Abstract
Background:
Prior controlled cannabis research has mostly focused on smoked cannabis and predominantly included frequent cannabis users. Oral cannabis products (“edibles”) make up a large and growing segment of the retail cannabis market. This study sought to characterize the pharmacodynamic effects of oral cannabis among infrequent cannabis users.
Methods:
Seventeen healthy adults who had not used cannabis for at least 60 days completed four experimental sessions in which they consumed a cannabis-infused brownie that contained 0, 10, 25, or 50 mg THC. Subjective effects, vital signs, cognitive/psychomotor performance, and blood THC concentrations were assessed before and for 8 h after dosing.
Results:
Relative to placebo, the 10 mg THC dose produced discriminable subjective drug effects and elevated heart rate but did not alter cognitive/psychomotor performance. The 25 and 50 mg THC doses elicited pronounced subjective effects and markedly impaired cognitive and psychomotor functioning compared with placebo. For all active doses, pharmacodynamic effects did not manifest until 30 – 60 min after ingestion, and peak effects occurred 1.5 – 3 h post-administration. Blood THC levels were significantly correlated with some pharmacodynamic drug effects, but were substantially lower than what is typically observed after cannabis inhalation.
Conclusion:
Ingestion of oral cannabis dose-dependently altered subjective drug effects and impaired cognitive performance. Unlike inhaled forms of cannabis for which acute effects occur almost immediately, effects of oral cannabis were considerably delayed. In an era of legalization, education about the time course of drug effects for cannabis edibles is needed to facilitate dose titration and reduce acute overdose incidents.
Keywords: Cannabis, Marijuana, Edibles, THC, Oral dosing, Pharmacodynamics
1. Introduction
Cannabis is one of the most widely used drugs in the world. Recent reforms to the policies governing the medicinal and non-medicinal (i.e., “recreational”) use of cannabis have greatly increased access to cannabis. Inhalation of smoked cannabis is the most common route of self-administration (Borodovsky et al., 2016; Knapp et al., 2018). However, the cannabis retail marketplace contains a vast array of products that can be administered via other routes (Russell et al., 2018; Spindle et al., 2019a). Oral cannabis products (a.k.a. “edibles”) have emerged as a popular alternative method of cannabis administration (Schauer et al., 2016; Steigerwald et al., 2018a). This subset of cannabis products includes cannabis-infused food (e.g., brownies, cookies, gummies) as well as various cannabis-containing beverages (Russell et al., 2018; Spindle et al., 2019a). Cannabis edibles are perceived to be healthier than smoked cannabis and can be used discreetly, which increases their appeal (Kostadinov and Roche, 2017; Lamy et al., 2016).
Δ-9-tetrahydrocannabinol (THC) is the primary psychoactive chemical constituent of the cannabis plant that is responsible for producing subjective “highs,” feelings of euphoria, as well as dysphoric effects such as panic, paranoia, and acute psychosis (Russo, 2011). Previous experimental examinations of the pharmacokinetic and pharmacodynamic effects of oral cannabis have typically administered pure THC, often in the form of dronabinol. These studies have shown that oral THC dose-dependently increases positive subjective drug effects ratings (e.g., ‘like drug effect’), subjective items associated with feelings of intoxication (e.g., ‘stoned’), and heart rate (Curran et al., 2002; Fogel et al., 2017; Lile et al., 2013; Vandrey et al., 2013). These studies also show dose-dependent impairment in attention, memory, and psychomotor performance following oral THC ingestion, but these effects are more consistently observed among less frequent cannabis users (Curran et al., 2002), likely due to the development of tolerance among heavier users. Though informative, these studies have limited ecological validity to current products available on the retail cannabis market. This is because most retail products are made with raw cannabis or whole-plant cannabis extracts and contain food ingredients that may affect drug absorption and alter pharmacodynamic effects relative to pharmaceutical formulations containing only THC (e.g., dronabinol).
Only a small subset of oral cannabis dosing studies have administered cannabis “edibles” (e.g., brownies) containing cannabis plant material (Cone et al., 1988; Newmeyer et al., 2017a; Niedbala et al., 2001; Vandrey et al., 2017; Wachtel et al., 2002). Several of these studies (Newmeyer et al., 2017a; Niedbala et al., 2001; Wachtel et al., 2002) compared the pharmacokinetic and pharmacodynamic effects of oral cannabis to a similar dose(s) of smoked or vaporized cannabis. The fourth study, conducted in our laboratory, examined the acute effects of oral cannabis among participants who were randomly assigned to receive a single oral cannabis dose (Vandrey et al., 2017). Collectively, these studies demonstrate that the acute pharmacodynamic effects of cannabis are substantially delayed following oral cannabis ingestion and often do not peak until several hours after administration, which is in stark contrast to inhaled forms of cannabis (either smoked or vaporized) for which cannabis effects peak within minutes (Newmeyer et al., 2017a; Niedbala et al., 2001; Vandrey et al., 2017; Wachtel et al., 2002). Regarding pharmacokinetic effects, peak concentrations of THC and its metabolites following oral cannabis ingestion are substantially lower compared with inhaled cannabis. The delayed onset of oral cannabis effects makes dose titration more difficult and increases the chances of acute overdose while the pharmacokinetics of oral cannabis make it difficult to identify individuals who are intoxicated based on blood THC concentrations alone (Allen et al., 2017; Barrus et al., 2016; Hudak et al., 2015).
There are some critical limitations of published laboratory studies involving controlled oral cannabis administration. First, most have only administered one or two doses, and as a result, the acute effects of oral cannabis have not been sufficiently characterized for the wide range of doses available in the retail marketplace (Steigerwald et al., 2018b). Participants in oral cannabis studies have included moderate to heavy users (Newmeyer et al., 2017a; Niedbala et al., 2001; Wachtel et al., 2002) whereas infrequent users of oral cannabis products represent a growing proportion of the user market and infrequent use may be associated with greater sensitivity to the acute pharmacodynamic effects of cannabis compared with frequent users that have developed tolerance. Second, prior studies have included male participants predominantly. Last, oral cannabis products represent a growing portion of the market share of cannabis products, which necessitates further understanding of the physiologic, subjective, and cognitive effects of these products.
The present study extends prior research by characterizing the pharmacodynamic effects of multiple oral cannabis doses (0, 10, 25, and 50 mg THC) in a sample of infrequent cannabis users. The current study utilizes a within-subjects crossover design as opposed to the between-subjects design used in our prior oral cannabis study (Vandrey et al., 2017), which allowed us to better control for the considerable inter-individual variability that exists in the pharmacokinetics and pharmacodynamics of oral cannabis because each participant served as their own control. The use of only infrequent cannabis users also minimized the potential that some participants would be tolerant to the effects of cannabis/THC.
2. Methods
2.1. Participants
Study participants were healthy adults between the ages of 18 and 45 recruited via media advertisements and word-of-mouth. Age was verified with government-issued photo ID. Participants were nontreatment seeking and endorsed a history of lifetime exposure to cannabis but denied use of cannabis and other illicit drugs in the past three months. Participants completed a brief telephone screen and those deemed initially eligible were invited to complete a laboratory screening session. At the screening assessment, participants provided written informed consent and completed a series of semi-structured interviews and self-report questionnaires that collected basic demographic information and assessed mental health (via DSM-IV Checklist for Adult Disorders), physical health, and history of recreational drug use. Participants were required to report no allergies to any of the ingredients used to prepare the cannabis brownies (e.g., chocolate, egg, wheat). Participants were required to test negative (via urinalysis) for cannabinoid metabolites and recent illicit drug use. Participants also underwent a physical exam during which routine clinical chemistry, hematology, and serology were assessed and an electrocardiogram (EKG) was obtained to determine cardiovascular health (participants who were deemed unhealthy based on results from any of these tests were not considered eligible for the study). A serum pregnancy test was also performed for females. At the end of the laboratory screening, participants completed training on subjective drug effect questionnaires and the computerized cognitive test battery to ensure task comprehension and to establish stable performance on the cognitive tasks.
2.2. Experimental session procedures
Participants completed four outpatient experimental laboratory visits, each lasting approximately 9 h, that were scheduled a minimum of one week apart to ensure sufficient elimination of cannabinoids between sessions. Participants arrived to the Johns Hopkins Behavioral Pharmacology Research Unit (BPRU) at 07:30 for each session. Upon arrival, participants were administered a breath alcohol test and provided a urine sample that was tested for recent illicit drug use and pregnancy (for females only); no positive tests occurred. A nurse inserted an indwelling intravenous catheter in the participant’s non-dominant arm to allow for repeated blood sampling. All participants consumed a standard low-fat breakfast prior to the collection of baseline measures and cannabis exposure (breakfast was consumed approximately 1 h prior to dosing).
2.3. Study drug
Placebo or active cannabis obtained from the National Institute on Drug Abuse (NIDA) Drug Supply Program was used to prepare brownies that contained 0, 10, 25, or 50 mg THC. Active cannabis (used to prepare 10, 25, and 50 mg THC brownies) contained 11 % total THC, 0.1 % cannabidiol, and 0.8 % cannabinol. Participants were told that they would receive cannabis brownies containing either 10, 25, or 50 mg THC (or placebo) but that neither they, nor the research staff, would know what dose they received on a given day. Placebo cannabis, from which THC had been extracted using ethanol, was used to prepare placebo brownies; the same quantity of plant material was used in active and placebo conditions to assist with blinding. The Johns Hopkins BPRU Pharmacy prepared the cannabis brownies 24 – 48 hours prior to administration. Individually-weighed doses of cannabis were ground into a powder using a food processor and were subsequently heated for 30 min at 250 °F (130 °C) to catalyze decarboxylation of tetra-hydrocannabinolic acid (THC-A) to THC. Following decarboxylation, cannabis plant material was mixed into the brownie batter. To ensure exact dosing, each dose was prepared separately in an individual baking tray. Prior to the study, sample brownies were made at each target THC dose (0, 10, 25, and 50 mg THC) and these sample brownies were sent for analytical testing to a designated laboratory (ElSohly Laboratories Inc., Oxford, MS). Results of this analytical testing confirmed the conversion of THC-A to THC and that the desired doses were reliably achieved in each product using our preparation procedures.
2.4. Assessment measures
Biological specimens, physiological measures, and subjective drug effects were assessed at baseline, 10 min after brownie consumption, and 0.5, 1, 1.5, 2, 3, 4, 5, 6, and 8 h post-exposure. Cognitive and psychomotor performance were also assessed at the same time points with the exception of the 10 min and 0.5 h post-exposure time points due to time constraints.
2.4.1. Biological specimens
Blood samples (10 mL/specimen) were collected, mixed by inversion, and transferred into two plastic cryotubes (5 mL each). Samples were frozen and stored at −60 °C until they were shipped to Immunalysis Corporation (Pomona, CA) for quantitative analysis of THC and its two primary metabolites: 11-hydroxy-THC (11-OH-THC) and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol (THCCOOH). The limit of quantitation (LOQ) for all analytes in blood was 1 ng/mL and the upper limit of linearity (ULOL) was 100 ng/mL; further details regarding the analytical method can be found elsewhere: (Spindle et al., 2019b; Vandrey et al., 2017).
2.4.2. Physiological measures
Vital signs (i.e., heart rate, systolic and diastolic blood pressure) were acquired via automated monitor while participants were seated in an upright position.
2.4.3. Subjective drug effects
Participants provided self-reported ratings of subjective drug effects with a 15-item Drug Effect Questionnaire (DEQ). Ratings of ‘Drug Effect,’ ‘Unpleasant Drug Effect,’ ‘Good Drug Effect,’ ‘Sick,’ ‘Heart Racing,’ ‘Anxious/Nervous,’ ‘Relaxed,’ ‘Paranoid,’ ‘Sleepy/Tired,’ ‘Alert,’ ‘Irritable,’ ‘Vigorous,’ ‘Restless,’ ‘Hungry/Have” Munchies”,’ and ‘Craving Marijuana’ were made with a 100 mm visual analog scale (VAS) that was anchored with ‘Not at All’ on the left and ‘Extremely’ on the right.
2.4.4. Cognitive task performance
Cognitive and psychomotor performance were assessed using three computerized tasks previously shown to be sensitive to acute cannabis intoxication (Arkell et al., 2019; Spindle et al., 2018; Vandrey et al., 2017). Performance on each of these tasks generalizes to the operation of a motor vehicle and functioning in the workplace. The Divided Attention Task (DAT) required participants to perform two visual tasks simultaneously (Casswell and Marks, 1973). Using the computer mouse, participants tracked a central stimulus that moved horizontally from side to side while simultaneously monitoring a target digit that appeared in the lower portion of the screen. Peripheral stimuli (single digit integers) appeared in each of the four corners of the screen and participants had to click the computer mouse during each instance when the digits in the corners of the screen matched the central target digit. Primary outcomes for the DAT were the total number of correctly identified peripheral targets, mean correct peripheral target reaction time (milliseconds), and average distance (number of pixels) of the cursor from the central horizontal stimulus.
Psychomotor performance was assessed with a computerized version of the Digit Symbol Substitution Task (DSST) that required participants to replicate a geometric pattern that was displayed on the monitor using the computer keyboard (McLeod et al., 1982). Nine different patterns were each comprised of three highlighted squares presented on a 3 × 3 grid and each unique pattern was numbered. When the number corresponding to one of the patterns appeared, participants replicated the shape of the pattern using an assigned 3 × 3 section of the computer keyboard. Primary outcomes for the DSST were the total number of trials attempted, total number of correct trials, and the percentage of attempted trials that were completed correctly.
The Paced Serial Addition Task (PASAT; Herrmann et al., 2015, a computerized adaptation of the Paced Auditory Serial Addition Task (Gronwall, 1977) was administered to assess working memory. During this task, a string of single-digit integers were displayed sequentially on the computer monitor. Participants were instructed to calculate the sum of each successive integer pair and choose the correct answer from a list of choices displayed on the screen. Primary outcomes for the PASAT were the number of correct trials and mean reaction time (milliseconds) during correct trials.
2.5. Cannabis exposure
During each visit, participants consumed one cannabis-containing brownie that had 1 of 4 possible THC doses (0, 10, 25, or 50 mg). They were instructed to consume the entire brownie within five minutes and were under direct staff observation during this time. Participants were permitted to drink water only as needed and were not permitted to consume any snack items for 30 min post-ingestion to minimize potential differences in absorption across participants. Drug administration was double-blind and dose order was counterbalanced and randomly assigned across study participants.
2.6. Data analytic plan
Demographic characteristics and whole blood THC data were summarized using descriptive statistics including means and standard deviations. Repeated-measures regressions were performed (with SAS PROC MIXED; version 9.4) to analyze data for vital signs, subjective drug effects, and cognitive performance tasks. Separate regressions were performed for each outcome and 3 factors were included in each model: time, dose, and participant sex. A first-order autoregressive AR (1) covariance structure was used for these analyses. Planned contrasts were conducted to compare mean peak scores between placebo (0 mg) to each active dose (10, 25, and 50 mg) for each outcome. Correlations (Pearson’s r) were performed to examine relations between concentrations of THC, 11-OH-THC, and THCCOOH and subjective ratings of ‘drug effect’ from the DEQ and scores on primary outcomes for the DSST, DAT, and PASAT within the three active dosing conditions. P values < .05 were considered statistically significant for all analyses.
3. Results
3.1. Participants and adverse events
Participant characteristics are displayed in Table 1. Table 2 shows mean (SD) peak scores for pharmacodynamic outcomes in each dosing condition. Two adverse events occurred in this study. One participant vomited following consumption of the 50 mg THC cannabis brownie and another vomited in the 25 mg THC condition (both participants vomited approximately 3 h after drug administration). In both instances, emesis was brief and immediately resolved feelings of nausea.
Table 1.
Participant Characteristics.
| Participants (N = 17) | |
|---|---|
| Age (Years), Mean (SD) | 25.2 (5.4) |
| Sex (% Female) | 47.1 |
| Race (% Caucasian) | 64.7 |
| Ethnicity (% Hispanic/Latino) | 17.6 |
| Body Mass Index (kg/M2), Mean (SD) | 25.8 (3.9) |
| Age of First Cannabis Use, Mean (SD) | 17.8 (2.1) |
| Days Since Last Cannabis Use, Mean (SD) | 856 (2032) |
| Education Level (% with at least some college) | 94 % |
| Average # of Drinks/Week, Mean (SD) | 5.3 (4.4) |
| Cigarette Smokers at Intake (n) | 1 |
Table 2.
Summary Table of Physiological, Subjective Drug Effects, and Cognitive Task Variables.
| Placebo | 10 mg | 25 mg | 50 mg | |
|---|---|---|---|---|
| Physiological | ||||
| Systolic Blood Pressure | 120.7 (0.8) | 118.6 (0.8) | 119.2 (0.9) | 118.2 (1.0) |
| Diastolic Blood pressure (mm/Hg) | 70.7 (0.6) | 70.7 (0.6) | 71.9 (0.7) | 71.7 (0.7) |
| Heart Rate (Beats per Minute) | 70.4 (0.7) | 76.2 (0.8) | 80.3 (1.2) | 83.2 (1.3) |
| Subjective Drug Effects (VAS, 0–100) | ||||
| Drug Effect | 2.3 (0.5) | 18.0 (1.8) | 38.3 (2.5) | 47.5 (2.7) |
| Unpleasant Drug Effect | 0.3 (0.3) | 3.2 (0.7) | 8.5 (1.2) | 13.3 (1.4) |
| Good Drug Effect | 3.5 (1.0) | 18.9 (1.9) | 34.9 (2.3) | 34.0 (2.3) |
| Sick | 0.4 (0.2) | 3.1 (0.6) | 3.3 (0.7) | 7.9 (1.0) |
| Heart Racing | 0.2 (0.1) | 1.7 (0.4) | 7.6 (1.2) | 14.9 (1.7) |
| Anxious/Nervous | 0.8 (0.2) | 1.8 (0.4) | 7.1 (1.1) | 12.5 (1.5) |
| Relaxed | 60.9 (2.4) | 65.1 (2.0) | 58.4 (2.2) | 52.1 (2.2) |
| Paranoid | 0.3 (0.1) | 1.5 (0.5) | 4.3 (1.0) | 11.0 (1.5) |
| Sleepy/Tired | 33.3 (2.1) | 39.4 (2.3) | 53.8 (2.0) | 57.0 (2.3) |
| Alert | 46.5 (2.4) | 42.6 (2.3) | 29.1 (2.0) | 30.3 (2.1) |
| Irritable | 1.2 (0.3) | 3.0 (0.7) | 3.0 (0.7) | 8.7 (1.1) |
| Vigorous | 28.6 (2.2) | 24.0 (1.9) | 16.5 (1.8) | 17.5 (1.7) |
| Restless | 3.7 (0.7) | 6.4 (1.0) | 10.5 (1.6) | 15.0 (1.9) |
| Hungry/Munchies | 7.6 (1.4) | 14.1 (1.7) | 17.1 (1.8) | 20.6 (2.0) |
| Craving Cannabis | 0.2 (0.1) | 0.7 (0.3) | 1.2 (0.5) | 0.7 (0.3) |
| Behavioral/Cognitive | ||||
| Divided Attention Task | ||||
| Average Distance from Target | 19.5 (0.6) | 23.6 (1.1) | 31.0 (1.7) | 35.0 (2.0) |
| Mean Overall Response | 1128.1 | 1168.4 | 1281.0 | 1286.3 |
| Time (ms) | (25.4) | (26.4) | (31.4) | (33.9) |
| Total Number Correct | 22.6 (0.1) | 21.9 (0.3) | 20.9 (0.3) | 20.5 (0.4) |
| Digit Symbol Substitution Task | ||||
| Total Attempted | 52.6 (0.6) | 52.9 (0.6) | 48.8 (0.7) | 46.0 (0.9) |
| Total Correct | 50.1 (0.6) | 49.2 (0.6) | 45.3 (0.7) | 41.9 (1.0) |
| Percent Correct | 95.3 (0.3) | 93.0 (0.5) | 92.6 (0.7) | 90.1 (1.1) |
| Paced Serial Addition Test | ||||
| Total Correct | 76.8 (1.1) | 73.8 (1.2) | 69.2 (1.7) | 64.4 (1.7) |
All values = mean (SD) peak scores.
3.2. Cardiovascular effects
Fig. 1 depicts heart rate (beats per minute) over time for each dosing condition. No significant main effects of cannabis Dose were observed for systolic or diastolic blood pressure (Fs < 1, ps > .70). In contrast, a significant main effect of Dose was observed for heart rate (F3,48 = 5.6, p < .01). Planned comparisons indicated that, compared to placebo, heart rate was significantly higher following ingestion of the 10 mg, 25 mg, and 50 mg dose (all ps < .05). There was a main effect of sex observed for systolic blood pressure and heart such, indicating that females had significantly lower systolic blood pressure (F1,15 = 10.37, p < .05) and higher heart rate compared with males (F1,15 = 28.69, p < .05) overall. However, there were no Sex x Dose interactions for cardiovascular outcomes.
Fig. 1.
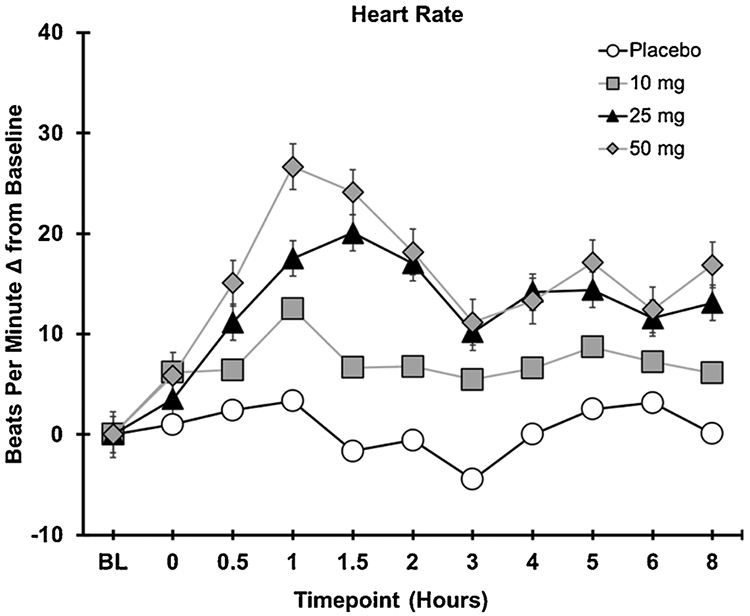
Heart rate as a function of dose and time.
3.3. Subjective drug effects
Significant effects of Dose were observed for ratings of drug effect (F3,48 = 38.9, p < .0001), unpleasant drug effect (F3,48 = 11.7, p < .0001), good drug effect (F3,48 = 25.9, p < .0001), sick (F3,48 < .001), heart racing (F3,48 = 12.2, p < .0001), anxious/nervous (F3,48 = 11.5, p < .0001), paranoid (F3,48 = 8.8, p < .0001), sleepy/tired (F3,48 = 7.2, p < .001), irritable (F3,48 = 7.1, p < .01), restless (F3,48 = 3.1, p < .05), and hungry/have munchies (F3,48 = 4.3, p < .01); see Fig. 2 for depiction of drug effect, unpleasant drug effect, and good drug effect, over time for each experimental condition. Planned comparisons revealed that, compared to placebo, the 10 mg dose significantly increased ratings of drug effect and good drug effect (both ps < .001). The 25 mg dose increased ratings of drug effect, unpleasant drug effect, good drug effect, heart racing, anxious/nervous, sleepy/tired, hungry/have munchies (all ps < .05), and decreased ratings of alert (p = .03). The 50 mg dose increased ratings of drug effect, unpleasant drug effect, good drug effect, sick, heart racing, anxious/nervous, paranoid, sleepy/tired, irritable, restless, hungry/have munchies (all ps < .01), and decreased ratings of alert (p = .05) compared to placebo. Main effects of sex were observed for sick, heart racing, and anxious/nervous, indicating that female participants had significantly higher ratings of sick (F1,15 = 4.71, p < .05), heart racing (F1,15 =5.04, p < .05), and anxious/nervous (F1,15 = 5.9, p < .05) compared with males. However, no Sex x Dose interactions were observed for subjective outcomes.
Fig. 2.
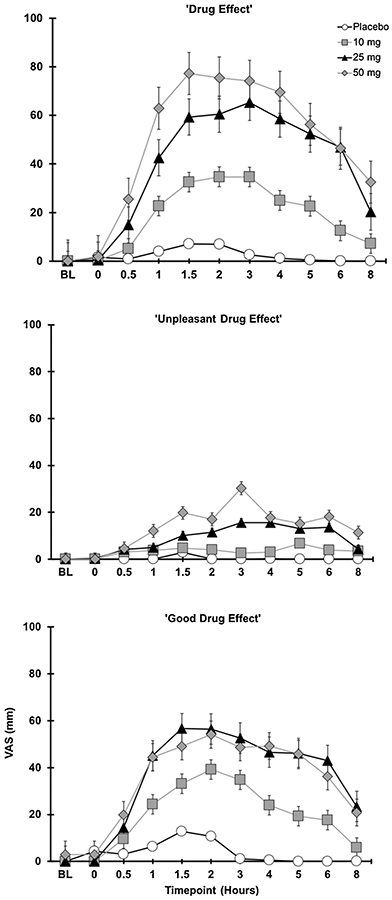
Subjective drug effects as a function of dose and time.
3.4. Cognitive task performance
3.4.1. Divided attention task (DAT)
For the DAT (Fig. 3, panel A), a significant main effect of Dose was observed on the average distance (pixels) from the central stimulus (F3,48 = 4.5, p < .01). Planned comparisons between placebo and active dose conditions indicated that both the 25 and 50 mg doses significantly increased the average distance from the target, indicating worse performance compared with placebo (ps < .05); performance in the placebo and the 10 mg conditions did not differ (p = .28). Main effects of Dose on the total number of correct targets (F3,48 = 2.3, p = .09) and average correct peripheral target RT (F3,48 = 2.3, p = .08) all failed to reach significance. However, planned contrasts revealed that participants correctly identified fewer peripheral integers in the 50 mg condition compared with the placebo condition (p = .02). No significant Sex x Dose interactions were observed.
Fig. 3.
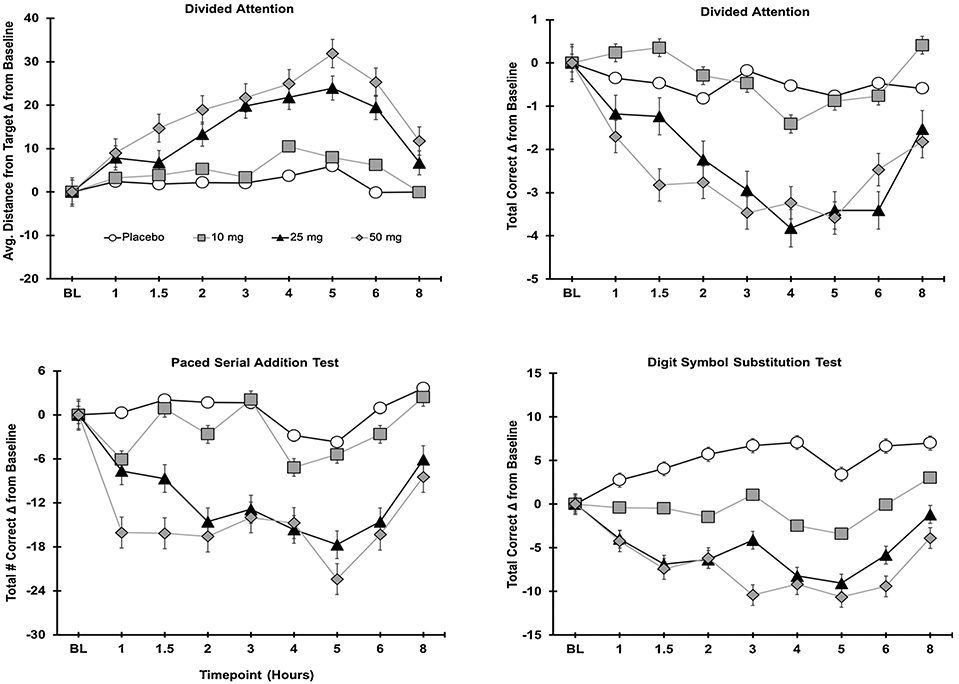
Cognitive task performance as a function of dose and time.
3.4.2. Digit symbol substitution test (DSST)
On the DSST (Fig. 3, panel B), a significant main effect of Dose was observed for the total number of trials attempted (F3,48 = 4.5, p < .01), total number of correct trials (F3,48 = 6.6, p = .001), and the percentage of attempted trials that were correct (F3,48 = 4.8, p < .01). Compared with placebo, the 50 mg dose significantly reduced the total number of trials attempted (p = .003); the 10 mg and 25 mg doses did not significantly differ from placebo (ps = .86, and .07, respectively). On the total number of correct responses, planned comparisons revealed that, compared to placebo, the 25 mg and 50 mg doses reduced the number of correct responses (ps < .001 and .05, respectively); a comparable number of correct responses were observed in the placebo and 10 mg conditions (p = .63). For the percentage of correct responses (total number of correct trials/total trials attempted), accuracy was significantly reduced following ingestion of the 50 mg dose compared with placebo (p < .001); the 10 mg and 25 mg doses did not significantly differ from placebo (ps = .10 and .06, respectively). No significant Sex x Dose interactions were observed.
3.4.3. Paced Serial Addition Test (PASAT)
On the PASAT (Fig. 3, panel C), a main effect of Dose for the total number of correct trials failed to reach statistical significance (F3,48 = 2.7, p = .06). However, planned contrasts revealed that participants provided fewer correct responses in the 50 mg dose condition compared with the placebo condition (p < .01). No significant Sex x Dose interactions were observed.
3.5. Correlations between blood cannabinoid concentrations and pharmacodynamic outcomes
Whole blood THC concentrations (as well as concentrations of 11-OH-THC and THCCOOH; Fig. 4) were positively correlated with VAS ratings of drug effect for each of the active oral cannabis doses (Pearson’s rvalues > 0.38). At the 10 mg dose, impaired performance on the DAT and PASAT was significantly correlated with blood concentrations of THC, 11-OH-THC, and THCOOH; DSST performance and blood cannabinoid concentrations were not significantly correlated at the 10 mg dose. At the 25 and 50 mg THC doses, impaired performance on all three cognitive tasks (DAT, DSST, and PASAT) was significantly correlated with blood concentrations of THC, 11-OH-THC, and THCOOH (see Table 3).
Fig. 4.
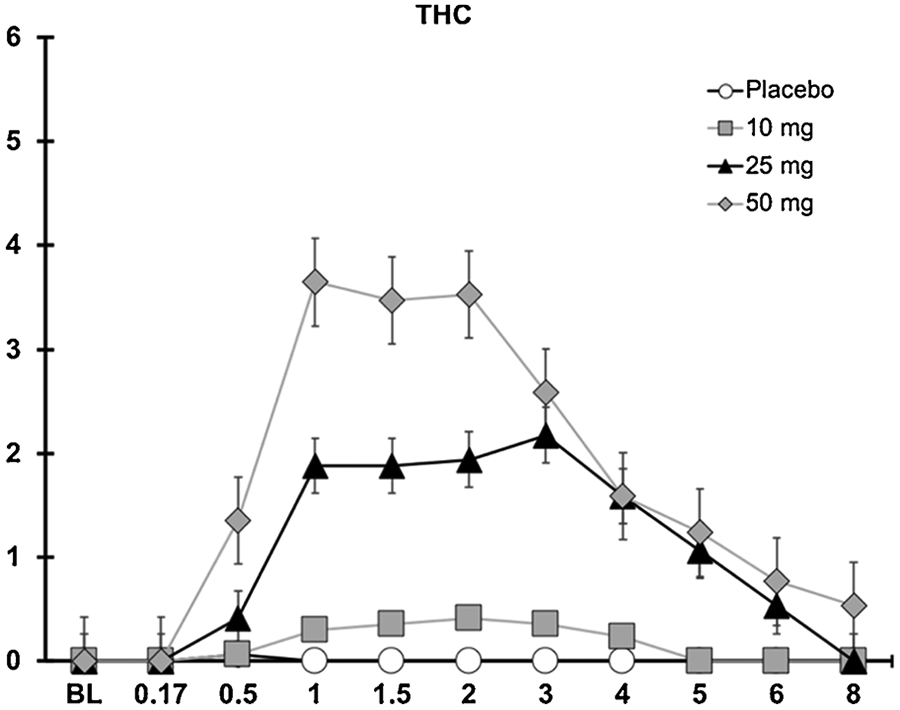
Whole Blood THC concentration as a function of dose and time.
Table 3.
Correlations Between Whole Blood Cannabinoid Levels and Pharmacodynamic Outcomes.
| THC | 11-OH-THC | THCCOOH | |
|---|---|---|---|
| 10 mg | |||
| VAS Drug Effect | 0.44** | 0.58** | 0.40** |
| DAT Distance from Target | 0.40** | 0.40** | 0.39** |
| DSST % Correct | −0.12 | −0.14 | −0.11 |
| PASAT Total Correct | −0.29** | −0.33** | −0.31** |
| 25 mg | |||
| VAS Drug Effect | 0.60** | 0.62** | 0.67** |
| DAT Distance from Target | 0.26** | 0.26** | 0.45** |
| DSST % Correct | −0.21** | −0.32** | −0.43** |
| PASAT Total Correct | −0.24** | −0.33** | −0.42** |
| 50 mg | |||
| VAS Drug Effect | 0.45** | 0.49** | 0.38** |
| DAT Distance from Target | 0.32** | 0.49** | 0.67** |
| DSST % Correct | −0.36** | −0.43** | −0.40** |
| PASAT Total Correct | −0.32** | −0.54** | −0.62** |
Correlation is significant at the 0.01 level (two-tailed).
4. Discussion
Detailed understanding of the pharmacodynamic dose effects of oral cannabis is vital given the increasing popularity of these products in the expanding legal cannabis market. Most prior controlled cannabis research studies have examined the acute effects of smoked cannabis in frequent cannabis users. Moreover, the few controlled examinations of oral cannabis were limited by the use of between-subjects designs, narrow dose ranges, the inclusion of mostly male participants, and/or the use of pure THC (as opposed to food or drinks infused with whole plant cannabis, which is more typical among retail cannabis edibles). The present study utilized a within-subjects design to characterize the pharmacodynamic profile of four doses of oral cannabis (i.e., 0, 10, 25, 50 mg THC) among 17 infrequent cannabis users (i.e., ≥ 2 months since last cannabis use at study entry).
Overall, ingestion of oral cannabis resulted in dose-dependent effects in attention, memory, and psychomotor performance. The 10 mg dose did not produce performance deficits relative to placebo. However, the 25 and 50 mg doses produced moderate to severe impairment on all performance measures included in this study when compared with placebo. These findings are noteworthy because the THC doses administered in this study are representative of common doses found in commercially-available oral cannabis products. That is, 10 mg has been set as the standard unit dose or “serving size” for cannabis edibles sold at retail outlets in several U.S. states (e.g., Colorado) as well as Canada, and many oral cannabis products contain 25–50 mg in a single package (Steigerwald et al., 2018b; Vandrey et al., 2015). Medicinal or non-medical (i.e., “recreational”) cannabis users who ingest similar oral cannabis doses (particularly those who use cannabis intermittently) should avoid operating a motor vehicle or performing other tasks that require peak functioning of motor skills and cognition in order to avoid accidental injury to themselves or others.
Consistent with prior controlled oral cannabis research, pharmacodynamic cannabis effects were substantially delayed in this study compared to what is typically observed following cannabis inhalation (Newmeyer et al., 2017b; Spindle et al., 2018). For example, on average, subjective drug effects became perceptible 30 min after brownie ingestion and peaked 1.5–3 hours post-administration. Effects on cognitive and psychomotor performance followed a similar pattern, as peak impairment on cognitive measures were detected between 2 – 5 hours post-administration, depending on the task and dose. In contrast, peak subjective and cognitive effects of inhaled cannabis occur within 10 – 30 min of inhalation (Newmeyer et al., 2017b; Spindle et al., 2018). Despite differences in the onset of effects, the magnitude of cognitive impairment observed in this study was comparable to that observed following inhalation of similar doses of smoked or vaporized cannabis. For example, ingestion of the 25 mg oral cannabis dose in this study decreased the number of correct responses on the PASAT by 18 on average, while the same dose (25 mg THC) of smoked and vaporized cannabis reduced correct PASAT responses by an average of 16 and 22, respectively, in a recent study (Spindle et al., 2018). Taken together, these findings highlight why oral cannabis products are at high risk for eliciting accidental acute overdose, particularly among infrequent users (Barrus et al., 2016; Hudak et al., 2015) and why these products are responsible for the majority of emergency room visits related to cannabis intoxication (Monte et al., 2019). Consumers of cannabis products, as well as cannabis vendors, should be aware of the delayed onset of effects for oral cannabis and difficulties with dose titration. This knowledge is particularly important for novice users, who may be most at risk for acute overdose from oral cannabis products.
Blood concentrations of THC and THC principal metabolites (11-OH-THC and THCCOOH) were significantly correlated with key subjective drug effect and cognitive performance outcomes in this study. Despite the significant correlations observed between blood cannabinoid levels and cognitive performance, blood THC concentrations in this study were far lower than peak concentrations observed after cannabis inhalation in prior studies (Fabritius et al., 2013; Newmeyer et al., 2016); see Fig. 4. Differences in blood THC concentrations between oral and inhaled cannabis may stem from orally-ingested THC undergoing first pass metabolism which does not occur when THC is inhaled [(for review see: (Huestis, 2007)], and also the fact that oral ingestion results in a slower rate of absorption which can result in lower systemic concentrations in the blood that may not reflect concentrations in the brain and other tissues. Indeed, despite the 25 and 50 mg THC doses producing marked impairment, mean peak blood THC concentrations were only 2.2 and 3.6 ng/mL, respectively. These are near or below per se quantitative THC cutoffs often used by law enforcement to detect impaired drivers. Moreover, when chronic cannabis users abstained from cannabis for 1 week in a monitored inpatient facility, several individuals had THC concentrations as high as 3 ng/mL on Day 7 (Karschner et al., 2009). Thus, blood THC is unlikely to suffice as a reliable indicator of cannabis intoxication for oral cannabis administration despite the positive correlation with pharmacodynamic endpoints observed in this study, and suggests that novel biomarkers (other than THC or its metabolites) and/or behavioral tests of impairment are needed to address concerns associated with drivers impaired due to acute cannabis exposure.
Another important finding from this study was the considerable inter-individual variability in pharmacodynamic effects of cannabis, despite the fact that all participants were infrequent users with little if any variability in pharmacologic tolerance to the effects of cannabis/THC. For example, some users displayed impaired performance after ingestion of the 10mg THC dose, even though, on average, this dose did not alter performance relative to placebo. In a similar vein, some users displayed little to no impairment following ingestion of the highest dose (50 mg THC), which produced drastic alterations in performance in the majority of participantsparticipant. Sex differences (i.e., main effects of sex) were observed for some subjective drug effect measures, but no Sex x Dose interactions were observed for any pharmacodynamic outcomes. However, we were likely underpowered to detect such interactions given the relatively small sample size (i.e., 9 males; 8 females). Additional controlled research, with larger sample sizes, is needed to identify individual-level factors (e.g., age, sex, genetics, prior experiences with cannabis) that may contribute to such variability in pharmacodynamic cannabis effects among non-tolerant individuals. Further exploration of potential sex differences on pharmacodynamic outcomes following oral cannabis dosing is particularly important, given that prior studies have found that women exhibit greater sensitivity to acute effects from smoked cannabis compared to men (Cooper and Haney, 2014).
This study had several limitations. First, THC-dominant cannabis was the only cannabis type used in this study, but the retail market contains many other variants of cannabis such as cannabidiol (CBD)-dominant and “hybrid” (i.e., equal proportions of THC and CBD) chemovars. Second, though we were adequately powered for detecting dose effects using within-subjects comparisons, our sample size was underpowered to test for individual-level participant characteristics (e.g., race, sex, age) that may have influenced our study outcomes. Thus, additional research is needed to evaluate between-subjects characteristics such as sex, prior experience with cannabis use, genetics, and other factors that may differentiate the acute pharmacodynamic response to oral cannabis. Third, this study was conducted in a controlled laboratory setting which may limit the generality of our findings to drug effects that may be influenced by physical or social context.
In conclusion, the present study characterized pharmacodynamic drug effects following consumption of oral cannabis in a sample of infrequent cannabis users; results indicated that pharmacodynamic effects were altered in a dose-dependent manner. That is, the smallest active cannabis dose administered (10 mg THC) produced discriminable drug effects and elevated heart rate relative to placebo but did not alter performance on a battery of cognitive and psychomotor tests. Conversely, the two highest oral cannabis doses administered (25 and 50 mg THC) produced substantial subjective drug effects and markedly impaired cognitive and psychomotor functioning. These acute cannabis effects often did not peak until several hours after dose administration, which stands in stark contrast to inhaled forms of cannabis where such effects occur almost immediately. Novice users should be educated about how to avoid acute overdose from cannabis edibles. In particular, knowledge regarding the delayed onset of effects for oral cannabis products should be widely disseminated in locations where cannabis can be purchased legally.
Acknowledgments
We thank the support staff of the Johns Hopkins University Behavioral Pharmacology Research Unit for outstanding contributions to the implementation of this study. We also thank Dr. Christine Moore and Ms. Cynthia Coulter at Immunalysis Inc., support staff at RTI International, and all the individuals involved in the NIDA Drug Supply Program.
Funding
This research was supported by the Substance Abuse and Mental Health Services Administration (SAMHSA) and the National Institute on Drug Abuse (NIDA; T32DA07209). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or SAMHSA.
Footnotes
Declaration of Competing Interest
Dr. Vandrey has served as a paid consultant for Zynerba Pharmaceuticals and Canopy Health Innovations Inc., and had received honoraria for serving on the scientific advisory boards of FSD Pharma and Present Life Corporation. The remaining authors have no conflicts of interest to declare.
References
- Allen JA, Davis KC, Duke JC, Nonnemaker JM, Bradfield BR, Farrelly MC, 2017. New product trial, use of edibles, and unexpected highs among marijuana and hashish users In Colorado. Drug Alcohol Depend. 176, 44–47. [DOI] [PubMed] [Google Scholar]
- Arkell TR, Lintzeris N, Kevin RC, Ramaekers JG, Vandrey R, Irwin C, Haber PS, McGregor IS, 2019. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 236, 2713–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrus DG, Capogrossi KL, Cates SC, Gourdet CK, Peiper NC, Novak SP, Lefever TW, Wiley JL, 2016. Tasty THC: Promises and Challenges of Cannabis Edibles. Methods Rep RTI Press; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borodovsky JT, Crosier BS, Lee DC, Sargent JD, Budney AJ, 2016. Smoking, vaping, eating: Is legalization Impacting the way people use cannabis? Int. J. Drug Policy 36, 141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casswell S, Marks D, 1973. Cannabis Induced Impairment of performance of a divided attention task. Nature 24, 60–61. [DOI] [PubMed] [Google Scholar]
- Cone EJ, Johnson RE, Paul BD, Mell LD, Mitchell J, 1988. Marijuana-laced brownies: behavioral effects, physiologic effects, and urinalysis In humans following Ingestion. J. Anal. Toxicol 12, 169–175. [DOI] [PubMed] [Google Scholar]
- Cooper ZD, Haney M, 2014. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend. 136, 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, Middleton P, Henry J, 2002. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 164, 61–70. [DOI] [PubMed] [Google Scholar]
- Fabritius M, Chtioui H, Battistella G, Annoni JM, Dao K, Favrat B, Fornari E, Lauer E, Maeder P, Giroud C, 2013. Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Anal. Bioanal. Chem 405, 9791–9803. [DOI] [PubMed] [Google Scholar]
- Fogel JS, Kelly TH, Westgate PM, Lile JA, 2017. Sex differences in the subjective effects of oral Delta(9)-THC in cannabis users. Pharmacol. Biochem. Behav 152, 44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronwall DM, 1977. Paced auditory serial-addition task: a measure of recovery from concussion. Percept. Mot. Skills 44, 367–373. [DOI] [PubMed] [Google Scholar]
- Herrmann ES, Cone EJ, Mitchell JM, Bigelow GE, LoDico C, Flegel R, Vandrey R, 2015. Non-smoker exposure to secondhand Cannabis Smoke II: effect of room ventilation on the physiological, subjective, and behavioral/cognitive effects. Drug Alcohol Depend. 151, 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudak M, Severn D, Nordstrom K, 2015. Edible cannabis-induced psychosis: intoxication and beyond. Am. J. Psychiatry 172, 911–912. [DOI] [PubMed] [Google Scholar]
- Huestis MA, 2007. Human cannabinoid pharmacokinetics. Chem. Biodivers 4, 1770–1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschner EL, Schwilke EW, Lowe RH, Darwin WD, Pope HG, Herning R, Cadet JL, Huestis MA, 2009. Do Δ9-tetrahydrocannabinol concentrations indicate recent use in chronic cannabis users? Addiction 104, 2041–2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp AA, Lee DC, Borodovsky JT, Auty SG, Gabrielli J, Budney AJ, 2018. Emerging trends in Cannabis administration among adolescent Cannabis users. J. Adolesc. Health 64, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostadinov V, Roche A, 2017. Bongs and baby boomers: trends in cannabis use among older Australians. Aust. J. Ageing 36, 56–59. [DOI] [PubMed] [Google Scholar]
- Lamy FR, Daniulaityte R, Sheth A, Nahhas RW, Martins SS, Boyer EW, Carlson RG, 2016. “Those edibles hit hard”: Exploration of Twitter data on cannabis edibles in the U.S. Drug Alcohol Depend. 164, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Charnigo RJ, Stinchcomb AL, Hays LR, 2013. Pharmacokinetic and pharmacodynamic profile of supratherapeutic oral doses of Delta(9) -THC in cannabis users. J. Clin. Pharmacol 53, 680–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling J, 1982. An automated version of the digit symbol substitution test (DSST). Behav. Res. Meth. Instr 14, 463–466. [Google Scholar]
- Monte AA, Shelton SK, Mills E, Saben J, Hopkinson A, Sonn B, Devivo M, Chang T, Fox J, Brevik C, Williamson K, Abbott D, 2019. Acute illness associated with Cannabis use, by route of exposure: an observational study. Ann. Intern. Med 170, 531–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Barnes AJ, Abulseoud OA, Scheidweiler KB, Huestis MA, 2016. Free and glucuronide whole blood cannabinoids’ pharmacokinetics after controlled smoked, vaporized, and oral Cannabis administration in frequent and occasional Cannabis users: identification of recent Cannabis intake. Clin. Chem 62, 1579–1592. [DOI] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Abulseoud OA, Huestis MA, 2017a. Subjective and physiological effects, and expired carbon monoxide concentrations in frequent and occasional cannabis smokers following smoked, vaporized, and oral cannabis administration. Drug Alcohol Depend. 175, 67–76. [DOI] [PubMed] [Google Scholar]
- Newmeyer MN, Swortwood MJ, Taylor ME, Abulseoud OA, Woodward TH, Huestis MA, 2017b. Evaluation of divided attention psychophysical task performance and effects on pupil sizes following smoked, vaporized and oral cannabis administration. J. Appl. Toxicol 37, 922–932. [DOI] [PubMed] [Google Scholar]
- Niedbala RS, Kardos KW, Fritch DF, Kardos S, Fries T, Waga J, Robb J, Cone EJ, 2001. Detection of marijuana use by oral fluid and urine analysis following single-dose administration of smoked and oral marijuana. J. Anal. Toxicol 25, 289–303. [DOI] [PubMed] [Google Scholar]
- Russell C, Rueda S, Room R, Tyndall M, Fischer B, 2018. Routes of administration for cannabis use - basic prevalence and related health outcomes: a scoping review and synthesis. Int. J. Drug Policy 52, 87–96. [DOI] [PubMed] [Google Scholar]
- Russo EB, 2011. Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol 163, 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer GL, King BA, Bunnell RE, Promoff G, McAfee TA, 2016. Toking, vaping, and eating for health or fun: marijuana use patterns in adults, U.S., 2014. Am. J. Prev. Med 50, 1–8. [DOI] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R, 2018. Acute effects of smoked and vaporized cannabis in healthy adults who infrequently use cannabis: a crossover trial. JAMA Netw. Open 1, e184841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Bonn-Miller MO, Vandrey R, 2019a. Changing landscape of cannabis: novel products, formulations, and methods of administration. Curr. Opin. Psychol 30, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindle TR, Cone EJ, Schlienz NJ, Mitchell JM, Bigelow GE, Flegel R, Hayes E, Vandrey R, 2019b. Acute pharmacokinetic profile of smoked and vaporized Cannabis in human blood and oral fluid. J. Anal. Toxicol 43, 233–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald S, Wong PO, Cohen BE, Ishida JH, Vali M, Madden E, Keyhani S, 2018a. Smoking, vaping, and use of edibles and other forms of marijuana among U.S. adults. Ann. Intern. Med 169, 890–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steigerwald S, Wong PO, Khorasani A, Keyhani S, 2018b. The form and content of cannabis products in the United States. J. Gen. Intern. Med 33, 1426–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Stitzer ML, Mintzer MZ, Huestis MA, Murray JA, Lee D, 2013. The dose effects of short-term dronabinol (oral THC) maintenance in daily cannabis users. Drug Alcohol Depend. 128, 64–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO, 2015. Cannabinoid dose and label accuracy in edible medical cannabis products. JAMA 313, 2491–2493. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Herrmann ES, Mitchell JM, Bigelow GE, Flegel R, LoDico C, Cone EJ, 2017. Pharmacokinetic profile of oral cannabis in humans: blood and oral fluid disposition and relation to pharmacodynamic outcomes. J. Anal. Toxicol 41, 83–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, Ambre J, de Wit H, 2002. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 161, 331–339. [DOI] [PubMed] [Google Scholar]


