Abstract
Background:
Although consensus guidelines generally discourage any surgical management (ASM; i.e. resection and/or transplantation) in patients with hepatocellular carcinoma (HCC) and portal vein thrombosis (PVT), recent series from Asia have challenged this paradigm.
Methods:
Patients from the US Safety Net Collaborative database (2012–2014) with localized HCC and radiographically-confirmed PVT were propensity-score matched based on demographic and clinicopathologic factors associated with receipt of ASM and overall survival (OS). OS was compared between patients undergoing ASM and those not selected for surgery.
Results:
Of 1910 HCC patients, 207 (14.5%) had localized disease and PVT. The majority received either liver directed therapies (LDT; 34%) and/or targeted systemic therapies (36%). Twenty-one patients (10.1%) underwent ASM (resection [n=11], transplantation [n=10]); a third experienced any complication with no 30-day mortalities. Independent predictors of undergoing ASM were younger age, recent hepatology consultation, and lower MELD score. After matching for age, comorbidities, MELD, tumor size, receipt of LDT or systemic therapy, OS was significantly longer for patients selected for ASM versus non-ASM patients (median not reached vs. 5.8 months, p<0.001).
Conclusion:
In a large North American multi-institutional cohort, a minority of HCC patients with PVT were selected for ASM. Resection or transplantation was associated with improved survival and may have a role in the multimodality management in selected patients.
Keywords: Outcomes, Hepatobiliary, Cancer
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common primary liver cancer worldwide, accounting for approximately 700,000 deaths annually,1 and also represents the fastest-rising cause of cancer-related death in the United States (US).2 The latter development is driven in large part by the epidemic of obesity-related non-alcoholic fatty liver disease (NAFLD) as well as progression to cirrhosis in individuals born in the peak era of Hepatitis C virus (HCV) infection.3 Regardless of HCC etiology, prognosis in HCC varies greatly by tumor stage. While curative options are available for patients diagnosed at early stages, with 5-year survival eclipsing 70% with any surgical management (ASM; i.e., surgical resection and/or liver transplantation),4,5 patients presenting with locally advanced or metastatic HCC have dismal outcomes.6
In patients presenting with locally advanced HCC, portal vein thrombosis (PVT) is a relatively common occurrence, observed in 35–50% of patients at the time of diagnosis7 and associated with hematogenous tumor dissemination.8 Not surprisingly, PVT is associated with poor prognosis, likely due to the associated intra- and extra-hepatic tumor extension as well as the sequelae of elevated portal pressures resulting in portal hypertension in these patients. 9,10 Left untreated, median survival for HCC patients with PVT ranges from 2–4 months.11 Moreover, several recent consensus HCC management guidelines (e.g., Barcelona Clinic Liver Cancer [BCLC], Hong-Kong Liver Cancer [HKLC], Liver Cancer Study Group of Japan, etc.) discourage surgical resection, liver transplantation, and/or transarterial liver-directed therapies (i.e., chemo- or radio-embolization, etc.) in HCC patients presenting with PVT.12,13 The consensus recommended treatment according to these guidelines entails systemic therapy with tyrosine kinase inhibitors (e.g., sorafenib, lenvatinib, etc.).14
More recently, however, evidence from experienced centers in Asia treating high volumes of HCC patients with endemic viral hepatitis has challenged this relatively well-established paradigm. Several retrospective single-institution studies, as well as reasonably conducted meta-analyses, suggest that surgical resection is associated with nearly a three-fold higher median survival in HCC patients with PVT without main trunk or contralateral vein involvement.15–17 Notwithstanding the careful selection of these patients for ASM, the applicability of these Asian data to Western/US populations—where the etiology and epidemiology of HCC vary substantially—remains uncertain. In this study, using the five-center US Safety Net and Academic Center Collaborative database, we sought to evaluate factors associated with selection of any surgical management (i.e., resection and/or transplantation), as well as its association with overall survival (OS), in HCC patients with PVT in a large multi-institutional North American cohort.
METHODS
Data Source
The United States Safety Net and Academic Center Consortium (USSNC) comprises five tertiary referral academic institutions with affiliated safety-net hospitals. The collaboration was started initially to extend the demographic base for cancer research, as well as to understand the effects of social determinants of health on cancer outcomes. The HCC database includes patients diagnosed with HCC at both academic and safety-net centers, and comprises data on patient demographics, screening and healthcare access, clinical characteristics, treatment modalities and cancer-related outcomes. Retrospective review of patient medical records and sharing of de-identified data was approved by the Institutional Review Boards at all collaborating institutions.
Patient Selection
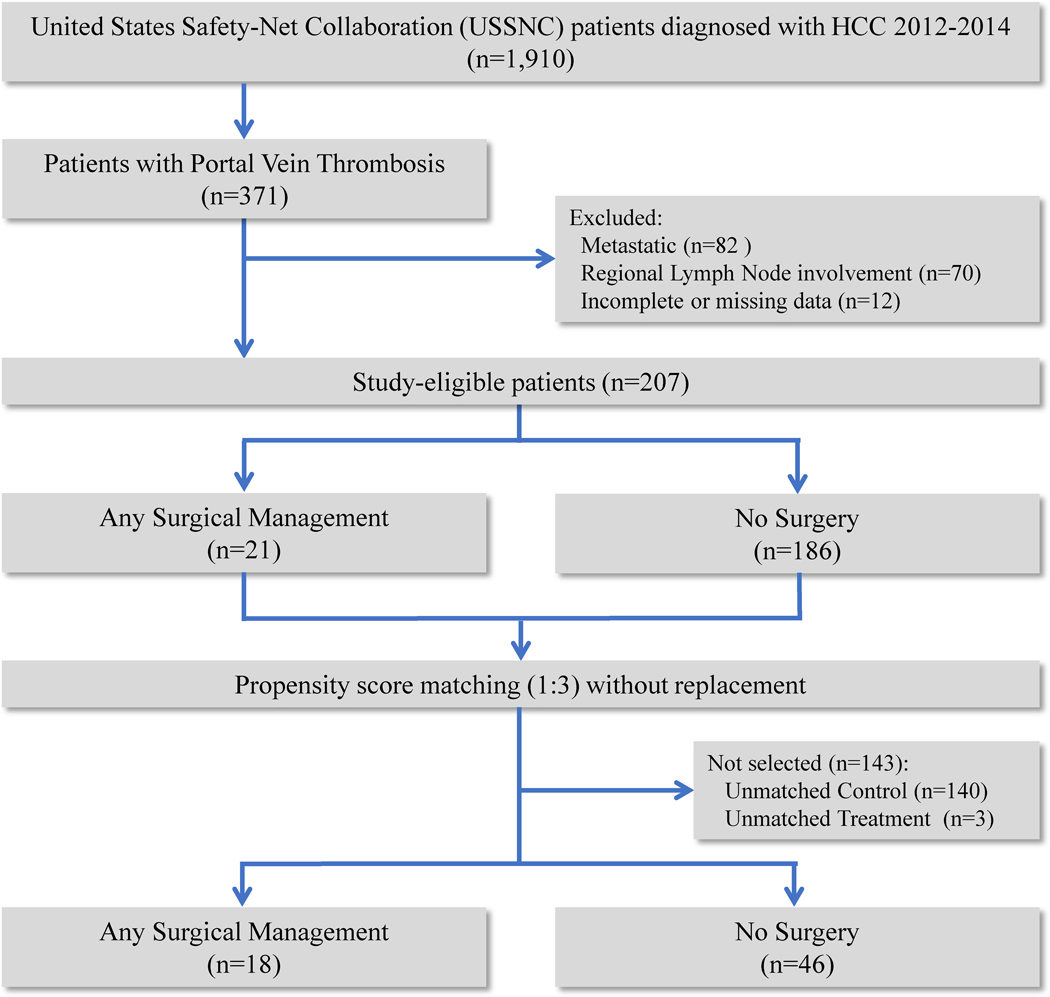
Patients over 18 years of age with hepatocellular carcinoma were identified in the USSNC database from 2012 to 2014 (n=1910). Patients with regional lymph node involvement, distant metastases, missing data or incomplete follow-up were excluded from the analysis (Figure 1). Patients with documented radiographically confirmed PVT of any portal venous branch (i.e., Liver Cancer Study Group of Japan portal vein thrombosis types Vp1–418 and AJCC 8th Edition Stage IIIB19) were included in this study.
Figure 1:
Patient selection criteria diagram
Variables
Patents were stratified into a “no surgery” cohort and an “any surgical management” (ASM) cohort. The ASM cohort included patients who underwent liver transplantation, major hepatectomy (i.e., ≥3 contiguous Couinaud segments) and minor or non-anatomic hepatectomy. We included demographic (i.e., age, gender, race, ethnicity, median income, insurance status) and clinical (i.e., body mass index [BMI], Charlson Comorbidity Index [CCI], functional status, hepatology visit within 1 year of diagnosis, treatment at an academic center, presentation at tumor board, previous hepatitis or cirrhosis, Model of End Stage Liver Disease [MELD] Score, Child-Pugh Score, radiographic tumor size, tumor number (i.e., dichotomized at solitary or multiple) and alpha fetoprotein level [AFP]) variables for analysis. Treatment variables included liver-directed therapies (LDT)—e.g., radio frequency ablation (RFA), transarterial chemoembolization (TACE), and Yttrium-90 radioembolization (Y90)—as well as systemic therapies (e.g., tyrosine kinase inhibitors sorafenib, lenvatinib, or regorafenib). The primary endpoint was overall survival, which was defined as time of diagnosis to date of death or last follow-up. Secondary endpoints included predictors of ASM as well as clinical outcomes such as postoperative complications, reoperation, 90-day readmission and 30-day mortality.
Statistical Analysis
Descriptive statistics were computed for demographic factors, clinical characteristics and treatment modalities. Frequencies (percentage) were reported for categorical data and mean (standard deviation) or median (interquartile range) for continuous data. Differences in percentages were compared across groups using chi-squared (X2) test for categorical variables and means of continuous variables were compared using Student’s t-test for parametric data or the Mann-Whitney U test for non-parametric data. Binomial logistic regression was performed to identify independent predictors of undergoing ASM and odds ratios were calculated.
Propensity score matching was performed in order to reduce selection bias among the treatment cohorts in this study. “Control” (i.e., no surgery) and “case” (i.e., ASM) sets were matched on a set of accrued variables that would otherwise confound comparisons between them. Once a matched sample was formed, the treatment effect could be estimated by directly comparing outcomes (e.g., overall survival) between control and case subjects in the matched sample.
Factors identified as predictors of undergoing ASM on multivariate regression, as well as factors known to affect overall survival were used to match the cohorts. The nearest neighbor matching algorithm was used with caliper width set at 0.2.20 The final propensity score model was based on a 1:3 case-to-control ratio, and patients were ultimately matched by age, comorbidities, tumor size, MELD score, receipt of liver directed therapy (LDT) and receipt of chemotherapy.
Kaplan-Meier survival analyses using the Klein-Moeschberger methodology21 were performed in the propensity-matched cohorts, and the log-rank test compared differences in overall survival between treatment groups. Statistical significance was determined at an alpha level of <0.05. Statistical analyses were carried out using statistical software SPSS v25, (SPSS Inc. Chicago, 2017) and Rv3.6.1, (The R Foundation, 2016).
RESULTS
Descriptive statistics
Of 1910 patients reviewed, 207 non-metastatic patients with PVT were identified (Figure 1). The median age was 59.8 [IQR: 55.6, 64.5] with 38 (18.4%) female, 80 (38.6%) black, and 33 (15.9%) Hispanic. Over a third of patients received liver-directed therapies—five patients (2.4%) underwent RFA, 42 (20.3%) underwent TACE and 33 (15.9%) underwent Y90 radioembolization. Seventy-five patients (36.2%) received targeted systemic therapy (i.e., either sorafenib or regorafenib) (Table 1).
Table 1.
Sociodemographics, clinical and treatment characteristics of hepatocellular carcinoma patients with portal vein thrombosis undergoing any surgical management versus no surgery
| No Surgery n = 186 N (%) | Any Surgical Management n = 21 N (%) | Uni-variate p-value | Multi-variate OR | Multi-variate p-value | ||
|---|---|---|---|---|---|---|
| Age, years (mean ± SD) | 61.2 (±8.46) | 55.2 (±9.38) | 0.002 | 0.75 | 0.008 | |
| Gender | Female | 35 (18.8) | 3 (14.3) | 0.611 | Ref | 0.525 |
| Male | 151 (81.2) | 18 (85.7) | 0.37 | |||
| Race* | White | 68 (42.5) | 11 (55.0) | 0.143 | Ref | 0.290 |
| Black | 75 (46.9) | 5 (25.0) | 4.75 | 0.261 | ||
| Asian | 17 (10.6) | 4 (20.0) | 10.3 | 0.623 | ||
| Hispanic Ethnicity | 30 (16.1) | 3 (14.3) | 1.00 | 1.27 | 0.915 | |
| Income (median, IQR) | 46,300 (36,900, 62,300) | 47,000 (42,600, 66,000) | 0.832 | 1.00 | 0.535 | |
| Insurance* | Private | 45 (26.3) | 7 (35.0) | 0.610 | Ref | 0.618 |
| Medicare/Medicaid | 90 (52.6) | 11 (55.0) | 0.39 | 0.523 | ||
| Hospital Card | 6 (3.5) | 0 (0) | -- | -- | ||
| Uninsured | 30 (17.5) | 2 (10.0) | -- | -- | ||
| BMI (mean ± SD) | 26.2 (±7.46) | 26.0 (±4.16) | 0.870 | 1.13 | 0.277 | |
| Charlson Comorbidity Index | 0 | 32 (26.4) | 7 (35.0) | 0.410 | Ref | 0.801 |
| 1 | 42 (34.7) | 4 (19.0) | 0.38 | 0.498 | ||
| 2 | 25 (20.7) | 6 (28.6) | 0.41 | 0.658 | ||
| ≥ 3 | 22 (18.2) | 3 (14.3) | 6.1 | 0.329 | ||
| Functional Status* | Independent | 137 (78.7) | 21 (100.0) | 0.064 | -- | -- |
| Partially Dependent | 32 (18.4) | 0 (0) | -- | -- | ||
| Totally Dependent | 5 (2.9) | 0 (0) | -- | -- | ||
| Hepatology visit within 1 year of Dx | 28 (15.1) | 11 (52.4) | <0.001 | 27.6 | 0.029 | |
| Treated at academic center | 96 (51.6) | 10 (47.6) | 0.729 | 0.83 | 0.897 | |
| Presented at tumor board | 85 (46.4) | 13 (61.9) | 0.159 | 8.86 | 0.083 | |
| Hepatitis | 143 (83.1) | 17 (81.0) | 0.802 | 1.95 | 0.750 | |
| Cirrhosis | 150 (87.2) | 17 (81.0) | 0.428 | 0.01 | 0.095 | |
| Radiologic Tumor Size, cm (median, IQR) | 7.9 (4.8,11.8) | 4.5 (3.0,10.0) | 0.153 | 0.80 | 0.315 | |
| Solitary tumor (vs. > 1 tumor) | 71 (38.2) | 12 (57.1) | 0.093 | 5.60 | 0.245 | |
| AFP, ng/dL (median, IQR) | 1100 (62, 7900) | 30.3 (9.0, 307) | 0.027 | 1.00 | 0.930 | |
| MELD Score (mean ± SD) | 13.5 (±6.70) | 10.3 (±3.35) | 0.030 | 0.55 | 0.031 | |
| Child-Pugh Class | A | 67 (36.4) | 12 (57.1) | 0.171 | Ref | 0.377 |
| B | 85 (46.2) | 6 (28.6) | 0.45 | 0.602 | ||
| C | 32 (17.4) | 3 (14.3) | 9.41 | 0.389 | ||
| Any Liver-Directed Therapy | 59 (31.7) | 11 (52.4)§ | 0.058 | 0.22 | 0.405 | |
| RFA* | 2 (1.1) | 3 (14.3) | <0.001 | -- | -- | |
| TACE | 35 (18.8) | 7 (38.1) | 0.039 | 5.03 | 0.192 | |
| Y90* | 31 (17.0) | 2 (10.0) | 0.397 | -- | -- | |
| Targeted Systemic Therapy° | 68 (36.6) | 7 (33.3) | 0.771 | 0.11 | 0.113 | |
OR=Odds Ratio, SD=Standard deviation, IQR=Interquartile Range, BMI=Body Mass Index, Dx=Diagnosis, AFP=Alpha fetoprotein, MELD=Model of End Organ Dysfunction, RFA=Radiofrequency ablation, TACE=transarterial chemoembolization, Y90=Yttrium-90;
Covariates with fewer than 3 patients not included in multivariate analysis;
Sorafenib or Regorafenib;
All liver-directed therapy in the ASM cohort was utilized prior to surgery (i.e., neoadjuvant setting)
Twenty-one patients (10.1%) were submitted for ASM; of these, 10 (47.6%) underwent liver transplantation, 7 (33.3%) underwent major hepatectomy, and 4 (19.0%) underwent minor or non-anatomic hepatectomy. In the perioperative period, 7 (33.3%) experienced any complication with 4 (19.0%) requiring additional procedures, 6 (28.6%) were readmitted within 90 days, and there were no 30-day mortalities. Of patients undergoing hepatectomy, 8 of 11 (88.9%) were R0 resections (Table 2).
Table 2.
Characteristics and outcomes of patients undergoing any surgical management
| Any Surgical Management n=21 N (%) | Resection n=11 N (%) | Transplant n=10 N (%) | P value | ||
|---|---|---|---|---|---|
| Surgical Approach | Open | 20 (95.2) | 10 (90.9) | 10 (100.0) | 0.279 |
| Laparoscopic | 1 (5.3) | 1 (11.1) | 0 (0) | ||
| EBL, Liters (median, IQR) | 0.55 (0.4, 2.1) | 0.40 (0.3, 0.7) | 1.0 (0.45, 3.0) | 1.00 | |
| Margin Status* | R0 | 18 (94.7) | 8 (88.9) | 10 (100.0) | 0.279 |
| R1 | 1 (5.3) | 1 (11.1) | 0 (0) | ||
| PVT on Final Pathology | 8 (38.1) | 5 (45.4) | 3 (30.0) | 0.260 | |
| Any Complication | 7 (33.3) | 2 (12.1) | 5 (50.0) | 0.210 | |
| Additional Operation | 4 (19.0) | 0 (0) | 4 (40.0) | 0.023 | |
| LOS, Days (median, IQR) | 7 (6,13) | 7 (6, 8) | 10 (6,13) | 0.370 | |
| Discharged Home | 18 (85.7) | 8 (72.7) | 10 (100.0) | 0.279 | |
| 90-Day Readmission | 6 (28.6) | 1 (0.1) | 5 (50.0) | 0.069 | |
| 30-Day Mortality | 0 (0) | 0 (0) | 0 (0) | 1.00 | |
| Recurrence | 11 (52.4) | 9 (81.8) | 2 (20.0) | 0.005 | |
EBL=Estimated Blood Loss, PVT=Portal Vein Thrombosis, LOS=Length of Stay
Patients with missing data not included in comparison
Predictors of Treatment Receipt
On univariate analysis, patients who underwent ASM were younger (55 vs. 61 years old), less often black (25% vs. 47%), more often had independent functional status (100% vs. 79%) and more often had a hepatology visit prior to diagnosis (52% vs. 15%). Moreover, patients submitted for ASM had lower MELD scores (10.3±3.4 vs.13.5±6.7), smaller tumors (median 7.9 [4.8,11.8] vs. 4.5 [3.0,10.0] cm) and lower initial AFP levels (median 30 [9,307] vs. 1100 [62, 7900] ng/mL). More ASM patients received any form of liver-directed therapy (52% vs. 32%), but not targeted systemic therapy (33% vs. 37%, p=0.77). Notably, there was no statistical differences between cohorts when comparing gender, ethnicity, median income, type of insurance, BMI, or comorbidities. Furthermore, patients treated at the academic university hospital were not more likely to undergo ASM compared with those treated at affiliated safety net hospitals (Table 1).
On multivariate analysis, after accounting for sociodemographic factors (i.e., age, gender, race, income, insurance status and previous hepatology visit), clinicopathologic factors (i.e., presence of cirrhosis, Child-Pugh Class, MELD Score, tumor size, tumor number, and AFP level), and previous therapy (i.e., receipt of any LDT or systemic therapies), patients who were older (OR 0.75, 95% CI 0.62–0.93, p=0.008) and had higher MELD scores (OR 0.55, 95% CI 0.32–0.95, p=0.031) were less likely to be selected for ASM. Conversely, patients who had hepatology consultation within one year of diagnosis (OR 27.6, 95% CI 1.94–342, p=0.029) were more likely to be submitted for ASM (Table 1).
Propensity score matching
To better control for the confounding inherent in the selection and/or completion of surgical management in this cohort of HCC patients with PVT, patients were matched 3:1 based upon the likelihood of either undergoing surgical management or factors associated with survival in the unmatched cohort (Tables 1 and 3). The propensity score-matched cohort comprised 64 patients – 18 (28.1%) ASM and 46 (71.9%) no surgery; 3 patients in the ASM cohort could not be successfully matched (Supplementary Figure S1). Table 4 lists the covariate imbalance between cohorts before and after matching. Previously observed covariate imbalances between ASM and no surgery cohorts with respect to age, comorbidities, MELD score, tumor size, receipt of liver targeted therapies and chemotherapy were alleviated following matching (Supplementary Table S1).
Table 3.
Cox proportional hazard model predicting mortality among unmatched and propensity score-matched cohorts
| Unmatched Cohort (n=207) | Propensity Score-Matched Cohort (n=64) | ||||
|---|---|---|---|---|---|
| HR | p-value | HR | p-value | ||
| Older age | 0.98 [0.95, 1.01] | 0.126 | 1.05 [0.93, 1.17] | 0.454 | |
| Male Gender | 1.46 [0.83, 2.57] | 0.194 | 0.52 [0.07, 3.77] | 0.521 | |
| Race | White | Ref | 0.022 | Ref | 0.052 |
| Black | 1.54 [0.97, 2.45] | 0.065 | 0.36 [0.08, 1.56] | 0.171 | |
| Asian | 2.87 [1.28, 6.42] | 0.010 | 6.84 [0.72, 64.8] | 0.094 | |
| Hispanic | 0.82 [0.34, 1.97] | 0.658 | 0.12 [0.014, 1.06] | 0.057 | |
| BMI | 1.03 [0.99, 1.08] | 0.990 | 1.02 [0.91, 1.14] | 0.770 | |
|
Charlson
Comorbidity Index |
0 | Ref | 0.551 | Ref | 0.540 |
| 1 | 1.20 [0.61, 2.36] | 0.650 | 0.63 [0.14, 2.81] | 0.558 | |
| 2 | 1.16 [0.55, 2.41] | 0.701 | 2.31 [0.52, 10.3] | 0.277 | |
| ≥3 | 0.78 [0.33, 1.86] | 0.585 | 0.39 [0.038, 4.06] | 0.431 | |
| Hepatology visit within 1 year of Dx | 1.38 [0.76, 2.48] | 0.297 | 1.86 [0.42, 8.22] | 0.427 | |
| Treated at academic center | 1.02 [0.64, 1.63] | 0.937 | 2.95 [0.49, 17.8] | 0.244 | |
| Presentation at tumor board | 0.69 [0.45, 1.06] | 0.093 | 0.15 [0.04, 0.67] | 0.013 | |
| MELD Score | 1.11 [1.07, 1.16] | <0.001 | 1.18 [0.94, 1.49] | 0.166 | |
| AFP | 1.0 [ 1.0, 1.0] | 0.862 | 1.0 [ 1.0, 1.0] | 0.354 | |
| Radiologic Tumor Size | 1.02 [1.00, 1.03] | 0.058 | 0.97 [0.84, 1.11] | 0.660 | |
| Any Liver Directed Therapy | 0.43 [0.25, 0.73] | 0.002 | 0.10 [0.01, 0.85] | 0.035 | |
| Targeted Systemic Therapy | 0.61 [0.37, 0.99] | 0.048 | 1.02 [0.27, 3.88] | 0.985 | |
| Any Surgical Management | 0.05 [0.02, 0.18] | <0.001 | 0.01 [0.00, 0.09] | <0.001 | |
HR=Hazard Ratio, BMI=Body Mass Index, Dx=Diagnosis, MELD=Model of End Organ Dysfunction, AFP=Alpha fetoprotein
Table 4.
Covariate balance in unmatched and propensity score-matched cohorts
| Unmatched Cohort (n=207) |
Propensity Score-Matched Cohort (n=64) | |||||
|---|---|---|---|---|---|---|
| No Surgery | ASM | p-value | No Surgery | ASM | p-value | |
| Age, years | 61.2±8.5 | 55.2±9.4 | 0.002 | 59.1±7.0 | 57.8±5.8 | 0.520 |
| CCI | 1.5±2.1 | 1.2±1.2 | 0.571 | 0.96±1.5 | 1.1±1.2 | 0.283 |
| MELD Score | 13.5±6.7 | 10.3±3.4 | 0.030 | 11.4±4.9 | 10.7±3.4 | 0.621 |
| Tumor Size, cm | 9.6±10.7 | 6.2 ±4.4 | 0.153 | 7.7±5.3 | 6.2±4.7 | 0.335 |
| Any Liver Directed Therapy | 33.3% | 52.4% | 0.083 | 58.7% | 61.1% | 0.862 |
| Receipt of Chemotherapy | 36.6% | 33.3% | 0.770 | 37.0% | 38.9% | 0.890 |
CCI= Charlson Comorbidity Index, MELD=Model of End Organ Dysfunction
Survival analysis
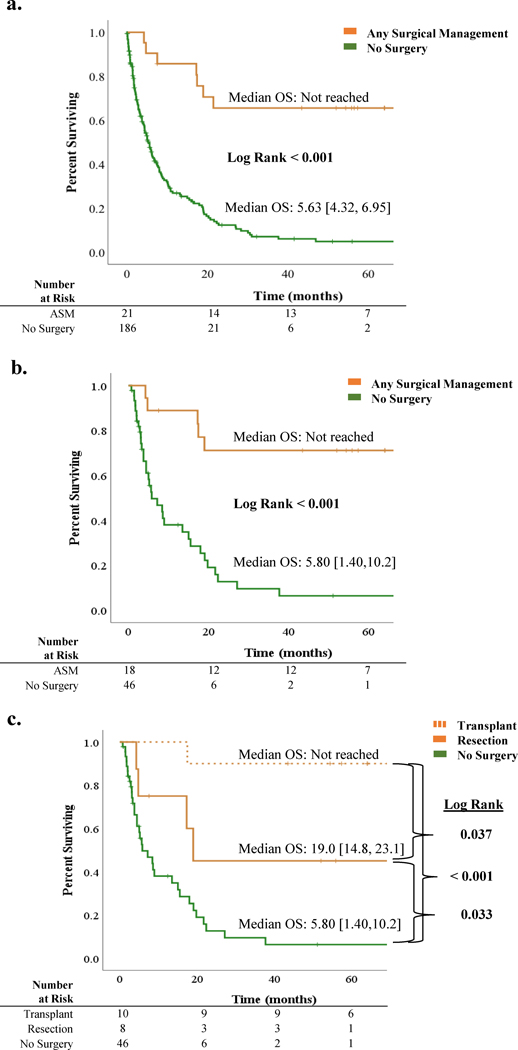
With a median follow up of 52 months [95% CI 37.7–66.3], median overall survival in the unmatched cohort was 6.5 months [95% CI 4.8, 8.2] compared with 15.5 months [95% CI 5.7, 25.3] in the matched cohort. Kaplan-Meier survival estimates (using Klein-Moschberger methodology) comparing the ASM and no surgery groups in the unmatched and propensity score-matched cohorts are illustrated in Figures 2A and 2B, respectively. Median OS of patients who underwent ASM was significantly improved compared with patients not selected for surgery (median not reached vs. 5.8 months, p<0.001). When stratified by type of surgical management (i.e., liver transplantation vs. resectional hepatectomy), median OS was improved for patients undergoing liver transplantation compared with resection (not reached vs. 19.0 months, p=0.037), and for patients undergoing resection versus no surgery (19.0 vs 5.80, p=0.033) (Figure 2C). On multivariate survival modeling, selection for ASM remained a significant predictor of improved survival in the propensity score-matched cohort (HR 0.01, 95% CI 0.00, 0.10; p<0.001; Table 3).
Figure 2:
Overall survival (OS) in hepatocellular patients with portal vein thrombosis: A. Unmatched cohort: OS in patients undergoing any surgical management (ASM) versus no surgery; B. Propensity score-matched cohort: OS in patients undergoing ASM versus no surgery; C. Propensity score-matched cohort: OS in patients undergoing transplant versus resection versus no surgery.
DISCUSSION
This is the first study, to our knowledge, to describe factors influencing selection of surgical management (i.e., hepatectomy or liver transplantation) in a contemporary multi-institutional North American cohort of HCC patients presenting with portal vein thrombosis. In this multi-institutional cohort comprising five tertiary referral academic medical centers affiliated with safety net hospital networks, a significant minority of HCC patients with PVT (10%) were selected for surgical management. Not surprisingly, factors associated with receipt of surgical management in this heavily pretreated population—such as younger age, prior hepatology consultation, and lower MELD scores—suggested careful surgical selection. In such patients, resection and/or transplantation was independently associated with improved survival after controlling for confounding via propensity score matching, suggesting that surgical strategies may have a role in the multimodality management of North American HCC patients presenting with PVT.
There is a substantial body of evidence from Asia—where viral hepatitis is endemic and the dominant contributor to HCC pathogenesis22—suggesting that surgical management may play a role in the multimodality management of select patients presenting with PVT. A retrospective review in over 6,000 Japanese Child-Pugh A patients with PVT (without main trunk or contralateral vein involvement) revealed an almost 3-fold higher median survival in patients undergoing resection compared with those not selected for resection.15 On similar lines, in a cohort of 172 Korean patients with segmental or main branch PVT, survival was nearly 3-fold higher with resection compared with transarterial chemoembolization (TACE) or sorafenib only.16 Furthermore, a recent meta-analysis pooling 7 retrospective studies from high-volume centers in Asia revealed an association with improved survival in 4,810 HCC patients with PVT selected for ASM.17 However, there are no clear data supporting this paradigm in North American patients, where the epidemiology is starkly different.23 A bi-institutional retrospective review by Pawlik and colleagues in 2005 reported sobering outcomes following surgical resection in HCC patients with major vascular invasion—median and 5-year survival was 11 months and 10%, respectively.24 The present study, therefore, represents not only the first multi-institutional North American series exploring this paradigm, but also provides a contemporary reappraisal of this controversial treatment approach in this patient population.
The comparative effectiveness of ASM with non-surgical therapies in this locally advanced HCC cohort, however, must be interpreted with a clear understanding of the careful selection inherent in submission for surgical management—either liver transplantation or resection—in the ASM cohort. Factors involved in this selection ranged from younger age, better medical fitness, less advanced liver disease, favorable socioeconomic status, and improved access to care. Other biologic and anatomic factors, not captured in this analysis, were likely contributory as well. It is impossible, for instance, to determine if radiographic PVT in the ASM cohort was indicative of tumor or bland thrombus. Moreover, it is plausible that patients selected for ASM were enriched for Vp1/Vp2 (or short-segment Vp3) PVT compared with patients not selected for surgery; these data cannot clarify these details. Conversely, it is unclear if the selection of ASM was intended to alleviate the deleterious consequences of portal hypertension from Vp3/Vp4 PVT in ASM patients. Notwithstanding, this study potentially outlines a paradigm whereby surgically fit HCC patients with PVT who initially receive liver-directed and/or systemic therapies may be considered for ASM if anatomic and biologic considerations allow.
Limitations of this retrospective study warrant discussion. First, despite a rigorous propensity score matching algorithm, our study cannot control for confounding from unaccrued covariates that account for the undoubted selection of patients with more favorable tumor biology for ASM. This is exemplified in the improved overall survival of patients undergoing hepatectomy despite near-universal recurrence of disease. Moreover, this study lacked data on treatments offered for salvage of recurrent disease, which undoubtedly contribute to survival. Second, only 8 of 21 patients undergoing ASM had macrovascular tumor invasion noted on final pathology. From these data, it is unclear whether is related to lack of tumor thrombus initially (i.e., bland thrombus manifesting as radiographic PVT) or tumor downstaging as a result of liver-directed or systemic treatments. There was no difference in survival, however, between patients who had or did not have PVT on final pathology (5-year survival 71% vs. 63%, respectively, p=0.721; Supplementary Figure S2). Finally, the outcomes following ASM in selected patients from the high-volume tertiary referral institutions included herein may not be generalizable to other practice settings nationally. As such, these findings warrant validation in larger national cancer registries. Notwithstanding, these data mirror findings from high-volume Asian centers expanding indications for ASM in locally advanced HCC patients, and provide a compelling rationale for a prospective randomized trial investigating the role of surgical management in HCC patients presenting with PVT. Indeed, the relatively small number of patients selected for resection or transplant in this contemporary patient sample suggests that surgical management may be underutilized in Western populations.
Supplementary Material
Synopsis.
In a multi-institutional North American cohort of hepatocellular cancer (HCC) patients with portal vein thrombosis, selection for resection or transplantation involved biologic and physiologic factors. When controlling for such factors, surgical management was associated with improved survival in this cohort.
ACKNOWLEDGEMENTS
The authors acknowledge support from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) Award—P30 CA240139 (to J. Datta and N.B. Merchant); KL2 career development grant by the Miami Clinical and Translational Science Institute (CTSI) under NIH Award—UL1TR002736 (to J. Datta); American College of Surgeons Franklin H. Martin Fellowship Award and Stanley Glaser Foundation Award (to J. Datta); NCI/NIH Award—2R01CA161976-06 (to N.B. Merchant); E. Ryon is supported by NCI/NIH Award—T32CA211034 (to N.B. Merchant)
Sources of Financial Support:
National Cancer Institute (NCI) of the National Institutes of Health (NIH) Award—P30 CA240139 (to J. Datta and N.B. Merchant); KL2 career development grant by the Miami Clinical and Translational Science Institute (CTSI) under NIH Award—UL1TR002736 (to J. Datta); American College of Surgeons Franklin H. Martin Fellowship Award and Stanley Glaser Foundation Award (to J. Datta); NCI/NIH Award—2R01CA161976-06 (to N.B. Merchant); E. Ryon was supported by NCI/NIH Award T32CA211034 (to N.B. Merchant).
Footnotes
Data Availability Statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015. March 1;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 2.Kulik L, El-Serag HB. Epidemiology and Management of Hepatocellular Carcinoma. Gastroenterology. 2019. January;156(2):477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotman Y B, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015. January;61(1):191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathan H, Hyder O, Mayo SC, et al. Surgical therapy for early hepatocellular carcinoma in the modern era: a 10-year SEER-medicare analysis. Ann Surg. 2013;258(6):1022–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah C, Mramba LK, Bishnoi R, et al. Survival differences among patients with hepatocellular carcinoma based on the stage of disease and therapy received: pre and post sorafenib era. J Gastrointest Oncol. 2017;8(5):789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2016, National Cancer Institute. Bethesda, MD; 2018. [Google Scholar]
- 7.Chan SL, Chong CC, Chan AW, et al. Management of hepatocellular carcinoma with portal vein tumor thrombosis: Review and update at 2016. World J Gastroenterol. 2016. August 28;22(32):7289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly GC, Chen R, Hyrien O, Mantry P, et al. Incidence, risk factors and consequences of portal vein and systemic thromboses in hepatocellular carcinoma. Thromb Res. 2008;122(3):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang JF, Lao YC, Yuan BH, et al. Treatment of hepatocellular carcinoma with portal vein tumor thrombus: advances and challenges. Oncotarget. 2017. May 16;8(20):33911–33921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minagawa M, Makuuchi M. Treatment of hepatocellular carcinoma accompanied by portal vein tumor thrombus. World J Gastroenterol. 2006;12(47):7561–7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999. January;29(1):62–7. [DOI] [PubMed] [Google Scholar]
- 12.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329–38. [DOI] [PubMed] [Google Scholar]
- 13.Yau T, Tang VY, Yao TJ, et al. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014. June;146(7):1691–700.e3. [DOI] [PubMed] [Google Scholar]
- 14.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- 15.Kokudo T, Hasegawa K, Matsuyama Y, et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J Hepatol 2016; 65: 938–943. [DOI] [PubMed] [Google Scholar]
- 16.Lee JM, Jang BK, Lee YJ, et al. Survival outcomes of hepatic resection compared with transarterial chemoembolization or sorafenib for hepatocellular carcinoma with portal vein tumor thrombosis. Clin Mol Hepatol. 2016. March;22(1):160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang L, Chen TH, Li C, et al. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB. 2018. December;20(12):1119–1129. [DOI] [PubMed] [Google Scholar]
- 18.Ikai I, Kudo M, Arii S, et al. Report of the 18th follow-up survey of primary liver cancer in Japan. Hepatol Res. 2010;40:1043–1059. [DOI] [PubMed] [Google Scholar]
- 19.Kamarajah SK, Frankel TL, Sonnenday C, et al. Critical evaluation of the American Joint Commission on Cancer (AJCC) 8th edition staging system for patients with Hepatocellular Carcinoma (HCC): A Surveillance, Epidemiology, EndResults (SEER) analysis. J Surg Oncol. 2018. March;117(4):644–650. [DOI] [PubMed] [Google Scholar]
- 20.Randolph JJ, Falbe K, Manuel AK, Balloun JL. A Step-by Step Guide to Propensity Score Matching in R. Practical Assessment, Research, and Evaluation. 2014. November: 19(18):1–6. [Google Scholar]
- 21.Klein JP and Moeschberger ML. Survival Analysis: Techniques for censored and truncated data. 2nd ed. Springer-Verlag, New York. 2003. [Google Scholar]
- 22.Perz JF, Armstrong GL, Farrington LA, et al. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006. October;45(4):529–38. [DOI] [PubMed] [Google Scholar]
- 23.Choo SP, Tan WL, Goh BKP, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer. 2016. November 15;122(22):3430–3446. [DOI] [PubMed] [Google Scholar]
- 24.Pawlik TM, Poon RT, Abdalla EK, et al. Hepatectomy for hepatocellular carcinoma with major portal or hepatic vein invasion: results of a multicenter study. Surgery. 2005. April;137(4):403–10. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.