Abstract
Background:
Data are limited on the burden of influenza and seasonal influenza vaccine effectiveness (VE) in children with sickle cell disease (SCD).
Methods:
We used a prospectively collected clinical registry of SCD patients 6-months to 21-years of age to determine the influenza cases per 100 patient-years, vaccination rates, and a test-negative case-control study design to estimate influenza vaccine effectiveness (VE) against medically-attended laboratory-confirmed influenza infection. Influenza-positive cases were randomly matched to test-negative controls on age and influenza season in 1:1 ratio. We used adjusted logistic regression models to compare odds ratio (OR) of vaccination in cases to controls. We calculated VE as [100% × (1-adjusted OR)] and computed 95% confidence intervals (CIs) around the estimate.
Results:
There were 1037 children with SCD who were tested for influenza, 307 children (29.6%) had at least one influenza infection (338 infections, incidence rate 3.7 per 100 person-years, 95% CI: 3.4, 4.1) and 56.2% of those tested received annual influenza vaccine. Overall vaccine effectiveness pooled over 5 seasons was 22.3% (95%CI −7.3, 43.7%). Adjusted vaccine effectiveness estimates ranged from 39.7% (95%CI −70.1, 78.6%) in 2015/2016 to −5.9% (95%CI: −88.4, 40.4%) in the 2016/17 seasons. Influenza VE varied by age and was highest in children 1–5 years of age (66.6%; 95%CI 30.3, 84.0). Adjusted vaccine effectiveness against acute chest syndrome during influenza infection was 39.4% (95% CI: −113.0, 82.8%).
Conclusions:
Influenza vaccine effectiveness in patients with SCD varies by season and age. Multi-center prospective studies are needed to better establish and monitor influenza vaccine effectiveness among children with SCD.
Keywords: Sickle cell disease, influenza, vaccine effectiveness, test-negative
1 |. INTRODUCTION
Sickle cell disease (SCD) is a common inherited condition and children with SCD are believed to be at increased risk of influenza-related morbidity and mortality.1 However minimal published data are available characterizing the burden of influenza infections in those with SCD. During the 2009 H1N1 influenza pandemic influenza-associated hospitalization rates were estimated to be 56 times greater in children with SCD compared to those without SCD and double that of children with cystic fibrosis.2 Inusa et al. described 21 PCR-confirmed H1N1 cases seen in children with SCD in London, England and estimated that 50% of all H1N1 cases seen in children with SCD presented to a hospital and 25% developed ACS.3 The reason for increased severity of influenza is unclear.3,4 It is thought to be in part due to an increased frequency of acute chest syndrome (ACS) triggered by respiratory infection and pre-existing pulmonary abnormalities related to SCD, such as reactive airway disease (RAD) or asthma, which have been shown to be correlated with an increased risk of influenza-related hospital admission in children with SCD.5
Vaccination is the most effective strategy for prevention of influenza-related illness.6 Advisory Committee on Immunization Practices (ACIP) recommends annual influenza vaccination for individuals aged >6 months.7 However, vaccination rates remain suboptimal and have been reported be as low as 22% in those with SCD.8,9 Nakamura et al. showed that SCD patients were less likely to get seasonal influenza vaccine compared to children with other high-risk conditions such as chronic metabolic diseases or cardiac disease.10 In addition to low vaccine uptake, vaccine response in children with SCD may be attenuated due to altered humoral and cell-mediated immune responses secondary to SCD and immunomodulatory effects of SCD treatments such as hydroxyurea or chronic transfusions.11–13
There are a paucity of data on influenza vaccine immunogenicity and effectiveness in children with SCD. One study examining the humoral and cell-mediated immune responses to monovalent H1N1/2009 and seasonal trivalent influenza vaccination (TIV) in healthy children compared to those with a high-risk condition found that the majority of children with SCD were able to achieve initial protective titers (defined as a serum hemagglutination inhibition (HI) antibody titer ≥1:40 or four-fold increase from baseline) after a single-dose of vaccine, however only 50% of SCD vaccinees had sustained protective antibody titers by 16 weeks after vaccination compared to 70% of healthy controls.14 Another study examining seroconversion after two doses of TIV in children with SCD found a wide range of serologic response with 54–84% of vaccinees having titers of 1:32 greater, which was considered to be protective titer in the study, in the convalescent sera.15 Factors such as age less than 3 years and receipt of chronic transfusions were associated with decreased response to an inactivated A/California/07/2009 H1N1 pandemic influenza vaccine.12 The exact mechanisms that account for this variability in influenza vaccine response are unclear, although recent data found impaired seroconversion and generation of CD8+ T-cell and memory B-cell responses in influenza-vaccinated SCD patients.16 Even less is known regarding influenza vaccine effectiveness (VE) in preventing or lessening the severity of medically-attended influenza infection. To address this knowledge gap, we aimed to determine the incidence of influenza infection in children with SCD and influenza VE against medically-attended laboratory-confirmed influenza infection.
2 |. METHODS
2.1 |. Patients
We initially identified all patients 6 months to 21 years of age who had at least one molecular PCR-based test for influenza performed at Children’s Healthcare of Atlanta (CHOA) during 5 consecutive influenza seasons. Relevant influenza seasons were defined as October 1 to April 30 for the years: 2012–2013, 2013–2014, 2014–2015, 2015–2016, and 2016–2017. CHOA houses one of the largest pediatric SCD programs in the country. The list of patients who were tested for influenza infection during relevant respiratory seasons was then referenced to the CHOA Sickle Cell Disease Clinical Database (SCDCD) to identify those with a diagnosis of SCD. The CHOA SCDCD is a comprehensive, prospective database capturing clinical and demographic information on 3,386 registered patients including their comprehensive clinics, emergency department and inpatient service utilizations at CHOA, SCD genotype, episodes of ACS within the same calendar year, and information on specific SCD therapies (hydroxyurea, chronic transfusion therapy, or hematopoietic stem cell transplantation). We restricted our study to those children with SCD disease who have had at least one healthcare encounter within 12-month periods in our network to minimize the number of children who were not receiving active medical care at our institution.
2.2 |. Study procedures and definitions
For all influenza-positive patients with SCD identified, we conducted a comprehensive review of the electronic medical record (EMR) to confirm the presence of acute respiratory illness at the time of the influenza testing, based on ≥1 of the following symptoms: fever, nasal congestion, rhinorrhea, hoarseness, new or increased-from-baseline cough, sputum production, dyspnea, wheezing of less than 7 days duration or an admitting diagnosis suggestive of ARI (pneumonia, upper respiratory infection, bronchitis, influenza, cough, asthma, viral illness, respiratory distress, respiratory failure). We also collected the information recorded in the medical charts on the duration of symptoms prior to presentation, duration of hospitalization, need for mechanical ventilation or admission to the intensive care unit, co-detection of other respiratory pathogens, use of and timing of administration of antiviral medication, past surgical history, and presence of co-morbid conditions including asthma or reactive airway disease (RAD). We calculated the Emergency Department Reliance (EDR) score at the time of presentation based on the total number of emergency department visits per season divided by the total of ambulatory (outpatient and emergency department) visits and defined a high score as >0.33 based on previously published studies.17 The Georgia Registry of Immunization Transactions and Services (GRITS) records were reviewed for all children with SCD to determine their influenza vaccination status. Under the Georgia Immunization Registry Law, all immunizations given in the State of Georgia are required to be entered into this database which has shown to be a powerful tool for capturing vaccines administered within the state.18 Institutional Review Board approval at CHOA was obtained.
2.3 |. Statistical Analyses
Cases were defined as children with SCD with a medically attended ARI who had laboratory-confirmed influenza infection by a molecular diagnostic test. Controls were defined as children with SCD who had a medically attended ARI and tested negative for influenza. Controls and cases were matched on age and influenza season with 1:1 ratio. Children with SCD who had not received all recommended doses of influenza vaccine per the ACIP or had no documentation of the seasonal influenza vaccine were considered unvaccinated. We excluded patients with missing vaccine records in GRITS, repeat encounters with molecular testing that detected the same pathogen within 30 days, receipt of influenza vaccine within 14 days prior to testing, antiviral use within 7 days prior to testing, and those without documented symptoms of an ARI on presentation.
Season specific and overall incidence rates were computed by dividing number of influenza infections by the time-at-risk i.e. person-years for corresponding populations. We defined person-years as the follow up time for each child until they are diagnosed with influenza or the end of respiratory season whichever comes first. The odds ratio of influenza vaccination during the current season between the influenza cases and controls was calculated using logistic regression. Potential confounders (age, gender, race/ethnicity, severity features of underlying disease such as previous ACS episodes, and need for chronic blood transfusion) were assessed and included in the adjusted model if the covariate changed the VE estimate by >5% or was thought clinically to potentially impact VE.19 In the final model these covariates included age, influenza season, duration of symptoms at the time of presentation, hospitalization and presence of asthma. Influenza VE was calculated as (1-adjusted OR) ×100%. Traditional model diagnostics (e.g., goodness-of-fit tests and residuals plots) were used to evaluate model fit. We considered p-values of <0.05 and 95% confidence intervals (CI) excluding 0% as statistically significant. Statistical analyses were conducted using SAS statistical software (version 9.3 SAS Institute, Cary, NC).
3 |. RESULTS
3.1 |. Influenza disease burden
Across five consecutive influenza seasons from 2012–2017, we captured 2,752 children and adolescents with SCD who had at least one healthcare encounter at CHOA in 12 months, with 9,055 person-years of follow-up. Over the five consecutive influenza seasons, a total of 1037 (37.7%) children with SCD were tested for influenza with a molecular test and 307 children (29.6%) had at least one episode of influenza infection (31 children had 2 infections, 2 children had 3 infections occurring ≥30 days apart) (Figure 1). We identified 342 influenza infections. We excluded 4 episodes either due to use of an antiviral before testing (n=2) or lack of ARI symptoms (n=2). This resulted in a total of 338 eligible influenza-positive “cases” who were matched to 338 influenza-negative “controls” on age and season (Figure 1). Influenza A was detected in 216 (63.9%), influenza B was detected in 110 (32.5%) and subgroup was unknown in 12 (3.6%) cases (Table 1). The overall incidence rate pooled over 5 seasons was 3.7/100 person-years (95% CI: 3.4, 4.1) and varied by season (Table 1).
Figure 1.
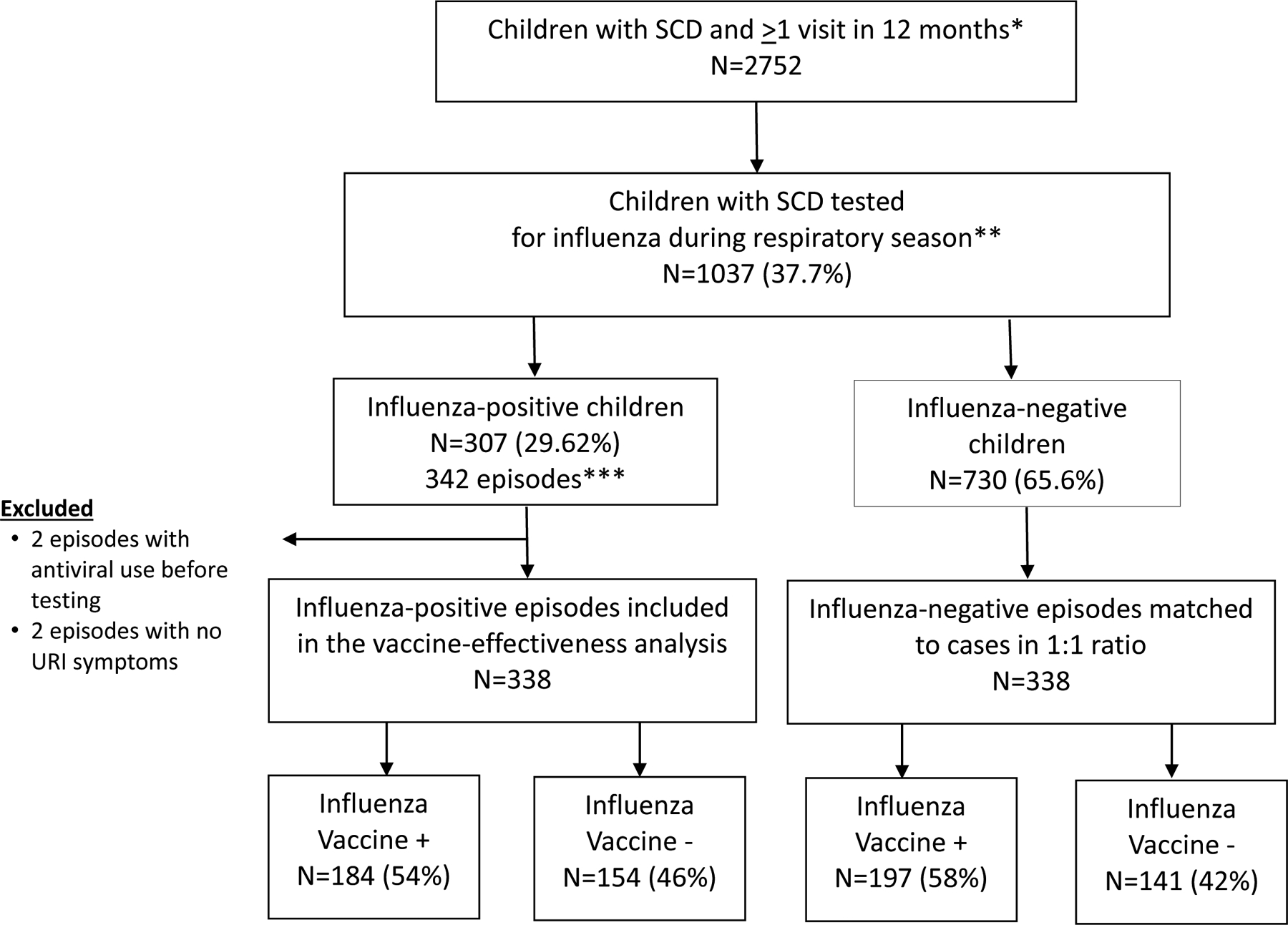
Study enrollment and influenza case status, 2012–2017
*Each 12-month period starts on July, 1 and ends on June, 30 in next calendar year and includes one influenza season.
**Influenza seasons were defined as October 1 to April 30 for the years: 2012–2013, 2013–2014, 2014–2015, 2015–2016, and 2016–2017
***33 children had >1 infection at least 30 days apart (31 children with 2 infections, 2 children with 3 infections)
Table 1:
Distribution of Influenza Positive Cases by Season and Influenza Subtype
| Season | Influenza A | Influenza B | Unknown | Total Influenza Cases | Total Person-Years | Incidence Rate (per 100 person-years) | 95% LCL | 95% UCL | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||||||
| 2012–2013 | 41 | 56.2% | 32 | 43.8% | 0 | 0.0% | 73 | 1743 | 4.2 | 3.4 | 5.2 |
| 2013–2014 | 12 | 80.0% | 3 | 20.0% | 0 | 0.0% | 15 | 1752 | 0.9 | 0.5 | 1.4 |
| 2014–2015 | 93 | 90.3% | 10 | 9.7% | 0 | 0.0% | 103 | 1795 | 5.7 | 4.76 | 6.9 |
| 2015–2016 | 19 | 54.3% | 14 | 40.0% | 2 | 5.7% | 35 | 1831 | 1.9 | 1.48 | 2.75 |
| 2016–2017 | 51 | 45.5% | 51 | 45.5% | 10 | 8.9% | 112 | 1934 | 5.8 | 4.8 | 6.9 |
| Overall | 338 | 9055 | 3.7 | 3.46 | 4.1 | ||||||
LCL Lower confidence limit, UCL upper confidence limit
Influenza-positive cases and influenza-negative controls did not differ significantly by age, sex, race/ethnicity or SCD genotype (Table 2). Influenza-positive children were more likely to present with <3 days of symptoms (86.1% vs 78.1%, p=0.0004). Likewise, influenza-positive children were less likely to be hospitalized (47.1% vs 69.1%, p<0.0001). Children with influenza and those without influenza were similar with regards to Emergency Department Reliance, receipt of transfusion or of hydoxyurea in the prior 3 months. Cases had fewer episodes of ACS within the previous calendar year compared with controls (mean number of ACS episodes 0.18 vs 0.32 respectively, p= 0.0016). Influenza-positive children were less likely to develop ACS during the enrollment encounter (4% vs 14%, p <0.0001). Oseltamivir was used in 312/338 (92.3%) of influenza-positive cases and 7/338 (2.1%) of influenza-negative controls (p <0.0001). There was not a significant difference in length of hospitalization, ICU admission, mortality, or need for mechanical ventilation between cases and controls. Among influenza-positive cases, 6/184 (3.3%) children who had received influenza vaccination developed ACS during the current encounter whereas 8/154 (5.2%) unvaccinated children had ACS (OR 0.62, 95% CI 0.21–1.81).
Table 2:
Demographic, Clinical, and Vaccination Features of Influenza Positive and Negative Children with Sickle Cell Disease
| Influenza Positive Cases (N=338) | Influenza Negative Controls (N=338) | P-value | |
|---|---|---|---|
| Age, years (median, IQR) | 8.1 (4.8–12.5) | 8.1 (4.4–12.5) | 0.96 |
| Female (n, %) | 166 (49.1%) | 151 (44.7%) | 0.25 |
| Race | |||
| Black or African-American (n, %) | 329 (97.6%) | 330 (97.6%) | 0.81 |
| White (n, %) | 4 (1.2%) | 6 (1.8%) | |
| Other or Unknown (n, %) | 5 (1.5%) | 2 (0.6%) | |
| Ethnicity* | |||
| Non-Hispanic or Latino (n, %) | 328 (97.0%) | 324 (95.9%) | 0.56 |
| Hispanic or Latino (n, %) | 9 (2.7%) | 14 (4.1%) | |
| Sickle Cell Genotype | |||
| SS/S Beta Zero Thalassemia (n, %) | 247 (73.1%) | 265 (78.4) | |
| Other genotypes (n, %) | 91 (27.9%) | 73 (22.6%) | |
| Days of symptoms prior to presentation | |||
| <3 days (n, %) | 291 (86.1%) | 264 (78.1%) | 0.0004 |
| 3–4 days (n, %) | 33 (9.8%) | 31 (9.2%) | |
| ≥5 days (n, %) | 14 (4.1%) | 43 (12.7%) | |
| EDR Higha (n, %) | 229 (69.6%) | 238 (74.4%) | 0.18 |
| Receipt of transfusion in prior 3 months (n, %) | 9 (2.7%) | 9 (2.7%) | 0.95 |
| Hydroxyurea use (n, %) | 148 (45.0%) | 123 (38.4%) | 0.09 |
| Reactive Airway Disease or Asthma (n, %) | 87 (25.7%) | 113 (33.4%) | 0.028 |
| ACSb episodes in prior year (mean, 95% CI) | 0.28 (0.1–0.2) | 0.3 (0.3–0.4) | 0.0016 |
| Receipt of Oseltamavir (n, %) | 312 (92.3%) | 7 (2.1%) | <0.0001 |
| Receipt of Seasonal Influenza Vaccine (n, %) | 184 (54.4%) | 197 (58.3%) | 0.31 |
| Hospitalization (n, %) | 155 (47.1%) | 233 (69.1%) | <0.0001 |
| Length of stay, days (mean, 95% CI) | 3.0 (2.4–3.6) | 3.7 (3.2–4.1) | 0.09 |
| ICU admission (n, %) | 2 (1.3%) | 8 (3.5%) | 0.18 |
| Mechanical ventilation (n, %) | 0 (0.0%) | 1 (0.3%) | 0.11 |
| ACSb during hospitalization (n, %) | 14 (4.3%) | 46 (14.4%) | <0.0001 |
Emergency Department Reliance (EDR), High EDR >0.33,
Acute Chest Syndrome (ACS),
Unknown 1 (0.3%)
Among children with influenza, 28 (8.6%) patients had other viral coinfections, while 107/338 (31.7%) who tested negative for influenza had at least one other identified viral infection (Table 3). The most common viral pathogens detected among influenza-negative controls were Rhinovirus/Enterovirus (48 patients, 14.2%), human metapneumovirus (HMPV) (26 patients, 7.7%) and respiratory syncytial virus (RSV) (17 patients, 5.0%).
Table 3.
Non-influenza respiratory pathogens detected in respiratory viral panel among children with SCD
| Influenza Positive Cases (N=338) | Influenza Negative Controls (N=338) | |||
|---|---|---|---|---|
| N | %* | N | % | |
| HMPV | 2 | 0.6 | 26 | 7.7 |
| Rhino/Enterovirus | 13 | 3.8 | 48 | 14.2 |
| RSV | 4 | 1.2 | 17 | 5.0 |
| Adenovirus | 1 | 0.3 | 9 | 2.7 |
| Parainfluenza_3 | 1 | 0.3 | 8 | 2.4 |
| Parainfluenza_4 | 1 | 0.3 | 4 | 1.2 |
| Coronavirus_229e | 1 | 0.3 | 2 | 0.6 |
| Coronavirus_oc43 | 4 | 1.2 | 9 | 2.7 |
| Chlamydia pneumoniae | 2 | 0.6 | 0 | 0.0 |
| Mycoplasma pneumoniae | 0 | 0.0 | 5 | 1.5 |
| Total | 29** | 8.6 | 128 | 37.9 |
% of patients,
28 patients, 29 viruses, 1 patient had >1 viral coinfection
3.2 |. Vaccine effectiveness
Overall, 54% of influenza cases and 58% of controls were vaccinated with seasonal influenza vaccine ≥14 days prior to illness onset (Table 4). Adjusted overall VE pooled for all seasons from 2012 to 2017 was estimated as 22.3% (95% CI: −7.3, 43.7%) against medically-attended influenza infection. Overall VE was 19.7% (95% CI: −15.6, 44.3%) against influenza A and 21.8% (95% CI: −23.0, 50.3%) against influenza B. Influenza VE among children with SCD varied between seasons (ranged −5.9% to 39.7%, Table 4). VE also varied by age groups and the highest point estimates were in SCD children 1–5 years old (66.6%, 95% CI: 30.3, 84.0%) and lowest estimates were among children aged 6–18 years (−3.9%, 95% CI: −52.9, 29.5%). Adjusted VE against developing ACS during influenza-related hospitalization was 39.4% (95% CI: −113.0, 82.8%).
Table 4.
Crude and Adjusted Influenza Vaccine Effectiveness Estimates by Influenza Type, Age Group and Season
| Number of Influenza Vaccinated Among Flu Test (+) Cases | Number of Influenza Vaccinated Among Flu Test (−) Controls | Crude VE | Adjusted VE*** | |
|---|---|---|---|---|
| (N, %) | (N, %) | (N, 95% CI) | (N, 95% CI) | |
| Overall | 184/338 (54.4%) | 197/338 (58.3%) | 14.5% (−15.9 to 36.9) | 22.3% (−7.3 to 43.7) |
| Influenza A* | 121 (65.8%)* | 8.4% (−29.4 to 35.1) | 19.7% (−15.6 to 44.3) | |
| Influenza B* | 59 (32.0%)* | 16.8% (−28.3 to 46.0) | 21.8% (−23.0 to 50.3) | |
| Age Group | ||||
| 6mo – < 1yr | 11 (40.7%) | 13 (54.2%) | 41.8% (−76.7 to 80.9) | 24.4% (−145.1 to 76.7) |
| 1yr – < 5yr | 28 (46.7%) | 53 (71.6%) | 65.3% (29.1 to 83.1) | 66.6% (30.3 to 84.0) |
| 6yr – < 18yr | 145 (57.8%) | 131 (54.6%) | −13.8% (−62.6 to 20.3) | −3.9% (−52.9 to 29.5) |
| Seasons** | ||||
| 2012–2013 | 44 (60.3%) | 47 (64.4%) | 16.1% (−64.1 to 57.1) | 22.1% (−60.3 to 61.7) |
| 2013–2014 | 8 (53.3%) | 10 (66.7%) | 42.9% (−150.3 to 87.0) | 26.1% (−288.9 to 86.0) |
| 2014–2015 | 52 (50.5%) | 60 (58.2%) | 26.9% (−26.7 to 57.8) | 33.7% (−18.6 to 63.0) |
| 2015–2016 | 15 (42.9%) | 18 (51.4%) | 29.2% (−81.7 to 72.4) | 39.7% (−70.1 to 78.6) |
| 2016–2017 | 65 (58.0%) | 62 (55.4%) | −11.5% (−89.2 to 34.3) | −5.9% (−88.4 to 40.4) |
4 cases with no subtype information available
2012–2013 49% (43–55), 2013–2014 52% (44–59), 2014–2015 19% (10–27), 2015–2016 48% (43–55), 2016–2017 40% (32–46) (Centers for Disease Control and Prevention. Seasonal Flu Vaccine Effectiveness Studies. 5 November 2019. Available at: https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm. Accessed 21 November 2019.)
Adjusted for age, influenza season, duration of symptoms at presentation, hospitalization and presence of asthma
4 |. DISCUSSION
Our study evaluated the burden of influenza infection and influenza vaccine effectiveness against medically attended ARI in children with SCD across 5 influenza seasons from 2012–2017. We found that half of the children with SCD had undergone testing for influenza at least once during the study period, and of those tested, approximately one third had at least one laboratory-confirmed influenza infection. The incidence rate of influenza infection among children with SCD was 3.7 per 100 person-years. Influenza-positive cases were more likely to present earlier for evaluation to a healthcare facility, within 3 days after symptom onset, and were more likely to receive oseltamivir compared to influenza-negative children with SCD. We speculate that seeking medical care earlier during influenza infection may be related to the presence of fever and myalgias, which are common early symptoms of influenza and are also symptoms that typically prompt individuals with SCD to seek emergency care. We did not find a statistically significant difference in length of hospitalization, and overall mortality and ICU admissions were similarly rare in both groups. This could be driven by the early presentation of patients with SCD to healthcare, since 86.1% of influenza-positive cases presented with <3 days of symptoms, and extensive use of oseltamivir (92.3%). Earlier presentation to care and initiation of oseltamivir have been shown to shorten hospitalization and decrease mortality in critically ill patients.20,21
We also found that influenza vaccine effectiveness against medically-attended illness had significant variability between age groups and seasons. Influenza VE can be impacted by many factors such as receipt of prior seasonal influenza vaccination, timing of influenza vaccination relative to the onset of infection, age, co-morbidities, and antigenic match between the vaccine strain and antigenically-drifted circulating influenza strains.22 For example, overall influenza VE reported as 38% (95%CI: 31%,43%) in 2017–2018 season but was 19% (95%CI: 10%,27%) in 2014–2015 season among children and adults enrolled in the U.S. Influenza Vaccine Effectiveness Network.23 In our study, point estimates of influenza VE for 2012–2017 seasons ranged from −5.9% to 39.7% against medically attended ARI. There are very limited data on the benefit of influenza vaccination in high-risk pediatric populations including the SCD population. However, humoral, cellular and cytokine immune responses to the influenza vaccination are known to be altered among children with SCD.16 The differences in immune profile including diminished lymphocyte subsets among SCD children, particularly among those who are receiving chronic transfusions, also suggests that these patients will likely have a reduced ability to overcome influenza once infected. In addition, a recent study of VE against laboratory-confirmed influenza in Canada found significant VE for fully vaccinated children except those with underlying asthma.24 Asthma or RAD was reported in approximately one third of our patients and we included these factors in our final adjusted models.
Data from surveillance networks showed that among hospitalized adults, influenza vaccination may reduce the risk of influenza-related death, ICU admission, and length of hospital stay.25,26 Approximately 80% of reported pediatric influenza-associated deaths have occurred in unvaccinated children.27,28 During the 2015–2016 season where influenza A(H1N1) was the predominant circulating influenza strain, vaccination was 51% and 53% effective in preventing hospitalization due to influenza A(H1N1)pdm09 and influenza B virus infection respectively.29 Recent evidence from the New Vaccine Surveillance Network found influenza VE against influenza-associated hospitalizations among vaccinated children was 49% and 51% during the 2016–2017 and 2017–2018 flu seasons respectively. Although we failed to show a statistical significance, influenza-positive children who had received influenza vaccination tended to have fewer episodes of ACS and ICU admission in our study compared to those who were influenza-positive but had not received annual vaccination. We found a lower rate of ACS in influenza positive children compared to prior studies (6.4% compared to 20.8–47.6%), although these were conducted during the H1N1 influenza pandemic and our data reflects cumulative rates across 5 seasons.3,5,31 These data are worth further investigations and highlight the important concept that even among patients with breakthrough influenza infection, vaccination can still attenuate disease severity.25,32
In our study approximately half of the children with SCD were not vaccinated against influenza prior to ARI-related medically-attended illness. This is a missed opportunity to improve outcomes among these high-risk children. In addition, we found that influenza vaccine effectiveness was 66.6% (95% CI: 30.3, 84.0%) among children with SCD aged 1–5 years, an age group considered a priority for influenza vaccination by the ACIP and World Health Organization. While our estimates for influenza vaccine effectiveness are not optimal, particularly compared with the effectiveness for most other vaccines in routine use, annual influenza vaccination remains our best strategy for preventing influenza infections in high risk and healthy individuals.
We were unable to evaluate the impact of chronic transfusion therapy and hydroxyurea use on influenza VE in our study. The use of chronic transfusions has been associated with decreased antibody response to H1N1 vaccination, although in a different study the use of hydroxyurea and chronic transfusions was found to be protective against ACS in hospitalized children.5,12 Given the widespread use of chronic transfusion therapy and hydroxyurea in children with SCD, their impact on vaccine response merits further evaluation.
A strength of our study is the large number of children with SCD captured through our center’s clinical database. We performed individual chart reviews on each study subject, and limited our analyses to those who met our strict case definition for acute respiratory infection upon presentation to care. We restricted our cases to those who had symptoms ≤7 days to avoid the impact of delayed hospital presentation and false-negative test results if the infection had been cleared before testing. We utilized a test-negative case-control design to estimate the seasonal influenza vaccine effectiveness, which controls for health-care seeking bias that may exist due to differences in access to care. This methodology is also utilized by others including the Centers for Disease Control and Prevention (CDC) to determine annual influenza vaccine effectiveness and provides an opportunity to compare the estimates between the studies.33
Our study has several limitations including the retrospective nature of the chart reviews which could have issues with misclassification and missing data. We also used clinical testing which does not include subtype information on the hemagglutinin and neuraminidase surface proteins, and our study is not able to provide subtype-specific VE estimates. Immunization data may not have been complete if immunization was obtained outside of the state of Georgia, or if the healthcare provider did not comply with Georgia law. We think that this is unlikely as prior data suggest that GRITS is highly accurate in comparison to vaccination records obtained from the patient’s healthcare provider.18 It is possible that some of our patients may have presented for medical attention for an ARI due to influenza outside of a CHOA healthcare facility, which would result in an underestimation of the true incidence of influenza in our population. It is unlikely, however, that this would differ between those that are vaccinated and unvaccinated. Another limitation is that the decision to test for influenza when a patient presents with ARI is provider-specific, and not all SCD patients with ARI may have been tested for influenza. In addition, children with SCD may be more likely to present for care with fever and to be hospitalized with fever than non-SCD children. However, we restricted our controls to only children with SCD to overcome this bias. Providers may be less likely to test for influenza if they are aware of influenza vaccination prior to presentation which could bias our vaccine effectiveness estimates. Finally, our study includes a specific cohort of children with SCD and the statistical significance of our analyses was impacted by our sample size (i.e. our confidence intervals are wide). Due to our study design, we were not able to determine which type of influenza vaccine (inactivated trivalent, quadrivalent, or live attenuated influenza vaccine) was administered, only whether the vaccine was given and the timing of vaccine.
In conclusion, this study contributes to our understanding of the healthcare burden of influenza in children with SCD, as well as the need to improve influenza vaccine uptake and vaccine effectiveness. Although VE against medically-attended laboratory-confirmed influenza is suboptimal, vaccination remains the best strategy to prevent influenza-related illness, hospitalizations, and deaths. Strategies such as administration of high-dose influenza vaccine, or a booster dose within the same season, have been shown to increase immunogenicity in solid-organ and hematopoietic stem cell transplant patients and similar strategies should be explored in children with SCD.34–36 To our knowledge, our study provides the estimates of influenza incidence and influenza VE based on the largest pediatric SCD cohort in the literature, however future, multi-center prospective studies are needed to determine influenza VE and determine the impact of implementing new strategies in the SCD population.
Funding:
NB received funding from the National Heart, Lung and Blood Institute of the National Institutes of Health under award number 1K23HL140142-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Battersby AJ, Knox-Macaulay HH, Carrol ED. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer 2010; 55(3): 401–6. [DOI] [PubMed] [Google Scholar]
- 2.Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics 2010; 125(2): 234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inusa B, Zuckerman M, Gadong N, et al. Pandemic influenza A (H1N1) virus infections in children with sickle cell disease. Blood 2010; 115(11): 2329–30. [DOI] [PubMed] [Google Scholar]
- 4.Caboot JB, Allen JL. Pulmonary complications of sickle cell disease in children. Curr Opin Pediatr 2008; 20(3): 279–87. [DOI] [PubMed] [Google Scholar]
- 5.George A, Benton J, Pratt J, et al. The impact of the 2009 H1N1 influenza pandemic on pediatric patients with sickle cell disease. Pediatr Blood Cancer 2011; 57(4): 648–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omer SB, Yildirim I. Editorial Commentary: Influenza Vaccine Effectiveness: A Glass Both Half Full and Half Empty. Clin Infect Dis 2016; 63(12): 1574–6. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Children & Flu. 2019. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm. (accessed 21 November 2019.
- 8.Beverung LM, Brousseau D, Hoffmann RG, Yan K, Panepinto JA. Ambulatory quality indicators to prevent infection in sickle cell disease. Am J Hematol 2014; 89(3): 256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hambidge SJ, Ross C, Glanz J, et al. Trivalent inactivated influenza vaccine is not associated with sickle cell crises in children. Pediatrics 2012; 129(1): e54–9. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura MM, Lee GM. Influenza vaccination in adolescents with high-risk conditions. Pediatrics 2008; 122(5): 920–8. [DOI] [PubMed] [Google Scholar]
- 11.Ballester OF, Abdallah JM, Prasad AS. Impaired IgM antibody responses to an influenza virus vaccine in adults with sickle cell anemia. Am J Hematol 1985; 20(4): 409–12. [DOI] [PubMed] [Google Scholar]
- 12.Purohit S, Alvarez O, O’Brien R, Andreansky S. Durable immune response to inactivated H1N1 vaccine is less likely in children with sickle cell anemia receiving chronic transfusions. Pediatr Blood Cancer 2012; 59(7): 1280–3. [DOI] [PubMed] [Google Scholar]
- 13.Balandya E, Reynolds T, Obaro S, Makani J. Alteration of lymphocyte phenotype and function in sickle cell anemia: Implications for vaccine responses. Am J Hematol 2016; 91(9): 938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Long CB, Ramos I, Rastogi D, et al. Humoral and cell-mediated immune responses to monovalent 2009 influenza A/H1N1 and seasonal trivalent influenza vaccines in high-risk children. J Pediatr 2012; 160(1): 74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glezen WP, Glezen LS, Alcorn R. Trivalent, inactivated influenza virus vaccine in children with sickle cell disease. Am J Dis Child 1983; 137(11): 1095–7. [DOI] [PubMed] [Google Scholar]
- 16.Nagant C, Barbezange C, Dedeken L, et al. Alteration of humoral, cellular and cytokine immune response to inactivated influenza vaccine in patients with Sickle Cell Disease. PLoS One 2019; 14(10): e0223991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alessandrini EA, Shaw KN, Bilker WB, Perry KA, Baker MD, Schwarz DF. Effects of Medicaid managed care on health care use: infant emergency department and ambulatory services. Pediatrics 2001; 108(1): 103–10. [DOI] [PubMed] [Google Scholar]
- 18.Cortese MM, Leblanc J, White KE, et al. Leveraging state immunization information systems to measure the effectiveness of rotavirus vaccine. Pediatrics 2011; 128(6): e1474–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrews N, Waight P, Yung CF, Miller E. Age-specific effectiveness of an oil-in-water adjuvanted pandemic (H1N1) 2009 vaccine against confirmed infection in high risk groups in England. J Infect Dis 2011; 203(1): 32–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lytras T, Mouratidou E, Andreopoulou A, Bonovas S, Tsiodras S. Effect of early oseltamivir treatment on mortality in critically ill patients with different types of influenza: a multi-season cohort study. Clin Infect Dis 2019. [DOI] [PubMed] [Google Scholar]
- 21.Katzen J, Kohn R, Houk JL, Ison MG. Early Oseltamivir After Hospital Admission Is Associated With Shortened Hospitalization: A 5-Year Analysis of Oseltamivir Timing and Clinical Outcomes. Clin Infect Dis 2019; 69(1): 52–8. [DOI] [PubMed] [Google Scholar]
- 22.Lewnard JA, Cobey S. Immune History and Influenza Vaccine Effectiveness. Vaccines (Basel) 2018; 6(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.CDC. Past Seasons Vaccine Effectiveness Estimates. April 5, 2019. https://www.cdc.gov/flu/vaccines-work/past-seasons-estimates.html (accessed December 20 2019).
- 24.Buchan SA, Chung H, Campitelli MA, et al. Vaccine effectiveness against laboratory-confirmed influenza hospitalizations among young children during the 2010–11 to 2013–14 influenza seasons in Ontario, Canada. PLoS One 2017; 12(11): e0187834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arriola C, Garg S, Anderson EJ, et al. Influenza Vaccination Modifies Disease Severity Among Community-dwelling Adults Hospitalized With Influenza. Clin Infect Dis 2017; 65(8): 1289–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thompson MG, Pierse N, Sue Huang Q, et al. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 2018; 36(39): 5916–25. [DOI] [PubMed] [Google Scholar]
- 27.Doyle JD, Chung JR, Kim SS, et al. Interim Estimates of 2018–19 Seasonal Influenza Vaccine Effectiveness - United States, February 2019. MMWR Morb Mortal Wkly Rep 2019; 68(6): 135–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flannery B, Reynolds SB, Blanton L, et al. Influenza Vaccine Effectiveness Against Pediatric Deaths: 2010–2014. Pediatrics 2017; 139(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferdinands JM, Gaglani M, Martin ET, et al. Prevention of Influenza Hospitalization Among Adults in the United States, 2015–2016: Results From the US Hospitalized Adult Influenza Vaccine Effectiveness Network (HAIVEN). J Infect Dis 2019; 220(8): 1265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campbell M Angela P, MPH, Ogokeh Constance E, MPH, McGowan Craig, MS, Rha Brian, MD, MSPH, Selvarangan Rangaraj, BVSc, PhD, Staat Mary A, MD, MPH, Weinberg Geoffrey A, MD, Boom Julie A, MD, Englund Janet A, MD, Williams John V, MD, Halasa Natasha B, MD, MPH, Szilagyi Peter G, MD, MPH, Harrison Christopher J, MD, Klein Eileen J, MD, MPH, McNeal Monica, MS, Michaels Marian G, MD, MPH, Sahni Leila C, PhD, MPH, Stewart Laura S, PhD, Lively Joana Y, MPH, Beacham Lauren, MA, Payne Daniel C, PhD, MSPH, Fry Alicia M, MD, MPH, Patel Manish, MD. Influenza Vaccine Effectiveness Against Laboratory-Confirmed Influenza in Children Hospitalized with Respiratory Illness in the United States, 2016–2017 and 2017–2018 Seasons. Open Forum Infect Dis 2019; 6(2): S26–S7. [Google Scholar]
- 31.Strouse JJ, Reller ME, Bundy DG, et al. Severe pandemic H1N1 and seasonal influenza in children and young adults with sickle cell disease. Blood 2010; 116(18): 3431–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arriola CS, Anderson EJ, Baumbach J, et al. Does Influenza Vaccination Modify Influenza Severity? Data on Older Adults Hospitalized With Influenza During the 2012–2013 Season in the United States. J Infect Dis 2015; 212(8): 1200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Seasonal Flu Vaccine Effectiveness Studies. 2019. https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm. (accessed 21 November 2019.
- 34.Halasa NB, Savani BN, Asokan I, et al. Randomized Double-Blind Study of the Safety and Immunogenicity of Standard-Dose Trivalent Inactivated Influenza Vaccine versus High-Dose Trivalent Inactivated Influenza Vaccine in Adult Hematopoietic Stem Cell Transplantation Patients. Biol Blood Marrow Transplant 2016; 22(3): 528–35. [DOI] [PubMed] [Google Scholar]
- 35.Natori Y, Shiotsuka M, Slomovic J, et al. A Double-Blind, Randomized Trial of High-Dose vs Standard-Dose Influenza Vaccine in Adult Solid-Organ Transplant Recipients. Clin Infect Dis 2018; 66(11): 1698–704. [DOI] [PubMed] [Google Scholar]
- 36.Cordero E, Roca-Oporto C, Bulnes-Ramos A, et al. Two Doses of Inactivated Influenza Vaccine Improve Immune Response in Solid Organ Transplant Recipients: Results of TRANSGRIPE 1–2, a Randomized Controlled Clinical Trial. Clin Infect Dis 2017; 64(7): 829–38. [DOI] [PubMed] [Google Scholar]


