Abstract
Binge drinking is a remarkably prevalent behavior. In 2015, 27% of U.S. residents 18 years old or older reported at least one episode of binge drinking in the previous month. Rodent models for binge drinking are widely used to study the mechanisms by which alcohol causes a variety of adverse health effects in humans. Concerns have been raised that many binge drinking studies in rodents involve alcohol doses that would be unrealistically high in humans. Allometric dosage scaling can be used to estimate the dose of a drug or chemical in mice that would be necessary to achieve similar biological effects at a realistic dose in humans. However, it has become apparent that no single allometric conversion factor is applicable for all drugs and chemicals, so it is necessary to evaluate each compound empirically. In the present study, we compared the area under the blood alcohol concentration vs. time curve (AUC) and the peak blood alcohol concentration following oral alcohol administration at various doses in mice and humans, using data from previously published studies. The results demonstrated that the oral dose of alcohol must be larger in mice (on a g of alcohol to kg of body weight basis) than in humans to achieve similar alcohol AUC values or to achieve similar peak concentrations in the blood. The dose required in mice was about 2-fold greater than the dose required in humans to achieve similar alcohol AUC and peak concentrations. The results shown here were substantially different from the average 5–12-fold difference between mice and humans calculated in previous studies using agents other than alcohol. Results shown here demonstrate that an empirical approach using data from several independent experiments provides information needed to determine the alcohol dose in mice that produces a similar level of exposure (AUC and peak concentration) as in humans. The results indicate that a single alcohol dose in the range of 5–6 g/kg, a range often used in mouse models for binge drinking, is not excessive when modeling human binge drinking. Results presented here illustrate that in mice both alcohol AUC and peak alcohol concentration correlate well with an important biological effect, activation of the hypothalamic-pituitary-adrenal axis, as indicated by increased corticosterone AUC values.
Introduction
Binge drinking has become a national problem. It is remarkable that 17% of Americans over 18 years of age report an average of 53 binge drinking episodes per year with an average of 7 standard drinks per episode (Kanny et al., 2018). Commonly used binge alcohol models in mice often involve oral administration (by gavage) (Carson and Pruett, 1996). A single dose from 3–6 g/kg of body weight does not cause histopathology in the stomach or duodenum, and 7g/kg causes relatively minor histopathological changes. A single dose of 3–6 g/kg does not cause alterations in clinical chemistry parameters, including liver enzymes that indicate overt hepatotoxicity. A single dose at 7 g/kg causes a small elevation in liver enzymes indicative of mild liver damage. Administration of alcohol by gavage in mice is suitable for assessing the effects of a single binge episode (Carson and Pruett, 1996) or daily binge drinking for as long as 30 days (Pruett et al., 2009). In the present study we used data from our original study (Carson and Pruett, 1996) to calculate the area under the alcohol concentration vs. time curve (AUC). To confirm that the results were comparable to results from other labs, we reported AUC and peak alcohol concentration using published data from another lab (Livy et al., 2003) and included those results in the present study. Data from human subjects are more limited, primarily because researchers and Institutional Review Boards have become more stringent, and controlled administration of alcohol to healthy volunteers is now limited to low doses. Experiments involving administration of alcohol to human subjects under controlled conditions rarely include doses greater than 1.0 g/kg. However, a dose-response series from 0.4 g/kg to 2.0 g/kg is available from one study, and it is ideally suited for the analysis reported here (Mizoi et al., 1985). Two other studies with human subjects in which the alcohol AUC values could be calculated were also identified (Bauer et al., 1992; Mitchell et al., 2014). These data were used in the present study to confirm that the primary study with human subjects (Mizoi et al., 1985) was not an outlier. Additional studies have been done using subjects in which extreme binge alcohol consumption occurred (Hammond et al., 1973; Wiener et al., 2013), and the results are suitable for determination of the relationship between alcohol dose and peak blood alcohol concentration and for comparison of this relationship in humans and in mice. It should be noted that mice can engage in binge-like drinking using a protocol in which water is withheld and then provided (with alcohol) in a “drinking in the dark” protocol (Barkley-Levenson and Crabbe, 2014). However, the voluntary consumption protocols in mice only yield blood alcohol concentrations in the lower end of the range reported in human binge drinkers (~25 mM). We sought to examine alcohol exposure in mice and humans over the whole range of blood alcohol noted in human binge drinkers, and this can only be achieved by gavage in mice.
One of the prominent biological effects of a single dose of alcohol in mice is a stress response characterized by increased concentrations of corticosterone in the blood (Carson and Pruett, 1996). A similar neuroendocrine stress response has been reported in humans (Kutscher et al., 2002; Merry and Marks, 1969), but published results from human subjects do not allow quantitative comparison of alcohol and cortisol AUC values. However, a complete set of published results in mice were used in the present study to calculate alcohol AUC, and alcohol peak concentration and to determine how well they correlate with an important biological effect, activation of the hypothalamic-pituitary-adrenal axis (Carson and Pruett, 1996). This was done primarily to determine if AUC or peak concentration (or both) were correlated with a biological effect of alcohol.
The simplest approach to estimate biologically equivalent dosages of drugs and chemicals in different species in acute exposure studies is the use of a dosage scaling metric. For some compounds, biologically equivalent dosages can be attained if the dosages are expressed as mg/m2 of body surface area rather than mg/kg of body weight. This was effectively illustrated by Freireich and colleagues (Freireich et al., 1966) and recalculated with additional data more recently by Travis and colleagues (Travis and White, 1988). The Freireich study is one of the most cited examples of the basic principle of allometric dosage scaling. This study was conducted with cancer chemotherapeutic drugs, for which comparable biological effects can be measured in both humans and various experimental animal species. The conclusion was that equal dosages of these drugs expressed as mg/kg of body weight yielded different biological effects in humans and mice. However, dosages that were equal when expressed as mg/m2 of body surface area, yielded similar biological effects in humans and mice.
To obtain a dosage in mice and humans that is equivalent in terms of body surface area, it is necessary to give mice a dosage in mg/kg that is 12.3 times larger than the dosage in humans (in mg/kg). This is based on results reported by Freireich and colleagues for chemotherapeutic drugs. Conversion constants for dosage on the basis of body weight and body surface area have been determined for most commonly used mammalian species (Freireich et al., 1966). A more recent and more comprehensive study using a variety of drugs (not just chemotherapeutic agents) indicates that an average dosage in humans expressed as mg/kg should be multiplied by 5.5 to obtain a dosage that yields equivalent biological effects in mice (Travis and White, 1988). Thus, if a dosage of 5 mg/kg in humans produced a 50% decrease in neutrophil concentration in the blood, a dosage of 27.5 mg/kg in mice would be required to produce a similar biological effect. The differences in the studies by Freireich (Freireich et al., 1966) and Travis (Travis and White, 1988) illustrate one of the major problems of simple allometric dosage scaling: different scaling factors are needed for different drugs and chemicals. For example, depending on whether the conversion factors proposed by Freireich or those proposed by Travis were used, a mouse would need to receive 12.3 g/kg or 5.5 g/kg, respectively, to produce biological effects comparable to those produced by a dose of 1 g/kg in a human. Results obtained in the current study indicate that neither of these conversion factors is appropriate for estimating the alcohol dose required in mice to yield comparable AUC or peak blood alcohol concentration in humans. Obtaining an empirically derived conversion factor for ethanol dose between mice and humans that can be used to plan new studies and to interpret previous studies was a major goal of work described in this paper.
Materials and Methods
In this study we have analyzed data from previous studies in mice (Carson and Pruett, 1996; Livy et al., 2003) and in humans (Bauer et al., 1992; Hammond et al., 1973; Mitchell et al., 2014; Mizoi et al., 1985; Wiener et al., 2013) to calculate the values of the area under the alcohol concentration vs. time curve (AUC) in the blood. This was done to determine the relationship between alcohol dose (g/kg) and AUC. We searched for papers using mice or human subjects in which the blood alcohol concentration and the exact alcohol dosage (administered orally) was stated and in which the blood alcohol concentration was measured over time to allow calculation of the AUC. The AUC was calculated by entering the data in Prism 7.0 Software (GraphPad, San Diego, CA) and selecting area under the curve from the analysis menu. In most cases, the alcohol concentrations reached 0 by the time the sampling was completed, but in some instances, alcohol was still detectable at the longest sampling time. In the latter instances, we did not extrapolate to 0, but used the data actually measured to determine the AUC. As can be seen in Figure 1, most values were 0 or very near 0 at the last time of sampling, so extrapolating would not have had a major effect on AUC values reported here. The expectation was that most biological effects of alcohol would correlate well with the alcohol AUC. Correlation between dose and AUC was evaluated using Prism 7.0, and the regression lines for human and mouse were compared for significant differences in slope and intercept using the method of Zar (Zar, 1984) as implemented by Prism 7.0 software.
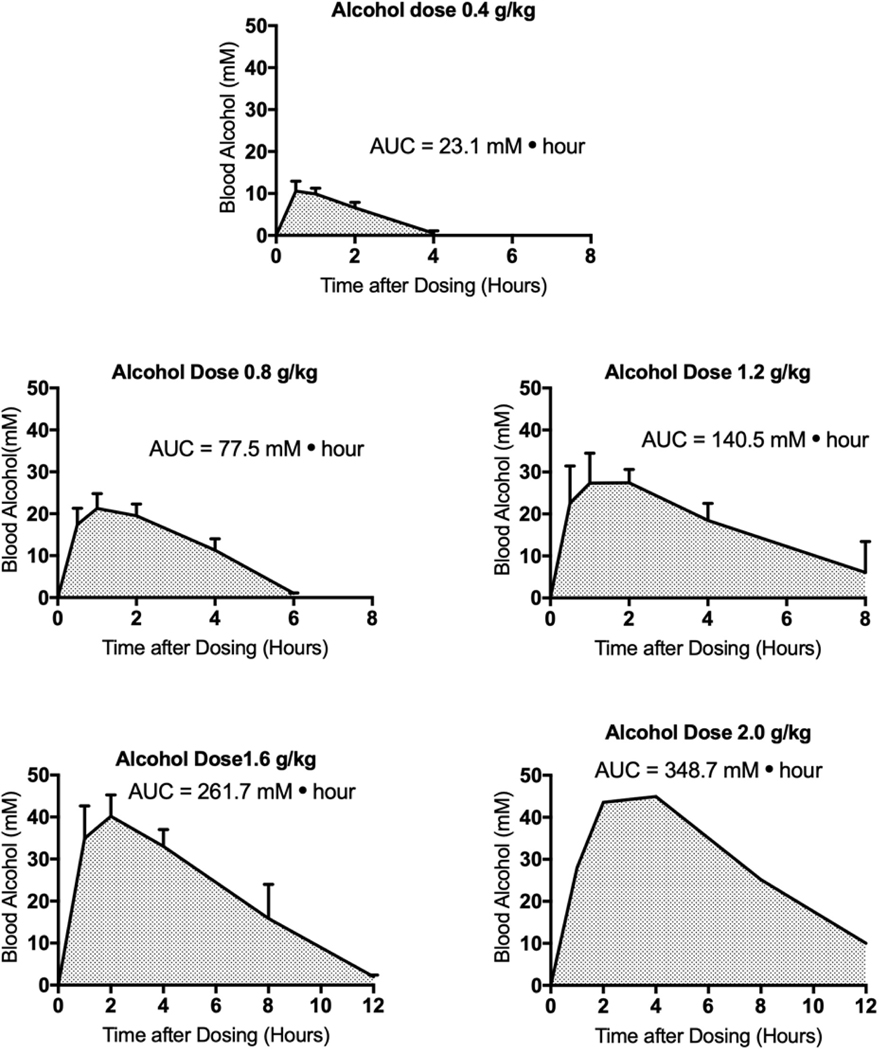
Figure 1.
Area under the alcohol concentration vs. time curve (AUC) for human subjects taken from data previously published by Mizoi and colleagues (Mizoi et al., 1985). This study included subjects with normal aldehyde dehydrogenase and subjects with a deficient aldehyde dehydrogenase phenotype. Only the subjects with normal aldehyde dehydrogenase were analyzed in this paper. The group size varies depending on dose and is specified in Table 1. The values shown are means ± standard error of the mean. Standard errors are not shown for the dose of 2.0 g/kg, because the group size was only 3, and the standard errors were not shown in the original paper. AUC values were calculated using Prism 7 (GraphPad, San Diego, CA) with the data shown. Values at late time points were not extrapolated to 0 alcohol concentration. The peak alcohol concentration as reported in this study is the highest average alcohol concentration reported, regardless of the time at which that concentration was reached.
The human studies involving oral alcohol exposure in a controlled setting used male subjects (Bauer et al., 1992; Hammond et al., 1973; Mitchell et al., 2014; Mizoi et al., 1985; Wiener et al., 2013). The human case study reports with unusually high blood alcohol concentrations and documentation of the dose consumed involved female subjects (Wiener et al., 2013; Hammond et al., 1973). The mouse studies by Carson and Pruett involved the use of female C57BL/6 × C3H F1 mice (Carson and Pruett, 1996). The other experiment with mice utilized 10 male and 8 female C57BL/6 mice (Carson and Pruett, 1996; Livy et al., 2003). Initial analysis of the data suggested that gender was not a major determinant of AUC or peak blood concentrations. This is supported by a study in which LS (long sleep) and SS (short sleep) mice were evaluated and peak alcohol concentration and AUC values were determined for male and female mice (Phillips et al., 1984). The results indicate that peak blood alcohol concentration and AUC values were remarkably similar for males and females. Although it would be preferable to have an approximately equal percentage of males and females in all studies, the results of Phillips and coworkers (Phillips et al., 1984) suggest that this would not substantively change the quantitative assessments reported here. Few studies involving controlled alcohol administration to human subjects utilize doses greater than 1.4 g/kg in humans, so the 2.0 g/kg top dose in the Mizoi study was important and provided data that overlap with data from mouse studies, which generally involve higher alcohol doses (3–6 g/kg).
Data from the study by Mizoi and colleagues (Mizoi et al., 1985) allowed us to also identify the peak blood alcohol concentration in human subjects, and these values were plotted against alcohol dose to determine the dose dependence of the peak blood alcohol concentration in humans. In addition, there were two case reports of very high blood alcohol concentrations in humans (Hammond et al., 1973; Wiener et al., 2013), and data from these studies exhibited a linear relationship with data from studies using much lower doses. The maximum alcohol dose in a human subject was ~5 g/kg (Wiener et al., 2013) and the maximum dose in mice was 7 g/kg (Carson and Pruett, 1996), so the dose response relationship for oral alcohol exposure and peak blood alcohol concentration could be determined for mice and humans with substantial overlap of the dose range in both species. Other parameters of importance in the human and mouse studies are shown in Table 1. The alcohol concentration used for dosing, the duration of the dosing period, the group size, and the alcohol doses are shown. Only studies with oral alcohol administration were considered because of the relevance of this route to typical human exposure.
Table 1.
| Reference | Species | Strain | Group Size | Route | Dose | Ethanol concentration | Time of ethanol administration | Duration of Blood Sampling |
|---|---|---|---|---|---|---|---|---|
| (Carson and Pruett, 1996) | mouse | C57BL/6 × C3H F1 | 5/dose | oral (gavage) | 3, 4, 5, 6, 7 g/kg | 32% | ~2 min | 12.0 hr |
| (Livy et al., 2003) | mouse | C57BL/6 | 10/group | oral (gavage) | 3.8 g/kg | 21% | ~2 min | 7.5 hr |
| (Mizoi et al., 1985) | human | NA | 52, 44, 15, 9, 3/ dose group | oral (drinking) | 0.4, 0.8, 1.2, 1.6, 2.0 g/kg | 0.4g/kg/ 10 min | 10–50 min | 10.0 hr |
| (Bauer et al., 1992) | human | NA | 10/group | oral (drinking) | 0.8 g/kg | Not specified | 10 min* | 12.0 hr |
| (Mitchell et al., 2014) | human | NA | 15/group | oral (drinking) | 0.5 g/kg | 20% | 10 min* | 8.0 hr |
| (Hammond et al., 1973) | human | NA | 1 (case study) | oral (drinking) | 5.4 g/kg | 50% (100 proof) | ~50 min* | 9.5 hr |
| (Wiener et al., 2013) | human | NA | 1 (case study) | oral (drinking) | 4.3–4.8 g/kg | 40–45% | ~50 min* | 22.0 hr |
Approximate values. Exact values were not stated.
To confirm alcohol AUC correlates with biological activity of alcohol, we evaluated the relationship between alcohol AUC and one of the biological effects of alcohol, increased glucocorticoid concentrations in the blood (Carson and Pruett, 1996). We used data from our original study (Carson and Pruett, 1996) but calculated the AUC values for both alcohol and serum corticosterone, which was not done in the original study. The alcohol AUC was calculated using the concentration vs. time data for several different alcohol doses, and these values were plotted against blood corticosterone concentration vs. time values (AUC) taken from our previous paper (Carson and Pruett, 1996).
Results
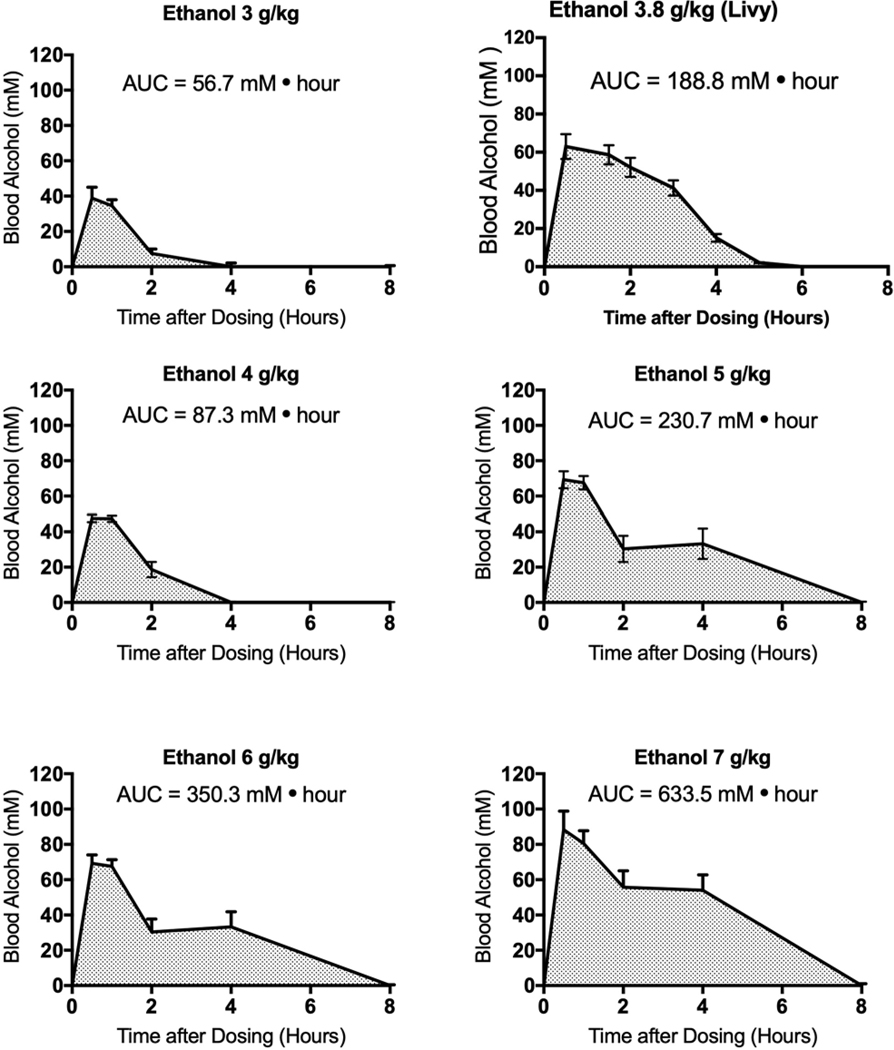
Figures 1 and 2 illustrate the use of previously published data to calculate area under the alcohol concentration vs. time curve (AUC) in blood from humans and mice, respectively. Results from humans (Figure 1) are from a study by Mizoi and colleagues (Mizoi et al., 1985). Alcohol AUC values were assessed in the same manner for two other studies in which human subjects were given alcohol at a known dosage under controlled conditions (Mitchell et al., 2014; Bauer et al., 1992). The AUC values from these studies were calculated as shown in Figure 1, and these AUC values are shown in later figures. The peak alcohol concentration in the blood in human subjects was determined by selecting the highest alcohol concentration measured during the time course of the study. In the Mizoi study as well as in the Mitchell and Bauer studies, the peak blood alcohol concentration occurred from 1 to 4 hr after dosing. In the two high dose case studies (Bauer et al., 1992; Hammond et al., 1973; Mitchell et al., 2014; Wiener et al., 2013), the peak alcohol concentration occurred at 3 or 4 hr after assessment began. In the mouse studies (Carson and Pruett, 1996; Livy et al., 2003), the peak blood alcohol concentration occurred at 1 hr after dosing. These case report data were not suitable for assessment of alcohol AUC values, because medical treatment was initiated to decrease blood alcohol concentrations rapidly, so alcohol concentration over time (as needed to assess AUC) would not represent a natural occurrence but would reflect medical intervention. However, peak blood alcohol concentrations could be determined as the highest blood alcohol concentration during the time after drinking had ceased. Although there are undoubtedly a number of additional studies in which blood alcohol concentration was measured over time in human subjects, very few included the information on alcohol dose, alcohol concentration in the blood, and the lack of medical intervention needed to determine alcohol AUC values. Results for alcohol AUC values in mice (Figure 2) include data from two independent studies (Carson and Pruett, 1996; Livy et al., 2003).
Figure 2.
Area under the alcohol concentration vs time curve for mice calculated using data from previously published studies (Carson and Pruett, 1996; Livy et al., 2003). The values shown are means ± standard error of the mean, and group sizes are shown in Table 1.
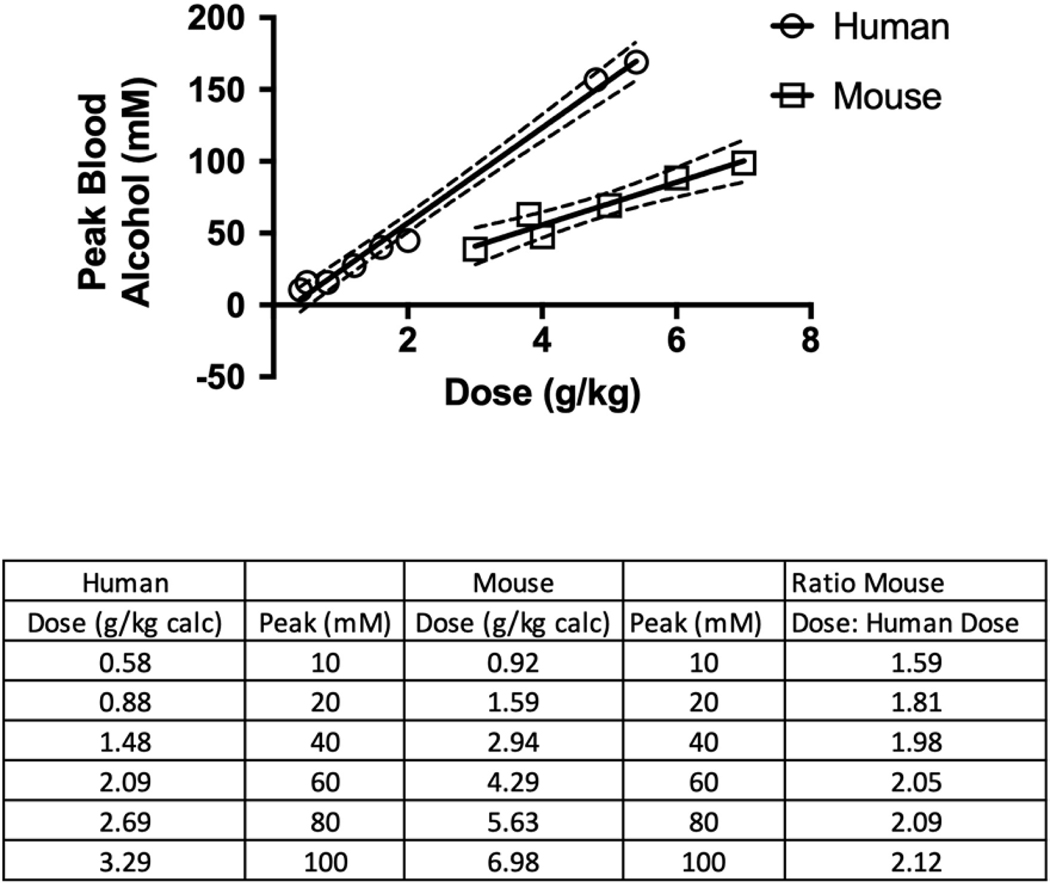
Results shown in Figure 3 indicate that a lower dose of alcohol is required in humans than in mice to produce comparable alcohol AUC values. For some drugs and chemicals, the correlation between AUC and biological effects is different from the correlation between peak blood alcohol concentration and biological effects. For example, sustained release lithium preparations yield 30–50% decreases in peak serum concentrations with no net effect on AUC. However, most clinicians use peak concentrations (not AUC) to adjust the dosage for best efficacy (Grandjean and Aubry, 2009). Thus, some effects of some drugs can best be predicted by peak blood concentrations, whereas biological effects of other drugs are more closely correlated to AUC values. Surprisingly, few adverse effects of alcohol have been studied in this regard, so we investigated whether the relationships between dose and alcohol AUC and between dose and peak alcohol concentration were similar or disparate. Results shown in Figures 3 and 4 indicate that both AUC and peak concentration were linearly dependent on alcohol dose in humans and in mice. Therefore, it would be expected that either relationship could be used as a predictor of biological outcomes. The ratio of alcohol dose to alcohol AUC in mice compared to humans is shown in Figure 3. Across the range of doses in these studies, the dose in mice required to produce comparable AUC or comparable peak concentrations (Figure 4) is approximately twice the dose required in humans. At lower blood ethanol concentrations (10 and 20 mM), the alcohol dose required to produce a particular alcohol AUC value is greater for mice than for humans (the ratio of dose required to produce 10 mM•hr AUC in mice is 5.49 × that in humans and for a 20 mM•hr AUC, the ratio is 4.10 × the human dose) (Figure 3). At higher blood alcohol concentrations, the ratio approaches 2.00. The relationship is similar for peak blood alcohol concentration (Figure 4). For doses of 1.5–3.3 g/kg in humans and 2.9–7.0 g/kg in mice, the doses required to achieve similar peak blood alcohol concentrations were approximately 2-fold greater for mice than for humans.
Figure 3.
Panel A illustrates the relationship between alcohol doses and area under the alcohol concentration vs. time curve (AUC, mM•hr) in humans from three independent studies (Bauer et al., 1992; Mitchell et al., 2014; Mizoi et al., 1985). Panel B indicates the same relationship for mice from two independent studies (Carson and Pruett, 1996; Livy et al., 2003). The r2 value for panel A is 0.989, and the r2 value for panel B is 0.929. These are both significantly different from a slope of 0 (p < 0.0001), and the slopes for humans and mice are significantly different (p = 0.014). The same values are shown in panel C for ease of comparison, and the slope of the line for human samples and the slope of the line for mouse samples are significantly different (p = 0.014). The Table at the bottom of this Figure (D.) indicates the ratio of the dose in mice that produces a particular AUC value to the dose in humans that produces that same AUC value (calculated from Figure 1 C). For humans, all doses above 2 g/kg are extrapolated; for mice all values except the dose corresponding to 600 mM•hr were measured. The solid lines represent the regression lines calculated for the values shown and the dotted lines are the upper and lower 95% confidence intervals.
Figure 4.
Relationship between dose and peak blood alcohol concentration in humans and mice. The results for humans were obtained using data from the same references as noted in Figure 3, except that the data for the two highest doses were obtained from two additional studies involving unusually large doses of alcohol (Hammond et al., 1973; Wiener et al., 2013). The r2 value for the human studies is 0.982, and the r2 value for the mouse studies is 0.893. The slopes of these lines are both significantly different from a slope of 0 (p < 0.0001), and the slope of the line for human samples is significantly different from the slope of the line for mouse samples (p < 0.01). The solid lines represent the regression line calculated for the values shown and the dotted lines are the upper and lower 95% confidence intervals. The data from human subjects is from a study by Mizoi (Mizoi et al., 1985), and case studies by Wiener (Wiener et al., 2013) and Hammond (Hammond et al., 1973). The peak blood alcohol concentration for each dose is the mean blood alcohol concentration at the time at which the concentration was largest, as described in the legend for Figure 1.
We have published a series of papers demonstrating that activation of the hypothalamic-pituitary-adrenal axis by alcohol in a binge drinking model in mice contributes to or fully explains the suppression of several important immunological parameters (Collier et al., 1998, 2000; Glover et al., 2011; Glover et al., 2009; Glover and Pruett, 2006; Han and Pruett, 1995; Han et al., 1993b; Pruett et al., 2007; Pruett et al., 2008; Pruett et al., 1999; Pruett et al., 1998; Pruett et al., 2003; Pruett et al., 2009; Schwab et al., 2005; Weiss et al., 1996; Wu and Pruett, 1997, 1999; Wu and Pruett, 1996). An increase in the area under the corticosterone concentration vs. time curve can be used to predict suppression of several immunological parameters (Collier et al., 1998; Glover et al., 2011; Glover et al., 2009; Glover and Pruett, 2006; Han et al., 1993a; Pruett et al., 2003; Schwab et al., 2005; Weiss et al., 1996; Wu and Pruett, 1999). In the results shown in Figure 5, we analyzed data from previous papers to determine the relationship between alcohol AUC and corticosterone AUC and between peak blood alcohol concentration and corticosterone AUC. The results indicate that both peak alcohol concentration and alcohol AUC exhibit excellent correlation with corticosterone AUC, a biological effect induced by alcohol, which has an impact on many other biological parameters. Although there are a number of papers in which increased cortisol (the major glucocorticoid hormone in humans) concentration has been observed following binge alcohol exposure (Kutscher et al., 2002; Merry and Marks, 1969), the complete time series of alcohol and cortisol concentrations and the alcohol doses are not available to allow a comparison of alcohol dose and cortisol AUC similar to that reported for mice in Figure 5. The results shown in Figures 3, 4, and 5 suggest that either alcohol AUC or peak blood alcohol concentration could be used to predict a biological effect of alcohol such as corticosterone AUC.
Figure 5.
Both alcohol AUC and peak blood alcohol values correlate well with an acute outcome of alcohol exposure in mice, a stress response resulting in increased serum corticosterone. The numbers near each symbol represent the dose of alcohol (g/kg of body weight) by gavage (Carson and Pruett, 1996). The r2 value for AUC is 0.980, and the r2 value for peak alcohol concentration is 0.930, and both of the slopes of both lines are significantly different from 0. The solid lines represent the regression line calculated for the values shown and the dotted lines are the upper and lower 95% confidence intervals.
Discussion
Rodent models for alcohol exposure have been used for decades, but most of the research in this area has been directed toward understanding the effects of chronic drinking. The remarkable prevalence of binge drinking in the U.S. (Kanny et al., 2018) has increased interest in the effects of binge drinking. In addition, recent studies have demonstrated that superimposing binge exposure and chronic exposure in rodents produces effects in the liver that are similar to the effects observed in some humans. For example, the “NIAAA model” involves chronic exposure to alcohol in a liquid diet and one or more binge exposures by gavage (Bertola et al., 2013). The binge doses in these models are typically in the 5–6 g/kg range. In a recent review article, the effects of acute alcohol exposure on the liver were compared in various animal models (Ghosh Dastidar et al., 2018).
Dosage scaling between two species using a single conversion factor for all chemicals or drugs has been discussed in two review articles published in 2008 (Reagan-Shaw et al., 2008) and 2015 (Blanchard and Smoliga, 2015) in the FASEB Journal, and different conclusions were reached in these articles. The former review emphasizes the effectiveness of the method of Freireich in which the dose in mice is assumed to be 12.3 times the dose required in humans to yield similar biological effects. The authors pointed out that the Food and Drug Administration (FDA) recommends using this conversion factor in determining the initial safe dose for human studies of new drugs. The authors imply that determining the appropriate dose in humans by dividing the safe dose in mice by 12.3 is suitable for all drugs and chemicals at pharmacologically active doses as well as minimal safe doses. However, Blanchard and Smoliga (Blanchard and Smoliga, 2015) pointed out that this particular conversion factor is only recommended to determine the lowest safe starting dose in human clinical trials. They point out that the FDA guidance does not recommend assuming that a 12.3 conversion factor will provide a general estimate of biologically equivalent doses (Blanchard and Smoliga, 2015). In their review, Blanchard and Smoliga provide examples of drugs for which simple allometric scaling using the 12.3 conversion factor does not yield equivalent biological effects.
In alcohol research using rodent models, many investigators have used alcohol doses (e.g., 1–2 g/kg) that would not be considered excessive in humans. Some investigators have used doses in mice that are comparable to doses that humans consume in binge drinking episodes (approximately 1–4 g/kg) (Jimenez et al., 2019). In some cases, these doses have significant effects on particular functions (Shults et al., 2015), and these effects correspond with effects reported in humans (Davis et al., 2013). However, 1–2 g/kg administered by gavage does not produce comparable effects in the liver in mice compared to effects observed in humans (Ghosh Dastidar et al., 2018). In fact, to recapitulate in rodents the effects seen in the liver in some humans with alcohol use disorders (AUD), it is necessary to use constant feeding of alcohol by gastric cannula (Tsukamoto et al., 1990) or by superimposing chronic alcohol exposure and acute exposure at doses higher than typical for humans (e.g., 6.0 g/kg) (Bertola et al., 2013). The “binge” doses by gavage in mice used in the “NIAAA model” and similar models are comparable to those we reported in a single dose binge exposure model in mice (Carson and Pruett, 1996). Results presented here indicate that both alcohol AUC and peak blood alcohol concentrations are induced in humans by doses that are approximately one-half the doses in mice required to produce comparable alcohol exposure. Thus, a dose of 6 g/kg in mice produces similar peak blood concentrations and similar exposure to alcohol over time (AUC) as a dose of 3 g/kg in humans.
In determining an appropriate dose of alcohol in mice to produce similar biological effects as noted in humans, some investigators seem to judge appropriate doses in mice as those that are comparable to doses that are consumed by a significant number of humans. In studies using binge drinking models in mice, 3–6 g/kg is a commonly used dose range (Ghosh Dastidar et al., 2018). To calculate the amount of alcohol that would have to be consumed to provide a dose of 6 g/kg in a human subject, we can use the following rationale. The volume of an alcoholic beverage needed to contain 6 g of alcohol must account for the density of alcohol (0.79 g/ml), so 6 g of alcohol would require 6 g × 1ml/0.79 g = 7.6 ml of 100% alcohol. The most concentrated alcoholic beverages are generally in the range of 50% alcohol (v/v), and 15.2 ml of a 100-proof beverage would contain 6 g of alcohol. To yield a dose of 6 g/kg, the volume containing 6 g of alcohol would need to be multiplied by the body weight (e.g., 70 kg for a typical male) to yield a volume of 1,064 ml of a 100-proof beverage. Obviously, most people do not consume a liter of a 100-proof beverage in a single episode. However, results shown here indicate that equivalent alcohol AUC or peak blood alcohol concentration produced by a 6 g/kg dose in mice will typically be produced with a 3 g/kg dose in humans (Figures 3 and 4). This could be attained by consumption of 532 ml of a 100-proof beverage. Several reports indicating consumption of doses this large or larger have been published (Hammond et al., 1973; O’Neill et al., 1984; Wiener et al., 2013). In two particularly relevant studies, the amount of alcohol consumed by a human subject was reported, and 709 ml of tequila (Wiener et al., 2013) or 750 ml of bourbon (Hammond et al., 1973) yielded blood alcohol concentrations greater than 150 mM. Two of these studies are included in the analysis of data reported in this manuscript (Figure 4). A blood alcohol concentration of 326 mM (~1500 mg/dl) was reported in another study in an individual who survived (O’Neill et al., 1984), but these results were considered to be of questionable utility for the present study due to the aggressive medical intervention that was done to save the life of this patient. In a study of blood alcohol concentrations in subjects driving under the influence, there were ~150 cases in which blood alcohol concentrations were more than 87 mM (400 mg/dl) (Jones and Harding, 2013). A study in which emergency department personnel were trained to recognize highly intoxicated persons indicates that persons with remarkably high blood alcohol concentrations are not rare and often cannot be identified by behavioral characteristics that are generally associated with excessive alcohol consumption. If it is considered that the oral alcohol dose required in mice to produce similar alcohol AUC values and similar peak alcohol concentrations is about twice the dose required in humans (Figure 3), it seems that a single dose in the range of 3–6 g/kg in mice would yield alcohol effects similar to those noted in humans exposed to a single episode in which a dose of 1.5–3.0 g/kg was consumed. The upper end of this range (5 or 6 g/kg) in mice has been criticized as an unrealistic dose that would be very rare in humans. However, doses that would produce similar alcohol AUC values and similar peak blood alcohol concentrations (2.5 or 3 g/kg) are not rare in humans.
The pharmacology/toxicology of alcohol is influenced by its rapid and relatively uniform distribution and by its elimination by 0-order kinetics, which means the elimination rate is not dependent on the concentration. Most other drugs and chemicals are not distributed and eliminated as rapidly as alcohol and the elimination kinetics of most drugs and chemicals is not 0-order. It is interesting that the rate of elimination of alcohol is 2–3 times greater in mice than in humans (Bruckner et al., 2013; Finn et al., 1989), suggesting that the clearance rate may be a major determinant of the difference in mice and humans that we identified in the present study. It would be expected that a higher dose would be needed in mice than in humans to achieve similar outcomes due to the more rapid clearance in mice. The focus in this paper has been comparison of humans (the species of interest) and mice (the most widely used animal model). However, there are two papers of particular interest that provide useful information with regard to modeling binge drinking in other species (Ghosh Dastidar et al., 2018) and also comparing mice with another commonly used model species, rats (Livy et al., 2003). The latter paper was also the source of some of the mouse data used in the present analysis.
A contributing factor to the observation that a higher dose of alcohol is required in mice than in humans to produce comparable peak and AUC values may be incomplete saturation of alcohol dehydrogenase (ADH) at lower alcohol concentrations. Below saturation, elimination kinetics would be more first order (dose-dependent) as opposed to the traditional zero order kinetics generally assumed for alcohol (Bosron et al., 1983). If the Km for human ADH was less than the Km for mouse ADH, first order kinetics would yield more rapid clearance in mice than in humans at low alcohol concentrations. This may be the case, but there are multiple isotypes of ADH in mice and humans and measuring the Km for a single isoenzyme in vivo is not practical, and the Km can be influenced by many factors. Regardless of the mechanism by which alcohol is cleared more rapidly in mice than in humans, this has been a consistent observation and likely plays a role in the observation that higher alcohol doses are needed in mice than in humans to yield similar quantitative levels of exposure.
It is often assumed that target organs and tissues in humans exhibit similar sensitivity to alcohol as those in mice. However, the LD50 of ethanol for mice is several times the LD50 for humans (Anonymous, 2019). It is generally accepted that the target that leads to a lethal outcome in both species is the brain, so it would seem that the brain in mice is less sensitive to lethal effects of alcohol than in humans. However, the effects of alcohol on immune system cells seem to be similar at similar concentrations for mice and humans (Szabo et al., 2007). The results reported here indicate that the cumulative exposure to alcohol over time (AUC) and the peak alcohol concentration in the blood are about twice as great in mice as compared to humans receiving the same dose of alcohol. However, this should not be used to conclude that all biological effects of alcohol will exhibit this two-fold relationship, because it remains possible that target cells, organs, and tissues may vary between mice and humans in their sensitivity to adverse effects of alcohol. It is worth noting, that if other systems are similar to the central nervous system in this regard (e.g., mice are less sensitive to adverse effects than humans), then doses even more than 2-fold greater would be needed in mice to produce similar biological effects as noted in humans. The present study does not address this issue, and alcohol sensitivity would need to be determined for each cell type, organ, or tissue of interest to make a comprehensive estimate of the biological effects of alcohol in mice and humans.
Based on studies with a wide range of drugs and chemicals, it was expected that the difference between the doses of alcohol needed to produce similar levels of exposure in mice would be more than 5-fold higher than doses in humans (Travis and White, 1988). However, the results of the present analysis are consistent with results reviewed by Blanchard and Smoglia (Blanchard and Smoliga, 2015), which indicated that simple allometric conversion factors do not effectively predict the doses needed to achieve comparable biological effects in mice and humans for some drugs and chemicals. The empirical findings reported here are relevant with regard to previous and future use of mice as a model of human binge drinking, because they indicate that the use of doses of 3–6 g/kg of alcohol in mice yield similar peak concentrations and exposure over time (AUC) as would be caused in humans by doses of 1.5–3.0 g/kg. Such doses in mice are widely used in studies of binge drinking and such doses in humans are not particularly rare among binge drinkers. The results presented here indicate that most studies in mice have used relevant alcohol doses to represent exposure that occurs in human binge drinkers, and to our knowledge, this is the first study in which this has been empirically demonstrated.
Acknowledgements:
Data from the Pruett laboratory used in this manuscript were obtained through support from the National Institute on Alcohol Abuse and Alcoholism (R01AA009505). Drs. Pruett, Nanduri, and Tan are currently supported by a Center of Biomedical Research Excellence grant from the National Institute for General Medical Sciences (P20GM103646-07).
Mouse models for binge drinking have been used for decades, but disagreement remains as to whether particular oral doses in mice (as g alcohol per kilogram of body weight) are appropriate as a model for human exposure. We used published reports and our own raw data to calculate area under the alcohol concentration vs. time curve (AUC) and to determine the peak blood alcohol concentration in mice and humans. We found that within the most widely used dose range in mice, a dose about twice as large as given to humans is necessary to achieve comparable peak blood alcohol concentrations and comparable alcohol AUC values. These findings have implications in interpretation of past studies and design of new studies in which mice will be used as an animal model for the effects of binge drinking in humans.
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Anonymous, (2019) Registration Dossier, Ethanol. European Chemicals Agency. URL, https://echa.europa.eu/registration-dossier/-/registered-dossier/16105/7/3/1. [Google Scholar]
- Anonymous (2019) Registration report: Ethanol. European Chemicals Agency. [Google Scholar]
- Barkley-Levenson AM and Crabbe JC (2014) High drinking in the dark mice: a genetic model of drinking to intoxication. Alcohol (Fayetteville, NY 48: 217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer LA, Schumock G, Horn J, Opheim K (1992) Verapamil inhibits ethanol elimination and prolongs the perception of intoxication. Clin Pharmacol Ther 52: 6–10. [DOI] [PubMed] [Google Scholar]
- Bertola A, Mathews S, Ki SH, Wang H, Gao B (2013) Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8: 627–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard OL and Smoliga JM (2015) Translating dosages from animal models to human clinical trials--revisiting body surface area scaling. FASEB J 29: 1629–34. [DOI] [PubMed] [Google Scholar]
- Bosron WF, Magnes LJ, Li TK (1983) Human liver alcohol dehydrogenase: ADH Indianapolis results from genetic polymorphism at the ADH2 gene locus. Biochem Genet 21: 735–44. [DOI] [PubMed] [Google Scholar]
- Bruckner VJ, Anand S, Warren DA (2013) Toxic effects of solvents and vapors. In Klaassen CDs (ed), Casarett and Doull’s Toxicology: The Basic Science of Poisons, pp. 1069–72. New York: Pergamon Press. [Google Scholar]
- Carson EJ and Pruett SB (1996) Development and characterization of a binge drinking model in mice for evaluation of the immunological effects of ethanol. Alcoholism, clinical and experimental research 20: 132–8. [DOI] [PubMed] [Google Scholar]
- Collier SD, Wu WJ, Pruett SB (1998) Endogenous glucocorticoids induced by a chemical stressor (ethanol) cause apoptosis in the spleen in B6C3F1 female mice. Toxicol Appl Pharmacol 148: 176–82. [DOI] [PubMed] [Google Scholar]
- Collier SD, Wu WJ, Pruett SB (2000) Ethanol suppresses NK cell activation by polyinosinic-polycytidylic acid (poly I:C) in female B6C3F1 mice: role of endogenous corticosterone. Alcoholism, clinical and experimental research 24: 291–9 [PubMed] [Google Scholar]
- Davis CS, Esposito TJ, Palladino-Davis AG et al. (2013) Implications of alcohol intoxication at the time of burn and smoke inhalation injury: an epidemiologic and clinical analysis. J Burn Care Res 34: 120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Bejanian M, Jones BL, Syapin PJ, Alkana RL (1989) Temperature affects ethanol lethality in C57BL/6, 129, LS, and SS mice. Pharmacol Biochem Behav 34: 375–80. [DOI] [PubMed] [Google Scholar]
- Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE (1966) Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep 50: 219–44. [PubMed] [Google Scholar]
- Ghosh Dastidar S, Warner JB, Warner DR, McClain CJ, Kirpich IA (2018) Rodent Models of Alcoholic Liver Disease: Role of Binge Ethanol Administration. Biomolecules 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover M, Cheng B, Deng X, Pruett S (2011) The role of glucococorticoids in the immediate vs. delayed effects of acute ethanol exposure on cytokine production in a binge drinking model. Int Immunopharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover M, Cheng B, Fan R, Pruett S (2009) The role of stress mediators in modulation of cytokine production by ethanol. Toxicol Appl Pharmacol 239: 98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover M and Pruett SB (2006) Role of corticosterone in immunosuppressive effects of acute ethanol exposure on Toll-like receptor mediated cytokine production. J Neuroimmune Pharmacol 1: 435–42. [DOI] [PubMed] [Google Scholar]
- Grandjean EM and Aubry JM (2009) Lithium: updated human knowledge using an evidence-based approach. Part II: Clinical pharmacology and therapeutic monitoring. CNS Drugs 23: 331–49. [DOI] [PubMed] [Google Scholar]
- Hammond KB, Rumack BH, Rodgerson DO (1973) Blood ethanol. A report of unusually high levels in a living patient. JAMA 226: 63–4. [DOI] [PubMed] [Google Scholar]
- Han Y-C and Pruett SB (1995) Mechanisms of ethanol-induced suppression of a primary antibody response in a mouse model for binge drinking. J Pharmacol Exp Ther 275: 950–57. [PubMed] [Google Scholar]
- Han YC, Lin T-L, Pruett SB (1993a) Thymic atrophy caused by ethanol in a mouse model for binge drinking: involvement of endogenous glucocorticoids. Toxicol Appl Pharmacol 123: 16–25. [DOI] [PubMed] [Google Scholar]
- Han YC, Lin TL, Pruett SB (1993b) Thymic atrophy caused by ethanol in a mouse model for binge drinking: involvement of endogenous glucocorticoids. Toxicol Appl Pharmacol 123: 16–25. [DOI] [PubMed] [Google Scholar]
- Jimenez VM Jr., Settles EW, Currie BJ, Keim PS, Monroy FP (2019) Persistence of Burkholderia thailandensis E264 in lung tissue after a single binge alcohol episode. PLoS One 14: e0218147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AW and Harding P (2013) Driving under the influence with blood alcohol concentrations over 0.4 g%. Forensic Sci Int 231: 349–53. [DOI] [PubMed] [Google Scholar]
- Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD (2018) Annual Total Binge Drinks Consumed by U.S. Adults, 2015. Am J Prev Med 54: 486–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutscher S, Heise DJ, Banger M et al. (2002) Concomitant endocrine and immune alterations during alcohol intoxication and acute withdrawal in alcohol-dependent subjects. Neuropsychobiology 45: 144–9. [DOI] [PubMed] [Google Scholar]
- Livy DJ, Parnell SE, West JR (2003) Blood ethanol concentration profiles: a comparison between rats and mice. Alcohol (Fayetteville, NY 29: 165–71. [DOI] [PubMed] [Google Scholar]
- Merry J and Marks V (1969) Plasma-hydrocortisone response to ethanol in chronic alcoholics. Lancet 1: 921–3. [DOI] [PubMed] [Google Scholar]
- Mitchell MC Jr., Teigen EL, Ramchandani VA (2014) Absorption and peak blood alcohol concentration after drinking beer, wine, or spirits. Alcoholism, clinical and experimental research 38: 1200–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoi Y, Kogame M, Fukunaga T, Ueno Y, Adachi J, Fujiwara S (1985) Polymorphism of aldehyde dehydrogenase and ethanol elimination. Alcohol (Fayetteville, NY 2: 393–6. [DOI] [PubMed] [Google Scholar]
- O’Neill S, Tipton KF, Prichard JS, Quinlan A (1984) Survival after high blood alcohol levels. Association with first-order elimination kinetics. Arch Intern Med 144: 641–2. [PubMed] [Google Scholar]
- Phillips TJ, Gilliam DM, Dudek BC (1984) An evaluation of the role of ethanol clearance rate in the differential response of long-sleep and short-sleep mice to ethanol. Alcohol (Fayetteville, NY 1: 373–8. [DOI] [PubMed] [Google Scholar]
- Pruett S, Hebert P, Lapointe JM, Reagan W, Lawton M, Kawabata TT (2007) Characterization of the action of drug-induced stress responses on the immune system: evaluation of biomarkers for drug-induced stress in rats. Journal of immunotoxicology 4: 25–38. [DOI] [PubMed] [Google Scholar]
- Pruett S, Lapointe JM, Reagan W, Lawton M, Kawabata TT (2008) Urinary corticosterone as an indicator of stress-mediated immunological changes in rats. Journal of immunotoxicology 5: 17–22. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Collier S, Wu WJ, Fan R (1999) Quantitative relationships between the suppression of selected immunological parameters and the area under the corticosterone concentration vs. time curve in B6C3F1 mice subjected to exogenous corticosterone or to restraint stress. Toxicol Sci 49: 272–80. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Collier SD, Wu W-J (1998) Ethanol-induced activation of the hypothalamic-pituitary-adrenal axis in a mouse model for binge drinking: role of Ro15–4513-sensitive gamma aminobutyric acid receptors, tolerance, and relevance to humans. Life Sci 63: 1137–46. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Myers LP, Hebert P (2003) Modeling and predicting immunological effects of chemical stressors: characterization of a quantitative biomarker for immunological changes caused by atrazine and ethanol. Toxicol Sci 75: 343–54. [DOI] [PubMed] [Google Scholar]
- Pruett SB, Fan R, Zheng Q, Schwab C (2009) Patterns of immunotoxicity associated with chronic as compared with acute exposure to chemical or physical stressors and their relevance with regard to the role of stress and with regard to immunotoxicity testing. Toxicol Sci 109: 265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reagan-Shaw S, Nihal M, Ahmad N (2008) Dose translation from animal to human studies revisited. FASEB J 22: 659–61. [DOI] [PubMed] [Google Scholar]
- Schwab CL, Fan R, Zheng Q, Myers LP, Hebert P, Pruett SB (2005) Modeling and predicting stress-induced immunosuppression in mice using blood parameters. Toxicol Sci 83: 101–13. [DOI] [PubMed] [Google Scholar]
- Shults JA, Curtis BJ, Chen MM, O’Halloran EB, Ramirez L, Kovacs EJ (2015) Impaired respiratory function and heightened pulmonary inflammation in episodic binge ethanol intoxication and burn injury. Alcohol (Fayetteville, NY 49: 713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo G, Dolganiuc A, Dai Q, Pruett SB (2007) TLR4, ethanol, and lipid rafts: a new mechanism of ethanol action with implications for other receptor-mediated effects. J Immunol 178: 1243–9. [DOI] [PubMed] [Google Scholar]
- Travis CC and White RK (1988) Interspecific scaling of toxicity data. Risk Anal 8: 119–25. [DOI] [PubMed] [Google Scholar]
- Tsukamoto H, Gaal K, French SW (1990) Insights into the pathogenesis of alcoholic liver necrosis and fibrosis: status report. Hepatology (Baltimore, Md 12: 599–608. [DOI] [PubMed] [Google Scholar]
- Weiss PA, Collier SD, Pruett SB (1996) Role of glucocorticoids in ethanol-induced decreases in expression of MHC class II molecules on B cells and selective decreases in spleen cell number. Toxicol Appl Pharmacol 139: 153–62. [DOI] [PubMed] [Google Scholar]
- Wiener SW, Olmedo R, Howland M, Nelson L, Hoffman R (2013) Ethanol elimination kinetics following massive ingestion in an ethanol naive child. Hum Exp Toxicol 32: 775–7. [DOI] [PubMed] [Google Scholar]
- Wu W-J and Pruett SB (1997) Involvement of catecholamines and glucocorticoids in ethanol-induced suppression of splenic natural killer cell activity in a mouse model for binge drinking. Alcohol Clin Exp Res 21: 1030–36. [PubMed] [Google Scholar]
- Wu W-J and Pruett SB (1999) Ethanol decreases host resistance to pulmonary metastases in a mouse model: role of natural killer cells and the ethanol-induced stress response. Int J Cancer 82: 886–92. [DOI] [PubMed] [Google Scholar]
- Wu WJ and Pruett SB (1996) Suppression of splenic natural killer cell activity in a mouse model for binge drinking. II. Role of the neuroendocrine system. J Pharmacol Exp Ther 278: 1331–9. [PubMed] [Google Scholar]
- Zar JH (1984) Comparing Simple Linear Regression Equations, Biostatistical Analysis, pp. 292–305. Englewood Cliffs, NJ: Prentice-Hall, Inc. [Google Scholar]