Abstract
Clinical reports suggest that the coronavirus disease-19 (COVID-19) pandemic caused by severe acute respiratory syndrome (SARS)-coronavirus-2 (CoV-2) has not only taken millions of lives, but has also created a major crisis of neurologic complications that persist even after recovery from the disease. Autopsies of patients confirm the presence of the coronaviruses in the CNS, especially in the brain. The invasion and transmission of SARS-CoV-2 in the CNS is not clearly defined, but, because the endocytic pathway has become an important target for the development of therapeutic strategies for COVID-19, it is necessary to understand endocytic processes in the CNS. In addition, mitochondria and mechanistic target of rapamycin (mTOR) signaling pathways play a critical role in the antiviral immune response, and may also be critical for endocytic activity. Furthermore, dysfunctions of mitochondria and mTOR signaling pathways have been associated with some high-risk conditions such as diabetes and immunodeficiency for developing severe complications observed in COVID-19 patients. However, the role of these pathways in SARS-CoV-2 infection and spread are largely unknown. In this review, we discuss the potential mechanisms of SARS-CoV-2 entry into the CNS and how mitochondria and mTOR pathways might regulate endocytic vesicle–mitochondria interactions and dynamics during SARS-CoV-2 infection. The mechanisms that plausibly account for severe neurologic complications with COVID-19 and potential treatments with Food and Drug Administration-approved drugs targeting mitochondria and the mTOR pathways are also addressed.
Keywords: coronavirus, SARS-CoV-2, COVID-19, mitochondria, lysosome, mTOR, ACE2, autophagy, hyperinflammation
Introduction
Coronaviruses (CoVs) are a group of viruses that cause severe respiratory diseases in humans, including severe acute respiratory syndrome (SARS) and the current coronavirus disease-19 (COVID-19; Diaz, 2020; Lukassen et al., 2020; Yan et al., 2020). The current SARS-CoV-2 vaccines have been very useful in combating COVID-19. However, there is still concern about the vaccine being less effective in some older individuals with immunosenescence, immunocompromised patients, and patients receiving treatment with immunosuppressive agents (Ramasamy et al., 2020; Sonani et al., 2021). Therefore, there is still an urgent need to fully understand COVID-19 neuronal invasion and complications to facilitate clinical management, treatments, and mitigation of the spread of the virus.
Patients with COVID-19 have been reported to exhibit neurologic symptoms, such as headache, nausea, brief loss of consciousness, loss of sense of smell and taste, strokes, confusion, brain inflammation encephalitis, meningitis, and seizures (Baig, 2020; Baig et al., 2020; Carod-Artal, 2020; Wu et al., 2020). Several studies of autopsies of patients infected with the SARS-CoV responsible for the 2003 SARS epidemic suggested that the virus was present in the hypothalamus and cortex (Hamming et al., 2004; Law et al., 2005; Tseng et al., 2007; Netland et al., 2008). Similarly, recent studies suggest that SARS-CoV-2 is present in the CNS in COVID-19 patients (Paniz-Mondolfi et al., 2020; Kumari et al., 2021). Although extensive studies have been performed on the invasion and transmission of CoVs in the respiratory system, not much is known about how these viruses invade and spread in the CNS.
CoVs enter the host cell using clathrin-mediated endocytosis (CME) that is triggered by the binding of the virion to host receptors, such as angiotensin-converting enzyme 2 (ACE2), and to host proteases, such as transmembrane serine protease 2 (TMPRSS2; Hoffmann et al., 2020) or furin (Kielian, 2020) that are present in the secretory pathway and CME compartments. Neurons use a CME mechanism similar to that used in non-neuronal cells, and endocytosis at the synapses involves neuron-specific isoforms of the CME endocytic proteins (Rappoport et al., 2004, 2005). In addition to ACE2 and TMPRSS2, neuropilin is suggested to increase virus infection and spread in the CNS (Kielian, 2020). This suggests a different or modified CME-dependent mechanism of viral entry into neurons and other brain cells.
While contacts between CME vesicles and organelles such as the lysosome and endoplasmic reticulum are well established, the interaction between mitochondria and CME vesicles has only recently been reported (Cioni et al., 2019; Todkar et al., 2019). Recent reports suggest that impaired mitochondria function contributes to severe complications in COVID-19 patients (Li et al., 2020; Lukassen et al., 2020; Malavolta et al., 2020), implying that mitochondria may play a key role in SARS-CoV-2 infection and spread. Recent studies also suggest that autophagy, which is tightly regulated by mitochondria and the mechanistic target of rapamycin (mTOR) signaling pathways (Scarffe et al., 2014; Zhao et al., 2015; Magalhaes et al., 2016), is an essential cellular process affected by SARS-CoV-2 infection (Appelberg et al., 2020; Maiese, 2020). CoVs have been shown to partially induce autophagy (Chen et al., 2014), but block the fusion of autophagosomes and lysosomes, thereby inhibiting the final stages of phagocytosis (Chen et al., 2014; Gassen et al., 2019). Since mitochondria and mTOR are essential in regulating autophagy, and dysfunction in these pathways is associated with many neurodegenerative and immunodeficiency disorders (Ryskalin et al., 2018), it is therefore necessary to understand the role of these processes in neurons during SARS-CoV-2 infection.
In this review, we will address potential mechanisms of SARS-CoV-2 entry into neurons and discuss how mitochondria and the mTOR pathways might regulate CME vesicle–mitochondria interactions and dynamics during SARS-CoV-2 infection in the CNS. The plausible mechanisms that account for severe neurologic complications with COVID-19 and potential treatments with some Federal Drug Administration-approved drugs targeting mitochondria and the mTOR pathways are discussed. We also propose studies to investigate the relevant pathophysiology of COVID-19 associated with neurologic complications and health, so that therapies to mitigate these effects can be developed.
Possible role of mitochondria in CME SARS-CoV-2 replication and/or clearance
Endocytosis-dependent host entry by CoV is triggered by binding of the virion to host receptors, such as ACE2, and TMPRSS2 (Fig. 1). TMPRSS2 then primes the viral spike protein (CoV-S) to allow viral entry into the host cell (Hamming et al., 2004; Hoffmann et al., 2020; Lukassen et al., 2020). The engagement of CoV-S with ACE2 causes adaptor proteins, such as AP2, to bind to clathrin to form a clathrin-coated pit (CCP). The CCP is further processed to form the virus-containing clathrin-coated vesicle (CCV), which then delivers its viral contents to early endosomes. The contents in early endosomes are sorted for the following two destinations: they are either retrieved for recycling to the secretory pathway or are delivered to the late endosome. In the late endosomes, the viral particles can escape into the cytosol and use the cellular machinery to synthesize viral proteins. Viral particles that do not escape are transferred to lysosomes via the fusion of the endosomes with lysosome to form endolysosomes for degradation (Magalhaes et al., 2016; Schultz et al., 2016; Liu et al., 2018). Lysosomes are also required for the maturation and degradation stage of autophagy, via autophagosome–lysosome fusion. Thus, lysosomes and autophagy are key components of the endocytic pathway and may play a critical role in SARS-CoV-2 clearance.
Figure 1.
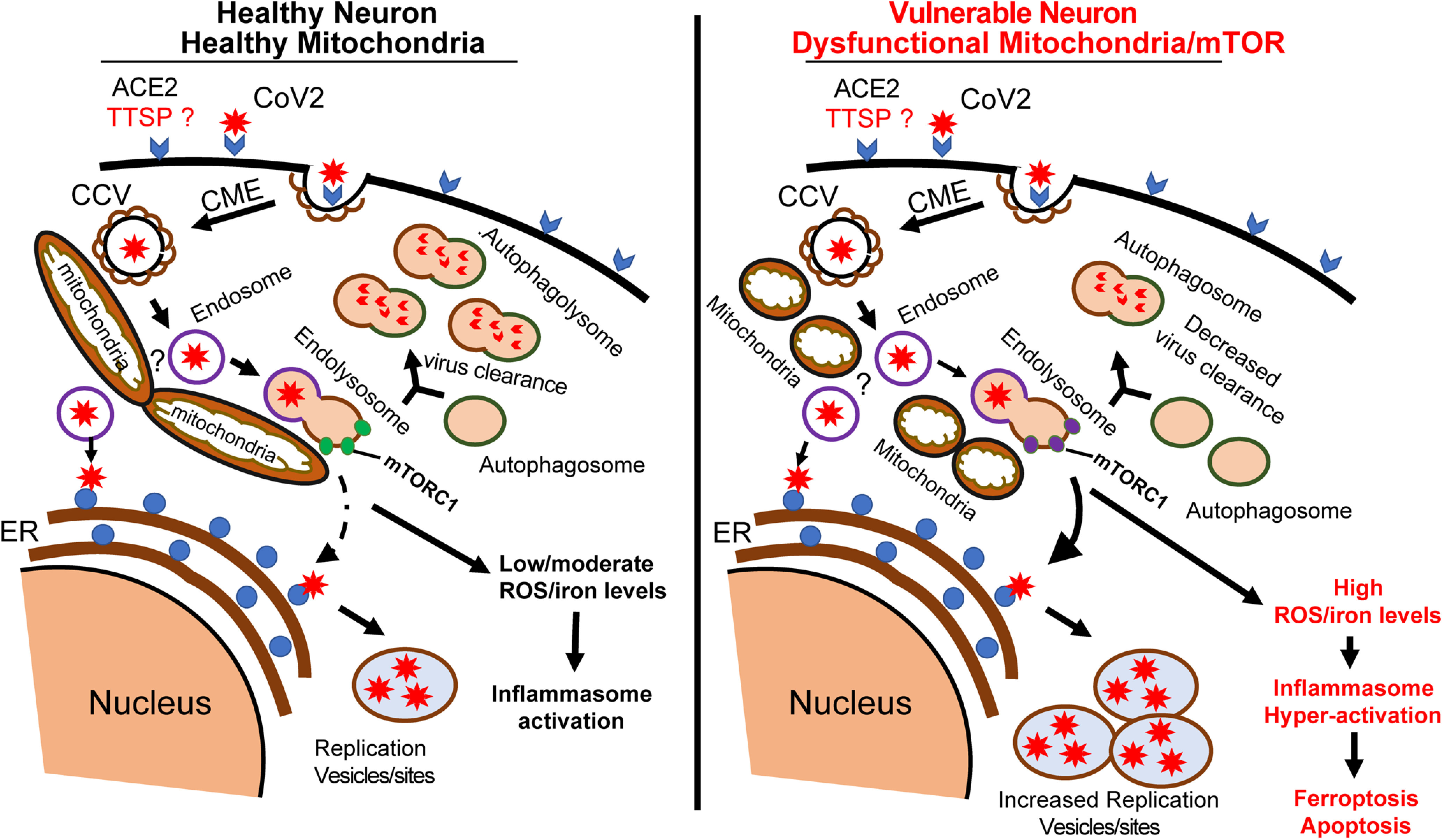
Schematic diagram of proposed SARS-CoV-2 entry into neurons and the role of mitochondria. In a healthy neuron, the initial binding of SARS-CoV-2 to receptors and unknown neuronal TTSP complex initiate CME. CCV containing the virus fuses with endosomes to expose the virus to the endoplasmic reticulum (ER) to make new viral proteins for viral replication. We hypothesize that at the mitochondria, the endosomes fuse with mTORC1-bound lysosomes to form a hybrid endolysosome, which can also interact with ER or fuse with autophagosomes in the autophagy pathway. The lysosome–autophagy pathway is active in healthy neurons, allowing degradation of CoV vesicles and virus clearance. In high-risk COVID patients, neurons have dysfunctional mitochondria and the lysosome–autophagy signaling is impaired, leading to increased virus replication and ROS/iron levels. The high levels of ROS/iron production initiate the induction of pathways such as apoptosis or ferroptosis.
Recently, mitochondria have been shown to interact with early endosomes (Li et al., 2015; Cioni et al., 2019; Todkar et al., 2019), and a growing number of studies have demonstrated delivery of specific proteins or molecules to mitochondria via endocytic trafficking (Cioni et al., 2019; Todkar et al., 2019). Other studies suggest that mitochondria–lysosome contacts allow the regulation of both mitochondrial and lysosomal functions (Wong et al., 2018; Bartel et al., 2019). Mitochondria and lysosomes are highly dynamic and dysfunction of both organelles has been associated with multiple diseases, (Abu-Remaileh et al., 2017; Wong et al., 2018). How mitochondria interactions with endosomes and lysosomes modulate degradation of the cargo of late endosomes remains largely unknown, but we hypothesize that mitochondria interaction with CME endosomes is critical for the degradative function of lysosomes (Fig. 1). Further studies to determine the possible cross talk among mitochondria, lysosomes, and endosomes that may create a robust network for degrading viral particles in the CME pathway will be important to develop therapeutic interventions to combat SARS-CoV-2 infection and spread.
Possible mechanisms of SARS-CoV-2 entry into the CNS
The tropism of CoV is determined by the presence of ACE2 and its accessory proteins, which are present on the cells of the lungs, kidneys, small intestines, airway, vascular endothelia (Hamming et al., 2004; Hoffmann et al., 2020; Lukassen et al., 2020) and the brain (Gallagher et al., 2006), including the substantia nigra, ventricles, and middle temporal gyrus (Xia and Lazartigues, 2008); astrocytes, glia, and oligodendrocytes (Chen et al., 2021). This raises the possibility of SARS-CoV-2 infection throughout the CNS. The potential mechanism of SARS-CoV-2 entry and spread in the CNS are discussed below according to the known routes of SARS-CoV (Netland et al., 2008) and in some recent experimental studies specific for SARS-CoV-2 (Baig et al., 2020; Hasanagic and Serdarevic, 2020; Keyhanian et al., 2020; Li et al., 2020; Zanin et al., 2020).
How SARS-CoV-2 invades the CNS is not clear and remains to be established, but possible neuroinvasive mechanisms include hematologic spread, retrograde transport from the peripheral nervous system (PNS), and blood–brain barrier (BBB)-mediated spread. Studies on CoVs strongly favor retrograde neuronal transport as a viable route for viral invasion of the CNS (Guadarrama-Ortiz et al., 2020; Li et al., 2020; Pennisi et al., 2020). SARS-CoV-2 can enter the CNS through direct infection of the olfactory peripheral nerve terminals and other cranial nerves followed by retrograde transport (Keyhanian et al., 2020; Zubair et al., 2020). Virus transport along axons can occur by microtubules, and transport across synapses can occur by vesicular release (Baig et al., 2020). In addition, ACE2 interaction with integrin β-1, which has been suggested to play a key role in neural functions such as myelination, axon guidance, and cell adhesion (Sardar et al., 2020), might play an important role in CoV-2 CNS entry and spread. This area of study should be considered to further understand how the ACE2 receptors in the CNS connect CoV-2 and neurologic manifestations (Baig, 2020; Ellul et al., 2020; Pennisi et al., 2020).
ACE2 is expressed in the capillary endothelium of the BBB (Hamming et al., 2004), providing another access point for CoVs into the CNS (Guadarrama-Ortiz et al., 2020; Pennisi et al., 2020; Zubair et al., 2020). It remains unclear whether SARS-CoV-2 can cross the BBB; however, a recent study showed that intravenously injected SARS-CoV-2 S1 protein crossed the BBB in mice and advanced to the parenchymal brain region. The S1 protein entry into the CNS via the BBB requires ACE2 (Rhea et al., 2021). This presents the possibility that SARS-CoV-2 infects ACE2-expressing vascular endothelial cells at the BBB to gain access to the CNS (Achar and Ghosh, 2020; Buzhdygan et al., 2020; Pellegrini et al., 2020). SARS-CoV-2 could also infect leukocytes and enter the BBB via these cells. (Guadarrama-Ortiz et al., 2020; Pennisi et al., 2020; Zubair et al., 2020). Systemic virus dissemination in the CNS could also occur via exosomal cellular transport and lymphatic spread following infection, immune activation, and production of granulocyte-macrophages (Alenquer and Amorim, 2015). Notably, the inflammation associated with COVID-19 increases the possibility that infected cells and the virus can enter the CNS through the BBB (Guadarrama-Ortiz et al., 2020; Pennisi et al., 2020; Zubair et al., 2020). Furthermore, SARS-CoV-2-induced BBB damage may contribute to the neurologic disorders associated with COVID-19. Studies are needed to understand this phenomenon.
After crossing the BBB, the virus might be taken up by brain cells via CME. Neurons use a CME mechanism similar to that used in non-neuronal cells (Fig. 1); however, endocytosis at the synapses involves different isoforms of these endocytic proteins, including splice variants of clathrin light and heavy chains, auxilin, AP180, intersectin, and dynamin-1 (Rappoport et al., 2004, 2005). In respiratory tissues, TMPRSS2 (Hoffmann et al., 2020), human airway trypsin-like protease (HAT), and the pH-dependent cysteine proteases cathepsin B/L are important for virus infectivity. HAT and TMPRSS2 are coexpressed with the viral host receptor ACE2 in bronchial epithelial cells and pneumocytes (Lukassen et al., 2020). These proteases belong to the family of type II transmembrane serine proteases (TTSPs) that share a common domain structure, but also possess variable domains that allow them to perform different physiological functions in different tissues (Bugge et al., 2009; Damalanka and Janetka, 2019; Hoffmann et al., 2020; Lukassen et al., 2020). The expression and activity of TTSPs in neurons are poorly characterized. It was previously shown that the CoV-S protein could only be cleaved and activated in host cells when coexpressed with TMPRSS2 (Hoffmann et al., 2020; Lukassen et al., 2020). Since TMPRSS2 expression is not detectable in the brain (Fagerberg et al., 2014), we hypothesize that different TTSPs might be involved in CoV-S protein activation during CME-dependent viral entry into neurons (Fig. 1). While both cathepsin and TMPRSS2/HAT can cleave and activate CoV-S, only TMPRSS2 activity seems to be required for viral spread in the host (Hoffmann et al., 2020). Other TTSPs such as TMPRSS1, TMPRSS3, and TMPRSS5 have been shown to be expressed in neurons (Bugge et al., 2009; Damalanka and Janetka, 2019), but have yet to be associated with SARS-CoV-2 infections. Further studies will be necessary to determine whether these TTSPs, and any other proteases expressed in the CNS, are necessary for SARS-CoV-2 infection in neurons. Identification of the CME components in the CNS (especially neurons) that are involved in SARS-CoV-2 entry will be critical for developing therapeutic interventions to prevent virus spread in the CNS and mitigate COVID-19 neurologic complications.
Neurologic manifestations
While there has been improvement in treatments of COVID-19 and mitigation of the spread by vaccination, there are still concerning reports of long-term neurologic sequelae that need urgent attention (Mao et al., 2020). The neurologic manifestations of COVID-19 vary extensively from patient to patient, and involve both the CNS and the PNS (Carod-Artal, 2020; Filatov et al., 2020; Guadarrama-Ortiz et al., 2020; Mao et al., 2020; Wang et al., 2020). We aim to provide an update and discuss the mechanisms involved in the development of some of the COVID-19 neurologic manifestations.
PNS effects: anosmia, ageusia, and Guillain Barre syndrome
Some patients reported anosmia and ageusia. The presence of anosmia may be caused by the direct viral infection of the olfactory nerve. This may also result in retronasal olfactory dysfunction, which could cause ageusia. However, other possibilities for the cause of ageusia remain, such as the presence of ACE2 in oral cavity mucosa and tongue epithelia, or some direct effect of SARS-CoV-2 on gustatory receptors (Baig, 2020; Carod-Artal, 2020; Filatov et al., 2020; Guadarrama-Ortiz et al., 2020; Kanjanaumporn et al., 2020). Some patients present with Guillain-Barré syndrome, also known as acute inflammatory demyelinating polyneuropathy (AIDP), or other peripheral nerve disorders, including acute motor and sensory axonal neuropathy (AMSAN) or acute motor axonal neuropathy (AMAN; Carod-Artal, 2020; Ellul et al., 2020). AIDP is suggested to be caused by an immune attack on the peripheral nerve myelin sheath, while AMSAN and AMAN are caused by an immune attack on peripheral nerve axons. The manifestations of these syndromes typically include muscle weakness, radicular or muscle pain, a reduction in reflexes, and a reduction in sensory abilities (Keyhanian et al., 2020). Guillain-Barré syndrome following SARS-CoV-2 infection may also be a result of a molecular mimicry mechanism (Yuki and Hartung, 2012), as SARS-CoV-2 may have epitopes (nucleocapsid and Orf1ab Polyprotein) similar in structure to components of peripheral nerves, especially ganglioside peptides or heat shock proteins, which are associated with Guillain-Barré syndrome and other autoimmune diseases (Ang et al., 2004; Lucchese and Flöel, 2020). Thus, antibodies generated to combat SARS-CoV-2 may also bind to peripheral nerves and cause neuronal dysfunction (Ang et al., 2004; Yuki and Hartung, 2012; Kajumba et al., 2020; Lucchese and Flöel, 2020). However, detailed experimental data are needed to further understand the presence and mechanisms of anosmia, ageusia, and Guillain-Barré syndrome in COVID-19 patients.
Seizure, stroke, and neuroinflammation
Seizures exhibited by patients following SARS-CoV-2 infection may be a result of infection-induced hyperinflammation (see discussion below), as the release of proinflammatory cytokines can allow for seizures to more easily occur (Devinsky et al., 2013; Rana and Musto, 2018; Najjar et al., 2020). These seizures may also be caused by other neurologic manifestations of COVID-19, including ischemic or hemorrhagic strokes (Myint et al., 2006; Najjar et al., 2020).
Recent clinical studies also show that stroke in patients may be the result of cerebral vasculature dysfunction following infection (Mao et al., 2020). Stroke might be prevalent in patients with severe COVID-19 that have a history of coagulation disorders. These coagulation disorders can increase the risk of thrombotic events and death (Myint et al., 2006; Guadarrama-Ortiz et al., 2020). Abnormal coagulation and thrombosis induced by the presence of SARS-CoV-2 have been observed in some COVID-19 patients (Levi et al., 2020), and these pathologies may also cause stroke and other neurologic disorders. Acute myocarditis, which has also been reported in cases of COVID-19, may also cause stroke through its promotion of brain embolization (Guadarrama-Ortiz et al., 2020; Inciardi et al., 2020; Sala et al., 2020).
In addition, some COVID-19 patients present with encephalopathy, including acute hemorrhagic necrotizing encephalopathy. The main manifestations of encephalopathy are impaired attention and arousal, resulting in delirium, confusion, lethargy, or coma (Frontera, 2012; Zubair et al., 2020). Some toxic and metabolic factors that can increase the risk of COVID-19-related encephalopathy are hyponatremia (an abnormally low sodium concentration in the blood), hypernatremia (an abnormally high concentration of sodium in the blood), hypocalcemia (an abnormally low concentration of calcium in the blood), hypercalcemia (an abnormally high concentration of calcium in the blood), hypoglycemia (an abnormally low concentration of glucose in the blood), hyperglycemia (an abnormally high concentration of glucose in the blood), renal dysfunction, and liver dysfunction (Krishnan et al., 2014; Mehta et al., 2020; Pennisi et al., 2020).
Psychiatric manifestations
A study by Mazza et al. (2020) found that 55% of a cohort of 402 COVID-19 patients presented with at least one psychiatric disorder. Some of these manifestations include delirium, cognitive impairments, mood alterations, anxiety disorders, depression, suicidal ideations, post-traumatic stress disorder (PTSD), insomnia, psychosis, and schizophrenia (Brown et al., 2020; Keyhanian et al., 2020; Mazza et al., 2020). In other clinical reports, COVID-19 patients have been shown to develop hypokinetic delirium (Rozzini et al., 2020). Self-rated symptoms in the psychopathological range include 30% for PTSD, 40% for depression, 40% for anxiety, 20% for obsessive-compulsive disorder symptoms, and 40% for insomnia (Mazza et al., 2020). A study by Brown et al. (2020) found that ∼4% of COVID-19 patients presented with psychosis. The neuroinflammation and hypoxia characteristic of COVID-19 may lead to psychiatric sequalae (Sultana and Ananthapur, 2020). Cognitive impairments may also be a result of encephalopathy or neuroinflammation. However, the psychiatric sequelae of COVID-19 may be a result of psychosocial factors such as social distancing and economic recession. The latter reason may explain the rise of mental health disorders in both the infected and noninfected populations (Dantzer, 2018; Baig, 2020; Baig et al., 2020; Brown et al., 2020; Sultana and Ananthapur, 2020; Zubair et al., 2020). Another commonly occurring complication is impaired consciousness (Ahmed et al., 2014; Filatov et al., 2020; Najjar et al., 2020; Pennisi et al., 2020; Zubair et al., 2020). The COVID-19 neurologic complications keep evolving, and there is an urgent need for treatments for patients even after recovering from the infection.
Mitochondria and immune response to SARS-CoV-2
Because of widely distributed axonal network and dense synaptic connections, neurons require high bioenergy production, suggesting a high degree of mitochondrial activity (Kim et al., 2013; Ando et al., 2017; Scott et al., 2017). Many neurons are vulnerable to degeneration because of the substantial mitochondrial stress (Scarffe et al., 2014). Thus, maintaining healthy active mitochondria is critical for neuronal survival, and targeting mitochondria for COVID-19 therapeutic treatment could potentially be a major contributor in preventing neuronal complications associated with COVID-19 (Malavolta et al., 2020; Singh et al., 2020).
The CoV open reading frame 9 (ORF-9) protein has been shown to interact with mitochondrial outer membrane receptors, including mitochondrial antiviral signaling systems (MAVS; Shi et al., 2014) and the translocase of the outer mitochondrial membrane (TOM) complex (Miserey-Lenkei et al., 2021). These CoV interacting mitochondrial proteins function as viral recognition receptors in the cytosol and at the mitochondria during viral replication (Malavolta et al., 2020). At the mitochondria, ORF-9 triggers the degradation of MAVS and Drp1, a protein involved in mitochondrial fission, leading to mitochondrial hyperelongation (Shi et al., 2014). CoV ORF-9 is also suggested to induce the release of mitochondrial DNA (mtDNA) and to activate mtDNA-induced inflammasome (Singh et al., 2020), limiting the initial host cell innate immune antiviral response (Shi et al., 2014; Fitzpatrick, 2019; Gordon et al., 2020). Thus, people with health conditions (e.g., type 2 diabetes, Parkinson's disease, and Alzheimer's disease) associated with declined mitochondrial functions and/or mitochondrial stress (Dawson and Dawson, 2017; Cenini and Voos, 2019) may be vulnerable to SARS-CoV-2 infection and COVID-19 health complications to mortality.
Mitochondrial ROS-dependent oxidative stress and hyperinflammation
Mitochondrial functions include oxidative phosphorylation, intracellular calcium and iron regulation, ATP synthesis, regulation of reactive oxygen species (ROS), and apoptosis (Kim et al., 2013; Roca-Agujetas et al., 2019). Several recent clinical reports and studies link increased progression of COVID-19 to hyperinflammatory states involving major systemic distress that may impact mitochondrial function and contribute to the progression and severity of the disease (Najjar et al., 2020; Terrazzano et al., 2020). In addition, oxidative stress has been associated with inflammation and the antiviral immune response (Roca-Agujetas et al., 2019; Korakas et al., 2020). Despite its critical role in regulating innate immunity and inflammatory responses, the role of mitochondria in COVID-19 pathogenesis and management is not well characterized.
We suggest that one of the major source of ROS that may be linked to cellular oxidative stress in COVID-19 is the mitochondria (Rossman et al., 2018; Roca-Agujetas et al., 2019). The increased levels of mitochondrial ROS during viral infection are induced by excessive production of inflammatory cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-6, and IL-10 (Singer, 2014) that are found in COVID-19 patient serum (Korakas et al., 2020; Mehta et al., 2020; Zabetakis et al., 2020). The early state of viral infection consists of an initial acute hyperinflammatory phase to kill and control the virus spread. The entry of virus induces mitochondria to release ROS, which stimulate proinflammatory cytokine production to help fight the virus. In healthy individuals, the acute hyperinflammatory phase is followed by an immune-tolerant phase to clear viral particles and cellular recovery (Singer, 2014; Fitzpatrick, 2019).
Mitochondria can be very vulnerable during the hyperinflammatory phase of sepsis, which involves increased oxygen consumption, elevated ATP production, hyperglycemia, stimulation of increased cytokine production recovery (Singer, 2014; Fitzpatrick, 2019). The high bioenergy demand during the hyperinflammatory phase of sepsis suggests a high degree of mitochondrial stress (Kim et al., 2013; Ando et al., 2017; Scott et al., 2017). In addition, innate immune cells prolonging the hyperinflammatory phase of sepsis can cause excessive mitochondrial stress and eventually cell death. The immune-tolerant phase is characterized by a reduced inflammatory response and increased mitochondrial biogenesis as cells enter into a hypometabolic state with reduced oxygen consumption and ATP production (Frontera, 2012; Singer, 2014; Fitzpatrick, 2019; Mehta et al., 2020).
However, COVID-19 patients with dysfunctional mitochondria are likely to exhibit a prolonged hyperinflammatory phase of sepsis, which may cause increased production of proinflammatory cytokines resulting in increased cell death (Mehta et al., 2020). The ROS-induced mitochondrial stress negatively affects mitochondrial metabolism and ATP synthesis and increases mitochondrial fragmentation (Scott et al., 2017; Ge et al., 2020). Thus, high-risk COVID-19 patients may be more susceptible to the spread of the virus because the dysfunctional mitochondria are unable to keep up with the sudden high energy demand associated with the prolonged hyperinflammatory phase. Cells with dysfunctional mitochondria may also have an impaired immune-tolerant phase repair responses, as well as reduced responsiveness to treatments (Conti et al., 2019; Mehta et al., 2020; Yang et al., 2020). Thus, the interplay between inflammation and mitochondrial ROS-dependent oxidative stress are important for regulating inflammatory and antiviral immune responses.
Excess ROS production can be counteracted by antioxidants, such as vitamins C and E, as well as redox-active formulas like the mitochondrial-targeted antioxidant MitoQ (Apostolova and Victor, 2015). MitoQ improves cellular function by reducing mitochondrial ROS and ameliorates ROS-associated complications in animal models (Rossman et al., 2018). It also has the potential to inactivate the immunocompromised state that plays a detrimental role in the viral pathway of COVID-19. Careful consideration will be necessary to evaluate these drugs in COVID-19 patients.
Iron and mitochondrial dysfunction
The role of iron in mitochondrial dysfunction is well characterized, and there is evidence that hyperferritinemia is directly associated with COVID-19 severity (Najjar et al., 2020). Alterations in iron and ferritin levels have been established as COVID-19 biomarkers to determine illness severity and outcomes of the disease (Gómez-Pastora et al., 2020; Perricone et al., 2020). Similar to ROS, iron-mediated oxidative stress plays an important role in the mitochondria-dependent immunity response; disruption of iron levels or mitochondrial iron metabolism can result in cellular stress and death (Ellul et al., 2020; Gómez-Pastora et al., 2020; Perricone et al., 2020). High levels of ferritin impair mitochondrial functions by reducing mitochondrial oxygen consumption and increasing ROS levels and cellular stress. Elevated levels of iron in COVID-19 patients interfere with cellular integrity, including membrane fluidity and permeability, and impair cellular respiratory function in alveolar and cardiac myocytes, which leads to respiratory failure and eventually cellular death (Zhou et al., 2020). The current clinical reports further confirm that SARS-CoV-2 is capable of triggering a form of programmed cell death, ferroptosis, that depends on iron accumulation in the bronchial epithelium and in macrophages via hyperferritinemia (Gómez-Pastora et al., 2020; Hanff et al., 2020; Najjar et al., 2020; Perricone et al., 2020).
Iron depletion therapy as potential treatment for COVID-19
Recent reports suggest that neurons are also vulnerable to ferroptosis, and that this might be one of the major causes of the neurologic manifestations of COVID-19 (Carod-Artal, 2020; Filatov et al., 2020; Guadarrama-Ortiz et al., 2020; Mao et al., 2020). We therefore propose iron depletion therapy, in addition to other therapeutic approaches, for COVID-19 patients, especially those in the intensive care unit. An iron depletion therapy for COVID-19 treatment using clinically approved chelating agents, such as deferoxamine, deferiprone, and deferasirox (van Asbeck et al., 2001; Traore and Meyer, 2004; Temraz et al., 2014), should be investigated. These chelating agents have been shown to effectively promote ferritin degradation in lysosomes or to chelate cytosolic iron for degradation by proteasomes. Furthermore, this therapeutic approach has been shown to have beneficial effects on other viral infections (van Asbeck et al., 2001; Traore and Meyer, 2004; Temraz et al., 2014). However, an analysis of the pharmacodynamic mechanisms of the treatments with these chelating agents should be carefully considered to avoid exploitation of the iron chelators by the virus, as observed in some studies (Lehmann et al., 2015).
mTOR, mitochondria–lysosome contacts in SARS-CoV-2 clearance
Malfunction of the mTOR pathway has been implicated in several neurologic disorders and has emerged as a key target in the search for drug targets for COVID-19 (Appelberg et al., 2020; Bolourian and Mojtahedi, 2020; Tang et al., 2021). mTOR is a ubiquitous and highly conserved protein kinase that associates with several proteins to achieve kinase activity through two distinct complexes, complex 1 (mTORC1) and complex 2 (mTORC2). The mTORC1 pathway is key in the regulation of neuronal function, growth, and other cellular processes, such as autophagy, related to virus clearance (Franz and Krueger, 2018; Ryskalin et al., 2018; Schubert-Bast et al., 2019). In addition to regulating cell survival, mTOR is suggested to have roles in cytoskeleton organization and cell migration (Jain et al., 2014; Bockaert and Marin, 2015; Abu-Remaileh et al., 2017; Ryskalin et al., 2018; Perucca and Perucca, 2019). Recent studies suggest that mTOR plays an important role in mitochondria–lysosome contacts that allow bidirectional regulation of both mitochondrial and lysosomal dynamics (Wong et al., 2018; Bartel et al., 2019). The mitochondria–lysosome contact is also suggested to play a key role in mTOR-dependent autophagy (Efeyan et al., 2013; Zhao et al., 2015; Ryskalin et al., 2018). Neurons with autophagic lysosome reformation dysfunction are unable to maintain functional lysosomes required for autophagic clearance (Magalhaes et al., 2016). The role of mTOR in controlling virus spread is not well documented, but hypothesizes that patients with mTOR-mediated lysosome reformation dysfunction are likely to have increased virus spread in the CNS, and this may result in severe neurologic complications. An investigation into the possibility of SARS-CoV-2 manipulating the mTOR-mediated mitochondria–lysosome contacts should lead to novel approaches to prevent and treat COVID-19.
Potential inhibition of SARS-CoV-2 cap-dependent translation modulated by mTORC1
CoVs protein synthesis is dependent on the hijacking of host cell cap-dependent translation mechanisms, and virus replication may affect mRNA translation and stability, impacting protein translation (Nakagawa et al., 2016). In the 5′ cap-dependent translation initiation process, the binding of ribosome complex to the 5′ end of mRNA is critical for protein synthesis. The eukaryotic initiation factor (eIF4F) complex assembles at the 5′ end of mRNA to mediate the proper binding of ribosomal subunits to mRNA. Given that coronavirus mRNAs have a 5′ cap structure, most coronaviruses are believed to undergo cap-dependent translation using eIF4F (Appelberg et al., 2020). Consistent with this, studies have found that blocking eIF4F assembly and activity by preventing the binding of the initiation factors that make up the complex (eIF4E and eIF4G) suppress human coronavirus-229E replication (Cencic et al., 2011). Thus, SARS-CoV-2 replication might be inhibited by blocking the assembly of the eIF4F complex.
The assembly of the eIF4F complex is modulated by the mTORC1 pathway. Specifically, mTORC1 modulates the activation of eIF4F by directly phosphorylating both 4EBP and the kinase S6K1. The phosphorylated 4EBP dissociates from eIF4E, thereby allowing eIF4F complex assembly (Sancak et al., 2008; Lawrence et al., 2018; Maiese, 2020). In addition, the phosphorylated S6K1 activates other substrates involved in translation initiation such as eIF4B, which promotes binding of the eIF4F complex to mRNA. Thus, therapeutic approaches (Kumar et al., 2021) to selectively inhibit these processes could potentially block 5′ cap-dependent mRNA translation-dependent SARS-CoV-2 replication.
mTOR/AK/MAPK as a therapeutic target for COVID-19
In more recent studies, dysregulated mTOR activity has been observed in COVID-19 patients with hyperactivation of the immune system response, which further calls for the need to effectively inhibit mTOR pathways in COVID-19 patients (Terrazzano et al., 2020). In fact, the mTOR pathway has been linked to the development of many viral diseases in vitro. For example, Kindrachuk et al. (2015) suggest that ERK/MAPK and PI3K/AKT/mTOR signaling are crucial to the pathogenesis of the Middle East Respiratory Syndrome (MERS)-CoV. In that study, treating cells with mTOR inhibitors inhibited MERS-CoV infection by 60% (Kindrachuk et al., 2015). Furthermore, a study by Appelberg et al. (2020) found that the SARS-CoV-2 can modulate the Akt–mTOR–HIF signaling pathway at various levels to promote its infection. Other studies have implicated the PI3K–Akt–mTOR pathway in the activation of type I IFNs, which are cytokines that regulate antiviral immunity.
Because of their roles in promoting the proliferation of proinflammatory cytokines, mTOR inhibition and p53 activation have been suggested as effective methods of controlling hyperinflammation in COVID-19 patients (Ramaiah, 2020). The mTOR–NLRP3-IL–1β pathway is suggested to be associated with the cytokine storm via IL-6, a cytokine that is associated with a more severe prognosis in COVID-19 patients (Korakas et al., 2020; Mehta et al., 2020; Zabetakis et al., 2020). In addition, inhibition of mTORC1 enhances the T-cell stimulatory activity of dendritic cells and promotes autophagy of macrophages, while also reducing the population of antigen-specific memory B cells after B-cell activation (Xing et al., 2019; Appelberg et al., 2020; Zheng et al., 2020). Moreover, a recent study conducted by Tang et al. (2021) found that mTORC1 is hyperactivated in lymphangioleimyomatosis (LAM), leading to an increased expression of IL-6 in LAM-associated fibroblasts as well as an upregulation of ACE2 in type II pneumocytes, making these cells susceptible to CoV-2 infection (Mehta et al., 2020; Zabetakis et al., 2020). It was observed that mTORC1 inhibitor treatment significantly downregulated both type I and type II IFN pathways, as well as downregulating IL-6-induced acute-phase response genes that were previously observed to be enriched in cells expressing ACE2. This further suggests that mTORC1 inhibition may be a viable treatment for COVID-19 because of its ability to decrease IFN and IL-6 pathways, promoting the expression of ACE2 (Conti et al., 2019; Mehta et al., 2020; Yang et al., 2020; Zabetakis et al., 2020).
Another possible treatment method for COVID-19 that has garnered attention is the possibility of inhibiting mTOR activity via both mTORC1 and mTORC2 pathways while promoting AMPK activation. The mTOR–AMPK pathway is associated with oxidative stress, mitochondrial dysfunction, and immune system maintenance. AMPK can inhibit mTORC1 via its interactions with other complexes that block mTORC1 activity, such as hamartin, which is associated with tuberous sclerosis (Appelberg et al., 2020; Zheng et al., 2020). In recent studies, IL-37 has been observed to be immunosuppressive through mTOR while promoting AMPK activity, which ameliorates hyperinflammation in severe COVID-19 symptoms (Maiese, 2020). Compounds such as metformin, which can block mTOR activity and may promote AMPK activity, have been suggested as possible treatments that target this pathway. However, AMPK/mTOR dysregulation, such as through metformin, have also been shown to worsen the prognosis for some diseases (Kim et al., 2013; Cuyas et al., 2014; Head et al., 2017; Azar et al., 2020). Thus, careful consideration of patients' medical conditions and history is critical in making the decision to use such treatments. Interestingly, rapamycin was shown to enhance the magnitude and quality of viral-specific CD4 T-cell responses. However, only Akt inhibitor MK-2206 showed significant inhibition of viral replication compared with other mTORC1 inhibitors that failed to block viral infections (Appelberg et al., 2020).
Use of mTOR inhibitors in treatment of COVID-19 patients
Other mTOR inhibitors that target the mTORC1 pathway, including everolimus, sirolimus, Torin-1, and MK-2206, are being investigated as potential drugs for COVID-19 patients. Sirolimus was previously shown to be effective in inhibiting MERS-CoV infection in rodents (Maiese, 2020). Everolimus is a second-generation rapamycin analog (Terrazzano et al., 2020) that inhibits mTORC1 and regulates mRNA translation, ribosome synthesis, protein synthesis, mitochondrial metabolism, and adipocyte formation (Dunlop and Tee, 2009). Further studies suggest that everolimus treatments could alleviate the hyper-reactivity of cytokine storm in COVID-19 patients and therefore reduce the severity of the viral infection. Everolimus has also been used to reduce viral replication in organ transplantation and cancer patients (Terrazzano et al., 2020). This makes everolimus a promising candidate for testing for COVID-19 therapy.
One alternative to using an mTOR inhibitor-based treatment is to devise a treatment for COVID-19 using drugs that mimic the effects of Niemann–Pick type C (NPC) disease. The NPC disease involves improper functioning of the NPC1 protein, which impairs the ability of many viruses to enter host cells (Sturley et al., 2020a,b). Alteration in lysosome and endoplasmic reticulum quality control pathways in NPC1 and its dependent late endosome/lysosome lipid pathway is suggested to play an important role in coronavirus invasion and spread (Sturley et al., 2020a,b). This association of NPC1 with viral infections makes the protein another novel target that could be used effectively to fight SARS-CoV-2 (Xu et al., 2010; Sturley et al., 2020b). The drug itraconazole targets the mitochondrial protein VDAC1 and inhibits the lysosomal protein NPC1 and stimulates AMPK activation and cholesterol trafficking, resulting in synergistic inhibition of mTOR (Schultz et al., 2016; Head et al., 2017). Another promising approach is using NPC1 inhibitors, which have been shown to block viral entry into the cell (Liu et al., 2020; Sturley et al., 2020a).
Although mTOR inhibitors, such as rapamycin and its analogs, are currently being used to treat certain disorders, there are still concerns about their effects on other nontargeted cellular functions. For example, Shi et al. (2018) show that rapamycin downregulates IFN-induced transmembrane (IFITM) proteins and enhances viral infection. IFITM2 and IFITM3 are localized at the plasma membrane and function as antiviral factors that inhibit virus infection through a lysosomal degradation pathway (Spence et al., 2019). Thus, the inhibition and/or downregulation of antiviral proteins by mTOR inhibitors in COVID-19 patients may pose the greatest challenge in using this therapeutic approach to combat COVID-19. Another major setback with an mTOR inhibitor, rapamycin, is its toxic side effects to the lungs. Biguanides, instead of rapamycin, is recommended, since biguanides is shown to have minimal toxic effects (Lehrer, 2020). Although this treatment has been successful in improving the prognosis of those infected with certain viruses, another approach may be needed to target specific substrates or downstream signaling in the mTOR pathway to ensure that treated patients are not left immunocompromised.
Other potential therapeutic approaches
The mTORC1 pathway is upregulated by the presence of nutrient signals and downregulated following nutrient starvation (Kim et al., 2002; Cuyas et al., 2014; Rakhmanova et al., 2018). Several studies have found that obesity and overnutrition lead to chronic hyperactivation of mTOR in various tissues, and to increased S6K activity and overphosphorylation of 4EBP (Zoncu et al., 2011; Efeyan et al., 2013; Kim et al., 2013; Cuyas et al., 2014; Demetriades et al., 2016). Diabetes caused by obesity is associated with low-grade chronic inflammation, which may escalate to hyperinflammation on infection with SARS-CoV-2 (Korakas et al., 2020; Zabetakis et al., 2020). While reports have shown a strong association between the severity of COVID-19 and obesity in the absence of comorbidities, it should also be noted that the noncommunicable diseases shown to be risk factors for more severe infection by COVID-19 are characterized by systemic inflammation. These include diabetes mellitus and cardiovascular diseases, both of which are common comorbidities in obese individuals (Bolourian and Mojtahedi, 2020; Korakas et al., 2020; Zabetakis et al., 2020). Cytokines TNF, IL-1, IL-6, and MCP-1 (monocyte chemoattractant protein-1) are produced in increased amounts, leading to oxidative stress and defective immunity in obese individuals with COVID-19. The mTOR antagonist tocilizumab has been used to reduce inflammation caused by cytokine storms, and one study associated its use with a significantly shorter duration of vasopressor support for patients with severe COVID-19 (Guaraldi et al., 2020; Kewan et al., 2020). In a retrospective, observational cohort study involving 544 patients admitted to care with severe COVID-19 pneumonia, 179 patients were treated with tocilizumab and 365 were treated with standard care. There was no significant difference in mechanical ventilation required by patients with or without tocilizumab treatments. However, 20% of patients in the standard care group died compared with 7% of those treated with tocilizumab (Guaraldi et al., 2020). The result is very promising; however, further clinical investigation is needed to characterize tocilizumab as a potential drug for treatment of COVID-19.
Proinflammatory cytokines such as IL-Iβ, which are expressed in microglia and astrocytes, are suggested to cause an increase in neurotransmitters (e.g., glutamate and aspartate) levels (Viviani et al., 2003; Alyu and Dikmen, 2017). We hypothesize that the cytokine storms observed in a COVID-19 patient may cause excessive increase levels of glutamate and other neurotransmitters. Since glutamate is an excitatory neurotransmitter that plays a key role in neural plasticity (Chater and Goda, 2014; Selvakumar et al., 2014; Pignatelli et al., 2017; Umanah et al., 2017), we suggest that excessive glutamate released by SARS-CoV-2-induced neuroinflammation correlates with the excitotoxic damage of neurons in COVID-19 patients. This excitotoxic damage is suggested to be associated with neurologic manifestations, such as epileptic seizures and encephalopathy, in COVID-19 patients (Moriguchi et al., 2020; Zanin et al., 2020). A recent study proposed glutamate receptor antagonists, such as memantine, a noncompetitive antagonist of NMDA glutamate receptors to reduce glutamate excitotoxicity in the CNS (Hasanagic and Serdarevic, 2020). Memantine has been used successfully to treat different neuropsychiatric disorders, such as dementia, epilepsy, autism, schizophrenia, and depression (Perucca and Perucca, 2019). Interestingly, in human cells and primary murine neuronal cultures, memantine was found to inhibit replication of the human coronavirus strain OC43 (HcoV-OC43; Brison et al., 2014). Memantine was also found to have anti-inflammatory effects by suppressing cytokine expression and the release of tumor necrosis factors, interleukin, and proinflammatory proteins. Thus, memantine would be an interesting drug to further study with SARS-CoV-2 as it has potential to regulate excitotoxic effects of glutamate and neuroinflammation (Povysheva and Johnson, 2016; Hasanagic and Serdarevic, 2020). Like memantine, perampanel is another drug that functions as an antagonist for AMPA glutamate receptors, regulating glutamate levels within the CNS. Our recent studies suggest that perampanel selectively inhibits overactivated AMPA receptors (Ahrens-Nicklas et al., 2017; Umanah et al., 2017). Thus, selective/specific modulators of neurotransmitters are other interesting drugs to study because of the potential positive effects they may have in treating SARS-CoV-2-induced neuroinflammation in COVID-19 patients with neurologic complications.
Summary
Based on the extensive neurologic and psychiatric sequelae of COVID-19 reported in many patients, it appears as though SARS-CoV-2 is neurotropic. Mechanisms of SARS-CoV-2 entry into the nervous system are suggested to include retrograde transport along peripheral nerves and the hematogenous pathway via the blood–brain barrier. These mechanisms are not clear and must be studied further to fully understand how SARS-CoV-2 invades the CNS. The presence of the virus in specific regions of the brain, but its absence in other areas, at the early stages of the disease could provide clues to the route of the virus from the respiratory system to the CNS. The neurologic manifestations of COVID-19 and post-COVID-19 neurologic complications keep evolving and need urgent attention. Further, studies to address whether the virus is able to infect certain regions of the brain, especially the substantia nigra, could provide critical information on a late development of other neurologic disorders such as Parkinson's disease in COVID-19 patients.
Although some COVID pathologies arise from defects in mitochondrial ion and ROS homeostasis, targeting mitochondrial dynamic and quality control systems has yet to be extensively studied in COVID-19. We hypothesize that several of the neuronal complications in COVID-19 patients may be because of substantial bioenergetic cost and overwhelming hyperinflammation resulting in mitochondrial damage and stress. In addition, the role of mitochondria in COVID-19 may be associated with the mTOR signaling pathway and lysosome–autophagy processes to clear virus at the early stage of sepsis.
We propose that mTOR and mitochondria-targeted modulators may play a major role in treating patients with COVID-19 through the following mechanisms. First, the chronic hyperactivation of mTOR may be reduced by downregulating mTOR, which might reduce the accelerated translation that occurs during sepsis, thereby reducing the rate of viral translation and replication. Second, selectively inhibiting specific mTOR targets may alleviate the hyperinflammation and improve the prognosis of patients with COVID-19. Third, modulators of mitochondria function and ROS/iron-chelating agents may be used to reduce the abnormal mitochondrial stress. Given the role of mitochondria and mTOR in preventing viral replication and dissemination, combinations of therapeutic approaches targeting these pathways will most likely be effective. However, careful consideration should be given to the stage of the disease at which treatment is administered. We also recommend extensive neurologic evaluations of all COVID-19 patients who recover from moderate to severe symptoms, especially those returning from intensive care units. Further studies are urgently required to help provide the necessary information and understanding of the mechanisms in SARS-CoV-2 invasion and spread in the nervous system. These may lead to the development of novel therapeutic approaches to combat the current COVID-19 pandemic and any future pandemic crisis.
Footnotes
This work was supported by grant from the National Institutes of Health/National Institute of Neurological Disorders and Stroke Grant NS-099362 to G.K.E.U. Support was also provided by the Neurology and Neuroscience Departments, The Johns Hopkins University, and The Johns Hopkins University School of Medicine Institute of Cell Engineering,.
The authors declare no competing financial interests.
References
- Abu-Remaileh M, Wyant GA, Kim C, Laqtom NN, Abbasi M, Chan SH, Freinkman E, Sabatini DM (2017) Lysosomal metabolomics reveals V-ATPase- and mTOR-dependent regulation of amino acid efflux from lysosomes. Science 358:807–813. 10.1126/science.aan6298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achar A, Ghosh C (2020) COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain relevance. Cells 9:2360. 10.3390/cells9112360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Leurent B, Sampson EL (2014) Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing 43:326–333. 10.1093/ageing/afu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahrens-Nicklas RC, Umanah GK, Sondheimer N, Deardorff MA, Wilkens AB, Conlin LK, Santani AB, Nesbitt A, Juulsola J, Ma E, Dawson TM, Dawson VL, Marsh ED (2017) Precision therapy for a new disorder of AMPA receptor recycling due to mutations in ATAD1. Neurol Genet 3:e130. 10.1212/NXG.0000000000000130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alenquer M, Amorim MJ (2015) Exosome biogenesis, regulation, and function in viral infection. Viruses 7:5066–5083. 10.3390/v7092862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alyu F, Dikmen M (2017) Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr 29:1–16. 10.1017/neu.2016.47 [DOI] [PubMed] [Google Scholar]
- Ando M, Fiesel FC, Hudec R, Caulfield TR, Ogaki K, Górka-Skoczylas P, Koziorowski D, Friedman A, Chen L, Dawson VL, Dawson TM, Bu G, Ross OA, Wszolek ZK, Springer W (2017) The PINK1 p.I368N mutation affects protein stability and ubiquitin kinase activity. Mol Neurodegener 12:32. 10.1186/s13024-017-0174-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang CW, Jacobs BC, Laman JD (2004) The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol 25:61–66. 10.1016/j.it.2003.12.004 [DOI] [PubMed] [Google Scholar]
- Apostolova N, Victor VM (2015) Molecular strategies for targeting antioxidants to mitochondria: therapeutic implications. Antioxid Redox Signal 22:686–729. 10.1089/ars.2014.5952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg S, Gupta S, Svensson Akusjärvi S, Ambikan AT, Mikaeloff F, Saccon E, Végvári Á, Benfeitas R, Sperk M, Ståhlberg M, Krishnan S, Singh K, Penninger JM, Mirazimi A, Neogi U (2020) Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells. Emerg Microbes Infect 9:1748–1760. 10.1080/22221751.2020.1799723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar WS, Njeim R, Fares AH, Azar NS, Azar ST, El Sayed M, Eid AA (2020) COVID-19 and diabetes mellitus: how one pandemic worsens the other. Rev Endocr Metab Disord 21:451–463. 10.1007/s11154-020-09573-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM (2020) Neurological manifestations in COVID-19 caused by SARS-CoV-2. CNS Neurosci Ther 26:499–501. 10.1111/cns.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM, Khaleeq A, Ali U, Syeda H (2020) Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci 11:995–998. 10.1021/acschemneuro.0c00122 [DOI] [PubMed] [Google Scholar]
- Bartel K, Pein H, Popper B, Schmitt S, Janaki-Raman S, Schulze A, Lengauer F, Koeberle A, Werz O, Zischka H, Müller R, Vollmar AM, von Schwarzenberg K (2019) Connecting lysosomes and mitochondria - a novel role for lipid metabolism in cancer cell death. Cell Commun Signal 17: 10.1186/s12964-019-0399-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockaert J, Marin P (2015) mTOR in brain physiology and pathologies. Physiol Rev 95:1157–1187. 10.1152/physrev.00038.2014 [DOI] [PubMed] [Google Scholar]
- Bolourian A, Mojtahedi Z (2020) Obesity and COVID-19: the mTOR pathway as a possible culprit. Obes Rev 21:e13084. 10.1111/obr.13084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison E, Jacomy H, Desforges M, Talbot PJ (2014) Novel treatment with neuroprotective and antiviral properties against a neuroinvasive human respiratory virus. J Virol 88:1548–1563. 10.1128/JVI.02972-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E, Gray R, Lo Monaco S, O'Donoghue B, Nelson B, Thompson A, Francey S, McGorry P (2020) The potential impact of COVID-19 on psychosis: a rapid review of contemporary epidemic and pandemic research. Schizophr Res 222:79–87. 10.1016/j.schres.2020.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugge TH, Antalis TM, Wu Q (2009) Type II transmembrane serine proteases. J Biol Chem 284:23177–23181. 10.1074/jbc.R109.021006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, Razmpour R, Hale JF, Galie PA, Potula R, Andrews AM, Ramirez SH (2020) The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol Dis 146:105131. 10.1016/j.nbd.2020.105131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carod-Artal FJ (2020) Neurological complications of coronavirus and COVID-19. Rev Neurol 70:311–322. 10.33588/rn.7009.2020179 [DOI] [PubMed] [Google Scholar]
- Cencic R, Desforges M, Hall DR, Kozakov D, Du Y, Min J, Dingledine R, Fu H, Vajda S, Talbot PJ, Pelletier J (2011) Blocking eIF4E-eIF4G interaction as a strategy to impair coronavirus replication. J Virol 85:6381–6389. 10.1128/JVI.00078-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenini G, Voos W (2019) Mitochondria as potential targets in Alzheimer disease therapy: an update. Front Pharmacol 10: 10.3389/fphar.2019.00902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chater TE, Goda Y (2014) The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci 8:401. 10.3389/fncel.2014.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wang K, Xing Y, Tu J, Yang X, Zhao Q, Li K, Chen Z (2014) Coronavirus membrane-associated papain-like proteases induce autophagy through interacting with Beclin1 to negatively regulate antiviral innate immunity. Protein Cell 5:912–927. 10.1007/s13238-014-0104-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Wang K, Yu J, Howard D, French L, Chen Z, Wen C, Xu Z (2021) The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol 11:573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioni JM, Lin JQ, Holtermann AV, Koppers M, Jakobs MAH, Azizi A, Turner-Bridger B, Shigeoka T, Franze K, Harris WA, Holt CE (2019) Late endosomes act as mRNA translation platforms and sustain mitochondria in axons. Cell 176:56–72.e15. 10.1016/j.cell.2018.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti P, D'Ovidio C, Conti C, Gallenga CE, Lauritano D, Caraffa A, Kritas SK, Ronconi G (2019) Progression in migraine: role of mast cells and pro-inflammatory and anti-inflammatory cytokines. Eur J Pharmacol 844:87–94. 10.1016/j.ejphar.2018.12.004 [DOI] [PubMed] [Google Scholar]
- Cuyas E, Corominas-Faja B, Joven J, Menendez JA (2014) Cell cycle regulation by the nutrient-sensing mammalian target of rapamycin (mTOR) pathway. Methods Mol Biol 1170:113–144. 10.1007/978-1-4939-0888-2_7 [DOI] [PubMed] [Google Scholar]
- Damalanka VC, Janetka JW (2019) Recent progress on inhibitors of the type II transmembrane serine proteases, hepsin, matriptase and matriptase-2. Future Med Chem 11:743–769. 10.4155/fmc-2018-0446 [DOI] [PubMed] [Google Scholar]
- Dantzer R (2018) Neuroimmune interactions: from the brain to the immune system and vice versa. Physiol Rev 98:477–504. 10.1152/physrev.00039.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL (2017) Mitochondrial mechanisms of neuronal cell death: potential therapeutics. Annu Rev Pharmacol Toxicol 57:437–454. 10.1146/annurev-pharmtox-010716-105001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetriades C, Plescher M, Teleman AA (2016) Lysosomal recruitment of TSC2 is a universal response to cellular stress. Nat Commun 7:10662. 10.1038/ncomms10662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Vezzani A, Najjar S, De Lanerolle NC, Rogawski MA (2013) Glia and epilepsy: excitability and inflammation. Trends Neurosci 36:174–184. 10.1016/j.tins.2012.11.008 [DOI] [PubMed] [Google Scholar]
- Diaz JH (2020) Hypothesis: angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J Travel Med 27:taaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop EA, Tee AR (2009) Mammalian target of rapamycin complex 1: signalling inputs, substrates and feedback mechanisms. Cell Signal 21:827–835. 10.1016/j.cellsig.2009.01.012 [DOI] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM (2013) Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493:679–683. 10.1038/nature11745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T (2020) Neurological associations of COVID-19. Lancet Neurol 19:767–783. 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg L, Hallstrom BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, Habuka M, Tahmasebpoor S, Danielsson A, Edlund K, Asplund A, Sjöstedt E, Lundberg E, Al-Khalili Szigyarto C, Skogs M, Takanen JO, Berling H, Tegel H, Mulder J, Nilsson P, Schwenk JM, et al. (2014) Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol Cell Proteomics 13:397–406. 10.1074/mcp.M113.035600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov A, Sharma P, Hindi F, Espinosa PS (2020) Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus 12:e7352. 10.7759/cureus.7352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick SF (2019) Immunometabolism and sepsis: a role for HIF? Front Mol Biosci 6:85. 10.3389/fmolb.2019.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz DN, Krueger DA (2018) mTOR inhibitor therapy as a disease modifying therapy for tuberous sclerosis complex. Am J Med Genet C Semin Med Genet 178:365–373. 10.1002/ajmg.c.31655 [DOI] [PubMed] [Google Scholar]
- Frontera JA (2012) Metabolic encephalopathies in the critical care unit. Continuum (Minneap Minn) 18:611–639. 10.1212/01.CON.0000415431.07019.c2 [DOI] [PubMed] [Google Scholar]
- Gallagher PE, Chappell MC, Ferrario CM, Tallant EA (2006) Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am J Physiol Cell Physiol 290:C420–426. 10.1152/ajpcell.00409.2004 [DOI] [PubMed] [Google Scholar]
- Gassen NC, Niemeyer D, Muth D, Corman VM, Martinelli S, Gassen A, Hafner K, Papies J, Mösbauer K, Zellner A, Zannas AS, Herrmann A, Holsboer F, Brack-Werner R, Boshart M, Müller-Myhsok B, Drosten C, Müller MA, Rein T (2019) SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun 10:5770. 10.1038/s41467-019-13659-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge P, Dawson VL, Dawson TM (2020) PINK1 and Parkin mitochondrial quality control: a source of regional vulnerability in Parkinson's disease. Mol Neurodegener 15:20. 10.1186/s13024-020-00367-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Pastora J, Weigand M, Kim J, Wu X, Strayer J, Palmer AF, Zborowski M, Yazer M, Chalmers JJ (2020) Hyperferritinemia in critically ill COVID-19 patients - Is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta 509:249–251. 10.1016/j.cca.2020.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, O'Meara MJ, Rezelj VV, Guo JZ, Swaney DL, Tummino TA, Hüttenhain R, Kaake RM, Richards AL, Tutuncuoglu, B, Foussard H, Batra J, Haas K, Modak M, Kim M, Haas P, et al. (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583:459–468. 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadarrama-Ortiz P, Choreno-Parra JA, Sanchez-Martinez CM, Pacheco-Sanchez FJ, Rodriguez-Nava AI, Garcia-Quintero G (2020) Neurological aspects of SARS-CoV-2 infection: mechanisms and manifestations. Front Neurol 11:1039. 10.3389/fneur.2020.01039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbì L, Girardis M, et al. (2020) Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol 2:e474–e484. 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203:631–637. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff TC, Mohareb AM, Giri J, Cohen JB, Chirinos JA (2020) Thrombosis in COVID-19. Am J Hematol 95:1578–1589. 10.1002/ajh.25982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanagic S, Serdarevic F (2020) Potential role of memantine in the prevention and treatment of COVID-19: its antagonism of nicotinic acetylcholine receptors and beyond. Eur Respir J 56:2001610. 10.1183/13993003.01610-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head SA, Shi WQ, Yang EJ, Nacev BA, Hong SY, Pasunooti KK, Li RJ, Shim JS, Liu JO (2017) Simultaneous targeting of NPC1 and VDAC1 by itraconazole leads to synergistic inhibition of mTOR signaling and angiogenesis. ACS Chem Biol 12:174–182. 10.1021/acschembio.6b00849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181:271–280.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D, Cani DS, Cerini M, Farina D, Gavazzi E, Maroldi R, Adamo M, Ammirati E, Sinagra G, Lombardi CM, Metra M (2020) Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 5:819–824. 10.1001/jamacardio.2020.1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain A, Arauz E, Aggarwal V, Ikon N, Chen J, Ha T (2014) Stoichiometry and assembly of mTOR complexes revealed by single-molecule pulldown. Proc Natl Acad Sci U S A 111:17833–17838. 10.1073/pnas.1419425111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajumba MM, Kolls BJ, Koltai DC, Kaddumukasa M, Kaddumukasa M, Laskowitz DT (2020) COVID-19-associated Guillain-Barre syndrome: atypical para-infectious profile, symptom overlap, and increased risk of severe neurological complications. SN Compr Clin Med. Advance online publication. Retrieved May 10, 2021. 10.1007/s42399-020-00646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjanaumporn J, Aeumjaturapat S, Snidvongs K, Seresirikachorn K, Chusakul S (2020) Smell and taste dysfunction in patients with SARS-CoV-2 infection: a review of epidemiology, pathogenesis, prognosis, and treatment options. Asian Pac J Allergy Immunol 38:69–77. 10.12932/AP-030520-0826 [DOI] [PubMed] [Google Scholar]
- Kewan T, Covut F, Al-Jaghbeer MJ, Rose L, Gopalakrishna KV, Akbik B (2020) Tocilizumab for treatment of patients with severe COVID-19: a retrospective cohort study. EClinicalMedicine 24:100418. 10.1016/j.eclinm.2020.100418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyhanian K, Umeton RP, Mohit B, Davoudi V, Hajighasemi F, Ghasemi M (2020) SARS-CoV-2 and nervous system: from pathogenesis to clinical manifestation. J Neuroimmunol 350:577436. 10.1016/j.jneuroim.2020.577436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielian M (2020) Enhancing host cell infection by SARS-CoV-2. Science 370:765–766. 10.1126/science.abf0732 [DOI] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175. 10.1016/S0092-8674(02)00808-5 [DOI] [PubMed] [Google Scholar]
- Kim SG, Hoffman GR, Poulogiannis G, Buel GR, Jang YJ, Lee KW, Kim BY, Erikson RL, Cantley LC, Choo AY, Blenis J (2013) Metabolic stress controls mTORC1 lysosomal localization and dimerization by regulating the TTT-RUVBL1/2 complex. Mol Cell 49:172–185. 10.1016/j.molcel.2012.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindrachuk J, Ork B, Hart BJ, Mazur S, Holbrook MR, Frieman MB, Traynor D, Johnson RF, Dyall J, Kuhn JH, Olinger GG, Hensley LE, Jahrling PB (2015) Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling modulation for Middle East respiratory syndrome coronavirus infection as identified by temporal kinome analysis. Antimicrob Agents Chemother 59:1088–1099. 10.1128/AAC.03659-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korakas E, Ikonomidis I, Kousathana F, Balampanis K, Kountouri A, Raptis A, Palaiodimou L, Kokkinos A, Lambadiari V (2020) Obesity and COVID-19: immune and metabolic derangement as a possible link to adverse clinical outcomes. Am J Physiol Endocrinol Metab 319:E105–E109. 10.1152/ajpendo.00198.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Leung LY, Caplan LR (2014) A neurologist's approach to delirium: diagnosis and management of toxic metabolic encephalopathies. Eur J Intern Med 25:112–116. 10.1016/j.ejim.2013.11.010 [DOI] [PubMed] [Google Scholar]
- Kumar R, Afsar M, Khandelwal N, Chander Y, Riyesh T, Dedar RK, Gulati BR, Pal Y, Barua S, Tripathi BN, Hussain T, Kumar N (2021) Emetine suppresses SARS-CoV-2 replication by inhibiting interaction of viral mRNA with eIF4E. Antiviral Res 189:105056. 10.1016/j.antiviral.2021.105056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari P, Rothan HA, Natekar JP, Stone S, Pathak H, Strate PG, Arora K, Brinton MA, Kumar M (2021) Neuroinvasion and encephalitis following intranasal inoculation of SARS-CoV-2 in K18-hACE2 mice. Viruses 13:132. 10.3390/v13010132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law HK, Cheung CY, Ng HY, Sia SF, Chan YO, Luk W, Nicholls JM, Peiris JS, Lau YL (2005) Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood 106:2366–2374. 10.1182/blood-2004-10-4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RE, Cho KF, Rappold R, Thrun A, Tofaute M, Kim DJ, Moldavski O, Hurley JH, Zoncu R (2018) A nutrient-induced affinity switch controls mTORC1 activation by its Rag GTPase-Ragulator lysosomal scaffold. Nat Cell Biol 20:1052–1063. 10.1038/s41556-018-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann C, Islam S, Jarosch S, Zhou J, Hoskin D, Greenshields A, Al-Banna N, Sharawy N, Sczcesniak A, Kelly M, Wafa K, Cheliak W, Holbein B (2015) The utility of iron chelators in the management of inflammatory disorders. Mediators Inflamm 2015:516740. 10.1155/2015/516740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer S (2020) Inhaled biguanides and mTOR inhibition for influenza and coronavirus (Review). World Acad Sci J 2:1–5. 10.3892/wasj.2020.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi M, Thachil J, Iba T, Levy JH (2020) Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol 7:e438–e440. 10.1016/S2352-3026(20)30145-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shao L, Chen BC, Zhang X, Zhang M, Moses B, Milkie DE, Beach JR, Hammer JA 3rd, Pasham M, Kirchhausen T, Baird MA, Davidson MW, Xu P, Betzig E (2015) ADVANCED IMAGING. Extended-resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science 349:aab3500. 10.1126/science.aab3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92:552–555. 10.1002/jmv.25728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, Smoot J, Gregg AC, Daniels AD, Jervey S, Albaiu D (2020) Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent Sci 6:315–331. 10.1021/acscentsci.0c00272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu C, Gui JF, Pang DW, Zhang QY (2018) Real-time dissecting the entry and intracellular dynamics of single reovirus particle. Front Microbiol 9:2797. 10.3389/fmicb.2018.02797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchese G, Flöel A (2020) SARS-CoV-2 and Guillain-Barré syndrome: molecular mimicry with human heat shock proteins as potential pathogenic mechanism. Cell Stress Chaperones 25:731–735. 10.1007/s12192-020-01145-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukassen S, Chua RL, Trefzer T, Kahn NC, Schneider MA, Muley T, Winter H, Meister M, Veith C, Boots AW, Hennig BP, Kreuter M, Conrad C, Eils R (2020) SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 39:e105114. 10.15252/embj.20105114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magalhaes J, Gegg ME, Migdalska-Richards A, Doherty MK, Whitfield PD, Schapira AH (2016) Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genet 25:3432–3445. 10.1093/hmg/ddw185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2020) The mechanistic target of rapamycin (mTOR): novel considerations as an antiviral treatment. Curr Neurovasc Res 17:332–337. 10.2174/1567202617666200425205122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavolta M, Giacconi R, Brunetti D, Provinciali M, Maggi F (2020) Exploring the relevance of senotherapeutics for the current SARS-CoV-2 emergency and similar future global health threats. Cells 9:909. 10.3390/cells9040909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77:683–690. 10.1001/jamaneurol.2020.1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza MG, De Lorenzo R, Conte C, Poletti S, Vai B, Bollettini I, Melloni EMT, Furlan R, Ciceri F, Rovere-Querini P (2020) Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun 89:594–600. 10.1016/j.bbi.2020.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395:1033–1034. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miserey-Lenkei S, Trajkovic K, D'Ambrosio JM, Patel AJ, Copic A, Mathur P, Schauer K, Goud B, Albanese V, Gautier R, Subra M, Kovacs D, Barelli H, Antonny B (2021) A comprehensive library of fluorescent constructs of SARS-CoV-2 proteins and their initial characterization in different cell types. Biol Cell. Advance online publication. Retrieved May 20, 2021. doi: 10.1111/boc.202000158. 10.1111/boc.202000158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, et al. (2020) A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis 94:55–58. 10.1016/j.ijid.2020.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint PK, Staufenberg EF, Sabanathan K (2006) Post-stroke seizure and post-stroke epilepsy. Postgrad Med J 82:568–572. 10.1136/pgmj.2005.041426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar S, Najjar A, Chong DJ, Pramanik BK, Kirsch C, Kuzniecky RI, Pacia SV, Azhar S (2020) Central nervous system complications associated with SARS-CoV-2 infection: integrative concepts of pathophysiology and case reports. J Neuroinflammation 17:231. 10.1186/s12974-020-01896-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa K, Lokugamage KG, Makino S (2016) Viral and cellular mRNA translation in coronavirus-infected cells. Adv Virus Res 96:165–192. 10.1016/bs.aivir.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82:7264–7275. 10.1128/JVI.00737-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paniz-Mondolfi A, Bryce C, Grimes Z, Gordon RE, Reidy J, Lednicky J, Sordillo EM, Fowkes M (2020) Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). J Med Virol 92:699–702. 10.1002/jmv.25915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini L, Albecka A, Mallery DL, Kellner MJ, Paul D, Carter AP, James LC, Lancaster MA (2020) SARS-CoV-2 infects the brain choroid plexus and disrupts the blood-CSF barrier in human brain organoids. Cell Stem Cell 27:951–961.e5. 10.1016/j.stem.2020.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi M, Lanza G, Falzone L, Fisicaro F, Ferri R, Bella R (2020) SARS-CoV-2 and the nervous system: from clinical features to molecular mechanisms. Int J Mol Sci 21:5475. 10.3390/ijms21155475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perricone C, Bartoloni E, Bursi R, Cafaro G, Guidelli GM, Shoenfeld Y, Gerli R (2020) COVID-19 as part of the hyperferritinemic syndromes: the role of iron depletion therapy. Immunol Res 68:213–224. 10.1007/s12026-020-09145-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucca P, Perucca E (2019) Identifying mutations in epilepsy genes: impact on treatment selection. Epilepsy Res 152:18–30. 10.1016/j.eplepsyres.2019.03.001 [DOI] [PubMed] [Google Scholar]
- Pignatelli M, Umanah GKE, Ribeiro SP, Chen R, Karuppagounder SS, Yau HJ, Eacker S, Dawson VL, Dawson TM, Bonci A (2017) Synaptic plasticity onto dopamine neurons shapes fear learning. Neuron 93:425–440. 10.1016/j.neuron.2016.12.030 [DOI] [PubMed] [Google Scholar]
- Povysheva NV, Johnson JW (2016) Effects of memantine on the excitation-inhibition balance in prefrontal cortex. Neurobiol Dis 96:75–83. 10.1016/j.nbd.2016.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhmanova V, Jin M, Shin J (2018) Inhibition of mast cell function and proliferation by mTOR activator MHY1485. Immune Netw 18:e18. 10.4110/in.2018.18.e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah MJ (2020) mTOR inhibition and p53 activation, microRNAs: the possible therapy against pandemic COVID-19. Gene Rep 20:100765. 10.1016/j.genrep.2020.100765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, Belij-Rammerstorfer S, Berry L, Bibi S, Bittaye M, Cathie K, Chappell H, Charlton S, Cicconi P, Clutterbuck EA, Colin-Jones R, et al. (2020) Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 396:1979–1993. 10.1016/S0140-6736(20)32466-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Musto AE (2018) The role of inflammation in the development of epilepsy. J Neuroinflammation 15:144. 10.1186/s12974-018-1192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappoport JZ, Simon SM, Benmerah A (2004) Understanding living clathrin-coated pits. Traffic 5:327–337. 10.1111/j.1398-9219.2004.00187.x [DOI] [PubMed] [Google Scholar]
- Rappoport JZ, Benmerah A, Simon SM (2005) Analysis of the AP-2 adaptor complex and cargo during clathrin-mediated endocytosis. Traffic 6:539–547. 10.1111/j.1600-0854.2005.00280.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, Holden SJ, Raber J, Banks WA, Erickson MA (2021) The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci 24:368–378. 10.1038/s41593-020-00771-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Agujetas V, de Dios C, Lestón L, Marí M, Morales A, Colell A (2019) Recent insights into the mitochondrial role in autophagy and its regulation by oxidative stress. Oxid Med Cell Longev 2019:3809308. 10.1155/2019/3809308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman MJ, Santos-Parker JR, Steward CAC, Bispham NZ, Cuevas LM, Rosenberg HL, Woodward KA, Chonchol M, Gioscia-Ryan RA, Murphy MP, Seals DR (2018) Chronic supplementation with a mitochondrial antioxidant (MitoQ) improves vascular function in healthy older adults. Hypertension 71:1056–1063. 10.1161/HYPERTENSIONAHA.117.10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozzini R, Bianchetti A, Mazzeo F, Cesaroni G, Bianchetti L, Trabucchi M (2020) Delirium: clinical presentation and outcomes in older COVID-19 patients. Front Psychiatry 11:586686. 10.3389/fpsyt.2020.586686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryskalin L, Limanaqi F, Frati A, Busceti CL, Fornai F (2018) mTOR-related brain dysfunctions in neuropsychiatric disorders. Int J Mol Sci 19:2226. 10.3390/ijms19082226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala S, Peretto G, Gramegna M, Palmisano A, Villatore A, Vignale D, De Cobelli F, Tresoldi M, Cappelletti AM, Basso C, Godino C, Esposito A (2020) Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J 41:1861–1862. 10.1093/eurheartj/ehaa286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320:1496–1501. 10.1126/science.1157535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardar R, Satish D, Gupta D (2020) Identification of novel SARS-CoV-2 drug targets by host microRNAs and transcription factors co-regulatory interaction network analysis. Front Genet 11:571274. 10.3389/fgene.2020.571274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarffe LA, Stevens DA, Dawson VL, Dawson TM (2014) Parkin and PINK1: much more than mitophagy. Trends Neurosci 37:315–324. 10.1016/j.tins.2014.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert-Bast S, Rosenow F, Klein KM, Reif PS, Kieslich M, Strzelczyk A (2019) The role of mTOR inhibitors in preventing epileptogenesis in patients with TSC: current evidence and future perspectives. Epilepsy Behav 91:94–98. 10.1016/j.yebeh.2018.05.039 [DOI] [PubMed] [Google Scholar]
- Schultz ML, Krus KL, Lieberman AP (2016) Lysosome and endoplasmic reticulum quality control pathways in Niemann-Pick type C disease. Brain Res 1649:181–188. 10.1016/j.brainres.2016.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott L, Dawson VL, Dawson TM (2017) Trumping neurodegeneration: targeting common pathways regulated by autosomal recessive Parkinson's disease genes. Exp Neurol 298:191–201. 10.1016/j.expneurol.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvakumar B, Campbell PW, Milovanovic M, Park DJ, West AR, Snyder SH, Wolf ME (2014) AMPA receptor upregulation in the nucleus accumbens shell of cocaine-sensitized rats depends upon S-nitrosylation of stargazin. Neuropharmacology 77:28–38. 10.1016/j.neuropharm.2013.08.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CS, Qi HY, Boularan C, Huang NN, Abu-Asab M, Shelhamer JH, Kehrl JH (2014) SARS-coronavirus open reading frame-9b suppresses innate immunity by targeting mitochondria and the MAVS/TRAF3/TRAF6 signalosome. J Immunol 193:3080–3089. 10.4049/jimmunol.1303196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi G, Ozog S, Torbett BE, Compton AA (2018) mTOR inhibitors lower an intrinsic barrier to virus infection mediated by IFITM3. Proc Natl Acad Sci U S A 115:E10069–E10078. 10.1073/pnas.1811892115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer M (2014) The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5:66–72. 10.4161/viru.26907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Chaubey G, Chen JY, Suravajhala P (2020) Decoding SARS-CoV-2 hijacking of host mitochondria in COVID-19 pathogenesis. Am J Physiol Cell Physiol 319:C258–C267. 10.1152/ajpcell.00224.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonani B, Aslam F, Goyal A, Patel J, Bansal P (2021) COVID-19 vaccination in immunocompromised patients. Clin Rheumatol 40:797–798. 10.1007/s10067-020-05547-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence JS, He R, Hoffmann HH, Das T, Thinon E, Rice CM, Peng T, Chandran K, Hang HC (2019) IFITM3 directly engages and shuttles incoming virus particles to lysosomes. Nat Chem Biol 15:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturley SL, Rajakumar T, Hammond N, Marka Z, Marka S, Munkacsi AB (2020a) Insights into the COVID-19 pandemic from a rare neurodegenerative disease. OSF Preprints. Advance online publication. Retrieved May 10, 2021. [Google Scholar]
- Sturley SL, Rajakumar T, Hammond N, Higaki K, Márka Z, Márka S, Munkacsi AB (2020b) Potential COVID-19 therapeutics from a rare disease: weaponizing lipid dysregulation to combat viral infectivity. J Lipid Res 61:972–982. 10.1194/jlr.R120000851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana S, Ananthapur V (2020) COVID-19 and its impact on neurological manifestations and mental health: the present scenario. Neurol Sci 41:3015–3020. 10.1007/s10072-020-04695-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y, Kwiatkowski DJ, Henske EP (2021) mTORC1 Hyperactivation in LAM leads to ACE2 upregulation in type II pneumocytes: implications for COVID-19. Eur Respir J 57:2002737. 10.1183/13993003.02737-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temraz S, Santini V, Musallam K, Taher A (2014) Iron overload and chelation therapy in myelodysplastic syndromes. Crit Rev Oncol Hematol 91:64–73. 10.1016/j.critrevonc.2014.01.006 [DOI] [PubMed] [Google Scholar]
- Terrazzano G, Rubino V, Palatucci AT, Giovazzino A, Carriero F, Ruggiero G (2020) An open question: is it rational to inhibit the mTor-dependent pathway as COVID-19 therapy? Front Pharmacol 11:856. 10.3389/fphar.2020.00856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todkar K, Chikhi L, Germain M (2019) Mitochondrial interaction with the endosomal compartment in endocytosis and mitochondrial transfer. Mitochondrion 49:284–288. 10.1016/j.mito.2019.05.003 [DOI] [PubMed] [Google Scholar]
- Traore HN, Meyer D (2004) The effect of iron overload on in vitro HIV-1 infection. J Clin Virol 31 [Suppl 1]:92–98. 10.1016/j.jcv.2004.09.011 [DOI] [PubMed] [Google Scholar]
- Tseng CT, Huang C, Newman P, Wang N, Narayanan K, Watts DM, Makino S, Packard MM, Zaki SR, Chan TS, Peters CJ (2007) Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human angiotensin-converting enzyme 2 virus receptor. J Virol 81:1162–1173. 10.1128/JVI.01702-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umanah GKE, Pignatelli M, Yin X, Chen R, Crawford J, Neifert S, Scarffe L, Behensky AA, Guiberson N, Chang M, Ma E, Kim JW, Castro CC, Mao X, Chen L, Andrabi SA, Pletnikov MV, Pulver AE, Avramopoulos D, Bonci A, et al. (2017) Thorase variants are associated with defects in glutamatergic neurotransmission that can be rescued by Perampanel. Sci Transl Med 9:eaah4985. 10.1126/scitranslmed.aah4985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Asbeck BS, Georgiou NA, van der Bruggen T, Oudshoorn M, Nottet HS, Marx JJ (2001) Anti-HIV effect of iron chelators: different mechanisms involved. J Clin Virol 20:141–147. 10.1016/s1386-6532(00)00122-0 [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M (2003) Interleukin-1β enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci 23:8692–8700. 10.1523/JNEUROSCI.23-25-08692.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Kream RM, Stefano GB (2020) Long-term respiratory and neurological sequelae of COVID-19. Med Sci Monit 26:e928996. 10.12659/MSM.928996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong YC, Ysselstein D, Krainc D (2018) Mitochondria-lysosome contacts regulate mitochondrial fission via RAB7 GTP hydrolysis. Nature 554:382–386. 10.1038/nature25486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, Liu C, Yang C (2020) Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun 87:18–22. 10.1016/j.bbi.2020.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Lazartigues E (2008) Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem 107:1482–1494. 10.1111/j.1471-4159.2008.05723.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing X, Zhang J, Wu K, Cao B, Li X, Jiang F, Hu Z, Xia K, Li JD (2019) Suppression of Akt-mTOR pathway rescued the social behavior in Cntnap2-deficient mice. Sci Rep 9:3041. 10.1038/s41598-019-39434-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Dang Y, Ren YR, Liu JO (2010) Cholesterol trafficking is required for mTOR activation in endothelial cells. Proc Natl Acad Sci U S A 107:4764–4769. 10.1073/pnas.0910872107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367:1444–1448. 10.1126/science.abb2762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Liu S, Liu J, Zhang Z, Wan X, Huang B, Chen Y, Zhang Y (2020) COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther 5:128. 10.1038/s41392-020-00243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki N, Hartung HP (2012) Guillain–Barré syndrome. N Engl J Med 366:2294–2304. 10.1056/NEJMra1114525 [DOI] [PubMed] [Google Scholar]
- Zabetakis I, Lordan R, Norton C, Tsoupras A (2020) COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients 12:1466. 10.3390/nu12051466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, Fontanella MM (2020) SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 162:1491–1494. 10.1007/s00701-020-04374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Zhai B, Gygi SP, Goldberg AL (2015) mTOR inhibition activates overall protein degradation by the ubiquitin proteasome system as well as by autophagy. Proc Natl Acad Sci U S A 112:15790–15797. 10.1073/pnas.1521919112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Li R, Liu S (2020) Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: a novel intervention strategy beyond vaccines and specific antiviral medicines. J Med Virol 92:1495–1500. 10.1002/jmv.26009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395:1054–1062. 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334:678–683. 10.1126/science.1207056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S (2020) Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 77:1018–1027. 10.1001/jamaneurol.2020.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]


