Abstract
Recent advances have re-defined a role for T cell factor 1 (Tcf1) that goes beyond T cell development and T memory formation and encompasses new functions in the regulation of T cell biology. Here, we discuss the multi-faceted and context-dependent role of Tcf1 in peripheral T cells, particularly during disease-induced inflammatory states such as autoimmunity, cancer, and chronic infections. Understanding how Tcf1 fine-tunes peripheral T cell biology holds the potential to tailor improved immune-targeted therapies.
One sentence summary:
This review discusses Tcf1, which plays context-dependent roles in T cell function during autoimmunity, cancer, and chronic infection.
Introduction
T cell factor 1 (Tcf1, encoded by Tcf7) is the key transcription factor of the canonical Wnt signaling pathway (Figure 1A). The Wnt signaling pathway is evolutionarily conserved and regulates a variety of fundamental processes such as development, cell-fate specification, and maintenance of tissue homeostasis. In mammals, this pathway comprises 19 Wnt ligands and 15 Frizzled (Fzd) receptors and co-receptors (https://web.stanford.edu/group/nusselab/cgi-bin/wnt/). Notably, the components of the Wnt signaling pathway are differentially expressed in a spatiotemporal manner across cell types, making its study particularly challenging. Tcf1 was cloned in 1991 as a T lymphocyte-specific transcript containing a DNA-binding high-mobility group (HMG) box (1). It is known to play a crucial role in T cell fate specification by initiating a T cell gene program downstream of Notch signaling (2). Accordingly, Tcf7 germline knockout mice display severely impaired T cell development (3, 4). In mature T cells, Tcf1 is known to be critical for the generation of the CD8+ T cell memory response (5). Several recent studies have uncovered new roles for Tcf1 in the regulation of peripheral T cell responses in autoimmunity, cancer, and chronic viral infection, leading to a resurgence of interest into this factor.
Figure 1. The Canonical Wnt Signaling Pathway and Tcf1.

(A) In the absence of Wnt ligands (left), intra-cytoplasmic β-catenin is sequestered in the “destruction complex”, which consists of the scaffold protein Axin2, APC, GSK3β, and CK1. This complex phosphorylates β-catenin marking it for ubiquitination and subsequent proteasomal degradation. Wnt ligand binding to its receptor complex (right), comprised of the Fzd receptor and the LRP 5 and 6 co-receptor proteins, leads to recruitment of the destruction complex to the cell membrane followed by its disassembly and accumulation of β-catenin in the cytoplasm. At this stage, β-catenin translocates into the nucleus where it displaces the transcriptional repressor proteins Groucho/TLE and binds to the nuclear Tcf1/Lef1 proteins to activate gene transcription. (B) Schematic view of long (p45) and short (p33) Tcf1 protein isoforms. The β-BD (red), HDAC (orange), and in HMG (blue) domains are depicted.
APC, adenomatous polyposis coli; GSK3β, glycogen synthase kinase 3β; CK1, casein kinase 1; Fzd, frizzled; LRP, low-density lipoprotein receptor-related proteins; TLE, transducin-like Enhancer of split; Tcf, T cell factor; Lef, Lymphoid enhancer-binding factor; β-BD, β-catenin binding domain; HDAC, histone deacetylase; HMG, high-mobility group.
Tcf1 has long and short isoforms, which differ by the presence or absence of the N-terminal β-catenin interacting domain, respectively (Figure 1B). Although it was initially suggested that short Tcf1 isoforms act as dominant negative regulators, studies employing knockout mice specific for the long isoform (p45) demonstrated that thymocytes were able to undergo maturation without any overt signs of developmental arrest, although with reduced survival (6). Accordingly, mice lacking both β- and γ-catenin, which bind to and activate the long Tcf1 isoform, display normal T cell development (7, 8). Lastly, in a model of acute viral infection, long isoforms were dispensable in CD8+ T cells during the effector phase, but necessary for optimal generation of the memory CD8+ T cell pool (9). Together these data question the negative regulatory role for the short isoforms and further indicate that Tcf1 can have β-catenin-independent functions.
In addition to its function as a transcription factor, Tcf1 has also been described to have chromatin remodeling functions. Tcf1 has an intrinsic histone deacetylase (HDAC) activity (10) and can act as a pioneering factor by opening condensed chromatin (11) (Figure 1B). Both of these chromatin remodeling activities of Tcf1 have been implicated in its role in promoting T cell fate specification. Whether the chromatin remodeling functions of Tcf1 also operate in its regulation of peripheral T cell responses is currently unknown. Thus, additional work is needed to further elucidate the role of Tcf1 isoforms, the β-catenin-dependent and -independent functions of Tcf1, and its transcriptional versus chromatin remodeling functions in T cells. Although many questions regarding how Tcf1 mediates its effects remain, it is clear that Tcf1 has critical roles in T cell biology and increasing evidence now points to other roles in peripheral CD4+ and CD8+ T cells. Indeed, Tcf1 appears to regulate both stemness and the differentiation pathways of CD4+ T cells in autoimmunity and of CD8+ T cells in chronic viral infection and cancer.
Tcf1 regulation of autoimmunity
Self-reactive CD4+ T cells are central in the development and progression of autoimmune diseases. In humans, it is estimated that random TCR rearrangement can potentially generate ~1018 different specificities (12). Although central and peripheral mechanisms of tolerance either eliminate or inactivate auto-reactive T cell clones (13), these mechanisms are imperfect. Moreover, autoimmune disorders such as multiple sclerosis (MS), psoriasis, type 1 diabetes (T1D), rheumatoid arthritis (RA) and inflammatory bowel disease (IBD) have steadily increased over the last four decades (14). Therefore, understanding the factors that regulate T cell-driven autoimmune inflammation is increasingly important. In this regard, genome-wide association studies have identified a nonsynonymous single nucleotide polymorphism (SNP) (C883A) in the coding region of TCF7 and multiple SNPs within the non-coding regulatory regions of the TCF7 gene locus as being associated with increased disease risk in T1D and MS, respectively (15, 16). Although it is currently unknown whether these polymorphisms impact TCF7/TCF1 expression and function, these data further support a role for TCF1 in the regulation of autoinflammatory T cell responses.
Naïve CD4+ T cells can differentiate into various T helper (Th) subsets depending on the local micro-milieu in which they are embedded. Four major CD4+ Th subsets have been described, each defined by their expression of a signature cytokine and a master transcription factor: IFNγ+ T-bet+ Th1, IL-4+ Gata3+ Th2, IL-17A+ Rorγt+ Th17, and IL-21+ Bcl6+ T follicular helper (Tfh) cells. In this review, we will focus on Tcf1-mediated regulation of autoinflammatory Th1 and Th17 cells. The role of Tcf1 in the biology of Th2 and Tfh cells has been extensively reviewed elsewhere (5, 17). Of note, Tcf1 has been demonstrated to have a critical role in the ability of Tfh to support B cell activation and antibody production during viral infection (18, 19). Although studies perturbing Tcf1 in Tfh cells in models of B cell-driven autoimmunity are lacking, it is likely that Tcf1 could impact autoantibody production through its actions in Tfh cells.
Both Th1 and Th17 cells have been implicated in autoimmunity. Initial studies showed a clear presence of IFNγ-producing Th cells in MS and RA patient samples and their respective mouse models: experimental autoimmune encephalomyelitis (EAE) and collagen-induced arthritis (CIA) (14). This observation led to the hypothesis that autoimmune diseases were Th1-mediated. However, mice deficient for key Th1 genes, such as IFNγ or the p35 subunit of IL12 (required to drive Th1 differentiation), were still susceptible to the development of EAE and CIA (20–22), raising the question of whether another Th subset was responsible for driving disease. This conundrum was partially resolved by the discovery of Th17 cells, which require IL-23 for their differentiation. Indeed, mice deficient for the p19 subunit of IL-23 were shown to be resistant to EAE (23). However, mice deficient for T-bet, the master transcription factor for Th1 cells, were also shown to be resistant to EAE, suggesting the existence of a more complex transcriptional network regulating auto-pathogenic T cells (24). Although the precise roles of Th1 and Th17 cells in autoimmunity is still debated, it is generally accepted that both IFNγ and IL-17A have important roles in driving damaging tissue inflammation.
A dual role for Tcf1 in Th1 cells
Evidence for a role for Tcf1 in Th1 cells came from the observation that CD4+ T cells from germline Tcf7 knock-out mice could produce higher levels of IFNγ. However, enforced expression of ICAT, which specifically inhibits the interaction between β-catenin and Tcf1, did not affect IFNγ production by CD4+ T cells (25). Subsequently, it was demonstrated that T-bet can recruit the transcriptional repressor Bcl6 to the Tcf7 promoter and inhibit its gene expression (26). Although these studies suggest reciprocal negative regulation between Tcf1 and key factors involved in Th1 differentiation or effector function, other studies revealed an indispensable role for Tcf1 in the generation of memory Th1 cells (9). Together these data indicate a model whereby Tcf1 plays a dual role in Th1 cells; an early negative regulatory role during Th1 differentiation and, later, a role in establishing memory Th1 cells (Figure 2A). Unfortunately, most of these studies either employed Tcf7 germline knock-out mice, which have dramatically impaired T cell development, or examined Tcf1 indirectly via disruption of β-catenin, which can have Tcf1-independent effects (27). Thus, further study is needed to fully elucidate the role of Tcf1 in Th1 cells.
Figure 2. Role of Tcf1 in Th1 and Th17 differentiation and function.

A) Naïve T cells express high levels of Tcf1, which are downregulated upon Th1 effector cell differentiation. A subset of effector Th1 will re-express Tcf1 acquiring a memory phenotype, which is later crucial to drive a second wave of Th1 effector response. B) Tcf1hi naïve T cells differentiate into Tcf1lo Th17 cells generating a first wave of effector cells. Tcf1 is then re-expressed in a subset of cells to generate a stem-like Th17 subset. This Th17 stem-like population can either self-renew (1); give rise to effector Th17, which mainly produce IL-17A, in response to environmental cues or asymmetric partitioning of Tcf1 (2); or produce highly tissue destructive Th17/Th1-like cells that are polyfunctional and can secrete IL-17A, IFNγ, GM-CSF, and TNFα (3).
Tcf, T cell factor; Th1, T helper 1; Th17, T helper 17; IL-17A, interleukin 17; IFNγ, interferon gamma; GM-CSF, granulocyte-macrophage colony-stimulating factor; TNFα, tumor necrosis factor alpha.
Role in Th17 differentiation, plasticity, and stem-like phenotype
Initial evidence for a role for Tcf1 in Th17 biology came from studies utilizing Tcf7 germline knockout mice showing that T cells in these mice had higher propensity to produce IL-17A and, consequently, exhibited exacerbated EAE (28, 29). However, the expression of several genes known to specify the Th17 lineage (Rorc, Irf4, Runx1) were unaffected in Tcf1−/− T cells. Further, enforced expression of Tcf1 in Tcf1−/− cells could not revert the increase in IL17A. Together these data indicate that Tcf1 deficiency impacts IL-17A production without affecting overall Th17 lineage commitment. In contrast, constitutively active β-catenin signaling in CD4+ T cells from CD4Cre-Ctnnb1ex3 mice was shown to drive the activation of a Th17 pro-inflammatory program. Although chromatin-immunoprecipitation (ChIP)-seq analysis revealed that Tcf1 binds to the Rorc locus in both WT (CD4Cre) and CD4Cre-Ctnnb1ex3 CD4+ T cells, accessible chromatin regions marked by lysine acetylation of histone 3 (H3KAc) were found only in the presence of constitutively active β-catenin signaling (30). This finding is in line with another report showing that Tcf1 regulates the survival of double-positive thymocytes by augmenting Rorγt expression (31). Thus, both pro- and anti-Th17 functions have been described for Tcf1.
In line with studies showing Tcf1-mediated inhibition of IL-17A production in murine Th17 cells, in vitro studies of human CD4+ T cells showed that Th17 differentiation was enhanced by the presence of Wnt pathway inhibitors, such as secreted frizzled-related protein-1 (sFRP1), dickkopf-related protein 1 (DKK-1), and Wnt inhibitory factor 1 (WIF-1), whereas it was reduced by both canonical and non-canonical Wnt signaling through stimulation with Wnt3a and Wnt5a, respectively (32). Of note, these studies did not directly examine TCF1. Further, the observed Wnt pathway-mediated inhibition of Th17 differentiation was suggested to involve repression of the TGFβ pathway, which is important for the generation of both human and murine Th17 cells, although Th17 cells can differentiate in the absence of TGFβ, particularly during inflammation (33, 34). Lastly, none of the above studies addressed the role of Tcf1 in regulating pro-inflammatory (pathogenic) versus homeostatic (non-pathogenic) Th17 subtypes (35, 36), which could have important implications for autoimmunity.
Tcf1 has also been implicated in Th17 longevity and plasticity. In vitro differentiated Th17 cells have been shown to be long-lived and adopt a stem-like phenotype characterized by high Tcf7 expression after transfer in vivo (37). By virtue of these properties, adoptively transferred antigen-specific Th17 cells were not only effective in eradicating established tumors but also drove autoimmune-like toxicity. Interestingly, the ability of Th17 cells to eradicate tumors, and by extension mediate autoimmune-like toxicity, was dependent on their capacity to further differentiate and acquire Th1-like features while retaining IL17A-production. Indeed, the emergence of Th17 cells bearing Th1-like properties (IL17A+IFNγ+) has been shown to be a key pathogenic determinant in different models of autoimmunity, such as EAE and IBD, and is highly dependent on IL-23 receptor signaling (38–40). Further supporting a role for Tcf1 in Th17 stemness, single-cell analysis of Th17 cells during EAE revealed high expression of Tcf7 and presence of a stem-like transcriptional signature in IL17A-producing cells harvested from the central nervous system (CNS)-draining lymph nodes (41). Lastly, Tcf1 expression has been shown to mark a Th17 population with stemness features and low anabolic metabolism that can subsequently give rise to a Th1-like effector subset responsible for sustaining neuro-inflammation during EAE progression (42). Although it was suggested that Tcf1 may act as a metabolic gatekeeper to stabilize Th17 cell lineage identity and effector function, perturbations of Tcf1 were not performed, leaving the precise role of Tcf1 unclear.
Thus, emerging data together with the association of SNPs in TCF7 with susceptibility to autoimmunity, point to a role for Tcf1 in regulating the differentiation, stemness, and pathogenic potential of Th1 and Th17 cells. However, its precise role remains unclear due to conflicting data. This could be due to several reasons: the use of germline knockout mice that have aberrant T cell development, examination of settings where the β-catenin/Tcf1 pathway is perturbed before T cell maturation, and Tcf1-independent actions mediated by constitutively-active β-catenin signaling (Table 1). Current data are consistent with a model whereby Tcf1 initially restrains the effector differentiation of Th17 cells but later its re-expression is required to maintain long-lived precursor cells harboring the potential of generating highly pathogenic IL17A+IFNγ+ cells (Figure 2B). In such a model, fluctuations in Tcf1 expression may finely tune effector functions, differentiation trajectory, as well as long-term fitness and persistence. Studies using sophisticated conditional and/or inducible lineage-specific knock-out mice will be required to test this model. Further, to what extent the transcriptional versus chromatin remodeling functions of Tcf1 and how Tcf1 is partitioned to daughter cells during cell division (discussed below) affect mature CD4+ T cell biology remains to be addressed.
Table I.
Studies examining modulation of Wnt signaling and Tcf1.
| T subsets | Experimental Models | Perturbations | Findings | Ref |
|---|---|---|---|---|
| Murine Th1 | In vitro | Tcf7 germline KO | Tcf1 represses IFNγ | 25 |
| Murine Th1/Tfh | LCMV | Tcf7 long isoforms KO | Tcf1 required for T memory formation | 9 |
| Murine Th17 | In vitro, EAE | Tcf7 germline KO | Tcf1 represses IL17a | 28, 29 |
| Murine Th17 | Colitis, colorectal cancer | Constitutively active β-catenin | Tcf1 binds Rorc locus in chromatin accessible regions | 30 |
| Murine Thymocytes | T cell development | Tcf7 germline KO, constitutively active β-catenin | Tcf1 promotes thymocyte survival via enhancement of Rorγt | 31 |
| Human Th17 | In vitro | Wnt inhibitors | Enhanced Th17 differentiation | 32 |
| Human Th17 | In vitro | Wnt3a, Wnt5a stimulation | Reduced Th17 differentiation | 32 |
| Murine Tregs | In vivo T cell development | Tcf7 hemizygous | Higher Treg percentage | 44 |
| Human and murine Tregs | In vitro | None | Tcf1 interacts with and inhibits Foxp3-mediated transcription | 45 |
| Human and murine Tregs | In vitro | Gsk3β inhibitor, Wnt3a stimulation | Reduced Treg suppressive capacity | 45 |
| Murine Tregs | In vivo steady state | Constitutively active β-catenin | Spontaneous colitis | 30 |
| Murine Tregs | In vivo steady state | Treg-specific Tcf1/Lef1 double KO | Spontaneous multi-organ autoimmunity | 46,47 |
Tcf1 modulation of regulatory T cells
Recent studies suggest an important role played by Tcf1 in modulating regulatory T cells (Treg) function. In the periphery, Treg are critical for preserving tolerance and resolving tissue inflammation. It has been proposed that strong and transient stimulation of TCRs with natural affinity against self-antigens drives the development of Tregs in the thymus (43). In this regard, in vitro studies suggested that Tcf7 hemizygosity lowered the TCR affinity threshold required for the engagement of a Treg developmental program (44). Accordingly, Tcf7+/− mice exhibited higher Treg frequencies in vivo. Another study involving Tcf7 germline knock-out mice demonstrated that Tcf1 can physically interact with and inhibit Foxp3-mediated transcription by a mechanism called transrepression. Moreover, activation of the β-catenin/Tcf1 pathway using GSK3β inhibitors or Wnt3a stimulation, which can have Wnt pathway-independent effects, impaired the suppressive capacity of human and murine Treg (45). Similarly, Foxp3CreCtnnb1ex3 mice bearing specific constitutive β-catenin activation in Tregs developed spontaneous colonic autoinflammation four months after birth (30). Together these studies suggest a role for Tcf1 in antagonizing Treg development and suppressive function.
However, other studies employing lineage-specific ablation of Tcf1 alone or together with Lymphoid enhancer binding factor 1 (Lef1) have indicated a central role for the Wnt pathway in sustaining Treg suppressive function and survival in vivo. Treg-specific ablation of Tcf1 and Lef1 did not impact Treg cell number but led to spontaneous multi-organ autoimmunity due to both the inability to maintain suppressive activity and to reduced survival downstream of impaired IL-2 -STAT5 signaling (46, 47). Notably, mice selectively lacking Tcf1 alone also displayed autoimmunity, although this was not investigated in detail and disease was milder than that observed in mice lacking both Tcf1 and Lef1 in Treg. Additionally, loss of either Tcf1 or Lef1 resulted in decreased generation of T follicular regulatory (Tfr) cells, which are essential to restrain Tfh and B cell immune responses in germinal centers. Notably, lack of both factors resulted in complete loss of Tfr.
As with conventional CD4+ T cells, the conflicting data regarding the role of Tcf1 in Treg is likely due to multiple factors: the examination of germline Tcf7 knockout mice versus mice that delete Tcf7 specifically in Treg, the examination of mice with concomitant Lef1 deletion or expressing constitutively active β-catenin, or the use of GSK3β inhibitors, which are known to have multiple effects beyond the canonical Wnt pathway (Table 1). The studies employing lineage-specific ablation of Tcf1 in Tregs are more likely to reflect the function of Tcf1 in mature peripheral Treg. Indeed, the fact that Tregs express lower levels of Tcf1 compared to other conventional CD4+ T cell subsets supports a model whereby low Tcf1 levels limit inhibition of FoxP3-driven transcription while maintaining sufficient activity needed for Treg persistence in tissues (46).
Tcf1 regulation of CD8+ T cells in cancer and chronic viral infection
CD8+ T cells are critical effectors required for combating chronic viral infections and cancer. However, the persistent exposure to antigens along with the environmental cues present in these chronic inflammatory diseases prime CD8+ T cells towards an altered differentiation trajectory, resulting in the acquisition of a dysfunctional or “exhausted” cell state. Preventing or mitigating this state has been considered to be the key to achieving successful control of cancer and chronic viral infections. Indeed, therapies that block immune inhibitory or checkpoint receptors (i.e. immune checkpoint blockade; ICB) that are highly expressed on dysfunctional CD8+ T cells have been shown to boost anti-tumor or anti-viral responses and have led to unprecedented durable responses in multiple cancers previously considered untreatable (48, 49). However, recent studies investigating how ICB affects the CD8+ T cell response in these disease contexts show limited and transient effects on dysfunctional CD8+ T cells. Instead, these studies identified populations of CD8+ T cells that rely on Tcf1 and are requisite for successful response to ICB.
Tcf1+ stem-like CD8+ T cells
Seminal studies in models of chronic viral infections identified a subset of Tcf1+ CD8+ T cells that is responsible for the proliferative burst upon anti-PD1 therapy and is critical for viral control (50–53). A major feature of these Tcf1+ T cells is their self-renewal potential. Adoptive transfer experiments demonstrated that only Tcf1+ T cells, but not their Tcf1− counterparts, had the capacity to both self-renew and give rise to a progeny of Tcf1− cells endowed with effector potentials and high levels of checkpoint receptor expression (50–54). Studies in tumor models further revealed that genetic deletion of Tcf7 in CD8+ T cells (55) or deletion of Tcf1-expressing cells (56) limited the efficacy of ICB in mouse models of cancer. Similar to the findings in chronic viral infection models, tumor-infiltrating Tcf1+ T cells are endowed with better long-term persistence, improved capacity to control tumor growth and are singly capable of both self-renewing as well as of giving rise to terminally dysfunctional T cells (54, 57). The clinical relevance of the TCF1+ T cell subset was further supported by the positive association between the presence of tumor-infiltrating TCF1+ T cells in melanoma patients and responses to ICB (58) as well as progression-free and overall survival (57). Moreover, TCF1+PD1+ cells were found to be expanded in patients with non-small cell lung cancer (NSCLC), colorectal tumors, and melanomas upon treatment with ICB (56, 57, 59).
In chronic infection models, three recent studies employing single-cell genomics have shed light on the relationship of Tcf1+ stem-like CD8+ T cells to other CD8+ T cell populations generated in this context (60–62). Four states of T cell differentiation have been described: 1) a Tcf1high stem-like precursor that is quiescent and tissue resident, 2) a Tcf1+ precursor that is blood accessible and highly proliferative; 3) an effector-like Tcf1−T-bethigh transitory population, which relies on CD4+ T cell-derived IL21 for its formation (61), retains high effector and killing capacity and is highly expanded upon ICB, and 4) a late dysfunctional Tcf1−Eomeshigh population that expresses high levels of checkpoint receptors, lacks proliferative capacity, and cytokine polyfunctionality but retains some killing capability (Figure 3). In tumors, similar CD8+ T cell states have been described with a naïve-like state in addition to the stem-like, effector, and dysfunctional states. The latter state comprises cells with differential functional and proliferative capacities akin to the transitory proliferating and late dysfunctional cells described in chronic viral infection (63–65). Of note, data from human studies suggest the existence of a cytotoxic-effector state that is largely composed of non-tumor reactive “bystander” cells (66, 67).
Figure 3. Tcf1 and its relationship to CD8+ T cell states in chronic viral infections and tumors.
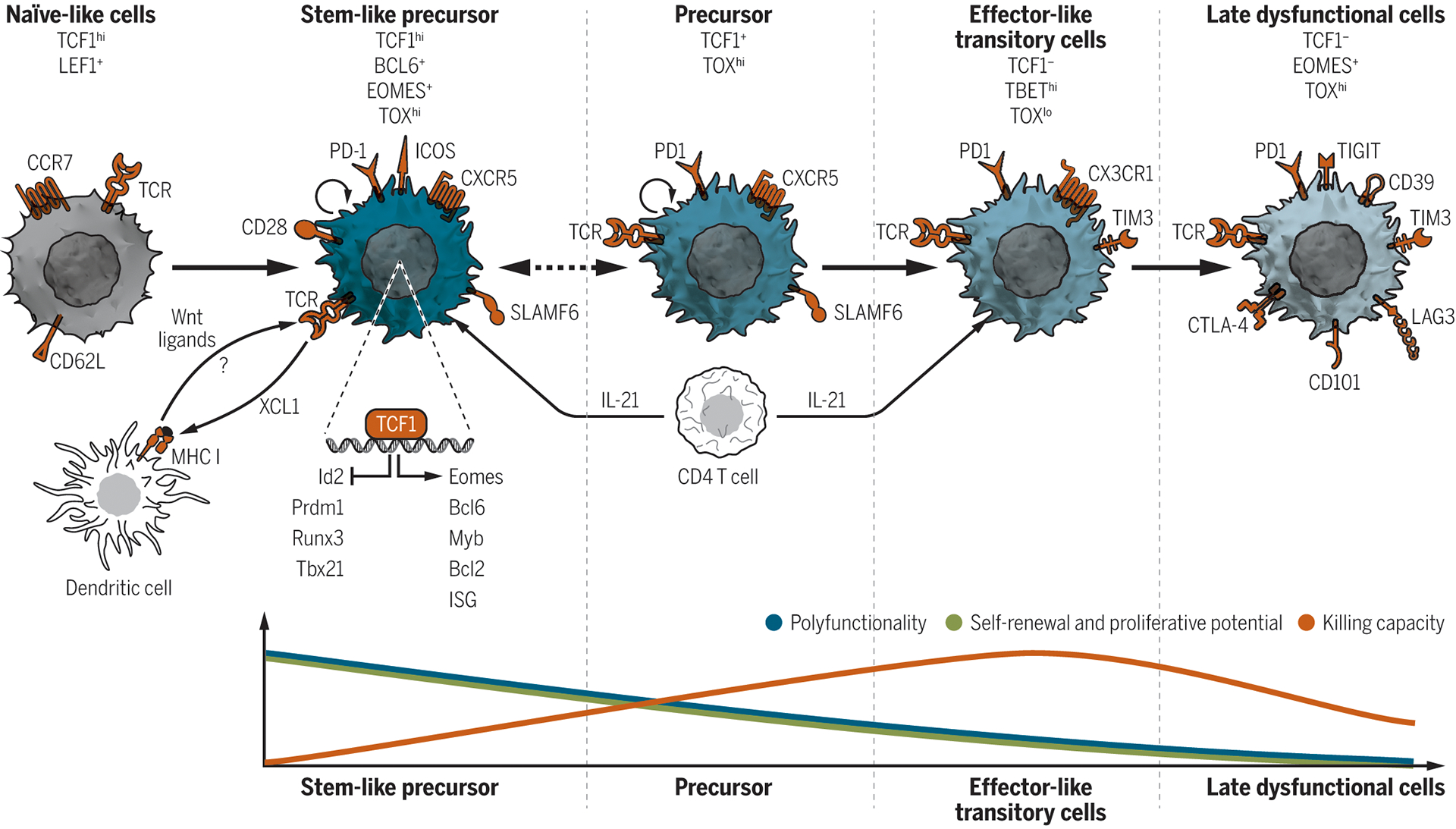
Proposed model of the differentiation trajectories and features of the CD8+ T cell states present in cancer and chronic viral infections. Naïve-like T cells express high levels of the transcription factors Tcf1 and Lef1 and the lymphocyte homing and adhesion molecules Ccr7 and CD62L. Tcf1 also marks a downstream population of stem-like precursors CD8+ T cells characterized by high self-renewal capacity, proliferative potential, and polyfunctionality. These cells can sustain the entire CD8+ T cell output and are mostly quiescent in vivo although retaining high proliferative potential. Tcf1 promotes the formation, maintenance and functions of these cells by inhibiting the expression of effector-associated genes such as Runx3, Prdm1, Id2, and Tbx21 and by promoting memory-associated genes such as Eomes, cMyb, Bcl6, ISGs, and the pro-survival gene Bcl2. Phenotypically stem-like precursor cells express Slamf6, Cxcr5, low or intermediate levels of Pd1 as well as the Cd28 and Icos co-stimulatory molecules. The ability of stem-like precursor cells to express the Xcl1 chemokine may favor their interaction with DCs. Wnt ligands produced by DCs may activate the Wnt-Tcf1 pathway in neighboring stem-like precursor cells further regulating their maintenance and functions. Tcf1 is further expressed, albeit at lower levels, in a second subset of precursor cells. These precursors express the Pd1 receptor, are blood-accessible, highly proliferative at steady state and give rise to downstream effector-like transitory cells. The transition from precursors to effector-like transitory cells is associated with downregulation of Tcf1 expression and up-regulation of the transcription factor T-bet, which antagonizes the Tox-mediated induction of late dysfunctional phenotype in transitory effector-like T cells. Phenotypically, transitory cells express the Pd1 and Tim3 checkpoint receptors and Cx3cr1. They expand upon ICB and contribute to viral and tumor control. These T cells ultimately convert into late-dysfunctional T cells that express high levels of checkpoint receptors (Pd1, Tim3, Lag3, Ctla4, Cd39, Tigit, Cd101), lack proliferative capacity, display low polyfunctionality, but retain killing capability. Late dysfunctional T cells increase Eomes and Tox expression, the latter antagonizing T-bet activity. CD4+ T cell-derived IL21 instructs the priming and differentiation of Tcf1+ stem-like T cells and is required for the generation of the Cx3cr1+ effector-like transitory T cells.
Ccr7, CC-chemokine receptor 7; Cx3cr1, Cx3 chemokine receptor 1; Ctla-4, cytotoxic lymphocyte-associated antigen 4; Lag3, lymphocyte activation gene 3; Pd-1, programmed cell death 1; Tigit, T cell immunoreceptor with Ig and ITIM domains; Tcf7, transcription factor 7; Lef, lymphoid enhancer-binding factor; Tox, thymocyte selection associated high mobility group box; Eomes, eomesodermin; ISG, interferon-stimulated genes; Prdm1, PR domain zinc finger protein 1; Bcl2, B-cell lymphoma 2; Id2, inhibitor of DNA binding 2; Bcl6, B-cell lymphoma 6 protein.
These observations have raised several questions such as: which T cell state is required for the response to ICB, what are the environmental and cell intrinsic factors driving the diverse functional states observed, and how can we promote the generation of selected T cell states by ex vivo T cell manipulation or through in vivo interventions to improve current clinical therapies? Late dysfunctional T cells, also referred to as “terminally exhausted” cells, are characterized by decreased effector functions, limited cytotoxic capacity, and high expression of several checkpoint receptors such as programmed cell death protein 1 (Pd-1), 2B4, T cell immunoglobulin mucin receptor 3 (Tim3), lymphocyte activation gene 3 protein (Lag3), cytotoxic T lymphocyte-associated antigen 4 (Ctla-4), and Tigit (68, 69). Although, the success of ICB was initially attributed to the reversion of the dysfunctional state of CD8+ tumor-infiltrating lymphocytes (TILs), subsequent studies have shown that ICB has limited capacity to reprogram dysfunctional CD8+ T cells given their acquisition of a fixed epigenetic state that cannot be remodeled by therapy (64, 70, 71). Indeed, both in tumors and in chronic viral infections, dysfunctional CD8+ T cells are known to harbor transcriptional and epigenetic programs distinct from those observed in conventional memory and effector populations (64, 70–73). As mentioned above, a population of stem-like CD8+ T cells endowed with self-renewal potential, polyfunctional effector, and proliferative capacity has been recently identified in these contexts. Importantly, these cells seem more amenable to reinvigoration by ICB and may be required for durable anti-tumor responses (50–53, 55–59, 64).
Phenotypically, stem-like cells express low or intermediate levels of Pd-1 and other checkpoint receptors and lack the expression of the Tim3 checkpoint receptor (Figure 3). Of note, and as opposed to dysfunctional CD8+ T cells, they uniformly express the transcription factor Tcf1, which is required for their formation, maintenance, and functions (50–53, 55, 56). Stem-like CD8+ T cells also share features with CD4+ Tfh cells, including expression of the inducible T cell co-stimulator (ICOS) molecule and the transcription factors Bcl6 and Id3, leading some groups to refer to these cells as follicular-like CD8+ T cells (50–53, 74). Similar to Tfh cells, stem-like CD8+ T cells in chronic LCMV, HIV, and SIV infection models infiltrate B cell follicles (51, 52, 74) and are characterized by the expression of Cxcr5, a well-known marker of Tfh cells (50–53, 74). Conversely, both in murine and human tumors, Slamf6 is highly expressed on stem-like CD8+ TILs and positively correlates with Tcf1 levels while expression of Cxcr5 has been reported in some studies on human TILs (57, 59, 75, 76).
The ability to self-renew and to give rise to more differentiated progeny are in line with Tcf1+ cells having features of progenitor or stem-like cells. For these reasons, these cells have been referred to as stem-like (50, 54, 56, 77) or progenitor exhausted (57, 73) cells, in accordance with their ability to specifically give rise to late dysfunctional T cells. Because the term “progenitor” implies the potential to give rise to multiple T-cell lineages, others have suggested the use of the term “precursor” (78) to emphasize that Tcf1+ T cells are capable to generate solely CD8+ T lineage cells. Indeed, an initial study showed restricted lineage potential of unsorted dysfunctional virus-specific CD8+ T cells (likely enriched for Tcf1− cells) generated in the context of chronic viral infections upon their transfer into naïve mice (79). However, a second study in tumors showed that bulk tumor-specific (Tag-I) CD8+ T cells, harvested early post tumor injection (and likely containing a higher portion of Tcf1+ cells), could instead differentiate into functional memory cells when transferred into secondary hosts immunized with listeria monocytogenes expressing Tag-I (80). A follow-up study from the same group further confirmed that tumor-specific T cells harvested at early but not late stages of tumor development retain a plastic epigenetic state amenable to reprogramming (64). Similarly, a recent study in chronic LCMV infection revealed that some exhaustion features are initiated early in Tcf1+ CD8+ T cells and thereafter are propagated to their T cell progeny with a fixed epigenetic imprint developing over time (81). Further investigation will be required to ultimately define the lineage potential, plasticity, and reprogrammability of selected subsets of Tcf1+ T cells.
How Tcf1 is partitioned within the progeny of Tcf1+ stem-like CD8+ T cells and whether signals that the T cells receive through the TCR will dictate which daughter cells will retain Tcf1 may be important for determining which cells will be predisposed to initiate differentiation towards dysfunctional cells. This decision may occur at a population level, a model that is commonly referred to as population asymmetry. Population asymmetry postulates that, stochastically, some Tcf1+ stem-like CD8+ T cells will give rise only to Tcf1+ daughter cells while others will give rise only to Tcf1− daughter cells depending on external or intrinsic signals. An alternative model, known as asymmetric cell division, postulates that asymmetric partitioning during cell division of factors present in the stem-like CD8+ T cell will determine which daughter cell will retain Tcf1 and which one will lose Tcf1 expression. A recent paper on CD4+ T cells also suggested that asymmetric cell division drives the fate of CD4+ T cells (82). While in vitro experiments showed no asymmetric partitioning of the available Tcf1 protein during metaphase, Tcf1 was retained only in one of the two daughter cells when conjoined sibling pairs were analyzed. Whether something similar is also occurring in stem-like CD8+ T cells is currently unknown. Lineage-tracing experiments and imaging studies will be required to address this question.
As discussed above, Tcf1 long and short isoforms do not have equal roles in the generation of conventional memory CD8+ T cells. Currently, the role of the Tcf1 short vs long isoforms in mature CD8+ T cells, as well as in stem-like CD8+ T cells generated in tumor and chronic viral infection models, remain largely unknown. Likewise, how the relative abundance of the two isoforms is regulated across CD8+ T cell subsets during differentiation and to what extent this impacts their effector functions remain unexplored. A recent study showed that both the long (p45) and the short (p33) Tcf1 isoforms, ectopically expressed by retroviral vectors in mature LCMV-specific CD8+ T cells, could equally restrain the formation of terminally differentiated CD39+Tim3+ T cells and favor the accumulation of stem-like CD8+ T cells at the early phase of chronic infection. Conversely, in established chronic infection only the long isoform of Tcf1 is able to promote the transcription of stem-like associated genes, such as CD127, CXCR5 and cMyb, and effectively preserve the pool of stem-like CD8+ T cells (73).
Transcriptional regulation of stem-like CD8+ T cells by Tcf1
The generation and maintenance of stem-like CD8+ T cells and the role of Tcf1 was initially studied in the context of chronic viral infection models and only more recently has become an intense area of investigation in the cancer field. Because the tumor microenvironment differs from that found in chronic viral infections, it is likely that while some biological processes will be shared between the two disease contexts, others will be likely shaped by local cues. In both contexts, Tcf1 can induce the expression of the transcription factor Bcl6, similarly to what has been shown in Tfh cells (18, 83), and together these factors synergize to maintain T cell stemness while restraining differentiation towards the dysfunctional T cell state. Accordingly, CD8+ T cells lacking Bcl6 or Tcf1 showed reduced frequency of stem-like precursors (52, 54). Conversely, overexpression of Tcf1 can promote the formation of Tcf1+ (Cxcr5+ or Slamf6+) stem-like precursor CD8+ T cells in the contexts of chronic viral infection and cancer (54, 73, 84, 85). Tcf1 maintains T cell stemness by directly inhibiting Prdm1 expression and allowing the transcription of memory related genes such as Ccr7, IL7ra, Sell, and Cxcr5, that are otherwise repressed by Prdm1. Accordingly, deletion of Prdm1 was shown to promote the expansion of stem-like Tcf1+ CD8+ T cells in the context of chronic viral infections (50, 52, 54). Similarly, the transcription factor Id2 can counteract the activity of Tcf1 and Bcl6, and loss of function studies in mice have shown increased numbers of stem-like CD8+ T cell precursors in the absence of Id2 in the chronic LCMV model (51, 52).
Other transcription factors regulate the maintenance of stem-like CD8+ T cells by either promoting or antagonizing Tcf1. Foxo1, which regulates T cell stemness and memory formation (86) is required for the expression of Tcf1 (87) and is essential for the generation and maintenance of Tcf1+ precursor T cells in chronic viral infection models (88). cMyb has also been shown to promote T cell stemness, memory potential, and survival programs in CD8+ T cells by directly inducing Tcf1 while simultaneously repressing Zeb2 expression, a transcription factor that regulates effector differentiation (89). Of note, over-expression of cMyb in CD8+ T cells could enhance memory formation, polyfunctional capacity, recall responses, and tumor eradication in a melanoma model of adoptive immunotherapy. Dysfunctional Tcf1− CD8+ T cells have been reported to share features with tissue resident memory T cells (TRM) in both tumors and chronic viral models (62, 90, 91). Accordingly, Runx3, a key transcriptional regulator of TRM cells (92), is also highly expressed in late dysfunctional T cells and was reported to negatively regulate Tcf1 expression. Runx3-deficient CD8+ T cells showed increased expression of Bcl6 and Tcf1 and subsequent reduction in the expression of cytotoxic molecules in effector T cells (93). In line with this, a recent report revealed that Tcf1 expression prevents the acquisition of a TRM phenotype in CD8+ T cells by antagonizing TGF-β-induced CD103 expression in a lung infection model (94), suggesting that Runx3 and Tcf1 may antagonize each other.
It has recently been proposed that in the context of chronic LCMV infection, Tcf1 initiates the T cell dysfunction program while antagonizing the conventional effector differentiation pathway (73). Specifically, Tcf1 opposes T-bet expression and favors Eomes and cMyb expression, the latter driving the transcription of the anti-apoptotic Bcl2 gene required for the survival of stem-like precursor CD8+ T cells. Thus, it has been suggested that Tcf1 could act as a fate-decision transcription factor which maintains the pool of precursors that give rise to dysfunctional CD8+ T cell progeny in the context of chronic activation. The dysfunctional program is further epigenetically imprinted by Tox, which is induced by chronic TCR stimulation and NFAT activation (95–100). Importantly, all of these studies revealed that in chronic diseases Tox is necessary for long-term persistence of T cells by preventing activation-induced cell death of overstimulated T cells and for mitigating tissue pathology by inducing the expression of checkpoint receptors on T cells. Accordingly, removal of Tox led to progressive loss of the Tcf1+ stem-like CD8+ T cell pool, transient control of viral load, and increased tissue pathology. Thus, the acquisition of a dysfunctional state by CD8+ T cells can be interpreted as an ‘adaptation state’ established under distinct environmental settings to prevent immunopathology while retaining some levels of effector functions. Nonetheless, it remains unclear how Tox expression regulates Tcf1 locus accessibility and transcription. Indeed, while in the autochthonous liver cancer model the lack of Tox was associated with increased Tcf1 locus chromatin accessibility and transcription (95), another study employing the chronic clone13 LCMV infection model showed opposite results (97).
Environmental and epigenetic regulation of Tcf1+ stem-like CD8+ T cells
Environmental cues may also instruct the generation and maintenance of stem-like Tcf1+ CD8+ T cells. High levels of extracellular potassium can promote increased expression of stemness and memory genes, including Bach2, Tcf7, Klf2, Il7r, and Bcl6, thus supporting the fitness of the stem-like CD8+ T cell pool and the subsequent differentiation of effector CD8+ T cells with better anti-tumor functions. Accordingly, adoptive transfer of T cells ex vivo exposed to high concentration of potassium showed improved cell persistence and melanoma clearance compared to control T cells in vivo (77). Genetic loss of the de novo methyltransferase Dnmt3a in CD8+ T cells can restore the transcription of memory genes, including Tcf1, and counteract terminal effector differentiation. In the chronic LCMV model, loss of Dnmt3a in CD8+ T cells increased the frequency of less dysfunctional T cells and improved viral control upon ICB (72). Cytokine cues may also have a role. For example, the inflammatory cytokine IL12 was shown to suppress Tcf1 transcription and promote effector differentiation in CD8+ T cells through a mechanism that involved, at least in part, the activation of the IL12Rb-STAT4 pathway and induction of Prdm1 (101). Likewise, pro-inflammatory type-I interferon signaling opposes the Tcf1-Bcl6 axis and the generation of stem-like CD8+ T cells (54). Similarly, the strength of TCR signaling and co-stimulation affects T cell fate (102). Accordingly, adaptations in antigen presenting cells (APCs) that result in diminished T cell signaling intensity and co-stimulation, as well as the presence of CD4+ T cell-derived IL21 upon T cell priming, appear to favor the generation of stem-like CD8+ T cells, which are endowed with improved persistence and superior responsiveness to ICB over terminally differentiated T cells in chronic viral infections (103) (Figure 3). Therefore, the combined action of environmental factors such as ions, cytokines, costimulatory molecules, antigen receptor signals, as well as the T cell epigenetic landscape can affect the expression of Tcf1 and thus the generation and function of stem-like CD8+ T cells and their progeny.
Role of Tcf1 in the cross-talk between stem-like CD8+ T cells and other immune cells
Epigenetic profiling of Tcf1+ stem-like versus dysfunctional CD8+ T cells in chronic viral models, revealed open chromatin regions at genes relevant for T cell-DC interactions such as Xcl1 (104), which promotes the recruitment of Xcr1+ conventional type 1 dendritic cells (DC1) that are endowed with superior capacity to process and cross-present antigens to CD8+ T cells (105). Moreover, Tcf1+ stem-like CD8+ T cells express high levels of the costimulatory molecule CD28, which is required for the proliferative burst induced upon PD1/PDL1 ICB (106, 107). Lack of CD28 on T cells or blockade of the B7 co-stimulatory molecules on APC, prevented effective responses to PD1/PDL1 ICB, revealing the importance of the B7/CD28 pathway and T cell-APC interactions for ICB efficacy (106). These findings raise interesting questions of whether Tcf1 has a role in shaping T cell- APC interactions as well as how these interactions sustain the immune response in the context of chronic viral infections and in tumors, particularly upon ICB. Notably, the presence of clusters of TCF1+ stem-like CD8+ T cells and APCs positively associates with high T cell infiltration in human tumors while the absence of such niches is associated with poor T cell infiltration and has been proposed as a mechanism of immune evasion (76). Similarly, the presence of tertiary lymphoid structures enriched in B cells and TCF1+ stem-like CD8+ T cells has also been positively correlated with responses to immunotherapy in sarcoma, melanoma, and renal cell carcinoma (75, 108, 109). Therefore, the interaction of Tcf1+ stem-like CD8+ T cells with DC and/or B cells in these niches may be instrumental for regulating the function of Tcf1+ stem-like CD8+ T cells and their capacity to sustain the output of terminally differentiated T cell progeny required for durable anti-tumor responses. In such instance, it remains currently unexplored whether DC or stromal cells in these immune niches may contribute to preserve stemness and self-renewal capacity of Tcf1+ T cells by locally releasing Wnt ligands, a mechanism analogous to the secretion of Wnt ligands by paracrine niche cells for the maintenance of intestinal stem cells (110) (Figure 3). It is noteworthy that Tcf1+ stem-like CD8+ T cells are enriched for interferon-stimulated genes (ISG) (62) and that overexpression of the Tcf1 long-isoform in CD8+ T cells further increased ISG expression (84). While the exact role of Tcf1-induced ISG transcription in stem-like CD8+ T cells is currently unknown, it is possible that low-level IFN signaling may influence stem-like CD8+ T cells or neighboring DCs in the context of chronic antigen stimulation.
Clinical perspective of TCF1+ stem-like CD8+ T cells
Studies of adoptive cell therapy in humans and mice have demonstrated the superior anti-tumor capacity of less-differentiated cells retaining memory potential and stemness over more differentiated subsets (111–116). Several strategies that favor the ex-vivo expansion of stem-like cells have been developed through different means such as by introducing IL-7, IL-15, and IL-21 in the T cell expansion protocols, which favor the generation of a memory T cell state and the expression of memory-associated genes including Tcf1, Eomes, and Bcl6 (117–120). Conversely, as discussed above, IL12 and type-I interferon signaling suppress Tcf1 transcription (54, 101). Other strategies employing drugs that induce Wnt-Tcf1 pathway activation (121) have been shown to maintain the expression of stemness genes, including Tcf1, thereby promoting the acquisition of a stem-like phenotype in T cells. Uncovering how Tcf1 mediates its functions in stem-like CD8+ T cells will open additional approaches for expanding this important subset either in vitro or in vivo for therapeutic benefit.
Concluding Remarks
Tcf1 plays a fundamental role in T cell development, stemness, and memory formation. Increasing data point to additional roles of this transcription factor in the regulation of peripheral T cell responses. The ability of Tcf1 to shape the effector response of peripheral CD4+ and CD8+ T cells has direct consequences in disease settings such as autoimmunity, cancer, and chronic viral infection. By means of its varied transcriptional and chromatin remodeling functions (Figure 4), Tcf1 can, either directly or indirectly, influence specific gene programs in distinct cell types. Because Tcf1 activity is likely to be context-dependent, it is important to place its function in relation with the T cell epigenetic landscape and the regulatory network expressed in each cell type at a given time. Moreover, small-scale variations in Tcf1 intra-cellular levels are likely to have major effects on the strength and type of downstream signaling pathways with obvious consequences on key biological processes such as differentiation, cell-fate decision, and function. Therefore, deciphering the diverse roles of Tcf1 will require tools that allow tracking of as well as perturbation of Tcf1 expression in a temporal and cell-type specific fashion. We are just beginning to uncover how Tcf1 regulates homeostatic T cell functions, modulates T cell responses during disease, and how its activity is influenced by intracellular and environmental stimuli. In the future, this knowledge will likely endow us with new means to efficiently modulate immune responses for therapeutic benefit.
Figure 4. Mechanisms of Tcf1-mediated regulation.
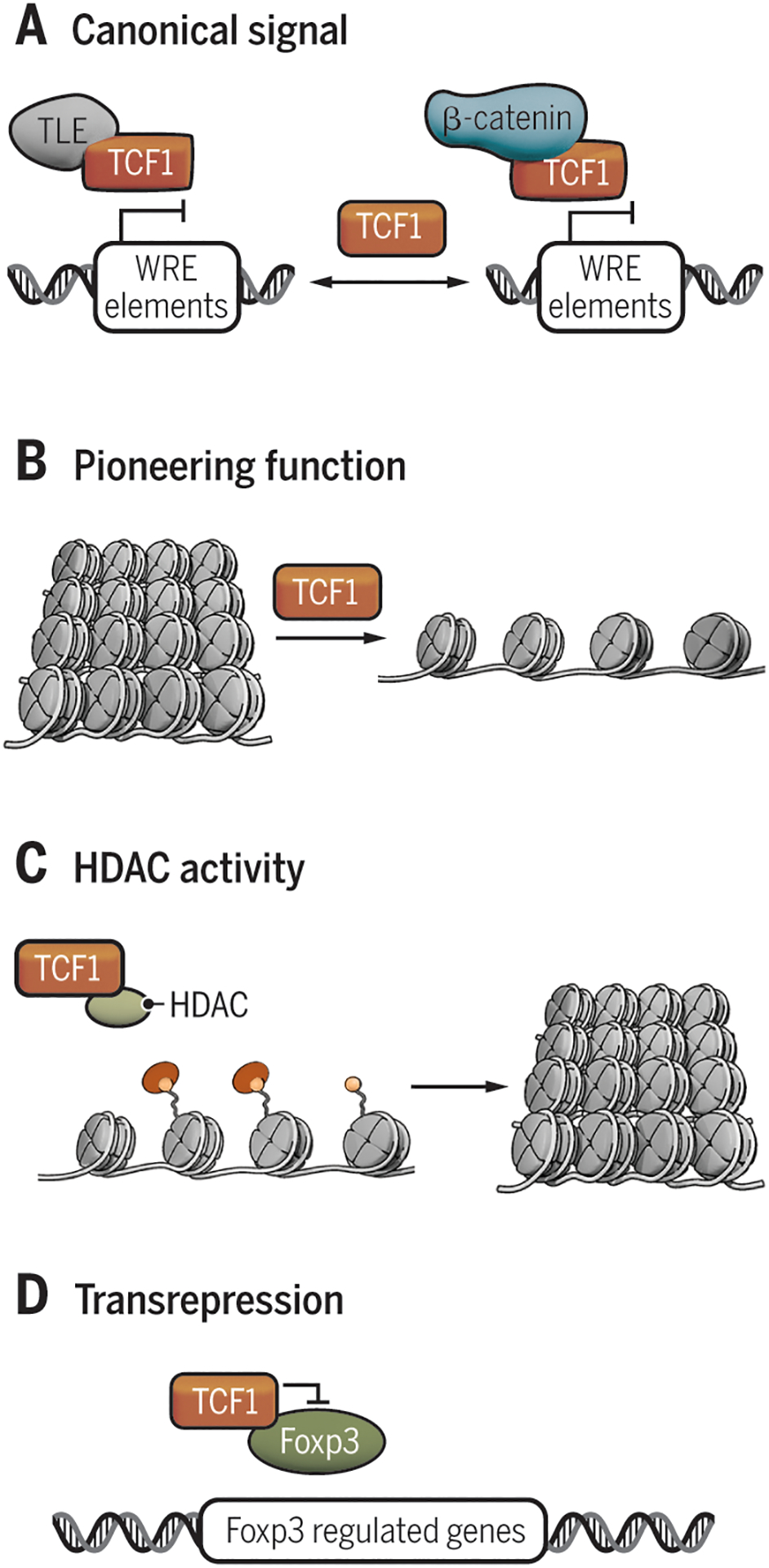
A) Canonical on/off model of Tcf1 activation. B) Tcf1 is able to act as a pioneering factor, targeting silent chromatin (11). C) Tcf1 bears an intrinsic HDAC activity to silence target genes (10). D) Tcf1 can physically bind target factors (e.g. Foxp3) inhibiting their activity, a process called transrepression (45).
TLE, transducin-like Enhancer of split; Tcf, T cell factor; βcat, β-catenin; WRE, Wnt responsive elements; HDAC, histone deacetylase; Ac, acetylation; Foxp3, forkhead box P3.
Funding
Work in the author’s laboratory (A.C.A.) is supported by grants from the National Institutes of Health (P01AI073748 and R01CA187975). A.C.A. is a recipient of the Brigham and Women’s Hospital President’s Scholar Award. D.M is supported by SNSF postdoc mobility (P400PB_183910). G.E. is supported by EMBO Long Term Fellowship (ALTF 182-2018) and the CRI Irvington Postdoctoral Fellowship (CRI 2934).
Footnotes
Competing interests
A.C.A. is a member of the SAB for Tizona Therapeutics, Compass Therapeutics, Zumutor Biologics, ImmuneOncia, and Astellas Global Pharma Development Inc., which have interests in cancer immunotherapy. A.C.A.’s interests were reviewed and managed by the Brigham and Women’s Hospital and Partners Healthcare in accordance with their conflict of interest policies. A.C.A. is an inventor on provisional patents related to Tcf1.
References
- 1.van de Wetering M, Oosterwegel M, Dooijes D, Clevers H, Identification and cloning of TCF-1, a T lymphocyte-specific transcription factor containing a sequence-specific HMG box. EMBO J 10, 123–132 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weber BN, Chi AW, Chavez A, Yashiro-Ohtani Y, Yang Q, Shestova O, Bhandoola A, A critical role for TCF-1 in T-lineage specification and differentiation. Nature 476, 63–68 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verbeek S, Izon D, Hofhuis F, Robanus-Maandag E, te Riele H, van de Wetering M, Oosterwegel M, Wilson A, MacDonald HR, Clevers H, An HMG-box-containing T-cell factor required for thymocyte differentiation. Nature 374, 70–74 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Schilham MW, Wilson A, Moerer P, Benaissa-Trouw BJ, Cumano A, Clevers HC, Critical involvement of Tcf-1 in expansion of thymocytes. J Immunol 161, 3984–3991 (1998). [PubMed] [Google Scholar]
- 5.Raghu D, Xue HH, Mielke LA, Control of Lymphocyte Fate, Infection, and Tumor Immunity by TCF-1. Trends Immunol 40, 1149–1162 (2019). [DOI] [PubMed] [Google Scholar]
- 6.Xu Z, Xing S, Shan Q, Gullicksrud JA, Bair TB, Du Y, Liu C, Xue HH, Cutting Edge: beta-Catenin-Interacting Tcf1 Isoforms Are Essential for Thymocyte Survival but Dispensable for Thymic Maturation Transitions. J Immunol 198, 3404–3409 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeannet G, Scheller M, Scarpellino L, Duboux S, Gardiol N, Back J, Kuttler F, Malanchi I, Birchmeier W, Leutz A, Huelsken J, Held W, Long-term, multilineage hematopoiesis occurs in the combined absence of beta-catenin and gamma-catenin. Blood 111, 142–149 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Cobas M, Wilson A, Ernst B, Mancini SJ, MacDonald HR, Kemler R, Radtke F, Beta-catenin is dispensable for hematopoiesis and lymphopoiesis. J Exp Med 199, 221–229 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gullicksrud JA, Li F, Xing S, Zeng Z, Peng W, Badovinac VP, Harty JT, Xue HH, Differential Requirements for Tcf1 Long Isoforms in CD8(+) and CD4(+) T Cell Responses to Acute Viral Infection. J Immunol 199, 911–919 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing S, Li F, Zeng Z, Zhao Y, Yu S, Shan Q, Li Y, Phillips FC, Maina PK, Qi HH, Liu C, Zhu J, Pope RM, Musselman CA, Zeng C, Peng W, Xue HH, Tcf1 and Lef1 transcription factors establish CD8(+) T cell identity through intrinsic HDAC activity. Nat Immunol 17, 695–703 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson JL, Georgakilas G, Petrovic J, Kurachi M, Cai S, Harly C, Pear WS, Bhandoola A, Wherry EJ, Vahedi G, Lineage-Determining Transcription Factor TCF-1 Initiates the Epigenetic Identity of T Cells. Immunity 48, 243–257 e210 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koch H, Starenki D, Cooper SJ, Myers RM, Li Q, powerTCR: A model-based approach to comparative analysis of the clone size distribution of the T cell receptor repertoire. PLoS Comput Biol 14, e1006571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y, Hogquist KA, T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol 4, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkett PR, Meyer zu Horste G, Kuchroo VK, Pouring fuel on the fire: Th17 cells, the environment, and autoimmunity. J Clin Invest 125, 2211–2219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.C. International Multiple Sclerosis Genetics, Beecham AH, Patsopoulos NA, Xifara DK, Davis MF, Kemppinen A, Cotsapas C, Shah TS, Spencer C, Booth D, Goris A, Oturai A, Saarela J, Fontaine B, Hemmer B, Martin C, Zipp F, D’Alfonso S, Martinelli-Boneschi F, Taylor B, Harbo HF, Kockum I, Hillert J, Olsson T, Ban M, Oksenberg JR, Hintzen R, Barcellos LF, Wellcome C, Trust Case Control, I. B. D. G. C. International, Agliardi C, Alfredsson L, Alizadeh M, Anderson C, Andrews R, Sondergaard HB, Baker A, Band G, Baranzini SE, Barizzone N, Barrett J, Bellenguez C, Bergamaschi L, Bernardinelli L, Berthele A, Biberacher V, Binder TM, Blackburn H, Bomfim IL, Brambilla P, Broadley S, Brochet B, Brundin L, Buck D, Butzkueven H, Caillier SJ, Camu W, Carpentier W, Cavalla P, Celius EG, Coman I, Comi G, Corrado L, Cosemans L, Cournu-Rebeix I, Cree BA, Cusi D, Damotte V, Defer G, Delgado SR, Deloukas P, di Sapio A, Dilthey AT, Donnelly P, Dubois B, Duddy M, Edkins S, Elovaara I, Esposito F, Evangelou N, Fiddes B, Field J, Franke A, Freeman C, Frohlich IY, Galimberti D, Gieger C, Gourraud PA, Graetz C, Graham A, Grummel V, Guaschino C, Hadjixenofontos A, Hakonarson H, Halfpenny C, Hall G, Hall P, Hamsten A, Harley J, Harrower T, Hawkins C, Hellenthal G, Hillier C, Hobart J, Hoshi M, Hunt SE, Jagodic M, Jelcic I, Jochim A, Kendall B, Kermode A, Kilpatrick T, Koivisto K, Konidari I, Korn T, Kronsbein H, Langford C, Larsson M, Lathrop M, Lebrun-Frenay C, Lechner-Scott J, Lee MH, Leone MA, Leppa V, Liberatore G, Lie BA, Lill CM, Linden M, Link J, Luessi F, Lycke J, Macciardi F, Mannisto S, Manrique CP, Martin R, Martinelli V, Mason D, Mazibrada G, McCabe C, Mero IL, Mescheriakova J, Moutsianas L, Myhr KM, Nagels G, Nicholas R, Nilsson P, Piehl F, Pirinen M, Price SE, Quach H, Reunanen M, Robberecht W, Robertson NP, Rodegher M, Rog D, Salvetti M, Schnetz-Boutaud NC, Sellebjerg F, Selter RC, Schaefer C, Shaunak S, Shen L, Shields S, Siffrin V, Slee M, Sorensen PS, Sorosina M, Sospedra M, Spurkland A, Strange A, Sundqvist E, Thijs V, Thorpe J, Ticca A, Tienari P, van Duijn C, Visser EM, Vucic S, Westerlind H, Wiley JS, Wilkins A, Wilson JF, Winkelmann J, Zajicek J, Zindler E, Haines JL, Pericak-Vance MA, Ivinson AJ, Stewart G, Hafler D, Hauser SL, Compston A, McVean G, De Jager P, Sawcer SJ, McCauley JL, Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 45, 1353–1360 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noble JA, White AM, Lazzeroni LC, Valdes AM, Mirel DB, Reynolds R, Grupe A, Aud D, Peltz G, Erlich HA, A polymorphism in the TCF7 gene, C883A, is associated with type 1 diabetes. Diabetes 52, 1579–1582 (2003). [DOI] [PubMed] [Google Scholar]
- 17.van Loosdregt J, Coffer PJ, The Role of WNT Signaling in Mature T Cells: T Cell Factor Is Coming Home. J Immunol 201, 2193–2200 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Xu L, Cao Y, Xie Z, Huang Q, Bai Q, Yang X, He R, Hao Y, Wang H, Zhao T, Fan Z, Qin A, Ye J, Zhou X, Ye L, Wu Y, The transcription factor TCF-1 initiates the differentiation of T(FH) cells during acute viral infection. Nat Immunol 16, 991–999 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Wu T, Shin HM, Moseman EA, Ji Y, Huang B, Harly C, Sen JM, Berg LJ, Gattinoni L, McGavern DB, Schwartzberg PL, TCF1 Is Required for the T Follicular Helper Cell Response to Viral Infection. Cell Rep 12, 2099–2110 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG, Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE). J Immunol 156, 5–7 (1996). [PubMed] [Google Scholar]
- 21.Becher B, Durell BG, Noelle RJ, Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest 110, 493–497 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthys P, Vermeire K, Mitera T, Heremans H, Huang S, Billiau A, Anti-IL-12 antibody prevents the development and progression of collagen-induced arthritis in IFN-gamma receptor-deficient mice. Eur J Immunol 28, 2143–2151 (1998). [DOI] [PubMed] [Google Scholar]
- 23.Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD, Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 (2003). [DOI] [PubMed] [Google Scholar]
- 24.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK, Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med 200, 79–87 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, Zhu Z, Sen JM, T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol 10, 992–999 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oestreich KJ, Huang AC, Weinmann AS, The lineage-defining factors T-bet and Bcl-6 collaborate to regulate Th1 gene expression patterns. J Exp Med 208, 1001–1013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valenta T, Hausmann G, Basler K, The many faces and functions of beta-catenin. EMBO J 31, 2714–2736 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q, Sharma A, Ghosh A, Sen JM, T cell factor-1 negatively regulates expression of IL-17 family of cytokines and protects mice from experimental autoimmune encephalomyelitis. J Immunol 186, 3946–3952 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma J, Wang R, Fang X, Ding Y, Sun Z, Critical role of TCF-1 in repression of the IL-17 gene. PLoS One 6, e24768 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keerthivasan S, Aghajani K, Dose M, Molinero L, Khan MW, Venkateswaran V, Weber C, Emmanuel AO, Sun T, Bentrem DJ, Mulcahy M, Keshavarzian A, Ramos EM, Blatner N, Khazaie K, Gounari F, beta-Catenin promotes colitis and colon cancer through imprinting of proinflammatory properties in T cells. Sci Transl Med 6, 225ra228 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Xie H, Huang Z, Ma J, Fang X, Ding Y, Sun Z, T cell factor 1 regulates thymocyte survival via a RORgammat-dependent pathway. J Immunol 187, 5964–5973 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YS, Lee KA, Yoon HB, Yoo SA, Park YW, Chung Y, Kim WU, Kang CY, The Wnt inhibitor secreted Frizzled-Related Protein 1 (sFRP1) promotes human Th17 differentiation. Eur J Immunol 42, 2564–2573 (2012). [DOI] [PubMed] [Google Scholar]
- 33.Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, Grainger JR, Chen Q, Kanno Y, Watford WT, Sun HW, Eberl G, Shevach EM, Belkaid Y, Cua DJ, Chen W, O’Shea JJ, Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature 467, 967–971 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brucklacher-Waldert V, Ferreira C, Stebegg M, Fesneau O, Innocentin S, Marie JC, Veldhoen M, Cellular Stress in the Context of an Inflammatory Environment Supports TGF-beta-Independent T Helper-17 Differentiation. Cell Rep 19, 2357–2370 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, Kuchroo V, Defining the functional states of Th17 cells. F1000Res 4, 132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stockinger B, Omenetti S, The dichotomous nature of T helper 17 cells. Nat Rev Immunol 17, 535–544 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP, Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 35, 972–985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B, Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol 12, 255–263 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT, Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A 112, 7061–7066 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F, Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 33, 279–288 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, Pandolfi PP, Mak T, Satija R, Shalek AK, Kuchroo VK, Park H, Regev A, Single-Cell Genomics Unveils Critical Regulators of Th17 Cell Pathogenicity. Cell 163, 1400–1412 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karmaus PWF, Chen X, Lim SA, Herrada AA, Nguyen TM, Xu B, Dhungana Y, Rankin S, Chen W, Rosencrance C, Yang K, Fan Y, Cheng Y, Easton J, Neale G, Vogel P, Chi H, Metabolic heterogeneity underlies reciprocal fates of TH17 cell stemness and plasticity. Nature 565, 101–105 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li MO, Rudensky AY, T cell receptor signalling in the control of regulatory T cell differentiation and function. Nat Rev Immunol 16, 220–233 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barra MM, Richards DM, Hansson J, Hofer AC, Delacher M, Hettinger J, Krijgsveld J, Feuerer M, Transcription Factor 7 Limits Regulatory T Cell Generation in the Thymus. J Immunol 195, 3058–3070 (2015). [DOI] [PubMed] [Google Scholar]
- 45.van Loosdregt J, Fleskens V, Fu J, Brenkman AB, Bekker CP, Pals CE, Meerding J, Berkers CR, Barbi J, Grone A, Sijts AJ, Maurice MM, Kalkhoven E, Prakken BJ, Ovaa H, Pan F, Zaiss DM, Coffer PJ, Stabilization of the transcription factor Foxp3 by the deubiquitinase USP7 increases Treg-cell-suppressive capacity. Immunity 39, 259–271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xing S, Gai K, Li X, Shao P, Zeng Z, Zhao X, Zhao X, Chen X, Paradee WJ, Meyerholz DK, Peng W, Xue HH, Tcf1 and Lef1 are required for the immunosuppressive function of regulatory T cells. J Exp Med 216, 847–866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang BH, Wang K, Wan S, Liang Y, Yuan X, Dong Y, Cho S, Xu W, Jepsen K, Feng GS, Lu LF, Xue HH, Fu W, TCF1 and LEF1 Control Treg Competitive Survival and Tfr Development to Prevent Autoimmune Diseases. Cell Rep 27, 3629–3645 e3626 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sharma P, Allison JP, Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Topalian SL, Drake CG, Pardoll DM, Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27, 450–461 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, Sharpe AH, Freeman GJ, Germain RN, Nakaya HI, Xue HH, Ahmed R, Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417–421 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.He R, Hou S, Liu C, Zhang A, Bai Q, Han M, Yang Y, Wei G, Shen T, Yang X, Xu L, Chen X, Hao Y, Wang P, Zhu C, Ou J, Liang H, Ni T, Zhang X, Zhou X, Deng K, Chen Y, Luo Y, Xu J, Qi H, Wu Y, Ye L, Follicular CXCR5- expressing CD8(+) T cells curtail chronic viral infection. Nature 537, 412–428 (2016). [DOI] [PubMed] [Google Scholar]
- 52.Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, Minnich M, Meckiff BJ, Wei Y, Hou Z, Zotos D, Fenix KA, Atnerkar A, Preston S, Chipman JG, Beilman GJ, Allison CC, Sun L, Wang P, Xu J, Toe JG, Lu HK, Tao Y, Palendira U, Dent AL, Landay AL, Pellegrini M, Comerford I, McColl SR, Schacker TW, Long HM, Estes JD, Busslinger M, Belz GT, Lewin SR, Kallies A, Yu D, CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol 17, 1187–1196 (2016). [DOI] [PubMed] [Google Scholar]
- 53.Utzschneider DT, Charmoy M, Chennupati V, Pousse L, Ferreira DP, Calderon-Copete S, Danilo M, Alfei F, Hofmann M, Wieland D, Pradervand S, Thimme R, Zehn D, Held W, T Cell Factor 1-Expressing Memory-like CD8(+) T Cells Sustain the Immune Response to Chronic Viral Infections. Immunity 45, 415–427 (2016). [DOI] [PubMed] [Google Scholar]
- 54.Wu T, Ji Y, Moseman EA, Xu HC, Manglani M, Kirby M, Anderson SM, Handon R, Kenyon E, Elkahloun A, Wu W, Lang PA, Gattinoni L, McGavern DB, Schwartzberg PL, The TCF1-Bcl6 axis counteracts type I interferon to repress exhaustion and maintain T cell stemness. Sci Immunol 1, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, Pawlak M, Dionne D, Xia J, Rozenblatt-Rosen O, Kuchroo VK, Regev A, Anderson AC, Checkpoint Blockade Immunotherapy Induces Dynamic Changes in PD-1(−)CD8(+) Tumor-Infiltrating T Cells. Immunity 50, 181–194 e186 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Siddiqui I, Schaeuble K, Chennupati V, Fuertes Marraco SA, Calderon-Copete S, Pais Ferreira D, Carmona SJ, Scarpellino L, Gfeller D, Pradervand S, Luther SA, Speiser DE, Held W, Intratumoral Tcf1(+)PD-1(+)CD8(+) T Cells with Stem-like Properties Promote Tumor Control in Response to Vaccination and Checkpoint Blockade Immunotherapy. Immunity 50, 195–211 e110 (2019). [DOI] [PubMed] [Google Scholar]
- 57.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, Yates KB, Lako A, Felt K, Naik GS, Manos M, Gjini E, Kuchroo JR, Ishizuka JJ, Collier JL, Griffin GK, Maleri S, Comstock DE, Weiss SA, Brown FD, Panda A, Zimmer MD, Manguso RT, Hodi FS, Rodig SJ, Sharpe AH, Haining WN, Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol 20, 326–336 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, Lieb DJ, Chen JH, Frederick DT, Barzily-Rokni M, Freeman SS, Reuben A, Hoover PJ, Villani AC, Ivanova E, Portell A, Lizotte PH, Aref AR, Eliane JP, Hammond MR, Vitzthum H, Blackmon SM, Li B, Gopalakrishnan V, Reddy SM, Cooper ZA, Paweletz CP, Barbie DA, Stemmer-Rachamimov A, Flaherty KT, Wargo JA, Boland GM, Sullivan RJ, Getz G, Hacohen N, Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013 e1020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brummelman J, Mazza EMC, Alvisi G, Colombo FS, Grilli A, Mikulak J, Mavilio D, Alloisio M, Ferrari F, Lopci E, Novellis P, Veronesi G, Lugli E, High-dimensional single cell analysis identifies stem-like cytotoxic CD8(+) T cells infiltrating human tumors. J Exp Med 215, 2520–2535 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hudson WH, Gensheimer J, Hashimoto M, Wieland A, Valanparambil RM, Li P, Lin JX, Konieczny BT, Im SJ, Freeman GJ, Leonard WJ, Kissick HT, Ahmed R, Proliferating Transitory T Cells with an Effector-like Transcriptional Signature Emerge from PD-1(+) Stem-like CD8(+) T Cells during Chronic Infection. Immunity 51, 1043–1058 e1044 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zander R, Schauder D, Xin G, Nguyen C, Wu X, Zajac A, Cui W, CD4(+) T Cell Help Is Required for the Formation of a Cytolytic CD8(+) T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 51, 1028–1042 e1024 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ, Yan P, Nzingha K, Xu W, Amaravadi RK, Xu X, Karakousis GC, Mitchell TC, Schuchter LM, Huang AC, Wherry EJ, Developmental Relationships of Four Exhausted CD8(+) T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van der Leun AM, Thommen DS, Schumacher TN, CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer 20, 218–232 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Philip M, Fairchild L, Sun L, Horste EL, Camara S, Shakiba M, Scott AC, Viale A, Lauer P, Merghoub T, Hellmann MD, Wolchok JD, Leslie CS, Schietinger A, Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 545, 452–456 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.LaFleur MW, Nguyen TH, Coxe MA, Miller BC, Yates KB, Gillis JE, Sen DR, Gaudiano EF, Al Abosy R, Freeman GJ, Haining WN, Sharpe AH, PTPN2 regulates the generation of exhausted CD8(+) T cell subpopulations and restrains tumor immunity. Nat Immunol 20, 1335–1347 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, Yeong JPS, Nahar R, Zhang T, Kared H, Duan K, Ang N, Poidinger M, Lee YY, Larbi A, Khng AJ, Tan E, Fu C, Mathew R, Teo M, Lim WT, Toh CK, Ong BH, Koh T, Hillmer AM, Takano A, Lim TKH, Tan EH, Zhai W, Tan DSW, Tan IB, Newell EW, Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 557, 575–579 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Scheper W, Kelderman S, Fanchi LF, Linnemann C, Bendle G, de Rooij MAJ, Hirt C, Mezzadra R, Slagter M, Dijkstra K, Kluin RJC, Snaebjornsson P, Milne K, Nelson BH, Zijlmans H, Kenter G, Voest EE, Haanen J, Schumacher TN, Low and variable tumor reactivity of the intratumoral TCR repertoire in human cancers. Nat Med 25, 89–94 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Thommen DS, Schumacher TN, T Cell Dysfunction in Cancer. Cancer Cell 33, 547–562 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Singer M, Anderson AC, Molecular Dissection of CD8(+) T-Cell Dysfunction. Trends Immunol 38, 567–576 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pauken KE, Sammons MA, Odorizzi PM, Manne S, Godec J, Khan O, Drake AM, Chen Z, Sen DR, Kurachi M, Barnitz RA, Bartman C, Bengsch B, Huang AC, Schenkel JM, Vahedi G, Haining WN, Berger SL, Wherry EJ, Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 354, 1160–1165 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sen DR, Kaminski J, Barnitz RA, Kurachi M, Gerdemann U, Yates KB, Tsao HW, Godec J, LaFleur MW, Brown FD, Tonnerre P, Chung RT, Tully DC, Allen TM, Frahm N, Lauer GM, Wherry EJ, Yosef N, Haining WN, The epigenetic landscape of T cell exhaustion. Science 354, 1165–1169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghoneim HE, Fan Y, Moustaki A, Abdelsamed HA, Dash P, Dogra P, Carter R, Awad W, Neale G, Thomas PG, Youngblood B, De Novo Epigenetic Programs Inhibit PD-1 Blockade-Mediated T Cell Rejuvenation. Cell 170, 142–157 e119 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, Johnson J, Staupe RP, Bengsch B, Xu C, Yu S, Kurachi M, Herati RS, Vella LA, Baxter AE, Wu JE, Khan O, Beltra JC, Giles JR, Stelekati E, McLane LM, Lau CW, Yang X, Berger SL, Vahedi G, Ji H, Wherry EJ, TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision. Immunity 51, 840–855 e845 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, Cooper A, Hataye J, Andrews S, Ambrozak D, Del Rio Estrada PM, Boritz E, Paris R, Moysi E, Boswell KL, Ruiz-Mateos E, Vagios I, Leal M, Ablanedo-Terrazas Y, Rivero A, Gonzalez-Hernandez LA, McDermott AB, Moir S, Reyes-Teran G, Docobo F, Pantaleo G, Douek DC, Betts MR, Estes JD, Germain RN, Mascola JR, Koup RA, Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K, Vallon-Christersson J, van Schoiack A, Lovgren K, Warren S, Jirstrom K, Olsson H, Pietras K, Ingvar C, Isaksson K, Schadendorf D, Schmidt H, Bastholt L, Carneiro A, Wargo JA, Svane IM, Jonsson G, Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 577, 561–565 (2020). [DOI] [PubMed] [Google Scholar]
- 76.Jansen CS, Prokhnevska N, Master VA, Sanda MG, Carlisle JW, Bilen MA, Cardenas M, Wilkinson S, Lake R, Sowalsky AG, Valanparambil RM, Hudson WH, McGuire D, Melnick K, Khan AI, Kim K, Chang YM, Kim A, Filson CP, Alemozaffar M, Osunkoya AO, Mullane P, Ellis C, Akondy R, Im SJ, Kamphorst AO, Reyes A, Liu Y, Kissick H, An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vodnala SK, Eil R, Kishton RJ, Sukumar M, Yamamoto TN, Ha NH, Lee PH, Shin M, Patel SJ, Yu Z, Palmer DC, Kruhlak MJ, Liu X, Locasale JW, Huang J, Roychoudhuri R, Finkel T, Klebanoff CA, Restifo NP, T cell stemness and dysfunction in tumors are triggered by a common mechanism. Science 363, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kallies A, Zehn D, Utzschneider DT, Precursor exhausted T cells: key to successful immunotherapy? Nat Rev Immunol 20, 128–136 (2020). [DOI] [PubMed] [Google Scholar]
- 79.Utzschneider DT, Legat A, Fuertes Marraco SA, Carrie L, Luescher I, Speiser DE, Zehn D, T cells maintain an exhausted phenotype after antigen withdrawal and population reexpansion. Nat Immunol 14, 603–610 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Schietinger A, Philip M, Krisnawan VE, Chiu EY, Delrow JJ, Basom RS, Lauer P, Brockstedt DG, Knoblaugh SE, Hammerling GJ, Schell TD, Garbi N, Greenberg PD, Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity 45, 389–401 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Utzschneider DT, Gabriel SS, Chisanga D, Gloury R, Gubser PM, Vasanthakumar A, Shi W, Kallies A, Early precursor T cells establish and propagate T cell exhaustion in chronic infection. Nat Immunol, (2020). [DOI] [PubMed] [Google Scholar]
- 82.Nish SA, Zens KD, Kratchmarov R, Lin WW, Adams WC, Chen YH, Yen B, Rothman NJ, Bhandoola A, Xue HH, Farber DL, Reiner SL, CD4+ T cell effector commitment coupled to self-renewal by asymmetric cell divisions. J Exp Med 214, 39–47 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choi YS, Gullicksrud JA, Xing S, Zeng Z, Shan Q, Li F, Love PE, Peng W, Xue HH, Crotty S, LEF-1 and TCF-1 orchestrate T(FH) differentiation by regulating differentiation circuits upstream of the transcriptional repressor Bcl6. Nat Immunol 16, 980–990 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shan Q, Hu S, Chen X, Danahy DB, Badovinac VP, Zang C, Xue HH, Ectopic Tcf1 expression instills a stem-like program in exhausted CD8(+) T cells to enhance viral and tumor immunity. Cell Mol Immunol, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y, Hu J, Li Y, Xiao M, Wang H, Tian Q, Li Z, Tang J, Hu L, Tan Y, Zhou X, He R, Wu Y, Ye L, Yin Z, Huang Q, Xu L, The Transcription Factor TCF1 Preserves the Effector Function of Exhausted CD8 T Cells During Chronic Viral Infection. Front Immunol 10, 169 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaech SM, Cui W, Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol 12, 749–761 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hess Michelini R, Doedens AL, Goldrath AW, Hedrick SM, Differentiation of CD8 memory T cells depends on Foxo1. J Exp Med 210, 1189–1200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Utzschneider DT, Delpoux A, Wieland D, Huang X, Lai CY, Hofmann M, Thimme R, Hedrick SM, Active Maintenance of T Cell Memory in Acute and Chronic Viral Infection Depends on Continuous Expression of FOXO1. Cell Rep 22, 3454–3467 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gautam S, Fioravanti J, Zhu W, Le Gall JB, Brohawn P, Lacey NE, Hu J, Hocker JD, Hawk NV, Kapoor V, Telford WG, Gurusamy D, Yu Z, Bhandoola A, Xue HH, Roychoudhuri R, Higgs BW, Restifo NP, Bender TP, Ji Y, Gattinoni L, The transcription factor c-Myb regulates CD8(+) T cell stemness and antitumor immunity. Nat Immunol 20, 337–349 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Clarke J, Panwar B, Madrigal A, Singh D, Gujar R, Wood O, Chee SJ, Eschweiler S, King EV, Awad AS, Hanley CJ, McCann KJ, Bhattacharyya S, Woo E, Alzetani A, Seumois G, Thomas GJ, Ganesan AP, Friedmann PS, Sanchez-Elsner T, Ay F, Ottensmeier CH, Vijayanand P, Single-cell transcriptomic analysis of tissue-resident memory T cells in human lung cancer. J Exp Med 216, 2128–2149 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, Salgado R, Byrne DJ, Teo ZL, Dushyanthen S, Byrne A, Wein L, Luen SJ, Poliness C, Nightingale SS, Skandarajah AS, Gyorki DE, Thornton CM, Beavis PA, Fox SB, C. Kathleen Cuningham Foundation Consortium for Research into Familial Breast, Darcy PK, Speed TP, Mackay LK, Neeson PJ, Loi S, Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med 24, 986–993 (2018). [DOI] [PubMed] [Google Scholar]
- 92.Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, Wang W, Pipkin ME, Goldrath AW, Runx3 programs CD8(+) T cell residency in non-lymphoid tissues and tumours. Nature 552, 253–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shan Q, Zeng Z, Xing S, Li F, Hartwig SM, Gullicksrud JA, Kurup SP, Van Braeckel-Budimir N, Su Y, Martin MD, Varga SM, Taniuchi I, Harty JT, Peng W, Badovinac VP, Xue HH, The transcription factor Runx3 guards cytotoxic CD8(+) effector T cells against deviation towards follicular helper T cell lineage. Nat Immunol 18, 931–939 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu J, Madi A, Mieg A, Hotz-Wagenblatt A, Weisshaar N, Ma S, Mohr K, Schlimbach T, Hering M, Borgers H, Cui G, T Cell Factor 1 Suppresses CD103+ Lung Tissue-Resident Memory T Cell Development. Cell Rep 31, 107484 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Scott AC, Dundar F, Zumbo P, Chandran SS, Klebanoff CA, Shakiba M, Trivedi P, Menocal L, Appleby H, Camara S, Zamarin D, Walther T, Snyder A, Femia MR, Comen EA, Wen HY, Hellmann MD, Anandasabapathy N, Liu Y, Altorki NK, Lauer P, Levy O, Glickman MS, Kaye J, Betel D, Philip M, Schietinger A, TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alfei F, Kanev K, Hofmann M, Wu M, Ghoneim HE, Roelli P, Utzschneider DT, von Hoesslin M, Cullen JG, Fan Y, Eisenberg V, Wohlleber D, Steiger K, Merkler D, Delorenzi M, Knolle PA, Cohen CJ, Thimme R, Youngblood B, Zehn D, TOX reinforces the phenotype and longevity of exhausted T cells in chronic viral infection. Nature 571, 265–269 (2019). [DOI] [PubMed] [Google Scholar]
- 97.Khan O, Giles JR, McDonald S, Manne S, Ngiow SF, Patel KP, Werner MT, Huang AC, Alexander KA, Wu JE, Attanasio J, Yan P, George SM, Bengsch B, Staupe RP, Donahue G, Xu W, Amaravadi RK, Xu X, Karakousis GC, Mitchell TC, Schuchter LM, Kaye J, Berger SL, Wherry EJ, TOX transcriptionally and epigenetically programs CD8(+) T cell exhaustion. Nature 571, 211–218 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yao C, Sun HW, Lacey NE, Ji Y, Moseman EA, Shih HY, Heuston EF, Kirby M, Anderson S, Cheng J, Khan O, Handon R, Reilley J, Fioravanti J, Hu J, Gossa S, Wherry EJ, Gattinoni L, McGavern DB, O’Shea JJ, Schwartzberg PL, Wu T, Single-cell RNA-seq reveals TOX as a key regulator of CD8(+) T cell persistence in chronic infection. Nat Immunol 20, 890–901 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seo H, Chen J, Gonzalez-Avalos E, Samaniego-Castruita D, Das A, Wang YH, Lopez-Moyado IF, Georges RO, Zhang W, Onodera A, Wu CJ, Lu LF, Hogan PG, Bhandoola A, Rao A, TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8(+) T cell exhaustion. Proc Natl Acad Sci U S A 116, 12410–12415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang X, He Q, Shen H, Xia A, Tian W, Yu W, Sun B, TOX promotes the exhaustion of antitumor CD8(+) T cells by preventing PD1 degradation in hepatocellular carcinoma. J Hepatol 71, 731–741 (2019). [DOI] [PubMed] [Google Scholar]
- 101.Danilo M, Chennupati V, Silva JG, Siegert S, Held W, Suppression of Tcf1 by Inflammatory Cytokines Facilitates Effector CD8 T Cell Differentiation. Cell Rep 22, 2107–2117 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Daniels MA, Teixeiro E, TCR Signaling in T Cell Memory. Front Immunol 6, 617 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Snell LM, MacLeod BL, Law JC, Osokine I, Elsaesser HJ, Hezaveh K, Dickson RJ, Gavin MA, Guidos CJ, McGaha TL, Brooks DG, CD8(+) T Cell Priming in Established Chronic Viral Infection Preferentially Directs Differentiation of Memory-like Cells for Sustained Immunity. Immunity 49, 678–694 e675 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jadhav RR, Im SJ, Hu B, Hashimoto M, Li P, Lin JX, Leonard WJ, Greenleaf WJ, Ahmed R, Goronzy JJ, Epigenetic signature of PD-1+ TCF1+ CD8 T cells that act as resource cells during chronic viral infection and respond to PD-1 blockade. Proc Natl Acad Sci U S A 116, 14113–14118 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, Barczak A, Rosenblum MD, Daud A, Barber DL, Amigorena S, Van’t Veer LJ, Sperling AI, Wolf DM, Krummel MF, Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell 26, 638–652 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kamphorst AO, Wieland A, Nasti T, Yang S, Zhang R, Barber DL, Konieczny BT, Daugherty CZ, Koenig L, Yu K, Sica GL, Sharpe AH, Freeman GJ, Blazar BR, Turka LA, Owonikoko TK, Pillai RN, Ramalingam SS, Araki K, Ahmed R, Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 355, 1423–1427 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hui E, Cheung J, Zhu J, Su X, Taylor MJ, Wallweber HA, Sasmal DK, Huang J, Kim JM, Mellman I, Vale RD, T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Helmink BA, Reddy SM, Gao J, Zhang S, Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, Gopalakrishnan V, Xi Y, Zhao H, Amaria RN, Tawbi HA, Cogdill AP, Liu W, LeBleu VS, Kugeratski FG, Patel S, Davies MA, Hwu P, Lee JE, Gershenwald JE, Lucci A, Arora R, Woodman S, Keung EZ, Gaudreau PO, Reuben A, Spencer CN, Burton EM, Haydu LE, Lazar AJ, Zapassodi R, Hudgens CW, Ledesma DA, Ong S, Bailey M, Warren S, Rao D, Krijgsman O, Rozeman EA, Peeper D, Blank CU, Schumacher TN, Butterfield LH, Zelazowska MA, McBride KM, Kalluri R, Allison J, Petitprez F, Fridman WH, Sautes-Fridman C, Hacohen N, Rezvani K, Sharma P, Tetzlaff MT, Wang L, Wargo JA, B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Petitprez F, de Reynies A, Keung EZ, Chen TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougouin A, Moreira M, Lacroix G, Natario I, Adam J, Lucchesi C, Laizet YH, Toulmonde M, Burgess MA, Bolejack V, Reinke D, Wani KM, Wang WL, Lazar AJ, Roland CL, Wargo JA, Italiano A, Sautes-Fridman C, Tawbi HA, Fridman WH, B cells are associated with survival and immunotherapy response in sarcoma. Nature 577, 556–560 (2020). [DOI] [PubMed] [Google Scholar]
- 110.Clevers H, Loh KM, Nusse R, Stem cell signaling. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 346, 1248012 (2014). [DOI] [PubMed] [Google Scholar]
- 111.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, Price DA, Waldmann TA, Restifo NP, Franchini G, Roederer M, Superior T memory stem cell persistence supports long-lived T cell memory. J Clin Invest 123, 594–599 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP, A human memory T cell subset with stem cell-like properties. Nat Med 17, 1290–1297 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gattinoni L, Klebanoff CA, Palmer DC, Wrzesinski C, Kerstann K, Yu Z, Finkelstein SE, Theoret MR, Rosenberg SA, Restifo NP, Acquisition of full effector function in vitro paradoxically impairs the in vivo antitumor efficacy of adoptively transferred CD8+ T cells. J Clin Invest 115, 1616–1626 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, Boesteanu AC, Wang Y, O’Connor RS, Hwang WT, Pequignot E, Ambrose DE, Zhang C, Wilcox N, Bedoya F, Dorfmeier C, Chen F, Tian L, Parakandi H, Gupta M, Young RM, Johnson FB, Kulikovskaya I, Liu L, Xu J, Kassim SH, Davis MM, Levine BL, Frey NV, Siegel DL, Huang AC, Wherry EJ, Bitter H, Brogdon JL, Porter DL, June CH, Melenhorst JJ, Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 24, 563–571 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, Sanchez-Perez L, Muranski P, Kern SJ, Logun C, Palmer DC, Ji Y, Reger RN, Leonard WJ, Danner RL, Rosenberg SA, Restifo NP, Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A 106, 17469–17474 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, Glass M, Johnson VE, McMannis JD, Shpall E, Prieto V, Papadopoulos N, Kim K, Homsi J, Bedikian A, Hwu WJ, Patel S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Davies MA, Mansaray R, Fulbright OJ, Toth C, Ramachandran R, Wardell S, Gonzalez A, Hwu P, Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res 18, 6758–6770 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cieri N, Camisa B, Cocchiarella F, Forcato M, Oliveira G, Provasi E, Bondanza A, Bordignon C, Peccatori J, Ciceri F, Lupo-Stanghellini MT, Mavilio F, Mondino A, Bicciato S, Recchia A, Bonini C, IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121, 573–584 (2013). [DOI] [PubMed] [Google Scholar]
- 118.Zanon V, Pilipow K, Scamardella E, De Paoli F, De Simone G, Price DA, Martinez Usatorre A, Romero P, Mavilio D, Roberto A, Lugli E, Curtailed T-cell activation curbs effector differentiation and generates CD8(+) T cells with a naturally-occurring memory stem cell phenotype. Eur J Immunol 47, 1468–1476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cui W, Liu Y, Weinstein JS, Craft J, Kaech SM, An interleukin-21-interleukin-10-STAT3 pathway is critical for functional maturation of memory CD8+ T cells. Immunity 35, 792–805 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Siegel AM, Heimall J, Freeman AF, Hsu AP, Brittain E, Brenchley JM, Douek DC, Fahle GH, Cohen JI, Holland SM, Milner JD, A critical role for STAT3 transcription factor signaling in the development and maintenance of human T cell memory. Immunity 35, 806–818 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, Paulos CM, Muranski P, Restifo NP, Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med 15, 808–813 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


