Abstract
One of the genes which has been linked to the onset of juvenile/early onset Parkinson’s disease (PD) is PINK1. There is evidence that supports the therapeutic potential of exercise in the alleviation of PD symptoms. It is possible that exercise may enhance synaptic plasticity, protect against neuro-inflammation and modulate L-Dopa regulated signalling pathways. We explored the effects of exercise on Pink1 deficient Drosophila melanogaster which undergo neurodegeneration and muscle degeneration. We used a ‘power-tower’ type exercise platform to deliver exercise activity to Pink1- and age matched wild-type Drosophila. Mitochondrial proteomic profiles responding to exercise were obtained. Of the 516 proteins identified, 105 proteins had different levels between Pink1- and wild-type non-exercised Drosophila. Gene ontology enrichment analysis and STRING network analysis highlighted proteins and pathways with altered expression within the mitochondrial proteome. Comparison of the Pink1- exercised proteome to wild-type proteomes showed that exercising the Pink1- Drosophila caused their proteomic profile to return towards wild-type levels.
Keywords: PINK1, Drosophila, mitochondria, exercise, proteomics
INTRODUCTION
Parkinson’s disease (PD) is a progressive, irreversible neurodegenerative condition which affects over 6 million people across the globe [1]. The greatest risk factor for PD is advancing age, and with the number of people over the age of 60 expected to exceed 2 billion by the year 2050 (currently estimated to be over 900 million) there will soon be significantly higher numbers of people living with PD [2]. The classical physical symptoms are known to be resting tremor, rigidity and bradykinesia. It is now also known that symptoms of PD include reduced quality of sleep as well as both cognitive impairments and poor mental health. In terms of the pathophysiology of the disease, the death of pigmented dopaminergic neurons in the substantia nigra pars compacta in PD patients is critical [3]. Molecular characteristics of PD include the aggregation of α-synuclein leading to Lewy body formation, alongside mitochondrial dysfunction [4, 5].
Hereditary forms of PD can be either autosomal dominant or autosomal recessive, dependent upon the mutant gene involved. In both dominant and recessive forms of hereditary PD, autophagic and lysosomal pathways are both mechanistically implicated [6–8]. One of the critical pathways which has been linked to the onset of juvenile/early onset PD is the PINK1/Parkin mitophagy pathway, a form of autophagy for the degradation of dysfunctional mitochondria. The role of mitophagy is to provide a quality control mechanism for the mitochondrial population within a cell, and this is a particularly crucial function in energetically demanding neuronal cells [9].
Mechanistically, PINK1 localizes with outer mitochondrial membrane (OMM) of depolarized mitochondria and then recruits and activates the E3 ubiquitin-ligase activity of Parkin via phosphorylation of its Ser65 residue [10, 11]. PINK1 has also been shown to phosphorylate the Ser65 residue of Ubiquitin, which aids in the activation of Parkin’s E3 ligase activity [12, 13]. Ultimately these events lead to the ubiquitination of OMM proteins by Parkin, the autophagic machinery then degrades the ubiquitinated mitochondria [14]. Mutations in PINK1 (and Parkin) can result in autosomal recessive juvenile onset PD, with onset before the age of 40 years old [15]. While this form of PD is rare, a comprehensive understanding of it can improve the outcomes of patients with PINK1 mutations and also those with idiopathic PD due to their shared pathophysiology [16]. A recent publication by Sliter et al. reported that Pink1 and Parkin mitigate STING induced inflammation, where both Pink1-/- and Prkn-/- mice under exhaustive exercise have a strong inflammatory phenotype that is rescued by the concurrent loss of the STING pathway [17]. In the same year, Zhong et al. reported that newly synthesised oxidised mitochondrial DNA is exported to the cytosol and stimulates another of the innate immune responses, the NLRP3 inflammasome [18].
Given the overlapping biology between PINK1 loss of function in PD and other forms of genetic and sporadic PD, PINK1 null mutants of animal models are hugely useful in studies of PD. Pink1 loss of function Drosophila models were first developed in 2006, shortly after the first parkin loss of function mutant Drosophila models were being utilised in research [19–21]. These first studies found that Pink1 loss of function resulted in mitochondrial dysfunction, compromised fertility in males, indirect flight muscle degeneration and associated locomotor defects, increased sensitivity to oxidative stress, and dopaminergic degeneration (Park et al., 2006) [19]. Parkin overexpression rescued many of the defects observed in the Pink1 mutants, indicating the downstream function of Parkin in the now established PINK1/Parkin mitophagy pathway, reviewed here [22]. More recent studies using Pink1 mutant Drosophila have found that their neurons exhibit decreased levels of synaptic transmission, defective fission and reduced ATP levels due to decreased COXI and COXIV activity as well as non-motor symptoms such as learning and memory deficits, weakened circadian rhythms and electrophysiological changes in clock-neurons [23–25].
It was first observed in 1992 that participation in exercise reduced the risk of the onset of PD in later years, while later data showed that this protection against PD risk is more obvious in males [26, 27]. Many groups have presented data that show the therapeutic potential of exercise in the alleviation of patient symptoms [28–30]. The biochemical mechanisms that would explain these observations are still unclear, but current evidence suggests that exercise may enhance synaptic plasticity, protect against neuroinflammation and modulate L-Dopa regulated signalling pathways [31–33].
In this study we aimed to analyse the biochemical changes induced by exercise in the mitochondrial proteome of the Pink1 loss of function mutant (Pink1-) Drosophila. As exercise is reported to both reduce the risk of onset and improve outcomes for Parkinson’s disease patients, we sought to characterise the biochemical changes that could underpin this improvement in our model of Parkinson’s disease. We focused on the mitochondria as their dysfunction is widely associated with Parkinson’s disease, and the Pink1- genetic model has a disrupted mitophagy pathway due to the absence of a functional PINK1 protein.
MATERIALS AND METHODS
Drosophila stocks
Fly stocks were kindly provided to NM by Miguel Martins (MRC Toxicology Unit) and Alex Whitworth (MRC Mitochondrial Biology Unit). Fly stocks and crosses were maintained on standard cornmeal agar media at 25° C in a 12:12 light-dark cycle. The experiments were performed on males: wild type (genotype w1118) and Pink1- (genotype Pink1B9/Y).
Drosophila exercise
Approximately twenty wild-type control or Pink1- Drosophila, 1-4 days post-eclosion, were separated into glass vials filled with 5ml food. Exercised group vials were stoppered with cotton wool 6cm from the food; non-exercised group vials were stopped with cotton wool 1cm from the food, creating a physical barrier to activity. Both exercised and non-exercised groups were placed in racks on the ICE machine (Supplementary Material) for 30 minutes per day for 7 days. The Drosophila were exercised in the morning each day and were sacrificed by freezing at -80° C one hour after the final exercise bout. Comparison groups were exercised and non-exercised wild-type and Pink1- Drosophila.
Mitochondrial isolation
Groups of twenty wild-type or Pink1- Drosophila were homogenised in 100-200μl mitochondrial extraction buffer (50mM Tris-Cl pH 7.4, 100mM KCL, 1.5mM MgCl2, 1mM EGTA, 50mM HEPES and 100mM sucrose) by 5 minutes of manual homogenisation using a 1.2-2ml Eppendorf micro-pestle (Sigma-Aldrich). The homogenate was centrifuged at 800g for 10 minutes, at 4° C, to remove the insoluble fraction. Supernatants from the first centrifugation were centrifuged at 1,000g for 10 minutes at 4° C to pellet the nuclear fraction. Supernatants from the second centrifugation were centrifuged at 13,200g for 30 minutes at 4° C to pellet the mitochondrial fraction. The protein content was determined by Bradford assay (μg/μl) and mitochondrial fractions were stored at -80° C.
2D-gel electrophoresis
50μg of the mitochondrial fraction were added to rehydration solution (8M urea, 2% CHAPS, 2% IPG Buffer, 0.1% bromophenol blue). 20mM DTT was added to an aliquot of rehydration solution directly before use. The standard protocol according to manufacturer instructions was followed [34]. Briefly, sample was applied to rehydrate ZOOM IPG strips for an hour at room temperature followed by iso-electric focusing using the ZOOM IPG (Life Technologies) system and pH 3-10 (non-linear) ZOOM IPG strips. Gels were stained (SimplyBlue™ SafeStain, Life Technologies) and imaged (ImageQuant 300, GE Healthcare Life Sciences). Analyses were performed using SameSpots software (Totallab) (one-way ANOVA). Three pooled biological replicates were included for each of the four groups.
Samples were analysed by the Centre of Excellence in Mass Spectrometry at University of York [35]. Briefly, proteins were reduced and alkylated, followed by digestion in-gel with trypsin. Matrix Assisted Laser Desorption Ionization Tandem Time-of-Flight mass spectrometry (MALDI-TOF/MS) was used to analyse the samples. The generated tandem MS data was compared against the NCBI database using the MASCOT search programme to identify the proteins. De novo sequence interpretation for individual peptides were inferred from peptide matches.
Label-free proteomics
30μg/μl of each mitochondrial fraction was prepared with 4X LDS sample buffer and 4mM DTT. Samples were run in triplicate on a 4-12% Bis-Tris gel in 1X MES SDS running buffer for 40 minutes at 200V (all Invitrogen). Three biological replicates were run for each of the four groups. The whole, individual gel lanes were excised and placed into separate Eppendorf tubes.
Samples were analysed by the Centre of Excellence in Mass Spectrometry at University of York [35]. Briefly, protein was in-gel digested post-reduction and alkylation. The resulting extracted peptides were analysed over 1-hour LC-MS acquisitions with elution from a 50cm, C18 PepMap column onto a Thermo Orbitrap Fusion Tribrid mass spectrometer using a Waters mClass UPLC. Extracted tandem mass spectra were searched against the combined Drosophila melanogaster and Saccharomyces cerevisiae subsets of the UniProt database. Protein identifications were filtered to achieve <1% false discovery rate as assessed against a reverse database. Identifications were further filtered to require a minimum of two unique peptides per protein group.
For relative label-free quantification, extracted ion chromatograms for identified peptides were extracted and integrated for all samples. A maximum mass deviation of 3 ppm and retention time drift of 3 mins were set. Resulting quantifications were further filtered to an arbitrary PEAKS quality factor of 5 for feature mapping and required a minimum of two aligned features from a minimum of two unique peptides per protein quantification. Protein abundances were normalised between samples based on total identified peptide ion area.
Gene ontology enrichment analysis
gProfiler was used to undertake Gene Ontology (GO) enrichment analysis for the label-free mass spectrometry identified proteins with significant expression differences between each of the four experimental groups [36]. KEGG, Molecular Function (MF), Biological Process (BP) and Cellular Compartment (CC) enrichment analyses are generated by gProfiler are presented.
Protein-protein interaction network analysis
Differences in expression of proteins between groups were further analysed using the STRING database v.11.0 [37]. The platform was used to create protein-protein interaction (PPI) networks based upon the differentially expressed proteins (DEPs) observed between groups.
RESULTS AND DISCUSSION
We subjected male Pink1- and wild-type Drosophila to a seven-day exercise regimen, whilst maintaining groups of unexercised Pink1- and wild-type as controls. The mitochondria of the four groups were then isolated and investigated using 2D gel electrophoresis and label-free mass spectrometry analyses to determine changes in their mitochondrial proteome. The 2D gel electrophoresis method allowed for a fast and simple separation of proteins, which served as a scoping method to identify some of the most significant changes in expression. Label-free mass spectrometry analyses generated proteomes that we used in network enrichment analysis. Previously we have used proteomic profiling to characterise mitochondrial populations in both mice and long-lived pipistrelle bats, and here we apply this to better understand the PD phenotype as well as possible changes due to exercise [38, 39].
2DE-MS identified a general reduction in protein expression post-exercise in the Pink1 mutant Drosophila
We isolated mitochondria from exercised and non-exercised Pink1- Drosophila and performed 2DE-MS on these fractions (Supplementary Figure 1). All proteins that were identified as changed in expression after the exercise intervention were reduced in expression (Table 1). PINK1 is recognised as having a central role in mitophagy, ensuring a healthy pool of mitochondria are maintained [19, 20]. It has recently been recognised that the regulation of mitophagy by the PINK1 system is age-dependent, with the Drosophila dependency on this pathway increasing with age [40]. Further, it has also been reported that earlier in the lifespan of the Drosophila there is a basal level of mitophagy that is not disrupted by the loss of PINK1 [41].
Table 1. Expression changes between exercised Pink1- Drosophila and non-exercised Pink1- Drosophila.
| Pink1- mitochondria - 2DE | |||
| Protein identity | ANOVA (p) | Fold change | Exercise related change |
| Tropomyosin-1, isoforms 33/34 | 0.022 | 1.4 | ↓ |
| Tropomyosin-2 | |||
| Acyl-coenzyme A dehydrogenase | 0.015 | 1.4 | ↓ |
| Isocitrate dehydrogenase | 0.008 | 1.4 | ↓ |
| Enolase | 0.022 | 1.3 | ↓ |
| Probable isocitrate dehydrogenase [NAD] subunit alpha | 0.015 | 1.5 | ↓ |
| Glycerol-3-phosphate dehydrogenase [NAD(+)] | |||
| Pyruvate dehydrogenase E1 component subunit beta | |||
| Pyruvate dehydrogenase E1 component subunit beta | 0.048 | 1.3 | ↓ |
| Aldo-keto reductase, isoform C | |||
| Alcohol dehydrogenase | 0.024 | 1.3 | ↓ |
| CG9992, isoform A |
Changes in expression were determined by 2DE-MS.
It is therefore possible that alternate mitophagy pathways can compensate for the loss of PINK1 and are upregulated during exercise, and this could account for the sweeping reductions in protein levels via 2DE-MS (Table 1). Indeed, alternative proteins have been identified and a pathway described in which they promote PINK1/PARKIN-independent mitophagy: AMBRA1, HUWE1 and IKKα [42]. It is also notable that ATG8, the final mediator of the AMBRA1 PINK1/PARKIN-independent mitophagy pathway, has been reported to be upregulated in response to exercise as a part of a broader upregulated autophagic response to exercise [43]. However, it could also be suggested that the energetic demands of the exercise result in less energy available to protein synthesis pathways. In this instance, exercise would likely affect proteostasis more broadly, beyond just the mitochondrial proteome.
The specific proteins identified within the table include structural proteins such as tropomyosin which is associated with the intracellular transport of mitochondria as well as mitochondrial metabolic proteins from a variety of pathways, such as acyl co-enzyme A dehydrogenase from the β-oxidation pathway, isocitrate dehydrogenase from the TCA cycle, and the metabolic gatekeeper pyruvate dehydrogenase. Due to the varied function of the proteins in Table 1, it is likely that their downregulation is representative of a broader decrease in protein expression levels in Pink1 Drosophila in response to exercise.
We proceeded to pursue the directionally homologous 2DE-MS results by obtaining a global topology of the mitochondrial protein changes that occur to Pink1- Drosophila due to exercise intervention using a label-free proteomics method.
GO annotation of identified label-free proteins and proportion identified that are localised to mitochondria
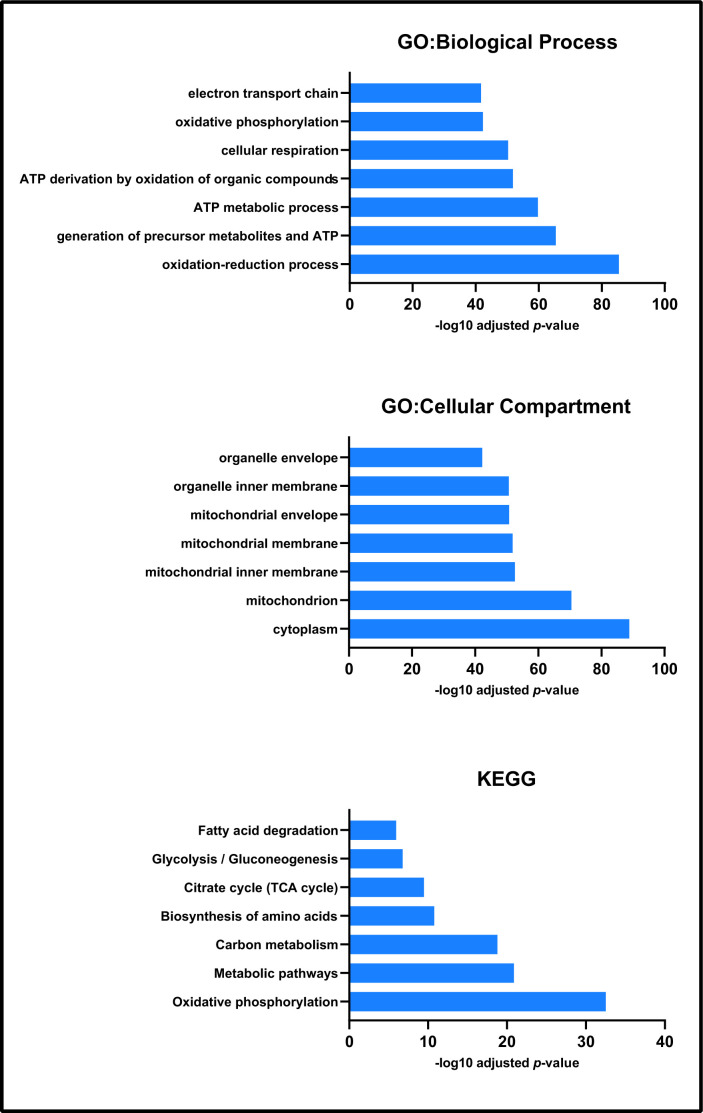
Non-gel-based label-free proteomic analyses identified 516 proteins from the mitochondrial fractions of Pink1- and wild-type Drosophila (Supplementary Table 1). GO and KEGG analyses showed that these fractions were enriched for mitochondrial processes and pathways, confirming the efficacy of our fractionation methodology (Figure 1). The top term of the GO cellular compartment analysis was cytoplasm, followed by mitochondrion and sub-mitochondrial compartments, many mitochondrial proteins are known to also localise to the cytoplasm [44, 45].
Figure 1.
Enrichment analysis of all proteins identified by label-free proteomics. Biological process, cellular compartment and KEGG enrichment analysis each presented processes associated with mitochondrial function and physiology.
Pink1- Drosophila have decreased levels of proteins from energy metabolism pathways compared with wild-type Drosophila
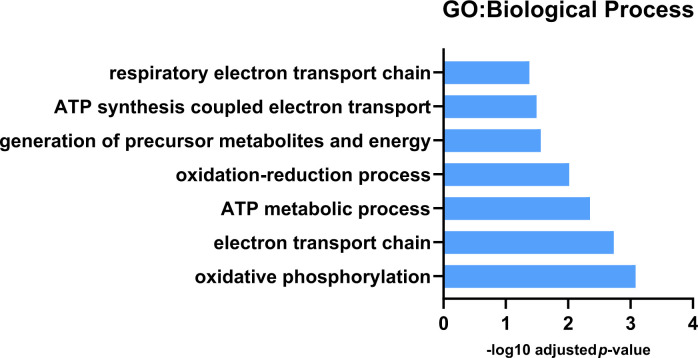
Label-free proteomics highlighted 105 differently expressed proteins between non-exercised wild-type Drosophila and non-exercised Pink1- Drosophila (Supplementary Table 2). Ten of the proteins were shown to be reduced in expression in Pink1- Drosophila compared to wild-type Drosophila. We found that Pink1- Drosophila have reductions in protein expression in proteins from mitochondrial processes associated with energy metabolism, with the top GO biological process terms being oxidative phosphorylation, electron transport chain, ATP metabolic process and oxidation-reduction process (Figure 2).
Figure 2.
GO: Biological process analysis for downregulated protein expression differences between Pink1 non-exercised and wild-type non-exercised files. Pink1 files have reduced expression of proteins involved in mitochondrial respiration and oxidative phosphorylation.
The deficiencies in mitochondrial oxidative phosphorylation, the electron transport chain and specifically in the activity of Complex I in Parkinson’s disease are well established, and this aligns with our findings from the GO analysis of the proteomics data [46–49]. Specific subunits of complexes within the electron transport chain that decreased in expression include NADH dehydrogenase (ubiquinone) 75 kDa subunit isoform B (complex I), GH01077p (complex III in Drosophila), HDC00331 (complex IV in Drosophila) and Levy isoform A (complex IV in Drosophila). While complex I and complex IV have been reported as dysfunctional in PD, reduced expression or decreased activity for complex III isn’t well documented [47, 50, 51]. However, decreased Complex II/III activity has been shown in platelets of untreated Parkinson’s disease patients [52].
It is interesting to note that most (95/105) of the differentially expressed proteins were more highly expressed in Pink1- Drosophila (Supplementary Table 2). GO biological process analysis showed these proteins to be enriched for redox processes, cytoplasmic translation, cellular amide metabolic processes and fatty acid derivative biosynthetic processes. GO cellular compartment analysis showed that the more highly expressed proteins in Pink1- were enriched for cytoplasmic ribosomes, organelle membranes and endoplasmic reticulum. KEGG pathway analysis paralleled these findings, highlighting the identified proteins as involved in fatty acid metabolism, ribosomes and one carbon pool by folate.
There is evidence linking fatty acid metabolism and function to Parkinson’s disease, with proteins identified by GWAS studies, suppressed β-oxidation, and physical interaction between α-synuclein and fatty acids potential being key factors [53–57]. Early studies into the effect of α-synuclein (SNCA) gene deletion on lipid metabolism in mice reported reduced palmate uptake and altered palmate metabolism in the brain, reduced acyl-CoA Synthetase activity that resulted in reduced arachidonic acid uptake and turnover, and increased docosahexaenoic acid brain mass, incorporation and turnover [58–60]. Our own work shows differences in arachidonic acid derivatives in Parkinson’s disease mitochondria [61].
The data presented here show an enrichment of the folate metabolic pathway, not reported previously. It may be that in Pink1- Drosophila this is a compensatory mechanism. KEGG analysis highlighted the metabolism of folate (vitamin B9) as enriched in Pink1- Drosophila. B-vitamins, in particular folate, are well studied in the context of Parkinson’s disease due to the observation of homocysteine (a methionine cycle metabolite) having neurotoxic effects [62–65]. It is hypothesised that the administration of B-vitamins can drive the synthesis of methionine, thus reducing intracellular homocysteine [66–68]. However, the relationship between B -vitamins, neurotoxicity and Parkinson’s disease is complex and a consensus has yet to be established. Some data show either little correlation between homocysteine levels and B vitamins including B6, folate and B12 while others show contradictory results, including elevated homocysteine levels and decreased folate levels in Parkinson’s disease patients [68–71].
Exercise reduces measured protein levels in Pink1- Drosophila
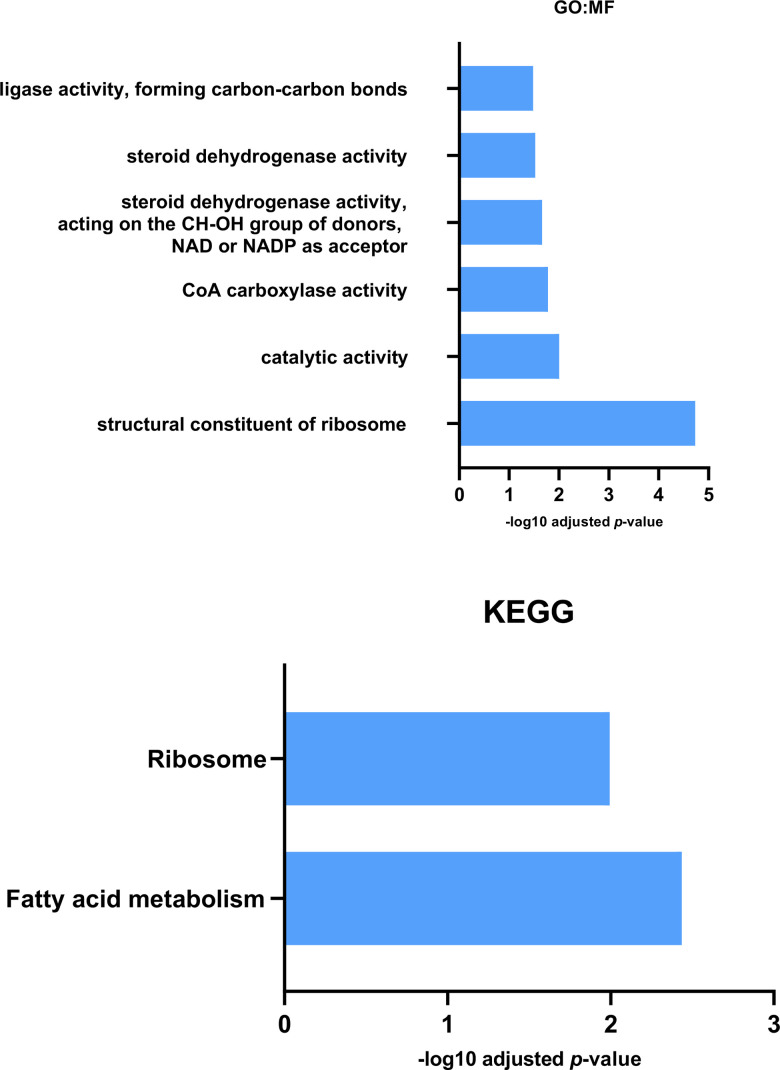
The 2DE-MS analysis of exercised and non-exercised Pink1- Drosophila revealed reductions in twelve proteins in response to exercise (Table 1). Label-free proteomics showed a similar pattern in Pink1- exercised Drosophila compared with non-exercised Pink1- Drosophila; of the fifty-seven protein expression differences, fifty-five were reductions of protein expression in response to exercise (Supplementary Table 3). GO:MF and KEGG pathway analysis determined that the terms ribosomal and fatty acid metabolism were significantly represented in the proteins with reduced expression (Figure 3). Interestingly, these terms were shown to be increased in Pink1- Drosophila compared to wild-type Drosophila that had not been exercised. This suggests that in Pink1- Drosophila exercise returns levels of protein expression towards wild-type values.
Figure 3.
GO: Molecular Function and KEGG analysis for proteins reduced in expression in Pink1- exercised files compared with Pink1- non-exercised files. Both Molecular Function and KEGG enrichment analysis indicated a decrease in expression of fatty acid metabolic proteins in the exercised Pink1- files.
It has been shown that PINK1 interacts with the protein translation pathway and that increased protein translation in Pink1- Drosophila causes an exacerbated Pink1- phenotype. Taking this a step further, it was shown that 40S ribosomal subunit S6 (RpS6) RNAi was able to mitigate the Pink1- phenotype [72]. These data suggest improper protein translation regulation is involved in the pathogenesis of PD and that inhibition of this pathway mitigates progression. Exercise appears to be able to reverse the upregulated protein translation pathways found in Pink1- Drosophila.
KEGG analysis identified fatty acid metabolism from proteins reduced in expression due to exercise, while GO:MF analysis highlighted CoA Carboxylase activity from the same protein data set. This reduction in the metabolism, and in particular the synthesis, of fatty acids can be contrasted with the KEGG analysis described earlier which identified elevated expression of fatty acid metabolism associated proteins in Pink1- Drosophila compared with wild-type Drosophila. It can be interpreted that exercise reverses the change in Pink1- Drosophila and returns the fatty acid metabolic profile back towards wild-type Drosophila.
The two proteins upregulated with exercise in Pink1- Drosophila were OCIA domain-containing protein 1 (OCIAD1) and dihydroorotate dehydrogenase (quinone) mitochondrial (DHODH), neither have previously been connected with exercise. OCIAD1 has been shown to localise to both endosomes and mitochondria and regulate pathways such as JAK/STAT, Notch and PI3K/AKT [73–75]. OCIAD1 has been shown to regulate mitochondrial ETC activity via control of complex I activity, which showed an inverse association with OCIAD1 overexpression [76]. Deregulated OCIAD1 levels have been linked to mitochondrial dysfunction, interaction with BCL-2 and Alzheimer’s disease [75].
DHODH is an inner mitochondrial membrane enzyme that catalyses the fourth step in de novo synthesis of pyrimidines [77]. A link between pyrimidine synthesis and mitochondrial morphology was shown with the addition of the drug leflunomide to muscle cells [78]. The group showed that leflunomide inhibited DHODH by binding to its ubiquinone binding channel, thereby preventing the production of pyrimidine ribonucleotide uridine monophosphate (UMP). DHODH inhibition induced upregulation of mitochondrial fusion and subsequent mitochondrial elongation, by depleting the cellular pyrimidine pool. As ubiquinone is reduced to ubiquinol in the DHODH-mediated catalysis of dihydroorotate to orotate, and as ubiquinol is a substrate of respiratory complex III, DHODH is important for the ETS. DHODH deficiency has been reported to partially inhibit complex III and increase ROS generation [79].
The same Pink1- Drosophila strain has previously been reported to have upregulated genes involved in nucleotide metabolism, which is also the case in brains of PD patients with PINK1 mutations [80]. Genetic and pharmacological upregulation of nucleotide metabolism and scavenging pathways restored mitochondrial function caused by PINK1 loss. Therefore, DHODH upregulation by exercise may act in a compensatory manner to manage metabolic stress due to the Pink1- phenotype.
Exercise reduces the difference in levels of protein expression between Pink1- and wild-type Drosophila
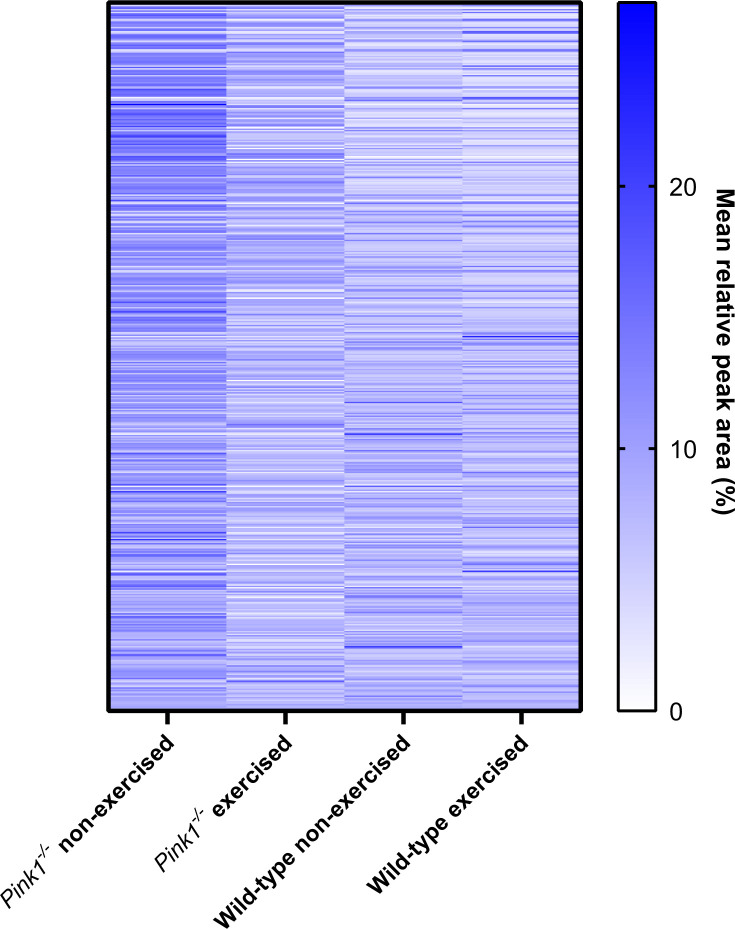
Of the 516 proteins identified, 105 protein had different levels between non-exercised Pink1- Drosophila and non-exercised wild-type Drosophila (Supplementary Table 2). Comparing the exercised Pink1- Drosophila group to either non-exercised wild-type Drosophila, or exercised wild-type Drosophila, showed close to half the number of differentially expressed proteins (55 and 56 proteins, respectively) (Supplementary Tables 4, 5). Qualitatively the heatmap of protein expression for exercised Pink1- Drosophila more closely resembles wild-type Drosophila (Figure 4). This suggests exercise can ameliorate the aberrant protein profile of Pink1- Drosophila towards a more wild-type profile.
Figure 4.
Heat map of protein expression levels (for proteins identified among all groups), determined by label-free mass spectrometry of mitochondrial fractions. Qualitatively, the identified Pink1- exercised fly proteome more closely resembles the two WT fly proteomes than does the Pink1 non-exercised fly proteome.
This is in agreement with findings from Cheedipudi et al. (2020) who reported gene expression data from an exercised mouse model of arrhythmogenic cardiomyopathy, that originally showed a dysregulation of near 800 genes, showed partial restoration of gene expression with regular exercise, with the greatest remedial effects on proteins involved in inflammation and oxidative phosphorylation [81].
Network analyses of differentially expressed proteins
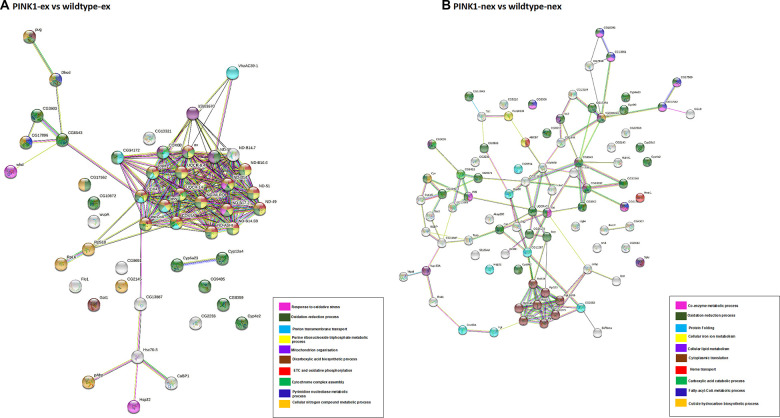
STRING database network analysis complemented the results seen with gProfiler. The protein-protein interaction (PPI) networks were generated using STRING, and Figure 5A shows that exercised Pink1- Drosophila, compared with the exercised wild-type Drosophila, have a differentially expressed proteins (DEPs) network that consisted of 49 nodes and 200 edges with average node degree 8.16 and PPI enrichment p-value of (P < 1.0e-16).
Figure 5.
Network analysis of differentially expressed proteins. (A) Pink1- exercised vs wild type exercised; (B) Pink1- non-exercised vs wild type non-exercised. Networks analysed using STRINGdb. The nodes are coloured according to the processes (legend) that the proteins are involved in by using GO Terms for Biological Processes. The edge shows type of interactions, experimentally determined interactions are pink and those obtained from databases are sky blue. Predicted interactions such as gene neighbourhood are blue, green and red for gene co-occurrence, gene neighbourhood and gene fusions. Co-expression interactions are shown in black, text-mining interactions are shown in light green and protein homology edges are purple.
Most of the proteins in the network have downregulated expression, with the following proteins found to be upregulated: endoplasmic reticulum chaperone BiP, enoyl-CoA hydratase short chain, glutamine synthetase, protein disulfide isomerase, methylmalonate-semialdehyde dehydrogenase, polyadenylate-binding protein, poly(U)-specific endoribonuclease, glutamine synthetase, flotillin-1, heat shock protein 22, phosphatidate cytidylyltransferase, fatty acyl-CoA reductase and dihydroorotate dehydrogenase.
The DEPs were found to be part of the ETC and OXPHOS processes, transmembrane transport and oxidative-reduction processes. The highly connected cluster in this network consisted of proteins from complex I: ND-18, ND-MLRQ, ND-ASHI, ND-B14.5B, ND-B14, ND-B16.6, ND-49, ND-51, ND-B17.2, ND-B14.7; complex IV: COX6B, COX4, mt: COII, COX5A; complex III: OX, and other proteins CYPE, UQCR-6.4, UQCR-14, levy and 40S ribosomal proteins S18. This cluster of proteins was downregulated in the exercised Pink1- Drosophila.
Figure 5B shows the non-exercised Pink1- Drosophila versus the non-exercised wild-type Drosophila PPI network, which consists of 86 nodes and 121 edges with average node degree 2.81 and PPI enrichment p-value (P < 1.0e-16). In contrast to exercised Pink1- Drosophila versus exercised wild-type Drosophila, all DEPs were upregulated, except for cytochrome c oxidase subunit 4, cyclope isoform A, flightin isoform B, cytochrome b-c1 complex subunit 7, NADH dehydrogenase 1 alpha subcomplex 12, Troponin 1, NADH dehydrogenase 18, cytochrome c oxidase subunit, cytochrome P450, NADH dehydrogenase 1 beta subcomplex subunit 8, cytochrome c oxidase subunit 5A, NADH dehydrogenase B14 and levy isoform A.
The DEPs were found to be a part of oxidation-reduction processes, fatty-acyl-CoA metabolic processes, translation and protein folding. The identification of DEPs from fatty-acyl-CoA metabolic process is concurrent with the enrichment analysis presented in Figure 3. The highly connected node in this network is that of upregulated ribosomal proteins RPS18, RPS23, RPS23, RPS7, RPS14b, RPL6, RPL5, RPL10AB and RPL9.
The DEPs were found to be a part of oxidation-reduction processes, fatty-acyl-CoA metabolic processes, translation and protein folding. The identification of DEPs from fatty-acyl-CoA metabolic process concurs with the enrichment analysis presented in Figure 3. The highly connected node in this network is that of upregulated ribosomal proteins RPS18, RPS23, RPS23, RPS7, RPS14b, RPL6, RPL5, RPL10AB and RPL9.
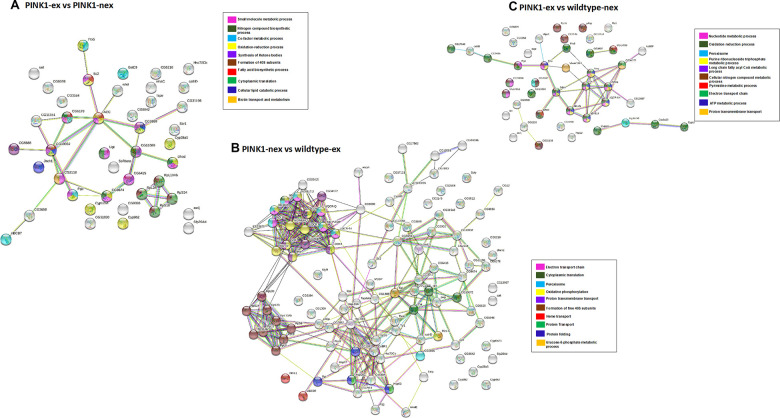
Similarly, for Figure 6A–6C, three additional PPI networks were generated for DEPs in pairwise group comparisons. For exercised Pink1- Drosophila compared with non-exercised Pink1- Drosophila the PPI network consisted of 43 nodes and 29 edges with average node degree 1.35 and PPI enrichment p-value <1.62e-06 (Figure 6A). All of the DEPs were downregulated except for OCIA domain containing protein 1 and dihydroorotate dehydrogenase. The DEPs were found to be involved in formation of 40S ribosomal subunit, oxidation reduction processes, synthesis of ketone bodies, translation and cellular lipid catabolic processes.
Figure 6.
Network analysis of differentially expressed proteins. (A) Pink1- exercised vs Pink1- non-exercised; (B) Pink1- non-exercised vs wild type exercised; (C) Pink1- exercised vs wild type non-exercised. Networks analysed using STRINGdb. The nodes are coloured according to the processes (legend) that the proteins are involved in by using GO Terms for Biological Processes. The edge shows type of interactions, experimentally determined interactions are pink and the one obtained from databases are sky blue. Predicted interactions such as gene neighbourhood are blue, green and red for gene co-occurrence, gene neighbourhood and gene fusions. Co-expression interactions are shown in black, text-mining interactions are shown in light green and protein homology edges are purple.
For non-exercised Pink1- Drosophila compared with exercised wild-type Drosophila the network has 107 nodes and 341 edges with average node degree 6.37 and PPI enrichment p-value (P <1.0e-16) (Figure 6B). The DEPs were found to be involved in ETC, translation, peroxisome, formation of 40S subunits and protein folding. The PPI network showed three highly connected clusters. Cluster 1 contained proteins from both complex I and complex IV, proteins that are downregulated like that of exercised Pink1- Drosophila compared with the exercised wild-type Drosophila. Cluster 2 contained ribosomal proteins that are upregulated, similar to that of non-exercised Pink1- Drosophila compared with the non-exercised wild-type Drosophila PPI network. Cluster 3 contains the proteins UGT, HSP22, HSP60C, CABP1, GP93, HSC70-5, HSC70Cb, HSP60A, RTNL1 and CNX99A. These proteins involved in proteostasis and protein folding and were found to be upregulated.
For the final PPI network, exercised Pink1- Drosophila compared with non-exercised wild-type Drosophila, there are 44 nodes and 40 edges with average node degree 1.82 and PPI enrichment p-value 6.79e-11 (Figure 6C). The exercised Pink1- Drosophila versus non-exercised wild-type Drosophila were involved in purine ribonucleotide triphosphate metabolic processes, ETC and peroxisomes.
For each of the three PPI networks, the number of edges is larger than expected and the nodes were more connected than for a random PPI network of the same size.
CONCLUSIONS
A picture of mitochondrial proteomic changes made in response to exercise was obtained by using two different mass spectrometry methodologies. We were able to measure changes in an organelle whose function in exercise, and dysfunction in Parkinson’s disease, is crucial.
2D-GE MS data comparison between Pink1- exercised and Pink1- non-exercised Drosophila revealed several proteins with decreased levels of expression in response to exercise. These data were indicative of a generalised reduction of expression of the mitochondrial proteome in Pink1- Drosophila in response to exercise. This was investigated further in exercised and non-exercised Drosophila, both wild-type and Pink1-, by label-free mass spectrometry.
GO and KEGG analyses of the label-free mass spectrometry proteomic data validated our mitochondrial isolation methodology by identifying the enrichment of mitochondrial processes and pathways. The comparison between non-exercised wild-type Drosophila and non-exercised Pink1- Drosophila revealed that proteins involved in bioenergetics had reduced expression in the mutant. Most strikingly, exercise of the Pink1- Drosophila caused a broad reduction in protein expression within the mitochondrial protein, resulting in the Pink1- Drosophila mitochondrial proteome to return to levels similar to the wild-type Drosophila.
GO, KEGG and STRING network analysis of the differentially expressed proteins from the mitochondrial proteome comparisons identified enrichment of bioenergetic pathways. The most significantly enriched pathways in the non-exercised Pink1- Drosophila included oxidation-reduction, fatty acid metabolism, and folate metabolism, all of which are associated with PD. Our data point to exercise aiding normalisation of these pathways. Specific proteins in the pathways may be candidates to develop therapeutic approaches in PD.
Supplementary Material
Footnotes
AUTHOR CONTRIBUTIONS: BE performed data analysis, assisted with experiments, and wrote the manuscript, TLI performed experimental work and helped prepare the manuscript, GK performed STRING analyses and helped prepare the manuscript, JRI developed and constructed the ICE machine for fly exercise, NM generated the Drosophila used for these experiments and was consulted on all aspects of the fly work, LC directed the research, supervised experiments, provided reagents and prepared the manuscript.
CONFLICTS OF INTEREST: The authors declare that they have no conflicts of interest.
FUNDING: This work was supported by the Biotechnology and Biological Sciences Research Council [grant number BB/J014508/1], via awards to BE and TLI. GK is supported by a University of Nottingham Vice-Chancellors International Scholarship award. NM and LC are funded by HEFCE.
REFERENCES
- 1.GBD 2016 Parkinson ’ s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018; 17:939–53. 10.1016/S1474-4422(18)30295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Storey A. Living longer - how our population is changing and why it matters. Off. Natl. Stat. 2018; 1–53. [Google Scholar]
- 3.Hirsch E, Graybiel AM, Agid YA. Melanized dopaminergic neurons are differentially susceptible to degeneration in Parkinson’s disease. Nature. 1988; 334:345–48. 10.1038/334345a0 [DOI] [PubMed] [Google Scholar]
- 4.Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010; 120:131–43. 10.1007/s00401-010-0711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JS, Davis RL, Sue CM. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Curr Neurol Neurosci Rep. 2018; 18:21. 10.1007/s11910-018-0829-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R. LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet. 2009; 18:4022–34. 10.1093/hmg/ddp346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thomas KJ, McCoy MK, Blackinton J, Beilina A, van der Brug M, Sandebring A, Miller D, Maric D, Cedazo-Minguez A, Cookson MR. DJ-1 acts in parallel to the PINK1/parkin pathway to control mitochondrial function and autophagy. Hum Mol Genet. 2011; 20:40–50. 10.1093/hmg/ddq430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC. Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun. 2014; 5:3828. 10.1038/ncomms4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palikaras K, Lionaki E, Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat Cell Biol. 2018; 20:1013–22. 10.1038/s41556-018-0176-2 [DOI] [PubMed] [Google Scholar]
- 10.Shiba-Fukushima K, Imai Y, Yoshida S, Ishihama Y, Kanao T, Sato S, Hattori N. PINK1-mediated phosphorylation of the Parkin ubiquitin-like domain primes mitochondrial translocation of Parkin and regulates mitophagy. Sci Rep. 2012; 2:1002. 10.1038/srep01002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kondapalli C, Kazlauskaite A, Zhang N, Woodroof HI, Campbell DG, Gourlay R, Burchell L, Walden H, Macartney TJ, Deak M, Knebel A, Alessi DR, Muqit MM. PINK1 is activated by mitochondrial membrane potential depolarization and stimulates Parkin E3 ligase activity by phosphorylating Serine 65. Open Biol. 2012; 2:120080. 10.1098/rsob.120080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, et al. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014; 510:162–66. 10.1038/nature13392 [DOI] [PubMed] [Google Scholar]
- 13.Kazlauskaite A, Kondapalli C, Gourlay R, Campbell DG, Ritorto MS, Hofmann K, Alessi DR, Knebel A, Trost M, Muqit MM. Parkin is activated by PINK1-dependent phosphorylation of ubiquitin at Ser65. Biochem J. 2014; 460:127–39. 10.1042/BJ20140334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ordureau A, Sarraf SA, Duda DM, Heo JM, Jedrychowski MP, Sviderskiy VO, Olszewski JL, Koerber JT, Xie T, Beausoleil SA, Wells JA, Gygi SP, Schulman BA, Harper JW. Quantitative proteomics reveal a feedforward mechanism for mitochondrial PARKIN translocation and ubiquitin chain synthesis. Mol Cell. 2014; 56:360–75. 10.1016/j.molcel.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cookson MR. Parkinsonism due to mutations in PINK1, parkin, and DJ-1 and oxidative stress and mitochondrial pathways. Cold Spring Harb Perspect Med. 2012; 2:a009415. 10.1101/cshperspect.a009415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pankratz N, Foroud T. Genetics of Parkinson disease. Genet Med. 2007; 9:801–11. 10.1097/gim.0b013e31815bf97c [DOI] [PubMed] [Google Scholar]
- 17.Sliter DA, Martinez J, Hao L, Chen X, Sun N, Fischer TD, Burman JL, Li Y, Zhang Z, Narendra DP, Cai H, Borsche M, Klein C, Youle RJ. Parkin and PINK1 mitigate STING-induced inflammation. Nature. 2018; 561:258–62. 10.1038/s41586-018-0448-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong Z, Liang S, Sanchez-Lopez E, He F, Shalapour S, Lin XJ, Wong J, Ding S, Seki E, Schnabl B, Hevener AL, Greenberg HB, Kisseleva T, Karin M. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature. 2018; 560:198–203. 10.1038/s41586-018-0372-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006; 441:1157–61. 10.1038/nature04788 [DOI] [PubMed] [Google Scholar]
- 20.Clark IE, Dodson MW, Jiang C, Cao JH, Huh JR, Seol JH, Yoo SJ, Hay BA, Guo M. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 2006; 441:1162–66. 10.1038/nature04779 [DOI] [PubMed] [Google Scholar]
- 21.Greene JC, Whitworth AJ, Kuo I, Andrews LA, Feany MB, Pallanck LJ. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA. 2003; 100:4078–83. 10.1073/pnas.0737556100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015; 85:257–73. 10.1016/j.neuron.2014.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009; 1:99–111. 10.1002/emmm.200900006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu W, Acín-Peréz R, Geghman KD, Manfredi G, Lu B, Li C. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proc Natl Acad Sci USA. 2011; 108:12920–24. 10.1073/pnas.1107332108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julienne H, Buhl E, Leslie DS, Hodge JJ. Drosophila PINK1 and parkin loss-of-function mutants display a range of non-motor Parkinson’s disease phenotypes. Neurobiol Dis. 2017; 104:15–23. 10.1016/j.nbd.2017.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasco AJ, Paffenbarger RS Jr, Gendre I, Wing AL. The role of physical exercise in the occurrence of Parkinson’s disease. Arch Neurol. 1992; 49:360–65. 10.1001/archneur.1992.00530280040020 [DOI] [PubMed] [Google Scholar]
- 27.Yang F, Trolle Lagerros Y, Bellocco R, Adami HO, Fang F, Pedersen NL, Wirdefeldt K. Physical activity and risk of Parkinson’s disease in the Swedish National March Cohort. Brain. 2015; 138:269–75. 10.1093/brain/awu323 [DOI] [PubMed] [Google Scholar]
- 28.Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007; 78:678–84. 10.1136/jnnp.2006.099333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008; 23:631–40. 10.1002/mds.21922 [DOI] [PubMed] [Google Scholar]
- 30.Murray DK, Sacheli MA, Eng JJ, Stoessl AJ. The effects of exercise on cognition in Parkinson’s disease: a systematic review. Transl Neurodegener. 2014; 3:5. 10.1186/2047-9158-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shin MS, Jeong HY, An DI, Lee HY, Sung YH. Treadmill exercise facilitates synaptic plasticity on dopaminergic neurons and fibers in the mouse model with Parkinson’s disease. Neurosci Lett. 2016; 621:28–33. 10.1016/j.neulet.2016.04.015 [DOI] [PubMed] [Google Scholar]
- 32.Jang Y, Koo JH, Kwon I, Kang EB, Um HS, Soya H, Lee Y, Cho JY. Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res. 2017; 1655:186–93. 10.1016/j.brainres.2016.10.029 [DOI] [PubMed] [Google Scholar]
- 33.Klemann CJ, Xicoy H, Poelmans G, Bloem BR, Martens GJ, Visser JE. Physical Exercise Modulates L-DOPA-Regulated Molecular Pathways in the MPTP Mouse Model of Parkinson’s Disease. Mol Neurobiol. 2018; 55:5639–57. 10.1007/s12035-017-0775-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Life Technologies. ZOOM ® IPGRunner TM System For isoelectric focusing of ZOOM ® Strips Catalog Numbers ZM0001, ZM0002, ZM0004. 2012. [Google Scholar]
- 35.Centre for Excellence in Mass Spectrometry. FAQs - Biology, The University of York. 2019. [Google Scholar]
- 36.Raudvere U, Kolberg L, Kuzmin I, Arak T, Adler P, Peterson H, Vilo J. g:Profiler: a web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019; 47:W191–98. 10.1093/nar/gkz369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szklarczyk D, Gable AL, Lyon D, Junge A, Wyder S, Huerta-Cepas J, Simonovic M, Doncheva NT, Morris JH, Bork P, Jensen LJ, Mering CV. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019; 47:D607–13. 10.1093/nar/gky1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard A, Shephard F, Freed J, Liddell S, Chakrabarti L. Mitochondrial proteomic profiling reveals increased carbonic anhydrase II in aging and neurodegeneration. Aging (Albany NY). 2016; 8:2425–36. 10.18632/aging.101064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollard AK, Ingram TL, Ortori CA, Shephard F, Brown M, Liddell S, Barrett DA, Chakrabarti L. A comparison of the mitochondrial proteome and lipidome in the mouse and long-lived Pipistrelle bats. Aging (Albany NY). 2019; 11:1664–85. 10.18632/aging.101861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cornelissen T, Vilain S, Vints K, Gounko N, Verstreken P, Vandenberghe W. Deficiency of parkin and PINK1 impairs age-dependent mitophagy in Drosophila. Elife. 2018; 7:e35878. 10.7554/eLife.35878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JJ, Sanchez-Martinez A, Martinez Zarate A, Benincá C, Mayor U, Clague MJ, Whitworth AJ. Basal mitophagy is widespread in Drosophila but minimally affected by loss of Pink1 or parkin. J Cell Biol. 2018; 217:1613–22. 10.1083/jcb.201801044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Di Rita A, Peschiaroli A, D Acunzo P, Strobbe D, Hu Z, Gruber J, Nygaard M, Lambrughi M, Melino G, Papaleo E, Dengjel J, El Alaoui S, Campanella M, et al. HUWE1 E3 ligase promotes PINK1/PARKIN-independent mitophagy by regulating AMBRA1 activation via IKKα. Nat Commun. 2018; 9:3755. 10.1038/s41467-018-05722-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lira VA, Okutsu M, Zhang M, Greene NP, Laker RC, Breen DS, Hoehn KL, Yan Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013; 27:4184–93. 10.1096/fj.13-228486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yogev O, Pines O. Dual targeting of mitochondrial proteins: mechanism, regulation and function. Biochim Biophys Acta. 2011; 1808:1012–20. 10.1016/j.bbamem.2010.07.004 [DOI] [PubMed] [Google Scholar]
- 45.Ben-Menachem R, Tal M, Shadur T, Pines O. A third of the yeast mitochondrial proteome is dual localized: a question of evolution. Proteomics. 2011; 11:4468–76. 10.1002/pmic.201100199 [DOI] [PubMed] [Google Scholar]
- 46.Parker WD Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989; 26:719–23. 10.1002/ana.410260606 [DOI] [PubMed] [Google Scholar]
- 47.Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990; 54:823–27. 10.1111/j.1471-4159.1990.tb02325.x [DOI] [PubMed] [Google Scholar]
- 48.Shoffner JM, Watts RL, Juncos JL, Torroni A, Wallace DC. Mitochondrial oxidative phosphorylation defects in Parkinson’s disease. Ann Neurol. 1991; 30:332–39. 10.1002/ana.410300304 [DOI] [PubMed] [Google Scholar]
- 49.Finsterer J, Jarius C, Baumgartner M. Parkinson’s disease associated with impaired oxidative phosphorylation. Neuroradiology. 2001; 43:997–1000. 10.1007/s002340100618 [DOI] [PubMed] [Google Scholar]
- 50.Keeney PM, Xie J, Capaldi RA, Bennett JP Jr. Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006; 26:5256–64. 10.1523/JNEUROSCI.0984-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Müftüoglu M, Elibol B, Dalmizrak O, Ercan A, Kulaksiz G, Ogüs H, Dalkara T, Ozer N. Mitochondrial complex I and IV activities in leukocytes from patients with parkin mutations. Mov Disord. 2004; 19:544–48. 10.1002/mds.10695 [DOI] [PubMed] [Google Scholar]
- 52.Haas RH, Nasirian F, Nakano K, Ward D, Pay M, Hill R, Shults CW. Low platelet mitochondrial complex I and complex II/III activity in early untreated Parkinson’s disease. Ann Neurol. 1995; 37:714–22. 10.1002/ana.410370604 [DOI] [PubMed] [Google Scholar]
- 53.Chang D, Nalls MA, Hallgrímsdóttir IB, Hunkapiller J, van der Brug M, Cai F, Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, et al. , International Parkinson ’ s Disease Genomics Consortium, and 23andMe Research Team. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet. 2017; 49:1511–16. 10.1038/ng.3955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li G, Cui S, Du J, Liu J, Zhang P, Fu Y, He Y, Zhou H, Ma J, Chen S. Association of GALC, ZNF184, IL1R2 and ELOVL7 With Parkinson’s Disease in Southern Chinese. Front Aging Neurosci. 2018; 10:402. 10.3389/fnagi.2018.00402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saiki S, Hatano T, Fujimaki M, Ishikawa KI, Mori A, Oji Y, Okuzumi A, Fukuhara T, Koinuma T, Imamichi Y, Nagumo M, Furuya N, Nojiri S, et al. Decreased long-chain acylcarnitines from insufficient β-oxidation as potential early diagnostic markers for Parkinson’s disease. Sci Rep. 2017; 7:7328. 10.1038/s41598-017-06767-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharon R, Goldberg MS, Bar-Josef I, Betensky RA, Shen J, Selkoe DJ. alpha-Synuclein occurs in lipid-rich high molecular weight complexes, binds fatty acids, and shows homology to the fatty acid-binding proteins. Proc Natl Acad Sci USA. 2001; 98:9110–15. 10.1073/pnas.171300598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karube H, Sakamoto M, Arawaka S, Hara S, Sato H, Ren CH, Goto S, Koyama S, Wada M, Kawanami T, Kurita K, Kato T. N-terminal region of alpha-synuclein is essential for the fatty acid-induced oligomerization of the molecules. FEBS Lett. 2008; 582:3693–700. 10.1016/j.febslet.2008.10.001 [DOI] [PubMed] [Google Scholar]
- 58.Golovko MY, Faergeman NJ, Cole NB, Castagnet PI, Nussbaum RL, Murphy EJ. Alpha-synuclein gene deletion decreases brain palmitate uptake and alters the palmitate metabolism in the absence of alpha-synuclein palmitate binding. Biochemistry. 2005; 44:8251–59. 10.1021/bi0502137 [DOI] [PubMed] [Google Scholar]
- 59.Golovko MY, Rosenberger TA, Faergeman NJ, Feddersen S, Cole NB, Pribill I, Berger J, Nussbaum RL, Murphy EJ. Acyl-CoA synthetase activity links wild-type but not mutant alpha-synuclein to brain arachidonate metabolism. Biochemistry. 2006; 45:6956–66. 10.1021/bi0600289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Golovko MY, Rosenberger TA, Feddersen S, Faergeman NJ, Murphy EJ. Alpha-synuclein gene ablation increases docosahexaenoic acid incorporation and turnover in brain phospholipids. J Neurochem. 2007; 101:201–11. 10.1111/j.1471-4159.2006.04357.x [DOI] [PubMed] [Google Scholar]
- 61.Ingram TL, Shephard F, Sarmad S, Ortori CA, Barrett DA, Chakrabarti L. Sex specific inflammatory profiles of cerebellar mitochondria are attenuated in Parkinson’s disease. Aging (Albany NY). 2020; 12:17713–37. 10.18632/aging.103937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lipton SA, Kim WK, Choi YB, Kumar S, D’Emilia DM, Rayudu PV, Arnelle DR, Stamler JS. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc Natl Acad Sci USA. 1997; 94:5923–28. 10.1073/pnas.94.11.5923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Müller T, Werne B, Fowler B, Kuhn W. Nigral endothelial dysfunction, homocysteine, and Parkinson’s disease. Lancet. 1999; 354:126–27. 10.1016/s0140-6736(99)01660-8 [DOI] [PubMed] [Google Scholar]
- 64.Duan W, Ladenheim B, Cutler RG, Kruman II, Cadet JL, Mattson MP. Dietary folate deficiency and elevated homocysteine levels endanger dopaminergic neurons in models of Parkinson’s disease. J Neurochem. 2002; 80:101–10. 10.1046/j.0022-3042.2001.00676.x [DOI] [PubMed] [Google Scholar]
- 65.Martignoni E, Tassorelli C, Nappi G, Zangaglia R, Pacchetti C, Blandini F. Homocysteine and Parkinson’s disease: a dangerous liaison? J Neurol Sci. 2007; 257:31–37. 10.1016/j.jns.2007.01.028 [DOI] [PubMed] [Google Scholar]
- 66.Mayer O, Filipovský J, Hromádka M, Svobodová V, Racek J, Mayer O Jr, Stehlík P, Trefil L, Zárybnická M. Treatment of hyperhomocysteinemia with folic acid: effects on homocysteine levels, coagulation status, and oxidative stress markers. J Cardiovasc Pharmacol. 2002; 39:851–57. 10.1097/00005344-200206000-00010 [DOI] [PubMed] [Google Scholar]
- 67.Maron BA, Loscalzo J. The treatment of hyperhomocysteinemia. Annu Rev Med. 2009; 60:39–54. 10.1146/annurev.med.60.041807.123308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen L. Associations between B Vitamins and Parkinson’s Disease. Nutrients. 2015; 7:7197–208. 10.3390/nu7095333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xie Y, Feng H, Peng S, Xiao J, Zhang J. Association of plasma homocysteine, vitamin B12 and folate levels with cognitive function in Parkinson’s disease: A meta-analysis. Neurosci Lett. 2017; 636:190–95. 10.1016/j.neulet.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 70.Shen Y, Dong ZF, Pan PL, Xu G, Huang JY, Liu CF. Association of homocysteine, folate, and white matter hyperintensities in Parkinson’s patients with different motor phenotypes. Neurol Sci. 2019; 40:1855–63. 10.1007/s10072-019-03906-3 [DOI] [PubMed] [Google Scholar]
- 71.Dong B, Wu R. Plasma homocysteine, folate and vitamin B12 levels in Parkinson’s disease in China: A meta-analysis. Clin Neurol Neurosurg. 2020; 188:105587. 10.1016/j.clineuro.2019.105587 [DOI] [PubMed] [Google Scholar]
- 72.Liu S, Lu B. Reduction of protein translation and activation of autophagy protect against PINK1 pathogenesis in Drosophila melanogaster. PLoS Genet. 2010; 6:e1001237. 10.1371/journal.pgen.1001237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C, Dall’Armi C, Simoes S, Point Du Jour KS, McCabe BD, Small SA, Di Paolo G. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat Commun. 2013; 4:2250. 10.1038/ncomms3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khadilkar RJ, Rodrigues D, Mote RD, Sinha AR, Kulkarni V, Magadi SS, Inamdar MS. ARF1-GTP regulates Asrij to provide endocytic control of Drosophila blood cell homeostasis. Proc Natl Acad Sci USA. 2014; 111:4898–903. 10.1073/pnas.1303559111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li X, Wang L, Cykowski M, He T, Liu T, Chakranarayan J, Rivera A, Zhao H, Powell S, Xia W, Wong ST. OCIAD1 contributes to neurodegeneration in Alzheimer’s disease by inducing mitochondria dysfunction, neuronal vulnerability and synaptic damages. EBioMedicine. 2020; 51:102569. 10.1016/j.ebiom.2019.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shetty DK, Kalamkar KP, Inamdar MS. OCIAD1 Controls Electron Transport Chain Complex I Activity to Regulate Energy Metabolism in Human Pluripotent Stem Cells. Stem Cell Reports. 2018; 11:128–41. 10.1016/j.stemcr.2018.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cherwinski HM, Cohn RG, Cheung P, Webster DJ, Xu YZ, Caulfield JP, Young JM, Nakano G, Ransom JT. The immunosuppressant leflunomide inhibits lymphocyte proliferation by inhibiting pyrimidine biosynthesis. J Pharmacol Exp Ther. 1995; 275:1043–49. [PubMed] [Google Scholar]
- 78.Miret-Casals L, Sebastián D, Brea J, Rico-Leo EM, Palacín M, Fernández-Salguero PM, Loza MI, Albericio F, Zorzano A. Identification of New Activators of Mitochondrial Fusion Reveals a Link between Mitochondrial Morphology and Pyrimidine Metabolism. Cell Chem Biol. 2018; 25:268–78.e4. 10.1016/j.chembiol.2017.12.001 [DOI] [PubMed] [Google Scholar]
- 79.Fang J, Uchiumi T, Yagi M, Matsumoto S, Amamoto R, Takazaki S, Yamaza H, Nonaka K, Kang D. Dihydro-orotate dehydrogenase is physically associated with the respiratory complex and its loss leads to mitochondrial dysfunction. Biosci Rep. 2013; 33:e00021. 10.1042/BSR20120097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tufi R, Gandhi S, de Castro IP, Lehmann S, Angelova PR, Dinsdale D, Deas E, Plun-Favreau H, Nicotera P, Abramov AY, Willis AE, Mallucci GR, Loh SH, Martins LM. Enhancing nucleotide metabolism protects against mitochondrial dysfunction and neurodegeneration in a PINK1 model of Parkinson’s disease. Nat Cell Biol. 2014; 16:157–66. 10.1038/ncb2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheedipudi SM, Hu J, Fan S, Yuan P, Karmouch J, Czernuszewicz G, Robertson MJ, Coarfa C, Hong K, Yao Y, Campbell H, Wehrens X, Gurha P, Marian AJ. Exercise restores dysregulated gene expression in a mouse model of arrhythmogenic cardiomyopathy. Cardiovasc Res. 2020; 116:1199–213. 10.1093/cvr/cvz199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Piazza N, Gosangi B, Devilla S, Arking R, Wessells R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS One. 2009; 4:e5886. 10.1371/journal.pone.0005886 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.