Abstract
Porcine epidemic diarrhea virus (PEDV) causes diarrhea in pigs leading to severe illnesses and high mortality rates. The development of medicinal agents to treat PEDV infection is therefore crucial. In this study, antiviral activities against PEDV of ethanol and aqueous extracts of 17 Vietnamese traditional medicinal plants were evaluated using the cytopathic effect-based assay. The results showed that 14 out of 17 medicinal plants could inhibit the cytopathic effect of PEDV. The ethanol extract of Stixis scandens was identified as the most active extract with its MIC (minimum inhibitory concentration) being 0.15 μg/mL. Other plant extracts also displayed strong antiviral activity against PEDV, including Anisomeles indica, Pericampylus glaucus and Croton kongensis. The results demonstrate that certain medicinal plants have a high antiviral potential and may serve as a lead to develop novel pharmaceutical agents to cure PED as well as the diseases caused by other coronaviruses.
Keywords: Porcine epidemic diarrhea virus, Coronavirus, Antiviral activity, Stixis scandens, Vietnamese medicinal plants
Introduction
To date, there is a very limited number of commercial antiviral agents and no cure has been found to treat many viral diseases. The issue is exacerbated by the fact that drug-resistant viral strains appear rapidly due to their high replication and mutation rate and hence highly variable genetics [1]. Therefore, there is a critical need for novel antiviral compounds.
Traditional medicinal plants have been used for thousands of years to treat various infectious diseases including viral diseases. Several hundred compounds derived from medicinal plants have been identified and proven for their antiviral activities [2]. The wide range of action mechanisms of the compounds as the result of their diversity as well as their relatively small molecular weights make them promising drug leads. Thus, screening for antiviral natural compounds from medicinal plants has become a promising approach [2, 3].
Vietnam is known to be a rich repository of natural products with more than 12,000 tropical plant species of which nearly 3,000 have been recorded to have over 8,000 therapeutic effects [4]. Traditional medicine in Vietnam has a long history and remains very popular in medical practice to treat numerous diseases including viral diseases [5]. However, insights into the medicinal functions, especially the antiviral effect of the plant extracts, are largely unknown.
Porcine epidemic diarrhea virus (PEDV) is an enveloped, single-stranded RNA coronavirus belonging to the family Coronaviridae. Since its first identification in Belgium and the United Kingdom in 1978, PEDV has spread to many countries worldwide and caused devastating economic losses in the swine industry, particularly in Europe and Asia [6, 7]. PEDV was also reported to cause an outbreak in the USA in 2013 and has spread through 30 states [8, 9]. PEDV causes vomiting, diarrhea and dehydration in pigs leading to severe illness and high mortality rates of up to 100% in piglets under 2 weeks old [10]. PEDV was first identified in Vietnam in 2009 and has become a severe threat in pig farming across many provinces [11, 12]. PEDV strains isolated in Vietnam were reported to have a nationwide distribution, and they belong to the G2b and G1b genotypes [13]. A recent study also reported PEDV strains from unvaccinated piglets in 11 provinces across Vietnam belonging to the G1 and G2 groups [12]. Although a PEDV vaccine has been used to control it, PED is still a highly contagious and devastating enteric disease in pig farms causing heavy economic losses. Therefore, the development of medicinal agents to treat PED is crucial. As mentioned earlier, Vietnamese traditional medicinal plants are a promising source of natural products with bioactivities, including antiviral activity. Thus, in this study, we investigated the virucidal activity against PEDV of ethanol and aqueous extracts of 17 different Vietnamese traditional medicinal plants, in an effort to progress towards the discovery of novel drugs that efficiently control PED.
Materials and methods
Media, cells and virus strain
Growth medium (GM) used for Vero cell culture was Dulbecco's Modified Eagle Medium (DMEM) supplemented with 5% heat-inactivated fetal bovine serum (FBS) and 1% antibiotic–antimycotic agent mixture (Gibco, Thermo Fisher Scientific, Waltham, USA). Maintenance medium (MM) contained DMEM, 0.3% tryptose phosphate broth (TPB), 0.02% yeast extract and 10 µg/ml of trypsin (Gibco) as a previous report [14]. The PEDV strain of HUA-14PED96 was propagated in Vero cells and used for viral inhibition test [15].
Plant materials
Samples of 17 different plant species were collected in the scope of an Académie de Recherche et d’Enseignement Supérieur (ARES) project about “Exploring the Medical and (Eco)-toxicological Potential of Natural Extracts in North Vietnam”, and deposited at the Botanical Museum of Hanoi (HNU), VNU University of Science (Table 1). We selected these 17 species because they are medicinal plants found in Vietnam and have not been studied much (based on SciFinder searches), plus that some of them, including Lactuca indica L. (S1), Gnetum montanum Markgr. (S7) and Tinospora sinensis (Lour.) Merr. (S15), have commercial potentials, according to our socio-economic survey (unpublished data).
Table 1.
List of medicinal plants selected in this study
| Sample name | Scientific name | Collected part | Place of collection | Deposited number |
|---|---|---|---|---|
| S1 | Lactuca indica L. | Stem, leaf | Lang Giang, Lang Son province | HNU 024108 |
| S2 | Glochidion eriocarpum Champ. ex Benth. | Leaf | Bat Xat, Lao Cai province | HNU 024776 |
| S3 | Anisomeles indica (L.) Kuntze | Stem, leaf | Bat Xat, Lao Cai province | HNU 024777 |
| S4 | Pericampylus glaucus (Lam.) Merr. | Stem, leaf | Bat Xat, Lao Cai province | HNU 024778 |
| S5 | Mahonia bealei (Fortune) Carrière | Stem | Bat Xat, Lao Cai province | HNU 024779 |
| S6 | Ficus semicordata Buch.-Ham. ex Sm. | Bark, leaf | Vi Xuyen, Ha Giang province | HNU 024780 |
| S7 | Gnetum montanum Markgr | Stem | Vi Xuyen, Ha Giang province | HNU 024781 |
| S8 | Tacca chantrieri André | Rhizome, root | Vi Xuyen, Ha Giang province | HNU 024782 |
| S9 | Crinum asiaticum L. | Leaf | Cam Pha, Quang Ninh province | HNU 024783 |
| S10 | Mallotus barbatus Müll.Arg. | Root | Lac Son, Hoa Binh province | HNU 024784 |
| S11 | Aganope balansae (Gagnep.) P.K.Loc | Stem | Bac Quang, Ha Giang province | HNU 024785 |
| S12 | Hedyotis capitellata Wall. ex G.Don | Stem, leaf | Bac Quang, Ha Giang province | HNU 024786 |
| S13 | Stixis scandens Lour. | Leaf | Bac Quang, Ha Giang province | HNU 024787 |
| S14 | Croton kongensis Gagnep. | Stem, leaf | Lac Son, Hoa Binh province | HNU 024789 |
| S15 | Tinospora sinensis (Lour.) Merr. | Stem | Lac Son, Hoa Binh province | HNU 024790 |
| S16 | Aristolochia xuanlienensis N.T.T. Huong, B. H. Quang & J. S. Ma | Stem, leaf | Cam Thuy, Thanh Hoa province | HNU 024791 |
| S17 | Aristolochia acuminata Lam. | Stem, leaf | Phuc Yen, Vinh Phuc province | HNU 024792 |
The plant samples were cleaned with water and dried for 72 h to a constant weight using a hot-air oven at 40 °C. The samples were then ground in a blender to obtain a fine powder and subjected to extraction.
Preparation of extracts
Dried plant powders were used for extraction in two different solvent systems, ethanol and double-distilled water. Each dried powder sample was suspended with a solvent at a ratio of 1:10 (w/v) and then incubated in an ultrasonic cleaner (S100H, Elma GmbH, Germany) for 30 min at 45 °C. The mixture was swirled at 200 rpm for 60 min at room temperature in a shaker (Incubator-shaker 747, Amerex Instruments, USA). To facilitate supernatant recovery, the mixture was centrifuged at 10,000 rpm for 20 min at 10 °C (Avanti J-E, Beckman Coulter, USA). The supernatant was filtered using Whatman No.1 filter paper and the filtrate was later completely evaporated either in a vacuum rotary evaporator (HS-2005S-N, Hahnshin S & T Co., Republic of Korea) if it was an ethanol solvent or lyophilized if it was a water solvent. The dried extracts were preserved at 4 °C for further use.
The ethanol extracts (EE) and aqueous extracts (AE) were dissolved in DMSO (dimethyl sulfoxide) to make a stock solution of 50 mg/mL. This stock solution was tenfold diluted in DMEM to make a working solution of 5 mg/mL before testing.
Cell toxicity test
The cytotoxicity assay was performed to evaluate the effects of EE and AE on proliferation and viability of Vero (African green monkey kidney) cells. Serial two-fold dilutions of EE and AE working solutions (50 mg/mL) were prepared in DMEM and then added to the Vero cell culture which had been previously prepared in a 96-well plate (100 µl/well). Each sample dilution was tested in three wells and the experiments were performed in duplicate. The plate was incubated for 1 h at 37 °C in 5% CO2. Then the samples were replaced by 200 μl Maintenance medium. Vero cell morphology was observed under the inverted microscope and checked daily for cytotoxic effects for a period of 5 days.
Both dead and alive Vero cells were determined by the identification of their morphological changes following the treatment with extracts. Maximum nontoxic concentration (MNTC) of an extract was determined as the maximum concentration of the extract at which the cells developed normally.
Viral inhibition test
In order to examine the inhibitory activity of the EE and AE against the PED virus, the MNTC of EE and AE was used as the starting concentration for the test. Serial two-fold dilutions of EE and AE were prepared in DMEM and then mixed with an equal volume of PED virus solution (400 TCID50/0.1 ml) [16]. The virus-extract mixture was incubated at 37 °C for 1 h and then added to the Vero cell culture that had been previously prepared in a 96-well plate (100 µl/well). Each sample dilution was tested in three wells and the experiments were performed in duplicate. The plate was incubated for 1 h at 37 °C in 5% CO2. Then the samples were replaced by 200 μl Maintenance medium. In parallel, infected and uninfected (mock) Vero cells with PEDV were used as control measures. The cytopathic effect (CPE) was monitored daily for five days. The antiviral activity of the samples was evaluated based on the inhibition of the CPE of the virus. The minimum inhibitory concentration (MIC) of the crude extract was determined as the minimum concentration of the extract at which the cells developed normally in the presence of the virus.
Results
Cell cytotoxic effect
The cytotoxic effects of ethanol (EE) and aqueous extracts (AE) of 17 medicinal plants were evaluated to determine the maximum nontoxic concentration (MNTC) of each extract (Tables 2 and S1). There were only two EEs of Lactuca indica (S1) and Tacca chantrieri (S8) having a high MNTC of 0.63 mg/mL and one EE of Aristolochia xuanlienensis (S16) having a MNTC of 1.25 mg/mL. All MNTCs of other EEs were smaller than 0.5 mg/mL. Representative microscopic images of treated Vero cells at a toxic concentration (0.08 mg/mL) and a non-toxic concentration (0.04 mg/mL) of S13 ethanol extract sample are shown in Fig. 1. Overall, the MNTC values varied between 0.04 mg/mL and 5 mg/mL. It is interesting to note that, in general, MNTCs of EE were significantly lower than those of AE of the same plant. Four AEs presented a MNTC of 5 mg/mL, including those of Stixis scandens (S13), Tinospora sinensis (S15), Aristolochia xuanlienensis (S16) and Aristolochia acuminata (S17); two AEs (of Lactuca indica—S1 and Croton kongensis—S14) had an MNTC of 2.5 mg/mL; and one AE (of Anisomeles indica—S3) had an MNTC of 1.25 mg/mL, to be compared to the MNTCs of the corresponding EEs, respectively 40, 80, 1250, 80, 630, 40, 40 µg/mL. The remaining AEs had MNTCs of less than 0.5 mg/mL, with the smallest value at 0.08 mg/mL, slightly higher or equal to the MNTCs of the corresponding EE. Only two AE extracts were more toxic than their corresponding EEs, with two-fold lower MNTCs: extracts of Mahonia bealei (S5) and Tacca chantrieri (S8).
Table 2.
Maximum non-toxic concentrations (MNTCs) on Vero cell line and minimum inhibitory concentrations (MICs) on PEDV-cytopathic effect of the ethanol extracts and the aqueous extracts of 17 medicinal plants in this study
| Plant samples | Scientific name | Ethanol extract | Aqueous extract | ||||
|---|---|---|---|---|---|---|---|
| MNTC (μg/mL) | MIC (μg/mL) | MNTC/MIC | MNTC (μg/mL) | MIC (μg/mL) | MNTC/MIC | ||
| S1 | Lactuca indica L. | 630 | 19.53 | 32.26 | 2500 | 312.5 | 8.00 |
| S2 | Glochidion eriocarpum Champ. ex Benth. | 40 | 2.44 | 16.39 | 160 | – | |
| S3 | Anisomeles indica (L.) Kuntze | 40 | 0.61 | 65.57 | 1250 | 78.13 | 16.00 |
| S4 | Pericampylus glaucus (Lam.) Merr. | 160 | 0.61 | 262.30 | 310 | – | |
| S5 | Mahonia bealei (Fortune) Carrière | 310 | 78.13 | 3.97 | 160 | 19.53 | 8.19 |
| S6 | Ficus semicordata Buch.-Ham. ex Sm. | 80 | – | 310 | – | ||
| S7 | Gnetum montanum Markgr | 160 | – | 310 | 39.06 | 7.94 | |
| S8 | Tacca chantrieri André | 630 | 2.44 | 258.20 | 310 | – | |
| S9 | Crinum asiaticum L. | 80 | 4.88 | 16.39 | 80 | – | |
| S10 | Mallotus barbatus Müll.Arg. | 40 | – | 80 | 9.77 | 8.19 | |
| S11 | Aganope balansae (Gagnep.) P.K.Loc | 310 | – | 310 | – | ||
| S12 | Hedyotis capitellata Wall. ex G.Don | 80 | – | 310 | – | ||
| S13 | Stixis scandens Lour. | 40 | 0.15 | 266.67 | 5000 | 1250 | 4.00 |
| S14 | Croton kongensis Gagnep. | 40 | 1.22 | 32.79 | 2500 | 625 | 4.00 |
| S15 | Tinospora sinensis (Lour.) Merr. | 80 | 2.44 | 32.79 | 5000 | 2500 | 2.00 |
| S16 | Aristolochia xuanlienensis N.T.T. Huong, B. H. Quang & J. S. Ma | 1250 | 4.88 | 256.15 | 5000 | 625 | 8.00 |
| S17 | Aristolochia acuminata Lam. | 80 | 2.44 | 32.79 | 5000 | 1250 | 4.00 |
The data represent the lowest MNTC and the highest MIC observed in all performed experiments for each sample
Fig. 1.
Representative microscopic pictures of Vero cell cultures exposed to toxic (0.08 mg/mL) (a) and maximum nontoxic (0.04 mg/mL) (b) concentrations (MNTC) of S13 ethanol extract
Antiviral effect
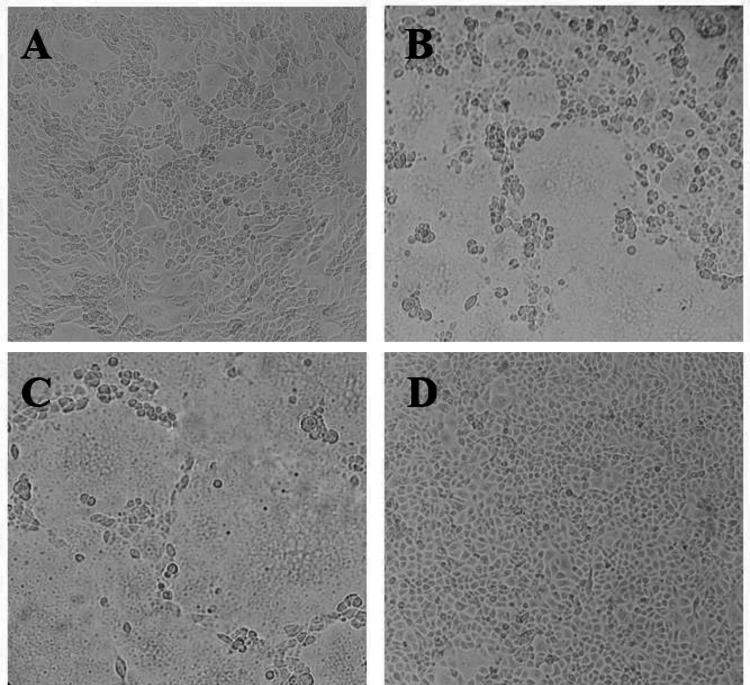
Ethanol and aqueous extracts of 17 plant species were screened for anti-PEDV activity by CPE-based assay, with the tested concentrations in serial twofold dilution starting from their respective MNTCs. The minimum inhibitory concentration (MIC) of the crude extract of each plant was determined (Tables 2 and S2). The antiviral effects of the extracts were clearly observed, as illustrated by representative microscopic images of Vero cells infected with PEDV pre-treated with S13 ethanol extract at the concentrations of 0.15 μg/mL and 0.07 μg/mL (Fig. 2, A and B). Out of the 17 plants, three plants, including Ficus semicordata (S6), Aganope balansae (S11) and Hedyotis capitellata (S12), could not inhibit the cytopathic effect of PEDV. In addition, there were four plants, including Glochidion eriocarpum (S2), Pericampylus glaucus (S4), Tacca chantrieri (S8), Crinum asiaticum (S9), that had only EEs showing anti-PEDV activities while their AEs did not. In contrast, there were two plants Gnetum montanum (S7) and Mallotus barbatus (S10) with only AEs showing anti-PEDV activities while their EEs did not. Among the remaining eight plants that had both AE and EE showing anti-PEDV activities, there was one plant, Mahonia bealei (S5), with the MIC of its AE lower than that of its EE. The remaining seven plants had low-MIC EEs and very high-MIC AEs, indicating that the antiviral active compounds of these plants were presumably dissolved in ethanol but not water. Notably, the EE of Stixis scandens (S13) had the lowest MIC of 0.15 μg/mL followed by the MICs of those of Anisomeles indica (S3) and Pericampylus glaucus (S4) which were the same at 0.61 μg/mL.
Fig. 2.
Representative microscopic pictures of antiviral activity (0.15 μg/mL) (a) and non-antiviral activity (0.07 μg/mL) (b) against PEDV of the S13 ethanol extract sample on Vero cells. Infected (c) and uninfected (mock) (d) Vero cells with PEDV were used as control measures
Discussion
PEDV, a single-stranded RNA coronavirus, has become a devastating enteric disease and caused significant economic losses in livestock worldwide. Consequently there is an urgent need to discover antiviral agents to fight against PEDV. Vietnam is home to a large collection of medicinal plants, especially in the mountainous areas of the north [5]. Many of these medicinal plants have been used for years to relieve symptoms of viral disease in humans even though the active compounds and their mechanisms of action are largely unknown [4, 5]. Nevertheless, Vietnamese medicinal plants are a potential source of novel antiviral agents.
In this study, the cytopathic effect (CPE)-based assay, which evaluates the ability to inhibit the CPE of PEDV on Vero cells, was utilized to screen for antiviral activities of ethanol and aqueous extracts of 17 medicinal plants collected from northern Vietnam. To our best knowledge, this is the first study to report the antiviral activities of medicinal plants against PEDV. Plus, our selected plants were those that have not yet been studied much. Apparently, our results were remarkably interesting, as they showed that 14 plants could inhibit the CPE of PEDV with a wide range of MIC from 0.15 to 78.13 μg/mL in the case of EE and from 9.77 to 2500 μg/mL in the case of AE. With almost every plant sample, the EE showed lower cytotoxicity as well as stronger antiviral activity than the AE, leading to higher MNTC/MIC. The stronger antiviral activities of ethanol/methanol extracts compared to aqueous extracts have also been previously reported [17, 18]. Many antiviral-screening studies have focused only on the ethanol/methanol extracts of medicinal plants [19, 20].
The most active extract was the EE of Stixis scandens (S13). The EE was active at a concentration as low as 0.15 μg/mL while the AE of this plant only displayed CPE inhibition from 1.25 mg/mL. This antiviral activity of the EE also compared well with the cytotoxic activity of the extract. The ratio of MNTC/MIC for the EE of Stixis scandens was 267, while it was only 4 for the AE and was also the highest MNTC/MIC among all samples. This strong antiviral activity compared to its cytotoxicity supports the great potential of developing antiviral agents from this plant. Stixis scandens Lour. belongs to the Capparaceae family and is traditionally used to treat rheumatism, hemoptysis and infected eye diseases in Vietnam [5]. There is no report on the natural compounds from Stixis scandens. However, chemical constituents of a closely related species Stixis suaveolens have been studied exclusively by Vietnamese research groups. Seven lignan compounds have been isolated and identified from an aqueous fraction [21], while ten compounds including lignans, lignan glycosides and phenolic glycosides were isolated from a methanol fraction [22]. Moreover, two new phenolic amides were also reported from ethylacetate extracts of Stixis suaveolens leaves by the same group [23]. However, no bioactivity of compounds extracted from Stixis suaveolens have been published. In particular, no report is available on the antiviral activity of Stixis genus.
Our results also revealed additional plants potentially displaying strong antiviral activity against PEDV, including Anisomeles indica and Pericampylus glaucus (with the MICs of 0.61 μg/mL); Croton kongensis (with the MIC of 1.22 μg/mL); Glochidion eriocarpum, Tacca chantrieri, Tinospora sinensis and Aristolochia acuminata (with MICs of 2.44 μg/mL). Anisomeles indica is an extensively studied plant with known pharmacological activities including antioxidant, antimicrobial, anti-Helicobacter-pylori and anti-cancer activity [24]. In particular, ovatodiolide – proven to have anti-HIV activity—has been purified from Anisomeles indica [25]. Alkaloids from Pericampylus glaucus were shown to be active against HBV and HIV-1 [26]. Here we show for the first time that these two plants are also active against PEDV.
None of the remaining active plants have been previously reported to have antiviral activities. Diterpens from Croton kongensis exhibited antimycobacterial and antimalarial activities [27]. Triterpenoid saponins from Glochidion eriocarpum exhibited cytotoxic activity against various cancer cell lines [28, 29]. Taccalonolides from Tacca chantrieri are well known as distinct microtubule stabilizers, expected to be promising anti-cancer agents [30]. Tinospora sinensis is also a popular medicinal plant in traditional medicine with proven immunomodulating, antitubercular, anti-cancer activities [31, 32]. The anti-inflammatory activity of Aristolochia acuminata has been reported by Battu et al. in 2011 [33]. Thus, our study is the first to report the antiviral activity of Croton kongensis, Glochidion eriocarpum, Tacca chantrieri, Tinospora sinensis and Aristolochia acuminata. Moreover, this is also the first report on the antiviral activity of medicinal plants against PEDV.
As an extension, the findings in this study could be a leading cue to develop antiviral medicine for the treatment of SARS-CoV-2, another member of the Coronaviridae family, which caused the COVID-19 pandemic.
Conclusions
In this study, 17 medicinal plants collected in Vietnam were screened for antiviral activity against PEDV. We found 14 plants that could inhibit the CPE of PEDV. The EE of Stixis scandens (S13) was identified as the most active extract with its MIC being 0.15 μg/mL. To the best of our knowledge, this is the first study reporting the antiviral activity of Stixis scandens, Croton kongensis, Glochidion eriocarpum, Tacca chantrieri, Tinospora sinensis and Aristolochia acuminata. This is also the first report on the antiviral activity of medicinal plants against PEDV. The data may also serve as a lead to develop novel pharmaceutical agents to cure PED, as well as the diseases caused by other coronaviruses including SARS-CoV-2.
Acknowledgements
This study was supported by ARES (Académie de Recherche et d’Enseignement Supérieur) and DGD (Direction Générale de la Coopération au Développement) through the funding of the project “Exploring the Medical and (Eco)-toxicological Potential of Natural Extracts in North Vietnam”. M. Muller is a "Maître de Recherche" at the "Fonds National de Recherche Scientifique (F.R.S.-FNRS)"
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Van Phan Le, Email: letranphan@vnua.edu.vn.
Thao Kim Nu Nguyen, Email: thaonkn@vnu.edu.vn.
References
- 1.Pillay D, Schinazi RF. Antiviral drug resistance: from the laboratory to the patient. Antivir Ther. 1998;3(4):237. [PubMed] [Google Scholar]
- 2.Kitazato K, Wang Y, Kobayashi N. Viral infectious disease and natural products with antiviral activity. Drug Discov Ther. 2007;1(1):14. [PubMed] [Google Scholar]
- 3.Jassim SA, Naji MA. Novel antiviral agents: a medicinal plant perspective. J Appl Microbiol. 2003;95(3):412. doi: 10.1046/j.1365-2672.2003.02026.x. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen-Vo T-H, Le T, Pham D, Nguyen T, Le P, Nguyen A, Nguyen T, Nguyen T-N, Nguyen V, Do H, Trinh K, Duong HT, Le L. VIETHERB: a database for vietnamese herbal species. J Chem Inf Model. 2019;59(1):1. doi: 10.1021/acs.jcim.8b00399. [DOI] [PubMed] [Google Scholar]
- 5.Chi VV. Dictionary of medicinal plants in Vietnam. Hanoi: Medical Publishing House; 2011. [Google Scholar]
- 6.Carvajal A, Argüello H, Martínez-Lobo FJ, Costillas S, Miranda R, de Nova PJG, Rubio P. Porcine epidemic diarrhoea: new insights into an old disease. Porcine Health Manag. 2015;1(1):12. doi: 10.1186/s40813-015-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pensaert MB, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978;58(3):243. doi: 10.1007/BF01317606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Li G, Stasko J, Thomas JT, Stensland WR, Pillatzki AE, Gauger PC, Schwartz KJ, Madson D, Yoon K-J, Stevenson GW, Burrough ER, Harmon KM, Main RG, Zhang J. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J Clin Microbiol. 2014;52(1):234. doi: 10.1128/JCM.02820-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jung K, Saif LJ. Porcine epidemic diarrhea virus infection: etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet J. 2015;204(2):134. doi: 10.1016/j.tvjl.2015.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibata I, Tsuda T, Mori M, Ono M, Sueyoshi M, Uruno K. Isolation of porcine epidemic diarrhea virus in porcine cell cultures and experimental infection of pigs of different ages. Vet Microbiol. 2000;72(3):173. doi: 10.1016/S0378-1135(99)00199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Do D, Toan N, Puranaveja S, Thanawongnuwech R. Genetic characterization of porcine epidemic diarrhea virus (PEDV) isolates from Southern Vietnam during 2009–2010 outbreaks. Isr J Vet Med. 2011;41:9. [Google Scholar]
- 12.Than VT, Choe SE, Vu TTH, Do TD, Nguyen TL, Bui TTN, Mai TN, Cha RM, Song D, An DJ, Le VP. Genetic characterization of the spike gene of porcine epidemic diarrhea viruses (PEDVs) circulating in Vietnam from 2015 to 2016. Vet Med Sci. 2020;6(3):535–542. doi: 10.1002/vms3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim YK, Lim SI, Lim JA, Cho IS, Park EH, Le VP, Hien NB, Thach PN, Quynh do H, Vui TQ, Tien NT, An DJ. A novel strain of porcine epidemic diarrhea virus in Vietnamese pigs. Arch Virol. 2015;160(6):1573. doi: 10.1007/s00705-015-2411-5. [DOI] [PubMed] [Google Scholar]
- 14.Hofmann M, Wyler R. Propagation of the virus of porcine epidemic diarrhea in cell culture. J Clin Microbiol. 1988;26(11):2235. doi: 10.1128/jcm.26.11.2235-2239.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe SE, Park KH, Lim SI, Le VP, Hien NB, Thach PN, Phuong le HT, An BH, Han SH, Cho IS, An DJ. Complete genome sequence of a porcine epidemic diarrhea virus strain from Vietnam, HUA-14PED96, with a large genomic deletion. Genome Announc. 2016;4(1):11. doi: 10.1128/genomeA.00002-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh JS, Song DS, Yang JS, Song JY, Moon HJ, Kim TY, Park BK. Comparison of an enzyme-linked immunosorbent assay with serum neutralization test for serodiagnosis of porcine epidemic diarrhea virus infection. J Vet Sci. 2005;6(4):349. doi: 10.4142/jvs.2005.6.4.349. [DOI] [PubMed] [Google Scholar]
- 17.Arbab AH, Parvez MK, Al-Dosari MS, Al-Rehaily AJ. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp Ther Med. 2017;14(1):626. doi: 10.3892/etm.2017.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SJ, Huang SH, Lin YJ, Tsou YY, Lin CW. Antiviral activity of Rheum palmatum methanol extract and chrysophanol against Japanese encephalitis virus. Arch Pharm Res. 2014;37(9):1117. doi: 10.1007/s12272-013-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogbole OO, Akinleye TE, Segun PA, Faleye TC, Adeniji AJ. In vitro antiviral activity of twenty-seven medicinal plant extracts from Southwest Nigeria against three serotypes of echoviruses. Virol J. 2018;15(1):110. doi: 10.1186/s12985-018-1022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rumschlag-Booms E, Zhang H, Soejarto DD, Fong HH, Rong L. Development of an antiviral screening protocol: one-stone-two-birds. J Antivir Antiretrovir. 2011;3:8. doi: 10.4172/jaa.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anh NQ, Yen TT, Hang NT, Anh DH, Viet PH, Hoang NH, Van Doan V, Van Kiem P. Lignan compounds from Stixis suaveolens. Vietnam J Chem. 2019;57(3):304. doi: 10.1002/vjch.201900016. [DOI] [Google Scholar]
- 22.Anh NQ, Yen TT, Hang NT, Anh DH, Viet PH, Hoang NH, Van Doan V, Van Kiem P. Phenolic and lignan compounds from Stixis suaveolens. Vietnam J Chem. 2019;57(3):311. doi: 10.1002/vjch.201900019. [DOI] [Google Scholar]
- 23.Ngo QA, Tran TY, Nguyen TH, Nguyen VT, Duong HA, Pham HV. Stixilamides A and B, two new phenolic amides from the leaves of Stixis suaveolens. Nat Prod Res. 2019;35(8):1384–1387. doi: 10.1080/14786419.2019.1647424. [DOI] [PubMed] [Google Scholar]
- 24.Baranwal V, Irchhaiya R, Singh S. Anisomeles indica: an overview. Int Res J Pharmacy. 2012;3:84. [Google Scholar]
- 25.Shahidul Alam M, Quader MA, Rashid MA. HIV-inhibitory diterpenoid from Anisomeles indica. Fitoterapia. 2000;71(5):574. doi: 10.1016/S0367-326X(00)00197-0. [DOI] [PubMed] [Google Scholar]
- 26.Yan M-H, Cheng P, Jiang Z-Y, Ma Y-B, Zhang X-M, Zhang F-X, Yang L-M, Zheng Y-T, Chen J-J. Periglaucines A−D, Anti-HBV and -HIV-1 alkaloids from Pericampylus glaucus. J Nat Prod. 2008;71(5):760. doi: 10.1021/np070479+. [DOI] [PubMed] [Google Scholar]
- 27.Thongtan J, Kittakoop P, Ruangrungsi N, Saenboonrueng J, Thebtaranonth Y. New antimycobacterial and antimalarial 8,9-secokaurane diterpenes from Croton kongensis. J Nat Prod. 2003;66(6):868. doi: 10.1021/np030067a. [DOI] [PubMed] [Google Scholar]
- 28.Kiem PV, Thu VK, Yen PH, Nhiem NX, Tung NH, Cuong NX, Minh CV, Huong HT, Hyun J-H, Kang H-K, Kim YH. New triterpenoid Saponins from Glochidion eriocarpum and their cytotoxic activity. Chem Pharm Bull. 2009;57(1):102. doi: 10.1248/cpb.57.102. [DOI] [PubMed] [Google Scholar]
- 29.Nhiem NX, Thu VK, Van Kiem P, Van Minh C, Tai BH, Quang TH, Cuong NX, Yen PH, Boo H-J, Kang J-I, Kang H-K, Kim YH. Cytotoxic oleane-type triterpene saponins from Glochidion eriocarpum. Arch Pharmacal Res. 2012;35(1):19. doi: 10.1007/s12272-012-0102-2. [DOI] [PubMed] [Google Scholar]
- 30.Risinger AL, Jackson EM, Polin LA, Helms GL, LeBoeuf DA, Joe PA, Hopper-Borge E, Ludueña RF, Kruh GD, Mooberry SL. The taccalonolides: microtubule stabilizers that circumvent clinically relevant taxane resistance mechanisms. Can Res. 2008;68(21):8881. doi: 10.1158/0008-5472.CAN-08-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badavenkatappa SG, Peraman R. In vitro antitubercular, anticancer activities and IL-10 expression in HCT-116 cells of Tinospora sinensis (Lour.) Merr. leaves extract. Nat Prod Res. 2019 doi: 10.1080/14786419.2019.1705814. [DOI] [PubMed] [Google Scholar]
- 32.Xiong H, Ding X, Wang H, Jiang H, Wu X, Tu C, Wu C, Pi Y, Yang G, Zhao Z, Mei Z. Tibetan medicine Kuan-Jin-Teng exerts anti-arthritic effects on collagen-induced arthritis rats via inhibition the production of pro-inflammatory cytokines and down-regulation of MAPK signaling pathway. Phytomedicine. 2019;57:271. doi: 10.1016/j.phymed.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 33.Battu GR, Parimi R, Chandra Shekar KB. In vivo and in vitro pharmacological activity of Aristolochia tagala (syn: Aristolochia acuminata) root extracts. Pharm Biol. 2011;49(11):1210. doi: 10.3109/13880209.2011.589855. [DOI] [PubMed] [Google Scholar]