Abstract
With antibiotic-resistant bacteria becoming increasingly prevalent, biomaterials capable of targeted, in situ drug delivery are urgently needed. The synthetic polymer Poloxamer 407 (P407) is of particular interest due to its thermoreversible gelation. Clinical use of P407 typically involves sterilization via autoclaving, but the effects of these extreme environmental conditions on hydrogel water content, rheological properties and efficacy as a drug delivery vehicle remain unknown. The aim of this study was to investigate the effects of autoclaving on the properties of P407 hydrogel. Autoclaving reduced hydrogel water content due to evaporation, thus increasing the polymer weight fraction of the hydrogels. In contrast, except for a reduction in gelation temperature following autoclaving, autoclaved hydrogels had similar rheological properties as nonautoclaved hydrogels. In vitro, autoclaving did not hinder the hydrogel’s efficacy as a carrier for vancomycin antibiotic, and P407 (with and without vancomycin) had a bactericidal effect on planktonic Staphylococcus aureus. An in vivo pilot study using P407 to deliver bacteriophage highlighted the need for additional understanding of the functionality of the hydrogel for surgical applications. In conclusion, P407 hydrogel water content and gelation temperature were reduced by autoclave sterilization, while other rheological properties and the efficacy of the biomaterial as a delivery vehicle for vancomycin in vitro were unaffected.
Keywords: autoclave, drug delivery, hydrogel, Poloxamer 407, sterilization
1 |. INTRODUCTION
Poloxamer 407 (P407), also referred to as Pluronic F-127, is a tri-block copolymer, comprised of polyethylene oxide (PEO, ~70%) and polypropylene oxide (PPO, ~30%).1–3 When dissolved in aqueous solution, P407 forms a hydrogel useful for a range of biomedical applications. Originally P407 was developed as an emulsifying agent in cosmetic and industrial products due to its amphipathic nature. Other research has focused on its use in ophthalmic injuries,4 skin wounds,4 and the surgical stabilization of vascular structures.5 The most compelling property of P407 is its reversible thermoresponsive nature, allowing it to undergo gelation near body temperature (~37°C) and remain at the site of implantation as a sustainable carrier.6,7 Under colder conditions (<15–25°C depending on polymer weight fraction), P407 exists in a liquid form, during which it can be loaded with therapeutics for later release from its gel state. Previously, P407 has been investigated as a drug delivery vehicle for analgesics (lidocaine,2 ziconotide8) and antibiotics (vancomycin,3 levofloxacin,1 and metronidazole1), or muscarinic antagonists for glaucoma treatment (pilocarpine nitrate9). The polymer matrix of P407, and ultimately its drug release kinetics, can be altered by combining it with other polymers for a hybrid formulation or through the addition of salt-form molecules such as chitosan salts.8,10 These modifications cause variation in the matrix structure, erosion, and swelling of the hydrogel, changing both the rate of polymer degradation and the rate of drug release. The molecular weight and end group identity of P407 can be tailored to modulate the tissue adhesivity and gelation properties, which influence performance as an injectable drug delivery vehicle.11
Clinicians in both the medical and veterinary fields have found many applications for P407, and its use in drug delivery is becoming more widespread. For wound and surgical applications particularly, sterile preparation of P407 is necessary. Sterile P407 has reportedly been prepared through the use of sterile solvent, autoclaving, or a combination of both.2,3,12 A range of autoclaving times (15–30 min) have been reported for P407 at an autoclave temperature of 121°C.2,13 We hypothesized that autoclaving conditions would affect the thermoresponsive properties of P407, and thus sought to determine the effects of: (a) autoclaving time and temperature on hydrogel water content, and (b) autoclaving (with fixed parameters) on the rheological and in vitro antimicrobial properties of vancomycin-loaded hydrogel against Staphylococcus aureus (S. aureus) (Figure 1).
FIGURE 1.
The physical effects of autoclaving on Poloxamer 407 hydrogels was analyzed by measuring water content and rheological properties of the gels after autoclaving. Additionally, the ability of autoclaved P407 to release antibiotics was observed through a direct contact, planktonic bacterial assay with optical density readings
Clinically, we have demonstrated the efficacy of autoclaved P407 to deliver amikacin, clindamycin, and/or vancomycin in cases of multidrug resistant soft tissue infections in dogs.12 Others have also used P407 for local delivery of drugs.1,11 Collectively, these results motivated our initial, pilot investigation of (nonautoclaved) P407 as a delivery vehicle for bacteriophage for infection mitigation in a rat model of femoral osteomyelitis and soft tissue infection by S. aureus.14 A better understanding of the effects of extreme heat conditions (i.e., autoclaving) on P407’s utility, including evaluation of the autoclave parameters currently used clinically, would inform researchers and clinicians of potential loss in water content and hydrogel function when preparing sterile P407 for effective drug delivery.
2 |. MATERIALS AND METHODS
2.1 |. Hydrogel preparation
P407 solutions of polymer weight fractions 30%, 35%, and 40% weight per volume (w/v) were prepared by dissolving P407 powder in nonsterile distilled water at 25°C. To facilitate the generation of a homogenous, evenly mixed solution, the P407 and distilled water solution(s) were manually stirred then placed in a −20°C freezer for 5–10 min increments to prevent gelation at room temperature. The solutions were alternately stirred and cooled approximately 3–5 times until all P407 was dissolved. Following gel synthesis, the P407 hydrogels were stored at 4°C. P407 used for the Kirby-Bauer and planktonic assays was dissolved in distilled water at a higher polymer weight fraction before dilution with vancomycin solution toa polymer weight fraction of 30% w/v. Hydrogels used for the in vivo study were not autoclaved, but were prepared by dissolving aseptically maintained P407 powder into sterile water, then diluting with bacteriophage therapeutic solution to a polymer weight fraction of 30% w/v. All autoclaved hydrogels were sterilized in a Tuttnauer Autoclave Steam Sterilizer (Model #2540 M) at 3% humidity per manufacturer’s specifications, with the timer set to either 20, 30, or 45 min. These times included the short (5–10 min) warm up period of the autoclave prior to reaching final temperature.
2.2 |. P407 for delivery of bacteriophage in a rat osteomyelitis femoral defect model
All procedures were performed in accordance with the Institutional animal care and use committee (IACUC) of Mississippi State University (see Figure 2 for an overview of in vivo procedures). Charles River female SASCO Sprague–Dawley rats aged 13 weeks (skeletally mature) were used for all animal studies. For all surgical procedures, rats were anesthetized with isoflurane in oxygen at an initial concentration of 2%–3%, then kept at 1%–2% for maintenance. Rats were administered extended release buprenorphine (1.0–1.2 mg/kg, ZooPharm) preoperatively for pain relief. Hind limbs were cleaned with alcohol and chlorhexidine and sterilely draped. Surgical approach and defect creation were performed as previously described.14 The skin was incised on the lateral aspect of the femur over the femur from the greater trochanter to the stifle. The incision was continued through the superficial and deep fascia between the biceps femoris and vastus lateralis muscles to expose the craniolateral aspect of the diaphysis of the femur. A 1.2 mm (diam) bicortical defect in the mid-diaphysis of the femur was created with a pneumatic drill (Conmed Hall #PRO6150). Ten microliters of ~106 colony forming units (CFU) of ATCC 6538-GFP in phosphate buffered saline (PBS) was injected into the defect, and a sterile orthopedic screw (Antrin Miniature Specialties, #00–90) was placed into the defect. At 14 days, the surgical site was reopened and the orthopedic screw was removed for bacterial counts (n = 10) or scanning electron microscopy (n = 1). Of these eleven, animals were either sacrificed for baseline bacterial counts (n = 6), or treated with CRISPR-Cas9 modified bacteriophage (MOI ~5)14,15 in nonautoclaved, aseptically-prepared16 30% P407 hydrogels (n = 5), and new sterile screws were placed into the defect. At day 20 (6 days posttreatment), screws (n = 5) were collected from the phage treated animals and animals were sacrificed. All animals were sacrificed by carbon dioxide asphyxiation. ex vivo bacterial counts of bone and surrounding soft tissue were performed to quantify bacterial load in untreated animals at day 14, and treated animals at day 20.
FIGURE 2.
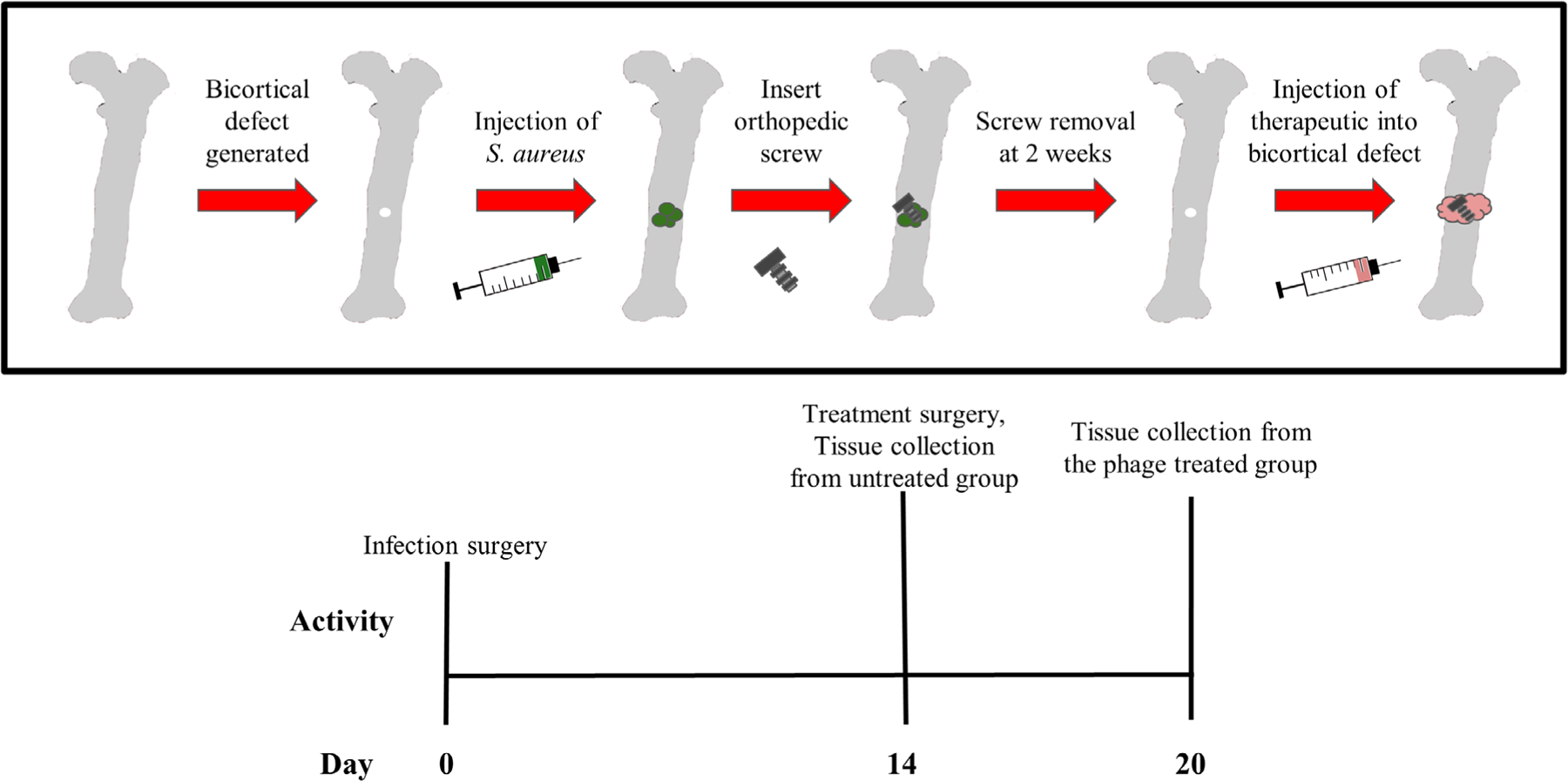
Overview of in vivo osteomyelitis model. Day 1: Staphylococcus aureus (S. aureus) was injected into the bone defect and screw was placed. Day 14: contaminated screw was removed, bacteriophage treatment and a new (sterile) screw were applied to the treatment group; tissues from the untreated group were collected for bacterial counting. Day 20: treatment group was imaged, and tissues were collected for bacterial counting
2.3 |. Water content analysis
P407 samples (polymer weight fractions 30%, 35%, and 40% (w/v)) were prepared for autoclaving by pipetting 100 μl of each hydrogel into a 20 ml scintillation vial. The vials were weighed prior to autoclaving and loosely covered with aluminum foil. Samples of each P407 polymer weight fraction were autoclaved in triplicate (n = 3) at temperatures 100°C, 121°C, or 134°C for 20, 30, or 45 min. Samples were removed from the autoclave after a short (5 min) exhaust time, in order to prevent excess solution evaporation during the drying cycle. Samples were immediately weighed postautoclave. The percent weight loss (P) was then calculated based on the preautoclave weight (W1) and postautoclaving weight (W2) (see formula).
2.4 |. Rheological analyses
Rheological tests including flow temperature ramp, oscillation frequency sweep, and flow sweep were performed on autoclaved (121°C for 20 min) and nonautoclaved P407 samples at polymer weight fractions of 30%, 35%, and 40% (n = 3) using a stress-controlled rheometer (DHR 2, TA Instruments). Temperature was precisely controlled with a Peltier system. The loading temperature for the samples was 0°C. For each sample loaded, all three tests were performed in sequential order of flow temperature ramp, oscillation frequency sweep, and flow sweep. Temperature ramp tests (temperature ranging from 0–40°C, shear rate = 0.1 s−1, heating rate = 5°C/min) were performed to study the sol–gel transition.17 Gelation temperatures of each experimental group were collected at the point where viscosity began to plateau after a sharp increase. After the flow temperature ramp test was completed, samples were equilibrated at 37°C for 10 min before performing the oscillation frequency test at a strain of 0.1% with the angular frequency ranging from 0.1–100 rad/s.17 The storage modulus obtained at the maximum applied frequency (100 rad/s) was compared for the different polymer weight fractions. Then flow sweep was performed at 37°C with the applied shear rate ranging from 1–100 s−1.17
2.5 |. Kirby-Bauer Assay
To evaluate the antibacterial efficacy of autoclaved and nonautoclaved P407 hydrogels, a Kirby-Bauer assay was performed as previously described,18 with a few modifications.14 First, a colony of S. aureus ATCC 6538 modified to express green fluorescent protein (ATCC 6538-GFP) was cultured into 4 ml Brain Heart Infusion (BHI, BBL™ Brain Heart Infusion, #211059) for 24 hr at 37°C, 150 RPM.14 Bacteria were then evenly spread across BHI agar plates. Then, 10 μl samples were directly pipetted onto the bacterial lawns.14 Five experimental groups (n = 5) were evaluated: nonautoclaved 30% P407, autoclaved 30% P407, autoclaved 30% P407 loaded with vancomycin (10 mg/ml), nonautoclaved 30% P407 loaded with vancomycin (10 mg/ml), and PBS loaded with vancomycin (10 mg/ml). The autoclaved samples were sterilized at 121°C for 20 min. Plates were incubated for 24 hr statically at 37°C (agar side up). Zones of inhibition produced by the therapeutics were measured and recorded.
2.6 |. Planktonic antibacterial assay
A vancomycin stock of 175 mg/ml was prepared in 1 ml of PBS. The vancomycin stock, syringes, and 35% nonautoclaved and autoclaved P407 were cooled to 4°C then kept on ice. Nonautoclaved or autoclaved P407 hydrogel and vancomycin were mixed in a dual syringe method to create a 2.5 mg vancomycin per 100 μl of 30% P407 solution. The empty hydrogel was prepared using the same procedure but with PBS to dilute the gel to 30%. A colony of S. aureus (ATCC 6538-GFP) was grown for 16 hr overnight in 4 ml of BHI at 37°C with 150 RPM shaking. Four culture tubes were filled with 4 ml BHI and 200 μl of the overnight culture was added to each tube. These cultures were each grown to an optical density (OD) 600 nm of 0.2 (μQuant, BioTek Instruments, Inc.).19 In a 48-well nontreated plate, 100 μl of each gel (nonautoclaved 30% P407, nonautoclaved 30% P407 loaded with vancomycin (25 mg/ml), and autoclaved 30% P407 loaded with vancomycin (25 mg/ml), n = 6) was incubated at 37°C until the P407 solutions solidified. The cultures grown to an OD 600 of 0.2 were spun down at 4000 RPM for 4 min. The supernatant was removed and the pellets resuspended in 4 ml of 1% glucose BHI. In each well, 400 μl of the cultures were gently pipetted over the gels. Then, 400 μl of the cultures were placed in blank wells for the untreated control and PBS loaded with vancomycin (100 μl, 25 mg/ml) groups. After 24 hr, the solutions for each well were collected, spun down at 5000 RPM, resuspended in PBS, and plated for bacterial counting.
2.7 |. Statistical methods
The in vivo bacterial counts (soft tissue, bone, and screw) were analyzed using two-tailed t-tests with unequal variance due to unequal sample sizes. Two-way analyses of variance (ANOVAs) were used to analyze the effects of autoclave temperature and duration on water content for each P407 polymer weight fraction. Gelation temperature and the recorded storage modulus at 100 rad/s were analyzed using two-way ANOVAs. For the flow sweep test, a two-way ANOVA and Tukey’s multiple comparisons were run for each shear rate. The Kirby-Bauer and planktonic antibacterial assays were each evaluated using a one-way ANOVA with Šídák’s multiple comparisons. Error bars for the flow sweep analysis and in vivo bacterial counts are represented as standard error of mean due to large variations and small sample sizes. All other error bars are standard deviation.
3 |. RESULTS
3.1 |. P407 for delivery of bacteriophage in a rat osteomyelitis femoral defect model
Initial bacterial counts from ex vivo screws were collected on day 14 from the control group and the bacteriophage treatment group prior to treatment. The average bacterial loads were 4.073 × 104 CFU/ml and 7.019 × 104 CFU/ml for the control and phage treatment (pretreatment) groups, respectively. On day 20 (6 days after treatment with phage), bacterial counts were taken from the ex vivo screws, which averaged 1.06 × 105 CFU/ml (Figure 3a). Bacterial counts on untreated soft tissues and bones collected at day 14 revealed an average load of 2.41 × 106 and 9.08 × 105 CFU/ml, respectively (Figure 4). Bacterial counts on phage-treated soft tissues and bones collected on day 20 revealed an average load of 2.75 × 106 CFU/ml and 2.06 × 06 CFU/ml, respectively. No differences in bacterial counts were observed in soft tissue or bone between day 14 untreated controls and day 20 phage-treated samples.
FIGURE 3.
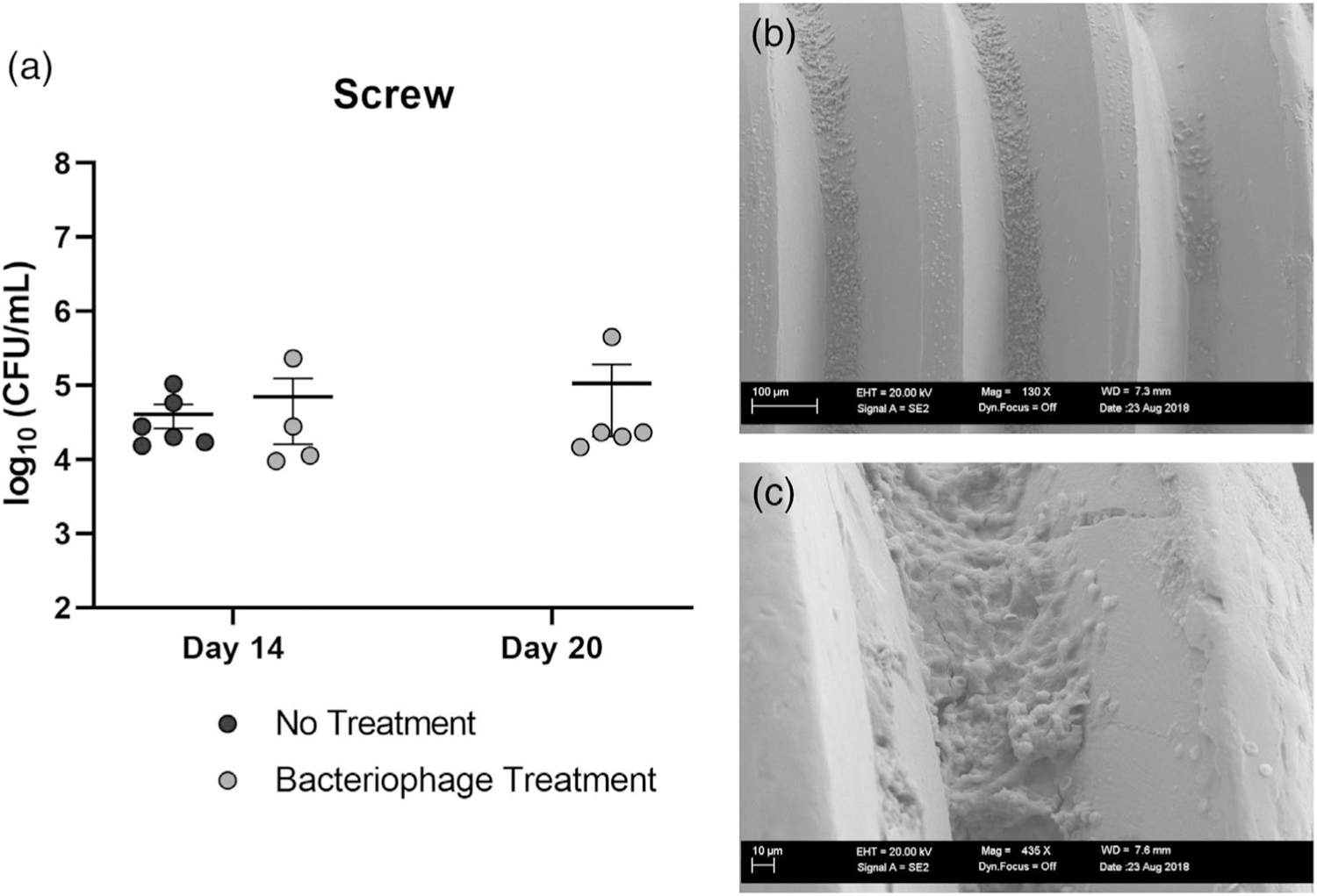
(a) Bacterial counts of screws at day 14 indicated a similar bacterial load between the untreated group sacrificed at day 14, and what would become the bacteriophage treated group. Bacterial load in screws from the phage-treated group at day 14 (pretreatment) was not different from screws collected at day 20 (6 days posttreatment) (n = 4–6). (b) Scanning electron microscopy (SEM) of excised screw at 130× magnification. S. aureus cocci are visible between screw threads. (c) SEM of excised screw at 716×. Bacterial growth exhibiting hallmarks of biofilm
FIGURE 4.
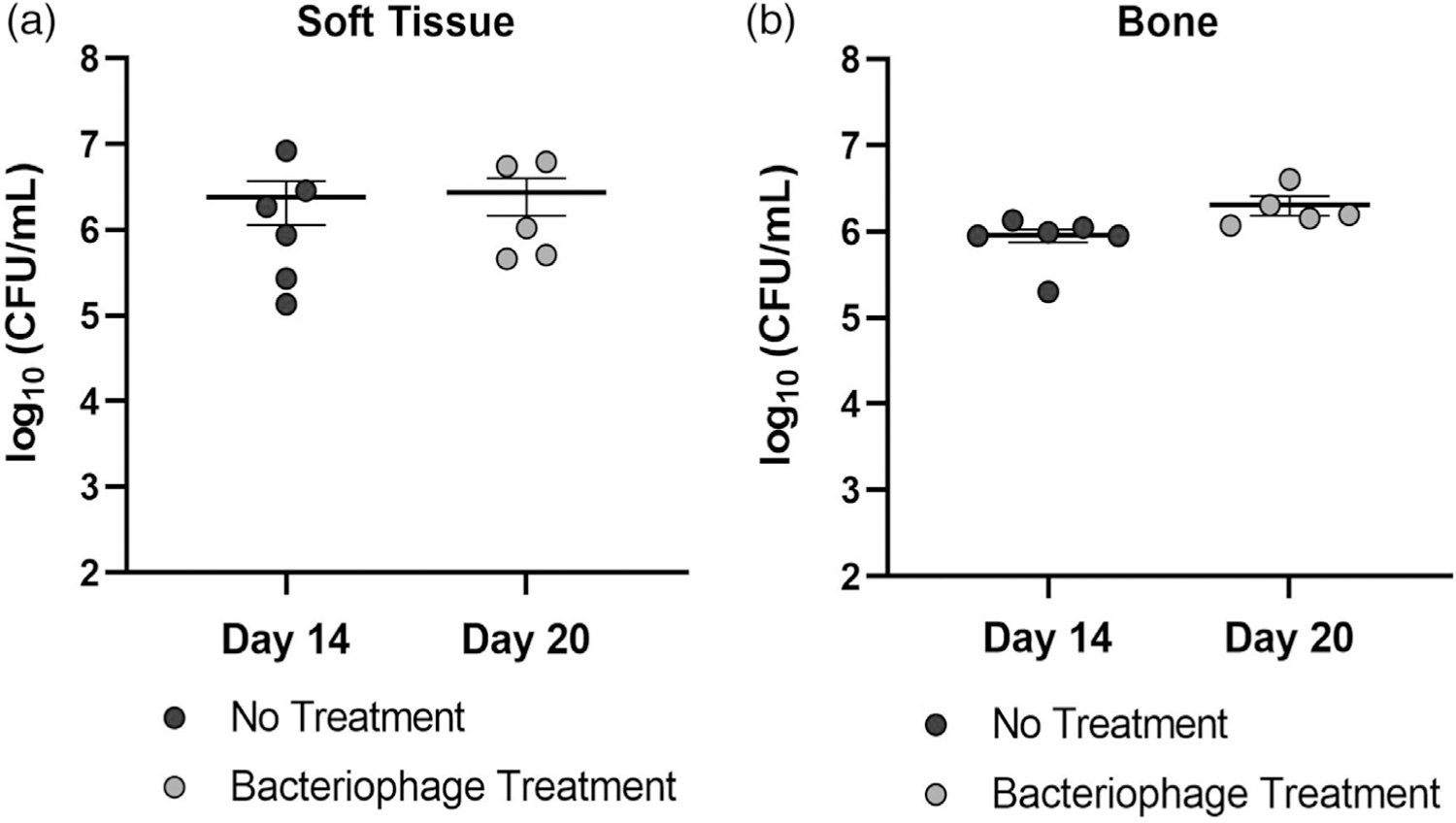
Bacterial counts of untreated and bacteriophage treated (a) soft tissue and (b) bone specimens (n = 5–6). Tissues excised from the untreated group on day 14 are not significantly different from those collected from bacteriophage-treated tissues collected at day 20 (6 days posttreatment)
3.2 |. Effect of autoclave parameters on hydrogel water content
Analysis of the effects of autoclave time and temperature was performed for 30%, 35%, and 40% P407 polymer weight fractions (Figure 5). The results of this analysis suggested that autoclave temperature played a larger role in water evaporation than autoclave time. Hydrogel samples autoclaved at 100°C for any given time (20, 30, or 45 min) showed the least amount of water loss, with less than 25% weight loss after autoclaving for all groups. All polymer weight fractions (30%, 35%, and 40%) experienced significant increases in weight loss when autoclaved at either 121°C or 134°C in comparison to 100°C. Some groups showed significant differences between autoclaving at 121°C versus 134°C, primarily when the autoclave time was 30 min or longer. Some differences as a function of autoclave time were also observed, especially at 134°C.
FIGURE 5.
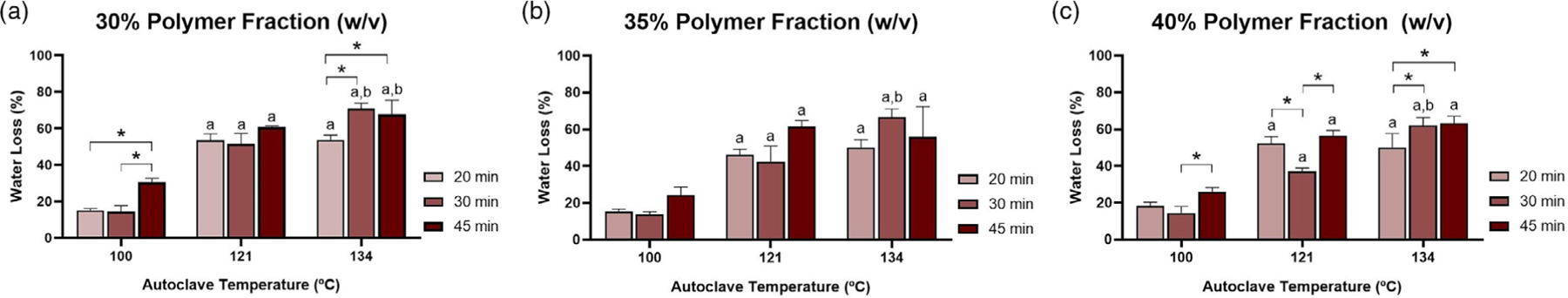
Percent water loss as a function of polymer weight fraction: (a) 30%, (b) 35%, and (c) 40%. “a” denotes significantly higher weight loss than group exposed to an identical autoclave time at 100°C. “b” denotes significantly higher weight loss than group exposed to an identical autoclave time at 121°C. The asterisks denote significant differences as indicated. p < .05 for all differences indicated
3.3 |. Effect of autoclaving on rheological properties
The results of the oscillation frequency sweep showed a higher storage modulus (G’) than loss modulus (G”) over the range of frequencies tested (Figure 6a–c). No effect of autoclaving on loss modulus or storage modulus was observed for any of the P407 polymer weight fractions. From flow sweep tests, power law behavior was observed, although the data are presented on a linear scale for visualization of differences. Regardless of shear rate, few differences were observed in viscosity between autoclaved and nonautoclaved samples for each polymer weight fraction (Figure 7a). Specifically, the only differences from autoclaving were in the 40% gel at 1.00 s−1 and the 35% gel at 1.26 s−1. For the lower shear rates (1.00–3.16 s−1), some differences were found between polymer weight fractions under the same autoclave conditions (e.g., 30% autoclaved and 35% autoclaved). For shear rates 1.00–3.16 s−1, differences were found between 30% and 40% nonautoclaved gel. In general, at the lower shear rates, a higher polymer weight fraction resulted in greater viscosity, but these differences were resolved as shear rate increased beyond 3.16 s−1. Both polymer weight fraction and autoclaving had an effect on gelation temperature, as expected (Figure 7b). Gelation temperature was higher for nonautoclaved gels compared to autoclaved gels at 35% polymer weight fraction, but no differences were found in either the 30% or 40% groups. For both autoclaved and nonautoclaved groups, gelation temperature was lowered as polymer weight fraction increased.
FIGURE 6.
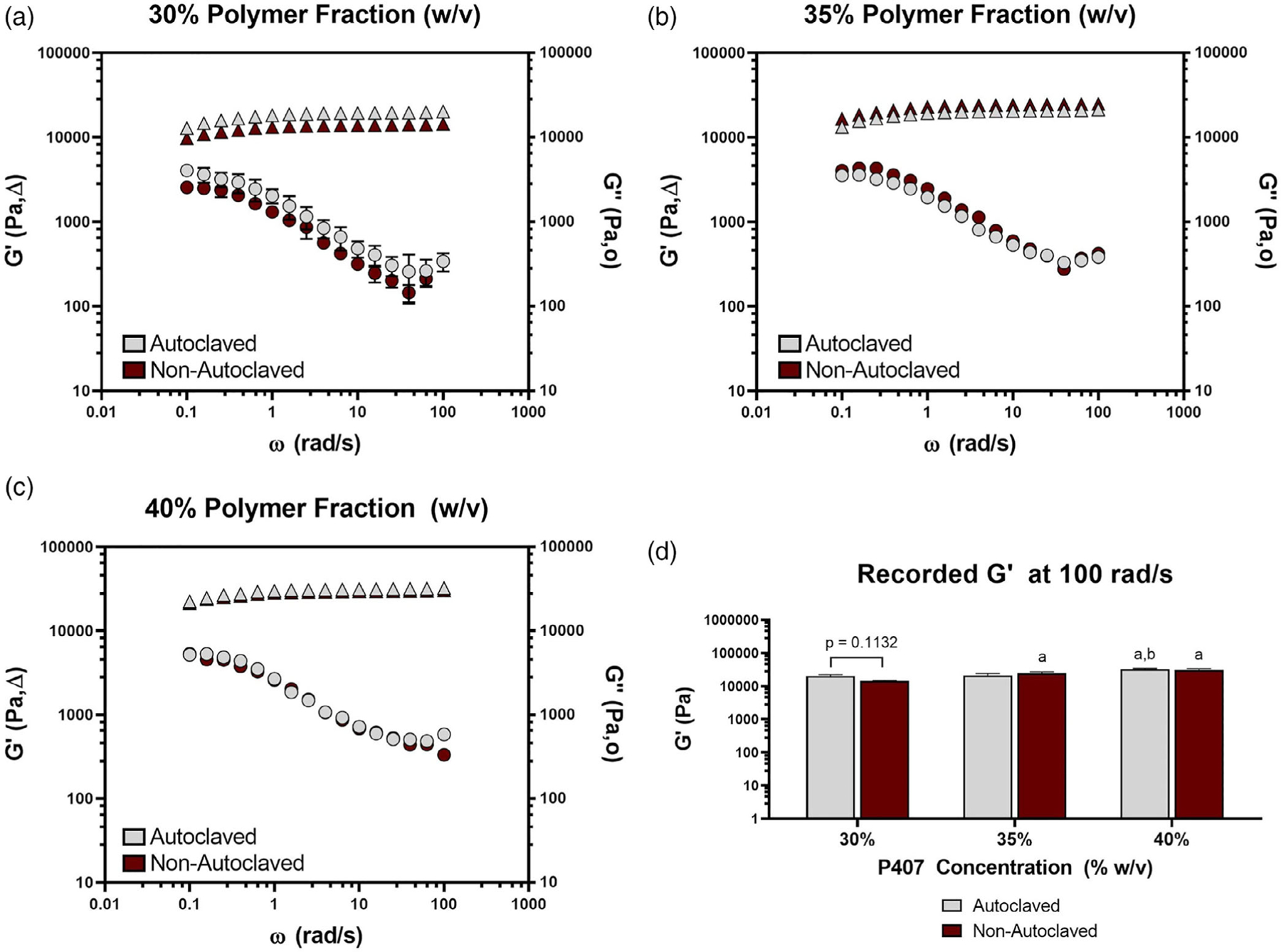
Loss and storage modulus (Pa) measured against an oscillation frequency (ω) range of 0 to 100 rad/s for autoclaved and nonautoclaved polymer weight fractions of (a) 30%, (b) 35%, and (c) 40%. (d) The recorded storage modulus (Pa) at 100 rad/s of autoclaved and nonautoclaved hydrogels with polymer weight fractions of 30%, 35%, and 40%. “a” denotes a significantly higher maximum recorded storage modulus than the 30% counterpart. “b” denotes a significantly higher maximum recorded storage modulus than the 35% counterpart. n = 3 for all groups. p < .05 for all differences indicated
FIGURE 7.
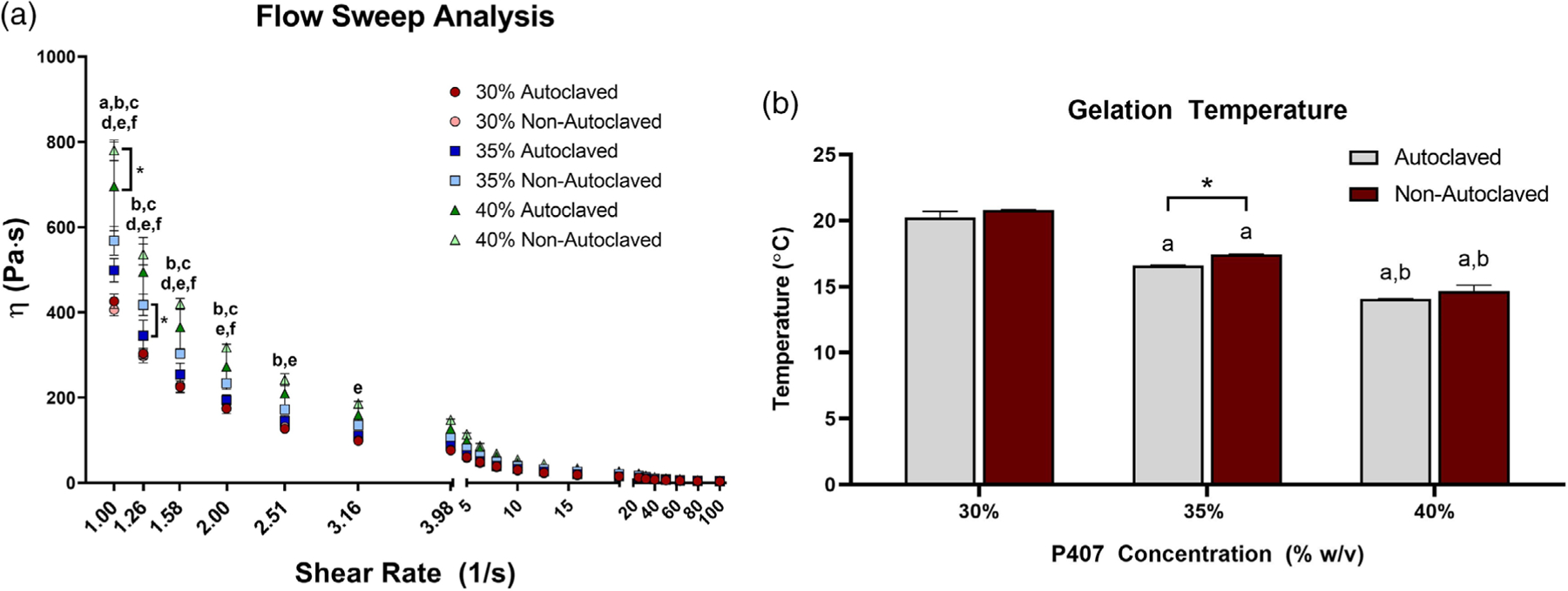
(A) Viscosity, η, (Pa·s) measured against shear rate from 0 to 100 s−1 for autoclaved and nonautoclaved polymer weight fractions of 30%, 35%, and 40%. This data is presented on a linear scale for visualization purposes. Error bars are reported as standard error of mean. Vertical brackets with asterisks indicate a significant difference due to autoclaving. The following key represents significant differences between the indicated groups denoted with the corresponding letters: (a) 30% autoclaved and 35% autoclaved, (b) 30% autoclaved and 40% autoclaved, (c) 35% autoclaved and 40% autoclaved, (d) 30% nonautoclaved and 35% nonautoclaved, (e) 30% nonautoclaved and 40% nonautoclaved, and (f) 35% nonautoclaved and 40% nonautoclaved. (B) Gelation temperatures recorded for autoclaved and nonautoclaved P407 polymer weight fractions of 30%, 35%, and 40%. The asterisk indicates a significant difference (p = .0079) between the 35% autoclaved and nonautoclaved groups. “a” denotes a significantly lower gelation temperature than the 30% counterpart. “b” denotes a significantly lower gelation temperature than the 35% counterpart. n = 3 for all groups. p < .05 for all differences indicated
3.4 |. Kirby-Bauer antibacterial assay
Empty (antibiotic-free) P407, both autoclaved and nonautoclaved, demonstrated no inhibition of bacterial growth (Figure 8a). Zones of inhibition of all vancomycin-loaded treatments (gels and PBS) were greater than those without vancomycin. No differences in zones of inhibition were found between any of the vancomycin-loaded treatments.
FIGURE 8.
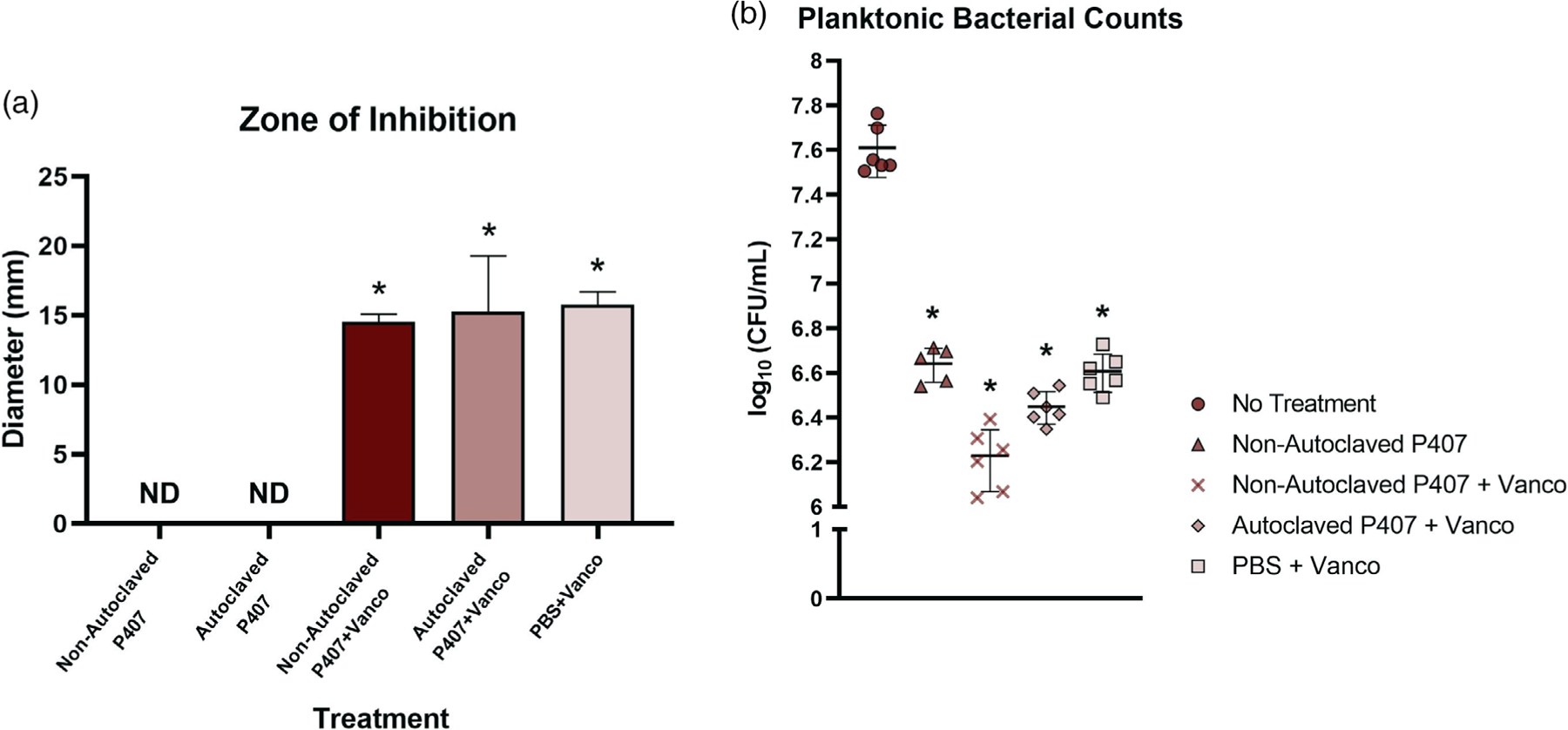
(a) Results from the Kirby-Bauer assay (n = 5) showed that empty gels, autoclaved and nonautoclaved, did not have antimicrobial activity. In addition, the vancomycin in both autoclaved and nonautoclaved P407 was as effective as vancomycin delivered in phosphate buffered saline (PBS). The vehicles (nonautoclaved P407, autoclaved P407, and PBS) loaded with vancomycin produced zones of inhibition that were significantly different than the blank autoclaved and nonautoclaved P407 (*p < .05, ND: not detected). (b) Colony forming units per milliliter 24 hr post-treatment (n = 5–6). Nonautoclaved P407, nonautoclaved P407 + vancomycin (vanco), autoclaved P407 + vancomycin, and PBS + vancomycin were significantly different from no treatment (*p < .05) but not significantly different from each other. CFU, colony forming units
3.5 |. Planktonic antibacterial assay
All groups containing vancomycin showed a reduction in bacterial growth versus the untreated wells (Figure 8b). Interestingly, the empty gel also significantly inhibited bacterial growth compared to no treatment.
4 |. DISCUSSION
In the in vivo pilot study, a nonautoclaved, aseptically-prepared 30% P407 formulation was used for delivery of bacteriophage therapeutic. The P407 solution was originally prepared at a higher polymer weight fraction before dilution to 30% (w/v) with the bacteriophage solution. Surprisingly, upon injection into the bone defect, the gel was rapidly dissolved in the blood that pooled in and around the defect. This phenomenon, combined with the need for a sterile hydrogel, prompted further investigation into the properties of P407 and whether autoclaving could be an acceptable sterilization method. Because of P407’s thermoresponsive characteristic, it was expected to become firm once in contact with the body tissues (37°C) following injection. Nonetheless, the incorporation of the phage solution may have reduced the ability of P407 to undergo gelation in vivo. The phage solution was PBS-based, and the high salt content may have destabilized the Poloxamer micellular matrix, as a result of salts binding to water molecules and/or directly to the P407 molecule. This “salting in” effect has been suggested previously for polymer-salt solution interactions.6,20
Clinical practices that involve autoclaving of P407 hydrogels generally call for autoclave parameters of 121°C for 15–30 min.2,3,12 It is assumed that any material removed from P407 hydrogel during an autoclave cycle is water only, with polymer weight fraction increasing as water evaporates out of the gel. Here, hydrogels autoclaved at 121°C for 20 or 30 min, conditions that are used clinically, experienced water weight losses between 30% and 45% of the original hydrogel weight. At this temperature, no differences were found between autoclave times of 20 and 30 min. The 30% (lowest) polymer weight fraction of P407 seemed most susceptible to water loss under these conditions, with its average weight loss greater than those of the 35% and 40% polymer weight fraction. Likely, this was attributed to the higher amount of water in the 30% gel prior to autoclaving. This equates to approximately one third to one half of the water content evaporating during autoclaving for the 40% and 30% gels, respectively, thus causing a significant change to the gel’s polymer weight fraction. As is typical for many polymers in hydrogel form, P407 polymer weight fraction has been directly linked to release kinetics of therapeutics such as lidocaine,2 which together with our findings, highlights the need for updating autoclave practices. The effects of autoclaving on other synthetic polymer-based hydrogels (polyethylene glycol [PEG] and cross-linked hydroxymethyl methacrylate [HEMA]) have been investigated, and similar concerns were raised regarding changes in hydrogel structure. Specifically, polymer aggregates and degradation of the gel may result from steam sterilization.21,22 One particular study supported that autoclaving P407, referenced as Pluronic® F127, was acceptable, but differences in gelation behavior were observed after autoclaving.23 Collectively, these results are indicative of polymer weight fraction changes occurring during the autoclave cycle.
Hydrogel water loss during autoclaving may vary with autoclave device, the container used for autoclaving the hydrogel, and the volume and surface area of hydrogel undergoing sterilization. To account for this, clinicians and researchers should test their specific autoclave and sample conditions to more accurately account for water loss experienced with their autoclaving procedures. The particular autoclave used in this study has a timer that is manually set and was started as soon as the machine began heating up. Therefore, the autoclave times in this study included the 5–10 min warm up period prior to reaching the set temperature. This brief period of time was not expected to have affected the results reported herein, but this is a limitation of the device and study, and future work should involve more precise autoclave times that begin once peak temperature is reached.
The polymer weight fractions tested in this study are informed by (and slightly higher than) those used in veterinary practice, due to our desire for a more rigid gel for the localization of therapeutics in the in vivo infection model.12,14 Here, as polymer weight fraction increased, gelation temperature steadily decreased. An effect of autoclaving on gelation temperature was observed for 35% P407, but not for 30% or 40%, although these polymer weight fractions indicated similar trends. Gelation temperature is a key property for the utility of P407 applied via syringe because any gelation temperature below room temperature can negatively influence gel injectability. The 30% formula had the highest gelation temperature, near 20°C, making it the most ideal of the three tested polymer weight fractions. Notably, the more concentrated formulas were difficult to work with at room temperature. No differences were found in storage modulus, a measure of gel elasticity, between autoclaved and nonautoclaved groups, meaning that the gel did not incur greater elasticity from water evaporation. Loss modulus and viscosity also remained generally unaffected after autoclaving. At all frequencies, storage modulus was higher than loss modulus for both groups at each polymer weight fraction, which demonstrates the viscoelastic behavior of P407 hydrogels. Rheologically, autoclaving had minimal effects on the hydrogel’s stability and gelation behavior, while the data suggests that gelation temperature may be susceptible to change as a function of polymer weight fraction.
The gelation process of poloxamer copolymer in water is understood to be a micellization process in which the packing of micelles and micelle entanglements are the driving force for gelation.24 The poloxamer copolymers exist as unimers in an aqueous media at low polymer weight fractions, and they self-assemble to form micelles with an increase in temperature and/or polymer weight fraction. The spherical micelles have dehydrated PPO cores surrounded by hydrated, swollen PEO chains, and gelation occurs when either temperature or polymer weight fraction reaches a threshold value. Although the loss of water during autoclaving increased the polymer weight fraction here, any further increase in temperature above the gelation temperature would not be expected to affect the molecular structure of the gel, which is controlled by the packing and entanglement of micelles. For P407, at low polymer weight fractions, the gel modulus would be expected to change with polymer weight fraction. However, at higher polymer weight fractions of 20–30%, gel modulus was found to be independent of polymer weight fraction.24–27 In this study, although water loss occurred during autoclaving, the lack of differences in moduli between autoclaved and nonautoclaved groups may be attributed to the relatively high polymer weight fractions tested. Furthermore, lack of differences as a function of polymer weight fraction is likely due to the small range of weight fractions tested (30%, 35%, and 40%).
P407 nonspecifically interferes with both bacterial adherence to surfaces and the ability of bacteria to form biofilm and has been shown to limit infection of S. aureus and S. epidermidis on poly(methyl methacrylate) (PMMA).16 The Kirby-Bauer results here demonstrated that P407 alone, nonautoclaved or autoclaved at 121°C for 20 min, did not have direct antimicrobial activity. When loaded with vancomycin, both autoclaved and nonautoclaved P407 deliver the drug with similar efficacy to PBS, suggesting that the gel does not adversely affect the antibacterial efficacy of vancomycin. The lack of differences between autoclaved and nonautoclaved P407 also suggested that autoclaving under clinically accepted protocols does not affect the polymer’s ability to act as an effective delivery vehicle. In contrast, results from the planktonic bacterial assay suggested that P407 alone inhibited bacterial growth, likely by interfering with bacterial adhesion to the well-plate and biofilm formation.16 It is also possible that the P407 and vancomycin could have synergistic antimicrobial effects. P407 is considered a surfactant, capable of disturbing the lipid membranes of bacteria and leading to cell death.16 Likely, the gel applied directly to the agar plates in the Kirby-Bauer assay was unable to remain as an intact solid on the surface long enough to kill the surrounding bacteria, but instead soaked into the agar, rendering it incapable of affecting the bacteria on the agar’s surface. This phenomenon likely explains why P407 failed to show intrinsic antimicrobial properties in the Kirby-Bauer assay but was able to prevent bacterial adhesion in the planktonic experiment. The bacterial experiments performed here provided evidence that P407, whether autoclaved or nonautoclaved, did not render vancomycin ineffective in its ability to kill bacteria. Due to the highly soluble nature of P407 in aqueous environments such as PBS, a vancomycin release kinetics experiment could not be completed (using either a direct contact or transwell assay). Thus, the effects of autoclaving on antibiotic release profiles remains unknown.
For the in vivo study, if P407 had instead been autoclaved similarly to other protocols in literature (121°C for 15–30 min),2,12 based on weight loss data here, the resulting gel would have become a 49–53% P407 formulation. From rheological data, the autoclaving procedure would also be expected to lower gelation temperature by 1–2°C for 35% gels. Thus, if an autoclaving procedure had been applied to the 30% P407, it could have functioned to concentrate the gel prior to any dilution with therapeutic and enabled greater delivery vehicle stability, which is likely the occurrence in the clinical scenario.2,12 Gelation time was not measured for gels used in vivo, but since autoclaving increased the polymer weight fraction of the gel in vitro, we would have expected autoclaving to also reduce the gelation time, which could have improved the efficacy of P407 in our surgical model. Although the pilot in vivo study had limited utility to demonstrate efficacy of P407 as a delivery vehicle, it motivated a thorough investigation into sterilization techniques for surgical application of P407. We have used P407 as a delivery vehicle for antibiotics to treat veterinary soft tissue infections, although in almost all cases, the gels were applied externally.12 Thus, it may be most appropriate to utilize the gels for topical applications. From our animal model, it is unclear whether the lack of efficacy of bacteriophage treatment against osteomyelitis and soft tissue infection was due to variability in bacterial load during initial infection, inadequate phage dosing, and/or poor retention of phage. It is possible that the delivery of S. aureus by PBS carrier failed to maintain the bacteria within the bony site. This model has since been modified to include a shorter infection period with a lower initial bacterial load delivered on the orthopedic screw, rather than via PBS injection, into the defect site.14 It is likely that the updated model is better suited to testing the efficacy of P407 in surgical applications because it minimizes the variability in bacterial load between animals. Further in vivo studies and optimization of the delivery vehicle and therapeutics must be performed with P407 to improve its utility in cases of osteomyelitis.
5 |. CONCLUSION
Although a widely accepted practice, the effects of autoclave sterilization on P407 hydrogels used in drug delivery are not well characterized. In the osteomyelitis model, P407 did not gel as expected but could have shown improved functionality had it been autoclaved prior to use. High temperatures (100–134°C) significantly altered the water content of the gel, thus increasing polymer weight fraction. Autoclaving did not greatly affect most rheological properties of P407, but had a small effect on gelation behavior. Autoclaving did not hinder the hydrogel’s efficacy as a carrier for vancomycin antibiotic in vitro, and notably, P407 alone had a bactericidal effect on planktonic S. aureus. Ultimately, autoclaving increased the hydrogel’s polymer weight fraction and reduced gelation temperature, but did not render P407 ineffective as a biomaterial carrier in vitro. Further investigation into the effects of autoclaving on drug release kinetics are warranted to improve the clinical utility of P407 as a drug delivery vehicle for antimicrobials.
ACKNOWLEDGMENTS
This research was funded by Mississippi State University’s Bagley College of Engineering Undergraduate Research Stipend, Office of Research and Economic Development, and Department of Clinical Sciences in the College of Veterinary Medicine. L. Priddy is supported in part by NIH grant P20GM103646-07. The authors would like to thank Dr. Robert Wills and Weitong Chen for assistance with statistical analyses.
Funding information
College of Veterinary Medicine; Bagley College of Engineering; Mississippi State University
REFERENCES
- 1.Bansal M, Mittal N, Yadav SK, et al. Periodontal thermoresponsive, mucoadhesive dual antimicrobial loaded in-situ gel for the treatment of periodontal disease: preparation, in-vitro characterization and antimicrobial study. J Oral Biol Craniofacial Res 2018;8(2):126–133. 10.1016/j.jobcr.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ricci EJ, Lunardi LO, Nanclares DMA, Marchetti JM. Sustained release of lidocaine from Poloxamer 407 gels. Int J Pharm 2005;288(2):235–244. 10.1016/j.ijpharm.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 3.Veyries ML, Couarraze G, Geiger S, et al. Controlled release of vancomycin from Poloxamer 407 gels. Int J Pharm 1999;192(2):183–193. 10.1016/S0378-5173(99)00307-5. [DOI] [PubMed] [Google Scholar]
- 4.Escobar-Chávez JJ, López-Cervantes M, Naïk A, Kalia YN, Quintanar-Guerrero D, Ganem-Quintanar A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J Pharm Pharm Sci 2006;9(3):339–358. [PubMed] [Google Scholar]
- 5.Bouchot O, Aubin MC, Carrier M, Cohn WE, Perrault LP. Temporary coronary artery occlusion during off-pump coronary artery bypass grafting with the new poloxamer P407 does not cause endothelial dysfunction in epicardial coronary arteries. J Thoracic Cardiovas Surg 2006; 132(5):1144–1149. 10.1016/j.jtcvs.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 6.Erfani Jabarian L, Rouini MR, Atyabi F, Foroumadi A, Nassiri SM, Dinarvand R. In vitro and in vivo evaluation of an in situ gel forming system for the delivery of PEGylated octreotide. Eur J Pharm Sci 2013;48(1–2):87–96. 10.1016/j.ejps.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Zhang K, Shi X, Lin X, Yao C, Shen L, Feng Y. Drug delivery Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats Poloxamer-based in situ hydrogels for controlled delivery of hydrophilic macromolecules after intramuscular injection in rats. Drug Deliv 2015;22(3): 375–382. 10.3109/10717544.2014.891272. [DOI] [PubMed] [Google Scholar]
- 8.Manda P, Kushwaha AS, Kundu S, Shivakumar HN, Jo SB, Murthy SN. Delivery of ziconotide to cerebrospinal fluid via intranasal pathway for the treatment of chronic pain. J Control Release 2016;224:69–76. 10.1016/j.jconrel.2015.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller S, & Donovan M (1982). Effect of Poloxamer 407 gel on the mitotic activity of pilocarpine nitrate in rabbits
- 10.Cafaggi S, Leardi R, Parodi B, Caviglioli G, Russo E, Bignardi G. Preparation and evaluation of a chitosan salt-poloxamer 407 based matrix for buccal drug delivery. J Control Release 2005;102(1):159–169. 10.1016/j.jconrel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Ci L, Huang Z, Liu Y, Liu Z, Wei G, Lu W. Amino-functionalized poloxamer 407 with both mucoadhesive and thermosensitive properties: preparation, characterization and application in a vaginal drug delivery system. Acta Pharmaceutica Sinica B 2017;7(5):593–602. 10.1016/j.apsb.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Swanson EA, Horstemeyer L, Foust B, Priddy LB. Novel use of Poloxamer 407 – antibiotic compounds to treat antimicrobial-resistant soft tissue infections in dogs. Biomed Sci Instrumen 2019;55(1):1–13. [Google Scholar]
- 13.Kandemir N, Xia Y, Duan P, Yang W, Chen J. Rheological characterization of Agarose and Poloxamer 407 (P407) based hydrogels. MRS Adv 2018;3(30):1719–1724. 10.1557/adv.2018.131. [DOI] [Google Scholar]
- 14.Cobb LH, Park JY, Swanson EA, et al. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS ONE 2019;14(11):e0220421. 10.1371/journal.pone.0220421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park JY, Moon BY, Park JW, Thornton JA, Park YH, Seo KS. Genetic engineering of a temperate phage-based delivery system for CRISPR/Cas9 antimicrobials against Staphylococcus aureus. Sci Rep 2017;7:1–13. 10.1038/srep44929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veyries ML, Faurisson F, Joly-Guillou ML, Rouveix B. Control of staphylococcal adhesion to polymethylmethacrylate and enhancement of susceptibility to antibiotics by poloxamer 407. Antimicrob Agents Chemother 2000;44(4):1093–1096. 10.1128/aac.44.4.1093-1096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gioffredi E, Boffito M, Calzone S, et al. Pluronic F127 hydrogel characterization and biofabrication in Cellularized constructs for tissue engineering applications. Procedia CIRP 2016;49:125–132. 10.1016/j.procir.2015.11.001. [DOI] [Google Scholar]
- 18.Bauer AW, Kirby WMM, Sherris JC, Turck AM, Von Graevenitz A. 40 microbiology: a centenary perspective 1966 antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pat-hol 1978;45(3):493–496. 10.1016/S0305-4179(78)80006-0. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson K, McVey AF, Clark IBN, Swain PS, Pilizota T. General calibration of microbial growth in microplate readers. Sci Rep 2016;6(1): 1–7. 10.1038/srep38828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexandridis P Poly(ethylene oxide)/poly(propylene oxide) block copolymer surfactants. Current Opinion Colloid Interface Sci 1997;2(5): 478–489. 10.1016/S1359-0294(97)80095-7. [DOI] [Google Scholar]
- 21.Eljarrat-Binstock E, Bentolila A, Kumar N, Harel H, Domb AJ. Preparation, characterization, and sterilization of hydrogel sponges for iontophoretic drug-delivery use. Polym Adv Technol 2007;18(9):720–730. 10.1002/pat.948. [DOI] [Google Scholar]
- 22.Karajanagi SS, Yoganathan R, Mammucari R, et al. Application of a dense gas technique for sterilizing soft biomaterials. Biotechnol Bio eng 2011;108(7):1716–1725. 10.1002/bit.23105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rafael D, Andrade F, Martinez-Trucharte F, et al. Sterilization procedure for temperature-sensitive hydrogels loaded with silver nanoparticles for clinical applications. Nanomaterials 2019;9(3):1–14. 10.3390/nano9030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojarunchitt T, Hook S, Rizwan S, Rades T, Baldursdottir S. Development and characterisation of modified poloxamer 407 thermoresponsive depot systems containing cubosomes. Int J Pharm 2011; 408(1–2):20–26. 10.1016/j.ijpharm.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 25.Cabana A, Aït-Kadi A, Juhász J. Study of the gelation process of polyethylene oxide(a)-polypropylene oxide(b)-polyethylene oxide, copolymer (poloxamer 407) aqueous solutions. J Colloid Interface Sci 1997; 190(2):307–312. 10.1006/jcis.1997.4880. [DOI] [PubMed] [Google Scholar]
- 26.Desai SD, Blanchard J. In vitro evaluation of pluronic F127-based controlled-release ocular delivery systems for pilocarpine. J Pharm Sci 1998;87(2):226–230. 10.1021/js970090e. [DOI] [PubMed] [Google Scholar]
- 27.Ricci EJ, Bentley MVLB, Farah M, Bretas RES, Marchetti JM. Rheological characterization of Poloxamer 407 lidocaine hydrochloride gels. Eur J Pharm Sci 2002;17(3):161–167. 10.1016/S0928-0987(02)00166-5. [DOI] [PubMed] [Google Scholar]


