Abstract
Scope
Xanthohumol, a prenylflavonoid from hops, has been extensively studied preclinically but has undergone limited research in human subjects. A triple-masked, placebo-controlled phase I clinical trial was conducted to examine the safety and tolerability of xanthohumol.
Methods and results
Thirty healthy volunteers were randomized to 24 mg/day xanthohumol (99.8% pure) or placebo for eight weeks. Comprehensive metabolic panels, complete blood counts, body weight, vital signs, and health-related quality of life questionnaires were assessed every two weeks. Participants were interviewed for adverse events (AEs) throughout the trial. Thirteen of 14 (93%) and 14 of 16 (88%) participants completed the trial in the placebo and xanthohumol groups, respectively. There were no withdrawals due to AEs. There were no clinically relevant, between-group differences in laboratory biomarkers, body weight, vital signs, or health-related quality of life. There were no severe or FDA-defined serious AEs, but non-serious AEs were documented in both the placebo (n=42) and xanthohumol (n=58) groups.
Conclusion
Over an eight-week period, 24 mg daily xanthohumol was safe and well-tolerated by healthy adults.
Keywords: clinical trial, phase I, safety, tolerability, xanthohumol
Graphical Abstract
“Xanthohumol, derived from hops, was administered by mouth in capsule form once daily for 8 weeks. Safety and tolerability were monitored by laboratory measurements, quality of life questionnaires, adherence, adverse events, and vital signs were followed throughout the trial. Future analyses on the metabolic pathways, impact on inflammatory markers, and impact on the gut microbiome will be reported.”
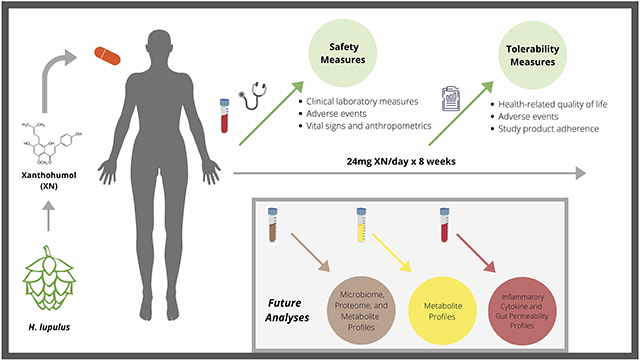
1. INTRODUCTION
Xanthohumol (XN), a prenylflavonoid from the flowers of hops (Humulus lupulus), exhibits multiple biological activities.[1,2] Specifically, antioxidant activity, including inhibition of LDL oxidation in vitro and DNA protective effects in humans have been demonstrated.[3,4] In mouse models, XN has exhibited anti-inflammatory effects including reductions in tissue inflammatory cytokine levels, as well as metabolic effects including alteration of bile acid metabolism, and improvements in impaired glucose metabolism.[5,6] Prebiotic effects of XN have been demonstrated in vitro, in a mouse model and in the human intestine.[5-8] XN is commonly consumed through beer, which contains up to 1 mg per liter, depending on brewing processes. XN is safe and well-tolerated when taken in amounts commonly ingested through the diet.[5, 6] However, XN can also be consumed in larger doses through dietary supplements and other botanical products. As with many botanically-sourced, concentrated constituents, limited human subject safety data is available regarding high doses of XN intake.
In clinical research, the terms "safety" and "tolerability" are often used synonymously despite representing different outcomes. Clinical safety is typically assessed by monitoring blood tests (including routine biochemical parameters), vital signs, and adverse events (AEs), including whether they are serious or non-serious.[7, 8] On the other hand, clinical tolerability can be assessed by monitoring the acceptability of AEs (including whether participants complete or elect to withdraw from a study due to AEs), impact on quality of life, and by monitoring adherence to a study intervention.[7]
Despite interest in and the potential of XN as a therapeutic agent, few clinical trials have prospectively studied it as an isolated constituent in human subjects and even fewer trials have reported on its safety and tolerability. The previous trials included a variety of outcome measures, a range of intervention lengths, a range of dosages, and XN given as an isolated constituent or in combination with other ingredients or hops constituents. In a study designed to evaluate the impact of 12 mg XN per day for two weeks on oxidative DNA damage in healthy adults, Ferk et al. also reported that XN did not impact plasma glucose, plasma cholesterol, serum estradiol, or serum progesterone.[9] Ryan et al. reported findings regarding a complex nutrition support beverage containing 12.5 mg XN administered to adults with inflammatory bowel disease daily for twelve weeks and found it was not associated with adverse changes in metabolic panel parameters, blood counts, or health-related quality of life.[10] In a placebo-controlled trial aimed at evaluating impact on markers of DNA damage and oxidative stress, Stevens et al. administered up to 24 mg XN per day for three weeks in healthy subjects; XN intake was not associated with adverse changes in routine metabolic panel parameters and no treatment-related AEs were observed during the conduct of the study (unpublished results of authors). In a pharmacokinetics study, van Breemen et al. evaluated escalating doses up to 85.2 mg XN per day of a standardized hops extract in menopausal women over 5-day intervals; the extract did not impact sex hormones or blood clotting.[12] In another pharmacokinetics study, Legette et al. evaluated single oral doses of 20, 60, or 180 mg XN and also reported that no intervention-related AEs were observed in the study.[13] Thus, of the trials performed, XN appears safe and well-tolerated without evidence of harm.
Although no evidence of acute or sub-chronic toxicity of XN has been reported, additional safety-focused research is necessary, particularly before evaluating XN as an isolated constituent and as a candidate long-term therapeutic in populations with disease. No previous studies have evaluated XN taken daily as an isolated constituent for a period longer than three weeks in human subjects. Therefore, the primary aims of this study were to assess the clinical safety and tolerability of 24 mg XN per day over an 8-week period.
2. EXPERIMENTAL SECTION
2.1. Study Design
This study was a phase I, two-arm, 1:1, randomized, triple-masked, placebo-controlled clinical trial. The detailed methodology has been published.[14] The protocol was approved by the institutional review board (IRB) at National University of Natural Medicine (IRB # RB9718), registered on ClinicalTrials.gov (NCT03735420), and was conducted according to the principles of the Declaration of Helsinki. Participants provided written informed consent prior to participation in the study. The study was conducted under an Investigational New Drug (IND #140626) application to the United States Food and Drug Administration (FDA). The FDA and the National Institutes of Health (NIH) reviewed and approved the Data and Safety Monitoring Plan (DSMP), which specified halting criteria and outlined the timeline and requirements for independent review of data by an independent Data and Safety Monitoring Board (DSMB).
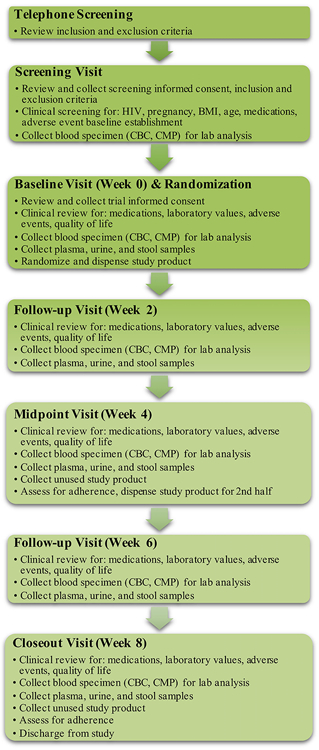
Flow of study procedures is illustrated in Figure 1. The trial was conducted in three phases including enrollment, allocation, and the interventional period. The enrollment period consisted of a telephone screen, an in-person screening visit, and an in-person baseline visit. Fasting blood samples were collected at the clinical screening and baseline visits. At the baseline visit, participants were provided with materials for at-home stool and urine collection, of which samples will serve as media for analysis described below. Allocation and group assignment were conducted on the day following the baseline visit, when participants returned with their baseline stool and urine samples. The interventional period included four additional clinical visits with blood, stool, and urine samples collected every two weeks. Body weight, heart rate, and blood pressure were measured at each study visit.
Figure 1.
Figure 1 depicts the flow of study procedures for paricipants from the conduct of the initial telephone screening to the conclusion of their participation in the trial.
HIV, human immunodeficiency virus; BMI, body mass index; CBC, complete blood count; CMP, comprehensive metabolic panel
The primary endpoints for the trial were the safety of daily XN supplementation in healthy adults through laboratory analysis, including a comprehensive metabolic panel (CMP) and a complete blood count (CBC), as well as its tolerability through AE monitoring, assessment for quality of life changes, and monitoring of adherence to the trial products. These data were measured every two weeks and AEs were assessed continuously throughout the conduct of the trial.
2.2. Study Subjects and Allocation
Healthy adults aged 21-50 years were recruited to the Helfgott Research Institute at the National University of Natural Medicine (NUNM) in Portland, OR between September 2019 and May 2020. Trial activities for all enrolled participants concluded on May 7, 2020. The approved protocol specified enrollment of up to sixteen participants per group (n=32 total), allowing up to 25% attrition to retain at least twelve participants in each group (n=24 total).
Participants were randomized and allocated to either XN or placebo, to be taken as a single capsule once per day with the first daily meal for eight weeks. The randomization and allocation concealment procedures were described in-depth in the published protocol.[14] To ensure equal representation, randomization was stratified by biologic sex to ensure equal allocation to each group. Participants were excluded if they had a history of any chronic disease, were taking any prescription medications, or if they were taking any dietary supplements which could potentially modulate inflammatory pathways (such as flavonoids, XN or hops, curcumin or turmeric, ginger, quercetin, rosemary, fenugreek, white willow, devil’s claw, or > 1g per day of fish oil). Participants were also screened for HIV, pregnancy, and other a priori specified abnormalities (e.g., liver function test abnormalities or thrombocytopenia) on a routine CMP and CBC. All eligibility criteria are detailed in the published protocol.[14]
2.3. Dosage Information and Regimen
The 24 mg daily XN dosage was selected based on previous human subject investigations, described above, including work that reported on safety-related parameters.[9-13] The 24 mg daily XN dosage evaluated in this study is achievable through commercially available supplements and nutrition support products but would not be achievable through a regular diet.
The experimental product capsules contained 24 mg 99+% pure XN, 288 mg of rice protein, 109.3 mg microcrystalline cellulose, 4.3 mg Aerosil® 200 fumed silica, and 4.3 mg magnesium stearate. The placebo capsules contained 288 mg of rice protein, 109.3 mg microcrystalline cellulose, 4.3 mg Aerosil® 200 fumed silica, and 4.3 mg magnesium stearate. Of note, XN was combined with rice protein because rice protein has been shown to significantly increase the bioavailability of XN and its metabolites in humans.[15] To maintain blinding, the study material was encapsulated in orange-colored gelatin capsules. The 99+% pure xanthohumol was provided by Hopsteiner (New York, NY, USA). Metagenics, Inc. (Gig Harbor, WA, USA), a United States Pharmacopeia Good Manufacturing Practices (GMP) verified dietary supplement manufacturer, encapsulated the study material and provided the rice protein, microcrystalline cellulose, silica, and magnesium stearate. Adherence was assessed by pill count upon return of unused capsules at the mid-point and final study visits. Consumption of at least 80% of the required capsules was considered adherent.
2.4. Clinical Biomarker Analyses
At all clinical visits, serum and whole blood were collected by venipuncture, and sent to Quest Diagnostics (Seattle, WA, USA) the day of collection for a routine CMP and CBC. Test results were typically available within one business day. As per the DSMP, test results were inspected upon receipt by the study team to determine if values were within or outside reference ranges according to age and biological sex. This allowed the study team to monitor for potential issues such as elevated liver enzymes, diminished kidney function, electrolyte abnormalities, anemia, lymphocytopenia, or thrombocytopenia in real time throughout the study. If study participants demonstrated an out-of-range laboratory value upon bi-weekly blood draw that did not contribute toward the trial’s halting criteria, they were monitored at the following visit for resolution of abnormal values. Participants with laboratory abnormalities contributing to halting criteria were requested to present for follow-up blood draws prior to their subsequent clinical visit. Stool, urine, and additional blood samples were also collected for identification and quantification of XN and XN-derived metabolites, analysis of effects on gut microbiota, fecal calprotectin, bile acid metabolism, and biomarkers of inflammation as described in the published protocol.[14] However, these analyses are initiated but incomplete at the time of article submission.
2.5. Quality of Life Assessment
To assess for effects on health-related quality of life, the Patient-Reported Outcomes Measurement Information System 29-item profile (PROMIS-29), a validated physical and mental health profile measure, was administered at baseline and every 2 weeks during follow up.[16] The questionnaire contains seven domains (physical functioning, anxiety, depression, fatigue, sleep disturbance, social functioning, and pain) and queries symptoms, rated on a 0-10 numeric scale with four items per domain. The PROMIS-29 was administered privately and electronically, which has been shown to be comparable to paper-based scoring.[17, 18] Generated reports with T-scores were uploaded to the Health Measures Scoring Service and compared to T-scores according to the general population without acute or chronic disease.[16, 19]
2.6. Adverse Event & Safety Monitoring
AEs were defined as any untoward medical occurrence in the clinical investigation. An AE could be an unfavorable and unintended sign, symptom, or disease temporally associated with the study, whether related to the study intervention or placebo. At each of the in-person study visits, participants were interviewed for AEs per the published protocol.[10] AEs would be considered “serious” if above a grade 3, or a participant outcome had included a life-threatening experience, inpatient hospitalization, disability or incapacity, death, a congenital anomaly, birth defect, or a medical or surgical intervention to prevent one of these outcomes.[8, 20] All other AEs were designated as “non-serious”. To allow for reporting of unprompted AEs, participants were also asked open-ended questions at each study visit and encouraged to contact the study team between study visits with any new, unusual, or bothersome symptoms.
2.7. Statistical Analyses
We compared changes in all outcome measures from baseline to week 8 between XN and placebo. A priori power calculations demonstrated, with a minimum of 12 participants allocated per group, the trial had 80% power at a threshold of α = 0.05 to detect an effect size of 1.1, corresponding to a 25% difference in change in values between groups, applying independent t-tests.
Given that our primary aim was to assess the safety and tolerability of high dose XN in those who took the product and not to assess efficacy or effectiveness, our primary analysis was a "per-protocol" analysis and excluded any participant without follow-up or week 8 data. Because one participant had missing data, at the final follow-up, we also conducted a sensitivity analysis and imputed data for this participant using a last observation carried forward (LOCF) approach. As no changes in the significance of differences between groups resulted, the non-imputed model data are reported.
Continuous biomarker data were analyzed using 2-sided independent t-tests to compare changes from baseline to each follow-up visit between study groups. Mean PROMIS-29 scores were calculated by group and compared to domain-level T-scores over the intervention period to baseline per domain. Independent t-tests were performed on change in T-scores from baseline to week 8 to determine significant differences between groups.
As most study outcomes were independent measures of safety rather than efficacy, uncorrected p-values for a large number of tests are reported. Although multiple comparisons may require a stricter threshold of interpretation of significance, in this case, we were interested in any suggestion of possible clinically significant change. Thus, biomarkers showing uncorrected significance for differences between the groups should be considered as indicative of possible effects, worthy of consideration in future research; given the high likelihood of false positives with so many comparisons; however, they should not be interpreted as providing good evidence for a real effect of treatment. All data collected were consolidated to the REDCap database and calculations and analyses were performed using R (The R Foundation for Statistical Computing, software version 3.6.0, Vienna, Austria).
3. RESULTS
3.1. Participant Characteristics
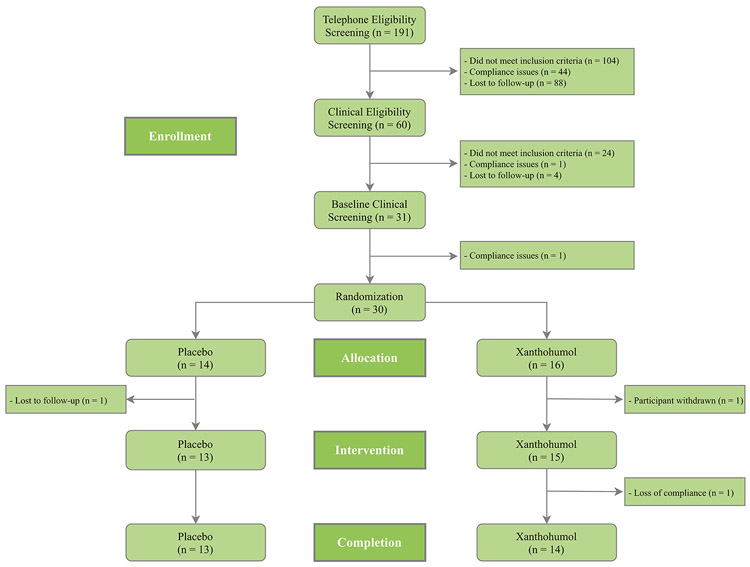
A total of 27 participants completed the trial, outlined in Figure 2. Baseline demographics, vitals and anthropometrics of the study participants are described in Table 1. Thirty participants were randomized into the trial with 14 (8 females, 6 males) allocated to placebo and 16 (8 females, 8 males) allocated to xanthohumol. Thirteen of 14 participants allocated to placebo completed the trial with one participant lost to follow-up. Fourteen of 16 participants allocated to xanthohumol completed the trial. One participant was withdrawn by the study team within days of randomization due to the discovery of restricted supplement use. Another elected to withdraw after the week 4 visit upon electing to initiate a restricted supplement. Of the three participants who did not complete the trial, participation ended for two before any data were collected beyond the baseline visit.
Figure 2.
Figure 2 depicts recruitment, eligibility determination, group allocation, attitrition, and completion for all participants throughout the trial. Pre-randomization compliance issues included social history expectations for trial participation, such as smoking or supplement use containing prohibited substances. Post-randomization loss of compliance included an elected, prohibited medication change.
Table 1.
Enrolled Participant Demographics by Group
| Placebo (n = 14) | Xanthohumol (n = 16) | ||
|---|---|---|---|
| Statistics: frequency (%) or mean (SD) | |||
| Age | 33.14 (5.36) | 29.06 (6.45) | |
| Weight (kg) | 70.60 (14.59) | 69.82 (10.33) | |
| Height (meters) | 1.73 (0.11) | 1.73 (0.12) | |
| Body mass index (kg/m2) | 23.30 (2.57) | 23.41 (2.23) | |
| Heart rate (bpm) | 61.07 (8.30) | 64.50 (11.78) | |
| Sex (female) | 8 (57.14%) | 8 (50.00%) | |
| Race | White/Caucasian | 12 | 16 |
| Asian | 2 | 0 | |
| Ethnicity | Non-Hispanic/Latino | 13 | 14 |
| Hispanic/Latino | 1 | 2 | |
Additional characteristics are found in subsequent tables, including baseline values for blood chemistries (Table 2), patient-reported quality of life measurements (Table 3), and pre-existing symptoms (Table 4).
Table 2.
Summary of Anthropometric and Clinical Laboratory Parameters by Group
| Placebo (n = 13) | Xanthohumol (n = 15) | |||||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Week 0 Mean (SD) |
Week 8 Mean (SD) |
Mean Δ** (SD) | Week 0 Mean (SD) |
Week 8 Mean* (SD) |
Mean Δ** (SD) | Pa | |
| Weight (kg) | 70.60 (14.59) | 71.87 (16.02) | −0.78 (1.70) | 69.82 (10.33) | 69.29 (11.58) | −0.57 (0.95) | 0.69 | |
| Body mass index (kg/m2) | 23.30 (2.57) | 23.54 (3.03) | −0.13 (0.66) | 23.41 (2.23) | 23.06 (2.12) | −0.21 (0.55) | 0.75 | |
| Heart rate (bpm) | 61.07 (8.30) | 63.73 (7.50) | 5.27 (6.99) | 64.50 (11.78) | 65.21 (9.35) | 0.86 (8.97) | 0.19 | |
| BP (mmHg) | Systolic | 107.21 (10.71) | 110.36 (10.95) | 3.82 (4.60) | 111.75 (14.17) | 113.71 (14.99) | 2.71 (7.55) | 0.67 |
| Diastolic | 61.21 (7.27) | 64.27 (5.62) | 4.64 (6.00) | 63.38 (8.97) | 61.07 (9.30) | 2.07 (7.11) | 0.35 | |
| CMP | Glucose | 86.86 (7.2) | 87.92 (7.43) | 1.23 (6.62) | 85.00 (8.07) | 88.71 (4.84) | 4.43 (6.52) | 0.22 |
| BUN | 11.29 (3.54) | 10.92 (2.84) | −0.38 (2.26) | 12.69 (3.63) | 12.07 (3.85) | −0.50 (2.50) | 0.90 | |
| Creatinine | 0.73 (0.09) | 0.76 (0.09) | 0.03 (0.06) | 0.82 (0.13) | 0.84 (0.14) | 0.01 (0.05) | 0.42 | |
| eGFR | 114.07 (8.72) | 111.77 (13.76) | −2.85 (9.91) | 110.50 (10.62) | 108.43 (11.95) | −1.93 (6.21) | 0.77 | |
| Sodium | 138.57 (1.34) | 139.23 (1.79) | 0.85 (1.68) | 138.62 (1.71) | 139.43 (1.28) | 0.71 (0.91) | 0.80 | |
| Potassium | 4.30 (0.16) | 4.28 (0.28) | −0.01 (0.34) | 4.22 (0.26) | 4.41 (0.35) | 0.19 (0.41) | 0.18 | |
| Chloride | 105.71 (2.02) | 105.31 (1.75) | −0.15 (1.41) | 104.69 (1.49) | 104.86 (1.66) | 0.14 (1.23) | 0.56 | |
| CO2 | 27.36 (1.78) | 27.85 (3.18) | 0.31 (2.21) | 27.69 (2.57) | 28.71 (1.77) | 1.21 (2.64) | 0.34 | |
| Calcium | 9.19 (0.26) | 9.28 (0.38) | 0.08 (0.30) | 9.44 (0.30) | 9.41 (0.36) | −0.06 (0.29) | 0.22 | |
| Protein | 6.70 (0.39) | 6.85 (0.41) | 0.12 (0.38) | 6.93 (0.35) | 6.83 (0.24) | −0.08 (0.25) | 0.11 | |
| Albumin | 4.29 (0.24) | 4.43 (0.22) | 0.12 (0.23) | 4.58 (0.30) | 4.54 (0.34) | −0.06 (0.20) | 0.04 | |
| Globulin | 2.41 (0.30) | 2.42 (0.37) | 0.01 (0.28) | 2.35 (0.31) | 2.29 (0.26) | −0.01 (0.12) | 0.79 | |
| A:G Ratio | 1.80 (0.28) | 1.88 (0.32) | 0.08 (0.23) | 1.99 (0.37) | 2.02 (0.41) | −0.01 (0.15) | 0.22 | |
| Bilirubin | 0.60 (0.21) | 0.68 (0.20) | 0.06 (0.21) | 0.71 (0.24) | 0.68 (0.23) | −0.06 (0.25) | 0.17 | |
| Alk, Phos. | 44.93 (11.4) | 47.92 (14.29) | 2.69 (6.30) | 47.56 (12.70) | 46.5 (11.22) | 1.21 (6.58) | 0.56 | |
| AST | 15.50 (2.14) | 15.46 (2.60) | −0.08 (2.10) | 17.31 (4.57) | 18.43 (5.84) | 1.21 (6.58) | 0.55 | |
| ALT | 13.36 (2.68) | 12.92 (2.84) | −0.62 (2.75) | 13.69 (6.35) | 15.14 (9.24) | 1.14 (6.22) | 0.36 | |
| GGT | 11.57 (4.16) | 11.85 (5.00) | 0.00 (2.27) | 12.31 (3.96) | 12.14 (4.52) | −0.43 (1.60) | 0.57 | |
| CBC | WBC | 5.34 (1.22) | 5.38 (1.48) | 0.12 (0.76) | 5.26 (1.29) | 4.91 (1.08) | −0.24 (1.44) | 0.43 |
| RBC | 4.49 (0.44) | 4.52 (0.49) | 0.00 (0.23) | 4.52 (0.41) | 4.56 (0.49) | 0.08 (0.24) | 0.36 | |
| Hemoglobin | 13.45 (1.01) | 13.58 (1.15) | 0.07 (0.62) | 13.74 (1.41) | 13.79 (1.83) | 0.15 (0.84) | 0.78 | |
| Hematocrit | 39.84 (2.72) | 39.99 (3.24) | −0.03 (2.16) | 40.20 (3.45) | 40.40 (4.26) | 0.44 (2.27) | 0.59 | |
| MCV | 89.04 (3.83) | 88.77 (4.03) | 0.04 (0.80) | 89.06 (2.79) | 88.74 (2.51) | −0.63 (1.88) | 0.25 | |
| MCH | 30.04 (1.30) | 30.12 (1.42) | 0.20 (0.52) | 30.40 (1.41) | 30.23 (1.68) | −0.23 (0.87) | 0.14 | |
| MCHC | 33.75 (0.52) | 33.95 (0.67) | 0.21 (0.48) | 34.14 (0.86) | 34.06 (1.26) | −0.03 (0.77) | 0.35 | |
| RDW | 12.46 (0.59) | 12.31 (0.44) | −0.17 (0.30) | 12.38 (0.38) | 12.40 (0.64) | 0.07 (0.43) | 0.11 | |
| Platelets | 245.64 (44.29) | 259.08 (54.72) | 10.46 (25.62) | 239.00 (47.31) | 244.86 (39.68) | 7.14 (20.47) | 0.71 | |
| MPV | 11.00 (0.75) | 10.98 (0.74) | 0.02 (0.24) | 10.81 (0.73) | 10.86 (0.78) | 0.01 (0.37) | 0.99 | |
| Neutrophils | 3001.86 (947.87) | 3048.08 (1266.78) | 144.38 (710.45) | 2899.94 (1245.64) | 2646.93 (773.23) | −169.21 (1464.23) | 0.49 | |
| Lymphocytes | 1740.5 (363.75) | 1719.92 (260.56) | −36.23 (344.29) | 1810.12 (404.74) | 1697.14 (484.27) | −84.93 (326.01) | 0.71 | |
| Monocytes | 434.86 (110.24) | 458 (133.39) | 15.38 (80.44) | 433.88 (73.01) | 437.07 (146.03) | 1.07 (129.12) | 0.73 | |
| Eosinophils | 119.21 (59.84) | 121.92 (89.54) | 0.31 (61.67) | 89.56 (46.85) | 102.29 (54.61) | 13.50 (33.48) | 0.49 | |
| Basophils | 39.64 (16.42) | 36.77 (15.19) | −1.08 (8.16) | 29.25 (12.55) | 31.00 (10.38) | 3.64 (10.55) | 0.21 | |
| % Neutrophils | 55.52 (6.27) | 54.85 (8.27) | 0.28 (7.98) | 53.49 (9.92) | 53.44 (8.10) | 0.30 (11.93) | 0.10 | |
| % Lymphocytes | 33.04 (5.44) | 33.39 (7.00) | −0.32 (7.02) | 35.52 (8.62) | 34.89 (8.65) | −0.78 (11.31) | 0.90 | |
| % Monocytes | 8.25 (1.67) | 8.58 (1.61) | 0.10 (0.89) | 8.52 (1.78) | 8.85 (1.96) | 0.11 (2.18) | 0.98 | |
| % Eosinophils | 2.40 (1.45) | 2.45 (2.12) | −0.03 (1.49) | 1.87 (1.11) | 2.16 (1.22) | 0.28 (0.64) | 0.48 | |
| % Basophils | 0.79 (0.35) | 0.74 (0.37) | −0.03 (0.22) | 0.59 (0.30) | 0.66 (0.25) | 0.09 (0.26) | 0.22 | |
One participant in Group B withdrew following Week 4 visit sample collection; thus, n=14 for Week 8 in Group B.
Δ is measured as mean change in value compared from Week 8 to Baseline.
p-values calculated as mean change in value from Baseline by unpaired, 2-sided t-test between groups.
BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; CO2, blood carbon dioxide; A:G Ratio, albumin-to-globulin ratio; Alk. Phos, alkaline phosphatase; AST, aspartate aminotransferase; ALT, alanine transaminase; GGT, gamma-glutamyl transferase; WBC, white blood cell count; RBC, red blood cell count; MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; RDW, red blood cell distribution width; MPV, mean platelet volume
Table 3.
Summary of PROMIS-29 Domains by Group
| Placebo (n = 13) | Xanthohumol (n = 15) | ||||
|---|---|---|---|---|---|
| Parameter | Week 0 Mean (SD) |
Week 8 Mean (SD) |
Week 0 Mean (SD) |
Week 8 Mean (SD) |
Pa |
| Anxiety/Fear | 48.38 (8.29) | 46.15 (8.00) | 47.27 (7.01) | 47.34 (7.60) | 0.69 |
| Depression | 42.06 (3.98) | 44.58 (6.1) | 44.96 (6.69) | 44.57 (6.12) | 0.31 |
| Fatigue | 41.72 (7.99) | 39.10 (7.83) | 41.98 (8.22) | 42.61 (9.73) | 0.33 |
| Pain Interference | 44.27 (7.24) | 45.85 (6.93) | 42.81 (3.34) | 44.36 (5.68) | 0.67 |
| Physical Function | 55.88 (3.82) | 54.90 (4.89) | 56.90 (0.00) | 56.26 (2.41) | 0.82 |
| Sleep Disturbance | 54.06 (1.83) | 52.97 (2.28) | 53.27 (3.28) | 52.93 (5.19) | 0.94 |
p-values calculated independent t-test comparing change between groups and considered significant <0.05.
p < .05 considered statistically significant
Table 4.
Adverse events in 28 randomized participants
| Placebo (n=13) | Xanthohumol (n=15) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (n=14) | Week 2 (n=13) | Week 4 (n=13) | Week 6 (n=13) | Week 8 (n=13) | TOTAL | Baseline (n=15) | Week 2 (n=15) | Week 4 (n=15) | Week 6 (n=14) | Week 8 (n=14) | TOTAL | |
| n | N/Nb/Np | 42 | n | N/Nb/Np | 58 | |||||||
| Expected Adverse Events (Prompted) | ||||||||||||
| Eyes, Ears, Nose, Throat | ||||||||||||
| Tinnitus | 2 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 1 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 |
| Congestion/Sinusitis | 1 | 1/0/0 | 0/0/0 | 2/0/0 | 0/1/1 | 5 | 0 | 0/0/0 | 1/0/0 | 3/0/0 | 0/0/0 | 4 |
| Allergy Symptoms | 0 | 0/0/0 | 2/0/0 | 1/0/0 | 0/0/0 | 3 | 0 | 0/0/0 | 2/0/0 | 1/0/1 | 0/0/0 | 4 |
| Miscellaneous EENT Symptoms* | 1 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 2 | 0/0/0 | 1/0/0 | 2/0/0 | 0/0/0 | 3 |
| Gastrointestinal | ||||||||||||
| Abdominal Pain | 0 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 | 1 | 2/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 2 |
| Decreased Appetite | 0 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 |
| Constipation | 0 | 3/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 3 | 0 | 1/0/0 | 1/0/0 | 1/0/0 | 1/0/0 | 4 |
| Diarrhea | 1 | 2/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 2 | 0 | 0/0/0 | 1/0/0 | 1/0/0 | 1/0/0 | 3 |
| Indigestion | 2 | 2/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 2 | 0 | 2/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 3 |
| Increased Thirst | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 1 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 |
| Neurological, Musculoskeletal | ||||||||||||
| Musculoskeletal Pain | 2 | 0/1/0 | 0/1/0 | 1/0/0 | 0/0/0 | 3 | 2 | 0/0/0 | 1/0/0 | 0/0/0 | 1/0/0 | 2 |
| Headache | 0 | 0/0/0 | 2/0/0 | 2/0/0 | 0/0/0 | 4 | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 2/0/0 | 3 |
| Restlessness | 1 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 |
| Numbness | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 1 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 |
| Agitation/Jitters | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 1 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 |
| Decreased Attention Span | 1 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 1 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 |
| Psychological, General | ||||||||||||
| Depression | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 | 1 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Irritability | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 | 0 | 1/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 2 |
| Lethargy | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 |
| Insomnia | 4 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 3 | 1/0/0 | 0/0/0 | 3/0/0 | 0/0/0 | 4 |
| Fatigue | 0 | 0/0/0 | 2/0/0 | 0/0/0 | 0/0/0 | 2 | 1 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 |
| Hyperactivity | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 |
| Dyspnea | 0 | 0/0/0 | 1/0/0 | 1/0/0 | 0/0/0 | 2 | 0 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 |
| Cardiopulmonary | ||||||||||||
| Hypotension | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Chest Pain | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Peripheral Edema | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 |
| Dermatological | ||||||||||||
| Acne | 3 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 |
| Itching/Dryness | 2 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 | 2 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 |
| Rash | 1 | 1/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 2 | 1 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Genitourinary | ||||||||||||
| Breast Swelling | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 |
| Other Genitourinary Symptoms** | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 1/0/0 | 1 |
| Constitutional/Whole Body | ||||||||||||
| Fever | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 |
| Sore Throat | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 1/0/0 | 1 | 0 | 0/0/0 | 2/0/0 | 1/0/1 | 0/0/0 | 4 |
| Weight Gain | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 1 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Generalized Body Pain | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 |
| Spontaneous Adverse Events (Unprompted) | ||||||||||||
| Eyes, Ears, Nose, Throat | ||||||||||||
| Sinusitis | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 |
| Cardiopulmonary | ||||||||||||
| Cough | 0 | 0/0/0 | 1/0/0 | 1/0/0 | 0/0/0 | 2 | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Constitutional/Whole Body | ||||||||||||
| Generalized Body Pain | 0 | 0/0/0 | 1/0/0 | 0/0/0 | 0/0/0 | 1 | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Fever | 0 | 0/0/0 | 0/0/0 | 1/0/0 | 0/0/0 | 1 | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 |
| Strong Body Odor | 0 | 0/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 0 | 0 | 1/0/0 | 0/0/0 | 0/0/0 | 0/0/0 | 1 |
EENT symptoms included periorbital twitching dry eyes
Genitourinary symptoms included cloudy urine.
N: number of participants with new onset symptoms; Nb: number of participants with worsening symptoms from baseline; Np: number of participants with worsening from previous visit.
3.2. Impact on Clinical Biomarkers and Anthropometrics
Mean body weight, BMI, heart rate, blood pressure, CMP and CBC values at the baseline and study end visits, as well as mean change from baseline to study end (week 8), are presented in Table 2. Intake of XN or placebo did not impact body weight, BMI, heart rate, or blood pressure. Mean albumin concentration increased slightly in the placebo group, and decreased slightly in the xanthohumol group, leading to a small statistically significant difference (p=0.04); the means for both groups stayed well within the clinically normal reference range.
3.3. Impact on Quality of Life
Group assignment had no significant effect on any of the seven PROMIS-29 scale scores over an 8-week period (Table 3).
3.4. Adverse Events
No participants withdrew from the study due to AEs. 100 non-serious AEs (signs or symptoms not present at or worsening since the baseline clinical visit) were documented throughout the trial and are delineated in Table 4. All recorded AEs were graded 1 (mild) or 2 (moderate) with none considered serious. A higher number of AEs were documented in the xathohumol group (n=58), than the placebo group (n=42).
3.5. Study Capsule Adherence
Although both groups achieved ≥80% adherence, adherence was significantly higher in the xanthohumol group (96.1% of capsules consumed, over eight weeks) than in the placebo group (87.2%, p=.044).
4. DISCUSSION
Main objectives of this study were to prospectively assess the safety and tolerability of 24 mg daily XN intake in healthy adults. Our results demonstrated XN was safe and well-tolerated during the 8-week intervention period. The lack of abnormal values in clinical biomarkers – including those of hepatic and renal function, electrolytes, fasting glucose, and blood counts – suggests general safety and a lack of harm to major organ systems. Where there were significant differences in laboratory values, the observed changes did not demonstrate a sustained or progressively-worsening pattern throughout the trial. All reported AEs were non-serious, and most AEs reported in both study groups were prompted by the investigators. Findings related to tolerability suggested XN did not negatively impact quality of life; participants taking XN had excellent adherence, adherence was higher in the xanthohumol group, and none of the participants taking XN elected to withdraw from the study due to AEs. The findings of this phase I trial are consistent with, yet expand upon, previous human subject XN research that monitored safety-related parameters and found that daily XN did not adversely impact biochemical parameters [9-12] and that daily intake of XN in a formula with other ingredients was not associated with serious AEs or study product adherence issues.[10] However, the phase I XMaS trial safety and tolerability data are the most thorough and rigorous generated on XN to date. These results add to the existing literature on XN by reporting extensive AE data and clinical laboratory monitoring, as well as impact on anthropometric measurements and quality of life, evaluated over the longest period of time evaluated to date.
Given that commercial XN-containing products are already sold and in use at dosages similar to the dosage evaluated in the present study, these data are relevant to public health and clinical care. Furthermore, these data aid in addressing the juxtaposition that exists between extensive and increasing use of botanical products by consumers and the limited available prospective clinical safety data on botanical products.[21-23] A common goal of stakeholders involved in researching, manufacturing, and regulating botanical products is having safe products available in the marketplace; as such, experts have called for coordination between these stakeholders.[21-23] The XMaS trial exemplifies such synchronization between researchers at several academic research institutions, material suppliers, a supplement manufacturer, governmental regulatory bodies, and governmental sponsors. Aspects of the XMaS phase I trial may serve as a model for safety-oriented trials that aim to address known challenges in botanical research.
The design of the phase I XMaS trial had both strengths and limitations. Strengths include a triple-masked, randomized, placebo-controlled trial designed to minimize bias and optimize causal inference. The robust trial design included a thorough, standardized method for monitoring safety and tolerability. Additional strengths include high retention of study participants and excellent adherence to the study capsules in both the intervention and placebo groups. Limitations of this trial include a small sample size for generalizability of findings, and the evaluation of a single daily dosage of XN, which may not reflect safety at other doses. However, considering that the dosage administered far exceeds what would be achievable through a regular diet, we maintain that the chosen dose was sufficiently large for undesirable effects to be detected by the extensive safety and tolerability measures monitored. Future analyses of stool, blood, and urine samples will elucidate pathways of XN metabolism and could improve our understanding about XN bioavailability through oral administration and its possible relationship to the development of AEs or laboratory changes. Similarly, the trial was designed to include conservative halting criteria for safety, which were not met, nor were any suggestions of toxicity evident in routine clinical biomarker assessments; both add confidence our findings were not greatly limited by statistical power.
In summary, 24 mg daily XN taken over an eight-week period was safe and well-tolerated by healthy adults. The results of the work reported here will inform future research evaluating XN in clinical populations. Expanding upon the present work, the safety, tolerability, metabolism, and biologic mechanisms of 24 mg daily XN is currently being evaluated in adults with Crohn’s disease via a formal phase II trial. Additional aims of the phase I trial were to generate data on XN metabolism, XN effects on gut microbial composition, and XN effects on additional biomarkers, which is currently under way.
Acknowledgements
We would like to thank the following institutions and individuals for their support during the conduct of this trial:
Research Coordinators and Assistants: Heather Schiffke, MATCM, Lita Buttolph, DSOM, Sara Guedry, and Tyler Weed. Ms. Schiffke served in multiple managerial roles including study product control and maintenance of blinding for the study team. Dr. Buttolph conducted telephone screenings, clinical visits, randomized participants, and collected biological samples for storage. Ms. Guedry and Mr. Weed conducted telephone screenings, performed quality control checks on participant data, and prepared biological sample kits for participant use.
Data and Safety Monitoring Board: Robyn Dreibelbis, DO, Jessica Minnier, PhD, Robert Martindale, MD, PhD. The DSMB reviewed an interim and final analysis of the reported data including laboratory values and reported adverse events.
Study Intervention Materials: We thank Hopsteiner for providing the xanthohumol and Metagenics, Inc. for contributing the rice protein, microcrystalline cellulose, silica, and magnesium stearate. We also thank Annalouise O’Connor, PhD and the Product Development team at Metagenics for encapsulating and shipping the xanthohumol and placebo capsules.
Financial Support:
Funding for the XMaS Trial was provided by the National Institutes of Health National Center for Complementary and Integrative Health (grants R01 AT010271 and R01 AT010271-02S1). Pacific Northwest National Laboratory is operated for the U.S. Department of Energy by Battelle Memorial Institute (contract DE-AC05-76RL01830). Study materials were provided Hopsteiner and by Metagenics, Inc. Hopsteiner and Metagenics, Inc. had no involvement in the research design, data analysis, or interpretation of the results.
Abbreviations:
- AE
adverse event
- CBC
complete blood count
- CMP
comprehensive metabolic panel
- DSMB
data and safety monitoring board
- DSMP
data and safety monitoring plan
- FDA
United States Food and Drug Administration
- GMP
Good Manufacturing Practice
- IND
investigational new drug
- IRB
institutional review board
- NIH
National Institutes of Health
- LOCF
last observation carried forward
- PROMIS-29
Patient-Reported Outcomes Measurement Information System 29-item profile
- XN
xanthohumol
Footnotes
Potential Conflicts of Interest: No conflicts of interest to report.
Resources
- [1].Gerhäuser C, Frank N. Mol. Nutr. Food Res 2005, 49(9), 821, DOI: 10.1002/mnfr.200590033. [DOI] [PubMed] [Google Scholar]
- [2].Stevens J, Revel J. ACS Publications. 2018, 283. 10.1021/bk-2018-1286.ch015. [DOI] [Google Scholar]
- [3].Miranda CL, Stevens JF, Ivanov V, McCall M, Frei B, Deinzer ML, Buhler DR. J. Agric. Food Chem 2000, 48(9), 3876. DOI: 10.1021/jf0002995. [DOI] [PubMed] [Google Scholar]
- [4].Ferk F, Mišík M, Nersesyan A, Pichler C, Jäger W, Szekeres T, Marculescu R, Poulson HE, Henriksen T, Bono R, Romanazzi V, Al-Serori H, Biendl M, Wagner KH, Kundi M, Knasmüller S. Mol. Nutr. Food Res 2016, 60(4), 773, DOI: 10.1002/mnfr.201500355. [DOI] [PubMed] [Google Scholar]
- [5].Zhang Y, Bobe G, Revel JS, Rodrigues RR, Sharpton TJ, Fantacone ML, Raslan K, Miranda CL, Lowry MB, Blakemore PR, Morgun A, Shulzhenko N, Maier CD, Stevens JF, Gombart AF. Mol. Nutr. Food Res 2020, 64(1), e1900789, DOI: 10.1002/mnfr.201900789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Miranda CL, Johnson LA, de Montgolfier O, Elias VD, Ullrich LA, Hay JJ, Paraiso IL, Choi J, Reed RL, Revel JS, Kioussi C, Bobe G, Iwaniec UT, Turner RT, Katzenellenbogen BS, Katzenellenbogen JA, Blakemore PR, Gombart AF, Maier CS, Raber J, Stevens JF. Scientific Reports. 2018, 8, 613 . DOI: 10.1038/s41598-017-18992-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Possemiers S, Heyerick A, Robbens V, de Keukeleire D, Verstraete W. J. Agric. Food Chem 2005, 53(16), 6281. DOI: 10.1021/jf0509714. [DOI] [PubMed] [Google Scholar]
- [8].Possemiers S, Bolca S, Grootaert C, Heyerick A, Decroos K, Dhooge W, de Keukeleire D, Rabot S, Verstrate W, Van de Wiele T. J. Nutr 2006, 136(7), 1862. DOI: 10.1093/jn/136.7.1862 [DOI] [PubMed] [Google Scholar]
- [9].JF Stevens, Maier CS. Phytochem. Rev 2016, 15(3), 425, DOI: 10.1007/s11101-016-9459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Stevens JF, Taylor AW, Deinzer ML, J. Chromatogr. A 1999, 832(1-2), 97, DOI: 10.1016/s0021-9673(98)01001-2. [DOI] [PubMed] [Google Scholar]
- [11].Oser B, Ford R, J. Food Tech 1973, 27(11), 56. [Google Scholar]
- [12].Shader RI, Clin. Ther 2018, 40(5), 672, DOI: 10.1016/j.clinthera.2018.04.003. [DOI] [PubMed] [Google Scholar]
- [13].RB Sherman, Woodcock J, Norden J, Grandinetti C, Temple RJ, N.Engl. J. Med 2011, 365(1), 3, DOI: 10.1056/NEJMp1103464. [DOI] [PubMed] [Google Scholar]
- [14].JJ Ryan, Hanes DA, Bradley RD. Glob. Adv. Health Med 2019, 8, DOI: 10.1177/2164956119867251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].van Breemen RB, Yuan Y, Banuvar S, Shulman LP, Qiu X, Alvarenga RF, Chen SN, Dietz BM, Bolton JL, Pauli GF, Krause E, Viana M, Nikolic D. Mol. Nutr. Food Res 2014, 58(10), 1962, DOI: 10.1002/mnfr.201400245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Legette L, Karnpracha C, Reed RL, Choi J, Bobe G, Christensen JM, Rodriguez-Proteau R, Purnell JQ, Stevens JF. Mol. Nutr. Food Res 2014, 58(2), 248, DOI: 10.1002/mnfr.201300333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bradley R, Langley BO, Ryan JJ, Phipps J, Hanes DA, Stack E, Jansson JK, Metz TO, Stevens JF. Trials. 2020, 21(1), 835, DOI: 10.1186/s13063-020-04769-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].O'Connor A, Konda V, Reed RL, Christensen JM, Stevens JF. Mol. Nutr.Food Res 2018, 62(6), e1700692, DOI: 10.1002/mnfr.201700692. [DOI] [PubMed] [Google Scholar]
- [19].Hays RD, Spritzer KL, Schalet BD, Cella D. Qual. Life Res 2018, 27(7), 1885, DOI: 10.1007/s11136-018-1842-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Katz P, Pedro S, Michaud. Arthritis Care Res. 2017, 69(9), 1312, DOI: 10.1002/acr.23183. [DOI] [PubMed] [Google Scholar]
- [21].Katz P, Kannowski CL, Sun L, Michaud K. ACR Open Rheumatol. 2020, 2(6), 320, DOI: 10.1002/acr2.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cook KF, Jensen SE, Schalet BD, Beaumont JL, Amtmann D, Czajkowski S, Dewalt DA, Fries JF, Pilkonis PA, Reeve BB, Stone AA, Weinfurt KP, Cella D. J. Clin. Epidemiol 2016, 73, 89, DOI: 10.1016/j.jclinepi.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Department of Health and Human Services. 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf [DOI] [PubMed]
- [24].Shipkowski KA, Bets JM, Birnbaum LS, Bucher JR, Coates PM, Hopp DC, MacKay D, Oketch-Rabah H, Walker NJ, Welch C, Rider CV. Food Chem. Toxicol 2018, 118, 963, DOI: 10.1016/j.fct.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dwyer JT, Coates PM, and Smith MJ. Nutrients. 2018, 10(1), DOI: 10.3390/nu10010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Swann JP. Drug Test Anal., 2016, 8(3-4), 271, DOI: 10.1002/dta.1919. [DOI] [PubMed] [Google Scholar]