Abstract
Exposure to ozone has been linked to reproductive outcomes, including preterm birth. In this systematic review, we summarize published epidemiologic cohort and case-control studies examining ozone exposures (estimated on a continuous scale) in early pregnancy (1st and 2nd trimesters (T1, T2)) and preterm birth using ratio measures, and perform a meta-analysis to evaluate the potential relationship between them. Studies were identified by searching PubMed and Web of Science, screened according to predefined inclusion/exclusion criteria, and evaluated for study quality. We extracted study data including effect estimates, confidence limits, study location, study years, ozone exposure assessment method, and mean or median ozone concentrations. Nineteen studies were identified and included, of which 18 examined T1 exposure (17 reported effect estimates), and 15 examined T2 exposure. Random effects meta-analysis was performed in the metafor package, R 3.5.3. The pooled OR (95% CI) for a 10 ppb increase in ozone exposure in T1 was 1.06 (1.03, 1.10) with a 95% prediction interval of 0.95, 1.19; for T2 it was 1.05 (1.02, 1.08) with a 95% prediction interval of 0.95, 1.16. Effect estimates for both exposure periods showed high heterogeneity. In meta-regression analyses of study characteristics, study location (continent) explained some (~20%) heterogeneity for T1 exposure studies, but no characteristic explained a substantial amount of heterogeneity for T2 exposure studies. Increased ozone exposure during early pregnancy is associated with preterm birth across studies.
Keywords: Air Pollution, Ozone, Preterm Birth, Systematic Review, Meta-Analysis, Meta-Regression
Introduction
Preterm birth (PTB1), delivery that occurs before 37 weeks of completed gestation, is a marker for fetal underdevelopment and is related to subsequent adverse health outcomes, including high infant mortality and adverse developmental outcomes later in childhood (Behrman and Butler 2007). PTB is the leading cause of perinatal morbidity and mortality and second most common cause of death, after pneumonia, in children under 5 years of age (Lawn et al. 2010; Liu et al. 2016). In 2016, the proportion of births delivered preterm in the United States was 9.85% (Martin and Osterman 2018). PTB is characterized by multiple etiologies (spontaneous, premature rupture of membranes (PROM), or medically induced), which may have either separate or shared mechanistic pathways. Known risk factors for PTB include low socio-economic factors, age, race, substance abuse, tobacco use during pregnancy, poor nutritional status, and the presence of a birth defect (Goldenberg et al. 2008).
Ozone, one of the criteria pollutants regulated under the Clean Air Act, is created in the troposphere as a result of chemical reactions between nitrogen oxides and volatile organic compounds in the presence of heat and sunlight. Exposure to ozone is associated with a variety of health outcomes, such as respiratory effects (US Environmental Protection Agency 2020). Studies have also investigated if exposure to ozone during pregnancy could affect fetal growth and development.
There is a growing body of epidemiologic studies examining associations between ozone and adverse birth outcomes, including PTB. Findings for the relationship between ozone and PTB have been inconsistent; associations between exposure to ozone and PTB are generally elevated when exposure is averaged over the first or second trimesters of pregnancy, with less consistent associations reported for exposures estimated for the third trimester or averaged over the entire pregnancy period (US Environmental Protection Agency 2020).
To address this question of PTB related to ozone exposure, we conducted a systematic review and meta-analysis of studies examining associations between ozone exposure (measured on a continuous, rather than categorical, scale) during the first or second trimester of pregnancy and PTB. This systematic review uses the Population, Exposure, Comparison, Outcome, Study Design (PECOS) statement (Morgan et al. 2018) shown in Figure 1
Figure 1:

Population, Exposure, Comparator, Outcome, Study Design (PECOS) statement used to define scope of systematic review
Methods
Data sources and searches
We searched PubMed and Web of Science literature databases, without beginning time restriction through January 31, 2021, using combinations of keywords related to ozone and PTB. Keywords for exposure included “ozone” and “O3”, while keywords for the outcome included “preterm birth”, “preterm labor”, and “preterm delivery”. Exact search terms used to query each database are provided in supplemental materials. Studies not captured in our search but captured in the 2013 or 2020 Ozone Integrated Science Assessments (ISAs) (US Environmental Protection Agency 2013, 2020) were also included. In addition, the reference lists of included studies were screened for any potentially relevant studies not previously identified in this review.
Study selection
After duplicate studies were removed from the initial query results, two investigators independently performed title and abstract screening using SWIFT-ActiveScreener (SWIFT-AS). We excluded studies if they did not examine ozone as an exposure, did not examine PTB as an outcome, were not case-control or cohort design, were reviews or abstract-only, or were not English language. Studies with unclear designations were kept for full-text screening. Two investigators then performed a full text screen to retain studies assessing continuous ozone exposure during the 1st or 2nd trimesters (exposure period must have covered entire trimester).
Data Extraction
Data from included studies were extracted into an excel spreadsheet independently by two investigators. Extracted information included: study design, cohort or study name, study population details (e.g., registry or hospital based), study size, study location, timing of study, definition of PTB used, exposure assessment method, exposure timing, ozone averaging method (e.g., 8 hr-max, 24 hr avg, etc.), distribution of ozone concentrations, confounders examined, co-pollutant correlations, multi-pollutant models evaluated and with which co-pollutants, effect measure, exposure contrast/increment, effect estimates, upper and lower confidence bounds, and modifiers or sub-strata examined. We requested quantitative results from the authors when articles indicated that analyses were performed but were not reported in the manuscript or supplemental materials.
Study Quality Evaluation
Using a modified Office of Health Assessment and Translation (OHAT) framework (Rooney et al. 2014), we developed guidance for evaluating study quality across domains including participant selection, outcome, exposure, confounding, analysis, selective reporting, sensitivity, and overall quality. Quality levels for individual domains were “good”, “adequate”, “deficient”, and “critically deficient” for all domains except sensitivity, which had levels of “adequate” or “deficient” only. Overall quality was rated as “high confidence”, “medium confidence”, “low confidence”, or “uninformative”. Guidance tables are presented in supplemental materials. Each study was independently evaluated by two investigators, notes and decisions were extracted into the Health Assessment Workspace Collaborative (HAWC) tool (Shapiro et al. 2018), and any conflicts were resolved in group discussion.
Statistical Analysis
For the purposes of meta-analysis, a single effect estimate was selected from each study and standardized to an incremental increase in ozone exposure equal to 10 ppb; preferentially, we chose those estimates that covered the full preterm period for the definition of PTB (e.g., <37 completed weeks of gestation versus <32 weeks) and included the full study population. As few studies reported co-pollutant adjusted effect estimates, we used effect estimates from single pollutant models. Effect estimates were standardized to reflect an incremental increase of 10 ppb by dividing natural logged effect estimates (and confidence limits) by the original exposure increment reported in the manuscript, multiplying by 10 and exponentiating. We then performed meta-analysis, using both fixed and random effects methods, and estimated pooled odds ratios and 95% confidence intervals for trimester 1 and/or trimester 2 using the metafor package in R with RStudio (Allaire 2012; Team 2013; Viechtbauer 2010). When random effects models were used, 95% prediction intervals were also estimated for each trimester – these convey where we expect to see individual study results given the heterogeneity observed. Publication bias was assessed through funnel plots and Egger’s regression tests, and when appropriate, trim and fill methods were used to assess the potential impact of publication bias. I2 statistics were used to evaluate heterogeneity between studies, and when heterogeneity was statistically significant (α < 0.05), meta-regression was performed using study characteristic variables including: study location (country or continent), study design, study size (≥100,000 births, or 10,000 to <100,000 births), ozone averaging period (8-hr max, daily average, other), mean ozone concentration, start year, end year), study quality domains of participant selection, outcome ascertainment, exposure assessment, confounding, and analysis (all deficient, good, adequate), and overall study quality confidence score (low, medium, or high). Leave-one-out analyses were performed as sensitivities to identify potential outlier and influential studies.
Results - ozone and preterm birth systematic review and meta-analysis
Figure 2 presents the literature flow diagram for study inclusion. Our search of PubMed and Web of Science databases returned 155 studies, and 3 additional studies from the Ozone ISAs were added by investigators (Hao et al. 2016; Ritz et al. 2007; Wilhelm and Ritz 2005). In reviewing references of included studies, it was determined that any potentially relevant references had been identified during the initial screening process and excluded or included. In the abstract screening phase, we excluded 115 studies for either not meeting the criteria of examining ozone (n=13), not examining PTB (n=62), or study design outside of consideration (n=40); this left 43 studies. In the process of full text screening, 13 studies were excluded as not examining 1st or 2nd trimester ozone exposures, including Brauer et al. (2008) which was discovered to not meet inclusion criteria after consultation with authors. Five studies were excluded as they did not examine continuous exposure contrasts, and another five were excluded for population overlap or effects reported in another included paper (these were largely aggregated reports). This included two studies (Liang et al. 2019; Yang et al. 2020) which covered the same population and did not present independent effect estimates; the study that focused on subpopulation analysis Liang et al. (2019) was excluded. Twenty studies were identified for inclusion (G Chen et al. 2018; Chen et al. 2021; Ha et al. 2014; Hansen et al. 2006; Hao et al. 2016; Jalaludin et al. 2007; Lavigne et al. 2016; Lee et al. 2013; Lin et al. 2015; Liu et al. 2019; Olsson et al. 2012; Olsson et al. 2013; Qian et al. 2016; Smith et al. 2020; Sun et al. 2019; Wang et al. 2018; Warren et al. 2012; Wilhelm and Ritz 2005; Wu et al. 2011; Yang et al. 2020). Within this group, one study, Chen et al. (2021), was identified as an extreme outlier, and because of this it was removed from the main analysis and included in analyses reported in supplemental materials. Of the remaining 19 studies, 18 investigated 1st trimester exposures and were identified for inclusion in the systematic review for that trimester, and 15 were identified for inclusion in the systematic review for the 2nd trimester exposure. One study (Wilhelm and Ritz 2005) examined both 1st and 2nd trimester exposures, but only reported quantitative effect estimates for 2nd trimester exposures and was not included in the meta-analysis for 1st trimester. This left a total of 17 studies included in the 1st trimester meta-analysis and 15 studies included in the 2nd trimester meta-analysis. Study details and extracted results are shown in supplemental Table S.1.
Figure 2:
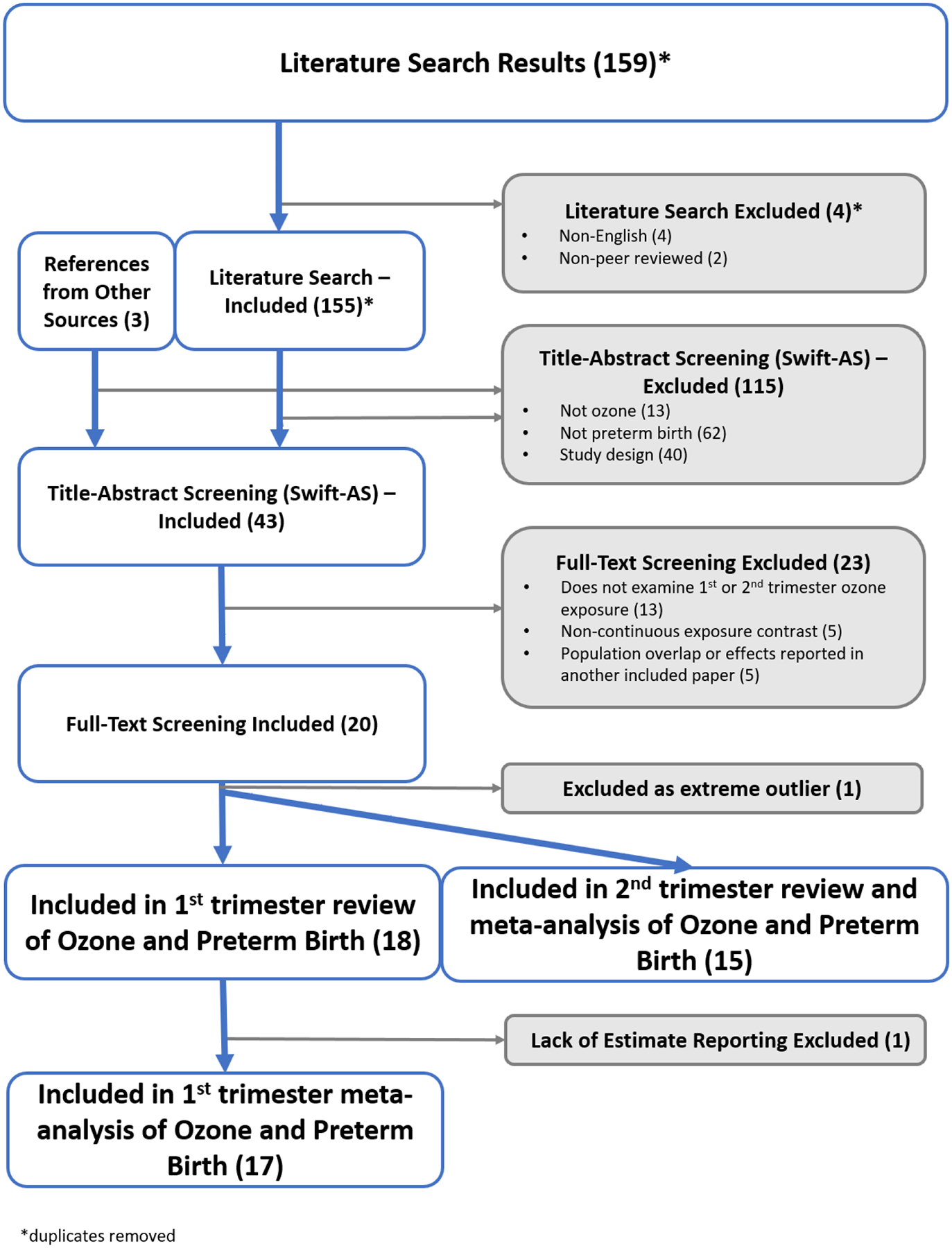
Flow chart of study inclusion for systematic review and meta-analysis of first or second trimester exposure to ozone and preterm
Included studies were primarily: cohort designs, with three case-control studies; estimated odds ratios (n = 15), with others estimating hazard ratios; had populations above 100,000 individuals (n = 12), followed by >10,000 – 100,000 (n=4); and used ozone concentration data from monitoring systems (n = 13) versus modeled concentrations (n = 6). There was large geographic variation with studies from several different countries and continents. The ozone metric used varied considerably across studies; nine studies used 8-hr daily maximum concentrations (i.e., the National Ambient Air Quality Standard (NAAQS) averaging time); however, four used 8-hr average concentration from 10am-6pm, four used daily average (i.e., 24-hr avg) concentration, one used a monthly predication (Smith et al. 2020) and one used daily 1-hr maximum concentration (Jalaludin et al. 2007). Reported mean ozone concentrations ranged from 17 ppb in Australia to 57 ppb in China. About half of the included studies reported some form of co-pollutant correlations with ozone, and six examined co-pollutant adjusted models (Ha et al. 2014; Olsson et al. 2012; Olsson et al. 2013; Warren et al. 2012; Wilhelm and Ritz 2005; Yang et al. 2020).
Study quality evaluation
A summary of study evaluations is presented in Figure 3 and additional details can be obtained by accessing the HAWC project page at https://hawcprd.epa.gov/assessment/100500027/. One study was ranked high confidence, with the rest being ranked medium (n=9) or low confidence (n=9) overall (Figure 3). In individual domains, deficient metric scores were often due to a lack of stated reasoning or underlying information within the text. Several lower scores were due to restrictions of the data, for example, exposure assessment scores were frequently lower when a single home residence from the time of birth was used for entire pregnancy exposure without knowledge of movement during pregnancy.
Figure 3:
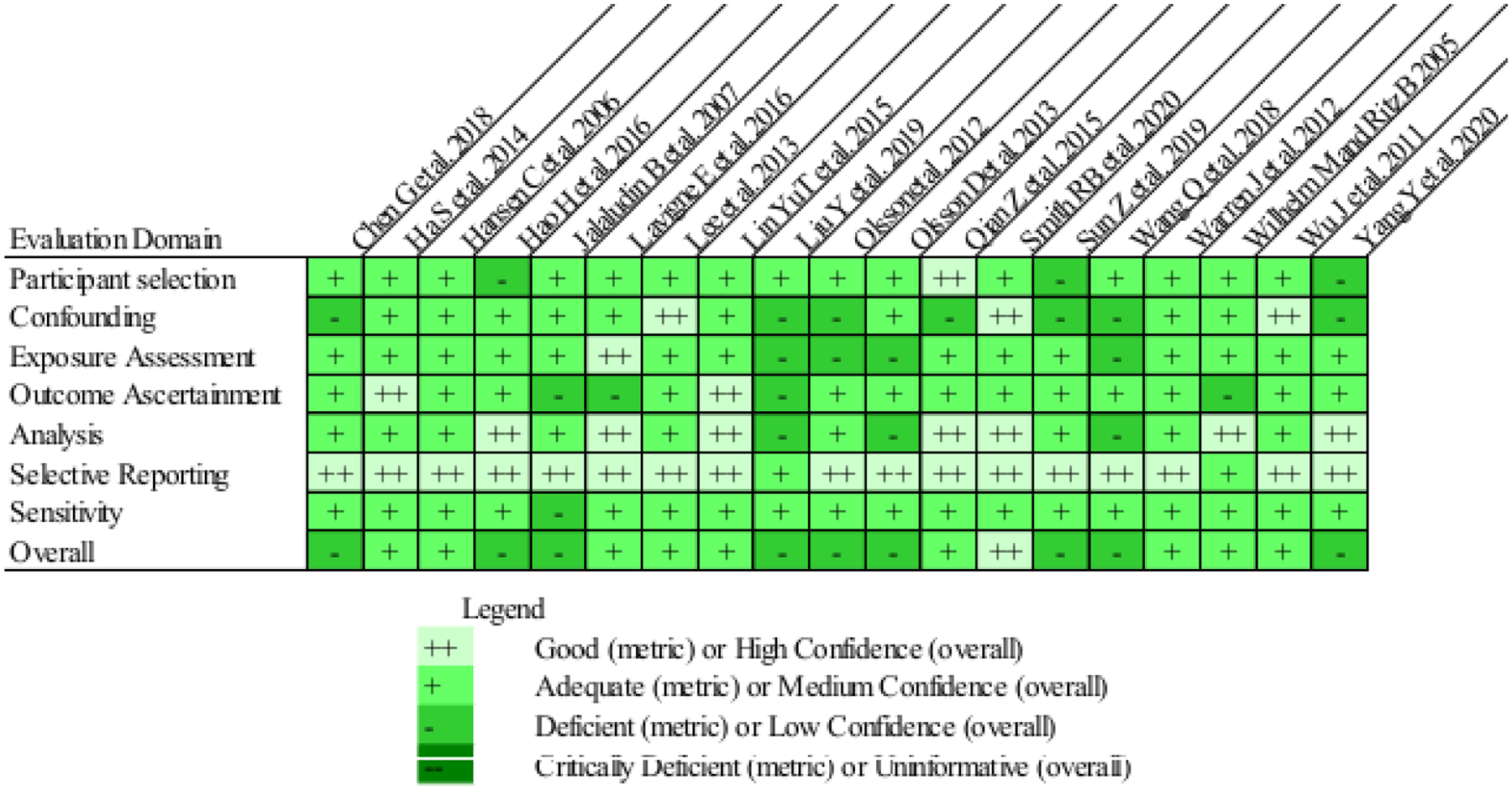
Study quality evaluation visualization showing metric scores for individual domains and confidence score for overall quality.
Meta-analysis trimester 1
In meta-analyses for 1st trimester ozone exposure (n=17), heterogeneity was determined to be present, with an I2 statistic of 97% and a Q-statistic p-value of <0.0001, therefore results from random effects models are presented. Effect estimates for 10 ppb increases in 1st trimester ozone exposure ranged from 0.79 to 1.38 in the 17 studies; the pooled odds ratio from the random effects model was 1.06 (1.03, 1.10) (Figure 4) with a prediction interval of 0.95 to 1.19. Examination of the funnel plot and Egger’s test (p<0.001) indicated the presence of potential publication bias, while a rank correlation test did not (p = 0.2); trim-and-fill analyses estimated three missing studies and resulted in a pooled odds ratio of 1.04 (1.00, 1.08) (Figure 5). In leave-one-out sensitivity analyses, pooled effect estimates ranged from 1.05 to 1.07 (Supplemental Figure S.1), indicating that no single study had a substantial influence on the pooled estimate. As Wu et al. (2011) reported estimates for both Los Angeles and Orange counties, we chose one (Los Angeles) effect estimate to include in the main analysis and performed a sensitivity analysis using the other to avoid double weighting the study. We observed no differences in the pooled estimate based on which county was included in our meta-analysis (not shown).
Figure 4:
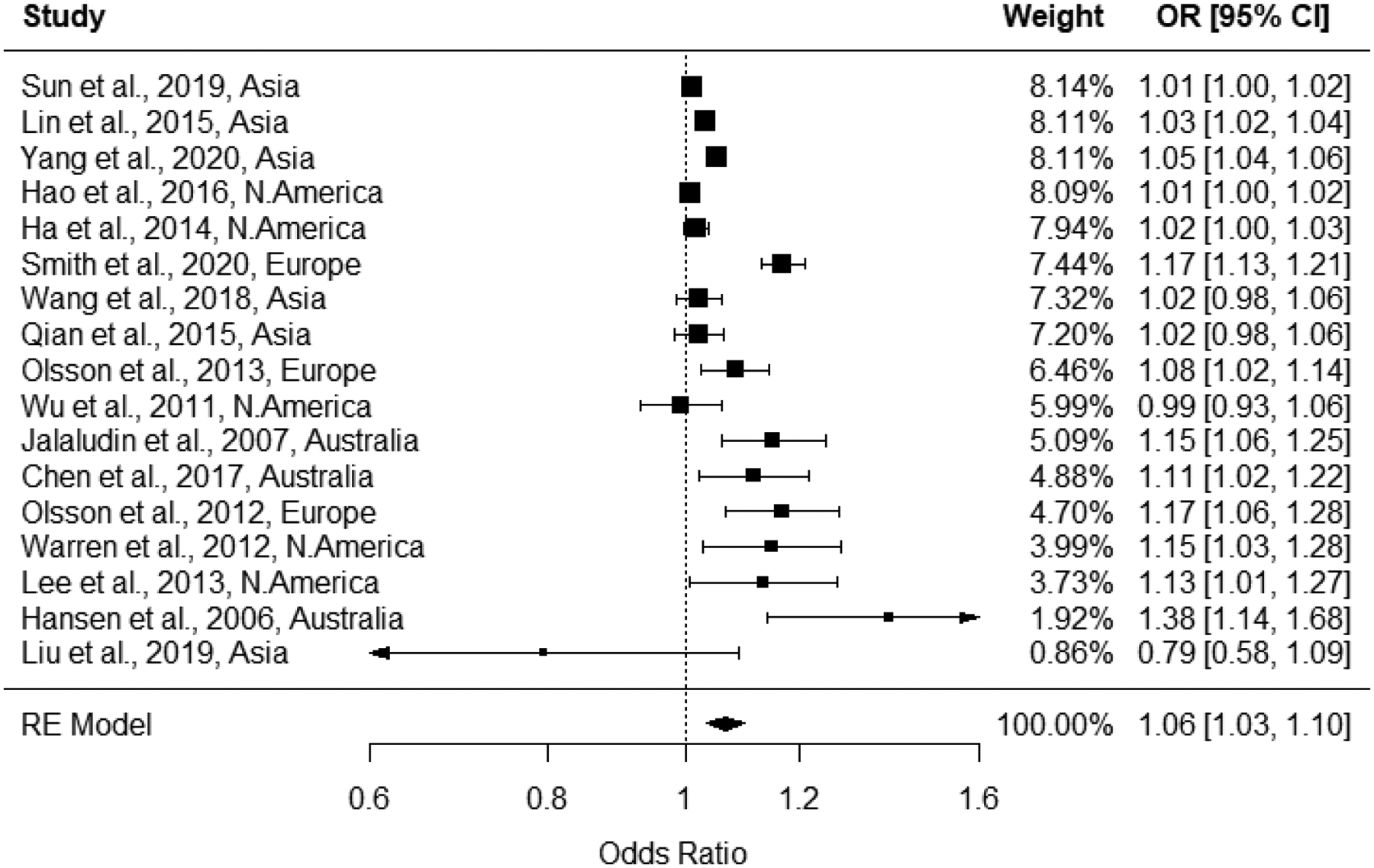
Forest plot for associations between 1st trimester ozone exposure and odds of preterm birth, with pooled odds ratio from random effects model.
Figure 5:
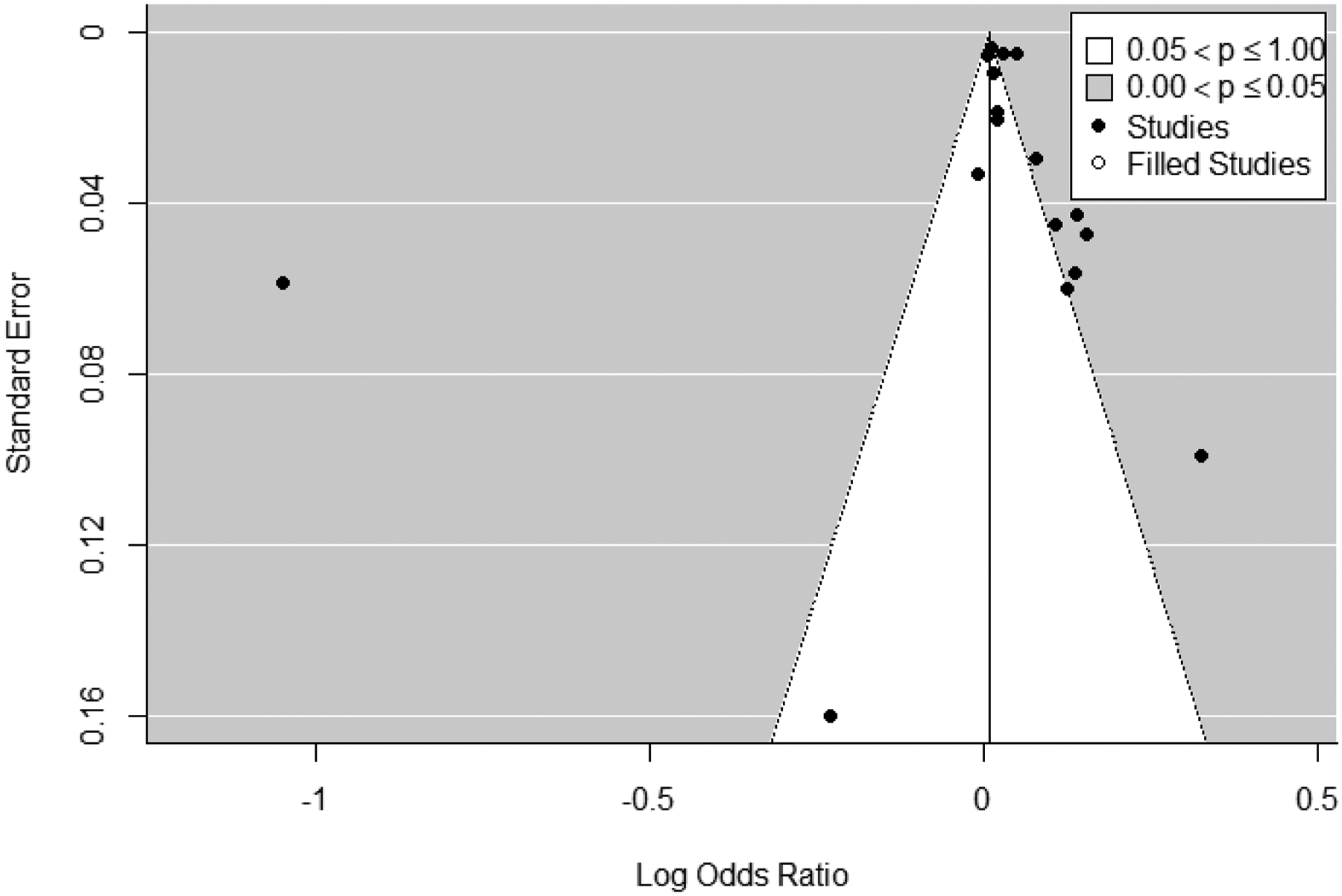
Trim and fill funnel plot for studies included in meta-analysis of associations between 1st trimester ozone exposure and odds of preterm birth. Actual studies are shown with black circles, while expected studies are white circles.
We conducted meta-regression that included potential explanatory variables: study location (continent), study design (cohort or case-control), study size (≥100,000 births, or 10,000 to <100,000 births), ozone averaging period (8-hr max, daily average, other), mean ozone concentration (continuous), study quality domains of participant selection, outcome ascertainment, exposure assessment, confounding, and analysis (all deficient, good, adequate), and overall study quality confidence score (low, medium, or high). Of these factors, only study location explained some heterogeneity for 1st trimester exposure associations; in meta-analysis modified by study location I2 was reduced to 74% (from 97%) (Figure 6). Location-specific pooled estimates ranged from 1.01 for the US to 1.15 for Australia (Table 1). Heterogeneity varied substantially within the continents, with Australian, and North American studies having low I2 values, while heterogeneity within Asian and European studies remained high (85% and 60%, respectively); note that the small number of studies on each continent may itself reduce heterogeneity.
Figure 6:
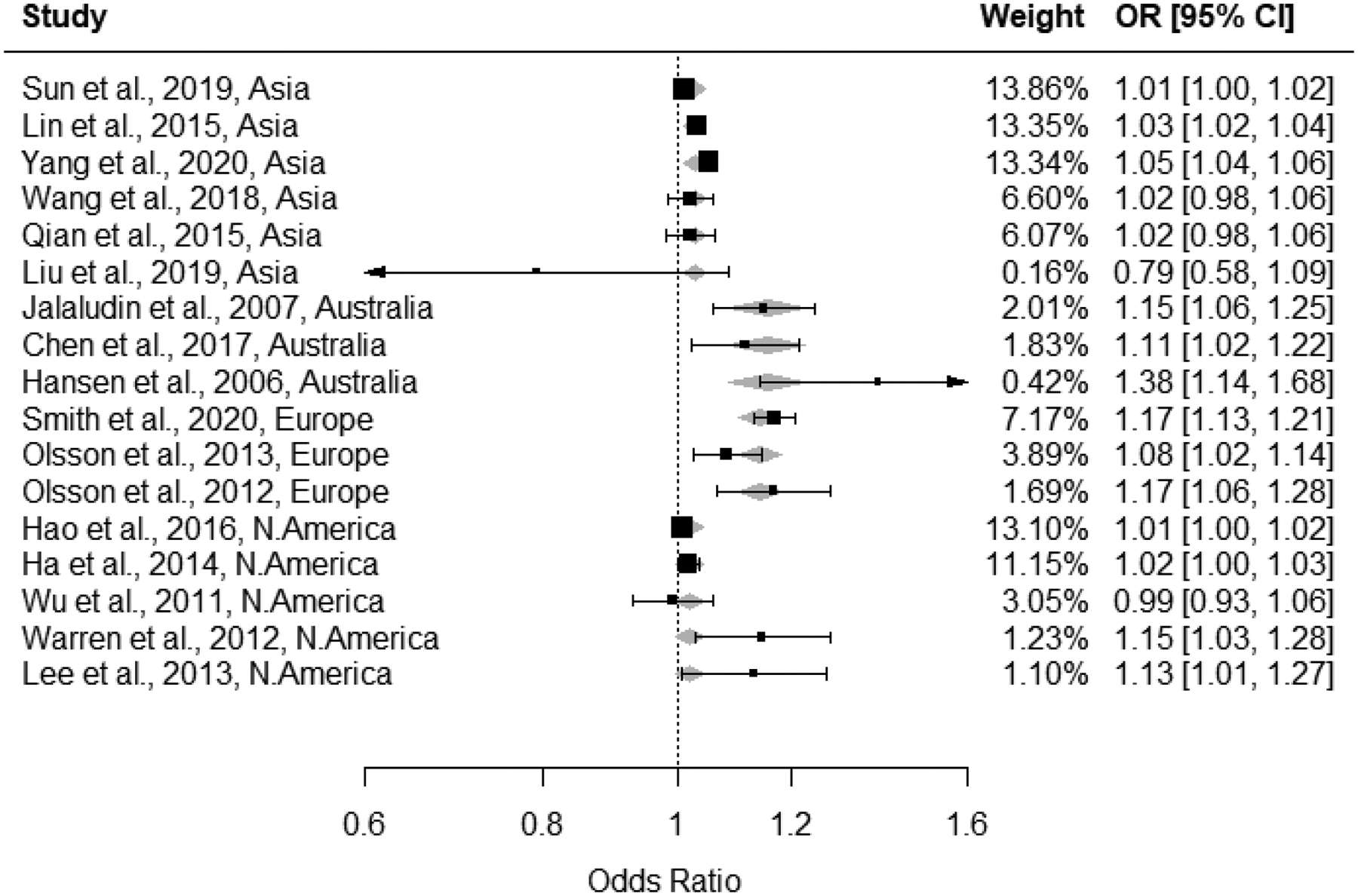
Forest plot for associations between 1st trimester ozone exposure and odds of preterm birth with modification by continent; continent-specific meta-estimates are shown with grey diamonds behind individual study effect estimates.
Table 1:
continent specific meta-effect estimates
| Continent | Continent-specific meta-effect | I2 |
|---|---|---|
| Australia | 1.15 (1.09, 1.22) | 0.24% |
| Asia | 1.03 (1.01, 1.04) | 84.58% |
| Europe | 1.14 (1.08, 1.20) | 60.39% |
| North America | 1.01 (1.00, 1.02) | 3.74% |
Meta-analysis trimester 2
All studies that examined 2nd trimester exposures were included in the 2nd trimester ozone exposure meta-analysis (n = 15), and heterogeneity was determined to be present, with an I2 statistic of 97% and a Q-statistic p-value of <0.001. Therefore, results from random effects models are presented for the 2nd trimester pooled estimate. Effect estimates for 10 ppb increases in 2nd trimester ozone exposure ranged from 0.87 to 1.38 in the 15 studies; the pooled estimate from the random effects model was 1.05 (1.02, 1.08) with a prediction interval of 0.95 to 1.16 (Figure 7). While the funnel plot appeared balanced (Figure 8), the Egger’s test (p<0.01) indicated evidence for potential publication bias, however trim-and-fill analysis estimated no missing studies and rank correlation testing was non-statistically significant (p=0.55). In leave-one-out sensitivity analyses, pooled effect estimates ranged from 1.04 to 1.06 (Supplemental Figure S.2), indicating that no single study had a substantial influence on the pooled estimate.
Figure 7:
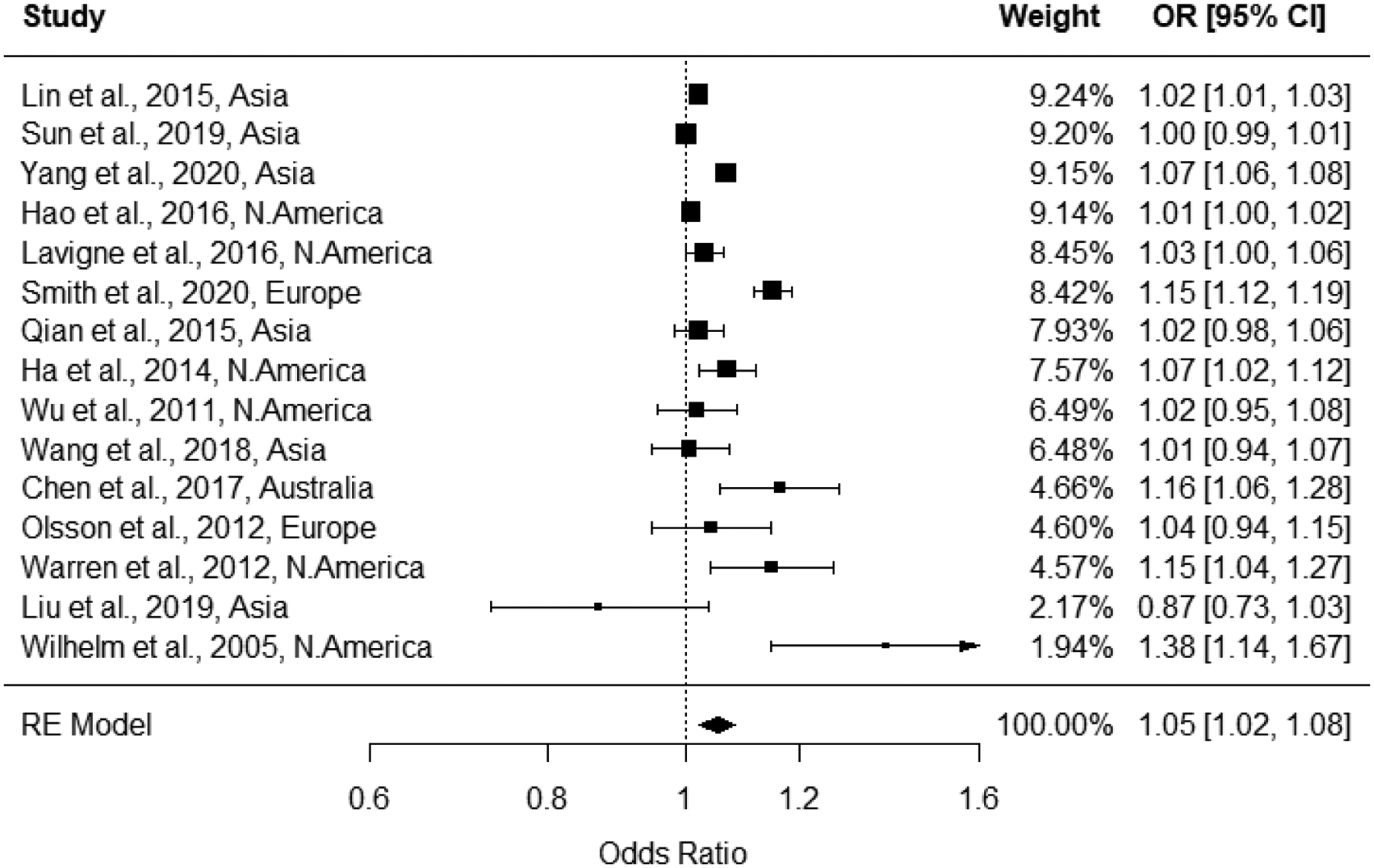
Forest plot for 2nd trimester ozone exposure and preterm birth associations, with pooled odds ratio from random effects model.
Figure 8:
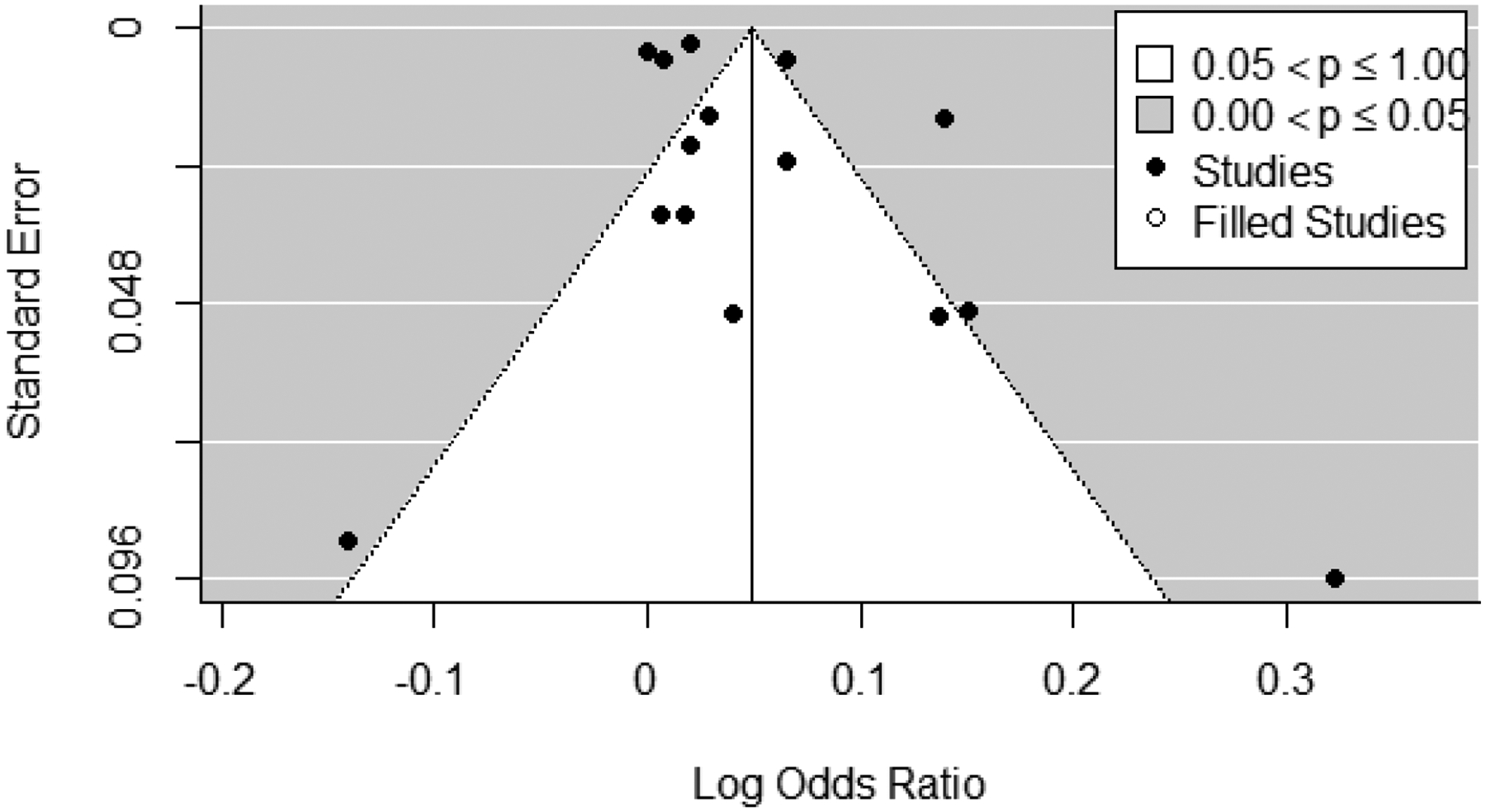
Trim and fill funnel plot for studies included in meta-analysis of associations between 2nd trimester ozone exposure and odds of preterm birth. Actual studies are shown with black circles, trim-and-fill analysis estimated no missing (i.e., “filled”) studies.
We conducted meta-regression that included potential explanatory variables: study location (continent), study design (cohort or case-control), study size (≥100,000 births, or 10,000 to <100,000 births), ozone averaging period (8-hr max, daily average, other), mean ozone concentration (continuous), study quality domains of participant selection, outcome ascertainment, exposure assessment, confounding, and analysis (all deficient, good, adequate), and overall study quality confidence score (low, medium, or high). In meta-regression analyses, study size (smaller studies tended to have lower magnitude of associations and larger studies higher) and mean ozone concentrations (studies with higher mean concentrations had lower magnitude of associations) were identified as potential contributors to heterogeneity, however neither reduced observed heterogeneity in meta-analyses when examined as modifiers. This was likely due to single studies in some modifier categories, as those technically had no within group heterogeneity.
Overall confidence
We also evaluated the overall confidence in the body of evidence (Table 2). We found no factors that were influential enough to decrease or increase overall confidence, leading to a final designation of moderate overall confidence. In general, we believe that in evaluating causality, inference should be drawn across multiple lines of evidence, for example across epidemiology, toxicology, and human exposure studies (when possible). While we rate our overall confidence here, it is important to note that this is for the studies included on the very specific topic of interest.
Table 2:
Evaluation of overall confidence in body of evidence
| Initial Confidence by Key Features of Study Design | Factors Increasing Confidence | Factors Decreasing Confidence | Confidence in the Body of the Evidence |
|---|---|---|---|
| Moderate Features: exposure occurs prior to outcome, individual outcome data, and comparison groups used | Risk of Bias – no studies were uninformative, half were considered low confidence, and half were medium to high (1) confidence (=) | Residual confounding – studies examining co-pollutant confounding did not observe an impact on observed associations. Not possible to evaluate if other residual confounding would bias toward or away from null without more information (=) | Moderate |
| Publication bias expected to have minimal impact (=) | Concentration response - not evaluated (=) | ||
| Indirectness – studies are of relevant populations and examine ambient exposures of interest (=) | Consistency – while there was substantial heterogeneity, associations are not so dissimilar to change interpretation, study design and population size did not contribute to heterogeneity (=) | ||
| Imprecision – confidence intervals are generally of reasonable to narrow range, except in very small study populations (=) | Magnitude of effect – magnitudes are small, as would be expected in epidemiology studies of environmental exposures and birth outcomes (=) |
Discussion
PTB is a public health concern because it is associated with high infant mortality and adverse developmental outcomes later in childhood. Previous reviews have concluded that short-term exposure to ozone during late pregnancy was consistently not associated with PTB and that associations with long-term exposures were inconsistent across studies, particularly across study locations (US Environmental Protection Agency 2013). In recent years, the number of studies examining ozone exposure and PTB has expanded greatly, with many focusing on exposures averaged over trimesters, and consistently report elevated associations for the 1st or 2nd trimester exposures (US Environmental Protection Agency 2020). To examine these associations in a systematic and transparent manner, we conducted a systematic review and meta-analysis of studies evaluating the relationship between ozone exposure during the 1st or 2nd trimester of pregnancy and PTB.
Generally, we observed associations elevated from the null between PTB and ozone concentrations averaged over the 1st or 2nd trimester of pregnancy. Pooled random-effects estimates from our meta-analysis were positive, though small in magnitude, when evaluating both 1st (1.06, 95% CI: 1.03, 1.10) and 2nd (1.05, 95% CI: 1.02, 1.08) trimester exposures. Correction for potential publication bias did not change our interpretation of the meta-analytic results for exposures during the 1st trimester. There appeared to be the potential for publication bias in the pooled estimate for exposures during the 2nd trimester, however trim-and-fill analysis estimated no missing studies and rank correlation testing was non-statistically significant (p=0.55). In addition to meta-analyses, we conducted meta-regression analyses to evaluate whether heterogeneity in the magnitude of the associations between ozone exposure and PTB could be explained by variability in covariates. The meta-regression indicated that a some of the variability in 1st trimester associations was explained by continent of study, though no factors explained the observed heterogeneity in associations between PTB and ozone exposure during the 2nd trimester. While the meta-regression identified continent as a source of heterogeneity among the 1st trimester effect estimates, Figure 6 and Table 1 demonstrate the consistent, positive associations observed across studies conducted in different continents. It is likely that the identified heterogeneity is due to variability in highly-powered studies reporting low-magnitude associations (i.e., <1.2; range 0.79–1.38).
An important assumption about the random effects models used is that effect estimates are taken from a series of sub-populations, and that differences between these populations, and structures within these populations, gives rise to observed heterogeneity. We identified study location as a source of heterogeneity in our meta-regression of 1st trimester ozone exposure and PTB. While there still appears to be some residual heterogeneity within continents, specifically studies occuring within Asian countries, this may be an artifact of large study samples and tight confidence intervals as actual point estimates are similar; specifically, we observed elevated ORs for Australia (OR=1.15) and Europe (OR=1.14) and lower, though still positive, ORs for Asia (OR=1.03) and North America (OR=1.01) (Table 2). PTB rates vary by location, with the lowest rates in Sweden (4%, (Murray et al. 2019)) and higher in China (7%, (C Chen et al. 2018)), Australia (8% (Australian Government Department of Health 2019)), Taiwan (9%,(Wang et al. 2014)), and the U.S. (10%, (Martin and Osterman 2018)). However, the variability in these rates does not explain the geographic heterogeneity that we observed. Other potential explanations for the heterogeneity could be related to the distribution across countries of other PTB risk factors, such as poverty and other socioeconomic status related factors, race/racism, maternal age, smoking and parental body mass index. Factors associated with atmospheric conditions and chemistry, such as presence and concentrations of co-pollutants, can differ by region and may contribute to observed heterogeneity. Noise and temperature, especially at extremes, have also been identified as potential risk factors for PTB, and may influence response to air pollutants (Smith et al. 2020; Sun et al. 2019). Additional research will provide useful insights into the geographic variability of PTB rates and the associations between ozone concentrations and PTB.
Biological modes of action by which ozone exposure may lead to adverse birth outcomes, including preterm birth, are largely through pathways initiated by systemic inflammation and oxidative stress responses (US Environmental Protection Agency 2020). These pathways could originate through activation of sensory nerves in the respiratory tract or respiratory inflammation and oxidative stress, and lead to altered thyroid, cardiovascular, and uterine function (US Environmental Protection Agency 2020). Evidence from animal models demonstrates altered circulating serum cytokines with ozone exposure, which may impact proper placentation, altered uterine artery vascularity, impaired trophoblast invasion and migration, and impaired trophoblast metabolic capacity (US Environmental Protection Agency 2020). There has also been evidence of altered thyroid hormone levels in non-pregnant animals (US Environmental Protection Agency 2013). These pathways provide a plausible biological mechanism by which ozone exposure in early pregnancy could contribute to preterm birth.
The results of our systematic review and meta-analyses build upon previous reviews. Earlier reviews were based on a relatively small number of studies and often reported qualitative observations rather than pooled results. One study (Stieb et al. 2012) conducted a meta-analysis of ozone and PTB, and reported a pooled estimate from 4 studies for first trimester ozone exposure of 1.22 (0.91, 1.64) and 0.94 (0.88, 1.00) for a single study of second trimester ozone exposure and PTB. Other previous reviews reported qualitative results (Shah et al. 2011; Šrám et al. 2005), and determined that the results for ozone and PTB were “inconclusive” and “insufficient”, respectively. More recently, (Bekkar et al. 2020) conducted a qualitative systematic review of exposures to air pollution or ambient heat with birth outcomes. They included seven studies that evaluated ozone exposures and PTB and did not restrict to any specific windows of exposure or exposure contrasts. Bekkar et al. (2020) concluded that most studies of air pollution and PTB observed positive results, with a focus on statistical significance and inclusion of all pregnancy-related exposure periods. This included four studies of ozone finding positive associations across various exposure periods. Additionally, (Guo et al. 2019) conducted a systematic review of air pollution and adverse birth outcomes. Their literature search covered the period through March 2017 and identified 3 studies of ozone exposure and PTB and reported a pooled estimate of 1.02 (95% CI: 1.00, 1.04) per 10 ppb increase in ozone concentration. In contrast, our systematic review and meta-analyses reflect the rapidly expanding literature base and provide updated pooled estimates for 1st and 2nd trimester ozone exposure from 17 and 15 studies, respectively.
We calculated and present both 95% confidence intervals and 95% prediction intervals for the pooled estimates from our meta-analyses. The confidence intervals provide information about the uncertainty around the pooled OR, while the prediction intervals represent the expected range for observed effects in similar studies and reflect the variation in exposure effects over different populations, conditions, and/or settings (IntHout et al. 2016). Thus, the confidence intervals for our pooled estimates provide increased certainty for an elevated OR (i.e., above 1.0) for both 1st and 2nd trimester ozone exposure and PTB. The prediction intervals reflect the heterogeneity of studies included in the meta-analyses and provide a range within which we would predict to see the OR for future studies of ozone exposure and PTB. Our prediction intervals include the null value (OR=1.0) for both 1st and 2nd trimester ozone exposure, indicating that it would be expected that some future studies observe null results. Therefore, as new studies are published, it will be important to consider underlying population characteristics and other sources of heterogeneity identified in this review.
A strength of our systematic review is the incorporation of an evaluation of study quality to our methods. This ensured critical evaluation of study design methods. Overall, we observed very similar results in our study quality evaluation across studies. Similarity in study design accounts for the majority of the consistency in the study quality ratings assigned across studies (Figure 3); studies are largely registry based and use standard metrics of outcome and exposure assessment. We would like to note that there are many complexities in both creating these studies/analyses and in evaluating them; often there are limitations in the available data that preclude achieving the highest possible study quality score based on our metrics, but this should not discount the overall value and utility of these studies. We determined that the overall quality of individual studies largely resulted in either medium or low confidence. Overall quality determinations did not appear to impact heterogeneity in meta-regression and sensitivity analyses. Often, lower study quality scores were assigned due to a lack of specificity, details, or clarity in the published manuscripts. Study location did appear to be correlated with study quality. The 19 included studies were judged to be of sufficient quality to support the conclusions reached in this review. The ability of the study quality analysis to identify specific influential components of the study quality scores is likely limited due to the large number of covariates adjusted for and other variability in the study designs and statistical analyses. An additional limitation of our study quality analysis is that it did not directly consider statistical power, though a qualitative examination of exposure variability was used to consider study sensitivity; insensitive studies can result in false negatives from underpowered studies and may be an additional source of heterogeneity in meta-analyses.
Other strengths of our meta-analysis on ozone and PTB were the inclusion of a larger number of studies compared to previous meta-analyses of ozone and PTB (Guo et al. 2019; Stieb et al. 2012), providing new summary information on potential effect measure modifiers of PTB and ozone exposure. We were also able to focus on specific time windows within pregnancy, and perform several sensitivity analyses (e.g., trim and fill, leave one out, sub-group analyses) to examine robustness of the pooled effect estimates.
There were several limitations that we could not address in our systematic review and meta-analyses. We were unable to adequately evaluate the role of copollutant confounding in each of the studies included in this review. Correlation of ozone concentrations with other co-pollutants can lead to inflation of the effect estimates reported in epidemiologic studies. Some studies included in our systematic review reported correlation coefficients with other monitored air pollutants, and there was a wide range reported for PM10 (−0.2 to −0.85), PM2.5 (−0.01 to −0.78), NO2 (−0.02 to −0.80), SO2 (−0.13 to −0.69) and CO (−0.06 to −0.74) (Table S.1). The inability to account for potential copollutant confounding is a limitation in the meta-analysis.. Though a notable exception was Chen et al. (2021) where correlations ranges from −0.63 with SO2 to −0.93 with NO2, potentially contributing to its extreme outlier status. Given that the majority of co-pollutant correlations are low, confounding of the relationship between ambient ozone exposure and a health effect by exposure to CO, SO2, NO2, PM10 or PM2.5 is not a substantial concern (US Environmental Protection Agency 2020).
Each of the studies included in this systematic review utilized a linear model and no studies specifically evaluated the concentration-response relationship using non-linear models or other statistical approaches. Thus, information about the concentration-response relationship for ozone exposure and preterm birth is unavailable and an additional limitation. In a recent assessment of ozone and other health effects (mainly mortality or respiratory morbidity), it was concluded that recent studies provide evidence to support a linear concentration-response relationship, but with less certainty in the shape of the curve at lower concentrations (i.e., below 30−40 ppb) (US Environmental Protection Agency 2020).
Another limitation is clear evidence for a biological pathway by which ozone exposure might induce PTB. PTB is characterized by multiple etiologies (spontaneous, premature rupture of membranes, or medically induced), and few studies distinguish between these three groups in examining associations between ozone and PTB. While there is some evidence that ozone inhalation could result in a series of physiological responses that could lead to PTB (US Environmental Protection Agency 2020), there is substantial uncertainty surrounding the biological mechanism leading to PTB, and multiple mechanisms may exist simultaneously. Moreover, while we focused on trimester length exposures to ozone, there is also research examining impacts of short-term ozone exposures (days or weeks) and PTB. Short-term ozone exposures may act on birth outcomes through different mechanistic pathways than long-term exposures, and thus were not included in this review.
In addition, although we investigated both averaging time and effect measure (OR, HR, RR) as potential sources of heterogeneity and neither were identified as substantial sources of heterogeneity, pooling estimates based on different averaging times likely contributes additional heterogeneity compared to analyses based on a consistent averaging time, and we did not adjust for effect measure in the meta-analysis.
Conclusion
Overall, the results of this systematic review and meta-analysis, based on 19 studies identified for overall inclusion, support an elevated risk of PTB associated with ozone exposure during the 1st and 2nd trimesters of pregnancy. The elevated pooled estimates from our meta-analyses were robust to trim-and-fill methods that account for potential publication bias, and after adjusting for heterogeneity due to study location in 1st trimester analysis. While we were able to refine examination of exposure periods (trimester vs. entire pregnancy) and potential sources of heterogeneity, there are still uncertainties that remain. Further exploration in studies of ozone and PTB could address uncertainties, particularly with more complete consideration of other PTB risk factors, such as socioeconomic status, and race/racism.
Supplementary Material
Acknowledgements
We acknowledge Ms. Danielle Moore for assistance in designing and conducting literature searches and Drs. Stephanie Deflorio-Barker and Lauren Wyatt for input on early versions of the manuscript.
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The research described in this article has been reviewed by the Center for Public Health and Environmental Assessment, US EPA, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does the mention of trade names of commercial products constitute endorsement or recommendation for use.
Abbreviations - PTB: preterm birth; PROM: premature rupture of membranes; PECOS: population, exposure, comparator, outcome, study design; SWIFT-AS: SWIFT-Active Screener; OHAT: Office of Health Assessment and Translation; HAWC: Health Assessment Workspace Collaborative; NAAQS: National Ambient Air Quality Standard
References
- Allaire J 2012. Rstudio: Integrated development environment for r. Boston, MA: 770:394. [Google Scholar]
- Australian Government Department of Health. 2019. Risk of preterm birth. Available: https://www.health.gov.au/resources/pregnancy-care-guidelines/part-d-clinical-assessments/risk-of-preterm-birth [accessed July 7 2020].
- Behrman RE, Butler AS. 2007. Preterm birth: Causes, consequences, and prevention: National Academies Press; Washington, DC. [PubMed] [Google Scholar]
- Bekkar B, Pacheco S, Basu R, DeNicola N. 2020. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the us: A systematic review. JAMA Network Open 3:e208243–e208243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer M, Lencar C, Tamburic L, Koehoorn M, Demers P, Karr C. 2008. A cohort study of traffic-related air pollution impacts on birth outcomes. Environmental Health Perspectives 116:680–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhang J, Xia H, Zhang H, Betran AP, Zhang L, et al. 2018. Epidemiology of preterm birth in china in 2015 and 2016: A nationwide survey. The Lancet 392:S73. [Google Scholar]
- Chen G, Guo Y, Abramson MJ, Williams G, Li S. 2018. Exposure to low concentrations of air pollutants and adverse birth outcomes in brisbane, australia, 2003–2013. Science of the Total Environment 622:721–726. [DOI] [PubMed] [Google Scholar]
- Chen J, Fang J, Zhang Y, Xu Z, Byun HM, Li PH, et al. 2021. Associations of adverse pregnancy outcomes with high ambient air pollution exposure: Results from the Project ELEFANT. Sci Total Environ 761:143218. [DOI] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. 2008. Epidemiology and causes of preterm birth. The Lancet 371:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L-Q, Chen Y, Mi B-B, Dang S-N, Zhao D-D, Liu R, et al. 2019. Ambient air pollution and adverse birth outcomes: A systematic review and meta-analysis. Journal of Zhejiang University-SCIENCE B 20:238–252. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ha S, Hu H, Roussos-Ross D, Haidong K, Roth J, Xu X. 2014. The effects of air pollution on adverse birth outcomes. Environmental Research 134:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen C, Neller A, Williams G, Simpson R. 2006. Maternal exposure to low levels of ambient air pollution and preterm birth in Brisbane, Australia. BJOG: An International Journal of Obstetrics & Gynaecology 113:935–941. [DOI] [PubMed] [Google Scholar]
- Hao H, Chang HH, Holmes HA, Mulholland JA, Klein M, Darrow LA, et al. 2016. Air pollution and preterm birth in the U.S. State of Georgia (2002–2006): Associations with concentrations of 11 ambient air pollutants estimated by combining community multiscale air quality model (cmaq) simulations with stationary monitor measurements. Environmental Health Perspectives 124:875–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IntHout J, Ioannidis JP, Rovers MM, Goeman JJ. 2016. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalaludin B, Mannes T, Morgan G, Lincoln D, Sheppeard V, Corbett S. 2007. Impact of ambient air pollution on gestational age is modified by season in Sydney, Australia. Environmental Health 6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavigne E, Yasseen III AS, Stieb DM, Hystad P, Van Donkelaar A, Martin RV, et al. 2016. Ambient air pollution and adverse birth outcomes: Differences by maternal comorbidities. Environmental Research 148:457–466. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Gravett MG, Nunes TM, Rubens CE, Stanton C, Group GR. 2010. Global report on preterm birth and stillbirth (1 of 7): Definitions, description of the burden and opportunities to improve data. BMC Pregnancy and Childbirth 10:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P-C, Roberts JM, Catov JM, Talbott EO, Ritz B. 2013. First trimester exposure to ambient air pollution, pregnancy complications and adverse birth outcomes in Allegheny County, PA. Maternal and Child Health Journal 17:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Yang Y, Li J, Zhu X, Ruan Z, Chen S, et al. 2019. Migrant population is more vulnerable to the effect of air pollution on preterm birth: Results from a birth cohort study in seven Chinese cities. International Journal of Hygiene and Environmental Health 222:1047–1053. [DOI] [PubMed] [Google Scholar]
- Lin Y-T, Jung C-R, Lee YL, Hwang B-F. 2015. Associations between ozone and preterm birth in women who develop gestational diabetes. American Journal of Epidemiology 181:280–287. [DOI] [PubMed] [Google Scholar]
- Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. 2016. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the sustainable development goals. The Lancet 388:3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Xu J, Chen D, Sun P, Ma X. 2019. The association between air pollution and preterm birth and low birth weight in guangdong, china. BMC Public Health 19:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J, Osterman M. 2018. Describing the increase in preterm births in the united states, 2014–16. [PubMed]
- Morgan RL, Whaley P, Thayer KA, Schünemann HJ. 2018. Identifying the peco: A framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environment International 121:1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray SR, Juodakis J, Bacelis J, Sand A, Norman JE, Sengpiel V, et al. 2019. Geographical differences in preterm delivery rates in Sweden: A population-based cohort study. Acta Obstetricia et Gynecologica Scandinavica 98:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson D, Ekström M, Forsberg B. 2012. Temporal variation in air pollution concentrations and preterm birth—a population based epidemiological study. International Journal of Environmental Research and Public Health 9:272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson D, Mogren I, Forsberg B. 2013. Air pollution exposure in early pregnancy and adverse pregnancy outcomes: A register-based cohort study. BMJ Open 3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Z, Liang S, Yang S, Trevathan E, Huang Z, Yang R, et al. 2016. Ambient air pollution and preterm birth: A prospective birth cohort study in wuhan, china. International Journal of Hygiene and Environmental Health 219:195–203. [DOI] [PubMed] [Google Scholar]
- Ritz B, Wilhelm M, Hoggatt KJ, Ghosh JKC. 2007. Ambient air pollution and preterm birth in the environment and pregnancy outcomes study at the University of California, Los Angeles. American Journal of Epidemiology 166:1045–1052. [DOI] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environmental Health Perspectives 122:711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah PS, Balkhair T, births KSGoDoPL. 2011. Air pollution and birth outcomes: A systematic review. Environment International 37:498–516. [DOI] [PubMed] [Google Scholar]
- Shapiro AJ, Antoni S, Guyton KZ, Lunn RM, Loomis D, Rusyn I, et al. 2018. Software tools to facilitate systematic review used for cancer hazard identification. Environmental Health Perspectives 126:104501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RB, Beevers SD, Gulliver J, Dajnak D, Fecht D, Blangiardo M, et al. 2020. Impacts of air pollution and noise on risk of preterm birth and stillbirth in London. Environment International 134:105290. [DOI] [PubMed] [Google Scholar]
- Šrám RJ, Binková B, Dejmek J, Bobak M. 2005. Ambient air pollution and pregnancy outcomes: A review of the literature. Environmental Health Perspectives 113:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieb DM, Chen L, Eshoul M, Judek S. 2012. Ambient air pollution, birth weight and preterm birth: A systematic review and meta-analysis. Environmental Research 117:100–111. [DOI] [PubMed] [Google Scholar]
- Sun Z, Yang L, Bai X, Du W, Shen G, Fei J, et al. 2019. Maternal ambient air pollution exposure with spatial-temporal variations and preterm birth risk assessment during 2013–2017 in Zhejiang Province, China. Environment International 133:105242. [DOI] [PubMed] [Google Scholar]
- Team RC. 2013. R: A language and environment for statistical computing.Vienna, Austria. [Google Scholar]
- US Environmental Protection Agency. 2013. Integrated science assessment for ozone and related photochemical oxidants. EPA 600/R-10/076F. [Google Scholar]
- US Environmental Protection Agency. 2020. Integrated science assessment for ozone and related photochemical oxidants. EPA/600/R-20/012. [Google Scholar]
- Viechtbauer W 2010. Conducting meta-analyses in r with the metafor package. Journal of Statistical Software 36:1–48. [Google Scholar]
- Wang LK, Chen WM, Chen CP. 2014. Preterm birth trend in Taiwan from 2001 to 2009. Journal of Obstetrics and Gynaecology Research 40:1547–1554. [DOI] [PubMed] [Google Scholar]
- Wang Q, Benmarhnia T, Zhang H, Knibbs LD, Sheridan P, Li C, et al. 2018. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environment International 121:317–324. [DOI] [PubMed] [Google Scholar]
- Warren J, Fuentes M, Herring A, Langlois P. 2012. Spatial-temporal modeling of the association between air pollution exposure and preterm birth: Identifying critical windows of exposure. Biometrics 68:1157–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Ritz B. 2005. Local variations in co and particulate air pollution and adverse birth outcomes in Los Angeles County, California, USA. Environmental Health Perspectives 113:1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wilhelm M, Chung J, Ritz B. 2011. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environmental Research 111:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Liang Z, Ruan Z, Zhang S, Zhao Q, Lin H. 2020. Estimating the attributable burden of preterm birth and low birth weight due to maternal ozone exposure in nine Chinese cities. Atmospheric Environment 222:117169. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


