Abstract
Development and application of nanotechnology-enabled medical products, including drugs, devices, and in vitro diagnostics, are rapidly expanding in the global marketplace. In this review, the focus is on providing the reader with an introduction to the landscape of commercially available nanotechnology-enabled medical products as well as an overview of the international documentary standards and reference materials that support and facilitate efficient regulatory evaluation and reliable manufacturing of this diverse group of medical products. We describe the materials, test methods, and standards development needs for emerging medical products. Scientific and measurement challenges involved in the development and application of innovative nanoenabled medical products motivate discussion throughout this review.
Keywords: documentary standards, drug products, in vitro diagnostics, measurement assurance, medical devices, nanomedicine
1. INTRODUCTION
Nanotechnology-enabled (nanoenabled) medical products consist of a broad and rapidly growing global catalog of human drug products, medical devices, and in vitro diagnostics (IVDs) that have been developed (or are in the process of being developed) for the main purpose of improving human health. These nanoenabled medical products may contain some fraction of engineered nanomaterials (ENMs), may consist totally of ENMs, and/or may be products that generate ENMs over time due to normal wear or degradation processes. Nanoenabled medical products may also simply present nanostructured features or topographical surface structures at the nanoscale size range. It is now well established that ENMs [e.g., liposomes, silver nanoparticles (AgNPs), carbon nanotubes (CNTs)] are intentionally manufactured materials that are increasingly incorporated into medical products because of their unique physical, chemical, mechanical, biological, and/or catalytic properties. However, a global, harmonized regulatory framework for approval and sustainable use of nanoenabled medical products, some of which actually contain ENMs, does not currently exist (1). Regulatory approval of nanoenabled drug products follows the regulatory pathway of medical products in conjunction with guidelines from the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH). In contrast, regulatory approval of nanoenabled medical devices and the medical device subcategory of nanoenabled IVDs may follow any one of several basic regulatory approval pathways depending on the technical application and the level of risk surrounding use of the medical device or IVD (assay or device) (2).
Three reasons for the lack of a specific, harmonized regulatory framework for nanoenabled medical products include (a) the large number of potential materials, surface coatings/features, targeting ligands, and physicochemical properties that can be varied during the development of novel ENMs; (b) the inherent complexity of characterizing, understanding, and reproducing ENM interactions with biological systems (nano-bio interactions) at both the in vitro and in vivo levels during preclinical and clinical product development stages; and (c) the critical shortage and technical gaps in the available international consensus standards (guidance documents, technical reports, test methods) and reference materials for characterizing the quality attributes of nanoenabled medical products (1,3,4). All three of these potential reasons contribute to the reality of the bottleneck where there are many more nanoenabled medical products in various stages of clinical trials than actual products in translation into the marketplace. In this critical review, we focus on the latter reason, i.e., the availability or rather the lack of availability of international standards to facilitate the development, manufacture, and regulatory approval of nanoenabled medical products. It is noted that in the area of ENM standard test methods, analytical chemistry and measurement science play an important role in the development of high-quality in vitro bioassays and of robust physicochemical characterization methods necessary for evaluating the quality attributes of medical products. Application and incorporation of in-process controls, robustness testing, and critical analysis of method figures of merit (e.g., analytical sensitivity, dose-response dynamic range) are just a few tools analytical chemistry and measurement science bring to bear in standards development. To this effect, we provide an overview of the existing international standards for nanoenabled drug products, medical devices, and IVDs. The scientific and measurement assurance challenges involved in development of consensus documentary standards for these innovative medical products are outlined. Finally, we describe and discuss recent innovations in nanoenabled medical products and provide a perspective of emerging international standards to support development of these products.
1.1. Nanoenabled Drug Products
Nanoenabled drug products, alternatively known as nanomaterial-containing drug products, nanoparticle-containing drug products, or nanomedicines are medical products that have been specifically formulated using nanotechnology tools and/or nanoscale materials for prevention and treatment of human disease. Nanoenabled drug products typically demonstrate size-dependent uptake into cells, increased drug bioavailability with prolonged circulation times, and drug localization at tumor sites via passive targeting on the basis of an enhanced permeability and retention effect, and/or active targeting on the basis of receptor binding on the surface of cells (5). The upward trajectory of nanoenabled drug products at the preclinical and clinical stages of the regulatory approval process is not expected to slow down, with more than 70 marketed nanoenabled drug products since 1974 (6, 7) and more than 110 nanoenabled drug products currently in active or recruiting status in clinical trials (see https://clinicaltrials.gov/). Nanoenabled drug products are developed primarily for intravenous drug delivery applications (8) where the ENM is utilized as the carrier for the active pharmaceutical ingredient (API), the API is formulated at the nanoscale size range, or the ENM is utilized as a formulation excipient (6). There exists a great and increasing diversity of ENM types, including liposomes, polymers, emulsions, micelles, dendrimers, nanocrystals, and gold nanoparticles (AuNPs). These ENMs are used in nanoenabled drug product formulations, and these formulations are primarily directed toward treatment of various types of cancers (e.g., Doxil) (9). However, other representative human health application areas include, but are not limited to, immune/inflammatory disorders, cardiovascular disorders, degenerative disorders, and infectious diseases. Several detailed overviews of the types and characteristics of ENMs used in nanoenabled drug products, routes of administration, and disease application areas have been recently published (6–8, 10). One of the first approved (1974) nanoenabled drug products that still maintains a market presence is INFeD (iron dextran injection; United States Pharmacopeia) (6). This ~15-nm-sized iron-polymer complex is administered via intramuscular injection and is indicated for iron replenishment of hemoglobin and depleted iron stores, i.e., treatment of anemia (11). One of the most recently approved (2018) nanoenabled drug products is Onpattro, which is suggested for treatment of peripheral nerve disease (12). The drug product consists of small-interfering RNA (siRNA) encased in lipid NPs for the delivery, via intravenous infusion, of the drug (sodium patisiran) into the liver where the drug interferes with RNA production of an abnormal form of transthyretin. Comprehensive reviews that provide further detailed descriptions of nanoenabled drug products currently in clinical trials and/or on the market have been recently published (13, 14).
1.2. Nanoenabled Medical Devices
Nanoenabled medical devices incorporate nanotechnology-produced structures and/or materials into medical devices in order to enhance human therapeutic outcomes or to treat an array of human diseases. European Commission (EC) Directive 2007/47/EC defines a medical device as “any instrument, apparatus, appliance, software, material, or other article, whether used alone or in combination, including the software intended by its manufacturer to be used specifically for diagnostic and/or therapeutic purposes, and necessary for its proper application, intended by the manufacturer to be used for human beings for the purpose of: (1) diagnosis, prevention, monitoring, treatment or alleviation of disease […] and which does not achieve its principal intended action in or on the human body by pharmacological, immunological or metabolic means…” (15, p. 11). This definition is quite similar to the definition expressed in the Federal Food Drug and Cosmetics Act [21 U.S.C. § 321 (h) (1938)] that the US Food and Drug Administration’s (FDA’s) Center for Devices and Radiological Health (CDRH) utilizes for regulating devices. ENMs and/or nanostructures are increasingly being utilized in medical devices to enhance the performance (16) and/or to improve the biocompatibility of the device (17). Among other functions, ENMs can function as the medical device (e.g., iron oxide NPs used for photothermal therapy) (18), can be discrete components of or utilized on the surface of medical devices (e.g., hydroxyapatite NPs on implanted bone scaffolds for enhancing biocompatibility) (19), can be incorporated into composite materials that are part of the medical device (e.g., titanium dioxide NPs for enhancing the mechanical strength of dental adhesives) (20) and/or can function as medical device coatings (e.g., AgNPs acting as antimicrobial agents on surgical tools) (21). The types of ENMs utilized in nanoenabled medical devices are typically different from the types of ENMs utilized in nanoenabled drug products. Whereas nanoenabled drug products generally incorporate the use of soft, biodegradable entities [e.g., liposomes, dendrimers, micelles (6)], nanoenabled medical devices are more likely to involve use of hard, not as biodegradable ENMs that contain cores based on silver, gold, iron oxide, titanium, and/or hydroxyapatite (10). Long-term physical stability and longer shelf lives of the hard NPs may be contributing factors in the increased presence of these types of ENMs in nanoenabled medical devices.
Commercialization of nanoenabled medical devices is not as prolific as the commercialization of nanoenabled drug products (22) because the market is inherently smaller for medical devices; however, the nanoenabled medical device market is growing (23). To date, more than 1,400 peer-reviewed scientific articles have been published on ENMs applied to medical device development (Web of Science search words were “nano” and “medical devices”), while the global market of medical device-containing nanotechnology is estimated to be valued currently at approximately $8.5 billion, increasing from $5 billion in 2014 (24). It is challenging to accurately determine the number of nanoenabled medical devices that are commercially available. One reason for this is because some manufacturers of nanoenabled medical devices use the term nano in their product name even though the product was not developed using nanotechnology tools, nor does the medical device contain ENMs or nanostructures (23). This is exemplified by fact that in the United States alone, 2,586 “nano* implantable devices” from 16 unique manufacturers are listed within the FDA’s database of medical devices sold in the country between 1980 and 2017 (22). Nevertheless, one estimate of marketed nanoenabled medical devices concluded that approximately 20 products had been approved through the FDA 510(k) regulatory process (10), whereas a more recent estimate reported at least 65 products (both estimates include products that are nominally considered nanoenabled IVDs) (23).
The current application areas for nanoenabled medical devices are extremely broad and include noninvasive human surface contacting devices [e.g., antibacterial zinc oxide NP-containing hospital fabrics (by Nano Textile)], invasive human surface contacting devices [e.g., antibacterial AgNP-containing wound dressings (Acticoat by Smith & Nephew)], invasive external communicating devices [e.g., plasma-polished diamond nanolayer coating (Diamaze by Cadence Blades) on surgical blades/scalpels that enable easier cutting through tissues], invasive implantable devices [e.g., bioceramic nanotube implant system (Nano FortiCore by Nanovis) for securing spinal implants in the intervertebral space], and injectable devices [e.g., iron oxide NPs (Magtrace by Endomag) for magnetic staining of lymph nodes] (15). These nanoenabled medical devices have primarily been utilized in applications related to performance enhancements and/or improved biocompatibility in cardiology, dentistry, neurology, oncology, orthopedics, surgery, and healthcare-related textile development (e.g., wound dressings) (23). As of this writing, there are no comprehensive reviews characterizing or exhaustively describing nanoenabled medical devices in clinical trials or in the marketplace. Further details expanding on the current and emerging application areas of nanoenabled medical devices are provided later in this review.
1.3. Nanoenabled In Vitro Diagnostics: Assays and Devices
Nanoenabled IVDs or nanodiagnostics are a subcategory of medical devices that are designed, developed, and specifically used to diagnose and/or monitor diseases or pathogens in specimens that are taken from human bodies. A primary factor driving the ongoing development of nanoenabled IVDs is the potential to use ENM probes in microarray formats to rapidly test for multiple disease biomarkers at very low (attomolar) levels in individual samples. The ENM probes can be conjugated to antibodies or oligonucleotides that are specific for the disease/pathogen biomarkers (proteins) of interest. In general, nanoenabled IVDs can be reagents, instruments, or systems that are intended for use in the collection, preparation, and examination of human specimens (21 C.F.R. 809.3). There are no explicit regulations related to use of ENMs, nanostructures, or nanotechnology in nanoenabled IVDs (25). However, these diagnostic products come under regulatory purview of the FDA’s CDRH and the Center for Biologics Evaluation and Research (CBER) in the United States and the European Union (EU) IVD Directive 98179/EC (IVDD), which was replaced in 2017 by EU IVD Medical Device Regulation 2017/746 (IVDR) (26). Nanoenabled IVDs encompass a broad range of nanotechnologies that are typically developed in the format of ENM probes, nanocantilever arrays, nanowires, nanopores, nanobarcodes, nanosensors, and nanoarrays (27–29). All of these IVD nanotechnologies share a central purpose: to enable development of in vitro bioassays and/or diagnostic devices that facilitate quicker, more sensitive, and less costly clinical testing of patient or population samples for pathogens and/or disease biomarkers than the current diagnostic products on the market.
Another important feature of nanoenabled IVDs is the possibility of utilizing them in point-of-care (POC) testing environments such as in a doctor’s office or a patient’s home. The most advanced nanoenabled IVD products are based on use of ENM probes owing to their unique physicochemical properties, ease of synthesis, and readily accessible surfaces for functionalization with nucleic acids, antibodies, peptides, etc. (25, 29, 30). ENMs, such as quantum dots (QDs), superparamagnetic NPs (Fe, Ni, Co), ferrofluids, AgNPs, and AuNPs have a substantial presence in current nanoenabled IVD products (see Figure 1) (25, 27). AuNPs have been found to be especially useful for enhanced low-level detection of health status biomarkers, such as measuring glucose levels in diabetes patients and prostate-specific antigen levels in prostate cancer patients, and for the detection of infectious disease pathogens (e.g., HIV, malaria) (30, 31). One of the most well-established AuNP-based IVD assays is the Verigene system that was created from the original development and use ofNP bio-barcodes (32). This system utilizes AuNP microarrays and magnetic beads for identifying disease biomarkers and/or pathogens circulating in blood or other body fluids. Silver enhancement of the microarray-bound AuNP probes allows optical detection of the targeted analytes. Many other nanoenabled IVDs are based on the use of AuNPs in lateral flow assays or in nanobiosensors (30).
Figure 1.

Current paradigm for medical applications involving nanoenabled IVD assays. (a–c) Typical applications of inorganic NPs (e.g., gold, quantum dots, paramagnetic) functionalized with specific antibodies in different types of IVDs. (a) Lateral flow assay often used in qualitative point-of-care testing applications. (b) Multiplexed quantum dot barcode assay with gated flow cytometry detection; different barcoded NPs capture individual biomolecules (31–33). (c) Isolation of paramagnetic NPs with specifically bound biomolecules destined for further investigation (34). (d) Theranostic organic NPs are loaded with API, and related DNA barcodes are applied to cancerous tissue. Treated tissue is extracted, and the efficacy of the different APIs is tested with help of the individual DNA barcodes (35). Abbreviations: API, active pharmaceutical ingredient; CL, control line; FACS, fluorescence activated cell sorting; IVD, in vitro diagnostics; NP, nanoparticle; TL, test line.
1.4. Current Landscape of Standards for Nanoenabled Medical Products
Innovation and development in the area of nanoenabled drug products, medical devices, and IVDs are growing steadily (16, 33). To facilitate the continued global development and efficient translation of nanoenabled medical products from the bench to the clinic and finally to the marketplace, consensus documentary standards that are recognized by regulatory authorities and accessible to product manufacturers/developers should be readily available (1, 3, 4, 34, 35). Availability of these standards (e.g., test methods, guides) will not only benefit nanoenabled medical product manufacturers but will also provide benefits to the entire healthcare enterprise by allowing entities such as big pharma to concentrate on drug innovation. Importantly, standards will enable more rapid and efficient regulatory decision making. Consensus documentary standards in such critical areas as nanotechnology terminology, ENM physicochemical characterization, methods for identifying and quantifying nano-bio effects/responses, methods for evaluating the safety of preclinical products, and general guidance documents, as well as fit-for-purpose reference materials, are needed to properly characterize the quality attributes of increasingly complex nanoenabled medical products.
After an extensive review of the literature (1, 3, 4, 34, 35), including the recent report from the Global Summit on Regulatory Science (GSRS16), “Nanotechnology standards and applications” (36), as well as searching the websites of the International Organization for Standardization (ISO) and ASTM International, we prepared a list (Supplemental Table 1) of the available documentary standards and regulatory guidelines that are most relevant for characterizing the quality attributes of nanoenabled medical products. There are many general nanotechnology-relevant standards, regulatory guidance documents, and publicly available protocols (4, 36), but not all of these standards and protocols address nanotechnology application areas directly related to nanoenabled medical products. Supplemental Table 1 shows that only a limited number of published documentary standards and general guidelines are highly applicable to nanoenabled medical products. Supplemental Table 2 provides a summary of published guidelines that are product specific; however, these guidelines only apply to nanoenabled drug products. There exist several published standards that define nanotechnology and nanoscale terminology that are both useful and important for nanoenabled medical products, but the debate over the true definition of nanomaterial continues (37). There are many standards devoted to the characterization of physicochemical properties represented in Supplemental Table 1. But it is noteworthy that many emerging standards are in various stages of preparation for the specific characterization of biological responses. More than half of the standards in this table are classified as “In Development,” which indicates that relevant standards development organizations, industry regulators, and industrial stakeholders recognize that standards for nanoenabled medical products are a priority investment (25, 38). Standards that are applicable to in vitro safety evaluation of nanoenabled medical products are sparse in number and, notably, no existing reference materials are currently available for benchmarking biological responses from or for characterizing nanoenabled medical products (4). It is also recognized that there are few reference materials available for characterizing ENM properties other than size. Supplemental Table 1 shows that both the US government and the European Commission have established regulatory guidelines that are pertinent for the manufacture and use of nanoenabled medical products, but gaps remain and are discussed further.
2. EMERGING NANOENABLED DRUG PRODUCTS
In the last two decades, governments across the world have recognized the potential benefits of nanoenabled medical products and have generously funded their research and development. Examples include the National Nanotechnology Initiative of the US National Institutes of Health (39) and dedicated calls within the 7th EU Framework Program and Horizon 2020 of the European Union (40). This financial support stimulated cooperation across the nanotechnology and medicine sectors and enabled the formulation of new concepts and materials for therapeutic applications. In some cases, nanoscience terminology (Supplemental Table 1) was also adopted to rebrand some existing formulations, the most famous example being liposome technology, whose first product, Doxil, was approved by the FDA in 1995. Other technologies that allowed nanoenabled drug products to enter the market are based on the use of PEGylated proteins and polypeptides, polymers, protein-drug conjugates, surfactant formulations, nanocrystals, virosomes, and metal-based NPs (41). As far as progress in research and development goes, the last 20 years have also seen a steady increase in nanoenabled drug product publications and patents worldwide (8). Analysis of these publications reveals that drug approval by regulatory authorities triggers significant research efforts in, for example, using the same formulation for new drugs or using the same drug to validate other nanoenabled drug products (42). However, this analysis also shows that barriers to commercialization of approved drugs that are reformulated in ENM carriers are significant. The increase in API performance, for example, may not be large enough for pharmaceutical companies to justify the financial investment. ENM drug carriers, in fact, add complexity to the new nanoenabled drug product candidates, which may translate into significant analytical and regulatory challenges. In this respect, the availability of consensus documentary standards with practical guidelines for the analysis of specific classes of nanoenabled drug products may pave the way to the commercialization of many new products that are still at the development stage. For products that are already commercially available, similar guidelines are needed for regulatory approval of generics.
In this challenging landscape, research and development efforts should focus on those unmet medical needs where nanoenabled drug products provide a unique therapeutic opportunity (40). Supplemental Table 3 shows the leading causes of deaths in developed countries according to the World Health Organization and provides examples of such opportunities. Among these leading causes of death, cancer is increasing its burden in low- and medium-income countries and is projected to account for two-thirds of all cases of mortality worldwide by 2050 (43). The following examples of emerging nanoenabled drug products illustrate and highlight some unique therapeutic opportunities delivered by nanotechnology platforms.
2.1. Ultrafine Bubbles
Fine bubbles are conventionally used as contrast agents in ultrasound imaging (44). FDA-approved contrast agents include Optison, Definity, and Imagent. According to ISO 20480–1:2017, fine bubbles have a diameter below 100 μM. They can be held in place by surface tension or be surrounded with a coating, such as phospholipid or albumin, and can contain air or another gas. Therapeutic agents are used in conjunction with fine bubbles to increase the uptake of the agents into cells by a phenomenon called sonoporation. Importantly, fine bubbles have been shown to open the blood-brain barrier (45). Fine bubbles can also be formulated to carry therapeutic agents through engineering of their membranes. This approach to drug delivery offers the ability to follow the circulation of drug carriers with ultrasound and also trigger the release (through bursting of the membranes) of therapeutic payloads to surrounding tissue. Ultrafine bubbles are a class of fine bubbles with sizes below 1 μm and they are also sometimes referred to as nanobubbles. Research has shown that their performance as contrast agents for ultrasound imaging is similar to conventional fine bubble products (46). Ultrafine bubbles are extremely stable because of their very low buoyancy. This enables them to remain suspended in liquids for extended periods of time, providing a larger window of opportunity for imaging and delivery; for example, 6 to 8 minutes versus 1 to 2 minutes with conventional fine bubbles (47).
Potential use of ultrafine bubbles for a range of industrial applications has recently prompted the development of relevant documentary standards. Currently these cover the sample preparation via dispersion in water (ISO 20298-1:2018) and their storage and transportation (ISO 21255:2018). These standards underpin the ability to perform reproducible measurements. Characteristics of interest are the bubble size, number concentration, and stability, among other physical and chemical properties. However, no documentary standards exist to support the development and application of ultrafine bubbles in therapeutics, representing a gap in the international standards framework that needs to be addressed in the near future.
2.2. Exosomes
Exosomes are a class of nanoscale extracellular vesicles that are produced from exocytosis of multivesicular bodies found in most eukaryotic cells (48). They can be ingested by target cells and have been shown to transfer biological signals between local or distant cells (49). Exosomes are involved in both physiological and pathological processes and for this reason they have been exploited as health status biomarkers. They have also been engineered for therapeutic intervention, for example, the delivery of small and high molecular weight therapeutic agents, including doxorubicin and siRNA (50). With respect to synthetic nanoenabled drug products, an exosome’s biological surface features are similar to the biological features on cell membranes. This similarity makes exosomes less likely to elicit potential toxic or immunogenic responses, while boosting their ability to target and penetrate specific organs. Furthermore, exosomes can cross biological barriers, including the blood-brain barrier, and transfer their contents through cell membranes in order to deliver their cargo in a biologically active form.
In 2018, 35 clinical trials investigated the relationship between cancer and exosomes, a third of which were focused on therapeutic use of exosomes (51). Although the market for exosome-based diagnostics is rapidly increasing, lack of regulatory guidelines is delaying the equivalent development of exosome-based therapeutic products. One of the major bottlenecks in developing exosome-based formulations is their low yield per cell, which directly impacts the final production cost and the development of clinical applications. This is coupled with the difficulty of isolating exosomes from complex biological matrices such as blood, urine, and cerebrospinal fluid. A further challenge is the lack of established strategies for exosome drug loading, while preserving their integrity, surface molecular makeup, and biological activity. The industrial use of exosomes also has challenges concerning their manufacturing and regulatory approval, including the need for robust guidelines for testing their purity, sterility, potency, and identity of manufacturing lots, as well as the need to characterize the in vivo toxicological profiles (52).
Exosomes have typical sizes ranging between 30 nm and 150 nm. However, this range is not unique to this class of vesicles, and there is currently no consensus on markers that distinguish origins of extracellular vesicles. Isolation strategies consist of differential ultracentrifugation-based techniques, size-based techniques, immunoaffinity capture-based techniques, exosome precipitation, and microfluidics-based techniques, all having different levels of maturity (53). The standard morphological and size characterization method is electron microscopy aided by negative staining (54), but other methods have also been employed (55). Although commercial interest in these types of materials is rapidly increasing (51), the complexity of their biological environment and their significant heterogeneity pose substantial barriers to development of an analytical methodology that can be used to adequately evaluate and characterize the quality attributes of exosome-based drug products. The Society for Extracellular Vesicles is addressing this gap by publishing guidelines on the minimal information required for studies of extracellular vesicles, which covers their terminology, preparation, analysis, and use (56). This effort is supported by the development of commercially available test materials for the control of the experimental procedures.
2.3. Virus-Based Drug Products
In nature, viruses are designed to insert their genetic information into mammalian cells. For this reason, biomedical engineers have tried to exploit them for gene therapy since the early 1980s (57). Recently, the application of nanoscience and nanotechnology in combination with viral engineering has facilitated testing the utility of viruses in innovative therapeutic products, such as vaccines and drug products. To date, different forms of virus-based drug products have been engineered (58), including virosomes (59), virus-like particles (60), and fully synthetic nanoenabled drug products that can mimic the functions of viruses (61). Viral delivery strategies for genomeediting systems have been developed to treat, for example, genetic diseases, with many currently in clinical trials and the first gene therapy product based on viral gene-transfer technology receiving marketing approval in Europe in 2012. The global gene therapy market value is estimated by Roots Analysts to exceed $10 billion by 2025. However, the translation of clinical development into licensed product requires viral vector product manufacturing to overcome several challenges, including increasing production volume while maintaining rigorous manufacturing practices (e.g., consistency and quality). Furthermore, there is a desire from industrial partners to meet Good Manufacturing Practice compliance at earlier stages of clinical development, which adds to the complexity of product development. Viral delivery vectors (mainly retroviruses, adenoviruses, and adeno-associated viruses) are used to protect the nucleic acid or protein cargo via encapsulation and to target specific cells for delivery of the desired payload. However, the same viral systems can also induce long-term transgene expression in humans with a single injection, raising serious safety concerns (62). Delivery vectors based on nonviral materials have the potential to be less toxic and immunogenic but present their own set of delivery challenges.
Virus-like particles are self-assembled, virus-derived structural antigens and have been exploited for the development of vaccines, such as for hepatitis B viruses and AIDS. Virosomes are their in vitro–reconstituted counterpart. Not dissimilar from liposomes in terms of lipid composition, they incorporate viral membrane proteins. These materials are noninfectious and nonreplicating particles and are therefore a safer option for the development of vaccines and nanoenabled drug products. Industrial adoption of these materials for manufacturing purposes requires the development of robust analytics at different stages of production, but previous work has mainly focused on demonstrating the immunogenicity of the materials. Analytical testing challenges and the need for regulatory guidelines and documentary standards are similar to requirements discussed previously for ultrafine bubbles, exosomes, and nanoenabled drug products in general. To harness the potential of these emerging drug delivery vectors in drug product development and manufacturing, documentary standards that can be used to effectively characterize the physicochemical properties and biological responses of these vectors under preclinical conditions require careful consideration and discussion among the relevant biopharmaceutical, governmental, and academic stakeholders.
3. EMERGING NANOENABLED MEDICAL DEVICES
Development of nanoenabled medical devices is being revolutionized as the fields of microfluidics, novel nanomaterials, nanomanufacturing, and microsensors with increasing sensitivity are accelerating. Use of nanotechnology within medical device development has not only resulted in the miniaturization of implantable product architecture, but has also led to the advent of next-generation multifunctional disease detection and monitoring systems that are smaller, require lower power management, and support prolonged implantation, owing to the unique physicochemical attributes offered by ENMs. This is therefore leading to significant advances in personalized POC diagnostics, implantable continuous monitoring systems, tissue replacements, and increasingly sophisticated orthopedic implant materials such as bone cements and electronic devices to better support clinical management of patients with a range of diseases (15, 63, 64).
3.1. Implantable Nanoelectronic Sensing Systems
Nanoenabled implantable devices offer a wide range of clinical benefits, including provision of detectors that undertake clinical tasks in a more cost-effective and rapid manner than standard methods. For example, diabetes monitoring can be enhanced through use of implantable glucose monitoring devices that are inserted either under the skin or intramuscularly and communicate with an external primary transmitter, supporting in vivo, real-time, continuous analysis (63). Aside from glucose detection, similar nanoelectronic sensing systems are also being developed to measure other biological molecules that represent important clinical parameters to remotely monitor disease progression and response to therapy, including oxygen, ions, proteins, and antibodies (65). These implantable systems function as a virtual alarm that is activated when the concentration of the moiety under analysis increases above or drops below a specified acceptable range. A key benefit of such monitoring systems is that they provide continuous data on the patient during their normal day-to-day life, allowing more comprehensive data sets to be generated without the need for hospital visits. Not only can this approach better support more accurate prognosis and patient clinical management to reduce healthcare costs, but it also offers patients an improved quality of life. While there are clear healthcare benefits, these novel devices present challenges with respect to regulatory approval processes, as there is a recognized shortage of documentary standards (test methods) for the ENM components in these implantable devices that needs to be urgently addressed.
3.2. Nanoenabled Medical Devices
One function of ENMs currently being explored in relation to novel nanoenabled medical devices is their use in antibacterial surface coatings and prevention of blood platelet adhesion to the surface of the implant. These coatings are necessary for the reduction of bacterial colonization and biofilm formation, which is of particular importance for dental and orthopedic implantology (63, 66–68). Biofouling can not only induce an inflammatory response but can also result in damage to surrounding tissues and mechanical dysfunction of the implanted device; this is therefore a key challenge to overcome when considering the necessity for developing products that can remain in the body for decades without failure to avoid the need for repeated surgeries. AgNPs are particularly beneficial for this purpose, as they offer prolonged antibacterial action and have been used to coat a variety of medical devices, including neurosurgical shunts, venous catheters, and cardiovascular implants. Furthermore, other ENMs such as gold, copper, titanium, and palladium NPs have also demonstrated strong antimicrobial properties suitable for use in long-term health care applications (66, 68–71). ENMs also offer other beneficial properties for implant products, including high tensile strength coupled to low weight and high conductivity that support the development of low-voltage and low-power electronics. Thus, materials such as silicone dioxide NPs are being utilized in composite materials for dental restoration purposes, while injectable bone-filling products are being fabricated from hydroxyapatite NPs, and bone cements have incorporated the use of CNTs (72). Titanium dioxide coated with functionalized CNTs is also promising as a novel material with great potential for orthopedic implantable electronic devices owing to its highly conductive properties (64). These examples therefore demonstrate how nanotechnology is applied to enhance biocompatibility and promote long-term stability of the medical device, in addition to advancing their functional properties. However, benefits provided by incorporation of nanotechnology into these novel devices are also the root cause of difficulties in regulatory approvals. It is widely accepted that ENMs cannot be treated in the same way as chemicals with respect to hazard assessment. Thus, current standards need to be adapted to address the additional considerations required to understand the biological impact of potential ENM exposure following the application of nanoenabled medical devices.
3.3. Regulatory Approval Processes for Emerging Nanoenabled Medical Devices
Given the aging population globally, which is associated with increased chronic degenerative disease, heavy burdens are being placed on the global healthcare systems. The potential to remotely monitor patients in real time that is offered by disruptive advances in medical device development based on nanotechnology is therefore highly attractive. However, the market has not grown as rapidly as earlier forecasts in which an annual growth of $2.4–24 billion has been predicted (73). Slow development of products is thought to be due, in part, to extensive, high-cost approval processes, regulatory uncertainty surrounding use of ENMs, and gaps in the availability of relevant documentary standards for nanoenabled medical devices (Supplemental Table 1).
Medical devices are typically categorized into one of three classes with respect to human safety evaluation and according to their intended use, which dictates subsequent risk assessment requirements to achieve regulatory approval (Supplemental Table 4). However, incorporation of ENMs into medical devices creates a challenge for safety evaluation such that specific characteristics of the ENMs used in the products need to be considered. In 2011, the European Commission released a definition of nanomaterials valid for all marketed goods (74). Although this definition is not legally binding, it is being increasingly applied as a reference in EU policy documents relating to nanotechnology. Hence, this definition has been utilized in recently updated regulations on medical devices, which now have specific requirements for nanoenabled products destined for medical healthcare purposes (75, 76). Additionally, all medical devices incorporating or consisting of ENMs have been classified as (Supplemental Table 4):
Class IIA if they present a negligible potential for internal exposure;
Class IIB if they present a low potential for internal exposure; and
Class III if they present a high or medium potential for internal exposure.
In support of the updated EU Regulation on Medical Devices, another important document that has been released by the Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) is Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices (15). This addresses the important safety assessment considerations that should be included when evaluating potential human health hazards associated with nanoenabled implantable devices (Supplemental Table 1). This recent guidance has imposed more stringent classification of medical devices with ENM components as a result of increased risk of introducing ENMs into the body. The FDA has also implemented adapted guidance for the regulatory approval of nanoenabled medical devices, which in many aspects is similar to the European approach (2, 22). Manufacturers now need to characterize the physicochemical characteristics of the ENMs incorporated into their products. Standards have recently become available to assist with this effort, such as the ISO/TR 10993–22:2017 “Biological evaluation of medical devices: Part 22: Guidance on nanomaterials” (Supplemental Table 1). This was introduced in 2017 into the ISO 10993 series of standards for evaluating the biocompatibility of medical devices and describes considerations for the biological evaluation of medical devices that are composed of or contain ENMs. The toxicological profile of ENMs within medical devices according to route of exposure and exposure time needs to be established and also provide data on the life cycle of the implant, including the potential for ENM release following wear and tear during the standard life span of the device.
It is, however, important to note that fundamental measurement challenges exist that complicate robust safety analysis of nanoenabled medical devices, as guidance documents to support this level of analysis are not available. To fully characterize the long-term stability and life cycle of nanoenabled medical devices following implantation, highly sensitive analytical technologies are required to evaluate the state of ENMs over time. This includes measurement of parameters such as ENM degradation embedded within a complex biological matrix and establishing release of free ENMs and/or release of ions over extended periods of time in the human body. Currently, such analytical tools with sufficient sensitivity and standard test methods are lacking and consequently, extensive biological evaluation is required, the first stage of which involves evaluating potential risks arising from release of ENMs from the implanted device when under use (15). This level of investigation needs to provide data leading to an understanding of what ENMs are released and what impact they would likely have on the sites of exposure (local effects). If there is evidence of ENM release from the implanted device, the next stage is to establish the biodistribution and biopersistence of any released ENMs. Toxicokinetic studies would therefore be important to establish which organs the ENMs reach and whether they are retained for extended periods of time in those organs or excreted. However, it is important to note that analytical tools, as well as standard test methods, to support nano-biodistribution studies are also lacking. The most effective means of tracking ENM localization in the body is through radio labeling, but this would not be suitable for evaluating low-level release of materials directly from an implanted medical device. There are, however, ongoing efforts by the Organisation for Economic Co-operation and Development (OECD) and ISO to create standards for ENM biodistribution studies under the current Working Party on Manufactured Nanomaterials and ISO/TC 229 Program of Work, respectively. Once the appropriate data sets as described above have been collated, a hazard profile of the released ENMs, based on exposure levels and organs in which they are retained and accumulate, would need to be produced and compiled for risk characterization (15, 72). The hazard profile of the released ENMs and other technical issues (see Supplemental Materials) need to be considered to allow nanoenabled medical devices to reach their commercial potential.
4. EMERGING NANOENABLED IN VITRO DIAGNOSTICS: ASSAYS AND DEVICES
Use of QDs (77, 78), AuNPs (79), and/or superparamagnetic ENMs has helped to substantially increase the sensitivity of existing IVDs and allowed them to attain the next level of performance. These types of nanoenabled IVDs are conjugated with specific binding moieties such as antibodies or oligonucleotides for the selective detection of health status biomarkers and pathogens. Various types of immunoassays, immunohistochemistry platforms, cellular imaging reagents, systems for separating specific cell populations, and DNA diagnostics now rely heavily on the use of inorganic ENMs.
4.1. Typical Development Process for In Vitro Diagnostics Products
Development of new nanoenabled in vitro assays or devices typically begins by first using simulated samples to determine the ability of the assay to detect relevant biomarkers in spiked samples through quantitative determinations of analytical sensitivity, limit of detection, linear dynamic range and selectivity, and any potential indications of cross-reactivity or interferences. Next, the in vitro assay is tested using a small number of clinical samples (<10 patient samples) to evaluate the performance of the in vitro assay under real-world conditions. However, these sample numbers are not sufficient to determine reliable false positive/false negative rates for the in vitro assay. Before advancing beyond basic research objectives, a full clinical evaluation of the in vitro assay (>50 patient samples) should be performed to obtain clinically relevant assay sensitivity and analyte specificity values. To date, a very limited number of studies at the clinical validation step have been published as compared to relatively large numbers of published studies on evaluating simulated samples (representing the initial step of method development). Consequently, the clinical performance capabilities of numerous nanoenabled IVD assays are still unknown (80).
4.2. Novel Development Trends in the In Vitro Diagnostics Field
Microscopy- and spectroscopy-based assays/techniques have also benefited from recent technological developments in nanoenabled IVDs. The unique physicochemical properties of ENMs have enabled microscopy- and spectroscopy-based assays to achieve new analytical specifications in terms of ultrahigh spatial and molecular resolution in combination with ultrahigh sensitivity (16, 81). This trend could lead to improved knowledge in many medical areas and might further stimulate the creation of advanced in situ and ex vivo diagnostics tools. For example, the detection of circulating tumor cells (CTCs), which are found in physiological fluids following release from primary tumors or metastatic tumors, have drawn a great deal of attention for the evaluation of cancer dissemination. Sensitive detection of CTCs via application of a novel nanoenabled IVD assay would be a potential beneficial alternative to invasive biopsies that require subsequent proteomic and functional genetic analyses. Isolation of CTCs from human fluids necessitates elaborate analytic procedures that often result in low yields and impure samples. Recently, biosensor-based strategies for the rapid detection of CTCs were developed using specific antibodies labeled with AuNPs and/or magnetic beads in liquid suspensions (Figure 1b,c) (82, 83). Other new developments have focused on harnessing technological advances in the coupling of microfluidics with nanoscale materials to enable high-purity collection and downstream functional characterization of CTCs. Examples include capture and subsequent release of CTCs for whole genome sequencing and RNA sequencing.
Development of novel theragnostic ENMs for personalized cancer medicine appear to be taking a similar path. With this emerging technological advancement, the diagnostic test is performed with the help of a multidrug screening assay precisely inside the tumor of interest, followed by extraction of biological activity data at the single-cell level. A recent study by Yaari et al. (84) described the use of liposomes loaded with different small-molecule cancer drugs and specific synthetic DNA barcodes as an example of an emerging theragnostic application. In this tumorcell-targeting study, a cocktail of DNA barcode-labeled and drug-carrying NPs was injected intravenously. Each API deployed its therapeutic mode of action inside different cells located mainly at the tumor site. After 2 days, a tissue biopsy was taken from the tumor site and dissected. Subsequent measurements revealed a correlation with cell viability, which was determined as a ratio between live and dead cells, the different APIs, and the correspondingly coloaded synthetic DNA barcodes. This correlation hints at the therapeutic potential of personalized anticancer medicines (84) and could foreshadow an emergent paradigm in personalized cancer treatment protocols (Figure 1d).
4.3. Primary Challenge for In Vitro Diagnostics: Qualitative or Semi-Quantitative Test Results?
One of the primary scientific challenges for nanoenabled IVDs, whether in the format of assays or devices, is that many of the diagnostic measurements are either qualitative or semiquantitative at best. This measurement reality is mostly driven by POC testing market forces. Many emerging nanoenabled IVDs are geared toward assays and devices that are rapid, cost-efficient, and portable so that tests for disease biomarkers and/or infectious pathogens can be conducted with relative ease either in doctors’ offices or in remote locations around the globe. Many of the remote locations are economically challenged, so it is of prime socioeconomic importance to develop IVDs that can obtain rapid yes/no results when a doctor cannot be physically present to interpret critical clinical results. Qualitative test methods comprise both identification and yes/no (confirmation) information. They also have distinctly different functional characteristics in comparison to classical quantitative test methods in physics/chemistry and are primarily utilized in the biological and medical fields. The nature of qualitative test methods is characterized by their binary behavior: presence/absence, positive sample/negative sample, or a yes/no response according to a preset threshold. There are basically two types of qualitative tests. The first type of qualitative test method deals with the selective identification, thus binding, of biomolecules, whereas the second type is referred to as sample classification. This second type has the objective of providing a rapid and reliable classification of the samples on the grounds of previously established criteria (e.g., a threshold value imposed by regulators) (85). These qualitative screening methods must be validated against more elaborate and hence higher-quality confirmatory reference methods, which themselves provide quantitative results and are therefore metrologically traceable to appropriate reference materials.
To unify the performance criteria for these different types of qualitative test methods, it is necessary to introduce a performance function for characterizing both qualitative and semiquantitative methods; in turn, this performance function must be correlated to quantitative methodology (86). False negative rates, false positive rates, sensitivity, and specificity are key characteristics of screening methods that can be determined from the pertinent performance curves. The performance characteristics of each method are related to the uncertainty region that is associated with each method, and the applicable uncertainty regions can be gleaned from the performance curves (87). A Eurachem position paper stresses the importance of assessing the risk of incorrect classification in qualitative test methods, moreover providing information on the risk of incorrect results (88). In this publication, the authors relate the terms dealing with false response rates (false-positive rates and false-negative rates) with the Bayes’ theorem. Moreover, estimation of false response rates often requires an extremely large number of tests to determine indicative values. As a potential solution, the authors propose the adaptation of a semiquantitative reporting system, which is based on the weight of evidence scale: indication, strong indication, and very strong indication.
In short, several first-generation nanoenabled IVDs that are based on simple ENM probes have received regulatory approval and are now commercially available. In stark contrast to this situation, many of the more complex and ambitious, second-generation IVD concepts remain ensconced in academically oriented research projects (16, 33). The reasons for this reality are multifold, and the complete lack of appropriate nanoenabled medical product reference materials (Supplemental Table 1) does not lead to easy solutions. The translational path seems to be quite laborious with the need to probe real-world clinical samples using the new nanoenabled IVD assays and devices under development combined with the requirement to compare the obtained performance results to the results of already established quantitative diagnostic methods. According to statistical considerations (86–88), the qualitative or semiquantitative nature of many of the newly developed nanoenabled IVDs inhibits the relative determination of the false-positive and false-negative rates, which also increases the number of required clinical samples that must be tested. This situation might improve with the development and release of IVD-focused documentary standards that are currently under development in ISO TC229 (89).
5. FOUNDATIONS OF MEASUREMENT ASSURANCE FOR NANOENABLED MEDICAL PRODUCTS
Consensus documentary standards for nanoenabled medical products require input of stakeholders from industry (e.g., ENM manufacturers, drug product and medical device manufacturers), academia (e.g., subject area experts) and government (e.g., staff scientists, regulators) during their development. The formal process of developing high-quality documentary standards (e.g., test methods, practices, and guides) also requires a diversity of technical opinions that reflect an accurate understanding of the measurement strategies that must be employed in the development of robust standards. One overarching goal in the development of, for example, a standard test method that describes an in vitro assay for measuring a specific biological response induced by a nanoenabled drug product, is to produce a test method in which there is a high level of measurement confidence (Figure 2). Over the course of many years, scientists at the National Institute of Standards and Technology (NIST) have developed and described a robust framework and enabling strategies for achieving confidence in measurements that are critical for manufacturing cell therapy products and for regenerative medicine applications (90–94). These same cell therapy measurement assurance strategies can be applied to the development of nanoenabled medical products. The ever-increasing complexity of nanoenabled medical products has stimulated the need for high-quality, robust, and validated measurements of quality attributes that can ensure manufacturing quality, safety, and efficacy. The required physicochemical and biological properties data demand increased levels of measurement confidence in order to facilitate efficient regulatory decision making and reliable manufacturing. Strategies for achieving measurement confidence in a measurement process entail understanding and reducing the measurement uncertainty at each step of the process (90–95). This is a foundational tenet of analytical method development. When this strategy is applied to the development of assays for characterizing the quality attributes of nanoenabled medical products, achieving good measurement accuracy, precision (repeatability, reproducibility), robustness, dynamic range, and limit of detection as well as properly incorporating in-process controls become essential (90–94, 96). Understanding and mitigating the sources of uncertainty in a measurement process will allow a routine analytical protocol (e.g., standard operating procedure) to evolve into a high-quality standard test method (documentary standard). Some measurement assurance tools that can be utilized to achieve measurement confidence in data that are generated by assays for nanoenabled medical products include use of cause and effect diagrams, design of experiments (DOE), in-process controls and performance specifications, interlaboratory comparisons, and reference materials (94, 97) (Figure 2). Cause and effect (Ishikawa) diagrams are graphical depictions of the steps in the measurement process that are potential sources of measurement variability (98–100). Sensitivity testing can be conducted on the steps that have been identified to be likely sources of variability to determine the level of variance contributed by each step to the assay result. DOE is a multifactorial statistical design method to determine how different steps (factors) of the measurement process affect each other (multiple experimental factors are studied at the same time). In-process controls are experimental controls at intermediate, critical steps in a measurement process that verify that the measurement process is functioning properly (usually linked to assay performance specifications). An interlaboratory comparison exercise is a tool that is implemented to characterize the performance and robustness of the assay among different laboratories. Interlaboratory comparison is usually conducted as one of the last steps in the measurement assurance process and enables an accounting of the assay precision and bias (with the use of an appropriate reference material if available) (97). These various tools work in tandem to reduce the uncertainty in the data generated by the assays; however, there is no one tool that is perfect for achieving measurement assurance in all circumstances (94).
Figure 2.
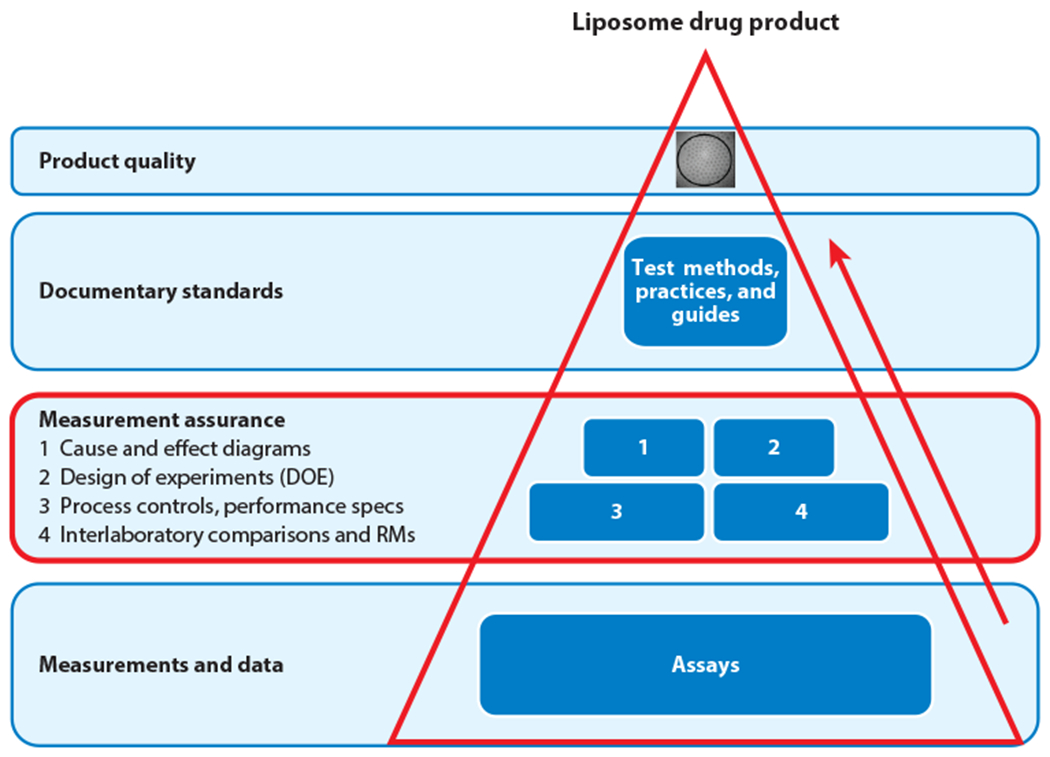
Process for achieving measurement assurance in a routine assay. This process enables the conversion of a routine in vitro assay for evaluating the quality attributes of a typical nanoenabled drug product (e.g., liposomal drug product) into a high-quality test method (documentary standard). Note that box 3 employs the use of multiple measurement assurance tools. Abbreviation: RM, reference material.
One final point concerns the need to ensure metrological traceability of the analytical results obtained via the measurement process. The measurand and the quantity of the measurand should be clearly defined, with final reported results based on an unbroken chain of calibrations that utilize calibration, reference, and international standards linked to the International System of Units (SI). Achieving metrological traceability for biological measurements is often more challenging than for chemical measurements because the measurand is often ill defined, and the biological property that is being measured is defined by the measurement. Nevertheless, prudent application of selected tools (Figure 2) during refinement and validation of the measurement process, in combination with measurement result traceability, will ensure that high-quality assays and recognized consensus documentary standards are produced.
6. FUTURE PERSPECTIVE
A substantial increase in both inter- and multidisciplinary interactions has occurred in recent years. These are vital to allowing cross-fertilization between scientific disciplines, which give rise to disruptive technological advances. In order to continue to rapidly advance the next generation of nanoenabled medical products, collaborations uniting health care professionals, bioengineers, biophysicists, chemists, materials scientists, and toxicologists are critical to ensuring that all aspects of scientific knowledge are included at an early stage. This process is essential to developing the necessary precompetitive test methods and documentary standards that will accelerate product pipelines. However, it is clear that uncertainty surrounding ENM safety has resulted in regulatory approaches that are causing prohibitive challenges in application of nanotechnology for advancing nanoenabled medical product development. It is important to strike a balance between effective regulation, while still supporting innovation and protecting public health; thus, continued open dialogue is needed among risk assessment groups, regulatory bodies, and standards development organizations, together with industrial stakeholders developing nanoenabled medical products, to minimize innovation bottlenecks.
Supplementary Material
ACKNOWLEDGMENTS
We thank John Elliott (NIST) for helpful discussions and presentations on measurement assurance and the importance of quality measurements.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that may be perceived as affecting objectivity of this review. Certain commercial equipment, instruments, and materials are identified in this article in order to specify an experimental procedure as completely as possible. In no case does identification of particular equipment or materials imply a recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that these materials, instruments, or equipment are necessarily the best available for a given purpose.
LITERATURE CITED
- 1.Hoffmann SB, Halamoda-Kenzaoui B, Borgos SE. 2018. Identification of regulatory needs for nanomedicines. J. Interdiscip. Nanomed. 3(1):4–15 [Google Scholar]
- 2.Van Norman GA. 2016. Drugs, devices, and the FDA: part 2. An overview of approval processes: FDA approval of medical devices. JACC Basic Transl. Sci. 1(4):277–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coty JB, Vauthier C 2018. Characterization of nanomedicines: a reflection on a field under construction needed for clinical translation success. J. Control. Release 275:254–68 [DOI] [PubMed] [Google Scholar]
- 4.Halamoda-Kenzaoui B, Holzwarth U, Roebben G, Bogni A, Bremer-Hoffmann S. 2019. Mapping of the available standards against the regulatory needs for nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 11(1):e1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain RK, Stylianopoulos T. 2010. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 7(11):653–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Mello SR, Cruz CN, Chen ML,Kapoor M,Lee SL,Tyner KM. 2017.The evolving landscape of drug products containing nanomaterials in the United States. Nat. Nanotechnol. 12(6):523–29 [DOI] [PubMed] [Google Scholar]
- 7.Noorlander CW, Kooi MW, Oomen AG, Park MVDZ, Vandebriel RJ, Geertsma RE. 2015. Horizon scan of nanomedicinal products. Nanomedicine 10(10):1599–608 [DOI] [PubMed] [Google Scholar]
- 8.Wagner V, Dullaart A, Bock AK, Zweck A. 2006. The emerging nanomedicine landscape. Nat. Biotechnol. 24(10):1211–17 [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Kantoff PW, Wooster R, Farokhzad OC. 2017. Cancer nanomedicine: progress, challenges and opportunities. Nat. Rev. Cancer 17(1):20–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etheridge ML, Campbell SA, Erdman AG, Haynes CL, Wolf SM, McCullough J. 2013. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomed. Nanotechnol. Biol. Med. 9(1):1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Petrochenko P, Chen L, Wong SY, Absar M, et al. 2016. Core size determination and structural characterization of intravenous iron complexes by cryogenic transmission electron microscopy. Int. J. Pharmaceut. 505(1-2):167–74 [DOI] [PubMed] [Google Scholar]
- 12.Editorial. 2018. A triumph of perseverance over interference. Nat. Biotechnol. 36(9):775. [DOI] [PubMed] [Google Scholar]
- 13.Anselmo AC, Mitragotri S. 2016. Nanoparticles in the clinic. Bioeng. Transl. Med. 1(1):10–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR. 2016. Nanoparticle-based medicines: a review of FDA-approved materials and clinical trials to date. Pharm. Res. 33(10):2373–87 [DOI] [PubMed] [Google Scholar]
- 15.Eur. Comm. 2015. Scientific Committee on Emerging and Newly Identified Health Risks SCENIHR Opinion on the Guidance on the Determination of Potential Health Effects of Nanomaterials Used in Medical Devices. Luxembourg: Eur. Comm. https://ec.europa.eu/health/scientific_committees/emerging/docs/scenihr_o_045.pdf [Google Scholar]
- 16.Pelaz B, Alexiou CH, Alvarez-Puebla RA, Alves F, Andrews AM, et al. 2017. Diverse applications of nanomedicine. ACS Nano 11(3):2313—81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furno F, Morley KS, Wong B, Sharp BL, Arnold PL, et al. 2004. Silver nanoparticles and polymeric medical devices: a new approach to prevention of infection? J. Antimicrob. Chemoth. 54(6):1019–24 [DOI] [PubMed] [Google Scholar]
- 18.Zhou ZG, Sun YA, Shen JC, Wei J, Yu C, et al. 2014. Iron/iron oxide core/shell nanoparticles for magnetic targeting MRI and near-infrared photothermal therapy. Biomaterials 35(26):7470–78 [DOI] [PubMed] [Google Scholar]
- 19.Dong ZH, Li YB, Zou Q. 2009. Degradation and biocompatibility of porous nano-hydroxyapatite/polyurethane composite scaffold for bone tissue engineering. Appl. Surf. Sci. 255(12):6087–91 [Google Scholar]
- 20.Sun J, Petersen EJ, Watson SS, Sims CM, Kassman A, et al. 2017. Biophysical characterization of functionalized titania nanoparticles and their application in dental adhesives. Acta Biomater. 535:85–97 [DOI] [PubMed] [Google Scholar]
- 21.Ge LP, Li QT, Wang M, Ouyang J, Li XJ, Xing MMQ. 2014. Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int. J. Nanomed. 92:399–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones AD 3rd, Mi G, Webster TJ. 2019. A status report on FDA approval of medical devices containing nanostructured materials. Trends Biotechnol. 37(2):117–20 [DOI] [PubMed] [Google Scholar]
- 23.Geertsma RE, Park MVDZ, Puts CF, Roszek B, van der Stijl R, de Jong WH. 2015. Nanotechnologies in medical devices. RIVM Rep. 2015-0149, Natl. Inst. Public Health Environ., Bilthoven, Neth. http://pdfs.semanticscholar.org/0ee9/49097779eaf41b0d944ecaea3ea7bd020af7.pdf [Google Scholar]
- 24.MarketandMarkets. 2015. Nanotechnology in medical devices market by product (biochip, implant materials, medical textiles, wound dressings, cardiac rhythm management devices, hearing aid), Application (therapeutic, diagnostic, research)—global forecast to 2019. Rep., MarketsandMarkets, Hadapsar, India. https://www.marketsandmarkets.com/PressReleases/nanotechnology-medical-device.asp [Google Scholar]
- 25.Baptista PV 2014. Nanodiagnostics: leaving the research lab to enter the clinics? Diagnosis 1(4):305–9 [DOI] [PubMed] [Google Scholar]
- 26.Commission Regulation 2017/746, Regulation of the European Parliament and of the Council of 5 April 2017 on in vitro diagnostic medical devices and repealing Directive 98/79/EC and Commission Decision 2010/227/EU, 2017 O.J. (L 117) 176 [Google Scholar]
- 27.Azzazy HM, Mansour MM. 2009. In vitro diagnostic prospects of nanoparticles. Clin. Chim. Acta 403(1–2):1–8 [DOI] [PubMed] [Google Scholar]
- 28.Azzazy HM, Mansour MM, Kazmierczak SC. 2006. Nanodiagnostics: a new frontier for clinical laboratory medicine. Clin. Chem. 52(7):1238–46 [DOI] [PubMed] [Google Scholar]
- 29.Jain KK. 2003. Nanodiagnostics: application of nanotechnology in molecular diagnostics. Expert. Rev. Mol. Diagn. 3(2):153–61 [DOI] [PubMed] [Google Scholar]
- 30.Cordeiro M, Carlos FF, Pedrosa P, Lopez A, Baptista PV. 2016. Gold nanoparticles for diagnostics:advances towards points of care. Diagnostics 6(4):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou W, Gao X, Liu DB, Chen XY. 2015. Gold nanoparticles for in vitro diagnostics. Chem. Rev. 115(19):10575–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nam JM, Thaxton CS, Mirkin CA. 2003. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 301(5641):1884–86 [DOI] [PubMed] [Google Scholar]
- 33.Alvarez MM, Aizenberg J, Analoui M, Andrews AM, Bisker G, et al. 2017. Emerging trends in micro- and nanoscale technologies in medicine: from basic discoveries to translation. ACS Nano 11(6):5195–214 [DOI] [PubMed] [Google Scholar]
- 34.Gioria S, Caputo F, Urbán P, Maguire CM, Bremer-Hoffmann S, et al. 2018. Are existing standard methods suitable for the evaluation of nanomedicines: some case studies. Nanomedicine 13(5):539–54 [DOI] [PubMed] [Google Scholar]
- 35.Halamoda-Kenzaoui B, Baconnier S, Bastogne T, Bazile D, Boisseau P, et al. 2019. Bridging communities in the field of nanomedicine. Regul. Toxicol. Pharmacol. 106:187–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glob. Summit Regul. Sci. 2016. 2016 Global Summit on Regulatory Science (GSRS16): nanotechnology standards and applications. Rep., Sept., Glob. Summit Regul. Sci. https://www.astm.org/COMMIT/GSRS16%20Final%20Report.pdf
- 37.Miernicki M, Hofmann T, Eisenberger I, von der Kammer F, Praetorius A. 2019. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat. Nanotechnol. 14(3):208–16 [DOI] [PubMed] [Google Scholar]
- 38.Leong HS, Butler KS, Brinker CJ, Azzawi M, Conlan S, et al. 2019. On the issue of transparency and reproducibility in nanomedicine. Nat. Nanotechnol. 14(7):629–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Natl. Res. Counc. 2001. Preliminary Comments. Review of the National Nanotechnology Initiative. Washington, DC: Natl. Acad. Press. 10.17226/10216 [DOI] [PubMed] [Google Scholar]
- 40.EuroNanoMed2/ETP Nanomedicine. 2016. Strategic research and innovation agenda for nanomedicine 2016–2030. Rep., EuroNanoMed2/ETP Nanomedicine, Paris. https://etp-nanomedicine.eu/wp-content/uploads/2018/09/Nanomedicine-SRIA-2016-2030.pdf [Google Scholar]
- 41.Weissig V, Pettinger TK,Murdock N. 2014. Nanopharmaceuticals (part 1): products on the market. Int. J. Nanomed. 94:357–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Venditto VJ, Szoka FC Jr. 2013. Cancer nanomedicines: so many papers and so few drugs! Adv. Drug Deliv. Rev. 65(1):80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bray F, Moller B. 2006. Predicting the future burden of cancer. Nat. Rev. Cancer 6(1):63–74 [DOI] [PubMed] [Google Scholar]
- 44.Blomley MJK, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. 2001. Science, medicine, and the future—microbubble contrast agents: a new era in ultrasound. BMJ 322(7296):1222–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qin SP, Caskey CF, Ferrara KW. 2009. Ultrasound contrast microbubbles in imaging and therapy:physical principles and engineering. Phys. Med. Biol. 54(6):R27–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oeffinger BE, Wheatley MA. 2004. Development and characterization of a nano-scale contrast agent. Ultrasonics 42(1–9):343–47 [DOI] [PubMed] [Google Scholar]
- 47.Bing CC, Hong Y, Hernandez C, Rich M, Cheng BB, et al. 2018. Characterization of different bubble formulations for blood-brain barrier opening using a focused ultrasound system with acoustic feedback control. Sci. Rep. 8:7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He CJ, Zheng S, Luo Y, Wang B. 2018. Exosome theranostics: biology and translational medicine. Theranostics 8(1):237–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.György B, Szabó TG, Pásztói M, Pál Z, Misják P, et al. 2011.Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell. Mol. Life Sci. 68(16):2667–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El Andaloussi S Mäger I, Breakefield XO,Wood MJA. 2013.Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 12(5):348–58 [DOI] [PubMed] [Google Scholar]
- 51.Roy S, Hochberg FH,Jones PS. 2018. Extracellular vesicles: the growth as diagnostics and therapeutics; a survey. J. Extracell. Vesicles 7(1):1438720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Batrakova EV, Kim MS. 2016. Development and regulation of exosome-based therapy products. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 8(5):744–57 [DOI] [PubMed] [Google Scholar]
- 53.Li P, Kaslan M, Lee SH, Yao J, Gao Z. 2017. Progress in exosome isolation techniques. Theranostics 7(3):789–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Höög JL.Lötvall J 2015. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 4(1):28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szatanek R, Baj-Krzyworzeka M,Zimoch J, Lekka M, Siedlar M, Baran J. 2017. The methods of choice for extracellular vesicles (EVs) characterization. Int. J. Mol. Sci. 18(6):1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, et al. 2018. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7(1):1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mercola KE, Stang HD, Browne J, Salser W, Cline MJ. 1980. Insertion of a new gene of viral origin into bone-marrow cells of mice. Science 208(4447):1033–35 [DOI] [PubMed] [Google Scholar]
- 58.Mateu MG. 2016. Assembly, engineering and applications of virus-based protein nanoparticles. In Protein-Based Engineered Nanostructures. Advances in Experimental Medicine and Biology, ed. Cortajarena A, Grove T, pp. 83–120. Cham, Switz.: Springer; [DOI] [PubMed] [Google Scholar]
- 59.Felnerova D, Viret JF, Glück R, Moser C. 2004. Liposomes and virosomes as delivery systems for antigens, nucleic acids and drugs. Curr. Opin. Biotech. 15(6):518–29 [DOI] [PubMed] [Google Scholar]
- 60.Ludwig C, Wagner R. 2007. Virus-like particles—universal molecular toolboxes. Curr. Opin. Biotech. 18(6):537—45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zuber G, Dauty E, Nothisen M, Belguise P, Behr JP. 2001. Towards synthetic viruses. Adv. Drug Deliv. Rev. 52(3):245–53 [DOI] [PubMed] [Google Scholar]
- 62.Bankiewicz KS, Forsayeth J, Eberling JL, Sanchez-Pernaute R, Pivirotto P, et al. 2006. Long-term clinical improvement in MPTP-lesioned primates after gene therapy with AAV-hAADC. Mol. Ther. 14(4):564–70 [DOI] [PubMed] [Google Scholar]
- 63.Juanola-Feliu E, Colomer-Farrarons J, Miribel-Catala P, Samitier J, Valls-Pasola J. 2012. Market challenges facing academic research in commercializing nanoenabled implantable devices for in-vivo biomedical analysis. Technovation 32(3–4):193–204 [Google Scholar]
- 64.Przekora A, Benko A, Nocun M, Wyrwa J, Blazewicz M, Ginalska G. 2014. Titanium coated with functionalized carbon nanotubes—a promising novel material for biomedical application as an implantable orthopaedic electronic device. Mater. Sci. Eng. C 45:287–96 [DOI] [PubMed] [Google Scholar]
- 65.Juanola-Feliu E, Miribel-Catala PL, Aviles CP, Colomer-Farrarons J, Gonzalez-Pinero M, Samitier J. 2014. Design of a customized multipurpose nanoenabled implantable system for in-vivo theranostics. Sensors 14(10):19275–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ferraris S, Spriano S. 2016. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 61:965–78 [DOI] [PubMed] [Google Scholar]
- 67.Marassi V, Di Cristo L, Smith SGJ, Ortelli S, Blosi M, et al. 2014. Silver nanoparticles as a medical device in healthcare settings: a five-step approach for candidate screening of coating agents. R. Soc. Open Sci. 5(1). 10.1098/rsos.171113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polivková M, Hubáček T, Staszek M, Švorčík V, Siegel J. 2017. Antimicrobial treatment of polymeric medical devices by silver nanomaterials and related technology. Int. J. Mol. Sci. 18(2):419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adams CP, Walker KA, Obare SO, Docherty KM. 2014. Size-dependent antimicrobial dffects of novel palladium nanoparticles. PLOS ONE 9(1):e85981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ben-Sasson M, Zodrow KR, Qi GG, Kang Y, Giannelis EP, Elimelech M. 2014. Surface functionalization of thin-film composite membranes with copper nanoparticles for antimicrobial surface properties. Environ. Sci. Technol. 48(1):384–93 [DOI] [PubMed] [Google Scholar]
- 71.Li XN, Robinson SM, Gupta A, Saha K, Jiang ZW, et al. 2014. Functional gold nanoparticles as potent antimicrobial agents against multi-drug-resistant bacteria. ACS Nano 8(10):10682–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Musazzi UM, Marini V, Casiraghi A, Minghetti P. 2017. Is the European regulatory framework sufficient to assure the safety of citizens using health products containing nanomaterials? Drug Discov. Today 22(6):870–82 [DOI] [PubMed] [Google Scholar]
- 73.Freedonia Group. 2010. Nanotechnology in health care. Ind. Study Rep. 2622, Freedonia Group, Cleveland, OH. https://www.freedoniagroup.com/industry-study/nanotechnology-in-health-care-2622.htm [Google Scholar]
- 74.Commission Recommendation 2011/696/EU, Recommendation of 18 October 2011 on the definition of nanomaterial, 2011 O.J. (L 275) 38 [Google Scholar]
- 75.Counc. Eur. Union. 2016. Proposal for a regulation of the European Parliament and of the council on medical devices, and amending Directive 2011/83/EC, Regulation (EC)No 178/2002 and Regulation (EC)No 1223/2009.Draft Rep., Eur. Parliam., Brussels. http://www.europarl.europa.eu/sides/getDoc.do?pubRef=-//EP//NONSGML+COMPARL+PE-510.767+01+DOC+PDF+V0//EN&language=EN [Google Scholar]
- 76.Commission Regulation 2017/745, Regulation of the European Parliament and of the Council on Medical Devices, and Amending Directive 2001/83/EC, Regulation (EC) No 178/2002 and Regulation (EC) No 1223/2009 and repealing Council Directives 90/385/EEC and 93/42/EEC, 2017 O.J. (L 117) 1 [Google Scholar]
- 77.Giri S, Sykes EA,Jennings TL, Chan WCW. 2011. Rapid screening of genetic biomarkers of infectious agents using quantum dot barcodes. ACS Nano 5(3):1580–87 [DOI] [PubMed] [Google Scholar]
- 78.Meng ZJ, Song RH, Chen Y, Zhu Y, Tian YH, et al.2013. Rapid screening and identification of dominant B cell epitopes of HBV surface antigen by quantum dot-based fluorescence polarization assay. Nanoscale Res. Lett. 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao ZQ, Ye HH, Tang DY, Tao J, Habibi S, et al. 2017. Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett. 17(9):5572–79 [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Biondi MJ, Feld JJ, Chan WCW. 2016. Clinical validation of quantum dot barcode diagnostic technology. ACS Nano 10(4):4742–53 [DOI] [PubMed] [Google Scholar]
- 81.Yan R, Moon S, Kenny SJ, Xu K. 2018. Spectrally resolved and functional super-resolution microscopy via ultrahigh-throughput single-molecule spectroscopy. Accounts Chem. Res. 51(3):697–705 [DOI] [PubMed] [Google Scholar]
- 82.Alix-Panabieres C, Pantel K. 2013. Circulating tumor cells: liquid biopsy of cancer. Clin. Chem. 59(1):110–18 [DOI] [PubMed] [Google Scholar]
- 83.Maltez-da Costa M, de la Escosura-Muniz A, Nogues C, Barrios L, Ibanez E,Merkoci A.2012. Simple monitoring of cancer cells using nanoparticles. Nano Lett. 12(8):4164–71 [DOI] [PubMed] [Google Scholar]
- 84.Yaari Z, da Silva D, Zinger A, Goldman E, Kajal A, et al. 2016. Theranostic barcoded nanoparticles for personalized cancer medicine. Nat. Commun. 7:13325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rios A, Barcelo D, Buydens L, Cardenas S, Heydorn K, et al. 2003. Quality assurance of qualitative analysis in the framework of the European project ‘MEQUALAN.’ Accredit. Qual. Assur. 8(2):68–77 [Google Scholar]
- 86.Song R, Schlecht PC, Ashley K. 2001. Field screening test methods: performance criteria and performance characteristics. J. Hazard Mater. 83(1–2):29–39 [DOI] [PubMed] [Google Scholar]
- 87.Pulido A, Ruisanchez I, Boque R, Ruis FX. 2003. Uncertainty of results in routine qualitative analysis. Trends Anal. Chem. 22(10):647–54 [Google Scholar]
- 88.Ellison SLR, Gregory S. 1998. Quantifying uncertainty in qualitative analysis. Analyst 123(5):1155–61 [Google Scholar]
- 89.Int. Organ. Stand. 2005. Program of Work and Status, Current ISO/TC 229 Program of Work (2/22/2019), WG 5 ISO/PWI.ISO/TC 229. Geneva: Int. Organ. Stand. https://www.iso.org/committee/381983.html [Google Scholar]
- 90.Lin-Gibson S, Sarkar S, Elliott J. 2018. Summary of the National Institute of Standards and Technology and US Food and Drug Administration cell counting workshop: sharing practices in cell counting measurements. Cytotherapy 20(6):785–95 [DOI] [PubMed] [Google Scholar]
- 91.Lin-Gibson S, Sarkar S, Elliott J, Plant A. 2016. Understanding and managing sources of variability in cell measurements. Cell Gene Ther. Insights 2(6):663–73 [Google Scholar]
- 92.Lin-Gibson S, Sarkar S, Ito Y. 2016. Defining quality attributes to enable measurement assurance for cell therapy products. Cytotherapy 18(10):1241–44 [DOI] [PubMed] [Google Scholar]
- 93.Sarkar S, Pierce L, Lin-Gibson S, Lund SP. 2019. Standards landscape in cell counting: implications for cell & gene therapy. Cell Gene Ther. Insights 5(1):117–31 [Google Scholar]
- 94.Simon CG, Lin-Gibson S, Elliott JT, Sarkar S, Plant AL. 2016. Strategies for achieving measurement assurance for cell therapy products. Stem Cells Transl. Med. 5(6):705–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Taverniers I, Van Bockstaele E, De Loose M. 2004. Trends in quality in the analytical laboratory. I. Traceability and measurement uncertainty of analytical results. Trend. Anal. Chem. 23(7):480–90 [Google Scholar]
- 96.Taverniers I, De Loose M, Van Bockstaele E. 2004. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal. Chem. 23(8):535–52 [Google Scholar]
- 97.Elliott JT, Rosslein M, Song NW, Toman B, Kinsner-Ovaskainen A, et al. 2017. Toward achieving harmonization in a nanocytotoxicity assay measurement through an interlaboratory comparison study. ALTEX 34(2):201–18 [DOI] [PubMed] [Google Scholar]
- 98.Hanna SK, Cooksey GA, Dong S, Nelson BC, Mao L, et al. 2016. Feasibility of using a standardized Caenorhabditis elegans toxicity test to assess nanomaterial toxicity. Environ. Sci. Nano 3(5):1080–89 [Google Scholar]
- 99.Rösslein M, Elliott JT, Salit M, Petersen EJ, Hirsch C, et al. 2015. Use of cause-and-effect analysis to design a high-quality nanocytotoxicology assay. Chem. Res. Toxicol. 28(1):21–30 [DOI] [PubMed] [Google Scholar]
- 100.Scanlan LD, Lund SP, Coskun SH, Hanna SK, Johnson ME, et al. 2018. Counting Caenorhabditis elegans:protocol optimization and applications for population growth and toxicity studies in liquid medium. Sci. Rep. 8:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


