Summary
Chronic kidney disease (CKD) is among the most prevalent and dire complications of diabetes mellitus in adults across the world. Diabetes substantially contributes to the burden of kidney disease, such that one third to one half of CKD in the United States and many other countries is attributable to diabetic kidney disease (DKD). As DKD progresses to end-stage renal disease (ESRD), patients are at heightened risk for atypical glycemic complications, including the development of burnt-out diabetes, manifested by hypoglycemic bouts and poor outcomes. Furthermore, even in the absence of diabetes, hypoglycemia is a frequent occurrence in CKD patients that may contribute to their high burden of cardiovascular disease and death. Extrapolation of data from clinical trials in high-cardiovascular-risk populations and observational studies in patients with non−dialysis-dependent (NDD) CKD and ESRD suggest that moderate glycemic targets defined by glycated hemoglobin levels of 6% to 8% and glucose levels of 100 to 150 mg/dL are associated with better survival in DKD patients. However, given the imprecision of glycated hemoglobin levels in kidney disease, further research is needed to determine the optimal glycemic metric and target in diabetic NDD-CKD and ESRD patients. Given their exceedingly high cardiovascular morbidity and mortality, there is a compelling need for further investigation of how to optimally manage dysglycemia in the NDD-CKD and ESRD populations.
Approximately 34.2 million people in the United States have diabetes, among whom approximately 20% are estimated to be undiagnosed.1
Among diagnosed cases, 90% to 95% are attributed to type 2 diabetes mellitus. As one of the most prevalent complications of this endocrine disorder, chronic kidney disease (CKD) affects 30% and 40% of patients with type 1 and type 2 diabetes, respectively.2 In turn, National Health and Nutrition Examination Surveys data have shown that diabetes substantially contributes to the burden of kidney disease in the United States, such that one quarter of CKD among US adults is attributable to diabetes after adjusting for demographics.3 Data from a large integrated health care delivery system (Kaiser Permanente Northern California) also suggest that CKD patients with diabetes have a 1.5-fold higher risk of rapid CKD progression versus those without diabetes.4
Consequently, diabetes is the leading cause of end–-stage renal disease (ESRD) in the United States, accounting for 47% and 39% of incident and prevalent cases, respectively.5 Globally, parts of Mexico (Jalisco), Taiwan, Singapore, and the United States manifest the highest incident rates of ESRD as a result of diabetes (ie, 279, 242, 230, and 185 new cases per million population, respectively).5 Furthermore, national trends continue to show a steady increase in the number of prevalent ESRD cases resulting from diabetes (ie, 298,697 patients as of 2018).5 Given the exceedingly high mortality rate of patients with ESRD as a result of diabetes compared with their nondiabetic counterparts (ie, annual mortality rates of 171, 144, versus 62 per 1,000 patient-years of follow-up evaluation for ESRD resulting from diabetes and hypertension versus glomerular disease, respectively5), these epidemiologic trends underscore the importance of identifying management strategies that improve the health and survival of this population.
BURNT-OUT DIABETES AND OTHER GLYCEMIC DERANGEMENTS IN KIDNEY DISEASE
Burnt-Out Diabetes Phenomenon
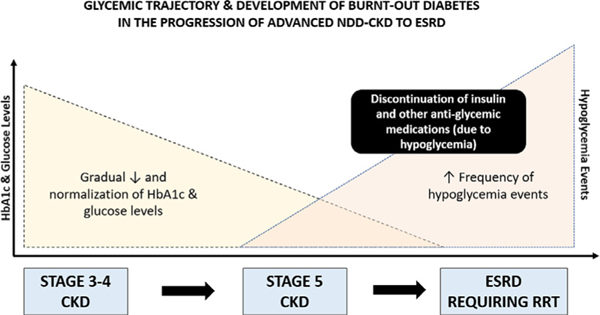
As diabetic patients with advanced CKD progress to ESRD, many will experience spontaneous resolution of hyperglycemia, normalization of glycated hemoglobin (HbA1c) levels, and frequent hypoglycemia in a phenomenon that has been described as “burnt-out diabetes (Fig 1).6–8 Reports from large population-based studies support these observations. For example, among 63,607 incident diabetic hemodialysis patients from a large dialysis organization (LDO) receiving treatment during 2007 to 2011, 38% of patients had HbA1c levels lower than 6% at the time of transitioning to dialysis.9,10 In another national study of 19,977 US veterans with diabetic kidney disease (DKD), mean HbA1c levels steadily decreased as patients transitioned to ESRD (delta, −0.8% over a 5-year period).11,12 As a result, diabetic patients transitioning to dialysis oftentimes require a reduction and/or cessation of their antiglycemic medications to avoid hypoglycemia.8
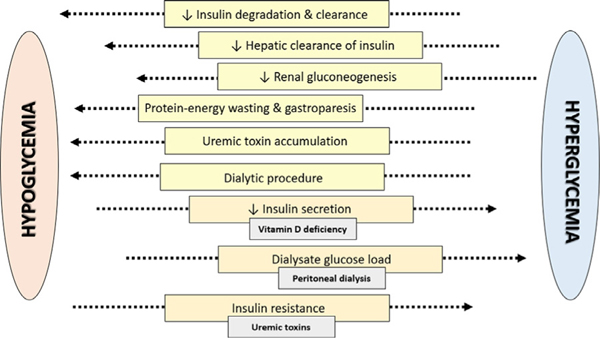
Figure 1.
Risk factors for hypoglycemia and hyperglycemia in chronic kidney disease patients.
Hypoglycemia
Even in the absence of diabetes, CKD patients are predisposed to glycemic derangements, and, in particular, hypoglycemia. Several large population-based studies have reported a high frequency of hypoglycemia events in CKD patients irrespective of diabetes status. In a study of 243,222 US veterans, hypoglycemia was frequent in CKD patients with and without diabetes (ie, 11 and 4 events/100 patient-months, respectively).13 In a national cohort of 58,304 incident hemodialysis patients, approximately 17% versus 7% of those with versus without diabetes, respectively, had at least one or more hypoglycemic events, as defined by monthly predialysis glucose levels.14 With respect to more severe hypoglycemia events necessitating medical attention, in a study of 30,156 US veterans with advanced CKD transitioning to ESRD, approximately 6% of patients experienced a hypoglycemia-related hospitalization in the 1- to 2-year period preceding dialysis initiation.15
Various intrinsic and extrinsic factors may engender hypoglycemia among advanced CKD patients progressing to ESRD (Fig. 2).6,8 First, other than the liver, the kidney is the only organ capable of generating sufficient glucose to release into the circulation16; subsequently, patients with advanced kidney dysfunction are susceptible to hypoglycemia as a result of impaired renal gluconeogenesis.17 Second, as kidney function decreases, CKD patients have reduced renal degradation/clearance and hepatic clearance of endogenous insulin.8 Third, in addition to endogenous insulin, decreased renal metabolism and clearance of exogenous insulin and other antiglycemic agents may lead to hypoglycemia. Epidemiologic data have shown that certain medication patterns are particularly associated with a heightened risk of hypoglycemia in DKD patients. In a study of 30,156 US veterans, examination of the 12 most commonly prescribed antiglycemic agents by drug class showed that regimens with sulfonylureas and/or insulin were associated with hypoglycemia.15 In addition, although there was a graded association between the number of prescribed oral antidiabetic drugs and hypoglycemia risk, insulin use alone had a similar magnitude of risk as compared with prescription of two or more oral antidiabetic drugs. Fourth, advanced CKD patients may manifest other co-existing comorbidities such as protein-energy wasting and diabetic gastroparesis that may exacerbate the risk of hypoglycemia.15 Fifth, as CKD patients transition to ESRD, it also has been hypothesized that uremic metabolites promote dysglycemia by suppressing hepatic glucose output and stimulating insulin secretion. Finally, among ESRD patients receiving renal replacement therapy, the hemodialysis procedure in and of itself may cause hypoglycemia owing to the following: (1) intradialytic uptake of glucose by erythrocytes during the procedure, which is exacerbated further by (2) secular changes in use of lower dialysate glucose concentrations over time (ie, transition from 200 to 100 mg/dL), and (3) restriction of food intake during the dialysis treatment session.18
Figure 2.
Glycemic status trajectory and the development of burntout diabetes in advanced chronic kidney disease (CKD) patients transitioning to end-stage renal disease (ESRD). Abbreviation: HbA1c, glycated hemoglobin; NDD-CKD, non−dialysis-dependent chronic kidney disease; RRT, renal replacement therapy.
Hyperglycemia
Conversely, CKD patients with and without diabetes are also at risk for hyperglycemia via multiple pathways (Fig. 2).6,8 In advanced kidney disease, impaired insulin secretion and insulin resistance may be observed, and although the precise underlying mechanisms have not been elucidated fully, it has been suggested that secondary hyperparathyroidism and activated vitamin D deficiency are contributory. In several small studies, treatment of hyperparathyroidism (ie, phosphate restriction or parathyroidectomy19,20) has resulted in improved insulin secretion and/or sensitivity. In addition, administration of activated vitamin D has been shown to correct glucose intolerance, insulin resistance, and hypoinsulinemia in hemodialysis patients independent of PTH suppression.21,22 It also has been suggested that uremic toxins may contribute to insulin resistance, given the observations of impaired insulin-stimulated glucose uptake in adipose and muscle tissue of uremic animal models, and improvement in glucose metabolism with hemodialysis.23–25 Finally, in peritoneal dialysis patients, exposure to high glucose loads via dextrose-based solutions also may contribute to hyperglycemia.8
GLYCEMIC TARGETS IN DIABETIC PATIENTS WITH AND WITHOUT CKD
In the general population, various clinical practice guidelines recommend HbA1c targets of less than 7.0% in patients with type 2 diabetes.26 These thresholds have been informed in part by early clinical trials of intensive versus standard glycemic targets, including the Diabetes Control and Complications Trial (DCCT)27 and the UK Prospective Diabetes Study (UKPDS)28 conducted in type 1 and type 2 diabetic patients, respectively (Table 1).
Table 1.
Landmark Clinical Trials of Glycemic Targets in Patients With Diabetes
| Study | Study Population | Study Arms | Achieved HbA1c, % | Outcomes |
|---|---|---|---|---|
| DCCT and EDIC studies27,30 | Type 1 diabetes | Intensive Fasting glucose 70–1 Post-meal glucose <180 mg/dL HbA1c <6.05% Conventional No target |
7.4 versus 9.1 7.9 versus 7.3 |
↓Microvascular disease ↓CV disease |
| UKPDS and post-trial follow-up studies28,31 | Newly diagnosed type 2 diabetes | Intensive Insulin or sulfonylurea § metformin Conventional No target |
7.0 versus 7.9 | ↓ Microvascular disease ↓CV disease/death(post-trial) |
| ACCORD trial32 | Type 2 diabetes + CV disease or CV risk factors | Intensive HbA1c <6.0% Standard HbA1c 7% to 9% |
6.4 versus 7.5 | ↑ CV events |
| ADVANCE trial33 | Type 2 diabetes + macrovascular/microvascular + vascular risk factors |
Intensive HbA1c ≤6.5% Standard Local guidelines |
6.5 versus 7.3 | No difference in CV outcomes |
| Veterans Affairs diabetes trial34 | Type 2 diabetes | Intensive –HbA1c <6.0% –Standard –HbA1c <9.0% |
6.9 versus 8.4 | No difference in CV outcomes |
Abbreviations: ACCORD, Action to Control Cardiovascular Risk in Diabetes; ADVANCE, Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation; CV, cardiovascular; DCCT, Diabetes Control and Complications Trial; EDIC, Epidemiology of Diabetes Interventions and Complications; HbA1c, glycated hemoglobin; UKPDS, United Kingdom Prospective Diabetes Study.
To determine whether the complications of diabetes could be prevented or delayed, the DCCT randomized type 1 diabetic patients with minimal end-organ damage to intensive versus conventional glycemic control, and, after a mean follow-up period of 6.5 years, intensive glycemic control was found to reduce the early stages of microvascular complications by 35% to 76% compared with conventional control.27,29 Notably, patients in the intensive glycemic arm experienced a three-fold higher risk of hypoglycemic events. In the long-term observational Epidemiology of Diabetes Interventions and Complications (EDIC) study that followed the DCCT, the first 4 years of the EDIC follow-up evaluation showed a further widening of microvascular outcome differences, as well as improved macrovascular outcomes in the intensive glycemic control arm, suggesting a persistent “legacy effect” over time.30 In the UKPDS trial, which randomized newly diagnosed type 2 diabetic patients to intensive versus conventional glycemic control, after a median follow-up period of 10 years the overall rate of microvascular complications was reduced by 25% in the intensive control arm.28 Epidemiologic follow-up data of the UKPDS also have shown a graded association between glycemic status and macrovascular risk, such that each percentage point decrease in HbA1c was associated with a 7% reduction in all-cause mortality and an 18% reduction in combined fatal and nonfatal myocardial infarction events.31
Although early clinical trials of intensive versus conventional glycemic control enrolled type 1 and type 2 diabetic patients with minimal to no kidney damage, subsequent trials have been conducted in long-standing type 2 diabetic patients with higher cardiovascular risk (Table 1). Among these include the Action to Control Cardiovascular Risk in Diabetes trial, which randomized type 2 diabetic patients with underlying cardiovascular risk (ie, age, 40–79 y with cardiovascular disease; or age, 55–79 years with atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two additional risk factors for cardiovascular disease) to intensive versus standard glycemic control. After a median follow-up period of 3.7 years, the trial was stopped early given that intensive glycemic control was found to confer increased all-cause and cardiovascular mortality risk.32 The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation trial randomized type 2 diabetic patients with macrovascular or microvascular disease or at least one other vascular disease risk factor to intensive versus standard glycemic control, and found no differences in major macrovascular events, cardiovascular death, or death from any cause across the two groups.33 Although patients in the intensive control arm had a modest reduction in albuminuria, they also experienced a higher frequency of severe hypoglycemia. Similarly, the Veterans Affairs Diabetes Trial, which randomized US veterans to intensive versus conventional glycemic control, did not show a cardiovascular benefit and observed a higher frequency of hypoglycemia events including coma in the intensive control arm.34
Given their high burden of cardiovascular disease, the latter trial cohorts may bear greater analogy to the CKD population. As such, clinical practice guidelines advise more conservative glycemic targets in patients with moderate-to-advanced kidney disease (Table 2). Although the National Kidney Foundation (NKF) Kidney Disease Outcomes Quality Initiative35 and Kidney Disease Improving Global Outcomes guidelines36 recommend a target HbA1c of 7% to prevent or reduce progression of microvascular complications of diabetes, they do not recommend an HbA1c level less than 7% in patients at risk of hypoglycemia, defined as patients with stages 4 to 5 CKD and/or those on insulin or sulfonylureas. Moreover, they advise a target HbA1c of greater than 7% in individuals with comorbidities, limited life expectancy, and risk of hypoglycemia. Although there are a growing number of tools available for assessment of glycemic status (eg, HbA1c, self-monitored blood glucose, fructosamine, glycated albumin, continuous glucose monitoring, 1,5-anhydroglucitol37,38), the optimal metric in CKD patients remains widely debated. The NKF Kidney Disease Outcomes Quality Initiative guidelines have recommended HbA1c as the best long-term metric of glycemic control when used in conjunction with self-monitored blood glucose.35 However, a consensus conference convened by the American Diabetes Association, the NKF, and the American Society of Nephrology advised that, although HbA1c levels between 7% and 8% appear to be associated with the highest survival rates in retrospective analyses of DKD patients, the imprecision of HbA1c measurements makes specific targets for people with DKD difficult to define.37,38 The following summary outlines findings from selected rigorous epidemiologic studies examining glycemic status and hard end points in NDD-CKD and ESRD patients.
Table 2.
Recommendations for Glycemic Targets in DKD Patients
| Professional Society/Group | Recommendations |
|---|---|
| KDOQI35 KDIGO36 |
Target HbA1c of 7% to prevent or # progression of microvascular complications of diabetesDo not recommend HbA1c <7% in individuals at risk of hypoglycemia Stages 4 and 5 CKD Patients on insulin or sulfonylureas Target HbA1c >7% in individuals with comorbidities, limited life expectancy, and risk of hypoglycemia |
| Consensus Conference on Chronic Kidney Disease and Diabetes: American Diabetes Association, American Society of Nephrology, and the National Kidney Foundation37,38 |
Given the limitations of the most frequently used glycemic biomarker, HbA1c, and the high risk of hypoglycemia, specific decisions on therapy should be based on SMBG. Specific glycemic targets must consider overtreatment as well as undertreatment of blood glucose. Both preprandial and postprandial glycemic targets need to be individualized based on a patient’s knowledge and drug regimen, especially if it includes insulin. Although HbA1c levels between 7% and 8% appear to be associated with the highest survival rates in retrospective analyses of DKD patients, the imprecision of HbA1c measurements makes specific targets for people with DKD difficult to define. However, measurement of HbA1c still should be performed because the trending of the levels can assist in therapy decisions. |
Abbreviations: CKD, chronic kidney disease; DKD, diabetic kidney disease; HbA1c, glycated hemoglobin; KDIGO, Kidney Disease Improving Global Outcomes; KDOQI, Kidney Disease Outcomes Quality Initiative; SMBG, self-monitoring of blood glucose.
GLYCEMIC STATUS AND OUTCOMES IN CKD AND ESRD
Glycemic Status and Survival in NDD-CKD
Several large population-based studies examining glycemic status and mortality in US NDD-CKD patients have supported more moderate glycemic targets. In a secondary analysis of type 2 diabetic patients from the Action to Control Cardiovascular Risk in Diabetes trial, among whom 3,636 versus 6,506 patients had mild-to-moderate (stages 1–3) CKD versus normal kidney function, respectively, in the CKD subgroup intensive glycemic control was associated with a 31% higher all-cause mortality and a 41% higher cardiovascular mortality risk versus standard control.39 Because the two arms achieved significant separation in HbA1c levels over the course of the study (ie, 4-month HbA1c levels of 6.7% versus 7.5% in the intensive versus standard arms, respectively) without significant differences between CKD versus non-CKD patients, it may be inferred that lower HbA1c levels were associated with worse survival in patients with CKD. In addition, hypoglycemia was more frequent with intensive versus standard therapy in patients with CKD (5.3% versus 2.0%, respectively) and in those without CKD (3.5% versus 1.1%, respectively). In a larger study of 23,296 stage 3 to 4 CKD patients across four centers in Canada, higher HbA1c levels greater than 9% were associated with worsening of kidney function (ie, doubling of creatinine, ESRD), cardiovascular events, and all-cause hospitalization.40 However, a U-shaped relationship between HbA1c and survival was observed, such that both lower and higher HbA1c levels (<6.5 and >8%, respectively) were associated with higher mortality risk. In a more recent study of 618 participants with NDD-CKD (ie, estimated glomerular filtration rate <60 mL/min per 1.73 m2 or a urinary protein to creatinine ratio ≥30 mg/g) from the Seattle Kidney Study, among whom approximately half had diabetes, higher HbA1c levels were not associated with the composite outcome of death and/or ESRD, supporting a more conservative glycemic target in advanced kidney disease patients.41
Glycemic Status and Survival in ESRD
To date, there have been multiple observational studies of glycemic status and mortality in the diabetic ESRD population that have shown differential findings across prevalent versus incident dialysis patients (Table 3). In a study of 24,875 prevalent hemodialysis patients with diabetes from a national LDO, extremes of glycemia as defined by time-varying HbA1c levels (HbA1c <6.5% and >11%) each were associated with higher mortality risk.42 Similarly, in a subsequent study of 54,757 prevalent hemodialysis patients with diabetes from another national LDO, both lower and higher time-averaged HbA1c levels (ie, <7% and ≥8%) also were associated with a higher death risk.43 In a subsequent study of 9,201 prevalent hemodialysis patients with diabetes from the US portion of the Dialysis Outcomes and Practice Patterns Study cohort, both lower and higher HbA1c levels averaged over the first 8 months (HbA1c <6% and >9%) were associated with higher mortality risk.44 In a meta-analysis of 83,684 prevalent hemodialysis patients with diabetes, higher and very low HbA1c levels (>8.5% and <5.4%, respectively) were associated with a higher death risk.45
Although studies of prevalent hemodialysis patients with diabetes have shown a fairly consistent U-shaped relationship between HbA1c levels and mortality risk, a J-shaped pattern has been observed among incident dialysis patients. In a study of 63,607 incident hemodialysis patients with diabetes from a national LDO, time-varying (ie, repeated measures) and baseline (ie, measured upon transition to ESRD) HbA1c levels were assessed as proxies of short-term and long-term associations of glycemic status and mortality, respectively.9,10 Time-varying analyses showed a U-shaped association with mortality (ie, suggesting that intensive and liberal glycemic control are associated with short-term death risk), similar to that of prevalent ESRD studies in which glycemic measurements were restricted to the period after which terminal kidney failure developed. However, analyses of baseline HbA1c measured on transition to ESRD showed that HbA1c levels of 9% or greater were associated with a higher death risk, whereas HbA1c levels lower than 9% were associated with similar or improved survival (ie, suggesting that only liberal glycemic control is associated with long-term death risk).
Given the differential HbA1c−mortality relationships observed among incident versus prevalent ESRD patients, there has been growing interest in whether glycemic management during earlier stages of DKD have a greater impact on health and survival compared with later stages, and if there is a sustained “legacy effect ” of pre-ESRD glycemic status on post-ESRD survival, similar to observations from the long-term follow-up studies of the DCCT/EDIC and UKPDS trials.30,31 Studies under the National Institutes of Health U01 “Transitions of Care in CKD” US Renal Data System Special Study Center have sought to address this knowledge gap.46 In a study of 15,549 US veterans with NDD-CKD and diabe-tes, pre-ESRD HbA1c levels of 8% or greater were associated with higher post-ESRD mortality, whereas HbA1c levels lower than 6% were not associated with a higher death risk compared with the reference group (ie, HbA1c 6% to <7%), signaling a J-shaped association between glycemic status and mortality.12 Similarly, preESRD random glucose levels of 200 mg/dL or greater were associated with higher post-ESRD mortality whereas glucose levels less than 125 mg/dL were not associated with a higher death risk compared with the reference group (ie, glucose, 125 to <150 mg/dL). In a subsequent study separately examining glycemic status and mortality across two matched diabetic NDD-CKD populations according to whether they transitioned versus did not transition to dialysis (ie, because of death before the development of ESRD, lack of CKD progression, and so forth), higher averaged random glucose levels of 200 mg/dL or greater and HbA1c levels of 8% or higher were associated with early dialysis mortality in the Transition Cohort (ie, those who progressed to ESRD).47 However, among the matched diabetic NDD-CKD patients in the Nontransition Cohort (ie, those who did not transition to ESRD), both lower (hypoglycemic and low-normal glucose levels of <80 and 80 to <100 mg/dL, respectively) and higher glucose levels of 160 mg/dL or greater were associated with higher mortality.
These divergent patterns potentially may be explained by a differential effect of glycemic status on long-term versus short-term survival in patients with advanced CKD. Although the Transition Cohort showed a J-shaped relationship between random glucose and HbA1c levels with mortality risk, suggesting that, among those who survive and progress to ESRD,47 liberal glycemic control may have long-term detrimental outcomes over time (ie, via generation of oxidative stress, activation of protein kinase C, accumulation of advanced glycosylation end products, and progressive microvascular and macrovascular damage48,49), the U-shaped association between glycemic status and mortality in the Nontransition Cohort also suggest that intensive glycemic control is associated with short-term death risk, possibly owing to hypoglycemia or low-normal glucose levels leading to central nervous system toxicity and subsequent encephalopathy, seizures, coma, disequilibrium, and subsequent falls,50,51 and/or adrenergic stimulation, resulting in coronary ischemia, ventricular arrhythmias, and sudden cardiac death.52
Hypoglycemia and Mortality Risk in ESRD
In the general population, hypoglycemia is an established risk factor for cardiovascular morbidity (eg, myocardial infarction, stroke, and sudden cardiac death) and mortality.53–57 Given the exceedingly high cardiovascular mortality of ESRD patients,5 this has prompted more direct study of hypoglycemia in the ESRD population. In a study of 30,156 US veterans with DKD transitioning to ESRD, having one or more hypoglycemia-related hospitalizations in the 1- to 2-year period preceding dialysis was associated with higher post-ESRD mortality risk.15 Moreover, an increasing frequency of hypoglycemia was associated with incrementally higher death risk, such that those with three or more pre-ESRD hypoglycemia-related hospitalizations had a 2.1-fold higher post-ESRD death risk. In a study of 58,304 incident hemodialysis patients from a national LDO comprising 51,924 versus 6,380 patients with versus without underlying diabetes, respectively, having one or more hypoglycemia events was associated with a higher mortality risk in both the diabetic and nondiabetic subgroups.14
CONCLUSIONS AND FUTURE DIRECTIONS
In summary, DKD patients progressing to ESRD are at heightened risk for glycemic complications, including development of the burnt-out diabetes phenomenon. Furthermore, hypoglycemia is a frequent occurrence associated with worse survival in CKD patients with and without diabetes. Although extrapolation of data from clinical trials in high-cardiovascular-risk populations and growing observational data in diabetic NDD-CKD and ESRD patients suggest that moderate glycemic targets are associated with better survival, clinical trials are needed to confirm the optimal glycemic target and metric in DKD patients. Given the cardiovascular and neurotoxicity of dysglycemia, further studies also are needed to identify modifiable and nonmodifiable targets for hypoglycemia and hyperglycemia in CKD patients. For example, comparative effectiveness studies of newer antiglycemic agents with a lower risk of hypoglycemia and potentially greater cardiovascular benefit as compared with traditional agents in CKD are needed. Further investigation of multifaceted approaches in the management of DKD, including dietary interventions and modulation of the dialysis prescription, also may mitigate hypoglycemia and hyperglycemia in NDD-CKD and ESRD patients. Finally, greater study of the short- and long-term implications of glycemic targets on other clinically relevant end points, including infection risk and patient-reported outcomes (ie, health-related quality of life, depression) are needed. Given their exceptionally high mortality rate, there is a compelling need for further investigation of how to optimally manage dysglycemia in NDD-CKD and ESRD patients.
Acknowledgments
Financial support: Supported by research grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases including K24-DK091419 (K.K.-Z.), U01-DK102163 (K.K.-Z., C.P.K.), R44-116383 (K.K.-Z.), R03-DK114642 (C.M.R.), R01-DK122767 (C.M.R.), and R01-DK124138 (C.M.R., K.K.-Z.). Funders of this study did not have any role in the study design; collection, analysis, or interpretation of data; writing of the report; or the decision to submit the report for publication.
Footnotes
Conflict of interest statement: none.
REFERENCES
- 1.Diabetes in the United States. [cited 2020 Nov 1]. Available from: https://www.cdc.gov/diabetes/pdfs/library/socialmedia/diabetes-infographic.pdf.
- 2.Alicic RZ, Tuttle KR. Management of the diabetic patient with advanced chronic kidney disease. Semin Dial. 2010;23:140–7. [DOI] [PubMed] [Google Scholar]
- 3.Zelnick LR, Weiss NS, Kestenbaum BR, et al. Diabetes and CKD in the United States population, 2009–2014. Clin J Am Soc Nephrol. 2017;12:1984–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go AS, Yang J, Tan TC, et al. Contemporary rates and predictors of fast progression of chronic kidney disease in adults with and without diabetes mellitus. BMC Nephrol. 2018;19:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United States Renal Data System. 2020 USRDS Annual Data Report: epidemiology of kidney disease in the United States. Bethesda, MD:: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases;; 2020. [Google Scholar]
- 6.Kovesdy CP, Park JC, Kalantar-Zadeh K. Glycemic control and burnt-out diabetes in ESRD. Semin Dial. 2010;23:148–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kovesdy CP, Sharma K, Kalantar-Zadeh K. Glycemic control in diabetic CKD patients: where do we stand? Am J Kidney Dis. 2008;52:766–77. [DOI] [PubMed] [Google Scholar]
- 8.Rhee CM, Leung AM, Kovesdy CP, Lynch KE, Brent GA, Kalantar-Zadeh K. Updates on the management of diabetes in dialysis patients. Semin Dial. 2014;27:135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhee CM, Kim SB, Mehrotra R, Streja E, Nguyen DV, Brunelli SM, et al. Predictors of glycemic status and associations with mortality in incident diabetic hemodialysis patients. Abstract presented at: 2015 American Society of Nephrology Kidney Week Meeting. [Google Scholar]
- 10.Rhee CM, Kim S, Mehrotra R, Gillen D, Brunelli SM, Kovesdy CP, et al. Glycated hemoglobin levels and mortality in a large US cohort of incident diabetic hemodialysis patients. Abstract presented at: 2015 European Renal Association-European Dialysis Transplantation Congress. [Google Scholar]
- 11.Rhee CM, Ravel VA, Streja E, Soohoo M, Jing J, Nguyen DV, et al. The impact of pre-ESRD glycemic status on early post-ESRD mortality among US veterans: a transition of care in CKD study. Abstract presented at: 2015 American Society of Nephrology Kidney Week Meeting. [Google Scholar]
- 12.Rhee CM, Kovesdy CP, Ravel VA, et al. Association of glycemic status during progression of chronic kidney disease with early dialysis mortality in patients with diabetes. Diabetes Care. 2017;40:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moen MF, Zhan M, Hsu VD, et al. Frequency of hypoglycemia and its significance in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang DH, Streja E, You AS, Lee YK, Kovesdy CP, Kalantar-Zadeh K, et al. Hypoglycemia and mortality risk in incident hemodialysis patients. Abstracted presented at: 2019 American Society of Nephrology Kidney Week Meeting. [Google Scholar]
- 15.Rhee CM, Kovesdy CP, You AS, et al. Hypoglycemia-related hospitalizations and mortality among patients with diabetes transitioning to dialysis. Am J Kidney Dis. 2018;72:701–10. [DOI] [PubMed] [Google Scholar]
- 16.Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001;24:382–91. [DOI] [PubMed] [Google Scholar]
- 17.Legouis D, Faivre A, Cippa PE, de Seigneux S. Renal gluconeogenesis: an underestimated role of the kidney in systemic glucose metabolism. Nephrol Dial Transplant. 2020. In press. [DOI] [PubMed] [Google Scholar]
- 18.Abe M, Kalantar-Zadeh K. Haemodialysis-induced hypoglycaemia and glycaemic disarrays. Nat Rev Nephrol. 2015;11:302–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mak RH, Bettinelli A, Turner C, Haycock GB, Chantler C. The influence of hyperparathyroidism on glucose metabolism in uremia. J Clin Endocrinol Metab. 1985;60:229–33. [DOI] [PubMed] [Google Scholar]
- 20.Mak RH, Turner C, Haycock GB, Chantler C. Secondary hyper-parathyroidism and glucose intolerance in children with uremia. Kidney Int Suppl. 1983;16:S128–33. [PubMed] [Google Scholar]
- 21.Mak RH. Intravenous 1,25 dihydroxycholecalciferol corrects glucose intolerance in hemodialysis patients. Kidney Int. 1992;41:1049–54. [DOI] [PubMed] [Google Scholar]
- 22.Mak RH. 1,25-Dihydroxyvitamin D3 corrects insulin and lipid abnormalities in uremia. Kidney Int. 1998;53:1353–7. [DOI] [PubMed] [Google Scholar]
- 23.Koppe L, Fouque D, Soulage CO. Metabolic abnormalities in diabetes and kidney disease: role of uremic toxins. Curr Diab Rep. 2018;18:97. [DOI] [PubMed] [Google Scholar]
- 24.Koppe L, Mafra D, Fouque D. Probiotics and chronic kidney disease. Kidney Int. 2015;88:958–66. [DOI] [PubMed] [Google Scholar]
- 25.McCaleb ML, Izzo MS, Lockwood DH. Characterization and partial purification of a factor from uremic human serum that induces insulin resistance. J Clin Invest. 1985;75:391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes A. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S66–76. [DOI] [PubMed] [Google Scholar]
- 27.Diabetes C, Complications Trial Research G, Nathan DM, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- 28.United Kingdom Prospective Diabetes Study (UKPDS). Relative efficacy of randomly allocated diet, sulphonylurea, insulin, or metformin in patients with newly diagnosed non-insulin dependent diabetes followed for three years. BMJ. 1995;310:83–8. [PMC free article] [PubMed] [Google Scholar]
- 29.Nathan DM, Group DER. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. [DOI] [PubMed] [Google Scholar]
- 32.Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet. 2010;376:419–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Group AC, Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–72. [DOI] [PubMed] [Google Scholar]
- 34.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. [DOI] [PubMed] [Google Scholar]
- 35.National Kidney F. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–86. [DOI] [PubMed] [Google Scholar]
- 36.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 37.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Am J Kidney Dis. 2014;64:510–33. [DOI] [PubMed] [Google Scholar]
- 38.Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papademetriou V, Lovato L, Doumas M, et al. Chronic kidney disease and intensive glycemic control increase cardiovascular risk in patients with type 2 diabetes. Kidney Int. 2015;87:649–59. [DOI] [PubMed] [Google Scholar]
- 40.Shurraw S, Hemmelgarn B, Lin M, et al. Association between glycemic control and adverse outcomes in people with diabetes mellitus and chronic kidney disease: a population-based cohort study. Arch Intern Med. 2011;171:1920–7. [DOI] [PubMed] [Google Scholar]
- 41.Limkunakul C, de Boer IH, Kestenbaum BR, Himmelfarb J, Ikizler TA, Robinson-Cohen C. The association of glycated hemoglobin with mortality and ESKD among persons with diabetes and chronic kidney disease. J Diabetes Complications. 2019;33:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams ME, Lacson E Jr., Wang W, Lazarus JM, Hakim R. Glycemic control and extended hemodialysis survival in patients with diabetes mellitus: comparative results of traditional and time-dependent Cox model analyses. Clin J Am Soc Nephrol. 2010;5:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ricks J, Molnar MZ, Kovesdy CP, et al. Glycemic control and cardiovascular mortality in hemodialysis patients with diabetes: a 6-year cohort study. Diabetes. 2012;61:708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ramirez SP, McCullough KP, Thumma JR, et al. Hemoglobin A (1c) levels and mortality in the diabetic hemodialysis population: findings from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Diabetes Care. 2012;35:2527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hill CJ, Maxwell AP, Cardwell CR, et al. Glycated hemoglobin and risk of death in diabetic patients treated with hemodialysis: a meta-analysis. Am J Kidney Dis. 2014;63:84–94. [DOI] [PubMed] [Google Scholar]
- 46.Kalantar-Zadeh K, Kovesdy CP, Streja E, et al. Transition of care from pre-dialysis prelude to renal replacement therapy: the blueprints of emerging research in advanced chronic kidney disease. Nephrol Dial Transplant. 2017;32(Suppl 2):ii91–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee CM, Kovesdy CP, Ravel VA, et al. Glycemic status and mortality in chronic kidney disease according to transition versus nontransition to dialysis. J Ren Nutr. 2019;29:82–90. [DOI] [PubMed] [Google Scholar]
- 48.Kawahito S, Kitahata H, Oshita S. Problems associated with glucose toxicity: role of hyperglycemia-induced oxidative stress. World J Gastroenterol. 2009;15:4137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reusch JE. Diabetes, microvascular complications, and cardiovascular complications: what is it about glucose? J Clin Invest. 2003;112:986–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cryer PE. Hypoglycemia, functional brain failure, and brain death. J Clin Invest. 2007;117:868–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gosmanov AR, Gosmanova EO, Kovesdy CP. Evaluation and management of diabetic and non-diabetic hypoglycemia in end-stage renal disease. Nephrol Dial Transplant. 2016;31:8–15. [DOI] [PubMed] [Google Scholar]
- 52.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes. 2003;52:1469–74. [DOI] [PubMed] [Google Scholar]
- 53.Cryer PE. The barrier of hypoglycemia in diabetes. Diabetes.2008;57:3169–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galindo RJ, Beck RW, Scioscia MF, Umpierrez GE, Tuttle KR. Glycemic monitoring and management in advanced chronic kidney disease. Endocr Rev. 2020;;41:756–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Novodvorsky P, Bernjak A, Chow E, et al. Diurnal differences in risk of cardiac arrhythmias during spontaneous hypoglycemia in young people with type 1 diabetes. Diabetes Care. 2017;40:655–62. [DOI] [PubMed] [Google Scholar]
- 56.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117:910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu TM, Lin CL, Chang SN, Sung FC, Kao CH. Increased risk of stroke in patients with chronic kidney disease after recurrent hypoglycemia. Neurology. 2014;83:686–94. [DOI] [PubMed] [Google Scholar]