Abstract
Background:
The general population is ubiquitously exposed to the toxic metal cadmium through the diet and smoking. Cadmium exposure is associated with increased morbidity and mortality in myocardial infarction and stroke. Atherosclerosis is the main underlying mechanism of myocardial infarction. However, associations between cadmium and coronary artery atherosclerosis have not been examined.
Objectives:
Our study sought to examine the hypothesis that blood cadmium (B-Cd) is positively associated with coronary artery calcification, as a measure of coronary artery atherosclerosis in the population-based Swedish SCAPIS study.
Methods:
Our analysis included 5,627 individuals (51% women), age 50–64 y, enrolled from 2013 to 2018. The coronary artery calcium score (CACS) was obtained from computed tomography. Blood cadmium was determined by inductively coupled plasma mass spectrometry (ICP-MS). Associations between B-Cd and coronary artery calcium score (CACS Agatston score) were evaluated using prevalence ratios (PRs) in models adjusted for sex, age, smoking, hypertension, diabetes, low-density cholesterol/high-density cholesterol ratio, and family history.
Results:
The median B-Cd concentration was . The prevalence of positive coronary artery calcium () was 41% and the prevalence of was 13%. Relative to the lowest quartile (Q) of B-Cd (), the highest quartile (median ) was associated with a small but significant increase in (PR 1.1; 95% CI: 1.0, 1.3), and a greater relative increase in (PR 1.6; 95% CI: 1.3, 2.0). When restricted to 2,446 never-smokers, corresponding PRs were 1.1 (95% CI 0.9, 1.3) for (63 cases in Q4) and 1.7 (95% CI 1.1, 2.7) for (17 cases in Q4).
Discussion:
Blood cadmium in the highest quartile was associated with CACS in a general population sample with low to moderate cadmium exposure. This supports the hypothesis that atherosclerosis is an important mechanism underlying the associations between cadmium and incident cardiovascular disease. The findings suggest that public health measures to reduce cadmium exposure are warranted. https://doi.org/10.1289/EHP8523
Introduction
Cadmium (Cd) is a toxic metal with ubiquitous exposure through the diet and smoking as the main sources. Cadmium accumulates mainly in the kidneys and has a half-life of decades, and therefore it usually increases with age. Exposure and body burden can be assessed by measuring blood or urine cadmium concentrations (Nordberg et al. 2015; Akerstrom et al. 2013).
Apart from being a well-known cause of kidney and skeletal damage, blood or urine cadmium has been reported to be an independent risk factor for cardiovascular disease, including coronary heart disease in several reviews (Tellez-Plaza et al. 2013a; Chowdhury et al. 2018; Tinkov et al. 2018). This is based on prospective general population studies demonstrating associations between cadmium biomarkers and cardiovascular mortality as well as incident cardiovascular disease, in the United States, Japan, and Europe (Nawrot et al. 2008; Menke et al. 2009; Li et al. 2011; Tellez-Plaza et al. 2012, 2013b; Barregard et al. 2016). Moreover, the Trial to Assess Chelation Therapy (TACT) trial, targeting toxic metals, such as cadmium, suggested a striking protective benefit of chelation therapy on the risk of cardiovascular events (Lamas et al. 2016).
Because atherosclerosis is the main cause of cardiovascular disease, it is also a likely mechanism mediating the association with cadmium exposure. Atherosclerosis is an inflammatory disease and cadmium is proinflammatory (Almenara et al. 2013; Olszowski et al. 2012; Fagerberg et al. 2017). Moreover, there is experimental support for the proatherogenic effects of cadmium in animal studies, indicating oxidative stress and proinflammatory endothelial dysfunction as important mechanisms (Messner et al. 2009; Knoflach et al. 2011; Oliveira et al. 2019). Indeed, two epidemiological studies showed associations between blood cadmium (B-Cd) and atherosclerotic plaque in the carotid artery (Fagerberg et al. 2012, 2015). Cadmium accumulates in the aortic wall (Abu-Hayyeh et al. 2001), and the content of cadmium in symptomatic carotid plaques, from carotid endarterectomy, was found to be 50 times higher than that in the blood and to be higher in the vulnerable part of the plaque where rupture often occurs (Bergström et al. 2015a). However, despite the association reported between cadmium exposure and clinical coronary heart disease, we are not aware of studies on the association between cadmium and coronary artery atherosclerosis.
Therefore, the aim of the present study was to examine the hypothesis that B-Cd is positively associated with coronary artery calcification, as a measure of coronary artery atherosclerosis in the large population-based Swedish CArdioPulmonary bioImage Study (SCAPIS).
Methods
Study Population
The present cross-sectional study was based on individuals from the SCAPIS project, recruiting 50- to 64-y-old individuals () randomly selected from the general population of six Swedish cities (Gothenburg, Linköping, Malmö, Stockholm, Umeå, and Uppsala) (Bergström et al. 2015b). No exclusion criteria were applied except for the inability to understand written and spoken Swedish for informed consent. Blood samples for cadmium analysis were collected from all study participants from two of the sites during two specified periods 2013–2017 in Gothenburg and 2017–2018 in Malmö, in total 5,903 participants. The participation rate was 52% of those invited. The external validity vs. the target population has been examined in the SCAPIS pilot study (Bonander et al. 2019) as well as in the total SCAPIS cohort. Data from Statistics Sweden and the National Patient Register showed that participants were only marginally different from nonparticipants with respect to age () and sex (). A history of cardiovascular disease was slightly less common in participants (14%) than in nonparticipants (16%) (Bonander et al. 2019). The unweighted prevalence of any coronary atherosclerosis in the SCAPIS cohort was identical (42%) with the estimated prevalence (42%) standardized to the catchment area population 50–64 years of age, using Inverse Probability of Participation Weighting. Out of the 5,903 participants, Cd could not be determined in 108 samples (1.8%; too small a volume, or clotting), and the calcium score was not examined in 168 of the remaining individuals (2.9%; previous coronary artery intervention invalidating coronary artery calcium estimation, ; technical failures in acquiring or reading images, ; and subject declined computed tomography (CT, ). Thus, the present study was based on 5,627 participants (Gothenburg: and Malmö: ).
Each participant underwent a thorough clinical examination, including extensive questionnaires on medical history and lifestyle factors, fasting venous blood sampling, and a physical examination. Additionally, extensive imaging was performed, including CT of the heart (Bergström et al. 2015b). Each participant gave written informed consent to participate in the study, which was approved by the Ethics Review Board of Umeå University.
Coronary Artery Calcification
At both study sites, coronary artery calcification was assessed in noncontrast-enhanced images from an identical ECG-gated multislice CT scanner (Siemens, Somatom Definition Flash, Siemens Healthineers). The calcium content in each coronary artery was measured using the same protocol at both sites, and summed to produce a total coronary artery calcification score (CACS; Agatston score) according to international standards (Agatston et al. 1990; McCollough et al. 2007). An Agatston score was defined as positive and as “high” (Tota-Maharaj et al. 2014; Greenland et al. 2018). High reproducibility at repeat readings of images has been reported in the SCAPIS pilot study (Ekblom-Bak et al. 2018). In repeat reading of images from 50 randomly selected subjects, the kappa measure of agreement was 0.91 in identifying subjects with and 1.00 for .
B-Cd
Prior to analysis, the blood samples were diluted 20 times with an alkaline solution as described previously (Fagerberg et al. 2015). Cadmium concentrations were determined by inductively coupled plasma mass spectrometry (ICP-MS; Gothenburg: 7700x, Agilent Technologies and Lund: iCAP Q, Thermo Fisher Scientific, GmbH) equipped with a collision cell with kinetic energy discrimination and with helium as the collision gas. The limit of detection was and the method imprecision was 9.8% (Gothenburg) and 6.2% (Lund). Only two samples had B-Cd below the detection limit, and for those samples, the measured values () were used. Analytical accuracy was verified, using certified reference materials: Seronorm Trace elements whole blood L-1 and L-2 (SERO AS), and showed good agreement between obtained and recommended concentrations. (For details, see Table S1). An interlaboratory comparison showed good agreement between the two laboratories (, Pearson , slope 0.96).
Covariates
Based on smoking data from the questionnaires, individuals were categorized as never, former, or current smokers. The smoking question was phrased “Do you smoke?” with alternatives “No, I have never smoked,” “Yes, I smoke regularly,” “Yes, I smoke sometimes,” “No, I stopped smoking,” and “I do not want to/cannot answer.” Those responding smoking “regularly” or “sometimes” were classified as current smokers, without any requirement on a specific length of time since start of smoking. All who chose the alternative “No, I stopped smoking” were classified as former smokers, and those responding “I do not want to/cannot answer” were coded as missing. Blood pressure was measured twice in each arm in the supine position, after 5 min of rest, with an automatic device (Omron M10-IT, Omron Health Care Co.), and the mean of the measurements was used. Hypertension (yes/no) was based on an affirmative answer to the question on physician-diagnosed hypertension in the questionnaire (86% of which reported ongoing antihypertensive medication), a systolic blood pressure , or a diastolic blood pressure at the physical examination. Diabetes mellitus (yes/no) was assessed based on medical history (questionnaire and interview; known diagnosis of diabetes), as well as fasting plasma glucose and HbA1c (either , or ; new diagnosis). Low-density lipoprotein (LDL), high-density lipoprotein (HDL), and C-reactive protein (CRP) were analyzed in fasting blood samples using standard methods at the clinical laboratories of the university hospitals. Height, weight, and waist circumference were measured. The following additional information was collected from the questionnaire: education [three categories: primary school or less (low), secondary school (medium), university (high)]; physical activity during leisure time (classified as “sedentary” if mainly sedentary, with walks, cycling, or similar ), otherwise nonsedentary; country of birth (born in or outside Sweden); family history of early cardiovascular disease (“family history of CVD” classified as present if a parent was diagnosed with myocardial infarction before age 60 y or stroke before age 65 y); and regular use of lipid-lowering medications. Participants listed all medications taken “regularly,” and the authors selected lipid-lowering medications among those listed.
Statistical Methods
Cross-sectional associations between B-Cd (in quartiles) and the CACS were assessed primarily as prevalence ratios (PRs) using Poisson regression models, but also as odds ratios (ORs) derived from logistic regression models. Because a positive calcium score was relatively common, the PRs would reflect the relative risk better than the ORs, which would be inflated for conditions with high prevalence (Grant 2014). Confidence intervals (CIs) for the Poisson regression models were calculated according to the method described by Zou (2004), using a robust error variance procedure known as sandwich estimation, with the REPEATED statement in the SAS GENMOD procedure (SAS Institute Inc.). Most previous studies used ORs as the measure of effect size; so to facilitate comparison with those studies, the results from logistic regression models are also presented.
Potential confounders were assessed from the literature. Model 1 included only age and sex. Model 2 also included risk factors for atherosclerosis that were associated with B-Cd, and changed regression estimates for B-Cd by : smoking habits (3 categories), hypertension (yes/no), diabetes (yes/no), family history of CVD (yes/no), and LDL/HDL ratio. Because there were missing data for some covariates, the number of participants and cases in Model 2 () was somewhat less than in Model 1 ().
Blood cadmium levels are usually higher in women than in men as reviewed by Nordberg et al. (2015), while atherosclerosis is more common in men. Therefore, for all the models, sex-stratified results are also presented. Smoking was adjusted for in the models, but in addition separate analyses were performed in never-smokers.
Sensitivity analyses were performed with additional adjustment of Model 2 for the following atherosclerosis risk factors: waist circumference (continuous), use of lipid-lowering medications (yes/no), education (low, medium, or high), low leisure time physical activity (yes/no), born outside Sweden (yes/no), systolic blood pressure (continuous), and log-transformed CRP. We also repeated Model 2 with additional adjustment for site (Gothenburg or Malmö), and with additional adjustment for smoking pack-years (continuous, in addition to never, former, or current smoking), and after excluding individuals with B-Cd concentrations above the 99.5th percentile (, ) and below the 0.5th percentile (, ).
B-Cd was categorized into quartiles (Qs), because associations with atherosclerosis were assumed not to be found at low levels of B-Cd. For example studies of peripheral artery disease (Tellez-Plaza et al. 2010) and carotid artery atherosclerotic plaques (Fagerberg et al. 2015) showed no associations below . We used the same quartile cut points also in models stratified for gender and in analyses restricted to never-smokers. The rationale for this is that we can compare the same B-Cd concentrations by quartiles when we present results for women, men, and both sexes combined as well as by smoking category. However, we also modeled associations with B-Cd as an untransformed continuous variable, and, after excluding the 52 participants with the highest and lowest B-Cd concentrations, using unrestricted cubic splines with knots at the 25th, 50th, and 75th percentiles of B-Cd.
To assess potential effect modification, we used stratified models, as well as a multiplicative interaction term between B-Cd and each stratification factor. Stratification was done by covariates in Model 2: sex, age, smoking, hypertension, diabetes, family history, and LDL/HDL ratio. The PR estimates were obtained in separate stratum-specific models. The p-values for interaction between B-Cd (as a continuous variable) and stratification variables were obtained in separate models for each stratification variable.
Statistical analyses were performed using SAS software package, version 9.4 (SAS Institute Inc.) and Stata/SE (release 16.1; Stata-Corp LLC). A two-tailed was considered statistically significant.
Results
As shown in Table 1, the mean age of the participants was 57 y, 51% were women, and 16% were current smokers. The median B-Cd concentration was , higher in women () than in men (). In never-smokers the median B-Cd was , again higher in women () than in men (). More details on the distribution of B-Cd are shown in supplemental Table S2.
Table 1.
Characteristics of a Swedish population-based cohort () by quartiles of blood cadmium. Quartiles are mutually exclusive, but cut points in the column are rounded.
| Variable | All | Women | Men | Quartiles of blood cadmium () | |||
|---|---|---|---|---|---|---|---|
| 0.16 to 0.24 | 0.24 to 0.39 | 0.39 to 8.5 | |||||
| Number of participants | 5,627 | 2,893 | 2,734 | 1,407 | 1,406 | 1,407 | 1,407 |
| Blood cadmium, mean (median) | 0.38 (0.24) | 0.43 (0.29) | 0.32 (0.19) | 0.12 (0.12) | 0.20 (0.20) | 0.30 (0.30) | 0.89 (0.63) |
| Age (y), | |||||||
| Women [ (%)] | 2,893 (51) | 355 (25) | 678 (48) | 939 (67) | 921 (65) | ||
| Men [ (%)] | 2,734 (49) | 1,052 (75) | 728 (52) | 468 (33) | 486 (35) | ||
| Never smoker [ (%)] | 2,520 (46) | 1,227 (43) | 1,293 (49) | 950 (69) | 727 (53) | 589 (43) | 254 (18) |
| Ex-smoker [ (%)] | 2,100 (38) | 1,156 (41) | 944 (35) | 392 (29) | 601 (44) | 690 (50) | 417 (30) |
| Current smoker [ (%)] | 883 (16) | 454 (16) | 429 (16) | 29 (2.1) | 45 (3.3) | 96 (7.0) | 713 (52) |
| Missing data on smoking category, | 124 | 56 | 68 | 36 | 33 | 32 | 23 |
| Cigarette pack-years, a (missing data as for smoking category) | |||||||
| BMI (), | |||||||
| Waist (cm), ( missing) | (1) | (1) | (1) | ||||
| Hypertension [ (%)] (n missing) | 1,610 (29) (154) | 761 (27) (63) | 849 (32) (91) | 396 (29) (31) | 388 (28) (26) | 372 (27) (39) | 454 (34) (58) |
| Diabetes mellitus [ (%)] | 376 (6.7) | 144 (5.0) | 232 (8.5) | 88 (6.3) | 91 (6.5) | 87 (6.2) | 110 (7.8) |
| Lipid lowering treatment [ (%)] | 361 (6.4) | 154 (5.3) | 207 (7.6) | 74 (5.3) | 92 (6.5) | 96 (6.8) | 99 (7.0) |
| Systolic BP (mm Hg), ( missing) | (3) | (1) | (2) | (3) | |||
| Diastolic BP (mm Hg), ( missing) | (3) | (1) | (2) | (3) | |||
| LDL cholesterol (mmol/L), ( missing) | (10) | (4) | (6) | (4) | (1) | (3) | (2) |
| HDL cholesterol (mmol/L), ( missing) | (10) | (5) | (5) | (3) | (1) | (4) | (2) |
| LDL/HDL ratio, ( missing) | (11) | (5) | (6) | (4) | (1) | (4) | (2) |
| CRP (mg/L), mean (median) ( missing) | 2.0 (1.0) (9) | 2.0 (1.0) (4) | 2.0 (1.0) (5) | 1.9 (1.0) (3) | 1.8 (0.9) (1) | 1.9 (0.9) (3) | 2.3 (1.3) (2) |
| Family history, AMI or strokeb [ (%)] ( missing) | 592 (11) (188) | 344 (12) (85) | 248 (9.4) (103) | 145 (11) (46) | 140 (10) (35) | 151 (11) (43) | 156 (12) (63) |
| Born outside Sweden [ (%)] ( missing) | 1,145 (21) (99) | 592 (21) (42) | 553 (21) (57) | 156 (11) (21) | 225 (16) (19) | 314 (23) (20) | 450 (33) (39) |
| Low education level [ (%)] | 578 (11) | 264 (9.3) | 314 (12) | 112 (8.1) | 128 (9.3) | 131 (9.5) | 207 (15) |
| Medium education level [ (%)] | 2,460 (45) | 1,189 (42) | 1,271 (48) | 602 (44) | 584 (42) | 595 (43) | 679 (50) |
| High education level [ (%)] | 2,480 (45) | 1,392 (49) | 1,088 (41) | 670 (48) | 672 (49) | 657 (48) | 481 (35) |
| Missing data on education level, | (109) | (48) | (61) | (23) | (22) | (24) | (40) |
| Sedentary leisure time [ (%)] | 676 (12) | 299 (11) | 377 (14) | 148 (11) | 125 (9.1) | 159 (12) | 244 (19) |
| Non-sedentary leisure time [ (%)] | 4,735 (88) | 2,501 (89) | 2,234 (86) | 1,218 (89) | 1,246 (91) | 1,199 (88) | 1,072 (81) |
| Missing data on leisure time, | (216) | (93) | (123) | (41) | (35) | (49) | (91) |
| Calcium score , (%)c | 2,301 (41) | 781 (27) | 1,520 (56) | 622 (44) | 561 (40) | 492 (35) | 626 (44) |
| Calcium score , (%)d | 705 (13) | 181 (6.3) | 524 (19) | 164 (12) | 161 (11) | 138 (9.8) | 242 (17) |
Note: BMI, body mass index; BP, blood pressure; CRP, C-reactive protein; LDL, low-density lipoprotein cholesterol; HDL, high-density lipoprotein cholesterol; SE, standard error.
Among current or former smokers.
If either myocardial infarction at age , or stroke at age .
Agatston score , sum of scores in three coronary arteries.
Agatston score , sum of scores in three coronary arteries.
The prevalence of a positive calcium score () was 41%, and was found in 13% of the participants. Outcomes and cardiovascular risk factors, stratified by sex and B-Cd quartiles, are shown in Table 1.
The PR for positive calcium score () was slightly, but significantly increased in the highest quartile (Q4) of B-Cd (PR 1.1; 95% CI 1.0, 1.3) compared with Q1, whereas PRs comparing the second and third quartiles with the first were null. When stratified by gender, associations between categorical B-Cd and were similar for men and women (Table 2, Model 2). A increase in B-Cd was also associated with a small but significant PR for overall (PR 1.1; 95% CI: 1.1, 1.2), and when stratified by gender. When modeled using cubic splines (after excluding 52 participants with the highest and lowest exposures) the overall association was positive for , close to the 75th percentile cut point for the highest quartile (Figure S1), whereas associations were positive beginning at slightly higher and lower concentrations for women and men, respectively (Figure S2).
Table 2.
Prevalence ratios (95% confidence intervals) for coronary artery calcium score (Agatston score) , and by sex and blood cadmium concentration in a Swedish cohort (). Quartiles are mutually-exclusive, but cut points in the columns are rounded.
| Outcome, group, and model | Number of cases/subjects | PR per | PR (95% CI) and number of cases/total number per quartile of blood cadmium (Q1–Q4) in | |||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| 0.16 to 0.24 | 0.24 to 0.39 | 0.39 to 8.5 | ||||
| Calcium score | ||||||
| All | ||||||
| Model 1 (age and sex) | 2,301/5,627 | 1.2 (1.1, 1.3) | 1.0 (622/1,407) | 1.0 (0.9, 1.1) (561/1,406) | 1.0 (0.9, 1.1) (492/1,407) | 1.3 (1.2, 1.5) 626/1,407) |
| Model 2a | 2,143/5,295 | 1.1 (1.1, 1.2) | 1.0 (574/1,329) | 1.0 (0.9, 1.1) (527/1,342) | 0.9 (0.9, 1.0) (454/1,320) | 1.1 (1.0, 1.3) (588/1,304) |
| Women | ||||||
| Model 1 (age) | 781/2,893 | 1.2 (1.1, 1.4) | 1.0 (82/355) | 1.0 (0.8, 1.3) (172/678) | 0.9 (0.8, 1.2) (222/939) | 1.4 (1.1, 1.7) (305/921) |
| Model 2a | 735/2,745 | 1.1 (1.0, 1.2) | 1.0 (73/338) | 1.0 (0.8, 1.3) (164/659) | 0.9 (0.7, 1.2) (206/883) | 1.2 (0.9, 1.5) (292/865) |
| Men | ||||||
| Model 1 (age) | 1,520/2,734 | 1.2 (1.1, 1.2) | 1.0 (540/1,042) | 1.0 (0.9, 1.1) 389/728 | 1.0 (0.9, 1.1) 270/468 | 1.3 (1.2, 1.3) 321/486 |
| Model 2a | 1,408/2,550 | 1.1 (1.0, 1.2) | 1.0 (501/991) | 1.0 (0.9, 1.1) 363/683 | 1.0 (0.9, 1.1) 248/437 | 1.2 (1.0, 1.3) 296/439 |
| Calcium score | ||||||
| All | ||||||
| Model 1 (age and sex) | 702/5,627 | 1.5 (1.4, 1.6) | 1.0 (163/1,407) | 1.1 (0.9, 1.3) 161/1,406 | 1.1 (0.9, 1.4) 138/1,407 | 2.0 (1.7, 2.4) 240/1,407 |
| Model 2a | 654/5,295 | 1.3 (1.1, 1.4) | 1.0 (148/1,329) | 1.1 (0.9, 1.3) 152/1,342 | 1.0 (0.8, 1.3) 126/1,320 | 1.6 (1.3, 2.0) 228/1,304 |
| Women | ||||||
| Model 1 (age) | 181/2,893 | 1.5 (1.3, 1.7) | 1.0 (10/355) | 1.3 (0.6, 2.6) 28/678 | 1.6 (0.8, 3.1) 48/939 | 3.4 (1.8, 6.4) 95/921 |
| Model 2a | 170/2,745 | 1.2 (1.1, 1.4) | 1.0 (8/338) | 1.4 (0.6, 3.1) 27/659 | 1.6 (0.7, 3.3) 45/883 | 2.6 (1.3, 5.5) 90/865 |
| Men | ||||||
| Model 1 (age) | 521/2,734 | 1.5 (1.3, 1.6) | 1.0 (153/1,052) | 1.1 (0.9, 1.4) 133/728 | 1.1 (0.9, 1.4) 90/468 | 1.8 (1.5, 2.2) 145/486 |
| Model 2a | 484/2,550 | 1.2 (1.1, 1.4) | 1.0 (140/991) | 1.1 (0.9, 1.4) 125/683 | 1.0 (0.8, 1.3) 81/437 | 1.5 (1.1, 1.9) 138/439 |
Note: CI, confidence interval; HDL: , high-density lipoprotein; LDL, low-density lipoprotein; PR, prevalence ratio; Q, quartile.
Model 2: (never, former, current), hypertension, diabetes, family history, LDL/HDL ratio.
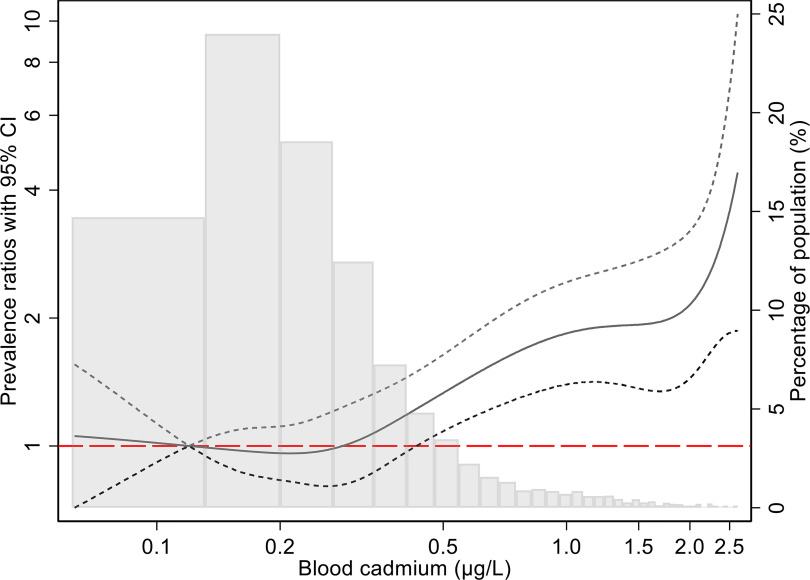
The association with the highest vs. lowest B-Cd quartile was stronger for (PR 1.6; 95% CI: 1.3, 2.0), whereas PRs for the second and third quartiles remained null. In men, PRs for were null for the second and third quartiles relative to the first, whereas the PR for the highest quartile was 1.5 (95% CI: 1.1, 1.9). Only 8 women had among those in the lowest quartile of B-Cd, resulting in imprecise estimates. However, although PRs for the second and third quartiles were not significant, they increased monotonically to a PR of 2.6 (95% CI: 1.3, 5.5) for the highest vs. lowest quartile comparison. When B-Cd was modeled as a continuous variable, a increase was associated with a significantly higher prevalence of overall (PR 1.25; 95% CI: 1.13, 1.38), with very similar PRs for men and women ( for interaction) (Figure 1). Similar to , when B-Cd was modeled using cubic splines, the association was positive for concentrations near and above the 75th percentile in men and women combined (Figure 2) and men only (Figure S3A). In women spline model estimates for were imprecise but had a positive slope throughout the B-Cd exposure distribution (Figure S3B).
Figure 1.
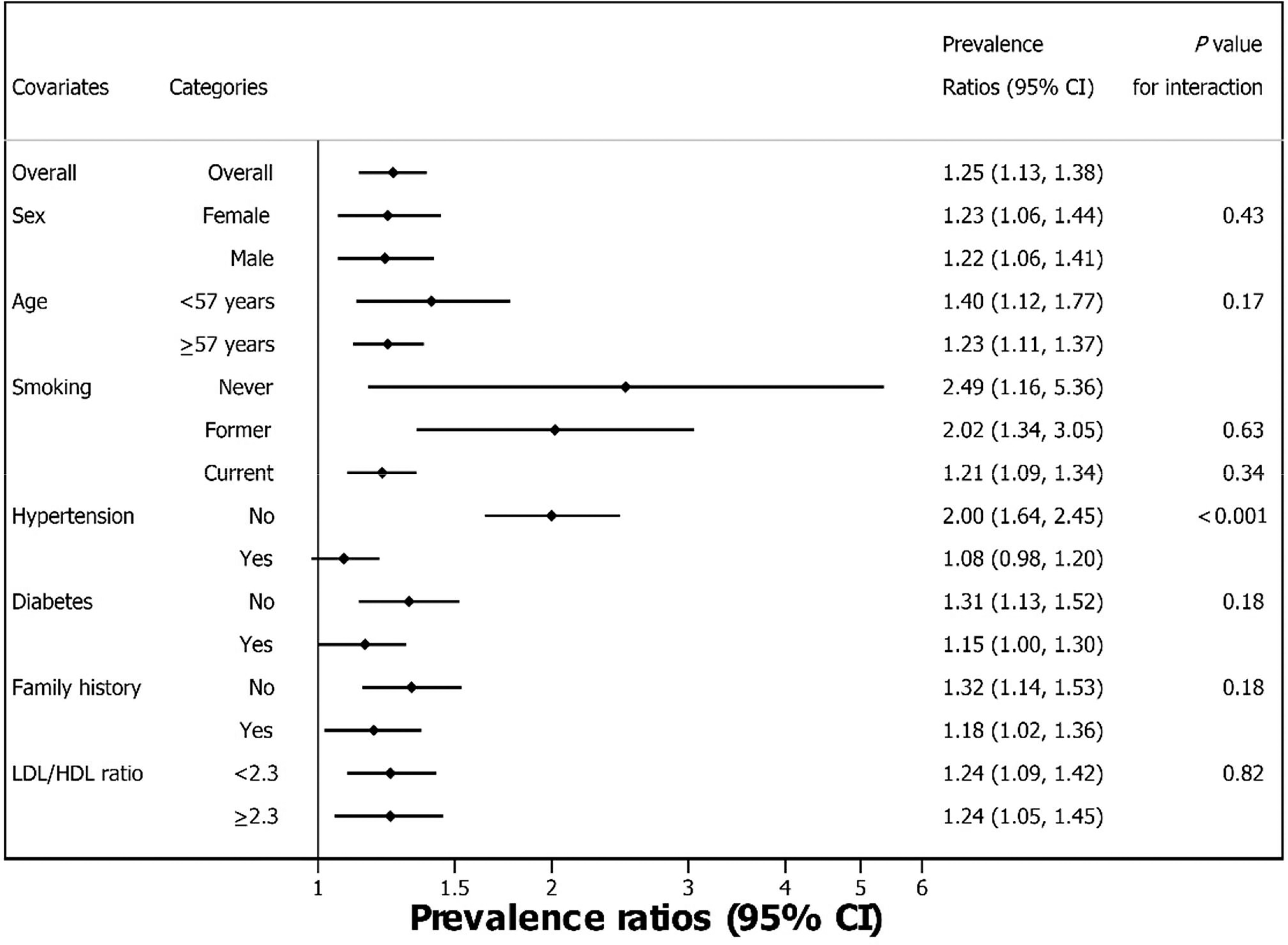
Forest plot of overall and stratified prevalence ratios and 95% confidence intervals for , adjusted for age, sex, smoking, hypertension, diabetes mellitus, family history and LDL/HDL ratio (Model 2), in a Swedish population-based cohort, using blood cadmium as continuous variable (). The PR estimates were obtained in separate stratum-specific models. The -values for interaction between blood cadmium (as a continuous variable) and stratification variables were obtained in separate models for each stratification variable. The interaction -values for smoking categories are for comparison with never-smokers. Some corresponding numerical data are also shown in Table 2. Note: CACS, coronary artery calcium score; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PR, prevalence ratio.
Figure 2.
Adjusted prevalence ratio (solid line) with 95% CI (short dashed lines) for the relation between blood cadmium concentrations () and the prevalence of (Model 2, all individuals), in a Swedish population-based cohort. The PRs were modeled using unrestricted cubic splines with three knots (0.16, 0.24, and 0.38) at blood cadmium percentiles 25%, 50%, and 75%, in a Poisson regression model adjusted for age, sex, smoking, hypertension, diabetes, family history, and LDL/HDL ratio. The reference value for blood cadmium was (median for Q1). The histogram shows the frequency distribution of blood cadmium concentrations. Corresponding numerical data are shown in Table 2. Note: CACS, coronary artery calcium score; CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PR, prevalence ratio.
The PR for in association with a increase in B-Cd was significantly stronger for participants without hypertension (PR 2.00; 95% CI: 1.64, 2.45) than in those with hypertension (PR 1.08; 95% CI: 0.98, 1.20; -interaction ) (Figure 1). Similarly, associations were stronger for participants without (vs. with) diabetes and without (vs. with) a history of myocardial infarction or stroke in a parent, and in younger () compared with older () participants, though differences between groups were not significant (interaction -values 0.17–0.18). The PR for was closer to the null for current smokers than never or former smokers, but estimates were imprecise for never and former smokers, and interaction p-values were large.
When restricted to never-smokers, there were 2,446 observations for Model 2, and the median B-Cd concentration was . When evaluated as a categorical variable using the same quartile cut points as for the total population, the Q4 vs. Q1 comparison was not significant for (PR 1.1; 95% CI: 0.9, 1.3; 63 and 384 cases, respectively), with a similar but less precise PR for a increase in B-Cd (PR 1.1; 95% CI: 0.7, 1.6) (Table 3), and a flat exposure–response curve for all but the highest exposures when modeled using cubic splines (Figure S4). Although the Q4 vs. Q1 comparison was significant for (PR 1.7; 95% CI: 1.1, 2.7), there were only 17 and 93 cases in the highest and lowest quartiles, respectively. The PR for with a increase in continuous B-Cd was also positive (PR 2.4; 95% CI: 1.2, 5.4) (Table 3), whereas the cubic spline model suggested an imprecise but weak positive exposure–response for B-Cd concentrations above approximately (Figure S5).
Table 3.
Prevalence ratios, with 95% confidence intervals for coronary artery calcium score (Agatston score) , and by sex and blood cadmium concentration in never-smokers () from a Swedish population-based cohort. Quartiles are mutually exclusive, but cut points in the columns are rounded.
| Outcome, group, and model | Number of cases/subjects | PR per | PR (95% CI) and number of cases/total number per quartile of blood cadmium (Q1–Q4) in | |||
|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | |||
| 0.16 to 0.24 | 0.24 to 0.39 | 0.39 to 8.5 | ||||
| Calcium score | ||||||
| All | ||||||
| Model 1 (age and sex) | 874/2,520 | 1.0 (0.7, 1.6) | 1.0 (395/950) | 1.0 (0.9, 1.1) 251/727 | 0.9 (0.8, 1.1) 163/589 | 1.0 (0.8, 1.3) 65/254 |
| Model 2a | 848/2,446 | 1.1 (0.7, 1.6) | 1.0 (384/925) | 1.0 (0.9, 1.1) 245/710 | 1.0 (0.8, 1.1) 156/365 | 1.1 (0.9, 1.3) 63/246 |
| Women | ||||||
| Model 1 (age) | 237/1,227 | 1.0 (0.5, 1.9) | 1.0 (44/224) | 1.0 (0.7, 1.4) 73/363 | 0.9 (0.6, 1.2) 75/422 | 1.0 (0.7, 1.4) 45/218 |
| Model 2a | 228/1,193 | 0.9 (0.4, 1.7) | 1.0 (42/216) | 1.0 (0.7, 1.4) 72/359 | 0.9 (0.6, 1.2) 71/406 | 1.0 (0.7, 1.4) 43/212 |
| Men | ||||||
| Model 1 (age) | 637/1,293 | 1.1 (0.6, 1.9) | 1.0 (351/726) | 1.0 (0.9, 1.1) 178/364 | 1.0 (0.9, 1.2) 88/167 | 1.0 (0.8, 1.4) 20/36 |
| Model 2a | 620/1,253 | 1.3 (0.8, 2.1) | 1.0 (342/709) | 1.0 (0.9, 1.1) 173/351 | 1.0 (0.9, 1.2) 85/159 | 1.2 (0.9, 1.5) 20/34 |
| Calcium score | ||||||
| All | ||||||
| Model 1 (age and sex) | 208/2,520 | 2.2 (1.0, 4.9) | 1.0 (96/950) | 0.9 (0.7, 1.3) 55/727 | 1.1 (0.8, 1.6) 40/589 | 1.5 (0.9, 2.4) 17/254 |
| Model 2a | 200/2,446 | 2.4 (1.2, 5.4) | 1.0 (93/925) | 1.0 (0.7, 1.3) 53/710 | 1.1 (0.8, 1.6) 37/565 | 1.7 (1.1, 2.7) 17/246 |
| Women | ||||||
| Model 1 (age) | 33/1,227 | 3.4 (1.7, 7.5) | 1.0 (3/224) | 1.9 (0.5, 6.8) 10/363 | 1.5 (0.4, 5.3) 9/422 | 3.5 (1.0, 12) 11/218 |
| Model 2a | 32/1,193 | 3.3 (1.5, 7.1) | 1.0 (3/216) | 1.9 (0.5, 6.8) 10/359 | 1.4 (0.4, 5.2) 8/406 | 3.2 (0.9, 11) 11/212 |
| Men | ||||||
| Model 1 (age) | 175/1,293 | 1.5 (1.3, 1.6) | 1.0 (93/726) | 0.9 (0.6, 1.2) 45/364 | 1.2 (0.8, 1.7) 31/167 | 1.0 (0.5, 2.1) 6/36 |
| Model 2a | 168/1,253 | 1.5 (0.5, 4.9) | 1.0 (90/709) | 0.9 (0.6, 1.3) 43/351 | 1.2 (0.8, 1.8) 29/159 | 1.2 (0.6, 2.3) 6/34 |
Note: CI, confidence interval; HDL, high-density lipoprotein; LDL, low-density lipoprotein; PR, prevalence ratio; Q, quartile: .
Model 2: , diabetes, family history, LDL/HDL ratio.
When stratified by gender (for Model 2, 1,193 observations and 228 cases in women, 1,253 cases and 620 cases in men) PRs for in never-smokers were null or close to the null for all comparisons, with the exception of a weak nonsignificant PR for a increase in B-Cd in men (PR 1.3; 95% CI: 0.8, 2.1 vs. PR 0.9; 95% CI: 0.4, 1.7 in women) (Table 3). For in female never-smokers (32 cases), there were too few cases per quartile for meaningful comparisons. The PR for a increase in B-Cd was positive but imprecise (PR 3.3; 95% CI: 1.5, 7.1) (Table 3). PRs were also imprecise for in male never-smokers (168 cases total), and closer to the null than corresponding PRs for women (e.g., for a increase in B-Cd PR 1.5; 95% CI: 0.5, 4.9).
As expected (Grant 2014) ORs were larger than corresponding PRs, but CIs were wider (Table S3). For example, in men and women combined Model 2 ORs for the highest vs. lowest quartile of B-Cd were 1.3 (95% CI: 1.1, 1.6) for and 1.9 (95% CI: 1.4, 2.5) for . ORs were also slightly larger and less precise than corresponding PRs when the analysis was restricted to never-smokers (Table S4).
Sensitivity Analyses
Additional adjustment of Model 2 for cardiovascular risk factors, study site, and cigarette pack-years (respectively) had little effect on associations overall, or when stratified by gender (Table S5). We also performed sensitivity analyses after excluding 26 participants with B-Cd below the 0.5th percentile (2 women, 1 of whom had , and 24 men, including 14 with , 2 of whom had ), and 26 with B-Cd above the 99.5th percentile (18 women, including 8 with , 5 of whom had , and 8 men, all of whom had , including 4 with ). Results were similar to the primary model estimates for and for associations between B-Cd quartiles and (Table S5). However, PRs for a increase in B-Cd were stronger for men and women combined (PR 1.7; 95% CI: 1.4, 2.0 compared with PR 1.3; 95% CI: 1.1, 1.4 before excluding the 52 individuals) and for women (PR 2.6; 95% CI: 1.8, 3.6 compared with PR 1.2; 95% CI: 1.1, 1.4) (Table S5).
Discussion
The present population-based study showed a statistically significant positive association between CACS and B-Cd for both calcium score and Agatston units. Compared with the lowest quartile B-Cd, the estimated prevalence of was 60% (95% CI: 30%, 100%) higher for those in the highest quartile in the main model. The study adjusted for lifestyle habits together with traditional cardiovascular risk factors in the statistical analyses, and used a well-established measure of cadmium exposure.
Our findings support the hypothesis that high cadmium exposure is accompanied by increased coronary calcification. To our knowledge, this is the first study to report on coronary atherosclerosis, measured as coronary artery calcium, in relation to cadmium exposure. These findings suggest that coronary atherosclerosis is a mechanism behind the association between blood or urine cadmium and incident coronary heart disease found in prospective population studies (Tellez-Plaza et al. 2013b; Barregard et al. 2016). Associations with cadmium have also been reported for stroke and cardiovascular mortality (Nawrot et al. 2008; Menke et al. 2009; Li et al. 2011; Tellez-Plaza et al. 2012, 2013b; Barregard et al. 2016).
CACS vs. Coronary Artery Plaque
The present study used the Agatston score as a proxy for coronary atherosclerosis. The CACS is widely used both in clinical risk evaluation as well as in research, and it closely reflects the occurrence of coronary atherosclerosis, as evidenced by studies of coronary CT angiography and autopsy reports (Greenland et al. 2007, Mori et al. 2018). Furthermore, CACS provides additional predictive capacity for cardiovascular disease on top of traditional risk scores (Elias-Smale et al. 2010; Tota-Maharaj et al. 2014; Greenland et al. 2018). For example, in the Multi-Ethnic Study of Atherosclerosis (MESA) cohort the risk of future CHD events was about 12 times higher at compared with (Tota-Maharaj et al. 2014).
Arterial calcification occurs with increasing age, atherosclerosis, and metabolic disorders such as diabetes mellitus (Wu et al. 2013; Heymann et al. 2012). The chronic inflammatory process, which is a driver of atherosclerosis, activates not only the innate inflammatory system and immune cells but also vascular cells, leading to vascular remodeling that includes fibrosis and calcification.
Cadmium and Proatherosclerotic Mechanisms
Previous clinical studies on the direct proatherosclerotic effect of cadmium exposure have focused on subclinical as well as symptomatic carotid plaques and peripheral artery disease (Fagerberg et al. 2012, 2013, 2015; Bergström 2015a; Tellez-Plaza et al. 2010). These studies showed significant cross-sectional associations between B-Cd and carotid plaques or decreased ankle-brachial index. In a prospective study, urinary cadmium concentration was associated with the progression of carotid atherosclerosis (Fagerberg 2012).
Atherosclerosis is an inflammatory disease that is induced by subintimal accumulation of pro-atherogenic lipids (Hansson et al. 2015) and is closely related to aging. At the age of the present study participants (50–64 y), about half of the men from the United States and Sweden have coronary atherosclerosis, as assessed by positive CACS, with lower but still considerable levels in women (McClelland et al. 2006; Östgren et al. 2020). The high prevalence makes it obvious that the majority of these plaques do not cause myocardial infarction or acute coronary syndromes. Transformation into a vulnerable plaque is a necessary prerequisite before causing thrombosis and blood flow perturbations (Thim et al. 2008). Hence, an overview of the proatherosclerotic effects of cadmium exposure should include mechanisms related to both the occurrence and growth of plaques and the development of plaque vulnerability.
First, observations in humans and experimental studies (in animals and in vitro) suggest that cadmium exposure is associated with multiple proatherosclerotic effects, acting simultaneously and leading to the occurrence of atherosclerosis (as reviewed by Tinkov et al. 2018). There are experimental studies and population studies, mainly from Asian countries, suggesting that cadmium exposure is associated with a proatherosclerotic lipid profile (Tinkov et al. 2018). In mice models, cadmium accelerates the progression of atherosclerosis and cause endothelial dysfunction and damage (Messner et al. 2009; Tinkov et al. 2018). Studies in humans, animals and cell culture experiments show that cadmium is associated with hyperlipidemia, lipid retention, LDL cholesterol oxidation, endothelial dysfunction and damage with migration of inflammatory cells into the arterial wall as reviewed by (Tinkov et al. (2018). However, the proinflammatory effect of cadmium is still unclear, and it appears that the most consistent data concerning mechanisms include increased levels of reactive oxygen species and endothelial dysfunction (Santos-Gallego and Jialal 2016). A key step in plaque formation is the trapping of LDL-containing lipoprotein particles by proteoglycans in the arterial wall. It has been shown in experimental studies that cadmium promotes such retention of atherogenic lipoproteins (Tinkov et al. 2018).
Second, inflammation is a central feature in plaque vulnerability (Tinkov et al. 2018). There are a few observations linking cadmium to plaque vulnerability. In endarterectomies from patients operated on for symptomatic carotid stenosis, the intraplaque cadmium concentration was highest in the part of the plaque that is most prone to rupture and the intraplaque concentration of macrophages in this part of the plaque correlated with the circulating B-Cd levels (Bergström et al. 2015a; Fagerberg et al. 2016).
Smoking
Smoking is a potential confounder because it is a major risk factor for atherosclerosis and cigarette smoke contributes to cadmium exposure. Our results were, however, adjusted for smoking category, and in a sensitivity analysis we also added the number of pack-years to the statistical models. Restricting the analyses to never-smokers reduced the total number of cases with by two-thirds and reduced the number of participants in the highest quartile by , resulting in imprecise estimates (Table 3). The positive association between high B-Cd (Q4 vs. Q1) and in never-smokers (PR 1.7; 95% CI: 1.1, 2.7) was similar to the estimated association in all participants (PR 1.6; 95% CI: 1.3, 2.0). However, this was driven by a stronger association in never smoking women (PR 3.2; 95% CI: 0.9, 11 based on only 11 cases in Q4 and 3 cases in Q1) whereas the corresponding estimate was close to the null for male never-smokers (PR 1.2; 95% CI: 0.6, 2.3 based on 6 cases in Q4 and 90 in Q1). Additional studies with larger numbers of observations are needed to confirm associations overall and in never-smokers specifically, and clarify possible differences between women and men.
The present study focused on CACS as a measure of coronary atherosclerosis. When considering coronary heart disease events, risk estimates for blood or urine cadmium in never-smokers have been similar to risk estimates based on all smoking categories, although more imprecise (Tellez-Plaza et al. 2013b; Barregard et al. 2016). It should be noted that cadmium may at least partly be responsible for the well-known association between smoking and atherosclerosis. This issue has been examined using mediation analysis of atherosclerotic plaques in the carotid artery in a cross-sectional study of 4,009 Swedish individuals with varying smoking habits and blood cadmium concentrations (Andersson et al. 2018). In summary, the authors estimated that 60%–65% of the association between smoking and the prevalence of plaque was mediated by B-Cd.
We used B-Cd as a marker of exposure to this metal. B-Cd is affected by both current exposure to cadmium, from diet and smoking, and body burden (Akerstrom et al. 2013; Nordberg et al. 2015). Urinary cadmium is commonly used as a marker of body burden. An analysis from the U.S. National Health and Nutrition Examination Survey can be used to compare the validity of these two measures in relation to smoking, the largest contributor in individuals with high cadmium exposure (Hecht et al. 2016). This study showed that in former smokers, the correlation between pack-years, as a measure of duration, and amount of smoking, was higher for B-Cd compared with urinary cadmium. In two studies of atherosclerosis, the association with both blood and urine cadmium can be compared. For peripheral artery disease, the association was stronger with urinary cadmium in men, whereas in women, the association was stronger with B-Cd (Tellez-Plaza et al. 2010). In a study of carotid artery atherosclerosis, associations with blood and urinary cadmium were similar (Fagerberg et al. 2012).
Dose–Response and Effect Modification
As shown in Table 2 and Figure 2, the prevalence of positive CACS as well as starts to increase mainly when B-Cd concentration is higher than , suggesting that there may be a threshold for the association between B-Cd and CACS. Apart from in women, there was little evidence of a monotonic increase of PRs across the whole range of B-Cd, and therefore we consider the PR estimates for Q4 more relevant than those for B-Cd included as a continuous variable. In the present population age 50–64 y, the median B-Cd was , and concentrations above were found in about 20% of men (2.5% in never-smokers) and 30% of women (16% in never-smokers). B-Cd levels in Sweden are similar to those in the United States (Miao and Ji 2019) and many European countries (Meltzer et al. 2010; González-Estecha et al. 2011). The subgroup analyses suggested that the association between B-Cd and PR for high CAC score was more pronounced among participants without hypertension. These results were, however, based on B-Cd as a continuous variable, and we have no obvious explanation for this subgroup result.
Study Limitations
The participation rate of 52% is low, but previous estimates in the SCAPIS-pilot cohort suggested only a small selective participation bias in estimates of history of cardiovascular disease (Bonander et al. 2019). B-Cd is usually a good marker of long-term cadmium exposure, but in participants who recently changed their smoking habits, B-Cd will only partly reflect long-term exposure. Although the B-Cd levels are similar to those found in Europe (Meltzer et al. 2010; González-Estecha et al. 2011) and the United States (Miao and Ji 2019), the findings in this Swedish cohort are not necessarily relevant for populations with higher dietary cadmium exposure, as found for example in many Asian countries (Jeong et al. 2020). Coronary calcification is a good marker for coronary atherosclerosis in general, but the calcium score does not provide information on the distribution of atherosclerosis, the noncalcified portions of atherosclerosis, degree of stenosis, and other plaque features associated with high-risk coronary atherosclerosis (Maurovich-Horvat et al. 2014). Consequently, it is a limitation that coronary plaques were not directly imaged. A valid CAC score could not be determined for 101 participants who had previously undergone coronary interventions. Another limitation is the limited samples sizes in never-smokers, especially when stratified for gender. This was partly due to the use of quartile cut points for the population as a whole also when stratifying by gender and when restricting the analyses to never-smokers. The fact that most covariates in adjusted models were dichotomous is also a limitation. Finally, but importantly, this was a cross-sectional study.
Conclusions
B-Cd was positively associated with coronary calcium score in Swedish middle-aged adults, adding further support to evidence that Cd has proatherosclerotic effects on coronary arteries. These results agree well with previous epidemiological studies showing associations between cadmium exposure and fatal and nonfatal coronary heart disease. Exposure is mainly via diet and smoking (Nordberg et al. 2015; Tinkov et al. 2018) and can therefore be mitigated by implementing public health policies. Lowering the population level exposure to cadmium has the potential to result in substantial reductions in cardiovascular disease. This is supported by the TACT trial suggesting beneficial effects on cardiovascular risk of chelation therapy targeting lead and cadmium burdens (Lamas et al. 2016). Future research should focus on cadmium exposure and the prevalence and development of atherosclerotic coronary plaques as assessed by direct imaging techniques. A wider goal is to examine whether cadmium exposure is involved in the transformation of atherosclerotic lesions into vulnerable plaques.
Supplementary Material
Acknowledgments
The authors acknowledge the outstanding contributions by all staff at the SCAPIS test centers.
The main funding body of the SCAPIS is the Swedish Heart-Lung Foundation. The study SCAPIS study is also funded by the Knut and Alice Wallenberg Foundation, the Swedish Research Council, and VINNOVA (Sweden’s Innovation Agency). The present study was also funded by the University of Gothenburg and Sahlgrenska University Hospital, Lund University and Skåne University Hospital. G.B. was supported by the Heart and Lung foundation, the Swedish Research Council, FORTE, and the Sahlgrenska University Hospital under the ALF (Avtal om Läkarutbildning och Forskning) agreement.
References
- Abu-Hayyeh S, Sian M, Jones KG, Manuel A, Powell JT. 2001. Cadmium accumulation in aortas of smokers. Arterioscler Thromb Vasc Biol 21(5):863–867, PMID: 11348888, 10.1161/01.ATV.21.5.863. [DOI] [PubMed] [Google Scholar]
- Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. 1990. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15(4):827–832, PMID: 2407762, 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- Akerstrom M, Barregard L, Lundh T, Sallsten G. 2013. The relationship between cadmium in kidney and cadmium in urine and blood in an environmentally exposed population. Toxicol Appl Pharmacol 268(3):286–293, PMID: 23454399, 10.1016/j.taap.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Almenara CCP, Broseghini-Filho GB, Vescovi MVA, Angeli JK, Faria T. D O, Stefanon I, et al. 2013. Chronic cadmium treatment promotes oxidative stress and endothelial damage in isolated rat aorta. PLoS One 8(7):e68418, PMID: 23874620, 10.1371/journal.pone.0068418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson EM, Fagerberg B, Sallsten G, Borné Y, Hedblad B, Engström G, et al. 2018. Partial mediation by cadmium exposure of the association between tobacco smoking and atherosclerotic plaques in the carotid artery. Am J Epidemiol 187(4):806–816, PMID: 29020130, 10.1093/aje/kwx306. [DOI] [PubMed] [Google Scholar]
- Barregard L, Sallsten G, Fagerberg B, Borné Y, Persson M, Hedblad B, et al. 2016. Blood cadmium levels and incident cardiovascular events during follow-up in a population-based cohort of Swedish adults: the Malmö Diet and Cancer Study. Environ Health Perspect 124(5):594–600, PMID: 26517380, 10.1289/ehp.1509735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström G, Berglund G, Blomberg A, Brandberg J, Engström G, Engvall J, et al. 2015b. The Swedish CArdioPulmonary BioImage Study: objectives and design. J Intern Med 278(6):645–659, PMID: 26096600, 10.1111/joim.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström G, Fagerberg B, Sallsten G, Lundh T, Barregard L. 2015a. Is cadmium exposure associated with the burden, vulnerability and rupture of human atherosclerotic plaques? PLoS One 10(3):e0121240, PMID: 25816093, 10.1371/journal.pone.0121240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonander C, Nilsson A, Björk J, Bergström GML, Strömberg U. 2019. Participation weighting based on sociodemographic register data improved external validity in a population-based cohort study. J Clin Epidemiol 108:54–63, PMID: 30562543, 10.1016/j.jclinepi.2018.12.011. [DOI] [PubMed] [Google Scholar]
- Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. 2018. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362:k3310, PMID: 30158148, 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom-Bak E, Ekblom Ö, Fagman E, Angerås O, Schmidt C, Rosengren A, et al. 2018. Fitness attenuates the prevalence of increased coronary artery calcium in individuals with metabolic syndrome. Eur J Prev Cardiol 25(3):309–316, PMID: 29171773, 10.1177/2047487317745177. [DOI] [PubMed] [Google Scholar]
- Elias-Smale SE, Proença RV, Koller MT, Kavousi M, van Rooij FJA, Hunink MG, et al. 2010. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol 56(17):1407–1414, PMID: 20946998, 10.1016/j.jacc.2010.06.029. [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Barregard L, Sallsten G, Forsgard N, Östling G, Persson M, et al. 2015. Cadmium exposure and atherosclerotic plaques – results from the Malmö Diet and Cancer Study. Env Res 136:67–74, PMID: 25460622, 10.1016/j.envres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Bergström B, Borén J, Barregard L. 2012. Cadmium exposure is accompanied by increased prevalence and future growth of atherosclerotic plaques in 64-year-old women. J Intern Med 272(6):601–610, PMID: 22812670, 10.1111/j.1365-2796.2012.02578.x. [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Bergström G, Borén J, Barregard L. 2013. Cadmium exposure, intercellular adhesion molecule-1 and peripheral artery disease: a cohort and an experimental study. BMJ Open 3(3):e002489, 10.1136/bmjopen-2012-002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg B, Borné Y, Barregard L, Sallsten G, Forsgard N, Hedblad B, et al. 2017. Cadmium exposure is associated with soluble urokinase plasminogen activator receptor, a circulating marker of inflammation and future cardiovascular disease. Environ Res 152:185–191, 10.1016/j.envres.2016.10.019. [DOI] [PubMed] [Google Scholar]
- Fagerberg B, Kjelldahl J, Sallsten G, Barregard L, Forsgard N, Österberg K, et al. 2016. Cadmium exposure as measured in blood in relation to macrophage density in symptomatic atherosclerotic plaques from human carotid artery. Atherosclerosis 249:209–214, PMID: 27156912, 10.1016/j.atherosclerosis.2016.01.011. [DOI] [PubMed] [Google Scholar]
- González-Estecha M, Trasobares E, Fuentes M, Martínez MJ, Cano S, Vergara N, et al. 2011. Blood lead and cadmium levels in a six hospital employee population. PESA study, 2009. J Trace Elem Med Biol 25(suppl 1):S22–S29, PMID: 21129942, 10.1016/j.jtemb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Grant RL. 2014. Converting an odds ratio to a range of plausible relative risks for better communication of research findings. BMJ 348:f7450, PMID: 24464277, 10.1136/bmj.f7450. [DOI] [PubMed] [Google Scholar]
- Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. 2018. Coronary calcium score and cardiovascular risk. J Am Coll Cardiol 72(4):434–447, PMID: 30025580, 10.1016/j.jacc.2018.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland P, Bonow RO, Brundage BH, et al. 2007. ACCF/AHA 2007 clinical expert consensus document on coronary artery calcium scoring by computed tomography in global cardiovascular risk assessment and in evaluation of patients with chest pain: a report of the American College of Cardiology Foundation Clinical Expert Consensus Task Force (ACCF/AHA writing committee to update the 2000 expert consensus document on electron beam computed tomography). Circulation 115:402–426, PMID: 17220398, 10.1161/CIRCULATIONAHA.107.181425. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P, Tabas I. 2015. Inflammation and plaque vulnerability. J Intern Med 278(5):483–493, PMID: 26260307, 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht EM, Arheart K, Lee DJ, Hennekens CH, Hlaing WM. 2016. A cross-sectional survey of cadmium biomarkers and cigarette smoking. Biomarkers 21(5):429–435, PMID: 26983064, 10.3109/1354750X.2016.1153717. [DOI] [PubMed] [Google Scholar]
- Heymann M-F, Herisson F, Davaine J-M, Charrier C, Battaglia S, Passuti N, et al. 2012. Role of the OPG/RANK/RANKL triad in calcifications of the atheromatous plaques: comparison between carotid and femoral beds. Cytokine 58(2):300–306, PMID: 22402034, 10.1016/j.cyto.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Jeong J, Yun SM, Kim M, Koh YH. 2020. Association of blood cadmium with cardiovascular disease in Korea: from the Korea National Health and Nutrition Examination Survey 2008–2013 and 2016. Int J Environ Res Public Health 17(17):6288, PMID: 32872339, 10.3390/ijerph17176288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoflach M, Messner B, Shen YH, Frotschnig S, Liu G, Pfaller K, et al. 2011. Non-toxic cadmium concentrations induce vascular inflammation and promote atherosclerosis. Circ J 75(10):2491–2495, PMID: 21799275, 10.1253/circj.cj-11-0196. [DOI] [PubMed] [Google Scholar]
- Lamas GA, Navas-Acien A, Mark DB, Lee KL. 2016. Heavy metals, cardiovascular disease, and unexpected benefits of chelation therapy. J Am Coll Cardiol 67(20):2411–2418, PMID: 27199065, 10.1016/j.jacc.2016.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nishijo M, Nakagawa H, et al. 2011. Relationship between urinary cadmium and mortality in habitants of a cadmium-polluted area: a 22-year follow-up study in Japan. Chin Med J 124(21):3504–3509. PMID: 22340168.24755916 [PubMed] [Google Scholar]
- Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. 2014. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 11(7):390–402, PMID: 24755916, 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. 2006. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation 113(1):30–37, PMID: 16365194, 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- McCollough CH, Ulzheimer S, Halliburton SS, Shanneik K, White RD, Kalender WA. 2007. Coronary artery calcium: a multi-institutional, multimanufacturer international standard for quantification at cardiac CT. Radiology 243(2):527–538, PMID: 17456875, 10.1148/radiol.2432050808. [DOI] [PubMed] [Google Scholar]
- Meltzer HM, Lise Brantsaeter A, Borch-Iohnsen B, Ellingsen DG, Alexander J, Thomassen Y, et al. 2010. Low iron stores are related to higher blood concentrations of manganese, cobalt and cadmium in non-smoking, Norwegian women in the HUNT 2 study. Environ Res 110(5):497–504, PMID: 20381026, 10.1016/j.envres.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Menke A, Muntner P, Silbergeld EK, Platz EA, Guallar E. 2009. Cadmium levels in urine and mortality among U.S. adults. Environ Health Perspect 117(2):190–196, PMID: 19270787, 10.1289/ehp.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messner B, Knoflach M, Seubert A, Ritsch A, Pfaller K, Henderson B, et al. 2009. Cadmium is a novel and independent risk factor for early atherosclerosis mechanisms and in vivo relevance. Arterioscler Thromb Vasc Biol 29(9):1392–1398, PMID: 19556524, 10.1161/ATVBAHA.109.190082. [DOI] [PubMed] [Google Scholar]
- Miao H, Ji JS. 2019. Trends of blood cadmium concentration among workers and non-workers in the United States (NHANES 2003 to 2012). J Occup Environ Med 61(12):e503–e509, PMID: 31626072, 10.1097/JOM.0000000000001742. [DOI] [PubMed] [Google Scholar]
- Mori H, Torii S, Kutyna M, Sakamoto A, Finn AV, Virmani R. 2018. Coronary artery calcification and its progression: what does it really mean? JACC Cardiovasc Imaging 11(1):127–142, PMID: 29301708, 10.1016/j.jcmg.2017.10.012. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Van Hecke E, Thijs L, Richart T, Kuznetsova T, Jin Y, et al. 2008. Cadmium-related mortality and long-term secular trends in the cadmium body burden of an environmentally exposed population. Environ Health Perspect 116(12):1620–1628, PMID: 19079711, 10.1289/ehp.11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordberg GF, Nogawa K, Nordberg M. 2015. Cadmium. In: Handbook on the Toxicology of Metals. Nordberg GF, Nordberg M, eds. Amsterdam, Netherlands: Elsevier, 667–716. [Google Scholar]
- Oliveira TF, Batista PR, Leal MA, Campagnaro BP, Nogueira BV, Vassallo DV, et al. 2019. Chronic cadmium exposure accelerates the development of atherosclerosis and induces vascular dysfunction in the aorta of ApoE-/- mice. Biol Trace Elem Res 187(1):163–171, PMID: 29707746, 10.1007/s12011-018-1359-1. [DOI] [PubMed] [Google Scholar]
- Olszowski T, Baranowska-Bosiacka I, Gutowska I, Chlubek D. 2012. Pro-inflammatory properties of cadmium. Acta Biochim Pol 59(4):475–482, PMID: 23240106, 10.18388/abp.2012_2080. [DOI] [PubMed] [Google Scholar]
- Östgren CJ, Söderberg S, Festin K, et al. 2020. Systematic coronary risk evaluation estimated risk and prevalent subclinical atherosclerosis in coronary and carotid arteries: a population-based cohort analysis from the Swedish cardiopulmonary bioimage study. Eur J Prev Cardiol 2047487320909300, PMID: 32126830. [DOI] [PubMed] [Google Scholar]
- Santos-Gallego CG, Jialal I. 2016. Cadmium and atherosclerosis: heavy metal or singing the blues? Atherosclerosis 249:230–232, PMID: 27012656, 10.1016/j.atherosclerosis.2016.01.041. [DOI] [PubMed] [Google Scholar]
- Tellez-Plaza M, Jones MR, Dominguez-Lucas A, Guallar E, Navas-Acien A. 2013a. Cadmium exposure and clinical cardiovascular disease: a systematic review. Curr Atheroscler Rep 15(10):356, PMID: 23955722, 10.1007/s11883-013-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Guallar E, Howard BV, Umans JG, Francesconi KA, Goessler W, et al. 2013b. Cadmium exposure and incident cardiovascular disease. Epidemiology 24(3):421–429, PMID: 23514838, 10.1097/EDE.0b013e31828b0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Crainiceanu CM, Sharrett AR, Guallar E. 2010. Cadmium and peripheral arterial disease: gender differences in the 1999–2004 US national health and nutrition examination survey. Am J Epidemiol 172(6):671–681, PMID: 20693268, 10.1093/aje/kwq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Menke A, Crainiceanu CM, Pastor-Barriuso R, Guallar E. 2012. Cadmium exposure and all-cause and cardiovascular mortality in the U.S. general population. Environ Health Perspect 120(7):1017–1022, PMID: 22472185, 10.1289/ehp.1104352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thim T, Hagensen MK, Bentzon JF, Falk E. 2008. From vulnerable plaque to atherothrombosis. J Intern Med 263(5):506–516, PMID: 18410594, 10.1111/j.1365-2796.2008.01947.x. [DOI] [PubMed] [Google Scholar]
- Tinkov AA, Filippini T, Ajsuvakova OP, Skalnaya MG, Aaseth J, Bjørklund G, et al. 2018. Cadmium and atherosclerosis: a review of toxicological mechanisms and a Meta-analysis of epidemiologic studies. Environ Res 162:240–260, PMID: 29358116, 10.1016/j.envres.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Tota-Maharaj R, Blaha MJ, Blankstein R, Silverman MG, Eng J, Shaw LJ, et al. 2014. The relationship of coronary artery calcium to coronary heart disease events is similar in young and elderly participants in the multi-ethnic study of atherosclerosis: a secondary analysis of a prospective population-based cohort. Mayo Clin Proc 89(10):1350–1359, PMID: 25236430, 10.1016/j.mayocp.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Rementer C, Giachelli CM. 2013. Vascular calcification: an update on mechanisms and challenges in treatment. Calcif Tissue Int 93(4):365–373, PMID: 23456027, 10.1007/s00223-013-9712-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou G. 2004. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 159(7):702–706, PMID: 15033648, 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.