Abstract
Background
Glass ampoules are widely used to contain injection medications because of their properties. However, the existing literature reports that glass particle contamination is found in opening glass ampoules. To date, nursing practice standards on this issue have not gained attention in terms of the manual breaking methods generally used for opening ampoules in a clinical setting that can minimize the risk of glass particle contamination and, therefore, increase patient safety.
Objective
This study aimed to compare manual breaking methods commonly used to open ampoules in clinical practice that affected the number of glass particles and to identify the factors influencing glass particle contamination.
Methods
We used a comparative research design to evaluate the manual breaking methods for opening medication ampoules among 56 registered nurses from diverse clinical areas in a university hospital. Each participant broke 12 ampoules in two sizes using six methods, each method combined with wrapping material and one breaking direction. We measured the number of glass particles in five sizes for each method and the factors influencing glass particle contamination.
Results
In total, 449 of 672 ampoules were contaminated with glass particles. Breaking the ampoule with a cotton ball (partial ampoule neck wrapping) from an outward direction resulted in the fewest glass particles, while breaking the ampoule with a gauze pad (entire ampoule neck wrapping) from an inward direction resulted in the most glass particles. Breaking method, ampoule size and clinical experience significantly influence glass particle contamination (P < 0.05).
Conclusions
The method (wrapping technique and breaking direction) that nurses use to break ampoules affects the number of glass particles. Therefore, improving the standard guidelines for preparing medication injections and training in breaking methods for opening ampoules is essential.
Keywords: glass ampoule, glass particle contamination, opening ampoules, patient safety, quality improvement
Introduction
Contamination of medication contents by glass particles is a common phenomenon following the opening of glass ampoules [1, 2]. Glass particles may be injected into the body by intramuscular and intravenous administration routes, which have been reported to be dangerous or harmful to the patients [3, 4]. These particles circulating in a blood vessel may cause pulmonary emboli, infusion phlebitis, granuloma formation or nodular liver fibrosis [5–8]. Such contamination in intramuscular injections may also cause complications, including pain, hematoma formation, acute inflammation and transient nodules [4]. As a hazard, plastic containers have been introduced and recommended to prevent glass particle contamination and promote safety; however, they are not compatible with all medications [9]. Glass ampoules are still commonly used for containing medications because their properties do not cause reactions with medications [3, 10]. Therefore, ampoule breaking methods remain the primary process in preparing medication injections.
Several methods are used to reduce the glass particle contamination associated with breaking ampoules, such as a filter needle syringe, in-line filtering of an infusion set [3, 11] and time delay where nurses draw medication from the ampoule and wait for glass particles to settle before administering the medication [12]. Although filters can protect against glass particles, their uses are limited because of cost constraints and time-consuming drug administration preparation techniques [4, 9, 13]. Using filters and time delays are often not practical in clinical settings, especially in developing countries where resources are limited with overwhelming workloads from nursing shortages [9, 14]. Glass particle contamination remains a crucial issue in this context.
Furthermore, glass particle contamination has not received the necessary attention in education, and avoidance of contamination regarding the breaking techniques may not be standard practice in clinical settings [13]. To date, medication preparation remains unchanged. Research and education about adherence to safe methods for opening glass ampoules are imperative. The methods used to break ampoules vary across clinical settings. The factors that have been associated with the occurrence of glass particle contamination focus on ampoule size and aspiration technique, while less is known about other factors in clinical contexts [2, 4, 10, 15, 16]. This study’s results may inform clinical practice to provide a better quality for preparing medication injections to enhance patient safety and remove other devices’ needs in situations where resources are limited. Therefore, this study aimed to evaluate the influence of ampoule breaking methods on the number and size of glass particles. We also evaluated factors potentially influencing the occurrence of glass particle contamination.
Methods
Sample and setting
This study used a comparative research design to evaluate glass particle contamination. It was part of a larger project that aimed to compare methods for breaking medication ampoules among registered nurses. This study obtained ethics approval from the Hospital’s Institutional Review Board (IRB No. MURA2016/444 S3; part of IRB No. MURA2016/444 S1–3). We calculated the sample size based on the statistical analysis described by Hulley, Cummings, Browner, Grady and Newman [17]. The required sample size with a 10% attrition rate for this study was 52 participants. We initially approached 58 registered nurses from seven different areas using convenience sampling. The participants who had been engaged in clinical practice for at least 2 years were included in the study. In total, 56 registered nurses who were willing to participate in this study were asked to provide written informed consent. Given that the procedures used for breaking ampoules were manual, the researchers were concerned about participants’ safety. First aid care was available as required, and the researchers closely observed all participants.
Instruments
This study used data from the Ampoule Breaking Record that focused on glass particles. The instrument that three experts confirmed as appropriate for assessing and measuring glass particle contamination comprises three parts.
Part A covered participants’ characteristics and skills in terms of breaking direction. An outward direction referred to an ampoule positioned with a dot marker in the front, and the ampoule tip was broken away from the body. In an inward direction, the dot marker was at the back of the ampoule, and the tip was broken towards the body.
Part B focused on the time needed to break the ampoule in each method. This part covered six breaking methods for two ampoule sizes (2 and 10 ml). Breaking Methods 1 and 2 used a gauze pad, Methods 3 and 4 used a cotton ball, and Methods 5 and 6 used a syringe wrapper. Manually breaking in an outward direction was employed for Methods 1, 3 and 5, with an inward direction for Methods 2, 4 and 6. Each method was assessed by one question for the 2 ml ampoule and one for the 10 ml ampoule, which evaluated the time taken to break the ampoule (in seconds), starting with cleaning the ampoule neck and finishing when the ampoule tip was off.
Part C was used to record the number of glass particles found in each ampoule size for each method. The glass particles were categorized into five size groups based on the needle gauges’ inner diameters typically used to infiltrate medication from glass ampoules in a clinical setting. The particle sizes were diameter <60 µm (G 20); diameter 61–68 µm (G19); diameter 69–83 µm (G18); diameter 84–120 µm and diameter >120 µm. The number of glass particles found in each broken ampoule was scored against these five sizes. Glass particle contamination was recorded as occurring if glass particles were found or non-occurring if no glass particles were found in the broken ampoule.
Data collection
The data were collected in the nursing laboratory. The participants were asked to randomly choose a number from one to six times (one number per time), which specified the sequence in which they performed the breaking method. Each participant broke 12 ampoules in total, using each method with two sizes of ampoules. Before participants broke an ampoule using each method, they washed their hands and put on gloves. Next, the ampoule neck was cleaned with 70% alcohol and broken as per each technique. Then, participants rested for 5 min before starting the next method. The time to complete the assignment for each participant was 1.5–2 h. Each broken ampoule was sealed in a plastic container and sent for an examination of glass particles. Scientists who had experienced measuring glass particles under a scanning electron microscope with grids and a calibrated ocular micrometre measured all content from each broken ampoule to determine any glass particles’ number and size (Figure 1). Any glass participles smaller than 20 µm were marked by the examiner as ‘sandy particles’ and not precisely measured.
Figure 1.
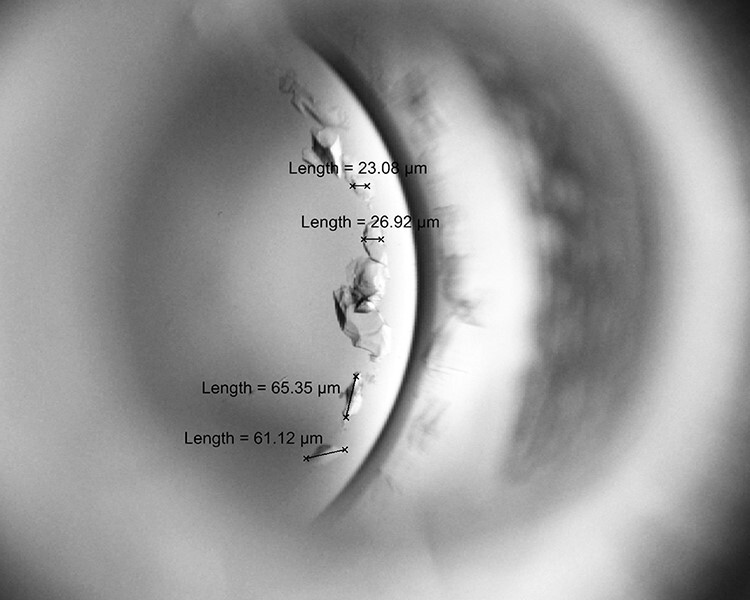
Capture glass particles in broken ampoule to measure number and size.
Data analyses
We analysed data with SPSS version 21 and used descriptive statistics to explore participants’ characteristics, number and size of glass particles, and occurrence of glass particle contamination. Univariate analysis was used to examine the association between the predicting factors (breaking method, ampoule size, breaking direction, area of speciality, work experience and time taken to break the ampoule) and glass particle contamination. We included the factors with a P-value < 0.25 in the logistic regression model. Binary logistic regression was used to predict the occurrence of glass particle contamination using Stata (Version 13).
Results
Participants’ characteristics
In total, 75% of participants were aged over 30 years, with a mean age of 28.80 (SD 4.65) years. Most participants had undergraduate degrees (89.3%) and <5 years of work experience (57.1%). The most common speciality was recorded as medical nursing (25.0%), and the least common was the emergency department (10.7%). Participants had relatively equal skills in terms of ampoule breaking direction (inward and outward).
Effects of ampoule size and breaking method
Of the 672 ampoules examined, 449 ampoules were contaminated with glass particles. The total number of glass particles in these 449 ampoules was 2744 (Table 1). Both the 10 and 2 ml ampoules had more small glass particles (≤83 µm) than large glass particles (>83 µm). In the 10 ml ampoules, 1673 glass particles were ≤83 µm and 234 were >83 µm. In the 2 ml ampoules, 757 glass particles were ≤83 µm and 80 were >83 µm.
Table 1.
Number and size of glass particles by ampoule size and breaking method (n = 672)
| Number of glass particles | ||||||||
|---|---|---|---|---|---|---|---|---|
| Breaking method | Ampoule size (ml) | <20G <60 µm |
19G 61–68 µm |
18G 69–83 µm |
84–120 µm | >120 µm | Total | Mean (SD) |
| Method 1 | 10 | 246 | 5 | 2 | 13 | 22 | 288 | 5.14 (4.97) |
| 2 | 142 | 1 | 3 | 7 | 11 | 164 | 2.93 (4.24) | |
| 388 | 6 | 5 | 20 | 33 | 452 | 4.04 (4.73) | ||
| Method 2 | 10 | 401 | 6 | 8 | 21 | 46 | 482 | 8.61 (6.37) |
| 2 | 173 | 0 | 3 | 2 | 11 | 189 | 3.38 (4.06) | |
| 574 | 6 | 11 | 23 | 57 | 671 | 5.99 (5.93) | ||
| Method 3 | 10 | 189 | 1 | 1 | 6 | 14 | 211 | 3.77 (4.06) |
| 2 | 47 | 0 | 0 | 2 | 9 | 59 | 1.05 (2.08) | |
| 236 | 1 | 2 | 8 | 23 | 270 | 2.41 (3.99) | ||
| Method 4 | 10 | 219 | 0 | 6 | 18 | 19 | 262 | 4.68 (5.76) |
| 2 | 64 | 0 | 2 | 7 | 1 | 74 | 1.32 (1.97) | |
| 283 | 0 | 8 | 25 | 20 | 336 | 3.00 (4.61) | ||
| Method 5 | 10 | 235 | 2 | 5 | 12 | 28 | 282 | 5.04 (4.26) |
| 2 | 141 | 2 | 2 | 6 | 6 | 157 | 2.80 (5.68) | |
| 376 | 4 | 7 | 18 | 34 | 439 | 3.92 (5.12) | ||
| Method 6 | 10 | 343 | 0 | 4 | 12 | 23 | 382 | 6.82 (6.86) |
| 2 | 175 | 0 | 0 | 3 | 15 | 194 | 3.46 (6.27) | |
| 518 | 0 | 5 | 15 | 38 | 576 | 5.14 (6.75) | ||
| Total | 10 | 1633 | 14 | 26 | 82 | 152 | 1907 | 5.68 (5.04) |
| 2 | 742 | 3 | 12 | 27 | 53 | 837 | 2.99 (4.44) | |
Ampoules with more glass particles were broken with Methods 1 and 2, and those broken with Methods 3 and 4 had fewer particles (Table 1). The sizes of glass particles did not differ across the six breaking methods. Method 2 had the most significant number of glass particles that were ≤83 µm, and Method 3 had the lowest number of particles of this size. Similarly, Method 2 had the most glass particles >83 µm, and Method 3 had the least number of particles of this size.
Factors influencing glass particle contamination
The univariate analyses showed a significant association between the occurrence of glass particle contamination and breaking method, ampoule size and clinical work experience. Breaking direction, area of speciality and breaking time did not show significant associations; however, breaking time had a P-value <0.25 and was retained for the logistic regression model. When all factors were analysed together, they showed significant and insignificant odds ratios (ORs) for glass particle contamination (n = 672, LR χ2(8) = 173.39, P > χ2 < 0.001). The breaking method, ampoule size and clinical work experience showed significant associations with glass particle contamination (Table 2). Among the breaking methods, Methods 3 and 4 had lower ORs for glass particle contamination (0.35 and 0.37, respectively) compared with Method 1. Breaking time was not significantly associated with the OR for glass particle contamination.
Table 2.
Factors predicting the occurrence of glass particle contamination (n = 672)
| Particle | ||||
|---|---|---|---|---|
| Factors | Occurrence (n = 456) n (%) |
Non-occurrence (n = 216) n (%) |
OR (95% CI) | P |
| Method | ||||
| Method 1 (n = 112) | 84 (75.0) | 28 (25.0) | Reference | – |
| Method 2 (n = 112) | 87 (77.7) | 25 (22.3) | 1.19 (0.61–2.35) | 0.610 |
| Method 3 (n = 112) | 64 (57.1) | 48 (42.9) | 0.35 (0.18 − 0.67) | 0.002 |
| Method 4 (n = 112) | 65 (58.0) | 47 (42.0) | 0.37 (0.20 − 0.71) | 0.003 |
| Method 5 (n = 112) | 78 (69.6) | 34 (30.4) | 0.73 (0.38 − 1.40) | 0.338 |
| Method 6 (n = 112) | 78 (69.6) | 34 (30.4) | 0.72 (0.37 − 1.39) | 0.329 |
| Ampoule size | ||||
| 2 ml (n = 336) | 158(47.0) | 178 (53.0) | Reference | – |
| 10 ml (n = 336) | 298 (88.7) | 38 (11.3) | 9.53 (6.19 − 14.69) | 0.000 |
| Work experience, years | ||||
| ≤5 (n = 325) | 235 (72.3) | 90 (27.7) | Reference | – |
| >5 (n = 347) | 221 (63.7) | 126 (36.3) | 0.60 (0.41 − 0.88) | 0.008 |
| Breaking time; mean (SD) | 16.67 (10.60) | 13.38 (7.79) | 1.01 (0.99–1.03) | 0.535 |
Methods: Method 1 = gauze pad and outward direction, Method 2 = gauze pad and inward direction, Method 3 = cotton ball and outward direction, Method 4 = cotton ball and inward direction, Method 5 = syringe wrapper and outward direction and Method 6 = syringe wrapper and inward direction.
CI, confidence interval.
Discussion
Principle findings
The breaking method (wrapping technique and breaking direction) affected the number of glass particles. Wrapping technique, ampoule size and work experience were associated with glass particle contamination.
Interpretation within the context of the broader literature
We found that the number of glass particles differed by breaking method and ampoule size. Breaking the ampoule using a cotton ball (Methods 3 and 4) resulted in fewer glass particles than breaking the ampoule using a gauze pad (Methods 1 and 2) or a syringe wrapper (Methods 5 and 6). An explanation for this finding is that the material used to wrap the ampoule neck affected the number of glass particles. In Methods 1 and 2 (gauze pad) and 5 and 6 (syringe wrapper), the ampoule neck was entirely covered by a protective wall, meaning the broken glass particles fell from all around the ampoule neck into the broken ampoule. In contrast, in Methods 3 and 4, a cotton ball was used to wrap the ampoule neck on only one side, meaning the glass particles drop into the broken ampoule from that side. In general, because of glass ampoule sealing, the pressure inside a glass ampoule is negative; when the glass ampoule is opened, the glass particles are drawn into the ampoule [12]. Our findings indicated that material that was entirely or partially covering the ampoule neck affected the number of glass particles.
Furthermore, breaking the ampoule in an outward direction (Methods 1, 3 and 5) resulted in fewer glass particles than breaking the ampoule in an inward direction (Methods 2, 4 and 6). This finding suggested that the direction of ampoule breaking was associated with the number of glass particles. Therefore, the number of glass particles depends on both the wrapping technique and the breaking direction. Consistent with prior studies, our findings showed that the breaking method affected the number of glass particles [4, 10].
Our logistic regression analysis confirmed that the breaking method was associated with glass particle contamination; breaking the ampoule using Methods 3 and 4 was associated with lower glass particle contamination than Method 1. However, Methods 3 and 4 both used a cotton ball as the wrapping material while differing in breaking direction. These results suggest that glass particle contamination depends on how the ampoule neck is covered, whereas the number of glass particles depends on how the ampoule neck is covered and the breaking direction. Therefore, a practical process for preventing glass particle contamination may involve breaking the ampoule in an outward direction, which is safer from ampoule injury [14]. We also found that the larger ampoule size (10 ml) had more glass particles than the small ampoule (2 ml). This finding was confirmed with logistic regression, which showed that ampoule size was associated with glass particle contamination. Compared with the 2 ml ampoule, breaking a 10 ml ampoule resulted in an OR for glass particle contamination 9.53. Consistent with the previous study, larger ampoules had more glass particle contamination than smaller ampoules [18, 19]. A possible explanation for this is that large glass ampoules have a wider ampoule neck than small ones. The diameter and thickness of the ampoule neck may also impact the number of glass particles. Consistent with previous studies, our results showed that breaking a large ampoule resulted in more glass particles than a small ampoule [2, 15]. Therefore, patients who receive injectable medication from a large ampoule have a greater risk for glass particle accumulation in the body compared with injection from a smaller ampoule [12]. Therefore, it may be necessary to produce ampoules with small necks. However, our results’ average number of glass particles was lower than reported in previous studies [3, 4]. A possible explanation is that previous studies measured glass particles by aspirating medication content and centrifuging to precipitate glass particles before counting, which differed from our research [11].
The size of the glass particles did not differ by ampoule size and breaking methods. Similarly, a prior study reported that the particle size did not vary with ampoule size [6]. Particles smaller than 83 µm can be aspirated through an 18G needle in a more significant number and size than aspirated through a 20G needle (diameter 60 µm). From previous studies, it is observed that withdrawing injected medication through a smaller-sized needle can minimize glass particles’ number and size [6, 11, 20–22]. Therefore, using a smaller needle can expel larger-sized glass particles better than using a larger needle.
Participants’ work experience was also associated with glass particle contamination. Ampoules broken by nurses with clinical work experience of >5 years had 0.60 times lower glass particle contamination than those who had ≤5 years of work experience. It may be that most nurses with clinical experience of >5 years have advanced skills and techniques for breaking ampoules. Our finding was consistent with previous studies finding nurses who had sufficient clinical experience to possibly be at lower risk for needle and sharp-edge injuries. In contrast, nurses with less experience may have more insufficient knowledge and inadequate practices than experienced nurses [23, 24]. However, the frequency with which each nurse breaks ampoules may be a factor that affects glass particle contamination. Increasing nurses’ skills will provide nurses with more experience in preparing injectable drugs, which will reduce glass particle contamination.
Implications for practice and research
The wrapping technique on the ampoule neck and breaking direction affect glass particles, while the wrapping technique predicts glass particle contamination. Therefore, partially wrapping the ampoule and breaking it outward should be implied in standard guidelines for preparing medication injections. Training methods for breaking ampoules to reduce glass particle contamination should be addressed in educational institutes and hospital policy. Further research should investigate which wrapping technique and breaking direction can reduce glass particle contamination and ensure that healthcare providers are safe from sharp-edge injury.
Strengths and limitations
Two scientists recorded and confirmed the numbers and sizes of glass particles, which made the data very consistent, precise and reliable.
Our study involved a small sample size different from other binary logistic regression sample size calculations because this study had a comparative research design, was conducted in a private centre and had a process for manipulating glass ampoules, such as physical treatment, which limited recruitment possibilities [25]. However, we used the data collection method to eliminate bias by recording the participants one at a time and using a randomization technique to choose the breaking method to prevent sample correlation. Although the sample size seems small, the primary samples used for the investigation were 672 broken ampoules that were large enough in comparison with previous studies (100–108 broken ampoules) [4, 12]. Our study created in an artificial situation that may not represent real clinical settings. Promoting better-informed practice in all healthcare settings where such ampoule breaking is practised and to confirms generalizability requires further research needs to evaluate the ampoule breaking direction in other hospitals and countries in real clinical settings.
Acknowledgements
The authors thank all nurses in the medical hospital who participated in this study.
Contributor Information
Natthacha Chiannilkulchai, Ramathibodi School of Nursing, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama 6 Road, Ratchathewi, Bangkok 10400, Thailand.
Siranee Kejkornkaew, Ramathibodi School of Nursing, Faculty of Medicine Ramathibodi Hospital, Mahidol University, 270 Rama 6 Road, Ratchathewi, Bangkok 10400, Thailand.
Conclusion
In the present study, breaking an ampoule with a cotton ball from an outward direction resulted in the fewest glass particles. Nurses’ specific techniques for breaking medication ampoules may be an essential factor in reducing glass particle contamination. Our study found that the wrapping technique and breaking direction affected the number of glass particles, but previous research focused on this type of finding is limited. However, the primary concern remains opening glass ampoules to ensure that patients are safe from glass particle contamination, while nurses and other healthcare personnel are safe from injury due to sharp edges while preparing medication injections.
Funding
This study was financially supported by Ramathibodi School of Nursing, Faculty of Medicine Ramathibodi Hospital, Mahidol University, Thailand.
Authors’ contributor
N.C. and S.K. designed the study and data collection. N.C. completed the analysis and drafted the manuscript. S.K. critically revised the manuscript. N.C. and S.K. revised the manuscript and approved the final version.
Ethics and other permissions
This study obtained ethics approval from Ramathibodi Hospital’s Research Ethics Committee (IRB No. MURA2016/444 S3).
Data availability statement
The data underlying this article will be shared at a reasonable request to the corresponding author.
References
- 1. Stoker R. Preventing InjuriesfromGlass Ampoule Shards. https://sofia.medicalistes.fr/spip/IMG/pdf/Preventing_injuries_from_glass_ampoule_shards.pdf (30 December 2020, date last accessed).
- 2. Zabir AF, Choy CY, Rushdan R. Glass particle contamination of parenteral preparations of intravenous drugs in anaesthetic practice. SAJAA 2008;14:17–9.doi: 10.1080/22201173.2008.10872550. [DOI] [Google Scholar]
- 3. Joo GE, Sohng K-Y, Park MY. The effect of different methods of intravenous injection on glass particle contamination from ampules. SpringerPlus 2016;5:1–8.doi: 10.1186/s40064-015-1632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee K-R, Chae Y-J, Cho S-E et al. A strategy for reducing particulate contamination on opening glass ampoules and development of evaluation methods for its application. Drug Dev Ind Pharm 2011;37:1394–401.doi: 10.3109/03639045.2011.580349. [DOI] [PubMed] [Google Scholar]
- 5. Brewer JH, Dunning JH. An in vitro and in vivo study of glass particles in ampules. J Am Pharm Assoc 1947;36:289–93.doi: 10.1002/jps.3030361002. [DOI] [PubMed] [Google Scholar]
- 6. Carbone-Traber KB, Shanks CA. Glass particle contamination in single-dose ampules. Anesth Analg 1986;65:1361–3. [PubMed] [Google Scholar]
- 7. Garvan JM, Gunner BW. The harmful effects of particles in intravenous fluids. Med J Aust 1964;2:1–6.doi: 10.5694/j.1326-5377.1964.tb114892.x. [DOI] [PubMed] [Google Scholar]
- 8. Puntis JW, Wilkins KM, Ball PA et al. Hazards of parenteral treatment: do particles count? Arch Dis Child 1992;67:1475–7.doi: 10.1136/adc.67.12.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Merry AF, Gargiulo DA, Fry LE. What are we injecting with our drugs? Anaesth Intensive Care 2017;45:539–42.doi: 10.1177/0310057X1704500503. [DOI] [PubMed] [Google Scholar]
- 10. Carraretto AR, Curi EF, de Almeida CED et al. Glass ampoules: risks and benefits. Braz J Anesthesiol 2011;61:513–21.doi: 10.1016/S0034-7094(11)70059-9. [DOI] [PubMed] [Google Scholar]
- 11. Sabon RL, Cheng EY, Stommel KA et al. Glass particle contamination: influence of aspiration methods and ampule types. Anesthesiology 1989;70:859–62. [PubMed] [Google Scholar]
- 12. Yorioka K, Oie S, Kamiya A. Comparison of particulate contamination in glass and plastic ampoules of glycyrrhizin injections after ampoule cutting. J Food Drug Anal 2009;17:225–8.doi: 10.38212/2224-6614.2606. [DOI] [Google Scholar]
- 13. Stein HG. Glass ampules and filter needles: an example of implementing the sixth ‘r’ in medication administration. Medsurg Nurs 2006;15:290–4. [PubMed] [Google Scholar]
- 14. Chiannilkulchai N, Kejkornkaew S. A comparative study of ampoule breaking and resultant injury among registered nurses. Pac Rim Int J Nurs Res 2020;24:89–101. [Google Scholar]
- 15. Park JS, Oh HR, Seo BH et al. Comparison of glass particle contamination according to method of ampule cutting and needle aspiration. J Korean Acad Nurs 2006;36:1033–41. [DOI] [PubMed] [Google Scholar]
- 16. Özlü ZK, Yayla AÇ, Gümüş K et al. The safe use of sharps and needlestick among nurses working in surgical clinics, Turkey. Eurasian J Emerg Med 2016;15:187–92.doi: 10.5152/eajem.2016.85856. [DOI] [Google Scholar]
- 17. Hulley SB, Cummings SR, Browner WS et al. Designing Clinical Research: An Epidemiologic Approach. 4th edn. Philadelphia, PA: Lippincott Williams & Wilkins, 2013. [Google Scholar]
- 18. Kempen PM, Sulkowski E, Sawyer RA. Glass ampules and associated hazards. Crit Care Med 1989;17:812–3.doi: 10.1097/00003246-198908000-00018. [DOI] [PubMed] [Google Scholar]
- 19. Preston ST, Hegadoren K. Glass contamination in parenterally administered medication. J Adv Nurs 2004;48:266–70.doi: 10.1111/j.1365-2648.2004.03195.x. [DOI] [PubMed] [Google Scholar]
- 20. Lye ST, Hwang NC. Glass particle contamination: is it here to stay? Anaesthesia 2003;58:93–4.doi: 10.1046/j.1365-2044.2003.296812.x. [DOI] [PubMed] [Google Scholar]
- 21. Katz H, Borden H, Hirscher D. Glass-particle contamination of color-break ampules. Anesthesiology 1973;39:354.doi: 10.1097/00000542-197309000-00025. [DOI] [PubMed] [Google Scholar]
- 22. Shaw NJ, Lyall EG. Hazards of glass ampoules. Br Med J (Clin Res Ed) 1985;291:1390.doi: 10.1136/bmj.291.6506.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cho E, Lee H, Choi M et al. Factors associated with needlestick and sharp injuries among hospital nurses: a cross-sectional questionnaire survey. Int J Nurs Stud 2013;50:1025–32.doi: 10.1016/j.ijnurstu.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hinkin J, Cutter J. How do university education and clinical experience influence pre-registration nursing students’ infection control practice? A descriptive, cross-sectional survey. Nurse Educ Today 2014;34:196–201.doi: 10.1016/j.nedt.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 25. Alvarez G, Núñez-Cortés R, Solà I et al. Sample size, study length, and inadequate controls were the most common self-acknowledged limitations in manual therapy trials: a methodological review. J Clin Epidemiol 2021;130:96–106.doi: 10.1016/j.jclinepi.2020.10.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared at a reasonable request to the corresponding author.


