1. Introduction
The word “biocompatibility” has two roots: bio-, “a word-forming element meaning life…” and compatibility, “capable of existing in harmony” (from the Latin, compatibilis).[1] Life implies “alive” and harmony implies a mutually beneficial co-existence. If you enter this word, “biocompatibility,” in Google Scholar, you will get 689,000 citation results. The vast majority of the millions of medical implants in humans today, presumably “biocompatible,” are walled off by a dense, avascular, crosslinked collagen capsule, effectively similar to leather and hardly suggestive of life or compatibility. In contrast, we are now seeing examples of implant biomaterials that lead to a vascularized reconstruction of localized tissue, a biological reaction different from traditional biocompatible materials that generate a foreign body capsule. Both the encapsulated biomaterials and the reconstructive biomaterials qualify as “biocompatible” by present day measurements of biocompatibility. Yet, this new generation of materials would seem to heal “compatibly” with the living organism, whereas older biomaterials are isolated from the living organism by the dense capsule. This review/perspective article will explore this biocompatibility etymological conundrum by reviewing the history of the concepts around biocompatibility, today’s standard methods for assessing biocompatibility, a contemporary view of the foreign body reaction and finally, a compendium of new biomaterials that heal without the foreign body capsule and the clinical translation of some of these newer materials.
2. History of Biocompatibility Leading to Contemporary Standards for Biocompatibility
-. The Era of Phenomenology (the Pre-history)
In 1996, a reasonably complete skeleton of an individual, judged to have been vigorous and healthy, was found on the banks of the Columbia River near the town of Kennewick, WA dated to about 9000 years ago.[2] There was a spear point in the hip that was apparently present during much of the adult life of the individual, presumably without impacting his normal activities. This would be an early example of an “implant” that was, at some level, healed into a person.
The use of implanted materials for medical/dental applications can be traced back thousands of years before the common era (sutures and teeth, in particular). An 1891 paper described the repair of cranial defects with celluloid (cellulose nitrate). In 1930, the use of glass balls for augmentation mammaplasty was introduced. Other materials used in early surgical implants include wood, leather, gold, rubber, magnesium, zinc, wax, aluminum and cellulose.
In the late 1930’s, the first commercial, synthetic plastics were manufactured. Polyethylene and poly(methyl methacrylate) (PMMA) were first used in surgery in that time period. The use of PMMA in cranioplasty was discussed in a few papers in the 1930’s and 1940’s. A review article by J. Bing described the reaction of PMMA used in cranioplasty as “a moderate tissue reaction with fibroblasts and macrophages, which surrounded the plate with a connective tissue capsule … after it has been inserted in the tissues of animals and man, it has been found that a thin membrane of connective tissue may be formed around it, but that no foreign body reaction is produced.”[3] As will be elaborated upon shortly, this disclaimer about the foreign body reaction would seem almost opposite of how we today describe the FBR.
One of the earliest observations about the biological fate of implanted objects in living, soft tissue was made by Ilya Ilyich (Élie) Metchnikoff, the scientist to whom we attribute the discovery of the macrophage, in 1884.[4] To quote Professor Metchnikoff,
“I hypothesized that if my presumption was correct, a thorn introduced into the body of a starfish larva, devoid of blood vessels and nervous system, would have to be rapidly encircled by the motile cells, similarly to what happens to a human finger with a splinter.”[4]
Metchnikoff’s observations using starfish larva supported his hypothesis of phagocytic cells’ attack of foreign bodies and, in 1887, he named these cells (in German) “macrophagen,” or, in English, macrophages. His observations of phagocytosis remain accurate and relevant for the reaction of the body to implanted solids we observe today. He received the Nobel prize in Physiology or Medicine in 1908 “in recognition of his work on immunity.”
-. Contemporary Thoughts on the FBR and Biocompatibility – 1970 to Today
By the 1960’s, the encapsulation of implanted synthetic materials, and the involvement of macrophages, was well-known. Aberrant inflammatory reactions and extreme fibrotic reactions with implants were also observed in that time period – i.e., not all implanted materials behaved the same in vivo. The reasons for these differences were not clear in this early era of materials implantation.
In the early 1960’s the words “biomaterial” and “biocompatibility” were not found in the scientific literature and only began to appear by the late 1960’s. One of the earliest uses of the word “biocompatibility” was in papers by Charles Homsy.[5,6] Homsy described experiments in which a range of materials were extracted with solvents to remove “migratable” components. These extracts were assessed by infrared spectrometry and applied to cells in culture. Higher levels of organic material in the extracts (suggested by C-H infrared absorbances) correlated with more cell damage and death in culture (interestingly, the cell line used was not specified). These experiments are the root of today’s technologies for assessing the biocompatibility of materials. Most of the International Organization for Standardization (ISO)-defined tests for biocompatibility (ISO10993), widely accepted now by regulatory agencies, involve extracting “migratable chemical moieties”[5] from materials and assessing their effects on cells in culture and on living research animals. Typically, a material with no measurable extractables will be classified as biocompatible. Note that this concept had precedents even before the work of Homsy. As pointed out by Bing in 1950, earlier papers emphasized that “it is important that the plastics used in medicine are pure, and contain no admixtures of tissue irritants.”[3]
Another important event in the history of biocompatibility is the contemporary appreciation of the role of the macrophage cell. The writings of James Anderson[7] alerted the biomaterials community to the significance of these cells. Their role in contemporary thinking on biocompatibility is elaborated upon in sections 3. and 4. of this article.
The first widely accepted definition of the word “biocompatibility” was ratified at a biomaterials consensus conference held in Chester, England in 1986 and subsequently published in 1987.[8]
“the ability of a material to perform with an appropriate host response in a specific application”
Though accurate, the definition did not suggest ways to assess biocompatibility, nor did it offer insights as to how to improve or enhance biocompatibility. In spite of these short-comings, the definition was reaffirmed in a recent conference on biomaterial definitions.[9]
Today, biocompatibility of implant devices and materials is assessed using ISO-defined tests for extractable components and also tests for local and systemic effects in vivo.[10] For example, if a material (or medical device) is (1) found to pass ISO tests that suggest little or no biological reaction from extractable components, then, (2) upon one month implantation in soft tissue is found to have a thin, fibrous capsule (typically in the range 50-200 microns in thickness) with little evidence of acute on-going inflammatory reaction (i.e., the reaction site is quiescent as suggested in ISO 10993-6), that implant might be considered “biocompatible.” This assessment scheme has led to the regulatory agency approval for human implantation of thousands of medical devices implanted in millions of humans, largely with acceptable outcomes.
The most recent updates to the ISO10993 standards also emphasize the risk to the patient that a device might present. Biological risk is defined in ISO10993-1 as “combination of the probability of harm to health occurring as a result of adverse reactions associated with medical device or material interactions, and the severity of that harm.” Though materials used in medical devices might pass the specific “biocompatibility” tests in ISO10993, the response observed might still increase the risk to the patient, particularly associated with device failure and the potential risks associated with re-operation. These points are emphasized in the next paragraph and in the section of this review titled “Device Failure Induced by the Foreign Body Reaction.” A broad consideration of risk and ISO standards is presented in a review article.[11]
Given the overall success of medical devices assessed with these ISO tests and approved by the U.S. FDA, it is interesting to consider the limitations for device performance using today’s “biocompatibility” definition. For implanted electrodes, the fibrotic scar inhibits electrical communication between electrode and body. For implanted drug delivery systems, the fibrotic scar slows transport of drugs to the body. For implanted sensors, the scar impedes diffusion of the analyte being measured to the sensor. For breast implants, the scar can be painful and deforming. For heart pacemaker implants, the scar complicates revision surgery. For vascular prostheses, the scar capsule inhibits the mechanical compliance of the vascular conduit. The high rates of infection of implanted devices may be associated with inhibition of vascularity by the traditionally biocompatible implant, thus preventing phagocytic cells from reaching the site of infection. Many other examples can be given where the scar and low vascularity associated with FBR has deleterious effects on device performance, efficacy and safety (this is elaborated upon in section 3. of this article).
It might seem that, since all solid implants made from biocompatible biomaterials lead to this classic FBR, we are constrained to deal with this reaction. This article will demonstrate that the classic FBR is not inevitable. We now have strategies and novel biomaterials to construct implants that heal in a facile manner into the body with good vascularity, regeneration of local tissue, and little or no fibrosis. Furthermore, refinements in the understanding of the biology of the reaction to implanted foreign materials provide us with insights into the mechanism of the FBR and strategies to address this reaction.
In the next sections of this article, we describe in detail the FBR, and particularly the role of leukocyte cells in this reaction*. We focus primarily on polymeric biomaterials, though our comments are equally relevant to metals and ceramics, especially where corrosion products and solubilized components are present. We then show that materials and strategies exist that can diminish or eliminate the scar capsule and lead to vascularized, reconstructive healing. Such pro-healing biomaterials will ultimately lead to a redefinition of the term “biocompatibility.”
In this perspective article we often use the terms, healing, integration and regeneration. We define them in this article as follows. Healing is the process of the repair of living tissue(s), organs and the biological system as a whole and resumption of (normal) functioning (Wikipedia). In the context of this article, we are particularly focused on a “scar-free,” vascularized healing necessary for normal functioning. Integration, in the context of this article, will, after healing, leave an implant in intimate contact with vascularized tissue without a barrier (scar, foreign body capsule) separating implant and body. Regeneration is defined, for this article, as the process of creating new, vascularized, physiologically normal tissue, particularly at the site of implantation where tissue has been destroyed or damaged by the surgical procedure.
3. The Foreign Body Reaction: A Contemporary View
The implantation of biomaterials causes injury to the body’s tissues which initiates a nonspecific host immune response known as the foreign body reaction (FBR).[12] The acute phase of the FBR is similar to natural wound healing, however the characteristics of biomaterials, even those considered biocompatible, often lead to chronic inflammation which is a hallmark of the FBR. In response to vascular injury, increases are observed in the permeability of local blood vessels, and platelets accumulate and aggregate at the injury site. The aggregation of platelets is coupled with the release of cytokines and other factors that attract neutrophils to the site in the acute inflammation phase.[13,14] Next, mononuclear cells migrate to the site and remain at the biomaterial-tissue interface during the chronic inflammation phase.[13,14] The persistent inflammatory stimulus that is the implanted biomaterial causes these interfacial mononuclear cells to eventually fuse into multinucleated foreign body giant cells (FBGCs). Finally, fibrosis occurs as granulation tissue forms around the implant.[13–16] The FBR can lead to poor implant outcomes and, in some cases, failure of implanted biomedical devices.[13,15,17,18]
-. Phases of the Foreign Body Reaction
The FBR can be broken down into 5 sequential phases: (i) protein adsorption, (ii) acute inflammation, (iii) chronic inflammation, (iv) foreign body giant cell formation, and (v) fibrous encapsulation. These phases are summarized in Figure 1. The FBR is initiated within seconds of biomaterial implantation as blood-[ISP]and interstitium-derived proteins with high surface affinity adsorb to the biomaterial, forming a surface-localized protein matrix. The types of proteins that initially adsorb are dependent on the surface characteristics of the biomaterial.[13–15,19,20] Protein adsorption is a critical component of the FBR as host cells have been shown to interact with the protein matrix rather than directly with the surface of the biomaterial.[20–22] However, the initial adsorption of proteins often does not reflect the composition of the long-term protein matrix as surface proteins are replaced with other proteins over time in what is known as the Vroman effect.[23] Of particular importance in this context is the binding of blood-derived fibrinogen and complement proteins to the biomaterial surface. The hydrolysis of fibrinogen to fibrin on a biomaterial surface releases factors that promote inflammation and leukocyte adhesion.[14,22] Complement proteins are a part of the host immune system that play a role in detecting foreign substances and labeling them for removal from the body.[24] Activation of complement proteins on the biomaterial surface increases inflammation through the attraction of phagocytes and degranulation of neutrophils. As a result, complement activation is indicated in the failure of blood-contacting devices.[13,17,25]
Figure 1.
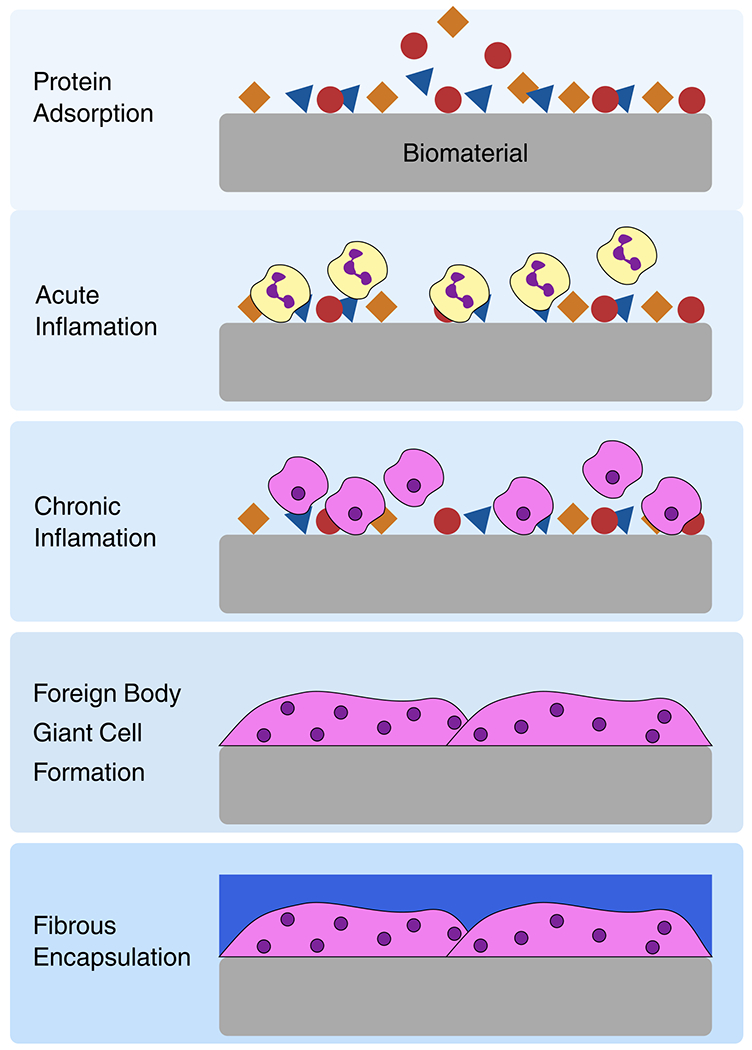
Phases of the foreign body reaction. Proteins (including fibrinogen, albumin and complement proteins) adsorb to the biomaterial upon implantation. During acute inflammation neutrophils, shown in yellow, accumulate at the implantation site. In chronic inflammation macrophages, shown in pink, are attracted to the implantation site. Macrophages fuse into foreign body giant cells following frustrated phagocytosis. Finally, the biomaterial is encapsulated by fibrous tissue, shown in blue.
Following protein adsorption, neutrophils infiltrate the implantation site.[12] This acute inflammation phase only lasts a few days and is triggered by tissue damage, cellular interaction with the adsorbed protein matrix, and the presence of bacteria.[12,14,22] Activated neutrophils degranulate at the biomaterials surface, releasing inflammatory mediators that recruit more leukocytes and monocytes to the site. They also engage in “frustrated” phagocytosis as they attempt to degrade and resorb the biomaterial and, as a result, can damage the surrounding tissue.[22,26,27] There is also evidence that neutrophils form extracellular traps (NETs) on the surface of biomaterials.[18] NETs can lead to complications from increased inflammation.[19]
In the chronic inflammation stage of the FBR, monocytes infiltrate the biomaterial site. Chemoattractants released by neutrophils and platelets recruit circulating monocytes and macrophages. At the implant site, macrophages primarily adhere to fibrinogen, fibronectin and complement fragments [13] which activates them. Activated macrophages exist on a spectrum from M1, classically activated, to M2, alternatively activated. See section 4. of this article for more details and references on the role of the macrophage and its phenotypes.
Macrophage fusion into FBGCs is another hallmark of the FBR. Most FBGCs are formed from some type of myeloid cell, although there is evidence of FBGCs formed from other cell types in exotic pathologies. Most evidence suggests that monocytes do not directly form FBCGs but rather have to differentiate into macrophages, osteoclasts, Touton giant cells, and Langhans giant cells. With regard to the FBR to biomaterials, FBGCs are large, multinucleated cells formed by fusion of macrophages after frustrated phagocytosis of an implant[13,28,29] FBGCs are found at the implant surface and can mediate device degradation and failure over time.[30] FBGCs are metabolically active to varying degrees depending on the cell’s origin and local pathology. The microenvironment created at the site of FBGC adhesion to the biomaterial surface enhances the activity of reactive oxygen species, acid and degradation enzymes released by FBGCs which accelerates implant degradation.[13,22]
The final phase of the FBR is fibrous encapsulation of the biomaterial. Production of TGF-β, other pro-fibrotic factors, and matrix metalloproteinases by macrophages attracts fibroblasts and endothelial cells to the implant surface.[14,16,22,31] These cells deposit collagen bundles that are cross linked into a matrix. Eventually this matrix matures into an acellular, fibrotic capsule composed predominantly of collagen 1[14,32] that has several deleterious implant outcomes including: deformation and mechanical stress of the biomaterial via fibrotic contraction;[22] separation from the surrounding tissues which can lead to failure of devices dependent on tissue interaction;[15,18] poor vascularization of the capsule and surrounding tissue and increased infection risk.[14,33]
-. Device Failure Induced by the Foreign Body Reaction
The FBR can cause suboptimal performance, malfunction or failure of biomaterials and devices through degradation, occlusion, mechanical distortion, extrusion, and infection.[13,14,33,34] Biomaterial and device performance is jeopardized by all phases of the foreign body reaction: protein adsorption, acute inflammation, chronic inflammation, FBGC formation, and fibrous encapsulation. The FBR continues for as long as the biomaterial implant remains in the body, impeding proper healing and tissue integration.
The protein and cell adhesion components of the FBR can impact the performance of biomedical devices that rely on sensor elements or porous filters to function. Implantable glucose sensors are an example of one such device. FBR and occlusion of fibrotic material decrease the stability of glucose readings and limit the lifetime of percutaneous sensor elements to 3-7 days.[35,36] Adsorption of blood-activating proteins leads to thrombotic failure of blood contacting medical devices. For example, adsorption of fibrinogen onto biomaterials can lead to platelet adherence and thrombosis.[37] Additionally, conformational changes to proteins such as FXII, FXI, and high-molecular weight kininogen during the process of surface adsorption can lead to thrombus formation.[38] Thrombus formation is a major cause of failure for central venous catheters, stents, ventricular assist devices, heart valves, extracorporeal membrane oxygenation devices and vascular grafts.[39,40]s Porous filters or membranes within devices can also foul and fail due to accumulation of aggregated protein and cellular elements.
The acute, chronic, and FBGC phases of the FBR can cause device failure over time through the release of degradation mediators that break down the biomaterial. During the acute phase of the FBR, activated neutrophils undergo respiratory burst and degranulation that releases degradation mediators.[26] Labow, et al. demonstrated that certain polyurethane formulations are sensitive to neutrophil-mediated breakdown.[41] In the chronic and FBGC phases of the FBR, macrophages and FBGCs adhere to the biomaterial surface and release reactive oxygen species which degrade the biomaterial.[13] This process has been demonstrated to cause failure of multiple clinical devices such as the pacemaker leads and temporomandibular joint implants. [13,42] The release of reactive oxygen species by macrophages also poses a problem for implanted sensors as they can alter sensor responses to stimuli.[36]
As a consequence of fibrous capsule formation during the FBR, biomaterials do not effectively integrate with the body’s tissues. This can cause secondary problems like infection and extrusion,[18,42] which are particularly problematic for percutaneous devices.[43] The fibrotic capsule is also problematic for drug-releasing biomaterials as it can inhibit drug release and dispersion into surrounding tissues,[44] thereby limiting drug efficacy.[18,45,46] The contraction of fibrotic tissue surrounding an implant can cause distortion of the implant and pain for the patient. This failure mode is commonly observed in silicone breast implants.[47,48]
-. Exploiting the Foreign Body Reaction: Osseointegration
While most advances in biomaterials aim to minimize the impact of the foreign body reaction, other bioengineering applications use it to their engineering advantage. For example, the mild, controlled encapsulation of implants in mineralized tissue, known as osseointegration, is considered a successful outcome.[49,50] The foreign body capsule that surrounds a titanium implant in vascularized bone is predominately collagen I which nucleates bone formation. Thus, in the bony environment, the foreign body capsule may mineralize leading to the close apposition of bone to titanium.[51] FBGCs are found on the surface of osseointegrated titanium implants years after implantation, highlighting the connection between the FBR and osseointegration.[52] However, if the capsule becomes too thick (i.e., from a persistent inflammatory reaction), it may not fully mineralize, which can lead to implant loosening. Titanium and Ti-6Al-4V are most prominently used for osseointegrative materials because of their extreme inertness (lack of leachables), though zirconium also shows promise.[53,54] It is not fully understood why certain materials are better at promoting osseointegration while others promote soft tissue capsule formation. There is evidence of prolonged presence of neutrophils at the implant surface and the upregulation of M2 type macrophages at the site of titanium implants, indicating that the modulation of these cells by the biomaterial during the chronic phase of the FBR plays a role in successful osseointegration.[55]
4. Leukocytic Cells Response to Implant Materials
Implantation of a biomedical device, in the presence or absence of trauma, will initiate recruitment of immune cells.[13,56] The “biocompatibility” of implants, the primary theme of this article, is dictated by the response of immune cells to the implant (and the proteins that have adsorbed to it). For decades, this immune response was viewed as a destructive phenomenon that ultimately ended in either fibrous capsule formation (i.e., the FBR, see section 3.) or chronic inflammation and scaffold degradation.[57–60] More contemporary thinking also notes that the immune cells contribute to healing and regeneration.
Medical implants composed of so-called biocompatible biomaterials are constantly at odds with the host’s FBR.[61] While progress has been made in mitigating the FBR (see Section 5.), many critically important biomaterials are still compromised by the FBR upon implantation.[62] Subsequent chronic inflammation, loss of tissue function, collagenous encapsulation, and inadequate vascularization can seriously affect implant efficacy and patient health.[62,63]
-. The Usual Suspects: Leukocytic Cells Important to Implant Healing and Biocompatibility
Inflammation is a complex and essential homeostatic response to extrinsic and intrinsic tissue damage. Host defenses mobilize immune cells and molecules to vascularized tissues to eliminate the source of cell injury and prepare for tissue regeneration. The main cellular participants in acute and chronic inflammatory responses to biomaterial implantation are leukocytic cells that comprise myeloid cells (mast cells, neutrophils, monocytes/macrophages, and dendritic cells) and lymphoid cells (T cells, B cells, and innate lymphoid cells - ILCs) (Figure 2 A–C).
Figure 2.
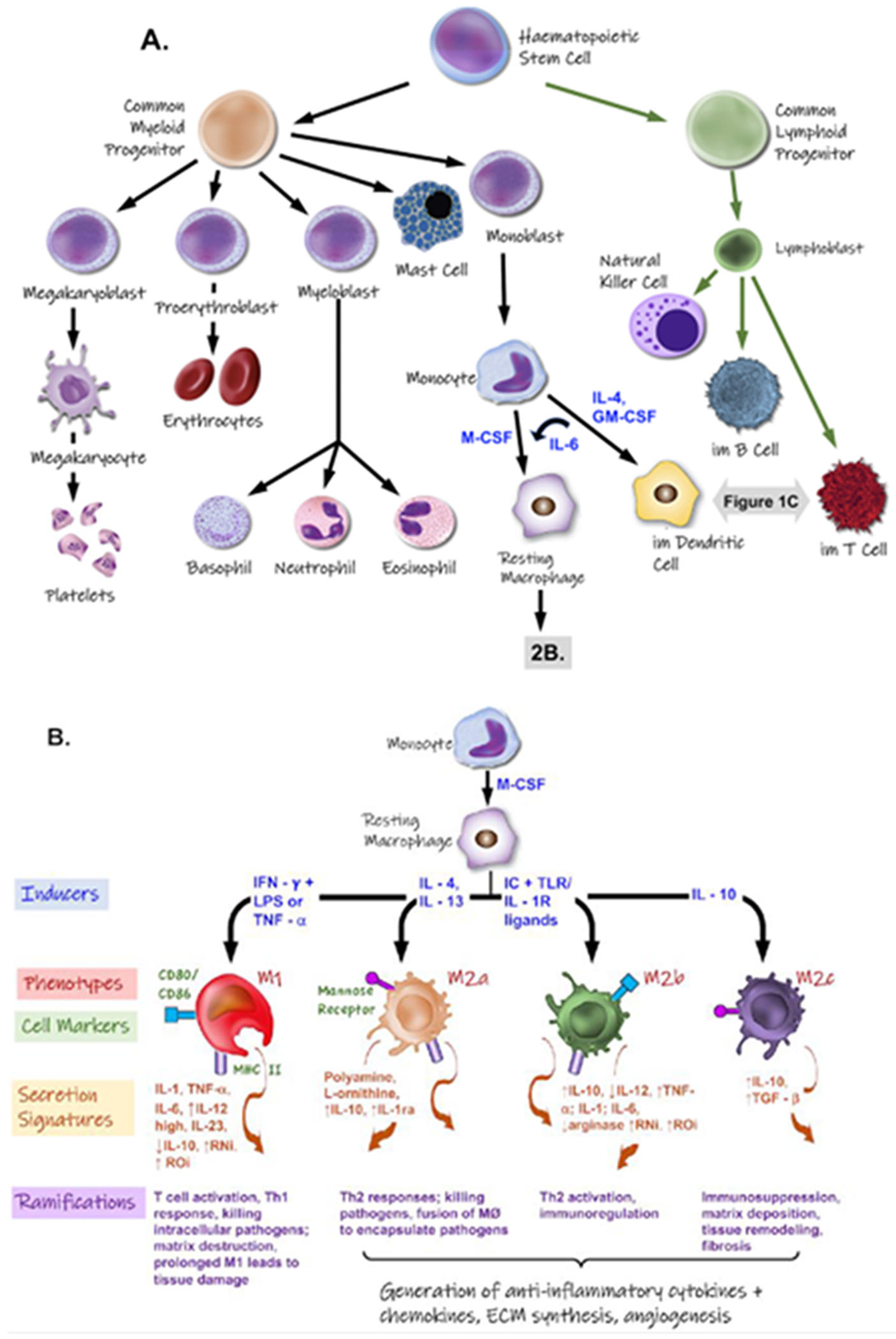
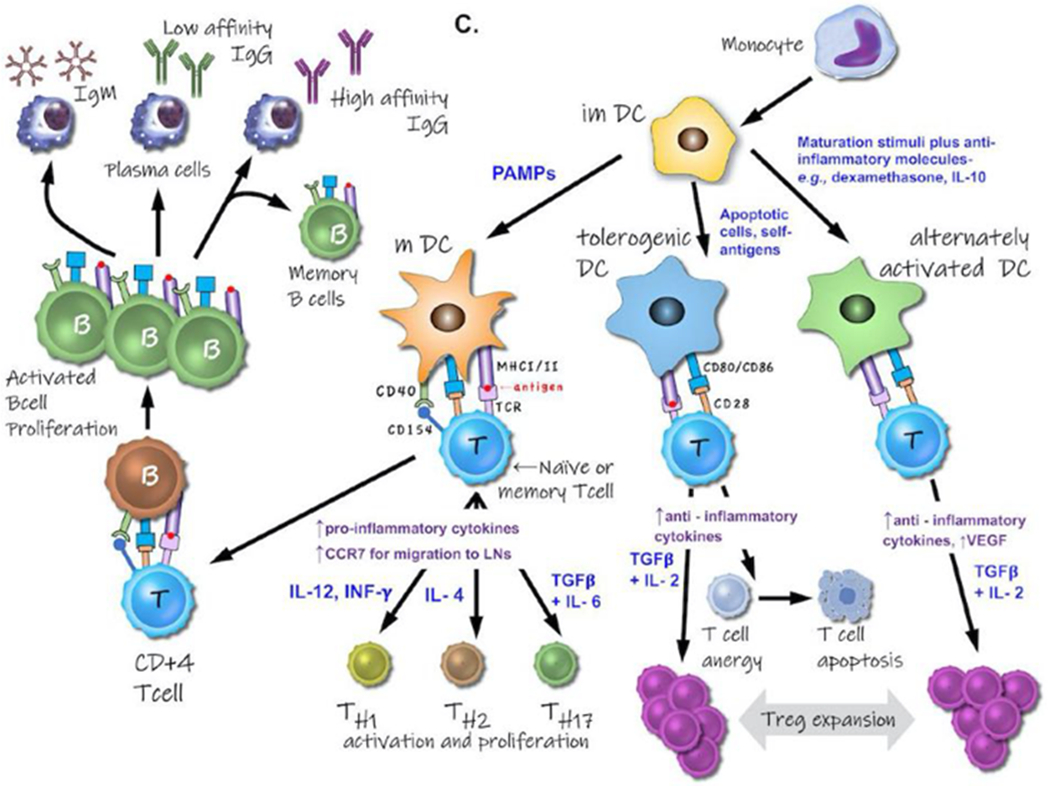
A. Genesis and differentiation of leukocytes. B. Inducers (blue font) and responses (orange font) of various polarized macrophage populations. Exposure to IFN-γ and LPS drives macrophages to a M1 phenotype, exhibiting cytotoxic and antitumoral traits. M2a- (IL-4 and IL-13 induced) and M2b-macrophages (TLR or IL-1R agonist induced) exert immunoregulatory functions and drive type II T cell responses. Induced by IL-10, M2c macrophages suppress certain immune responses and aid in tissue remodeling. Abbreviations: IL- = interleukin; IFN-γ = interferon-γ; LPS =lipopolysaccharide; RNi =reactive nitrogen intermediates; ROi =reactive oxygen intermediates; TLR = Toll-like receptors. C. Different subsets of monocyte-derived dendritic cells (DC), their inducers (in blue font), phenotype, and interactions and influences on both T cells and B cells.
○. Mast Cells
Mast cells (MCs) are tissue resident granulocytes classically associated in host defense, proinflammatory responses, and various immune disorders. MCs affect wound healing by recognizing antigens through pattern recognition receptors and the immunoglobulin E receptor (FceRI) and by releasing contents of their numerous granules that affect cell recruitment, fibrosis, extracellular matrix deposition, angiogenesis, and vasculogenesis. MCs participate in all three stages of wound healing: inflammation, proliferation, and remodeling.[64] MCs are activated by antigens and pathogens through FceRI.[65] MCs migrate to the site of injury and mature by way of mediators released by keratinocytes, platelets, and macrophages during the initial inflammatory response.[66–69] In turn, MCs release signals to further recruit neutrophils, monocytes, and other immune cell types. Cell access to the wound site is facilitated by MC released histamine and tryptase that dilate vasculature and increase capillary permeability.[70,71]MCs proliferation begins by continuing to release mediators that influence fibrosis, angiogenesis, and neovascularization. MCs release products that not only lead to the development of fibrosis but recruit and activate monocytes.[72] MCs also participate in the remodeling phase, as MCs are present and accumulate in fibrotic capsules and during scar formation.[73–76]
While most studies evaluating tissue response to implanted biomaterials has focused on the role of macrophage and T cell populations (see below), the contribution of mast cells in an immune response to biomaterials has been understudied. Most studies have mainly quantified just shifts in mast cell numbers associated with biomaterial implantation, the results of which are suspect given the general low numbers of mast cells in tissue.[77] The reader is directed to an excellent review by Ozpinar et al.,[78] on the influence of various biomedical implant design factors (i.e., natural versus synthetic material of construction, topography/porosity, biologically activated or decorated) on MC responses.
○. Neutrophils
Neutrophils are the dominant immune cell in human blood. During infection, neutrophils are mostly viewed as playing a beneficial role to the host. In cases where the inflammatory process is generated by injury itself or device implantation, it becomes more controversial as to whether neutrophils themselves play any beneficial role.
Neutrophils develop from bone marrow hematopoietic stem cells. Neutrophils circulate within the blood stream until they encounter inflammatory signals released from damaged and necrotic cells after tissue injury or device implantation. These soluble molecules are likely to exhibit damage-associated molecular patterns (DAMPs).[79] DAMP molecules comprise: DNA, histones, high mobility group protein B1 (HMGB1), N-formyl peptides, ATP, interleukin-1α (IL-1α) and many others.[80] Various DAMPs are chemoattractants detected by neutrophils G-protein coupled receptors (GPCRs). Alternatively, damaged tissue cells can release DAMPs that activate surrounding tissues inducing the secretion of chemokines and lipid mediators (e.g., leukotriene B4 and C-X-C motif chemokine ligand 8),[81,82] both strong inducers of neutrophil chemotaxis. Once released, C-X-C motif chemokine ligand 8 can bind to glycosaminoglycans present on cell walls and in the extracellular matrix, thus creating immobilized chemokine gradients along which neutrophils navigate.[83]
There are three possible strategies neutrophils adopt to repair a damaged tissue. First, as professional phagocytes, neutrophils can remove necrotic tissue debris at the site of injury. Second, mature neutrophils can rapidly release upon activation up to 700 unique proteins stored in their segmented nucleus and granules.[84] Release of these compounds (e.g., growth factors or pro-angiogenic factors) directly contributes to regeneration and revascularization. Vascular endothelial growth factor (VEGF), angiopoietin, fibroblast growth factor (FGF) and TGFβ are among the most potent angiogenic cytokines. Both human and murine neutrophils have been demonstrated to be a source of VEGF.[85,86] Matrix metalloproteinases instigate the degradation of ECM components and the release of various growth factors bound to the ECM. Neutrophils are uniquely capable of releasing MMP-9 free of its endogenous inhibitor to angiogenic sites.[87] The third most studied role in neutrophil healing involves neutrophil apoptosis and subsequent clearance by macrophages,[88] a process that initiates a feed-forward, pro-resolution program characterized by the release of TGF-β and IL-10. However, recent evidence suggests that some neutrophils accumulating at inflamed sites may not undergo apoptosis followed by phagocytosis. In a rat model of glomerular capillary injury, the majority of radiolabeled neutrophils attracted to the inflamed site returned to circulation versus becoming apoptotic.[89] Recent literature reports that neutrophils can leave damaged tissue in a process termed neutrophil reverse migration.[90,91]
○. Macrophages
Macrophages are a ubiquitous cellular component present in all tissues and body compartments under homeostatic physiological conditions.[92–94] The eponymous function of the macrophage lineage is phagocytosis. Macrophages express a myriad of pattern recognition receptors, including Toll-like receptors (TLRs), inflammasomes, and lectin-like receptors, located on the cell membrane, in the cytoplasm, and endosomal compartment. Macrophages exert an effector function on other cells of the innate immune system, including: neutrophils, ILCs, and natural killer (NK) cells.[95] Macrophages are thus the main constituent of humoral innate immunity, secreting complement and fluid-phase pattern recognition molecules (PRMs).[96] In turn, humoral immunity components collaborate with macrophages in effector functions and regulate the activity of mononuclear phagocytes. Classic adaptive responses of macrophages include tolerance, priming, and a wide spectrum of activation states. Moreover, macrophages, once activated, have the ability to mount a stronger transcriptional response that is qualitatively and quantitatively different from that mounted by untrained macrophages. (i.e., trained innate immunity).
It is now clear that macrophages can display a plethora of activation/differentiation phenotypes,[97] in addition to fusing to form foreign body giant cells. This heterogeneity is arises during differentiation of macrophages from circulating monocytes in response to ambient signals.[98] “Classically” activated macrophage cells, (i.e., M1 macrophages), arise in response to molecules released by TH1 cells or NK cells, (e.g., IFN-γ), either alone or in combination with microbial PRMs (e.g., lipopolysaccharide, LPS). The M1 phenotype (Figure 2B) is characterized by IL-12 and IL-23 production, elevated levels of reactive nitrogen and oxygen species (NO, superoxides, hydrogen peroxide) and secretion of pro-inflammatory cytokines (TNF, IL-1, IL-6).[98–100] M1 macrophages are associated with strong TH1 cell responses along with causing anti-proliferation and cytotoxic behavior. In their attack on invading pathogens, M1 cells can also destroy local tissue. Without resolution, M1 activation can lead to chronic inflammation, autoimmune diseases, and other pathologies.[101]
“Alternatively” activated macrophages (M2 macrophages) are considered “anti-inflammatory” because they release high levels of the passivating cytokine IL-10 and transforming growth factor-β (TGF-β) and low amounts of inflammatory cytokines. Signal transducer and activator of transcription (STAT3) is activated by IL-10, thereby inhibiting pro-inflammatory cytokine secretion and nitric oxide release.[101,102] M2 polarized cells induce arginase activity in mice, which acts upon nitric oxide synthase-2. M2 macrophages also aid in tissue remodeling by secreting components of the ECM, including: fibronectin, osteopontin, and fibrin crosslinker, transglutaminase.[103,104] M2 macrophages therefore are generally associated with anti-inflammatory, immunosuppressive, and protective properties.
In reality the didactic classification of macrophages as either M1 or M2 are only absolutes created artificially under controlled in vitro conditions. In vivo, the phenotypes of macrophages are regulated by complex, dynamic tissue environments, making it unlikely that a limited number of markers and cytokine expression patterns can define myeloid cell heterogeneity. Indeed, it is generally accepted that the utility of widely used myeloid markers such as F4/80, CD11b, and CD11c is highly tissue dependent. Rather, macrophages possess a continuum of phenotypes for distinct biological functions.[105] Literature suggests that a broad spectrum of macrophage phenotypes exists based on three homeostatic activities: host defense, wound healing, and immune regulation.
Finally, evidence for monocyte/macrophage plasticity is growing. Peripheral blood monocytes have been reported to give rise to endothelial[106,107] and myofibroblast lineages.[108] Furthermore, peritoneal macrophages, elicited by foreign body implantation, exhibited evidence of transdifferentiation to mesenchymal phenotypes. The most convincing in vivo data supporting a transdifferentiation hypothesis were reported in a series of papers by the Badylak group.[109–111] Brown et al.,[110,111] examined the role of MØ in the remodeling process following implantation of 14 different biologically-derived de-cellularized surgical mesh materials in the rat abdominal wall. Their results showed increased numbers of M2-MØ and these higher ratios of M2-to-M1 MØ within the site of remodeling at 14 days were associated with more positive remodeling outcomes. Further, their results suggest the constructive remodeling may be due to the M2-MØ preferential recruitment and survival of different progenitor-like cell populations.
Tissue engineering and regenerative medicine are strongly associated with the controlled application of cell therapy, often in combination with biocompatible materials. Such methodologies have myopically emphasized the role of stem cells as the active biological ingredient. However, recent reports show that the actual structural contribution of implanted stem cells to regenerated tissues is very limited and that stimulating factors exchanged between tissue and stem-or progenitor cells, including growth factors, cytokines, and extracellular vesicles (EVs) maybe the more important driving mechanism.[112] EVs are lipid membrane vesicles, containing a variety of RNA species (including mRNAs, miRNAs), transcription factors, soluble (cytosolic) proteins, and trans-membrane proteins presented in their appropriate functional orientation.[113] EVs play a role in many processes, including intercellular communication, recycling of membrane proteins and lipids, immune modulation, senescence, angiogenesis, cellular proliferation, and differentiation.[114,115] Cells release several types of EVs with different physiological properties, content, and function, as a result of their different mechanisms of generation, including: exosomes, microvesicles, and apoptotic bodies.[116] Exosomes are ~40-150nm in size with a density ranging 1.09-1.18 gm/mL; their most common markers are tetraspanins (CD9, CD63, CD81, and CD82).[113,117] Microvesicles are shed directly from the plasma membrane and can be larger than exosomes (50–1000 nm).[118] Like exosomes, micro-vesicles are able to transfer functional genomic and proteomic content to target cells.[119]
The Badylak group recently examined[120] both laboratory-produced and commercially available de-cellularized biologic scaffolds demonstrating that EVs were present on the scaffolds and could be released upon enzymatic digestion, similar to in vivo implantation conditions. These recovered EVs contained miRNAs capable of exerting phenotypical and functional effects on macrophage activation and cell differentiation,[120] suggesting a possible mechanism behind the inductive properties of ECM bioscaffolds. Growing evidence suggests that transcriptional regulators and miRNA molecules encapsulated within membrane vesicles (i.e., exosomes, microvesicles) that are released by a parent cell can modify the phenotype of recipient cells. EVs can present on their surfaces host membrane cell markers and can internally carry proteins (e.g., transcription factors), bioactive lipids, and nucleic acids (mRNAs, miRNAs). Somatic cells can be reprogrammed to reach an embryonic stem cell-like state by overexpression of certain factors, such as miRNAs.[121]
Eto et al.,[122] reports on a novel subpopulation of CD34+/CD206 + Adipose Tissue macrophages - (ATMs) (11.1% of CD206+ ATMs) that share characteristics with adipose stem/stromal cells (ASCs) and circulating monocytes, in that they exhibited adherent cell growth and differentiated into multiple mesenchymal (adipogenic, osteogenic, and chondrogenic) lineages, similar to adipose stem cells. This novel ATM subpopulation (CD45 +/CD14+/CD34 +/CD206 +) showed biological properties distinct from other ATMs and circulating monocytes/macrophages, including: adherence, localization, morphology, and mesenchymal multipotency suggesting a role in tissue maintenance and regeneration.
Kouri and Ancheta (1972),[123] first proposed that macrophages have the capacity to trans-differentiate by demonstrating existence of cells with intermediate morphologies (between macrophages and fibroblasts) within tissue capsules that formed around implanted polymer discs. The Campbell lab also identified these macrophage-fibroblast intermediary cells in the tissue capsule around intraperitoneal blood clots, further supporting the possibility of macrophage trans-differentiation.[124] More recently, peripheral blood monocytes have been reported to give rise in vivo to endothelial cells[106,107] and myofibroblast lineages[108] in vitro. Qu et al.,[125] demonstrated that a committed macrophage cell line, RAW 264.7 cells, could de- and re-differentiate into mesenchymal-like cells (osteoblasts) upon exposure to a purine analog that acts through the MyD88 pathway. Cooke and coworkers[126] found in vivo that activation of the inflammatory pathway in macrophages is required for efficient nuclear re-programming for induction of pluripotency. Similarly, human peripheral blood monocytes cultured in vitro on stainless steel stents were shown to undergo transition into myo-fibroblasts-like cells.[127] Medina and Ghahary[128] quantified cell transdifferentiation of circulating CD14(+) monocytes into keratinocyte-like cells, which not only exhibited an anti-fibrotic profile, but also released EVs that induced MMP-1 up-regulation in dermal fibroblasts, thus aiding wound healing. Mooney et al. provides evidence for myeloid trans-differentiation using transgenic MacGreen mice to quantify the accumulation of monocyte and neutrophilic granulocytes in the infiltrate and the tissue surrounding a foreign implant.[129] They reported the differential expression of the (a) tcsf1r-EGFP transgene, (b) F4/80 (pan macrophage marker), and (c) Ly6C. As the tissue capsule developed, GFP-positive cells changed from rounded to spindle-shaped morphology and co-expressed myofibroblast α-smooth muscle actin. Flow cytometry analysis of cells isolated from tissue capsules around the implants showed a steady increase in myofibroblast marker α-SM actin, with >80% of these cells also co-expressing GFP. Immunohistochemical assays at Day 14 and 28 also confirmed co-expression of GFP and α-SM actin confirming established monocyte-to-macrophage differentiation, but also the possibility of maturation from granulocyte through monocyte/macrophage to myofibroblast.
○. Dendritic cells
Dendritic cell (DC) response to biomaterials has grown in importance due to the emergence of a large number of tissue engineering products that combine synthetic or natural polymeric biomaterials with biologics (i.e., cells, nucleic acids, and/or proteins). While the synthetic biomaterial may elicit a non-specific inflammatory response involving platelets, polymorphonuclear cells, and macrophages, the cellular or biological component may induce an antigen specific immune response.[130] Since DCs are central in inducing regulatory T cells (Tregs), their ability to induce tolerance is emerging as a significant area for research for tissue regeneration applications.
Babensee’s group,[131] incorporated the antigen ovalbumin (OVA) into two different PLGA biomaterial constructs: either in PLGA microparticles or in PLGA porous scaffolds. Constructs with antigen were then injected (microparticles, MPs) or implanted (scaffolds) into mice and the subsequent immune response quantified. The amount of polymer and antigen delivered was maintained constant. OVA-specific IgGs were significantly higher and persisted longer for antigen released from implanted scaffolds versus when polymer/antigen was delivered by injected particles. Ali et al.,[132] reports similar results for PLG scaffolds versus PLG microparticles, both containing tumor extracted antigens.
Exposure of human derive DCs with PLGA microparticles versus solid films induced increased expression levels of costimulatory molecules (CD40, CD80, and CD86), MHC Class II molecules (HLA-DQ and HLA-DR) and the DC maturation marker (CD83) versus untreated immature DC controls. PLGA-treated DCs exhibited responses resembling those in mature DCs and an enhanced capability to stimulate T-cell proliferation.[133] Similarly, different synthetic materials can exert different effects on DC maturation. For example, PLGA or chitosan films induced DC maturation, whereas alginate and agarose did not; hyaluronic acid (HA) films elicited suppressed DC maturation.[134] Compared to immature DCs, DCs treated with PLGA or chitosan films supported higher levels of T-cell proliferation; DCs treated with hyaluronic acid films induced lower levels of T-cell proliferation; and DCs treated with agarose and alginate films did not differ in response from iDCs.[135]
Exact mechanisms by which DCs recognize biomaterials remain to be identified. This probably occurs via adsorption of complement factors, plasma proteins (with associated carbohydrate modifications), and other danger signals to the biomaterials, which are then recognized by the pattern recognition receptors (PRRs).[136] Use of MyD88 and TLR knocked out mice demonstrated that DCs employ TLR2, TLR4, and TLR6 to sense chemically and physically diverse biomaterials. Mice lacking TLRs or MyD88 exhibited reduced expression of both activation markers and proinflammatory cytokines versus wild-type controls.[137] Receptors such as FcR, complement receptors, and integrins may also influence biomaterial induced DC phenotype.
-. Other Cells Implicated in Biocompatibility: “The New Kids on the Block”
The limited success achieved in regenerating tissues is due in part to the tendency of therapeutic approaches to target late-stage processes in healing and regeneration. Conversely, the immune system is a highly complex network that orchestrates tissue integrity and immediately adapts to features of the local microenvironment throughout the healing process. While previous studies have investigated the host immune response to biomaterials within the context of the foreign body response (FBR), various leukocytes (T cells, B cells, and ILCs) have been identified as important mediators of scaffold-driven tissue remodeling.[111,138,139]
○. T cells
Differential T cell subtypes significantly affect wound healing outcomes.[140] CD4+ T cell populations are particularly important due to their presence within porous scaffolds and influential role in deciding implant fate through phenotypically determined key cell signaling modalities.[141] T Helper 1 (TH1, indicated by TNFα, TBX21, and IFN-γ) cells inhibit collagen deposition and traditionally pro-reparative effects by increasing local inflammatory signaling, while regulatory T cells (Tregs indicated by FoxP3 and IL-10) are instrumental in initiating and maintaining pro-regenerative responses.[141–143].
Analysis of the cytokine profile of T cells extracted from injured or infected tissues demonstrates that TH1 cytokines predominate in moderately inflammatory conditions compared with TH2 cytokines in aggressive inflammation.[144] In addition to the TH1 and TH2 cell subsets, there are two closely related T-helper cell subsets with opposing functions namely, regulatory T cells (Tregs) and TH-17 cells.[145–147]
TH17 cells are an independent T-helper cell subset that plays a critical role in the development of autoimmunity, allergic reactions and host defense.[148] TH17 cells were named after their production of the signature cytokine IL-17. IL-17 alone or in synergy with either IL1β, tumor necrosis factor (TNFα), interferon-γ, or Toll-like receptor (TLR) ligands can stimulate human fibroblasts, epithelial cells and macrophages to produce various pro-inflammatory mediators.[149–153] Evidence from these studies suggests that IL17 secreted by TH17 cells, may play a role in the breakdown of infected or injured tissues.
Conversely, Tregs - a specialized subset of T-helper cells - express the transcription factor Forkhead box P3 (FoxP3). Tregs participate in inducing effector T cells, therefore controlling elevated immune responses and the initiation of autoimmunity.[153,154] Tregs use cell contact dependent (mediated by surface molecules; glucocorticoid-induced tumor necrosis factor receptor [GITR], cytotoxic T-lymphocyte-associated antigen [CTLA]-4 and membrane-bound TGFβ) and contact independent mechanisms (sensing cytokines such as TGFβ and IL10) to suppress the effector T cells (TH1/TH2 and possibly TH17) and antigen-presenting cells.[153] Unresolved inflammation during tissue injury can impair healing and tissue remodeling. In many tissues, Tregs are attracted to the damaged site to facilitate inflammation resolution and regulate immunity. Tregs can indirectly modulate regeneration by controlling neutrophils,[155–157] inducing macrophage polarization,[158,159] and regulating helper T-cells.[157,160] Moreover, Tregs have been shown to directly facilitate regeneration by activating progenitor cells locally.[161,162] Tregs can actively release immunosuppressive exosomes that inhibit IFN-γ secretion and growth of Th1 effector cells.[163] Treg-derived exosomes can also induce the differentiation of other T cells to the Treg phenotype.[164]
Similar to findings for decellularized biological scaffolds by the Badylak group,[120] Hady et al., [165] reports for poly(HEMA) porous precision templated scaffolds PTS (where all pores are approximately the same size throughout the scaffold) that PTS pore size (40- vs. 100 μm) did not affect scaffold resident immune cell population ratios or the proportion of myeloid EVs generated from explanted PTS. However, quantitative transcriptomic assessment indicated cell and EV phenotype were pore size dependent. In vitro experiments demonstrated the ability of PTS cell derived EVs to stimulate T cells transcriptionally and proliferatively. Specifically, EVs isolated from cells inhabiting explanted 100μm PTS significantly upregulated TH1 inflammatory gene expression in immortalized T cells. EVs generated from cells inhabiting both 40μm and 100μm PTS upregulated essential Treg transcriptional markers in both primary and immortalized T cells. Finally, the effects of Treg depletion on explanted PTS resident cells were quantified. FoxP3+ cell depletion suggests Tregs play a unique role in balancing T cell subset ratios, thus driving host response in 40μm PTS. These results indicate that predominantly 40μm PTS myeloid cell-derived EVs affect T cells through a distinct, pore size mediated modality.
Particularly, Tregs both influence T cell subtype and modulate neutrophil, macrophage, and local progenitor cell activation.[156,157,161,162] Recent evidence suggests that Th2 cells (indicated by GATA3), which modulate classical Th1 driven inflammation while enhancing fibrosis, eosinophil activation, and IgE mediated responses, are required to elicit the pro-healing response intrinsic to certain biomaterial scaffolds.[166,167] Th3 and Th17 cells (indicated by TGF-β1 and RORγ, respectively) are traditionally ambiguous in wound healing: Th3 cells can release varying amounts of IL-10 and TGF-β1 depending on external stimuli, and Th17 cells can release both classically pro-inflammatory and anti-inflammatory cytokines.[168,169] Despite this ambiguity, recent evidence suggests that IL-17 released by Th17 cells is instrumental in enabling the inflammatory FBR.[170]
○. B cells
There is extensive literature linking B cells to chronic inflammation a swell as bone destruction.[171] Following biomaterial implantation coupled with bacterial infection or the presence of antigenic materials within scaffolds, B cells are thought to infiltrate and dominate the site.[172,173] Considering their chief role in innate-like and adaptive immune responses, B cells do provide residual protection against infections. In most cases, the bacteria-specific antibody response is generally unable to halt disease progression (e.g., periodontal disease) suggesting that the antibodies produced exhibit low anti-bacterial blocking functions or opsonophagocytic potential, and/or unfavorable effects. Further, endogenous antigens associated with cell-seeded scaffolds may also contribute to chronic inflammation. Scant evidence on the role of B cells in tissue healing seems to suggest that their depletion also represents a promising strategy to augment bone regeneration, since adaptive immune system deficient mice exhibit faster bone healing.[174] However, much is still to be discovered on the role of B cells in the repair and regeneration of various tissues.
○. Innate lymphoid cells
Innate lymphoid cells (ILCs) are a recently recognized subgroup of immune cells that mimic the phenotypes and functions of T cells. ILCs, defined by the lack of expression of T or B cell receptors, have been divided into three classes (ILC1, 2, 3), denoted by their canonical transcription factors and cytokine expression.[175] These ILC subsets mimic the expression of Th1, Th2, and Th17, respectively. ILC2s, similar to Th2 cells, produce IL-4, IL-5, and IL-13, are anti-inflammatory and secrete cell signals, mediators and metabolites associated with wound healing. ILC2s also promote CD4 T cell differentiation to Th2 cells via direct inhibition of Th1 cells.[176–178] ILCs are activated by stress signals, microbial compounds, and the cytokines, rather than by antigens: similar to the activation of memory or “innate” T cells, such as invariant NKT cells and subsets of gdT cells. This mode of activation makes ILCs highly reactive and early effectors during the immune response. Since ILCs are activated early in response to infection or injury, producing type 1, type 2, and type 3 cytokines, it is expected that ILCs regulate the developing adaptive immune response.[179] ILCs do this in two ways: directly by expressing MHC Class II molecules (MHC II), and indirectly by regulating DCs. ILC2s orchestrate tissue-repair responses by producing amphiregulin (a ligand of the epidermal growth factor receptor) and IL-13. By producing LTa1b2 and IL-22, ILC3s can promote tissue protective and repair responses. In a model of graft-versus-host disease (GvHD), ILC3s protect intestinal epithelial stem cells from GvHD-induced cell death.[180] Since the development of a pro-regenerative response to biomaterials may require type 2 cell populations[139] and that the interaction between Th2/ILC2 is central to tissue pro-regenerative responses, ILC2 activity may be a potential target for new biomaterial designs.
5. Contemporary Approaches to Mitigating the Foreign Body Response
The unification of an ever-expanding insight into human biology and physiology coupled with the rich, state-of-the-art toolbox of sophisticated and broadly applicable methods for material design, synthesis, and modification have paved the way for the modern age of biomaterials. A contemporary approach to biomaterials design is aimed at actively modulating the host response toward tissue reconstruction and integration as opposed to mere fibrotic-encapsulated coexistence. In this way, the field has made strides in actively reimagining and restructuring the definition of “biocompatibility.” Still, the path toward this more integrative and regenerative form of biocompatibility has elucidated the major causes of less-than-optimal, though still traditionally “biocompatible”, outcomes of implanted biomaterials:
Chronic tissue damage due to the inappropriate physical properties (e.g., mechanical) of the biomaterial within the body.
Implant-induced bacterial colonization, particularly with percutaneous biomaterials.
An immune attack (i.e., the classical foreign body reaction) which can disrupt adjacent tissue due to the persistence and hyperinflammatory nature of the cell types involved.
Immune separation from the tissues of the body in the formation of a densely crosslinked fibrotic capsule, which can significantly impair biomaterial functionality.
In recognition of these sub-optimal modes of biomaterials healing, the field has pursued contemporary solutions involving specific and engineered control along three major design axes: 1) control over the physicochemical biomaterial properties, 2) control over bioactive molecular release into the host tissue environment, and 3) control over surface-mediated interactions at the tissue-material interface. Themes relevant to this modern conception of biocompatibility include: the phenotypic polarization and distribution of key cell types that drive the tissue response;[105,111,139,167,181,182] the incorporation of a wide range of components from both natural and synthetic origins;[183,184] a keen awareness of microbiological processes;[185,186] and, perhaps most significantly, those approaches that integrate multiple solutions in the creation of multifunctional materials that address these various issues in a simultaneous or sequential fashion.[187–189]
The contemporary approach to the design of biocompatible biomaterials, then, begins with an understanding, from first principles, of the physiological requirements (i.e., mechanical, electrochemical, structural, etc.) of the target tissue and proceeds with the design, selection, and integration of materials along the three design axes. Ultimately, to maximize successful biological outcomes, the final design of the biomaterial must address these physiological requirements with careful consideration of the engineering trade-offs inherent to the material/synthesis choices made and their effect on biomaterial function.
It is in consideration of this contemporary approach and its underlying themes that we will present a curated set of recently published articles that demonstrate this active redefining of the term “biocompatible.” As a testament to the expansive research effort underway in the field of biomaterials, numerous review papers have been published focusing on virtually every topic covered here that the reader is encouraged to explore to deepen their understanding of these new biocompatibility strategies. The reader is also directed to the emerging space of biomaterial-assisted methods for immune engineering that are designed to induce immune tolerance as opposed to specifically limit foreign body reactivity.[190–192]
-. Control Over the Physical Properties of Biomaterials
○. Mechanical
The mechanical properties of human tissues range over several orders of magnitude[193–195] and individual cells have shown sensitivity to substrate stiffness, altering their phenotypic activation or differentiation profiles in response.[196,197] Mechanical mismatch at the tissue-device interface can induce chronic tissue damage as a result of persistent tissue strain in both soft[198] and hard tissues.[199] Matching mechanical properties with the target tissue is thus crucially important to provide appropriate cellular mechanical cues to promote functional regeneration of tissues and for minimizing tissue damage which can exacerbate fibrotic encapsulation. Modern approaches include materials with adaptive mechanical compliance for brain implants, which are initially stiff to aid implantation but then soften within the tissue,[200] and new fabrication techniques to tune the mechanical stiffness of porous metals to match the mechanical properties of specific bone defects.[201]
○. Porosity
An interconnected porous network provides a supportive, three-dimensional structure for the infiltration of cells, organization and deposition of functional extracellular matrix, and growth of new blood vessels and other tissue-specific structures.[202] This 3D network also promotes a heterogeneous spatial distribution of cellular phenotypes (i.e., macrophage M1 vs M2) to promote vascularization and remodeling across the material-tissue boundary as opposed to unresolved inflammation localized at the interface.[63] Porosity has been seen to impact biomaterials healing since at least the 1970s.[203] More recently, Bartneck et al.,[51] compared macrophage responses to flat 2-D PLGA films versus 3-D electrospun PLGA nanofiber meshes of three different average pore sizes (230, 100, 30 μm). Flat 2-D films lead to an increased number of M1 macrophages that released large amounts of pro-inflammatory cytokines. In contrast, 3D nanofibers with identical surface chemistry yielded varying macrophage phenotype, depending on pore size; 230μm exhibited M1 response, 100μm a mixed M1/M2 phenotype, and 30μm exhibited M2 responses. Taking this one step further, porous networks with precise, highly uniform spherical pores on the order of 40μm in diameter have shown extensive vascularization in the adjacent tissue and throughout the porous network along with a reduction in foreign body capsule thickness and density.[62,63] Consistent with with Bartneck et al.,’s findings, larger and smaller pore sizes show a response more akin to the classic FBR with low vascularity, cellularity and more fibrosis (Figure 3).
Figure 3:
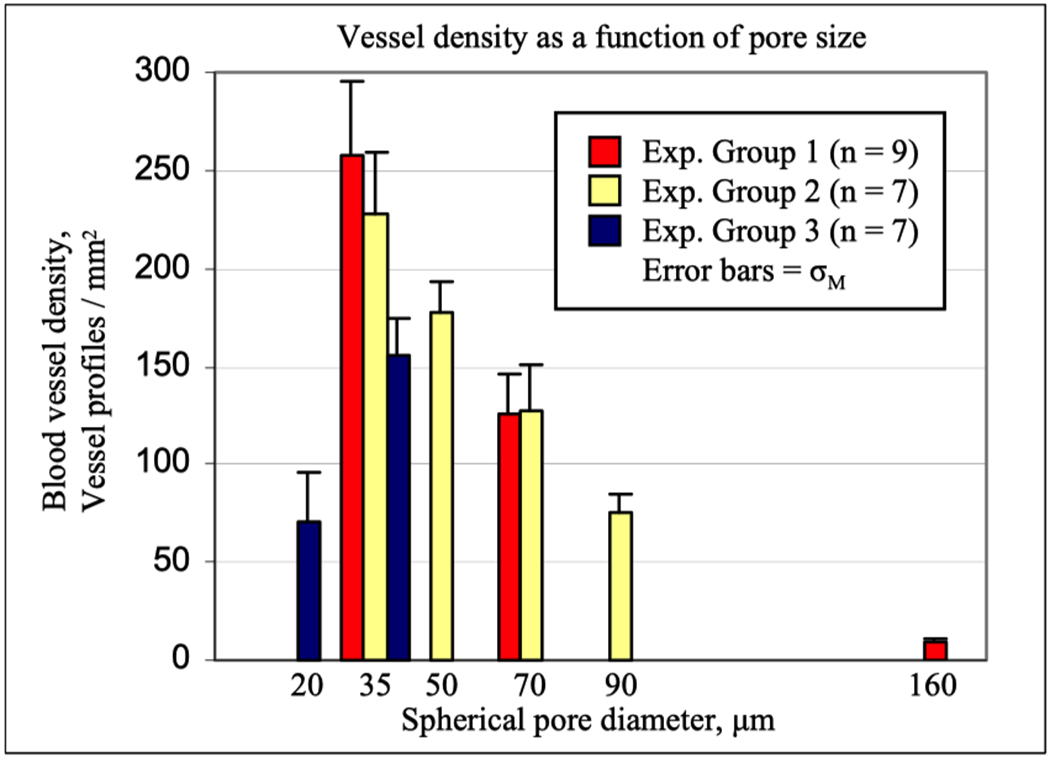
Pore size impacts vascularity and healing of implanted structures with uniform, interconnect pore sizes after one month implantation.[204]
Spatial confinement has been shown to directly impact macrophage phenotypic polarization by limiting actin polymerization, thus reducing the expression of inflammatory genes regulated by downstream actin-dependent transcription factors.[205] Pore size has also demonstrated an impact on the composition of EVs released from macrophages and their capacity to modulate T cell phenotype.[165] More generally, the generation of a microenvironment that resembles native tissue architecture to support and regulate cellular behavior and function serves as a central design feature for engineering the regeneration of native tissue in and around the implanted material. One final item worth mentioning on the topic of porosity involves the generation of a porous cytokine sink/trap whereby the porosity of the material is specifically targeted at sequestering inflammatory cytokines upon implantation to limit inflammatory reactivity of immune cells.[206]
○. Geometry
Device geometry (size, shape, presence of sharp edges, etc.) has also been shown to have a direct impact on the foreign body response.[207,208] For example, it was demonstrated that fibers less than 5 μm in diameter gave almost no FBR while larger fibers showed collagenous encapsulation.[209] This is also exemplified with the implantation of probes into highly delicate nervous tissue where both an increase in implant diameter and stiffness results in greater degrees of vascular and parenchymal tissue damage, blood-brain barrier permeability, microglia (tissue-resident macrophages) activation, neuronal cell death, and fibrotic scar formation.[210] Soft hydrogel coatings on traditional devices limit mechanically induced tissue strain but also increase device footprint, a clear example of engineering design tradeoffs. However, modern nanofabrication techniques have enabled the development neural probes with sub-cellular dimensions composed of flexible materials.[211] These devices, coupled with new automated precision implantation techniques, have demonstrated efficacy in long-term neural recording applications with little to no foreign body capsule formation.
Interestingly, Veiseh et al., have demonstrated that small spheres (300μm diameter) produce an increase in fibrotic tissue formation as compared to larger spheres (1500μm diameter), regardless of material type, stiffness, surface roughness, or exposed surface area.[19] Although the complete mechanism for these observations is still unknown, the authors consistently found fewer macrophage cells around the larger spheres and hypothesized this reduced population size produced a limited overall inflammatory response. Pancreatic islet cells were encapsulated in 1500μm diameter alginate spheres and implanted intraperitoneally in a mouse model of diabetes, demonstrating a prolonged return to normoglycemia compared to other groups. Further elucidation of this strategy for biomaterials design will need to be established since it is well known that larger objects (a pacemaker with a rounded-edge geometry, for example) would be completely fibrotically-encapsulated upon soft tissue implantation.
○. Electroactivity
Electroactivity is a core functional aspect of several tissues, most notably muscle and nervous tissue, but many respond to electrical stimuli. The incorporation of electroactive components into biomaterial scaffolds has demonstrated a significant impact in their functional integration with these target tissues.[212] Electroactive nanomaterials like metal nanoparticles, graphene, carbon nanotubes, and conductive polymers have been employed owing to their high conductivity and ease of incorporation.[213] Low levels of electrical current have been shown to increase progenitor cell recruitment and differentiation,[214] axonal growth,[215] and may modulate the phenotypic polarization of immune cells.[216] He et al., have developed an injectable material composed of the natural polymer chitosan and synthetic conductive polymer poly(pyrrole) that promoted remodeling of myocardial infarct-induced fibrotic scar tissue and rescued conduction pathways, thereby improving ventricular ejection volume and other functional cardiac metrics.[217]
○. Degradation
While the persistence of a device/material within the tissue is often necessary (e.g., sensors, stimulators, prostheses), controlled degradation is a powerful method for promoting tissue regeneration and remodeling. Depending on the intended application of the biomaterial/device and the degree to which it must persist within the tissue, degradable materials may be incorporated to target tissue ingrowth/integration to improve device function, or replace the device completely with regenerated, scar free tissue. Factors impacting degradation include the mechanism (hydrolytic, enzymatic) and material physicochemistry (porous/monolithic, hydrophobic/hydrophilic, molecular weight, crosslinking density etc.).[218] An extensive array of organic and inorganic degradable materials with control over these factors has been developed.[219] Degradation rate and products are crucially important to control to match tissue reconstruction, ensure appropriate load transfer from material to tissue, and promote beneficial physiochemical cellular signaling.
-. Molecular Release to Control Biological Reactions
The toolbox of bioactive molecules continues to grow as basic science research elucidates key players in various endogenous signaling pathways and new anti-inflammatory drugs are developed. These molecules can be loaded into the biomaterial bulk or bound to their surface (physically adsorbed,[220] entrapped in nanotopology,[221] etc.). Controlled release is an important factor as release rate has been shown to impact degree of angiogenesis and fibrotic encapsulation.[222] It should be noted that delivery of bioactive molecules to the local tissue environment in conjunction with an implanted biomaterial to reduce foreign body reactivity is limited as the molecules are typically consumed, degraded, or diffuse away within acute time periods. However, when integrated with other biomaterial design strategies, bioactive molecules may provide a powerful immunomodulatory signal early during the FBR to promote better chronic outcomes.
○. Single, Multipotent Bioactive Molecular Release
Several naturally derived small molecules and soluble factors have evolved to affect multi-potent bioactivity. For example, nitric oxide (NO) is a free radical naturally released by macrophages and endothelial cells and has been implicated in resolution of inflammation, inhibition of platelet adhesion and activation, stimulation of angiogenesis, and as a powerful antimicrobial agent.[223] Polymeric coatings composed of NO-bound carriers such as diazeniumdiolates or S-nitrosothiols that enable sustained release of NO have shown improvements in biomedical device performance (e.g. glucose sensors, vascular grafts, etc.) over clinically relevant time scales.[224,225] Another interesting molecule that has been integrated to biomaterials with controlled release dynamics is itaconate (ITA), a powerful modulator of macrophage inflammatory reactivity.[226] Recently, Huyer et al., developed a synthesis scheme to incorporate ITA into the backbone of polyester-based biomaterials to enable sustained, hydrolytic release of ITA and observed improved resolution of inflammation compared to silicone controls in a mouse intraperitoneal injection model.[227]
○. Sequential Molecular Release
The sequential or multi-modal delivery of multiple immunomodulatory cytokines has been shown to augment the response of macrophages.[220,228] Spiller et al., physically adsorbed interferon-gamma (IFNγ) for rapid release coupled with tighter biotin-streptavidin binding and slow release of interleukin-4 (IL4) to stimulate an early M1 macrophage response followed by a switch to M2.[220] This sequential presentation caused an increase in M2 expression over those initially stimulated toward M2 or switched from M2 to M1, which correlated with greater degrees of vascularization. The incorporation of tissue specific cells or stem cells into scaffolds is another powerful method for making use of endogenous release of immunomodulatory and regenerative bioactive molecules.[229,230] Another example involves the timed, sequential delivery of VEGF and PDGF growth factors, which generated robust blood vessel networks, whereas release of single components was not as effective in stimulating healthy blood vessels formation.[231]
○. Stimulus Responsive Release
Stimulus-responsive release of bioactive molecules enables a degree ‘programable’ biomaterial functionality.[232–234] For example, Boehler et al., electrodeposited the conductive polymer PEDOT loaded with the anti-inflammatory drug dexamethasone onto electrodes implanted into rat brains.[235] Release of dexamethasone was controlled via cyclic voltammetry and inhibited neuronal death around the electrode to improve long-term recording capabilities.
-. Control Over Surface Mediated Interactions
Surface-mediated interactions are also crucially important to consider as the possibility for frustrated phagocytosis at the tissue-device interface is ever present. Fortunately, the toolbox of easily addressable and broadly applicable surface modification techniques is also continually increasing.
○. Surface Topography
The surface topology of materials in the form of nano- or microscale surface roughness and patterning has been shown to have a direct impact on the adhesion and activation of cells[236,237]. For example, Luu et al., have shown that macrophages cultured on linear micropatterned surfaces adopt an elongated morphology serving as a driving stimulus for the release of pro-regenerative cytokines.[238] Similarly, Raw 264.7 macrophages, seeded on rough epoxy exhibited cell surface markers and secreted cytokine profiles of M2 macrophage phenotype as compared to those seeded on polished smooth resin surfaces.[239] Padmanabhan et al., have also demonstrated the impact of precise nanotopography, in the form of nanorod arrays, serves as a primary signal in modulating macrophage fusion via attenuation of cytoskeletal remodeling.[240]
○. Stealth Materials
Stealth surfaces incorporate materials that are essentially invisible or present a ‘self’ signal to cells. Super-hydrophilic, non-fouling synthetic zwitterionic materials have demonstrated extreme resistance to adsorption of proteins, thereby limiting the presentation of a ‘foreign’ signal to cells at the material surface and effectively eliminating the initial steps of the foreign body response.[241,242] The tissue response to these materials has demonstrated low macrophage cell count and inflammatory cytokine release, little to no fibrotic encapsulation, and is instead dominated by growth of healthy, vascularized tissue. Though much has been written about poly(ethylene glycol) (PEG) as a non-fouling surface, PEG surfaces are relatively poor at inhibiting the FBR, probably due to their activation of the complement system.[243,244] Recent work from the Stachelek lab has also demonstrated that the immobilization of CD47, a universal cell-surface marker designating ‘self’, was able to inhibit cellular attachment and inflammatory reactivity of macrophages when immobilized on surfaces [245] and to limit fibrin, platelet, and THP-1 macrophage adhesion when immobilized onto cardiovascular stents[246].
○. Bioactive Coatings
Surface coatings with targeted bioactivity modulate the local population of immune cells at a material surface, thereby curtailing the severity of the foreign body reaction.[247] Hsieh et al., reports that a surface coating of a layer by layer film of hyaluronic acid (HA) and type 1 collagen on PDMS reduced FBGC formation and reduced fibrous encapsulation after 3 weeks of implantation in a rat subcutaneous implant model compared to uncoated PDMS samples.[248] Similarly, Wu et al., reports that a HA and polydopamine (PDA) surface coating limits macrophage adhesion and activation while also stimulating endothelialization in a manner dependent on HA content when cultured in vitro and limited adhesion and activation of platelets when tested in a flow chamber with whole blood.[249] Wu et al., documents implanting optimized HA/PDA coated stainless steel discs into the femoral artery of pigs for 1 month; they observed reductions in coagulation and an increase in surface endothelialization compared to samples with excess HA and bare stainless steel. Qian et al., coated PCL fiber scaffolds with silk-fibroin and heparin-disaccharide and IL4, drastically reducing protein adsorption, FBGC formation, and fibrotic encapsulation with an increase in pro-regenerative macrophage phenotypes and growth of new vasculature.[250] From a synthetic standpoint, Coindre et al., coated a cell pouch device with poly(methacrylic acid-co-isodecyl acrylate), which increased vascularization and reduced the inflammatory macrophage population number by modulating an insulin growth factor signaling pathway.[251] This highly vascularized coated cell pouch supported therapeutic transplantation of islet cells in a diabetic mouse model and rescued normoglycemia.
○. High Throughput Biomaterial Design and Novel Chemical Structures
The advent of high-throughput biomaterial design endeavors has sped discovery of unique chemical structures that modulate the bioreaction to implants.[19,252] For example, Vegas et al., reports on identifying an optimal set of chemical modifications to alginate that could limit fibrotic encapsulation.[252] Their approach began with a large array of alginate analogues with various chemical modifications. This set was narrowed down through a rapid subcutaneous mouse implant model to measure levels of acute inflammation with a non-invasive fluorescent marker for immune cell activation. The top performers were then recombined with another set of structurally diverse alginates to produce microspheres for implantation with a more detailed post-mortem histological evaluation of fibrosis. The result of this process yielded a small set of triazole-modified alginates that demonstrated little to no fibrotic deposition or cellular adherence after 6 months of implantation. The authors concluded that the chemical modification presented at the surface of the material was the driving force behind the findings given the uniform mechanical properties, surface roughness, and protein adsorption across the materials tested.
-. Exemplary Multifunctional Biomaterials
As another testament to the progress in the field, many biomaterials designed today employ a multifunctional approach by thoughtfully integrating design features from the 3 design axes. Tian et al., engineered a HA-based biomaterial crosslinked with iron(III) ions and EDTA, with the ratios of each tuned precisely to match the mechanical properties of skin.[253] The physical crosslinking enabled release of Fe3+ ions upon bacterial-induced degradation which, once labile, could act as a potent antimicrobial and/or re-crosslink the material to maintain its integrity. Loading with PDGF also improved neovascularization and downregulation of pro-inflammatory activation of material-adjacent cells as the material degraded.
Qu et al., designed a hybrid scaffold for connective tissue regeneration by electrospinning fibers of PCL, poly(ethylene oxide) (PEO) infused with collagenase, and HA infused with PDGF.[254] The slow, hydrolytic degradation and mechanical properties of PCL were matched to that of connective tissue, providing the primary structural support. Rapid dissolution of PEO post-implantation imparted porosity and released collagenase to loosen the dense, implant-adjacent connective tissue to promote cellular infiltration. Enzymatic degradation of HA sustained over a 5-week period, enabling controlled release of PDGF to recruit stem cells, resulting in an increase in functional deposition and integration of collagen.
Bakhsheshi-Rad et al., engineered a biomaterial for regeneration of cortical bone by coating degradable magnesium substrates with electrospun PCL nanofibers infused with the mineral åkermanite and antibiotic doxycycline.[255] The magnesium substrate served as the primary mechanical support, matching that of bone, while the polymeric coating provided control over magnesium degradation and deleterious elution of H2. The addition of åkermanite particles enabled controlled release of silicon, calcium, phosphorous, and magnesium ions to facilitate the formation of bioactive apatite, a mineral central to bone regrowth, on the material surface. Åkermanite was also able to promote the proliferation and osteogenic differentiation of infiltrating adipose and mesenchymal stem cells to further improve bone regeneration. Lastly, the extended release of doxycycline as the PCL degraded enabled a sustained bacterial inhibition zone.
The decellularization of tissues has produced extracellular matrix (ECM) materials from a wide array of tissues.[256–258] The ECM scaffold provides an enzymatically degradable, porous, 3D scaffold comprised of natural polymers which contain binding domains for cellular adhesion and migration. Perhaps most strikingly, their degradation also incurs the release of small peptides, termed matricryptic peptides, and EVs containing a plethora of pro-regenerative nucleic acids and proteins. Brown et al.,[111] recently examined the role of macrophages in the remodeling process following implantation of 14 different biologically derived decellularized surgical mesh materials in the rat abdominal wall. Their results showed increased numbers of M2 macrophages and these higher ratios of M2-to-M1 macrophages within the site of remodeling at 14 days were associated with more positive remodeling outcomes. Further, their results suggest the constructive remodeling may be due to the M2 macrophage preferential recruitment and survival of different progenitor-like cell populations. These ECM materials have also demonstrated remarkable success in functional regeneration of human tissues.[138,259]
As a concluding remark to this section, it should be noted that the expansion of the biomaterials design toolbox and its application in the development of multifunctional biomaterials present an increased need for multivariate analysis techniques to elucidate correlations between material properties and biological outcomes. One example worth highlighting here is the application of multivariate statistical techniques, such as principal component analysis, to the analysis of time-of-flight secondary ion mass spectrometry (ToF-SIMS) data to determine the impact of various biomaterial properties on protein adsorption. [260–262]
6. Translation of New Biocompatibility Concepts from Lab to Clinic
Though most of the concepts for biomaterials that heal in a non-fibrotic, integrative manner, presented in section 5 of this article, are being explored in laboratory settings or pre-clinical models, there are some examples of strategies to ameliorate the FBR that have translated from bench to clinic. Possibly the earliest example of a biomaterial to reduce fibrosis upon implantation was the steroid-releasing pacemaker lead.[263] Decellularized extracellular matrix (ECM) biomaterials heal without fibrosis and with restoration of new tissue and vasculature. Millions of such ECM-based biomaterials have been used in surgical procedures.[264,265] Precision porous polymers with pores in 30-40 μm range have been used for a number of years as glaucoma draining devices to reduce intraocular pressure.[266] A vascular prosthesis that heals without the fibrotic sheath that impedes graft mechanical compliance is now in clinical trials (Evaluation of STARgraft AV for Hemodialysis Access, ClinicalTrials.gov Identifier: NCT03916731). Implanted glucose sensors fail in providing accurate, long-term glucose measurements, probably due to fibrotic blockage of the sensing element. A clinical trial is in progress to assess implanted sensor longevity. The sensor, described in a published article describes their electrode protection as “a thin electrolyte layer” without further elaboration. The clinical trial is described in the clinicaltrials.gov web site (PROMISE Study: An Evaluation of an Implantable Continuous Glucose Sensor up to 180 Days, ClinicalTrials.gov Identifier: NCT03808376).[267] Another concept addressing insulin production for diabetics, a protected implant for a cell therapy, is undergoing clinical trials, The device is protected by some combination of non-fouling zwitterionic polymers and small molecules found to inhibit the fibrotic response.[268] This clinical trial is described in the clinicaltrials.gov web site (ClinicalTrials.gov Study Number: NCT04541628).
7. Conclusions
The concept of biocompatibility started with simple toxicology and evolved to standardized ISO tests and histological examination. It is now time to further advance the ideas of biocompatibility beyond their historical roots and beyond the present definition dating from 1986 which, though accurate, offers no insights to testing and advancing biocompatibility. The biology of 1986 knew no vesicular communication (exosome communication), toll-like receptors were just discovered and there was only one “flavor” of macrophage (no knowledge of the macrophage polarizations), as just a few examples. How can we integrate encyclopedias of new discoveries and understanding into our understanding and application biocompatibility? For example, consider crosslinked poly(hydroxyethyl methacrylate)(pHEMA) hydrogels cast a solid slab or fabricated into a porous structure with interconnected 40 μm diameter pores. Though the chemistry of both specimens is identical, and though both will pass all ISO tests appropriate for regulatory clearance, the porous specimen will heal in many tissues in the body with good vascularity, tissue reconstruction, and little fibrosis while the slab will be surrounded by a dense, avascular fibrotic capsule (the classic FBR).[63] How can we call both these reactions “biocompatible?” The authors of this paper here offer up for consideration a new definition of biocompatibility, reflecting the advances in biomaterials and biology that have taken place in the past 34 years.
“BIOCOMPATIBILITY: the ability of a material to locally trigger and guide the proteins and cells of the host toward a non-fibrotic, vascularized reconstruction and functional tissue integration”
In fact, the potential exists today to even develop a quantitative measure of just how biocompatible an implant material might be. A scheme for such quantitative assessment has been presented.[33]
Footnotes
note that this review does not address blood compatibility or implants in non-vascularized tissues. The subject of blood compatibility is outside the scope of this review and has many other considerations not relevant to soft tissue reaction to implants. Also, this article is particularly focused on vascularized tissues that permit the egress of inflammatory cells to the site of injury (the implantation). Reactions in cartilage or other tissues with low vascularity can be different.
Bibliography
- [1].“Etymonline.com,” 2020. [Google Scholar]
- [2].Downey R, Sci. Race Story Kennewick Man N. Y. Copernic 2000. [Google Scholar]
- [3].Bing J, Acta Chir. Scand 1950, 100, 293. [PubMed] [Google Scholar]
- [4].Gordon S, J. Innate Immun 2016, 8, 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Homsy CA, J. Biomed. Mater. Res 1970, 4, 341. [DOI] [PubMed] [Google Scholar]
- [6].Homsy CA, Ansevin KD, O’bannon W, Thompson SA, Hodge R, Estrella ME, J. Macromol. Sci 1970, 4, 615. [Google Scholar]
- [7].Anderson JM, Miller KM, Biomaterials 1984, 5, 5. [DOI] [PubMed] [Google Scholar]
- [8].Williams DF, Progress in Biomedical Engineering: Definitions in Biomaterials, Elsevier, 1987. [Google Scholar]
- [9].Zhang X, Williams D, Definitions of Biomaterials for the Twenty-First Century, Elsevier, 2019. [Google Scholar]
- [10].Biological Evaluation of Medical Devices - Part 1: Evaluation and Testing within a Risk Management Process (ISO 10993-1:2009), The British Standards Institution, 2009. [Google Scholar]
- [11].Bernard M, Jubeli E, Pungente MD, Yagoubi N, Biomater. Sci 2018, 6, 2025. [DOI] [PubMed] [Google Scholar]
- [12].Anderson JM, Annu. Rev. Mater. Res 2001, 31, 81. [Google Scholar]
- [13].Anderson JM, Rodriguez A, Chang DT, Semin. Immunol 2008, 20, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Luttikhuizen DT, Harmsen MC, Luyn MJAV, Tissue Eng 2006, 12, 1955. [DOI] [PubMed] [Google Scholar]
- [15].Williams DF, Biomaterials 2008, 29, 2941. [DOI] [PubMed] [Google Scholar]
- [16].Ward WK, Diabetes Sci J Technol 2008, 2, 768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nilsson B, Ekdahl KN, Mollnes TE, Lambris JD, Mol. Immunol 2007, 44, 82. [DOI] [PubMed] [Google Scholar]
- [18].Ratner BD, Controlled Release J 2002, 78, 211. [DOI] [PubMed] [Google Scholar]
- [19].Veiseh O, Doloff JC, Ma M, Vegas AJ, Tam HH, Bader AR, Li J, Langan E, Wyckoff J, Loo WS, et al. , Nat. Mater 2015, 14, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang L, Eaton JW, J. Exp. Med 1993, 178, 2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jenney CR, Anderson JM, J. Biomed. Mater. Res 2000, 49, 435. [DOI] [PubMed] [Google Scholar]
- [22].Klopfleisch R, Jung F, J. Biomed. Mater. Res. A 2017, 105, 927. [DOI] [PubMed] [Google Scholar]
- [23].Vroman L, Adams AL, Fischer GC, Munoz PC, Blood 1980, 55, 156. [PubMed] [Google Scholar]
- [24].Sahu A, Lambris JD, Immunopharmacology 2000, 49, 133. [DOI] [PubMed] [Google Scholar]
- [25].Gorbet MB, Sefton MV, in Biomater. Silver Jubil. Compend (Ed: Williams DF), Elsevier Science, Oxford, 2004, pp. 219–241. [Google Scholar]
- [26].Labow RS, Meek E, Santerre JP, J. Cell. Physiol 2001, 186, 95. [DOI] [PubMed] [Google Scholar]
- [27].Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA, Immunol. Rev 2000, 177, 195. [DOI] [PubMed] [Google Scholar]
- [28].Negishi H, Ohba Y, Yanai H, Takaoka A, Honma K, Yui K, Matsuyama T, Taniguchi T, Honda K, Proc. Natl. Acad. Sci 2005, 102, 15989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Honma T, Hamasaki T, Virchows Arch 1996, 428, 165. [DOI] [PubMed] [Google Scholar]
- [30].Brodbeck WG, Shive MS, Colton E, Nakayama Y, Matsuda T, Anderson JM, J. Biomed. Mater. Res 2001, 55, 661. [DOI] [PubMed] [Google Scholar]
- [31].Koh TJ, DiPietro LA, Expert Rev. Mol. Med 2011, 13, DOI 10.1017/S1462399411001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].DiEgidio P, Friedman HI, Gourdie RG, Riley AE, Yost MJ, Goodwin RL, Ann. Plast. Surg 2014, 73, 451. [DOI] [PubMed] [Google Scholar]
- [33].Ratner BD, Regen. Biomater 2016, 3, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mooney V, Schwartz SA, Roth AM, Gorniowsky MJ, Ann. Biomed. Eng 1977, 5, 34. [DOI] [PubMed] [Google Scholar]
- [35].Avula M, Jones D, Rao AN, McClain D, McGill LD, Grainger DW, Solzbacher F, Biosens. Bioelectron 2016, 77, 149. [DOI] [PubMed] [Google Scholar]
- [36].Wilson GS, Hu Y, Chem. Rev 2000, 100, 2693. [DOI] [PubMed] [Google Scholar]
- [37].Jaffer IH, Weitz JI, Acta Biomater 2019, 94, 2. [DOI] [PubMed] [Google Scholar]
- [38].Sperling C, Fischer M, Maitz MF, Werner C, Biomaterials 2009, 30, 4447. [DOI] [PubMed] [Google Scholar]
- [39].Jaffer IH, Fredenburgh JC, Hirsh J, Weitz JI, J. Thromb. Haemost 2015, 13, S72. [DOI] [PubMed] [Google Scholar]
- [40].Ukita R, Wu K, Lin X, Carleton NM, Naito N, Lai A, Do-Nguyen CC, Demarest CT, Jiang S, Cook KE, Acta Biomater 2019, 92, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Labow RS, Erfle DJ, Santerre JP, Biomaterials 1995, 16, 51. [DOI] [PubMed] [Google Scholar]
- [42].Peramo A, Marcelo C, Ann. Biomed. Eng 2010, 38, 2013. [DOI] [PubMed] [Google Scholar]
- [43].von Recum AF, J. Biomed. Mater. Res 1984, 18, 323. [DOI] [PubMed] [Google Scholar]
- [44].Wood RC, Lecluyse EL, Fix JA, Biomaterials 1995, 16, 957. [DOI] [PubMed] [Google Scholar]
- [45].Sharkawy AA, Klitzman B, Truskey GA, Reichert WM, J. Biomed. Mater. Res 1997, 37, 401. [DOI] [PubMed] [Google Scholar]
- [46].Fournier E, Passirani C, Montero-Menei CN, Benoit JP, Biomaterials 2003, 24, 3311. [DOI] [PubMed] [Google Scholar]
- [47].Galdiero M, Larocca F, Iovene MR, Francesca M, Pieretti G, D’Oriano V, Franci G, Ferraro G, d’Andrea F, Nicoletti GF, Plast. Reconstr. Surg 2018, 141, 23. [DOI] [PubMed] [Google Scholar]
- [48].Bachour Y, Verweij SP, Gibbs S, Ket JCF, Ritt MJPF, Niessen FB, Mullender MG, J. Plast. Reconstr. Aesthet. Surg 2018, 71, 307. [DOI] [PubMed] [Google Scholar]
- [49].Spriano S, Yamaguchi S, Baino F, Ferraris S, Acta Biomater 2018, 79, 1. [DOI] [PubMed] [Google Scholar]
- [50].Albrektsson T, Dahlin C, Jemt T, Sennerby L, Turri A, Wennerberg A, Clin. Implant Dent. Relat. Res 2014, 16, 155. [DOI] [PubMed] [Google Scholar]
- [51].Ratner BD, in Titan. Med. Mater. Sci. Surf. Sci. Eng. Biol. Responses Med. Appl (Eds: Brunette DM, Tengvall P, Textor M, Thomsen P), Springer, Berlin, Heidelberg, 2001, pp. 1–12. [Google Scholar]
- [52].Bolind P, Johansson CB, Balshi TJ, Langer B, Albrektsson T, Int. J. Periodontics Restorative Dent 2005, 25, 425. [PubMed] [Google Scholar]
- [53].Guglielmotti MB, Olmedo DG, Cabrini RL, Periodontol. 2000 2019, 79, 178. [DOI] [PubMed] [Google Scholar]
- [54].Sivaraman K, Chopra A, Narayan AI, Balakrishnan D, J. Prosthodont. Res 2018, 62, 121. [DOI] [PubMed] [Google Scholar]
- [55].Trindade R, Albrektsson T, Galli S, Prgomet Z, Tengvall P, Wennerberg A, Clin. Implant Dent. Relat. Res 2018, 20, 82. [DOI] [PubMed] [Google Scholar]
- [56].Anderson JM, ASAIO J. 1988, 34, 101. [DOI] [PubMed] [Google Scholar]
- [57].Marchant RE, Anderson JM, Dillingham EO, J. Biomed. Mater. Res 1986, 20, 37. [DOI] [PubMed] [Google Scholar]
- [58].Marchant RE, Miller KM, Anderson JM, J. Biomed. Mater. Res 1984, 18, 1169. [DOI] [PubMed] [Google Scholar]
- [59].Marchant RE, Anderson JM, Phua K, Hiltner A, J. Biomed. Mater. Res 1984, 18, 309. [DOI] [PubMed] [Google Scholar]
- [60].Marchant R, Hiltner A, Hamlin C, Rabinovitch A, Slobodkin R, Anderson JM, J. Biomed. Mater. Res 1983, 17, 301. [DOI] [PubMed] [Google Scholar]
- [61].Ratner BD, Bryant SJ, Annu Rev Biomed Eng 2004, 6, 41. [DOI] [PubMed] [Google Scholar]
- [62].Bryers JD, Giachelli CM, Ratner BD, Biotechnol. Bioeng 2012, 109, 1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sussman EM, Halpin MC, Muster J, Moon RT, Ratner BD, Ann. Biomed. Eng 2014, 42, 1508. [DOI] [PubMed] [Google Scholar]
- [64].Komi DEA, Khomtchouk K, Santa Maria PL, Clin. Rev. Allergy Immunol 2020, 58, 298. [DOI] [PubMed] [Google Scholar]
- [65].Agier J, Pastwińska J, Brzezińska-Błaszczyk E, Inflamm. Res 2018, 67, 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Collington SJ, Hallgren J, Pease JE, Jones TG, Rollins BJ, Westwick J, Austen KF, Williams TJ, Gurish MF, Weller CL, J. Immunol 2010, 184, 6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].de Cássia Campos MR, Toso VD, de Souza DA, Vieira GV, da Silva EZM, Oliver C, Jamur MC, Cell. Immunol 2014, 289, 86. [DOI] [PubMed] [Google Scholar]
- [68].Karhausen J, Choi HW, Maddipati KR, Mathew JP, Ma Q, Boulaftali Y, Lee RH, Bergmeier W, Abraham SN, Sci. Adv 2020, 6, eaay6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kajiwara N, Sasaki T, Bradding P, Cruse G, Sagara H, Ohmori K, Saito H, Ra C, Okayama Y, Allergy Clin J Immunol 2010, 125, 1137. [DOI] [PubMed] [Google Scholar]
- [70].Ashina K, Tsubosaka Y, Nakamura T, Omori K, Kobayashi K, Hori M, Ozaki H, Murata T, PLOS ONE 2015, 10, e0132367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Kunder CA, John AL, Abraham SN, Blood 2011, 118, 5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Conti P, Caraffa A, Mastrangelo F, Tettamanti L, Ronconi G, Frydas I, Kritas SK, Theoharides TC, Cell Prolif 2018, 51, e12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wulff BC, Parent AE, Meleski MA, DiPietro LA, Schrementi ME, Wilgus TA, J. Invest. Dermatol 2012, 132, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen L, Schrementi ME, Ranzer MJ, Wilgus TA, DiPietro LA, PLOS ONE 2014, 9, e85226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Weiskirchen R, Meurer SK, Liedtke C, Huber M, Cells 2019, 8, 1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Vangansewinkel T, Lemmens S, Geurts N, Quanten K, Dooley D, Pejler G, Hendrix S, Sci. Rep 2019, 9, 3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Methe K, Nayakawde NB, Banerjee D, Sihlbom C, Agbajogu C, Travnikova G, Olausson M, Tissue Eng. Part A 2020, 26, 1180. [DOI] [PubMed] [Google Scholar]
- [78].Ozpinar EW, Frey AL, Cruse G, Freytes DO, Tissue Eng. Part B Rev 2020, DOI 10.1089/ten.teb.2020.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Pittman K, Kubes P, J. Innate Immun 2013, 5, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Chen GY, Nuñez G, Nat. Rev. Immunol 2010, 10, 826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Heijink IH, Pouwels SD, Leijendekker C, de Bruin HG, Zijlstra GJ, van der Vaart H, ten Hacken NH, van Oosterhout AJ, Nawijn MC, van der Toorn M, Am. J. Respir. Cell Mol. Biol 2015, 52, 554. [DOI] [PubMed] [Google Scholar]
- [82].Lämmermann T, Afonso PV, Angermann BR, Wang JM, Kastenmüller W, Parent CA, Germain RN, Nature 2013, 498, 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Webb Lm., Ehrengruber MU, Clark-Lewis I, Baggiolini M, Rot A, Proc. Natl. Acad. Sci 1993, 90, 7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Dalli J, Montero-Melendez T, Norling LV, Yin X, Hinds C, Haskard D, Mayr M, Perretti M, Mol. Cell. Proteomics 2013, 12, 2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Gaudry M, Brégerie O, Andrieu V, El Benna J, Pocidalo M-A, Hakim J, Blood J Am. Soc. Hematol 1997, 90, 4153. [PubMed] [Google Scholar]
- [86].Gong Y, Koh D-R, Cell Tissue Res 2010, 339, 437. [DOI] [PubMed] [Google Scholar]
- [87].Ardi VC, Kupriyanova TA, Deryugina EI, Quigley JP, Proc. Natl. Acad. Sci 2007, 104, 20262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Soehnlein O, Lindbom L, Nat. Rev. Immunol 2010, 10, 427. [DOI] [PubMed] [Google Scholar]
- [89].Hughes J, Johnson RJ, Mooney A, Hugo C, Gordon K, Savill J, Am. J. Pathol 1997, 150, 223. [PMC free article] [PubMed] [Google Scholar]
- [90].Powell D, Tauzin S, Hind LE, Deng Q, Beebe DJ, Huttenlocher A, Cell Rep 2017, 19, 1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Robertson AL, Holmes GR, Bojarczuk AN, Burgon J, Loynes CA, Chimen M, Sawtell AK, Hamza B, Willson J, Walmsley SR, Sci. Transl. Med 2014, 6, 225ra29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gordon S, Taylor PR, Nat. Rev. Immunol 2005, 5, 953. [DOI] [PubMed] [Google Scholar]
- [93].Ginhoux F, Guilliams M, Immunity 2016, 44, 439. [DOI] [PubMed] [Google Scholar]
- [94].Pollard JW, Nat. Rev. Immunol 2009, 9, 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Molgora M, Supino D, Mavilio D, Santoni A, Moretta L, Mantovani A, Garlanda C, Scand. J. Immunol 2018, 88, e12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Garlanda C, Bottazzi B, Bastone A, Mantovani A, Annu Rev Immunol 2005, 23, 337. [DOI] [PubMed] [Google Scholar]
- [97].Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M, Trends Immunol 2004, 25, 677. [DOI] [PubMed] [Google Scholar]
- [98].Mytar B, Siedlar M, Woloszyn M, Ruggiero I, Pryjma J, Zembala M, Br. J. Cancer 1999, 79, 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mosser DM, J. Leukoc. Biol 2003, 73, 209. [DOI] [PubMed] [Google Scholar]
- [100].Verreck FA, de Boer T, Langenberg DM, Hoeve MA, Kramer M, Vaisberg E, Kastelein R, Kolk A, de Waal-Malefyt R, Ottenhoff TH, Proc. Natl. Acad. Sci 2004, 101, 4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Gordon S, Nat. Rev. Immunol 2003, 3, 23. [DOI] [PubMed] [Google Scholar]
- [102].Murray PJ, Curr. Opin. Pharmacol 2006, 6, 379. [DOI] [PubMed] [Google Scholar]
- [103].Munder M, Eichmann K, Modolell M, J. Immunol 1998, 160, 5347. [PubMed] [Google Scholar]
- [104].Gratchev A, Kzhyshkowska J, Köthe K, Muller-Molinet I, Kannookadan S, Utikal J, Goerdt S, Immunobiology 2006, 211, 473. [DOI] [PubMed] [Google Scholar]
- [105].Brundu S, Polarization and Repolarization of Macrophages, 2015. [Google Scholar]
- [106].Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y, Stem Cells 2006, 24, 2733. [DOI] [PubMed] [Google Scholar]
- [107].Sharifi BG, Zeng Z, Wang L, Song L, Chen H, Qin M, Sierra-Honigmann MR, Wachsmann-Hogiu S, Shah PK, Arterioscler. Thromb. Vasc. Biol 2006, 26, 1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Jabs A, Moncada GA, Nichols CE, Waller EK, Wilcox JN, J. Vasc. Res 2005, 42, 174. [DOI] [PubMed] [Google Scholar]
- [109].Badylak SF, Valentin JE, Ravindra AK, McCabe GP, Stewart-Akers AM, Tissue Eng. Part A 2008, 14, 1835. [DOI] [PubMed] [Google Scholar]
- [110].Brown BN, Valentin JE, Stewart-Akers AM, McCabe GP, Badylak SF, Biomaterials 2009, 30, 1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Brown BN, Londono R, Tottey S, Zhang L, Kukla KA, Wolf MT, Daly KA, Reing JE, Badylak SF, Acta Biomater 2012, 8, 978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringnér M, Mörgelin M, Bourseau-Guilmain E, Bengzon J, Belting M, Proc. Natl. Acad. Sci 2013, 110, 7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G, Traffic 2002, 3, 321. [DOI] [PubMed] [Google Scholar]
- [114].Janowska[ISP]‐Wieczorek A, Wysoczynski M, Kijowski J, Marquez[ISP]‐Curtis L, Machalinski B, Ratajczak J, Ratajczak MZ, Int. J. Cancer 2005, 113, 752. [DOI] [PubMed] [Google Scholar]
- [115].van Balkom BWM, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MAJ, Pegtel DM, Stoorvogel W, Würdinger T, et al. , Blood 2013, 121, 3997. [DOI] [PubMed] [Google Scholar]
- [116].EL Andaloussi S, Lakhal S, Mäger I, Wood MJA, Adv. Drug Deliv. Rev 2013, 65, 391. [DOI] [PubMed] [Google Scholar]
- [117].Théry C, Zitvogel L, Amigorena S, Nat. Rev. Immunol 2002, 2, 569. [DOI] [PubMed] [Google Scholar]
- [118].Cocucci E, Racchetti G, Meldolesi J, Trends Cell Biol 2009, 19, 43. [DOI] [PubMed] [Google Scholar]
- [119].Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ, Leukemia 2006, 20, 1487. [DOI] [PubMed] [Google Scholar]
- [120].Huleihel L, Bartolacci JG, Dziki JL, Vorobyov T, Arnold B, Scarritt ME, Pineda Molina C, LoPresti ST, Brown BN, Naranjo JD, Tissue Eng. Part A 2017, 23, 1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Li Z, Dang J, Chang K-Y, Rana TM, Rna 2014, 20, 1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Eto H, Ishimine H, Kinoshita K, Watanabe-Susaki K, Kato H, Doi K, Kuno S, Kurisaki A, Yoshimura K, Stem Cells Dev 2013, 22, 985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kouri J, Ancheta O, Exp. Cell Res 1972, 71, 168. [DOI] [PubMed] [Google Scholar]
- [124].Mosse PR, Campbell GR, Ryan GB, Pathology (Phila.) 1985, 17, 401. [DOI] [PubMed] [Google Scholar]
- [125].Qu G, von Schroeder HP, Tissue Eng. Part A 2012, 18, 1890. [DOI] [PubMed] [Google Scholar]
- [126].Lee J, Sayed N, Hunter A, Au KF, Wong WH, Mocarski ES, Pera RR, Yakubov E, Cooke JP, Cell 2012, 151, 547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Stewart HJS, Guildford AL, Lawrence[ISP]‐Watt DJ, Santin M, J. Biomed. Mater. Res. Part Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater 2009, 90, 465. [DOI] [PubMed] [Google Scholar]
- [128].Medina A, Ghahary A, Wound Repair Regen 2010, 18, 245. [DOI] [PubMed] [Google Scholar]
- [129].Mooney JE, Summers KM, Gongora M, Grimmond SM, Campbell JH, Hume DA, Rolfe BE, Immunol. Cell Biol 2014, 92, 518. [DOI] [PubMed] [Google Scholar]
- [130].Babensee JE, Anderson JM, McIntire LV, Mikos AG, Adv. Drug Deliv. Rev 1998, 33, 111. [DOI] [PubMed] [Google Scholar]
- [131].Bennewitz NL, Babensee JE, Biomaterials 2005, 26, 2991. [DOI] [PubMed] [Google Scholar]
- [132].Ali OA, Huebsch N, Cao L, Dranoff G, Mooney DJ, Nat. Mater 2009, 8, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Yoshida M, Babensee JE, J. Biomed. Mater. Res. Part Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater 2004, 71, 45. [Google Scholar]
- [134].Babensee JE, Paranjpe A, J. Biomed. Mater. Res. Part Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater 2005, 74, 503. [DOI] [PubMed] [Google Scholar]
- [135].Park J, Babensee JE, Acta Biomater 2012, 8, 3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Reddy ST, Swartz MA, Hubbell JA, Trends Immunol 2006, 27, 573. [DOI] [PubMed] [Google Scholar]
- [137].Shokouhi B, Coban C, Hasirci V, Aydin E, Dhanasingh A, Shi N, Koyama S, Akira S, Zenke M, Sechi AS, Biomaterials 2010, 31, 5759. [DOI] [PubMed] [Google Scholar]
- [138].Sicari BM, Rubin JP, Dearth CL, Wolf MT, Ambrosio F, Boninger M, Turner NJ, Weber DJ, Simpson TW, Wyse A, et al. , Sci. Transl. Med 2014, 6, 234ra58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, et al. , Science 2016, 352, 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Engelhardt E, Toksoy A, Goebeler M, Debus S, Bröcker E-B, Gillitzer R, Am. J. Pathol 1998, 153, 1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Julier Z, Park AJ, Briquez PS, Martino MM, Acta Biomater 2017, 53, 13. [DOI] [PubMed] [Google Scholar]
- [142].Liu Y, Wang L, Kikuiri T, Akiyama K, Chen C, Xu X, Yang R, Chen W, Wang S, Shi S, Nat. Med 2011, 17, 1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Nosbaum A, Prevel N, Truong H-A, Mehta P, Ettinger M, Scharschmidt TC, Ali NH, Pauli ML, Abbas AK, Rosenblum MD, J. Immunol 2016, 196, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Yamazaki K, Yoshie H, Seymour GJ, Histol. Histopathol 2003. [DOI] [PubMed] [Google Scholar]
- [145].Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT, Nat. Immunol 2005, 6, 1123. [DOI] [PubMed] [Google Scholar]
- [146].Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang Y-H, Wang Y, Hood L, Zhu Z, Tian Q, Nat. Immunol 2005, 6, 1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M, J. Immunol 1995, 155, 1151. [PubMed] [Google Scholar]
- [148].Bettelli E, Oukka M, Kuchroo VK, Nat. Immunol 2007, 8, 345. [DOI] [PubMed] [Google Scholar]
- [149].Cardoso CR, Garlet GP, Crippa GE, Rosa AL, Junior WM, Rossi MA, Silva JS, Oral Microbiol. Immunol 2009, 24, 1. [DOI] [PubMed] [Google Scholar]
- [150].Beklen A, Ainola M, Hukkanen M, Gürgan C, Sorsa T, Konttinen YT, J. Dent. Res 2007, 86, 347. [DOI] [PubMed] [Google Scholar]
- [151].Beklen A, Sorsa T, Konttinen YT, Oral Microbiol. Immunol 2009, 24, 38. [DOI] [PubMed] [Google Scholar]
- [152].Mahanonda R, Jitprasertwong P, Sa-Ard-Iam N, Rerkyen P, Charatkulangkun O, Jansisyanont P, Nisapakultorn K, Yongvanichit K, Pichyangkul S, J. Dent. Res 2008, 87, 267. [DOI] [PubMed] [Google Scholar]
- [153].Sakaguchi S, Yamaguchi T, Nomura T, Ono M, Cell 2008, 133, 775. [DOI] [PubMed] [Google Scholar]
- [154].Mahmud SA, Manlove LS, Farrar MA, Jak-Stat 2013, 2, e23154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Carbone F, Nencioni A, Mach F, Vuilleumier N, Montecucco F, Thromb. Haemost 2013, 110, 501. [DOI] [PubMed] [Google Scholar]
- [156].D’Alessio FR, Tsushima K, Aggarwal NR, West EE, Willett MH, Britos MF, Pipeling MR, Brower RG, Tuder RM, McDyer JF, J. Clin. Invest 2009, 119, 2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S, Circ. Res 2014, 115, 55. [DOI] [PubMed] [Google Scholar]
- [158].Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL, Proc. Natl. Acad. Sci 2014, 111, 16029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN, J. Clin. Invest 2014, 124, 1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Dombrowski Y, O’hagan T, Dittmer M, Penalva R, Mayoral SR, Bankhead P, Fleville S, Eleftheriadis G, Zhao C, Naughton M, Nat. Neurosci 2017, 20, 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Castiglioni A, Corna G, Rigamonti E, Basso V, Vezzoli M, Monno A, Almada AE, Mondino A, Wagers AJ, Manfredi AA, PloS One 2015, 10, e0128094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H-A, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Cell 2017, 169, 1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Okoye IS, Coomes SM, Pelly VS, Czieso S, Papayannopoulos V, Tolmachova T, Seabra MC, Wilson MS, Immunity 2014, 41, 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Aiello S, Rocchetta F, Longaretti L, Faravelli S, Todeschini M, Cassis L, Pezzuto F, Tomasoni S, Azzollini N, Mister M, Sci. Rep 2017, 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hady TF, Hwang B, Pusic AD, Waworuntu RL, Mulligan M, Ratner BD, Bryers JD, J. Tissue Eng. Regen. Med. n.d., n/a, DOI 10.1002/term.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Allen JE, Wynn TA, PLoS Pathog 2011, 7, e1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Sadtler K, Allen BW, Estrellas K, Housseau F, Pardoll DM, Elisseeff JH, Tissue Eng. Part A 2016, 23, 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Beissert S, Schwarz A, Schwarz T, J. Invest. Dermatol 2006, 126, 15. [DOI] [PubMed] [Google Scholar]
- [169].Brockmann L, Giannou AD, Gagliani N, Huber S, Int. J. Mol. Sci 2017, 18, 1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Chung L, Maestas DR, Lebid A, Mageau A, Rosson GD, Wu X, Wolf MT, Tam AJ, Vanderzee I, Wang X, Sci. Transl. Med 2020, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Zouali M, Autoimmunity 2017, 50, 61. [DOI] [PubMed] [Google Scholar]
- [172].Seymour GJ, Powell RN, Cole KL, Aitken JF, Brooks D, Beckman I, Zola H, Bradley J, Burns GF, J. Periodontal Res 1983, 18, 375. [DOI] [PubMed] [Google Scholar]
- [173].Zhu M, Belkina AC, DeFuria J, Carr JD, Van Dyke TE, Gyurko R, Nikolajczyk BS, J. Leukoc. Biol 2014, 96, 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Könnecke I, Serra A, El Khassawna T, Schlundt C, Schell H, Hauser A, Ellinghaus A, Volk H-D, Radbruch A, Duda GN, Bone 2014, 64, 155. [DOI] [PubMed] [Google Scholar]
- [175].Klose CS, Artis D, Nat. Immunol 2016, 17, 765. [DOI] [PubMed] [Google Scholar]
- [176].Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F, Immunity 2014, 40, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Mirchandani AS, Besnard A-G, Yip E, Scott C, Bain CC, Cerovic V, Salmond RJ, Liew FY, J. Immunol 2014, 192, 2442. [DOI] [PubMed] [Google Scholar]
- [178].Huang Y, Paul WE, Int. Immunol 2016, 28, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Gasteiger G, Rudensky AY, Nat. Rev. Immunol 2014, 14, 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Hanash AM, Dudakov JA, Hua G, O’Connor MH, Young LF, Singer NV, West ML, Jenq RR, Holland AM, Kappel LW, Immunity 2012, 37, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Sridharan R, Cameron AR, Kelly DJ, Kearney CJ, O’Brien FJ, Mater. Today 2015, 18, 313. [Google Scholar]
- [182].Dziki JL, Badylak SF, Curr. Opin. Biomed. Eng 2018, 6, 51. [Google Scholar]
- [183].Sionkowska A, Prog. Polym. Sci 2011, 36, 1254. [Google Scholar]
- [184].Vats A, Tolley NS, Polak JM, Gough JE, Clin. Otolaryngol. Allied Sci 2003, 28, 165. [DOI] [PubMed] [Google Scholar]
- [185].Zaat S, Broekhuizen C, Riool M, Future Microbiol 2010, 5, 1149. [DOI] [PubMed] [Google Scholar]
- [186].Katsikogianni M, Missirlis Y, Eur. Cell. Mater 2004, 8, 37. [DOI] [PubMed] [Google Scholar]
- [187].Mertgen A-S, Trossmann VT, Guex AG, Maniura-Weber K, Scheibel T, Rottmar M, ACS Appl. Mater. Interfaces 2020, 12, 21342. [DOI] [PubMed] [Google Scholar]
- [188].Follmann HDM, Naves AF, Araujo RA, Dubovoy V, Huang X, Asefa T, Silva R, Oliveira ON, Curr. Pharm. Des 2017, 23, 3794. [DOI] [PubMed] [Google Scholar]
- [189].Freudenberg U, Liang Y, Kiick KL, Werner C, Adv. Mater 2016, 28, 8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Emerson AE, Slaby EM, Hiremath SC, Weaver JD, Biomater. Sci 2020, 8, 7014. [DOI] [PubMed] [Google Scholar]
- [191].Gammon JM, Jewell CM, Adv. Healthc. Mater 2019, 8, 1801419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Soni SS, Rodell CB, Acta Biomater. 2021, DOI 10.1016/j.actbio.2021.01.016. [DOI] [PubMed] [Google Scholar]
- [193].Finan JD, Sundaresh SN, Elkin BS, McKhann GM, Morrison B, Acta Biomater 2017, 55, 333. [DOI] [PubMed] [Google Scholar]
- [194].Joodaki H, Panzer MB, Proc. Inst. Mech. Eng. [H] 2018, 232, 323. [DOI] [PubMed] [Google Scholar]
- [195].Morgan EF, Unnikrisnan GU, Hussein AI, Annu. Rev. Biomed. Eng 2018, 20, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Engler AJ, Sen S, Sweeney HL, Discher DE, Cell 2006, 126, 677. [DOI] [PubMed] [Google Scholar]
- [197].Sridharan R, Cavanagh B, Cameron AR, Kelly DJ, O’Brien FJ, Acta Biomater 2019, 89, 47. [DOI] [PubMed] [Google Scholar]
- [198].Lee H, Bellamkonda RV, Sun W, Levenston ME, J. Neural Eng 2005, 2, 81. [DOI] [PubMed] [Google Scholar]
- [199].Pilliar RM, Davies JE, Smith DC, MRS Bull 1991, 16, 55. [Google Scholar]
- [200].Harris JP, Hess AE, Rowan SJ, Weder C, Zorman CA, Tyler DJ, Capadona JR, J. Neural Eng 2011, 8, 046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Jung H-D, Jang T-S, Wang L, Kim H-E, Koh Y-H, Song J, Biomaterials 2015, 37, 49. [DOI] [PubMed] [Google Scholar]
- [202].Dhandayuthapani B, Yoshida Y, Maekawa T, Kumar DS, “Polymeric Scaffolds in Tissue Engineering Application: A Review,” 2011. [Google Scholar]
- [203].Karp RD, Johnson KH, Buoen LC, Ghobrial HKG, Brand I, Brand KG, JNCI J. Natl. Cancer Inst 1973, 51, 1275. [DOI] [PubMed] [Google Scholar]
- [204].MARSHALL A, Polym. Prepr 2004, 45, 100. [Google Scholar]
- [205].Jain N, Vogel V, Nat. Mater 2018, 17, 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Zamecnik CR, Levy ES, Lowe MM, Zirak B, Rosenblum MD, Desai TA, Biomaterials 2020, 230, 119626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Zandstra J, Hiemstra C, Petersen AH, Zuidema J, van Beuge MM, Rodriguez S, Lathuile AAR, Veldhuis GJ, Steendam R, Bank RA, et al. , 2014, 28, 335. [DOI] [PubMed] [Google Scholar]
- [208].Matlaga BF, Yasenchak LP, Salthouse TN, J. Biomed. Mater. Res 1976, 10, 391. [DOI] [PubMed] [Google Scholar]
- [209].Sanders JE, Cassisi DV, Neumann T, Golledge SL, Zachariah SG, Ratner BD, Bale SD, J. Biomed. Mater. Res. A 2003, 65A, 462. [DOI] [PubMed] [Google Scholar]
- [210].Spencer KC, Sy JC, Ramadi KB, Graybiel AM, Langer R, Cima MJ, Sci. Rep 2017, 7, 1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Wei X, Luan L, Zhao Z, Li X, Zhu H, Potnis O, Xie C, Adv. Sci 2018, 5, 1700625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Qazi TH, Rai R, Boccaccini AR, Biomaterials 2014, 35, 9068. [DOI] [PubMed] [Google Scholar]
- [213].Khan MA, Cantù E, Tonello S, Serpelloni M, Lopomo NF, Sardini E, Appl. Sci 2019, 9, 961. [Google Scholar]
- [214].Gelmi A, Schutt CE, Adv. Healthc. Mater. n.d., n/a, 2001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Cangellaris OV, Gillette MU, Front. Mater 2018, 5, DOI 10.3389/fmats.2018.00021. [DOI] [Google Scholar]
- [216].Zhou W, Ni Y, Jin Z, Zhang M, Wu J, Zhu Y, Xu G, Gan D, Exp. Neurol 2012, 238, 192. [DOI] [PubMed] [Google Scholar]
- [217].He S, Wu J, Li S-H, Wang L, Sun Y, Xie J, Ramnath D, Weisel RD, Yau TM, Sung H-W, et al. , Biomaterials 2020, 258, 120285. [DOI] [PubMed] [Google Scholar]
- [218].Li C, Guo C, Fitzpatrick V, Ibrahim A, Zwierstra MJ, Hanna P, Lechtig A, Nazarian A, Lin SJ, Kaplan DL, Nat. Rev. Mater 2020, 5, 61. [Google Scholar]
- [219].Prakasam M, Locs J, Salma-Ancane K, Loca D, Largeteau A, Berzina-Cimdina L, J. Funct. Biomater 2017, 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, Biomaterials 2015, 37, 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Gulati K, Ivanovski S, Expert Opin. Drug Deliv 2017, 14, 1009. [DOI] [PubMed] [Google Scholar]
- [222].Soto RJ, Merricks EP, Bellinger DA, Nichols TC, Schoenfisch MH, Biomaterials 2018, 157, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Shin JH, Schoenfisch MH, Analyst 2006, 131, 609. [DOI] [PubMed] [Google Scholar]
- [224].Gifford R, Batchelor MM, Lee Y, Gokulrangan G, Meyerhoff ME, Wilson GS, J. Biomed. Mater. Res. A 2005, 75A, 755. [DOI] [PubMed] [Google Scholar]
- [225].Hopkins SP, Pant J, Goudie MJ, Schmiedt C, Handa H, ACS Appl. Mater. Interfaces 2018, 10, 27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Lampropoulou V, Sergushichev A, Bambouskova M, Nair S, Vincent EE, Loginicheva E, Cervantes-Barragan L, Ma X, Huang SC-C, Griss T, et al. , Cell Metab 2016, 24, 158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Huyer LD, Mandla S, Wang Y, Campbell SB, Yee B, Euler C, Lai BF, Bannerman AD, Lin DSY, Montgomery M, et al. , Adv. Funct. Mater 2021, 31, 2003341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Reyes-Ortega F, Cifuentes A, Rodríguez G, Aguilar MR, González-Gómez Á, Solis R, García-Honduvilla N, Buján J, García-Sanmartin J, Martínez A, et al. , Acta Biomater 2015, 23, 103. [DOI] [PubMed] [Google Scholar]
- [229].Wang X, Rivera[ISP]‐Bolanos N, Jiang B, Ameer GA, Adv. Funct. Mater 2019, 29, 1809009. [Google Scholar]
- [230].Hanson S, D’Souza RN, Hematti P, Tissue Eng. Part A 2014, 20, 2162. [DOI] [PubMed] [Google Scholar]
- [231].Richardson TP, Peters MC, Ennett AB, Mooney DJ, Nat. Biotechnol 2001, 19, 1029. [DOI] [PubMed] [Google Scholar]
- [232].Badeau BA, DeForest CA, Annu. Rev. Biomed. Eng 2019, 21, 241. [DOI] [PubMed] [Google Scholar]
- [233].Ooi HW, Hafeez S, van Blitterswijk CA, Moroni L, Baker MB, Mater. Horiz 2017, 4, 1020. [Google Scholar]
- [234].Wells CM, Harris M, Choi L, Murali VP, Guerra FD, Jennings JA, J. Funct. Biomater 2019, 10, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Boehler C, Kleber C, Martini N, Xie Y, Dryg I, Stieglitz T, Hofmann UG, Asplund M, Biomaterials 2017, 129, 176. [DOI] [PubMed] [Google Scholar]
- [236].McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF, Proc. Natl. Acad. Sci 2013, 110, 17253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].McWhorter FY, Davis CT, Liu WF, Cell. Mol. Life Sci 2015, 72, 1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Luu TU, Gott SC, Woo BWK, Rao MP, Liu WF, ACS Appl. Mater. Interfaces 2015, 7, 28665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Barth KA, Waterfield JD, Brunette DM, J. Biomed. Mater. Res. A 2013, 101A, 2679. [DOI] [PubMed] [Google Scholar]
- [240].Padmanabhan J, Augelli MJ, Cheung B, Kinser ER, Cleary B, Kumar P, Wang R, Sawyer AJ, Li R, Schwarz UD, et al. , Sci. Rep 2016, 6, 33277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Jiang S, Cao Z, Adv. Mater 2010, 22, 920. [DOI] [PubMed] [Google Scholar]
- [242].Zhang L, Cao Z, Bai T, Carr L, Ella-Menye J-R, Irvin C, Ratner BD, Jiang S, Nat. Biotechnol 2013, 31, 553. [DOI] [PubMed] [Google Scholar]
- [243].Hamad I, Hunter AC, Szebeni J, Moghimi SM, Mol. Immunol 2008, 46, 225. [DOI] [PubMed] [Google Scholar]
- [244].Zhang P, Sun F, Hung H-C, Jain P, Leger KJ, Jiang S, Anal. Chem 2017, 89, 8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Stachelek SJ, Finley MJ, Alferiev IS, Wang F, Tsai RK, Eckells EC, Tomczyk N, Connolly JM, Discher DE, Eckmann DM, et al. , Biomaterials 2011, 32, 4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Slee JB, Alferiev IS, Nagaswami C, Weisel JW, Levy RJ, Fishbein I, Stachelek SJ, Biomaterials 2016, 87, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Meyers SR, Grinstaff MW, Chem. Rev 2012, 112, 1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Hsieh CYC, Hu F-W, Chen W-S, Tsai W-B, ISRN Biomater 2014, 2014, 1. [Google Scholar]
- [249].Wu F, Li J, Zhang K, He Z, Yang P, Zou D, Huang N, ACS Appl. Mater. Interfaces 2016, 8, 109. [DOI] [PubMed] [Google Scholar]
- [250].Qian Y, Li L, Song Y, Dong L, Chen P, Li X, Cai K, Germershaus O, Yang L, Fan Y, Biomaterials 2018, 164, 22. [DOI] [PubMed] [Google Scholar]
- [251].Coindre VF, Kinney SM, Sefton MV, Biomaterials 2020, 259, 120324. [DOI] [PubMed] [Google Scholar]
- [252].Vegas AJ, Veiseh O, Doloff JC, Ma M, Tam HH, Bratlie K, Li J, Bader AR, Langan E, Olejnik K, et al. , Nat. Biotechnol 2016, 34, 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Tian R, Qiu X, Yuan P, Lei K, Wang L, Bai Y, Liu S, Chen X, ACS Appl. Mater. Interfaces 2018, 10, 17018. [DOI] [PubMed] [Google Scholar]
- [254].Qu F, Holloway JL, Esterhai JL, Burdick JA, Mauck RL, Nat. Commun 2017, 8, 1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Bakhsheshi-Rad HR, Akbari M, Ismail AF, Aziz M, Hadisi Z, Pagan E, Daroonparvar M, Chen X, Surf. Coat. Technol 2019, 377, 124898. [Google Scholar]
- [256].Badylak SF, Ann. Biomed. Eng 2014, 42, 1517. [DOI] [PubMed] [Google Scholar]
- [257].Badylak SF, Biomaterials 2007, 28, 3587. [DOI] [PubMed] [Google Scholar]
- [258].Sackett SD, Tremmel DM, Ma F, Feeney AK, Maguire RM, Brown ME, Zhou Y, Li X, O’Brien C, Li L, et al. , Sci. Rep 2018, 8, 10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Mase VJ, Hsu JR, Wolf SE, Wenke JC, Baer DG, Owens J, Badylak SF, Walters TJ, Orthopedics 2010, 33. [DOI] [PubMed] [Google Scholar]
- [260].Pérez[ISP]‐Luna VH, Horbett TA, Ratner BD, J. Biomed. Mater. Res 1994, 28, 1111. [DOI] [PubMed] [Google Scholar]
- [261].Tyler BJ, Rayal G, Castner DG, Biomaterials 2007, 28, 2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Gajos K, Awsiuk K, Budkowski A, Colloid Polym. Sci 2020, DOI 10.1007/s00396-020-04711-7. [DOI] [Google Scholar]
- [263].Stokes KB, Pacing Clin. Electrophysiol 1988, 11, 1797. [DOI] [PubMed] [Google Scholar]
- [264].Hussey GS, Dziki JL, Badylak SF, Nat. Rev. Mater 2018, 3, 159. [Google Scholar]
- [265].Parmaksiz M, Dogan A, Odabas S, Elçin AE, Elçin YM, Biomed. Mater 2016, 11, 022003. [DOI] [PubMed] [Google Scholar]
- [266].Denis P, Hirneiß C, Durr GM, Reddy KP, Kamarthy A, Calvo E, Hussain Z, Ahmed IK, Br. J. Ophthalmol 2020, DOI 10.1136/bjophthalmol-2020-316888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [267].Gough DA, Kumosa LS, Routh TL, Lin JT, Lucisano JY, Sci. Transl. Med 2010, 2, 42ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Bose S, Volpatti LR, Thiono D, Yesilyurt V, McGladrigan C, Tang Y, Facklam A, Wang A, Jhunjhunwala S, Veiseh O, et al. , Nat. Biomed. Eng 2020, 4, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]


