Abstract
Sex differences in the anatomy and physiology of the respiratory system have been widely reported. These intrinsic sex differences have also been shown to modulate the pathophysiology, incidence, morbidity, and mortality of several lung diseases across the lifespan. In this chapter, we describe the epidemiology of sexually dimorphic respiratory diseases including neonatal lung disease (respiratory distress syndrome, bronchopulmonary dysplasia) and pediatric and adult disease (including asthma, cystic fibrosis, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, lung cancer, lymphangioleiomyomatosis, obstructive sleep apnea, pulmonary arterial hypertension, and respiratory viral infections such as respiratory syncytial virus, influenza, and SARS-CoV-2). We also discuss the current state of research on the mechanisms underlying the observed sex differences in lung disease susceptibility and severity, and the importance of considering both sex and gender variables in research studies’ design and analysis.
Keywords: Sex, Gender, Lung Disease, Hormones, Chronic Disease
Introduction
Sex-related differences exist in many lung diseases throughout the lifespan (Carey et al. 2007a; Townsend et al. 2012). In neonates, the male disadvantage is a well-established clinical fact, especially in the preterm population (Bancalari and Jain 2019) to the point that guidelines to predict outcomes from the National Institutes of Child Health and Disease (NICHD) and Neonatal research network (NRN) for extremely preterm birth outcomes include sex as a critical biological variable (Rysavy et al. 2020). In children and adults, some lung conditions are more commonly found in women and men, respectively, and can present with different degrees of severity and symptoms. Overall, the literature shows that most lung diseases are more commonly found, or present with higher degree of severity, exacerbation rate, hospitalizations, and mortality in women than men (Han et al. 2018). These include asthma, chronic obstructive pulmonary disease (COPD), pulmonary hypertension, and some types of lung cancer such as adenocarcinoma (Zein and Erzurum 2015; Raghavan and Jain 2016; Sathish et al. 2015). Furthermore, some rare and less-understood lung conditions such as lymphangioleiomyomatosis (LAM), are almost exclusively found in women (Xu et al. 2020).
Although the terms sex and gender are commonly used interchangeably, they represent different concepts. According to the National Institutes of Health (NIH) Office for Research on Women’s Health (ORWH), “sex” refers to the biological differences between females and males, including chromosomal, anatomical, hormonal, and other physiological and functional differences. “Gender”, on the other hand, refers to the characteristics that a society or culture delineates as masculine or feminine, including social, environmental, cultural, and behavioral factors and choices that influence an individual’s self-identity. As opposed to sex, gender is a social construct and not defined biologically. Importantly, an individual’s gender does not necessarily need to be consistent with their biological sex given at birth, nor be fixed or binary. However, because the health of men and women are influences by both sex and gender, including these variables in research studies is crucial. In basic science, this means including both male and female cells and/or experimental animal models in study designs, as well as examining the influence of sex hormones down to the molecular level. For clinical, behavioral and outcomes research, this means considering gender-specific social influences and their impact on health and disease. Only when we incorporate sex and gender factors in research studies, we will be able to understand the mechanisms underlying the numerous sex disparities observed in lung disease prevalence and severity, and provide more efficient and personalized sex- and gender-specific medicine.
Sex and Gender differences in respiratory disease
It is not possible to talk about sex differences in respiratory disease without discussing first sex differences in lung biology. From the 16th week of gestation to adult life, significant differences exist in the male and female lung. In addition, changes in sex hormone levels throughout development, puberty, and physiological events such as pregnancy and menopause, also influence lung function and health. Early in life, while female sex hormones are beneficial, promoting lung development and maturation, androgens appear to exert the opposite effect (Silveyra 2016). After puberty, the opposite occurs in diseases such as severe asthma, where improvement is observed with increasing androgen levels, and fluctuations in female hormones throughout the menstrual cycle promote asthma exacerbations. Overall, the available body of research shows that the effect of sex hormones on lung health appears to depend on the timing of exposure, and thus differentially affect disease prevalence and severity in males and females throughout the lifespan. Table 1 summarizes the information available on some of the most common lung diseases affecting men and women disproportionately. In the sections below, we describe the epidemiological information available as well as the status of the research aiming to understand the mechanisms behind the sexual dimorphism for each disease.
Table 1.
Sex differences in neonatal, pediatric, and adult lung disease prevalence
| Disease | Population | Sex differences | References |
|---|---|---|---|
| Asthma | children | Boys > girls | (Newcomb 2016; Carey et al. 2007a; Akinbami et al. 2012) |
| adults | Women > men | ||
| Bronchopulmonary dysplasia (BPD) | neonates | Boys > girls | (Townsel et al. 2017; Bancalari and Jobe 2012; Gortner et al. 2013) |
| Chronic Bronchitis | adults | Women > men | (Aryal et al. 2014) |
| Chronic Cough | children | Boys > girls | (Benscoter 2018) |
| Chronic Obstructive Pulmonary Disease (COPD) | adults | Women > men | (Jenkins et al. 2017; Akinbami and Liu 2011) |
| Cystic Fibrosis | children | Girls > boys* | (Vidaillac et al. 2018; Harness-Brumley et al. 2014) |
| adults | Women > men | ||
| Coronavirus disease 19 (COVID-19) | adults | Men > women | (Scully et al. 2020) |
| Emphysema | adults | Men > women | (Martinez et al. 2007) |
| Idiopathic Pulmonary Fibrosis | adults | Men > women | (Zaman et al. 2020; Raghu et al. 2006) |
| Lung cancer | adults | Women > men | (Stabile and Siegfried 2003; Radkiewicz et al. 2019) |
| adults | Women > men | (Xu et al. 2020) | |
| Pulmonary arterial hypertension | adults | Women > men | (Mehari et al. 2014) |
| Obstructive Sleep Apnea | adults | Men > women | (Lin et al. 2008; Nevšímalová 2019) |
| Respiratory distress syndrome (RDS) | neonates | Boys> girls | (Townsel et al. 2017; Bancalari and Jobe 2012; Gortner et al. 2013) |
| Respiratory syncytial virus infection (RSV) | neonates/children | Boys> girls | (Nair et al. 2010) |
infection rates and outcomes worse in girls than boys, but no sex differences in incidence
Neonatal lung disease
Infants born prematurely are at higher risk for cardiopulmonary and neurological comorbidities such as retinopathy, pulmonary hypoplasia, respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), intraventricular hemorrhage (IVH), and chronic neuro-cognitive developmental disorders (O’Driscoll et al. 2018). Many of these comorbidities exhibit significant sex disparities that could be a consequence of differences in lung development, and/or caused by a complex interaction between immunological, hormonal and genetic factors earlier in life (O’Driscoll et al. 2017). Overall, male infants are presumed to have an intrinsic disadvantage, and to be more sensitive to adverse environmental exposures during development and after birth (Naeye et al. 1971). This sex-related disparity is particularly manifested during the neonatal period, and is more pronounced in prematurely born infants.
Sex differences in lung development
The development of the male and female lung is a highly regulated process controlled by genetic, epigenetic, hormonal, and environmental factors. This process is divided into five stages: embryonic, pseudoglandular, canalicular, saccular, and alveolar (Table 2). Each stage is characterized by specific cellular and structural events that are controlled by the expression of multiple developmental genes (Warburton et al. 1999; Warburton and Bellusci 2004). Sex differences in structural, mechanical, and functional aspects of lung development, as well as in its control by sex hormones, have been widely documented (Carey et al. 2007a; Carey et al. 2007b; Massaro et al. 1995). These differences are thought to be associated with the sexual dimorphism observed not only in neonatal lung disease, but also later in life (Silveyra 2016). Respiratory diseases such as RDS and BPD contribute to a large proportion of the morbidity and mortality of prematurely born infants (Townsel et al. 2017). Importantly, even late preterm infants, born at gestational ages of 34–36 weeks have been found to be greater risk for adverse respiratory morbidity and mortality than infants born at term (Gyamfi-Bannerman et al. 2016).
Table 2.
Stages of human lung development
| Developmental Stage (gestational age) |
Main events | Sex Differences | References |
|---|---|---|---|
| Embryonic (3–6 weeks) |
Lung buds emerge (foregut), trachea and bronchial buds form. | None reported | - |
| Pseudoglandular (6–16 weeks) |
Bronchial development, airway branching. | Fetal growth and breathing movements are detected earlier in the female fetus. AMH delays branching by promoting apoptosis in males. | (Hepper et al. 1997) (Catlin et al. 1990) |
| Canalicular (16–26 weeks) |
Subdivision of distal airways into canaliculi, vascularization. Differentiation of type I and II cells, surfactant production. |
Surfactant secretion and phospholipid maturation are inhibited in males (androgens) and promoted in females (estrogens). | (Seaborn et al. 2010; Provost et al. 2004; Torday and Nielsen 1987) |
| Saccular (26–36 weeks) |
Cell differentiation, type II cell maturation, surfactant secretion. Formation of sacs and primary septa. | Surfactant production and phospholipid profile remain more advanced in females. | (Fleisher et al. 1985) |
| Alveolar (36 weeks - adolescence) |
Alveolar multiplication, enlargement, and maturation. Lung growth continues and lung function increases with age and peaks in adolescence. |
Faster alveolarization in females. Higher flow rate per lung volume, but smaller lung size in girls. Better response to surfactant therapy in female newborns with RDS. FEV1 peaks earlier in females than males. | (Boezen et al. 2004; Becklake and Kauffmann 1999; Thurlbeck 1990) (Schrader et al. 1988) |
AMH: anti-Müllerian hormone, FEV1: forced expiratory volume in 1 minute
During the fetal period, male lung maturation is usually delayed in comparison to female maturation. Pulmonary surfactant production initiates later in the male vs. the female lung (Seaborn et al. 2010; Carey et al. 2007a). Consequently, male neonates are at increased risk of developing respiratory distress syndrome (RDS) and a higher risk of morbidity and mortality due to RDS compared with female neonates of similar gestational age (Ishak et al. 2014; Thurlbeck 1975). Furthermore, sex differences in overall neonatal survival and pulmonary outcomes have been described with a significantly higher incidence in males versus females (Henderson-Smart et al. 2006). One example is the high incidence observed in males for the development of bronchopulmonary dysplasia (BPD), a pulmonary pathology of the neonate for which RDS is not always an anterior event (Provost et al.). Differences in gene expression, particularly at the late developmental stages, have been shown to play significant roles in this sex disparity in lung health outcomes (Gortner et al. 2013; Ali and Greenough 2012; Becklake and Kauffmann 1999).
Respiratory Distress Syndrome
Respiratory distress syndrome (RDS) is a condition of the premature born characterized by a deficiency in pulmonary surfactant (Angus et al. 2001). Infants presenting with RDS show widespread alveolar atelectasis and a reduction in lung compliance, with secondary complications such as pneumothorax. Prior to the introduction of antenatal corticosteroids and postnatal surfactant replacement therapy, RDS was a major contributor to neonatal mortality, particularly in male newborns (Papageorgiou et al. 1981).
The main factors involved in the pathophysiology of RDS are surfactant deficiency and dysfunction in the immature lung, which occur at higher rates in males than females of the same gestational age (Naeye et al. 1971). Thus, the less developed or mature the lung, the higher the chance of disease manifestation after birth. As mentioned earlier, pulmonary surfactant is produced earlier in females than males during gestation, and its production is stimulated by female sex hormones, and inhibited by male sex hormones (Trotter et al. 2009; Sweezey et al. 1998). A meta-analysis of data from over 500,000 preterm newborn infants found that RDS was between 1.56–1.84 times higher to occur in newborn males than females (Liptzin et al. 2015). This report indicated that males were also at higher risk for other diseases of the newborn, such as bronchopulmonary dysplasia (BPD), as well as lower respiratory tract infections, bronchiolitis, and pneumonia.
Preventative and treatment options for RDS include postnatal surfactant and antenatal corticosteroid therapy (Townsel et al. 2017). For a long time, corticosteroids (e.g. betamethasone) have been reported to have sex-specific effects on placental on microvascular blood flow (Stark et al. 2011b; Stark et al. 2011a), as well as to improve the subsequent response of infants to surfactant administration (Kari et al. 1994), with more beneficial effects in females than males (Papageorgiou et al. 1981). However, a more recent systematic review and meta-analysis, did not find sex-specific differences, although the type of antenatal glucocorticoid used (betamethasone vs. dexamethasone) displayed a sex-specific effect (Roberge et al. 2011).
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia is a lung disease of the prematurely newborn, characterized by an arrest in alveolarization and aberrant pulmonary vascular development (Bancalari and Jobe 2012; Jobe and Bancalari 2001). The disease diagnosis is performed by assessing the need for mechanical ventilation and oxygen respiratory support at 36 weeks postmenstrual age (Bancalari and Jain 2019), and displays a higher incidence in extremely low birth weight neonates (Carlo et al. 2002).
The widespread use of antenatal corticosteroids, neonatal exogenous surfactant, and protective ventilation strategies, have led to increased survival of more extremely preterm infants, with a consequent increase in BPD incidence in the past few decades (Jensen and Schmidt 2014; Jobe and Bancalari 2001). While mortality from the disease has declined in the past several decades, children diagnosed with BPD still display long-term complications in lung health ranging from need for tracheostomy and mechanical ventilation to pediatric pulmonary arterial hypertension and poor neurodevelopmental outcomes (Davidson and Berkelhamer 2017; Rivera et al. 2016). Recent studies have also reported that adults who were born preterm display a higher incidence of airflow obstruction, gas trapping and reduced gas exchange than those born term (Yang et al. 2020), and that worsening of lung function persists throughout childhood, particularly in males (Harris et al. 2020).
Multiple clinical studies have reported sex differences in BPD, including a higher incidence in males vs. females that persists after adjusting for other confounders. Moreover, males display higher death and oxygen dependency rates, as well as pulmonary hemorrhage and use of postnatal steroids (Jobe and Bancalari 2001; Jensen and Schmidt 2014; Binet et al. 2012). Male sex is not only considered an independent major risk predictor of BPD, but also to worsening of lung function during the neonatal and early childhood periods (Thomas et al. 2006). However, despite the well-established sexual dimorphism in the incidence of BPD, the mechanisms associated with these disparities are not completely understood. Recent studies in animal models have suggested a role for microRNAs (miRNAs) in mediating sex biases in BPD (Leary et al. 2019; Zhang et al. 2019; Raffay et al. 2016). Others have related the sexual dimorphism of PBD to sex-specific differential activation of hypoxia inducible factors and genes related to angiogenesis, supporting the pulmonary angiogenesis dysregulation in the pathobiology of BPD (Leary et al. 2019; Coarfa et al. 2017).
Pediatric and adult lung disease
Sex differences in lung and airway development persist throughout infancy and early childhood (Thurlbeck 1982; Wailoo and Emery 1982). While females display larger airways than males, the number of alveoli per unit area, and the alveolar size do not differ between sexes. The the age- and height-adjusted lung volume, however, is higher in boys than girls, which may result in a larger alveolar surface area and a higher diffusion capacity of carbon monoxide (DLCO) in males (Barre et al. 2016; Schwartz et al. 1988; Thurlbeck 1982). With age, differences in lung volumes, as well as in lung size and shape, become more evident (Thurlbeck 1982; Torres-Tamayo et al. 2018). Together with differences in the distending forces of the lungs, these result in differences in the recoil pressure between males and females (Colebatch et al. 1979). This sexual dimorphism in human lung morphometrics and function, together with physiological differences observed by pulmonary function testing, spirometry, and other techniques have been used to partially explain the observed sex disparity in multiple pulmonary conditions.
Overall, while the majority of lung diseases presented below affect more adult women than men, several conditions are observed at higher rates in men than women, and/or show opposite trends in childhood vs. adult life. Contributions of sex hormones, genetic and epigenetic factors, as well as comorbidities and other extrinsic factors have been suggested to contribute to these trends. In the next sections, we summarize the recent epidemiological data, as well as research aiming to understand the mechanisms behind sex disparities in lung disease throughout the life span.
Asthma
Asthma is a heterogeneous disease characterized by chronic airway inflammation. Some of its symptoms are wheezing, shortness of breath, chest tightness, cough and airflow limitation (GINA 2019). Asthma is one of the most prevalent inflammatory diseases of the lung, affecting a significant portion of the world’s population. The World Health Organization reported that more than 339 million people suffer from asthma, resulting in more than 400,000 deaths per year (Centers for Disease Control and Prevention 2020).
While asthma imposes a substantial public health burden in terms of impaired quality of life and mortality in men and women, a clear sexual dimorphism exists in their risk, prevalence, and severity across life span (Falagas et al. 2007a; Naeem and Silveyra 2019; Han et al. 2018; Shah and Newcomb 2018). Depending on the sex and age of the patient, striking differences exist in asthma incidence, prevalence, and severity (Masoli et al. 2004). An interesting fact is that asthma in children is more prevalent in boys than girls, studies in adult populations frequently report more negative lung health outcomes for women than men, suggesting an involvement of sex hormones in mediating these effects (Koper et al. 2017; Sathish et al. 2015).
Epidemiological studies of childhood asthma have shown that prepubertal boys have more asthma than girls, especially at younger ages (Licari et al. 2018; Fuchs et al. 2018). Chronic cough in early childhood, whether from asthma or other causes, is also more common in boys than girls (Benscoter 2018). According to the Centers for Disease Control and Prevention, in the United States, it is estimated that 8.3% of boys and 6.7% of girls under 18 years old currently suffer from asthma. Interestingly, these patterns are reversed after puberty, where asthma prevalence rates for women are almost twice as those for men (5.5% vs 9.8% for women and men over 18 years of age, respectively) (Centers for Disease Control and Prevention. 2020). These statistics have led investigators to hypothesize that hormonal changes starting in puberty contribute to asthma development. This notion is further supported by studies showing that girls who undergo menarche at an earlier age have a higher risk of developing asthma after puberty than girls in which menarche occurs later (Salam et al. 2006).
Studies showing variations in asthma symptoms and hospitalization rates throughout the menstrual cycle and a decline in asthma incidence in women after menopause also support this hypothesis (Postma 2007; Brenner et al. 2005). Also, women are more susceptible to asthma induced by air pollution and show worse adverse pulmonary health outcomes than men (Li et al. 2019; Liu et al. 2019). In this regard, clinical studies and experimental evidence from mouse models have reported that female hormones such as estrogen can trigger lung inflammatory and allergic reactions, while male hormones such as androgens play the opposite role (WULFSOHN et al. 1964; Newcomb and Peebles 2013; Fuentes et al. 2019). Interestingly, the severity of asthma in men increases later in life when androgens levels decrease (Canguven and Albayrak 2011). Overall, more research is needed to elucidate the mechanisms underlying the observed sex differences in disease susceptibility and progression.
Sex differences in asthma have been linked to immunological factors, lung physiology and growth, and behavioral factors (Fuchs et al. 2012; von Mutius and Vercelli 2010; Kosti et al. 2012), as well as exposure to air pollutants (Hafkamp-de Groen et al. 2013; Balmes et al. 2014). Human studies and in vivo models of asthma have shown that female hormones can trigger lung inflammatory and allergic reactions, and male hormones usually play the opposite role (Fuentes and Silveyra 2018). Interestingly, researchers have discovered that sex hormones can alter macrophage polarization and other immune-related cells such as the group 2 innate lymphocytes (ILC2s) and airway smooth muscle cells (Becerra-Díaz et al. 2018). ILC2s that lack a killer-cell lectin like receptor G1 accumulate in the lungs of females after they have reached reproductive age but not in males (Kadel et al. 2018). Others have found that estrogen and testosterone increases and decreases Th2-mediated airway inflammation, respectively (Fuseini and Newcomb 2017). The authors also concluded that females have augmented IL-17A-mediated airway inflammation compared to males.
Genetic associations with asthma have also been reported, and found to be sex specific (Hunninghake et al. 2010). Two single nucleotide polymorphisms (SNPs) in the thymic stromal lymphopoietin (TSLP) gene (rs1837253 and rs2289276) have been associated with asthma in a sex-specific manner. Specifically, rs1837253 is associated with a lower risk for asthma in men, and rs2289276) is associated with a higher risk of asthma in women. While the underlying mechanisms for these sex-specific associations have not been elucidated, these genetic variants have been associated with changes in immunoglobulin E (IgE) levels, which in children are correlated with higher airway resistance and exacerbations triggered by dust, pollen, and pets (Haselkorn et al. 2010).
Exercise-induced bronchoconstriction
Exercise-induced bronchospasm (EIB) is a phenomenon of acute airway narrowing that occurs during or after exercise or physical exertion. As such, EIB can occur in the presence or absence of asthma. Traditionally, the terms exercise induced-asthma (EIA) and EIB have been used interchangeably. However, the current consensus is that EIB represents a more accurate reflection of the underlying pathophysiology of the condition, since exercise is not an independent risk factor for asthma, but rather a trigger of bronchoconstriction in patients with underlying asthma (Molis and Molis 2010; Storms 2005; Parsons et al. 2013).
As mentioned above, asthma prevalence is higher in boys than in girls; however, after puberty the prevalence is around 20% higher in women than men, indicating a potential contribution of hormones after puberty (Papi et al. 2018). Moreover, sex and gender differences in response to exercise have clear implications for understanding gender specific adaptations to exercise for athletic performance and overall health (Northoff et al. 2008).
The estimated prevalence of exercise-induced bronchoconstriction (EIB) varies from approximately 5% to 20% in the general population, to perhaps 30% to 70% in elite winter athletes and athletes who participate in summer endurance sports, to at least 90% in individuals with persistent asthma (Weiler et al. 2007). This condition has been reported in a range of sporting activities but is most common in participants of cold-weather sports (e.g., Nordic skiing) and indoor sports (e.g., ice-skating and swimming). Shinohara et al. investigated whether sex differences influence the prevalence and severity of EIB in prepubertal children aged 5–6 years. They found that the prevalence of EIB was higher in girls than in boys. In addition, the time to maximal bronchoconstriction was slower in girls than in boys, and the pattern of recovery after exercise was also faster in females than males (Shinohara et al. 2019). Therefore, it is recommended that when evaluating the prevalence and severity of EIB in prepubertal children, the influence of sex is considered.
The pathogenesis of EIA is not fully elucidated. Minute ventilation, the volume of air inhaled or exhaled from a person’s lungs per minute, rises with exercise. It is believed that EIB probably results from changes in airway physiology triggered by the large volume of relatively cool, dry air inhaled during vigorous activity (McFadden and Ingram 1979; Anderson et al. 1985). One of the major triggers for bronchoconstriction is water loss during periods of high ventilation. Strenuous exercise creates a hyperosmolar environment by introducing dry air into the airway with compensatory water loss, leading to transient osmotic changes in the airway surface. This hyperosmolar environment leads to mast cell degranulation and eosinophil activation with consequent release of inflammatory mediators, including leukotrienes. This process triggers bronchoconstriction and inflammation of the airway, as well as stimulation of sensory nerves and release of neurokinin and mucins. (Weiler et al. 2016). All this is supported by several research findings concluding that it is not the type of exercise, but the ventilation demand, and humidity of the inspired air, that are the main determinants of the occurrence and degree of bronchoconstriction (Deal et al. 1979; Kallings et al. 1999). Therefore, the diagnosis of asthma in athletes should be confirmed by lung function test, usually with bronchial provocation testing (McFadden 1990) in association with a history consistent with EIB, because self-reported symptoms are not adequate. Varsity athletes show a high incidence of EIB when objectively diagnosed by a variety of pulmonary function criteria. The use of symptoms to diagnose EIB is not predictive of whether athletes have objectively documented EIB. (Parsons et al. 2007).
Management of EIB should be based on the understanding that EIB susceptibility varies widely among asthmatic patients, and it could also be present in individuals without underlying asthma. A study by Parsons et al. found that 36 out of 42 EIB-positive athletes (86%) had no prior history of EIB or asthma (Parsons et al. 2007). In patients with asthma, EIB can also be an indicator of poorly controlled disease, and underlying asthma should be treated prior to controlling EIB (Weiler et al. 2016). As mentioned above, asthma can deteriorate during the peri-menstrual period, a phenomenon known as peri-menstrual asthma (PMA) which is usually much more severe and troublesome than the reported periovulatory worsening of asthma (Skoczyński et al. 2014). In this context, Stanford et al. demonstrated for the first time that the menstrual cycle phase is an important determinant of the severity of EIB in female asthmatic athletes (Stanford et al. 2006). This study reported deterioration in the severity of EIB during the mid-luteal phase accompanied by worsening asthma symptoms and increased bronchodilator use (Stanford et al. 2006). Aiming that exercise is not avoided by patients with EIB, general measures and pharmacologic interventions can be assessed subjectively in terms of symptom control and exercise tolerance, considering the fact that sex hormones play an important role in lung inflammation. Thus, medical evaluation and medication adjustment would likely be based on the understanding of sex differences.
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) affects an estimated 174 million people worldwide (104.7 million males and 69.7 million females) (Collaborators 2020). For many years, it was considered a disease of older men (Raghavan and Jain 2016). However, over the past 20 years its prevalence and rates of hospitalization have increased among women, closing this prevalence gap (Aryal et al. 2014; de Torres et al. 2009). This phenomenon is due in part to increased rates of tobacco use – the single largest risk factor for the development of COPD – among women, together with recent evidence demonstrating that first and secondhand tobacco smoke has more severe effects in women than men (Ben-Zaken Cohen et al. 2007; Han et al. 2018). Moreover, there is an increased recognition that the clinical presentation of COPD is different in women than men, which has led to better and more accurate diagnosis in women in the past few decades (Jenkins et al. 2017; Lamprecht et al. 2015). It has been shown that women with COPD have different disease burden, symptoms, and clinical trajectory than men (Han et al. 2018; Perez et al. 2020), and that women tend to develop COPD earlier in life and have more frequent respiratory exacerbations than men (Perez et al. 2020).
While asthma remains the most prevalent respiratory disease in the world, COPD is the fourth leading cause of death in the United States, and the eighth leading cause of disability worldwide (Soriano 2017). Recently, the World Health Organization has projected that COPD will be the third leading cause of death worldwide by 2030. Moreover, although the overall prevalence of COPD is increasing in both men and women (Soriano et al. 2020), recent data from United States Center for Disease Control’s National Center for Health Statistics has shown that COPD prevalence in the United States not only is higher in women, but it is also increasing at a higher rate among women than men (Akinbami and Liu 2011). Epidemiological data show that since the year 2000, the number of women in the US dying from COPD has surpassed the number of men (Aryal et al. 2013; Mannino and Buist 2007). Some studies have suggested that both asthma and the so-called “Asthma COPD Overlap” (ACOS), which are more common among adult women than men, can predispose women to develop COPD (Dodd et al. 2020; To et al. 2018).
It is possible that the increased prevalence of COPD in women is due not only to increased tobacco use, but also related to longer life expectancy for women in general, as well as changes in women’s occupational exposures over the past few decades (Aryal et al. 2013). Historically, professions that predispose to lung disease were predominantly held by men. However, due to the reassignment of sex roles and more single-parent households in recent decades, a higher number of women are found in these jobs (Aryal et al. 2014). This may play a role in the increasing prevalence of the disease among women. It is estimated that 15% of COPD is work-related. In addition, it has long been theorized that indoor air pollution resulting from smoke from biomass fuel combustion for cooking and other purposes also contributes to the development of COPD in never smokers, with women being disproportionately exposed (Varkey 2004) and affected (Pope et al. 2015). It is estimated that 50% of deaths from COPD are associated with indoor air pollution in developing countries, and about 75% of these are in women (Salvi and Barnes 2009).
In recent years, there have been several clinical and experimental studies aiming to understand the contribution of sex to the biologic pathogenesis of COPD (Barnes 2016). Levels of pro-inflammatory cytokines, including C reactive protein, interleukin-6 (IL-6), tumor necrosis factor alpha (TNFa), matrix metalloproteinase 9 (MMP-9), pulmonary and activation-regulated chemokine (PARC), and vascular endothelial growth factor (VEGF), have been theorized to contribute to the development of COPD. VEGF helps regulate growth of new vessels and vascular leak, and was found to be elevated in patients with COPD compared to healthy controls. In patients with COPD, statistically significant higher levels of VEGF and IL-6 have been found in men vs. women (Aryal et al. 2013). Additionally, studies in mouse models of chronic cigarette smoke have indicated that sex hormones may be contributing to the greater COPD susceptibility in females. Exposure to cigarette smoke in female mice results in higher peripheral airway obstruction and airway remodeling, and less emphysema than male mice, an effect that is mediated by estrogens (Tam et al. 2011). It was also found that in female mice, cigarette smoke was associated with activation of transforming growth factor-β (TGF-β), decreased expression of antioxidant genes, and the transcription factor Nrf2 (nuclear factor erythroid-derived 2-like 2), as well as increased oxidative stress (Tam et al. 2011). Overall, more research is needed to better understand the mechanisms behind sex differences in COPD susceptibility, as well as in the response of men and women to COPD available therapies.
Cystic Fibrosis
Cystic fibrosis (CF), an autosomal recessive multiorgan disease caused by mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, also displays sex differences. Epidemiological studies have reported a sex-based disparity in CF outcomes, where females experience higher rates of pulmonary exacerbations and a shortened life expectancy than males (Vidaillac et al. 2018). While the etiology of this disparity is not fully elucidated, it appears to be multifactorial. Studies have associated the sexual dimorphism in CF outcomes to bias in diagnosis (Lai et al. 2002), anatomical differences between males and females (Diab Cáceres et al. 2020), socioenvironmental factors (Rho et al. 2018), medication adherence (Shakkottai et al. 2015), physical activity level (Savi et al. 2015), and actions of male and female sex hormones (Abid et al. 2017; Sweezey and Ratjen 2014). A combination of poor perception of disease prognosis, withdrawal, anxiety, decreased adherence to therapies, and decrease in physical activity after puberty have been associated with increased morbidity and mortality in CF females (Selvadurai et al. 2004). Moreover, despite reported earlier referral to lung transplantation in females than males, survival time after transplantation does not show sex differences. Not only females with CF experience higher rates of infection and exacerbations than males, they also require more intensified treatment regarding antibiotics, macrolides, steroids and days of hospitalization than their male counterparts (Mehta et al. 2010; Olesen et al. 2010). However, despite earlier referral to lung transplantation in females than males, survival time after transplantation does not show sex differences.
As with other lung diseases described earlier, the sexual dimorphism in CF outcomes is also age dependent. In females, the predisposition to worse outcomes in CF has been found at a young age, where girls are more susceptible to bacterial infection than boys (Cogen et al. 2015). Females not only show higher lung bacterial colonization with Pseudomonas aeruginosa, Burkholderia cepacia and Methicillin Resistant Staphylococcus aureus, than males (Maselli et al. 2003; Courtney et al. 2007; Hubert et al. 2013; Ren et al. 2007), but also earlier colonization in life, which is a predictor of negative outcomes and decline in survival for females (Demko et al. 1995). Females have also been found to acquire Methicillin Sensitive Staphylococcus aureus, Methicillin Resistant Staphylococcus aureus, Haemophilus influenzae, Achromobacter xylosoxidans, Aspergillus species, and nontuberculous mycobacteria at earlier ages than males, and often even prior to puberty (Harness-Brumley et al. 2014).
During puberty and reproductive years, the predisposition to infection is enhanced in females, as well as an increased risk for pulmonary exacerbations and extrapulmonary complications (Maselli et al. 2003). Females also show a steeper decline in lung function, one of the key predictors of long-term health in CF patients, than males (Cogen et al. 2015). Because of the reduced life expectancy of patients with CF, little is known about the influence of menopause in the course of the disease (Nick et al. 2010). A study in long-term survivors (older than 40 years old) showed that females with CF are also less likely to live to the age of 40 than males (Nick et al. 2010).
While the mechanisms underlying these sex-disparities have not been fully elucidated, a role of sex hormones on pathogens has been suggested (Tyrrell and Harvey 2020). A study by Chotirmall et al. showed that the female hormones 17β-estradiol and estriol can induce conversion of Pseudomonas aeruginosa from a non-mucoid to mucoid phenotype in females with CF. The same study suggested that high levels of 17β-estradiol in females results in higher capture of more mucoid strains of Pseudomonas aeruginosa and subsequent pulmonary exacerbations (Chotirmall et al. 2012). In addition, not only post-pubertal increases in pulmonary exacerbations are reported in females (Sutton et al. 2014), but women also display cyclical symptoms in relation to their menstrual cycle, with higher lung function measures during the luteal phase than other cycle phases (Johannesson et al. 2000). Studies in bronchial epithelial cells also showed that 17β-estradiol reduces expression of proinflammatory cytokines via upregulation of the secretory leucoprotease inhibitor (SLPI), which could contribute to the higher infection rate observed in females vs. males (Chotirmall et al. 2012). In mouse models of CF, 17β-estradiol stimulates expression of toll-like receptor 2, IL-23 and IL-17A, and results in higher lung inflammatory infiltrates and mucin (Wang et al. 2010). Abid et al. showed that female mice inoculated with Pseudomonas aeruginosa died earlier and showed slower bacterial clearance than male mice (Abid et al. 2017). This effect was reversed by treatment with the estrogen receptor (ER) antagonist ICI 182,780 and ovariectomy, and recapitulated in ovariectomized females treated with exogenous 17β-estradiol (Abid et al. 2017).
Very few studies have evaluated the role of progesterone and testosterone in mediating sex differences in CF infection rates and outcomes. One study in human tracheal epithelial cells showed that exposure to progesterone results in decreased cilia beat frequency, an effect that was attenuated with the addition of 17β-estradiol (Jain et al. 2012). While women with CF are able to carry on pregnancies, the role of progesterone in lung function and CF outcomes has not been studied in detail (Ødegaard et al. 2002). With regard to androgens, a few reports have indicated that adolescent and adult males with CF have lower salivary and serum levels of testosterone than healthy controls (Boas et al. 1996; Leifke et al. 2003), as well as higher rates of male infertility (Yoon et al. 2019). In rodent studies, testosterone was found to enhance expression and functional activity of epithelial sodium channels (Mokhtar et al. 2014). Overall, the direct impact of sex hormones on disease progression in patients with CF remains unknown.
Idiopathic Pulmonary Fibrosis
Idiopathic pulmonary fibrosis (IPF) is a specific form of chronic, progressive fibrosing interstitial pneumonia of unknown cause, occurring primarily in older adults, and limited to the lungs (Raghu et al. 2011).With a median survival of 3–5 years following diagnosis, IPF is characterized by a progressive worsening of dyspnea and decline in lung function and quality of life in most patients (Fernández Fabrellas et al. 2018). Sex discrepancies in this disorder have been suggested for some time. The incidence and prevalence of disease have been reported in multiple studies to be higher in males than in females, with ratios ranging from ~1.6:1 to 2:1. Prior reports have also suggested that female sex is associated with better survival (Han et al. 2008).
Although our current understanding of the pathogenesis of IPF is incomplete, recent advances have delineated specific clinical and pathologic features. Epithelial cell dysfunction and aberrant epithelial-mesenchymal signaling lead to the activation of fibroblasts and extracellular matrix deposition and remodeling. This chronic activation appears to lead to profibrotic, pathologic changes in IPF fibroblasts. The myofibroblast is the classic pathologic fibroblast phenotype described in IPF lungs. Several mediators, including TGF-β, can elicit the differentiation of fibroblasts to myofibroblasts. Compared with resident lung fibroblasts, myofibroblasts secrete excessive amounts of matrix, including type I collagen. This excess matrix deposition may lead to pathologic lung fibrosis and remodeling (Wolters et al. 2014). Although these mechanisms have provided significant advances in our understanding of the disease, there is limited information on the molecular basis underlying the observed sex disparities in IPF. A study by Smith et al. suggested that estrogen may modulate the expression of genes involved in chromatin remodeling pathways, as well as the expression of genes in extracellular matrix turnover (Smith et al. 2018). However, results from animal studies have provided mixed results. Genome wide association studies have pointed to genetic influences mediating sex differences, including SNP polymorphisms in mucin 5B, near A-kinase anchoring protein 13, and desmoplakin genes (Allen et al. 2017).
Sex differences in IPF have been studied in the clinic. Han et al. studied whether the rate of increase in desaturation during serial 6-min walk testing, as well as survival, displayed sex differences. They noted several important observations: 1) males with IPF demonstrate more rapid deterioration in exertional desaturation over time when compared with females; 2) survival was worse in males than females; and 3) better survival for females persisted after additional adjustment for relative change in exertional desaturation and forced vital capacity (FVC) (Han et al. 2008).
Among the clinical conditions that have been associated with a worse IPF prognosis is the presence of comorbidities and complications such as emphysema, pulmonary hypertension, cardiovascular diseases, and bronchogenic carcinoma (Fernández Fabrellas et al. 2018). As mentioned in other sections, some of these conditions also present a sexual dimorphism, which could potentially influence the progression and outcomes of IPF. Finally, prompt treatment of IPF is critical to preserving the patients’ lung function, reducing the risk of acute exacerbations and improving outcomes (Maher and Strek 2019). Currently, two drugs are approved for the treatment of IPF in the United States and Europe: nintedanib and pirfenidone. In vitro studies have shown that by inhibiting signaling mediated via tyrosine kinases, nintedanib inhibits fundamental processes of fibrosis, such as the recruitment, proliferation and differentiation of fibroblasts and fibrocytes and the deposition of extracellular matrix. Data from animal models of fibrosis suggest that nintedanib may also act to normalize the distorted microvascular architecture in the lungs. The mechanism of action of pirfenidone is less well defined, as its target remains unknown, but non-clinical studies suggest that it inhibits pro-fibrotic behaviors in fibroblasts and fibrocytes. Both drugs have been shown to slow the disease progression but not significantly impact mortality (Maher and Strek 2019). However, studies have not addressed sex differences in the effectiveness of these and other therapies for IPF. Current efforts are directed at identifying key biomarkers that may direct more customized patient-centered healthcare to improve outcomes for these patients in the future, and it is essential that they address sex-specific mechanisms (Barratt et al. 2018).
Lung Cancer
Lung cancer is a major public health problem worldwide and is the world’s leading cause of cancer death (Barta et al. 2019). Approximately 95% of all lung cancers are classified as either small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC) (Sher et al. 2008; Suster and Mino-Kenudson 2020). This distinction is essential for staging, treatment, and prognosis. Lung cancer is relatively rare before the fifth decade of life, and risk increases with age thereafter. Over the past decade, the cancer incidence rate (2005‐2014) has been found stable in women and declined by approximately 2% annually in men, while the overall cancer death rate (2006‐2015) declined by about 1.5% annually in both men and women (Mattiuzzi and Lippi 2019). Lung adenocarcinoma is also more common among women than men (Zang and Wynder 1996).
Environmental risk factors for lung cancer include smoking cigarettes and other tobacco products, as well as exposure to secondhand tobacco smoke, occupational lung carcinogens, radiation, and indoor and outdoor air pollution (Alberg et al. 2013; Kurt et al. 2016; Pope et al. 2002). However, lung cancer incidence patterns reflect trends in sex behaviors associated with cigarette smoking (Rivera 2013). Generally speaking, any form of smoking exposure increases the lung cancer risk (Yang et al. 2016; Alberg et al. 2013). A recent United States report indicated that lung cancer incidence and death rates among women has increased in 18 states. Interestingly, the states with higher prevalence of smoking among adult women had the highest rates of lung cancer. This report showed that only one state had decreasing lung cancer incidence and death rates in women (Jemal et al. 2008). Currently, lung cancer incidence rates are declining about twice as fast in men as in women, reflecting historical differences in tobacco uptake and cessation, as well as upturns in female smoking prevalence in some birth cohorts (Escobedo and Peddicord 1996). In addition, the implementation of widespread lung cancer screening holds promise for the future.
Zang et al found that the odds of developing major lung cancer types are consistently higher for women than for men at every level of exposure to cigarette smoke (Zang and Wynder 1996). This sex difference, however, cannot be explained by differences in baseline exposure, smoking history, or body size, but it is likely due to the higher susceptibility to tobacco carcinogens in women (Peng et al. 2017; Ben-Zaken Cohen et al. 2007). In this regard, higher levels of polycyclic aromatic hydrocarbon-derived DNA adducts have been reported in female smokers vs. male smokers (Mollerup et al. 2006). A potential mechanism associated with these outcomes is related to the fact that estrogen synergizes with some tobacco compounds through the induction of CYP1B1, an enzyme responsible for estrogenic metabolism, which leads to enhanced reactive oxygen species formation and carcinogenesis (Hsu et al. 2017). Moreover, Kure et al found a higher frequency of G:C-->T:A mutations and a higher average hydrophobic DNA adduct level in female patients than males, even though the level of exposure to carcinogens from cigarette smoke was lower among females than males. These findings lend support to epidemiological evidence that women are at greater risk than men of contracting tobacco-induced lung cancer (Kure et al. 1996).
As mentioned earlier, there is considerable evidence indicating that sex hormones can influence respiratory function throughout life (Townsend et al. 2012; Behan and Wenninger 2008). As with other lung diseases, sex hormones have also been implicated in lung cancer (Mollerup et al. 2002). For example, estrogen, known to be a risk factor for the development of adenocarcinoma of the breast, ovary, and endometrium, has been postulated to contribute to lung cancer development and progression (Zang and Wynder 1996; Siegfried 2001). Furthermore, estrogen has also been implicated in lung cancer therapy (Baik and Eaton 2012). Women with advanced NSCLC survive longer than men after adjustment for other prognostic factors in the modern chemotherapy era, suggesting that estrogen levels may interact with the efficacy of current chemotherapy prescriptions or other as yet undefined factors. This finding, if validated, could be potentially exploited in designing new therapies (Wakelee et al. 2006; Schiller et al. 2002).
Regarding estrogen receptors (ERs), Kadota et al reported that stage I lung adenocarcinoma cells express higher levels of ERα in females than males (19% vs. 14%), and that ERα expression correlates with smaller tumor size. The authors concluded that nuclear ERα expression is an independent predictor of recurrence in pT1a stage lung adenocarcinoma (i.e., tumor size of 2 cm or less), and correlates with poor prognostic immune microenvironments (Kadota et al. 2015). In addition, non–small cell lung cancer lines (both squamous cell and adenocarcinoma) have been found to express estrogen receptors (Olak and Colson 2004).
Hormone replacement therapy (HRT) is a common treatment used in postmenopausal women. To date, there are several controversies in the relationship between the HRT and lung cancer. The Women’s Health Initiative trial concluded that treatment with estrogen plus progestin in postmenopausal women did not increase the incidence of lung cancer. However, HRT was found to increase the number of deaths from lung cancer, in particular deaths from non-small-cell lung cancer (Chlebowski et al. 2009). These findings should be incorporated into risk-benefit discussions with women considering combined hormone therapy, especially those with a high risk of lung cancer.
In summary, there is accumulating evidence to support the notion that the risk of development of lung cancer is different among women than among men. As expressed earlier, women may be more susceptible to the effects of carcinogens in tobacco and tobacco smoke as a result of hormonal, genetic, and metabolic differences between the sexes. Thus, the significance of sex as a separate contributing factor shall be consider in prognosis and therapeutic management.
Lymphangioleiomyomatosis
Lymphangioleiomyomatosis (LAM) is a rare progressive lung disease that occurs almost exclusively in women (Xu et al. 2020). The incidence of LAM is estimated to range between 1 and 8 per million women, and the disease mostly affects women of childbearing age (Harknett et al. 2011). The average age of symptom onset among LAM patients in the United States and Europe ranges between 34–37 years of age (Collaborators 2017). LAM is characterized by infiltration of specific dysregulated smooth muscle-like cells (LAM cells) in various organs and tissues, including lymph nodes, kidneys, and the lungs. As a result, LAM patients experience a progressive decline in lung function due to parenchymal destruction and development of cysts in lung tissue.
The mechanisms underlying LAM development, and the marked sex disparity in its incidence, have not been fully elucidated (Han et al. 2018). However, the neoplastic phenotype of LAM cells is known to occur as a consequence of constitutive activation of the mechanistic target of rapamycin (mTOR) due to loss of heterozygosity in the tuberous sclerosis genes (TSC1 or TSC2) (Henske and McCormack 2012). Advances in the understanding of TSC biology have provided critical clues to LAM pathogenesis and treatment, and led to the use of the mTOR inhibitor sirolimus (i.e. rapamycin) as an effective suppressive therapy. Alternatively, lung transplantation is also an established option for women with severe pulmonary impairment due to LAM.
The striking sex disparity observed in LAM has led multiple investigators to consider a role of sex hormones, and specifically estrogen, in the development, progression, and severity of LAM disease (El-Chemaly and Henske 2015). LAM clinical presentation occurs after puberty, accelerated progression is frequently observed during pregnancy, and menopause is associated with attenuated progression (Makrigiannis and Anderson). Animal models and in vitro studies have also shown that estrogen increases cell proliferation and migration (Yu et al. 2004). Moreover, LAM cells are known to express both estrogen and progesterone receptors. However, definitive evidence is lacking regarding manipulating sex hormones as a potential therapeutic approach, and additional efforts are needed to develop strategies for disease prevention and treatment.
Obstructive Sleep Apnea
The prevalence of OSA is similar between the sexes before puberty, but becomes more common in boys than girls after puberty (Redline et al. 2007). This sexual dimorphism persists throughout adulthood, where both the rate and severity of OSA are higher in men compared to women. These differences have been attributed to anatomical differences in the upper airway and increased accumulation in the neck of fluid displaced from the legs during recumbency while sleeping (Kasai et al. 2014; Malhotra et al. 2002). Other risk factors include craniofacial abnormalities, genetic conditions and neuromuscular disorders. Studies in hypogonadal men and obese adolescents with low testosterone levels have suggested a role of male sex hormones in the observed sex differences in OSA (Matsumoto et al. 1985; Mogri et al. 2013). In females, progesterone has been found to increase the tone of upper airway muscles and stimulate ventilation via chemoreceptor responses to hypoxia and hypercapnia (Shahar et al. 2003; Popovic and White 1998). These sex hormone mediated mechanisms have been proposed to contribute to the lower risk and severity of OSA observed in girls and women after puberty.
Pulmonary Arterial Hypertension
Pulmonary arterial hypertension (PAH) is a progressive and devastating disease of the pulmonary vasculature characterized by extreme elevation of pulmonary arterial pressure and subsequent right ventricular failure (Foderaro and Ventetuolo 2016). PAH is also characterized by progressive obstruction of the pulmonary arterial circulation due to formation of vaso-occlusive lesions arising from vigorous proliferation and migration of endothelial cells (Ranchoux et al. 2015). As a result of the increased pulmonary vascular resistance, higher right ventricular (RV) afterload causes adaptive RV hypertrophy that often progresses to maladaptive RV hypertrophy and fibrosis, leading to eventual premature death from RV failure. Despite improvements in the diagnosis and management of PAH, the disease continues to have a poor prognosis. A recent analysis showed that the 5-year survival for PAH is approximately 60% (Medrek et al. 2020).
Accumulating evidence shows that more females than men are diagnosed with PAH; however, epidemiological data show that survival among females is better than among males, especially in older patients (Marra et al. 2016; Jacobs et al. 2014; Lahm et al. 2014; Badesch et al. 2010; Humbert et al. 2006). Interestingly, the survival benefit for females appears to decline with age (Shapiro et al. 2012), and correlate with declines in estradiol levels (Ventetuolo et al. 2014). This discrepancy in incidence and disease outcomes in men and women is commonly referred to as the PAH “estrogen paradox”, and has prompted research into the sex-based differences and hormonal regulation mechanisms in PAH. While the mechanisms behind the sex disparity are far from being understood, they likely involve contributions of genetics, as well as sex hormones and their metabolites.
A few studies have suggested a genetic component in PAH. Mutations in the gene encoding for the bone morphogenetic protein (BMP) receptor type 2 (BMPR2) has been shown to increase PAH penetrance and severity in mouse models (Fessel et al. 2013). Moreover, mutations in the BMPR2 gene are the most common genetic cause of familial PAH (Evans et al. 2016; Machado et al. 2009). By using the “four core genotypes” mouse model (De Vries et al. 2002), it was found that the Y chromosome, and specifically upregulation of Y chromosomal genes in the lung, was protective against pulmonary vascular remodeling (Umar et al. 2018) irrespective of gonadal sex. More recent studies have investigated whether genes encoding for enzymes that mediate estrogen metabolism, such as CYP1B1 are associated with PAH in males and females. West et. al found that CYP1B1 expression was markedly downregulated in female, but not male patients with PAH due to BMPR2 mutations (West et al. 2008).
Regarding sex hormones, there are multiple studies demonstrating that estrogens exerts complex and context-dependent effects on the pulmonary vasculature (Earley and Resta 2002; McMurtry et al. 1973; Rabinovitch et al. 1981; Lahm et al. 2012; Xu et al. 2010). Microarray analyses in animal models identified diverse set of pathways regulated by estradiol, including steroid metabolism, immune response, cytoskeletal function, extracellular matrix composition, bone morphogenetic protein (BMP), Notch, Wnt and calcium signaling (Frump et al. 2017). Studies in vascular cells have shown that estradiol affects proliferation (Mair et al. 2014; Mair et al. 2015; White et al. 2012). In a rescue approach experimental animal model, it was shown that estradiol treatment reversed pulmonary vascular remodeling, fibrosis, and inflammatory signaling (Umar et al. 2011). Overall, while some studies in animals have demonstrated that both exogenous and endogenous estradiol can be protective against PAH, others have suggested a more causative role (Mair et al. 2014; Kawut et al. 2017; Lahm and Frump 2017). Collectively, these studies demonstrate that both endogenous and exogenous estradiol can act as potent regulators of pulmonary vascular homeostasis and greatly impact the progression or resolution of vascular injury. However, these models do not display sex differences nor point to a female predisposition, indicating that more research is needed to fully understand the roles played by hormones in PAH in men and women. Accumulating evidence indicates that estrogen metabolites can also modulate PAH pathogenesis (Tofovic et al. 2010). Thus, it is important to consider the role of metabolites when investigating the effects of estrogen in PAH. Interestingly, low levels of dehydroepiandrosterone (DHEA), a precursor for estrogens that can bind estrogen receptors, are associated with PA development in men (Ventetuolo et al. 2016a). A recent study in post-menopausal women also showed that women with PAH had lower levels of DHEA and higher levels of estradiol than those without cardiopulmonary disease (Baird et al. 2018). In patients with PAH, low DHEA and high estradiol were also associated with worse prognosis and increased risk for death (Baird et al. 2018). Whether DHEA is a marker or mediator of PAH remains under investigation. Overall, more research is needed to understand the mechanisms mediating sex differences in PAH, in order to develop sex-specific therapies to prevent and treat this devastating disease.
Respiratory Infection
Respiratory infection remains a leading cause of morbidity and mortality across all age groups. While overall, males are disadvantaged in the occurrence and severity of lower respiratory tract infections, females appear to be more susceptible to upper respiratory infections (Falagas et al. 2007b). Multiple studies have suggested that a complex interplay of genetics, sex hormones, host immunity, anatomical and physiological differences, as well as sociocultural and behavioral are likely to underlie the observed sex differences in infection rates and severity (Ben-Shmuel et al. 2018; Chamekh et al. 2017; Kadioglu et al. 2011; Ingersoll 2017). In the following sections, we describe the epidemiology and current knowledge on respiratory diseases that present with a sexual dimorphism.
Respiratory syncytial virus
During infancy and early childhood, infection with respiratory syncytial virus (RSV) occurs more frequently in boys than girls, especially those born prematurely (Mahmoud et al. 2018). Resulting from RSV infection, bronchiolitis is also more frequent and severe in male infants and young children, and is often associated with higher risk of wheezing and childhood asthma, as well as higher risk of hospitalization (Nair et al. 2013; Henderson et al. 2005).
Sex differences in RSV infection and bronchiolitis have been attributed to anatomical and immunological factors, including smaller airway diameter in males than females (Carvajal et al. 2019), as well as sex differences the Th2/Th17 response to viral infection. Animal mouse models of RSV infection show that infected female mice display better viral control than males, via mechanisms involving interferon-β expression. In addition, male mice show persistent immune alterations in Th2/Th17 cells, dendritic cells, and type 2 innate lymphoid cell responses that result in delayed control of viremia (Malinczak et al. 2019). Similar studies have indicated that sex hormones and their receptors can also mediate these mechanisms, although their contributions to infant and pediatric infectious disease remains unclear (Kadel and Kovats 2018).
Influenza
Influenza is an acute respiratory infectious disease caused by influenza viruses. According to the World Health Organization, there are 3 to 5 million cases annually of severe illness, and about 290,000 to 650,000 respiratory deaths (World Health Organization 2020). The severity and mortality of influenza disease is worse for young children, the elderly, individuals with chronic and immunocompromised medical conditions, and pregnant women (Morgan and Klein 2019).
Researchers have reported sex differences in influenza severity, mortality, vaccine tolerance, responses and outcomes (Klein and Flanagan 2016). Interestingly, males are more susceptible to infection than females and females have greater immune responses but experience more adverse reactions to influenza vaccines than males (Klein et al. 2012; vom Steeg and Klein 2016). In addition, females of reproductive age have the worst outcome during pandemic influenza (World Health Organization 2010). However, the causes and mechanisms for these discrepancies in susceptibility are not well-known. Research groups have reported that immunity to viruses can vary with changes in hormone concentrations caused by fluctuations over the menstrual cycle, contraception use, pregnancy, and menopause (Brabin 2002).
Most experiments using murine models have shown that young adult females develop greater respiratory inflammatory responses and have a more severe outcome from influenza infection than males, despite the sexes having similar virus titers (Hoffmann et al. 2015; Robinson et al. 2011b; Robinson et al. 2011a). For instance, proinflammatory cytokines (e.g., TNFα, IFNγ, IL6, and IL12) and chemokines (e.g. CCL2, CCL5, and CCL12) are higher in the lungs of influenza-infected females when compared to males (Hoffmann et al. 2015). It was also discovered that increased levels of testosterone and amphiregulin, which is an epidermal growth factor that mediates lung tissue repair, improve repair and recovery of lung damage in males (Vermillion et al. 2018b). Moreover, infection of female mice of reproductive age with influenza decreases ovarian function and levels of sex hormones suggesting that inhibition of sex hormones may contribute to severe outcomes in female mice (Robinson et al. 2011b; Vermillion et al. 2018a). Independent research groups discovered that female mice with influenza that were treated with estrogen showed a decrease in the inflammatory response (e.g., CCL2, IFNγ, TNFα) and an increase in antibody response to influenza vaccine (Vom Steeg et al. 2016; Celestino et al. 2018). Importantly, the expression of toll-like receptor-7 is higher in B cells from vaccinated females than males and its deletion decreased sex differences in vaccine-induced antibody responses and protection (Fink et al. 2018). Future research should focus on the molecular mechanisms that regulate how hormones and genes affect immunity to influenza and vaccines in males vs. females.
Coronavirus Disease 2019
The coronavirus disease 2019 (COVID-19) is a public health crisis caused by the novel severe respiratory syndrome coronavirus 2 (SARS-CoV-2). As of this writing, there have been over 33 million confirmed COVID-19 cases and more than 985,000 deaths worldwide. Importantly, demographic, and clinical data gathered by multiple health agencies around the globe have demonstrated profound sex differences in COVID-19 outcomes. While the rate of SARS-CoV-2 infection is similar between males and females, male patients infected with the SARS-CoV-2 virus have a significantly higher risk of developing severe COVID-19, being admitted to an intensive care unit (ICU), and dying when compared to female infected patients (Klein et al. 2020b).
As mentioned earlier, sex-specific immune responses to a diverse array of viral pathogens have been reported (Bongen et al. 2019; Ghosh and Klein 2017; Piasecka et al. 2018; Schurz et al. 2019; vom Steeg and Klein 2016). In addition, there are also prominent sex differences in the immune responses mounted by individuals receiving viral vaccines (Klein and Flanagan 2016; Markle and Fish 2014). In the case of infection with coronaviruses, there have been reports of sex differences during prior outbreaks, including the 2003 severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) epidemics, that had a higher case fatality rate and number of deaths in males than females (Karlberg et al. 2004; Leong et al. 2006; Alghamdi et al. 2014).
While not all countries provide sex-disaggregated data, the Sex, Gender and Covid-19 Project (Global Health 2020) has combined efforts from agencies located in several continents to increase reporting of data by sex for confirmed cases, testing, hospitalizations, ICU admissions, confirmed cases among healthcare workers, and deaths. In almost all countries, a significant male predominance in COVID-19 morbidity and mortality has been reported, suggesting a biological mechanism involved (Scully et al. 2020). In the United States, most of the states have made public sex-disaggregated data on COVID-19 morbidity and mortality. In an article published in June of 2020, Klein et al. reported that in states providing sex-disaggregated information, data shows that men are twice as likely to die from COVID-19 than women (Klein et al. 2020b). Moreover, sex differences in the immune response to SARS-CoV-2 have also been reported, where males with mild disease had higher plasma levels of pro-inflammatory cytokines and chemokines than females, but females had higher CD4 and CD8 T cell activation than males (Takahashi et al. 2020). A study comparing responses to convalescent plasma also showed higher microneutralization and IgG responses to SARS-CoV-2 in males than to females, which correlated with worse COVID-19 outcomes (Klein et al. 2020a).
Some of these sex effects have been attributed to chromosomal differences, since the X chromosome has been shown to express a large number of immune related genes, including some involved in cytokine and toll-like receptor (TLR) signaling, NF-kB and MAPK signaling (Spolarics et al. 2017). In addition, the gene encoding the human angiotensin-converting enzyme 2 (ACE2), which serves as the receptor for the spike (S) protein of SARS-CoV-2 (Chen et al. 2020) is also expressed in the X chromosome, and can escape X inactivation and be expressed from both the active and inactive X chromosome (Carrel and Willard 2005). This has been shown to lead to sex differences in ACE2 gene expression (Gemmati et al. 2020; Li et al. 2020; Tukiainen et al. 2017), which has potential consequences for the vulnerability to SARS-CoV-2.
As with other inflammatory lung diseases and infectious processes, a role of sex hormones has been postulated in mediating sex differences in COVID-19 (Spagnolo et al. 2020; Strope et al. 2020). Estrogen can regulate the expression of SARS-CoV-2 viral entry receptors, including ACE2 and the transmembrane protease, serine 2 (TMPRSS2) (Hoffmann et al. 2020; Majdic 2020). In this context, a recent report showed that post-pubertal females have lower levels of serum ACE2 when compared to age-matched males (Sward et al. 2020). Furthermore, the serum activity of ACE2 is higher in post-menopausal women when compared to younger women, suggesting a regulation by sex hormones like estrogen (Gebhard et al. 2020). Interestingly, Stelzig et al. recently showed that estrogen can downregulate the expression of ACE2 in normal human bronchial epithelial (NHBE) cells, but had no effect on TMPRSS2 (Stelzig et al. 2020). This correlates with prior work conducted in the four core genotypes model indicating that sex differences in enzymatic activity of ACE2 in mice are estrogen-dependent and sex chromosome-independent (Liu et al. 2010).
Regarding male sex hormones, it is unclear whether androgen levels contribute to SARS-CoV-2 or COVID-19 outcomes. A recent report showed that in males with SARS-CoV-2 pneumonia, low testosterone levels were associated with higher rates of ICU admission and death (Rastrelli et al. 2020). This correlates with prior studies showing that testosterone can upregulate IL-1 and downregulate IL-1β, IL-6, and TNF-α leading to a suppression of inflammation (Mohamad et al. 2019; Pozzilli and Lenzi 2020). Future studies investigating the effects of androgen levels on COVID-19 should consider the timing of the androgen measurement in the course of the SARS-CoV-2 infection (Strope et al. 2020). Testosterone can also regulate the expression of TMPRSS2 (Giagulli et al. 2020; Stopsack et al. 2020), thus contributing to viral infection and disease outcomes. Interestingly, TMPRSS2 is also highly expressed in urogenital organs, such as the prostate (Chakravarty et al. 2020).
Finally, it has been hypothesized that gender factors, i.e. smoking habits, handwashing, caregiver gender roles, etc. can influence the outcome of SARS-CoV-2 infections (Cai 2020; Vardavas and Nikitara 2020; Wenham et al. 2020; Gebhard et al. 2020). There are also significant sex and gender differences in comorbidities that have been associated with COVID-19 progression and outcomes (Sharma et al. 2020). In general, these comorbidities tend to be more prevalent in men than women (Wu and McGoogan 2020). Thus, several structural gender health disparities will need to be addressed in order to effectively mitigate the negative effects of the COVID-19 pandemic (Spagnolo et al. 2020).
Conclusion
Gender and sex differences in the prevalence, severity, and susceptibility to a variety of lung diseases have been reported across the lifespan (Fig. 1). While the causes of these disparities have not been fully elucidated, a lot has been accomplished in the past few decades. These investigations have revealed associations of biological factors (sex) such as airway anatomy and physiology, chromosomal contributions, genetics and epigenetics, and sex hormones with lung disease onset and outcomes in men and women. Others have shown that sociocultural and environmental factors (gender) can also influence differential outcomes in lung disease. Understanding the contributions of sex and gender, as well as their complex interplay in the context of respiratory health, represent a fundamental step toward precision medicine and the future development of more effective options to prevent and treat lung disease.
Fig. 1. Sex differences in lung disease progression across lifespan.
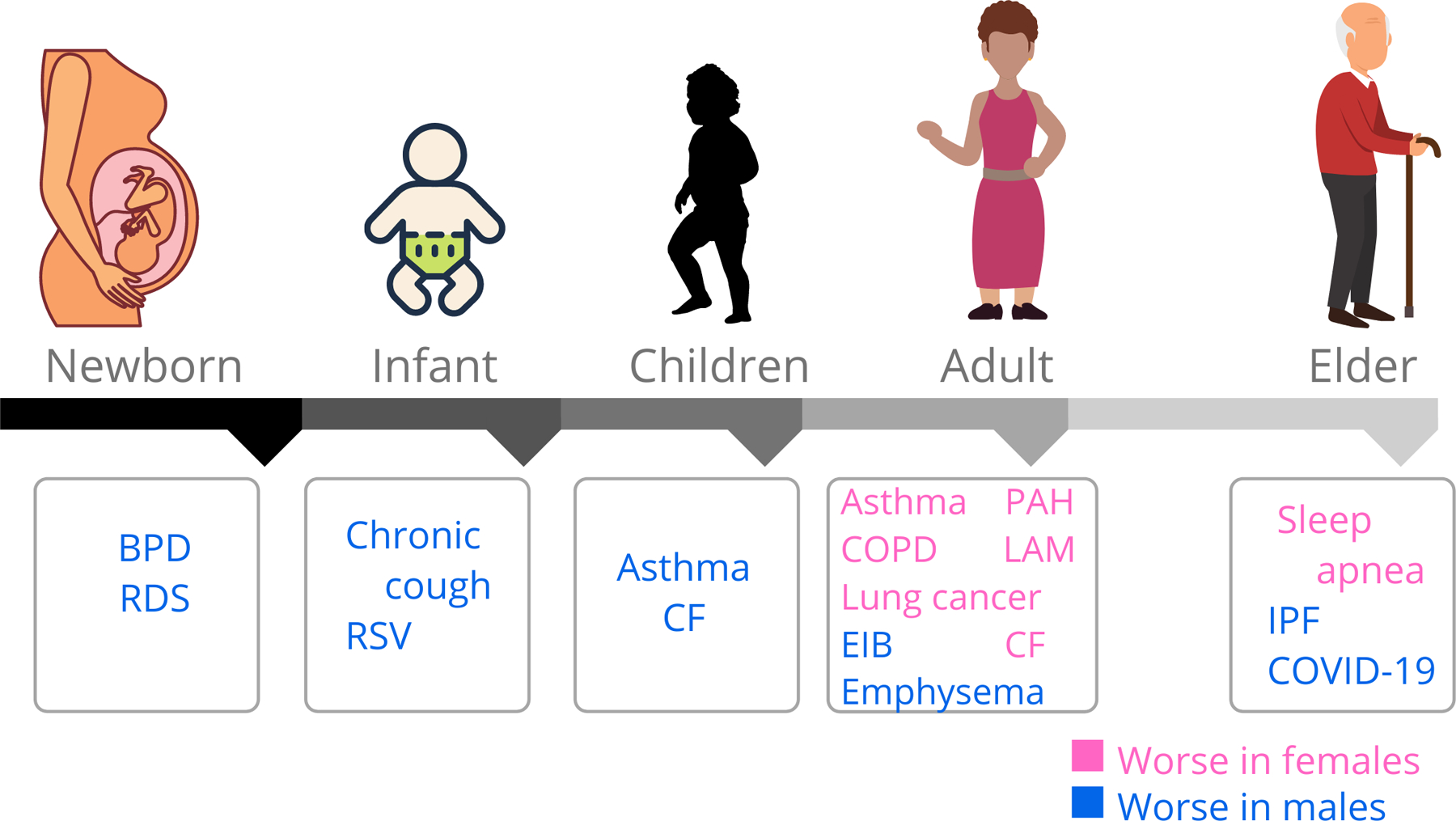
There are sex differences in the prevalence of several lung diseases across the lifespan. In pink are lung diseases that are more prevalent in females than males (blue). (Abbreviations- BPD: Bronchopulmonary dysplasia; RDS: Respiratory distress syndrome; RSV: Respiratory syncytial virus; CF: Cystic fibrosis; PAH: Pulmonary arterial hypertension; COPD: Chronic obstructive pulmonary disease; LAM: Lymphangioleiomyomatosis; EIB: Exercise-induced bronchoconstriction; IPF: Idiopathic pulmonary fibrosis; COVID-19: Coronavirus disease 2019)
Acknowledgments
This research was supported by the National Heart Lung and Blood Institute of the National Institutes of Health under awards number HL133520 and HL141618 (PS).
Contributor Information
Patricia Silveyra, Biobehavioral Laboratory, School of Nursing, University of North Carolina at Chapel Hill, 4013 Carrington Hall, Chapel Hill, NC, USA 27599,.
Nathalie Fuentes, National Institute of Allergy, Asthma, and Infectious Diseases, Bethesda, MD,.
Daniel Rodriguez Bauza, Clinical Simulation Center, The Pennsylvania State University College of Medicine, 500 University Drive, Hershey, PA 17033 USA,.
References
- Abid S, Xie S, Bose M, Shaul PW, Terada LS, Brody SL, Thomas PJ, Katzenellenbogen JA, Kim SH, Greenberg DE, Jain R (2017) 17β-estradiol dysregulates innate immune responses to Pseudomonas aeruginosa respiratory infection and is modulated by estrogen receptor antagonism. Infect Immun. doi: 10.1128/IAI.00422-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akinbami LJ, Liu X (2011) Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS Data Brief (63):1–8 [PubMed] [Google Scholar]
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X (2012) Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief (94):1–8 [PubMed] [Google Scholar]
- Alberg AJ, Brock MV, Ford JG, Samet JM, Spivack SD (2013) Epidemiology of lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 143 (5 Suppl):e1S–e29S. doi: 10.1378/chest.12-2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alghamdi IG, Hussain II, Almalki SS, Alghamdi MS, Alghamdi MM, El-Sheemy MA (2014) The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med 7:417–423. doi: 10.2147/IJGM.S67061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali K, Greenough A (2012) Long-term respiratory outcome of babies born prematurely. Ther Adv Respir Dis 6 (2):115–120. doi: 10.1177/1753465812436803 [DOI] [PubMed] [Google Scholar]
- Allen RJ, Porte J, Braybrooke R, Flores C, Fingerlin TE, Oldham JM, Guillen-Guio B, Ma SF, Okamoto T, John AE, Obeidat M, Yang IV, Henry A, Hubbard RB, Navaratnam V, Saini G, Thompson N, Booth HL, Hart SP, Hill MR, Hirani N, Maher TM, McAnulty RJ, Millar AB, Molyneaux PL, Parfrey H, Rassl DM, Whyte MKB, Fahy WA, Marshall RP, Oballa E, Bosse Y, Nickle DC, Sin DD, Timens W, Shrine N, Sayers I, Hall IP, Noth I, Schwartz DA, Tobin MD, Wain LV, Jenkins RG (2017) Genetic variants associated with susceptibility to idiopathic pulmonary fibrosis in people of European ancestry: a genome-wide association study. Lancet Respir Med 5 (11):869–880. doi: 10.1016/s2213-2600(17)30387-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SD, Schoeffel RE, Black JL, Daviskas E (1985) Airway cooling as the stimulus to exercise-induced asthma--a re-evaluation. Eur J Respir Dis 67 (1):20–30 [PubMed] [Google Scholar]
- Angus DC, Linde-Zwirble WT, Clermont G, Griffin MF, Clark RH (2001) Epidemiology of neonatal respiratory failure in the United States: projections from California and New York. American journal of respiratory and critical care medicine 164 (7):1154–1160. doi: 10.1164/ajrccm.164.7.2012126 [DOI] [PubMed] [Google Scholar]
- Aryal S, Diaz-Guzman E, Mannino DM (2013) COPD and gender differences: an update. Transl Res 162 (4):208–218. doi: 10.1016/j.trsl.2013.04.003 [DOI] [PubMed] [Google Scholar]
- Aryal S, Diaz-Guzman E, Mannino DM (2014) Influence of sex on chronic obstructive pulmonary disease risk and treatment outcomes. Int J Chron Obstruct Pulmon Dis 9:1145–1154. doi: 10.2147/COPD.S54476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD (2010) Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 137 (2):376–387. doi: 10.1378/chest.09-1140 [DOI] [PubMed] [Google Scholar]
- Baik CS, Eaton KD (2012) Estrogen signaling in lung cancer: an opportunity for novel therapy. Cancers (Basel) 4 (4):969–988. doi: 10.3390/cancers4040969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancalari E, Jain D (2019) Bronchopulmonary Dysplasia: 50 Years after the Original Description. Neonatology 115 (4):384–391. doi: 10.1159/000497422 [DOI] [PubMed] [Google Scholar]
- Bancalari EH, Jobe AH (2012) The respiratory course of extremely preterm infants: a dilemma for diagnosis and terminology. J Pediatr 161 (4):585–588. doi: 10.1016/j.jpeds.2012.05.054 [DOI] [PubMed] [Google Scholar]
- Barnes PJ (2016) Sex Differences in Chronic Obstructive Pulmonary Disease Mechanisms. Am J Respir Crit Care Med 193 (8):813–814. doi: 10.1164/rccm.201512-2379ED [DOI] [PubMed] [Google Scholar]
- Barratt SL, Creamer A, Hayton C, Chaudhuri N (2018) Idiopathic Pulmonary Fibrosis (IPF): An Overview. J Clin Med 7 (8). doi: 10.3390/jcm7080201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre SF, Haberthur D, Cremona TP, Stampanoni M, Schittny JC (2016) The total number of acini remains constant throughout postnatal rat lung development. Am J Physiol Lung Cell Mol Physiol 311 (6):L1082–L1089. doi: 10.1152/ajplung.00325.2016 [DOI] [PubMed] [Google Scholar]
- Barta JA, Powell CA, Wisnivesky JP (2019) Global Epidemiology of Lung Cancer. Ann Glob Health 85 (1). doi: 10.5334/aogh.2419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra-Díaz M, Strickland AB, Keselman A, Heller NM (2018) Androgen and Androgen Receptor as Enhancers of M2 Macrophage Polarization in Allergic Lung Inflammation. J Immunol. doi: 10.4049/jimmunol.1800352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becklake MR, Kauffmann F (1999) Gender differences in airway behaviour over the human life span. Thorax 54 (12):1119–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan M, Wenninger JM (2008) Sex steroidal hormones and respiratory control. Respir Physiol Neurobiol 164 (1–2):213–221. doi: 10.1016/j.resp.2008.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shmuel A, Sheiner E, Wainstock T, Landau D, Vaknin F, Walfisch A (2018) The association between gender and pediatric respiratory morbidity. Pediatric pulmonology 53 (9):1225–1230. doi: 10.1002/ppul.24083 [DOI] [PubMed] [Google Scholar]
- Ben-Zaken Cohen S, Paré PD, Man SF, Sin DD (2007) The growing burden of chronic obstructive pulmonary disease and lung cancer in women: examining sex differences in cigarette smoke metabolism. Am J Respir Crit Care Med 176 (2):113–120. doi:200611–1655PP. doi: 10.1164/rccm.200611-1655PP [DOI] [PubMed] [Google Scholar]
- Benscoter DT (2018) Bronchiectasis, Chronic Suppurative Lung Disease and Protracted Bacterial Bronchitis. Curr Probl Pediatr Adolesc Health Care 48 (4):119–123. doi: 10.1016/j.cppeds.2018.03.003 [DOI] [PubMed] [Google Scholar]
- Binet ME, Bujold E, Lefebvre F, Tremblay Y, Piedboeuf B, Canadian Neonatal N (2012) Role of gender in morbidity and mortality of extremely premature neonates. Am J Perinatol 29 (3):159–166. doi: 10.1055/s-0031-1284225 [DOI] [PubMed] [Google Scholar]
- Boas SR, Cleary DA, Lee PA, Orenstein DM (1996) Salivary testosterone levels in male adolescents with cystic fibrosis. Pediatrics 97 (3):361–363 [PubMed] [Google Scholar]
- Boezen HM, Jansen DF, Postma DS (2004) Sex and gender differences in lung development and their clinical significance. Clin Chest Med 25 (2):237–245. doi: 10.1016/j.ccm.2004.01.012 [DOI] [PubMed] [Google Scholar]
- Bongen E, Lucian H, Khatri A, Fragiadakis GK, Bjornson ZB, Nolan GP, Utz PJ, Khatri P (2019) Sex Differences in the Blood Transcriptome Identify Robust Changes in Immune Cell Proportions with Aging and Influenza Infection. Cell Rep 29 (7):1961–1973 e1964. doi: 10.1016/j.celrep.2019.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brabin L (2002) Interactions of the female hormonal environment, susceptibility to viral infections, and disease progression. AIDS Patient Care STDS 16 (5):211–221. doi: 10.1089/10872910252972267 [DOI] [PubMed] [Google Scholar]
- Brenner BE, Holmes TM, Mazal B, Camargo CA (2005) Relation between phase of the menstrual cycle and asthma presentations in the emergency department. Thorax 60 (10):806–809. doi: 10.1136/thx.2004.033928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H (2020) Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 8 (4):e20. doi: 10.1016/S2213-2600(20)30117-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canguven O, Albayrak S (2011) Do low testosterone levels contribute to the pathogenesis of asthma? Med Hypotheses 76 (4):585–588. doi: 10.1016/j.mehy.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Arbes SJ, Germolec DR, Korach KS, Zeldin DC (2007a) It’s all about sex: gender, lung development and lung disease. Trends Endocrinol Metab 18 (8):308–313. doi:S1043–2760(07)00132–4. doi: 10.1016/j.tem.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC (2007b) The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol 293 (2):L272–278. doi:00174.2007. doi: 10.1152/ajplung.00174.2007 [DOI] [PubMed] [Google Scholar]
- Carlo WA, Stark AR, Wright LL, Tyson JE, Papile LA, Shankaran S, Donovan EF, Oh W, Bauer CR, Saha S, Poole WK, Stoll B (2002) Minimal ventilation to prevent bronchopulmonary dysplasia in extremely-low-birth-weight infants. J Pediatr 141 (3):370–374 [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF (2005) X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434 (7031):400–404. doi: 10.1038/nature03479 [DOI] [PubMed] [Google Scholar]
- Carvajal JJ, Avellaneda AM, Salazar-Ardiles C, Maya JE, Kalergis AM, Lay MK (2019) Host Components Contributing to Respiratory Syncytial Virus Pathogenesis. Front Immunol 10:2152. doi: 10.3389/fimmu.2019.02152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin EA, Powell SM, Manganaro TF, Hudson PL, Ragin RC, Epstein J, Donahoe PK (1990) Sex-specific fetal lung development and müllerian inhibiting substance. The American review of respiratory disease 141 (2):466–470. doi: 10.1164/ajrccm/141.2.466 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2020) Most Recent Asthma Data. https://www.cdc.gov/asthma/most_recent_data.htm.
- Celestino I, Checconi P, Amatore D, De Angelis M, Coluccio P, Dattilo R, Alunni Fegatelli D, Clemente AM, Matarrese P, Torcia MG, Mancinelli R, Mammola CL, Garaci E, Vestri AR, Malorni W, Palamara AT, Nencioni L (2018) Differential Redox State Contributes to Sex Disparities in the Response to Influenza Virus Infection in Male and Female Mice. Front Immunol 9:1747. doi: 10.3389/fimmu.2018.01747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, Mohamed N, Lundon D, Dovey Z, Kyprianou N, Tewari AK (2020) Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 3 (1):374. doi: 10.1038/s42003-020-1088-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamekh M, Deny M, Romano M, Lefèvre N, Corazza F, Duchateau J, Casimir G (2017) Differential Susceptibility to Infectious Respiratory Diseases between Males and Females Linked to Sex-Specific Innate Immune Inflammatory Response. Frontiers in Immunology 8:1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Guo Y, Pan Y, Zhao ZJ (2020) Structure analysis of the receptor binding of 2019-nCoV. Biochem Biophys Res Commun. doi: 10.1016/j.bbrc.2020.02.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlebowski RT, Schwartz AG, Wakelee H, Anderson GL, Stefanick ML, Manson JE, Rodabough RJ, Chien JW, Wactawski-Wende J, Gass M, Kotchen JM, Johnson KC, O’Sullivan MJ, Ockene JK, Chen C, Hubbell FA, Investigators WsHI (2009) Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet 374 (9697):1243–1251. doi: 10.1016/S0140-6736(09)61526-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotirmall SH, Greene CM, McElvaney NG (2012) Immune, inflammatory and infectious consequences of estrogen in women with cystic fibrosis. Expert Rev Respir Med 6 (6):573–575. doi: 10.1586/ers.12.59 [DOI] [PubMed] [Google Scholar]
- Coarfa C, Zhang Y, Maity S, Perera DN, Jiang W, Wang L, Couroucli X, Moorthy B, Lingappan K (2017) Sexual dimorphism of the pulmonary transcriptome in neonatal hyperoxic lung injury: identification of angiogenesis as a key pathway. Am J Physiol Lung Cell Mol Physiol 313 (6):L991–L1005. doi: 10.1152/ajplung.00230.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen J, Emerson J, Sanders DB, Ren C, Schechter MS, Gibson RL, Morgan W, Rosenfeld M, Group ES (2015) Risk factors for lung function decline in a large cohort of young cystic fibrosis patients. Pediatr Pulmonol 50 (8):763–770. doi: 10.1002/ppul.23217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebatch HJ, Greaves IA, Ng CK (1979) Exponential analysis of elastic recoil and aging in healthy males and females. J Appl Physiol Respir Environ Exerc Physiol 47 (4):683–691. doi: 10.1152/jappl.1979.47.4.683 [DOI] [PubMed] [Google Scholar]
- Collaborators GCRD (2017) Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med 5 (9):691–706. doi: 10.1016/S2213-2600(17)30293-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborators GCRD (2020) Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 8 (6):585–596. doi: 10.1016/S2213-2600(20)30105-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney JM, Bradley J, Mccaughan J, O’Connor TM, Shortt C, Bredin CP, Bradbury I, Elborn JS (2007) Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol 42 (6):525–532. doi: 10.1002/ppul.20619 [DOI] [PubMed] [Google Scholar]
- Davidson LM, Berkelhamer SK (2017) Bronchopulmonary Dysplasia: Chronic Lung Disease of Infancy and Long-Term Pulmonary Outcomes. J Clin Med 6 (1). doi: 10.3390/jcm6010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Torres JP, Cote CG, Lopez MV, Casanova C, Diaz O, Marin JM, Pinto-Plata V, de Oca MM, Nekach H, Dordelly LJ, Aguirre-Jaime A, Celli BR (2009) Sex differences in mortality in patients with COPD. Eur Respir J 33 (3):528–535. doi:09031936.00096108. doi: 10.1183/09031936.00096108 [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Rissman EF, Simerly RB, Yang L-Y, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP (2002) A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. J Neurosci 22 (20):9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal EC, McFadden ER, Ingram RH, Strauss RH, Jaeger JJ (1979) Role of respiratory heat exchange in production of exercise-induced asthma. J Appl Physiol Respir Environ Exerc Physiol 46 (3):467–475. doi: 10.1152/jappl.1979.46.3.467 [DOI] [PubMed] [Google Scholar]
- Demko CA, Byard PJ, Davis PB (1995) Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol 48 (8):1041–1049. doi: 10.1016/0895-4356(94)00230-n [DOI] [PubMed] [Google Scholar]
- Diab Cáceres L, Girón Moreno RM, García Castillo E, Pastor Sanz MT, Olveira C, García Clemente M, Nieto Royo R, Prados Sánchez C, Caballero Sánchez P, Olivera Serrano MJ, Padilla Galo A, Nava Tomas E, Esteban Peris A, Fernández Velilla M, Torres MI, Ancochea Bermúdez J (2020) Effect of Sex Differences on Computed Tomography Findings in Adults With Cystic Fibrosis: A Multicenter Study. Arch Bronconeumol. doi: 10.1016/j.arbres.2019.12.028 [DOI] [PubMed] [Google Scholar]
- Dodd KE, Wood J, Mazurek JM (2020) Mortality Among Persons with Both Asthma and Chronic Obstructive Pulmonary Disease Aged ≥25 Years, by Industry and Occupation - United States, 1999–2016. MMWR Morb Mortal Wkly Rep 69 (22):670–679. doi: 10.15585/mmwr.mm6922a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley S, Resta TC (2002) Estradiol attenuates hypoxia-induced pulmonary endothelin-1 gene expression. Am J Physiol Lung Cell Mol Physiol 283 (1):L86–93. doi: 10.1152/ajplung.00476.2001 [DOI] [PubMed] [Google Scholar]
- El-Chemaly S, Henske EP (2015) The next breakthrough in LAM clinical trials may be their design: challenges in design and execution of future LAM clinical trials. Expert Rev Respir Med 9 (2):195–204. doi: 10.1586/17476348.2015.1024663 [DOI] [PubMed] [Google Scholar]
- Escobedo LG, Peddicord JP (1996) Smoking prevalence in US birth cohorts: the influence of gender and education. Am J Public Health 86 (2):231–236. doi: 10.2105/ajph.86.2.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Girerd B, Montani D, Wang XJ, Galiè N, Austin ED, Elliott G, Asano K, Grünig E, Yan Y, Jing ZC, Manes A, Palazzini M, Wheeler LA, Nakayama I, Satoh T, Eichstaedt C, Hinderhofer K, Wolf M, Rosenzweig EB, Chung WK, Soubrier F, Simonneau G, Sitbon O, Gräf S, Kaptoge S, Di Angelantonio E, Humbert M, Morrell NW (2016) BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med 4 (2):129–137. doi: 10.1016/s2213-2600(15)00544-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falagas ME, Mourtzoukou EG, Vardakas KZ (2007a) Sex differences in the incidence and severity of respiratory tract infections. Respir Med 101 (9):1845–1863 [DOI] [PubMed] [Google Scholar]
- Falagas ME, Mourtzoukou EG, Vardakas KZ (2007b) Sex differences in the incidence and severity of respiratory tract infections. Respiratory Medicine 101 (9):1845–1863. doi: 10.1016/j.rmed.2007.04.011 [DOI] [PubMed] [Google Scholar]
- Fernández Fabrellas E, Peris Sánchez R, Sabater Abad C, Juan Samper G (2018) Prognosis and Follow-Up of Idiopathic Pulmonary Fibrosis. Med Sci (Basel) 6 (2). doi: 10.3390/medsci6020051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessel JP, Chen X, Frump A, Gladson S, Blackwell T, Kang C, Johnson J, Loyd JE, Hemnes A, Austin E, West J (2013) Interaction between bone morphogenetic protein receptor type 2 and estrogenic compounds in pulmonary arterial hypertension. Pulm Circ 3 (3):564–577. doi: 10.1086/674312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink AL, Engle K, Ursin RL, Tang WY, Klein SL (2018) Biological sex affects vaccine efficacy and protection against influenza in mice. Proc Natl Acad Sci U S A 115 (49):12477–12482. doi: 10.1073/pnas.1805268115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher B, Kulovich MV, Hallman M, Gluck L (1985) Lung profile: sex differences in normal pregnancy. Obstet Gynecol 66 (3):327–330 [PubMed] [Google Scholar]
- Foderaro A, Ventetuolo CE (2016) Pulmonary Arterial Hypertension and the Sex Hormone Paradox. Curr Hypertens Rep 18 (11):84. doi: 10.1007/s11906-016-0689-7 [DOI] [PubMed] [Google Scholar]
- Frump AL, Albrecht ME, McClintick JN, Lahm T (2017) Estrogen receptor-dependent attenuation of hypoxia-induced changes in the lung genome of pulmonary hypertension rats. Pulm Circ 7 (1):232–243. doi: 10.1177/2045893217702055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs O, Bahmer T, Weckmann M, Dittrich AM, Schaub B, Rösler B, Happle C, Brinkmann F, Ricklefs I, König IR, Watz H, Rabe KF, Kopp MV, Hansen G, von Mutius E, (DZL) ASGapotGCfLR (2018) The all age asthma cohort (ALLIANCE) - from early beginnings to chronic disease: a longitudinal cohort study. BMC Pulm Med 18 (1):140. doi: 10.1186/s12890-018-0705-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes N, Nicoleau M, Cabello N, Montes D, Zomorodi N, Chroneos ZC, Silveyra P (2019) 17b-estradiol affects lung function and inflammation following ozone exposure in a sex-specific manner. Am J Physiol Lung Cell Mol Physiol. doi: 10.1152/ajplung.00176.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes N, Silveyra P (2018) Endocrine regulation of lung disease and inflammation. Exp Biol Med (Maywood):1535370218816653. doi: 10.1177/1535370218816653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuseini H, Newcomb DC (2017) Mechanisms Driving Gender Differences in Asthma. Curr Allergy Asthma Rep 17 (3):19. doi: 10.1007/s11882-017-0686-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard C, Regitz-Zagrosek V, Neuhauser HK, Morgan R, Klein SL (2020) Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ 11 (1):29. doi: 10.1186/s13293-020-00304-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemmati D, Bramanti B, Serino ML, Secchiero P, Zauli G, Tisato V (2020) COVID-19 and Individual Genetic Susceptibility/Receptivity: Role of ACE1/ACE2 Genes, Immunity, Inflammation and Coagulation. Might the Double X-chromosome in Females Be Protective against SARS-CoV-2 Compared to the Single X-Chromosome in Males? Int J Mol Sci 21 (10). doi: 10.3390/ijms21103474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Klein RS (2017) Sex Drives Dimorphic Immune Responses to Viral Infections. J Immunol 198 (5):1782–1790. doi: 10.4049/jimmunol.1601166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giagulli VA, Guastamacchia E, Magrone T, Jirillo E, Lisco G, De Pergola G, Triggiani V (2020) Worse progression of COVID-19 in men: Is testosterone a key factor? Andrology. doi: 10.1111/andr.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINA (2019). Retrieved from: https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed September 24, 2020.
- Global Health 50/50 (2020) The COVID-19 Sex-Disaggregated Data Tracker. https://globalhealth5050.org/the-sex-gender-and-covid-19-project/. Accessed September 25, 2020
- Gortner L, Shen J, Tutdibi E (2013) Sexual dimorphism of neonatal lung development. Klin Padiatr 225 (2):64–69. doi: 10.1055/s-0033-1333758 [DOI] [PubMed] [Google Scholar]
- Gyamfi-Bannerman C, Thom EA, Blackwell SC, Tita AT, Reddy UM, Saade GR, Rouse DJ, McKenna DS, Clark EA, Thorp JM Jr., Chien EK, Peaceman AM, Gibbs RS, Swamy GK, Norton ME, Casey BM, Caritis SN, Tolosa JE, Sorokin Y, VanDorsten JP, Jain L, Network NM-FMU (2016) Antenatal Betamethasone for Women at Risk for Late Preterm Delivery. The New England journal of medicine 374 (14):1311–1320. doi: 10.1056/NEJMoa1516783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Arteaga-Solis E, Blenis J, Bourjeily G, Clegg DJ, DeMeo D, Duffy J, Gaston B, Heller NM, Hemnes A, Henske EP, Jain R, Lahm T, Lancaster LH, Lee J, Legato MJ, McKee S, Mehra R, Morris A, Prakash YS, Stampfli MR, Gopal-Srivastava R, Laposky AD, Punturieri A, Reineck L, Tigno X, Clayton J (2018) Female Sex and Gender in Lung/Sleep Health and Disease. Increased Understanding of Basic Biological, Pathophysiological, and Behavioral Mechanisms Leading to Better Health for Female Patients with Lung Disease. Am J Respir Crit Care Med 198 (7):850–858. doi: 10.1164/rccm.201801-0168WS [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han MK, Murray S, Fell CD, Flaherty KR, Toews GB, Myers J, Colby TV, Travis WD, Kazerooni EA, Gross BH, Martinez FJ (2008) Sex differences in physiological progression of idiopathic pulmonary fibrosis. Eur Respir J 31 (6):1183–1188. doi: 10.1183/09031936.00165207 [DOI] [PubMed] [Google Scholar]
- Harknett EC, Chang WY, Byrnes S, Johnson J, Lazor R, Cohen MM, Gray B, Geiling S, Telford H, Tattersfield AE, Hubbard RB, Johnson SR (2011) Use of variability in national and regional data to estimate the prevalence of lymphangioleiomyomatosis. QJM 104 (11):971–979. doi: 10.1093/qjmed/hcr116 [DOI] [PubMed] [Google Scholar]
- Harness-Brumley CL, Elliott AC, Rosenbluth DB, Raghavan D, Jain R (2014) Gender differences in outcomes of patients with cystic fibrosis. J Womens Health (Larchmt) 23 (12):1012–1020. doi: 10.1089/jwh.2014.4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris C, Zivanovic S, Lunt A, Calvert S, Bisquera A, Marlow N, Peacock JL, Greenough A (2020) Lung function and respiratory outcomes in teenage boys and girls born very prematurely. Pediatr Pulmonol 55 (3):682–689. doi: 10.1002/ppul.24631 [DOI] [PubMed] [Google Scholar]
- Henderson J, Hilliard TN, Sherriff A, Stalker D, Al Shammari N, Thomas HM (2005) Hospitalization for RSV bronchiolitis before 12 months of age and subsequent asthma, atopy and wheeze: a longitudinal birth cohort study. Pediatric allergy and immunology : official publication of the European Society of Pediatric Allergy and Immunology 16 (5):386–392. doi: 10.1111/j.1399-3038.2005.00298.x [DOI] [PubMed] [Google Scholar]
- Henderson-Smart DJ, Hutchinson JL, Donoghue DA, Evans NJ, Simpson JM, Wright I, Network AaNZN (2006) Prenatal predictors of chronic lung disease in very preterm infants. Arch Dis Child Fetal Neonatal Ed 91 (1):F40–45. doi: 10.1136/adc.2005.072264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henske EP, McCormack FX (2012) Lymphangioleiomyomatosis - a wolf in sheep’s clothing. J Clin Invest 122 (11):3807–3816. doi: 10.1172/JCI58709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepper PG, Shannon EA, Dornan JC (1997) Sex differences in fetal mouth movements. Lancet 350 (9094):1820. doi: 10.1016/S0140-6736(05)63635-5 [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Otte A, Thiele S, Lotter H, Shu Y, Gabriel G (2015) Sex differences in H7N9 influenza A virus pathogenesis. Vaccine 33 (49):6949–6954. doi: 10.1016/j.vaccine.2015.08.044 [DOI] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Muller MA, Drosten C, Pohlmann S (2020) SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert D, Réglier-Poupet H, Sermet-Gaudelus I, Ferroni A, Le Bourgeois M, Burgel PR, Serreau R, Dusser D, Poyart C, Coste J (2013) Association between Staphylococcus aureus alone or combined with Pseudomonas aeruginosa and the clinical condition of patients with cystic fibrosis. J Cyst Fibros 12 (5):497–503. doi: 10.1016/j.jcf.2012.12.003 [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, Yaici A, Weitzenblum E, Cordier JF, Chabot F, Dromer C, Pison C, Reynaud-Gaubert M, Haloun A, Laurent M, Hachulla E, Simonneau G (2006) Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 173 (9):1023–1030. doi: 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- Ingersoll MA (2017) Sex differences shape the response to infectious diseases. PLoS Pathog 13 (12):e1006688. doi: 10.1371/journal.ppat.1006688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishak N, Sozo F, Harding R, De Matteo R (2014) Does lung development differ in male and female fetuses? Experimental lung research 40 (1):30–39. doi: 10.3109/01902148.2013.858197 [DOI] [PubMed] [Google Scholar]
- Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, Kawut SM, Bogaard HJ, Boonstra A, Vonk Noordegraaf A (2014) The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest 145 (6):1230–1236. doi: 10.1378/chest.13-1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Ray JM, Pan JH, Brody SL (2012) Sex hormone-dependent regulation of cilia beat frequency in airway epithelium. Am J Respir Cell Mol Biol 46 (4):446–453. doi: 10.1165/rcmb.2011-0107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK (2008) Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100 (23):1672–1694. doi: 10.1093/jnci/djn389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins CR, Chapman KR, Donohue JF, Roche N, Tsiligianni I, Han MK (2017) Improving the Management of COPD in Women. Chest 151 (3):686–696. doi: 10.1016/j.chest.2016.10.031 [DOI] [PubMed] [Google Scholar]
- Jensen EA, Schmidt B (2014) Epidemiology of bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol 100 (3):145–157. doi: 10.1002/bdra.23235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobe AH, Bancalari E (2001) Bronchopulmonary dysplasia. Am J Respir Crit Care Med 163 (7):1723–1729. doi: 10.1164/ajrccm.163.7.2011060 [DOI] [PubMed] [Google Scholar]
- Johannesson M, Lúdvíksdóttir D, Janson C (2000) Lung function changes in relation to menstrual cycle in females with cystic fibrosis. Respir Med 94 (11):1043–1046. doi: 10.1053/rmed.2000.0891 [DOI] [PubMed] [Google Scholar]
- Kadel S, Ainsua-Enrich E, Hatipoglu I, Turner S, Singh S, Khan S, Kovats S (2018) A Major Population of Functional KLRG1. Immunohorizons 2 (2):74–86. doi: 10.4049/immunohorizons.1800008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadel S, Kovats S (2018) Sex Hormones Regulate Innate Immune Cells and Promote Sex Differences in Respiratory Virus Infection. Front Immunol 9:1653. doi: 10.3389/fimmu.2018.01653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadioglu A, Cuppone AM, Trappetti C, List T, Spreafico A, Pozzi G, Andrew PW, Oggioni MR (2011) Sex-Based Differences in Susceptibility to Respiratory and Systemic Pneumococcal Disease in Mice. The Journal of Infectious Diseases 204 (12):1971–1979. doi: 10.1093/infdis/jir657 [DOI] [PubMed] [Google Scholar]
- Kadota K, Eguchi T, Villena-Vargas J, Woo KM, Sima CS, Jones DR, Travis WD, Adusumilli PS (2015) Nuclear estrogen receptor-α expression is an independent predictor of recurrence in male patients with pT1aN0 lung adenocarcinomas, and correlates with regulatory T-cell infiltration. Oncotarget 6 (29):27505–27518. doi: 10.18632/oncotarget.4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallings LV, Emtner M, Bäcklund L (1999) Exercise-induced bronchoconstriction in adults with asthma--comparison between running and cycling and between cycling at different air conditions. Ups J Med Sci 104 (3):191–198. doi: 10.3109/03009739909178962 [DOI] [PubMed] [Google Scholar]
- Kari MA, Hallman M, Eronen M, Teramo K, Virtanen M, Koivisto M, Ikonen RS (1994) Prenatal dexamethasone treatment in conjunction with rescue therapy of human surfactant: a randomized placebo-controlled multicenter study. Pediatrics 93 (5):730–736 [PubMed] [Google Scholar]
- Karlberg J, Chong DS, Lai WY (2004) Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am J Epidemiol 159 (3):229–231. doi: 10.1093/aje/kwh056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T, Motwani SS, Elias RM, Gabriel JM, Taranto Montemurro L, Yanagisawa N, Spiller N, Paul N, Bradley TD (2014) Influence of rostral fluid shift on upper airway size and mucosal water content. J Clin Sleep Med 10 (10):1069–1074. doi: 10.5664/jcsm.4102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, Palevsky HI, Palmisciano AJ, Patel M, Pinder D, Propert KJ, Smith KA, Stanczyk F, Tracy R, Vaidya A, Whittenhall ME, Ventetuolo CE (2017) Anastrozole in Pulmonary Arterial Hypertension. A Randomized, Double-Blind, Placebo-controlled Trial. Am J Respir Crit Care Med 195 (3):360–368. doi: 10.1164/rccm.201605-1024OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein S, Pekosz A, Park HS, Ursin R, Shapiro J, Benner S, Littlefield K, Kumar S, Naik HM, Betenbaugh M, Shrestha R, Wu A, Hughes R, Burgess I, Caturegli P, Laeyendecker O, Quinn T, Sullivan D, Shoham S, Redd A, Bloch E, Casadevall A, Tobian A (2020a) Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. medRxiv. doi: 10.1101/2020.06.26.20139063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Dhakal S, Ursin RL, Deshpande S, Sandberg K, Mauvais-Jarvis F (2020b) Biological sex impacts COVID-19 outcomes. PLoS Pathog 16 (6):e1008570. doi: 10.1371/journal.ppat.1008570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL, Flanagan KL (2016) Sex differences in immune responses. Nat Rev Immunol 16 (10):626–638. doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- Klein SL, Hodgson A, Robinson DP (2012) Mechanisms of sex disparities in influenza pathogenesis. J Leukoc Biol 92 (1):67–73. doi: 10.1189/jlb.0811427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koper I, Hufnagl K, Ehmann R (2017) Gender aspects and influence of hormones on bronchial asthma - Secondary publication and update. World Allergy Organ J 10 (1):46. doi: 10.1186/s40413-017-0177-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt OK, Zhang J, Pinkerton KE (2016) Pulmonary health effects of air pollution. Curr Opin Pulm Med 22 (2):138–143. doi: 10.1097/MCP.0000000000000248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm T, Albrecht M, Fisher AJ, Selej M, Patel NG, Brown JA, Justice MJ, Brown MB, Van Demark M, Trulock KM, Dieudonne D, Reddy JG, Presson RG, Petrache I (2012) 17β-Estradiol attenuates hypoxic pulmonary hypertension via estrogen receptor-mediated effects. Am J Respir Crit Care Med 185 (9):965–980. doi: 10.1164/rccm.201107-1293OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm T, Frump AL (2017) Toward Harnessing Sex Steroid Signaling as a Therapeutic Target in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 195 (3):284–286. doi: 10.1164/rccm.201609-1906ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm T, Tuder RM, Petrache I (2014) Progress in solving the sex hormone paradox in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 307 (1):L7–26. doi: 10.1152/ajplung.00337.2013 [DOI] [PubMed] [Google Scholar]
- Lai HC, Kosorok MR, Laxova A, Makholm LM, Farrell PM (2002) Delayed diagnosis of US females with cystic fibrosis. Am J Epidemiol 156 (2):165–173. doi: 10.1093/aje/kwf014 [DOI] [PubMed] [Google Scholar]
- Lamprecht B, Soriano JB, Studnicka M, Kaiser B, Vanfleteren LE, Gnatiuc L, Burney P, Miravitlles M, García-Rio F, Akbari K, Ancochea J, Menezes AM, Perez-Padilla R, Montes de Oca M, Torres-Duque CA, Caballero A, González-García M, Buist S, BOLD Collaborative Research Group tE-ST, the PLATINO Team, and the PREPOCOL Study Group (2015) Determinants of underdiagnosis of COPD in national and international surveys. Chest 148 (4):971–985. doi: 10.1378/chest.14-2535 [DOI] [PubMed] [Google Scholar]
- Leary S, Das P, Ponnalagu D, Singh H, Bhandari V (2019) Genetic Strain and Sex Differences in a Hyperoxia-Induced Mouse Model of Varying Severity of Bronchopulmonary Dysplasia. Am J Pathol 189 (5):999–1014. doi: 10.1016/j.ajpath.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifke E, Friemert M, Heilmann M, Puvogel N, Smaczny C, von zur Muhlen A, Brabant G (2003) Sex steroids and body composition in men with cystic fibrosis. Eur J Endocrinol 148 (5):551–557. doi: 10.1530/eje.0.1480551 [DOI] [PubMed] [Google Scholar]
- Leong HN, Earnest A, Lim HH, Chin CF, Tan C, Puhaindran ME, Tan A, Chen MI, Leo YS (2006) SARS in Singapore--predictors of disease severity. Ann Acad Med Singapore 35 (5):326–331 [PubMed] [Google Scholar]
- Li X, Chen Q, Zheng X, Li Y, Han M, Liu T, Xiao J, Guo L, Zeng W, Zhang J, Ma W (2019) Effects of ambient ozone concentrations with different averaging times on asthma exacerbations: A meta-analysis. Sci Total Environ 691:549–561. doi: 10.1016/j.scitotenv.2019.06.382 [DOI] [PubMed] [Google Scholar]
- Li Y, Jerkic M, Slutsky AS, Zhang H (2020) Molecular mechanisms of sex bias differences in COVID-19 mortality. Crit Care 24 (1):405. doi: 10.1186/s13054-020-03118-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licari A, Castagnoli R, Brambilla I, Marseglia A, Tosca MA, Marseglia GL, Ciprandi G (2018) Asthma Endotyping and Biomarkers in Childhood Asthma. Pediatr Allergy Immunol Pulmonol 31 (2):44–55. doi: 10.1089/ped.2018.0886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CM, Davidson TM, Ancoli-Israel S (2008) Gender differences in obstructive sleep apnea and treatment implications. Sleep Med Rev 12 (6):481–496. doi: 10.1016/j.smrv.2007.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liptzin DR, Landau LI, Taussig LM (2015) Sex and the lung: Observations, hypotheses, and future directions. Pediatr Pulmonol 50 (12):1159–1169. doi: 10.1002/ppul.23178 [DOI] [PubMed] [Google Scholar]
- Liu J, Ji H, Zheng W, Wu X, Zhu JJ, Arnold AP, Sandberg K (2010) Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17beta-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ 1 (1):6. doi: 10.1186/2042-6410-1-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Pan J, Zhang H, Shi C, Li G, Peng Z, Ma J, Zhou Y, Zhang L (2019) Short-Term Exposure to Ambient Air Pollution and Asthma Mortality. Am J Respir Crit Care Med 200 (1):24–32. doi: 10.1164/rccm.201810-1823OC [DOI] [PubMed] [Google Scholar]
- Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, Newman JH, Phillips JA, Soubrier F, Trembath RC, Chung WK (2009) Genetics and Genomics of Pulmonary Arterial Hypertension. Journal of the American College of Cardiology 54 (1 Supplement):S32. doi: 10.1016/j.jacc.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher TM, Strek ME (2019) Antifibrotic therapy for idiopathic pulmonary fibrosis: time to treat. Respir Res 20 (1):205. doi: 10.1186/s12931-019-1161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud O, Granell R, Tilling K, Minelli C, Garcia-Aymerich J, Holloway JW, Custovic A, Jarvis D, Sterne J, Henderson J (2018) Association of Height Growth in Puberty with Lung Function: A Longitudinal Study. American journal of respiratory and critical care medicine. doi: 10.1164/rccm.201802-0274OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair KM, Wright AF, Duggan N, Rowlands DJ, Hussey MJ, Roberts S, Fullerton J, Nilsen M, Loughlin L, Thomas M, MacLean MR (2014) Sex-dependent influence of endogenous estrogen in pulmonary hypertension. Am J Respir Crit Care Med 190 (4):456–467. doi: 10.1164/rccm.201403-0483OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair KM, Yang XD, Long L, White K, Wallace E, Ewart M-A, Docherty CK, Morrell NW, MacLean MR (2015) Sex affects bone morphogenetic protein type II receptor signaling in pulmonary artery smooth muscle cells. Am J Respir Crit Care Med 191 (6):693–703. doi: 10.1164/rccm.201410-1802OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G (2020) Could Sex/Gender Differences in ACE2 Expression in the Lungs Contribute to the Large Gender Disparity in the Morbidity and Mortality of Patients Infected With the SARS-CoV-2 Virus? Front Cell Infect Microbiol 10:327. doi: 10.3389/fcimb.2020.00327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrigiannis AP, Anderson SK (2001) The murine Ly49 family: form and function. Arch Immunol Ther Exp (Warsz) 49 (1):47–50 [PubMed] [Google Scholar]
- Malhotra A, Huang Y, Fogel RB, Pillar G, Edwards JK, Kikinis R, Loring SH, White DP (2002) The male predisposition to pharyngeal collapse: importance of airway length. American journal of respiratory and critical care medicine 166 (10):1388–1395. doi: 10.1164/rccm.2112072 [DOI] [PubMed] [Google Scholar]
- Malinczak CA, Fonseca W, Rasky AJ, Ptaschinski C, Morris S, Ziegler SF, Lukacs NW (2019) Sex-associated TSLP-induced immune alterations following early-life RSV infection leads to enhanced allergic disease. Mucosal Immunol 12 (4):969–979. doi: 10.1038/s41385-019-0171-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannino DM, Buist AS (2007) Global burden of COPD: risk factors, prevalence, and future trends. Lancet 370 (9589):765–773. doi:S0140–6736(07)61380–4. doi: 10.1016/S0140-6736(07)61380-4 [DOI] [PubMed] [Google Scholar]
- Markle JG, Fish EN (2014) SeXX matters in immunity. Trends Immunol 35 (3):97–104. doi: 10.1016/j.it.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Marra AM, Benjamin N, Eichstaedt C, Salzano A, Arcopinto M, Gargani L, DA M, Argiento P, Falsetti L, Di Giosia P, Isidori AM, Ferrara F, Bossone E, Cittadini A, Grünig E (2016) Gender-related differences in pulmonary arterial hypertension targeted drugs administration. Pharmacol Res 114:103–109. doi: 10.1016/j.phrs.2016.10.018 [DOI] [PubMed] [Google Scholar]
- Martinez FJ, Curtis JL, Sciurba F, Mumford J, Giardino ND, Weinmann G, Kazerooni E, Murray S, Criner GJ, Sin DD, Hogg J, Ries AL, Han M, Fishman AP, Make B, Hoffman EA, Mohsenifar Z, Wise R, Group NETTR (2007) Sex differences in severe pulmonary emphysema. Am J Respir Crit Care Med 176 (3):243–252. doi:200606–828OC. doi: 10.1164/rccm.200606-828OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maselli JH, Sontag MK, Norris JM, MacKenzie T, Wagener JS, Accurso FJ (2003) Risk factors for initial acquisition of Pseudomonas aeruginosa in children with cystic fibrosis identified by newborn screening. Pediatr Pulmonol 35 (4):257–262. doi: 10.1002/ppul.10230 [DOI] [PubMed] [Google Scholar]
- Massaro GD, Mortola JP, Massaro D (1995) Sexual dimorphism in the architecture of the lung’s gas-exchange region. Proceedings of the National Academy of Sciences of the United States of America 92 (4):1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto AM, Sandblom RE, Schoene RB, Lee KA, Giblin EC, Pierson DJ, Bremner WJ (1985) Testosterone replacement in hypogonadal men: effects on obstructive sleep apnoea, respiratory drives, and sleep. Clinical endocrinology 22 (6):713–721. doi: 10.1111/j.1365-2265.1985.tb00161.x [DOI] [PubMed] [Google Scholar]
- Mattiuzzi C, Lippi G (2019) Current Cancer Epidemiology. J Epidemiol Glob Health 9 (4):217–222. doi: 10.2991/jegh.k.191008.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden ER (1990) Hypothesis: exercise-induced asthma as a vascular phenomenon. Lancet 335 (8694):880–883. doi: 10.1016/0140-6736(90)90478-n [DOI] [PubMed] [Google Scholar]
- McFadden ER, Ingram RH (1979) Exercise-induced asthma: Observations on the initiating stimulus. N Engl J Med 301 (14):763–769. doi: 10.1056/NEJM197910043011406 [DOI] [PubMed] [Google Scholar]
- McMurtry IF, Frith CH, Will DH (1973) Cardiopulmonary responses of male and female swine to simulated high altitude. J Appl Physiol 35 (4):459–462. doi: 10.1152/jappl.1973.35.4.459 [DOI] [PubMed] [Google Scholar]
- Medrek S, Sahay S, Zhao C, Selej M, Frost A (2020) Impact of race on survival in pulmonary arterial hypertension: Results from the REVEAL registry. J Heart Lung Transplant 39 (4):321–330. doi: 10.1016/j.healun.2019.11.024 [DOI] [PubMed] [Google Scholar]
- Mehari A, Valle O, Gillum RF (2014) Trends in pulmonary hypertension mortality and morbidity. Pulm Med 2014:105864. doi: 10.1155/2014/105864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta G, Macek M, Mehta A, Group ERW (2010) Cystic fibrosis across Europe: EuroCareCF analysis of demographic data from 35 countries. J Cyst Fibros 9 Suppl 2:S5–S21. doi: 10.1016/j.jcf.2010.08.002 [DOI] [PubMed] [Google Scholar]
- Mogri M, Dhindsa S, Quattrin T, Ghanim H, Dandona P (2013) Testosterone concentrations in young pubertal and post-pubertal obese males. Clinical endocrinology 78 (4):593–599. doi: 10.1111/cen.12018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamad NV, Wong SK, Wan Hasan WN, Jolly JJ, Nur-Farhana MF, Ima-Nirwana S, Chin KY (2019) The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male 22 (2):129–140. doi: 10.1080/13685538.2018.1482487 [DOI] [PubMed] [Google Scholar]
- Mokhtar HM, Giribabu N, Muniandy S, Salleh N (2014) Testosterone decreases the expression of endometrial pinopode and L-selectin ligand (MECA-79) in adult female rats during uterine receptivity period. Int J Clin Exp Pathol 7 (5):1967–1976 [PMC free article] [PubMed] [Google Scholar]
- Molis MA, Molis WE (2010) Exercise-induced bronchospasm. Sports Health 2 (4):311–317. doi: 10.1177/1941738110373735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollerup S, Jørgensen K, Berge G, Haugen A (2002) Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer 37 (2):153–159 [DOI] [PubMed] [Google Scholar]
- Morgan R, Klein SL (2019) The intersection of sex and gender in the treatment of influenza. Curr Opin Virol 35:35–41. doi: 10.1016/j.coviro.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeem A, Silveyra P (2019) Sex Differences in Paediatric and Adult Asthma. Eur Med J (Chelmsf) 4 (2):27–35 [PMC free article] [PubMed] [Google Scholar]
- Naeye RL, Burt LS, Wright DL, Blanc WA, Tatter D (1971) Neonatal mortality, the male disadvantage. Pediatrics 48 (6):902–906 [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simões EA, Rudan I, Weber MW, Campbell H (2010) Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375 (9725):1545–1555. doi: 10.1016/S0140-6736(10)60206-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair H, Simoes EA, Rudan I, Gessner BD, Azziz-Baumgartner E, Zhang JSF, Feikin DR, Mackenzie GA, Moiisi JC, Roca A, Baggett HC, Zaman SM, Singleton RJ, Lucero MG, Chandran A, Gentile A, Cohen C, Krishnan A, Bhutta ZA, Arguedas A, Clara AW, Andrade AL, Ope M, Ruvinsky RO, Hortal M, McCracken JP, Madhi SA, Bruce N, Qazi SA, Morris SS, El Arifeen S, Weber MW, Scott JAG, Brooks WA, Breiman RF, Campbell H, Severe Acute Lower Respiratory Infections Working G (2013) Global and regional burden of hospital admissions for severe acute lower respiratory infections in young children in 2010: a systematic analysis. Lancet 381 (9875):1380–1390. doi: 10.1016/S0140-6736(12)61901-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevšímalová S (2019) Sleep and sleep-related disorders in women. Cas Lek Cesk 158 (7–8):321–322 [PubMed] [Google Scholar]
- Newcomb D (2016) Chapter 4. Sex, Gender, and Asthma. In: Hemnes AH (ed) Gender, Sex Hormones and Respiratory Disease. A comprehensive guide. pp 87–103. doi: 10.1007/978-3-319-23998-9 [DOI] [Google Scholar]
- Newcomb DC, Peebles RS Jr. (2013) Th17-mediated inflammation in asthma. Curr Opin Immunol 25 (6):755–760. doi: 10.1016/j.coi.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick JA, Chacon CS, Brayshaw SJ, Jones MC, Barboa CM, St Clair CG, Young RL, Nichols DP, Janssen JS, Huitt GA, Iseman MD, Daley CL, Taylor-Cousar JL, Accurso FJ, Saavedra MT, Sontag MK (2010) Effects of gender and age at diagnosis on disease progression in long-term survivors of cystic fibrosis. Am J Respir Crit Care Med 182 (5):614–626. doi: 10.1164/rccm.201001-0092OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff H, Symons S, Zieker D, Schaible EV, Schäfer K, Thoma S, Löffler M, Abbasi A, Simon P, Niess AM, Fehrenbach E (2008) Gender- and menstrual phase dependent regulation of inflammatory gene expression in response to aerobic exercise. Exerc Immunol Rev 14:86–103 [PubMed] [Google Scholar]
- O’Driscoll DN, Greene CM, Molloy EJ (2017) Immune function? A missing link in the gender disparity in preterm neonatal outcomes. Expert Rev Clin Immunol 13 (11):1061–1071. doi: 10.1080/1744666X.2017.1386555 [DOI] [PubMed] [Google Scholar]
- O’Driscoll DN, McGovern M, Greene CM, Molloy EJ (2018) Gender disparities in preterm neonatal outcomes. Acta Paediatr. doi: 10.1111/apa.14390 [DOI] [PubMed] [Google Scholar]
- Ødegaard I, Stray-Pedersen B, Hallberg K, Haanaes OC, Storrøsten OT, Johannesson M (2002) Prevalence and outcome of pregnancies in Norwegian and Swedish women with cystic fibrosis. Acta Obstet Gynecol Scand 81 (8):693–697 [PubMed] [Google Scholar]
- Olak J, Colson Y (2004) Gender differences in lung cancer: have we really come a long way, baby? J Thorac Cardiovasc Surg 128 (3):346–351. doi: 10.1016/j.jtcvs.2004.05.025 [DOI] [PubMed] [Google Scholar]
- Olesen HV, Pressler T, Hjelte L, Mared L, Lindblad A, Knudsen PK, Laerum BN, Johannesson M, Consortium SCFS (2010) Gender differences in the Scandinavian cystic fibrosis population. Pediatr Pulmonol 45 (10):959–965. doi: 10.1002/ppul.21265 [DOI] [PubMed] [Google Scholar]
- Papageorgiou AN, Colle E, Farri-Kostopoulos E, Gelfand MM (1981) Incidence of respiratory distress syndrome following antenatal betamethasone: role of sex, type of delivery, and prolonged rupture of membranes. Pediatrics 67 (5):614–617 [PubMed] [Google Scholar]
- Papi A, Brightling C, Pedersen SE, Reddel HK (2018) Asthma. Lancet 391 (10122):783–800. doi: 10.1016/S0140-6736(17)33311-1 [DOI] [PubMed] [Google Scholar]
- Parsons JP, Hallstrand TS, Mastronarde JG, Kaminsky DA, Rundell KW, Hull JH, Storms WW, Weiler JM, Cheek FM, Wilson KC, Anderson SD, Bronchoconstriction ATSSoE-i (2013) An official American Thoracic Society clinical practice guideline: exercise-induced bronchoconstriction. Am J Respir Crit Care Med 187 (9):1016–1027. doi: 10.1164/rccm.201303-0437ST [DOI] [PubMed] [Google Scholar]
- Parsons JP, Kaeding C, Phillips G, Jarjoura D, Wadley G, Mastronarde JG (2007) Prevalence of exercise-induced bronchospasm in a cohort of varsity college athletes. Med Sci Sports Exerc 39 (9):1487–1492. doi: 10.1249/mss.0b013e3180986e45 [DOI] [PubMed] [Google Scholar]
- Perez TA, Castillo EG, Ancochea J, Pastor Sanz MT, Almagro P, Martínez-Camblor P, Miravitlles M, Rodríguez-Carballeira M, Navarro A, Lamprecht B, Ramírez-García Luna AS, Kaiser B, Alfageme I, Casanova C, Esteban C, Soler-Cataluña JJ, De-Torres JP, Celli BR, Marin JM, Lopez-Campos JL, Riet GT, Sobradillo P, Lange P, Garcia-Aymerich J, Anto JM, Turner AM, Han MK, Langhammer A, Sternberg A, Leivseth L, Bakke P, Johannessen A, Oga T, Cosío B, Echazarreta A, Roche N, Burgel PR, Sin DD, Puhan MA, Soriano JB (2020) Sex differences between women and men with COPD: A new analysis of the 3CIA study. Respir Med 171:106105. doi: 10.1016/j.rmed.2020.106105 [DOI] [PubMed] [Google Scholar]
- Piasecka B, Duffy D, Urrutia A, Quach H, Patin E, Posseme C, Bergstedt J, Charbit B, Rouilly V, MacPherson CR, Hasan M, Albaud B, Gentien D, Fellay J, Albert ML, Quintana-Murci L, Milieu Interieur C (2018) Distinctive roles of age, sex, and genetics in shaping transcriptional variation of human immune responses to microbial challenges. Proc Natl Acad Sci U S A 115 (3):E488–E497. doi: 10.1073/pnas.1714765115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD (2002) Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 287 (9):1132–1141. doi: 10.1001/jama.287.9.1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope D, Diaz E, Smith-Sivertsen T, Lie RT, Bakke P, Balmes JR, Smith KR, Bruce NG (2015) Exposure to household air pollution from wood combustion and association with respiratory symptoms and lung function in nonsmoking women: results from the RESPIRE trial, Guatemala. Environ Health Perspect 123 (4):285–292. doi: 10.1289/ehp.1408200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic RM, White DP (1998) Upper airway muscle activity in normal women: influence of hormonal status. J Appl Physiol (1985) 84 (3):1055–1062. doi: 10.1152/jappl.1998.84.3.1055 [DOI] [PubMed] [Google Scholar]
- Postma DS (2007) Gender differences in asthma development and progression. Gend Med 4 Suppl B:S133–146 [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Lenzi A (2020) Commentary: Testosterone, a key hormone in the context of COVID-19 pandemic. Metabolism 108:154252. doi: 10.1016/j.metabol.2020.154252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost PR, Simard M, Tremblay Y (2004) A link between lung androgen metabolism and the emergence of mature epithelial type II cells. Am J Respir Crit Care Med 170 (3):296–305. doi: 10.1164/rccm.200312-1680OC [DOI] [PubMed] [Google Scholar]
- Rabinovitch M, Gamble WJ, Miettinen OS, Reid L (1981) Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol 240 (1):H62–72. doi: 10.1152/ajpheart.1981.240.1.H62 [DOI] [PubMed] [Google Scholar]
- Radkiewicz C, Dickman PW, Johansson ALV, Wagenius G, Edgren G, Lambe M (2019) Sex and survival in non-small cell lung cancer: A nationwide cohort study. PLoS One 14 (6):e0219206. doi: 10.1371/journal.pone.0219206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffay TM, Dylag AM, Di Fiore JM, Smith LA, Einisman HJ, Li Y, Lakner MM, Khalil AM, MacFarlane PM, Martin RJ, Gaston B (2016) S-Nitrosoglutathione Attenuates Airway Hyperresponsiveness in Murine Bronchopulmonary Dysplasia. Mol Pharmacol 90 (4):418–426. doi: 10.1124/mol.116.104125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan D, Jain R (2016) Increasing awareness of sex differences in airway diseases. Respirology 21 (3):449–459. doi: 10.1111/resp.12702 [DOI] [PubMed] [Google Scholar]
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ, Fibrosis AEJACoIP (2011) An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183 (6):788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G (2006) Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 174 (7):810–816. doi: 10.1164/rccm.200602-163OC [DOI] [PubMed] [Google Scholar]
- Ranchoux B, Antigny F, Rucker-Martin C, Hautefort A, Péchoux C, Bogaard HJ, Dorfmüller P, Remy S, Lecerf F, Planté S, Chat S, Fadel E, Houssaini A, Anegon I, Adnot S, Simonneau G, Humbert M, Cohen-Kaminsky S, Perros F (2015) Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation 131 (11):1006–1018. doi: 10.1161/circulationaha.114.008750 [DOI] [PubMed] [Google Scholar]
- Rastrelli G, Di Stasi V, Inglese F, Beccaria M, Garuti M, Di Costanzo D, Spreafico F, Greco GF, Cervi G, Pecoriello A, Magini A, Todisco T, Cipriani S, Maseroli E, Corona G, Salonia A, Lenzi A, Maggi M, De Donno G, Vignozzi L (2020) Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology. doi: 10.1111/andr.12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redline S, Storfer-Isser A, Rosen CL, Johnson NL, Kirchner HL, Emancipator J, Kibler AM (2007) Association between metabolic syndrome and sleep-disordered breathing in adolescents. American journal of respiratory and critical care medicine 176 (4):401–408. doi: 10.1164/rccm.200703-375OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren CL, Morgan WJ, Konstan MW, Schechter MS, Wagener JS, Fisher KA, Regelmann WE, Fibrosis IaCotESoC (2007) Presence of methicillin resistant Staphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr Pulmonol 42 (6):513–518. doi: 10.1002/ppul.20604 [DOI] [PubMed] [Google Scholar]
- Rho J, Ahn C, Gao A, Sawicki GS, Keller A, Jain R (2018) Disparities in Mortality of Hispanic Patients with Cystic Fibrosis in the United States. A National and Regional Cohort Study. Am J Respir Crit Care Med 198 (8):1055–1063. doi: 10.1164/rccm.201711-2357OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera L, Siddaiah R, Oji-Mmuo C, Silveyra GR, Silveyra P (2016) Biomarkers for Bronchopulmonary Dysplasia in the Preterm Infant. Front Pediatr 4:33. doi: 10.3389/fped.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera MP (2013) Lung cancer in women: differences in epidemiology, biology, histology, and treatment outcomes. Semin Respir Crit Care Med 34 (6):792–801. doi: 10.1055/s-0033-1358550 [DOI] [PubMed] [Google Scholar]
- Roberge S, Lacasse Y, Tapp S, Tremblay Y, Kari A, Liu J, Fekih M, Qublan HS, Amorim MM, Bujold E (2011) Role of fetal sex in the outcome of antenatal glucocorticoid treatment to prevent respiratory distress syndrome: systematic review and meta-analysis. J Obstet Gynaecol Can 33 (3):216–226. doi: 10.1016/s1701-2163(16)34822-8 [DOI] [PubMed] [Google Scholar]
- Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL (2011a) Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ 2:8. doi: 10.1186/2042-6410-2-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DP, Lorenzo ME, Jian W, Klein SL (2011b) Elevated 17β-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7 (7):e1002149. doi: 10.1371/journal.ppat.1002149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rysavy MA, Horbar JD, Bell EF, Li L, Greenberg LT, Tyson JE, Patel RM, Carlo WA, Younge NE, Green CE, Edwards EM, Hintz SR, Walsh MC, Buzas JS, Das A, Higgins RD, Network EKSNIoCHaHDNRNaVO (2020) Assessment of an Updated Neonatal Research Network Extremely Preterm Birth Outcome Model in the Vermont Oxford Network. JAMA Pediatr 174 (5):e196294. doi: 10.1001/jamapediatrics.2019.6294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam MT, Wenten M, Gilliland FD (2006) Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol 117 (5):1001–1007. doi: 10.1016/j.jaci.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Salvi SS, Barnes PJ (2009) Chronic obstructive pulmonary disease in non-smokers. Lancet 374 (9691):733–743. doi: 10.1016/S0140-6736(09)61303-9 [DOI] [PubMed] [Google Scholar]
- Sathish V, Martin YN, Prakash YS (2015) Sex steroid signaling: implications for lung diseases. Pharmacol Ther 150:94–108. doi: 10.1016/j.pharmthera.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savi D, Simmonds N, Di Paolo M, Quattrucci S, Palange P, Banya W, Hopkinson NS, Bilton D (2015) Relationship between pulmonary exacerbations and daily physical activity in adults with cystic fibrosis. BMC Pulm Med 15:151. doi: 10.1186/s12890-015-0151-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH, Group ECO (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346 (2):92–98. doi: 10.1056/NEJMoa011954 [DOI] [PubMed] [Google Scholar]
- Schrader PC, Quanjer PH, Olievier IC (1988) Respiratory muscle force and ventilatory function in adolescents. Eur Respir J 1 (4):368–375 [PubMed] [Google Scholar]
- Schurz H, Salie M, Tromp G, Hoal EG, Kinnear CJ, Moller M (2019) The X chromosome and sex-specific effects in infectious disease susceptibility. Hum Genomics 13 (1):2. doi: 10.1186/s40246-018-0185-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J, Katz SA, Fegley RW, Tockman MS (1988) Sex and race differences in the development of lung function. The American review of respiratory disease 138 (6):1415–1421. doi: 10.1164/ajrccm/138.6.1415 [DOI] [PubMed] [Google Scholar]
- Scully EP, Haverfield J, Ursin RL, Tannenbaum C, Klein SL (2020) Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol 20 (7):442–447. doi: 10.1038/s41577-020-0348-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaborn T, Simard M, Provost PR, Piedboeuf B, Tremblay Y (2010) Sex hormone metabolism in lung development and maturation. Trends Endocrinol Metab 21 (12):729–738. doi: 10.1016/j.tem.2010.09.001 [DOI] [PubMed] [Google Scholar]
- Selvadurai HC, Blimkie CJ, Cooper PJ, Mellis CM, Van Asperen PP (2004) Gender differences in habitual activity in children with cystic fibrosis. Arch Dis Child 89 (10):928–933. doi: 10.1136/adc.2003.034249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R, Newcomb DC (2018) Sex Bias in Asthma Prevalence and Pathogenesis. Front Immunol 9:2997. doi: 10.3389/fimmu.2018.02997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar E, Redline S, Young T, Boland LL, Baldwin CM, Nieto FJ, O’Connor GT, Rapoport DM, Robbins JA (2003) Hormone replacement therapy and sleep-disordered breathing. American journal of respiratory and critical care medicine 167 (9):1186–1192. doi: 10.1164/rccm.200210-1238OC [DOI] [PubMed] [Google Scholar]
- Shakkottai A, Kidwell KM, Townsend M, Nasr SZ (2015) A five-year retrospective analysis of adherence in cystic fibrosis. Pediatr Pulmonol 50 (12):1224–1229. doi: 10.1002/ppul.23307 [DOI] [PubMed] [Google Scholar]
- Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ (2012) Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest 141 (2):363–373. doi: 10.1378/chest.10-3114 [DOI] [PubMed] [Google Scholar]
- Sharma G, Volgman AS, Michos ED (2020) Sex Differences in Mortality from COVID-19 Pandemic: Are Men Vulnerable and Women Protected? JACC Case Rep. doi: 10.1016/j.jaccas.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher T, Dy GK, Adjei AA (2008) Small cell lung cancer. Mayo Clin Proc 83 (3):355–367. doi: 10.4065/83.3.355 [DOI] [PubMed] [Google Scholar]
- Shinohara M, Ogawa S, Nakaya T, Niino R, Ito M, Haro K, Ishii E (2019) Sex Differences in the Prevalence and Severity of Exercise-Induced Bronchoconstriction in Kindergarteners in Japan. J Gen Fam Med 20 (6):221–229. doi: 10.1002/jgf2.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried JM (2001) Women and lung cancer: does oestrogen play a role? Lancet Oncol 2 (8):506–513. doi: 10.1016/S1470-2045(01)00457-0 [DOI] [PubMed] [Google Scholar]
- Silveyra P (2016) Chapter 9: Developmental Lung Disease. In: Hemnes AR (ed) Gender, Sex Hormones and Respiratory Disease. A comprehensive guide. p 243. doi: 10.1007/978-3-319-23998-9 [DOI] [Google Scholar]
- Skoczyński S, Semik-Orzech A, Szanecki W, Majewski M, Kołodziejczyk K, Sozańska E, Witek A, Pierzchała W (2014) Perimenstrual asthma as a gynecological and pulmonological clinical problem. Adv Clin Exp Med 23 (4):665–668. doi: 10.17219/acem/37250 [DOI] [PubMed] [Google Scholar]
- Smith LC, Moreno S, Robertson L, Robinson S, Gant K, Bryant AJ, Sabo-Attwood T (2018) Transforming growth factor beta1 targets estrogen receptor signaling in bronchial epithelial cells. Respir Res 19 (1):160. doi: 10.1186/s12931-018-0861-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano JB (2017) An Epidemiological Overview of Chronic Obstructive Pulmonary Disease: What Can Real-Life Data Tell Us about Disease Management? COPD 14 (sup1):S3–S7. doi: 10.1080/15412555.2017.1286165 [DOI] [PubMed] [Google Scholar]
- Soriano JB, Alfageme I, Miravitlles M, de Lucas P, Soler-Cataluña JJ, García-Río F, Casanova C, Rodríguez González-Moro JM, Cosío BG, Sánchez G, Ancochea J (2020) Prevalence and Determinants of COPD in Spain: EPISCAN II. Arch Bronconeumol. doi: 10.1016/j.arbres.2020.07.024 [DOI] [PubMed] [Google Scholar]
- Spagnolo PA, Manson JE, Joffe H (2020) Sex and Gender Differences in Health: What the COVID-19 Pandemic Can Teach Us. Ann Intern Med. doi: 10.7326/M20-1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolarics Z, Pena G, Qin Y, Donnelly RJ, Livingston DH (2017) Inherent X-Linked Genetic Variability and Cellular Mosaicism Unique to Females Contribute to Sex-Related Differences in the Innate Immune Response. Front Immunol 8:1455. doi: 10.3389/fimmu.2017.01455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabile LP, Siegfried JM (2003) Sex and gender differences in lung cancer. J Gend Specif Med 6 (1):37–48 [PubMed] [Google Scholar]
- Stanford KI, Mickleborough TD, Ray S, Lindley MR, Koceja DM, Stager JM (2006) Influence of menstrual cycle phase on pulmonary function in asthmatic athletes. Eur J Appl Physiol 96 (6):703–710. doi: 10.1007/s00421-005-0067-7 [DOI] [PubMed] [Google Scholar]
- Stark MJ, Hodyl NA, Wright IM, Clifton V (2011a) The influence of sex and antenatal betamethasone exposure on vasoconstrictors and the preterm microvasculature. J Matern Fetal Neonatal Med 24 (10):1215–1220. doi: 10.3109/14767058.2011.569618 [DOI] [PubMed] [Google Scholar]
- Stark MJ, Hodyl NA, Wright IM, Clifton VL (2011b) Influence of sex and glucocorticoid exposure on preterm placental pro-oxidant-antioxidant balance. Placenta 32 (11):865–870. doi: 10.1016/j.placenta.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Stelzig KE, Canepa-Escaro F, Schiliro M, Berdnikovs S, Prakash YS, Chiarella SE (2020) Estrogen regulates the expression of SARS-CoV-2 receptor ACE2 in differentiated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 318 (6):L1280–L1281. doi: 10.1152/ajplung.00153.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW (2020) TMPRSS2 and COVID-19: Serendipity or Opportunity for Intervention? Cancer Discov 10 (6):779–782. doi: 10.1158/2159-8290.CD-20-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms WW (2005) Asthma associated with exercise. Immunol Allergy Clin North Am 25 (1):31–43. doi: 10.1016/j.iac.2004.09.007 [DOI] [PubMed] [Google Scholar]
- Strope JD, Chau CH, Figg WD (2020) Are sex discordant outcomes in COVID-19 related to sex hormones? Semin Oncol. doi: 10.1053/j.seminoncol.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suster DI, Mino-Kenudson M (2020) Molecular Pathology of Primary Non-small Cell Lung Cancer. Arch Med Res. doi: 10.1016/j.arcmed.2020.08.004 [DOI] [PubMed] [Google Scholar]
- Sutton S, Rosenbluth D, Raghavan D, Zheng J, Jain R (2014) Effects of puberty on cystic fibrosis related pulmonary exacerbations in women versus men. Pediatr Pulmonol 49 (1):28–35. doi: 10.1002/ppul.22767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sward P, Edsfeldt A, Reepalu A, Jehpsson L, Rosengren BE, Karlsson MK (2020) Age and sex differences in soluble ACE2 may give insights for COVID-19. Crit Care 24 (1):221. doi: 10.1186/s13054-020-02942-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweezey NB, Ghibu F, Gagnon S, Schotman E, Hamid Q (1998) Glucocorticoid receptor mRNA and protein in fetal rat lung in vivo: modulation by glucocorticoid and androgen. Am J Physiol 275 (1):L103–109. doi: 10.1152/ajplung.1998.275.1.L103 [DOI] [PubMed] [Google Scholar]
- Sweezey NB, Ratjen F (2014) The cystic fibrosis gender gap: potential roles of estrogen. Pediatr Pulmonol 49 (4):309–317. doi: 10.1002/ppul.22967 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Ellingson MK, Wong P, Israelow B, Lucas C, Klein J, Silva J, Mao T, Oh JE, Tokuyama M, Lu P, Venkataraman A, Park A, Liu F, Meir A, Sun J, Wang EY, Casanovas-Massana A, Wyllie AL, Vogels CBF, Earnest R, Lapidus S, Ott IM, Moore AJ, Yale Irt, Shaw A, Fournier JB,Odio CD, Farhadian S, Dela Cruz C, Grubaugh ND, Schulz WL, Ring AM, Ko AI, Omer SB, Iwasaki A (2020) Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature. doi: 10.1038/s41586-020-2700-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam A, Morrish D, Wadsworth S, Dorscheid D, Man SF, Sin DD (2011) The role of female hormones on lung function in chronic lung diseases. BMC Womens Health 11:24. doi:1472–6874-11–24. doi: 10.1186/1472-6874-11-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MR, Marston L, Rafferty GF, Calvert S, Marlow N, Peacock JL, Greenough A (2006) Respiratory function of very prematurely born infants at follow up: influence of sex. Arch Dis Child Fetal Neonatal Ed 91 (3):F197–201. doi: 10.1136/adc.2005.081927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM (1975) Lung growth and alveolar multiplication. Pathobiol Annu 5:1–34 [PubMed] [Google Scholar]
- Thurlbeck WM (1982) Postnatal human lung growth. Thorax 37 (8):564–571. doi: 10.1136/thx.37.8.564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurlbeck WM (1990) Pathology of chronic airflow obstruction. Chest 97 (2 Suppl):6S–10S [PubMed] [Google Scholar]
- To T, Zhu J, Gray N, Feldman LY, Villeneuve PJ, Licskai C, Gershon A, Miller AB (2018) Asthma and Chronic Obstructive Pulmonary Disease Overlap in Women. Incidence and Risk Factors. Ann Am Thorac Soc 15 (11):1304–1310. doi: 10.1513/AnnalsATS.201802-078OC [DOI] [PubMed] [Google Scholar]
- Tofovic SP, Jones TJ, Bilan VP, Jackson EK, Petrusevska G (2010) Synergistic therapeutic effects of 2-methoxyestradiol with either sildenafil or bosentan on amelioration of monocrotaline-induced pulmonary hypertension and vascular remodeling. J Cardiovasc Pharmacol 56 (5):475–483. doi: 10.1097/FJC.0b013e3181f215e7 [DOI] [PubMed] [Google Scholar]
- Torday JS, Nielsen HC (1987) The sex difference in fetal lung surfactant production. Experimental lung research 12 (1):1–19 [DOI] [PubMed] [Google Scholar]
- Torres-Tamayo N, Garcia-Martinez D, Lois Zlolniski S, Torres-Sanchez I, Garcia-Rio F, Bastir M (2018) 3D analysis of sexual dimorphism in size, shape and breathing kinematics of human lungs. J Anat 232 (2):227–237. doi: 10.1111/joa.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsel CD, Emmer SF, Campbell WA, Hussain N (2017) Gender Differences in Respiratory Morbidity and Mortality of Preterm Neonates. Front Pediatr 5:6. doi: 10.3389/fped.2017.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend EA, Miller VM, Prakash YS (2012) Sex differences and sex steroids in lung health and disease. Endocr Rev 33 (1):1–47. doi: 10.1210/er.2010-0031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter A, Kipp M, Schrader RM, Beyer C (2009) Combined application of 17beta-estradiol and progesterone enhance vascular endothelial growth factor and surfactant protein expression in cultured embryonic lung cells of mice. Int J Pediatr 2009:170491. doi: 10.1155/2009/170491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukiainen T, Villani AC, Yen A, Rivas MA, Marshall JL, Satija R, Aguirre M, Gauthier L, Fleharty M, Kirby A, Cummings BB, Castel SE, Karczewski KJ, Aguet F, Byrnes A, Consortium GT, Laboratory DA, Coordinating Center -Analysis Working G, Statistical Methods groups-Analysis Working G, Enhancing Gg, Fund NIHC, Nih/Nci, Nih/Nhgri, Nih/Nimh, Nih/Nida, Biospecimen Collection Source Site N, Biospecimen Collection Source Site R, Biospecimen Core Resource V, Brain Bank Repository-University of Miami Brain Endowment B, Leidos Biomedical-Project M, Study E, Genome Browser Data I, Visualization EBI, Genome Browser Data I, Visualization-Ucsc Genomics Institute UoCSC, Lappalainen T, Regev A, Ardlie KG, Hacohen N, MacArthur DG (2017) Landscape of X chromosome inactivation across human tissues. Nature 550 (7675):244–248. doi: 10.1038/nature24265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell J, Harvey BJ (2020) Sexual dimorphism in the microbiology of the CF ‘Gender Gap’: Estrogen modulation of Pseudomonas aeruginosa virulence. Steroids 156:108575. doi: 10.1016/j.steroids.2019.108575 [DOI] [PubMed] [Google Scholar]
- Umar S, Cunningham CM, Itoh Y, Moazeni S, Vaillancourt M, Sarji S, Centala A, Arnold AP, Eghbali M (2018) The Y Chromosome Plays a Protective Role in Experimental Hypoxic Pulmonary Hypertension. Am J Respir Crit Care Med 197 (7):952–955. doi: 10.1164/rccm.201707-1345LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar S, Iorga A, Matori H, Nadadur RD, Li J, Maltese F, van der Laarse A, Eghbali M (2011) Estrogen rescues preexisting severe pulmonary hypertension in rats. Am J Respir Crit Care Med 184 (6):715–723. doi: 10.1164/rccm.201101-0078OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Nikitara K (2020) COVID-19 and smoking: A systematic review of the evidence. Tob Induc Dis 18:20. doi: 10.18332/tid/119324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varkey AB (2004) Chronic obstructive pulmonary disease in women: exploring gender differences. Curr Opin Pulm Med 10 (2):98–103. doi:00063198-200403000-00003. [DOI] [PubMed] [Google Scholar]
- Ventetuolo CE, Praestgaard A, Palevsky HI, Klinger JR, Halpern SD, Kawut SM (2014) Sex and haemodynamics in pulmonary arterial hypertension. Eur Respir J 43 (2):523–530. doi: 10.1183/09031936.00027613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermillion MS, Ursin RL, Attreed SE, Klein SL (2018a) Estriol Reduces Pulmonary Immune Cell Recruitment and Inflammation to Protect Female Mice From Severe Influenza. Endocrinology 159 (9):3306–3320. doi: 10.1210/en.2018-00486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermillion MS, Ursin RL, Kuok DIT, Vom Steeg LG, Wohlgemuth N, Hall OJ, Fink AL, Sasse E, Nelson A, Ndeh R, McGrath-Morrow S, Mitzner W, Chan MCW, Pekosz A, Klein SL (2018b) Production of amphiregulin and recovery from influenza is greater in males than females. Biol Sex Differ 9 (1):24. doi: 10.1186/s13293-018-0184-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaillac C, Yong VFL, Jaggi TK, Soh MM, Chotirmall SH (2018) Gender differences in bronchiectasis: a real issue? Breathe (Sheff) 14 (2):108–121. doi: 10.1183/20734735.000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Steeg LG, Klein SL (2016) SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog 12 (2):e1005374. doi: 10.1371/journal.ppat.1005374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin B, Klein SL (2016) Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol 311 (6):L1234–L1244. doi: 10.1152/ajplung.00352.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wailoo MP, Emery JL (1982) Normal growth and development of the trachea. Thorax 37 (8):584–587. doi: 10.1136/thx.37.8.584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakelee HA, Wang W, Schiller JH, Langer CJ, Sandler AB, Belani CP, Johnson DH, Group ECO (2006) Survival differences by sex for patients with advanced non-small cell lung cancer on Eastern Cooperative Oncology Group trial 1594. J Thorac Oncol 1 (5):441–446 [PubMed] [Google Scholar]
- Wang Y, Cela E, Gagnon S, Sweezey NB (2010) Estrogen aggravates inflammation in Pseudomonas aeruginosa pneumonia in cystic fibrosis mice. Respir Res 11:166. doi:1465–9921-11–166. doi: 10.1186/1465-9921-11-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D, Bellusci S (2004) The molecular genetics of lung morphogenesis and injury repair. Paediatr Respir Rev 5 Suppl A:S283–287 [DOI] [PubMed] [Google Scholar]
- Warburton D, Zhao J, Berberich MA, Bernfield M (1999) Molecular embryology of the lung: then, now, and in the future. Am J Physiol 276 (5 Pt 1):L697–704 [DOI] [PubMed] [Google Scholar]
- Weiler JM, Bonini S, Coifman R, Craig T, Delgado L, Capão-Filipe M, Passali D, Randolph C, Storms W, Ad Hoc Committee of Sports Medicine Committee of American Academy of Allergy AtI (2007) American Academy of Allergy, Asthma & Immunology Work Group report: exercise-induced asthma. J Allergy Clin Immunol 119 (6):1349–1358. doi: 10.1016/j.jaci.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Weiler JM, Brannan JD, Randolph CC, Hallstrand TS, Parsons J, Silvers W, Storms W, Zeiger J, Bernstein DI, Blessing-Moore J, Greenhawt M, Khan D, Lang D, Nicklas RA, Oppenheimer J, Portnoy JM, Schuller DE, Tilles SA, Wallace D (2016) Exercise-induced bronchoconstriction update-2016. J Allergy Clin Immunol 138 (5):1292–1295.e1236. doi: 10.1016/j.jaci.2016.05.029 [DOI] [PubMed] [Google Scholar]
- Wenham C, Smith J, Morgan R, Gender, Group C-W (2020) COVID-19: the gendered impacts of the outbreak. Lancet 395 (10227):846–848. doi: 10.1016/S0140-6736(20)30526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J, Cogan J, Geraci M, Robinson L, Newman J, Phillips JA, Lane K, Meyrick B, Loyd J (2008) Gene expression in BMPR2 mutation carriers with and without evidence of pulmonary arterial hypertension suggests pathways relevant to disease penetrance. BMC Med Genomics 1:45. doi: 10.1186/1755-8794-1-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K, Johansen Anne K, Nilsen M, Ciuclan L, Wallace E, Paton L, Campbell A, Morecroft I, Loughlin L, McClure John D, Thomas M, Mair Kirsty M, MacLean Margaret R (2012) Activity of the Estrogen-Metabolizing Enzyme Cytochrome P450 1B1 Influences the Development of Pulmonary Arterial Hypertension. Circulation 126 (9):1087–1098. doi: 10.1161/CIRCULATIONAHA.111.062927 [DOI] [PubMed] [Google Scholar]
- Wolters PJ, Collard HR, Jones KD (2014) Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol 9:157–179. doi: 10.1146/annurev-pathol-012513-104706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2010) Sex, gender and influenza. http://apps.who.int/iris/bitstream/10665/44401/1/9789241500111_eng.pdf.
- World Health Organization (2020) Influenza (Seasonal). https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal). Accessed September 26. 2020
- Wu Z, McGoogan JM (2020) Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- Wulfsohn NL, Politzer WM, Henrico JS (1964) Testosterone Therapy in Bronchial Asthma. S Afr Med J 38:170–172 [PubMed] [Google Scholar]
- Xu D-Q, Luo Y, Liu Y, Wang J, Zhang B, Xu M, Wang Y-X, Dong H-Y, Dong M-Q, Zhao P-T, Niu W, Liu M-L, Gao Y-Q, Li Z-C (2010) Beta-estradiol attenuates hypoxic pulmonary hypertension by stabilizing the expression of p27kip1 in rats. Respiratory Research 11 (1):182. doi: 10.1186/1465-9921-11-182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu KF, Xu W, Liu S, Yu J, Tian X, Yang Y, Wang ST, Zhang W, Feng R, Zhang T (2020) Lymphangioleiomyomatosis. Semin Respir Crit Care Med 41 (2):256–268. doi: 10.1055/s-0040-1702195 [DOI] [PubMed] [Google Scholar]
- Yang J, Kingsford RA, Horwood J, Epton MJ, Swanney MP, Stanton J, Darlow BA (2020) Lung Function of Adults Born at Very Low Birth Weight. Pediatrics 145 (2). doi: 10.1542/peds.2019-2359 [DOI] [PubMed] [Google Scholar]
- Yang P, Wang Y, Wampfler JA, Xie D, Stoddard SM, She J, Midthun DE (2016) Trends in Subpopulations at High Risk for Lung Cancer. J Thorac Oncol 11 (2):194–202. doi: 10.1016/j.jtho.2015.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JC, Casella JL, Litvin M, Dobs AS (2019) Male reproductive health in cystic fibrosis. J Cyst Fibros 18 Suppl 2:S105–S110. doi: 10.1016/j.jcf.2019.08.007 [DOI] [PubMed] [Google Scholar]
- Yu J, Astrinidis A, Howard S, Henske EP (2004) Estradiol and tamoxifen stimulate LAM-associated angiomyolipoma cell growth and activate both genomic and nongenomic signaling pathways. Am J Physiol Lung Cell Mol Physiol 286 (4):L694–700. doi: 10.1152/ajplung.00204.2003 [DOI] [PubMed] [Google Scholar]
- Zaman T, Moua T, Vittinghoff E, Ryu JH, Collard HR, Lee JS (2020) Differences in Clinical Characteristics and Outcomes Between Men and Women With Idiopathic Pulmonary Fibrosis: A Multicenter Retrospective Cohort Study. Chest 158 (1):245–251. doi: 10.1016/j.chest.2020.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang EA, Wynder EL (1996) Differences in lung cancer risk between men and women: examination of the evidence. J Natl Cancer Inst 88 (3–4):183–192. doi: 10.1093/jnci/88.3-4.183 [DOI] [PubMed] [Google Scholar]
- Zein JG, Erzurum SC (2015) Asthma is Different in Women. Curr Allergy Asthma Rep 15 (6):28. doi: 10.1007/s11882-015-0528-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Coarfa C, Dong X, Jiang W, Hayward-Piatkovskyi B, Gleghorn JP, Lingappan K (2019) MicroRNA-30a as a candidate underlying sex-specific differences in neonatal hyperoxic lung injury: implications for BPD. Am J Physiol Lung Cell Mol Physiol 316 (1):L144–L156. doi: 10.1152/ajplung.00372.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]


