Abstract
With increasing pathogenic bacterial infection that is occurring worldwide, antibacterial therapy has become an important research field. There is great antimicrobial potential in the nanomaterial-based metal–organic framework (MOF) platform because it is highly biocompatible, biodegradable, and nontoxic, and it is now widely used in the anticancer agent industry and in the production of medical products. This review summarizes the possible mechanisms of representative MOF-based nanomaterials, and recounts recent progress in the design and development of MOF-based antibacterial materials for the remedy of postoperative infection. The existing shortcomings and future perspectives of the rapidly growing field of antimicrobial therapy addressing patient quality of life issues are also briefly discussed. Because of their wide applicability, further studies on the use of different MOF antimicrobial therapies will be of great interest.
The existing shortcomings and future perspectives of the rapidly growing field of antimicrobial therapy addressing patient quality of life issues are also discussed.
Introduction
Pathogenic bacterial infection is one of the most severe threats to global public health because it results in millions of deaths annually. Due to the high morbidity and mortality rates caused by pathogenic bacteria, numerous broad-spectrum antibiotics have been widely used in large doses for bacterial infection therapy, which leads to increasing antibiotic resistance and a subsequent lack of effective treatment.1–5 Obviously, it is of crucial importance to develop effective strategies for inactivating bacteria with multidrug-resistance through noninvasive therapeutic approaches.
To date, substantial efforts have been dedicated to exploring new antimicrobial materials for bacterial infection therapy, such as metal/metal oxide nanoparticles,6–8 nanozymes,9 cationic polymers,10 and metal–organic frameworks (MOFs).11–13 Among these emerging nanomaterials, MOFs are a class of inorganic–organic hybrid porous polymers that have been developed as unique nanoplatforms for biomedical applications.14,15 MOFs are organic–inorganic hybrid materials that can be formed into a one-dimensional, two-dimensional, or three-dimensional structure according to need, and considerable research has been performed in recent years to elucidate their properties.16–18 The ultra-high specific surface area, satisfactory biocompatibility and biodegradability, and excellent physical and chemical properties of MOFs can be regulated and tailored through the selection of different metals and organic ligands. In addition, MOFs possess a porous structure and numerous channels, and they have high density with uniformly distributed catalytic active sites.
These properties of MOFs can promote and induce most small molecular substrates to fully enter the active sites, which is conducive to the transportation and diffusion of products, so as to achieve the antibacterial effect of MOF materials. Moreover, MOFs can maximize the use of metal atoms, which improves the catalytic properties and selectivity of the material.19 Additionally, an MOF-derived cascaded reactive oxygen species (ROS) catalytic core has been explored and synthesized.19 Because of the exceptional features of MOFs within diverse compositions that distinguish them from other materials, including their high specific surface area, permanent porosity, and versatile functionality, they are highly desirable for bacterial infection therapy.20,21
MOFs are very promising candidates for therapy because they can control and release antibacterial agents by several mechanisms including (i) release of the antimicrobial ligands from the MOF;22 (ii) release of antibacterial metal ions from the MOF, e.g., Ag, Cu, and Zn;23 (iii) release of antibacterial active species entrapped within the MOF;24 or (iv) release of antibacterial agents from flexible post-modification of the MOF.25 In this review article, we summarize the recent progress in developing MOF-based nanoplatforms for antibacterial application, and we envision the challenges and outlook for the development of MOFs in this exciting field (Fig. 1).
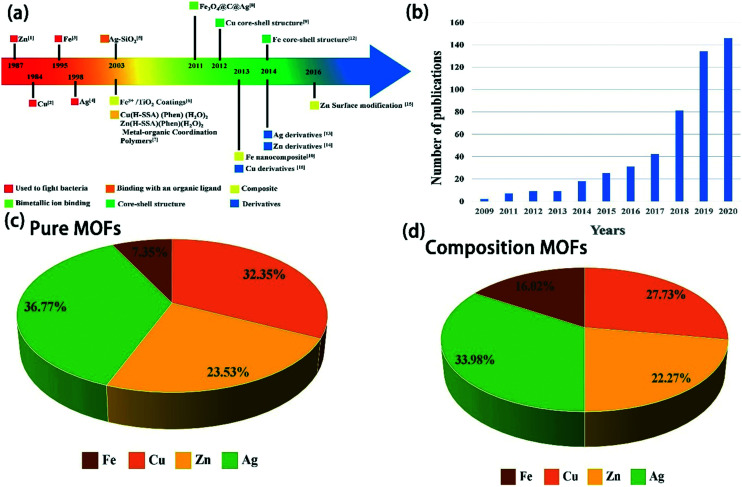
Fig. 1. (a) Milestones in the development of MOF-based materials for antimicrobial therapy systems. (b) The bar graph represents overall publication details on MOF antibacterial therapy using the keyword “MOFs” and “antimicrobial” from 2010–2020. The pie charts show the percentage sharing of (c) different pure MOFs, and (d) composite MOFs for antibacterial therapy.
MOFs for antibacterial therapy
Based on the current global research being conducted on metal organic skeleton materials, the metal ions of silver, zinc, iron, magnesium, manganese, and copper are used for their antibacterial functions. In this study, four common metal ions were mainly used to prepare nanomaterials with antibacterial properties. The selected MOF materials based on these four metal ions are shown in Table 1.
Overview of the antibacterial properties for selected MOF particles.
| Metal ion | Formula | Application | Ref. |
|---|---|---|---|
| Zn | Fe3O4@PAA@ZIF-8 | Load, transport, and release drugs | 25 |
| Inhibit bacterial growth | |||
| Improve antibacterial properties of drugs | |||
| Zn-MOF | Biological catalyst | 26 | |
| [Zn4(bdc)4(ppmh)2(H2O)]n | Antibacterial agent | 27 | |
| IRMOF-3 | Antimicrobial agent, drug additive | 28 | |
| ZnO@ZIF-8 | Load, transport, and release drugs | 29 | |
| Inhibit bacterial growth | |||
| ZIF-8/OCBs | Microbial inactivation | 30 | |
| [Ag2L(NO3)2]n | Antibacterial action against Gram-negative (Escherichia coli, Pseudomonas aeruginosa) and Gram-positive (Staphylococcus aureus, Bacillus subtilis) bacteria | 31 | |
| {[Ag6L4(CF3SO3)4][CF3SO3]2}n | |||
| {[Ag2L(NO3)][ClO4]}n | |||
| {[Ag2L(NO3)][BF4]}n | |||
| {[Ag2L(NO3)][PF6]}n | |||
| {[Ag2L(NO3)][SbF6]}n | |||
| ZnO-CNP-TRGL | Skin pathogen infection, implantation sterilization | 32 | |
| BSA@ZIF-8 | Anti-bacterial, anti-infectious | 33 | |
| Tet@ZIF-8@HA | Targets bacteria inside the cell | 34 | |
| Restores the effectiveness of antibiotics | |||
| Mg/Zn-MOF-74 | Antibacterial, anti-inflammatory, pro-osteogenesis | 35 | |
| TRB-ZnO@G | Augmentive anti-infection therapy | 36 | |
| o-NBA@ZIF-8 | Promotes wound healing | 37 | |
| Cu | Cur@Cu/ZIF-8 | Antimicrobial and anti-biofilm, enhances the production of ROS | 38 |
| Cu-BTC/PVA | Inhibits E. coli | 39 | |
| Cu-BPDCA-ty | Inhibits E. coli | 40 | |
| CuS@HKUST-1 | Excellent antibacterial properties, especially against S. aureus | 41 | |
| Wool@HKUST-1 | Inhibits E. coli | 42 | |
| HKUST-1@CMCS | Antibacterial carrier | 43 | |
| HKUST-1/OCBs | Antibacterial activity, microbial inactivation | 30 | |
| [Cu(μ2-Amp)2]n | Inhibits E. coli and S. aureus | 44 | |
| Ag | C–Zn/Ag | Drug carriers | 45 |
| Resistant to pathogen infection | |||
| Antibiotic substitutes | |||
| Ag2[HBTC] (im) | Wound dressing | 46 | |
| Anti-infection treatment | |||
| Control of bacterial skin infections | |||
| Ag@CD-MOF | Promotes wound healing | 47 | |
| Cap-FeIII-HMOF-5 | Inhibits the activity of E. coli | 48 | |
| Ag NPs@UiO-66(DMF) | Combats the current antimicrobial resistance crisis | 49 | |
| Fe | D-AzAla@MIL-100 (Fe) NPs | Bacterial detection, antimicrobial therapy | 50 |
| Sr | MG-MOF-74 | Anti-bacterial, promotes the proliferation of osteoblasts | 51 |
1. Zn-MOFs
1.1. Zinc ion-ligand binding MOFs
Researchers suggest that multifunctional 3D Zn(ii)-MOFs may be potential candidates to greatly improve human health.64 Chandra et al. designed a novel hybrid ligand coordination polymer [Zn4(bdc)4(ppmh)2(H2O)]n(1)(H2bdc = 1,4-benzene dicarboxylic acid), using pyridine HYDR and 1,4-benzenedicarboxylic acid (H2bdc), both of which were used as bridging agents combined with Zn(ii). It has been characterized by single crystal X-ray diffraction (SCXRD). This molecule undergoes a higher dimension from the combination of arrangement of organic ligands and different supramolecular interactions, mainly H-bonding and π⋯π interaction. Fascinatingly, the molecule exhibits enhanced electrical conductivity when exposed to light. Thus, the compound may be a useful candidate for optical device fabrication. The coordination polymer has demonstrated effective antibacterial and anticancer activity.27
IRMOF-3 is an efficient and reusable heterogeneous catalyst that is widely used in antibacterial property detection.57 Abdelhameed et al. explored the potential of post-synthetic modification to enhance the biological activity of IRMOF-3.28 Specifically, this material was modified with 2,5-dimethoxytetrahydrofuran, N,N′-disuccinimidyl carbonate, acryloyl chloride, and phthalaldehyde, and tested against various bacterial and fungal strains. The antimicrobial studies showed that the post-synthetic modification of MOF materials is a suitable method to develop very active antimicrobial agents. Indeed, IRMOF-3 derivatives exhibit bactericidal and fungicidal activity and can be used as drug additives to control the spread of microbial pathogens.
1.2. Composite MOFs based on zinc(ii)
1.2.1. Surface modification of MOFs based on zinc(ii)
Zeolitic imidazolate frameworks (ZIFs) are a new MOF with unique properties such as crystalline nature, tunable pore size, large surface area, and because of their biocompatibility and chemical stability, they are suitable for use in biomedical applications.65 ZIFs have been used in gas separation, and as catalysts and carriers for metal nanoparticles and drugs. Additionally, ZIF-8 is stable under physiological conditions and decomposable under acidic conditions, and thus, it can be used to construct pH-sensitive drug delivery systems.58
Currently, there is an ever-growing demand for controllable drug release. It was reported that they designed a pH-dependent MOF based on a 2-nitrobenzaldehyde (o-NBA)-modified MOF that was designated as o-NBA@ZIF-8. Light responsive o-NBA (a pH-jump reagent) was incorporated into the porous structure of ZIF nanoparticles as a ‘gatekeeper’,49 allowing the UV-light (365 nm)-responsive in situ production of acid, which subsequently induces pH-dependent degradation of ZIF and promotes the release of the antibiotic loaded in the mesopores.
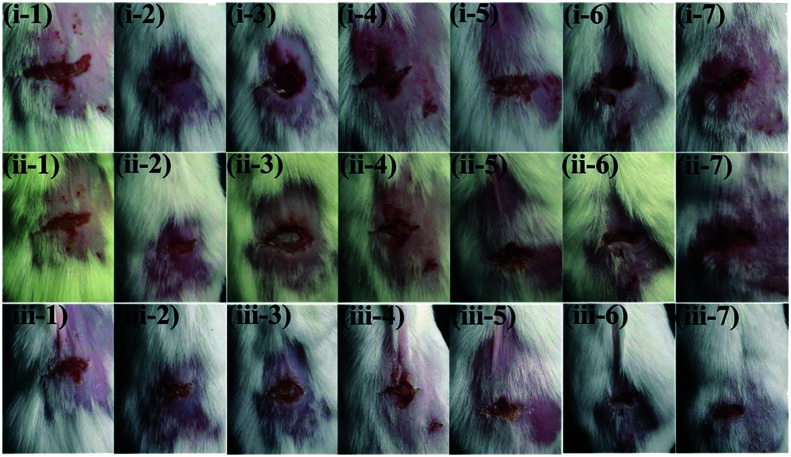
Song et al. fabricated an injury model on the backs of mice to demonstrate the antibacterial action of o-NBA@ZIF-8 and its ability to treat infected wounds. The mice were divided into seven groups according to different separate treatments (Fig. 2), and we observed that the most effective wound antibacterial therapy among the seven treatments was achieved by RFP&o-NBA@ZIF-8 + light. The combination of the UV light, the precise pH-triggered antibiotic release, and the zinc ions enables the light-activated nanocomposite to significantly inhibit bacteria-induced wound infection and accelerate wound healing, indicating a switchable and synergistic antibacterial effect. This rational design was successfully used to develop a light-responsive antibiotic-delivery system, and its therapeutic potential for infected wounds was also demonstrated.
Fig. 2. Photographs of wounds infected by MRSA. (1) PBS + light, (2) ZIF-8 + light, (3) o-NBA@ZIF-8 + dark, (4) o-NBA@ZIF-8 + light, (5) RFP@ZIF-8 + light, (6) RFP&o-NBA@ZIF-8 + dark, (7) RFP&o-NBA@ZIF-8 + light. (i) 0 d (infected for 12 h), (ii) 1 d, and (iii) 3 d. Reprinted from ref. 49. Copyright 2018 Adv. Funct. Mater.
Similarly, Zhang et al. have reported the design of a pH-responsive metal organic framework (MOF)/antibiotic synergistic system for the targeted highly efficient elimination of intracellular bacteria.62 The cell types used in the study were bacterial cells, Escherichia coli (F 1693) and Staphylococcus aureus (F 1557). The obtained tetracycline (Tet)@ZIF-8@hyaluronic acid (HA) system (abbreviated as TZH) was able to penetrate the biological cell membrane barrier via the HA-mediated pathway to achieve targeted elimination of the intracellular bacteria and reduce the antibiotic dose. Fig. 3 shows that antibiotics can be adeptly utilized in response to intracellular bacterial infections via TZH, a targeted drug delivery and controllable release system with satisfactory stability and hypotoxicity. Thus, this novel platform will be able to revitalize traditional antibiotics that have become ineffective over time and will contribute to further overcoming antibiotic resistance.
Fig. 3. (A) Scheme showing macrophages (RAW 264.7) containing intracellular bacteria that are infected with S. aureus (ATCC 29213) and also carry TZH. (B) Co-localization using fluorescence microscopy: (a) macrophages stained with 4′,6-diamidino-2-phenylindole (DAPI, blue), (b) bacteria marked by fluorescein isothiocyanate (FITC, green), (c) acidic compartment located by lysosome tracker red (red), and (d) the overlay image. All scale bars in (B) are 50 μm. (C) The statistical clearance rate of intracellular bacteria treated with TZH (50 μg mL−1) or control groups. (*P < 0.05; **P < 0.01; ***P < 0.001; NS, not significant.) (D) Photographs corresponding to (C). Reprinted from ref. 62. Copyright 2019 RSC.
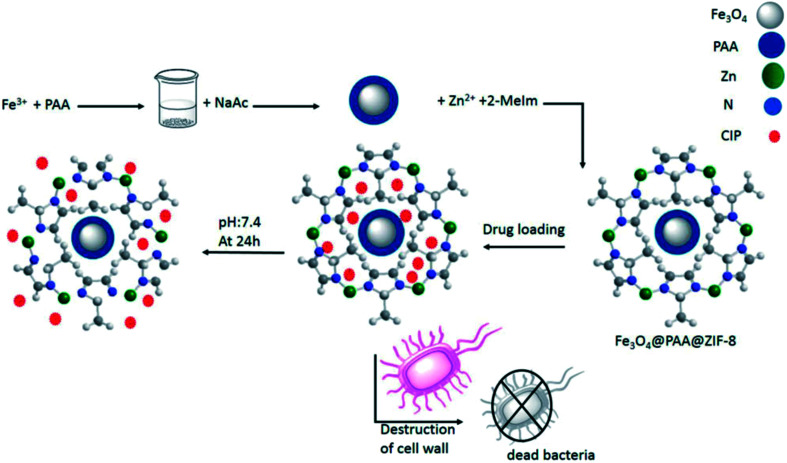
Recently, Esfahanian et al. loaded the anti-inflammation drug (CIP) into Fe3O4@PAA@ZIF-8,25 and the successful release of the drug at pH 2 showed that the constructed framework has the capability of shipping and releasing the drug under the physiological conditions of the body. Finally, E. coli and S. aureus were used for antibacterial testing, and the results showed that the antibiotic ciprofloxacin loaded on the framework further inhibited bacterial growth. As a result, Fe3O4@PAA@ZIF-8@CIP exhibits stronger antibacterial activity than pure CIP (Fig. 4).
Fig. 4. Simulation of the preparation of the Fe3O4@PAA@ZIF-8 framework, drug loading, release, and antibacterial testing. Reprinted from ref. 25. Copyright 2019 Informa Healthcare.
A successful scheme with a MOF was developed to improve the drug loading efficiency of the antitumor drug curcumin.52 For the first time, Dutta et al. applied mixed-metal MOF copper (Cu2+)-doped zeolitic imidazolate framework-8 (Cu/ZIF-8) to simultaneously improve the stability of the drug (by metallization with zinc (Zn2+)) and function (through complexation with Cu2+).38 This work illustrates a unique strategy for the co-amplification of the bio-availability and functionality of curcumin using Cu2+-doped ZIF-8 as a drug delivery system. It should be noted that Cur@Cu/ZIF-8 also exhibits greater anti-bacterial and anti-biofilm effects than free curcumin, and thus, this combination is best suited for biomedical application. Additionally, Cur@Cu/ZIF-8 exhibited a three-fold increase in the generation of ROS. The simple and convenient synthetic method developed in this study can be extrapolated to synthesize other functional metal–organic hybrid systems with desired functionality for drug delivery and other biological applications.
ZIF-8 is from a large category of MOFs that possesses a large surface area and porous structure, enabling it to absorb a large number of ions in a closed area while being subject to microwave radiation. Therefore, ZIF-8 is an excellent source of heat that is absorbed under microwave radiation, and can be used as a microwave sensitizer. Based on ZIF-8 with strong antibacterial properties, Wu et al. have successfully developed a new therapeutic strategy based on biodegradable BSA@ZIF-8 for the first time. Their in vivo experimental results showed that BSA@ZIF-8 protected 80% of mice from lethal challenges with tumors and accompanying infection. Overall, they present a novel strategy using biodegradable ZIFs for microwave ablation therapy with simultaneous antibacterial and anti-infection effects, and their method has achieved satisfactory tumor treatment outcomes.
Electrospinning is a simple, low-cost, effective technique for the production of polymer micro-nanofibers. Polyvinyl alcohol (PVA) is a water-soluble polymer with good chemical and thermal stability.53 Because it is highly biocompatible and non-toxic, can be easily processed, and has high water permeability, PVA is used in functional or medical textile applications. Many studies have focused on the electrospinning of a polymer solution of PVA and mixed nanoparticles to produce antibacterial fibers. The incorporation of high-performance MOFs into polymer fibers is a novel strategy for the production of functional textiles. Singbumrung et al. successfully prepared a Cu-1,3,5-benzene tricarboxylate (BTC) crystal using a sonochemical method and incorporated it into PVA to produce electrospun fibers.39 The antibacterial properties of the Cu-BTC-modified PVA fibers against S. aureus and E. coli were investigated. The modified fibers exhibited strong antibacterial characteristics, providing complete inhibition of microorganism growth, and were especially active against S. aureus. The results also confirmed that longer sonication produced stronger antibacterial activity. Their study demonstrates that the incorporation of Cu-BTC into PVA fiber produces a functional antibacterial fabric with a wide range of potential applications.
Because of the high surface area, structural porosity and dynamics, and high mechanical and physiological properties of MOFs, they can be potentially applied in the fields of gas storage and separation, chemical sensors, heterogeneous catalysts, and also in antimicrobial fields. During the formation of these nanomaterials, their properties are affected, which is highly important. In recent years, MOFs have been produced by the rapid, affordable, and effective ultrasonic-assisted reverse micelle (UARM) method. Recently, Akbarzadeh et al. found that Zn-MOF samples have favorable physicochemical properties. The impact of experimental parameters of the UARM method is effective on the resulting properties,54 such as the high surface area of the products that increases the interactions between the Zn-MOF nanostructure and bacteria. Antibacterial activities were investigated using diffusion methods in agar and also with dilutions of Zn-MOF samples. Antibiotics (tetracycline and ampicillin) and their anti-biofilm effects were evaluated using the microplate method. The obtained results revealed that the Zn-MOF nanostructures are highly antibacterial, which could be due to the nature of the applied Zn-MOF as well as the optimization process. The Zn-MOF nanostructures could act as novel antibacterial biocatalysts.55,63
1.2.2. Zinc-based MOF core–shell structure
Combination therapy with the use of nanomaterials and antibiotics is an effective approach to combat increasing antibiotic resistance. For example, the application of antibiotic-loaded core–shell ZnO@ZIF-8 particles was studied by Mohanta et al.29 Zinc oxide (ZnO) nanomaterials have been extensively explored in biomedical applications such as bio-imaging, drug delivery, photodynamic therapy (PDT), and UV filters in cosmetics. Intriguingly, ZnO nanoparticles also possess potent antimicrobial properties due to their ability to produce reactive oxygen species (ROS) when photo-excited. The generated ROS subsequently results in membrane disruption and microbial cell death. When combined with conventional antibiotics, ZnO enhances the efficacy of antibiotics by several fold through permeation of the bacterial cell membrane, which results in the increased uptake of antibiotics. It is also speculated that zinc ions released during the gradual disintegration of ZnO possess antibiotic properties.
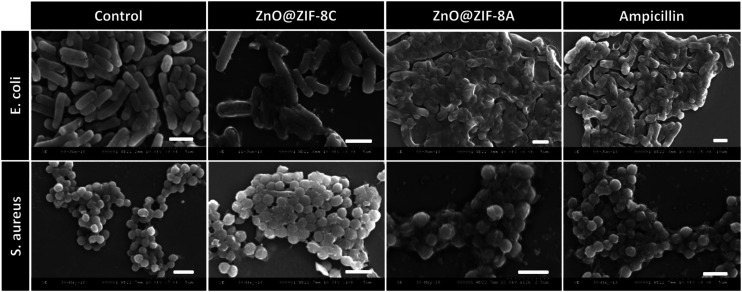
Herein, Mohanta et al. synthesized ZnO@ZIF-8 by polyol synthesis, and carried ampicillin on ZnO@ZIF-8.29 Then, through the pH response, the antibiotic contained in the core–shell material was released in a controlled manner, with successful local application and sustained release of the drug. Studies have shown that the combination of ZnO@ZIF-8 and ampicillin results in a product with an enhanced antibacterial effect. In addition, the safety of naked and ampicillin-encapsulated ZnO@ZIF-8 particles was studied through mammalian cell viability analysis using HeLa cancer cells, and broad-spectrum antibacterial activity was observed (Fig. 5).
Fig. 5. FESEM micrographs of microorganisms treated with bare (ZnO@ZIF-8 C) and ampicillin-loaded (ZnO@ZIF-8 A) particles (scale 2 μm). The analysis indicates that membrane disruption occurred. Reprinted from ref. 29. Copyright 2019 ACS.
1.3. MOF derivatives based on zinc ions
The antibiotic resistance of numerous bacteria has increased in the last decade. The mecA gene plays an important role in the pathogenicity of methicillin-resistant Staphylococcus aureus (MRSA) by increasing antibiotic resistance. Recent studies have indicated that nanotechnology can be combined with antimicrobial agents, and promising results have been observed. Askarinia et al. used Cu-BPDCA-Ty as a combination antibacterial against MRSA and also induced the downregulation of expression of the mecA gene, which ultimately led to the decrease of resistance to beta-lactam antibiotics.40 Therefore, further studies should be conducted to identify action mechanisms and toxicity of active compounds. Nanomaterials are expected to be recognized as a natural source for the development of new antibacterial components.
According to current research, the synthesis of MOF derivatives generally includes MOF pyrolysis, direct carbonization, and the sol–gel method. Inspired by these recent developments with MOF-derived nanocarbons, we proposed the construction of near infrared (NIR)-responsive and size transformable MOF-derived nanocarbons for rapid, safe, and synergistic chemo-photothermal bacterial disinfection.32
MOF derivatives have been documented in recent studies. For example, Fan et al. prepared new 2D carbon nanosheets (2D-CNs) directed from MOFs and proposed that 2D-CNs can be used for localized bacterial eradication to augment anti-infective therapy.36 TRB-ZnO@G was synthesized by in situ polymerization anchored with ZnO-doped carbon on graphene (ZnO@G). Notably, TRB-ZnO@G triggered by NIR can cause bacteria to aggregate, allowing massive Zn2+ ions to locally infiltrate, and then physically cut and kill the bacteria by hyperthermia, which synergistically enhances the destruction of the bacterial membrane and intracellular material.
2. Cu-MOFs
2.1. Copper ion-ligand binding MOFs
Copper is a trace element essential for the maintenance of bioactivity, and is one of the earliest known and used inorganic long-acting antibacterial materials. Cu-BTC, also known as HKUST 1 Basolite C 300 or MOF-199, is one of the most popular copper-based MOF materials.56
According to Su et al.,41 CuS@HKUST-1 composites have been successfully fabricated via a simple in situ sulfuration process, in which HKUST-1 acts as a tunable carrier platform for CuS nanoparticles (NPs). In this way, the photo-responsive properties of CuS, under NIR irradiation, can be fully utilized. After 20 min of light irradiation, the composites exhibited an excellent antibacterial effect of over 99.70% and 99.80% against S. aureus and E. coli, respectively, on account of the tripartite synergy of PTA, PDA, and released Cu ions. Additionally, even if it is in contact with mammalian cells for a lengthy period of time, CuS@HKUST-1 shows acceptable effects on cell growth. This study may provide a promising paradigm for biomedical applications of HKUST-1 because rapid and effective sterilization can be achieved in a polluted bacterial environment.
Another antibacterial material associated with MOF-199 is wool. Su et al. conducted an experimental study on the direct synthesis of HKUST in wool fibers, so as to obtain durable products that can be extensively washed. They introduced a MOF composed of copper and acid into wool fabric to improve its antibacterial properties. The wool@HKUST-1 fabric completely inhibited the tested microorganism. The results after washing showed a slight decrease in the level of inhibition of growth of the microorganisms; nevertheless, the inhibition remained almost complete, at 99.97% after 24 h and 99.99% after 48 h of treatment. Fabrics treated with HKUST-1 for 48 h during synthesis are noteworthy, with more optimal antimicrobial performance.
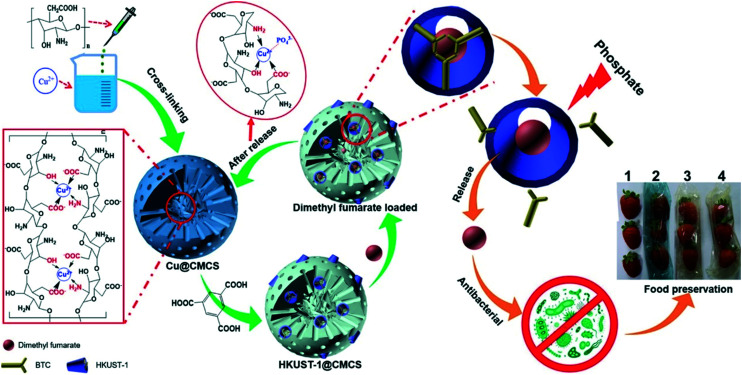
In one study, a new strategy of mutual encoding of carboxyl chitosan (CMCS) and HKUST-1 was proposed to construct an eco-friendly, recyclable, long-acting intelligent antibacterial vector HKUST-1@CMCS (Fig. 6). The comprehensive characterization showed that the structure of HKUST-1@CMCS was gradually destroyed by phosphate stimulation at different intensities, thus realizing the intelligent release of antibacterial drugs.43
Fig. 6. Schematic description of the preparation of HKUST-1@CMCS, the regeneration of HKUST-1@CMCS, phosphate-stimulated drug release without Cu(ii) ion residues, and antimicrobial activities and food preservation of dimethyl fumarate-loaded HKUST-1@CMCS film. Reprinted from ref. 43. Copyright 2020 Elsevier Ltd.
Some materials also possess antibacterial properties and the ability to absorb dyes. For example, biodegradable cellulose-based biomaterials (MOFs/oxidized cellulose beads (OCBs)) have been prepared from oxidized corn. The results showed that MOFs, namely HKUST-1 and IF-8, increased the capacity of OCBs to adsorb methyl orange (8% of OCBS-55% of HKUST-1/OCBs and 84% of IF-8/OCBs) and demonstrated excellent antibacterial activity (0 of OCBS-90.2% of HKUST-1/OCBs and 44.8% of IF-8/OCBs). This concept may provide a new approach for the preparation of cost-effective biological pesticides for environmental remediation purposes.30
2.2. Surface modification of a MOF based on copper(ii)
Curcumin was loaded onto Cu2+-doped ZIF-8 as a drug delivery system, which amplified the bioavailability of curcumin. We have shown that by adding a second transition metal ion (copper) to the tetrahedral site of ZIF-8, it is possible to adjust its antibacterial effect by adding phenolic antioxidants or drugs, composite charge carriers, or by changing its light absorption characteristics, and therefore its therapeutic efficacy.38
3. Ag-MOFs
3.1. Silver ion-ligand binding MOFs
Modern research indicates that the chemical structure of the metallic silver ion determines its high catalytic capacity, and the reduction potential of highly oxidized silver is sufficiently high to generate atomic oxygen in the surrounding space. The strong oxidizability of atomic oxygen can sterilize, and Ag+ can strongly attract the sulfhydryl group (–SH) on the protease in the bacteria's body and quickly combine with it, thus inactivating the protease and killing the bacteria. When the bacteria are killed by Ag+, Ag+ is released from the dead body of the bacteria, and then comes into contact with other bacterial colonies. This process is repeated over and over again, which indicates that the bactericidal properties of the metal silver ion are very durable.
Because of the application of MOFs in ion pair recognition, especially in anion exchange, their role in polymer chemistry has attracted much attention. Although many metal-mixed ligand complexes have been reported, to our knowledge, there are few reports on the synthesis of metal-mixed anion complexes. There has been much research on mixed anion complexes due to their novel structures and potential applications.32
In recent years, many researchers have undertaken considerable work on the synthesis of new silver(i) coordination polymers with flexible dithioether ligands. The literature reports that on the basis of the obtained results, it was found that in a competition between the nitrate ion and other anions, the non-coordinating anions BF4−, PF6−, and SbF6− and weakly coordinating ClO4− were directed toward the synthesis of the studied cationic coordination polymers with two types of anions, while moderately coordinating anion CF3SO3− built up a cationic structure with only one type of anion. The differences between the coordination ability of the coanions can be considered as the most effective factor for determining the coordination and linkage modes of the ligand and nitrate anion and hence for constructing different coordination polymers with variable structural topologies. Apart from interesting structural and topological features, these compounds exhibited significant antibacterial activity.30
3.2. Surface modification of MOFs based on the silver ion
A functional wound dressing can be used in anti-infective therapy to control bacterial skin infections.
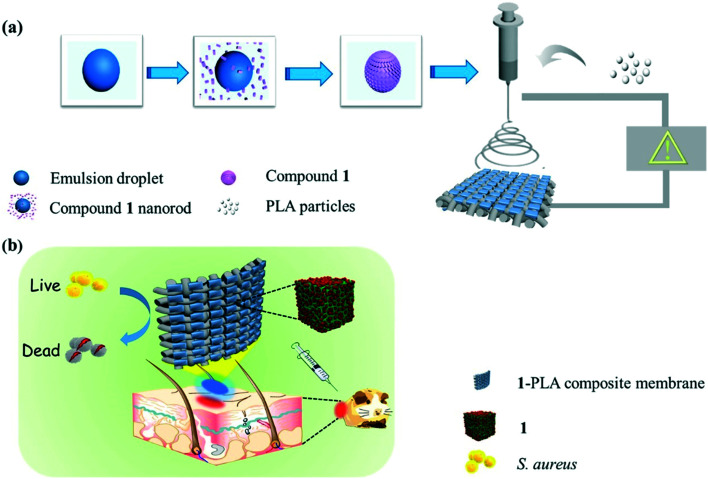
The experimental results showed that in vitro broad-spectrum bactericidal efficacy against Gram-negative E. coli, Pseudomonas aeruginosa, Gram-positive S. aureus, and Mycobacterium smegmatis was very good, with an antibacterial rate of more than 95.0%. The wound healing rate of S. aureus-infected wounds in vivo was 99.9%, demonstrating the potential of 1-polylactic acid (1-PLA) for further application in antibiotic-free wound dressings (Fig. 7).46
Fig. 7. Schematic illustration of the preparation of (a) 1-PLA and (b) corresponding antibacterial applications for wound dressing through a mouse skin wound model. Reprinted from ref. 46. Copyright 2020 Elsevier.
In addition, ultrafine silver nanoparticles were embedded in a Gly-Arg-Gly-Asp-Ser (GRGDS) peptide cyclodextrin (CD) metal–organic framework to promote antibacterial and wound healing applications. The use of silver nanoparticles (Ag NPs) is a promising approach in the development of antimicrobial alternatives. In this study, ultrafine Ag NPs were synthesized in a 1.7 nm void by the reaction–diffusion method using the medium porosity of a CD-MOF. Ag NPs were successfully immobilized to avoid direct interaction with the external environment and improve their stability. Further cross-linking and surface modification of Ag NP-embedded CD-MOF (Ag@CD-MOF) with GRGDS peptide can effectively improve the hemostatic effect in the wound area.46
3.3. MOF derivatives based on silver ions
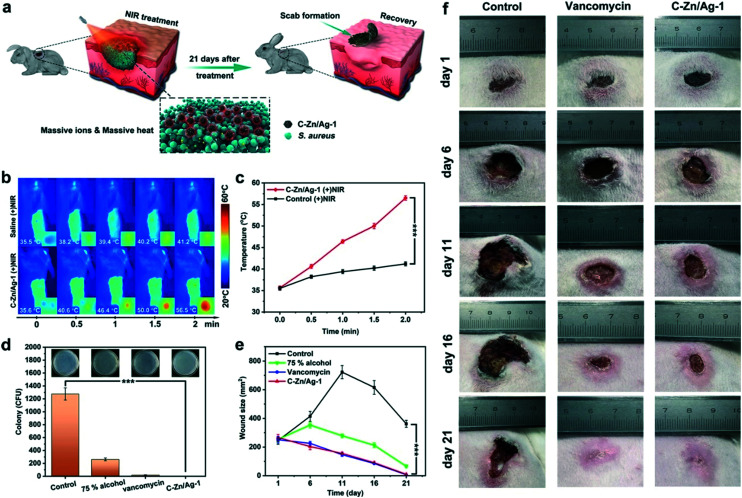
In addition, there is a structurally engineered silver-doped MOF derivative (C–Zn/Ag) that has exhibited high ion release performance and photothermal conversion ability and can be used for broad-spectrum sterilization. Systematic antibacterial experiments (Fig. 8) showed that C–Zn/Ag nanocomposites had a bactericidal rate of nearly 100% within 10 min against S. aureus and E. coli (107 CFU mL−1) in high concentration. Combined with the inactivation of intracellular protein and destruction of the bacterial membrane, C–Zn/Ag nanocomposites exhibited excellent antibacterial properties at low concentration. In addition, wound-healing evaluations in vivo showed rapid disinfection with vancomycin-like effects.45 Overall, nano-agents derived from silver-based MOFs exhibit high chemical and photothermal antibacterial efficiency even at a very low concentration, because they are governed by synergistic dual antibacterial mechanisms. Silver-based MOFs can be used for broad-spectrum sterilization, and also can be utilized in novel strategies for broad-spectrum bacterial sterilization in biological applications.
Fig. 8. (a) Schematic illustrations of in vivo assessment of wound sterilization and healing. (b) Photothermal images and (c) temperature change curves for wounds treated with saline and C–Zn/Ag-1 under NIR irradiation. The inset photos are magnified photothermal images for the NIR-treated sites. (d) S. aureus colonies counted on agar plates. The inset photos are agar plate images of S. aureus removed from the wound after different treatments on day 1. (e) The wound sizes change after different treatments. (f) Photographs of the infected wound after different treatments on day 1, day 6, day 11, day 16, and day 21. The asterisks indicate significant differences (P values: *P < 0.05, **P < 0.01, ***P < 0.001). Values are expressed as the mean ± SD, n = 5. Reprinted from ref. 45. Copyright 2020 ACS.
4. Fe-MOFs
4.1. Surface modification of MOFs based on iron(ii)
The capsaicin from red pepper (Capsicum frutescens) also has the potential to be used as an antibacterial material. In this study, FeIII-doped hollow metal–organic frameworks (FeIII-HMOF-5) were prepared as promising nanocarriers for capsaicin and incorporated into a gelatin–chitosan matrix to fabricate antibacterial packaging films.
Capsaicin, the extract of red pepper, is a common food coloring and flavoring agent, and has many advantageous properties such as analgesic and anti-inflammatory. More importantly, capsaicin has strong anti-microbial effects against Gram-negative and Gram-positive bacteria, and fungi, and thus, it has great potential to be used in food preservation. Composite films that incorporated Cap-FeIII-HMOF-5 were prepared with FeIII-HMOF-5 as the nanocarriers to load capsaicin. The hydrophilicity of FeIII-HMOF-5 effectively improved the compatibility of capsaicin inside the gelatin–chitosan matrix. The addition of Cap-FeIII-HMOF-5 significantly enhanced the mechanical properties, water vapor permeability, and UV-light-blocking properties. Moreover, Cap-FeIII-HMOF-5 endowed efficient antimicrobial activity against E. coli to gelatin–chitosan composite films, as demonstrated by practical application on fresh apple cubes. This work provides a novel strategy for the preparation of antibacterial food packaging film.48
4.2. Iron-based MOF core–shell structure
Bacterial infection is one of the most prominent underlying causes of many severe diseases, such as septic arthritis, dermatosis, and inflammatory bowel disease. Up to now, rapid and effective in vivo bacterial detection and treatment has not been realized due to a lack of well-developed methods. In recent years, the abuse of antibiotics has caused several infections that are difficult to treat. Therefore, there is an urgent need for alternative methods that can selectively and non-invasively detect, target, and kill bacteria without the risk of antibiotic resistance.
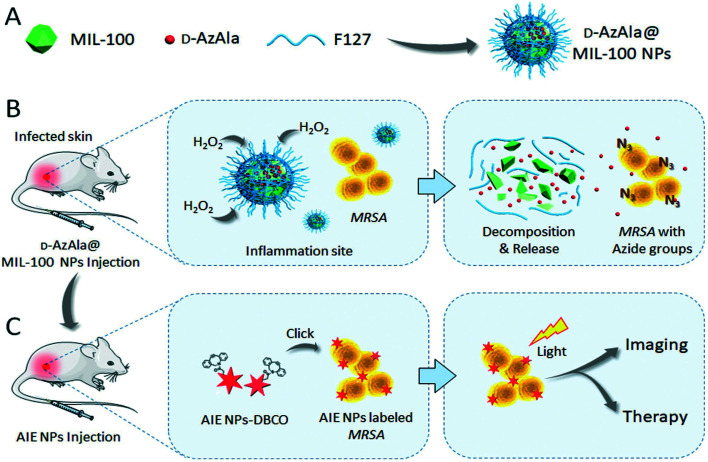
Monoclonal antibodies and antimicrobial peptides have been proven to be effective bacterial recognition systems through surface antigen binding or electrostatic interaction. However, the high cost and rapid body clearance have hindered the development of antigen- and peptide-based in vivo specific recognition and killing of bacteria. Metabolic biomolecular labeling technology has the ability to modify the surface of cells and pathogens with chemical functional groups, and therefore, it has become a powerful potential cell- and pathogen-targeting technology. Mao et al. reported a new strategy using MIL-100 (Fe) NPs as nanocarriers for precise delivery of 3-azido-d-alanine (d-AzAla) that will interfere with the metabolism of bacteria in the body.50 After intravenous injection, MIL-100 (Fe) NPs can preferentially accumulate and rapidly degrade within the high H2O2 inflammatory environment, releasing d-AzAla in the process. d-AzAla is selectively integrated into the cell walls of bacteria, which was confirmed by fluorescence signals from clickable dibenzocyclooctyne-cyanine 5 (DBCO-Cy5) (Fig. 9). Ultrasmall photosensitizer NPs with aggregation-induced emission characteristics were subsequently designed to react with the modified bacteria through in vivo click chemistry. Through photodynamic therapy, the amount of bacteria on infected tissue was significantly reduced. Overall, this study demonstrates the advantages of a MOF-assisted bacteria metabolic labeling strategy for precise bacterial detection and therapy guided by fluorescence imaging.
Fig. 9. Schematic illustration of the proposed strategy for bacterial diagnosis and therapy by H2O2-responsive MOF-assisted in vivo metabolic labeling of bacteria. (A) d-AzAla@MIL-100 (Fe) NPs are synthesized using Pluronic F-127 as a matrix to encapsulate the d-AzAla-loaded MIL-100 (Fe). (B) d-AzAla@MIL-100 (Fe) NPs accumulate at the site of the infected tissue and are decomposed in the presence of H2O2. The invading bacteria internalize these released d-AzAla and express azide groups on their cell walls. (C) Ultrasmall US-TPETM NPs with dibenzocyclooctyne (DBCO) groups bind with bacteria through click reaction, and specific tracking and effective photodynamic therapy (PDT) of bacteria can be achieved in the infected tissue. Reprinted from ref. 50. Copyright 2018 Wiley.
5. Other types of metal-MOFs
Some common MOFs with magnesium,51 cobalt,66 and zirconium67 have also been used as antibacterial agents. In the early stage of implant surgery, residual osteosarcoma cells or infectious bacteria can severely hinder the fusion of implants made with natural bone. Zhang et al. studied a MOF-based magnesium and strontium composite for treating local injuries. They constructed composite coatings of magnesium-mediated metal–organic frameworks (Mg-MOF74) and strontium-substituted hydroxyapatite (Sr-HA) on titanium surfaces for increasing the efficiency of local injury treatment. The experiment proved that Mg-MOF74 could effectively kill the surrounding S. aureus, E. coli, and Saos-2 cells, and promote the proliferation and osteogenic differentiation of osteoblasts.
Aguado et al. discovered a straightforward way to compound Co-SIM-1, a novel analogue of its zinc-based parent SIM-1. The results showed that the material exhibited significant antimicrobial activity and durability due to controlling the release of cobalt ions in a biocide solution. Cobalt-based organic frameworks are possible as antibacterial materials. Cobalt-based materials can be prepared with simple, inexpensive, and readily available commercial ligands, and therefore, future applications as antimicrobial materials will be economical and feasible.66 Unamuno et al. synthesized a new MOF with UiO-66(Zr)_COOH NPs (UiO for University of Oslo) composed of zirconium(iv) hexaoxoclusters, and 1,2,4-benzene-tricarboxylate was used to load gentamicin (GM, a broad spectrum aminoglycoside antibiotic, widely used for the treatment of bacterial infections caused by susceptible strains). GM coated with modified materials can be used as an effective oral tool for the treatment of different types of bacterial infections, thus improving the bioavailability of GM.67
Conclusion
MOFs have been well developed in anti-inflammatory and anti-bacterial applications. In the current study, the methods and applications of different types of transition-based MOFs combined with various ligands were reviewed. First, the developmental status of the antibacterial properties of MOFs was reviewed. Then, the antibacterial properties of these MOFs were mainly analyzed, and the antibacterial mechanism was also discussed.68–80 For example, researchers have detected the most representative ZIF-8 with photocatalytic activity. Under the impact of sunlight catalysis in the Zn-MOF system, ZIF-8 generates photogenerated electrons through the charge transfer between ligands and metals, and the photogenerated electrons can effectively activate O2 to form O2 and H2O2, causing the oxidation of pathogenic bacteria in the air and killing them. As for Ag-MOFs, the long-term sustained release of Ag+ may effectively kill pathogenic bacteria in the body. Similarly, the antibacterial mechanism of Cu-MOFs can also be tuned to slowly release Cu2+ from MOF materials, which destroys the bacterial structure at the same time. Moreover, the hydrogen-bonding interaction with ligand functional groups and cell protein can increase the cellular fat-soluble materials, which induces Cu2+ to penetrate the cell surface and increase the bacteriostatic activity.
These MOFs are highly advantageous because of their wide antibacterial spectrum, high efficiency, high stability, rapid acting time, and orientation. As is well-known, the antibacterial properties of MOFs are predominantly determined by the metal ions, and their release control is attributed to their highly ordered stereo structure. Although great progress has been made in the antimicrobial performance of MOFs, there are still some unresolved issues that require solutions in the form of the development of59–61 (i) acceptable toxicology of matrix materials for use in antibacterial applications, (ii) controllable degradation of the solids, (iii) efficient encapsulation of antibacterial molecules with proper loading amounts, and (iv) controllable release of encapsulated cargos. Otherwise, MOFs under complex physiological conditions will easily decompose. Although this is their advantage, the shortcomings cannot also be ignored, and therefore, according to the actual demand of MOFs, stability is very important. Eventually, the small-batch syntheses of MOFs will limit their large-scale application, especially for MOF composites with harsh preparation conditions and complex processes.81–91 Further study will be required to enable the stable synthesis of easily available MOFs and improve on their potential for large-scale clinical applications.92–95 The comprehensive discussion herein provides some new ideas for the future research of organic measurement and its application to the resistance of pathogenic microorganisms and bacteria.
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
This research was partially funded by the Science Foundation of Guangdong Medical University (GDMUZ2019008 and GDMUM2019010), Featured Innovation Project of Guangdong Province (2019KTSCX052 and 2019KTSCX053) and Special Funds for Scientific Technological Innovation of Undergraduates in Guangdong Province (pdjh2020b0265,pdjh2020a0252, pdjh2020b0260, pdjh2020b0267 and pdjh2021a0218), Guangdong Basic and Applied Basic Research Foundation (2019A1515110260), and PHD researchers of Guangdong Medical University in 2019, the Public Research and Capacity Building Projects of Department of Guangdong Province grant number (2017A010103022), and Key scientific research project of Colleges and Universities of Education Department of Guangdong Province (20202ZDZX2046) and Students Innovation Experimental Project (ZYDM011, ZZDM003 and ZYDM010) and the Shenzhen Peacock Plan (Research and Development and Industrialization of in vitro diagnostic nanomaterials for tumors (KQTD2016022920325195)).
References
- Maleki A. Shahbazi M. L. Alinezhad V. Santos H. A. Adv. Healthcare Mater. 2020;9:2000248. doi: 10.1002/adhm.202000248. [DOI] [PubMed] [Google Scholar]
- Que Y. A. Haefliger J. A. Piroth L. Francois P. Widmer E. Entenza J. M. Sinha B. Herrmann M. Francioli P. Vaudaux P. Moreillon P. J. Exp. Med. 2005;201:1627–1635. doi: 10.1084/jem.20050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K. Coopmeiners J. Graspeuntner S. Dalhoff K. Rupp J. FEBS Lett. 2016;590:3887–3904. doi: 10.1002/1873-3468.12353. [DOI] [PubMed] [Google Scholar]
- Courtney C. M. Goodman S. M. McDaniel J. A. Madinger N. E. Chatterjee A. Nagpal P. Nat. Mater. 2016;15:529. doi: 10.1038/nmat4542. [DOI] [PubMed] [Google Scholar]
- (a) Zhang X. Liu L. Huang L. Zhang W. Wang R. Yue T. Sun J. Li G. Wang J. Nanoscale. 2019;11:9468–9477. doi: 10.1039/c9nr01284b. [DOI] [PubMed] [Google Scholar]; (b) Liu J. and Pan Y., Metal-Organic Frameworks for Biomedical Applications, 2020, pp. 45–68, 10.1016/b978-0-12-816984-1.00004-4 [DOI] [Google Scholar]
- Fasciani C. Silvero M. J. Anghel M. A. Argüello G. A. Becerra M. C. Scaiano J. C. J. Am. Chem. Soc. 2014;136:17394. doi: 10.1021/ja510435u. [DOI] [PubMed] [Google Scholar]
- Zhao Y. Ye C. Liu W. Chen R. Jiang X. Angew. Chem., Int. Ed. 2014;53:8127. doi: 10.1002/anie.201401035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Z. Tian Q. Ye J. Zhang S. Wang G. Qi Y. Che Y. Ning G. J. Mater. Sci. 2020;55:4408. [Google Scholar]
- (a) Chen Z. Wang Z. Ren J. Qu X. Acc. Chem. Res. 2018;51:789. doi: 10.1021/acs.accounts.8b00011. [DOI] [PubMed] [Google Scholar]; (b) Han Y. Liu W. Huang J. Qiu S. Zhong H. Liu D. Liu J. Pharmaceutics. 2018;10:271. doi: 10.3390/pharmaceutics10040271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.-Q. Luo Z.-D. Pan Y. Singh A. K. Trivedi M. Kumar A. Coord. Chem. Rev. 2020;406:213245. [Google Scholar]
- (a) Song Z. Wu Y. Cao Q. Wang H. Wang X. Han H. Adv. Funct. Mater. 2018;28:1800011. [Google Scholar]; (b) Tan G. Zhong Y. Yang L. Jiang Y. Liu J. Ren F. Chem. Eng. J. 2020;390:124446. [Google Scholar]
- Liu M. Wang L. Zheng X. H. Xie Z. G. ACS Appl. Mater. Interfaces. 2017;9:41512–41520. doi: 10.1021/acsami.7b15826. [DOI] [PubMed] [Google Scholar]
- Ren X. Y. Yang C. Y. Zhang L. Li S. H. Shi S. Wang R. Zhang X. Yue T. L. Sun J. Wang J. L. Nanoscale. 2019;11:11830–11838. doi: 10.1039/c9nr03612a. [DOI] [PubMed] [Google Scholar]
- (a) Liu W. Pan Y. Xiao W. Xu H. Liu D. Ren F. Peng X. Liu J. Med. Chem. Commun. 2019;10:2038–2051. doi: 10.1039/c9md00400a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu J.-Q. Li X.-F. Gu C.-Y. Silva J. C. S. D. Barros A. L. Alves-Jr S. Li B.-H. Ren F. Batten S. R. Soares T. A. Dalton Trans. 2015;44:19370–19382. doi: 10.1039/c5dt02171e. [DOI] [PubMed] [Google Scholar]; (c) Ma D.-Y. Li Z. Xiao J.-X. Deng R. Lin P.-F. Chen R.-Q. Liang Y.-Q. Guo H.-F. Liu B. Liu J.-Q. Inorg. Chem. 2015;54:6719–6726. doi: 10.1021/acs.inorgchem.5b00335. [DOI] [PubMed] [Google Scholar]
- (a) Pan Y. Luo Z. Wang X. Chen Q. Chen J. Guan Y. Liu D. Xu H. Liu J. Q. Dalton Trans. 2020;49:5291–5301. doi: 10.1039/c9dt04804a. [DOI] [PubMed] [Google Scholar]; (b) Liu W. C. Zhong Y. Y. Wang X. X. Zhuang C. F. Chen J. H. Liu D. Xiao W. W. Pan Y. Huang J. J. Liu J. Q. Inorg. Chem. Commun. 2020;111:107675. [Google Scholar]
- (a) Chen Y. Li P. Modica J. A. Drout R. J. Farha O. K. J. Am. Chem. Soc. 2018;140:5678–5681. doi: 10.1021/jacs.8b02089. [DOI] [PubMed] [Google Scholar]; (b) Hu M. L. Razavi S. A. Piroozzadeh M. Morsali A. Inorg. Chem. Front. 2020;7:1598–1632. [Google Scholar]; (c) Xue J. J. Bigdeli F. Liu J. P. Hu M. L. Morsali A. Nanomedicine. 2018;13:691–2708. doi: 10.2217/nnm-2018-0174. [DOI] [PubMed] [Google Scholar]
- (a) Wu Q. Niu M. Chen X. Tan L. Fu C. Ren X. Ren J. Li L. Xu K. Zhong H. Meng X. Biomaterials. 2018;162:132–143. doi: 10.1016/j.biomaterials.2018.02.022. [DOI] [PubMed] [Google Scholar]; (b) Liu W. C. Pan Y. Zhong Y. T. Li B. H. Ding Q. J. Xu H. J. Qiu Y. Z. Ren F. Li B. Muddassir M. Liu J. Q. Chem. Eng. J. 2021;412:127899. [Google Scholar]; (c) Dutta A. Singh A. Wang X. X. Kumar A. Liu J. Q. CrystEngComm. 2020;22:7736–7781. [Google Scholar]; (d) Liu K. G. Gao X. M. Liu T. Hu M. L. Jiang D. E. J. Am. Chem. Soc. 2020;142:16905–16909. doi: 10.1021/jacs.0c06682. [DOI] [PubMed] [Google Scholar]
- (a) McKinlay A. C. Morris R. E. Horcajada P. Férey G. Gref R. Couvreur P. Serre C. Angew. Chem., Int. Ed. 2010;49:6260–6266. doi: 10.1002/anie.201000048. [DOI] [PubMed] [Google Scholar]; (b) Zhong Y. Y. Li X. S. Chen J. H. Wang X. X. Wei L. T. Fang L. Q. Kumar A. Zhuang S. Z. Liu J. Q. Dalton Trans. 2020;49:11045–11058. doi: 10.1039/d0dt01882a. [DOI] [PubMed] [Google Scholar]; (c) Liu K. G. Wei X. W. Bigdeli F. Gao X. M. Li J. Z. Yan X. W. Hu M. L. Morsali A. Inorg. Chem. 2020;59:2248–2254. doi: 10.1021/acs.inorgchem.9b02956. [DOI] [PubMed] [Google Scholar]; (d) Zhang C. Zhao H. Li Z. Liang Z. Qi S. Cai M. Zhang S. Jia X. Zhang G. Hu M. L. Chem. Commun. 2020;56:9521–9524. doi: 10.1039/d0cc03789c. [DOI] [PubMed] [Google Scholar]
- (a) Ma L. Jiang F. Fan X. Wang L. Y. He C. Zhou M. Li S. Luo H. R. Cheng C. Qiu L. Adv. Mater. 2020;32:2003065. doi: 10.1002/adma.202003065. [DOI] [PubMed] [Google Scholar]; (b) Yang Y. Wu X. Z. Ma L. He C. Cao S. J. Long Y. P. Huang J. B. Rodriguez R. D. Cheng C. Zhao C. S. Qiu L. Adv. Mater. 2021;33:2005477. doi: 10.1002/adma.202005477. [DOI] [PubMed] [Google Scholar]
- Mao D. Hu F. Kenry S. Ji W. Wu. Ding D. Kong D. Liu B. Adv. Mater. 2018;30:1706831. doi: 10.1002/adma.201706831. [DOI] [PubMed] [Google Scholar]
- Zhou W. Begum S. Wang Z. Krolla P. Wagner D. Bräse S. Wöll C. Tsotsalas M. ACS Appl. Mater. Interfaces. 2018;10:1528. doi: 10.1021/acsami.7b14866. [DOI] [PubMed] [Google Scholar]
- Au-Duong A.-N. Lee C.-K. Mater. Sci. Eng., C. 2017;76:477. doi: 10.1016/j.msec.2017.03.114. [DOI] [PubMed] [Google Scholar]
- Sava Gallis D. F. Butler K. S. Agola J. O. Pearce C. J. McBride A. A. ACS Appl. Mater. Interfaces. 2019;11:7782. doi: 10.1021/acsami.8b21698. [DOI] [PubMed] [Google Scholar]
- Chowdhuri A. R. Das B. Kumar A. Tripathy S. Roy S. Sahu S. K. Nanotechnology. 2017;28:095102. doi: 10.1088/1361-6528/aa57af. [DOI] [PubMed] [Google Scholar]
- Esfahanian M. Ghasemzadeh M. A. Razavian S. M. H. Artif. Cells, Nanomed., Biotechnol. 2019;47:2024. doi: 10.1080/21691401.2019.1617729. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh F. Motaghi M. Chauhan N. P. S. Sargazi G. Heliyon. 2020;6(1):e03231. doi: 10.1016/j.heliyon.2020.e03231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A. Das M. Pal K. Jana S. Dutta B. Ray P. P. Jana K. Sinha C. ACS Omega. 2019;4(18):17649. doi: 10.1021/acsomega.9b01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhameed R. M. Darwesh O. M. Rocha J. Silva A. M. S. Eur. J. Inorg. Chem. 2019;2019:1243. [Google Scholar]
- Mohanta G. C. Pandey S. K. Maurya I. K. Sahota T. S. Mondal S. K. Deep A. ChemistrySelect. 2019;4(41):12002. [Google Scholar]
- Duan C. Meng X. Liu C. Lu W. Liu J. Dai L. Wang W. Zhao W. Xiong C. Ni Y. Carbohydr. Polym. 2019;222:115042. doi: 10.1016/j.carbpol.2019.115042. [DOI] [PubMed] [Google Scholar]
- Soleymani-Babadi S. Beheshti A. Bahrani-Pour M. Mayer P. Motamedi H. Trzybiński D. Wozniak K. Cryst. Growth Des. 2019;19(9):4934. [Google Scholar]
- (a) Yang Y. Deng Y. Huang J. Fan X. Cheng C. Nie C. Ma L. Zhao W. Zhao C. Adv. Funct. Mater. 2019;29:1900143. [Google Scholar]; (b) Jo J. H. Kim H. C. Huh S. Kim Y. Lee D. N. Dalton Trans. 2019;48:8084–8093. doi: 10.1039/c9dt00791a. [DOI] [PubMed] [Google Scholar]; (c) Xia Y. Fan X. Yang H. Li L. He C. Cheng C. Haag R. Small. 2020;16:2003010. doi: 10.1002/smll.202003010. [DOI] [PubMed] [Google Scholar]; (d) Gao Y. Yang C. D. Zhou M. He C. Cao S. J. Long Y. P. Li S. Lin Y. Zhu P. X. Cheng C. Small. 2020;16:2005060. doi: 10.1002/smll.202005060. [DOI] [PubMed] [Google Scholar]
- Wu Q. Li M. Tan L. Yu J. Chen Z. Su L. Ren X. Fu C. Ren J. Li L. Cao F. Liang P. Zhang Y. Meng X. Nanoscale Horiz. 2018;3(6):606–615. doi: 10.1039/c8nh00113h. [DOI] [PubMed] [Google Scholar]
- Zhang X. Liu L. Huang L. Zhang W. Wang R. Yue T. Sun J. Li G. Wang J. Nanoscale. 2019;11:9468. doi: 10.1039/c9nr01284b. [DOI] [PubMed] [Google Scholar]
- Shen X. Zhang Y. Ma P. Sutrisno L. Luo Z. Hu Y. Yu Y. Tao B. Li C. Cai K. Biomaterials. 2019;212:1–16. doi: 10.1016/j.biomaterials.2019.05.008. [DOI] [PubMed] [Google Scholar]
- Fan X. Yang F. Huang J. B. Yang Y. Nie C. X. Zhao W. F. Ma L. Cheng C. Zhao C. S. Haag R. Nano Lett. 2019;19:5885–5896. doi: 10.1021/acs.nanolett.9b01400. [DOI] [PubMed] [Google Scholar]
- Song Z. Wu Y. Cao Q. Wang H. Wang X. Han H. Adv. Funct. Mater. 2018;28(23):23351. [Google Scholar]
- Dutta T. Bagchi D. Pal K. Biomed. Phys. Eng. Express. 2018;4:055004. [Google Scholar]
- Singbumrung K. Motina K. Pisitsak P. Chitichotpanya P. Wongkasemjit S. Inprasit T. Fibers Polym. 2018;19(7):1373. [Google Scholar]
- Askarinia M. Ghaedi M. Manzouri L. Khoramrooz S. S. Sharifi A. Ghalamfarsa G. Jannesar R. Sadri F. Khosravani S. A. Jundishapur J. Microbiol. 2018;11(3):e60680. [Google Scholar]
- Su M. Zhang R. Li H. Jin X. Li J. Yue X. Qin D. RSC Adv. 2019;9(69):40277. doi: 10.1039/c9ra07046j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis M. J. Caruzi B. B. Gil G. A. Samulewski R. B. Bail A. Scacchetti F. A. P. Moises M. P. Bezerra F. M. Polymer. 2019;11:713. doi: 10.3390/polym11040713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang G. Li Y. Qin Z. Liang Q. Xu C. Lin B. Carbohydr. Polym. 2020;233:115848. doi: 10.1016/j.carbpol.2020.115848. [DOI] [PubMed] [Google Scholar]
- Usefi S. Akhbari K. White J. J. Solid State Chem. 2019;276:61. [Google Scholar]
- Yang Y. Wu X. He C. Huang J. Yin S. Zhou M. Ma L. Zhao W. Qiu L. Cheng C. Zhao C. ACS Appl. Mater. Interfaces. 2020;12(12):13698. doi: 10.1021/acsami.0c01666. [DOI] [PubMed] [Google Scholar]
- Zhang S. Ye J. Sun Y. Kang J. Liu J. Wang Y. Li Y. Zhang L. Ning G. Chem. Eng. J. 2020;390:124523. [Google Scholar]
- Shakya S. He Y. Ren X. Guo T. Maharjan A. Luo T. Wang T. Dhakhwa R. Regmi B. Li H. Gref R. Zhang J. Small. 2019;15(27):e1901065. doi: 10.1002/smll.201901065. [DOI] [PubMed] [Google Scholar]
- Zhao J. Wei F. Xu W. Han X. Appl. Surf. Sci. 2020;510:145418. [Google Scholar]
- Han C. Yang J. Gu J. J. Nanopart. Res. 2018;20(3):375–385. [Google Scholar]
- Mao D. Hu F. Kenry S. Ji W. Wu. Ding D. Kong D. Liu B. Adv. Mater. 2018;30:1706831. doi: 10.1002/adma.201706831. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Y. Shen X. K. Ma P. P. Peng Z. H. Cai K. Y. Mater. Lett. 2019;241:18–22. [Google Scholar]
- (a) Xia Z. H. Jiang X. Li K. Li L. X. Chen W. B. Wang Y. X. Liu Y. Q. J. Biochem. Mol. Toxicol. 2020;34(7):667. doi: 10.1002/jbt.22499. [DOI] [PubMed] [Google Scholar]; (b) Qiao W. Huiling G. Frank V. Zexun L. Jie H. Hao L. Mater. Sci. Eng., C. 2020;115:890. [Google Scholar]; (c) Galli G. M. Griss L. G. Boiago M. M. Petrolli T. G. Glombowsky P. Bissacotti B. F. Copetti P. M. Silva A. D. D. Schetinger M. R. Sareta L. Mendes R. E. Mesadri J. Wagner R. Gundel S. Ourique A. F. Silva A. S. D. Res. Vet. Sci. 2020;132:1156. doi: 10.1016/j.rvsc.2020.06.008. [DOI] [PubMed] [Google Scholar]
- (a) Lima G. G. G. Ferreira B. D. Matos M. Pereira B. L. Nugent M. J. D. Hansel F. A. Magalhães W. L. E. Carbohydr. Polym. 2020;245:116612. doi: 10.1016/j.carbpol.2020.116612. [DOI] [PubMed] [Google Scholar]; (b) Jia Y. Hu C. Shi P. Xu Q. Q. Zhu W. Liu R. Int. J. Biol. Macromol. 2020;161:223. doi: 10.1016/j.ijbiomac.2020.06.013. [DOI] [PubMed] [Google Scholar]
- Akbarzadeh F. Motaghi M. Chauhan N. P. S. Sargazi G. Heliyon. 2020;6(1):e03231. doi: 10.1016/j.heliyon.2020.e03231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaut M. Steffen A. Contreras J.-M. Morice C. Paulen A. Schalk I. J. Plésiat P. Mislin G. L. A. Bioorg. Med. Chem. Lett. 2020;30(9):127098. doi: 10.1016/j.bmcl.2020.127098. [DOI] [PubMed] [Google Scholar]
- Chandra A. Das M. Pal K. Jana S. Dutta B. Ray P. P. Jana K. Sinha C. ACS Omega. 2019;4(18):17649. doi: 10.1021/acsomega.9b01745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan N. T. S. Nguyen T. T. Luu Q. H. Nguyen L. T. L. J. Mol. Catal. A: Chem. 2012:363. [Google Scholar]
- Ma S. Zhang M. Nie J. Tan J. Yang B. Song S. Carbohydr. Polym. 2019;203:415. doi: 10.1016/j.carbpol.2018.09.039. [DOI] [PubMed] [Google Scholar]
- Wang B. Yan B. Talanta. 2020;208:120438. doi: 10.1016/j.talanta.2019.120438. [DOI] [PubMed] [Google Scholar]
- Kuar N. Tiwari P. Kapoor K. S. Saini A. K. Sharma V. Mobin S. M. CrystEngComm. 2020;22:7513. [Google Scholar]
- Guo A. Durymanov M. Permyakova A. Sene S. Serre C. Reineke J. Pharm. Res. 2019;36:53. doi: 10.1007/s11095-019-2589-4. [DOI] [PubMed] [Google Scholar]
- Rauf A. Ye J. Zhang S. Qi Y. Wang G. Che Y. Ning G. Dalton Trans. 2019;48:17810. doi: 10.1039/c9dt03649k. [DOI] [PubMed] [Google Scholar]
- Ju D. Ibrahim M. E. S. Hugh D. S. AAPS PharmSciTech. 2014;15(6):1535. doi: 10.1208/s12249-014-0176-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. N. Jin L. N. Wang H. T. Kang X. H. Bian S. W. J. Colloid Interface Sci. 2018;530:29. doi: 10.1016/j.jcis.2018.06.062. [DOI] [PubMed] [Google Scholar]
- Rajua P. Arivalagan P. Natarajan S. J. Photochem. Photobiol., B. 2020;203:111774. doi: 10.1016/j.jphotobiol.2019.111774. [DOI] [PubMed] [Google Scholar]
- Aguado S. Quirós J. Canivet J. Farrusseng D. Boltes K. Rosal R. Chemosphere. 2014;113:188–192. doi: 10.1016/j.chemosphere.2014.05.029. [DOI] [PubMed] [Google Scholar]
- Unamuno X. Imbuluzqueta E. Salles F. Horcajada P. Blanco-Prieto M. J. Eur. J. Pharm. Biopharm. 2018;132:11–18. doi: 10.1016/j.ejpb.2018.08.013. [DOI] [PubMed] [Google Scholar]
- Wang X. J. Zhou X. J. Yang K. Li Q. Wan R. X. Hu G. Y. Ye J. Zhang Y. He J. H. Gu H. Q. Yang Y. F. Zhu L. Biomater. Sci. 2021 doi: 10.1039/d0bm01960g. [DOI] [PubMed] [Google Scholar]
- Mao K. Zhu Y. Rong J. Qiu F. X. Chen H. Y. Xu J. H. Yang D. Y. Zhang T. Zhong L. Colloids Surf., A. 2021;611:125888. [Google Scholar]
- Nadar S. S. Rathod V. K. Enzyme Microb. Technol. 2018;108:11–20. doi: 10.1016/j.enzmictec.2017.08.008. [DOI] [PubMed] [Google Scholar]
- Nie X. Wu S. Mensah A. Wang Q. Huang F. Li D. Wei Q. J. Colloid Interface Sci. 2020;579:233–242. doi: 10.1016/j.jcis.2020.06.038. [DOI] [PubMed] [Google Scholar]
- Rong J. Qiu F. Zhang T. Zhu Y. Xu J. Guo Q. Peng X. Appl. Surf. Sci. 2018;447:222–234. [Google Scholar]
- Fan X. Yang F. Huang J. Yang Y. Nie C. Zhao W. Ma L. Cheng C. Zhao C. Haag R. Nano Lett. 2019;19:5885–5896. doi: 10.1021/acs.nanolett.9b01400. [DOI] [PubMed] [Google Scholar]
- Yang Y. Wu X. He C. Huang J. Yin S. Zhou M. Ma L. Zhao W. Qiu L. Cheng C. Zhao C. ACS Appl. Mater. Interfaces. 2020;12:13698–13708. doi: 10.1021/acsami.0c01666. [DOI] [PubMed] [Google Scholar]
- He T. Chen S. Ni B. Gong Y. Wu Z. Song L. Gu L. Hu W. Wang X. Angew. Chem., Int. Ed. 2018;57:3493–3498. doi: 10.1002/anie.201800817. [DOI] [PubMed] [Google Scholar]
- Hu K. Chen C. Zhu Y. Zeng G. Huang B. Chen W. Liu S. Lei C. Li B. Yang Y. J. Colloid Interface Sci. 2019;540:115–125. doi: 10.1016/j.jcis.2019.01.013. [DOI] [PubMed] [Google Scholar]
- Usov P. M. Ahrenholtz S. R. Maza W. A. Stratakes B. Epley C. C. Kessinger M. C. Zhu J. Morris A. J. J. Mater. Chem. A. 2016;4:16818–16823. [Google Scholar]
- Cherian S. Wamser C. C. J. Phys. Chem. B. 2000;104:3624–3629. [Google Scholar]
- Yella A. Lee H. Tsao H. N. Yi C. Chandiran A. K. Science. 2011;334:1203–1203. doi: 10.1126/science.1209688. [DOI] [PubMed] [Google Scholar]
- Wang L. Jin P. Duan S. She H. Huang J. Wang Q. Sci. Bull. 2019;64:926–933. doi: 10.1016/j.scib.2019.05.012. [DOI] [PubMed] [Google Scholar]
- Wang J. Fan Y. Tan Y. Zhao X. Zhang Y. Cheng C. Yang M. ACS Appl. Mater. Interfaces. 2018;10:36615–36621. doi: 10.1021/acsami.8b15452. [DOI] [PubMed] [Google Scholar]
- Feng D. Gu Z.-Y. Li J.-R. Jiang H.-L. Wei Z. Zhou H. C. Angew. Chem., Int. Ed. 2012;51:10307–10310. doi: 10.1002/anie.201204475. [DOI] [PubMed] [Google Scholar]
- Alavijeh R. K. Beheshti S. Akhbari K. Morsali A. Polyhedron. 2018;156:257–278. [Google Scholar]
- Firouzjaei M. D. Shamsabadi A. A. Aktij S. A. Seyedpour S. F. Sharifian G. M. Rahimpour A. Rabbani E. M. Ulbricht M. Soroush M. ACS Appl. Mater. Interfaces. 2018;10(49):42967–42978. doi: 10.1021/acsami.8b12714. [DOI] [PubMed] [Google Scholar]
- Rubin H. N. Neufeld B. H. Reynolds M. M. ACS Appl. Mater. Interfaces. 2018;10(17):15189–15199. doi: 10.1021/acsami.7b19455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallis D. F. S. Butler K. S. Agola J. O. Pearce C. J. McBride A. A. ACS Appl. Mater. Interfaces. 2019;11(8):7782–7791. doi: 10.1021/acsami.8b21698. [DOI] [PubMed] [Google Scholar]
- Yu M. F. You D. Q. Zhuang J. J. Lin S. Y. Dong L. Q. Weng S. T. Zhang B. Cheng K. Weng W. J. Wang H. M. ACS Appl. Mater. Interfaces. 2017;9(23):19698–19705. doi: 10.1021/acsami.7b05296. [DOI] [PubMed] [Google Scholar]
- Lazaro I. A. Forgan R. S. Coord. Chem. Rev. 2019;380:230–259. [Google Scholar]
- Li P. Li J. Z. Feng X. Li J. Hao Y. C. Zhang J. W. Wang H. Yin A. X. Zhou J. W. Ma X. J. Wang B. Nat. Commun. 2019;10:2177. doi: 10.1038/s41467-019-10218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M. Wang L. Zheng X. H. Xie Z. G. ACS Appl. Mater. Interfaces. 2017;9:41512–41520. doi: 10.1021/acsami.7b15826. [DOI] [PubMed] [Google Scholar]
- Zhang M. Wang G. H. Wang D. Zheng Y. Q. Li Y. X. Meng W. Q. Zhang X. Du F. F. Lee S. X. Int. J. Biol. Macromol. 2021;175:481–494. doi: 10.1016/j.ijbiomac.2021.02.045. [DOI] [PubMed] [Google Scholar]
- Lu X. Ye J. Zhang D. Xie R. Bogale R. F. Sun Y. Zhao L. Zhao Q. Ning G. J. Inorg. Biochem. 2014;138:114–121. doi: 10.1016/j.jinorgbio.2014.05.005. [DOI] [PubMed] [Google Scholar]
- Zhu Y. Zhang Z. Li W. Lei Z. Cheng N. Tan Y. Mu S. Sun X. ACS Sustainable Chem. Eng. 2019;7:17855–17862. [Google Scholar]
- Tăbăcaru A. Pettinari C. Marchetti F. di Nicola C. Domasevitch K. V. Galli S. Masciocchi N. Scuri S. Grappasonni I. Cocchioni M. Inorg. Chem. 2012;51(18):9775–9788. doi: 10.1021/ic3011635. [DOI] [PubMed] [Google Scholar]
- Travlou N. A. Algarra M. Alcoholado C. Cifuentes-Rueda M. Labella A. M. Lázaro-Martínez J. M. Rodríguez-Castellón E. Bandosz T. J. ACS Appl. Bio Mater. 2018;1(3):693–707. doi: 10.1021/acsabm.8b00166. [DOI] [PubMed] [Google Scholar]