Abstract
Objective:
Clozapine remains the most effective intervention for treatment resistant schizophrenia; however, its use is prohibited following neutropenias. We review neutrophil biology as applied to clozapine and describe the strategies to initiate clozapine following neutropenia used in a case series of 14 consecutive patients rechallenged in a United Kingdom (UK) high-secure psychiatric hospital. We examine outcomes including the use of seclusion and transfer.
Methods:
A case series of 14 male patients with treatment resistant schizophrenia treated with clozapine despite previous episodes of neutropenia between 2006 and 2015 is presented. Data were collected during 2015 and 2019. Using this routinely collected clinical data, we describe the patient characteristics, causes of neutropenia, the strategies used for rechallenging with clozapine and clinical outcomes.
Results:
Previous neutropenias were the result of benign ethnic neutropenia, clozapine, other medications and autoimmune-related. Our risk mitigation strategies included: granulocyte-colony stimulating factor (G-CSF), lithium and watch-and-wait. There were no serious adverse events; at follow up half of the patient’s had improved sufficiently to transfer them to conditions of lesser security. There were dramatic reductions in the use of seclusion.
Conclusion:
Even in this extreme group, clozapine can be safely and effectively re/initiated following neutropenias, resulting in marked benefits for patients. This requires careful planning based on an understanding of neutrophil biology and the aetiology of the specific episode of neutropenia.
Keywords: agranulocytosis, antipsychotic agents, clozapine, forensic, granulocyte-colony-stimulating factor, lithium, neutrophils, schizophrenia, seclusion
Introduction
Forensic psychiatric services in the United Kingdom (UK) differ from those in many other developed countries. Both criminally and civilly detained patients are admitted on the basis of clinical need alone, which may be in the absence of a criminal conviction; detention is not time limited. There are three high-secure hospitals in England, 57 medium-secure units and a number of low-secure hospitals. Approximately 550 men are detained in the English high-secure hospitals. These care for both high-risk offender patients as well as those civilly detained but deemed uncontainable elsewhere. 1 This latter patient group often present with long psychiatric histories in the context of treatment-resistant psychosis combined with adverse drug reactions (ADRs) limiting treatment options.
Offender patients in high secure hospitals present with similar difficulties combined with a history of serious (life threatening or ending) violence. Lengths of stay can be prolonged and in many cases exceed a decade. Management may include very long periods of seclusion: long-term seclusion (LTS) is used in UK hospitals to describe solitary confinement based on risk to others. 2 LTS has attracted international criticism, as its use may amount to inhuman and degrading treatment3,4 and approximately 15% of the men detained in English high secure hospitals are managed in LTS, with another 4% managed with shorter term seclusion. (To avoid confusion among readers outside the UK we have used the term seclusion to include both LTS and seclusion.)
Most men in high-secure hospitals have schizophrenia and there is no reluctance to use clozapine.5,6 The benefits of clozapine over alternative antipsychotics include improved symptom control and function, as well as reduced violence. 7 However, clozapine may not be used within license when there is a history of neutropenia from any cause.
We review the risk-mitigation strategies that can be used when considering the use of clozapine complicated by a previous neutropenia.
We then present 14 cases in which we have used clozapine after neutropenia from any cause, with outcomes including: duration of continued high-secure placement, reduction in seclusion and LTS, measures of clinical improvement, duration of clozapine treatment and ADRs. Our approach evolved over time and we have presented a practical guide elsewhere. 8
Neutrophil biology
Neutrophils primarily protect the host from bacterial infections. They are produced in the bone marrow, where the neutrophil reserve is 10–20 times the number of circulating cells. This reserve can be mobilized in acute infection or inflammation. Neutrophil counts in blood samples vary regardless of their absolute numbers.9,10 Within individuals there is diurnal variation due to variation in granulocyte-colony stimulating factor (G-CSF)11,12 and both exercise and smoking lead to increases in circulating neutrophils.13,14 The lower limit for absolute neutrophil count (ANC) is set somewhat arbitrarily at 1.5 × 109/l, two standard deviations from the mean in white populations, rather than a necessarily pathological value. It is well above that at which there is significant risk of infection (ANC < 0.5 × 109/l). 15 Neutropenia can be caused by reduced production, accelerated destruction, sequestration or egress from the circulation and can be inherited or acquired. 16 In the haematology literature, agranulocytosis is defined as a complete absence of neutrophils; however, the term is often used to indicate severe neutropenia (ANC < 0.5 × 109/l) further specified as with or without sepsis. 17
Idiosyncratic drug-induced neutropenia
Many non-chemotherapy drugs can cause idiosyncratic drug-induced neutropenia (IDIN); while the individual mechanisms are not all elucidated, it is hypothesized that oxidized drug metabolites combine with human leukocyte antigens (HLAs) to act as haptens, inducing the production of antibodies mediated by T-helper cells; this has recently been demonstrated for clozapine. 18
Differentiating IDIN from other causes of neutropenia can be difficult as, although the majority of cases occur within weeks or months of drug initiation, some can occur after years of uneventful therapy. Whilst clozapine is one of the most commonly implicated drugs, many widely used drugs have significant effects; therefore, careful attention to the temporal relationships between drug initiation and side effects as well as scrutiny of concomitant medication is required. Curtis et al. 19 provide a helpful review of IDIN and other implicated drugs. 19
Clozapine-induced neutropenia and clozapine-induced agranulocytosis
Clozapine has unique efficacy in treatment-resistant schizophrenia (TRS). 20 Although good evidence suggests that clozapine might be best used as a second-line drug, it remains a third-line treatment. 21 As with response to other antipsychotics, 22 patients with TRS who show a good clinical response to clozapine frequently do so early; within days or weeks of initiation.23–25 This will be significant in determining the potential utility of rechallenge on account of the increased risk of clozapine-induced neutropenia (ANC < 1.5 × 109/l) and agranulocytosis (ANC < 0.5 × 109/l). These occur at rates of 3.8% and 0.4%, respectively, with the majority occurring within 18 weeks of treatment initiation and almost all within a year. The risk monitoring and mitigation systems in place result in deaths from agranulocytosis being extremely rare, occurring in approximately 1 in 7700 clozapine treated patients.26,27 UK pharmacovigilance data records eight deaths from clozapine-induced blood dyscrasias to date, compared with hundreds from cardiac and gastrointestinal effects. 28 After 1 year of treatment, the risk of blood dyscrasias is similar to that of other antipsychotics. The likelihood of neutropenia due to clozapine as opposed to any other cause is a function of the time between clozapine initiation and the adverse event.
The Liverpool Adverse Drug Reaction Causality Assessment Tool 29 may be useful in guiding decision making about the likelihood of clozapine-related neutropenia compared with other possibilities such as a viral illness or another drug. However, if clozapine cessation prompts recovery, then, regardless of the previous length of clozapine therapy, causation should be assumed. In the UK clozapine may only be used within licence as a third-line treatment if there is no previous history of neutropenia from any cause and with blood monitoring.
The UK clozapine blood-monitoring requirements are summarized in Table 1. Neutrophil counts are categorized according to a colour-coded system 10 and if a patient has ANC < 1.5 × 109/l (red alert) clozapine treatment must be stopped immediately. The UK requirements have not yet aligned to the lower neutrophil thresholds used in the US. 30
Table 1.
Clozapine license and monitoring and risk management requirements.
| Status | Current UK guidelines |
Current US guidelines |
||
|---|---|---|---|---|
| General population | Benign ethnic neutropenia (BEN) criteria | General population | BEN criteria | |
| Green * | ||||
| White cell count (WCC) (× 109/l) | >3.5 | >3.0 | Not required | Not required |
| Absolute neutrophil count (ANC) (× 109/l) | >2.0 | >1.5 | >2.0 | >2.0 |
| Amber # | ||||
| WCC (× 109/l) | 3.0–3.5 | 2.5–3.0 | Not required | Not required |
| ANC (× 109/l) | 1.5–2.0 | 1.0–1.5 | 1.0–1.5 | 0.5–1.0 |
| Red ^ | ||||
| WCC (× 109/l) | <3.0 | <2.5 | Not required | Not required |
| ANC (× 109/l) | <1.5 | <1.0 | <1.0 | <0.5 |
Continue treatment.
Increase monitoring frequency.
Discontinue treatment.
ANC, absolute neutrophil count; BEN criteria, benign ethnic neutropenia criteria; WCC, white cell count.
Clozapine blood testing may be optimized by afternoon blood sampling, ideally after exercise. This alone has been demonstrated to be sufficient to improve outcomes. 31 The mechanisms of clozapine-induced neutropenia (CIN) and clozapine-induced agranulocytosis (CIA) remain elusive and may be distinct; 32 they are idiosyncratic, unpredictable type B ADRs. 33 The hypothesis of an immune-mediated mechanism is supported by the timing of both initial adverse effects and the tendency to have faster and more severe problems on rechallenge. 34 Genetic linkage studies suggest both immune and anion transporter gene related mechanisms35–38 but are not sufficiently predictive to be clinically useful. 39 In addition, it has been suggested that the metabolites of clozapine may be toxic to neutrophils. 40
Clozapine re-challenge after CIN and CIA
The current available evidence regarding repeat dyscrasias with clozapine is conflicting. A 2006 publication of all available clozapine rechallenges in the UK and Ireland found that the majority did not experience a repeat dyscrasia: 38% of patients (n = 20) experienced a repeat dyscrasia with a significant increase in severity, duration and speed of onset. However, there were no deaths and all repeat dyscrasias resolved when clozapine was discontinued. The majority of dyscrasias occurred within 18 weeks of clozapine treatment; four occurred after this. 41
In 2016, data from a nationwide pharmacovigilance study in Argentina presented the outcomes of 19 patients rechallenged with clozapine, approximately one third of patients experienced a second dyscrasia which mostly occurred sooner during treatment but were less severe and of shorter duration. 42 Evidence from case reports shows positive outcomes in more than 60% of clozapine rechallenges after neutropenia (128 positive outcomes from 203 cases) but there is markedly less reported success following agranulocytosis (3 positive outcomes from 17 cases). 43 Publication bias limits the utility of this information.
Benign ethnic neutropenia and other ethnic variation
Asymptomatic or benign reductions in neutrophils are observed in individuals of all ethnic backgrounds. Benign ethnic neutropenia (BEN), neutropenia without immune dysfunction or increased liability to infection is not due to abnormal neutrophil production; although, the exact aetiology of the reduction in circulating cells remains unknown. BEN is associated with several ethnic groups, but in particular those with Black African and West African ancestry. 44 In studies of Black US populations, 4.5% of adults had ANC < 1.5 × 109/l; 0.6% had ANC < 1.0 × 109/l. 13
The Duffy–Null polymorphism, which protects against some types of malaria, is predictive of BEN 45 and is associated with CIN in UK patients of African genetic ancestry. 38 Since 2002, clozapine monitoring services in the UK have used reference ranges 0.5 × 109/l lower for patients with haematologically confirmed BEN (Table 1). 46 Therefore, some patients prescribed clozapine before 2002 will have had clozapine discontinued on account of neutrophil levels that can, in retrospect, be re-evaluated. The adjusted limits available in the US are significantly lower. Afro-Caribbean patients are less likely than their peers to be prescribed clozapine.47,48
Lithium and neutrophils
In haematological practice, lithium has been used to treat idiopathic neutropenia. 49 It enhances production of endogenous G-CSF, directly stimulating differentiation of stem cells and protecting neutrophils from the toxicity of some drugs. The case reports of lithium to support clozapine rechallenge were reviewed by Manu et al. 50 and Boazak et al., 51 showing high rates of success.
However, in the few reported cases where lithium was withdrawn following an initially successful rechallenge, a second dyscrasia followed.52,53 As an adjunct to clozapine rechallenge, the familiarity psychiatrists have with the use of lithium, patient acceptability, low cost and the attitude of downstream services are helpful.50,51,54 However, lithium’s effect on neutrophil production, although significant, is markedly less than the alternative of G-CSF and there is an uncertain risk of reversible neurotoxicity. 55 There is a case report of the use of lithium with clozapine resulting in fatal agranulocytosis. 56
G-CSF and neutrophils
G-CSF is a cytokine glycoprotein that stimulates the differentiation, release and survival of neutrophils and other granulocytes. Filgrastim (recombinant G-CSF) has been available since the early 1990s; it was originally used to treat patients with chemotherapy-induced neutropenia. The indications expanded to include severe chronic neutropenia and for the mobilization of haematopoietic progenitors for stem cell transplantation.17,57,58 It is also used to treat drug-induced neutropenia. 59 There are multiple case reports of recombinant G-CSF use in relation to CIN and CIA57,60–72 reviewed by Lally et al..73,74 the reported cases of rechallenge supported by either regular G-CSF or as required (plus rescue) G-CSF were successful in over 70% of initial CIN cases. Success was markedly reduced when used to support rechallenge following CIA. However, there is no randomized controlled trial (RCT) evidence. Common side effects of recombinant G-CSF include flu-like symptoms, bone pain, headache, pyrexia and fatigue. Rare complications include splenic rupture, glomerulonephritis, alveolar haemorrhage, thrombocytopenia and capillary leak syndrome.
Long-term safety data following short-term G-CSF use is available from the 20,000 annual healthy volunteer donors of peripheral blood progenitor cells. 75 Appropriate precautions for longer term use include the management of bone pain with analgesia, monitoring for splenomegaly 76 and bone mineral density assessment for the detection of subtle bone changes. 77 Initial concerns that G-CSF may cause an increased leukaemia risk have been alleviated by long-term follow up. 78
Methods
Pharmacy and medical records of inpatients between 1 January 2006 and 4 June 2015 were screened to identify cases in which clozapine was used or continued despite neutropenia from any cause. Data regarding response to treatment were collected, including the change in patient adverse incidents and necessity for patient seclusion due to risk to others.
Previous incidents and episodes of LTS were collected at 4 June 2015, in addition to data regarding progress out of high-secure hospital and continuation of clozapine.
Further data regarding patient progress and continuation of clozapine were collected on 31 May 2019. By the first data collection point, the average length of total follow up was 34.4 months (min 4.1, max 75.1). At the second census date, the average total follow up was 61.4 months (min 17.5, max 122.9). Over this period, patients had been followed up on clozapine following neutropenia for an average of 56.6 months (min 0.7, max 119.6).
Results
Patient characteristics
Fourteen subjects were identified; all were male with a primary diagnosis of schizophrenia (ICD-10 F20). Age at diagnosis was on average 19.9 years (min 16.0, max 26.8); average age at admission to high secure service was 28.4 years (min 21.0, max 39.9). The indication for clozapine in each case was treatment resistance, having been in high security for an average 5.1 years (min 0.4, max 23.8) prior to the interventions described. All but two patients had been nursed in seclusion or LTS for an average of 486 days; for two patients, this had been more than 2000 days on account of persistent and extreme psychotically driven violence. All had failed to adequately respond to multiple non-clozapine antipsychotics (min 3, max 15, average 7.8) alone and as polypharmacy; 12 had received doses above the recommended UK maxima. Ten patients had been prescribed clozapine before for an average of 394 days (min 35, max 1785). Seven patients had shown previous improvement with clozapine; the remaining three patients had taken clozapine on average for 77 days only (min 35, max 150).
During previous clozapine treatment, a change to alternative antipsychotic treatment was necessary following a period of neutropenia (n = 8) or non-compliance (n = 2). The decision to rechallenge was made for all patients following failure of other antipsychotic treatment regimens and the severity of the patients’ psychoses. The risk of possible treatment complications; in particular a further episode of neutropenia, was considered against likely benefits of clozapine treatment.
Neutropenia
All patients had previous neutropenia (ANC < 1.5 × 109/l) (Table 2). Unfortunately, the data were incomplete, as a small number of the adverse events occurred several years previously in other healthcare settings. Where data was available, previous clozapine-related neutropenic episodes had a duration of 7 days on average (min 2, max 21) with an average ANC nadir of 1.0 × 109/l (min 0.7, max 1.3) mostly on the second neutropenic day (n = 4).
Table 2.
Aetiology and mitigation of neutropenia; clinical outcomes.
| Patient number | Aetiology of previous neutropenia | Initial management of neutropenia | Outcome | Progress |
|---|---|---|---|---|
| 1 | CIN | G-CSF as required | Discharged: 54.0 months after starting clozapine | Mental state improved with clozapine. The patient had six red results during the first two months of treatment with a neutrophil nadir of 0.4 × 109/l; these episodes responded to PRN (as required) G-CSF before the strategy was switched to regular G-CSF. The patient was prescribed regular filgrastim 15 million units weekly. There were no further red results. The patient was discharged to conditions of lesser security. |
| 2 | CIN | G-CSF regular | Death: 59.1 months after starting clozapine | Mental state improved with clozapine. The patient was prescribed regular filgrastim 30 million units twice weekly. There were no reported side effects associated with G-CSF. The patient died of an unrelated myocardial infarction 59.1 months after restarting clozapine |
| 3 | CIN | G-CSF as required | Clozapine discontinued: 72.6 months after starting clozapine | Mental state improved with clozapine; psychotic symptoms were more manageable. Time nursed in LTS reduced on clozapine from 88% to 4%. The patient was transferred to a less restrictive ward environment. PRN G-CSF was available in the event of neutropenia; this was not needed. Clozapine was discontinued after 72.6 months due to poor clozapine absorption related to an inflammatory bowel condition. At the second census date, the patient remained detained in conditions of high security. |
| 4 | CIN | G-CSF regular | Clozapine discontinued: 119.6 months after starting clozapine | Mental state improved with clozapine. The patient was initially prescribed regular filgrastim 15 million units three times per week. The patient refused regular G-CSF after approximately five years. PRN (as required) G-CSF was subsequently available in the event of neutropenia; this was not needed. Splenomegaly was noted on ultrasound imaging; this continues to be monitored by haematology colleagues; no other intervention has been required. Clozapine was discontinued after 119.6 months due to patient refusal. At the second census date, the patient remained detained in conditions of high security. |
| 5 | CIN | G-CSF regular | Discharged: 28.6 months after starting clozapine | Mental state improved with clozapine. Time nursed in LTS reduced on clozapine from 87% to 6%. The patient was prescribed regular filgrastim 48 million units weekly. There were no reported adverse effects associated with G-CSF. The patient was discharged to conditions of lesser security 28.6 months after restarting clozapine. |
| 6 | Medication induced | G-CSF as required | Continues on clozapine in HSS | Mental state improved with clozapine. The patient developed neutropenia after 55 months of clozapine treatment when lamotrigine was introduced. Two doses of filgrastim 30 million units were given over three days; lamotrigine was withdrawn and clozapine continued. There has been no further neutropenia. At the second census date, the patient remained on clozapine in conditions of high security. |
| 7 | Medication induced | Watch and wait | Clozapine discontinued: 0.7 months after starting clozapine | The patient developed neutropenia on risperidone prior to clozapine use. Mental state improved with clozapine which titrated to 200 mg. However, a neutropenia of 1.3 × 109/l within the first month of treatment occurred and clozapine was withdrawn. The rechallenge had occurred in December, at the time of the neutropenia the treating psychiatrist was on leave, decision making passed to the covering psychiatrist who took a cautious approach. |
| 8 | Medication induced | Watch and wait | Discharged: 52.6 months after starting clozapine | The patient developed neutropenia on sodium valproate prior to clozapine use. Mental state improved with clozapine. There were no episodes of neutropenia following clozapine rechallenge. The patient was discharged to conditions of lesser security 52.6 months after restarting clozapine. |
| 9 | Autoimmune | G-CSF as required | Continues on clozapine in HSS | Mental state improved with clozapine. The patient was monitored using the BEN reference ranges with provision for G-CSF in the event of neutropenia; this has not been needed. As at the second census date, the patient remained on clozapine in conditions of high security. |
| 10 | BEN | Lithium | Discharged: 52.7 months after starting clozapine | Mental state improved with clozapine. The patient was initially prescribed lithium at 400 mg per day to increase neutrophil count; this was not tolerated by the patient due to sedation. G-CSF was subsequently made available in the event of a neutropenia; this was not required. The patient was discharged to conditions of lesser security 52.7 months after starting clozapine. |
| 11 | BEN | G-CSF as required | Continues on clozapine in HSS | Patient had previous exposure to clozapine prior to BEN diagnosis; clozapine was previously stopped when neutrophils fell below the non-BEN levels. Mental state improved with clozapine rechallenge. The patient was prescribed lenogastrim 263 µg if neutrophils fell below 1.0 × 109/l; this was required only once within one month of restarting clozapine when neutrophil count fell to 0.9 × 109/l. No side effects were reported due to use of G-CSF. At the second census the patient remained on clozapine in conditions of high security. |
| 12 | BEN | Watch and wait | Discharged: 48.8 months after starting clozapine | Mental state improved with clozapine. Neutropenia was initially managed by optimizing neutrophil counts with afternoon sampling and exercise. The patient rapidly developed repeated low neutrophil counts with a nadir of 0.4 × 109/l. Episodes of neutropenia were initially treated with PRN (as required) G-CSF, which was later converted to regular G-CSF. The patient was prescribed regular filgrastim 15 million units on alternate days. There were no reported adverse effects associated with G-CSF. The patient was discharged to conditions of lesser security 48.8 months after starting clozapine. |
| 13 | BEN | G-CSF regular | Discharged: 17.4 months after starting clozapine | Patient had previous exposure to clozapine prior to BEN diagnosis; clozapine was previously stopped when neutrophils fell below the non-BEN levels. Mental state improved with clozapine rechallenge. The patient was prescribed regular filgrastim 30 million units twice weekly. There were no reported adverse effects associated with G-CSF. The patient was discharged to conditions of lesser security 17.4 months after starting clozapine. |
| 14 | BEN | Lithium | Discharged: 30.5 months after starting clozapine | Mental state improved with clozapine. The patient was initially prescribed lithium at 800 mg per day to increase neutrophil count: this predated clozapine treatment. There were no reported side effects associated with lithium. There were no episodes of neutropenia. The patient was discharged to conditions of lesser security 30.5 months after starting clozapine. |
BEN, benign ethnic neutropenia; CIN, clozapine-induced neutropenia; G-CSF, granulocyte-colony stimulating factor; HSS, high secure services; LTS, long-term seclusion; PRN, pro re nata.
Decision making and ethical considerations
For all patients detailed analyses of the indication for clozapine, the severity of the patient’s current presentation, likelihood of benefit and a detailed re/examination of the presence of, severity and aetiology of the previous neutropenia was performed. Our approach is detailed elsewhere. 8
To assist with decision making related to the overall approach and to provide scrutiny in relation to balancing the possible risks of the status quo without clozapine against the possible benefits in terms of their psychosis, internal peer reviews were held and for several cases external advice was taken. These decisions became refined and informed by the fortunate successes of the initial cases in which the improvements far exceeded expectations, with patients previously subject to years of confinement improving to the extent that this could end, and they could move out of high security all together.
Our local haematology and pharmacy colleagues assisted regarding the possible origins and significance of the blood dyscrasias, including the possibility of the effects of other prescribed medications or physical health comorbidities. In addition, haematology support includes guidance relating to risk monitoring and mitigation.
Where available, patients and their relatives were involved in decision-making and all treatments were authorized within the appropriate legal framework.
For our group, the status quo was universally of extreme severity with prolonged periods of detention, meaning for years and decades and invariably with the use of the most severe restrictive practices available, usually seclusion, again for extremely long periods.79,80 Multiple other treatments had failed, often for over a decade or more. For these men alternatives had been pursued, usually for many years, but with persistent failure. Although inaction and the avoidance of risk-taking is a common approach when benefit is probable, but harm is possible, this was explicitly recognized and so teams were able to make difficult decisions. Our team approach to managing ethical dilemmas is set out previously in relation to the use of enforced clozapine. 81
Risk mitigation strategies
The aetiology of the previous neutropenia was considered when setting the risk mitigation strategy (Table 2). A watch and wait approach was taken with the patients who had previous non-clozapine related drug-induced neutropenia. These plans were completely flexible. Although provision was made for the availability of G-CSF to be used if required, no strict pre-determined criteria were made. Rather, the plans were to be pragmatic, dependent on the patient’s clinical presentation including the clinical response to clozapine during the rechallenge, the speed and severity of any future dyscrasia should it arise and any subsequent mental health deterioration on clozapine withdrawal. In addition, anticipated difficulties and actual management problems in the event of physical health deteriorations such as infection were considered. For the cases of established CIN, regular or as-required G-CSF was used. A variety of approaches including lithium, as-required and regular G-CSF were used, considering the challenges of the patients with BEN.
Medication and dose
Clozapine dose and initial support strategies were assessed at the first data collection point when the average dose of clozapine (n = 13) was 354 mg/day (min 225 mg/day, max 450 mg/day). Where regular G-CSF was used, doses varied between 15 and 60 million units per week; all G-CSF prescription was made following haematological advice. One patient was prescribed lithium at 800 mg/day for both his mental disorder and to stimulate neutrophil counts. A lower dose of lithium at 400 mg/day was used for another patient when only a neutrophil effect was needed.
Progress
Treating clinicians considered that all patients had demonstrated clinical improvement on clozapine. By the first census point 13 patients remained on clozapine and four had been transferred to lesser security. The proportion of time spent in LTS decreased significantly and in some cases years of seclusion ended.
Prior to starting clozapine, the studied patients had collectively spent 31% of their time nursed in LTS; this reduced to 13% following treatment with clozapine as at the first census date (paired t-test p < 0.001) (Figure 1). The adverse behavioural incidents (including aggression, violence and deliberate self-harm) for the ten patients who remained at June 2015 decreased from 16.7 per 100 days per patient before clozapine initiation to 6.0 per 100 days per patient after starting clozapine (paired t-test p < 0.005) (Figure 2).
Figure 1.
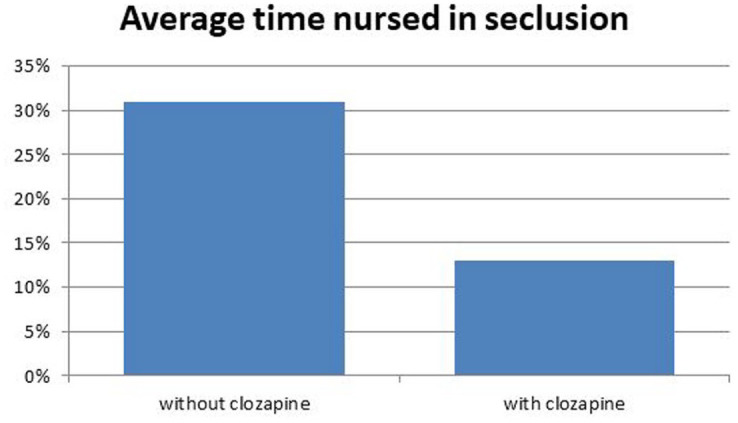
Clinical outcome; effect of treatment on restrictive practice.
Figure 2.
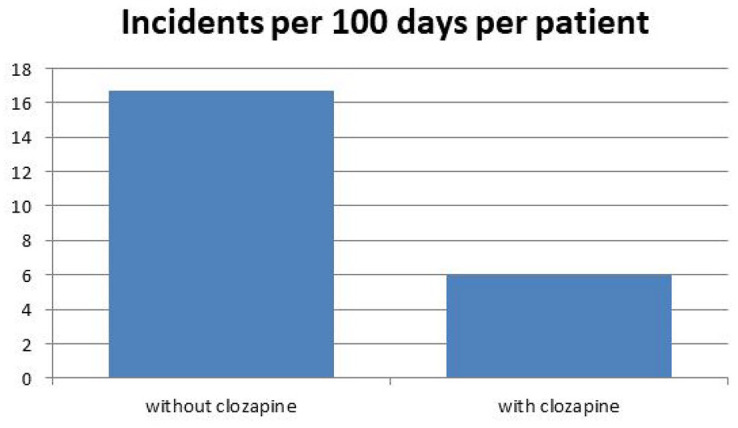
Clinical outcome; effect of treatment on clinical (adverse behavioural) incidents.
By the second census date, half of the original 14 patients had improved sufficiently to transfer to lesser security. The transfer process was slow, taking on average 40.7 months following initiation of the current clozapine trial (min 17.5, max 54.0). Seclusion and incident data were not collected at the second census date. At the second data collection point a further three patients had discontinued clozapine treatment: one patient had died of an unrelated cause; one had refused clozapine for unspecified reasons; and clozapine had been discontinued in another patient due to an inflammatory bowel condition. A further three patients continued on clozapine and improved sufficiently to transfer to less secure services. The remaining three patients showed variable progress but remained on clozapine; one patient had progressed sufficiently to allow termination of a prolonged period of LTS.
Adverse effects
Four patients had anomalous blood results (Table 2). No patient developed infection or required transfer to general hospital. Of those prescribed G-CSF (as required or regular), only Patient 4 (CIN) was noted to have splenomegaly on ultrasound imaging. This continues to be monitored by haematology colleagues; no other intervention has been required. No patient has complained of bone pain while on G-CSF. Lithium was discontinued in Patient 10 (BEN) due to sedation.
Discussion
Whilst larger series exist, 43,72 ours is still considerable in terms of numbers and exceptional in terms of length of follow-up. Moreover, we report the outcomes that have the most meaning to patients, their families and the teams that care for them; getting out of seclusion, reduced incidents and moving out of a high secure hospital, as well as the conventional maintenance of neutrophil count and the continuation of clozapine.
Patients in UK high secure hospitals are, as a whole, extreme, with average lengths of stay measured in years and with offending and institutional behaviour that excludes them from every other care setting. Our subgroup with clozapine prohibited on account of neutropenias, presented even more significant challenges. Their psychoses had failed to respond to multiple alternate antipsychotics and they were among the most severely unwell in the county. Nine being managed in seclusion for periods measured in the hundreds of days or even longer. Whilst therapeutic nihilism is an understandable response, our assertive approach evolved as we attempted to help our patients and the results far exceeded our expectations. The use of seclusion is of national and international concern;3,82 therapeutic persistence and the assertive use of clozapine are recognized as essential aspects of any attempt to reduce its use.83,84 It is recognized that the best treatment for psychosis after clozapine has been discontinued is a repeated trial of clozapine. 85
Given the risks associated with treatment failure in our patient group, we took an active approach to supporting neutrophil counts, often using G-CSF. Our decision-making considered the risk of the likely adverse outcomes linked with clozapine cessation: at worst, life-threatening violence. We were mindful of the difficulties involved in caring for an acutely psychotic man with severe neutropenia requiring treatment in reverse barrier isolation on a haematology ward; we took what we considered to be necessary steps to avoid this scenario. We are pleased to report that there were no serious adverse events but instead marked improvements in the quality of life for our patients. Careful mitigation of further neutropenia, with a flexible approach, is needed to ensure the patient is offered the best opportunity for improvement. We advocate close working relationships with haematology colleagues when prescribing clozapine to patients with previous neutropenia.
Clozapine rechallenge is a rare event and so even awareness of the possibility will be dependent on clinician, as opposed to patient, variables. This was certainly or own experience. Prior to 2006 it simply had not crossed our minds. Following that, the two first authors initially proceeded with clozapine rechallenge, with several other treating psychiatrists adopting it some years later. Since the only advice regarding clozapine and neutropenia is to never use clozapine, it is only the arguments to contradict this, rather than to not rechallenge, that will be actively made. Our case selection was dependent on factors other than simply the possible aetiologies of the neutropenia and risk of further adverse effects in particular subjective judgements at the feasibility of proceeding in the face of a usually highly uncooperative and problematic patient.
Our case series demonstrates that rechallenge with clozapine following neutropenia should not be dismissed without full consideration of the potential benefits even in the most challenging patient groups. In the UK, clozapine rechallenge has almost exclusively been reported from national centres, it is far from normal practice. 72 None of our patients could possibly have been transferred on account of their security needs and the severity of their behavioural disturbances. Since a number of our sample graduated to HSS on account of their treatment resistance combined with a history of neutropenia thought to contraindicate clozapine and then persistent assaults as inpatients, it is important that the possibility of rechallenge is widely recognized and actively pursued to avoid that sort of escalation through security. all levels of secure psychiatric settings could benefit from developing the skills to deliver these interventions, either alone or in collaboration with national centres.
This sort of assertive approach has already been demonstrated at various levels of security and in rehabilitation and other psychiatric settings with the use of intramuscular clozapine, which until very recently was not used in the UK.86,87 We are unaware of a pervious economic analysis but think that this would be unnecessary. In the UK one dose of G-CSF costs approximately £50 (US $70), whereas a day in a UK high secure hospital bed costs on average £800 (US $1100) but more in the highly staffed wards in which our patients were managed.
Further challenges remain regarding the international variation in clozapine monitoring requirements, the understanding of the underlying causes and therefore predictability of blood dyscrasias, as well as improving the literature describing rechallenge. Despite progress, understanding of the aetiology of life-threatening clozapine blood dyscrasias remains insufficient.
There are many causes of neutropenia; the definition is not uncontroversial and a low neutrophil count is not necessarily indicative of significant pathology. The existing neutrophil thresholds for the continuation of clozapine are effective at safeguarding patients, but may restrict opportunity to benefit in some, particularly those of African ancestry. Psychiatric literature relating to clozapine has previously defined agranulocytosis as a neutrophil count of less than 0.5 × 109/l; 88 this remains convention. But at the time of the first Finnish cases, agranulocytosis was described as the presence of very few or no granulocytes in the peripheral blood. 89
Our haematology colleagues subdivide neutropenia into mild (ANC 1.5–1.0 × 109/l) moderate (ANC 1.0–0.5 × 109/l) and severe (ANC 0.5–0.2 × 109/l) with or without sepsis. Agranulocytosis is strictly the complete absence of neutrophils (ANC < 0.2 × 109/l) at which infection is most likely. 17 ANC has rarely been set out in the reported clozapine rechallenge cases; it is possible that these have included subjects with benign mild neutropenia.
In 2015 the US FDA guidelines for interruption of clozapine treatment changed: white blood cell count (WBC) monitoring was no longer required and once clozapine is established the threshold for an ANC requiring treatment interruption was lowered to below 1.0 × 109/l (below 0.5 × 109/l in cases of BEN).90–92
Adopting the same monitoring internationally would be likely to provide a simple, in license solution for a significant number of patients. 30 Whilst HLA related mechanisms are implicated in the aetiology of CIN and CIA, the current associations, although now studied for 25 years, are not sufficient as to be clinically useful.39,93 In addition, as described by Legge and Walters, 38 we hope that a wider understanding of the role of the Duffy-null genotype in BEN will help better predict and understand the significance of reductions in neutrophil counts following the use of clozapine. The general issues related to clozapine rechallenge are summarised in Figure 3.
Figure 3.
Learning points.
Given that a significant proportion of cases of CIN and CIA are likely to have an immunological mechanism, it may be that slower rates of titration, both on initial use and in the event of rechallenge, result in reductions in adverse events. 94 This has been effective for the rechallenge of lamotrigine and carbamazepine following adverse serious side effects.95–97 As yet, there is no evidence to support such an approach, either for initial clozapine titrations let alone for rechallenge. 98
Given that controlled trials are highly unlikely, teams embarking on rechallenge should attempt to enrol their patients into the worldwide efforts to understand the aetiologias of CIN and CIA and report their results, ideally including the variables which may be significant; the exact details of the initial neutropenia, concomitant drug use, patient demographics and clinical details including ethnicity and Duffy type as well as the rechallenge titration schedule.
Case reports and series such as ours lack the certainty of outcome of a trial, but in this patient population we see no likelihood this becoming available. As with most case series, our methodology has a number of limitations. We retrospectively examined the cases where rechallenge was used and have not presented comparative data regarding those men who may have also had previous clozapine or other neutropenias. A preprint version of an earlier draft is available. 99
Acknowledgments
The authors would like to thank Ruth Massey and Gail Cooper of the Pharmacy Department, Ashworth Hospital, for their help in data collection. The contribution of Dr Ade, formerly Consultant Haematologist at Aintree University Hospital, is greatly acknowledged; his initial help and willingness to engage with a challenging patient group is laudable.
Footnotes
Author contribution: All authors made substantial contributions to the conception and design of the work, revisions for important intellectual content, approved the final version for publication and are accountable for all aspects of the work. ES drafted the work.
Conflict of interest statement: Dr Silva has received consultancy fees from Zarodex Therapeutics Ltd.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics statement: Exemption from ethics approval and waiver of informed consent granted: the associate medical director for research, development and innovation at Mersey Care NHS FT waived the need for seeking ethics approval and the need to obtain consent for the collection, analysis and publication of the retrospectively obtained and anonymized data for this non-interventional study.
Professional medical writer: None
ORCID iD: Edward Silva  https://orcid.org/0000-0002-7254-6307
https://orcid.org/0000-0002-7254-6307
Availability of data and materials: Data is available from the authors on request.
Contributor Information
Edward Silva, Rathbone Low Secure Unit, Mersey Care NHS Foundation Trust, Rathbone Hospital, Mill Lane, Liverpool, L13 4AW, UK.
Melanie Higgins, Ashworth Hospital, Mersey Care NHS Foundation Trust, Liverpool, UK.
Barbara Hammer, Arrowe Park Hospital, Merseyside, Wirral, UK.
Paul Stephenson, Health Education England- North West, Manchester, UK.
References
- 1. Völlm BA, Edworthy R, Huband N, et al. Characteristics and pathways of long-stay patients in high and medium secure settings in England; a secondary publication from a large mixed-methods study. Front Psychiatry 2018; 9: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Department of Health. Mental health act 1983: code of practice. London: TSO, 2015. [Google Scholar]
- 3. ECPT. Report to the Government of the United Kingdom on the visit to the United Kingdom carried out by the European Committee for the Prevention of Torture and Inhuman or Degrading Treatment or Punishment (CPT): from 30 March to 12 April 2016. Strasbourg: Council of Europe, 2017. [Google Scholar]
- 4. Care Quality Commission. Brief guide: long-term segregation. https://www.cqc.org.uk/sites/default/files/20190412_briefguide-longtermsegregation.pdf (2019, accessed 11 November 2020).
- 5. Thomson LDG. Management of schizophrenia in conditions of high security. Adv Psychiatr Treat 2000; 6: 252–260. [Google Scholar]
- 6. Darjee R, Øfstegaard M, Thomson L. Schizophrenia in a high-security hospital: long-term forensic, clinical, administrative & social outcomes. J Forens Psychiatry Psychol 2017; 28: 525–547. [Google Scholar]
- 7. Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol 2012; 15: 1351–1371. [DOI] [PubMed] [Google Scholar]
- 8. Silva E, Higgins M, Hammer B, et al. Clozapine rechallenge and initiation despite neutropenia- a practical, step-by-step guide. BMC Psychiatry 2020; 20: 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. von Vietinghoff S, Ley K. Homeostatic regulation of blood neutrophil counts. J Immunol 2008; 181: 5183–5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Souto Filho JTD, Portugal RD, Nucci M. Effect of circadian variation on neutrophil mobilization to the peripheral blood in benign constitutional neutropenia. Exp Hematol 2019; 69: 22–26. [DOI] [PubMed] [Google Scholar]
- 11. Ahokas A, Elonen E. Circadian rhythm of white blood cells during clozapine treatment. Psychopharmacology (Berl) 1999; 144: 301–302. [DOI] [PubMed] [Google Scholar]
- 12. McKee JR, Wall T, Owensby J. Impact of complete blood count sampling time change on white blood cell and absolute neutrophil count values in clozapine recipients. Clin Schizophr Relat Psychoses 2011; 5: 26–32. [DOI] [PubMed] [Google Scholar]
- 13. Hsieh MM, Everhart JE, Byrd-Holt DD, et al. Prevalence of neutropenia in the U.S. population: age, sex, smoking status, and ethnic differences. Ann Intern Med 2007; 146: 486–492. [DOI] [PubMed] [Google Scholar]
- 14. McCarthy DA, Dale MM. The leucocytosis of exercise. Sports Med 1988; 6: 333–363. [DOI] [PubMed] [Google Scholar]
- 15. Phillips R, Hancock B, Graham J, et al. Prevention and management of neutropenic sepsis in patients with cancer: summary of NICE guidance. BMJ 2012; 345: e5368. [DOI] [PubMed] [Google Scholar]
- 16. Dale DC. How I diagnose and treat neutropenia. Curr Opin Hematol 2016; 23: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Newburger PE, Dale DC. Evaluation and management of patients with isolated neutropenia. Semin Hematol 2013; 50: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ogese MO, Lister A, Jenkins RE, et al. Characterization of clozapine-responsive human T cells. J Immunol 2020; 205: ji2000646. [DOI] [PubMed] [Google Scholar]
- 19. Curtis BR. Non-chemotherapy drug-induced neutropenia: key points to manage the challenges. Hematology Am Soc Hematol Educ Program 2017; 2017: 187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry 2016; 209: 385–392. [DOI] [PubMed] [Google Scholar]
- 21. Kahn RS, Winter van Rossum I, Leucht S, et al. Amisulpride and olanzapine followed by open-label treatment with clozapine in first-episode schizophrenia and schizophreniform disorder (OPTiMiSE): a three-phase switching study. Lancet Psychiatry 2018; 5: 797–807. [DOI] [PubMed] [Google Scholar]
- 22. Kapur S, Arenovich T, Agid O, et al. Evidence for onset of antipsychotic effects within the first 24 hours of treatment. Am J Psychiatry 2005; 162: 939–946. [DOI] [PubMed] [Google Scholar]
- 23. Honer WG, Jones AA, Thornton AE, et al. Response trajectories to clozapine in a secondary analysis of pivotal trials support using treatment response to subtype schizophrenia. Can J Psychiatry 2015; 60(Suppl. 2): S19–S25. [PMC free article] [PubMed] [Google Scholar]
- 24. Suzuki T, Remington G, Arenovich T, et al. Time course of improvement with antipsychotic medication in treatment-resistant schizophrenia. Br J Psychiatry 2011; 199: 275–280. [DOI] [PubMed] [Google Scholar]
- 25. Schulte P. What is an adequate trial with clozapine?: therapeutic drug monitoring and time to response in treatment-refractory schizophrenia. Clin Pharmacokinet 2003; 42: 607–618. [DOI] [PubMed] [Google Scholar]
- 26. Myles N, Myles H, Xia S, et al. Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr Scand 2018; 138: 101–109. [DOI] [PubMed] [Google Scholar]
- 27. Li X-H, Zhong X-M, Lu L, et al. The prevalence of agranulocytosis and related death in clozapine-treated patients: a comprehensive meta-analysis of observational studies. Psychol Med 2020; 50: 583–594. [DOI] [PubMed] [Google Scholar]
- 28. MHRA. Interactive drug analysis profile: Clozapine [Internet]. https://info.mhra.gov.uk/drug-analysis-profiles/dap.html?drug=./UK_EXTERNAL/NONCOMBINED/UK_NON_000299949199.zipagency=MHRA (2019, accessed 11 November 2020).
- 29. Gallagher RM, Kirkham JJ, Mason JR, et al. Development and inter-rater reliability of the liverpool adverse drug reaction causality assessment tool. PLoS One 2011; 6: e28096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Whiskey E, Dzahini O, Ramsay R, et al. Need to bleed? Clozapine haematological monitoring approaches a time for change. Int Clin Psychopharmacol 2019; 34: 264–268. [DOI] [PubMed] [Google Scholar]
- 31. Jakobsen MI, Larsen JR, Svensson CK, et al. The significance of sampling time in therapeutic drug monitoring of clozapine. Acta Psychiatr Scand 2017; 135: 159–169. [DOI] [PubMed] [Google Scholar]
- 32. Flanagan RJ, Dunk L. Haematological toxicity of drugs used in psychiatry. Hum Psychopharmacol 2008; 23(Suppl. 1): S27–S41. [DOI] [PubMed] [Google Scholar]
- 33. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 34. Roge R, Moller BK, Andersen CR, et al. Immunomodulatory effects of clozapine and their clinical implications: what have we learned so far? Schizophr Res 2012; 140: 204–213. [DOI] [PubMed] [Google Scholar]
- 35. Spencer BW, Prainsack B, Rujescu D, et al. Opening Pandora’s box in the UK: a hypothetical pharmacogenetic test for clozapine. Pharmacogenomics 2013; 14: 1907–1914. [DOI] [PubMed] [Google Scholar]
- 36. Sriretnakumar V, Huang E, Müller DJ. Pharmacogenetics of clozapine treatment response and side-effects in schizophrenia: an update. Expert Opin Drug Metab Toxicol 2015; 11: 1–23. [DOI] [PubMed] [Google Scholar]
- 37. Legge SE, Hamshere ML, Ripke S, et al. Genome-wide common and rare variant analysis provides novel insights into clozapine-associated neutropenia. Mol Psychiatry 2017; 23: 162–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Legge SE, Walters JT. Genetics of clozapine-associated neutropenia: recent advances, challenges and future perspective. Pharmacogenomics 2019; 20: 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Verbelen M, Collier DA, Cohen D, et al. Establishing the characteristics of an effective pharmacogenetic test for clozapine-induced agranulocytosis. Pharmacogenomics J 2015; 15: 461–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pirmohamed M, Park K. Mechanism of clozapine-induced agranulocytosis: current status of research and implications for drug development. CNS Drugs 1997; 7: 139–158. [DOI] [PubMed] [Google Scholar]
- 41. Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry 2006; 188: 255–263. [DOI] [PubMed] [Google Scholar]
- 42. Prokopez CR, Armesto AR, Gil Aguer MF, et al. Clozapine rechallenge after neutropenia or leucopenia. J Clin Psychopharmacol 2016; 36: 377–380. [DOI] [PubMed] [Google Scholar]
- 43. Manu P, Lapitskaya Y, Shaikh A, et al. Clozapine rechallenge after major adverse effects: clinical guidelines based on 259 cases. Am J Ther 2018; 25: e218–e223. [DOI] [PubMed] [Google Scholar]
- 44. Atallah-Yunes SA, Ready A, Newburger PE. Benign ethnic neutropenia. Blood Rev 2019; 37: 100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Reich D, Nalls MA, Kao WHL, et al. Reduced neutrophil count in people of African descent is due to a regulatory variant in the duffy antigen receptor for chemokines gene. PLoS Genet 2009; 5: e1000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Clozaril-Connect. Clozapine and benign ethnic neutropenia. Mylan Products Ltd. https://www.hcpinfo.clozaril.co.uk/-/media/clozarilcouk/pdf/factsheet-benign-ethnic-neutropenia.pdf (2018, accessed 11 November 2020). [Google Scholar]
- 47. Kuno E, Rothbard AB. Racial disparities in antipsychotic prescription patterns for patients with schizophrenia. Am J Psychiatry 2002; 159: 567–572. [DOI] [PubMed] [Google Scholar]
- 48. Whiskey E, Olofinjana O, Taylor D. The importance of the recognition of benign ethnic neutropenia in black patients during treatment with clozapine: case reports and database study. J Psychopharmacol 2011; 25: 842–845. [DOI] [PubMed] [Google Scholar]
- 49. Focosi D, Azzarà A, Kast RE, et al. Lithium and hematology: established and proposed uses. J Leukoc Biol 2009; 85: 20–28. [DOI] [PubMed] [Google Scholar]
- 50. Manu P, Sarpal D, Muir O, et al. When can patients with potentially life-threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophr Res 2012; 134: 180–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boazak M, Goldsmith DR, Cotes RO. Mask off? Lithium augmentation for clozapine rechallenge after neutropenia or agranulocytosis: discontinuation might be risky. Prim Care Companion CNS Disord 2018; 20: 18l02282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dumas R, Bardin P, Vedie C. Long-term treatment of clozapine-induced leukopenia with lithium: fast-onset agranulocytosis following lithium discontinuation. Prim Care Companion CNS Disord 2016; 18: 10.4088/PCC.15l01841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mattai A, Fung L, Bakalar J, et al. Adjunctive use of lithium carbonate for the management of neutropenia in clozapine-treated children. Hum Psychopharmacol 2009; 24: 584–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kanaan RA, Kerwin RW. Lithium and clozapine rechallenge: a retrospective case analysis. J Clin Psychiatry 2006; 67: 756–760. [DOI] [PubMed] [Google Scholar]
- 55. Small JG, Klapper MH, Malloy FW, et al. Tolerability and efficacy of clozapine combined with lithium in schizophrenia and schizoaffective disorder. J Clin Psychopharmacol 2003; 23: 223–228. [DOI] [PubMed] [Google Scholar]
- 56. Gerson SL, Lieberman JA, Friedenberg WR, et al. Polypharmacy in fatal clozapine-associated agranulocytosis. Lancet 1991; 338: 262–263. [DOI] [PubMed] [Google Scholar]
- 57. Wickramanayake PD, Scheid C, Josting A, et al. Use of granulocyte colony-stimulating factor (filgrastim) in the treatment of non-cytotoxic drug-induced agranulocytosis. Eur J Med Res 1995; 1: 153–156. [PubMed] [Google Scholar]
- 58. Pamphilon D, Nacheva E, Navarrete C, et al. The use of granulocyte-colony-stimulating factor in volunteer unrelated hemopoietic stem cell donors. Transfusion 2008; 48: 1495–1501. [DOI] [PubMed] [Google Scholar]
- 59. Blier P, Slater S, Measham T, et al. Lithium and clozapine-induced neutropenia/agranulocytosis. Int Clin Psychopharmacol 1998; 13: 137–140. [DOI] [PubMed] [Google Scholar]
- 60. Weide R, Koppler H, Heymanns J, et al. Successful treatment of clozapine induced agranulocytosis with granulocyte-colony stimulating factor (G-CSF). Br J Haematol 1992; 80: 557–559. [DOI] [PubMed] [Google Scholar]
- 61. Sperner-Unterweger B, Czeipek I, Gaggl S, et al. Treatment of severe clozapine-induced neutropenia with granulocyte colony-stimulating factor (G-CSF). Remission despite continuous treatment with clozapine. Br J Psychiatry 1998; 172: 82–84. [DOI] [PubMed] [Google Scholar]
- 62. Spencer BW, Williams HR, Gee SH, et al. Granulocyte Colony Stimulating Factor (G-CSF) can allow treatment with clozapine in a patient with severe Benign Ethnic Neutropaenia (BEN): a case report. J Psychopharmacol 2012; 26: 1280–1282. [DOI] [PubMed] [Google Scholar]
- 63. Pasquale D, Newton M, Goss JB, et al. Granulocyte colony-stimulating factor treatment of clozapine-induced agranulocytosis. Am J Psychiatry 1996; 153: 1503–1504. [DOI] [PubMed] [Google Scholar]
- 64. Nielsen H. Recombinant human granulocyte colony-stimulating factor (rhG-CSF; filgrastim) treatment of clozapine-induced agranulocytosis. J Intern Med 1993; 234: 529–531. [DOI] [PubMed] [Google Scholar]
- 65. Majczenko TG, Stewart JT. Failure of filgrastim to prevent severe clozapine-induced agranulocytosis. South Med J 2008; 101: 639–640. [DOI] [PubMed] [Google Scholar]
- 66. Khan AA, Harvey J, Sengupta S. Continuing clozapine with granulocyte colony-stimulating factor in patients with neutropenia. Ther Adv Psychopharmacol 2013; 3: 266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Joffe G, Eskelinen S, Sailas E. Add-on filgrastim during clozapine rechallenge in patients with a history of clozapine-related granulocytopenia/agranulocytosis. Am J Psychiatry 2009; 166: 236. [DOI] [PubMed] [Google Scholar]
- 68. Heinz P, Muller H, Bauer M. [Treatment of clozapine-induced agranulocytosis with granulocyte colony-stimulating factor G-CSF]. Psychiatrische Praxis 1994; 21: 81. [PubMed] [Google Scholar]
- 69. Gullion G, Yeh HS. Treatment of clozapine-induced agranulocytosis with recombinant granulocyte colony-stimulating factor. J Clin Psychiatry 1994; 55: 401–405. [PubMed] [Google Scholar]
- 70. Gerson SL. G-CSF and the management of clozapine-induced agranulocytosis. J Clin Psychiatry 1994; 55(Suppl. B): 139–142. [PubMed] [Google Scholar]
- 71. Gerson SL, Gullion G, Yeh HS, et al. Granulocyte colony-stimulating factor for clozapine-induced agranulocytosis. Lancet 1992; 340: 1097. [DOI] [PubMed] [Google Scholar]
- 72. Meyer N, Gee S, Whiskey E, et al. Optimizing outcomes in clozapine rechallenge following neutropenia: a cohort analysis. J Clin Psychiatry 2015; 76: e1410–e1416. [DOI] [PubMed] [Google Scholar]
- 73. Lally J, Malik S, Krivoy A, et al. The use of granulocyte colony-stimulating factor in clozapine rechallenge: a systematic review. J Clin Psychopharmacol 2017; 37: 600–604. [DOI] [PubMed] [Google Scholar]
- 74. Lally J, Malik S, Whiskey E, et al. Clozapine-associated agranulocytosis treatment with granulocyte colony-stimulating factor/granulocyte-macrophage colony-stimulating factor: a systematic review. J Clin Psychopharmacol 2017; 37: 441–446. [DOI] [PubMed] [Google Scholar]
- 75. Gratwohl A, Baldomero H, Gratwohl M, et al. Quantitative and qualitative differences in use and trends of hematopoietic stem cell transplantation: a global observational study. Haematologica 2013; 98: 1282–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Platzbecker U, Prange-Krex G, Bornhauser M, et al. Spleen enlargement in healthy donors during G-CSF mobilization of PBPCs. Transfusion 2001; 41: 184–189. [DOI] [PubMed] [Google Scholar]
- 77. DiMeglio LA, Bolyard AA, Marrero TM, et al. The risk of low bone mineral density with long-term G-CSF therapy for severe chronic neutropenia. Blood 2010; 116: 1484. [Google Scholar]
- 78. Shaw BE, Confer DL, Hwang W, et al. A review of the genetic and long-term effects of G-CSF injections in healthy donors: a reassuring lack of evidence for the development of haematological malignancies. Bone Marrow Transplant 2015; 50: 334–340. [DOI] [PubMed] [Google Scholar]
- 79. Bowers L, van der Werf B, Vokkolainen A, et al. International variation in containment measures for disturbed psychiatric inpatients: a comparative questionnaire survey. Int J Nurs Stud 2007; 44: 357–364. [DOI] [PubMed] [Google Scholar]
- 80. Whittington R, Bowers L, Nolan P, et al. Approval ratings of inpatient coercive interventions in a national sample of mental health service users and staff in England. Psychiatr Serv 2009; 60: 792–798. [DOI] [PubMed] [Google Scholar]
- 81. Silva E, Till A, Adshead G. Ethical dilemmas in psychiatry: when teams disagree. BJPsych Adv 2017; 23: 231–239. [Google Scholar]
- 82. Ahalt C, Williams B. Reforming solitary-confinement policy — heeding a presidential call to action. N Engl J Med 2016; 374: 1704–1706. [DOI] [PubMed] [Google Scholar]
- 83. Fisher WA. Elements of successful restraint and seclusion reduction programs and their application in a large, urban, state psychiatric hospital. J Psychiatr Pract 2003; 9: 7–15. [DOI] [PubMed] [Google Scholar]
- 84. Till A, Selwood J, Silva E. The assertive approach to clozapine: nasogastric administration. BJPsych Bull 2019; 43: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Luykx JJ, Stam N, Tanskanen A, et al. In the aftermath of clozapine discontinuation: comparative effectiveness and safety of antipsychotics in patients with schizophrenia who discontinue clozapine. Br J Psychiatry 2020; 217: 498–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Casetta C, Oloyede E, Whiskey E, et al. A retrospective study of intramuscular clozapine prescription for treatment initiation and maintenance in treatment-resistant psychosis. Br J Psychiatry 2020; 217: 506–513. [DOI] [PubMed] [Google Scholar]
- 87. Henry R, Massey R, Morgan K, et al. Evaluation of the effectiveness and acceptability of intramuscular clozapine injection: illustrative case series. BJPsych Bull 2020; 44: 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Alvir JMJ, Lieberman JA, Safferman AZ, et al. Clozapine-induced agranulocytosis – incidence and risk factors in the United States. N Engl J Med 1993; 329: 162–167. [DOI] [PubMed] [Google Scholar]
- 89. Anderman B, Griffith RW. Clozapine-induced agranulocytosis: a situation report up to August 1976. Eur J Clin Pharmacol 1977; 11: 199–201. [DOI] [PubMed] [Google Scholar]
- 90. Sultan RS, Olfson M, Correll CU, et al. Evaluating the effect of the changes in FDA guidelines for clozapine monitoring. J Clin Psychiatry 2017; 78: e933–e939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nielsen J, Young C, Ifteni P, et al. Worldwide differences in regulations of clozapine use. CNS Drugs 2016; 30: 149–161. [DOI] [PubMed] [Google Scholar]
- 92. Bastiampillai T, Gupta A, Chan SK, et al. Changes for clozapine monitoring in the United States. Mol Psychiatry 2016; 21: 858–860. [DOI] [PubMed] [Google Scholar]
- 93. de With SAJ, Pulit SL, Staal WG, et al. More than 25 years of genetic studies of clozapine-induced agranulocytosis. Pharmacogenomics J 2017; 17: 304–311. [DOI] [PubMed] [Google Scholar]
- 94. Brown D, Larkin F, Sengupta S, et al. Clozapine: an effective treatment for seriously violent and psychopathic men with antisocial personality disorder in a UK high-security hospital. CNS Spectr 2014; 19: 391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Besag FM, Ng GY, Pool F. Successful re-introduction of lamotrigine after initial rash. Seizure 2000; 9: 282–286. [DOI] [PubMed] [Google Scholar]
- 96. Aiken CB, Orr C. Rechallenge with lamotrigine after a rash: a prospective case series and review of the literature. Psychiatry (Edgmont) 2010; 7: 27–32. [PMC free article] [PubMed] [Google Scholar]
- 97. Eames P. Adverse reactions to carbamazepine managed by desensitisation. Lancet 1989; 1: 509–510. [DOI] [PubMed] [Google Scholar]
- 98. Tsukahara M, So R, Yada Y, et al. Clinical utility and safety of slower-than-recommended titration of clozapine for treatment-resistant schizophrenia: a retrospective cohort study. Psychiatr Q. Epub ahead of print 5 September 2020. DOI: 10.1007/s11126-020-09841-3. [DOI] [PubMed] [Google Scholar]
- 99. Silva E, Higgins M, Hammer B, et al. Clozapine re-challenge and initiation despite neutropenia and outcome from 14 patients. (In peer review 2019). [Google Scholar]