Abstract
The prevalence of cardiovascular disease (CVD) in pregnancy, both diagnosed and previously unknown, is rising and a leading cause of maternal morbidity and mortality. Historically, women of child-bearing potential have been under-represented in research leading to lasting knowledge gaps in the cardiovascular care of pregnant and lactating women. Despite these limitations, clinicians should be familiar with the safety of frequently used diagnostic and therapeutic interventions to adequately care for this at-risk population. This document, the fourth of a five-part series, provides evidence-based recommendations regarding the use of common cardiovascular diagnostic tests and medications in pregnant and lactating women.
Keywords: cardio-obstetrics, pregnancy, lactation, imaging, medication
Tweet:
Obtaining accurate diagnostic data for appropriate maternal care is necessary for optimal fetal outcomes. Modalities such as ultrasound and MRI that minimize risk are preferred. If exposure to ionizing radiation is indicated, ALARA principles should be employed.
Condensed Abstract
The prevalence of cardiovascular (CV) disease in pregnancy, both diagnosed and previously unknown, is on the rise. Familiarity with the safety profile of frequently used diagnostic and therapeutic interventions is essential to adequately care for this at-risk population. This document, the fourth of a five-part series, provides evidence-based recommendations regarding the use of common CV diagnostic tests and medications in pregnant and lactating women.
Previously in this 5-part review series of cardiovascular disease (CVD) in pregnancy, we covered the approach to the cardio-obstetrics patient from risk stratification and delivery planning through postpartum care (Part 1), congenital and heritable disorders (Part 2), and acquired cardiovascular diseases (Part 3). In Part 4 of this review series, we now discuss the use of diagnostic cardiac imaging in pregnancy and considerations for the use of cardiovascular contrast agents and medications in pregnancy and lactation. We conclude with a brief review of the history of the inclusion of pregnant women in research studies to better understand the current state of the evidence, and the clinical dilemmas facing the cardio-obstetrics team when weighing maternal and fetal risks related to diagnostic testing and therapeutic interventions.
Diagnostic Cardiac Imaging in Pregnancy
This section will highlight the current approaches to the use of cardiac imaging modalities during pregnancy. In general, diagnostic testing strategies to evaluate known or suspected CVD in pregnancy are similar to those used in non-pregnant women, but should take into consideration additional safety measures to maximize fetal well-being.(1, 2) Detailed guidance on the use of diagnostic imaging during pregnancy and lactation are available from the American College of Obstetricians and Gynecologists (ACOG).(3) A basic principle that guides the use of imaging is a primary assessment of the patient’s clinical need. If the indication for imaging is appropriate and will alter clinical management, then imaging during pregnancy should be considered. Obtaining accurate diagnostic data for appropriate maternal care is necessary for optimal fetal outcomes. When pregnancy-safe imaging procedures such as ultrasound and magnetic resonance imaging (MRI) without gadolinium-based contrast agents (GBCA) are available and can provide the appropriate diagnostic data, they should be the preferred modality. For computed tomography (CT), nuclear medicine, or invasive coronary angiography, exposure to ionizing radiation is often the chief consideration when weighing the balance of risks and benefits. For these modalities, a frank discussion with the patient may help to facilitate the decision to use an imaging modality that would expose her and the fetus to ionizing radiation (Central Illustration).(4) In addition, if the decision is made to proceed with imaging, protocols should be adapted and individualized to minimize radiation exposure. Any elective procedures involving exposure to ionizing radiation are not recommended during pregnancy and should be deferred.
Central Illustration.
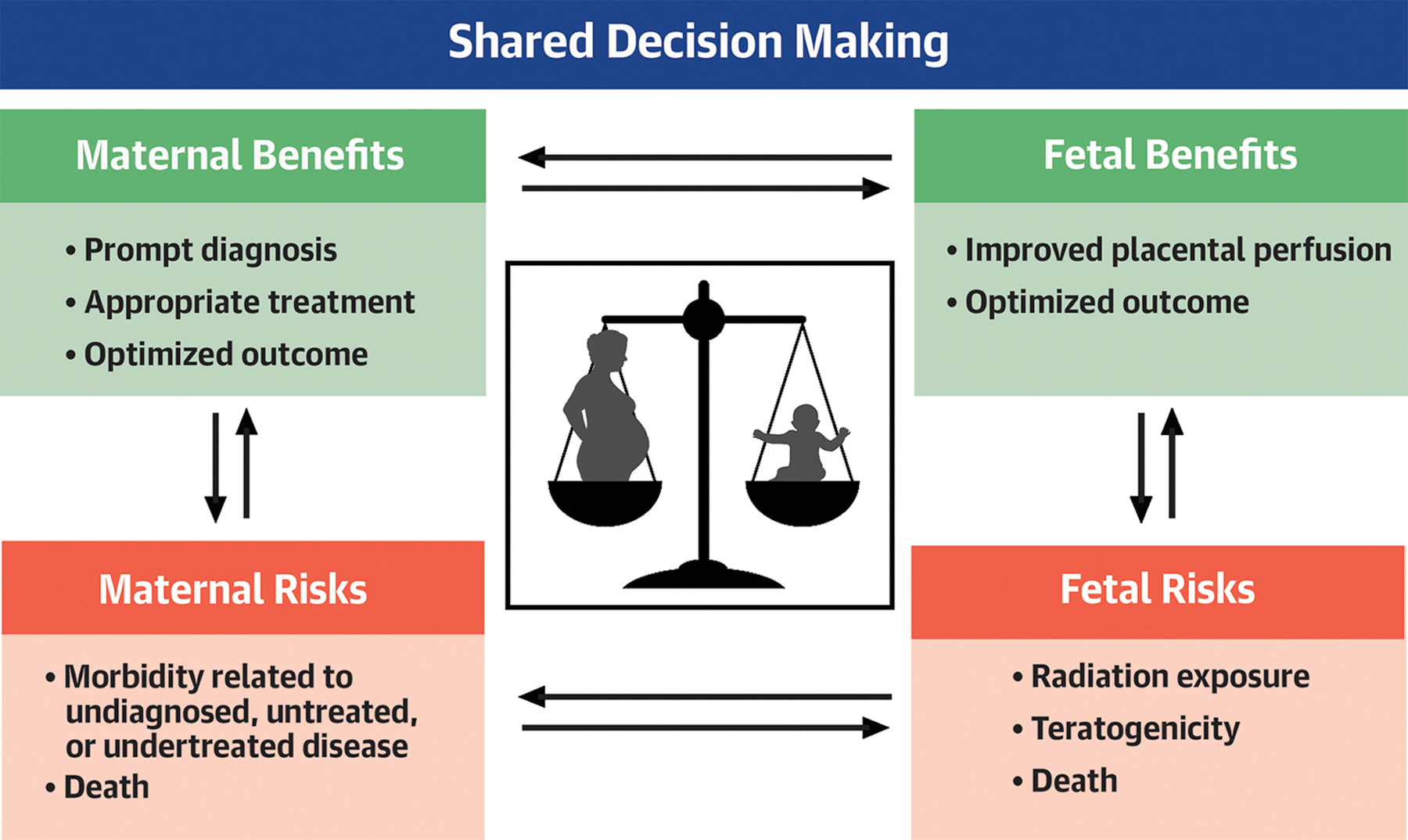
Shared Decision Making is the Cornerstone of Providing Optimal Care to Pregnant Women.
When choosing diagnostic tests and medications for pregnant and lactating women the interplay between maternal and fetal/infant risks and benefits must be carefully considered. Shared decision making in consultation with the patient and cardio-obstetrics team is essential for optimal outcomes.
Considerations for Positioning a Pregnant Patient During Diagnostic Testing
The maternal vascular system undergoes a number of physiologic adaptions to the pregnant state which vary by trimester and have been described in Part 1 of this series. An important additional hemodynamic phenomenon which must be taken into consideration when performing diagnostic imaging studies such as echocardiography, is the positional effect of the gravid uterus on the inferior vena cava (IVC). It has been shown that in the supine position, IVC compression occurs in the majority of women during the third trimester and maternal cardiac output decreases by up to 30% compared to left lateral decubitus position as a result. Decreases in maternal blood pressure and concomitant increases in heart rate are also seen, and in a minority of patients (8%) symptomatic supine hypotensive syndrome can occur.(5, 6) While the size and position of the uterus and fetus affect the degree of IVC compression, additional factors such as azygos venous flow rate also impact maternal hemodynamics and subsequently placental and fetal perfusion.(7, 8)
The combination of displacement of the heart (upward and lateral) secondary to the enlarged uterus along with preferred positioning in the left lateral decubitus may improve echocardiographic image quality, though this can be offset by a decrease the intracostal space leading to a limited acoustic window. For examinations performed during the first two trimesters when fundal height is usually below the umbilicus and IVC compression is minimal, patient positioning is rarely an issue. However, during the third trimester and at term an avoidance of supine positioning and subcostal imaging is recommended. If necessary the supine positioning should be limited to brief periods of time (<3–7 minutes).(5) Traditionally in both pregnancy and echocardiography, the left lateral decubitus position is preferred, though recent studies suggest that, if necessary, maternal cardiac output does not drop significantly in the right lateral position compared to the left.(9)
Transthoracic Echocardiography (TTE)
As in non-pregnant patients, TTE is the mainstay of cardiac imaging in the pregnant patient, and the indications for its use vary widely based on the clinical scenario. ACOG recommends a TTE be performed in all pregnant women with pulmonary hypertension, valvular disease, congenital heart disease or aortopathies (even if presumed corrected), cardiomyopathies, as well as those with a history of exposure to cardiotoxic chemotherapeutic agents.(10). Additional common uses include the evaluation of wall motion and ventricular function, as well as for serial monitoring of valvular and congenital disorders. An individualized approach to the timing of serial exams during pregnancy and in the postpartum period is recommended.(10)
There are remarkable physiologic adaptions in cardiac structure that occur in response to the increased plasma volume that is a hallmark of pregnancy (Figure 1).(11–13) Most initially become apparent in the second trimester and peak during the third trimester resulting in a pattern of concentric remodeling which should not be mistaken for pathology. A greater increase in left ventricular mass and relative wall thickness is seen in pregnancies affected by hypertensive disorders compared to normotensive pregnancies. (12) The effects of pregnancy on stenotic and regurgitant valvular diseases are reviewed in detail in Part 2 of this series.
Figure 1.
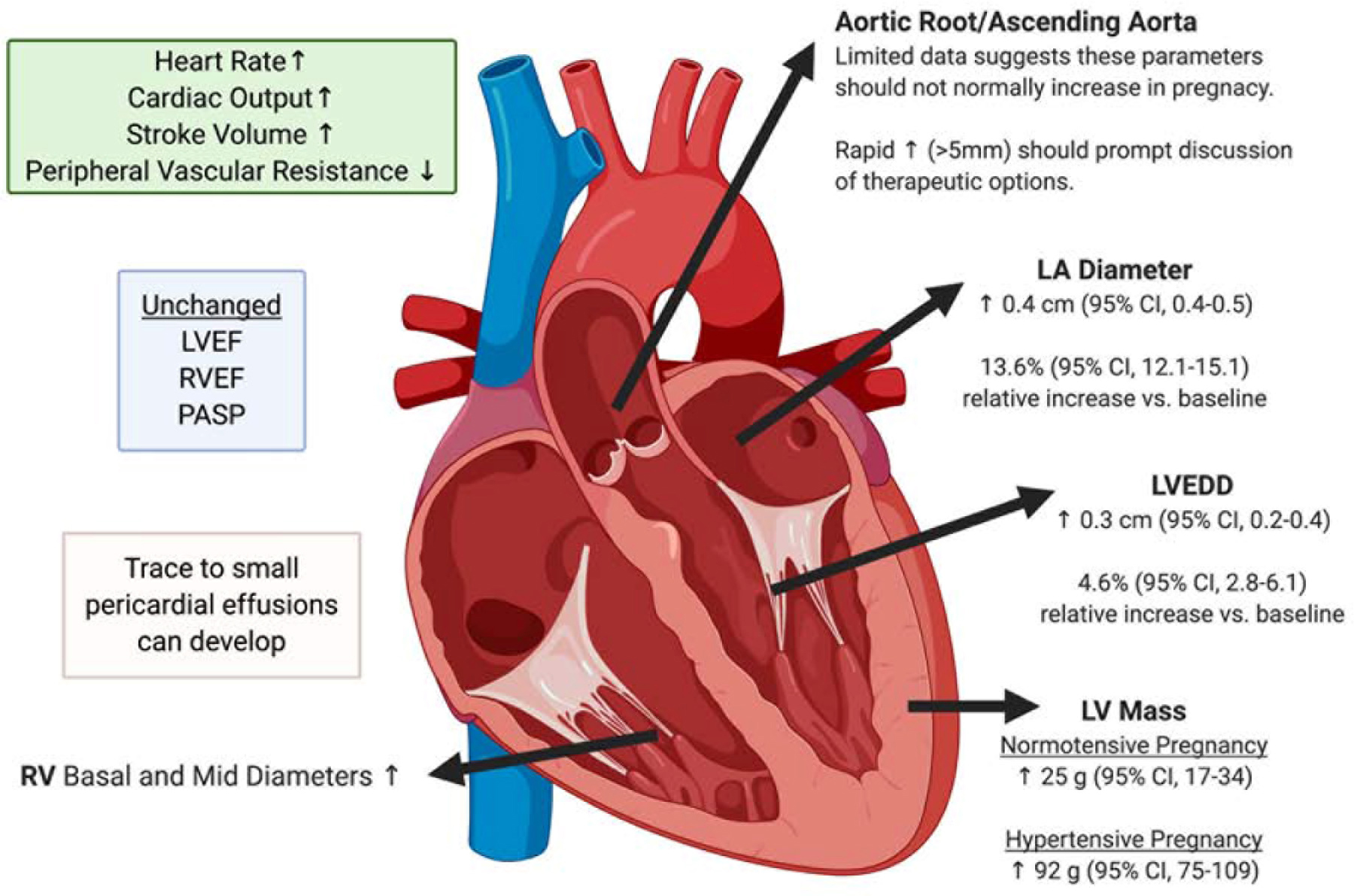
Changes to cardiac structure and function in pregnancy.
Expected alterations in commonly measured cardiac dimensions and hemodynamic parameters during pregnancy. CI = confidence interval; EDD = end diastolic diameter; EF = ejection fraction; g = grams; LA = left atrium; LV = left ventricle; PASP = pulmonary arterial systolic pressure; RV = right ventricle; ↑ indicates increase; ↓ indicates decrease. Figure created with BioRender.com
Transesophageal Echocardiography (TEE)
The elevation in progesterone during pregnancy decreases gastric motility and increases relaxation of the lower esophageal sphincter which in concert with increased intraabdominal pressure from a gravid uterus lead to a heightened risk of emesis and aspiration in pregnancy.(14) As a result, after 18 weeks gestation, anesthesiologists consider pregnant women’s fasting status to be “full stomach” regardless of the actual duration of fasting. While the potential risks and benefits must be weighed on an individual basis, endotracheal intubation is frequently recommended for TEE after the first trimester due to increased risk of aspiration of gastric contents in pregnant women, and consultation with a cardiac anesthesiologist is recommended. An additional maternal consideration when providing either general or monitored anesthesia care is the coupling of increased oxygen requirement/maternal hyperventilation with a reduced functional residual capacity can result in a very rapid onset of hypoxemia and acidosis during periods of apnea.
TEE is otherwise performed as in non-pregnant patients, though in late pregnancy care should be taken to ensure the patient is in the left lateral decubitus position and not supine to avoid unnecessary pressure on the IVC as previously discussed. Additional fetal concerns during the administration of anesthesia for a TEE are threefold and include potential teratogenesis from anesthetic agents, fetal hypoxia, and miscarriage/preterm birth. Midazolam is not recommended for use during the first trimester, though other sedatives and hypnotic agents can be used.(15) Maternal fetal medicine consultation for fetal monitoring should be considered if the fetus is a viable age (>22–24 weeks gestation).
Exercise Stress Testing
The use of submaximal exercise testing during pregnancy can be a useful assessment of cardiovascular response to exertion. In addition to the standard precautions and contraindications to exercise stress testing that apply to non-pregnant adults, Table 1 lists absolute and relative contraindications to exercise specific to pregnancy according to ACOG.(16) It should also be taken into consideration that non-weight bearing exercise on a recumbent bike is preferred to the treadmill, particularly in women who are unaccustomed to exertion. This will remove the influence of gestational weight gain and allow for better stability and reduction of fall risk. A minimum of 4 minutes of exercise are necessary for a diagnostic test, and the intensity of exercise should be individualized with subjective assessments of perceived exertion used to guide the testing. There are currently no normative values to identify a dysfunctional response to aerobic exercise in pregnant women and the use of standard age- and sex- matched referent values, or comparison to the patient’s pre-pregnancy response is recommended.(17, 18)
Table 1.
Contraindications to submaximal exercise stress testing in pregnant women
| Absolute |
|---|
| Persistent vaginal bleeding, especially in the 2nd and 3rd trimester |
| Incompetent cervix, history of cerclage placement |
| Known hemodynamically significant CVD |
| Multiple gestation |
| Placenta previa after 26 weeks |
| Preeclampsia/gestational hypertension |
| Preterm labor |
| Premature rupture of membranes/amniotic fluid leakage |
| Restrictive lung disease |
| Relative |
| Severe anemia |
| Bronchitis |
| Poorly controlled diabetes or hypertension |
| Dyspnea before exertion |
| Dizziness/presyncope |
Cardiac MRI
Cardiac MRI is used less frequently but can provide important supplement information about cardiac and in particular vascular structures and function. It is most commonly utilized in pregnant women to measure aortic dimensions,(19) though it is also useful for the assessment of wall motion and ventricular function when ultrasound is non-diagnostic. Similar to echocardiography, normative values for cardiac indices in pregnancy and the postpartum state have been published.(13) GBCAs are not necessary for the imaging of the aorta or the majority of other indications in pregnancy, and should be used with extreme caution and only in situations in which the potential benefit clearly justifies the potential risk to the fetus. The potential risks associated with GBCA use in pregnancy are discussed in further detail later in this review.
Cardiac Imaging that Utilizes Ionizing Radiation
Ionizing radiation includes gamma rays from nuclear medicine procedures such as single photon computed tomography (SPECT) or positron emission tomography (PET) and x-ray radiation from CT, general radiography, or fluoroscopy. The primary concern when exposing a pregnant woman to ionizing radiation at the levels expected from medical imaging is fetal exposure resulting in an elevated risk of childhood cancer, not teratogenesis.(20, 21) For exposure to a newborn child, the lifetime attributable risk of developing cancer is estimated to be 0.4% per 10 mGy (1 rad) dose to the baby. The potential risks in utero for the second and third trimesters and part of the first trimester may be comparable, but this is only an estimate.(22) The relative risk of childhood cancer in an irradiated fetus may be as high as two-fold, but this must be weighed against the failure to diagnose a potentially serious disease.(20) It is also important to keep in mind that the absolute risk to the fetus remains small (Table 2). Exposure to the fetus primarily occurs when the uterus is within the direct beam, though indirect scatter can also result in fetal exposure. From an Image Wisely Taskforce, the added cancer risk to the fetus following exposure to 10 mGy of radiation is estimated to be in the range of 0.022% to 0.06% (Table 3).
Table 2.
Potential fetal effects of radiation exposure and excess risk of cancer by fetal radiation dose.
| Dose (mGy) | Low-risk Model | Intermediate-risk Model | High-risk Model |
|---|---|---|---|
| 10 | 1 in 4545 | 1 in 3571 | 1 in 1667 |
| 20 | 1 in 2272 | 1 in 1786 | 1 in 834 |
| 30 | 1 in 1515 | 1 in 1190 | 1 in 556 |
| 40 | 1 in 1136 | 1 in 892 | 1 in 417 |
| 50 | 1 in 909 | 1 in 714 | 1 in 334 |
Table 3.
Summary of possible in-utero induced deterministic radiation effects by gestational age and radiation dosage.
| Gestational Age (weeks) | <50 mGy | 50–100 mGy | >100 mGy |
|---|---|---|---|
| 0–2 | None | None | None |
| 3–4 | Probably none | Possible spontaneous abortion | |
| 5–10 | Scientifically uncertain and probably too subtle to be clinically detectable | Possible malformation risk increases with increasing dose | |
| 11–17 | Risk of diminished IQ increases with increasing dose | ||
| 18–27 | None | IQ deficits not detectable at diagnostic doses | |
| >27 | None | None applicable to diagnostic medicine |
Radiation exposure exceeding a threshold of 50 mGy should be avoided in order to avert potential non-stochastic effects of radiation to the fetus.(23) Table 4 displays dose ranges associated with commonly performed cardiac diagnostic and therapeutic imaging techniques including chest x-ray, fluoroscopy, CT angiography, and ventilation-perfusion scan.(23, 24) For every patient, pregnant or not, the lowest possible exposure dose should be a goal (i.e., the principle of ALARA or As Low As Reasonably Achievable), and the Image Wisely website from the American College of Radiology has extensive material for dose modification suggestions for CT imaging of the pregnant patient.(22) The reductions in radiation exposure must be balanced to avoid reduced image quality whereby there is insufficient information for the diagnosis in question. Importantly, it is standard practice for all imaging laboratories to have operating procedures for exposing women of childbearing age to ionizing radiation. These should be added, if none currently exist. When time allows, consultation with a radiology or imaging specialist can be considered, particularly when considering the use of nuclear medicine compounds in pregnant or lactating women.(3, 25)
Table 4.
Average Radiation exposure from common cardiac imaging procedures
| Imaging Modality | Fetal Dose (mGy) |
|---|---|
| Ultrasound | 0 |
| MRI | 0 |
| CXR | 0.002–0.1 |
| CT Chest or CT pulmonary angiography | 0.03–0.66 |
| V/Q | 0.32–0.74 |
| Low dose perfusion scintigraphy | 0.1–0.5 |
| Fluoroscopy (diagnostic/therapeutic angiography, balloon valvuloplasty) | 3–20 |
| PET CT | 10–50 |
Cardiac Catheterization and Invasive Electrophysiology Procedures
An in-depth description of diagnostic and therapeutic cardiac procedures during pregnancy is covered in Part 3 of this series.
CVD Medication Use during Pregnancy and Lactation
While a comprehensive evaluation of all cardiovascular medications used during pregnancy is beyond the scope of this paper and was recently reviewed,(26) this section will highlight important physiologic effects of pregnancy on pharmacokinetics and pharmacodynamics and will discuss commonly used cardiovascular medications and contrast agents.
Pharmacokinetic Considerations for Pregnant and Lactating Women
Pregnancy is a hemodynamically complex period with marked increases in volume of distribution and cardiac output. As a result of these changes, as well as delays in gastric emptying, increased hepatic and renal clearance, and decreased albumin and plasma binding proteins, pharmacokinetics are altered in the pregnant state. Given the complex interplay between these variables, the effective dose of a drug during pregnancy may be higher or lower than in the non-pregnant state and may change throughout the course of pregnancy with increased clearance of certain medications requiring escalation of dose. Slow titration of medications, as well as use of the lowest possible effective dose are recommended to minimize adverse maternal and fetal effects of necessary medications.
Knowledge of the safety, efficacy and side effects of cardiac medications on both mother and fetus is essential when caring for pregnant and postpartum patients. The placenta is a complex organ that serves many roles including nutrient transfer, waste removal, and hormone secretion. While almost every drug administered to a mother eventually crosses the placenta, fetal drug concentration may be similar, higher, or lower than the maternal concentration. It is also important to note that in addition to direct fetal drug effects, medications may also impact the fetus through alterations of uteroplacental blood flow.(27) In 2015, the FDA implemented an updated Pregnancy and Lactation Labeling Rule eliminating the letter category system (A, B, C, D, and X). The new system requires summaries describing the risks and benefits of drugs and biological products when used in pregnancy and lactation to be described in the package insert.(28) LactMed, an online frequently updated database, is a useful reference for checking medication compatibility with lactation.(29)
Use of Common Cardiovascular Medications in Pregnant and Lactating Women
Hypertension and heart failure are the most commonly encountered cardiovascular diseases which require medication use in pregnancy, and they can be associated with significant risk to both mother and fetus. Several evidence-based medical therapies that are the foundation of treatment for non-pregnant patients are contraindicated for use in pregnancy due to their potential teratogenic effects during the first trimester. Table 5 lists recommended medications to treat these conditions. Anticoagulants are another commonly used medication class during pregnancy (Table 6). While many women receiving therapeutic anticoagulation during pregnancy have a chronic indication for its use, there will also be women who will require initiation of one of these agents during gestation. Additional information regarding anticoagulant use around the time of delivery can be found in Part 2 of this review series and a recent comprehensive review of venous thromboembolism associated with pregnancy.
Table 5.
Antihypertensives and heart failure medications in pregnancy
| Hypertension | Heart Failure |
|---|---|
| First Line | Metoprolol |
| Nifedipine ER | Carvedilol |
| Labetalol | Furosemide* |
| Alpha-methyldopa | Bumetanide* |
| Torsemide* | |
| Second Line | Metolazone* |
| Hydralazine | Hydralazine |
| Isosorbide dinitrate | Isosorbide dinitrate |
| Nitroglycerine | Nitroglycerine |
| Amlodipine | Dopamine |
| Furosemide* | Dobutamine |
| HCTZ* | Norepinephrine |
| Clonidine transdermal patch** | Digoxin |
| Contraindicated- ACE-I, ARBs, Direct Renin Inhibitors, Aldosterone Antagonists | Contraindicated- ACE-I, ARBs, Aldosterone Antagonists, Ivrabradine, Sacubitril/Valsartan |
Monitor for volume depletion to ensure adequate placental perfusion
Consider using in women with hyperemesis gravidarum who are unable to tolerate oral medications
Table 6.
Anticoagulants for use in pregnancy and lactation
| Advantages | Disadvantages | Special Consideration | Lactation | |
|---|---|---|---|---|
| Unfractionated heparin (UFH) | UFH does not cross the placenta, has an acute reversal agent (protamine) and is favored is patients with renal failure. | Requires frequent monitoring of PTT to determine therapeutic window. | UFH should be used 36 hours before induction or cesarean section since it has a shorter half-life than LWMH. UFH drip should be stopped 4–6 hours before anticipated delivery and restarted 6 hours after delivery if no bleeding complications. | UFH is not found in breast milk in any significant amount. There is no contraindication to its use in lactation. |
| UFH is also favored in patients with pulmonary embolism and hemodynamic compromise. | ||||
| Enoxaparin (LMWH) | Does not cross the placenta, and is convenient for outpatient use. | Requires twice daily injections | Metabolism is primarily by renal excretion and caution should be used in patients with impaired renal function. As pregnancy progresses there is altered metabolism and frequent dose adjustments may be required; follow peak and trough anti-Xa levels meticulously during pregnancy. | It is not found in breast milk in any significant amount therefore there is no contraindication to its use in lactation. |
| Lower risk of heparin induced thrombocytopenia, major bleeding and osteoporosis compared to UFH. | Cost | |||
| Fondaparinux | Fondaparinux is associated with minimal transplacental passage. Recommended by ACOG in the setting of heparin-induced thrombocytopenia or heparin allergy |
Little data on its use in pregnancy | ||
| Vitamin K Antagonists (VKA) | Pregnant women can be switched back to warfarin in the second and third trimester until delivery. | Crosses the placenta and is a known teratogen; administration >5 mg/day associated with neurodevelopmental deficits, fetal bleeding and miscarriage. | Women who are on VKAs prior to pregnancy generally need to be switched to LMWH as soon as pregnancy is confirmed with very few exceptions. An alternative approach is switching to LMWH prior to conception. | Warfarin is not found in breast milk in any significant amount and can be resumed postpartum. |
Contraindicated- DOACs Rivaroxaban crosses the placental barrier and is therefore contraindicated in pregnancy; other direct oral anticoagulants have not been evaluated. Pregnant women were excluded from direct oral anticoagulant trials. If a patient has an appropriate indication for anticoagulation, they should be switched to heparin products or warfarin during pregnancy and during lactation.
Use of Imaging Contrast Agents in Pregnant and Lactating Women
Agitated saline
The administration of intravenous agitated saline is routinely used in the non-pregnant adult to provide right heart contrast and is an integral part of the diagnostic workup of right to left cardiac shunting and evaluation of extracardiac shunt due to pulmonary arteriovenous malformation.(30) Because the air microbubbles are short-lived and diffuse into the lungs from the pulmonary circulation, their administration is quite safe in the absence of shunting. In pregnancy there is a concern that in the presence of a shunt the microbubbles of air could travel to the placenta resulting in placental infarction and fetal distress. In general, the recommendation is to defer the use of agitated saline contrast until the postpartum period, or to use alternative imaging modalities such as cardiac MRI without GBCA if needed to diagnose intra- and extra-cardiac shunting in pregnancy.(30)
Left ventricular (LV) ultrasound enhancing agents
LV contrast can be clinically useful to evaluate for intracardiac thrombus and to improve endocardial border definition and assessment of systolic function and regional wall motion. There is no empiric data about the safety of any of the three LV contrast agents – Definity™ (Lantheus Medical Imaging), Optison™ (GE Healthcare), or Lumason™ (Bracco Diagnostics Inc.) – in human pregnancy or lactation, though no evidence of fetal harm has been seen in animal studies.(31) Prescribing information suggests that due to their very short half-life relevant fetal exposure is not expected and recommend their use be considered only if clinically needed by the mother during pregnancy and lactation.(32–35)
Iodinated Contrast
Intravenous iodinated contrast can cross through the placenta and enter the fetal circulation and amniotic fluid; therefore, its use in pregnancy should be limited to situations in which the diagnostic information will alter care.(36) Importantly, intravenous contrast should not be withheld if an additional scan involving contrast might need to be performed in follow-up, thus exposing the woman and fetus to additional radiation. For breastfeeding women, ACOG recommends that breastfeeding be continued without interruption as these agents are excreted into the breast milk at very low levels.(3, 35) Oral contrast agents are not absorbed and, though rarely indicated for cardiovascular imaging procedures, do not have any potential for real or theoretical harm to a pregnant woman or her fetus.
Gadolinium-based Contrast Agents
Gadavist (Bayer Healthcare) (37) is the only contrast agent that is FDA approved for use specifically in cardiac MRI, though in practice many other GBCAs are used. There are no studies of Gadavist in pregnant women, and while it is unknown if Gadavist crosses the human placenta, other GBCAs are water soluble, do cross the placenta and transfer into the fetal circulation and amniotic fluid where they remain for an indefinite amount of time. Limited published human data on exposure to GBCAs during pregnancy did not show adverse effects in exposed neonates. Because GBCAs are water soluble there is little excretion into breast milk and breastfeeding should not be interrupted after GBCA administration.(3, 35)
Knowledge Gaps in the Care of Pregnant Women with CVD
Pregnant women and women of child-bearing age have often been under-represented in or excluded from CVD clinical trials (Figure 2).(38) This has led to persistent knowledge gaps in the CVD evidence base, both relating to the treatment of pregnancy-specific CVD as well as the treatment of CVD in women who are pregnant, postpartum, or of reproductive age;(38, 39) as exemplified throughout this review series by the paucity of large trials in the evidence base supporting the use of various pharmacologic therapies and diagnostics. The conduct of both clinical care and research on CVD in pregnancy is complex, but these complexities must be confronted, and the ethical obligation to improve care for pregnant women must not be ignored. In recognition of low levels of enrollment of women in clinical trials, in 1993 the NIH Revitalization Act (Public Law 103–43) was passed mandating the inclusion of women and minorities in all NIH-funded research, however this has does little to encourage the conduct of research specifically addressing CVD in the context of pregnancy.
Figure 2.
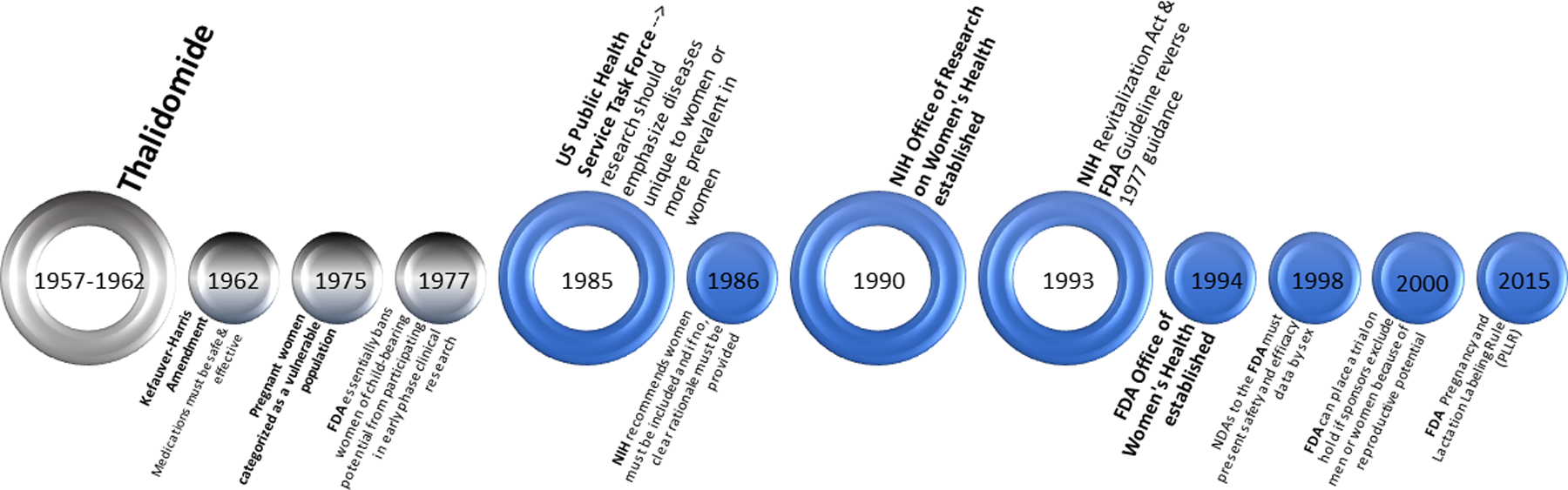
Timeline of Important U.S. Milestones Affecting Women’s Participation in Research. This timeline displays key milestones related to the inclusion or exclusion of women from participation as research subjects as well as important regulations related to the study of pregnancy and lactation. FDA = federal drug administration; NIH = national institutes of health
While pregnant women are ‘scientifically complex,’ secondary to physiologic differences intrinsic to the state of pregnancy and the potential for fetal harm,(40) they are not necessarily a ‘vulnerable’ population. Vulnerability implies a compromised ability to protect one’s interests and provide informed consent, a definition which the majority of pregnant women do not meet. It is also important to recognize that both involvement in clinical trials and the provision of nonevidence-based clinical care are not without risk. Thus, as the prevalence of CVD in pregnancy continues to grow over time, efforts to improve the quality of care delivered to these women must also intensify. The cost of an overly conservative approach is failure to improve care for anyone.(41)
Conclusions
The choice of diagnostic testing and therapeutic medications for pregnant and lactating women can be complex, but it is important to recognize that it is inappropriate to deem pregnant women a vulnerable population by default. When considering a non-elective diagnostic imaging procedure or therapeutic medication as part of the care for a pregnant or lactating woman, the choice of strategy must weigh both maternal and fetal/infant health and should factor in the patient’s preferences in a process of shared decision making. As described in this five-part series, delivering care to pregnant and lactating women is best done in the context of a cardio-obstetrics team-based setting. However, all clinicians should be familiar with the safety of frequently used diagnostic and therapeutic interventions for women with and at high risk for CVD to adequately care for this growing population.
Highlights.
Pregnant women represent a complex but not necessarily vulnerable population.
Ultrasound and magnetic resonance without gadolinium-based contrast are preferred over other imaging modalities for pregnant woman to avoid radiation exposure.
When imaging that involves ionizing radiation is necessary during pregnancy, the strategy should be designed to minimize exposure.
While almost every drug administered to a mother crosses the placenta, fetal drug concentration may be similar to, higher or lower than the maternal concentration.
Funding:
Bello – National Institutes of Health (NIH)/ National Heart Lung and Blood Institute (NHLBI) (K23 HL136853–03, R01 HL153382–01)
Quesada – NIH/NHLBI (K23 HL151867–01)
Volgman – NIH/ National Institute of Nursing Research (NINR) (R01 NR018443); Novartis TQJ230A12001, epidemiological study on lipoprotein a in patients with CVD
Abbreviations
- ACC
American College of Cardiology
- ACOG
American College of Obstetricians and Gynecologists
- AHA
American Heart Association
- ALARA
As Low As Reasonably Achievable
- CT
Computed Tomography
- CVD
Cardiovascular Disease
- GBCA
Gadolinium-Based Contrast Agents
- MRI
Magnetic Resonance Imaging
- LV
Left Ventricular
- TEE
Transesophageal Echocardiography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
Dr. Bairey Merz serves as Board of Director for iRhythm and receives personal fees paid through CSMC from Abbott Diagnostics, Sanofi. Dr. Brown is a co-author on Up to Date- Maternal Mortality. Dr. Volgman reports stock ownership in Apple Inc.
The other authors have nothing to disclose.
REFERENCES
- 1.Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;70(13):1647–72. [DOI] [PubMed] [Google Scholar]
- 2.Doherty JU, Kort S, Mehran R, Schoenhagen P, Soman P, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2019 Appropriate Use Criteria for Multimodality Imaging in the Assessment of Cardiac Structure and Function in Nonvalvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and the Society of Thoracic Surgeons. J Am Coll Cardiol 2019;73(4):488–516. [DOI] [PubMed] [Google Scholar]
- 3.Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 2017;130(4):e210–e6. [DOI] [PubMed] [Google Scholar]
- 4.Einstein AJ, Berman DS, Min JK, Hendel RC, Gerber TC, Carr JJ, et al. Patient-Centered Imaging: Shared Decision Making for Cardiac Imaging Procedures With Exposure to Ionizing Radiation. Journal of the American College of Cardiology 2014;63(15):1480–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard BK, Goodson JH, Mengert WF. Supine hypotensive syndrome in late pregnancy. Obstet Gynecol 1953;1(4):371–7. [PubMed] [Google Scholar]
- 6.Kinsella SM, Lohmann G. Supine hypotensive syndrome. Obstet Gynecol 1994;83(5 Pt 1):774–88. [PubMed] [Google Scholar]
- 7.Humphries A, Mirjalili SA, Tarr GP, Thompson JMD, Stone P. Hemodynamic changes in women with symptoms of supine hypotensive syndrome. Acta Obstet Gynecol Scand 2020;99(5):631–6. [DOI] [PubMed] [Google Scholar]
- 8.Humphries A, Mirjalili SA, Tarr GP, Thompson JMD, Stone P. The effect of supine positioning on maternal hemodynamics during late pregnancy. J Matern Fetal Neonatal Med 2019;32(23):3923–30. [DOI] [PubMed] [Google Scholar]
- 9.Humphries A, Thompson JMD, Stone P, Mirjalili SA. The effect of positioning on maternal anatomy and hemodynamics during late pregnancy. Clin Anat 2020;33(6):943–9. [DOI] [PubMed] [Google Scholar]
- 10.ACOG Practice Bulletin No. 212: Pregnancy and Heart Disease. Obstetrics & Gynecology 2019;133(5):e320–e56. [DOI] [PubMed] [Google Scholar]
- 11.Savu O, Jurcuţ R, Giuşcă S, Mieghem Tv, Gussi I, Popescu BA, et al. Morphological and Functional Adaptation of the Maternal Heart During Pregnancy. Circulation: Cardiovascular Imaging 2012;5(3):289–97. [DOI] [PubMed] [Google Scholar]
- 12.De Haas S, Ghossein-Doha C, Geerts L, van Kuijk SMJ, van Drongelen J, Spaanderman MEA. Cardiac remodeling in normotensive pregnancy and in pregnancy complicated by hypertension: systematic review and meta-analysis. Ultrasound Obstet Gynecol 2017;50(6):683–96. [DOI] [PubMed] [Google Scholar]
- 13.Ducas RA, Elliott JE, Melnyk SF, Premecz S, daSilva M, Cleverley K, et al. Cardiovascular magnetic resonance in pregnancy: insights from the cardiac hemodynamic imaging and remodeling in pregnancy (CHIRP) study. J Cardiovasc Magn Reson 2014;16(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang J, Streitman D. Physiologic Adaptations to Pregnancy. Neurologic Clinics 2012;30(3):781–9. [DOI] [PubMed] [Google Scholar]
- 15.Bonnet MP. Sedation and anaesthesia for non-obstetric surgery. Anaesth Crit Care Pain Med 2016;35 Suppl 1:S35–s41. [DOI] [PubMed] [Google Scholar]
- 16.ACOG Committee Opinion No. 650: Physical Activity and Exercise During Pregnancy and the Postpartum Period. Obstet Gynecol 2015;126(6):e135–42. [DOI] [PubMed] [Google Scholar]
- 17.Evenson KR, Barakat R, Brown WJ, Dargent-Molina P, Haruna M, Mikkelsen EM, et al. Guidelines for Physical Activity during Pregnancy: Comparisons From Around the World. Am J Lifestyle Med 2014;8(2):102–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meah VL, Backx K, Davenport MH. Functional hemodynamic testing in pregnancy: recommendations of the International Working Group on Maternal Hemodynamics. Ultrasound Obstet Gynecol 2018;51(3):331–40. [DOI] [PubMed] [Google Scholar]
- 19.Kuijpers JM, Mulder BJ. Aortopathies in adult congenital heart disease and genetic aortopathy syndromes: management strategies and indications for surgery. Heart 2017;103(12):952–66. [DOI] [PubMed] [Google Scholar]
- 20.https://www.imagewisely.org/imaging-modalities/computed-tomography/imaging-physicians/articles/the-pregnant-patient. Accessed on September 28, 2020.
- 21.Williams PM, Fletcher S. Health effects of prenatal radiation exposure. Am Fam Physician 2010;82(5):488–93. [PubMed] [Google Scholar]
- 22.https://www.acr.org/-/media/ACR/Files/Practice-Parameters/pregnant-pts.pdf. Acessed on October 2, 2020.
- 23.Wieseler KM, Bhargava P, Kanal KM, Vaidya S, Stewart BK, Dighe MK. Imaging in pregnant patients: examination appropriateness. Radiographics 2010;30(5):1215–29; discussion 30–3. [DOI] [PubMed] [Google Scholar]
- 24.Tremblay E, Thérasse E, Thomassin-Naggara I, Trop I. Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics 2012;32(3):897–911. [DOI] [PubMed] [Google Scholar]
- 25.Jain C ACOG Committee Opinion No. 723: Guidelines for Diagnostic Imaging During Pregnancy and Lactation. Obstet Gynecol 2019;133(1):186. [DOI] [PubMed] [Google Scholar]
- 26.Halpern DG, Weinberg CR, Pinnelas R, Mehta-Lee S, Economy KE, Valente AM. Use of Medication for Cardiovascular Disease During Pregnancy: JACC State-of-the-Art Review. J Am Coll Cardiol 2019. February 5;73(4):457–476. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths SK, Campbell JP. Placental structure, function and drug transfer. Continuing Education in Anaesthesia Critical Care & Pain 2014;15(2):84–9. [Google Scholar]
- 28.Food and Drug Administration. Federal Register. Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. 79 FR 72063 [online]. Federal Register 2014;79(9233 Pt. 2):72064–72102. https://www.federalregister.gov/documents/2014/12/04/2014-28241/content-and-format-of-labeling-for-human-prescription-drug-and-biological-products-requirements-for#citation-3-p72066. Accessed on October 8, 2020. [PubMed] [Google Scholar]
- 29.Drugs and Lactation Database (LactMed) [Internet] Bethesda (MD): National Library of Medicine (US); 2006-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK501922/. Accessed on October 5, 2020. [Google Scholar]
- 30.Mitchell C, Rahko PS, Blauwet LA, Canaday B, Finstuen JA, Foster MC, et al. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J Am Soc Echocardiogr 2019;32(1):1–64. [DOI] [PubMed] [Google Scholar]
- 31.Porter TR, Mulvagh SL, Abdelmoneim SS, Becher H, Belcik JT, Bierig M, et al. Clinical Applications of Ultrasonic Enhancing Agents in Echocardiography: 2018 American Society of Echocardiography Guidelines Update. J Am Soc Echocardiogr 2018;31(3):241–74. [DOI] [PubMed] [Google Scholar]
- 32.https://www.definityimaging.com/pdf/DEFINITY%20Marketing%20PI%205159870820.pdf Accessed on January 12, 2021.
- 33.http://www3.gehealthcare.com/~/media/documents/MarketoPDFsnogating/OPT-1H-OSLO_Optison_BK#:~:text=Optison%20is%20indicated%20for%20usetlveb. Accessed on September 23, 2020.
- 34.https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/203684s002lbl.pdf. Accessed on September 23, 2020.
- 35.Cova MA, Stacul F, Quaranta R, Guastalla P, Salvatori G, Banderali G, et al. Radiological contrast media in the breastfeeding woman: a position paper of the Italian Society of Radiology (SIRM), the Italian Society of Paediatrics (SIP), the Italian Society of Neonatology (SIN) and the Task Force on Breastfeeding, Ministry of Health, Italy. Eur Radiol 2014;24(8):2012–22. [DOI] [PubMed] [Google Scholar]
- 36.Webb JA, Thomsen HS, Morcos SK, Members of Contrast Media Safety Committee of European Society of Urogenital R. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol 2005;15(6):1234–40. [DOI] [PubMed] [Google Scholar]
- 37.https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/201277s000lbl.pdf. Accessed on September 23, 2020.
- 38.Liu KA, Mager NA. Women’s involvement in clinical trials: historical perspective and future implications. Pharm Pract (Granada) 2016;14(1):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.https://orwh.od.nih.gov/toolkit/recruitment/history#:~:text=NIH%20ensures%20that%20women%20and,minorities%20differently%20than%20other%20participants. Accessed on September 28, 2020.
- 40.ACOG Committee Opinion No. 646: Ethical Considerations for Including Women as Research Participants. Obstet Gynecol 2015;126(5):e100–7. [DOI] [PubMed] [Google Scholar]
- 41.Lyerly AD, Little MO, Faden RR. Reframing the framework: toward fair inclusion of pregnant women as participants in research. Am J Bioeth 2011;11(5):50–2. [DOI] [PubMed] [Google Scholar]


