Abstract
The tumor microenvironment (TME) is an ecosystem that contains various cell types, including cancer cells, immune cells, stromal cells, and many others. In the TME, cancer cells aggressively proliferate, evolve, transmigrate to the circulation system and other organs, and frequently communicate with adjacent immune cells to suppress local tumor immunity. It is essential to delineate this ecosystem’s complex cellular compositions and their dynamic intercellular interactions to understand cancer biology and tumor immunology and to benefit tumor immunotherapy. But technically, this is extremely challenging due to the high complexities of the TME. The rapid developments of single-cell techniques provide us powerful means to systemically profile the multiple omics status of the TME at a single-cell resolution, shedding light on the pathogenic mechanisms of cancers and dysfunctions of tumor immunity in an unprecedently resolution. Furthermore, more advanced techniques have been developed to simultaneously characterize multi-omics and even spatial information at the single-cell level, helping us reveal the phenotypes and functionalities of disease-specific cell populations more comprehensively. Meanwhile, the connections between single-cell data and clinical characteristics are also intensively interrogated to achieve better clinical diagnosis and prognosis. In this review, we summarize recent progress in single-cell techniques, discuss their technical advantages, limitations, and applications, particularly in tumor biology and immunology, aiming to promote the research of cancer pathogenesis, clinically relevant cancer diagnosis, prognosis, and immunotherapy design with the help of single-cell techniques.
Keywords: single-cell omics, immunotherapy, cancer, TCR (T cell receptor), biomarkers
Introduction
The tumor microenvironment (TME) is a complex ecosystem that consists of many different cell types, including tumor cells, immune cells, and many others. All these cells are tightly inter-associated and interact with each other. The heterogeneous milieu of TME induces various progression patterns of different cancers and leads to distinct treatment responses across different patients (1). Among that, the levels of T cell infiltration, the polarization of tumor-associated macrophages (TAM) can be varied, thereby affecting the prognosis of patients differently, the expression of PD-1 and PD-L1 in TME, the mutational landscapes, and the drug responses of malignant cells can also be distinct in different patients, relating to different efficacies of immune checkpoint blockade (ICB) therapies (2–4).
The previous genomic, transcriptomic, and proteomic cancer studies have helped develop multiple mutational- or molecular-target therapies and elevate treatment responses across different patients (5). However, the clinical benefits of these target-directed therapies are still limited. Only a small subset of patients is treatable, leading to emergent demand of using more precise methods to dissect characteristics of individual patients for developing better cancer treatments especially personalized tumor immunotherapy.
In this review, we introduce the state-of-art technological advances of single-cell omics and discuss corresponding computational methods for single-cell data analysis and their applications in cancer research. All of these further inspire and guide the design of and applications of single-cell techniques in basic and translational clinical cancer research.
The Development of Single-Cell Technologies
The development of methods in single-cell isolation, indexing, and sequencing allows in-depth profiling of the tumor milieu from different cellular scales with extremely high dimensions (6). Based on single omics, methods for integrated omics have also been developed for simultaneous detection of different omics, including genomic, transcriptomic, proteomic, and spatial information of single cells (7). Despite multiple challenges that remain to be addressed, these methods have been very powerful in uncovering the cellular basis of the heterogeneous tumor microenvironment and greatly expanded our understanding of cancers and tumor immunology in many aspects. In this session, we introduce the technical details of single-cell methods applied in understanding the tumor microenvironment ( Figure 1 ).
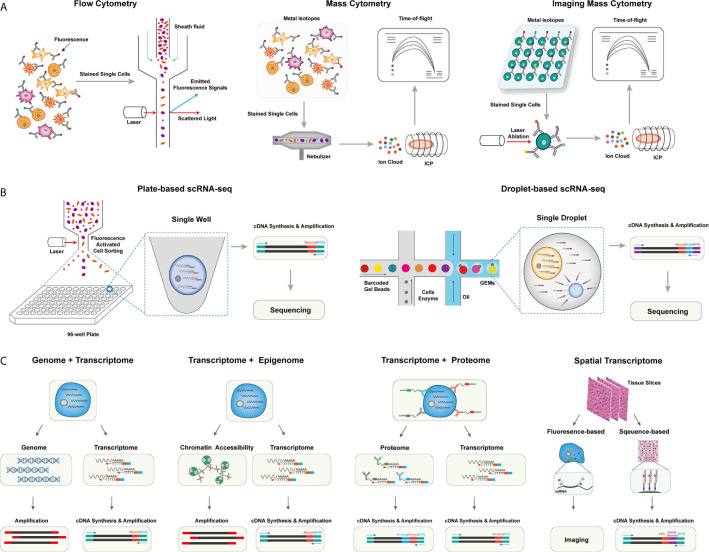
Figure 1.
The overview of single-cell omics techniques. (A) The overview of single-cell cytometry systems, including flow cytometry with fluorescence-labeled antibodies for single isolated cells (left), mass cytometry with metal isotope-conjugated antibodies isolated single cells (middle), and imaging mass cytometry with metal isotope-conjugated antibodies labeled on tissues (right). (B) The overview of two canonical scRNA-seq platforms, including plate-based scRNA-seq methods with sorted cells barcoded within each well (left), and droplet-based scRNA-seq method, single cells were barcoded within individual droplets (right). (C) The overview of single-cell multi-omics techniques, including library preparing for genomic, epigenomic, proteomic, and spatial indexing with transcriptomic of single cells simultaneously.
Mass Cytometry and Imaging Mass Cytometry
Flow cytometry, as a widely used single-cell labeling and sorting technique, has facilitated the understanding of cellular composition and diversity in various tissues (8), but its spectral overlap between nearby channels limits the number of detected markers and unable to unveil many functionally important cell subsets (9). To overcome this problem, mass cytometry, also named cytometry by time of flight mass spectrometry (CyTOF), was developed with a more specific channel signal (10, 11). CyTOF uses rare element isotopes to replace the commonly used fluorochrome to conjugate monoclonal antibodies (mAbs) in flow cytometry. These isotopes usually do not exist in cells, and the purity of rare element isotopes and their accurate detection by mass spectrometry significantly increase the detectable dimension of a single cell to over 100 markers theoretically, and due to the technical limits of isotope labeling onto mAbs, the marker number on single cells to date can only reach 45 (10–12). Meanwhile, CyTOF has already demonstrated its power and accuracy over flow cytometry in cell profiling when applied to analyze fresh and frozen PBMC or tumor tissues at the single-cell level (13). Unfortunately, CyTOF cannot be used for cell sorting, and its throughput is 25~50 times lower than flow cytometry due to extra time expense for isotope quantification (8).
Beyond profiling the homogenously stained single cells isolated from tissue samples, imaging CyTOF was developed to profile the cells’ spatial information in the target tissue (14). Similar to the multicolor immunofluorescence staining, imaging CyTOF can simultaneously detect over 30 types of rare element isotopes conjugated on antibodies to stain tissue sections. A high-resolution laser is used to ablate the target tissue section point by point, and the ionized elements were streamed into the ICP-MS for isotope measurement. Finally, a high-dimensional tissue imaging is reconstructed by integrating the subcellular spatial information of each point on the tissue sample (14).
Moreover, CyTOF can also be used in the quantification of epigenetic modification (e.g., phosphorylation, histone modification) (15), transcripts (16), and antigen-specific T cells (17) at the single-cell level by designated mAbs that target chromatin marks, ligation assay for RNA, and multiplexed peptide-major-histocompatibility-complex (pMHC)-tetramer staining for antigen-specific T cells, respectively, allowing an integrated inspection of cellular functionality in a multi-omics manner.
Single-Cell RNA Sequencing
Methods for profiling single-cell transcriptome have been developed and rapidly evolved to overcome limited markers detected on individual cells by CyTOF, improve the single-cell resolution of traditional bulk RNA sequencing (RNA-seq), and identify rare cell populations and their functional dynamics at the transcriptomic level (18). The first published single-cell RNA sequencing (scRNA-seq) method successfully detected 5,270 more genes in one blastomere compared to the microarray assay using hundreds of blastomeres, allowing the precise whole-transcriptome characterization at a single-cell level (19). And integrating the ‘cell-specific barcodes’ into the synthesized cDNA sequences (20, 21), the throughput of scRNA-seq improved from a few hundreds of cells to thousands of cells.
Multiple scRNA-seq or sc-nucleus RNA-seq protocols were developed to enhance the scale, the sensitivity, or the accuracy of single-cell transcriptome quantification (22). These methods can be categorized into plate-based or microfluidic-based platforms. For the plate-based platform, the representative method is Smart-seq, which is currently upgraded into a third-generation, Smart-seq3 (23). In Smart-seq3, a 5’ unique molecular identifier (UMI) is integrated into the full-length cDNA for counting transcripts, achieving the precise quantification of transcript isoforms. Other plate-based platforms, such as cell expression by linear amplification and sequencing (CEL-seq2) and massively parallel single-cell RNA sequencing (MARS-seq2), integrate the Fluidigm C1 system or liquid-handling robot to improve the data quality and reduce labor cost, respectively (24, 25). Applying a bead-based microfluidic system has dramatically pushed the field into a real high-throughput area for the microfluidic platform. In a single experiment, the microfluidic system can capture 3,000~10,000 droplets, each of which encapsulates a single-cell and a single-bead carrying specific DNA-barcoded primers (26–28). The transcripts in single droplets are then captured, reversely transcribed, and barcoded with cell barcodes and UMIs. These procedures well replace the single-well-based cell sorting and library construction steps in the plate-based platform and dramatically increase the detection number of single cells in each sample. Other strategies have also been used to profile the transcripts in single cells, such as the split-pool-based cell barcoding strategy (29–31) and the integration of beads with the microwell-based platform (32). Although there are still challenges in different aspects, such as cost, sequencing depth, and gene coverages, these scRNA-seq methods have enabled the profiling of single cells with more than thousands of genes per cell. The data dimension is significantly higher than the cytometry-based systems.
Single-Cell Multi-Omics Technologies
The interconnections and relations of genome, epigenome, transcriptome, and proteome determine the function of single cells, which requires a comprehensive understanding of the biology process across multi-omics simultaneously at the single-cell level (33). In the following session, we will focus on reviewing single-cell multi-omics technologies, that could simultaneously measure at least two of different omics including genomics, transcriptomics, epigenomics, proteomics, and spatial information at the single-cell level.
Yin et al. introduced the sci-L3-RNA/DNA co-assay to simultaneously measure the genomics and transcriptomics in single cells (34). In sci-L3-RNA-/DNA co-assay, single cellular DNA and mRNA were respectively barcoded by Tn5 transposon intersection and by poly-T primer, both of them carrying barcoding sequences and UMIs. Both libraries were prepared with three-level split-pool indexing and linear amplification for downstream analysis. Another strategy is to physically separate the nucleus and cytosol of a single cell and construct the library for each component individually. Following this strategy, direct nuclear tagmentation and RNA sequencing (DNTR-seq) (35) separately obtained the whole-genome sequencing and full-length cDNA sequencing from single cells with ultra-high resolutions. Besides directly obtaining the whole genome of single cells, the cDNA sequences from mRNA could also be used to detect the mutation status of single cells (36, 37), especially for identifying tumor-specific mutations across different tumor cell populations.
Open chromatin regions are also important functional characteristics for revealing cellular genomic regulations. With the assay development for transposase-accessible chromatin using sequencing (ATAC-seq), exploring open chromatin in single cells becomes possible. ATAC-seq enables fast and precise epigenomic profiling by integrating the sequencing adaptors into the accessible chromatin by prokaryotic Tn5 enzyme (38). Combining ATAC-seq with single-cell isolation and barcoding techniques enables access to open chromatin in single cells (39). Moreover, as both the transposed chromatin fragments and the synthesized cDNA fragments of cellular transcripts can be adapted into the same cell barcoding ID, Cao et al. and Chen et al. successfully detected chromatin accessibility and transcriptome simultaneously at the single-cell level (40, 41). Simultaneous high-throughput ATAC and RNA expression with sequencing (SHARE-seq) is another method to evaluate the relationship between chromatin accessibility and gene expression in single cells and identify the priming role of chrome accessibility in transcriptomic regulation, which is helpful to infer cell differentiation (42). Meanwhile, multiple in silico algorithms, such as model-based analyses of transcriptome and regulome (MAESTRO) and Signac (43, 44), have been correspondingly developed to integrated analyze scRNA-seq and scATAC-seq data in single cells.
The protein expression can directly reflect the functionality and biological states of cells. As a result, flow cytometry and CyTOF have been broadly used in biological researches for protein expression quantification despite their limited dimensions compared to scRNA-seq. To overcome this limitation, Stoeckius et al. came up with the idea of using specifically designed DNA sequences to label and barcode the protein-specific mAbs (45). The detection number of antibody-labeled proteins is significantly increased to more than 200 (46), which is five times more than the detection number in CyTOF. CITE-seq uses a poly-A tail in the antibodies-conjugated oligonucleotides to achieve compatibility with the mRNA capturing system (45). And in the commercial platform (e.g., Feature Barcoding by 10X Genomics), the barcoding strategy is further improved so that the transcriptomic and proteomic libraries are barcoded with poly-A capture sequences and antibody-specific capture sequences separately (47). Besides, Zhang et al. used DNA-barcoded pMHC tetramers to specifically label and sequence antigen-specific T cells (48). A similar strategy was also used to remove experimental and amplification bias by staining oligo-labeled surface proteins ubiquitously expressed on cells from different samples (49).
The in-situ cellular spatial information is essential to accurately capture the biological functions of cells in their physiological context. It is particularly important to investigate the spatial information in the tumor microenvironment (TME), such as tissue-specific T cell infiltration, the spatial distribution, and interaction of cellular ligands and receptors, and the distribution of malignant cells, to improve our understanding of tumorigenesis and tumor-specific immune escape in TME (50). The spatial transcriptome methods can be mainly classified into fluorescence or sequencing-based methods, which have also been comprehensively reviewed by Asp et al. (51). Based on the technique of fluorescence in situ hybridization (FISH) (52), seqFISH+ enables visualization transcripts at local sites and can image more than 10,000 genes at subcellular resolution with upgraded optical resolution and barcoding strategy (53). Despite the high spatial resolution, the applications of fluorescence-based platforms are usually hampered by the intensive experiment procedures and the design of the transcript probe. In contrast, the application of cellular barcoding strategies in scRNA-seq enables in-situ barcoding of local cells in tissues. The most challenging for this strategy is to demultiplex the physical locations with the detected barcoding sequences. In Slide-seq and high-definition spatial transcriptomics (HDST) assays (54–56), arrayed barcoding beads are used to capture spatial whole-transcriptomes, and the resolution of the reconstructed spatial map depends on the designed bead arrays. In another microfluidic-based method (57), the tissue slide was separately barcoded by parallel microfluidic channels within different directions, and the different combinations of barcodes can recover the spatial information.
Innovative Computational Methods for Single-Cell Analysis
Accompanied by the increased capability of generating high-dimensional and high-throughput single-cell data in one experiment, interpreting the biological functions of cells and functional alterations in disease status becomes even more challenging (58). Hie et al. summarized the typical computational workflow for single-cell RNA-seq data analysis, including data preprocessing, batch correction, clustering, and functional annotation of single cells (59). Among that, the methods for inferring cell lineage trajectories under different stimuli are broadly applied to understand cellular dynamics and interactions. Besides, with the development of single-cell multi-omics techniques, integrating multi-omics single-cell data is also computationally challenging (7).
Saelens et al. comprehensively benchmarked the performance of 45 trajectory inference methods (60), highlighting that the preset trajectory topology of computational methods can affect the inference results and that the performance of different methods can vary with different datasets. The most limitation of these methods, including Monocle3 (61), partition-based graph abstraction (PAGA) (62), and Slingshot (63), is that the cell trajectory estimation calculated by the cell-cell distance ignores the inherited cellular information. Instead, the innovative method RNA Velocity (64, 65) addresses this issue by quantifying the spliced and unspliced transcripts of single cells and connecting cells with similar transcripts splicing states. Another method, CytoTRACE, leverages the number of detected genes to reflect the developmental potential of single cells, providing a robust performance to delineate cellular trajectories (66). Furthermore, the DNA sequencing information, including T cell receptor sequencing, can also be used as cellular labels for inferring cellular dynamics and lineage tracing (67, 68).
The integrative analyses of single-cell multi-omics data consider the status of single cells from different scales of biological features and delineate cell types based on cell similarities in higher feature space, which are challenged by the different characteristics of single omic data and batch effects across multiple data samples. Ma et al. comprehensively summarized the data integration methods for analyzing single-cell multi-omics data (7). One strategy is to estimate the cellular distances within individual omics and then calculate the “weighted-nearest neighbor “ distance for integrated analysis of multiple-omics data (46). Another one exploits a modified statistic framework to identify low-dimensional variations across data modalities for data integration (69). In other methods, multi-view machine learning (70), canonical correlation (71), and deep generative model (72) have also been used for multi-omic single-cell data integration.
Application of Single-Cell Omics in Tumor Immunology
With the aid of single-cell methods, the heterogeneity of tumor cells and their interaction in the local microenvironment have been deeply and comprehensively interrogated. The single-cell data has been extensively used for identifying biomarkers for cancer diagnosis, prognosis prediction, and new treatable targets in designated clinical cohorts. The Human Tumor Atlas Network (HTAN) project (73) has put forward a framework of mapping tumor atlases in molecular, cellular, anatomical, and clinical fields, aiming to interrogate the single-cell data for clinical transitions thoroughly. In the following session, we mainly focus on applying different single-cell multi-omics techniques in establishing the cellular atlas of tumor ecosystem, T cell dynamics, and their interactions contributing to tumor diagnosis, treatment, and prognosis. The typical applications are correspondingly listed ( Figure 2 and Table 1 ).
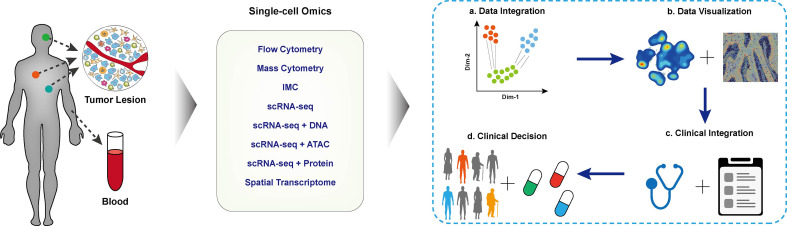
Figure 2.
Applications of single-cell techniques in clinical cancer research. The schematic diagram of cancer research with single-cell techniques, blood or tissue samples of the designated patient cohort was collected and performed single-cell profiling. The collected data were integrated for downstream analysis and visualization. With in-depth integration with clinical characteristics, biomarkers for clinical decisions, disease prognosis, and tumor immunotherapy.
Table 1.
Selected cancer research with Single-Cell omic technologies.
| Cancer type | Single-cell methods | Highlights | Ref |
|---|---|---|---|
| Early lung adenocarcinoma | CyTOF, scRNA-seq | Comparing the paired immune signatures across tumor lesion, normal lung tissue, and blood, Lavin et al. Identified the tumor lesion-specific immune regulations, especially the modifications of innate immune cells | (74) |
| Breast cancer | Imaging mass cytometry | The high-dimensional pathology images of breast cancers characterized the disease-related spatial resolved cellular signatures. | (77) |
| Hepatocellular carcinoma | scRNA-seq | In-depth integration of single-cell data with bioinformatic methods, Zhang et al. identified the migration of immune cells, especially the LAMP3+ dendritic cells, potentially contributing to lymphocyte activation. | (87) |
| Myeloproliferative neoplasms | scRNA-seq + genotyping | Integrating the cellular mutation genotypes and transcriptomic data, Nam et al. revealed the upregulation of NF-κB and IRE1-XBP1 pathways in mutated cells. And the modifications of mutations in transcriptomic outputs. | (82) |
| Mixed-phenotype acute leukemias | scRNA-seq + protein, scATAC-seq | By comparing the transcriptomic and epigenetic blood development maps between healthy and MPAL patients, Granja et al. uncovered the patient-specific regulatory networks, such as the RUNX1 regulation of CD69 in tumor patients. | (47) |
| Primary pancreatic tumors | scRNA-seq, spatial transcriptomics | With intersection analyses of scRNA-seq data and spatial transcriptomic data, Moncada et al. revealed the interactions of different cells in tumor microenvironments, especially the colocalization of inflammatory fibroblasts and cancer cells. | (86) |
| Basal or squamous cell carcinoma | scRNA-seq + TCR | Comparisons between the tissue TCR repertoires before and after immunotherapies, Yost et al. uncovered the new entered T cell clonotypes rather than the exhausted T cell clonotypes that may respond to immunotherapy. | (105) |
| Hepatocellular carcinoma | scRNA-seq | By comparing the immune landscape between primary and early-relapse HCC patients, Sun et al. indicated the innate-like CD8 T cells might contribute to an early relapse of HCC. | (119) |
| Pancreatic ductal adenocarcinoma | snRNA-seq, spatial transcriptomics | Comparisons of the PDAC samples before and after chemoradiotherapy, Hwang et al. revealed the basal rather than the classical phenotype of malignant cells might benefit the therapy efficiency with single-cell and spatial transcriptomic inspections. | (121) |
Dissecting Tumor Microenvironments at the Single-Cell Level
Taking advantage of high throughput and high dimensional proteomic single-cell analysis, CyTOF has been used to dissect the immune composition of TME in different types of tumors. In the study of early lung adenocarcinoma (74), Lavin et al. profiled the immune atlas in paired tumor lesions, normal lung tissues, and peripheral blood. They revealed a tumor-specific depletion of CD8+ T effector cells and the tumor-enriched macrophages with the expression of PPARγ potentially contributing to immune suppression in TME. This study provides potential immunotherapies for targeting macrophages in lung cancer. By comparing the immune atlas of clear cell renal cell carcinoma (ccRCC) and normal renal tissues (82), Chevrier et al. identified the polymorphic expressions of exhausted markers and CD38 on PD-1+ exhausted T cells in tumors and a special subset of CD38+ tumor-associated macrophages (TAM) highly associated with the immunosuppressed T cell subsets. Further integrating the tumor-infiltrating frequencies of immune cell subsets with clinical outcomes, they identified the abundance of several TAM subsets that can predict the progression-free survival of patients. Additionally, CyTOF and imaging CyTOF have also been combined with profiling the ecosystem of malignant cells and immune cells in breast cancer. Wagner et al. simultaneously compared the immune and malignant cell components of breast tumor, juxta-tumor, and mammoplasty tissue samples. The phenotypic abnormality of tumor cells and dynamics of immune cells suggests the tumor-immune combined phenotypes of breast tumor patients independent of the clinical grade and subtypes, suggesting the local interactions could be more critical for prognosis treatment efficacy (83). Using imaging CyTOF, Jackson et al. established the highly multiplexed molecular spatial maps of breast tumor microenvironments with different clinical subtypes and grades. Interrogating the single-cell pathology features, they further proposed that the subgroups of patients by pathology features in the tumor microenvironment could better predict patients’ overall survival and provide new strategies for clinical subtyping (75).
With higher dimension capability, scRNA-seq provides an opportunity to more broadly and systematically profile TME and its associated immune atlas with many more critical functional aspects (84). For example, with scRNA-seq, Azizi et al. identified the continuous cellular states of T cells and myeloid cells in breast cancers (85). They proposed that both TCR signals and environmental stimuli could module T cell functionality to combine TCR clonotypes with T cell phenotypes. scRNA-seq also enables more comprehensive lineage analyses to reflect the dynamic immune cell responses during tumorigenesis. With RNA Velocity analysis (64) and mitochondrial-based lineage tracing (86), Zhang et al. revealed that a subset of LAMP3+ dendritic cells could migrate from tumors to hepatic lymph nodes to trigger systematic adaptive immune responses (76).
Integrating the genotyping and single-cell transcriptomic data of myeloproliferative neoplasm cells, Nam et al. comprehensively delineated the contributions of CALR mutation in the differentiation of hematopoietic stem and progenitor cells (HSPCs). They also revealed that the CALR mutation more affected the cellular gene expressions at a later differentiation stage and further identified the mutation-specific activation of the IRE1-XBP1 pathway in HSPCs as a potential therapeutic target (77). With single-cell sequencing the genomics and transcriptomics of acute myeloid leukemia (AML) malignant cells, van Galen et al. identified six subsets of malignant AML cells across developmental hierarchies. They revealed the determination role of genotype in the compositions of AML cells in patients and further determined that the differentiated AML cells could suppress the function of T cells. The genotype-specific phenotype of AML cells and the immunosuppressive functionality of differentiated AML cells could further guide the genotype-specific immunotherapies in AML (87). Single-cell triple omics sequencing (scTrio-seq), a platform that simultaneously profiles genomic, epigenomic, and transcriptomic on individual cells, is able to delineate the more complex insights of the coordinated regulations of copy number variations, DNA methylation, and gene expressions in malignant cells of hepatocellular carcinomas and colorectal cancer (88, 89). Moreover, comparing the epigenomic regulatory networks of bone marrow and peripheral blood mononuclear cells between healthy and mixed-phenotype acute leukemia (MPAL) patients, Granja et al. uncovered the common regulation factors and revealed RUNX1 as an oncogene to upregulate CD69 in MPAL (47). Integrating scRNA-seq and spatial transcriptomic data in pancreatic ductal adenocarcinoma, Moncada et al. intersected the region-specific gene expression with cell type-specific gene expression. They revealed that the stress-response cancer cells were colocalized with IL-6 releasing inflammatory fibroblasts, supporting the IL-6 induced stress-response mechanism in cancers (78). Thus, the integration of single-cell multi-omics allows a more comprehensive exploration of cancer evolution, local cellular interactions, and immune regulations in the tumor microenvironment, strengthening our understanding of cancer pathogenesis and immune suppression (90, 91).
Evolution of Cancer Cells in Tumorigenesis and Drug Resistance
In-depth single-cell characterization of cancer cells in the TME, and their dynamic regulations in tumorigenesis, metastasis, and drug responses can uncover the heterogeneity of cancer cells and their causality with clinical outcomes (90, 92). The scRNA-seq profiling of diverse cancer cells in oligodendroglioma patients revealed a subset of undifferentiated malignant cells with stem cell phenotypes and proliferating potentials, suggesting the primary roles of cancer stem cells (CSCs) in cancer evolution (93). Integrating the genetically engineered mouse models (GEMMs) and scRNA-seq, Marjanovic et al. mimicked and profiled the progression of human lung adenoma and adenocarcinoma. They identified a subset of TIGIT+ cells with the high-plasticity cell state (HPCS) and annotated these cells as transitioning tumor cells that contributed to tumor progression and chemoresistance (94). Neftel et al. also characterized four malignant cell subsets of glioblastoma using scRNA-seq with specific molecular features, and that the cellular transitions demonstrated the plasticity of malignant cells across distinct malignant cell subsets with additional cell barcoding and lineage tracing (95). All of these findings highlighted the impact of high-resolution single-cell profiling in understanding tumorigenesis and the evolution of cancer cells.
Metastasis is the dominant cause of the deaths of cancer patients, and its process is stochastic and dynamic (96). scRNA-seq study in human metastatic lung adenocarcinoma (LUAD) revealed a subset of cancer cells with distinct differentiation trajectory and gene signature of aggressive cell movement, proliferation, and apoptosis. And the gene signature of this cancer cell subset is enriched in later and metastatic tumor tissues and associated with a worse prognosis (97). Meanwhile, the applications of scRNA-seq and Cas9-enabled high-resolution lineage tracing of the xenograft model of LUAD cell line delineated comprehensive disseminate routes of metastatic cancer cells. And combining the phenotypical inspection of scRNA-seq data, Quinn et al. uncovered the characteristics of cancer cells with different metastatic ability and quantified their specific transcriptomic regulation in modulating metastasis (98).
The drug resistance of cancer cells severely limits the efficacy of chemotherapy or molecularly targeted therapies, and the cellular states and responses during treatments can determine further disease progression (4). In breast cancer, the single-cell profiling of docetaxel-resistant MCF7 breast cancer cells revealed a subset of cells with a stem-like phenotype and identified LEF1 as the critical molecule regulator in drug resistance (99). In melanoma, an immune evasion-specific malignant cell program identified by scRNA-seq can predict the clinical responses of immune checkpoint inhibitors (ICIs). Targeting the signal activation of CDK4/6 in this program can repress the drug resistance program and enhance the ICI efficacy (100). Meanwhile, a multimodal method (Perturb-CITE-seq) was applied to characterize the mechanisms of resistance of ICIs. Integrating the simultaneously RNA and protein profiling with Cas9 genomic knockout screens, Frangieh et al. validated the known mechanisms of resistance to ICIs, and further revealed a novel CD58 related resistance mechanism. Specifically, they found that downregulating the expression of CD58 could induce the expression of PD-L1 on malignant cells and reduce the co-stimulatory signal of the CD58-CD2 axis on CD8+ T cells (101). Overall, the comprehensive interrogating of the cellular responses and drug resistances in malignant cells could uncover the new treatable targets and guide the combined therapies for cancer treatments.
T Cell Responses and TCR Repertoire in Tumor Immunity
T cells are essential adaptive immune cells that mediate tumor immunity. The promising immune checkpoint blockade (ICB) therapies mainly target T cells and recover T cell immunity through disrupting PD-1/PD-L1 and CTLA-4/CD80 or CD86 interactions or specifically activating tumor-antigen-specific T cell clones (102, 103). Unfortunately, only a small part of patients has beneficial responses with recovered anti-tumor T cell responses. Improving the ICB efficacy requires a more comprehensive understanding of dynamical T cell responses in patients during tumorigenesis and ICB treatments (104).
Platforms that integrate scRNA-seq data and scTCR-seq in individual T cells, such as Smart-seq3 and 10X Genomics single-cell immune profiling, enable a more precise delineation of immune responses and lineage tracking of T cells in tumorigenesis or under immunotherapy treatments (105). Smart-seq3, a representative of full-length sequencing platform, could read full-length CDR3 sequences of TCRαβ chains in single cells but with limited throughput (23). 10X single-cell immune profiling, a commercial droplet-based platform that integrates TCR enrichment procedures, enables more efficient immune profiling of T cells (68).
Every T cell owns a unique TCR, which provides a valuable lineage tracking marker to investigate the dynamics of T cells, including T cell clonal expansion, functional changes of a TCR clonotype, and T cell migration across different tissues. The T cell landscape with the information of paired TCR α and β chains in liver cancers comprehensively discloses the transition route of exhausted CD8+ T cells in HCC and highlights that a subset of CD8+ T cells with intermediate levels of PDCD1 and TIGIT can be the target cells for immunotherapies (106). In another work, Zhang et al. developed an analysis algorithm (STRATRAC) to quantify the T cell expansion, migration, and transition with paired TCR repertoires (107). With the T cell transition analysis of exhausted CD8+ T cells in colorectal tumors, Zhang et al. revealed a tight association of these cells with effector memory CD8+ T cells but independence of the development trajectory of effector memory and recently activated effector memory CD8+ T cells, suggesting a TCR-dependent fate decision in tumorigenesis. These works strengthen our understanding of the dynamics of T cell exhaustion in tumorigenesis. Furthermore, in-depth profiling of T cell dynamics before and after anti-PD-1 therapy in basal or squamous cell carcinoma suggests the newly entered T cell clonotypes, rather than the exhausted T cell clonotypes, respond to anti-PD-1 immunotherapy (79).
T cells are the dominant targets of immunotherapies, and their responses after immunotherapy treatments are critical to evaluate the clinical efficacy (108). Thereby, the clonal expansions and the accordant changes of TCR repertoires in tumors, normal adjacent tissue, and peripheral blood can be used for predicting the clinical responses to immunotherapies (109). Meanwhile, multiple computational methods have been developed to connect the similarities of TCR sequences with T cell functionalities, which would expand the applications of TCR repertoires in cancer research (110–112). Besides, the comprehensive inspection of T cells in tumors also directs adoptive T cell transfer (ACT) in cancer therapies to identify tumor-responsive T cells (104).
The Molecular Biomarkers for Tumor Diagnosis and Prognosis
The heterogeneities of the tumor microenvironment and strikingly different clinical outcomes in tumor patients require comprehensive molecular profiling to guide the personalized therapies. Multiple initiatives have been founded to identify tumor-specific biomarkers to facilitate better clinical decisions using integrative single-cell omics data analyses (73, 113, 114).
Several groups focused on seeking potential disease or prognosis-related biomarkers using CyTOF. Comparing the peripheral immune atlas of 20 melanoma patients before and after anti-PD-1 immunotherapy, Krieg et al. found that the frequency of CD14+CD16-HLA-DR+ monocytes in peripheral blood before treatment was highly correlated to the response of anti-PD-1 immunotherapy and thus could help to stratify patients before anti-PD-1 immunotherapy treatment (115). In a similar study of dissecting immune profiling in classical Hodgkin lymphoma (116), the peripheral TCR diversities in CD4+ T cells at baseline and during PD-1 blockade therapy were related to the clinical responses. Meanwhile, comparing the development of B cells in B cell precursor acute lymphoblastic leukemia patients and healthy controls, Good et al. revealed that the abnormal expansions of specific B cell subsets during development could predict disease relapse at the time of diagnosis (117). Although implemented in a small patient cohort, all of these strongly suggest the predictive capability of cellular composition changes in prognosis prediction and disease monitoring. Besides, the spatial inspection by imaging CyTOF in molecular colocalization of metastatic melanoma highlighted the association between the prior expression of β2m in TME and clinical outcomes of immunotherapy (118). Profiling the subcellular molecular maps of 483 breast tumor samples using imaging CyTOF in the METABRIC cohort, Ali et al. uncovered the genomic regulation of local tumor ecosystems, including cellular compositions and cellular neighborhoods. They intensively examined their clinical predictive roles in the prognosis of breast cancer (119). All these studies demonstrate the power of the single-cell CyTOF system in finding potential molecular biomarkers for cancer prognosis and predicting treatment efficacy.
scRNA-seq data has also been used in seeking molecular and cellular basis of TME. The distinct transcriptional signatures of malignant cells with different genomic backgrounds help classify tumor subtypes and the design of targeted treatments in a higher resolution (120). Comparing the ecosystems of primary and early-relapse HCC tissues, Sun et al. indicated and validated the enrichment of innate-like CD161+CD8+ T cells with limited cytotoxic ability in relapsed HCC tissues and may have poor response to the subclonal neoantigens in early-relapsed tumor cells, providing new targets to restrain HCC relapse (80). With the single-cell inspection of tumor-infiltrating lymphocytes in breast cancers, Savas et al. revealed a gene signature of tissue-resident memory CD8+ T cells rather than the CD8 alone could better predict the patient’s survival, suggesting these cells are potential regulatory targets of immunotherapy in breast cancer (121). More recently, Hwang et al. delineated the molecular taxonomy changes of TME in pancreatic ductal adenocarcinoma patients treated with or without neoadjuvant chemotherapy and radiotherapy by using the integrated single-nucleus RNA sequencing and spatially resolved transcriptomics analyses (81). They found that the basal-like or classical-like reprogramming of malignant cells was associated with distinct immune infiltration in tumors and further affected the treatment outcomes and clinical decisions.
Despite the durable clinical responses of chimeric antigen receptor T cell (CAR-T) therapy in treating hematological malignancies, the response rate, adverse events, and neurotoxicity during CAR-T treatment can vary across patients (122, 123). Single-cell omics have been applied to uncover the molecular biomarkers of clinical responses and monitor CAR-T cells’ functional changes for better clinical application (124, 125). Using scRNA-seq, Deng et al. intensively interrogated the transcriptomic phenotypes of CAR-T cells in infusion products (IPs) with their consequent clinical outcomes on large B cell lymphoma patients (124). They revealed that the enrichment of the memory phenotype of CAR-T cells within IPs lead to positive clinical responses but that the enrichment of exhaustion phenotype of CAR-T cells associated with disease progression. Moreover, they also identified a subset of monocyte-like cells in IPs significantly related to high-grade immune effector cell-associated neurotoxicity syndrome (ICANS). Furthermore, Sheih et al. comprehensively profiled the temporal changes of CD8+ CAR-T cells within IPs, peripheral blood early after infusion and after the peak of CAR-T cell expansion (125). Using the paired scRNA-seq and scTCR-seq, they identified the CD8+ CAR-T cells, within timely increased relative frequency (IRF) clonotypes, highly expressed the gene signatures of T cell cytotoxicity and proliferation, suggesting their effective roles in anti-tumor responses. These studies guide the further applications of single-cell omics to deeper understand the mechanistic insights of effective CAR-T therapy, which would shed light on optimizing CAR-T therapy and uncovering the molecular biomarkers for predicting clinical outcomes.
Furthermore, a new concept of a three-dimensional cell atlas during tumor evolutions has been introduced by Human Tumor Atlas Network (HTAN) project (73), indicating the molecular, spatial, and clinical inspections of human tumors, which would help uncover the fundamental mechanisms of tumorigenesis and new biomarkers for cancer screening, tumor metastasis, cancer immunotherapy, and drug responses in the future.
Perspectives
In this review, we comprehensively summarize the development of multiple single-cell omics techniques and their applications in cancer biology and cancer immunology. These innovative methods have extensively enhanced our understanding of tumorigenesis, the mechanisms of tumor-induced immune escape, and the dynamic responses to different tumor treatments. Although significant progress has been made, multiple challenges still exist, which could limit current studies and need to be further solved. In the CyTOF system, the preset and limited number of designated markers hinders the identification of novel or rare cell populations. Additional rare elements to increase the detectable number of channels are required to further assist their applications in the clinical field. In the scRNA-seq system, as the number of detected genes, the transcript-length coverage, and the measurement throughput varied across different platforms and assays, it is challenging to integrate and compare single-cell data from different systems. Meanwhile, the limited transcript capture efficiency of scRNA-seq methods leads to a high dropout of scRNA-seq data, resulting in a higher noise level than bulk RNA-seq (126). The common usage of 3’ end transcript capture in scRNA-seq methods involves many non-informative transcripts, making the specific examination of interested transcripts infeasible and wasting the sequencing cost (127). Thus, an optimized system that is able to economically and efficiently generate scRNA-seq data with high data quality and uniform data format is emergently desired to achieve robust analysis of larger sample cohorts. Meanwhile, a more prospective direction in the future is to profile single cells with integrated multi-omics to enable better and deeper profiling of the complicated tumor ecosystem. Moreover, new computational tools to improve the integrated data quality, facilitate the biological interpretation, and speed up the analysis procedures are valuable to be developed.
Single-cell data-driven clinical translation is important and promising in cancer diagnosis and treatment. Due to the expensive cost of single-cell methods, the enrolled patient cohort in current cancer research is very small, leading to inconsistent and non-repeatable biological findings. How to interrogate enormous single-cell features with clinical outcomes is computationally challenging and requires more external validations. Moreover, the tissue sites, sample status, isolation methods, and timepoint for sample resections can be varied across different clinical studies, leading to unstable and non-repeatable single-cell biomarkers found in the clinical field. Thus, a more feasible single-cell framework for performing large-scale clinical studies and the resources for sharing and exploiting the published single-cell data mainly in the cancer field, are urgently needed for better clinical translation in the future.
In summary, single-cell omics techniques will be indispensable for investigating both basic and clinical problems in tumor biology, tumor immunology, and tumor immunotherapy in the future, as they provide broader and deeper insights in large patient cohort to inspire more precise and personalized medicine in cancer treatments.
Author Contributions
JL, WY, WC, and SQ wrote the manuscript. TZ helped with the preparation of the figure, and all authors provided thoughtful advice to revise the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This project was supported by the National Natural Science Foundation of China 31600751 to WY and the Ministry of Science and Technology of China No.2017ZX10203205 to WC.
Conflict of Interest
HS and WY are both co-founders of, and WC is the scientific consultant of Zhejiang Puluoting Health Technology Co., Ltd (PLT). HS is the CEO of the PLT.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank the contributions of all authors and the support fundings in this project.
References
- 1. Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front Immunol (2020) 11:940. 10.3389/fimmu.2020.00940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the Tumor Immune Microenvironment (Time) for Effective Therapy. Nat Med (2018) 24:541–50. 10.1038/s41591-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petitprez F, Meylan M, de Reynies A, Sautes-Fridman C, Fridman WH. The Tumor Microenvironment in the Response to Immune Checkpoint Blockade Therapies. Front Immunol (2020) 11:784. 10.3389/fimmu.2020.00784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer Drug Resistance: An Evolving Paradigm. Nat Rev Cancer (2013) 13:714–26. 10.1038/nrc3599 [DOI] [PubMed] [Google Scholar]
- 5. Chae YK, Pan AP, Davis AA, Patel SP, Carneiro BA, Kurzrock R, et al. Path Toward Precision Oncology: Review of Targeted Therapy Studies and Tools to Aid in Defining “Actionability” of a Molecular Lesion and Patient Management Support. Mol Cancer Ther (2017) 16:2645–55. 10.1158/1535-7163.MCT-17-0597 [DOI] [PubMed] [Google Scholar]
- 6. Lim B, Lin Y, Navin N. Advancing Cancer Research and Medicine With Single-Cell Genomics. Cancer Cell (2020) 37:456–70. 10.1016/j.ccell.2020.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ma A, McDermaid A, Xu J, Chang Y, Ma Q. Integrative Methods and Practical Challenges for Single-Cell Multi-Omics. Trends Biotechnol (2020) 38:1007–22. 10.1016/j.tibtech.2020.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bendall SC, Nolan GP, Roederer M, Chattopadhyay PK. A Deep Profiler’s Guide to Cytometry. Trends Immunol (2012) 33:323–32. 10.1016/j.it.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szaloki G, Goda K. Compensation in Multicolor Flow Cytometry. Cytometry. Part A J Int Soc Analytical Cytology (2015) 87:982–5. 10.1002/cyto.a.22736 [DOI] [PubMed] [Google Scholar]
- 10. Bandura DR, Baranov VI, Ornatsky OI, Antonov A, Kinach R, Lou X, et al. Mass Cytometry: Technique for Real Time Single Cell Multitarget Immunoassay Based on Inductively Coupled Plasma Time-of-Flight Mass Spectrometry. Analytical Chem (2009) 81:6813–22. 10.1021/ac901049w [DOI] [PubMed] [Google Scholar]
- 11. Spitzer MH, Nolan GP. Mass Cytometry: Single Cells, Many Features. Cell (2016) 165:780–91. 10.1016/j.cell.2016.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Newell EW, Cheng Y. Mass Cytometry: Blessed With the Curse of Dimensionality. Nat Immunol (2016) 17:890–5. 10.1038/ni.3485 [DOI] [PubMed] [Google Scholar]
- 13. Gadalla R, Noamani B, MacLeod BL, Dickson RJ, Guo M, Xu W, et al. Validation of Cytof Against Flow Cytometry for Immunological Studies and Monitoring of Human Cancer Clinical Trials. Front Oncol (2019) 9:415. 10.3389/fonc.2019.00415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giesen C, Wang HA, Schapiro D, Zivanovic N, Jacobs A, Hattendorf B, et al. Highly Multiplexed Imaging of Tumor Tissues With Subcellular Resolution by Mass Cytometry. Nat Methods (2014) 11:417–22. 10.1038/nmeth.2869 [DOI] [PubMed] [Google Scholar]
- 15. Cheung P, Vallania F, Dvorak M, Chang SE, Schaffert S, Donato M, et al. Single-Cell Epigenetics–Chromatin Modification Atlas Unveiled by Mass Cytometry. Clin Immunol (2018) 196:40–8. 10.1016/j.clim.2018.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frei AP, Bava FA, Zunder ER, Hsieh EW, Chen SY, Nolan GP, et al. Highly Multiplexed Simultaneous Detection of Rnas and Proteins in Single Cells. Nat Methods (2016) 13:269–75. 10.1038/nmeth.3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leong ML, Newell EW. Multiplexed Peptide-Mhc Tetramer Staining With Mass Cytometry. Methods Mol Biol (2015) 1346:115–31. 10.1007/978-1-4939-2987-0_9 [DOI] [PubMed] [Google Scholar]
- 18. Stark R, Grzelak M, Hadfield J. Rna Sequencing: The Teenage Years. Nat Rev Genet (2019) 20:631–56. 10.1038/s41576-019-0150-2 [DOI] [PubMed] [Google Scholar]
- 19. Tang F, Barbacioru C, Wang Y, Nordman E, Lee C, Xu N, et al. Mrna-Seq Whole-Transcriptome Analysis of a Single Cell. Nat Methods (2009) 6:377–82. 10.1038/nmeth.1315 [DOI] [PubMed] [Google Scholar]
- 20. Schmidt WM, Mueller MW. Capselect: A Highly Sensitive Method for 5’ Cap-Dependent Enrichment of Full-Length Cdna in Pcr-Mediated Analysis of Mrnas. Nucleic Acids Res (1999) 27:e31. 10.1093/nar/27.21.e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Islam S, Kjallquist U, Moliner A, Zajac P, Fan JB, Lonnerberg P, et al. Characterization of the Single-Cell Transcriptional Landscape by Highly Multiplex Rna-Seq. Genome Res (2011) 21:1160–7. 10.1101/gr.110882.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mereu E, Lafzi A, Moutinho C, Ziegenhain C, McCarthy DJ, Alvarez-Varela A, et al. Benchmarking Single-Cell Rna-Sequencing Protocols for Cell Atlas Projects. Nat Biotechnol (2020) 38:747–55. 10.1038/s41587-020-0469-4 [DOI] [PubMed] [Google Scholar]
- 23. Hagemann-Jensen M, Ziegenhain C, Chen P, Ramskold D, Hendriks GJ, Larsson AJM, et al. Single-Cell Rna Counting at Allele and Isoform Resolution Using Smart-Seq3. Nat Biotechnol (2020) 38(6):708–14. 10.1038/s41587-020-0497-0 [DOI] [PubMed] [Google Scholar]
- 24. Keren-Shaul H, Kenigsberg E, Jaitin DA, David E, Paul F, Tanay A, et al. Mars-Seq2.0: An Experimental and Analytical Pipeline for Indexed Sorting Combined With Single-Cell Rna Sequencing. Nat Protoc (2019) 14:1841–62. 10.1038/s41596-019-0164-4 [DOI] [PubMed] [Google Scholar]
- 25. Hashimshony T, Senderovich N, Avital G, Klochendler A, de Leeuw Y, Anavy L, et al. Cel-Seq2: Sensitive Highly-Multiplexed Single-Cell Rna-Seq. Genome Biol (2016) 17:77. 10.1186/s13059-016-0938-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Macosko EZ, Basu A, Satija R, Nemesh J, Shekhar K, Goldman M, et al. Highly Parallel Genome-Wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell (2015) 161:1202–14. 10.1016/j.cell.2015.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klein AM, Mazutis L, Akartuna I, Tallapragada N, Veres A, Li V, et al. Droplet Barcoding for Single-Cell Transcriptomics Applied to Embryonic Stem Cells. Cell (2015) 161:1187–201. 10.1016/j.cell.2015.04.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng GX, Terry JM, Belgrader P, Ryvkin P, Bent ZW, Wilson R, et al. Massively Parallel Digital Transcriptional Profiling of Single Cells. Nat Commun (2017) 8:14049. 10.1038/ncomms14049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cao J, Packer JS, Ramani V, Cusanovich DA, Huynh C, Daza R, et al. Comprehensive Single-Cell Transcriptional Profiling of a Multicellular Organism. Science (2017) 357:661–7. 10.1126/science.aam8940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kuchina A, Brettner LM, Paleologu L, Roco CM, Rosenberg AB, Carignano A, et al. Microbial Single-Cell Rna Sequencing by Split-Pool Barcoding. Science (2020) 371:6531. 10.1101/869248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenberg AB, Roco CM, Muscat RA, Kuchina A, Sample P, Yao Z, et al. Single-Cell Profiling of the Developing Mouse Brain and Spinal Cord With Split-Pool Barcoding. Science (2018) 360:176–82. 10.1126/science.aam8999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hughes TK, Wadsworth MH, 2nd, Gierahn TM, Do T, Weiss D, Andrade PR, et al. Second-Strand Synthesis-Based Massively Parallel Scrna-Seq Reveals Cellular States and Molecular Features of Human Inflammatory Skin Pathologies. Immunity (2020) 53:878–894 e877. 10.1016/j.immuni.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu C, Preissl S, Ren B. Single-Cell Multimodal Omics: The Power of Many. Nat Methods (2020) 17:11–4. 10.1038/s41592-019-0691-5 [DOI] [PubMed] [Google Scholar]
- 34. Yin Y, Jiang Y, Lam KG, Berletch JB, Disteche CM, Noble WS, et al. High-Throughput Single-Cell Sequencing With Linear Amplification. Mol Cell (2019) 76:676–690 e610. 10.1016/j.molcel.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zachariadis V, Cheng H, Andrews N, Enge M. A Highly Scalable Method for Joint Whole-Genome Sequencing and Gene-Expression Profiling of Single Cells. Mol Cell (2020) 80:541–53.e545. 10.1016/j.molcel.2020.09.025 [DOI] [PubMed] [Google Scholar]
- 36. Petti AA, Williams SR, Miller CA, Fiddes IT, Srivatsari SN, Chen DY, et al. A General Approach for Detecting Expressed Mutations in Aml Cells Using Single Cell Rna-Sequencing. Nat Commun (2019) 10(1):1–16. 10.1038/s41467-019-11591-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vu TN, Nguyen HN, Calza S, Kalari KR, Wang L, Pawitan Y. Cell-Level Somatic Mutation Detection From Single-Cell Rna Sequencing. Bioinformatics (2019) 35:4679–87. 10.1093/bioinformatics/btz288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Buenrostro JD, Giresi PG, Zaba LC, Chang HY, Greenleaf WJ. Transposition of Native Chromatin for Fast and Sensitive Epigenomic Profiling of Open Chromatin, DNA-Binding Proteins and Nucleosome Position. Nat Methods (2013) 10:1213–8. 10.1038/nmeth.2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Buenrostro JD, Wu B, Litzenburger UM, Ruff D, Gonzales ML, Snyder MP, et al. Single-Cell Chromatin Accessibility Reveals Principles of Regulatory Variation. Nature (2015) 523:486–90. 10.1038/nature14590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cao J, Cusanovich DA, Ramani V, Aghamirzaie D, Pliner HA, Hill AJ, et al. Joint Profiling of Chromatin Accessibility and Gene Expression in Thousands of Single Cells. Science (2018) 361:1380–5. 10.1126/science.aau0730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen S, Lake BB, Zhang K. High-Throughput Sequencing of the Transcriptome and Chromatin Accessibility in the Same Cell. Nat Biotechnol (2019) 37:1452–7. 10.1038/s41587-019-0290-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ma S, Zhang B, LaFave LM, Earl AS, Chiang Z, Hu Y, et al. Chromatin Potential Identified by Shared Single-Cell Profiling of Rna and Chromatin. Cell (2020) 183(4):1103–16. 10.1101/2020.06.17.156943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang C, Sun D, Huang X, Wan C, Li Z, Han Y, et al. Integrative Analyses of Single-Cell Transcriptome and Regulome Using Maestro. Genome Biol (2020) 21:1–28. 10.1186/s13059-020-02116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stuart T, Srivastava A, Lareau C, Satija R. Multimodal Single-Cell Chromatin Analysis With Signac. bioRxiv (2020). 10.1101/2020.11.09.373613 [DOI] [Google Scholar]
- 45. Stoeckius M, Hafemeister C, Stephenson W, Houck-Loomis B, Chattopadhyay PK, Swerdlow H, et al. Simultaneous Epitope and Transcriptome Measurement in Single Cells. Nat Methods (2017) 14:865–8. 10.1038/nmeth.4380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hao Y, Hao S, Andersen-Nissen E, Mauck WM, Zheng S, Butler A, et al. Integrated Analysis of Multimodal Single-Cell Data. bioRxiv (2020). 10.1101/2020.10.12.335331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Granja JM, Klemm S, McGinnis LM, Kathiria AS, Mezger A, Corces MR, et al. Single-Cell Multiomic Analysis Identifies Regulatory Programs in Mixed-Phenotype Acute Leukemia. Nat Biotechnol (2019) 37:1458–65. 10.1038/s41587-019-0332-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang SQ, Ma KY, Schonnesen AA, Zhang M, He C, Sun E, et al. High-Throughput Determination of the Antigen Specificities of T Cell Receptors in Single Cells. Nat Biotechnol (2018) 36(12):1156–9. 10.1101/457069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stoeckius M, Zheng S, Houck-Loomis B, Hao S, Yeung BZ, Mauck WM, 3rd, et al. Cell Hashing With Barcoded Antibodies Enables Multiplexing and Doublet Detection for Single Cell Genomics. Genome Biol (2018) 19:224. 10.1186/s13059-018-1603-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Crosetto N, Bienko M, van Oudenaarden A. Spatially Resolved Transcriptomics and Beyond. Nat Rev Genet (2015) 16:57–66. 10.1038/nrg3832 [DOI] [PubMed] [Google Scholar]
- 51. Asp M, Bergenstrahle J, Lundeberg J. Spatially Resolved Transcriptomes-Next Generation Tools for Tissue Exploration. Bioessays (2020) 42:e1900221. 10.1002/bies.201900221 [DOI] [PubMed] [Google Scholar]
- 52. Raj A, Van Den Bogaard P, Rifkin SA, Van Oudenaarden A, Tyagi S. Imaging Individual Mrna Molecules Using Multiple Singly Labeled Probes. Nat Methods (2008) 5:877–9. 10.1038/nmeth.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eng CL, Lawson M, Zhu Q, Dries R, Koulena N, Takei Y, et al. Transcriptome-Scale Super-Resolved Imaging in Tissues by Rna Seqfish. Nature (2019) 568:235–9. 10.1038/s41586-019-1049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rodriques SG, Stickels RR, Goeva A, Martin CA, Murray E, Vanderburg CR, et al. Slide-Seq: A Scalable Technology for Measuring Genome-Wide Expression at High Spatial Resolution. Science (2019) 363:1463–7. 10.1126/science.aaw1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stickels RR, Murray E, Kumar P, Li JL, Marshall JL, Di Bella DJ, et al. Highly Sensitive Spatial Transcriptomics at Near-Cellular Resolution With Slide-Seqv2. Nat Biotechnol (2020) 39(3):313–9. 10.1038/s41587-020-0739-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vickovic S, Eraslan G, Salmen F, Klughammer J, Stenbeck L, Schapiro D, et al. High-Definition Spatial Transcriptomics for in Situ Tissue Profiling. Nat Methods (2019) 16:987–90. 10.1038/s41592-019-0548-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Liu Y, Yang M, Deng Y, Su G, Enninful A, Guo CC, et al. High-Spatial-Resolution Multi-Omics Sequencing Via Deterministic Barcoding in Tissue. Cell (2020) 183:1665–81.e1618. 10.1016/j.cell.2020.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stegle O, Teichmann SA, Marioni JC. Computational and Analytical Challenges in Single-Cell Transcriptomics. Nat Rev Genet (2015) 16:133–45. 10.1038/nrg3833 [DOI] [PubMed] [Google Scholar]
- 59. Hie B, Peters J, Nyquist SK, Shalek AK, Berger B, Bryson BD. Computational Methods for Single-Cell Rna Sequencing. Annu Rev Biomed Data Sci (2020) 3:339–64. 10.1146/annurev-biodatasci-012220-100601 [DOI] [Google Scholar]
- 60. Saelens W, Cannoodt R, Todorov H, Saeys Y. A Comparison of Single-Cell Trajectory Inference Methods. Nat Biotechnol (2019) 37:547–54. 10.1038/s41587-019-0071-9 [DOI] [PubMed] [Google Scholar]
- 61. Cao J, Spielmann M, Qiu X, Huang X, Ibrahim DM, Hill AJ, et al. The Single-Cell Transcriptional Landscape of Mammalian Organogenesis. Nature (2019) 566:496–502. 10.1038/s41586-019-0969-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wolf FA, Hamey FK, Plass M, Solana J, Dahlin JS, Gottgens B, et al. Paga: Graph Abstraction Reconciles Clustering With Trajectory Inference Through a Topology Preserving Map of Single Cells. Genome Biol (2019) 20(1):1–9. 10.1186/s13059-019-1663-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Street K, Risso D, Fletcher RB, Das D, Ngai J, Yosef N, et al. Slingshot: Cell Lineage and Pseudotime Inference for Single-Cell Transcriptomics. BMC Genomics (2018) 19(1):1–16. 10.1186/s12864-018-4772-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, et al. Rna Velocity of Single Cells. Nature (2018) 560:494–8. 10.1038/s41586-018-0414-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bergen V, Lange M, Peidli S, Wolf FA, Theis FJ. Generalizing Rna Velocity to Transient Cell States Through Dynamical Modeling. Nature Biotechnol (2020) 38:1408–14. 10.1038/s41587-020-0591-3 [DOI] [PubMed] [Google Scholar]
- 66. Gulati GS, Sikandar SS, Wesche DJ, Manjunath A, Bharadwaj A, Berger MJ, et al. Single-Cell Transcriptional Diversity is a Hallmark of Developmental Potential. Science (2020) 367(6476):405–11. 10.1126/science.aax0249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wagner DE, Klein AM. Lineage Tracing Meets Single-Cell Omics: Opportunities and Challenges. Nat Rev Genet (2020) 21:410–27. 10.1038/s41576-020-0223-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. De Simone M, Rossetti G, Pagani M. Single Cell T Cell Receptor Sequencing: Techniques and Future Challenges. Front Immunol (2018) 9:1638. 10.3389/fimmu.2018.01638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Argelaguet R, Arnol D, Bredikhin D, Deloro Y, Velten B, Marioni JC, et al. Mofa+: A Statistical Framework for Comprehensive Integration of Multi-Modal Single-Cell Data. Genome Biol (2020) 21:111. 10.1186/s13059-020-02015-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nguyen ND, Wang D. Multiview Learning for Understanding Functional Multiomics. PloS Comput Biol (2020) 16:e1007677. 10.1371/journal.pcbi.1007677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Zhang F, Wei K, Slowikowski K, Fonseka CY, Rao DA, Kelly S, et al. Defining Inflammatory Cell States in Rheumatoid Arthritis Joint Synovial Tissues by Integrating Single-Cell Transcriptomics and Mass Cytometry. Nat Immunol (2019) 20:928–42. 10.1038/s41590-019-0378-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gayoso A, Steier Z, Lopez R, Regier J, Nazor KL, Streets A, et al. Joint Probabilistic Modeling of Single-Cell Multi-Omic Data With Totalvi. Nat Methods (2021) 18:272–82. 10.1038/s41592-020-01050-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rozenblatt-Rosen O, Regev A, Oberdoerffer P, Nawy T, Hupalowska A, Rood JE, et al. The Human Tumor Atlas Network: Charting Tumor Transitions Across Space and Time at Single-Cell Resolution. Cell (2020) 181:236–49. 10.1016/j.cell.2020.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell (2017) 169:750–765 e717. 10.1016/j.cell.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jackson HW, Fischer JR, Zanotelli VRT, Ali HR, Mechera R, Soysal SD, et al. The Single-Cell Pathology Landscape of Breast Cancer. Nature (2020) 578:615–20. 10.1038/s41586-019-1876-x [DOI] [PubMed] [Google Scholar]
- 76. Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell (2019) 179:829–845 e820. 10.1016/j.cell.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 77. Nam AS, Kim KT, Chaligne R, Izzo F, Ang C, Taylor J, et al. Somatic Mutations and Cell Identity Linked by Genotyping of Transcriptomes. Nature (2019) 571:355–60. 10.1038/s41586-019-1367-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Moncada R, Barkley D, Wagner F, Chiodin M, Devlin JC, Baron M, et al. Integrating Microarray-Based Spatial Transcriptomics and Single-Cell Rna-Seq Reveals Tissue Architecture in Pancreatic Ductal Adenocarcinomas. Nat Biotechnol (2020) 38:333–42. 10.1038/s41587-019-0392-8 [DOI] [PubMed] [Google Scholar]
- 79. Yost KE, Satpathy AT, Wells DK, Qi Y, Wang C, Kageyama R, et al. Clonal Replacement of Tumor-Specific T Cells Following Pd-1 Blockade. Nat Med (2019) 25:1251–9. 10.1038/s41591-019-0522-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sun Y, Wu L, Zhong Y, Zhou K, Hou Y, Wang Z, et al. Single-Cell Landscape of the Ecosystem in Early-Relapse Hepatocellular Carcinoma. Cell (2020) 184(2):404–21.e16. 10.1016/j.cell.2020.11.041 [DOI] [PubMed] [Google Scholar]
- 81. Hwang WL, Jagadeesh KA, Guo JA, Hoffman HI, Yadollahpour P, Mohan R, et al. Single-Nucleus and Spatial Transcriptomics of Archival Pancreatic Cancer Reveals Multi-Compartment Reprogramming After Neoadjuvant Treatment. BioRxiv (2020). 10.1158/1538-7445.PANCA20-PR-007 [DOI] [Google Scholar]
- 82. Chevrier S, Levine JH, Zanotelli VRT, Silina K, Schulz D, Bacac M, et al. An Immune Atlas of Clear Cell Renal Cell Carcinoma. Cell (2017) 169:736–49.e718. 10.1016/j.cell.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wagner J, Rapsomaniki MA, Chevrier S, Anzeneder T, Langwieder C, Dykgers A, et al. Bodenmiller B. A Single-Cell Atlas of the Tumor and Immune Ecosystem of Human Breast Cancer. Cell (2019) 177:1330–1345 e1318. 10.1016/j.cell.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gohil SH, Iorgulescu JB, Braun DA, Keskin DB, Livak KJ. Applying High-Dimensional Single-Cell Technologies to the Analysis of Cancer Immunotherapy. Nat Rev Clin Oncol (2020) 18(4):244–56. 10.1038/s41571-020-00449-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell (2018) 174:1293–308.e1236. 10.1016/j.cell.2018.05.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Ludwig LS, Lareau CA, Ulirsch JC, Christian E, Muus C, Li LH, et al. Lineage Tracing in Humans Enabled by Mitochondrial Mutations and Single-Cell Genomics. Cell (2019) 176:1325–39.e1322. 10.1016/j.cell.2019.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. van Galen P, Hovestadt V, Wadsworth Ii MH, Hughes TK, Griffin GK, Battaglia S, et al. Single-Cell Rna-Seq Reveals Aml Hierarchies Relevant to Disease Progression and Immunity. Cell (2019) 176:1265–81.e1224. 10.1016/j.cell.2019.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bian S, Hou Y, Zhou X, Li X, Yong J, Wang Y, et al. Single-Cell Multiomics Sequencing and Analyses of Human Colorectal Cancer. Science (2018) 362:1060–3. 10.1126/science.aao3791 [DOI] [PubMed] [Google Scholar]
- 89. Hou Y, Guo H, Cao C, Li X, Hu B, Zhu P, et al. Single-Cell Triple Omics Sequencing Reveals Genetic, Epigenetic, and Transcriptomic Heterogeneity in Hepatocellular Carcinomas. Cell Res (2016) 26:304–19. 10.1038/cr.2016.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Nam AS, Chaligne R, Landau DA. Integrating Genetic and non-Genetic Determinants of Cancer Evolution by Single-Cell Multi-Omics. Nat Rev Genet (2021) 22:3–18. 10.1038/s41576-020-0265-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Stuart T, Satija R. Integrative Single-Cell Analysis. Nat Rev Genet (2019) 20:257–72. 10.1038/s41576-019-0093-7 [DOI] [PubMed] [Google Scholar]
- 92. Castro LNG, Tirosh I, Suvà ML. Decoding Cancer Biology One Cell at a Time. Cancer Discovery (2021) 11:960–70. 10.1158/2159-8290.CD-20-1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Tirosh I, Venteicher AS, Hebert C, Escalante LE, Patel AP, Yizhak K, et al. Single-Cell Rna-Seq Supports a Developmental Hierarchy in Human Oligodendroglioma. Nature (2016) 539:309–13. 10.1038/nature20123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Marjanovic ND, Hofree M, Chan JE, Canner D, Wu K, Trakala M, et al. Emergence of a High-Plasticity Cell State During Lung Cancer Evolution. Cancer Cell (2020) 38:229–246. e213. 10.1016/j.ccell.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Neftel C, Laffy J, Filbin MG, Hara T, Shore ME, Rahme GJ, et al. An Integrative Model of Cellular States, Plasticity, and Genetics for Glioblastoma. Cell (2019) 178:835–49.e821. 10.1016/j.cell.2019.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Science (2011) 331:1559–64. 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 97. Kim N, Kim HK, Lee K, Hong Y, Cho JH, Choi JW, et al. Single-Cell Rna Sequencing Demonstrates the Molecular and Cellular Reprogramming of Metastatic Lung Adenocarcinoma. Nat Commun (2020) 11:1–15. 10.1038/s41467-020-16164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Quinn JJ, Jones MG, Okimoto RA, Nanjo S, Chan MM, Yosef N, et al. Single-Cell Lineages Reveal the Rates, Routes, and Drivers of Metastasis in Cancer Xenografts. Science (2021) 371(6532):eabc1944. 10.1126/science.abc1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Prieto-Vila M, Usuba W, Takahashi R-U, Shimomura I, Sasaki H, Ochiya T, et al. Single-Cell Analysis Reveals a Preexisting Drug-Resistant Subpopulation in the Luminal Breast Cancer Subtype. Cancer Res (2019) 79:4412–25. 10.1158/0008-5472.CAN-19-0122 [DOI] [PubMed] [Google Scholar]
- 100. Jerby-Arnon L, Shah P, Cuoco MS, Rodman C, Su M-J, Melms JC, et al. A Cancer Cell Program Promotes T Cell Exclusion and Resistance to Checkpoint Blockade. Cell (2018) 175:984–97.e924. 10.1016/j.cell.2018.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Frangieh CJ, Melms JC, Thakore PI, Geiger-Schuller KR, Ho P, Luoma AM, et al. Multimodal Pooled Perturb-Cite-Seq Screens in Patient Models Define Mechanisms of Cancer Immune Evasion. Nat Genet (2021) 53:332–41. 10.1038/s41588-021-00779-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discovery (2018) 8:1069–86. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 103. Manfredi F, Cianciotti BC, Potenza A, Tassi E, Noviello M, Biondi A, et al. Tcr Redirected T Cells for Cancer Treatment: Achievements, Hurdles, and Goals. Front Immunol (2020) 11:1689. 10.3389/fimmu.2020.01689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Waldman AD, Fritz JM, Lenardo MJ. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat Rev Immunol (2020) 20:651–68. 10.1038/s41577-020-0306-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Redmond D, Poran A, Elemento O. Single-Cell Tcrseq: Paired Recovery of Entire T-Cell Alpha and Beta Chain Transcripts in T-Cell Receptors From Single-Cell Rnaseq. Genome Med (2016) 8:80. 10.1186/s13073-016-0335-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zheng C, Zheng L, Yoo JK, Guo H, Zhang Y, Guo X, et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell (2017) 169:1342–56.e1316. 10.1016/j.cell.2017.05.035 [DOI] [PubMed] [Google Scholar]
- 107. Zhang L, Yu X, Zheng L, Zhang Y, Li Y, Fang Q, et al. Lineage Tracking Reveals Dynamic Relationships of T Cells in Colorectal Cancer. Nature (2018) 564:268–72. 10.1038/s41586-018-0694-x [DOI] [PubMed] [Google Scholar]
- 108. Kidman J, Principe N, Watson M, Lassmann T, Holt RA, Nowak AK, et al. Characteristics of Tcr Repertoire Associated With Successful Immune Checkpoint Therapy Responses. Front Immunol (2020) 11:587014. 10.3389/fimmu.2020.587014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wu TD, Madireddi S, de Almeida PE, Banchereau R, Chen YJ, Chitre AS, et al. Peripheral T Cell Expansion Predicts Tumour Infiltration and Clinical Response. Nature (2020) 579:274–8. 10.1038/s41586-020-2056-8 [DOI] [PubMed] [Google Scholar]
- 110. Depuydt MAC, Prange KHM, Slenders L, Ord T, Elbersen D, Boltjes A, et al. Microanatomy of the Human Atherosclerotic Plaque by Single-Cell Transcriptomics. Circ Res (2020) 127:1437–55. 10.1161/CIRCRESAHA.120.316770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Huang H, Wang C, Rubelt F, Scriba TJ, Davis MM. Analyzing the Mycobacterium Tuberculosis Immune Response by T-Cell Receptor Clustering With Gliph2 and Genome-Wide Antigen Screening. Nat Biotechnol (2020) 38(10):1194–202. 10.1038/s41587-020-0505-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Dash P, Fiore-Gartland AJ, Hertz T, Wang GC, Sharma S, Souquette A, et al. Quantifiable Predictive Features Define Epitope-Specific T Cell Receptor Repertoires. Nature (2017) 547:89–93. 10.1038/nature22383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Irmisch A, Bonilla X, Chevrier S, Lehmann KV, Singer F, Toussaint NC, et al. The Tumor Profiler Study: Integrated, Multi-Omic, Functional Tumor Profiling for Clinical Decision Support. Cancer Cell (2021) 39(3):288–93. 10.1016/j.ccell.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 114. Rajewsky N, Almouzni G, Gorski SA, Aerts S, Amit I, Bertero MG, et al. Lifetime and Improving European Healthcare Through Cell-Based Interceptive Medicine. Nature (2020) 587:377–86. 10.1038/s41586-020-2715-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-Dimensional Single-Cell Analysis Predicts Response to Anti-Pd-1 Immunotherapy. Nat Med (2018) 24:144–53. 10.1038/nm.4466 [DOI] [PubMed] [Google Scholar]
- 116. Cader FZ, Hu X, Goh WL, Wienand K, Ouyang J, Mandato E, et al. A Peripheral Immune Signature of Responsiveness to Pd-1 Blockade in Patients With Classical Hodgkin Lymphoma. Nat Med (2020) 26(9):1468–79. 10.1038/s41591-020-1006-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Good Z, Sarno J, Jager A, Samusik N, Aghaeepour N, Simonds EF, et al. Single-Cell Developmental Classification of B Cell Precursor Acute Lymphoblastic Leukemia at Diagnosis Reveals Predictors of Relapse. Nat Med (2018) 24:474–83. 10.1038/nm.4505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Martinez-Morilla S, Villarroel-Espindola F, Wong PF, Toki MI, Aung TN, Pelekanou V, et al. Biomarker Discovery in Patients With Immunotherapy-Treated Melanoma With Imaging Mass Cytometry. Clin Cancer Res (2021) 27(7):1987–96. 10.1158/1538-7445.AM2020-2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Ali HR, Jackson HW, Zanotelli VRT, Danenberg E, Fischer JR, Bardwell H, et al. Imaging Mass Cytometry and Multiplatform Genomics Define the Phenogenomic Landscape of Breast Cancer. Nat Cancer (2020) 1:163–75. 10.1038/s43018-020-0026-6 [DOI] [PubMed] [Google Scholar]
- 120. Gonzalez-Silva L, Quevedo L, Varela I. Tumor Functional Heterogeneity Unraveled by Scrna-Seq Technologies. Trends Cancer (2020) 6:13–9. 10.1016/j.trecan.2019.11.010 [DOI] [PubMed] [Google Scholar]
- 121. Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-Cell Profiling of Breast Cancer T Cells Reveals a Tissue-Resident Memory Subset Associated With Improved Prognosis. Nat Med (2018) 24:986–93. 10.1038/s41591-018-0078-7 [DOI] [PubMed] [Google Scholar]
- 122. Ramakrishna S, Shah NN. Using Single-Cell Analysis to Predict Car T Cell Outcomes. Nat Med (2020) 26(12):1813–4. 10.1038/s41591-020-01157-w [DOI] [PubMed] [Google Scholar]
- 123. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel Car T-Cell Therapy in Refractory Large B-Cell Lymphoma. New Engl J Med (2017) 377:2531–44. 10.1056/NEJMoa1707447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, et al. Characteristics of Anti-Cd19 Car T Cell Infusion Products Associated With Efficacy and Toxicity in Patients With Large B Cell Lymphomas. Nat Med (2020) 26:1878–87. 10.1038/s41591-020-1061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sheih A, Voillet V, Hanafi L-A, DeBerg HA, Yajima M, Hawkins R, et al. Clonal Kinetics and Single-Cell Transcriptional Profiling of Car-T Cells in Patients Undergoing Cd19 Car-T Immunotherapy. Nat Commun (2020) 11:1–13. 10.1038/s41467-019-13880-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gong W, Kwak I-Y, Pota P, Koyano-Nakagawa N, Garry DJ. Drimpute: Imputing Dropout Events in Single Cell Rna Sequencing Data. BMC Bioinf (2018) 19:1–10. 10.1186/s12859-018-2226-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kanev K, Roelli P, Wu M, Wurmser C, Delorenzi M, Pfaffl MW, et al. Tailoring the Resolution of Single-Cell Rna Sequencing for Primary Cytotoxic T Cells. Nat Commun (2021) 12:1–11. 10.1038/s41467-020-20751-7 [DOI] [PMC free article] [PubMed] [Google Scholar]