Abstract
Premature intrapancreatic trypsinogen activation is widely regarded as an initiating event for acute pancreatitis. Previous studies have alternatively implicated secretory vesicles, endosomes, lysosomes, or autophagosomes/autophagolysosomes as the primary site of trypsinogen activation, from which a cell-damaging proteolytic cascade originates. To identify the subcellular compartment of initial trypsinogen activation we performed a time-resolution analysis of the first 12 h of caerulein-induced pancreatitis in transgenic light chain 3 (LC3)-GFP autophagy reporter mice. Intrapancreatic trypsin activity increased within 60 min and serum amylase within 2 h, but fluorescent autophagosome formation only by 4 h of pancreatitis in parallel with a shift from cytosolic LC3-I to membranous LC3-II on Western blots. At 60 min, activated trypsin in heavier subcellular fractions was co-distributed with cathepsin B, but not with the autophagy markers LC3 or autophagy protein 16 (ATG16). Supramaximal caerulein stimulation of primary pancreatic acini derived from LC3-GFP mice revealed that trypsinogen activation is independent of autophagolysosome formation already during the first 15 min of exposure to caerulein. Co-localization studies (with GFP-LC3 autophagosomes versus Ile–Pro–Arg–AMC trypsin activity and immunogold-labelling of lysosomal-associated membrane protein 2 [LAMP-2] versus trypsinogen activation peptide [TAP]) indicated active trypsin in autophagolysosomes only at the later timepoints. In conclusion, during the initiating phase of caerulein-induced pancreatitis, premature protease activation develops independently of autophagolysosome formation and in vesicles arising from the secretory pathway. However, autophagy is likely to regulate overall intracellular trypsin activity during the later stages of this disease.
Keywords: Acute pancreatitis, Autophagy, Premature trypsin activation, LC-3, Gastrointestinal disorder, Protease, Secretory vesicle, Endosome, Organelle
Introduction
Pancreatitis is a common gastrointestinal disorder for whose inherited and idiopathic variety a strong association with mutations in the genes for trypsinogen (PRSS1) or its inhibitor PSTI (SPINK1) was reported [1–3]. These human genetic studies, therefore, suggest that pancreatic proteases and their inhibitors, trypsin, and PSTI, in particular, play a critical role in the aetiology of the disease. Animal studies in which members of the trypsin family were either overexpressed [4] or deleted [5] appear to confirm this hypothesis, at least for the acute form of pancreatitis. Acute pancreatitis in humans is not only burdened with considerable hospital mortality, but also associated with an increase in tissue, serum, and urinary concentrations of either trypsin activity or of its activation peptide TAP (trypsinogen activation peptide) [6]. This not only provides further evidence for an involvement of trypsin in the pathogenesis of pancreatitis, but the extent of its activation appears to parallel the severity of the disease [6].
Experimental studies using animal models of pancreatitis [7–9] or isolated acini stimulated with supramaximal secretagogue concentrations [10–12] have shown that premature and intracellular activation of trypsinogen is not only a very early, but also an initiating event in the disease process. What has remained the subject of ongoing investigations are the mechanisms that trigger premature intracellular protease activation [5, 12–14] as well as the subcellular compartment in which it begins. Identifying the latter would not only allow for conclusions regarding the underlying activation mechanisms, but also suggest potential drug targets to prevent the disease onset [15, 16]. Several lines of carefully obtained experimental evidence have alternatively identified lysosomes [9, 14, 17], endosomes [18, 19], cytoplasmic vesicles [7, 14, 20], or autophagosomes/autophagolysosomes [14, 21, 22] as the principal intracellular organelle, in which active trypsin was identified [23]. While these intracellular compartments may not be mutually exclusive as trypsinogen activation organelles, the timepoints at which active trypsin were identified inside those vesicles were often relatively late, i.e., up to 8 h into the disease process. The respective organelles may, therefore, alternatively represent sites of elimination/degradation of active trypsin, rather than for its activation.
While the cellular mechanisms that explain the dominant role of trypsin in pancreatitis are still being debated [5, 13, 24], several groups have shown that the activation of trypsin depends on the activity of cathepsin B [9, 14, 25, 26], a lysosomal hydrolase. The problem with this transactivation of trypsinogen by cathepsin B is the fact that the former, as secretory enzyme, and the latter, as a lysosomal hydrolase, ought to be sorted and secreted via completely separate subcellular pathways and in distinct organelles. The discovery that the start of experimental pancreatitis is characterized by the formation of prominent cytoplasmic vacuoles [27], that these vacuoles/vesicles show a highly unregulated membrane–fusion, fission, and exocytosis kinetic [28, 29], and that in these vacuoles lysosomal enzymes co-localize with trypsinogen [7] and trypsin activity [8], has prompted many laboratories to search for the mechanisms by which these vacuoles arise and which organelle they represent.
Given the fact that cathepsin B is a lysosomal enzyme that is sorted into lysosomes via a mannose-6-phosphate (M6PR) receptor-dependent mechanism, the first suspects were, accordingly, lysosomes. While this remains a possibility, it became less resounding when it was discovered that a very large proportion of ER-synthesized cathepsin B bypasses the M6PR pathway and is sorted into secretory vesicles under physiological conditions [17, 25]. The fact that pharmacological interference with M6PR sorting of cathepsin B [30] or deletion of the M6PR, which induces massive accumulation and co-localization of trypsinogen and cathepsin B into lysosomes [31], does not induce spontaneous trypsinogen activation or pancreatitis failed to lend further support to the assumption that lysosomes represent the initial activation compartment.
The endosome is an organelle that originates at the cell surface and can thus carry extracellular cargo (including already secreted digestive enzymes) into the cytoplasm, where fusion with other vesicles would lead to co-localization of different protein classes [28, 32]. Elegant functional studies have shown that trypsin activity can, indeed, be detected in pancreatic acinar cell endosomes [19] but often, this co-localization occurred relatively late in the experiment [18], allowing for the possibility that it affects the degradation, rather than the activation of trypsin. Interestingly, endosome-associated trypsin activity was associated with a prominent disturbance in the intracellular fusion of secretory vesicles, termed compound exocytosis, that is distinct from the direct fusion of zymogen granules with the cell surface [20].
A forth mechanism that has recently been proposed to be involved in premature trypsinogen activation is autophagy [22, 33, 34]. Autophagy is a fundamental cellular mechanism employed to degrade defective or excess cellular proteins and to recycle their components for further use. It involves the formation of specified vesicles, so-called autophagosomes, that encircle the cellular constituents targeted for degradation, and, in later stages of the process, fuse with lysosomes and endosomes. Autophagy and autophagosome formation have been shown to play a critical role in experimental pancreatitis [33, 34] as well as in regulating intracellular trypsin activity levels [35]. Specifically, genetic deletion of atg5, a key component of the autophagosome formation mechanism, reduced intracellular trypsin levels in caerulein-induced pancreatitis by 8 h into the disease process, but also results in the development of chronic pancreatitis and pancreatic inflammation [35]. It has since been confirmed that autophagy is involved in regulating intracellular protease levels [22, 33], but these studies also allowed for the interpretation that a delayed degradation of activated trypsin or a specific form of macroautophagy that targets secretory vesicles and was termed ‘zymophagy’ [36] execute this regulation. Autophagic clearance of proteins or damaged organelles is impaired after induction of pancreatitis [22, 37]. Because of the degradation of LAMP2, a critical protein which is involved in the process of fusion of autophagosomes and lysosomes, the autophagic flux is defective and degradation of proteins and organelles is delayed [33, 37]. The pancreas specific deletion of Rab7, another protein which is involved in vesicular fusion process, also results in impaired autophagic flux [38]. In all of these animal models, an increased trypsin activity can be observed, which indicates that autophagic flux is involved in the degradation of proteases rather than its activation. Fusion of lysosomes with autophagosomes results in a co-localisation of lysosomal enzymes with secretory proteases, not only of cathepsin B, the major activator of trypsinogen [9, 14], but also of cathepsin L a lysosomal hydrolase which degrades trypsinogen as well as active trypsin [39], co-localize with pancreatic zymogens.
We have used techniques for the in situ localisation of active trypsin (TAP antibodies, fluorogenic substrates in living cells), as well as density gradient centrifugation to isolate organelles, to characterize the compartments in which active trypsin appears at first in pancreatic acinar cells during experimental pancreatitis. We specifically attempted to confirm or refute whether the formation of those trypsinogen activation compartments requires autophagy. We found that at timepoints below 60 min, trypsinogen activation neither requires autophagy nor does it need to occur in autophagosomes or autophagolysosomes. Conversely, vesicles carrying no autophago-/lysosomal marker contain active trypsin in the early phase after supramaximal secretagogue stimulation, move to a heavier subcellular fraction previously believed to contain only zymogen granules, and thus represent an alternative, secretory organelle in which trypsinogen activation is initiated.
Materials and methods
Materials
Caerulein was obtained from Sigma (Taufkirchen, Germany). Collagenase from clostridium histolyticum (EC.3.4.24.3) was from Serva (lot # 14007, Heidelberg, Germany). Human myeloperoxidase was from Calbiochem (San Diego, CA, USA). The substrate R110-(CBZ-Ile-Pro-Arg)2 was obtained from Invitrogen (Eugene, OR, USA) and AMC-(Suc-Ala2-Pro-Phe) from Bachem (Heidelberg, Germany). The Amylase quantification kit Amyl was purchased from Roche (Grenzach-Whylen, Germany). The biologically active, phosphorylated CCK octapeptide [Tyr(SO3H)27]–cholecystokinin fragment, Boc–Glutamine–Alanine–Arginine–AMC. HCl as well as caerulein was obtained from Bachem (Bubendorf, Switzerland) and Vinblastine from Sigma-Aldrich (St. Louis, US). All the other chemicals were of the highest purity and obtained either from Sigma-Aldrich (Eppelheim, Germany), Merck (Darmstadt, Germany), Amersham Pharmacia Biotech (Buckinghamshire, UK), or Bio-Rad (Hercules, CA, USA).
Animal experiments
All animal experiments were conducted after institutional review board (IRB) approval and according to the guidelines of the American Physiological Society. C57Bl/6n mice were purchased by Charles river (Sulzfeld, Germany) and used for experiments at an age of 8–12 weeks. For all experiments, 5 or more mice were used for each timepoint. Mice were kept in Nalgene shoebox cages with 12 h light/dark cycle and used after an overnight fast with water ad libitum. Pancreatitis was induced by 8 intraperitoneal caerulein injection (50 µg/kg/h) at hourly intervals with saline-treated animals serving as controls. Caerulein acts as a Cholecystokinin (CCK) analogue. Stimulation with supramaximal concentrations of either Caerulein of CCK leads to a secretion blockage in pancreatic acinar cells which directly results in intracellular protease activation and acinar cell injury in vivo as well as in vitro when using isolated acini [14]. For induction of autophagy vinblastine (50 mg/kg), intraperitoneally was used and the pancreas was harvested after 4 h. C57BL/6 male mice or transgenic littermates [40] were used throughout to isolate pancreatic acini as previously described [12, 14]. Animal breeding and selection of GFP LC3 animals: the GFP-LC3 positive animals were crossed with C57BL/6 and maintained as heterozygous for GFP-LC3 transgene. Genotyping was carried out by PCR analysis from cDNA using the following primers 5I-ATAACTTGCTGGCCTTTCCACT-3I and 5I-CGGGCCATTTACCGTAAGTTAT-3I and internal primer 5I-CAGCTCATTGCTGTTCCTCA-3I. The mice are left for minimum of 1 week to adjust to the laboratory conditions before conducting any experiments.
Subcellular fractionation
Subcellular fractions were prepared immediately after sacrifice as previously described [14, 26]. Pancreas was trimmed off fat and dissected into small pieces in homogenisation buffer (250 mM sucrose, 10 mM EDTA, 10 mM HEPES; pH 7.4; 4 °C) and dounced with pestle A and B for one stroke each in a tissue grind douncer (Kontes, USA). The pancreatic homogenate was cleared from nuclei and tissue debris by centrifugation at 150×g for 10 min. The supernatant was termed post-nuclear supernatant (PNS) and centrifugation at 610×g generated a heavier H-fraction mostly containing secretory vesicles, at 3000×g for 15 min a lighter L-fraction mostly containing lysosomes, and at 12,000×g 12 min a microsomal fraction and cytosolic supernatant. All the centrifugation steps were carried out at 4 °C. Protein content was determined according to Bradford using a commercially available Bradford reagent kit Sigma-Aldrich (Eppelheim, Germany).
Enzyme activity measurement
Trypsin activity was measured fluorometrically using the Rhodamine110 coupled bis-(CBZ-l-isoleucyl-l-prolyl-l-arginine amide) dihydrogen chloride, and catalytic activity in units was quantified using microtitre plate reader (Fluostar OPTIMA, BMG Labtech, Ortenberg, Germany). The activities were calculated and represented as U/mg of protein [13]. Pancreatic tissue was homogenized on ice in measurement buffer containing 100 mM TRIS, 5 mM CaCl2, and pH 8.0. The samples were immediately measured by adding substrate solution in a final concentration of 10 µM. Trypsin activity was studied as a kinetic over 60 min at 37 °C and pH 8.0.
Measurement of trypsin activity in isolated acini
The acinar cells were isolated from overnight fasted mice by collagenase (Serva, Heidelberg, Germany) digestion as previously reported [10, 14]. Acini were maintained in isolation medium (DMEM high Glucose, 100 mM HEPES containing 5% BSA) at 37 °C and stimulated with 0.001 mM CCK (Sigma-Albrecht, Germany). Intracellular protease activity was measured using Boc–Glutamine–Alanine–Arginine–AMC–HCl. Living acini were transferred to measurement buffer (24.5 mM HEPES, 96 mM NaCl, 11.5 mM glucose, 6 mM KCl, 1 mM MgCl2 6H2O, 0.5 mM CaCl2 2H2O, 2.5 mM NaH2PO4 H2O, 5 mM sodium fumarate, 5 mM sodium glutamate, 5 mM sodium pyruvate, and 1% BSA and DMEM) containing 10 µM AMC. Intracellular protease activity was measured as a kinetic over 1 h using microtitre plate reader (Fluostar OPTIMA, BMG Labtech, Ortenberg, Germany).
Amylase secretion assay
Serum was separated from the blood using Vacutainer (BD Pharmingen, Plymouth, UK). Amylase was measured colorimetrically using the AMYL®kit in accordance with the manufacturers’ instructions. Amylase activity is presented as units/ml of serum.
Western blot analysis
Tissue samples and subcellular fractions for Western blot experiments were homogenized on ice in lysis buffer containing 25 mM HEPES (pH7.5), 75 mM NaCl, 0.5% Triton X-100, 5% Glycerol, and 1 mM EDTA in the presence of different protease inhibitors (10 mM NaF, 5 mM Na4P2O7, 1 mM PMFS, and 1 µg/ml aprotinin). Protein content was determined by Bradford assay. 50 µg of total protein were loaded on 12.5% polyacrylamide gels and transferred to nitrocellulose membranes for immunoblotting as previously described [14]. Blots were developed in enhanced chemiluminescence kit (Thermo Scientific, Rockford, IL, USA) and captured by Fluorchem™SP image capture system (Alpha Innotech, Santa Clara, CA, USA). The bands were analysed using AlphaEaseFC for densitometry and plotted as increase in density in arbitrary values compared to 0 h [14]. Antibodies used for immunoblotting were Rabbit polyclonal anti-trypsin originally raised against human Trypsin-1, but cross-reactive with mouse Trypsin-7 (Lot# 0606632271, Cat# AB1823 [now discontinued], Chemicon, Temecula, CA, USA), rabbit polyclonal anti-LC3 (Clone#5, Cat#2755), rabbit monoclonal anti-LAMP1 (C54H11, Cat#3243), rabbit polyclonal anti-Rab5 (Cat#2143),Cell Signaling, Danvers, MA, USA, mouse monoclonal anti-GAPDH (Clone#6C5, Meridian Lifesciences, Memphis, TN, USA), mouse monoclonal anti-Syncollin (Cat#612468, BD Transduction laboratories, San Diego, CA, USA), mouse monoclonal anti-chymotrypsin (Cat#130601-13604, QED Biosciences, San Diego, CA, USA), rabbit mAb anti-ATG16 (Cat #8089 (D6D5) Cell Signaling Technology), mouse monoclonal anti-p115 (Cat#612261, BD biosciences, San Diego, CA, USA), goat polyclonal anti-58 k (Cat#KL1275131, Thermo Scientific, Rockford, IL, USA), mouse monoclonal anti-Syntaxin 6 (Cat#610635, BD biosciences), rabbit polyclonal anti-GP2 (Cat#LS-C80665, Lifespan Biosciences, Seattle, USA), rabbit polyclonal anti-LIMP2 (Lot#B3, Cat#NB400-129, Novus Biologicals, Cambridge, UK), rabbit polyclonal anti-GRP.78 (sc-13968) and anti-TGN38 (sc-33784, Santa Cruz Biotechnol. Inc., Dallas, TX, USA), and rabbit polyclonal anti-Rab9a (ABIN715331, antibodies online GmbH, Aachen, Germany). The following secondary antibodies were used: anti-mouse IgG (NA931V, GE Healthcare UK), anti-rabbit IgG (NA934V, GE Healthcare), goat anti-mouse IgG-HRP (Cat-nr. sc-2005, Santa Cruz), goat anti-rabbit IgG-HRP (Cat-nr. sc-2054, Santa Cruz), and anti-goat IgG (305-035-045, Jackson ImmunReasearch, Suffolk, UK). Primary antibodies were used at a dilution of 1:100, whereas secondary antibodies were used at a dilution of 1:125,000.
Immunofluorescence
Pancreatic tissue was fixed in 4% paraformaldehyde, and after overnight fixation, tissues were embedded in paraffin. The tissue slides were de-paraffinized in xylol for 30 min and rehydrated in decreasing concentrations of ethanol before they were transferred to 1x phosphate buffer saline (PBS). Antigen was retrieved by cooking in 10 mM citrate buffer in a pressure cooker for 5 min. Tissue was blocked overnight in 1x aurion BSA. Tissue slides were incubated with primary antibodies a combination of trypsin (AB 1823, Chemicon, CA, USA) with GFP (Cat no # MAB3580, Millipore), and LAMP2 (cat no # L0668, Sigma-aldrich, St. Louis, Germany) with GFP for 2 h followed by corresponding secondary antibodies Cy3-conjugated goat anti-rabbit IgG (Jackson ImmunReasearch, Suffolk, UK) and FITC-conjugated goat anti-mouse IgG (Jackson ImmunReasearch, Suffolk, UK) for 1 h, respectively. Primary antibodies were used at a dilution of 1:100, and secondary antibodies were used at a dilution of 1:200. DAPI is used to stain nuclei. Tissues were mounted with mowiol embedding media. Images were taken by Leica SP5 at 400× magnification. Co-localization was analysed with ImageJ using JACoP plug-in. The percentage of trypsin or LAMP2 positive dots were plotted against LC3 positive dots and graphically shown as co-localized [33].
Confocal imaging of pancreatic acinar cells
For simultaneous measurement of autophagy and trypsinogen activation, a high-resolution digital imaging system (Inverse Microscope IX70 (Olympus, Hamburg, Germany) with CCD camera (Imago) was used. Trypsinogen activation was measured by cleavage of AMC-coupled-bis-(CBZ-l-isoleucyl-l-prolyl-l-arginine amide) dihydrogen chloride (Invitrogen, Eugene, OR, USA) at Ex340 Em460 and autophagy by GFP dots (Ex488 Em507). The emitted fluorescent units were measured by ratio-imaging system (TILL Photonics) and fluorescent units were calculated with TILLvision software (4.0) as previously reported [10].
Electron microscopy
For electron microscopy, small blocks (2 mm in diameter) of pancreatic tissue from controls and pancreatitis animals after 30 and 60 min were fixed in 5% (wt/vol) paraformaldehyde in 0.2 mol/L piperazine-N,N′-bis [2-ethanesulfonic acid], pH 7.0, cryoprotected with polyvinylpyrrolidone/sucrose, and frozen in liquid nitrogen. Ultrathin frozen sections (60 nm) were prepared using a Leica (Wetzlar, Germany) Cryoultramicrotome (block temperature − 110 °C, knife temperature − 100 °C). The sections on formvar-coated copper grids were blocked with 5% (wt/vol) fetal calf serum (Life Technologies, Rockville, MD) in phosphate-buffered saline (PBS), pH 7.4, and then incubated with mouse monoclonal anti-LAMP2 antibody (1:10–1:30; Clone#Gl2A9, Cat# ab13524, Abcam, Cambridge, UK) for 45 min at room temperature. After washing with PBS, the sections were incubated with 10-nm gold-conjugated goat anti-mouse antibody (dilution 1:10; Dianova, Hamburg, Germany), washed again with PBS and water, and subsequently contrasted and embedded by incubation with methylcellulose/uranyl acetate on ice (9:1 mixture of 2% methylcellulose and 4% uranyl acetate). For co-labelling experiments, anti-TAP antibody (6 nm gold) was used as previously described [7]. Samples were examined on a Philips (Eindhoven, The Netherlands) 400 electron microscope and photographed at 30,000 × magnification.
High-resolution density gradient centrifugation
Multiple fractions were prepared from total mouse pancreas that was rinsed in 0.9% NaCl and homogenized with a Potter-Elvehjem in HS buffer (250 mM sucrose/10 mM citric acid/0.5 mM EGTA/0.1 mM MgSO4 pH 6.0). The post-nuclear supernatant (at 500 g) was loaded onto a 50% (v/v) Percoll solution/2× HS-buffer mix and centrifuged at 50,000g for 45 min at 4 °C. Percoll gradients were fractionated in 46 fractions using a peristaltic pump (Pharmacia, LKB Pump P-1) beginning from bottom using a needle.
Measurement of protease activity from multiple fractions
Trypsinogen upon enterokinase activation and free trypsin activity from the fractions were fluorimetrically measured in 50 mM Tris/150 mM NaCl/1 mM CaCl2 containing 100 µg/ml BSA at 37 °C using 64 µM BOC–Gln–Ala–Arg-7-amido-4-methylcoumarin as a substrate Bachem (Bubendorf, Switzerland) in a microtitre plate reader Saphire/Tecan. Cathepsin B was measured as relative fluorescent units (RFU) in 50 mM Na-acetate/1 mM EDTA/1 mM DTT pH 5.5 using 10 µM Z-Phe-Arg-7-amido-4-methylcoumarin.
Results
Supramaximal secretagogue stimulation in vivo induces trypsinogen activation before autophagy develops
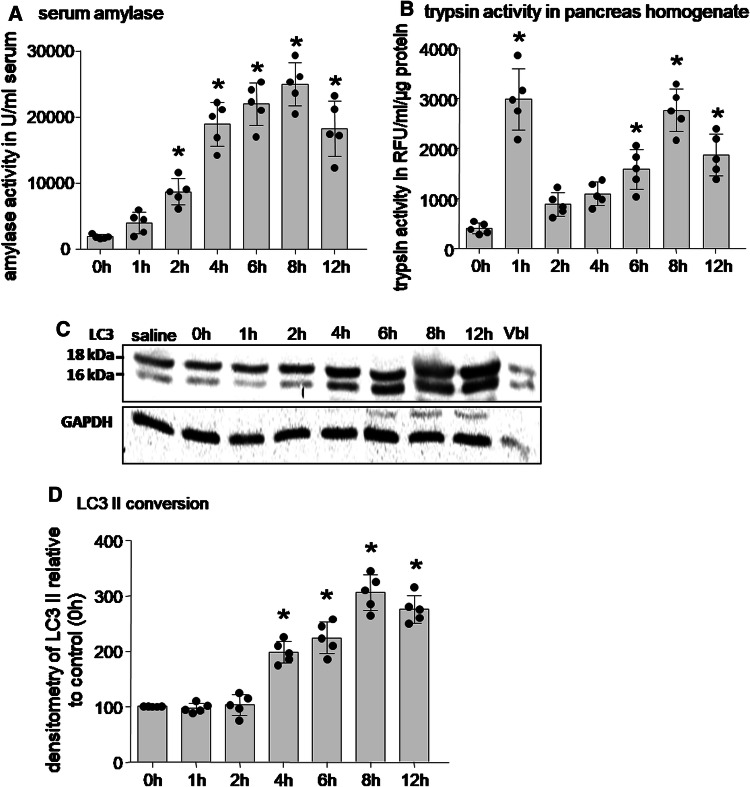
Pancreatitis was induced in mice using caerulein (50 µg/kg/h i.p. every hour for 8 h). At hourly intervals, we measured amylase activity in serum as an indicator of pancreatitis (Fig. 1a), trypsin activity in pancreatic homogenates of sacrificed animals (Fig. 1b), and microtubule-associated protein1 light chain 3 (MAP-LC3) as a marker of autophagosome formation and autophagy [33, 35] in homogenates (Fig. 1c, d). MAP-LC3 is a mammalian homologue of the yeast autophagy protein Atg8 of which the cytosolic form (LC3-I; 18 kDa) is conjugated and lipidated by phosphatidyl-ethanolamine and thus converted into the membranous form LC3-II (16 kDa). The shift from the heavier to the lighter band indicates autophagosome formation [41]. During the course of pancreatitis in mice, trypsin activity in the pancreas was found as early as 1 h after the start of supramaximal caerulein incubation (Fig. 1b), elevated serum amylase concentration was first found at 2 h (Fig. 1a), and the membranous form LC3-II as indicator of autophagy was significantly increased only at timepoints later than 4 h of pancreatitis on densitometry (Fig. 1d) from Western blots (Fig. 1c). This suggests that trypsinogen activation is an earlier process than autophagosome formation in pancreatitis and even cellular injury precedes autophagosome formation.
Fig. 1.
Induction of caerulein pancreatitis in mice triggers autophagy and protease activation. a Serum amylase levels, a marker of pancreatic damage, showed an increase in a time-dependent manner after the onset of disease. b Significant rise in trypsin activity was detected as early as 1 h after the onset of pancreatitis and was paralleled by a decrease in trypsinogen. Trypsin activity rise followed a biphasic pattern over the course of the disease. c Immunoblotting of LC3 in pancreatic homogenates from caerulein-treated mice revealed a time-dependent conversion of the cytosolic LC3 I (18 kDa) to the membranous form of LC3 II (16 kDa) and indicates autophagosome formation. Vinblastine (Vbl) treatment which induces autophagy served as positive control. GAPDH was used as loading control. d Densitometric quantification analysis of LC3-II expression in caerulein-treated mice indicated a significant increase in LC3 conversion only 4 h after the first caerulein injection. Data expressed as mean ± SEM. *p < 0.05 vs respective control, one-away ANOVA test. Number of animals employed in the experiment equals > 5
Autophagosomes form and contain trypsin only 4 h into the disease course
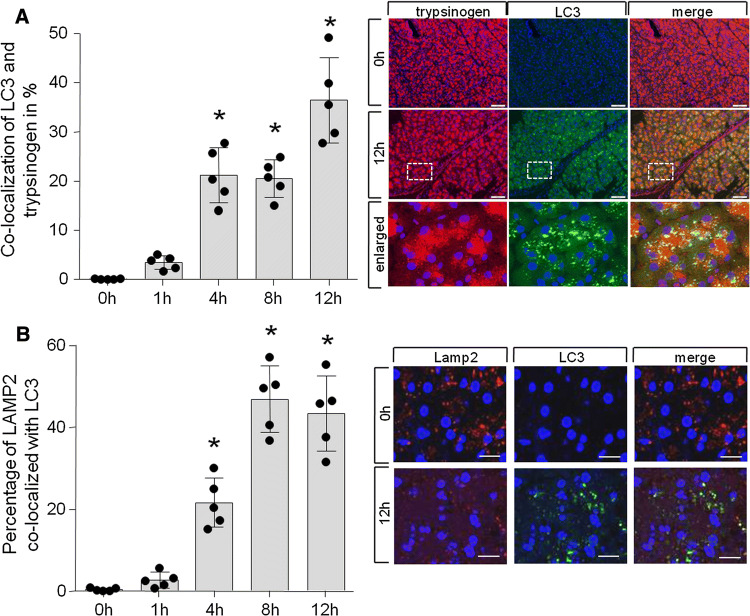
As a second line of evidence for the different timepoints of autophagosome formation and the presence of active trypsin in cytosolic vesicles, we used co-localisation studies for fluorescent microscopy. As we used mice which express a GFP-labelled LC3 which is not fluorescent when present in cytosolic form, but appears as distinct fluorescent foci when incorporated in the membranes of newly formed autophagosomes [40], autophagy can be studied on a subcellular level. In methanol-fixed sections, the detection of this fluorescence allows the characterization of forming autophagosomes and we used it for co-labelling experiments with an antibody that detects trypsinogen (as well as trypsin), or an antibody that detects the lysosomal/autophagolysosomal membrane protein LAMP2. This allows us to distinguish between lysosomes (LAMP2+/LC3−), autophagosomes (LAMP2−/LC3+), and autophagolysosomes (LAMP2+/LC3+). The microscopic findings were quantitated morphometrically (Fig. 2a, b) and confirmed a significant co-localization of LC3-II positive autophagosomes/autophagolysosomes with either trypsin/trypsinogen (Fig. 2a) only at time intervals later than 4 h after the start of supramaximal secretagogue stimulation, with a maximum 12 h after induction of pancreatitis (Fig. 2a). In addition, the maturation of autophagosomes to autophagolysosomes follows this time course, shown by the co-localisation of LC3 and the lysosomal marker LAMP2 (Fig. 2b).
Fig. 2.
Trypsinogen processing precedes LC-3 processing. a Percentage of trypsin co-localization with LC3 shows significance after 4 h and later in disease development with a maximum at 12 h after induction of pancreatitis. Data are derived from 4 independent experiment and 5 random view fields per animal. Percentage of co-localization is shown as Mender overlap coefficient value calculated using JACoP plug-in in ImageJ. b Similar kinetic was found for LAMP2 co-localization with LC3. Earliest trypsin co-localization with autophagosome markers (LC3) can be detected after 4 h. At this timepoint, also autophagosomal and lysosomal markers are co-localized (a, b). (Bar 20 µM)
Trypsinogen processing precedes LC-3 processing
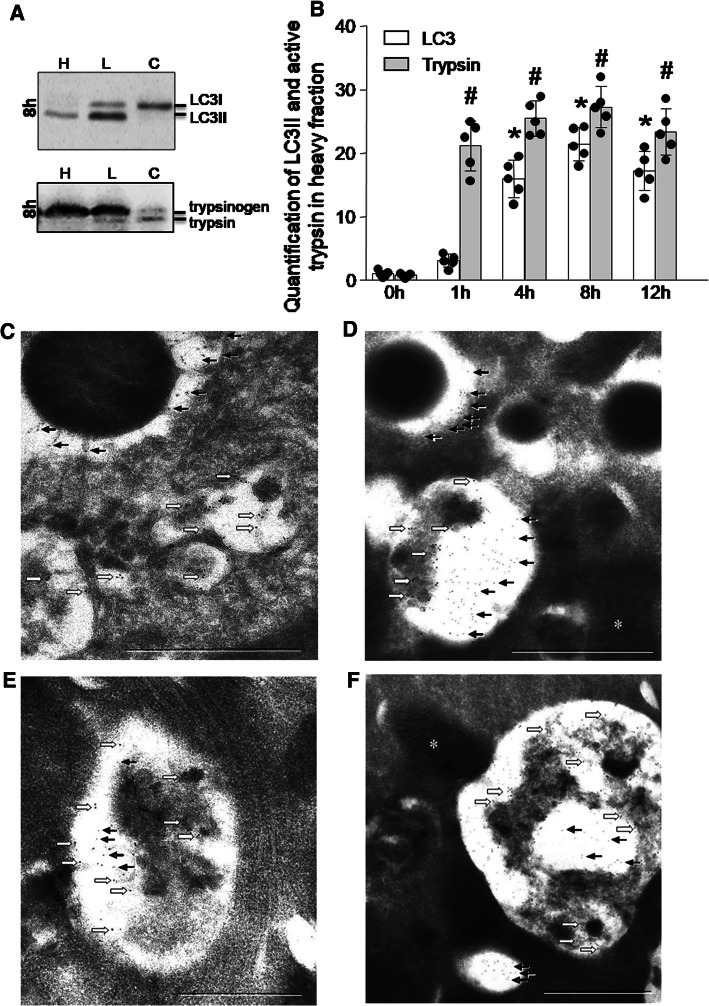
When trypsinogen is processed, this can be detected on Western blot analysis by a shift from the heavier 26 kDa trypsinogen band to the lighter 24 kDa band. The proteolytic cleavage of TAP by enterokinase or cathepsin B results in a shift of protein size which can be detected by Western blot [13]. Beside the proteolytic activation of trypsinogen, also the proteolytic degradation by lysosomal cathepsin L results in a reduced protein size in nearly the same range [39]. While this band-shift method for the detection of protease activation has been firmly established for detecting the activation of Pro-carboxypeptidase A1 [8], it can also be used for trypsinogen processing in subcellular fractions. A not dissimilar molecular weight shift can be detected on Western blots for the conversion of cytosolic LC3-I (18 kDa) to membranous LC3-II (16 kDa). We, therefore, used subcellular fractions generated by the method of differential centrifugation resulting in a heavy H-fraction, a 3000 g lighter L-fraction enriched in endo-lysosomal and autophagosomal markers and a 12,000 g supernatant C-fraction containing mostly cytosol. As shown in Fig. 3a, LC3 in its cytosolic form predominates in the cytosolic supernatant throughout the 12 h experiment. The lower molecular weight, membrane-bound LC3-II begins to appear in the lighter L-fraction only after 4 h of the experiment and increases thereafter. In the heavier H-fraction, it becomes more clearly detectable after 4 h. Conversely, activated trypsin appears as a lower molecular weight band as early as 1 h into the experiment and predominates in the heavier H-fraction. Densitometry of proteins bands in the secretory vesicle H-fractions confirmed the non-parallel processing of trypsinogen and LC3 during the course of pancreatitis (Fig. 3a). These observations are further evidence for the non-parallel spatial as well as temporal development of trypsin activation and autophagosome formation.
Fig. 3.
Processing and localization of Trypsin and LC-3. a Immunoblotting for LC3 (upper blot) and trypsinogen/trypsin (lower blot) was performed during the time course of caerulein-induced pancreatitis in pancreatic subcellular fractions: secretory vesicles (heavier H-fraction), lysosomes (lighter L-fraction), and cytosol (C-fraction) prepared by density gradient centrifugation. b Densitometric quantification of LC3 II and active Trypsin in the heavier secretory vesicle fraction was done with Image J software. (*) Indicates significant differences with respect to LC3, whereas (#) indicates significant differences with respect to trypsin at 0 h. Data confirm that early trypsinogen activation takes place in a secretory compartment, while late trypsin activity can be found in a LC3 positive subcellular compartment (Data are expressed as % mean ± SEM. *p < 0.05, vs respective controls, one-away ANOVA test for respective groups). Ultrastructural evidence of trypsin activity in autophagosomes. Shown on the micrographs is the early phase of caerulein-induced pancreatitis at 30 min (c, d) and 60 min (e, f) after the first caerulein injection. 6 nm gold particles (black arrows) label TAP (trypsinogen activation peptide) and 12 nm gold particles (white block arrow) label LAMP2. Bars indicate 1 µm. c In a secretory vesicle in the top left corner active trypsin is labelled by TAP but no LAMP2 signal can be detected. The autophagosome in the lower right corner contains LAMP2 but not TAP. In d, top left corner the small arrows indicate the presence of TAP in a secretory vesicle without co-labelling of LAMP2. The autophagolysosome at the bottom of d contains both, TAP and LAMP2. Both Autophagosomes (e, f) contain both markers after 1 h of pancreatitis. At the bottom of figure f another vesicle without LAMP2 is visible that contains TAP and, morphologically would be consistent with a Golgi-derived secretory vesicle but has no features of an autophagosome. Mitochondria (asterisk) contain neither TAP nor LAMP2 and thus served as internal controls
Ultrastructural evidence of trypsin activity in secretory vesicles and autophagosomes
To confirm the presence of a non-autophagosomal compartment of trypsinogen activation, we also used ultrathin pancreatic cryosections after 30 and 60 min of supramaximal caerulein stimulation. We labelled them for electron microscopy with an antibody against the lysosomal/autophagolysosomal marker LAMP2 (12 nm gold particles) and co-labelled them with an antibody directed against the activation peptide of trypsinogen (TAP, 6 nm gold particles). At 30 min, we could, indeed, find subcellular, membrane-confined vesicles, that contained TAP (and thus by implication active trypsin) without evidence of LAMP2. While these vesicles could represent secretory vesicles, they are clearly not autophagolysosomes or lysosomes and, therefore, represent a possible activation compartment that is independent of autophagy (Fig. 3b, c). At 60 min after caerulein infusion, we found occasional evidence for some TAP-containing structures that were also labelled for LAMP2 and thus represent formation of autophagolysosomes in later stages of the experiment (Fig. 3d, e).
Early protease activation and autophagosome formation determined by high-resolution Percoll gradient
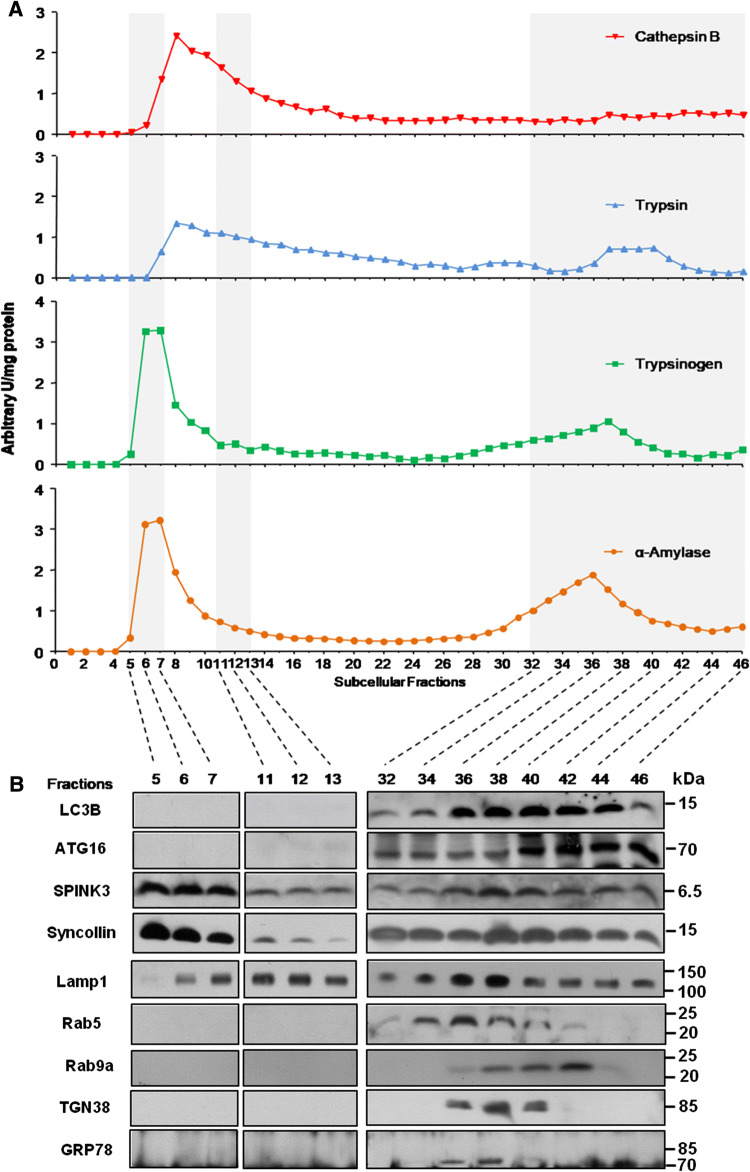
To confirm the non-parallel development of trypsinogen activation and autophagosome formation, we used high-resolution Percoll density gradient separation of 46 subcellular fractions and compared the distribution of trypsinogen content and trypsin activity, amylase activity (as secretory enzyme) and cathepsin B activity (as a lysosomal enzyme) together with Western blots of the respective fractions for markers of the autophagosome compartment (LC3-1, ATG16). While activities are shown in Fig. 4a, respective protein blots are found in Fig. 4b and the lower fraction number (1 or higher) refers to the heavier fractions (mostly zymogen granules), whereas the higher fraction number (up to 46) to lighter organelles such as microsomes. Activity measurements showed that fractions 5-7 had the highest activity of trypsin, trypsinogen content, and alpha-amylase. The complete absence of LC3-1 and ATG16 indicates that trypsin activity is not only clearly present after 1 h of supramaximal caerulein stimulation, but also predominantly confined to a vesicular compartment that clearly corresponds to secretory vesicles (as these fractions contain Spink3 and syncollin) and has no features of autophagomes. A second, but lower activity peak was observed for trypsin, trypsinogen content and alpha-amylase activity in fractions 32-46. In these lighter fractions, only the cytosolic form of LC3 as well as ATG16 was detected, suggesting that autophagosome formation had barely started by 1 h. The presence of Lamp1 in these fractions suggests an autophagolysosomal redistribution of the second peak of trypsin activity. In addition to autophagosomal markers, also markers for early and late endosomes (Rab5 and Rab9a) were present within these fractions [18, 42]. The trypsin activity-containing fraction shows a clear signal for Rab5, an early endosomal marker, but not for Rab9a, a late endosomal marker. The early timepoint of 1 h after onset of pancreatitis may be responsible for the absence of trypsin activity in the late endosomal fraction, Sherwood et al. [18] reported in isolated acini trypsin activity in endocytic vesicles 112 min after stimulation, an interval nearly 1 h later than our observation. In addition, trans-Golgi (TRP58) or ER markers (GRP78) were only present in the very light fractions and can, therefore, largely be excluded as initial activation compartment of trypsinogen. That leaves secretory vesicles (i.e., condensing vacuoles or zymogen granules) as the primary suspect.
Fig. 4.
Activation of trypsin during the early phase of pancreatitis is independent of autophagy. a Multiple subcellular fractions were prepared after 1 h of caerulein-induced pancreatitis from pancreatic homogenate by differential Percoll centrifugation. Trypsinogen, α-amylase, trypsin and cathepsin B activity was measured using respective fluorogenic substrates. b Immunoblotting was performed in selected fractions for LC3 and ATG16 as markers for autophagosomes and Spink3 as well as syncollin as markers for secretory vesicles. Lamp1 indicates lysosomal compartment, Rab5 refer as marker for early and Rab9a for late endosomes. TGN38 is a marker for the trans-Golgi fraction and GRP78 markers the endoplasmic reticulum containing fractions. Data indicate that zymogen activity peaks in the heavier fractions representing the secretory compartment (lanes 5–13), while markers of autophagosomes were detected in the lighter fractions (lanes 34–36)
Interestingly, activity of the lysosomal hydrolase cathepsin B (predominantly in fractions 11–13) had some overlap with the secretory vesicle compartment containing the bulk of trypsin activity, but also some overlap with the lighter activation compartment. While it is known that the presence of cathepsin B is required for trypsinogen activation to occur [30, 39], we cannot distinguish to what extent this co-distribution represents an overlap of heavy lysosomes with secretory vesicles in the same fraction or the known default-missorting of cathepsin B into the secretory compartment [17]. What is now unequivocal is that the early subcellular trypsinogen activation compartment is part of the secretory pathway and displays no evidence of belonging to the autophagosomal pathway.
Trypsinogen activation and autophagy are independent processes in CCK-stimulated acinar cells
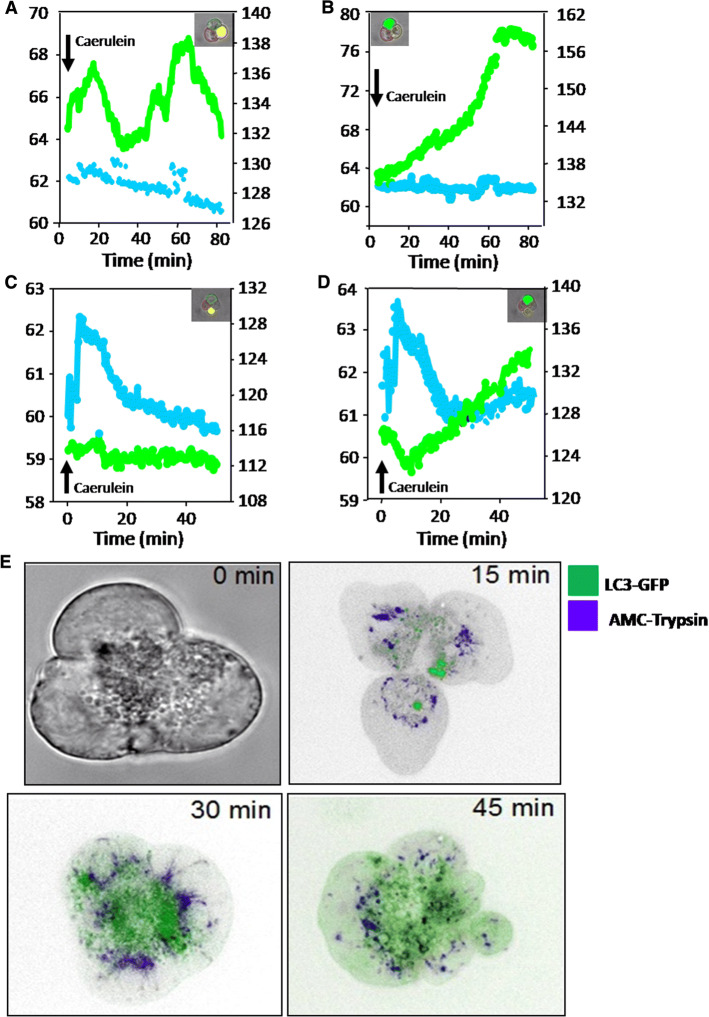
As a fifth line of evidence, we investigated isolated, living acini that were stimulated in vitro with supramaximal CCK (10 nM) in the presence of a specific and cell permeant trypsin substrate (Boc–Glutamine–Alanine–Arginine–AMC–HCl). Upon cleavage, the substrate emits fluorescence indicating the extent and the site of active trypsin (trypsinogen activation). Acinar cells were prepared from overnight fasted animals according to previously reported protocols [26, 39, 43]. We again used GFP-LC3 transgenic mice [40] to allow the visualization and quantification of autophagosome formation in living cells. When LC3-I, the cytosolic form converts to LC3-II, the membranous form, it appeared as green fluorescent (GFP) dots upon incorporation into the membrane of autophagosomes. The simultaneous registration of the trypsin activity signal (AMC substrate) with the autophagosome formation signal (GFP) allowed us to investigate the spatial and temporal distributions of the two processes in the same acinus at high resolution. Upon supramaximal CCK stimulation and as early as after 3 min, we observed a rapid increase in AMC fluorescence indicating the generation of active trypsin which, at that timepoint, was distinct and spatially separate from most GFP-LC3 dots. AMC and GFP fluorescence recorded in the same acinus and plotted against time revealed the following: some acinar cells emit increasing GFP fluorescence upon supramaximal CCK and thus undergo autophagy. At the same time, they remain negative for trypsin activity fluorescence (no AMC emission, Fig. 5a, the first and second measurements). Conversely, some cells develop prominent AMC fluorescence (trypsin activity), but remain negative for GFP florescence (absent LC3-II conversion and thus no autophagy, Fig. 5b, the third measurement). This indicates clearly that autophagosome formation and trypsinogen activation are independent processes and intracellular trypsin activity can arise in the complete absence of autophagy. It needs to be mentioned, however, that a significant number of cell also developed both types of fluorescence at later timepoints (AMC and GFP), indicating that the processes are not mutually exclusive and can develop in parallel. The earliest timepoint at which acinar cells were positive for both AMC and GFP occurred at around 25–30 min after CCK stimulation (Fig. 5d), whereas earlier signals were usually not co-localized (Fig. 5c). At later timepoints, a parallel increase in fluorescence was more common (Fig. 5d, e). This confirms that trypsinogen activation and autophagy are independent processes at the very early timepoints of supramaximal stimulation and can develop in separate and distinct compartments. During the later course of the disease, an effect of autophagy on trypsin activity cannot be ruled out.
Fig. 5.
Live cell fluorometry of acinar cells. Simultaneous fluorometry in individual acinar cells for trypsin activity (by cleavage of Boc–Glutamine–Alanine–Arginine–AMC. HCl) and LC3-GFP upon caerulein stimulation shows that LC3 conversion (autophagosome formation) develops in the absence of trypsin activity (a, b) and trypsin activity arises in the absence of LC3 conversion (c, d). Whole cell view time course is shown in e. This indicates that in the early phase of pancreatitis, autophagy and trypsinogen activation can develop independently
Discussion
Acute pancreatitis begins in pancreatic acinar (exocrine) cells which are packed with stored digestive proteases [44]. An involvement of these proteases in the pathogenesis of the disease has already been proposed more than a century ago [45] and was based on the observation that the post-mortem changes in acute pancreatitis suggest autodigestion of the gland by proteolytic enzymes. This led to the hypothesis that a premature and intracellular activation of digestive proteases represents an initial and initiating event for the development of acute pancreatitis [7, 13]. Because of the known and dominant position of trypsin in the protease activation cascade [7, 8, 14], it was proposed that activation of trypsin is the starting point of this process [46]. Evidence from human pancreatitis appears to support this assumption: measurements of free trypsin activity in blood or tissue of pancreatitis patients or, more specifically, detection of the activation peptide that is cleaved when trypsinogen is converted to active trypsin (TAP) with mono-specific antibodies, indicated that trypsinogen activation cannot only be demonstrated in early human pancreatitis, but also that the severity of acute pancreatitis corresponds to the degree of trypsinogen activation [6]. Another line of evidence comes from genetic studies: while it had long been established that pancreatitis can affect whole families in an apparently autosomal dominant manner [47], genetic linkage studies identified mutations in cationic trypsinogen (PRSS1) as the first genetic change to be associated with hereditary pancreatitis [1]. Later studies found not only additional mutations in the same gene [47, 48], but also mutations in other genes. What remains consistent with the premature protease activation hypothesis of pancreatitis, however, is the fact that, with few exceptions [49–51], the associated gene mutations involve either varieties of trypsin [1, 47, 52], other digestive proteases that are either regulated by trypsin or control trypsin activity [53, 54], or a trypsin inhibitor [2, 55]. The transgenic expression of active trypsin within the pancreas of mice also results in increased disease severity in experimental pancreatitis [4, 56], which underlines the crucial role of trypsinogen activation in the disease process. Recent studies of genetically engineered mice could demonstrate that mutations within the activation site of trypsinogen that lead to a dramatically increased autoactivation of trypsinogen results in the development of acute and chronic pancreatitis [57]. In contrast to a p.D23A-mutated T7 trypsinogen, wild-type trypsinogen does not exhibit such extensive autoactivation [23, 57]. Therefore, autoactivation appears to play a critical role in hereditary pancreatitis, but these cases represent only a minority, whereas most patients with pancreatitis do not carry trypsin (PRSS1) mutations and other induction mechanisms must be operative. It has been well established that the lysosomal hydrolase cathepsin B is essential for the activation of trypsinogen during experimental pancreatitis [9, 14, 23]. Trypsinogen activation is almost completely abolished in mice deficient for CTSB [9, 14] and pharmacological inhibition of CTSB reduces trypsinogen activation and the severity of pancreatitis dramatically [14, 23, 58]. As a defence against CTSB-mediated trypsin activation, the two enzymes are stored in different subcellular compartment under physiological conditions and need to undergo co-localization for trypsinogen activation to occur. The cellular compartment where they co-localize and in which the initial trypsinogen activation arises has long been a matter of debate.
We have addressed the question whether autophagosomes represent the initial activation compartment and whether autophagy is a required process for premature trypsinogen activation to occur in acinar cells. Autophagolysosomes have been suggested to represent a subcellular compartment in which secretory and lysosomal proteases are co-localized. Autophagy is induced during pancreatitis and in direct response to CCK and the Ca2+ signaling [59]. Previous studies could clearly show that dysfunctional autophagy is associated with pancreatitis and influences the disease severity [22, 33, 35]. Our data show that, in vitro as well as in vivo, trypsinogen activation precedes autophagosome formation by significant time intervals. Moreover, on immunogold-labelling for electron microscopy and in live cell fluorescence imaging, where the development of trypsin activity can be monitored in real time and in parallel with autophagosome formation, abundant examples can be found in which protease activation occurs independently of autophagosome formation and vice versa. Neither seems protease activation to be a precondition for autophagy, nor autophagy a precondition for intracellular protease activation. High-resolution subcellular fractions further confirm that the initial trypsinogen activation compartment is characterized by proteins consistent with secretory organelles, rather than autophagosomes.
Taken together, these data clearly indicate that the initial activation of trypsin in response to supramaximal caerulein stimulation occurs in a subcellular compartment of the secretory pathways and neither lysosomes, endosomes nor autophagosomes. Trypsin activation in the pancreas and during pancreatitis follows, however, a biphasic curve. While the first peak is clearly independent of autophagy and autophagosome formation, the second is not. We and others have shown that at this timepoint, during experimental pancreatitis, inflammatory cells have infiltrated the pancreas [11, 60] and that they induce the activation of trypsin in acinar cells directly via TNF-α [12]. Moreover, trypsin activation can even occur completely independent of acinar cells in infiltrating macrophages when they have phagocytosed either trypsinogen, debris from damaged acinar cells, or intact zymogen granules [60]. Interestingly, in all of the above processes, activation of trypsin is consistently mediated via Cathepsin B [9, 14, 26]. To what extend subcellular fractions in the later phases of pancreatitis contain autophagosomes or autophagolysosomes from inflammatory cells, in addition to those of acinar cells, cannot be answered at this point. Whether the initial autophagy-independent trypsin activation or the second autophagy-dependent wave of trypsin activation contributes to a greater extend to pancreatic damage and local or systemic inflammatory responses will also have to be elucidated. What appears to be clear at this point is that the earliest premature and intracellular trypsin activation begins in acinar cells, requires no autophagy or autophagosome formation, and is initiated in membrane-confined vesicles that arise in the secretory pathway. A strategy that intends to prevent the earliest cellular events of pancreatitis will, therefore, have to target these very organelles.
Abbreviations
- Em
Emission
- Ex
Excitation
- AMC
7-Amido 4-methylcoumarin
- MAP-LC3
Microtubule-associated protein 1-light chain 3
- GFP
Green fluorescent protein
- FITC
Fluorescein isothiocyanate
- h
Hour
Author contributions
SRM, BK, TW, MS, UMM, FGT, and CDB were involved in data acquisition, analysis, and interpretation of data, and drafting and revision of the manuscript. FUW, FSG, WH, TR, AA, and AG were involved in critical analysis of data, interpretation, and manuscript revision; JM, SRM, WH, TR, and MML: study concept and design, drafting of the manuscript, and obtained funding, study supervision.
Funding
This work was supported by the Deutsche Krebshilfe/Dr. Mildred-Scheel-Stiftung (109102), the Deutsche Forschungsgemeinschaft (DFG GRK840-D2/E3/E4, GRK1947-A3, MA 4115/1-2/3, AG 203/2-1, SE 2702/2-1), the Federal Ministry of Education and Research (BMBF GANI-MED 03IS2061A and BMBF 0314107, 01ZZ9603, 01ZZ0103, 01ZZ0403, 03ZIK012) and the European Union (EU-FP-7: EPC-TM and EU-FP7-REGPOT-2010-1, TBI-V-1-083-VBW-028, PePPP center of excellence MV ESF/14-BM-A55-0045/16; ESF MV V-630-S-150-2012/132/133),). FSG and AG are supported by Veterans Administration Merit Awards.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Markus M. Lerch and Julia Mayerle equally contributed to the study.
Walter Halangk passed away on August 20th 2018. This manuscript is dedicated to his memory.
References
- 1.Whitcomb DC, Gorry MC, Preston RA, et al. Hereditary pancreatitis is caused by a mutation in the cationic trypsinogen gene. Nat Genet. 1996;14:141–145. doi: 10.1038/ng1096-141. [DOI] [PubMed] [Google Scholar]
- 2.Witt H, Luck W, Hennies HC, et al. Mutations in the gene encoding the serine protease inhibitor, Kazal type 1 are associated with chronic pancreatitis. Nat Genet. 2000;25:213–216. doi: 10.1038/76088. [DOI] [PubMed] [Google Scholar]
- 3.Whitcomb DC, LaRusch J, Krasinskas AM, et al. Common genetic variants in the CLDN2 and PRSS1-PRSS2 loci alter risk for alcohol-related and sporadic pancreatitis. Nat Genet. 2012;44:1349–1354. doi: 10.1038/ng.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gaiser S, Daniluk J, Liu Y, et al. Intracellular activation of trypsinogen in transgenic mice induces acute but not chronic pancreatitis. Gut. 2011;60:1379–1388. doi: 10.1136/gut.2010.226175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawra R, Sah RP, Dudeja V, et al. Intra-acinar trypsinogen activation mediates early stages of pancreatic injury but not inflammation in mice with acute pancreatitis. Gastroenterology. 2011;141:2210–2217.e2. doi: 10.1053/j.gastro.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neoptolemos JP, Kemppainen EA, Mayer JM, et al. Early prediction of severity in acute pancreatitis by urinary trypsinogen activation peptide: a multicentre study. Lancet Lond Engl. 2000;355:1955–1960. doi: 10.1016/S0140-6736(00)02327-8. [DOI] [PubMed] [Google Scholar]
- 7.Hofbauer B, Saluja AK, Lerch MM, et al. Intra-acinar cell activation of trypsinogen during caerulein-induced pancreatitis in rats. Am J Physiol. 1998;275:G352–G362. doi: 10.1152/ajpcell.1998.275.2.C352. [DOI] [PubMed] [Google Scholar]
- 8.Grady T, Mah’Moud M, Otani T, et al. Zymogen proteolysis within the pancreatic acinar cell is associated with cellular injury. Am J Physiol. 1998;275:G1010–G1017. doi: 10.1152/ajpgi.1998.275.5.G1010. [DOI] [PubMed] [Google Scholar]
- 9.Halangk W, Lerch MM, Brandt-Nedelev B, et al. Role of cathepsin B in intracellular trypsinogen activation and the onset of acute pancreatitis. J Clin Invest. 2000;106:773–781. doi: 10.1172/JCI9411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krüger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol. 2000;157:43–50. doi: 10.1016/S0002-9440(10)64515-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mayerle J, Schnekenburger J, Krüger B, et al. Extracellular cleavage of E-cadherin by leukocyte elastase during acute experimental pancreatitis in rats. Gastroenterology. 2005;129:1251–1267. doi: 10.1053/j.gastro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Sendler M, Dummer A, Weiss FU, et al. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut. 2013;62:430–439. doi: 10.1136/gutjnl-2011-300771. [DOI] [PubMed] [Google Scholar]
- 13.Halangk W, Krüger B, Ruthenbürger M, et al. Trypsin activity is not involved in premature, intrapancreatic trypsinogen activation. Am J Physiol Gastrointest Liver Physiol. 2002;282:G367–G374. doi: 10.1152/ajpgi.00315.2001. [DOI] [PubMed] [Google Scholar]
- 14.Sendler M, Maertin S, John D, et al. Cathepsin B activity initiates apoptosis via digestive protease activation in pancreatic acinar cells and experimental pancreatitis. J Biol Chem. 2016;291:14717–14731. doi: 10.1074/jbc.M116.718999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mooren FC, Turi S, Gunzel D, et al. Calcium-magnesium interactions in pancreatic acinar cells. FASEB J Off Publ Fed Am Soc Exp Biol. 2001;15:659–672. doi: 10.1096/fj.00-0172com. [DOI] [PubMed] [Google Scholar]
- 16.Schick V, Scheiber JA, Mooren FC, et al. Effect of magnesium supplementation and depletion on the onset and course of acute experimental pancreatitis. Gut. 2014;63:1469–1480. doi: 10.1136/gutjnl-2012-304274. [DOI] [PubMed] [Google Scholar]
- 17.Hirano T, Saluja A, Ramarao P, et al. Apical secretion of lysosomal enzymes in rabbit pancreas occurs via a secretagogue regulated pathway and is increased after pancreatic duct obstruction. J Clin Invest. 1991;87:865–869. doi: 10.1172/JCI115091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sherwood MW, Prior IA, Voronina SG, et al. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA. 2007;104:5674–5679. doi: 10.1073/pnas.0700951104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Behrendorff N, Floetenmeyer M, Schwiening C, Thorn P. Protons released during pancreatic acinar cell secretion acidify the lumen and contribute to pancreatitis in mice. Gastroenterology. 2010;139(1711–1720):1720–1725. doi: 10.1053/j.gastro.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 20.Behrendorff N, Dolai S, Hong W, et al. Vesicle-associated membrane protein 8 (VAMP8) is a SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) selectively required for sequential granule-to-granule fusion. J Biol Chem. 2011;286:29627–29634. doi: 10.1074/jbc.M111.265199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hashimoto D, Ohmuraya M, Hirota M, et al. Involvement of autophagy in trypsinogen activation within the pancreatic acinar cells. J Cell Biol. 2008;181:1065–1072. doi: 10.1083/jcb.200712156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mareninova OA, Hermann K, French SW, et al. Impaired autophagic flux mediates acinar cell vacuole formation and trypsinogen activation in rodent models of acute pancreatitis. J Clin Invest. 2009;119:3340–3355. doi: 10.1172/JCI38674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mayerle J, Sendler M, Hegyi E, et al. Genetics, Cell Biology, and Pathophysiology of Pancreatitis. Gastroenterology. 2019;156:1951–1968.e1. doi: 10.1053/j.gastro.2018.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lerch MM, Gorelick FS. Early trypsinogen activation in acute pancreatitis. Med Clin North Am. 2000;84:549–563. doi: 10.1016/S0025-7125(05)70239-X. [DOI] [PubMed] [Google Scholar]
- 25.Kukor Z, Mayerle J, Krüger B, et al. Presence of cathepsin B in the human pancreatic secretory pathway and its role in trypsinogen activation during hereditary pancreatitis. J Biol Chem. 2002;277:21389–21396. doi: 10.1074/jbc.M200878200. [DOI] [PubMed] [Google Scholar]
- 26.Aghdassi AA, John DS, Sendler M, et al. Cathepsin D regulates cathepsin B activation and disease severity predominantly in inflammatory cells during experimental pancreatitis. J Biol Chem. 2018;293:1018–1029. doi: 10.1074/jbc.M117.814772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lampel M, Kern HF. Acute interstitial pancreatitis in the rat induced by excessive doses of a pancreatic secretagogue. Virchows Arch A Pathol Anat Histol. 1977;373:97–117. doi: 10.1007/BF00432156. [DOI] [PubMed] [Google Scholar]
- 28.Lerch MM, Saluja AK, Rünzi M, et al. Luminal endocytosis and intracellular targeting by acinar cells during early biliary pancreatitis in the opossum. J Clin Invest. 1995;95:2222–2231. doi: 10.1172/JCI117912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheele G, Adler G, Kern H. Exocytosis occurs at the lateral plasma membrane of the pancreatic acinar cell during supramaximal secretagogue stimulation. Gastroenterology. 1987;92:345–353. doi: 10.1016/0016-5085(87)90127-2. [DOI] [PubMed] [Google Scholar]
- 30.Lerch MM, Saluja AK, Dawra R, et al. The effect of chloroquine administration on two experimental models of acute pancreatitis. Gastroenterology. 1993;104:1768–1779. doi: 10.1016/0016-5085(93)90658-Y. [DOI] [PubMed] [Google Scholar]
- 31.Meister T, Niehues R, Hahn D, et al. Missorting of cathepsin B into the secretory compartment of CI-MPR/IGFII-deficient mice does not induce spontaneous trypsinogen activation but leads to enhanced trypsin activity during experimental pancreatitis—without affecting disease severity. J Physiol Pharmacol Off J Pol Physiol Soc. 2010;61:565–575. [PubMed] [Google Scholar]
- 32.Tooze J, Hollinshead M, Ludwig T, et al. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol. 1990;111:329–345. doi: 10.1083/jcb.111.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mareninova OA, Sendler M, Malla SR, et al. Lysosome associated membrane proteins maintain pancreatic acinar cell homeostasis: LAMP-2 deficient mice develop pancreatitis. Cell Mol Gastroenterol Hepatol. 2015;1:678. doi: 10.1016/j.jcmgh.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortunato F, Bürgers H, Bergmann F, et al. Impaired autolysosome formation correlates with Lamp-2 depletion: role of apoptosis, autophagy, and necrosis in pancreatitis. Gastroenterology. 2009;137(350–360):360–365. doi: 10.1053/j.gastro.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Diakopoulos KN, Lesina M, Wörmann S, et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–638.e17. doi: 10.1053/j.gastro.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 36.Grasso D, Ropolo A, Lo Ré A, et al. Zymophagy, a novel selective autophagy pathway mediated by VMP1-USP9x-p62, prevents pancreatic cell death. J Biol Chem. 2011;286:8308–8324. doi: 10.1074/jbc.M110.197301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gukovsky I, Li N, Todoric J, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–1209.e4. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi K, Mashima H, Miura K, et al. Disruption of small GTPase Rab7 exacerbates the severity of acute pancreatitis in experimental mouse models. Sci Rep. 2017;7:2817. doi: 10.1038/s41598-017-02988-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wartmann T, Mayerle J, Kähne T, et al. Cathepsin L inactivates human trypsinogen, whereas cathepsin L-deletion reduces the severity of pancreatitis in mice. Gastroenterology. 2010;138:726–737. doi: 10.1053/j.gastro.2009.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mizushima N, Kuma A. Autophagosomes in GFP-LC3 transgenic mice. Methods Mol Biol Clifton NJ. 2008;445:119–124. doi: 10.1007/978-1-59745-157-4_7. [DOI] [PubMed] [Google Scholar]
- 41.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chvanov M, De Faveri F, Moore D, et al. Intracellular rupture, exocytosis and actin interaction of endocytic vacuoles in pancreatic acinar cells: initiating events in acute pancreatitis. J Physiol. 2018;596:2547–2564. doi: 10.1113/JP275879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zenker M, Mayerle J, Lerch MM, et al. Deficiency of UBR1, a ubiquitin ligase of the N-end rule pathway, causes pancreatic dysfunction, malformations and mental retardation (Johanson-Blizzard syndrome) Nat Genet. 2005;37:1345–1350. doi: 10.1038/ng1681. [DOI] [PubMed] [Google Scholar]
- 44.Lerch MM, Saluja AK, Dawra R, et al. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992;103:205–213. doi: 10.1016/0016-5085(92)91114-J. [DOI] [PubMed] [Google Scholar]
- 45.Chiari H (1896) Über die Selbstverdauung des menschlichen Pankreas. Z Für Heilkd 69–96
- 46.Saluja AK, Lerch MM, Phillips PA, Dudeja V. Why does pancreatic overstimulation cause pancreatitis? Annu Rev Physiol. 2007;69:249–269. doi: 10.1146/annurev.physiol.69.031905.161253. [DOI] [PubMed] [Google Scholar]
- 47.Grocock CJ, Rebours V, Delhaye MN, et al. The variable phenotype of the p. A16V mutation of cationic trypsinogen (PRSS1) in pancreatitis families. Gut. 2010;59:357–363. doi: 10.1136/gut.2009.186817. [DOI] [PubMed] [Google Scholar]
- 48.Simon P, Weiss FU, Sahin-Toth M, et al. Hereditary pancreatitis caused by a novel PRSS1 mutation (Arg-122 – > Cys) that alters autoactivation and autodegradation of cationic trypsinogen. J Biol Chem. 2002;277:5404–5410. doi: 10.1074/jbc.M108073200. [DOI] [PubMed] [Google Scholar]
- 49.Weiss FU, Simon P, Bogdanova N, et al. Complete cystic fibrosis transmembrane conductance regulator gene sequencing in patients with idiopathic chronic pancreatitis and controls. Gut. 2005;54:1456–1460. doi: 10.1136/gut.2005.064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss FU, Schurmann C, Guenther A, et al. Fucosyltransferase 2 (FUT2) non-secretor status and blood group B are associated with elevated serum lipase activity in asymptomatic subjects, and an increased risk for chronic pancreatitis: a genetic association study. Gut. 2015;64:646–656. doi: 10.1136/gutjnl-2014-306930. [DOI] [PubMed] [Google Scholar]
- 51.Fjeld K, Weiss FU, Lasher D, et al. A recombined allele of the lipase gene CEL and its pseudogene CELP confers susceptibility to chronic pancreatitis. Nat Genet. 2015;47:518–522. doi: 10.1038/ng.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Witt H, Sahin-Tóth M, Landt O, et al. A degradation-sensitive anionic trypsinogen (PRSS2) variant protects against chronic pancreatitis. Nat Genet. 2006;38:668–673. doi: 10.1038/ng1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosendahl J, Witt H, Szmola R, et al. Chymotrypsin C (CTRC) variants that diminish activity or secretion are associated with chronic pancreatitis. Nat Genet. 2008;40:78–82. doi: 10.1038/ng.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Witt H, Beer S, Rosendahl J, et al. Variants in CPA1 are strongly associated with early onset chronic pancreatitis. Nat Genet. 2013;45:1216–1220. doi: 10.1038/ng.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weiss FU, Simon P, Witt H, et al. SPINK1 mutations and phenotypic expression in patients with pancreatitis associated with trypsinogen mutations. J Med Genet. 2003;40:e40. doi: 10.1136/jmg.40.4.e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhan X, Wan J, Zhang G, et al. Elevated intracellular trypsin exacerbates acute pancreatitis and chronic pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol. 2019;316:G816–G825. doi: 10.1152/ajpgi.00004.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geisz A, Sahin-Tóth M. A preclinical model of chronic pancreatitis driven by trypsinogen autoactivation. Nat Commun. 2018;9:5033. doi: 10.1038/s41467-018-07347-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saluja AK, Donovan EA, Yamanaka K, et al. Cerulein-induced in vitro activation of trypsinogen in rat pancreatic acini is mediated by cathepsin B. Gastroenterology. 1997;113:304–310. doi: 10.1016/s0016-5085(97)70108-2. [DOI] [PubMed] [Google Scholar]
- 59.Zhu Z-D, Yu T, Liu H-J, et al. SOCE induced calcium overload regulates autophagy in acute pancreatitis via calcineurin activation. Cell Death Dis. 2018;9:50. doi: 10.1038/s41419-017-0073-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sendler M, Weiss F-U, Golchert J, et al. Cathepsin B-mediated activation of trypsinogen in endocytosing macrophages increases severity of pancreatitis in mice. Gastroenterology. 2018;154:704–718.e10. doi: 10.1053/j.gastro.2017.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]