Abstract
In contrast to traditional laboratory animals, prairie voles form socially monogamous partnerships in the wild, and exhibit lasting social preferences for familiar individuals—both mates and same-sex peers—in the lab. Decades of research into the mechanisms supporting pair bonding behavior have made prairie voles an important model organism for the study of social relationships. The partner preference test is a laboratory test of familiarity preference that takes place over an extended interval (typically 3 hours), during which test subjects can directly interact with conspecifics and often engage in resting side-by-side contact, a.k.a. huddling. The use of this test has enabled study of the neural pathways and mechanisms involved in promoting or impairing relationship formation. The tendency to form partner preferences is also used as a behavioral indicator of the effects of early life experiences and environmental exposures. While this test was developed to assess the extent of social preference for mates in prairie voles, it has been adapted for use in other social contexts including same-sex peer relationships and in multiple other species. This protocol provides instructions for conducting the classic partner preference test, as well as variations including same-sex “peer” partner preference tests. The effects of several protocol variations are examined, including duration of cohousing, separation interval, the use of tethers versus barriers, linear versus branched apparatus configuration, and duration of the test. We then consider the role of social variables including sex of the focal individual, sex of conspecifics, reproductive state, other social choices, and the use of the test in other species. Finally, we examine sample data and discuss scoring and statistical analysis of partner preference tests.
Keywords: social behavior, partner preference, partner preference test, prairie vole, social recognition, familiarity preference
INTRODUCTION:
Social interactions may take place with familiar or novel individuals, and these interactions may be either nonselective or selective. For selective relationships, it is common to use familiarity preference as an index of social relationship formation or “bond” (Carter, 1998). For species such as humans and prairie voles that form relationships, selective preference for specific individuals is a foundation of these relationships, and can be assessed with behavioral tests in the laboratory. While the vast majority of rodents mate promiscuously and do not form social bonds, prairie voles (Microtus ochrogaster) form lasting “pair bonds” with mates in the wild (Getz et al., 1981; Madrid et al., 2020). In the laboratory, both males and females exhibit selective partner preferences for a mate over an unfamiliar opposite-sex vole (Williams et al., 1992b), and for familiar same-sex peers over novel ones (DeVries et al., 1997; Beery et al., 2018; Lee et al., 2019). The expression of a suite of social behaviors including peer and mate relationships and biparental care that are shared with humans have brought prairie voles to prominence within the realm of social neuroscience; see the accompanying review article by Kenkel et al. (this issue) for a guide to distinctive behavioral and physiological features of prairie voles.
Social preferences in voles are primarily assessed using the partner preference test (PPT), developed in the laboratory of Dr. Sue Carter (Williams et al., 1992b), and described in this protocol. This three-hour test is used to analyze the behavior of a focal subject that can roam freely between a tethered mate “partner” and a novel “stranger” conspecific. The extended interval allows for exploration and habituation to occur, with stable huddling occurring predominantly in the second and third hours. Since the 1990s, this test has been in continuous use in prairie voles to assess selective relationships with mates. A same-sex “peer” version of this test (DeVries et al., 1997) has been used extensively to assess the selectivity of social relationships between group members of multiple species (Beery and Shambaugh, 2021). These behavioral characterizations have laid the foundation for myriad manipulation studies that probe the neurobiological basis of these social preferences (reviewed in, e.g.: (Carter et al., 2008; Anacker and Beery, 2013; Lieberwirth and Wang, 2016; Walum and Young, 2018). Behavior in the PPT can also be used as an indicator of the effects of life experience and exposures on social preferences.
This article includes a detailed protocol for conducting the partner preference test, and an accompanying support protocol for behavioral scoring. The Critical Parameters section provides data and commentary on the effects of alterations of key variables including cohabitation duration prior to the test, separation following cohabitation, test duration, apparatus configuration (linear versus branched), as well the use of tethers versus barriers. It also contains a survey of versions of the PPT with different social behavior choices including the same-sex peer PPT, and discussion of applications of the PPT and peer PPT in species beyond prairie voles. The Background section provides broader historical context for the test, and discussion of potential future directions and applications.
STRATEGIC PLANNING
In planning the partner preference test, it is essential to consider which parameters will support the goals of the study. For instance, research focused on factors that may enhance relationship formation should use short cohousing durations that are insufficient for most voles to form a preference, while manipulations that may impair preference formation should be tested in animals cohoused for durations that are sufficient to induce partner preference. Several such decisions are reviewed in the CRITICAL PARAMETERS section, including: cohabitation duration prior to testing, whether there is a separation interval prior to the test, effects of altering the duration of the test, and effects of apparatus design (branched versus linear). Decisions related to the test subjects are discussed, including the reproductive state of the individuals tested, and sex of the subject and stimulus voles. Familiarity preference testing with the same-sex peer partner preference test is introduced, along with other social comparisons that have been made with the partner preference test setup, and species beyond voles that have been tested. Review of each of these considerations prior to conducting research should be sufficient to determine the desired test setup.
BASIC PROTOCOL 1
Partner Preference Test
In this protocol the researcher will set up a behavioral choice test, allow it to run for 3 hours undisturbed while it is video recorded, then take down the test. If conducted properly, the researcher will return to find the two stimulus voles tethered and may encounter the focal vole huddling, but test results will not be known until behavioral scoring occurs. Handling and tethering voles can be challenging, and if done inappropriately may interfere with the behavior of the animal or even alter the outcome of a given study. Thus, practice with these activities and use of bite-proof gloves is beneficial.
Materials:
For each PPT setup:
Three voles:
Focal test subject
Tethered partner (familiar cagemate)
-
Tethered stranger (novel conspecific)
Subjects are typically adults between 60–120 days of age, and age-matched within a study. In the original partner preference test, both the partner and the stranger are of the opposite-sex from the focal vole, but see Critical Parameters for the same-sex “peer PPT” and other variants.
Vole tethers (2 per setup) – constructed from the following parts:
-
Plastic zip tie (8” length)
If videos are scored by a computer, the use of different colors of zip ties, or attachment of colored pipe cleaners to the top of the zip tie can assist with individual identification.
3 to 4 swivels: e.g. Bass Pro Barrel Swivel with Interlock Snap
- Anchor: wire loop with slide-in washer system (Figure 1A, B, D) as in (Ahern et al., 2009)
- wire cable (1/16”)
- aluminum cable clamp (3/32”)
- 2 washers (3/8 by 1½”)
- 3 nuts (1/4”)
Figure 1:
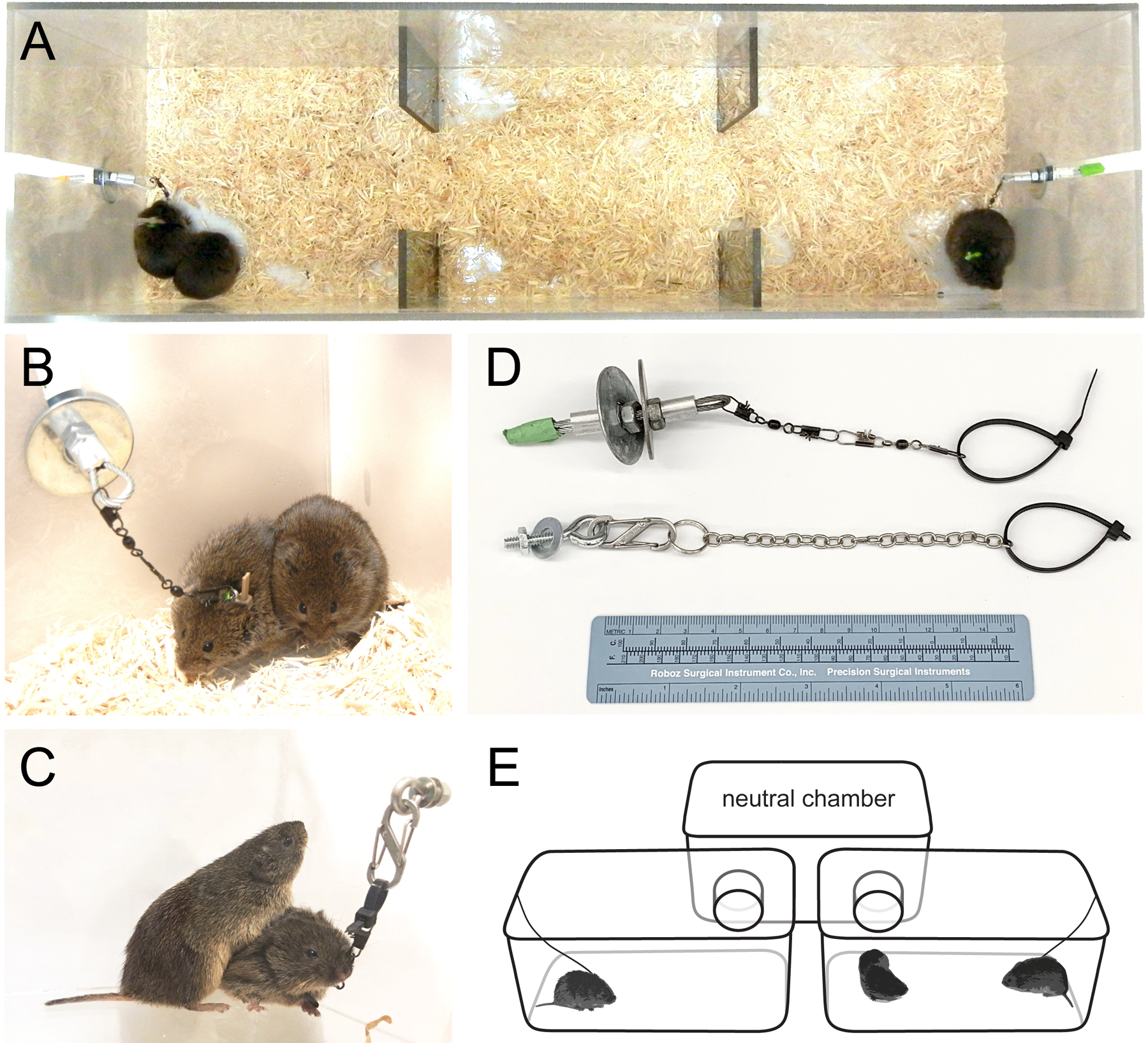
Apparatus and tethering setup for partner preference test. A: Linear apparatus and tether setup. One familiar and one novel individual are tethered in the end chambers. In the standard PPT, the focal animal is of one sex and the partner and stranger are of the opposite sex. In the peer partner preference test (pPPT), all animals are of the same sex. B: Close-up of tethered meadow vole showing attachment of the swivels to the crimped wire loop of an anchor. Metal components are used for the reusable portion of the tether; plastic zip-ties are cut at the end of each test. C. Close-up on a prairie vole, tethered to a fixed anchor. D. Slide-in and clip-on tether attachment systems. E. Schematic of a branched PPT setup. Branched apparatuses may be constructed of cages covered with clear, perforated lids, or as tall-sided custom chambers.
Plastic, three-chambered apparatus with attachment point for tethers. For a discussion of choice of apparatus type see Critical Parameters.
-
Linear version: 20 cm wide × 30 cm long × 75 cm high (Figure 1A; also see Ahern et al., 2009).
High sides obviate an apparatus cover, improving aerial video footage. Sides can be clear to allow video from the sides, or opaque to allow simultaneous aerial recording of multiple adjacent chambers.
-
Branched version: assembled from three equal sized chambers, (e.g. 17 cm × 28 cm × 12.5 cm) and arranged as a rear “neutral” chamber connected by tubes (≥5 cm diameter, 5 cm length) to two front chambers (Figure 1E).
If this version is made of low-height animal caging, it should be covered with clear perforated lids to prevent animal escape.
Clipper or scissors for removing zip ties after the test (e.g. side-cutting sprue wire clipper, diagonal wire cutter)
Video camera and mount: either a digital video camera with high capacity SD card, set to long play mode, or a computer connected camera (e.g. Basler Ace) and recording software.
Test cards (index cards, or pre-printed sheets) to display on-camera with test information.
Timer
Cleaning supplies (spray bottle with dilute soap or 70% ethanol, paper shop towels, cloths, or baby wipes)
Bite-resistant vole handling gloves.
Tight-fitting deerskin leather gloves (e.g. Wells Lamont 987), or nitrile coated gardening gloves over disposable gloves (e.g. Wonder Grip’s Nicely Nimble gloves or Wildflower Tools Gardening Gloves) work well.
Optional: hanging water bottles or gel water
Optional: bedding
Scoring software.
A variety of tools are available for scoring, including those listed below and discussed in the support protocol Behavioral Scoring.
IntervoleTimer (https://github.com/BeeryLab/intervole_timer/)
JWatcher (Blumstein and Daniel, 2007)
BORIS (Friard and Gamba, 2016)
SocialScan (CleverSys Inc.)
Ethovision XT (Noldus, Inc)
idTracker (Pérez-Escudero et al., 2014)
NOTE: All protocols using live animals must be reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) and follow officially approved procedures for the care and use of laboratory animals.
Protocol steps — Step annotations:
One day prior to testing, separate the focal vole and its partner to solo housing if using a separation step (see Critical Parameters).
Move cages of all voles to be tested to the testing area for habituation during setup (~30 min prior to test start).
-
Set up PPT apparatuses on a low surface, within view of camera. Lightly cover the floor of the chambers with clean bedding.
Placement of apparatuses inside a frame or cabinet can ensure consistent positioning below the camera. If filming from the side, apparatuses can be placed on shelves in a rack or on a cart.
Set up or check video recording equipment. Portable video cameras should be plugged in, and show >3 hours of remaining recording time. Computer-attached cameras should have recording software launched.
-
Prepare a test card to hold in view of the camera at the start of the test.
An index card and marker work fine, or pre-printed cards can be filled out with unique test ID, date of the test, and study name. The IDs of the left and right voles can be written on an image of the PPT apparatus. It is desirable to omit the ID number of the focal vole in case a human scorer could infer the identify of the partner from this information, but to include a second copy of the test card with the focal vole ID in a laboratory notebook.
Assemble all tethers needed (and one spare). Thread one of the zip ties through the end snap of the 3–8 unit long swivel-interlock snap chain. The other end of the chain should be connected to whatever hardware will anchor it to the apparatus. Close the zip tie loosely so that it forms a loop ~2 fingers wide.
-
Apply tether to the first “stranger” vole. Wearing bite-proof gloves and holding the vole securely in one hand by the scruff or along the back, slide the zip tie loop over the head of the vole, and tighten. Check to make sure that the tether is loose around the neck, but not wide enough to pass over the skull.
Make sure no ears, mouth, or legs are caught in the tether. If ears are under the tether, they can be loosely pulled forward. Tethering can be challenging at first, and may benefit from a second individual to separate holding and tethering roles. When a portable isoflurane rig is available and permitted for use, tethering can also take place following brief induction of anaesthesia. We tether strangers first to postpone disturbing the cage of the focal vole.
Clip the extra plastic material from the tether.
-
Place the tethered vole at one end of the apparatus, securing the tether to its anchor.
The position within the apparatus should be pre-determined for each test, and the relative position of the partner (e.g. left or right chamber) should either be alternated between tests or randomized, as there could be room side preferences, and learning across multiple tests.
If setting up more than one test, repeat the tethering process with the other stranger voles.
Tether the partner voles following the same procedure, placing them at the opposite ends of the apparatuses from the strangers.
-
Once all stimulus voles are tethered, allow a minimum of 5 minutes for them to habituate to their tethers. During this time, observe the voles to ensure they are active and breathing without difficulty.
Voles are typically highly active after tethering. If a vole appears lethargic, remove the tether and do not use that vole in testing. If a vole appears particularly active/jumpy following tethering, additional habituation time may be helpful.
Activate the video recording session and place testing cards in view of their cameras.
-
Place focal voles in the unoccupied chamber of each apparatus and start a three-hour timer when the last focal vole is in place.
Dividers may be used to block access to the partner and stranger chambers to allow the focal vole to habituate to the apparatus prior to test start, otherwise the early portion of the test functions partly as a habituation period.
-
Observe voles for a few minutes to ensure the test is running smoothly.
Intermittent aggressive interactions may occur, especially between the focal and stranger voles. This should be limited to rearing/squawking/chasing. If more direct aggression such as biting is observed, the test should be stopped or actively monitored to make sure aggression does not exceed a pre-determined and institutionally approved threshold.
Exit the testing room and place a sign on the door, e.g. “testing in progress – do not disturb,”
After three hours have elapsed, enter the testing room and stop the video recording.
Remove focal (untethered voles) to their cages. Check the IDs carefully from the testing card, and check individual identifying markers if available (ear tags or ear punches).
Remove tethered voles. Holding the vole securely in one hand, unanchor the tether from the cage. Use a clipper/scissors to clip the zip-tie collar off the vole before returning it to its home cage.
-
Clean the apparatuses. Dump soiled bedding in the waste bin. Clean the apparatus with soapy water or 70% ethanol and dry with soft towels.
Solvents such as ethanol and acetone can damage some plastics such as acrylic, so consider which plastic the apparatus was constructed from. Non-solvent based antimicrobial and disinfecting cleaners can be used as needed. Substances with strong odors also should be avoided.
-
Transfer videos to a permanent storage location and rename with study identifiers.
A sample naming scheme would be [YYYY-MM-DD-study abbreviation-PPT-testID]. Videos can then be stored on a server with cloud backup. Some older camera models split large video files into 1.5 hour segments. This is generally no longer an issue, but split videos can be merged with many video editing programs.
Delete videos from camera storage.
Tape testing cards in study notebook and record additional test information.
Score tests (see support protocol Behavioral Scoring). At minimum, the output should provide time spent in each chamber (e.g. Figure 2a), and time spent huddling with each vole (Figure 2b). Scoring may also provide a timeline of preference development (Figure 2c), measures of activity, counts of aggression, and other behaviors of interest. See also Statistical Analysis.
Figure 2.
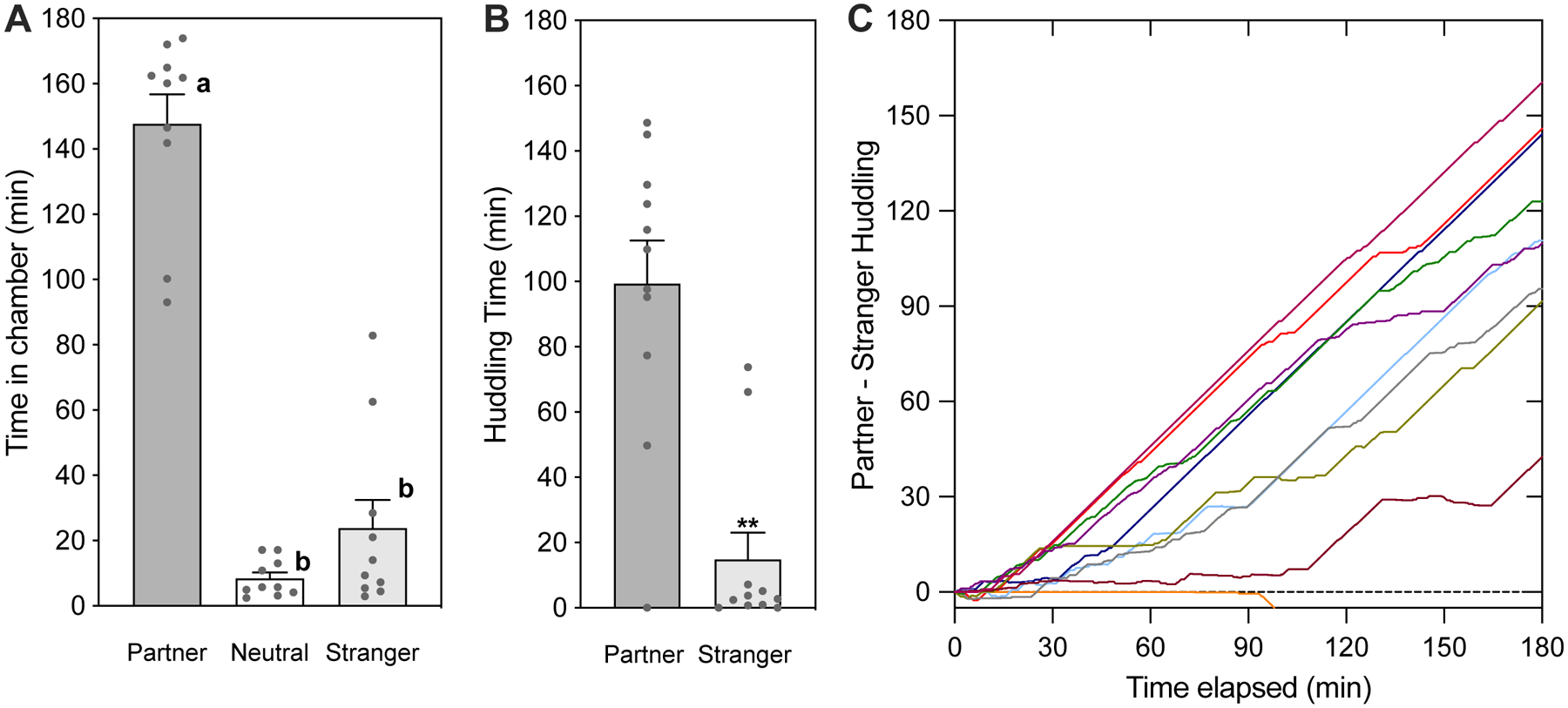
Sample partner preference test data and timeline analysis. Sample data are from female prairie voles tested with their male partner and a male stranger (data from Lee and Beery, 2021 supplementary materials). Females were housed with their male partners for 6 hours prior to testing. A. Time spent in each of the three apparatus chambers indicates focal voles typically spent more time in the partner chamber than in other chambers. B. Time spent huddling with the partner is significantly higher than with the stranger, as is typical for prairie voles that have formed a preference (paired t-test). C. Cumulative preference (shown here as partner-stranger huddling) over the course of the test. Note one vole (of 11) did not huddle with the partner.
SUPPORT PROTOCOL 1
BEHAVIORAL SCORING
Partner preference test scoring should quantify dependent variables including time huddling with the partner, time huddling with the stranger, and time in each of the three chambers. Additional variables that may be recorded as part of the scoring process include bouts of aggression, and activity or distance traveled. Composite variables and timeline analyses can be constructed from these metrics (see Data Analysis). Any scoring that is not fully automated should be performed by researchers who are a) unaware of the position of the partner vs. stranger within the test being scored, and b) unaware of the experimental group within a study (unless this is impossible because the groups are visually different). When multiple researchers score videos from a single study, they should be trained on a common set of practice videos that allow assessment of inter-rater reliability.
There is a wide range of options for behavioral scoring tools. General behavioral scoring labeling tools such as BORIS (Friard and Gamba, 2016) and JWatcher (Blumstein and Daniel, 2007) can be time-consuming for scoring large numbers of PPTs, but have the advantage of the scorer to annotate behavior at any level of detail. Two commercial software products that provide fully automated scoring solutions are SocialScan (CleverSys Inc.), and Ethovision XT (Noldus, Inc), and have been successfully used to score PPTs recorded under uniform conditions (Ahern et al., 2009). idTracker is a free, multi-animal tracking system (Pérez-Escudero et al., 2014) that has been used successfully for PPTs (Kenkel et al., 2019; Scribner et al., 2020), but does not provide detailed assessments of behavior. Efforts are underway in multiple labs to use open-source pose estimation software such as DeepLabCut (Mathis et al., 2018) and SLEAP (Pereira et al., 2020) to score PPTs, but this work is ongoing. IntervoleTimer (described here) is an open-source PPT-specific script that runs on Macs without additional software installation; it allows an observer to move a vole icon to match an accelerated or time-lapse video to score a three-hour test in 20–45 minutes.
It would be impossible to provide a detailed protocol for each scoring option, so this support protocol details the procedure for getting started with scoring using IntervoleTimer. This older script (e.g. Beery et al., 2008) is basic but effective, and customized for partner preference tests. It was used to score all tests shown in this protocol. To use IntervoleTimer, the scorer watches a video at 3–5x speed and uses keyboard strokes (< and >) to move an icon of a vole around in a picture of an apparatus (Figure 3). Transitions between each state the icon is in (left huddling, left not huddling, center chamber, right not huddling, right huddling) are recorded. The script outputs a text file with user input data on subjects, time stamps of each transition and cumulative durations in each state, and summary data including partner and stranger huddling times, chamber times, and the number of transitions between chambers (a measure of activity). Scorers can add additional narrative information or counts of other tallied behaviors such as bouts of aggression. Further processing on the raw transition time stamps can be performed to track preference development over time (as in figure 2C).
Figure 3.
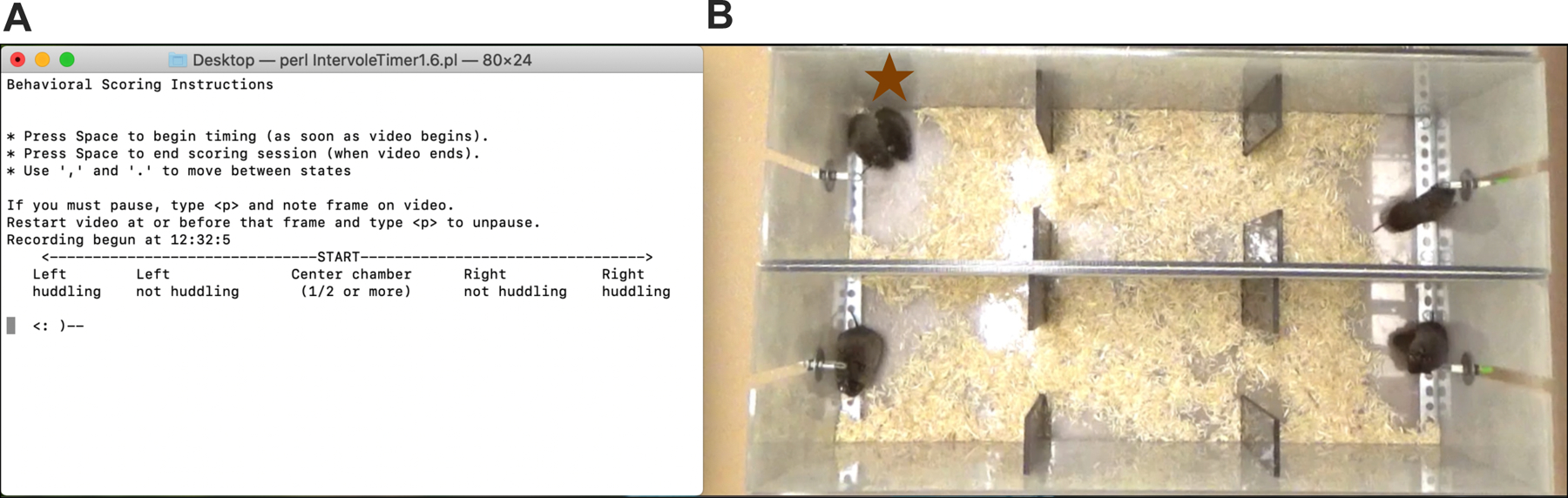
Scoring with IntervoleTimer.
A. During scoring in the Terminal app, the vole icon <:)— is moved between states to match the video using the < and > keys. B. The video for scoring is played at accelerated speed adjacent to the Terminal scoring window. In this case, the focal vole (indicated by star) is huddling on the left side of the chamber. The second PPT below would be scored separately; in that test the focal vole can be seen huddling on top of the right tethered vole.
Materials:
Computer running MacOS (or Unix or Linux; these instructions assume a Mac)
Software:
A copy of IntervoleTimer1.6.pl or later update
Video playback software with highspeed playback (e.g. Quicktime player, VLC)
Terminal (standard installation on Macs)
Video recorded during a partner preference test
Optional: timer with audible beep (any brand)
Optional: handheld tally counter (any brand) if measuring aggressive interactions
Protocol steps:
Download IntervoleTimer (https://github.com/BeeryLab/intervole_timer/) and store the file (e.g. IntervoleTimer1.6.pl) on the Desktop.
Open the video to be scored and size it to ~half the screen width available. Scroll to the frame with the test information card and pause there.
Open Terminal (~/Applications/Utilities/Terminal.app) and in the Terminal window type “cd Desktop/” to change directory to the Desktop.
Launch the perl script by typing in the Terminal window “perl IntervoleTimer1.6.pl”
Terminal should now display the opening screen of the scoring script with an ASCII text rodent image. At the prompts, enter the relevant information about the test.
-
Return to the video player. Hit play, set the playback speed to the desired accelerated pace. There will probably be a brief delay before the vole is placed in the middle of the apparatus. During this time, select the Terminal window with the scoring program and get the stopwatch ready.
You can rewind all the way to the beginning, or start partway into the video, as long as there is enough time to have clicked back over to Terminal before the focal vole is placed in the arena.
-
When the vole in the video is placed in the central chamber, hit the spacebar to begin scoring. A small vole image will appear. Use the < and > keys (i.e. “,” and “.”) to move the vole icon to match the image in the video (Figure 3).
Additional detail about scoring is available in the Instructions file in the Github directory with the scoring code.
When your stopwatch indicates the test is done (or the vole is removed from the apparatus), hit <spacebar> in Terminal to end scoring. You will be prompted for the scorer’s name, and provided a free text area where you can describe unusual behaviors, record counts of aggression or other behaviors tallied with the hand counter, etc.
-
Locate the scoring file generated (on the Desktop). Test information appears at the top, followed by raw data (timestamps of each state transition), followed by summary data for analysis. Enter the summary data into a data file for analysis with your software of choice, or automatically extract this information. Raw timestamps can also be extracted for additional analyses, such as timeline plots.
Summary data are provided at scoring speed, as well as scaled to the 180 minute test duration. If the test is not run for 180 minutes, either edit the scoring code to scale to the appropriate duration, or work with the data from scoring speed and scale it manually.
COMMENTARY
BACKGROUND INFORMATION:
Contemporary neuroscience uses a variety of tests to assess social behaviors from recognition to social approach (Winslow, 2003; Crawley, 2007). Elements of the partner preference test have been employed since the 1980s, as social choice experiments began to make use of two tethered subjects and a focal individual making a social choice (e.g. (Pomerantz et al., 1983; Slob et al., 1987). The partner preference test in voles was developed in the laboratory of C. Sue Carter as the first test in which familiarity of the conspecific was the variable that differed between tethered stimulus voles (Williams et al., 1992a, 1992b). At the same time the test was lengthened to three hours — a duration that reliably allows animals to settle into huddling behaviors, and reduces interference from exploratory behaviors.
Since its inception, the partner preference test in voles has been used to demonstrate the roles of neuropeptides in modulating social behavior, especially oxytocin and vasopressin acting within the brain (Cho et al., 1999), specifically in the nucleus accumbens and ventral pallidum (Young et al., 2001; Lim and Young, 2004). Several additional neurochemical systems have been found to influence partner preference in prairie voles, including dopamine signaling (Aragona et al., 2003, 2006; Gingrich et al., 2000; Liu and Wang, 2003; Resendez et al., 2016), opioid signaling (Burkett et al., 2011; Resendez et al., 2012, 2016), CRF (DeVries et al., 2002) and glucocorticoid signaling (DeVries et al., 1996; Blondel and Phelps, 2016). Sex differences in the mechanisms underlying pair bond formation, especially factors related to prior stressors or exposure to stress hormones, have also become apparent (DeVries et al., 1996; Carter et al., 2009), despite outward similarity of behavior (De Vries, 2004).
Several behavioral manipulations affect preference formation, including duration of cohabitation, access to mating, and experience of stress—which enhances partner preference formation in males, while decreasing it in females (Williams et al., 1992a; DeVries et al., 1996). Early life experiences including early handling, maternal care, and family structure also influence the expression of partner preference and other social behaviors (Bales et al., 2007; Perkeybile et al., 2013; Ahern and Young, 2009; Ahern et al., 2021). The effects of early exposures on adult partner preference and social behaviors are also being assessed, from exposure to endocrine disruptors to antidepressants and other pharmacological agents (Bales et al., 2013; Hostetler et al., 2011; Sullivan et al., 2014; Gillera et al., 2019; Lawrence et al., 2020).
CRITICAL PARAMETERS:
Cohabitation duration:
Partner preferences typically form within 6–12 hours in females and within 24 hours in males (DeVries and Carter, 2011). Partner preferences can be assessed following short durations of cohabitation (e.g. 1 or 6 hours) to determine the impact of factors that may accelerate their formation, or they may be assessed following extended cohabitation intervals (24 hours or longer) to measure reduction in preference. For example, infusion of oxytocin increases partner preference following 6 hour cohabitation (Williams et al., 1992a, 1994), while infusion of oxytocin receptor antagonist decreases partner preferences following 14 hours of mated cohabitation (Insel and Hulihan, 1995). There is some variation over time, by lab, by colony origin, or by sex of the focal subjects in the typical cohabitation threshold for partner preference formation, thus preliminary tests may need to be run in the local environment to establish baseline cohousing intervals that are and are not sufficient.
Separation between cohabitation and testing:
Early studies separated partnered voles for at least 24h prior to the partner preference test, in order to demonstrate that partner preferences are a durable indicator of relationship formation. Subsequent studies showed that partner preference for a mate endures for at least 6 days in male and female prairie voles and sometimes longer (Insel and Hulihan, 1995; DeVries and Carter, 2011), and that same-sex “peer” partner preferences in meadow voles persisted through three weeks of separation (Parker and Lee, 2003). Separations are still in used in some studies (e.g. (Burkett et al., 2011; Johnson et al., 2016) but are no longer the norm, and may be used for alternate purposes, such as to allow drug dissipation before testing.
Test duration:
The classic partner preference test is three hours long, although variations in length have been used in several studies. The long duration is important as partner preferences do not emerge during brief (i.e. 10 minute) social preference tests (Beery et al., 2018). Depending on the cohort, preference typically takes between 1–3 hours to be clearly demonstrated (Figure 4A; Williams et al., 1992a), and tends to grow in strength over the course of the test (Figure 4A). Metrics that are scaled by huddling time such as preference score (partner/total huddling, or %partner/total huddling) tend to reveal preferences by one hour, but do not grow much or at all with test duration (Figure 4B), and have been used in assessing preference in shorter PPTs (Scribner et al., 2020).
Figure 4.
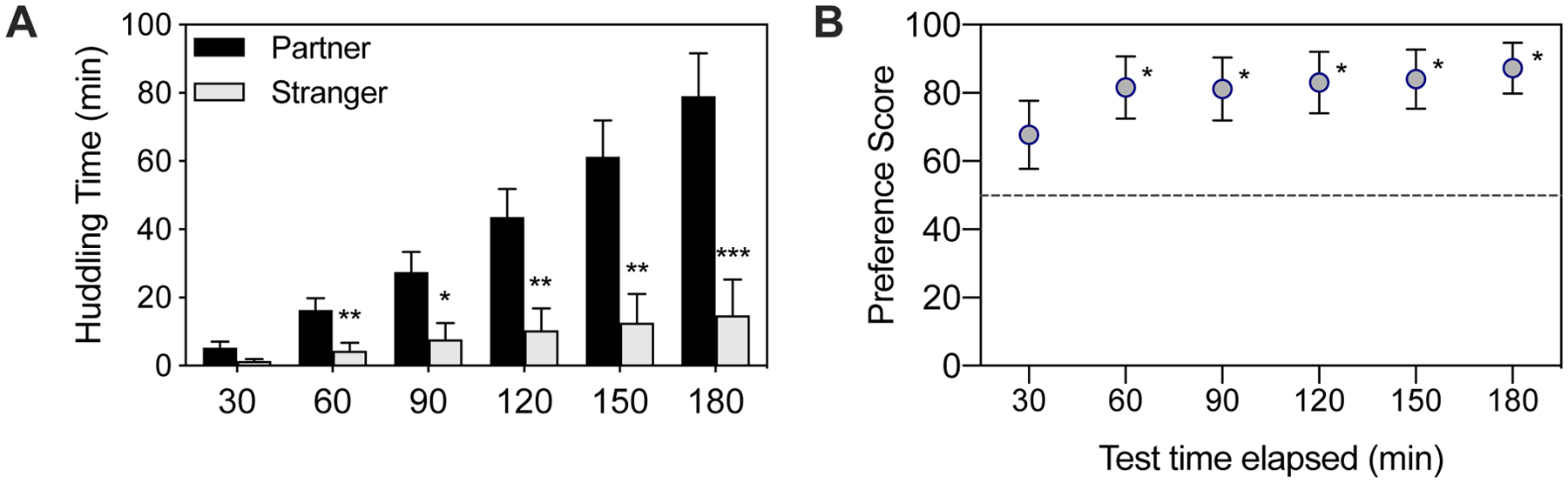
A. Partner vs. Stranger huddling difference increases over the course of the test and is typically significant by three hours if not earlier. B. Preference score, which reflects relative huddling (% P huddling/total huddling) stabilizes early but does not tend to increase much over the course of the test. Data are from female prairie voles tested in peer PPTs with a long-term female cagemate (Lee et al., 2019); timeline analysis was performed in Beery and Shambaugh (2021).
Apparatus configuration:
Both branched and linear versions of the partner preference testing apparatus are in common usage (Figure 1), and the choice of apparatus is largely up to the experimenter. A study of meadow voles tested in same-sex partner preference tests in both apparatus types (in counterbalanced order) revealed no difference in preference scores (partner/total huddling) by apparatus type. Testing in the linear apparatus was associated with more time huddling with the partner and less time in the empty center chamber, possibly because the subject voles were more readily accessible (Beery and Shambaugh, 2021). This study also showed that within apparatus types, results were highly consistent across control groups for different studies. This indicates that as long as the apparatus type is kept consistent within a lab, results remain comparable.
While the partner preference test allows direct access to huddling with a tethered conspecific, the majority of 10–20 minute social preference tests used in rats and mice utilize a perforated barrier surrounding each of the stimulus animals (e.g. Yang et al., 2011; Smith et al., 2015). The effect of tethering versus barrier use in the PPT was recently assessed (Beery et al., 2018). We found that housing the target vole behind a barrier (an inverted wire pencil cup) greatly reduced the amount of time spent in close proximity to the partner and resulting partner preference in adjacent social time, but partner preference continued to be evident in chamber times (Figure 5; Beery et al., 2018)
Figure 5.
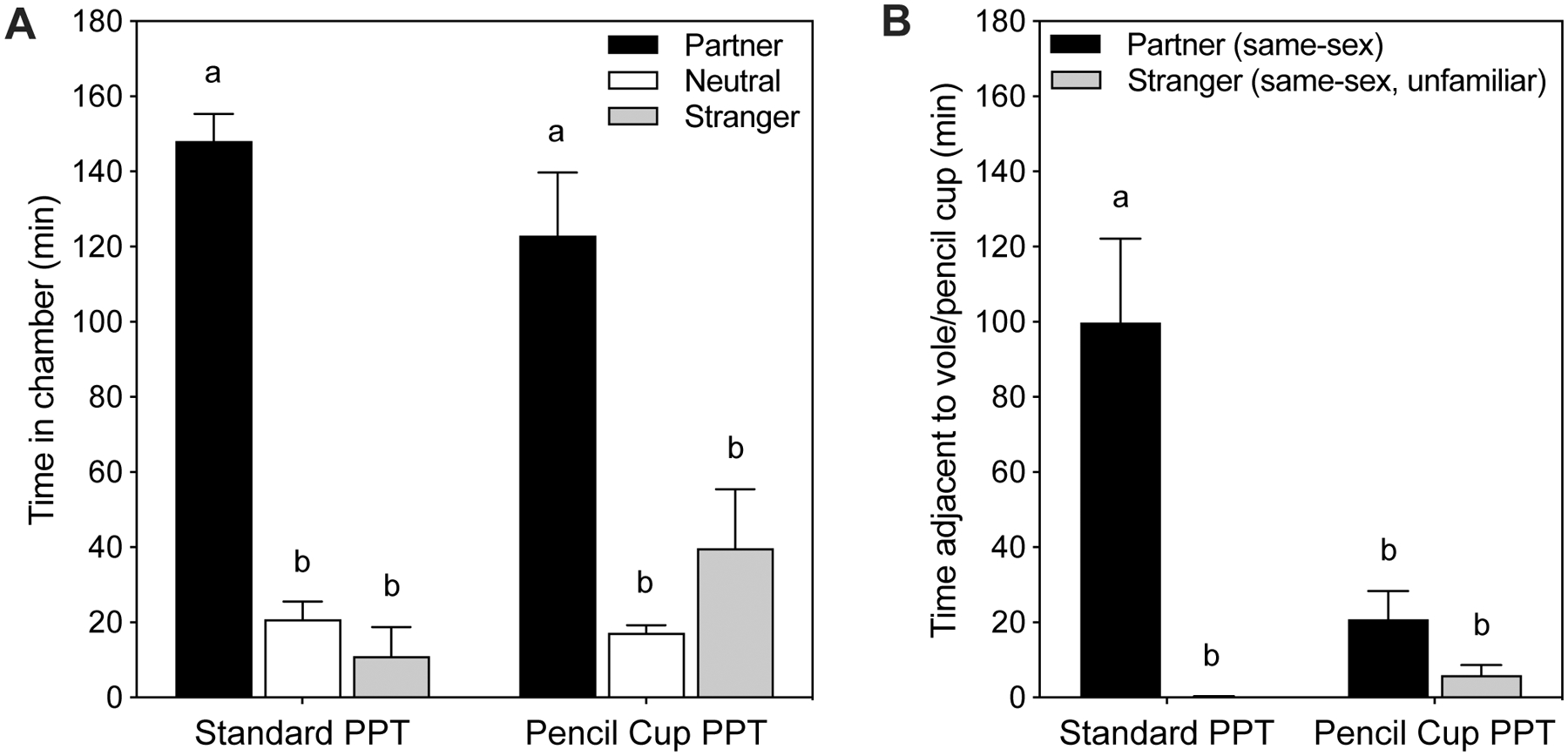
Comparison of the standard PPT (left bars) to a PPT in which the stimulus voles were enclosed by a wire pencil cup barrier in place of tethering (right bars). A. Time spent in different chambers was not affected by use of the pencil cup. B. Time spent in close proximity to the subject voles shows striking differences in the standard PPT but not the pencil cup PPT, indicating the importance of access to physical contact for close proximity. Subjects were female and male prairie voles tested with same-sex partners and strangers in the peer PPT. Data redrawn from (Beery et al., 2018).
Subject considerations: reproductive state
Female prairie voles are induced into estrus by social olfactory cues and ovulate only after mating, which is convenient for this kind of testing (see further discussion in Kenkel et al., this issue). In some studies of partner preference in male prairie voles, females are hormonally primed to bring them into behavioral estrus prior to pairing. For studies for which rapid mating upon pairing is desirable, females can be prepared for mating by exposure to male bedding cues, or primed with injections of estradiol benzoate (Roberts et al., 1998; Donaldson et al., 2010). When females are paired with males, these males may be sexually naïve or experienced (e.g. Burkett et al., 2011). The hormonal condition of either partner, stranger, and focal test animal should be taken into consideration in the design of these studies.
Subject considerations: the peer partner preference test (peer PPT)
The original, canonical version of the PPT tests a male or female focal vole with an opposite-sex partner (i.e. mate) and an opposite-sex stranger. Since then, same-sex “peer” partner preference tests have yielded complementary information on the formation and maintenance of non-mate social relationships. The same-sex peer partner preference test is regularly used in meadow voles, which exhibit group living and peer social preferences in winter in the wild, and under conditions of short (winter-like) photoperiods in the lab (Beery, 2019). This test has been used extensively to probe the basis of non-reproductive social relationships in both adult meadow voles (Parker and Lee, 2003; Beery et al., 2008; Beery and Zucker, 2010; Beery et al., 2014; Ondrasek et al., 2015; Anacker et al., 2016a, 2016b; Goodwin et al., 2019; Lee et al., 2019; Lee and Beery, 2021) and prairie voles (DeVries et al., 1997; Beery et al., 2018; Goodwin et al., 2019; Lee et al., 2019; Lee and Beery, 2021). The peer partner preference test differs from the opposite-sex partner preference test only in that all three subjects are of the same sex. The familiar partner is a same-sex cage-mate (sibling or non-sibling) and the stranger is a novel individual.
Subject considerations: other species
The same-sex peer partner preference test has been used comparatively across a variety of rodent species (Beery and Shambaugh, 2021). In addition to prairie and meadow voles, the peer partner preference test has been used in mice (Beery et al., 2018), degus (Insel et al., 2020), and rats (Beery and Shambaugh, 2021). Of these rodents, only the two vole species demonstrate selective preferences for familiar same-sex individuals, although degus exhibited high levels of nonselective huddling behavior (Insel et al., 2020).
Opposite-sex partner preference has also been assessed across a range of rodents of differing mating systems, including prairie voles and montane voles (Insel and Hulihan, 1995), meadow voles (Parker et al., 2001), California mice (Kowalczyk et al., 2018), mice (Cymerblit-Sabba et al., 2020), striped mice (Garnier and Schradin, 2019), and three gerbil species (Tchabovsky et al., 2019). Of these, partner preferences were most evident in prairie voles, meadow voles, female striped mice, and male Mongolian gerbils under control conditions. Other species, such as mice, showed partner preferences following specific neural manipulations (Cymerblit-Sabba et al., 2020). Partner preference for a familiar mate has also been assessed with varied testing setups in other taxonomic groups including birds (zebra finches: Smiley et al., 2012; Kingsbury and Goodson, 2014) and primates (marmosets: Carp et al., 2016). Adaptation of the PPT to other species may involve modifications of tethers, apparatus size, and other variables.
Subject considerations: other social comparisons
The extended duration partner preference test has also been adapted to assess preference for individuals exhibiting different characteristics related to but beyond basic familiarity. For example the test has been used to test preference for new versus former partners in prairie voles (Harbert et al., 2020), preference for huddling with a group versus a single partner in same-sex meadow voles (Ondrasek et al., 2015), preference for gonadally intact versus gonadectomized subjects, preference for same- versus opposite-sex individuals (DeVries et al., 1997), and changes with age and repeated pairing (Kenkel et al., 2019).
STATISTICAL ANALYSIS:
Partner preference within a group is defined as the subjects spending significantly more time adjacent to the partner than to the stranger, usually assessed using paired t-tests or a non-parametric alternative. Partner preference can also be described in terms of cage preference. Differences between groups can be assessed by generalized linear models or 2-way ANOVA on huddling time with familiarity [partner/stranger] as a within-subjects factor and manipulation [A/B] as a between-subjects factor (e.g. Donaldson et al., 2010). Partner preference has also been described on the individual level, typically defined when an individual spends at least 2x as much time huddling with the partner as with the stranger (Insel et al., 1995; Parker and Lee, 2003).
Relative preference for the partner is often expressed as a preference score, defined as (time adjacent to the partner)/(time adjacent to the partner+stranger). This results in a number between 0 and 1, or can be expressed as a percentage (0–100%) (Lim et al., 2004; Beery and Zucker, 2010; Anacker et al., 2016b; Harbert et al., 2020). Preference scores are useful for three purposes: First they allow group comparisons of preference using a single number (e.g. by one-way ANOVA), as does comparison of only partner huddling or only stranger huddling across groups. They also avoid the violation of independence of samples assumed by t-tests (as partner and stranger huddling time are not independent when measured in a choice test). Finally, preference scores tend to stabilize earlier in the test than huddling time, which is useful for comparison of groups when full-length tests are not feasible (Figure 4B). Preference scores can be compared between groups, or within groups relative to the expected mean when there is no preference (50%) with a one-sample t-test or Wilcoxon ranked sum for non-normal data.
UNDERSTANDING RESULTS:
Comparison of partner vs. stranger huddling within groups can be used to establish whether a stimulus is sufficient to promote preference, for example 6 hours of cohabitation was sufficient to achieve significant opposite-sex partner preferences in the female prairie voles shown in figure 2, although shorter periods of prior cohabitation are sometimes sufficient if the intent of the experiment is to discover factors that augment preference formation. Comparison of huddling times can also be made across groups or within groups over time, with preference scores capturing relative partner and stranger huddling within a single variable (Figure 4).
TIME CONSIDERATIONS:
Basic protocol 1 (partner preference test) can be completed in a morning or afternoon. The core of the partner preference test lasts for three hours, during which the experimenter is absent. Setup and take-down may vary with the number of tests being run, experience of the experimenter, and availability of an assistant but we typically budget ~30 minutes for each activity.
ACKNOWLEDGEMENTS:
I am grateful to Sue Carter, Will Kenkel, Morgan Gustison, and Nikki Lee for their comments and feedback on earlier versions of this protocol. AB is supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R15MH113085.
Footnotes
INTERNET RESOURCES:
VoleBase https://osf.io/yej35/ is a collection of resources for working with voles hosted through the Open Science Foundation. Among other resources it contains behavioral scoring guides for different software setups. Membership can be requested by vole researchers, and the site will offer some public resources in the future.
LITERATURE CITED:
- Ahern TH, Modi ME, Burkett JP, and Young LJ 2009. Evaluation of two automated metrics for analyzing partner preference tests. Journal of Neuroscience Methods 182:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, Olsen S, Tudino R, and Beery AK 2021. Natural variation in the oxytocin receptor gene and rearing interact to influence reproductive and nonreproductive social behavior and receptor binding. Psychoneuroendocrinology 128:105209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern TH, and Young LJ 2009. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (Microtus ochrogaster). Frontiers in Behavioral Neuroscience 3:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, and Beery AK 2013. Life in groups: the roles of oxytocin in mammalian sociality. Frontiers in Behavioral Neuroscience 7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Christensen JD, LaFlamme EM, Grunberg DM, and Beery AK 2016a. Septal oxytocin administration impairs peer affiliation via V1a receptors in female meadow voles. Psychoneuroendocrinology 68:156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker AMJ, Reitz KM, Goodwin NL, and Beery AK 2016b. Stress impairs new but not established relationships in seasonally social voles. Hormones and Behavior 79:52–57. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, and Wang Z 2003. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci 23:3483–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, and Wang Z 2006. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci 9:133–9. [DOI] [PubMed] [Google Scholar]
- Bales KL, Lewis-Reese AD, Pfeifer LA, Kramer KM, and Carter CS 2007. Early experience affects the traits of monogamy in a sexually dimorphic manner. Developmental Psychobiology 49:335–342. [DOI] [PubMed] [Google Scholar]
- Bales KL, Perkeybile AM, Conley OG, Lee MH, Guoynes CD, Downing GM, Yun CR, Solomon M, Jacob S, and Mendoza SP 2013. Chronic Intranasal Oxytocin Causes Long-Term Impairments in Partner Preference Formation in Male Prairie Voles. Biological Psychiatry 74:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK 2019. Frank Beach award winner: Neuroendocrinology of group living. Hormones and Behavior 107:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Christensen JD, Lee NS, and Blandino KL 2018. Specificity in Sociality: Mice and Prairie Voles Exhibit Different Patterns of Peer Affiliation. Frontiers in Behavioral Neuroscience 12. Available at: https://www.frontiersin.org/articles/10.3389/fnbeh.2018.00050/full [Accessed June 28, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Loo TJ, and Zucker I 2008. Day length and estradiol affect same-sex affiliative behavior in the female meadow vole. Hormones and behavior 54:153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, and Shambaugh KL 2021. Comparative assessment of familiarity/novelty preferences in rodents. Frontiers in Behavioral Neuroscience 15. Available at: https://www.frontiersin.org/articles/10.3389/fnbeh.2021.648830/abstract [Accessed March 25, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Vahaba DM, and Grunberg DM 2014. Corticotropin-releasing factor receptor densities vary with photoperiod and sociality. Hormones and Behavior 66:779–786. [DOI] [PubMed] [Google Scholar]
- Beery AK, and Zucker I 2010. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience 169:665–673. [DOI] [PubMed] [Google Scholar]
- Blondel DV, and Phelps SM 2016. Effects of acute corticosterone treatment on male prairie voles (Microtus ochrogaster): Territorial aggression does not accompany induced social preference. Journal of Comparative Psychology (Washington, D.C.: 1983) 130:400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein D, and Daniel JC 2007. Quantifying Behavior the Jwatcher Way, illustrated edition. Sinauer Associates, Sunderland, Mass. [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, and Young LJ 2011. Activation of μ-Opioid Receptors in the Dorsal Striatum is Necessary for Adult Social Attachment in Monogamous Prairie Voles. Neuropsychopharmacology 36:2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, and Bales KL 2016. Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus). American Journal of Primatology 78:326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C, Grippo A, Pournajafinazarloo H, Ruscio M, and Porges S 2008. Oxytocin, vasopressin and sociality. In Progress in Brain Research pp. 331–336. Elsevier; Available at: http://linkinghub.elsevier.com/retrieve/pii/S0079612308004275 [Accessed March 9, 2015]. [DOI] [PubMed] [Google Scholar]
- Carter CS 1998. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology 23:779–818. [DOI] [PubMed] [Google Scholar]
- Carter CS, Boone EM, Pournajafi-Nazarloo H, and Bales KL 2009. Consequences of Early Experiences and Exposure to Oxytocin and Vasopressin Are Sexually Dimorphic. Developmental Neuroscience 31:332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, and Carter CS 1999. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster). Behavioral neuroscience 113:1071. [DOI] [PubMed] [Google Scholar]
- Crawley JN 2007. Social behavior tests for mice. Available at: http://ccsystems.us/v2mag/L018.pdf [Accessed August 15, 2014].
- Cymerblit-Sabba A, Smith AS, Williams Avram SK, Stackmann M, Korgan AC, Tickerhoof MC, and Young WS 2020. Inducing Partner Preference in Mice by Chemogenetic Stimulation of CA2 Hippocampal Subfield. Frontiers in Molecular Neuroscience 13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries GJ 2004. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology 145:1063–1068. [DOI] [PubMed] [Google Scholar]
- DeVries AC, and Carter CS 2011. Sex differences in temporal parameters of partner preference in prairie voles (Microtus ochrogaster). Canadian Journal of Zoology. Available at: https://cdnsciencepub.com/doi/abs/10.1139/z99-054 [Accessed March 13, 2021]. [Google Scholar]
- DeVries AC, DeVries MB, Taymans SE, and Carter CS 1996. The effects of stress on social preferences are sexually dimorphic in prairie voles. Proceedings of the National Academy of Sciences 93:11980–11984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Guptaa T, Cardillo S, Cho M, and Carter CS 2002. Corticotropin-releasing factor induces social preferences in male prairie voles. Psychoneuroendocrinology 27:705–714. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Johnson CL, and Carter CS 1997. Familiarity and gender influence social preferences in prairie voles (Microtus ochrogaster). Can J Zool 75:295–301. [Google Scholar]
- Donaldson ZR, Spiegel L, and Young LJ 2010. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behavioral Neuroscience 124:159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friard O, and Gamba M 2016. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods in Ecology and Evolution 7:1325–1330. [Google Scholar]
- Garnier L, and Schradin C 2019. Pair bonding in monogamously and polygynously kept African striped mice, Rhabdomys pumilio. Animal Behaviour 150:69–76. [Google Scholar]
- Getz LL, Carter CS, and Gavish L 1981. The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behav Ecol Sociobiol 8:189–194. [Google Scholar]
- Gillera SEA, Marinello WP, Horman BM, Phillips AL, Ruis MT, Stapleton HM, Reif DM, and Patisaul HB 2019. Sex-specific effects of perinatal FireMaster® 550 (FM 550) exposure on socioemotional behavior in prairie voles. Neurotoxicology and Teratology:106840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, and Insel TR 2000. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behav Neurosci 114:173–83. [DOI] [PubMed] [Google Scholar]
- Goodwin NL, Lopez SA, Lee NS, and Beery AK 2019. Comparative role of reward in long-term peer and mate relationships in voles. Hormones and Behavior 111:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbert KJ, Pellegrini M, Gordon KM, and Donaldson ZR 2020. How prior pair-bonding experience affects future bonding behavior in monogamous prairie voles. Hormones and Behavior:104847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetler CM, Harkey SL, Krzywosinski TB, Aragona BJ, and Bales KL 2011. Neonatal exposure to the D1 agonist SKF38393 inhibits pair bonding in the adult prairie vole: Behavioural Pharmacology 22:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel N, Shambaugh KL, and Beery AK 2020. Female degus show high sociality but no preference for familiar peers. Behavioural Processes 174:104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, and Hulihan TJ 1995. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci 109:782–9. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, and Winslow JT 1995. Mating in the monogamous male: behavioral consequences. Physiol Behav 57:615–27. [DOI] [PubMed] [Google Scholar]
- Johnson ZV, Walum H, Jamal YA, Xiao Y, Keebaugh AC, Inoue K, and Young LJ 2016. Central oxytocin receptors mediate mating-induced partner preferences and enhance correlated activation across forebrain nuclei in male prairie voles. Hormones and Behavior 79:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel WM, Perkeybile AM, Yee JR, and Carter CS 2019. Rewritable fidelity: How repeated pairings and age influence subsequent pair-bond formation in male prairie voles. Hormones and Behavior 113:47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury MA, and Goodson JL 2014. Pair bond formation is impaired by VPAC receptor antagonism in the socially monogamous zebra finch. Behavioural Brain Research 272:264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AS, Davila RF, and Trainor BC 2018. Effects of social defeat on paternal behavior and pair bonding behavior in male California mice (Peromyscus californicus). Hormones and Behavior 98:88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RH, Palumbo MC, Freeman SM, Guoynes CD, and Bales KL 2020. Developmental Fluoxetine Exposure Alters Behavior and Neuropeptide Receptors in the Prairie Vole. Frontiers in Behavioral Neuroscience 14:584731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, and Beery AK 2021. The role of dopamine signaling in prairie vole peer relationships. Hormones and Behavior 127:104876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NS, Goodwin NL, Freitas KE, and Beery AK 2019. Affiliation, Aggression, and Selectivity of Peer Relationships in Meadow and Prairie Voles. Frontiers in Behavioral Neuroscience 13. Available at: https://www.frontiersin.org/articles/10.3389/fnbeh.2019.00052/full?&utm_source=Email_to_authors_&utm_medium=Email&utm_content=T1_11.5e1_author&utm_campaign=Email_publication&field=&journalName=Frontiers_in_Behavioral_Neuroscience&id=447143 [Accessed March 19, 2019]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, and Wang Z 2016. The neurobiology of pair bond formation, bond disruption, and social buffering. Current Opinion in Neurobiology 40:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazábal DE, Ren X, Terwilliger EF, and Young LJ 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429:754–7. [DOI] [PubMed] [Google Scholar]
- Lim MM, and Young LJ 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neuroscience 125:35–45. [DOI] [PubMed] [Google Scholar]
- Liu Y, and Wang ZX 2003. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience 121:537–44. [DOI] [PubMed] [Google Scholar]
- Madrid JE, Parker KJ, and Ophir AG 2020. Variation, plasticity, and alternative mating tactics: Revisiting what we know about the socially monogamous prairie vole. In Advances in the Study of Behavior pp. 203–242. Elsevier; Available at: https://linkinghub.elsevier.com/retrieve/pii/S0065345420300036 [Accessed December 16, 2020]. [Google Scholar]
- Mathis A, Mamidanna P, Cury KM, Abe T, Murthy VN, Mathis MW, and Bethge M 2018. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nature Neuroscience 21:1281–1289. [DOI] [PubMed] [Google Scholar]
- Ondrasek NR, Wade A, Burkhard T, Hsu K, Nguyen T, Post J, and Zucker I 2015. Environmental modulation of same-sex affiliative behavior in female meadow voles (Microtus pennsylvanicus). Physiology & Behavior 140:118–126. [DOI] [PubMed] [Google Scholar]
- Parker KJ, and Lee TM 2003. Female meadow voles (Microtus pennsylvanicus) demonstrate same-sex partner preferences. Journal of comparative psychology 117:283–9. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, and Lee TM 2001. Development of selective partner preferences in captive male and female meadow voles, Microtus pennsylvanicus. Animal Behaviour 61:1217–1226. [Google Scholar]
- Pereira TD, Tabris N, Li J, Ravindranath S, Papadoyannis ES, Wang ZY, Turner DM, McKenzie-Smith G, Kocher SD, Falkner AL, et al. 2020. SLEAP: Multi-animal pose tracking. bioRxiv:2020.08.31.276246 [Google Scholar]
- Pérez-Escudero A, Vicente-Page J, Hinz RC, Arganda S, and de Polavieja GG 2014. idTracker: tracking individuals in a group by automatic identification of unmarked animals. Nature Methods 11:743–748. [DOI] [PubMed] [Google Scholar]
- Perkeybile AM, Griffin LL, and Bales KL 2013. Natural variation in early parental care correlates with social behaviors in adolescent prairie voles (Microtus ochrogaster). Front Behav Neurosci 7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantz SM, Nunez AA, and Jay Bean N 1983. Female behavior is affected by male ultrasonic vocalizations in house mice. Physiology & Behavior 31:91–96. [DOI] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Eidson LN, Porter-Stransky KA, Nevárez N, McLean JW, et al. 2016. Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. eLife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, and Aragona BJ 2012. -Opioid Receptors within the Nucleus Accumbens Shell Mediate Pair Bond Maintenance. Journal of Neuroscience 32:6771–6784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts RL, Cushing BS, and Carter CS 1998. Intraspecific Variation in the Induction of Female Sexual Receptivity in Prairie Voles. Physiology & Behavior 64:209–212. [DOI] [PubMed] [Google Scholar]
- Scribner JL, Vance EA, Protter DSW, Sheeran WM, Saslow E, Cameron RT, Klein EM, Jimenez JC, Kheirbek MA, and Donaldson ZR 2020. A neuronal signature for monogamous reunion. Proceedings of the National Academy of Sciences 117:11076–11084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slob AK, deKlerk LWL, and Brand T 1987. Homosexual and heterosexual partner preference in ovariectomized female rats: Effects of testosterone, estradiol and mating experience. Physiology & Behavior 41:571–576. [DOI] [PubMed] [Google Scholar]
- Smiley KO, Vahaba DM, and Tomaszycki ML 2012. Behavioral effects of progesterone on pair bonding and partner preference in the female zebra finch (Taeniopygia guttata). Behavioural Processes 90:210–216. [DOI] [PubMed] [Google Scholar]
- Smith CJW, Wilkins KB, Mogavero JN, and Veenema AH 2015. Social Novelty Investigation in the Juvenile Rat: Modulation by the μ-Opioid System. Journal of Neuroendocrinology 27:752–764. [DOI] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, and Patisaul HB 2014. A Novel Model for Neuroendocrine Toxicology: Neurobehavioral Effects of BPA Exposure in a Prosocial Species, the Prairie Vole (Microtus ochrogaster). Endocrinology 155:3867–3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchabovsky AV, Savinetskaya LE, Ovchinnikova NL, Safonova AV, Ilchenko ON, Sapozhnikova SR, and Vasilieva NA 2019. Sociability and pair-bonding in gerbils: a comparative experimental study. In Current zoology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, and Young LJ 2018. The neural mechanisms and circuitry of the pair bond. Nature Reviews Neuroscience:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Carter CS, and Insel T 1992a. Partner Preference Development in Female Prairie Voles Is Facilitated by Mating or the Central Infusion of Oxytocina. Annals of the New York Academy of Sciences 652:487–489. [DOI] [PubMed] [Google Scholar]
- Williams JR, Catania KC, and Carter CS 1992b. Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav 26:339–49. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, and Carter CS 1994. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster). Journal of neuroendocrinology 6:247–250. [DOI] [PubMed] [Google Scholar]
- Winslow JT 2003. Mouse Social Recognition and Preference. Current Protocols in Neuroscience 22:8.16.1–8.16.16. [DOI] [PubMed] [Google Scholar]
- Yang M, Silverman JL, and Crawley JN 2011. Automated Three-Chambered Social Approach Task for Mice. In Current Protocols in Neuroscience (Crawley JN, Gerfen CR, Rogawski MA, Sibley DR, Skolnick P, and Wray S, eds.) John Wiley & Sons, Inc., Hoboken, NJ, USA: Available at: http://doi.wiley.com/10.1002/0471142301.ns0826s56 [Accessed October 26, 2016]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, and Insel TR 2001. Cellular mechanisms of social attachment. Horm Behav 40:133–8. [DOI] [PubMed] [Google Scholar]


