Abstract
Resting-state connectivity studies, which examine unconstrained low frequency BOLD fluctuations, have reported inconsistent abnormalities in bipolar disorder (BP). In this study, we investigated intrinsic brain connectivity under the constraints of a Continuous Emotion Regulation Task (CERT) in BP patients in depressed (BPD) and manic (BPM) states, along with healthy control participants.
Medication-free participants, with either a diagnosis of BP (BPD = 27, BPM = 30) or healthy controls (N = 33) were included. We collected 2 fMRI scans using the CERT paradigm, in which participants continuously watched negative pictures and either maintained emotions (MAINTAIN) or suppressed emotion using reappraisal techniques (SUPPRESS). Network-based statistic and graph theory analyses were examined for (i) the main effect of condition (within-group) and (ii) group and condition interactions.
In healthy participants, MAINTAIN largely involved occipital and parietal cortices (p<.001), whereas SUPPRESS also recruited the frontal and cingulate cortices (p=.023). The interaction between group (BPD vs. BPM) and condition revealed a network involving the inferior frontal lobe which was stronger during MAINTAIN for BPD and during SUPPRESS for BPM (p=.037). Graph theory properties (i.e., clustering coefficient) for key nodes also evidenced significant group by condition interactions. We observed BP-related changes in network properties involved in normal and abnormal emotion regulation, which provide insights into the neural bases for affective disturbances in BP.
Keywords: Bipolar disorder, emotion regulation, mania, depression, brain network
INTRODUCTION
Bipolar disorder (BP) is a chronic condition that remains one of the most debilitating forms of mental illness (Ormel et al., 2008). BP is characterized by shifts into states of seemingly opposing affective valence – episodes of (hypo)mania and depression. Thus, understanding BP-related neural circuitry requires identifying disturbances characteristic of each of these states, potentially providing insight into the mechanisms by which each mood state is triggered.
BP research implicates dysfunction in brain networks supporting affect regulation (Strakowski et al, 2012; Brady et al, 2014; Wessa et al, 2014). For example, mounting evidence supports the existence of BP-related disturbances in functional networks (mapped via fMRI) (Vargas et al, 2013). However, these studies have produced inconsistent findings, and thus the nature of network disturbances in BP remains poorly understood. Several shortcomings in these studies may explain these inconsistencies. For example, the majority did not examine mania and depression simultaneously, making it impossible to parse BP-related disturbances (i) specific to (hypo)mania or (ii) specific to depression. Furthermore, participants were taking psychotropic medications, which can impact fMRI (Anand et al, 2007), making it difficult to disentangle the effects of pathology vs. medication.
Finally, the methodology employed in these studies has several key limitations. First, most studies examine connectivity with only a small a set of ‘seed’ regions (and different sets across studies), potentially overlooking important connections. Furthermore, typical methods examine only pairwise coupling, ignoring the role of that connection within the greater network. Most importantly, these methods do not provide insight into whether BP is associated with restructuring of the functional organization of networks. Methods such as graph theory address these concerns by assaying emergent properties (e.g., network cohesiveness) of both the global network and specific regions.
Recently, we used a functional connectomic approach in a sample of both hypomanic/manic and depressed BP patients to identify brain network disturbances associated with BP (Spielberg et al., 2016). Employing graph-theory techniques, we identified unique connectome disturbances associated with each BP mood state: mania was related to hyperconnectivity in an amygdala network and disruption in the ‘small worldness’ of the global network, whereas depression was related differences in an orbital frontal cortex (OFC) network and decreased resilience of the global network (Spielberg et al., 2016).
Although promising, this study examined low-frequency (<0.1Hz) blood oxygen level-dependent (BOLD) fluctuations (LFBF) obtained while participants were in a resting state (i.e., participants were instructed to think about nothing in particular). Thus, this study could not provide insight into the network responses observed when regulation of affect is actually required. However, LFBF correlations may be conceptualized as a steady-state continuous measure of brain network function and can therefore also be measured while participants are engaged in a continuous performance task (CPT) (e.g., regulation of affect). For example, investigators have reported on the organization of functional networks while participants continuously perform motor (finger tapping) (Lowe et al., 2002), memory (Fornito et al., 2012), and attentional tasks (Spadone et al., 2015; Tomasi et al., 2014). Importantly, steady-state connectivity changes that occur when participants are continuously engaged in a task may be more akin to real life functioning, as compared to either block or event related task designs or the commonly used resting state studies. Therefore, in this respect, a continuous performance, steady-state design may be much more reflective of brain connectivity in real-life settings.
One paradigm often used to engage mood circuitry involves exposing participants to negative emotional stimuli and asking them to either maintain or regulate (i.e., with cognitive reappraisal) their emotional response (Ochsner & Gross, 2005) Using this strategy in a traditional activation paradigm, it has been reported that maintenance of emotion is associated with increased amygdala activation, as well as activation of the medial prefrontal cortex, whereas down-regulation of emotion involves increased activation of the orbital prefrontal cortex (Ochsner et al., 2004). However, importantly, the neural network-level dynamics associated with maintenance vs. down-regulation of emotional response via reappraisal have not been studied using the steady-state LFBF correlation paradigm, either in healthy participants or patients with BP.
To address these issues described above, we used graph theory to examine brain network changes in medication-free manic and depressed BP patients and health controls while they performed a continuous emotion regulation task (CERT) in which they continuously looking at pictures showing negative emotional stimuli and either maintained emotion or regulated using reappraisal strategies. We hypothesized differences in brain networks and their properties during maintenance and reappraisal of continuously presented emotional stimuli when bipolar participants are compared to healthy participants and when manic and depressed bipolar groups are compared to each other (Hummer et al., 2013).
MATERIALS AND METHOD
Participants:
Individuals with BP who were medication-free for at least 2 weeks, along with psychiatrically healthy adults, were recruited from the outpatient psychiatry clinic at Indiana University Hospital and by advertisement to the community. After complete description of the study to the participants, written informed consent, approved by the Institutional Review Board (IRB) at the Indiana University School of Medicine, was obtained. Both patients and healthy control participants were paid $75 for screening and $75 for an MRI scan. Participants underwent the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998), as well as a clinical interview by a psychiatrist (AA) to determine the appropriate Diagnostic and Statistical Manual 4th Edition Text Revision (DSM-IV-TR; First et al., 2002) diagnoses. Participants also completed the 17-item Hamilton Depression Rating Scale (HDRS; Hamilton, 1960) and the Young Mania Rating Scale (YMRS; Young et al., 1978) at the time of the scan.
BP participants aged 18–60 years were included in the study if, at the time of scan, they satisfied DSM-IV-TR criteria for BP and satisfied DSM-IV TR criteria for a hypomanic, manic, or depressed episode. In addition, to further delineate the two BP groups, BPD group was required to have HDRS ≥ 15 and YMRS ≤ 10 and the BPM group an HDRS ≤ 12 and a YMRS ≥ 12. Exclusion criteria for participants included: lifetime diagnosis of schizophrenia or schizoaffective disorder; a current primary anxiety disorder; use of psychotropic medications in the past 2 weeks; fluoxetine use over the past 4 weeks; acute suicidal or homicidal ideation or behavior; recent (< 1 week) or current inpatient hospitalization; meeting DSM-IV-TR criteria for substance dependence within the past year (except nicotine); positive urinary toxicology screening at baseline; use of alcohol in the past 1 week; serious medical or neurological illness; current pregnancy or breast feeding; and metallic implants or other contraindications to MRI.
Healthy controls (HC) (18–60 years) were also required to have no personal or family history of psychiatric illness or alcohol or substance abuse/dependence; current use of any centrally acting medications; use of alcohol in the past week; serious medical or neurological illness; pregnant or breast-feeding; and metallic implants or other contraindication to MRI.
MRI Acquisition:
Scans were performed using a Siemens 3T Tim Trio. After a short scout imaging scan to survey head position and center the field of view (FOV), a high-resolution 3D magnetization prepared rapid gradient echo (MPRAGE) scan was collected (160 sagittal slices, 1.0×1.0×1.2 mm). Subsequently, functional scans were acquired using T2*-weighted gradient echo echo-planar imaging (EPI) (TR/TE 2250/29fms; 39 axial slices; FOV 220×220 mm; 2.5×2.5×3.5 mm). An integrated parallel acquisition technique was implemented using a generalized auto-calibrating partially parallel acquisition (GRAPPA) with a reduction factor of 2 to improve spatial resolution, reduce geometric distortion, and decrease scan time. Two scans, one during emotion maintenance and one during emotional reappraisal were collected, each lasting 5:33 min (145 volumes) (Van Dijk et al., 2010).
Continuous Emotion Regulation Task (CERT):
In each scan, participants were continuously shown pictures with negative emotional valence derived from the International Picture System (IAPS; Lang et al., 1997). Each picture was shown for 15 seconds for a total of 21 pictures in each scan. The pictures for the two scans were matched for valence and arousal ratings. In the first scan, participants were instructed to maintain their emotional response to the pictures (MAINTAIN). For the second scan, participants were asked to suppress their emotional response to the pictures using appraisal techniques taught during the training session before the scan (SUPPRESS). These training techniques included trying to distance themselves from what was happening and imagining that it was not real. At the end of each scan, participants were asked to rate the entire picture set on a 1–5 scale for emotional valence with 5 being the most negative.
Image Analysis:
Preprocessing and motion correction:
The images were corrected for physiologic noise (Beall, 2010; Glover et al., 2000) using signals obtained with PESTICA (Physiologic Estimation by Temporal ICA; Beall & Lowe, 2007). Special attention was paid to motion correction, because both linear and non-linear motion artifacts have been shown to affect functional results (Van Dijk et al., 2012; Power et al., 2012). Motion correction was performed using SLice-Oriented MOtion COrrection (SLOMOCO) (Beall & Lowe, 2014). SLOMOCO first performs an in-plane slicewise motion registration followed by an out-of-plane motion parameter estimation and regularization. The regularized out-of-plane and residual in-plane motion parameters are used in a slice-specific second-order motion model that accounts for the effect of adjacent slice motion into or out of the slice of interest as well as the present slice. Finally, the software regresses the physiologic noise model in parallel with the slice-wise second-order motion model, and this regression correction comprises the last stage of SLOMOCO to produce data that has been corrected for physiologic noise and motion.
After motion correction, variance due to picture onset was regressed from the timeseries in order to ensure that lower-level stimulus responses did not drive connectivity estimates. Next, images were corrected for non-neural sources of variance using a regression-based correction with time series obtained from eroded white matter and ventricular masks (Jo et al., 2010). The corrected images were normalized to Montreal Neurological Institute (MNI) space and resampled to 2mm isotropic voxels using Statistical Parametric Mapping Version 5 (SPM5; Penny et al., 2011) and finally, bandpass filtered to retain low-frequency fluctuations (.008-.08Hz). For every scan, the number of motion-corrupted volumes was identified using the Jiang average voxel displacement measurement (Jiang et al., 1995) computed from the slice-wise motion parameters from SLOMOCO. A corrupted volume was defined as a volume where at least one slice within that volume experienced greater than 1mm of out of plane motion. Any participant with 15 or more volumes with greater than 1mm of out of plane motion were excluded from the analysis (Beall & Lowe, 2014; Jiang et al., 1995). Note, corrupted volumes were not removed from the timeseries, and this was only used to identify participants with high levels of motion-related variance.
Evaluation of changes in network connectivity:
Time series were extracted using principal component analysis (1st eigenvariate extracted) for a 195 regions of interest (ROI) atlas (Craddock et al., 2012) and connectivity matrices were obtained via Pearson correlations within the Graph Theory GLM (GTG) toolbox (Spielberg et al., 2015).
Connectivity matrices were then entered as dependent variables into the Network Based Statistic toolbox (NBS; Zalesky et al., 2010). In NBS, the regression model is first tested for each link, following which a t-threshold is applied across the network to remove unassociated links. Next, clusters of supra-threshold links (links sharing a node with ≥1 other cluster links) are identified and the corrected significance of each cluster computed. Specifically, the supra-threshold network’s intensity is calculated by summing the test statistic for each included connection and a corrected p-value is calculated by comparing observed cluster intensity against a null distribution of maximal supra-threshold sizes intensities via permutation (5000 randomizations), resulting in an overall corrected α<.05. For all analyses, mean DVARS (linear and quadratic) and motion (volumetric and slice-wise) were used as regression covariates. Given age differences between groups, age was also used as a covariate for analyses with multiple groups. For each calculation, permutations were restricted to repeated measures of each participant using exchange blocks.
Three main analyses of interest were carried out. We first examined the within-subject effect of CERT in (i) healthy controls, (ii) all bipolar patients, (iii) BPM, (iv) BPD, and (v) all participants. Given that these tests were within-group, and thus more powerful and produce denser networks, a higher NBS t threshold of 4.06 was used to provide more interpretable networks. A second analysis examined the interaction between a BP, BPM or BPD vs. HC group factor and CERT (MAINTAIN vs. SUPPRESS). The third analysis of interest examined the interaction between BP group (BPD vs. BPM) and CERT. A threshold of 3.40 was used in NBS for these between-group analyses, consistent with similar studies in BP (e.g., Wang et al., 2017). To better understand what was driving significant interaction effects, follow-up t-tests were conducted to examine whether connectivity differed by condition within group (paired t-test) and whether connectivity differed by group within each condition (independent samples t-test).
Identification of disturbed graph properties.
Connectivity matrices were entered into the Graph Theoretic GLM (GTG) toolbox v.44 (Spielberg et al., 2015), which computes graph properties for each participant (via Brain Connectivity Toolbox; Rubinov & Sporns, 2010). Each matrix was first thresholded to include only positive weights.
One global property was examined: Mean Connectivity Strength, which indexes the overall level of coupling). Two node-specific properties were examined: Betweenness Centrality and Clustering Coefficient. Betweenness Centrality indexes the extent to which a node functions as a hub for communication (i.e., number of shortest paths that pass through the node). Clustering Coefficient reflects the amount of clustering around a node (i.e., extent to which neighbors of a node are connected to each other). Properties were entered as dependent variables in repeated measures GLM in GTG (5,000 permutations, same predictors as above).
To limit the number of comparisons, properties were examined for only the nodes in the equivalent NBS analysis. False discovery rate (FDR) correction was used to account for the multiple nodes examined. As well, only the relevant analysis (i.e., BPvHC x condition or BPMvBPD x condition) was examined.
RESULTS
Seventy six participants were enrolled and 19 participants were not included in the analysis for the following reasons: 6 participants for motion, 3 participants for unreliable/unclear information, 4 participants for not meeting the scan day HAMD and YMRS criteria, 1 participant did not do the scan, 1 participant closed eyes during the scan, 1 participant was not able to see the monitor during the scan, 2 participants had technical difficulties in presentation of the task, 1 participant did only the MAINTAIN condition. 57 bipolar participants were included in the analysis out of which 27 were BPD and 30 were BPM, along with 33 HC. The demographic and illness characteristics of the participants are presented in Table 1.
Table1:
Clinical and demographic information by group
| Measures | BPD (N=27) | BPM (N=30) | HC (N=33) | ANOVA |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | ||
| Age (years) | 34 (11) | 35(11) | 33(11) | .775 |
| Age at first episode (years) | 16 (8) | 14(4) | - | .395 |
| Hamilton Depression Rating Scale (17 item) | 20 (3) | 6(3) | - | <.001 |
| Young Mania Rating Scale | 2 (3) | 16(2) | - | <.001 |
| Period off medication prior to scan (months) | 27 (32) | 53 (70) | - | .395 |
| Number of prior mood episodes (depression) | >20 | >20 | - | |
| Number of prior mood episodes ((hypo) mania) | >20 | >20 | - | |
| Weeks Since Last Manic Episode | 23 (25) | 29 (48) | - | .539 |
| Weeks Since Last Depressive Episode | 37 (29) | 37 (86) | - | .986 |
| Duration of Current Episode (weeks) | 7 (6) | 2(1) | - | <.001 |
| N (%) | N (%) | N (%) | X2 | |
| Female | 15 (56%) | 18 (60%) | 21 (64%) | .817 |
| Caucasian | 22 (82%) | 29 (97%) | 31 (94%) | .102 |
| History of Trauma | 13 (48%) | 16 (53%) | 1 (3%) | .696 |
| History of Psychosis | 10 (37%) | 8 (27%) | - | .400 |
| Bipolar I (vs. II) | 9 (33%) | 14 (47%) | - | .306 |
| History of Alcohol Abuse | 11 (41%) | 11 (37%) | - | .752 |
| History of Drug Abuse | 9 (33%) | 13 (43%) | - | .439 |
| Right Handedness | 17 (81%) | 21 (84%) | 30 (91%) | .551 |
NOTE: Tests compare bipolar groups with the exception of age, sex, race, and handedness, which include all three groups. The calculation of weeks since last manic/depressive episode do not include the current episode. Due to a data collection error, handedness was not collected for 11 participants (6 BPD, 5 BPM).
Behavioral Measures: Participants were asked at the end of MAINTAIN and SUPPRESS picture to rate the valence of each picture. All groups reported decreased negative valence for the SUPPRESS picture set compared to MAINTAIN picture set and the difference was statistically significant for the BP groups as a whole, BPM group and HC but only trend level significant for the BPD group (Table 2a). Between-group and Group x Condition effects however were not significant for any of the comparisons (Table 2b).
Table 2a:
Within group differences in picture-set valence ratings
| Group | Maintain (mean(SD)) | Suppress (mean(SD)) | Significance |
|---|---|---|---|
| BPD (N=23) | 2.61 (.84) | 2.13 (.87) | .064 |
| BPM (N=28) | 2.86 (.71) | 2.36 (1.1) | .047 |
| BP (N=51) | 2.75 (.77) | 2.25 (1.0) | .007 |
| HC (N=31) | 2.81 (.70) | 2.32 (.87) | .016 |
Table 2b:
Between Group and Group x Conditions tests for picture-valence set ratings.
| Condition | Groups | Test | Significance |
|---|---|---|---|
| Maintain only | BP,HC | Group | .719 |
| Suppress only | BP,HC | Group | .756 |
| Maintain only | BPM,HC | Group | .783 |
| Suppress only | BPM,HC | Group | .893 |
| Maintain only | BPD,HC | Group | .351 |
| Suppress only | BPD.HC | Group | .426 |
| Maintain only | BPD,BPM | Group | .256 |
| Suppress only | BPD,BPM | Group | .425 |
| Maintain only | BPD, BPM, HC | Group | .467 |
| Suppress only | BPD, BPM, HC | Group | .668 |
| Maintain & Suppress | BP,HC | Group X Condition | .982 |
| Maintain & Suppress | BPM, HC | Group X Condition | .959 |
| Maintain & Suppress | BPD,HC | Group X Condition | .986 |
| Maintain & Suppress | BPD, BPM | Group X Condition | .951 |
| Maintain & Suppress | BPD, BPM, HC | Group X Condition | .998 |
Within-Group CERT Effects on Network Connections:
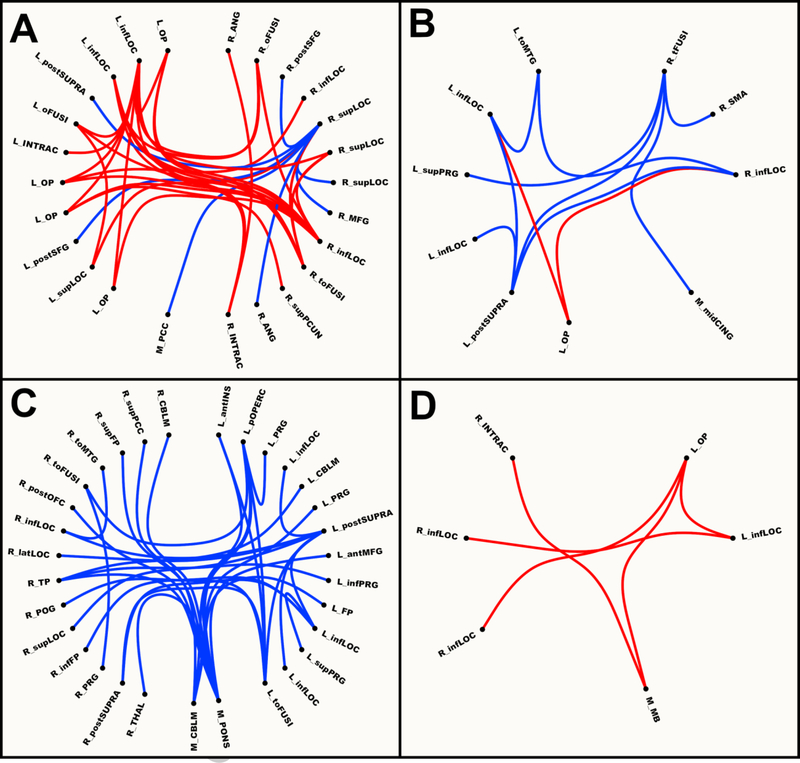
Two networks emerged from the NBS analyses for healthy controls (Fig. 1A). In the first, connectivity was stronger during MAINTAIN (p<.001, 18 nodes, 28 links), and it consisted of differential connections within and between bilateral occipital regions (i.e., inferior lateral occipital cortex, occipital pole) and right superior parietal regions (e.g., precuneus, angular), with the largest number of differential connections being with left inferior lateral occipital cortex. In the second network, connectivity was stronger during SUPPRESS (p=.023, 8 nodes, 7 links), and it consisted of differential connections between a region of right superior lateral occipital cortex and other regions in superior lateral occipital cortex, bilateral prefrontal (middle and superior frontal gyri), medial posterior cingulate cortex, and parietal regions (e.g., angular).
Figure 1.
Network based connectivity maps for Maintain vs. Suppress Contrast in A: Healthy; B: All Bipolar; C: Manic; and D: Depressed Groups. Red = maintain > suppress. Blue = suppress > maintain. L = left; M = medial; R = right; ant = anterior; inf = inferior; lat = lateral; mid = middle; post = posterior; sup = superior; ANG = angular gyrus; CBLM = cerebellum; CING = cingulate gyrus; FP = frontal pole; INS = insula; INTRAC = intracalcarine gyrus; LOC = later occipital cortex; MB = midbrain; MFG = middle frontal gyrus; OFC = orbitofrontal gyrus; oFUSI = occipital fusiform gyrus; OP = occipital pole; PCC = posterior cingulate gyrus; PCUN = precuneus; POG = postcentral gyrus; pOPERC = parietal operculum; PRG = precentral gyrus; SFG = superior frontal gyrus; SMA = supplementary motor area; SUPRA = supramarginal gyrus; tFUSI = temporal fusiform gyrus; THAL = thalamus; toFUSI = temporal-occipital fusiform gyrus; toMTG = temporal-occipital middle temporal gyrus; TP = temporal pole.
Two networks emerged for BP patients (Fig. 1B). In the first, connectivity was stronger during MAINTAIN (p=.009, 3 nodes, 2 links), and it consisted of differential links between left occipital pole and bilateral inferior lateral occipital cortex. In the second network, connectivity was stronger during SUPPRESS (p=.012, 8 nodes, 9 links), and consisted of differential connections between temporal areas (e.g., temporal fusiform), mid-line regions (e.g., mid-cingulate cortex, supplementary motor area), and bilateral occipital regions (e.g., inferior lateral occipital cortex, occipital pole), with the largest number of differential links being with right temporal fusiform and with left posterior supramarginal.
One network emerged for the BPM group (Fig. 1C) in which connectivity was stronger during SUPPRESS than MAINTAIN (p<.001, 31 nodes, 32 links), and it consisted of differential connections within and between pons, prefrontal regions (e.g., frontal pole, middle frontal gyrus), parietal regions (e.g., precuneus, parietal operculum), temporal regions (e.g., temporal-occipital middle temporal gyrus, temporal pole), insula, cerebellum, later occipital cortex, posterior cingulate gyrus, and thalamus, with the largest number of differential connections being with pons.
One network emerged for the BPD group (Fig. 1D) in which connectivity was stronger during MAINTAIN than SUPPRESS (p=.036, 6 nodes, 5 links), and it consisted of differential links within and between occipital regions (e.g., occipital pole, inferior lateral occipital cortex) and midbrain, with the largest number of differential links being with left occipital pole.
Interestingly, four links were present in the cross-BP networks that were not found when looking at the groups separately. These links were between regions in left occipital pole and left inferior lateral occipital cortex for the MAINTAIN > SUPPRESS contrast and between left posterior supramarginal gyrus and three regions of inferior lateral occipital cortex (two in the left hemisphere, one in the right) for the opposite contrast. This may seem strange if one expects that the cross-BP networks should be the sum of their parts, and thus there should be no links in the BP networks that were not in the BPD and BPM networks. However, it is likely these effects were present at a lower magnitude in each group, and as such were not significant in the smaller groups but were in the combined sample.
Between-Group CERT Effects on Network Connections:
No findings emerged at a significant level in which connectivity shifts associated with CERT differed between BP patients and controls.
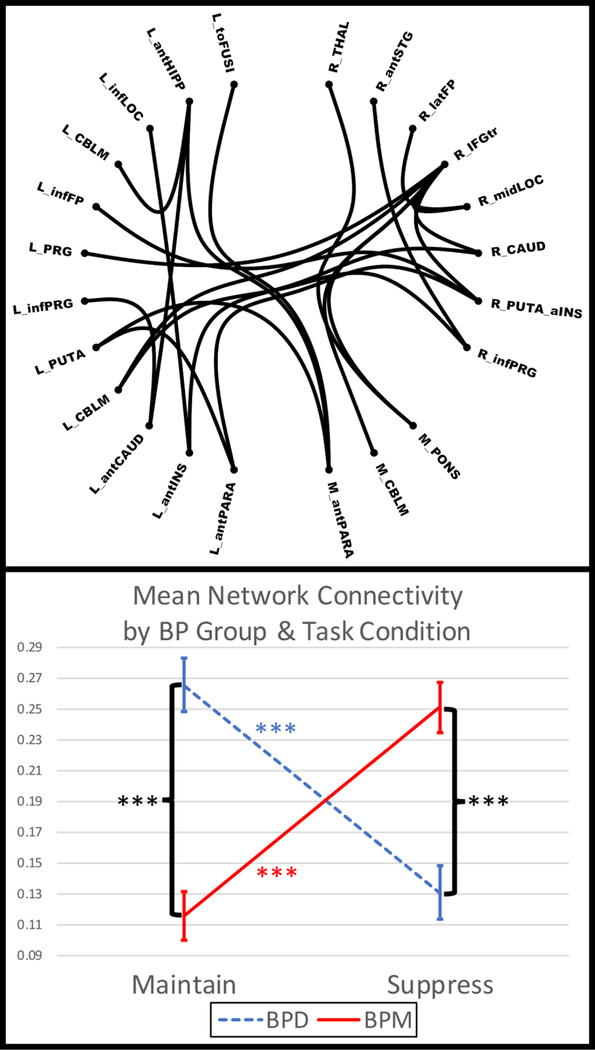
One network emerged in which connectivity shifts associated with CERT differed by BP group (Fig. 2, p=.037, 23 nodes, 23 links) and it consisted of differential links within and between subcortical structures (e.g., pons, putamen, thalamus), frontal regions (e.g., frontal pole, inferior frontal gyrus), insula, cerebellum, occipital regions (e.g., lateral occipital), temporal regions (e.g., superior temporal gyrus), and paracingulate, with the largest number of differential links being with right inferior frontal pole/inferior frontal gyrus pars triangularis (rIF). Paired t-tests revealed that mean connectivity in this network was significantly stronger during MAINTAIN relative to SUPPRESS in the BPD group (t26=5.2, p<.001), whereas the opposite was true for the BPM group (t29=6.3, p<.001, df=29). Independent samples t-tests revealed that BPD exhibited significantly stronger connectivity in this network than did BPM during MAINTAIN (t55=5.8, p<.001), whereas the opposite was true for SUPPRESS (t55=4.0, p<.001).
Figure 2.
Group (Mania vs. Depression) and Condition (Maintain vs. Suppress) Interaction for Network Based Connectivity. BP = bipolar disorder; BPD = depressed BP; BPM = manic/hypomanic BP; L = left; M = medial; R = right; ant = anterior; inf = inferior; mid = middle; CAUD = caudate; CBLM = cerebellum; FP = frontal pole; HIPP = hippocampus; IFGtr = inferior frontal gyrus pars triangularis; INS = insula; LOC = later occipital cortex; PRG = precentral gyrus; PUTA = putamen; PARA = paracingulate; STG = superior temporal gyrus; THAL = thalamus; toFUSI = temporal-occipital fusiform gyrus. *** = p<.001. Error bars reflect 1 standard deviation.
Disturbances in Graph Properties:
Analyses were carried out in the GTG toolbox examining the group (BPM vs. BPD) by CERT (MAINTAIN vs. SUPPRESS) interaction. These analyses revealed significant interaction effects for clustering coefficient for rIF (F(1,46)=9.1, p=.005 [corrected p=.039]), pons (F(1,46)=16.3, p=.002 [corrected p=.039]), right putamen/mid-insula (rP-aINS) (F(1,46)=8.6, p=.006 [corrected p=.039]), and right thalamus (F(1,46)=7.9, p=.007 [corrected p=.039]). Given that the graph properties used are influenced by the overall density and mean connection strength of the global network, we repeated significant analyses with the addition of density and mean connection strength (after median normalization) as covariates. All findings remained significant, further ensuring that these effects are not driven by basic network properties. Furthermore, given differences by group in the amount of time that participants had been off medication, analyses were repeated with time off medications as a covariate. Due to a data collection error, this information not collected for 5 participants (3 BPD, 2 BPM), and thus these analyses included only 52 participants. All analyses remained significant, with the exception of the right thalamus clustering coefficient (p=.065). However, given that the sample size was reduced by 9%, it is quite possible that this lack of significance is due to the reduction in power.
As shown in Fig. 3, rIF clustering coefficient evidenced a crossover pattern, although none of the follow-up t-tests were significant. Pons clustering coefficient for BPM (Fig. 3) during suppress was significantly higher than both BPD suppress (t55=2.4, p=.020) and BPM maintain (t29=3.8, p<.001). Right thalamus clustering coefficient (Fig. 3) had extremely similar values for the two groups during maintain, whereas BPM had greater clustering than BPD during suppress (t55=2.2, p=.030). Finally, right P-aINS clustering coefficient (Fig. 3) had similar values for BPD in both conditions and BPM in suppress, whereas BPM clustering was lower during maintain. BPM exhibited significantly greater clustering during suppress than maintain (t29=4.4, p<.001). However, the difference between BPD and BPM clustering during maintain was only marginally significant (t55=1.8, p=.081), and thus this pattern should be interpreted with caution.
Figure 3.
Graph theoretic metrics showing significant group (Mania vs Depression) and condition (Maintain vs Suppress) interactions. BP = bipolar disorder; BPD = depressed BP; BPM = manic/hypomanic BP; IF = inferior frontal pole/inferior frontal gyrus pars triangularis; P-aINS = putamen/anterior insula; * = p<.05; *** = p<.001. Error bars reflect 1 standard deviation.
DISCUSSION
In this study, the first of its kind, we examined brain network dynamics while hypomanic (BPM) and depressed (BPD) individuals with bipolar disorder and healthy controls (HC) continuously maintained or suppressed (via reappraisal) negatively valenced emotion. A network centered around right inferior frontal pole/inferior frontal gyrus pars triangularis (rIF) emerged in which BPD exhibited stronger connectivity during MAINTAIN than SUPPRESS, whereas the opposite was true for BPM (Fig. 2). Interestingly, four links were present in the cross-BP networks that were not found when looking at the groups separately. This may seem strange if one expects that the cross-BP networks should be the sum of their parts, and thus there should be no links in the BP networks that were not in the BPD and BPM networks. However, it is likely these effects were present at a lower magnitude in each group, and as such were not significant in the smaller groups but were in the combined sample.
Within-group analyses of condition provide further insight into the organization of emotion-regulation circuitry within these groups. No statistically significant corrected results were observed when comparing either all BP or the BP subgroups to HC, which may be due to the need for greater sample to obtain significant results after the stringent correction for multiple comparisons performed herein. Finally, examination of graph theory properties revealed significant group by task-condition interactions for rIF, pons, right putamen/mid-insula (rP-aINS), and right thalamus clustering coefficient.
Clustering coefficient reflects the extent to which the neighbors of a node form a cohesive local network, and greater clustering coefficient reflects more efficient communication in this local network. The highest levels of clustering coefficient for a node in pons was observed for BPM during SUPPRESS (relative to both BPD SUPPRESS and BPM MAINTAIN) (Fig. 3). Interestingly, mania following pontine strokes has been observed in a small number of cases (Satzer & Bond, 2016), although a study examining brainstem volumes in BP did not find any differences (Brambilla et al., 2001). Given the lack of spatial specificity of this ROI to structures within the pons, and that a number of small nuclei exist within this structure, it will be important to follow up this finding in future research using acquisitions with greater granularity (e.g., multiband EPI). Right thalamus clustering coefficient exhibited a somewhat similar pattern (Fig. 3), although in this case, all that can be inferred is that there is no group difference in clustering for MAINTAIN, whereas clustering becomes greater for BPM during SUPPRESS. Past work in a large sample found smaller thalamic volumes in BP, and evidence indicates that this might be prevented/reversed by lithium treatment (Hibar et al., 2016). Thus, clustering around thalamus may be an important target for future treatment research.
The lowest levels of clustering coefficient for rP-aINS were observed for BPM during MAINTAIN (relative to BPM SUPPRESS and, marginally, BPD MAINTAIN). Given the importance of insula and putamen in the generation of affective experience (e.g., interoception in mid-insula; Craig, 2003), this finding may indicate that interoceptive signals, for example, are less integrated within other pieces of the network during the experience of emotion in mania. This is perhaps surprising, given that deficits in BP are thought to be in the regulation of affect, not un-regulated experience (Phillips et al., 2008). However, meta-analyses of fMRI studies in BP have found consistent abnormalities in putamen (both hypo- and hyper-activation, depending on the particular region) (Houenou et al., 2011) supporting the involvement of this region. More generally, the observed reduction in cohesiveness in the network around rP-aINS may reflect a vulnerability wherein affective stimuli encountered during typical processing (i.e., without explicit regulation) are not integrated as thoroughly into ongoing processing. In turn, this may result in a failure to track the current state of the environment with reference to goals.
Finally, a cross-over pattern was observed for rIF clustering coefficient (Fig. 3), with levels for BPD MAINTAIN and BPM SUPPRESS being higher than the complementary group/conditions. Given that none of the follow-up tests were significant, only the relative shape of this pattern should be interpreted. Past meta-analytic work found that BP was associated with consistently reduced rIF activation during both affective and cognitive tasks, and this effect was found in BPM but not BPD or euthymic BP patients (Chen et al., 2011). Given that clustering coefficient reflects connectivity among the neighbors of a node, not the node’s activity, our finding complements this meta-analytic work. For example, our finding could indicate that one of the causes of lower IF activation in BPM may be less cohesive processing in its neighbors, at least during maintenance of affect. Alternatively, it is possible that the increased levels of cohesion during suppression of emotion may serve to down-regulate IF processing.
The present study benefited from a number of strengths, including a medication-free sample, examination of (hypo)manic and depressed BP patients simultaneously, a novel continuous performance and use of graph-theory methods. Several limitations must also be considered. Although participants were medication-free, they were not medication-naïve. As such, present findings may be driven, in part, by long-term medication use. Furthermore, this work was cross-sectional and could not differentiate between factors predisposing toward the development of BP from factors arising as a consequence. An ideal study would longitudinally examine the same individuals through both depressive and (hypo)manic episodes. However, such work is extremely difficult and it is unethical to keep patients medication-free. Therefore, present findings should be regarded as complementary to studies with medicated participants. As this study was conducted in medication-free outpatients, only two patients met criteria for a full manic episode (the rest hypomanic) and the majority of the depressed patients were only moderately depressed. Thus, the extent to which present findings are representative of more severe BP (i.e., mania, severe depression) remains unknown. The sample sizes are also relatively small, although they are commensurate with other studies in this field and reflect the inherent difficulty recruiting medication-free individuals with bipolar disorder. Thus, the study suffers from relatively low statistical power, and future research should at least replicate the study with similar sample sizes, and ideally with much larger samples. Finally, choice of individual-link thresholds in the NBS analysis can impact findings, and there is currently no agreed upon threshold or set of thresholds to use in the field. Although we have chosen thresholds in as principled a way as possible, inconsistency with other studies in threshold choice is still a limitation of the present work.
In summary, the results of this study suggest that a continuous emotional task can be used to identify networks involved in emotional regulation in healthy controls and individuals with mood disorders. The present findings suggest that posterior cortical areas appear to be involved in maintenance of emotion, whereas an anterior cortical network is involved in suppression of emotion. Finally, an inferior frontal network was implicated in differences between bipolar depression and mania.
Funding:
This project was funded by the National Institute of Mental Health to AA (R01MH075025).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest: Amit Anand declares that he has no conflict of interest. Jaykumar Grandhi declares that he has no conflict of interest. Harish Karne declares that he has no conflict of interest. Jeffrey Spielberg declares that he has no conflict of interest.
Ethical Approval: All procedures were performed in accordance with the ethical standards of the institutional review board and with the 1964 Helsinki declaration and its later amendments.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
REFERENCES:
- Anand A, Li Y, Wang Y, Gardner K, Lowe MJ (2007). Reciprocal effects of antidepressant treatment on activity and connectivity of the mood regulating circuit: an fMRI study. Journal of Neuropsychiatry & Clinical Neurosciences, 19, 274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall EB, & Lowe MJ. (2007). Isolating physiologic noise sources with independently determined spatial measures. Neuroimage, 37, 1286–1300. [DOI] [PubMed] [Google Scholar]
- Beall EB, & Lowe MJ. (2014). SimPACE: generating simulated motion corrupted BOLD data with synthetic-navigated acquisition for the development and evaluation of SLOMOCO: a new, highly effective slicewise motion correction. Neuroimage, 101, 21–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beall EB. (2010). Adaptive cyclic physiologic noise modeling and correction in functional MRI. Journal of Neuroscience Methods 187, 216–228. [DOI] [PubMed] [Google Scholar]
- Brady R Jr, Öngür D, & Keshavan M (2014). Neurobiology of mood-state shifts in bipolar disorder: a selective review and a hypothesis. Harvard Review of Psychiatry, 22, 23–30. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, … & Soares JC (2001). MRI study of posterior fossa structures and brain ventricles in bipolar patients. Journal of Psychiatric Research, 35(6), 313–322. [DOI] [PubMed] [Google Scholar]
- Chen CH, Suckling J, Lennox BR, Ooi C, & Bullmore ET (2011). A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disorders, 13(1), 1–15. [DOI] [PubMed] [Google Scholar]
- Craddock RC, James GA, Holtzheimer PE, Hu XP, & Mayberg HS. (2012). A whole brain fMRI atlas generated via spatially constrained spectral clustering. Human Brain Mapping, 33, 1914–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2003). Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology, 13, 500–505. [DOI] [PubMed] [Google Scholar]
- First MB, Frances A, & Pincus HA. (2002). DSM-IV-TR handbook of differential diagnosis, American Psychiatric Publishing, Inc. [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, & Simons JS. (2012). Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences, 109, 12788–12793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Li TQ, & Ress D. (2000). Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magnetic Resonance in Medicine, 44, 162–167. [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibar DP, Westlye LT, van Erp TG, Rasmussen J, Leonardo CD, Faskowitz J, … & Dale AM (2016). Subcortical volumetric abnormalities in bipolar disorder. Molecular Psychiatry, 21(12), 1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, & Wessa M (2011). Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. Journal of Affective Disorders, 132, 344–355. [DOI] [PubMed] [Google Scholar]
- Hummer TA, Hulvershorn LA, Karne HS, Gunn AD, Wang Y, & Anand A. (2013). Emotional response inhibition in bipolar disorder: A functional magnetic resonance imaging study of trait- and staterRelated abnormalities. Biological Psychiatry, 73, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang A, Kennedy DN, Baker JR, Weisskoff RM, Tootell RBH, Woods RP, Benson RR, Kwong KK, Brady TJ, Rosen BR, & Belliveau JW. (1995). Motion detection and correction in functional MR imaging. Human Brain Mapping, 3, 224–235. [Google Scholar]
- Jo HJ, Saad ZS, Simmons WK, Milbury LA, & Cox RW. (2010). Mapping sources of correlation in resting state FMRI, with artifact detection and removal. Neuroimage, 52, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, & Cuthbert BN (1997). International affective picture system (IAPS): Technical manual and affective ratings. NIMH Center for the Study of Emotion and Attention, 39–58. [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, & Mathews VP. (2002). Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology, 224, 184–192. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, & Gross JJ. (2005). The cognitive control of emotion. Trends in Cognitive Sciences, 9, 242–249. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, & Gross JJ. (2004). For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage, 23, 483–499. [DOI] [PubMed] [Google Scholar]
- Ormel J, Petukhova M, Chatterji S, Aguilar-Gaxiola S, Alonso J, Angermeyer MC, Bromet EJ, Burger H, Demyttenaere K, De Girolamo G & Haro JM (2008). Disability and treatment of specific mental and physical disorders across the world. The British Journal of Psychiatry, 192, 368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny WD, Friston KJ, Ashburner JT, Kiebel SJ, & Nichols TE. (2011). Statistical Parametric Mapping: The Analysis of Functional Brain Images: The Analysis of Functional Brain Images, Academic Press. [Google Scholar]
- Phillips ML, Ladouceur CD, & Drevets WC (2008). A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 829–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, & Petersen SE. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinov M, & Sporns O. (2010). Complex network measures of brain connectivity: uses and interpretations. Neuroimage, 52, 1059–1069. [DOI] [PubMed] [Google Scholar]
- Satzer D, & Bond DJ (2016). Mania secondary to focal brain lesions: implications for understanding the functional neuroanatomy of bipolar disorder. Bipolar Disorders, 18(3), 205–220. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, & Dunbar GC. (1989). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal Clinical Psychiatry, 59 Suppl 20, 22–33. [PubMed] [Google Scholar]
- Spadone S, Della Penna S, Sestieri C, Betti V, Tosoni A, Perrucci MG, Romani GL, & Corbetta M. (2015). Dynamic reorganization of human resting-state networks during visuospatial attention. Proceedings of the National Academy of Sciences, 112, 8112–8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg J, Beall E, Hulvershorn L, Altinay M, Karne H, & Anand A. (2016). Resting State Brain Network Disturbances Related to Hypomania & Depression in Medication-Free Bipolar Disorder. Neuropsychopharmacology, 41, 3016–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, McGlinchey RE, Milberg WP, & Salat DH. (2015). Brain network disturbance related to posttraumatic stress & traumatic brain injury in veterans. Biological Psychiatry, 78, 210–216. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, Delbello MP, & Adler CM. (2005). The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Molecular Psychiatry, 10, 105–116. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Wang R, Wang G-J, & Volkow ND. (2014). Functional connectivity and brain activation: A synergistic approach. Cerebral Cortex, 24, 2619–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, & Buckner RL. (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of Neurophysiology, 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, & Buckner RL. (2012). The influence of head motion on intrinsic functional connectivity MRI. Neuroimage, 59, 431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas C, López-Jaramillo C, & Vieta E (2013). A systematic literature review of resting state network-functional MRI in bipolar disorder. Journal of Affective Disorders 150, 727–735. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang J, Jia Y, Zhong S, Zhong M, Sun Y, … & Huang R (2017). Topologically convergent and divergent functional connectivity patterns in unmedicated unipolar depression and bipolar disorder. Translational Psychiatry, 7(7), e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessa M, Kanske P, & Linke J (2014). Bipolar disorder: a neural network perspective on a disorder of emotion and motivation. Restorative Neurology & Neuroscience, 32, 51–62. [DOI] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, & Meyer D. (1978). A rating scale for mania: reliability, validity and sensitivity. The British Journal of Psychiatry, 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Zalesky A, Fornito A, & Bullmore ET. (2010). Network-based statistic: Identifying differences in brain networks. Neuroimage, 53, 1197–1207. [DOI] [PubMed] [Google Scholar]