Article, see p 33
Myocardial injury is a common phenomenon in coronavirus disease 2019 (COVID-19).1 Initial clinical studies in China and later in the United States found that >30% of hospitalized patients with COVID-19 had myocardial injury as evidenced by elevated cardiac troponin I levels,2–4 and the elevation of troponin was associated with a higher risk of mortality.2–4 Although several mechanisms have been hypothesized, including direct infection of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) to cardiomyocytes5–7 leading to fulminant myocarditis, hypercoagulable state in a cytokine storm leading to vascular injuries such as coronary artery occlusion or microvascular thrombi,8 stress-induced cardiomyopathy,1,9,10 or type II myocardial infarction by supply-demand mismatch,1,9,10 the precise mechanisms are unclear and therapeutic targets to prevent or alleviate myocardial injury in COVID-19 are unknown.
In this issue of Circulation Research, Yang et al11 identified 2 drug candidates, ranolazine and tofacitinib, which can alleviate macrophage-mediated hyper-inflammation and myocardial injury in COVID-19 using clinical samples of COVID-19 patients and a coculture platform containing human pluripotent stem cell–derived cardiomyocytes and macrophages. First, by comparing RNA-seq (RNA-sequencing) data of autopsy heart samples from non–COVID-19 donors and COVID-19 patients, the authors found that the expression of CCL2 (C-C motif chemokine ligand 2), a well-known chemoattractant for migrating macrophages, was significantly up-regulated in COVID-19 hearts. Upregulation of CCL2 in the COVID-19 hearts were also confirmed at a protein level by immunostaining. Second, to explore the interaction between cardiomyocytes and macrophages, they utilized a coculture platform containing human pluripotent stem cell-derived cardiomyocytes and macrophages. They found that macrophages secrete IL (interleukin)-6 and TNF (tumor necrosis factor)-α after exposure to SARS-CoV-2, leading to reactive oxidative species production and apoptosis of cardiomyocytes. Third, to identify compounds that could abrogate macrophage-mediated reactive oxidative species production in cardiomyocytes, they performed a drug screening on their co-culture platform with a library of 1280 FDA (United States Food and Drug Administration)-approved drugs. One of the hits from the screening was ranolazine (a selective late-sodium current inhibitor used to treat chronic angina), and another was tofacitinib (a JAK [Janus kinase] inhibitor used to treat rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis). The 2 drugs were confirmed to alleviate reactive oxidative species production and apoptosis in cardiomyocytes either by blocking IL-6 and TNF-α expression in macrophage (tofacitinib), inhibiting JAK-STAT (JAK-signal transducer and activator of transcription) pathway in cardiomyocytes (tofacitinib), or lowering reactive oxidative species levels in cardiomyocytes (ranolazine; Figure).
Figure.
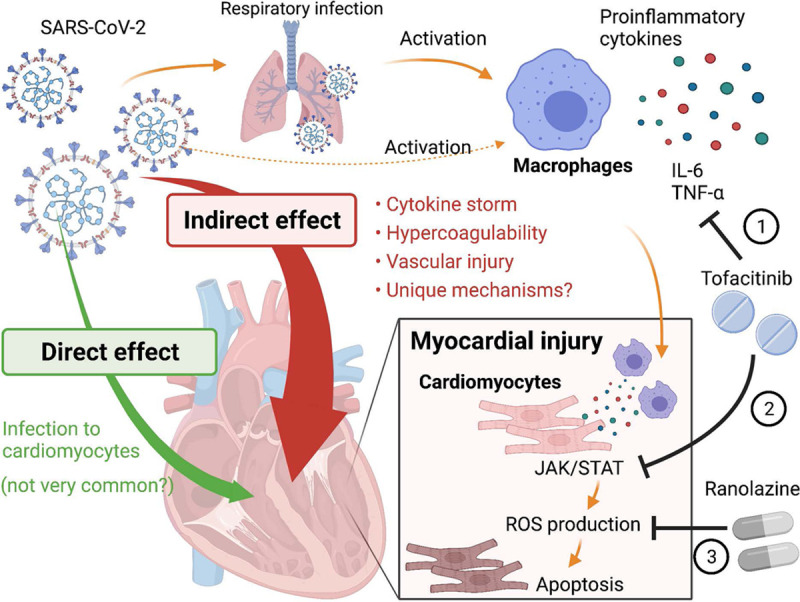
Indirect effect of coronavirus disease 2019 (COVID-19) on the heart through macrophage-mediated inflammation. COVID-19 causes myocardial injury through either direct infection or indirect inflammation-mediated pathways. Using a coculture system of cardiomyocytes (CMs) and macrophages, Yang et al11 demonstrated macrophage-mediated cardiac damage by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2). Tofacitinib and ranolazine are the 2 candidate drugs identified in this study that can alleviate this process by (1) inhibiting IL (interleukin)-6/TNF (tumor necrosis factor)-α production in macrophages (tofacitinib), (2) inhibiting JAK-STAT (JAK-signal transducer and activator of transcription) pathway in CMs (tofacitinib), or (3) inhibiting reactive oxidative species (ROS) production in CMs (ranolazine). Figure created with BioRender.com.
An important aspect of this study is that it is not focusing on the impact of direct infection to cardiomyocytes by SARS-CoV-2 but on inflammation-induced or macrophage-mediated myocardial injury because the myocardial injury often seen in COVID-19 is more likely due to indirect consequences of systemic inflammation or a cytokine storm than due to direct infection to cardiomyocytes. Although there are several reports showing that SARS-CoV-2 could infect human pluripotent stem cell–derived cardiomyocytes in vitro,5–7 and also human hearts directly in a small number of patients,1,7 the viral RNA or viral particles of SARS-CoV-2 are not typically detected in patients’ hearts in autopsy studies.12–14 Thus, direct infection of cardiomyocytes by SARS-CoV-2 is possible but rare in COVID-19 patients,13 which would not explain why as much as 30% of patients show myocardial injury. This suggests that systemic inflammation or a cytokine storm following the infection in respiratory organs is playing an important role in developing cardiovascular complications. This study by Yang et al11 focused on the indirect pathway, in which the upregulated expression of CCL2 in the heart (likely expressed by cardiomyocytes) attracts macrophages, leading to myocardial injury through secretion of IL-6 and TNF-α. Another study by Mills et al15 recently published in Cell also investigated the mechanisms of inflammation-induced cardiac dysfunction in COVID-19 using cardiac organoid models and showed that cardiac organoids treated with a combination of IL-1B, INF (interferon)-γ, and poly(1:C) are a promising platform that could mimic myocardial injury caused by COVID-19 cytokine storm. Using stem cell–derived cells and coculture systems or organoid models, these 2 studies identified potential drugs that could prevent or improve myocardial injury in COVID-19, including ranolazine, tofacitinib,11 and BET (bromodomain and extra-terminal motif) inhibitors.15 With the lack of animal models on development of myocardial injury after SARS-CoV-2 infection, the human stem cell-based platforms used by these studies are attractive ways to study the mechanisms of myocardial injury in COVID-19 and perform drug screening.
There are several remaining questions to be further studied. First, it is unknown whether there are any unique mechanisms in COVID-19 responsible for myocardial injury in up to 30% of hospitalized patients. Although the mechanism of cardiac injury through macrophage-mediated inflammation is a novel finding in COVID-19,11 the signaling molecules identified in this study were CCL-2, IL-6, and TNF-α, which are common proinflammatory cytokines generally seen in many types of inflammation. Other alternative mechanisms may also play important roles in COVID-19–induced myocardial injury because the frequency or extent of myocardial injury in COVID-19 is likely higher than that of other infectious diseases such as influenza or sepsis. Second, non-cardiomyocytes in the heart such as endothelial cells, smooth muscle cells, and fibroblasts also may be affected by the indirect effect of SARS-CoV-2 infection, including the macrophage-mediated inflammation and cytokine storms. These non-cardiomyocytes may contribute to the impaired cardiac function in patients with COVID-19. Third, it is also important to assess whether other organs could be damaged by the macrophage-mediated inflammation in COVID-19. Similar to the first point, the macrophage-mediated inflammation is commonly seen in many types of inflammatory diseases or conditions in other organs. Because COVID-19 develops diverse manifestations in many types of organs,1 the mechanism described in this study or unknown indirect effects could damage other organs, including brain, kidney, liver, arteries, etc
In conclusion, this work by Yang et al11 identified 2 candidate drugs that could alleviate the myocardial injury in COVID-19 by inhibiting the macrophage-mediated inflammation. It is important to study how infectious diseases such as COVID-19 and influenza cause cardiovascular damage through systemic inflammation or cytokine storms, because unknown pathogens that could lead to future pandemics may also provoke critical cardiovascular complications as we have seen with COVID-19 in the past year.
Sources of Funding
This publication was supported by research grants from the American Heart Association 17MERIT33610009 (J.C. Wu), National Institutes of Health R01 HL113006, R01 HL141851 (J.C. Wu), and Tobacco-Related Disease Research Program (TRDRP) postdoctoral fellowship (M. Nishiga).
Disclosures
None.
Footnotes
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Sources of Funding and Disclosures, see page 49.
References
- 1.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–558. doi: 10.1038/s41569-020-0413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F, Zhao S, Somani S, Van Vleck T, Vaid A, et al. ; Mount Sinai COVID Informatics Center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol. 2020;76:533–546. doi: 10.1016/j.jacc.2020.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi S, Qin M, Cai Y, Liu T, Shen B, Yang F, Cao S, Liu X, Xiang Y, Zhao Q, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41:2070–2079. doi: 10.1093/eurheartj/ehaa408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma A, Garcia G, Jr, Wang Y, Plummer JT, Morizono K, Arumugaswami V, Svendsen CN. Human iPSC-derived cardiomyocytes are susceptible to SARS-CoV-2 infection. Cell Rep Med. 2020;1:100052. doi: 10.1016/j.xcrm.2020.100052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Han Y, Nilsson-Payant BE, Gupta V, Wang P, Duan X, Tang X, Zhu J, Zhao Z, Jaffré F, et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136.e7. doi: 10.1016/j.stem.2020.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Bermejo JA, Kang S, Rockwood SJ, Simoneau CR, Joy DA, Silva AC, Ramadoss GN, Flanigan WR, Fozouni P, Li H, et al. SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci Transl Med. 2021;13:eabf7872. doi: 10.1126/scitranslmed.abf7872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, Nasr A, Kutys R, Guo L, Cornelissen A, et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828 [DOI] [PubMed] [Google Scholar]
- 9.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286 [DOI] [PubMed] [Google Scholar]
- 10.Hendren NS, Drazner MH, Bozkurt B, Cooper LT., Jr. Description and proposed management of the acute COVID-19 cardiovascular syndrome. Circulation. 2020;141:1903–1914. doi: 10.1161/CIRCULATIONAHA.120.047349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Han Y, Jaffre F, Nilsson -, Payant BE, Bram Y, Wang P, Zhu J, Zhang T, Redmond D, Houghton S, et al. An immuno-cardiac model for macrophage-mediated inflammation in COVID-19 hearts. Circ Res. 2021;129:33–46. doi: 10.1161/CIRCRESAHA.121.319060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ, Vander Heide RS. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465 [DOI] [PubMed] [Google Scholar]
- 13.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K, Scherschel K, Kirchhof P, Escher F, Schultheiss HP, et al. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mills RJ, Humphrey SJ, Fortuna PRJ, Lor M, Foster SR, Quaife-Ryan GA, Johnston RL, Dumenil T, Bishop C, Rudraraju R, et al. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell. 2021;184:2167–2182.e22. doi: 10.1016/j.cell.2021.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]


