Abstract
Objective:
Stroke is a debilitating disorder with significant annual mortality and morbidity rates worldwide. Immune cells are recruited to the injured brain within hours after stroke onset and can exhibit either protective or detrimental effects on recovery. However, immune cells, including CD8 T cells, persist in the injured brain for weeks, suggesting a longer-term role for the adaptive immune system during functional recovery. The aim of this study was to determine if the delayed secondary diapedesis of CD8 T cells into the ischemic brain negatively impacts functional recovery after transient ischemic stroke in male mice.
Results:
Mice exhibited an increased number of leukocytes in the ipsilesional hemispheres at 14 days (3-fold; p < 0.001) and 30 days (2.2-fold; p = 0.02) after transient middle cerebral artery occlusion (tMCAo) compared to 8 days post-tMCAo, at which time acute neuroinflammation predominantly resolves. Moreover, mice with higher ipsilesional CD8 T cells at 30 days (R2=0.52, p<0.01) exhibited worse functional recovery. To confirm a detrimental role of chronic CD8 T cell diapedesis on recovery, peripheral CD8 T cells were depleted beginning 10 days post-tMCAo. Delayed CD8 T cell depletion improved motor recovery on the rotarod (F(1,28)=4.264; p=0.048) compared to isotype control-treated mice. CD8 T cell-depleted mice also exhibited 2-fold (p< 0.001) reduced leukocyte infiltration at 30 days post-tMCAo. Specifically, macrophage, neutrophil, and CD4 T cell numbers were reduced in the ipsilesional hemisphere of the CD8 T cell-depleted mice independent of inflammatory status of the post-stroke CNS (e.g. microglial phenotype and cytokine production). RNAseq identified a unique profile for brain infiltrating CD8 T cells at 30 days post-tMCAo, with 46 genes differentially expressed relative to CD8 T cells at 3 days post-tMCAo.
Conclusion:
Our data reveal a role for CD8 T cells in the chronic phase post-stroke that can be therapeutically targeted. We demonstrate long-term CD8 T cell recruitment into the ipsilesional hemisphere that affects both immune cell numbers present in the injured brain and functional recovery through one month after stroke onset.
Keywords: Stroke, transient middle cerebral artery occlusion, CD8 T cells, inflammation, motor deficits, functional recovery
Introduction:
Stroke is a neurological disease that occurs when the blood supply to the brain is disrupted and is a leading cause of global morbidity and fatality (1). Currently, the therapeutic treatments available for stroke injury are limited and delivery is confined to the first 24 hours of stroke onset (2, 3). However, functional recovery after stroke extends for weeks to months, and patients partially or fully recover depending on the location, size, and severity of the lesion, as well as life-style factors (4). Therefore, identifying mechanisms that contribute to the evolution of stroke-related injury and repair—occurring over the course of weeks to months—could expand the available therapeutic interventions and the window of treatment beyond the initial few hours.
Inflammatory responses to tissue injury have been implicated as important pathological mechanisms early after stroke. However, studies demonstrate the persistence of immune cells for months after stroke injury in patients (5, 6). Specifically, significant numbers of adaptive immune cells, which can respond in a central nervous system (CNS) antigen-specific manner, are present in the ipsilesional hemisphere in the chronic phase after stroke (6). T cells, as well as activated and isotype-switched B cells, are also present in the lesion site, as well as sites of secondary degeneration, for weeks after experimental stroke in mice (5, 7, 8). Moreover, B cell-deficient mice exhibiting delayed impairments in cognition still exhibit consistent T cell egress, highlighting the complicated interplay between adaptive immune subsets (5). T cells secreting pro-inflammatory cytokines such as interleukin (IL)-17 are present in peripheral blood of stroke patients for months after stroke onset (6, 9), with IL-17 associated with poorer cognitive status and decreases in anti-inflammatory IL-10 with depressive symptoms (10). Finally, we and others have also directly confirmed a role for long-term adaptive immune populations in mediating motor recovery, with post-stroke interventions that modulate T cells showing efficacy in improving fine motor skill recovery over weeks after stroke onset in mice (11–14).
With conclusive evidence of significant immune cell numbers in the late phase of stroke recovery, it is still unclear which subset(s) are beneficial or detrimental to functional recovery. Besides the deleterious effects in acute phase following experimental transient middle cerebral artery occlusion (tMCAo), T cells are also beneficial in controlling inflammation, enhancing post-tMCAo repair, and promoting clearance of injured tissue debris during the chronic phases of recovery (8, 15, 16). While regulating acute phase neuroinflammation is an obvious beneficial therapeutic target, understanding long-term neuroinflammation that occurs during neuronal plasticity could also prove beneficial to post-stroke functional recovery. The goal of this study was to investigate the role of long-term post-tMCAo CD8 T cell immune responses in the recovering brain. We established chronic inflammation in the post-tMCAo brain weeks after tissue injury, and functionally characterized these chronic CD8 T cells using RNA sequencing. We used a targeted antibody-mediated CD8 T cell depletion model to assess behavioral recovery in the presence and absence of long-term post-tMCAo CD8 T cells. Finally, we determined the effect of CD8 T cells on microglial activation, CNS cytokine production, and recruitment of other immune cells in the post-tMCAo brain. Collectively, our findings demonstrate a role for CD8 T cells in the chronic phase of post-stroke functional recovery. Furthermore, these observations strongly implicate targeting long-term post-stroke adaptive immune cells for improving functional recovery after stroke.
Materials and methods
2.1. Animals:
Mouse husbandry and animal procedures were completed in conformation with guidelines and approved protocols by the Institutional Animal Care and Use Committee at UT Southwestern Medical Center. All experiments used male C57BL/6 mice (1.5 to 4 months old). Mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) or obtained from breeding pairs. Mice were maintained at the UT Southwestern Medical Center Animal Resource Center, in accordance with NIH guidelines for the care and use of laboratory animals. The breeding pairs were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and only mice within 5 generations of the delivered founders were used for experiments. Animals were maintained in standard animal housing with 12/12 light cycles (lights off at 6:00 PM), and food and water ad libitum.
2.2. Stroke surgeries (17):
Mice were anesthetized using 2% isoflurane/70% NO2/30% O2 during surgeries, with Buprenorphine and lidocaine used as analgesics, and body temperature was maintained at 37°C. Before and after transient middle cerebral artery occlusion (tMCAo) procedure, left middle cerebral artery was exposed to quantify cerebral blood flow (CBF) using transcranial laser Doppler flowmetry (Moore Instruments). Surgeons blinded to the cohort groups occluded the middle cerebral artery by advancing a silicon-tipped filament (6.0-gauge nylon, 12 mm), resulting in a CBF reduction of >80% (relative to baseline blood flow) deemed to be a successful occlusion. The occlusion period was 60 mins for all experimental cohorts and post-suture removal CBF values >50% of baseline pre-occlusion CBF were considered successful reperfusions. All surgeries were performed between 8-14:00 hours, with 10 mice/surgeon/day maximum. In total, 191 mice were used in experiments, with 175 mice undergoing stroke surgery. 61 mice were excluded, including 23 for poor blood flow and 34 for death during or after surgery.
2.3. Infarct volume quantification
2.3.1. Magnetic resonance imaging (MRI) was used for quantification of infarct volume in vivo at 6 days after tMCAo surgery.
Anesthetized animals were imaged in a supine position using a 7-Tesla small animal MRI system (Bruker, Rheinstetten, German), with a 400 mT/m gradient coil set and a 40-mm millipede coil (ExtendMR LLC, Milpitas, CA). Respiratory rates were monitored throughout the imaging session using respiratory sensor (SA Instruments, Stony Brook, NY). Parameters used for the imaging: slice thickness = 1mm, matrix size = 256 X 256, repetition time/echo time = 2500/60 ms, averages = 8, field of view = 25.6 X 25.6 mm, total scan time = 10 minutes and 45 seconds, and in-plane resolution = 100 μm. Fiji software (Image J; National Institutes of Health, Bethesda, MD) was used to determine hyper-intense areas in the axially obtained T2-weighted high-resolution images of the entire brain. Infarct volume was calculated by summing up all those hyper-intense areas and times the slice thickness (1 mm).
2.3.2. Cresyl violet staining was used for visualizing injured areas in the brain sections.
Brains were dissected after perfusion with 4% PFA and stored in 15% sucrose solution for 48hrs, followed by 30% sucrose solution until sectioning. Serially dissected 30μm thick brain sections were stained with cresyl violet dye. 40x digital images of the sections were obtained using whole slide imaging (Nanozoomer 2.0HT, Hamamatsu Photonics, Hamamatsu-shi, Japan). Infarcted tissue was defined as areas with pyknotic cells and infarct volumes were quantified using stereology software (Stereo Investigator, Microbrightfield Biosciences, Williston, VT). The Cavalieri Estimator probe was used for estimating the volume in a user defined region of interest.
2.4. Behavioral assessment
2.4.1. Rotarod behavioral test was used to quantify motor coordination.
Mice were allowed to stabilize their posture on a 10-inch elevated metal rod 1.25 inches in diameter with five semi-closed lanes (IITC Life Science, Woodland Hills, CA). Mice were consistently preferentially faced away from the investigator while walking. Parameters for rotarod testing were as follows: start speed of 4 rpm, acceleration rate of 0.2 rpm/s, top speed of 44 rpm, and maximum test duration of 300 seconds. End times were noted when the mouse fell from the rod or gripped the rod without moving. Average times from four trials were taken per day for each mouse.
2.4.2. Open-field behavioral test was used to assess locomotion and spontaneous activity.
Mice were placed individually in a single chamber open field made of transparent glass material. The chamber was thoroughly cleaned with 70% ethanol before each trial. The wooden base was divided into 9 large squares for quantification purposes. Observations from each mouse were recorded for a total of 10 mins. The attributes quantified for this study were number of crossings, rearings and grooming behavior and were manually evaluated from the recorded videos.
2.5. Flow cytometry:
Animals were sacrificed with isoflurane overdose and transcardially perfused with 30 ml 0.01M PBS prior to brain dissection. Brain hemispheres were dissociated using 70 μm mesh strainers and immune cells were isolated using Percoll gradient. Spleens were dissected prior to perfusion and also dissociated using 70 μm mesh strainers. The dissociated cells were washed with cell culture media and layered on top of Lympholyte-M cell separation media. The layer of leukocytes was collected, and hemocytometer count was quantified for all samples. Single cell suspensions of brains and spleens (106 cells per sample) were processed through fluorescence-activated cell sorting (FACS) using the following antibodies: CD45-APC-Cy7, BioLegend, 103116; TCRβ-BV510, BioLegend, 109233; CD19-PE, Tonbo biosciences, 50-0193-U100; CD8a-PE-Cy5, Tonbo biosciences, 55-0081-U100; CD4-PE/Dazzle 594, BioLegend, 100456; NK1.1-PerCP-Cy 5.5, Tonbo biosciences, 65-5941-U100; CD11b-APC, Tonbo biosciences, 20-0112-U100; Ly-6G and Ly-6C-FITC, BD biosciences, 553127. In Fig. 1 experiments, CD19-A700 fluorophore was used, but staining was not successful due to quality issues with the antibody, so no B cell data were acquired. Cells were incubated with blocking solution (FcRgII/III; BD Biosciences), followed by incubation with antibodies for 30 minutes at 4°C and fixed in 1% paraformaldehyde post-staining. For intracellular cytokine staining, the cells were treated to an additional permeabilization solution incubation after staining with surface antibody markers. The cells were then incubated with the corresponding cytokines specific antibody mix. All samples were analyzed using BD FACS Canto or Fortessa instrument (BD Biosciences, San Jose, CA). FlowJo 9.0 software (Tree Star, Ashland, OR) was used to gate cells into different leukocyte subsets. Hemocytometer counts were used to quantify absolute numbers.
Figure 1. Immune cell numbers are higher in the chronic phase post-stroke brain and correlate with behavioral recovery post-stroke.

(A) Total leukocytes; (B) CD4 T cells; (C) CD8 T cells increased at 14 days (blue circles) and 30 days (orange downward triangles) after tMCAo compared to either 8 days post-tMCAo (green upward triangles) or sham controls (red circles). (D) Schematic diagram for experimental setup; (E) Rotarod behavior of post-stroke mice (red dashed line, day of tMCAo); Correlation between rotarod behavior and (F) CD4 T cell numbers and (G) CD8 T cell numbers; (n = 6 to 12 per group). * = p<0.05; ** = p<0.01; *** =p<0.001; **** = p<0.0001 vs. contralesional hemisphere unless indicated by brackets. A-C, two-way ANOVA, Benjamini post-hoc; F-G, linear regression.
2.6. CD8 T cell depletion:
CD8α-specific antibodies (400μg, 2.43 clone, #BE0061, BioXCell) and the corresponding isotype control antibodies (400μg, Rat IgG2b, #BE0090, BioXCell) were diluted in sterile PBS (18) immediately prior to intraperitoneally injection 10 days after mice underwent tMCAo surgeries. Both antibody-treated cohort and isotype control-treated cohort received booster doses every 4 days (200ug, sterile PBS) to maintain CD8 T cell depletion.
2.7. RNA sequencing:
CD8 T cells were flow sorted (CD8a-PE-Cy5, Tonbo biosciences, 55-0081-U100; CD45-APC-Cy7, BioLegend, 103116; TCRβ-BV510, BioLegend, 109233) into lysis buffer from post-tMCAo brains. Samples were processed using services of Omega Bioservices. Clontech SMART-Seq_v4 Ultra Low Input RNA was used for library preparation. All samples were sequenced with 150bp paired-end reads. Approximately, 40 million reads were sequenced per library.-The Fastq files were subjected to quality check using fastqc (version 0.11.2, http://www.bioinformatics.babraham.ac.uk/projects/fastqc) and fastq_screen (version 0.4.4, http://www.bioinformatics.babraham.ac.uk/projects/fastq_screen), Fastq files were mapped to Mus musculus reference genome (mm10, UCSC version from igenomes) using STAR (19). Read counts were generated using featureCounts (20) and the differential expression analysis was performed using edgeR (21). Statistical cutoffs of FDR < 0.05, log2CPM > 0 were used to identify differentially expressed genes. Pathway and network analysis were performed using Qiagen’s IPA tool (www.qiagen.com/ingenuity ). Heat maps were generated using R (v3.5.2). The RNA-seq data has been deposited to the Gene Expression Omnibus (GSE155480).
2.8. Mouse Brain Protein Preparation and MULTI-SPOT Assay System Assay (MSD) V-Plex:
Unfrozen (collected and stored −80°C) hemispheres (no cerebellum and olfactory bulb) were homogenized (QUSONICA: 30s, pulse: 1s, amplitude 40%) on ice in 1ml buffer (M-PER Mammalian Protein Extraction Reagent – Thermoscientific cat# 78505 and Protease and Phosphatase inhibitor Thermoscientific cat# 78443 (1:100)) then centrifuged (15min, 4°C, 10,000xg). Supernatant was removed to a new tube, save pellet. Supernatant was centrifuged to remove debris at 4°C, max speed (~21,000xg) for 10 min. BCA assay determined protein concentration and all samples were normalized to 1mg/ml protein in diluent 41 (from the kit), and loaded 50ul per well. Each sample along with the standard curve were run in duplicate on each plate. The sample incubation step was run overnight at 4 degrees for a total of 24 hours. All other steps were as directed in the protocol. Cytokine Panel 1 (mouse) Kit VPLEX (#K15048D (IL-9, MCP-1, IL-33, IL-27p28/IL-30, IL-15, IL-17A/F, MIP-1a, IP-10, MIP-2) and Proinflammatory panel 1(mouse) Kit VPLEX (#K15245D) (IFN-g, IL-1b, IL-2, IL-4, IL-5, IL-6, KC/GRO, IL-10, IL-12p70, TNF-a). For final analyses in hemispheres harvested 30 days post-tMCAo, the following analytes were below the limit of assay detection: IFN-γ, IL-10, IL-12 p70, IL-2, IL-4, IL-5, IL-15, IL-17A/F, IL-27p28/IL-30, and IL-9.
2.9. Immunohistochemistry:
2.9.1. CD8α staining:
Brain sections mounted on slides were processed for antigen unmasking by using H-3300 Vector Antigen Unmasking Solution. Slides were blocked with blocking solution (3% Normal Rabbit Serum, 0.3% Triton-X 100, in 1x PBS) and incubated overnight with primary antibody anti-CD8α (1:100, Thermofisher #2025-05-31) for staining. Sections were then stained with secondary antibody biotinylated anti-Rat-IgG in 1.5% NRS for 60 mins at room temperature. Treatment with Avidin-biotin complex and DAB peroxidase substrate was used to visualize the staining. 40x digital images of the sections were obtained using whole slide imaging (Nanozoomer 2.0HT, Hamamatsu Photonics, Hamamatsu-shi, Japan) and positive cells were manually quantified using Image J software.
2.9.2. Microglial phenotyping:
100μm coronal sections (free floating) were washed, blocked and incubated over night with primary antibody, mouse anti-iNOS (1:200, Novus #NBP2-22119), rabbit anti-Arg1 (1:100, Novus, #NBP1-54621) at 4°C. Next day secondary antibodies antimouse Alexa-fluor 488, (1:200, Thermofisher #A-11001) and anti-rabbit Alexa-fluor 647, (1:200, Thermofisher #A-21245) were used, 2h RT. Section were washed, blocked and incubated with rabbit anti-Iba1 (1:200, Wako #019-19741). Secondary antibody anti-rabbit Alexa-fluor 594 (1:200, Thermofisher #A-110121) was used. After washing, nucleus was stained using DAPI (NucBlue, Invitrogen #R37605). Sections were transferred onto microscope slides and mounted with Fluoromount: VectaShied Hard set (Vector laboratories, H-1400). The slides were then scanned with the Zeiss Axio Scan Z1 digital slide scanner (Carl Zeiss Microscopy, Jena, Germany). These scans were analyzed with the HALO image analysis platform (Indica labs, Albuquerque, New Mexico, USA) shared kindly by Dr. Keith R. Pennypacker. Analysis settings were based on Indica Labs Area Quantification FL v1.2. The infarct was outlined and 3 layers were chosen 300μm away from the infarct. The Iba1 immuno-positive area was tagged with Alexa Fluor 594. The iNOS immune-positive area was tagged with Alexa-fluor 488, while the Arg1 positive area was tagged with Alexa-fluor 647. Each representative square had a uniform area of 65,025μm2. The Area Quantification FL v1.2 program in HALO then identified the positive signal and identified colocalization in the images. On the ipsilesional (injured) side, the average of 3 analysis squares was taken. One square on the contralesional (uninjured) side was analyzed for comparison. Please note confocal images shown in Fig. 5 used Alexa-350 as the Arg-1 secondary.
Figure 5. CD8 T cells depletion did not have an effect on the microglial activation at 30 days post-stroke.

Activated microglia were quantified in (A) contralesional hemispheres and ipsilesional hemispheres of (B) CD8 T cell-depleted mice (orange downward triangles), and Isotype (Iso)-treated control mice (blue circles). Quantification of activated microglia at different distances from the infarct (n = 6 or 7) show no effect on morphology with CD8 T cell depletion beginning 10 days post-tMCAo. (C-F) Quantification of (C) iNOS and (D) Arginase (Arg)-1 colocalization with Iba1 in adjacent sections show no differences in phenotype between cohorts. (G-J) Cytokines and chemokines were quantified by MSD V-plex assay in hemispheres of CD8 T cell-depleted and Iso-treated mice 30 days after tMCAo. While ipsilesional levels were significantly elevated over contralesional, there again was no effect of CD8 T cell depletion on CNS production of cytokines and chemokines. * = p<0.05; ** = p<0.01; *** =p<0.001; vs. contralesional values. (A) Two-way repeated measure ANOVA, Benjamini post-hoc; (E-H) Two-way ANOVA, Benjamini post-hoc; (I,J) Mann-Whitney t-test
2.10. Statistical Analysis:
All experiments were conducted in a randomized and blinded fashion and all data analyzed by blinded observers. The data sets were analyzed for normality, and parametric or nonparametric post hoc statistical analysis was used correspondingly to confirm a priori power calculations. GraphPad Prism 7.0 software (La Jolla, CA) was used for analysis, and values with p<0.05 were a priori termed significant. The data are reported as mean ± SD. Statistical tests include Two-way ANOVA, Benjamini post-hoc (flow cytometry and immunostaining data); linear regression (motor coordination vs. cell number), non-parametric Mann-Whitney t-test (cytokines, flow cytometry), or repeated measures Two-way ANOVA (rotarod data with/without CD8 T cell depletion), as also indicated in figure legends.
Results
3.1. Temporal distribution of immune cells in the post-tMCAo brain:
Immune cell numbers were quantified in the post-tMCAo brain at different time points (8 days, 14 days, and 30 days) after stroke injury. Increased numbers of CD45+ leukocytes were present in the ipsilesional hemispheres at 14 days (3-fold; p<0.001) and 30 days (2.2-fold; p=0.02) after stroke compared to both the ipsilesional hemispheres 8 days after stroke, or to corresponding contralesional hemispheres (Fig. 1A). Ipsilesional leukocyte populations were also significantly higher than the leukocytes present in naïve brain hemispheres, with the values at day 8 post-tMCAo only slightly higher than our previous quantification at day 2 post-tMCAo (17). CD4 T cell numbers were higher in the ipsilesional hemispheres at 14 days (5.45-fold; p<0.001) and 30 days (4.2-fold; p=0.002) compared to 8 days post-tMCAo (Fig. 1B). CD8 T cells were also increased in the ipsilesional hemispheres at 14 days (2.8-fold; p<0.001) and 30 days (1.9-fold; p=0.04) compared to 8 days after tMCAo (Fig. 1C). These results demonstrate that there is a secondary diapedesis of T cells into the brain during the chronic phase post-stroke elevated above either acute (i.e. day 2, (17)) or day 8 post-tMCAo values. Within the same brains, monocytes, macrophages and NK cells peaked at 14 days post-tMCAo, though neutrophil counts remained elevated through 30 days post-tMCAo (Supplemental Fig. 1).
3.2. CD8 T cells negatively correlate with functional recovery after stroke:
To determine how long-term T cell diapedesis affected motor recovery, post-tMCAo motor deficits were quantified using rotarod behavioral test in mice with infarction confirmed by MRI day 6 post-tMCAo (Fig. 1D). Mice were trained for 2 weeks prior to tMCAo (Fig. 1E), and motor function was tested through 4 weeks post-tMCAo. Mice with greater diapedesis of CD4 T cells (Fig. 1F; R2=0.33, p=0.05) and CD8 T cells (Fig. 1G; R2=0.52, p=0.008) in the ipsilesional hemispheres at 30 days after stroke exhibited worse functional recovery. Furthermore, T cell diapedesis at 30 days post-tMCAo only correlated with motor coordination at 27 days, though there was a trend for functional recovery at earlier testing days post-tMCAo for CD8 T cells (R2=0.30 p=0.06; Supplemental Fig. 2B). This correlation between T cell diapedesis and motor impairment was independent of early infarct volume, as neither CD4 T cell (R2=0.11, p=0.30) or CD8 T cell (R2=0.01, p=0.73) diapedesis at 30 days was linked to severity of infarction at day 6 post-tMCAo MRI (Supplemental Fig. 2C,D), though it should be noted that infarct volume was not assessed in this cohort at 30 days post-stroke. Interestingly, day 6 infarction did not correlate with motor recovery assessed on day 7 (Supplemental Fig. 2E), suggesting a strong influence of neuroinflammation on long-term behavioral outcomes.
3.3. Localization of CD8 T cells in the post-tMCAo brain:
Few prior studies have focused on long-term CD8 T cell activation after stroke, though our prior work shows significant CD8 T cell diapedesis, as well as CD8 T cell autoreactivity to CNS antigens in both mice and pediatric stroke patients on life support (22–24). As this was the population significantly associated with functional deficits (Fig. 1G), we quantified post-tMCAo CD8 T cells in brain sections to determine the distribution pattern of long-term diapedesis. Infarcted tissue was first identified and outlined using cresyl violet to identify areas of neuronal death in adjacent serial sections (Fig. 2A, B). The majority of the CD8 T cells localized within the infarcted tissue and were not distributed evenly or randomly throughout the entire brain parenchyma of infarcted sections (Fig. 2C). In fact, within the ipsilesional hemisphere, 86% of CD8 T cells were significantly localized within the infarct compared to non-infarct ipsilesional (p=0.06) and contralesional (p=0.049) brain tissue. Quantification of CD8 T cells through 8 weeks following tMCAo show higher numbers of CD8 T cells in the ipsilesional hemispheres at 4 weeks (113-fold; p<0.001) and 8 weeks (71-fold; p< 0.001) after stroke compared to uninjured control (Fig. 2D). CD8 T cell numbers were also higher in the ipsilesional hemispheres compared to the corresponding contralesional hemispheres at 4 weeks (21-fold; p<0.001) and 8 weeks (39-fold; p<0.001) after stroke, consistent with our flow cytometry results. As expected, mice with higher infarct volumes had more CD8 T cell numbers in the brain tissue at 2 weeks (R2=0.85, p=0.009) and 8 weeks post-tMCAo (R2=0.51, p=0.048). However, we did not observe this correlation at the 4 weeks post-tMCAo time point (data not graphed).
Figure 2. CD8 T cells in the 30 days post-stroke brain are localized in the infarcted area.

Representative whole brain images of (A) CD8α+ cells, (B) cresyl violet staining, and magnified (C) infarct region, non-infarct region, and contralesional region at 10X magnification show CD8 T cells localize to the infarct. (D) CD8α+ T cells increase in the ipsilesional brain post-stroke (red circles) compared to contralesional cortex (green squares; n = 6 to 10 per group). *** =p<0.001; **** = p<0.0001 vs. contralesional hemispheres unless indicated by brackets. Two-way ANOVA, Benjamini post-hoc.
3.4. Depletion of the secondary wave of CD8 T cells improves functional recovery post-tMCAo:
While it is well-known that T cells, including CD8 T cells, contribute to acute neurologic injury after stroke in the first hours to days (25), we wanted to assess whether the secondary wave of CD8 T cell diapedesis beginning ~2 weeks after tMCAo exhibited an independent pathological role in recovery. To this end, a selective antibody-mediated depletion model was used with CD8α or isotype IgG control antibodies, with administration beginning 10 days post-tMCAo (Fig. 3A)(26). Mice underwent 2 weeks of rotarod training before tMCAo surgeries and motor recovery was assessed through 4 weeks post-tMCAo. The open field test measured anxiety and was performed at day 27 post-tMCAo. Mice were distributed evenly into isotype control and anti-CD8 T cell depletion groups based on the average of day 7 and day 10 post-tMCAo rotarod values to ensure equal distribution of motor deficits at the start of depletion. Both isotype control and anti-CD8 treated groups did not have a difference (p=0.89) in their initial tissue infarction, as quantified by MRI at day 6 post-tMCAo, before the depletion treatment was initiated (Fig. 3B, C).
Figure 3. Mice with CD8 T cells depletion demonstrated fewer deficits in the post-stroke behavior tests.
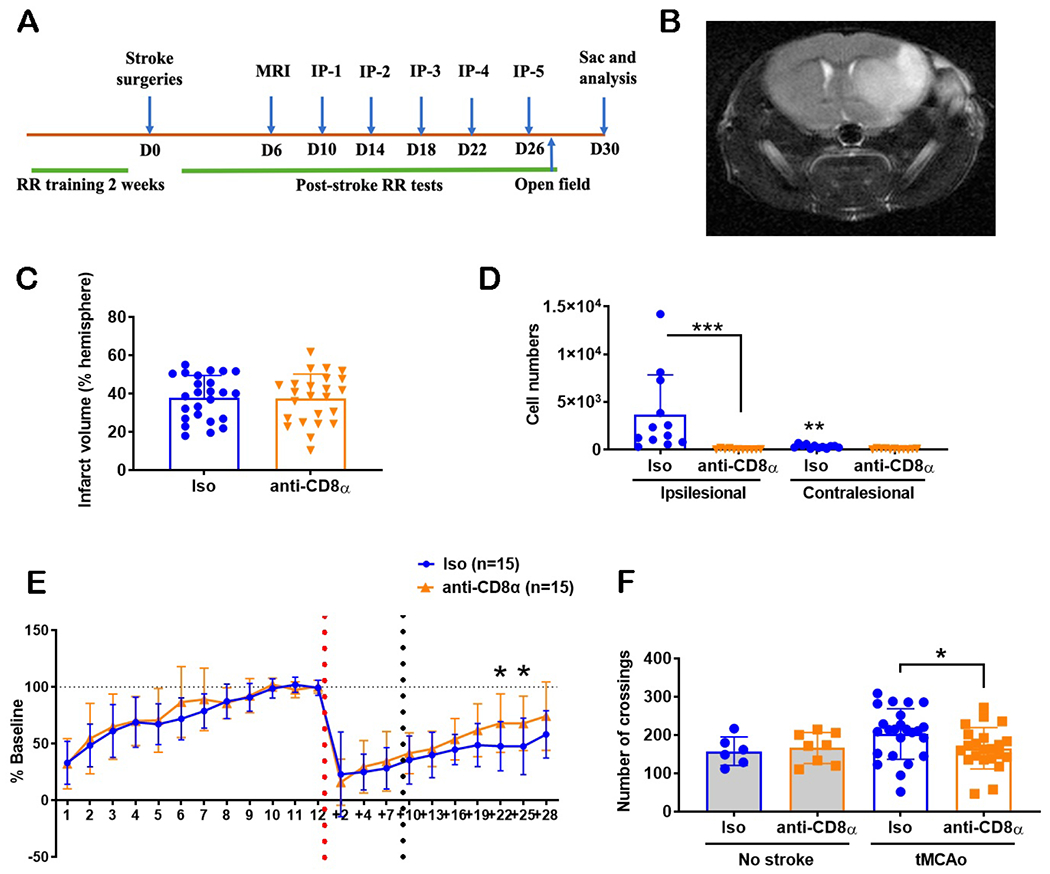
(A) Schematic for CD8 T cell depletion (IP, intraperitoneal injection) and post-stroke behavior analysis (RR, rotarod); (B) Representative MRI image at 6 days post-stroke; (C) No difference in infarct volumes (6 days post-stroke) between isotype (Iso) antibody-treated controls (blue circles) and CD8 T cell-depleted (orange downward triangles) mice prior to CD8 T cell depletion. Note: not all mice quantified by MRI underwent behavioral training; (D) CD8 T cell numbers were elevated in the post-stroke ipsilesional brain of Iso-treated control mice; ** = p<0.01; *** =p<0.001; vs. contralesional hemisphere unless indicated by brackets. (E) Rotarod behavior of both cohorts of mice, with a significant between-group difference on days 22 and 25 post-tMCAo (red dashed line, day of tMCAo) (F) Open field behavior of mice at 27 days post-stroke (i.e. crossings) show a hyperactivity in control mice. * = p<0.05 between groups. For all data, n = 12 to 25 per group. C, Mann-Whitney t-test; D,F Two-way ANOVA, Benjamini post-hoc; E, repeated measures Two-way ANOVA, Benjamini post-hoc.
CD8 T cell depletion was verified in the brain, spleen, and peripheral blood of post-tMCAo mice using antibody staining with CD8β-specific antibodies, as the depletion used CD8α-specific antibodies (Supplemental Fig. 2A). Anti-CD8 treated mice had lower CD8 T cells (67-fold; p<0.001) in the ipsilesional hemispheres compared to the isotype control treated mice day 30 post-tMCAo (Fig. 3D). Isotype control treated mice had more CD8 T cells in the ipsilesional hemispheres (11-fold; p=0.001) compared to the respective contralesional hemispheres. This difference was not observed in the anti-CD8 treated mice group.
For evaluation of motor function and coordination, no significant differences were observed between the two groups in the training time period (1 to 12 days pre-stroke) and the post-tMCAo, pre-treatment time period (+2 to +10 days post-tMCAo; Fig. 3E). After the antibody treatment, mice with delayed CD8 T cell depletion showed improved rotarod performance (F(1,28)=4.264; p=0.048) compared to the isotype control group from day 13 through day 28 post-tMCAo. Specifically, CD8 T cell-depleted mice demonstrated better performance on days 22 (p=0.012) and 25 (p=0.01) post-tMCAo compared to the control mice. Both groups of mice also had significant differences in their recovery with increasing number of days post-tMCAo (F(5,140)=12.4; p<0.001). Of note, in a subgroup analysis of brains collected on day 30 for histology (see microglial phenotyping below), there was no effect of CD8 T cell depletion beginning at 10 days post-stroke on infarct volume (p=0.744), again confirming changes in long-term behavior are independent of severity of initial infarction.
In addition to the rotarod motor coordination test, we also used the open field test to understand the effects of delayed CD8 T cell depletion on general locomotor activity, exploratory behavior, and anxiety. Uninjured naive control mice were also included to delineate the effect of stroke injury on the behavioral outcome. We quantified the crossings, rears, and grooming of post-tMCAo mice for a period of 10 mins on day 27 post-tMCAo. Post-tMCAo isotype control treated mice had higher square-to-square crossings compared to the post-tMCAo anti-CD8 treated mice (1.2-fold; p=0.03) and the healthy (i.e. without stroke) isotype control treated mice (Fig. 3F; 1.3-fold; p=0.09). We did not observe differences in crossings between healthy anti-CD8 treated mice and post-tMCAo anti-CD8 treated mice groups. We also did not observe differences in crossings between the healthy, uninjured cohorts. Both in isotype control treated cohorts (0.5-fold; p<0.001) and anti-CD8 treated cohorts (0.4-fold; p<0.001), post-tMCAo mice had lower numbers of rearing compared to the uninjured mice independent of treatment effect (Supplemental Fig. 3B). We also did not observe differences in the grooming behavior between the four cohorts (Supplemental Fig. 3C).
3.5. Effect of CD8 T cell depletion on long-term post-tMCAo recruitment of immune cells:
Delayed CD8 T cell depletion reduced leukocyte infiltration in the ipsilesional hemispheres (2-fold; p<0.001) compared to the isotype control treatment at 30 days post-tMCAo (Fig. 4A), though both control mice (12-fold; p<0.001) and CD8 T cell-depleted mice (8-fold; p=0.002) had higher immune cell numbers in the ipsilesional hemispheres compared to the corresponding contralesional hemispheres. More specifically, CD4 T cells (Fig. 4B; 2.3-fold; p=0.005), B cells (Fig. 4C; 2.3-fold; p=0.02), NK cells (Fig. 4D; 1.5-fold; p= 0.02), neutrophils (Fig. 4E; 1.75-fold; p= 0.03) and macrophages and monocytes (Fig. 4F; 2-fold; p= 0.02) were lower in the ipsilesional hemispheres of anti-CD8 treated mice. These cell numbers were also higher in the ipsilesional compared to corresponding contralesional hemispheres in both anti-CD8 and isotype control treated mice groups, apart from CD4 T cell and neutrophils in CD8 depleted mice. Of note, both circulating and splenic cell populations of leukocytes, B cells, CD4 T cells, NK cells, neutrophils, monocytes and macrophages within the same mice were not different between the anti-CD8 depleted and isotype control-treated mice cohorts (Supplemental Figure 4). These results suggest that long-term CD8 T cells in the brain contribute to chronic inflammation, with fewer general immune cell populations recruited post-tMCAo upon delayed CD8 T cell depletion.
Figure 4. CD8 T cell depletion decreased the number of immune cells present in the brain at 30 days post-stroke (i.e. 20 days post-treatment initiation).

(A) Total leukocytes; (B) CD4 T cells; (C) B cells; (D) NK cells; (E) Neutrophils; (F) Monocytes and macrophages (n = 11 or 12 per group) for isotype (Iso)-treated control mice (blue circles) and CD8 T cell-depleted mice (orange downward triangles) show a significant reduction in all immune cell subsets with CD8 T cell depletion. * = p<0.05; ** = p<0.01; *** =p<0.001; **** = p<0.0001; vs. contralesional hemisphere unless indicated by brackets. Two-way ANOVA, Benjamini post-hoc.
3.6. Effect of CD8 T cell depletion on long-term post-tMCAo microglial activation and CNS cytokine production:
Based on prior studies (7), we determined the effect of delayed CD8 T cell depletion on microglial activation to identify potential mechanisms by which CD8 T cells regulate long-term immune cell recruitment into the brain parenchyma. Microglia have a ramified shape when they are resting or surveying and an amoeboid shape when they are activated (27). Thus, we quantified microglial activation at different distances (300μm, 600μm, and 900μm) from the infarct by estimating the surface area of Iba-1 staining in 30 days post-tMCAo mice brain sections in the ipsilesional and contralesional hemispheres (Fig. 5A,B). We observed a significant increase in microglial activation at 300μm and 600μm in both CD8 T cell-depleted (4-fold; p=0.003 and 3-fold; p=0.04, respectively) and isotype control mice cohorts (6-fold; p<0.001 and 3.6-fold; p=0.046, respectively), but not at 900μm, from the infarct volume when compared to the contralesional area independent of treatment. In a separate cohort we colocalized Iba-1+ microglia with either iNOS or Arginase (Arg)-1, as indicative of pro-and anti-inflammatory phenotypes, respectively (28). For both populations, there were significant differences between hemispheres (both p<0.05, two-way ANOVA), but again there was no effect of peripheral CD8 T cell depletion on post-stroke microglial phenotype at 30 days post-tMCAo, with the exception of higher Arg-1+ microglia in the contralesional hemisphere in CD8 T cell-depleted mice (Fig. 5 C,D). These results suggest that CD8 T cells in the brain parenchyma do not affect chronic microglial activation or phenotype at 30 days post-tMCAo.
As there was no difference in microglial phenotype, we wanted to determine if other resident CNS cells (e.g. astrocytes, neurons, pericytes) exhibited altered pro-or anti-inflammatory protein secretion upon prolonged CD8 T cell depletion. An MSD mouse inflammation panel, quantifying 19 cytokine and chemokines, was used to assess CNS inflammatory status at 30 days post-tMCAo in CD8 T cell-depleted and control cohorts. Of the proteins detected by the MSD assay, 3 chemokines (CCL2, CCL3, CXCL1) and 1 cytokine (IL-33) were detected in naïve brains, as well as in ipsi- and contralesional hemispheres (Fig. 5G.H; Supplemental Fig. 5). Additional cytokines (TNF-α, IL-6, IL-1β) and chemokines (CXCL2, CXCL10) were only detected in the ipsilesional hemispheres (Fig. 5I,J; Supplemental Fig. 5). While there was a clear upregulation in the ipsilesional hemispheres, there was no effect on the production of any cytokine or chemokine by peripheral CD8 T cell depletion.
3.7. Functional characterization of long-term CD8 T cells in the post-stroke brain:
To determine functional differences between CD8 T cells that diapedese into the brain during the acute phase versus the chronic phase after stroke, we conducted RNA-Seq analyses of CD8 T cells isolated from ipsilesional hemispheres 3 days and 30 days post-tMCAo (Table 1, Supplemental Tables 1–3). CD8 T cells isolated from the brain and blood of uninjured naïve mice served as controls. Principal component analysis (PCA) of the gene expression values was used to determine the validity of the data sets (Fig. 6A; FDR<0.05 and log2CPM>0). CD8 T cells isolated from the 30 days post-tMCAo ipsilesional hemispheres differentially expressed only 46 genes relative to CD8 T cells isolated from 3 days post-tMCAo brains (Fig. 6B, Table1; FDR<0.05 and log2CPM>0). Interestingly, there was a similar low difference in expression between 30 days post-tMCAo brain-localized CD8 T cells vs. those isolated form naïve (i.e. uninjured) brain (26 genes; Fig. 6C). In contrast, CD8 T cells isolated from the brain at any time (i.e. naïve, 3 days or 30 days post-tMCAo) differentially expressed 2272, 298 and 176 genes, respectively, relative to CD8 T cells isolated from peripheral blood of naïve mice (Fig. 6D, Supplemental Figure 6). Among other genes, CD8 T cells in the 30 days post-tMCAo brain expressed significantly higher levels of Fas Ligand (FasL) (log2FC=3.98; p<0.001; FDR=0.03) compared to CD8 T cells in the 3 days post-tMCAo brain.
Table 1:
Top up-regulated and down-regulated genes in the CD8 T cells isolated from the 30 days post-stroke brain compared to the 3 days post-stroke brains (FDR < = 0.05; log2CPM > 0)
| Up-regulated genes | Down-regulated genes |
|---|---|
| Klra19 | Cxcl1 |
| Klra6 | Mt2 |
| Fasl | Hbb-bt |
| Klra13-ps | Hbb-b2 |
| Klra23 | Hba-a1 |
| Sh2d1a | Hba-a2 |
| Klra12 | Cxcl2 |
| Gpr174 | Ifitm1 |
| Hsd11b1 | Hbb-bs |
| Trnt1 | Hbb-b1 |
Figure 6. CD8 T cells in the 30 day post-stroke brain are functionally different compared to 3 day post-stroke brain.

(A) PCA plot for CD8 T cells enriched from the blood or left hemisphere of naïve mice compared to CD8 T cells isolated form the ipsilesional hemisphere 3 or 30 dasy post-tMCAo. Heat map of the differentially expressed genes (B) 30 days post-stroke brain and 3 days post-stroke brain; (C) naïve brain and 30days post-stroke brain; (D) naïve blood and 30 days post-stroke brain; (E) upstream regulator analysis; (F) Cytokine profile of CD8 T cells in the 30 days post-stroke brain. (n = 3 to 12 per group). **** = p<0.0001 vs. ipsilesional hemisphere unless otherwise indicated by brackets. Two-way ANOVA, Benjamini post-hoc.
To test for differentially-activated pathways in CD8 T cells isolated from the 3 days post-tMCAo brain compared to 30 days post-tMCAo brain, we performed Ingenuity Pathway Analysis (IPA). Functional pathway analysis revealed strong activation of autoimmune pathways, including 4/5 top pathways activated (Supplemental Fig. 7; rheumatoid arthritis, graft-versus-host disease, autoimmune thyroid disease, allograft rejection). Furthermore, multiple biological pathways involving hematopoietic development, cell signaling, differentiation, and diapedesis were differentially regulated (Supplemental Fig. 8). Among others, upstream regulator analysis demonstrated the effects on IL-6 and Interferon-γ (IFNγ) on the differentially expressed genes (Fig. 6E). To determine if these long-term post-tMCAo CD8 T cells secreted IFNγ, we used intracellular cytokine staining for flow cytometry analysis. CD8 T cells in the brain at 30 days after stroke primarily secrete IFNγ, with 68% of CD8 T cells in the ischemic hemisphere IFNγ positive (Fig. 6F). Also, IFNγ-secreting CD8 T cells in the ipsilesional hemisphere were significantly higher (17.6-fold; p<0.001) than the IFNγ-secreting cells in the contralesional hemisphere. This suggests that CD8 T cells are potentially signaling through IFNγ secretion in the long-term chronic phase post-tMCAo.
Discussion:
Our findings in mice demonstrate a significant role for CD8 T cells in the post-stroke chronic phase of recovery. Ischemic stroke triggers an inflammatory cascade, resulting in the recruitment of immune cells from the peripheral circulation to the brain parenchyma. In the immediate acute phase, accumulation of immune cells in the injured brain peaks about 3 to 4 days after stroke onset, with cell numbers decreasing as days progress (29). However, significant numbers of B and general T cells in mice parenchyma as late as 7 weeks post-stroke (5), with higher numbers of brain invading CD4 and CD8 T cells for at least a month after stroke onset (8, 30). Ahnstedt et al. demonstrated higher CD8 T cells in the brain at 15 days post-stroke when compared to 3 days and 7 days post-stroke (31) similar to our results. Importantly, Miró-Mur et al. recently confirmed CD3+ T cells in the ischemic parenchyma of a stroke patient months after onset, highlighting the potentially broad therapeutic window for targeting detrimental immune cell diapedesis into the injured brain (6).
Currently, it is still unclear if long-term immune cells in the brain parenchyma contribute or impair functional recovery after stroke. This work demonstrates that long-term CD8 T cells negatively correlate with functional recovery independent of acute infarct volume. Furthermore, selective depletion of CD8 T cells using CD8α-specific antibodies improved functional recovery and limited chronic immune cell diapedesis into the post-tMCAo parenchyma. This benefit was observed even when CD8 T cell depletion was initiated 10 days after stroke onset in equally injured cohorts. The clear effect of delayed depletion of CD8 T cells not only on recovery but the diapedeses of other immune cells suggests that it is the continual diapedesis of circulating CD8 T cells for weeks after stroke onset that contribute to the detrimental effects on functional recovery. Aging, however, induces high levels of parenchymal CD8 T cell populations that exhibit effector memory markers in the absence of major antigen-mediated activation as they establish residence in the CNS (32). As we used young mice, future studies should confirm if systemic depletion after stroke is efficacious in aged mice considering the resident CD8 T cell populations present in aged individuals at the highest risk for stroke.
Our prior work found an early recruitment of CD8 T cells to subcortical areas supporting motor recovery at 4 days after tMCAo (23), including sensory- and motor-related nuclei in the midbrain and pons. Jones et al. also identified ipsilesional thalamic CD8 T cell diapedesis at 14 days after photothrombotic stroke (7). These regions are outside of the cortical areas quantified in Figure 2 by our histology, which focused on rostral infarct and peri-infarct regions following tMCAo. However, our flow cytometry analysis routinely includes processing whole hemispheres except for the olfactory bulb and cerebellum, which means we may have missed relevant CD8 T cell diapedesis in our histology that was identified by flow cytometry. Long-term and region-specific diapedesis of CD8 T cells into reorganizing areas of the brain, including brain stem, may thus be better quantified by “whole brain” quantification using other methods (e.g. serial two-photon tomography (23)). These remote areas may underlie the association of long-term CD8 T cell diapedesis with motor recovery that is independent of acute infarction, but instead reflective of reorganization patterns that support chronic behavioral outcomes (11).
In contrast to the benefits observed after stroke, CD8 T cell depletion in naïve mice did not impact motor coordination but did limit enriched environment-induced plasticity in otherwise healthy mice on another study (33). SCID mice, deficient in lymphocytes including CD8 T cells, also did not demonstrate deficits in rotarod behavior but exhibited cognitive deficits (34). The differences in benefit vs. harm for CD8 T cell-depletion could be attributed to the difference in CNS milieu before and after ischemic injury, with other immune cells recruited to the ischemic brain parenchyma and participating in CD8 T cell-mediated effects. This suggests that the functional role of CD8 T cells is distinct in naïve healthy brains compared to injured post-tMCAo brains and, as mentioned above, may impact plasticity dependent on region of diapedesis.
We assessed functional recovery using open field behavior test, which measures motor activity, exploratory behavior, and anxiety (35). In our study, the post-stroke control mice demonstrated a hyperactive behavior (36) absent in the CD8 T cell-depleted mice, confirming a negative influence of post-stroke CD8 T cells. Unfortunately, we could not conclusively link the reduction in anxiety on CD8 T cell depletion due to the caveat that we used transparent walls for the open field test, not opaque walls (35). This limits the ability to assess anxiety accurately, as it limits the visual difference between the periphery and the middle, “open” field. Thus, the role of CD8 T cells in post-stroke anxiety remains unclear. RAG-1−/− mice lacking T cell and B cells exhibited increases in anxiety-like behaviors, which was rescued upon reconstituting with CD4 T cells but not CD8 T cells (37). Depression and anxiety levels were also associated with increased CD8 T cell numbers in cirrhosis patients (38). Thus, further studies including delayed adoptive transfer of CD8 T cells during the weeks following stroke onset (14), could clarify the role of long-term CD8 T cells in post-tMCAo anxiety, as well as other cognitive behaviors detrimentally affected by stroke.
Consistent with the previous studies, our results demonstrated chronic elevations of T cells, as well as other innate and adaptive immune cells, long-term after stroke onset. Moreover, levels of pro-inflammatory cytokines (e.g. IFNγ, TNFα, IL-17) and chemokines (e.g. CCL2, CCL3, CCL7) are elevated in the lesion area as late as 7 weeks after stroke (5). We did not identify differences in CNS cytokine/chemokine upregulation between CD8 T cell-depleted and isotype-treated control mice. We also did not observe a difference in microglial activation, nor any pro-inflammatory or anti-inflammatory phenotype that would suggest either beneficial or detrimental effects on post-stroke functional recovery (39). Interestingly, glial fibrillary acidic protein (GFAP) expression, indicative of astroglial activation and gliosis, is elevated in the ipsilesional hemispheres in both male and female mice as late as day 42 post-stroke (40), which is in accordance with our time frame of persistent microglia activation. These findings suggest that during the chronic phase of stroke recovery, immune cells are actively recruited from the periphery by the chemokine secretion of resident or infiltrating CNS cells in the brain parenchyma. It is, however, still unclear if the concentration of chemokines and cytokines in the parenchyma correlates with the tissue injury. Notably, apart from the vascular invasion route, these long-term post-tMCAo CD8 T cells are also potentially recruited intra-cerebrally through the CCR2-ligand gradient between the ipsilesional cortex and the choroid plexus (41). Further studies are required to delineate the specific route(s) by which chronic immune cell populations are continually recruited into the infarct region and how depletion of this specific lymphocyte population can have such a distinct influence on CNS diapedesis independent of splenic and circulating immune populations..
T cells present in the brain at one month after ischemic stroke have been shown to be proliferating cells and express higher levels of Ki67 and adhesion receptor CD44 compared to the splenic T cells (30). Furthermore, post-stroke brain-invading CD8 T cells also express higher levels of inflammatory cytokines TNFα and IFNγ and cytotoxic perforin at 30 days after stroke compared to 3 days post-tMCAo time point and splenic CD8 T cells (30). Consistent with these results, we observed higher numbers of IFNγ-secreting CD8 T cells in the ipsilesional hemispheres compared to the contralesional hemispheres at 30 days post-tMCAo. IFNγ signaling regulates several microglial functions such as phagocytic activity, expression of MHC class II and co-stimulatory molecules (42) and secretion of inflammatory cytokines and chemokines (43, 44), but CNS-derived IFNγ was not detectable at 30 days post-tMCAo in our model. These observations suggest that IFNγ secretion by the long-term post-stroke CD8 T cells could potentially regulate inflammatory responses in the chronic phase of recovery, as was shown to occur in the aged brain (32). We found that CD8 T cells in the 30 days post-tMCAo brain also expressed significantly higher levels of FasL compared to CD8 T cells in the 3 days post-tMCAo brain. This observation suggests that the long-term CD8 T cells in the post-tMCAo brain could potentially induce apoptosis in the interacting CNS cells through the Fas–FasL pathway (45). Considering the lack of change in overall CNS cytokine/chemokine production in CD8 T cell-depleted mice, and the fact that FasL-deficient mice exhibit improved functional recovery and lower pro-inflammatory cytokine/chemokine upregulation 1 week post-tMCAo (14), FasL may be a critical mediator of CD8 T cell-induced inflammation for weeks after stroke onset that may be a potentially unique mechanisms for stroke recovery independent of the role of CD8 T cells in chronic pathology in other CNS injuries (46).
It is becoming increasingly evident that stroke pathology induces a chronic inflammatory response in the brain parenchyma, while functional recovery after stroke is a long and dynamic process ranging from weeks to months after tissue injury (47). However, apart from physical therapy there are no available treatments targeting and improving functional recovery post-stroke (48). The data presented here, along with recent studies (5, 6, 8, 14), suggest the importance of targeting chronic phase inflammatory responses for improving functional recovery. Our study provides a critical foundation to examine the significance of CD8 T cell regulation in enhancing post-stroke functional recovery for weeks after onset and may be translational to other CNS injuries including traumatic brain injury (TBI) (46). Identifying pleiotropic mechanisms that can be targeted by adjunctive therapies to rehabilitation could help millions that live with long-term functional deficits after devastating illnesses such as stroke or TBI.
Supplementary Material
Acknowledgments:
This study was funded by grants to A.M.S. from the American Heart Association (14SDG18410020), NIH/NINDS (NS088555), Texas Institute for Brain Injury and Repair, and The Haggerty Center for Brain Injury and Repair (UTSW), to U.M.S. from the American Heart Association (17PRE33660147), to T.A.U. from NIH/NINDS (5T32 NS077889), and to D.M.W. from NIH/NIA (P30 AG02838).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim J, Thayabaranathan T, Donnan GA, Howard G, Howard VJ, Rothwell PM, et al. Global Stroke Statistics 2019. Int J Stroke. 2020:1747493020909545. [DOI] [PubMed] [Google Scholar]
- 2.Kellner CP, Awad AJ, Mocco J. Developing New Stroke Treatments Using Preclinical Randomized Controlled Trials. World Neurosurg. 2016;86:13–4. [DOI] [PubMed] [Google Scholar]
- 3.Evans MRB, White P, Cowley P, Werring DJ. Revolution in acute ischaemic stroke care: a practical guide to mechanical thrombectomy. Pract Neurol. 2017;17(4):252–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seitz RJ, Donnan GA. Recovery Potential After Acute Stroke. Front Neurol. 2015;6:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doyle KP, Quach LN, Sole M, Axtell RC, Nguyen TV, Soler-Llavina GJ, et al. B-lymphocyte-mediated delayed cognitive impairment following stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015;35(5):2133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miro-Mur F, Urra X, Ruiz-Jaen F, Pedragosa J, Chamorro A, Planas AM. Antigen-Dependent T Cell Response to Neural Peptides After Human Ischemic Stroke. Front Cell Neurosci. 2020;14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jones KA, Maltby S, Plank MW, Kluge M, Nilsson M, Foster PS, et al. Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke. Brain Behav Immun. 2018;67:299–307. [DOI] [PubMed] [Google Scholar]
- 8.Weitbrecht L, Berchtold D, Zhang T, Jagdmann S, Dames C, Winek K, et al. CD4(+) T cells promote delayed B cell responses in the ischemic brain after experimental stroke. Brain Behav Immun. 2021;91:601–14. [DOI] [PubMed] [Google Scholar]
- 9.Yin Y, Li G. Hypoxia induces T Helper 17 cell upregulation in cultured peripheral blood mononuclear cells from chronic stage patients of severe cerebral infarction. Microbiol Immunol. 2011;55(2):130–4. [DOI] [PubMed] [Google Scholar]
- 10.Swardfager W, Herrmann N, Andreazza AC, Swartz RH, Khan MM, Black SE, et al. Poststroke neuropsychiatric symptoms: relationships with IL-17 and oxidative stress. Biomed Res Int. 2014;2014:245210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ortega SB, Torres VO, Latchney SE, Whoolery CW, Noorbhai IZ, Poinsatte K, et al. B cells migrate into remote brain areas and support neurogenesis and functional recovery after focal stroke in mice. Proceedings of the National Academy of Sciences of the United States of America. 2020;117(9):4983–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadler R, Cramer JV, Heindl S, Kostidis S, Betz D, Zuurbier KR, et al. Short-chain fatty acids improve post-stroke recovery via immunological mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou YX, Wang X, Tang D, Li Y, Jiao YF, Gan Y, et al. IL-2mAb reduces demyelination after focal cerebral ischemia by suppressing CD8(+) T cells. CNS Neurosci Ther. 2019;25(4):532–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan L, Zhang CJ, Zhu L, Chen J, Zhang Z, Liu P, et al. FasL-PDPK1 Pathway Promotes the Cytotoxicity of CD8(+) T Cells During Ischemic Stroke. Transl Stroke Res. 2020;11(4):747–61. [DOI] [PubMed] [Google Scholar]
- 15.Peruzzotti-Jametti L, Donega M, Giusto E, Mallucci G, Marchetti B, Pluchino S. The role of the immune system in central nervous system plasticity after acute injury. Neuroscience. 2014;283:210–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klebe D, McBride D, Flores JJ, Zhang JH, Tang J. Modulating the Immune Response Towards a Neuroregenerative Peri-injury Milieu After Cerebral Hemorrhage. J Neuroimmune Pharmacol. 2015;10(4):576–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvaraj UM, Ortega SB, Hu R, Gilchrist R, Kong X, Partin A, et al. Preconditioning-induced CXCL12 upregulation minimizes leukocyte infiltration after stroke in ischemia-tolerant mice. J Cereb Blood Flow Metab. 2017;37(3):801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu KYP, Lu XJD, Zhu YS, Le N, Kim H, Poh CF. Plasma-Derived Inflammatory Proteins Predict Oral Squamous Cell Carcinoma. Front Oncol. 2018;8:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–30. [DOI] [PubMed] [Google Scholar]
- 21.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26(1):139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega SB, Noorbhai I, Poinsatte K, Kong X, Anderson A, Monson NL, et al. Stroke induces a rapid adaptive autoimmune response to novel neuronal antigens. Discov Med. 2015;19(106):381–92. [PMC free article] [PubMed] [Google Scholar]
- 23.Poinsatte K, Betz D, Torres VO, Ajay AD, Mirza S, Selvaraj UM, et al. Visualization and Quantification of Post-stroke Neural Connectivity and Neuroinflammation Using Serial Two-Photon Tomography in the Whole Mouse Brain. Front Neurosci. 2019;13:1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortega SB, Pandiyan P, Windsor J, Torres VO, Selvaraj UM, Lee A, et al. A pilot study identifying brain-targeting adaptive immunity in pediatric Extracorporeal Membrane Oxygenation (ECMO) patients with acquired brain injury. Critical Care Medicine. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brait VH, Arumugam TV, Drummond GR, Sobey CG. Importance of T lymphocytes in brain injury, immunodeficiency, and recovery after cerebral ischemia. J Cereb Blood Flow Metab. 2012;32(4):598–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mracsko E, Liesz A, Stojanovic A, Lou WP, Osswald M, Zhou W, et al. Antigen dependently activated cluster of differentiation 8-positive T cells cause perforin-mediated neurotoxicity in experimental stroke. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34(50):16784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A. Physiology of microglia. Physiol Rev. 2011;91(2):461–553. [DOI] [PubMed] [Google Scholar]
- 28.Xiong XY, Liu L, Yang QW. Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol. 2016;142:23–44. [DOI] [PubMed] [Google Scholar]
- 29.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40(5):1849–57. [DOI] [PubMed] [Google Scholar]
- 30.Xie L, Li W, Hersh J, Liu R, Yang SH. Experimental ischemic stroke induces long-term T cell activation in the brain. J Cereb Blood Flow Metab. 2018:271678X18792372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahnstedt H, Patrizz A, Chauhan A, Roy-O’Reilly M, Furr JW, Spychala MS, et al. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav Immun. 2020;87:556–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritzel RM, Crapser J, Patel AR, Verma R, Grenier JM, Chauhan A, et al. Age-Associated Resident Memory CD8 T Cells in the Central Nervous System Are Primed To Potentiate Inflammation after Ischemic Brain Injury. J Immunol. 2016;196(8):3318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zarif H, Nicolas S, Guyot M, Hosseiny S, Lazzari A, Canali MM, et al. CD8(+) T cells are essential for the effects of enriched environment on hippocampus-dependent behavior, hippocampal neurogenesis and synaptic plasticity. Brain Behav Immun. 2018;69:235–54. [DOI] [PubMed] [Google Scholar]
- 34.Filiano AJ, Xu Y, Tustison NJ, Marsh RL, Baker W, Smirnov I, et al. Unexpected role of interferon-gamma in regulating neuronal connectivity and social behaviour. Nature. 2016;535(7612):425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seibenhener ML, Wooten MC. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. Jove-J Vis Exp. 2015(96). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manwani B, Liu F, Xu Y, Persky R, Li J, McCullough LD. Functional recovery in aging mice after experimental stroke. Brain Behav Immun. 2011;25(8):1689–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattazzi L, Piras G, Ono M, Deacon R, Pariante CM, D’Acquisto F. CD4(+) but not CD8(+) T cells revert the impaired emotional behavior of immunocompromised RAG-1-deficient mice. Transl Psychiat. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ko FY, Tsai SJ, Yang AC, Zhou Y, Xu LM. Association of Cd8 T Cells with Depression and Anxiety in Patients with Liver Cirrhosis. Int J Psychiat Med. 2013;45(1):15–29. [DOI] [PubMed] [Google Scholar]
- 39.Nakagawa Y, Chiba K. Role of microglial m1/m2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals (Basel). 2014;7(12):1028–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahnstedt H, Patrizz A, Chauhan A, Roy-O’Reilly M, Furr JW, Spychala MS, et al. Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav Immun. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llovera G, Benakis C, Enzmann G, Cai R, Arzberger T, Ghasemigharagoz A, et al. The choroid plexus is a key cerebral invasion route for T cells after stroke. Acta Neuropathol. 2017;134(6):851–68. [DOI] [PubMed] [Google Scholar]
- 42.Ottum PA, Arellano G, Reyes LI, Iruretagoyena M, Naves R. Opposing Roles of Interferon-Gamma on Cells of the Central Nervous System in Autoimmune Neuroinflammation. Front Immunol. 2015;6:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin AA, Tripathi PK, Sholl A, Jordan MB, Hildeman DA. Gamma interferon signaling in macrophage lineage cells regulates central nervous system inflammation and chemokine production. J Virol. 2009;83(17):8604–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ellis SL, Gysbers V, Manders PM, Li W, Hofer MJ, Muller M, et al. The cell-specific induction of CXC chemokine ligand 9 mediated by IFN-gamma in microglia of the central nervous system is determined by the myeloid transcription factor PU.1. J Immunol. 2010;185(3):1864–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shrestha B, Diamond MS. Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol. 2007;81(21):11749–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Daglas M, Draxler DF, Ho H, McCutcheon F, Galle A, Au AE, et al. Activated CD8(+) T Cells Cause Long-Term Neurological Impairment after Traumatic Brain Injury in Mice. Cell reports. 2019;29(5):1178–91 e6. [DOI] [PubMed] [Google Scholar]
- 47.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet. 2011;377(9778):1693–702. [DOI] [PubMed] [Google Scholar]
- 48.Veltkamp R, Gill D. Clinical Trials of Immunomodulation in Ischemic Stroke. Neurotherapeutics. 2016;13(4):791–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


