) | 22 (23.9) |
| No | 57 (52.8) | 82 (75.3) | 70 (76.1) |
| Reuse syringes | |||
| Yes | 93 (94.9) | 89 (80.9) | 76 (78.4) |
| No | 5 (5.10) | 21 (19.1) | 21 (21.6) |
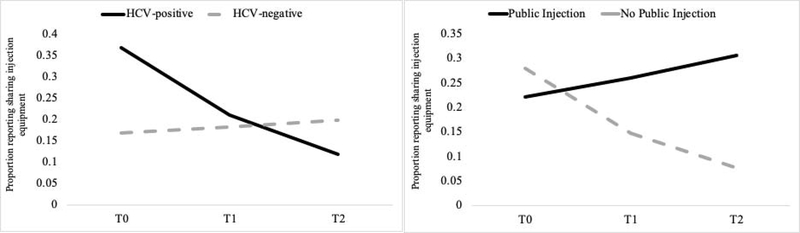
After adjusting for demographics and substance use in the regression models, HCV-positive clients were more likely to report sharing injection equipment (aRR=2.18, 95% CI: 1.40–3.38); however, there was a significant HCV by time interaction, suggesting a significant decreasing trend in sharing injection equipment over time for those who were HCV-positive (aRR=0.38, 95% CI: 0.20–0.71). In addition, there was a significant time by public injection interaction, suggesting a significant increase in syringe sharing over time for those who reported injecting in public locations (aRR=1.62, 95% CI: 1.13–2.33). Figure 1 shows the trajectories of sharing injection equipment over time for HCV-positive clients and those reporting public injection.
Figure 1.
Sharing injection equipment trajectories by HCV status and reporting public injection.
In the multivariable analysis examining reuse of syringes, after adjusting for age, biological sex, and substance use, men were more likely to report reusing syringes (aRR=1.15, 95% CI: 1.01–1.37) and those who reported injecting in public were less likely to report reusing syringes (aRR=0.90, 95% CI: 0.82–0.99). Most importantly, increasing syringe coverage was associated with a decrease in both sharing injection equipment (aRR=0.42, 95% CI: 0.25–0.72) and reusing syringes (aRR=0.79, 95% CI: 0.66–0.95) (Table 2). However, when we examined sharing syringes (only) separately, syringe coverage was nonsignificant in the adjusted model (aRR=0.69, 95% CI: 0.37, 1.29). The proportion of sharing injection equipment and reusing syringes at last follow up was 23.9% and 78.4%, respectively.
Table 2.
Adjusted relative risk (aRR) from multivariable Poisson regression models predicting injection-related behaviors among SSP clients (N=115).
| Characteristic | Sharing Injection Equipment aRR (95% CI) | Reusing Syringes aRR (95% CI) |
|---|---|---|
| Age | ||
| 18–35 | 1.61 (0.85–3.08) | 1.16 (0.98–1.37) |
| 36–49 | 1.63 (0.86–3.10) | 1.08 (0.91–1.28) |
| 50 and older | Ref | Ref |
| Biological Sex | ||
| Male | 1.32 (0.93, 2.16) | 1.15 (1.01, 1.37) |
| Female | Ref | Ref |
| Substance Use | ||
| Heroin | 1.70 (0.95, 3.03) | 1.06 (0.93, 1.20) |
| Speedball | 1.03 (0.73, 1.45) | 1.04 (0.93, 1.17) |
| Fentanyl | 1.31 (0.84, 2.05) | 1.07 (0.95, 1.20) |
| Time | 0.72 (0.40, 1.31) | 0.91 (0.81, 1.03) |
| Injection in Public | 0.79 (0.54, 1.15) | 0.90 (0.82, 0.99) |
| HIV-positive (baseline) | 1.09 (0.64, 1.83) | 0.99 (0.85, 1.16) |
| HCV-positive (baseline) | 2.18 (1.40, 3.38) | 1.04 (0.93, 1.17) |
| Syringe Coverage | 0.42 (0.25, 0.72) | 0.79 (0.66, 0.95) |
| Time if HCV-positive | 0.38 (0.20, 0.71) | 0.88 (0.79, 0.99) |
| Time if Public Injection | 1.62 (1.13, 2.33) | 1.01 (0.89, 1.14) |
Note. Bolded p-value represent significance at P <0.05
4. Discussion
This study extends previous research on the role that SSPs play in reducing injection risk behavior among PWID who utilize SSP services, and it provides potential subgroups for targeted prevention efforts. However, 78.4% of SSP clients reported reusing syringes at one-year follow-up, suggesting that providing sterile injection equipment may not be sufficient to reduce reuse of syringes. In the adjusted model, those who tested HCV-positive showed a 62% reduction in sharing injection equipment compared to their HCV-negative counterparts. Previous research has demonstrated that PWID who become aware of their HCV-positive status change their drug use behavior (Bruneau et al., 2014). In addition, those who reported injecting in a public location saw a significant increasing trend in sharing injection equipment, suggesting that additional intervention (e.g., housing) and service delivery may be needed to reduce injection risk behaviors among this subgroup. Previous research has demonstrated the link between homelessness and high-risk injection behaviors (Hunter et al., 2018; Marshall, Kerr, Qi, Montaner, & Wood, 2010; Small, Rhodes, Wood, & Kerr, 2007; Trayner et al., 2019).
In both the adjusted models examining sharing injection equipment and reusing syringes, increasing syringe coverage was associated with a 58% reduction in sharing injection equipment and 21% reduction in reusing syringes. The IDEA SSP follows a restrictive one-for-one syringe exchange policy, mandated by state law. Previous studies have demonstrated an association between needs-based syringe distribution policies and reduced syringe reuse and HIV risk (Ricky N Bluthenthal, Anderson, et al., 2007; Ricky N Bluthenthal et al., 2004; Ricky N Bluthenthal, Ridgeway, et al., 2007; Kral, Anderson, Flynn, & Bluthenthal, 2004). Among this sample, the median syringe coverage at the last follow-up timepoint was only 39% (IQR=9%-83%), well below the threshold of sufficient coverage (≥100%) needed to use a new syringe for each injection. Insufficient syringe coverage (<100%) has been found to be positively associated with syringe reuse (O’Keefe et al., 2018). This finding of inadequate coverage among SSP participants corroborates previous research from the IDEA SSP (Bartholomew, Tookes, Bullock, et al., 2020) and expands on the critical role that increasing syringe coverage plays in reducing risk. With the expansion of SSPs in Florida, policy action must be taken at the state level to reform the state’s restrictive one-for-one exchange to an evidence-based syringe distribution policy to further reduce injection-related HIV and HCV risk.
4.1. Limitations
There are several limitations that must be noted when interpreting the results. First, participants self-reported behaviors longitudinally and may be subject to recall bias. However, in this case, the study collected data anonymously and trusted SSP staff conducted surveys in confidential settings, limiting potential bias in our study. Second, these data reflect SSP clients who had high utilization of services and retention. To date, more than 1,300 PWID have enrolled and provided baseline data at the SSP; however, unfortunately, more than 30% of clients never return after the initial enrollment to engage in the SSP services. The cohort in this study may differ from those no longer using the program or declining to participate in longitudinal assessments, leading to potential bias in these results and lack of generalizability to other populations of PWID. However, compared to those who only provided baseline data, this cohort reported higher rates of high-risk injection practices (e.g., sharing injection equipment, reusing syringes) and homelessness, presenting a higher-risk group of participants (Appendix A). Third, syringe coverage was a direct estimate of syringes and did not capture other injection equipment (e.g., cookers, cottons). However, due to the SSP distribution of syringes with additional injection equipment as harm reduction packs, we believe that syringe coverage serves as a good proxy for injection equipment. Fourth, this is an observational study and lacks a control condition for comparison. Keeping these limitations in mind, our study suggests that clients using the IDEA SSP program have seen reductions in high-risk injection practices when using our services, and that syringe coverage may play a key role in achieving those benefits.
5. Conclusion
This study provides preliminary evidence of reductions in injection-related risk behaviors from the IDEA SSP, the first legal SSP in Florida, and highlights potential high priority groups such as people experiencing homelessness that may need additional intervention. In addition, improving syringe coverage among SSP clients may be an important factor in reducing behaviors that place individuals at risk for contracting HIV and HCV. With more states authorizing SSPs in the U.S. South, SSPs utilizing evidence-based syringe distribution policies must remain an important component of a comprehensive strategy to prevent HIV and other viral infections among PWID.
Highlights.
Implementation of an SSP decreased injection equipment sharing and reuse of syringes
HCV-positive clients experienced significant reductions in injection risk over time
Increasing syringe coverage significantly decreased injection risk, suggested a needs-based distribution model over one-for-one exchange.
6. Funding
This project was funded by the National Cancer Institute (P30CA240139), National Institutes on Drug Abuse (R01DA045713, UG1DA13720) and National Institute on Mental Health (P30MH116867). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A.
Baseline characteristics of all clients enrolled in the syringe services program (N=1,059).
| Characteristic | Longitudinal cohort (n=115) (n,%) |
Baseline-only cohort (n=945) (n,%) |
p-value |
|---|---|---|---|
| Age (median, IQR) | 38 (32–47) | 36 (30–44) | 0.053 |
| Biological Sex | 0.71 | ||
| Male | 88 (76.5) | 700 (75.0) | |
| Female | 27 (23.5) | 234 (25.0) | |
| Race/Ethnicity | 0.46 | ||
| Non-Hispanic White | 57 (50.4) | 504 (55.5) | |
| Non-Hispanic Black | 5 (4.4) | 49 (5.4) | |
| Hispanic | 51 (45.1) | 356 (39.2) | |
| Housing Status | <0.01 | ||
| Currently experiencing homelessness | 56 (58.3) | 311 (34.3) | |
| Drugs Injected | |||
| Heroin | 97 (84.4) | 689 (72.5) | <0.01 |
| Cocaine | 30 (26.1) | 260 (27.5) | 0.79 |
| Methamphetamine | 10 (8.7) | 166 (17.6) | 0.02 |
| Crack-cocaine | 11 (9.6) | 80 (8.5) | 0.67 |
| Speedball | 25 (21.7) | 164 (17.4) | 0.23 |
| Fentanyl | 17 (14.8) | 160 (16.9) | 0.59 |
| HIV-positive | 11 (9.7) | 91 (9.8) | 0.76 |
| HCV-positive | 50 (45.1) | 386 (42.3) | 0.53 |
| Average number of injections per day | <0.01 | ||
| <5 | 64 (57.7) | 342 (39.5) | |
| ≥5 | 47 (42.3) | 523 (60.5) | |
| Injection in public places (e.g. streets) | <0.01 | ||
| Yes | 64 (55.7) | 329 (34.8) | |
| No | 51 (44.3) | 616 (65.2) | |
| Share injection equipment | <0.01 | ||
| Yes | 51 (47.2) | 282 (29.8) | |
| No | 57 (52.8) | 663 (70.2) | |
| Reuse syringes | <0.01 | ||
| Yes | 93 (94.9) | 600 (67.7) | |
| No | 5 (5.10) | 286 (32.3) | |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdul-Quader AS, Feelemyer J, Modi S, Stein ES, Briceno A, Semaan S, … Des Jarlais DC (2013). Effectiveness of structural-level needle/syringe programs to reduce HCV and HIV infection among people who inject drugs: a systematic review. AIDS Behav, 17(9), 2878–2892. doi: 10.1007/s10461-013-0593-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altice FL, Springer S, Buitrago M, Hunt DP, & Friedland GH (2003). Pilot study to enhance HIV care using needle exchange-based health services for out-of-treatment injecting drug users. Journal of Urban Health, 80(3), 416–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford RD, Curtis B, & Brown AM (2018). Peer-delivered harm reduction and recovery support services: initial evaluation from a hybrid recovery community drop-in center and syringe exchange program. Harm reduction journal, 15(1), 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachhuber MA, Thompson C, Prybylowski A, Benitez J, Mazzella S, & Barclay D (2018). Description and outcomes of a buprenorphine maintenance treatment program integrated within Prevention Point Philadelphia, an urban syringe exchange program. Substance abuse, 39(2), 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball LJ, Puka K, Speechley M, Wong R, Hallam B, Wiener JC, … Silverman MS (2019). Sharing of Injection Drug Preparation Equipment Is Associated With HIV Infection: A Cross-sectional Study. JAIDS Journal of Acquired Immune Deficiency Syndromes, 81(4), e99–e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew TS, Tookes HE, Bullock C, Onugha J, Forrest DW, & Feaster DJ (2020). Examining risk behavior and syringe coverage among people who inject drugs accessing a syringe services program: A latent class analysis. International Journal of Drug Policy, 78, 102716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholomew TS, Tookes HE, Serota DP, Behrends CN, Forrest DW, & Feaster DJ (2020). Impact of routine opt-out HIV/HCV screening on testing uptake at a syringe services program: An interrupted time series analysis. International Journal of Drug Policy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthenthal RN, Anderson R, Flynn NM, & Kral AH (2007). Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug and Alcohol Dependence, 89(2–3), 214–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluthenthal RN, Kral AH, Erringer EA, & Edlin BR (1998). Use of an illegal syringe exchange and injection-related risk behaviors among street-recruited injection drug users in Oakland, California, 1992 to 1995. J Acquir Immune Defic Syndr Hum Retrovirol, 18(5), 505–511. doi: 10.1097/00042560-199808150-00013 [DOI] [PubMed] [Google Scholar]
- Bluthenthal RN, Malik MR, Grau LE, Singer M, Marshall P, Heimer R, & Team, D. o. B. t. S. E. S. (2004). Sterile syringe access conditions and variations in HIV risk among drug injectors in three cities. Addiction, 99(9), 1136–1146. [DOI] [PubMed] [Google Scholar]
- Bluthenthal RN, Ridgeway G, Schell T, Anderson R, Flynn NM, & Kral AH (2007). Examination of the association between syringe exchange program (SEP) dispensation policy and SEP client-level syringe coverage among injection drug users. Addiction, 102(4), 638–646. [DOI] [PubMed] [Google Scholar]
- Bogart LM, Kral AH, Scott A, Anderson R, Flynn N, Gilbert ML, & Bluthenthal RN (2005). Sexual risk among injection drug users recruited from syringe exchange programs in California. Sex Transm Dis, 32(1), 27–34. doi: 10.1097/01.olq.0000148294.83012.d0 [DOI] [PubMed] [Google Scholar]
- Broz D, Pham H, Spiller M, Wejnert C, Le B, Neaigus A, & Paz-Bailey G (2014). Prevalence of HIV infection and risk behaviors among younger and older injecting drug users in the United States, 2009. AIDS Behav, 18 Suppl 3, 284–296. doi: 10.1007/s10461-013-0660-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneau J, Zang G, Abrahamowicz M, Jutras-Aswad D, Daniel M, & Roy É (2014). Sustained drug use changes after hepatitis C screening and counseling among recently infected persons who inject drugs: a longitudinal study. Clinical Infectious Diseases, 58(6), 755–761. [DOI] [PubMed] [Google Scholar]
- CDC. (2019a). HIV Surveillance Report, 2018.
- CDC. (2019b). HIV Surveillance Report, 2018. Retrieved from http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
- Colledge S, Larney S, Bruno R, Gibbs D, Degenhardt L, Yuen WS, … Peacock A (2020). Profile and correlates of injecting-related injuries and diseases among people who inject drugs in Australia. Drug and Alcohol Dependence, 108267. [DOI] [PubMed] [Google Scholar]
- Coye AE, Bornstein KJ, Bartholomew TS, Li H, Wong S, Janjua NZ, … St Onge JE (2020). Hospital Costs of Injection Drug Use in Florida. Clinical Infectious Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta S, Tie Y, Lemons A, Wu K, Burnett J, & Shouse RL (2019). Injection Practices and Sexual Behaviors Among Persons with Diagnosed HIV Infection Who Inject Drugs — United States, 2015–2017. In MMWR Morb Mortal Wkly Rep (Vol. 68, pp. 653–657). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Peacock A, Colledge S, Leung J, Grebely J, Vickerman P, … Dumchev K (2017). Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. The Lancet Global Health, 5(12), e1192–e1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Des Jarlais DC, Kerr T, Carrieri P, Feelemyer J, & Arasteh K (2016). HIV infection among persons who inject drugs: ending old epidemics and addressing new outbreaks. AIDS (London, England), 30(6), 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson-Gomez J, Twaibu W, Christenson E, Dan K, Anguzu R, Homedi E, & Mbona Tumwesigye N (2020). Injection and sexual risk among people who use or inject drugs in Kampala, Uganda: An exploratory qualitative study. PLoS One, 15(4), e0231969. doi: 10.1371/journal.pone.0231969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeza A, Bazzi A, Salhaney P, Biancarelli D, Childs E, Mimiaga MJ, … Biello K (2020). HIV Pre-exposure Prophylaxis for People Who Inject Drugs: The Context of Cooccurring Injection- and Sexual-Related HIV Risk in the U.S. Northeast. Subst Use Misuse, 55(4), 525–533. doi: 10.1080/10826084.2019.1673419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DR, Flynn NM, & Perales D (2001). Effectiveness of syringe exchange programs in reducing HIV risk behavior and HIV seroconversion among injecting drug users. Aids, 15(11), 1329–1341. doi: 10.1097/00002030-200107270-00002 [DOI] [PubMed] [Google Scholar]
- Hagan H, & Thiede H (2000). Changes in injection risk behavior associated with participation in the Seattle needle-exchange program. J Urban Health, 77(3), 369–382. doi: 10.1007/bf02386747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, & Alexander ER (2001). Sharing of drug preparation equipment as a risk factor for hepatitis C. American journal of public health, 91(1), 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics, 42(2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K, Park JN, Allen ST, Chaulk P, Frost T, Weir BW, & Sherman SG (2018). Safe and unsafe spaces: non-fatal overdose, arrest, and receptive syringe sharing among people who inject drugs in public and semi-public spaces in Baltimore City. International Journal of Drug Policy, 57, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo D, & Ouellet LJ (2007). Needle exchange and injection-related risk behaviors in Chicago: a longitudinal study. JAIDS Journal of Acquired Immune Deficiency Syndromes, 45(1), 108–114. [DOI] [PubMed] [Google Scholar]
- Huo D, & Ouellet LJ (2009). Needle Exchange and Sexual Risk Behaviors among a Cohort of Injection Drug Users in Chicago, Illinois. Sex Transm Dis, 36(1), 35–40. doi: 10.1097/OLQ.0b013e318186dee3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kåberg M, Karlsson N, Discacciati A, Widgren K, Weiland O, Ekström AM, & Hammarberg A (2020). Significant decrease in injection risk behaviours among participants in a needle exchange programme. Infectious Diseases, 52(5), 336–346. [DOI] [PubMed] [Google Scholar]
- Knol MJ, Le Cessie S, Algra A, Vandenbroucke JP, & Groenwold RH (2012). Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. Cmaj, 184(8), 895–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral AH, Anderson R, Flynn NM, & Bluthenthal RN (2004). Injection risk behaviors among clients of syringe exchange programs with different syringe dispensation policies. JAIDS Journal of Acquired Immune Deficiency Syndromes, 37(2), 1307–1312. [DOI] [PubMed] [Google Scholar]
- Larney S, Peacock A, Mathers BM, Hickman M, & Degenhardt L (2017). A systematic review of injecting-related injury and disease among people who inject drugs. Drug and Alcohol Dependence, 171, 39–49. [DOI] [PubMed] [Google Scholar]
- Marshall BD, Kerr T, Qi J, Montaner JS, & Wood E (2010). Public injecting and HIV risk behaviour among street-involved youth. Drug and alcohol dependence, 110(3), 254–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu-Gelabert P, Guarino H, Zibbell JE, Teubl J, Fong C, Goodbody E, … Friedman SR. (2020). Prescription opioid injection among young people who inject drugs in New York City: a mixed-methods description and associations with hepatitis C virus infection and overdose. Harm Reduct J, 17(1), 22. doi: 10.1186/s12954-020-00367-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mir MU, Akhtar F, Zhang M, Thomas NJ, & Shao H (2018). A Meta-analysis of the Association Between Needle Exchange Programs and HIV Seroconversion Among Injection Drug Users. Cureus, 10(9). doi: 10.7759/cureus.3328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaigus A, Reilly KH, Jenness SM, Hagan H, Wendel T, & Gelpi-Acosta C (2013). Dual HIV risk: receptive syringe sharing and unprotected sex among HIV-negative injection drug users in New York City. AIDS Behav, 17(7), 2501–2509. doi: 10.1007/s10461-013-0496-y [DOI] [PubMed] [Google Scholar]
- Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, & Degenhardt L (2011). Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet, 378(9791), 571–583. doi: 10.1016/s0140-6736(11)61097-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noroozi M, Mirzazadeh A, Noroozi A, Mehrabi Y, Hajebi A, Zamani S, … Soori H (2015). Client-level coverage of needle and syringe program and high-risk injection behaviors: a case study of people who inject drugs in Kermanshah, Iran. Addiction & health, 7(3-4), 164. [PMC free article] [PubMed] [Google Scholar]
- O’Keefe D, Aung SM, Pasricha N, Wun T, Linn SK, Lin N, … Dietze P (2018). Measuring individual-level needle and syringe coverage among people who inject drugs in Myanmar. International Journal of Drug Policy, 58, 22–30. [DOI] [PubMed] [Google Scholar]
- Palmateer NE, Taylor A, Goldberg DJ, Munro A, Aitken C, Shepherd SJ, … Hutchinson SJ (2014). Rapid Decline in HCV Incidence among People Who Inject Drugs Associated with National Scale-Up in Coverage of a Combination of Harm Reduction Interventions. In PLoS One (Vol. 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif S, Safley D, McAllaster C, Wilson E, & Whetten K (2017). State of HIV in the US Deep South. Journal of community health, 42(5), 844–853. [DOI] [PubMed] [Google Scholar]
- Reif SS, Whetten K, Wilson ER, McAllaster C, Pence BW, Legrand S, & Gong W (2014). HIV/AIDS in the Southern USA: a disproportionate epidemic. AIDS care, 26(3), 351–359. [DOI] [PubMed] [Google Scholar]
- SAS P, & Procedures R (2019). SAS/STAT 9.4 User’s Guide. Cary, NC: SAS Institute Inc. [Google Scholar]
- Schulkind J, Stephens B, Ahmad F, Johnston L, Hutchinson S, Thain D, … Dillon JF (2019). High response and re-infection rates among people who inject drugs treated for hepatitis C in a community needle and syringe programme. Journal of viral hepatitis, 26(5), 519–528. [DOI] [PubMed] [Google Scholar]
- Small W, Rhodes T, Wood E, & Kerr T (2007). Public injection settings in Vancouver: physical environment, social context and risk. International Journal of Drug Policy, 18(1), 27–36. [DOI] [PubMed] [Google Scholar]
- Spielberg F, Branson BM, Goldbaum GM, Lockhart D, Kurth A, Celum CL, … Wood RW (2003). Overcoming barriers to HIV testing: preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. JAIDS-HAGERSTOWN MD-, 32(3), 318–327. [DOI] [PubMed] [Google Scholar]
- Spiller MW, Broz D, Wejnert C, Nerlander L, & Paz-Bailey G (2015). HIV Infection and HIV-Associated Behaviors Among Persons Who Inject Drugs — 20 Cities, United States, 2012. In MMWR Morb Mortal Wkly Rep (Vol. 64, pp. 270–275). [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Abramovitz D, Lozada R, Martinez G, Rangel MG, Vera A, … Patterson TL (2013). Reductions in HIV/STI incidence and sharing of injection equipment among female sex workers who inject drugs: results from a randomized controlled trial. PloS one, 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Celentano DD, Shah N, Lyles C, Stambolis VA, Macalino G, … Vlahov, D. (1999). Needle-exchange attendance and health care utilization promote entry into detoxification. Journal of Urban Health, 76(4), 448–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathdee SA, Ricketts EP, Huettner S, Cornelius L, Bishai D, Havens JR, … Latkin CA (2006). Facilitating entry into drug treatment among injection drug users referred from a needle exchange program: Results from a community-based behavioral intervention trial. Drug and Alcohol Dependence, 83(3), 225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trayner KM, McAuley A, Palmateer NE, Goldberg DJ, Shepherd SJ, Gunson RN, … Hutchinson SJ (2019). Increased risk of HIV and other drug-related harms associated with injecting in public places among people who inject drugs in Scotland: a national bio-behavioural survey. The Lancet, 394, S91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CM (2017). Offering Community-Based Wound Care as Part of a Comprehensive Syringe Exchange Program. University of Hawai'i at Manoa. [Google Scholar]
- Wejnert C, Hess KL, Hall HI, Van Handel M, Hayes D, Fulton P Jr., … Valleroy LA (2016). Vital Signs: Trends in HIV Diagnoses, Risk Behaviors, and Prevention Among Persons Who Inject Drugs - United States. MMWR Morb Mortal Wkly Rep, 65(47), 1336–1342. doi: 10.15585/mmwr.mm6547e1 [DOI] [PubMed] [Google Scholar]
- WHO. (2017). Global hepatitis report 2017: World Health Organization. [Google Scholar]
- Zibbell JE, Asher AK, Patel RC, Kupronis B, Iqbal K, Ward JW, & Holtzman D (2018). Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. American journal of public health, 108(2), 175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]