Abstract
Background:
Determinants of childhood diarrhea in households with improved WASH (ie, households with improved drinking water sources, improved sanitation facilities, and those who practiced safe child stool disposal) are limited. This study aimed to identify the determinants of diarrhea among under-five children exclusively in households with improved Water, Sanitation, and Hygiene (WASH).
Methods:
A repeated cross-sectional study design was followed, and data from the Demographic and Health Survey (DHS) conducted between 2005 and 2016 in Ethiopia was used. A total of 1,975 child-mother pairs (257 children with diarrhea and 1718 children without diarrhea) in households with improved WASH were included in this study. Hierarchical conditional logistic regression models were used. Adjusted odds ratios (AOR) with corresponding 95% confidence intervals (CI) were estimated to determine the strength of association.
Results:
Children aged 13 to 24 months (Adjusted Odds Ratio [AOR] = 2.70, 95%CI: 1.69-4.32), children who did not receive the measles vaccine (AOR = 2.33, 95%CI: 1.60-3.39), and those residing in the agrarian region (AOR = 1.66, 95%CI: 1.10-2.49) were significantly more likely to develop diarrheal morbidity. The size of the child at birth was also found to be significantly associated with diarrheal morbidity.
Conclusion:
In this study, child factors (age of the child, vaccinated for measles, and the size of a child at birth), and household-related factors (contextual region) had a significant effect on the risk of childhood diarrheal morbidity in households with improved WASH in Ethiopia.
Keywords: WASH, diarrhea, sanitation and hygiene, Ethiopia
Introduction
Diarrhea is the second leading causes of under-five deaths and accounts for 1 in 9 deaths globally, 1 particularly in low-income countries. In addition to this enormous under-five loss of life, more than 910 million childhood cases of diarrhea per year are distributed unequally across the globe. 2 About 88% of diarrhea-associated deaths are attributable to unsafe water, inadequate sanitation, and insufficient hygiene.3,4 Diarrhea can have a detrimental impact on childhood growth and cognitive development. 5 According to the World Health Organization (WHO), the foremost key measures to prevent diarrhea disease include improved sanitation facilities, access to safe drinking-water, exclusive breastfeeding for the first 6 months of life, and good hygiene practice. 1 Several studies have also consistently reported that sanitation interventions are effective in preventing diarrhea.6-13 A systematic review by Freeman et al 8 found that improved sanitation was associated with lower odds of diarrhea.
In sub-Saharan Africa, under-five diarrhea is still more pervasive and poses a significant, long-standing public health problem. Almost three-quarters of diarrheal mortality was concentrated in 15 high-burden countries, and among these 15 countries, two-third were from sub-Saharan Africa that includes Ethiopia. 14 In this region of Africa, the proportion of diarrheal morbidity among under-five children varied considerably across the cohorts of birth from 10% to 35%. 15
In Ethiopia, diarrhea contributes to more than 1 in every 10 (13%) child deaths. 16 A recent review revealed that the pooled prevalence of diarrhea among under-five children in Ethiopia was 22% (evidence from 31 studies), 17 which was much higher than (12%) the prevalence reported recent 2016 Ethiopian Demographic and Health Survey (EDHS). 16
According to several epidemiologic studies, household-related factors: including improper refuse disposal practices, 18 lack of availability of latrine,17,19-21 sharing of a sanitation facility by more households, 22 wealth status,23,24 presence of feces and flies on the floor of and/or around sanitation facilities, 22 paternal factors: lack of maternal education,17,20 maternal handwashing practice after visiting a toilet,17,25,26 improper child stool disposal method, 27 child-related factors: the presence of 2 or more siblings in a household, 18 age of the child, 18 and use of water and soap to wash hands at, 28 child feeding practice and exclusively breastfed, 29 contribute to under-five diarrhea in Ethiopia. Although the prevalence of diarrhea is a common problem in Ethiopia, most of these studies were conducted at the local district level,20-27 and none of them conducted among households improved WASH facilities.18-21,25-28 To date, no study available on this aspect that identifies determinants of diarrhea at the national level among households with improved WASH.
It is acknowledged that households with access to water, sanitation, and hygiene (WASH) facilities have better child health and lower childhood diarrhea.11,28-31 However, recent reports have indicated that water, sanitation, and handwashing interventions alone not always more effective at reducing reduced diarrhea.32-34 For instance, evidence from 217 DHS surveys indicated sanitation had a greater effect than water infrastructure when all surveys were pooled. However, no evidence for benefits in improving drinking water or sanitation alone was observed. 32 Antecedent studies also reported that the availability of latrine was not significantly associated with childhood diarrhea.20,35,36 Even among households with improved sanitation facilities, frequent unsafe child feces disposal behavior was still reported,37,38 which was associated with childhood diarrhea.27,39,40 These and other recent studies have signified the mere presence of improved water and sanitation facilities did not necessarily result in favorable child health outcomes. And the customary linking of diarrheal with poor WASH facilities in low-income settings can mask the determinants of diarrhea in households with improved WASH facilities, and in many cases, this segment of the population was ignored in scientific literature, particularly in low-income settings.
With this in mind, no study so far has examined the determinants of childhood diarrhea exclusively among households with improved WASH (ie, households with improved drinking water sources, improved sanitation facilities, and those who practiced safe child stool disposal) in Ethiopia.22,23,28,29,31 Therefore, in this study, we aimed to investigate the various household, parental, and child-related factors influencing diarrheal morbidity of Ethiopian under-five children reside in households with improved WASH.
Materials and Methods
Study design, data source, and sampling procedures
A repeated cross-sectional study (ie, at 3-time points) was conducted using data from the Demographic and Health Survey (DHS) conducted between 2005 and 2016 in Ethiopia. The Ethiopian DHS survey is a country-representative survey providing quality information on a wide range of health and health-related indicators; representative data for the country as a whole and 9 regional states and 2 city administrations of Ethiopia. A 2-stage stratified cluster sampling was used in the EDHS. A representative sample of 14 500 households from 540 clusters in EDHS-2005, 17 817 households from 624 clusters in EDHS-2011, and 16 650 households from 645 clusters in EDHS-2016 were selected in the first stage from the sampling frame of the Ethiopian Population and Housing Census conducted in 1994 (for the EDHS-2005) and 2007 (for EDHS-2011 and EDHS-2016) through probability proportional to the unit size. Enumeration areas (EAs) were the sampling units for the first stage. Systematic random sampling was applied in the second stage to select households from each selected cluster. Details of the survey are described elsewhere.16,41,42 We retrieve the Children’s Recode (KR) dataset, which has 1 record for every child of interviewed women born in the 5 years preceding the survey. The EDHS data is available and accessible on the DHS program website: http://dhsprogram.com/data/dataset/Ethiopia.
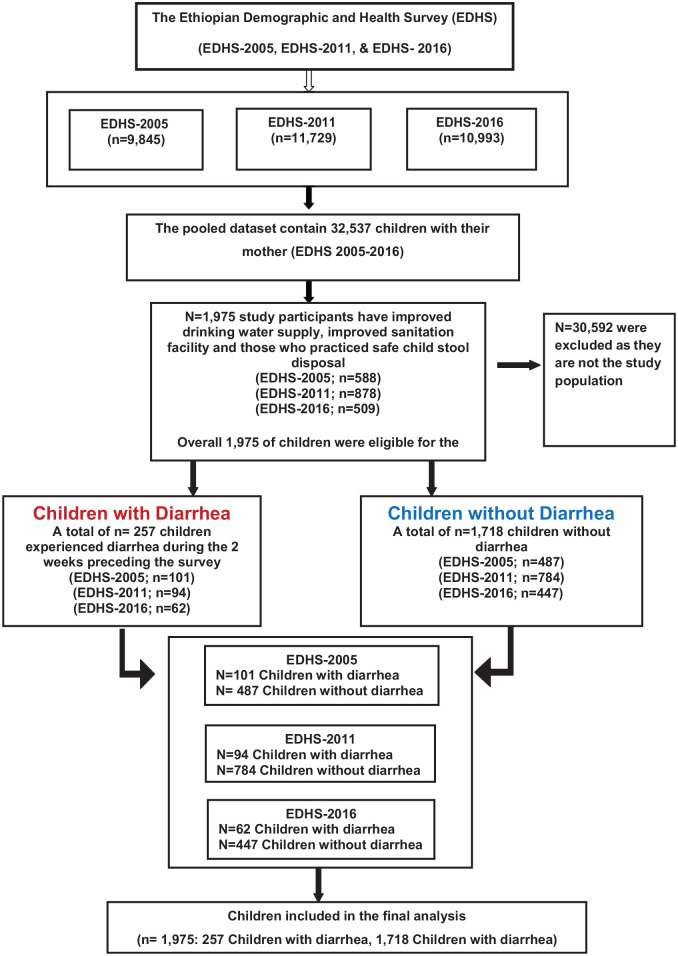
In this study, children age 0 to 59 months of age living with their mothers were included in the analysis. As illustrated in the schematic diagram of the sampling procedure (Figure 1), we used data from 3 rounds of EDHS conducted between 2005 and 2016. The pooled dataset contained data on 32 537 children under age 5 living with the mother. We created the Water, Sanitation, and Hygiene (WASH Index) from the variable such as sources of drinking water supply, sanitation facility, and child stool disposal. Accordingly, households were improved WASH (ie, improved drinking water supply, improved sanitation facility, and those who practiced safe child stool disposal) were included in the final analysis. Overall, 1,975 study participants (2005 EDHS [n = 588]; 2011 EDHS [n = 878], 2016 EDHS [n = 509]) have improved WASH facility were eligible for the study. Of the 1,975 study participants included in this study, 257 children experienced diarrhea during the 2 weeks preceding the survey (2005 EDHS [n = 101]; 2011 EDHS [n = 94], and 2016 EDHS [n = 62]) and 1,718 children without diarrhea (EDHS-2005; n = 487; EDHS-2011; n = 784; EDHS-2016; n = 447) were included all into the analysis (Figure 1).
Figure 1.
Schematic diagram of study participant selection.
Dependent variable
In the EDHS survey, diarrhea was assessed based on the women’s responses to the question: (a) has the child had diarrhea in the last 2 weeks? The women’s response to the above question was recorded as “yes” and “no” options. 16
Independent variables
Variables controlled as confounding variables include child-related factors: child’s age (0-12 months, 13-24 months, ⩾25 months), sex of child (male, female), currently breastfeeding (yes, no), measles vaccination received (yes, no), birth interval (⩽24 months, >24 months), and birth size (as reported subjectively by the mother of the child, grouped into 5 categories: very large, larger than average, average, smaller than average, and very small. Parental related factors: mother age (<18 years, 18-24, 25-34, and >35 years), mother educational level (no education, primary, secondary, and higher), mother’s exposure to media (yes, no), mother occupational status (working, not working), father’s educational level (no education, primary, secondary, and higher), and Father’s employment status (not working, working in agriculture, and working in non-agriculture). Household’s characteristics: the place of residence (urban, rural), contextual region (agrarian, pastoralist, and city dwellers), and cooking fuel type (modern, traditional). For household fuel type, electricity, natural gas, biogas, and kerosene were categorized as modern fuel. Charcoal, wood, animal dung, and other crops and straw were considered as traditional fuel. In the EDHS, households are given scores based on the number and kinds of consumer goods they own, ranging from a television to a bicycle or car, in addition to housing characteristics such as source of drinking water, toilet facilities, and flooring materials. These scores are derived using principal component analysis. National wealth quintiles are compiled by assigning the household score to each usual household member, ranking each person in the household population by her or his score, and then dividing the distribution into 5 equal categories (poorest, poorer, middle, rich, and richest), each comprising 20% of the population. In the present study, the wealth quintiles were categorized into poor, middle, and rich. Further, the flooring material of the household’s was classified as (cement made, earthen), access to electricity (yes, no), and time to get to a water source classified as (on-premises, 0-30 minutes, >30 minutes).17-26
In the current study, the regions were categorized into agrarian, pastoralist, and city. The regions of Tigray, Amhara, Oromiya, SNNP, Gambella, and Benishangul Gumuz were recorded as agrarian. The Somali and Afar regions were combined to form the pastoralist region and the city administrations – Addis Ababa, Dire Dawa, and Harar were combined as the city.
Data analysis
Data were analyzed using the STATA statistical software system package version 14.0 (StataCorp., College Station, TX, USA). Data analysis was based on the hierarchical conditional logistic regression model, which was developed based on similar methods described elsewhere.43,44 We fitted 5 different models (model 0-4) to reach the final model (model 5). Primarily, a bivariate logistic regression model (model 0) was fitted with each of the explanatory variables to select candidate variables for the subsequent multivariable models. Variables that had a significance level of P-value <.25 in the bivariate analysis, 45 were retained for inclusion into the multivariable analysis of hierarchical conditional logistic regression consisting of 4 different models.46-48 A hierarchical conditional logistic regression model was employed to identify potential confounders in a step-by-step fashion, by taking into account child-related, parental, and household factors. In the first model, all children-related explanatory variables with a P-value <.25 from a maximum model (model 0) were entered in model 1 without parental and household factors. In the second model, parental factors plus significant variables (ie, significant at P-value <.25) from model 1 were independently modeled (model 2). In the third model, household factors, and significant variables in model 2 (P-value <.25) were modeled (model 3). Finally, variables that remained significantly associated with diarrhea at P < .25 in the multivariable analysis of model 3 were included in the final model (model 4) to best explain the occurrence of diarrhea. In the final model (model 4), variables with a P-value <.05 were considered as independently associated with diarrhea. Adjusted odds ratios (AOR) with corresponding 95% confidence intervals (CI) were estimated to determine the strength of association.
Operational definitions
Improved WASH (WASH): In this particular study improved WASH refers to household’s improved drinking water sources, improved sanitation facilities, and those who practiced safe child stool disposal. Households only consider having improved WASH if they fulfill all 3 requirements.
Improved sources of drinking water: Include piped water, public taps, standpipes, tube wells, boreholes, protected dug wells and springs, and rainwater.
Improved toilet facilities: Include any non-shared toilet of the following types: flush/pour flush toilets to piped sewer systems, septic tanks, and pit latrines; ventilated improved pit (VIP) latrines; pit latrines with slabs; and composting toilets.
Safe child stool disposal: Disposing of child stools by putting or rinsing in a toilet or latrine, or a situation where the child used a toilet or latrine was regarded as safe disposal, otherwise unsafe.
Ethics statements
Our study is based on the Ethiopian Demographic and Health Survey (EDHS). The EDHS was implemented by the Central Statistical Agency (CSA) at the request of the Federal Ministry of Health (FMoH). Each of the surveys was conducted after ethical clearance was obtained from the Institutional Review Board (IRB) of ICF Macro, and Centers for Disease Control (CDC) in Atlanta, the Ethiopia Health and Nutrition Research Institute Review Board, and the National Research Ethics Review Committee at the Ministry of Science and Technology in Ethiopia. The datasets used in this study were obtained via online registration to the MDHS program which is readily available on the DHS website http://dhsprogram.com/data/available-datasets and can be accessed for research with prior permission. It does not have any identifiable information on the survey participants. DHS strictly follows all the ethical concerns, including informed consent, hence no ethical approval or informed consent was required for the current study.
Results
Socio-demographic characteristics of study participants
A total of 1,975 child-mother pairs (257 children with diarrhea and 1,718 children without diarrhea) in households with improved WASH were included in the final analyses. Table 1 shows a frequency distribution of selected characteristics of the study participants. The age distribution of children with the diarrheal disease was 63 (24.5%) between 0 and 12 months old, 105 (40.9%) between 13 and 24 months old, and 89 (34.6%) ⩾25 months old, while among children without diarrhea 400 (23.3%) between 0 and 12 months old, 408 (23.7%) between 13 and 24 months old, and 910 (52.9%) ⩾25 months old. Among children who had diarrhea, 51.0% have previously received a dose of measles vaccine, 17.5% were born within 24 months of a preceding birth, 41.8% live in a household with an earthen floor, and 67.7% were urban residence. Regarding their mother’s educational status, 39.3% and 30.6% of mothers among children with and without diarrhea, respectively, had no formal education.
Table 1.
Socio-demographic, child, and parental characteristics of cases and controls among under-five children in Ethiopia.
| Variables | Category | Diarrhea | |
|---|---|---|---|
| Yes (n = 257) | No (n = 1,718) | ||
| n (%) | n (%) | ||
| Child-related factors | |||
| Child’s sex | Male | 132 (51.4) | 833 (48.5) |
| Female | 125 (48.6) | 885 (51.5) | |
| Child’s age (in months) | 0-12 | 63 (24.5) | 400 (23.3) |
| 13-24 | 105 (40.9) | 408 (23.7) | |
| ⩾25 | 89 (34.6) | 910 (52.9) | |
| Number of under-five children | 0-1 | 130 (50.6) | 871 (50.7) |
| 2-3 | 124 (48.2) | 824 (47.9) | |
| >3 | 3 (1.2) | 23 (1.3) | |
| Currently breastfeeding | Yes | 171 (66.5) | 729 (42.4) |
| No | 86 (33.5) | 989 (57.6) | |
| Measles vaccination (n = 1,834) | Vaccinated a | 128 (51.0) | 1,081 (68.3) |
| Unvaccinated | 123 (49.0) | 502 (31.7) | |
| Birth interval (months) (n = 1,320) | ⩽24 | 33 (17.5) | 245 (21.7) |
| >24 | 156 (82.5) | 886 (78.3) | |
| Size of the child at birth | Very large | 67 (26.3) | 318 (18.6) |
| Larger than average | 16 (6.3) | 269 (15.7) | |
| Average | 116 (45.5) | 800 (46.7) | |
| Smaller than average | 9 (3.5) | 108 (6.3) | |
| Very small | 47 (18.4) | 217 (12.7) | |
| Parental factors | |||
| Mother’s age (in years) | <18 | 4 (1.5) | 26 (1.5) |
| 18-24 | 68(26.5) | 393 (22.9) | |
| 25-34 | 139 (54.1) | 997 (58.0) | |
| ⩾35 | 46 (17.9) | 302 (17.6) | |
| Mother’s education level | No education | 101 (39.3) | 526 (30.6) |
| Primary | 89 (34.6) | 566 (32.9) | |
| Secondary | 49 (19.1) | 427 (24.8) | |
| Higher | 18 (7.0) | 199 (11.6) | |
| Mother employment status | Not working | 148 (58.0) | 1,027 (59.9) |
| Working | 107 (42.0) | 686 (40.1) | |
| Paternal education (1913) | No education | 47 (18.7) | 322 (19.4) |
| Primary | 92 (36.6) | 513 (30.9) | |
| Secondary | 80 (31.9) | 517 (31.1) | |
| Higher | 32 (12.7) | 310 (18.6) | |
| Paternal occupation | Not working | 8 (3.1) | 71 (4.2) |
| Working in agriculture | 64 (25.2) | 225 (13.5) | |
| Working in non-agriculture | 182 (71.6) | 1,372 (82.3) | |
| Media exposure/watching TV/ | Yes b | 161 (62.6) | 1,258 (73.2) |
| No | 96 (37.3) | 460 (26.8) | |
| Household factors | |||
| Type of floor of the house | Cement made | 149 (58.2) | 1,293 (75.6) |
| Sand/Earth and others | 107 (41.8) | 417 (24.4) | |
| Type of fuel used | Modern | 64 (25.0) | 513 (29.9) |
| Traditional | 192 (75.0) | 1,205 (70.1) | |
| Household size | 1-4 | 91 (35.4) | 612 (35.6) |
| ⩾5 | 166 (64.6) | 1,106 (64.4) | |
| Time to get to a water source | On-premises | 111 (44.2) | 1,024 (59.6) |
| 0-30 min | 95 (37.9) | 491 (28.6) | |
| >30 min | 45 (17.9) | 202 (11.8) | |
| Place of residence | Urban | 174 (67.7) | 1,397 (81.3) |
| Rural | 83 (32.3) | 321 (18.7) | |
| Region | Agrarian | 109 (42.4) | 480 (27.9) |
| Pastoralist | 38 (14.8) | 198 (11.5) | |
| City | 110 (42.8) | 1,040 (60.5) | |
| Access to electricity | Yes | 178 (69.3) | 1,404 (81.7) |
| No | 79 (30.7) | 318 (18.3) | |
| Wealth quintiles | Poor | 16 (6.2) | 46 (2.7) |
| Middle | 17 (6.6) | 57 (3.3) | |
| Rich | 224 (87.2) | 1,615 (94.0) | |
| Survey year (EDHS) | 2005 | 101 (39.3) | 487 (28.3) |
| 2011 | 94 (36.6) | 784 (45.6) | |
| 2016 | 62 (24.1) | 447 (26.1) | |
Children who received 1 dose of measles vaccine at any time before the survey (according to a vaccination card, health facility, or the mother’s report).
Frequency of watching television was categorized as yes (less than once a week, at least once a week, and almost every day) and no (not at all).
Bivariate binary logistic regression analysis
Bivariate information that summarizes the association between predictors and response variables is presented in Table 2. In bivariate logistic regression analysis, child-related factors (child’s age, size of the child at birth, currently breastfeeding status, and measles vaccination receive), parental related factors (paternal education, father occupation, and media exposure), and household-related factors (place of residence, contextual region, wealth quintiles, type of floor of the house, time to get to a water source, and access to electricity) were identified variables associated with the occurrence of diarrhea (P < .05) (Table 2).
Table 2.
Socio-demographic, child and parental factors with acute diarrhea among under-five children in Ethiopia.
| Variables | Category | Diarrhea | Unadjusted OR (95%CI) | P-value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Child factors | |||||
| Sex | Male | 132 | 833 | 1 | |
| Female | 125 | 885 | 0.89 (0.68-1.16) | .390 | |
| Child’s age (months) | 0-12 | 63 | 400 | 1 | |
| 13-24 | 105 | 408 | 1.63 (1.16-2.29)* | .005 | |
| ⩾25 | 89 | 910 | 0.62 (0.44-0.87)* | .007 | |
| Number of under five children | 0-1 | 130 | 871 | 1 | |
| 2-3 | 124 | 824 | 1.01 (0.77-1.31) | .951 | |
| >3 | 3 | 23 | 0.87 (0.26-2.95) | .828 | |
| Currently breastfeeding | Yes | 171 | 729 | 1.75 (1.34-2.28)** | P < .001 |
| No | 86 | 989 | 1 | ||
| Measles vaccination | Vaccinated | 128 | 1,081 | 1 | |
| Unvaccinated | 123 | 502 | 2.07 (1.58-2.71)* | P < .001 | |
| Birth interval (months) | ⩽24 | 33 | 245 | 1 | |
| >24 | 156 | 886 | 1.31 (0.87-1.95) | .191 | |
| Size of the child at birth | Very large | 67 | 318 | 0.97 (0.64-1.47) | .895 |
| Larger than average | 16 | 269 | 0.27 (0.15-0.49)* | P < .001 | |
| Average | 116 | 800 | 0.67 (0.46-0.97)* | .034 | |
| Smaller than average | 9 | 108 | 0.38 (0.18-0.81)* | .013 | |
| Very small | 47 | 217 | 1 | ||
| Parental factors | |||||
| Age of the mother (years) | ⩽24 | 72 | 419 | 1 | |
| 25-34 | 139 | 997 | 0.81 (0.59-1.10) | .181 | |
| ⩾35 | 46 | 302 | 0.88 (0.59-1.32) | .553 | |
| Mother employment status | Not working | 148 | 1,027 | 0.92 (0.71-1.20) | .561 |
| Working | 107 | 686 | 1 | ||
| Mother education | No education | 101 | 526 | 1.22 (0.89-1.66) | .205 |
| Primary | 89 | 566 | 0.73 (0.50-1.06) | .096 | |
| Secondary | 49 | 427 | 0.57 (0.34-0.98) | .041 | |
| Higher | 18 | 199 | 1 | ||
| Media exposure/watching TV/ | Yes | 161 | 1,258 | 1 | |
| No | 96 | 460 | 1.63 (1.24-2.14)* | P < 001 | |
| Paternal education | No education | 47 | 322 | 1.41 (0.84-2.27) | .153 |
| Primary | 92 | 513 | 1.73 (1.13-2.66)* | .011 | |
| Secondary | 80 | 517 | 1.49 (0.97-2.31) | .067 | |
| Higher | 32 | 310 | 1 | ||
| Parental occupation | Not working | 8 | 71 | 0.85 (0.18-0.86) | .669 |
| Working in agriculture | 64 | 225 | 2.14 (1.56-2.94)* | P < .001 | |
| Working in non-agriculture | 182 | 1,372 | 1 | ||
| Household factors | |||||
| Place of residence | Urban | 174 | 1,397 | 1 | |
| Rural | 83 | 321 | 2.07 (1.55-2.77)* | P < .001 | |
| Type of floor of the house | Cement made | 149 | 1,293 | 1 | |
| Sand/Earth and others | 107 | 417 | 2.23 (1.69-2.92)* | P < .001 | |
| Household size | 1-4 | 91 | 612 | 1 | |
| ⩾5 | 166 | 1,106 | 1.01 (0.76-1.32) | .947 | |
| Access to electricity | Yes | 178 | 1,404 | 1 | |
| No | 79 | 318 | 1.98 (1.48-2.66)* | P < .001 | |
| Type of fuel used | Modern | 64 | 513 | 1 | |
| Traditional | 192 | 1,205 | 1.28 (0.94-1.73) | .111 | |
| Time to get to water source | On the premises | 111 | 1,024 | 1 | |
| 0-30 min | 95 | 491 | 1.78 (1.33-2.39)* | P < .001 | |
| >30 min | 45 | 202 | 2.05 (1.41-2.99)* | P < .001 | |
| Wealth quintiles | Poor | 16 | 46 | 2.51 (1.39-4.50)* | .002 |
| Middle | 17 | 57 | 2.15 (1.22-3.76)* | .007 | |
| Rich | 224 | 1,615 | 1 | ||
| Region | Agrarian | 109 | 480 | 2.14 (1.61-2.86)* | P < .001 |
| Pastoralist | 38 | 198 | 1.81 (1.21-2.70)* | .003 | |
| City | 110 | 1,040 | 1 | ||
Multivariable hierarchical conditional logistic regression analysis
Table 3 (model 4) shows the association between child-related, parental, and household factors and diarrhea. Using a model building process, we derived a multivariable model for under-five diarrhea that comprised, child age, size of child at birth, birth interval, measles vaccination received, contextual region, and type of floor of the house. Compared with children whose age was ⩽12 months, children 13 to 24 months of age (adjusted OR [AOR] = 2.70, 95%CI: 1.69-4.32) were 2.7 times more likely to experience diarrhea. The odds of having diarrhea were 2.33 times higher among children who did not previously receive a dose of measles vaccine (AOR = 2.33, 95%CI: 1.60-3.39) compared with children who received the measles vaccine. Children who were larger than average (AOR = 0.26; 95%CI: 0.12-0.57) and smaller than average size (AOR = 0.25; 95%CI: 0.08-0.77) at birth were less likely to experience diarrhea compared with children who were very small size at birth. A strong significant association was also detected between diarrhea morbidity and contextual region. Children who reside in the agrarian region were higher odds of developing diarrhea compared to children living in the city (AOR = 1.66, 95%CI: 1.10-2.49).
Table 3.
Multivariable analysis of factors independently associated with acute diarrhea among under-five children in Ethiopia in the hierarchical conditional logistic regression model.
| Variables | Category | Model 1a | Model 2b | Model 3c | Model 4d |
|---|---|---|---|---|---|
| AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | AOR (95%CI) | ||
| Child-related factors | |||||
| Child’s age (in months) | 0-12 | 1 | 1 | 1 | 1 |
| 13-24 | 2.79 (1.73-4.53)** | 2.47 (1.53-4.00)** | 2.28 (1.40-3.71)* | 2.70 (1.69-4.32)** | |
| ⩾25 | 1.24 (0.69-2.22) | 1.03 (0.64-1.66) | 0.99 (0.62-1.59) | 1.07 (0.67-1.69) | |
| Currently breastfeeding | Yes | 1.07 (0.69-1.66) | |||
| No | 1 | ||||
| Measles vaccination | Vaccinated | 1 | 1 | 1 | 1 |
| Unvaccinated | 2.66 (1.84-3.83)** | 2.39 (1.62-3.53)** | 2.17 (1.47-3.23)** | 2.33 (1.60-3.39)** | |
| Size of the child at birth | Very large | 0.81 (0.49-1.35) | 0.88 (0.52-1.47) | 0.77 (0.45-1.32) | 0.86 (0.51-1.44) |
| Larger than average | 0.22 (0.11-0.48)** | 0.24 (0.11-0.52)** | 0.26 (0.12-0.57)* | 0.26 (0.12-0.57)* | |
| Average | 0.66 (0.42-1.05) | 0.65 (0.41-1.05) | 0.72 (0.44-1.16) | 0.73 (0.46-1.15) | |
| Smaller than average | 0.22 (0.07-0.65)* | 0.25 (0.08-0.75)* | 0.26 (0.08-0.80)* | 0.25 (0.08-0.77)* | |
| Very small | 1 | 1 | 1 | ||
| Birth interval (months) | ⩽24 | 1 | 1 | 1 | 1 |
| >24 | 1.40 (0.91-2.14) | 1.36 (0.87-2.11) | 1.28 (0.82-1.99) | 1.33 (0.87-2.04) | |
| Parental related factors | |||||
| Mother’s educational level | No education | 1.50 (0.60-3.75) | |||
| Primary | 1.87 (0.78-4.48) | ||||
| Secondary | 1.82 (0.76-4.37) | ||||
| Higher | |||||
| Media exposure/watching TV/ | Yes | 1 | |||
| No | 0.95 (0.61-1.49) | ||||
| Father’s educational level | No education | 0.85 (0.39-1.82) | |||
| Primary | 1.01 (0.51-2.02) | ||||
| Secondary | 1.31 (0.68-2.52) | ||||
| Higher | 1 | ||||
| Father’s employment status | Not working | 1.02 (0.40-2.59) | 0.90 (0.35-2.28) | ||
| Working in agriculture | 2.00 (1.25-3.19)* | 0.80 (0.43-1.48) | |||
| Working in non-agriculture | 1 | 1 | |||
| Household related factors | |||||
| Place of residence | Urban | 1 | |||
| Rural | 1.18 (0.63-2.23) | ||||
| Region | Agrarian | 1.63 (1.03-2.59)* | 1.66 (1.10-2.49)* | ||
| Pastoralist | 0.99 (0.53-1.84) | 1.19 (0.71-1.98) | |||
| City | 1 | 1 | |||
| Time to get to a water source | On-premises | 1 | |||
| 0-30 min | 1.17 (0.74-1.87) | ||||
| >30 min | 1.25 (0.70-2.23) | ||||
| Type of fuel used | Modern | 1 | |||
| Traditional | 0.93 (0.58-1.47) | ||||
| Type of floor of the house | Cement made | 1 | 1 | ||
| Sand/Earth | 1.51 (0.93-2.47) | 1.43 (0.97-2.09) | |||
| Access to electricity | Yes | 1 | |||
| No | 0.74 (0.38-1.44) | ||||
| Wealth quintiles | Poor | 1.11 (0.51-2.42) | |||
| Middle | 1.76 (0.83-3.72) | ||||
| Rich | 1 | ||||
Model 1: Includes variables that had P < .25 from the bivariate analysis of child-related variables (Model 1: AIC = 978.86; BIC = 1,029.99; LL = −479.43).
Model 2: Includes variables that had P < .25 from model 1 and variables that had P < .25 from bivariate analysis of maternal and paternal related variables (Model 2: AIC = 958.14; BIC = 1,049.79; LL = −461.07).
Model 3: Includes variables that had P < .25 from model 2 and variables that had P < .25 from bivariate analysis of household-related variables (Model 3: AIC = 953.49; BIC = 1,060.37; LL = −455.74).
Model 4 (Final model): Includes variables that had P < .25 from model 3 (Model 4: AIC = 965.73; BIC = 1,027.07; LL = −470.86).
P-value < .05 (Adjusted). **P-value < .001 (Adjusted).
Discussion
This study used a repeated cross-sectional study that sought to establish the determinant factors for diarrhea among children less than 5 years old in households with improved WASH in Ethiopia. The finding of this study showed that both child (age of the child, size of child at birth, and vaccination status) and household-related factors (contextual region) had a significant effect on the risk of diarrheal morbidity.
Our findings confirm the results of previous studies that diarrhea prevalence was higher in children 13 to 24 months of age compared with their younger children counterparts; which is 2.70 times more likely as compared to younger children (⩽12 months). This finding was consistent with the recent EDHS report indicated that the prevalence of diarrhea increases after age 6 months, from 8% among children under age 6 months to 18% among those 12 to 23 months. 16 And was also consistent with previously conducted studies in Ethiopia.41,42,49-51 The other possible reason for this could be attributed to the fact that at this age children crawling and walking on the ground may have an increased probability of exposure to pathogenic microorganisms from the environment. This pattern was consistent within many low-income countries.15,52,53,54
In this study, the vaccination status of children was statistically significant for the occurrence of diarrheal disease in Ethiopia. Children who did not receive the measles vaccine had a higher risk of diarrhea than those who were given it. Meaning the receipt of the measles vaccine was associated with a decrease in diarrhea in children. This finding is in line with a recent study conducted in northwest Ethiopia, which reported children who did not receive measles vaccine were 3.81 times (AOR 3.81; 95%CI: 1.91-7.58) more likely to develop diarrhea than those children who received measles vaccine. 24 Similarly, Bawankule et al, 55 in their report revealed the protective effect of measles vaccination and decreased odds of developing diarrhea in children. The study further reported measles vaccination was associated with reducing diarrhea in vaccinated children by 22% in the Democratic Republic of Congo, 21% in Nigeria, 19% in Pakistan, and 12% in India. 50 The link between measles vaccination status and associated diarrheal morbidity reduction was in line with previously conducted studies in different corners of the globe, in India, 56 Zimbabwe, 57 and Brazil. 58 Moreover, in the integrated Global Action Plan for Pneumonia and Diarrhea (GAPPD) agenda which is initiated by WHO and UNICEF, measles vaccination has been introduced as a preventive measure to end preventable child deaths from pneumonia and diarrhea by 2025. 59
In this study, the occurrence of diarrhea varies by the size of the child at birth. Children who were large or average size at birth were less likely to experience diarrhea compared with children who were very small size at birth. This finding is also consistent with the studies from sub-Saharan Africa 15 and India, 60 where the size of the child at birth played an important role in child health and diarrheal morbidity.
The importance of residence in the prevalence of diarrheal morbidity is highlighted in this study. The probability of developing diarrhea among children living in agrarian regions was higher compared to their counterpart city dwellers. This association was noted in multiple studies in Ethiopia and elsewhere.15,18,32,61 The observed differences may reflect children who reside in the city have favored in several ways that benefit there well bring as compared to those children who reside in other places.
Limitations
The results of our study should be interpreted in light of the following limitations. First, the analyses were conducted using DHS data collected in a cross-sectional survey, which prevents causal inferences. Second, because the information on diarrhea was self-reported, there is the possibility of recall bias, although the recall period of illnesses, in this case, was limited to 2 weeks preceding the survey. In DHS surveys, the measurement of the prevalence of diarrhea is based on a 14-day recall period rather than the 24-hour recall, thus, reporting and recall bias in our study is likely. Third, due to the nature of DHS data incident diarrheal cases were not included; since the DHSs use a 14-day recall period to measure the prevalence of diarrhea in children. Fourth, some variables such as the size of the child at birth were reported subjectively by the mother. Hence, longitudinal data would enable better reducing recall bias and providing data to estimate the causal effect of exposure variables on diarrheal morbidity in households with improved WASH facilities. Fifth, we did not consider a random-effects model to account for clustering which may affect the result of our finding.
Despite the abovementioned limitations, the study has some strengths. First, the data used in this study derived from population-based studies that cover all the regions of Ethiopia. This allows for the generalizability of the study to the entire country. Second, we used a hierarchical conditional logistic regression model, which controls several different confounders at different levels. Third, we used pooled data from 3 DHS surveys, which is a blend characteristic of both cross-sectional and time-series data.
Conclusions
Based on multivariable analysis, child factors (children 13-24 months of age, not vaccinated for measles, and children who were very small size at birth), and household variables (residing in the agrarian region) were significant factors associated with higher odds of diarrhea in households with improved WASH in Ethiopia. The present finding highlighted the importance of tackling child diarrhea than improved WASH facilities. Therefore, health authorities should focus on identified factors to resolve diarrhea disease in this segment of the population and to sustain sanitation for everyone and everywhere.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: BS: Conceptualizes, design the study and data curation, performed the analysis, wrote, and approved the final manuscript. KA: critically reviewed the manuscript and approved the final manuscript. All authors read and approved the final manuscript before submission.
Availability of Data and Material: The data we used which is the “2005, 2011, and 2016 Ethiopian Demographic and Health Survey” were obtained from the DHS program (www.dhsprogram.com) but the “Dataset Terms of Use” do not permit us to distribute this data as per data access instructions (http://dhsprogram.com/data/Access-Instructions.cfm). To get access to the dataset you must first be a registered user of the website (www.dhsprogram.com) and download the 2016 Ethiopian Demographic and Health Survey.
Ethics Approval: DHS Programme granted permission to download and use the data for this study after being registered and submitting a request with briefly stated objectives of the study. The Institution Review Board approved procedures for DHS public-use data sets that do not in any way allow respondents, households, or sample communities to be identified. There are no names of individuals or household addresses in the data files. The detail of the ethical issues has been published in the 2005 to 2016 EDHS final report, which can be accessed at: http://www.dhsprogram.com/publications.
ORCID iD: Biniyam Sahiledengle  https://orcid.org/0000-0002-1114-4849
https://orcid.org/0000-0002-1114-4849
References
- 1. GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect Dis. 2017;17:909-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reiner RC, Jr, Wiens KE, Deshpande A, et al. Mapping geographical inequalities in childhood diarrhoeal morbidity and mortality in low-income and middle-income countries, 2000–17: analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:1779-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. UNICEF. Progress for Children: A Report Card on Water and Sanitation. External Number 5. UNICEF; 2006. [Google Scholar]
- 4. Black RE, Morris S, Bryce J. Where and why are 10 million children dying every year? Lancet. 2003;361:2226-2234. [DOI] [PubMed] [Google Scholar]
- 5. Bowen A, Agboatwalla M, Luby S, Tobery T, Ayers T, Hoekstra RM. Association between intensive handwashing and child development in Karachi, Pakistan: a cluster controlled trial. Arch Pediatr Adolesc Med. 2012;166:1037-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waddington H, Snilstveit B, White H, Fewtrell L. Water, sanitation and hygiene interventions to combat childhood diarrhoea in developing countries. Int Initiative Impact Eval. 2009;1:1-119. [Google Scholar]
- 7. Clasen TF, Bostoen K, Schmidt WP, et al. Interventions to improve disposal of human excreta for preventing diarrhoea. Cochrane Database Syst Rev. 2010;2010:CD007180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Freeman MC, Garn JV, Sclar GD, et al. The impact of sanitation on infectious disease and nutritional status: a systematic review and metaanalysis. Int J Hyg Environ Health. 2017;220:928-949. [DOI] [PubMed] [Google Scholar]
- 9. Cairncross S, Hunt C, Boisson S, et al. Water, sanitation and hygiene for the prevention of diarrhoea. Int J Epidemiol. 2010;39:i193-i205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Munos MK, Walker CLF, Black RE. The effect of oral rehydration solution and recommended home fluids on diarrhoea mortality. Int J Epidemiol. 2010;39:i75-i87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford JM., Jr. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42-52. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt WP, Cairncross S. Household water treatment in poor populations: is there enough evidence for scaling up now? Environ Sci Technol. 2009;43:986-992. [DOI] [PubMed] [Google Scholar]
- 13. Clasen T, Roberts I, Rabie T, Schmidt W, Cairncross S. Interventions to improve water quality for pre venting diarrhoea. Cochrane Database Syst Rev. 2006;3:CD004794. [DOI] [PubMed] [Google Scholar]
- 14. Walker CL, Rudan I, Liu L, et al. Global burden of childhood pneumonia and diarrhoea. Lancet. 2013;381:140516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bado AR, Susuman AS, Nebie EI. Trends and risk factors for childhood diarrhea in sub-Saharan countries (1990–2013): assessing the neighborhood inequalities. Glob Health Action. 2016;9:30166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Central Statistical Agency [Ethiopia] and ICF. Ethiopia Demographic and Health Survey 2016. CSA and ICF; 2016. [Google Scholar]
- 17. Alebel A, Tesema C, Temesgen B, Gebrie A, Petrucka P, Kibret GD. Prevalence and determinants of diarrhea among under-five children in Ethiopia: a systematic review and metaanalysis. PLoS One. 2018;13:e0199684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mengistie B, Berhane Y, Worku Y. Prevalence of diarrhea and associated risk factors among children under-five years of age in Eastern Ethiopia: a cross-sectional study. Open J Prev Med. 2013;3:446-453. [Google Scholar]
- 19. Angesom T. Prevalence and Associated Factors of Diarrhea among Under-Five Children in Laelay-Maychew District, Tigray Region, Ethiopia. Doctoral dissertation, Addis Ababa University; 2015. [Google Scholar]
- 20. Regassa W, Lemma S. Assessment of diarrheal disease prevalence and associated risk factors in children of 6-59 months old at Adama District rural Kebeles, eastern Ethiopia, January/2015. Ethiop J Health Sci. 2016;26:581-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hashi A, Kumie A, Gasana J. Prevalence of Diarrhoea and associated factors among under-five children in Jigjiga District, Somali Region, Eastern Ethiopia. Open J Prev Med. 2016;6:233-246. [Google Scholar]
- 22. Adane M, Mengistie B, Kloos H, Medhin G, Mulat W. Sanitation facilities, hygienic conditions, and prevalence of acute diarrhea among under-five children in slums of Addis Ababa, Ethiopia: baseline survey of a longitudinal study. PLoS One. 2017;12:e0182783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gebru T, Taha M, Kassahun W. Risk factors of diarrhoeal disease in under-five children among health extension model and non-model families in Sheko district rural community, Southwest Ethiopia: comparative cross-sectional study. BMC Public Health. 2014;14:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azage M, Kumie A, Worku A, Bagtzoglou AC. Childhood diarrhea in high and low hotspot districts of Amhara region, Northwest Ethiopia: a multilevel modeling. J Health Popul Nutr. 2016;35:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Solomon ET, Gari SR, Kloos H, Mengistie B. Diarrheal morbidity and predisposing factors among children under 5 years of age in rural East Ethiopia. Trop Med Health. 2020;48:1-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bitew BD, Woldu W, Gizaw Z. Childhood diarrheal morbidity and sanitation predictors in a nomadic community. Ital J Pediatr. 2017;43:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mihrete TS, Alemie GA, Teferra AS. Determinants of childhood diarrhea among underfive children in Benishangul Gumuz regional state, north West Ethiopia. BMC Pediatr. 2014;14:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Soboksa NE, Hailu AB, Gari SR, Alemu BM. Water supply, sanitation and hygiene interventions and childhood diarrhea in Kersa and Omo Nada districts of Jimma Zone, Ethiopia: a comparative cross sectional study. J Health Popul Nutr. 2019;38:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Asfaha KF, Tesfamichael FA, Fisseha GK, et al. Determinants of childhood diarrhea in Medebay Zana District, Northwest Tigray, Ethiopia: a community based unmatched case–control study. BMC Pediatr. 2018;18:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mengistie B, Berhane Y, Worku A. Household water chlorination reduces incidence of diarrhea among under-five children in rural Ethiopia: a cluster randomized controlled trial. PLoS One. 2013;8:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Megersa S, Benti T, Sahiledengle B. Prevalence of diarrhea and its associated factors among under-five children in open defecation free and non-open defecation free households in Goba District Southeast Ethiopia: a comparative cross-sectional study. Clin Mother Child Health. 2019;16:324. [Google Scholar]
- 32. Fuller JA, Westphal JA, Kenney B, Eisenberg JNS. The joint effects of water and sanitation on diarrhoeal disease: a multicountry analysis of the Demographic and Health Surveys. Trop Med Int Health. 2015;20:284-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eisenberg JN, Scott JC, Porco T. Integrating disease control strategies: balancing water sanitation and hygiene interventions to reduce diarrheal disease burden. Am J Public Health. 2007;97:846-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Null C, Stewart CP, Pickering AJ, et al. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health. 2018;6:e316-e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mohammed S, Tilahun M, Tamiru D. Morbidity and associated factors of diarrheal diseases among under five children in Arba-Minch district, Southern Ethiopia, 2012. Sci J Public Health. 2013;1:102-106. [Google Scholar]
- 36. Gedamu G, Kumie A, Haftu D. Magnitude and associated factors of diarrhea among under five children in Farta wereda, North West Ethiopia. Qual Prim Care. 2017;25:199-207. [Google Scholar]
- 37. Sahiledengle B. Prevalence and associated factors of safe and improved infant and young children stool disposal in Ethiopia: evidence from demographic and health survey. BMC Public Health. 2019;19:970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Morita T, Godfrey S, George CM. Systematic review of evidence on the effectiveness of safe child faeces disposal interventions. Trop Med Int Health. 2016;21:1403-1419. [DOI] [PubMed] [Google Scholar]
- 39. Lamichhane P, Sharma A, Mahal A. Does safe disposal of child faeces matter? An assessment of access to improved sanitation and child faeces disposal behaviour and diarrhoea in rural Nepal. Int Health. 2018;10:277-284. [DOI] [PubMed] [Google Scholar]
- 40. Aluko O, Afolabi O, Olaoye E, Adebayo A, Oyetola S, Abegunde O. The management of the faeces passed by under five children: an exploratory, cross-sectional research in an urban community in Southwest Nigeria. BMC Public Health. 2017;17:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mohammed AI, Zungu L. Environmental health factors associated with diarrhoeal diseases among under-five children in the Sebeta town of Ethiopia. S Afr J Infect Dis. 2016;31:122-129. [Google Scholar]
- 42. Central Statistical Agency [Ethiopia] and ORC Macro. Ethiopia Demographic and Health Survey 2005. Central Statistical Agency/ Ethiopia and ORC Macro; 2006. [Google Scholar]
- 43. Central Statistical Agency [Ethiopia] and ICF International. Ethiopia Demographic and Health Survey 2011. Central Statistical Agency and ICF International; 2012. [Google Scholar]
- 44. Adane M, Mengistie B, Medhin G, Kloos H, Mulat W. Piped water supply interruptions and acute diarrhea among under-five children in Addis Ababa slums, Ethiopia: a matched case-control study. PLoS One. 2017;12:e0181516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bekele T, Rahman B, Rawstorne P. The effect of access to water, sanitation and handwashing facilities on child growth indicators: evidence from the Ethiopia Demographic and Health Survey 2016. PLoS One. 2020;15:e0239313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vittinghoff E, Glidden DV, Shiboski SC, McCulloch CE. Logistic regression. In: Regression Methods in Biostatistics for Biology and Health. Springer, Boston, MA; 2012:139-202. 10.1007/978-1-4614-1353-0_5. [DOI] [Google Scholar]
- 47. McNamee R. Confounding and confounders. Occup Environ Med. 2003;60:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kirkwood BR, Sterne J. Medical statistics: chapter 29: regression modelling. In: Kirkwood BR, Sterne JAC, eds. Essential Medical Statistics. 2nd ed. Blackwell Science; 2003. [Google Scholar]
- 49. Dessalegn M, Kumie A, Tefera W. Predictors of under-five childhood diarrhea: Mecha District, west Gojam, Ethiopia. Ethiop J Health Dev. 2011;25:192-200. [Google Scholar]
- 50. Sisay MM, Atnafu A, Demissie GD, Tessema ZT. Geographical disparities and determinants of childhood diarrheal illness in Ethiopia: further analysis of 2016 Ethiopian Demographic and Health Survey. Trop Med Health. 2020;48:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Edwin P, Azage M. Geographical variations and factors associated with childhood diarrhea in Tanzania: a National Population Based Survey 2015-16. Ethiop J Health Sci. 2019;29:513-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Christa L, Walker L, Perin J, Martin J, Bochi-Pinto C, Robert E. Diarrhea incidence in low- and middle-income countries in 1990 and 2010: a systematic review. BMC Public Health. 2012;12:220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Siziya S, Muula AS, Rudatsikira E. Correlates of diarrhoea among children below the age of 5 years in Sudan. Afr Health Sci. 2013;13:376-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li R, Lai Y, Feng C, Dev R, Wang Y, Hao Y. Diarrhea in under five year-old children in Nepal: a spatiotemporal analysis based on Demographic and Health Survey data. Int J Environ Res Public Health. 2020;17:2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bawankule R, Singh A, Kumar K, Shetye S. Does measles vaccination reduce the risk of acute respiratory infection (ARI) and diarrhea in children: a multi-country study? PLoS One. 2017;12:e0169713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jain DL, Sarathi V, Jawalekar S. Predictors of treatment failure in hospitalized children [3–59 months] with severe and very severe pneumonia. Indian Pediatr. 2013;50:787-789. [DOI] [PubMed] [Google Scholar]
- 57. Marufu T, Siziya S, Tshimanga M, Murugasampillay S, Mason E, Manyume B. Factors associated with measles complications in Gweru, Zimbabwe. East Afr Med J. 2001;78:135-138. [DOI] [PubMed] [Google Scholar]
- 58. Santos CA, Strina A, Amorim LD, et al. Individual and contextual determinants of the duration of diarrhoeal episodes in preschool children: a longitudinal study in an urban setting. Epidemiol Infect. 2012;140:689-696. [DOI] [PubMed] [Google Scholar]
- 59. WHO, UNICEF. Ending Preventable Child Deaths from Pneumonia and Diarrhoea by 2025: The integrated Global Action Plan for Pneumonia and Diarrhoea (GAPPD). World Health Organization/The United Nations Children’s Fund (UNICEF); 2013. [Google Scholar]
- 60. Bawankule R, Shetye S, Singh A, Singh A, Kumar K. Epidemiological investigation and management of bloody diarrhea among children in India. PLoS One. 2019;14:e0222208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kumi-Kyereme A, Amo-Adjei J. Household wealth, residential status and the incidence of diarrhoea among children under-five years in Ghana. J Epidemiol Glob Health. 2016;6:131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]