Abstract
Non-steroidal anti-inflammatory drugs are not only potent analgesics and antipyretics but also nephrotoxins, and may cause electrolyte disarray. In addition to the commonly expected effects, including hyperkalemia, hyponatremia, acute renal injury, renal cortical necrosis, and volume retention, glomerular disease with or without nephrotic syndrome or nephritis can occur as well including after years of seemingly safe administration. Minimal change disease, secondary membranous glomerulonephritis, and acute interstitial nephritis are all reported glomerular lesions seen with non-steroidal anti-inflammatory use. We report a patient who used non-steroidal anti-inflammatory drugs for years without diabetes, chronic kidney disease, or proteinuria; he then developed severe nephrotic range proteinuria with 7 g of daily urinary protein excretion. Renal biopsy showed minimal change nephropathy, a likely secondary membranous glomerulonephritis, and acute interstitial nephritis present simultaneously in one biopsy. Cessation of non-steroidal anti-inflammatory drug use along with steroid treatment resulted in a moderate improvement in renal function, though residual impairment remained. Urine heavy metal screen returned with elevated levels of urine copper, but with normal ceruloplasmin level. Workup suggested that the elevated copper levels were due to cirrhosis from non-alcoholic fatty liver disease. The membranous glomerulonephritis is possibly linked to non-steroidal anti-inflammatory drug exposure, and possibly to heavy metal exposure, and is clinically and pathologically much less likely to be a primary membranous glomerulonephritis with negative serological markers.
Keywords: Minimal change disease, podocytopathy, secondary membranous glomerulonephritis, acute interstitial nephritis, non-steroidal anti-inflammatory drugs
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are some of the most widely used and readily available medications for common complaints like fever and joint pain. While these drugs can be convenient and effective ways of alleviating fever or pain, their increasing number of adverse effects can often be overlooked. In particular, NSAID’s effects on renal physiology can lead to serious nephrotoxicity. Up to 5% of patients using NSAIDs may develop renal injury. 1 The most well-described pathogenesis of NSAID-induced renal injury is the inhibition of homeostatic prostaglandin synthesis. This would result in unopposed renal arteriolar vasoconstriction in many clinical conditions, leading to acute renal insufficiency and acute tubular necrosis. 2
These mechanisms of injuries can be categorized into the following broad groups: acute and chronic renal failure, edema and electrolyte imbalance, nephrotic syndrome with interstitial nephritis, and renal papillary necrosis.3,4 Reports of NSAID-induced nephrotic syndrome with acute interstitial nephritis (AIN) have been sparse, with most of the renal histology reported to be minimal change disease (MCD) 5 with interstitial nephritis.6,7 There are reports of nephrotic syndrome due to membranous nephropathy (MN) as an idiosyncratic drug reaction to NSAIDs.6,8 At this time, the pathophysiology of NSAID-induced nephrotic syndrome with AIN has not been elucidated. Here, we describe an unusual case of NSAID-induced MN with concurrent MCD and interstitial nephritis.
Case presentation
Our patient is a 67-year-old Spanish-speaking male with history of hypertension, hyperlipidemia, and chronic bilateral osteoarthritis of the knees, who was admitted to our hospital for anasarca and shortness of breath. Over the 3 months prior to initial presentation, he noticed worsening bilateral lower extremity pitting edema, abdominal swelling, and orthopnea. He also endorsed having nausea, vomiting, and diarrhea. Furthermore, he reported making less urine than before, accompanied by urine frothiness.
On initial examination, his blood pressure was 168/67 mm Hg. He had notable anasarca and a brain natriuretic peptide in the 2000s. There was 4+ pitting edema of his lower extremities up to the thighs. His lungs were clear and there was no respiratory distress. Initial urinalysis showed elevated urine protein of >500 mg/dL with 5 to 10 white blood cells/high power field (WBC/HPF) and 20 to 30 granular casts/HPF. His baseline serum creatinine (SCr) in 2018 was 0.5 to 0.6 mg/dL, but his SCr on initial admission was 2.1 mg/dL. Albumin was 2.0 g/dL, and urine protein:creatinine ratio (UPCR) was 6132 mg/g. Extensive proteinuria workup was done, revealing of 7 g protein/24 h, 3600 mg/24-h albuminuria. The proteinuria was mostly albuminuria nearly 60% with 40% of proteinuria being non-albumin proteinuria.
Further workup, including HIV, hepatitis B, hepatitis C, antinuclear antibody, rapid plasma reagin, anti-Rho, double-stranded DNA, and complements C3 and C4, was all negative or within normal limits. Serum and urine protein electrophoresis showed no monoclonal spikes. Renal ultrasound demonstrated normal cortical echogenicity and contour for both kidneys, with right measuring 13.2 cm and left measuring 13.0 cm.
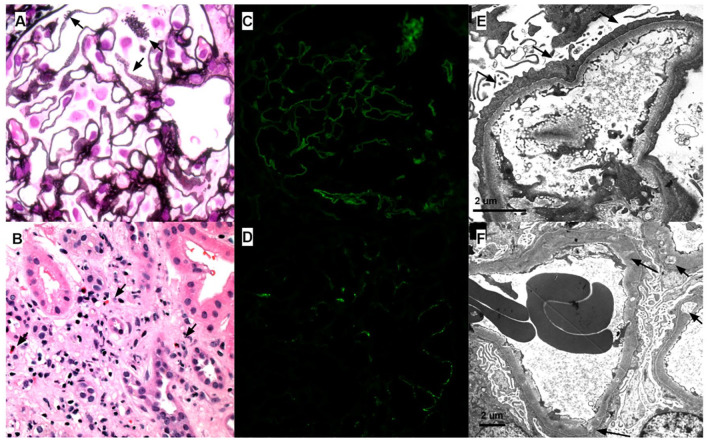
A percutaneous sonographic guided renal biopsy was done and was composed of renal cortex and contained at least 33 non-sclerotic glomeruli. The glomerular capillary loops were mildly thickened and exhibited segmental subtle spikes and pinholes. Podocytes appeared reactive. There was no mesangial or endocapillary hypercellularity. There were no large endocapillary deposits, necrotizing lesions, segments of sclerosis, or crescents. There was patchy acute tubular injury associated with mild patchy interstitial inflammation associated with foci of tubulitis and scattered eosinophils. There was mild interstitial fibrosis/tubular atrophy (~15%). Immunofluorescence studies demonstrated segmental granular glomerular capillary wall staining with immunoglobulin G (IgG, trace), immunoglobulin M (IgM, trace), C1q (trace-1+), kappa (trace), and lambda (trace) light chains. There was no extra-glomerular staining. The M-type phospholipase A2 receptor (PLA2R) stain was negative. Electron microscopic analysis demonstrated diffuse podocyte foot process effacement with frequent microvillous transformation and condensation of actin cytoskeletal filaments. Glomerular basement membranes were variable thickening with segmental subepithelial and intramembranous electron lucencies and subepithelial remodeling (spike/pinholes) and showed rare small granular subepithelial and intramembranous deposits. Minimal mesangial electron densities were present. No deposits displayed any significant fibrillary or microtubular substructure. There were no tubuloreticular inclusions or immune complex-type deposits present in any other location (see Figure 1).
Figure 1.
Three manifestations of NSAID-associated renal disease: (1) membranous nephropathy, (2) superimposed minimal change disease, and (3) acute interstitial nephritis. (A) Glomerular capillary loops with subtle spikes/pinholes consistent with membranous nephropathy (arrows; 600×; Jones Silver Stain). (B) Interstitial inflammation and edema with conspicuous eosinophils (arrows; 400×; H&E stain). (C, D) Immunofluorescence staining demonstrating segmental weak granular capillary loop staining with (C) IgG and (D) C1q (400×). (E) Diffuse podocyte foot process effacement (arrows), condensation of the podocyte actin cytoskeleton, and microvillous transformation with a capillary loop without evidence of membranous-type deposits. (F) Capillary loops with variable subepithelial and intramembranous electron lucency suggestive of old resorbed membranous-type deposits (class IV membranous nephropathy) and diffuse podocyte foot process effacement.
Diagnosis of MN (stage IV of IV) and mild AIN was rendered. Because the degree of podocyte foot process effacement was felt to be more extensive than would be expected given the somewhat segmental and low-grade MN, the possibility of a superimposed podocytopathy (MCD) was raised. The time course of the worsening proteinuria was also felt to be incompatible with the relatively chronic stage of the MN. The constellation of biopsy findings raised the suspicion for an underlying allergic/drug-induced etiology.
In light of this biopsy-proven diagnosis of MN, PLA2R [Anti Phospholipase A2 Receptor Antbiody] serology, anti-THSD7A [Anti Thrombospondin Type 1 Domain Contaning 7A Antibody], and anti-NELL [Anti Neural Epidermal Growth Factor-Like 1 Protein Antibody] were sent, and anti-PLA2R and anti-THSD7A came back negative. 9 Anti-NELL was sent, but after discussion with the Mayo Clinic laboratory, it could not be performed due to a lack of current approval as a commercially approved assay. The patient had no signs of systemic lupus erythematosus, no indications of malignancy (on recent computed tomography (CT) imaging studies and colonoscopy), and no evidence of chronic infections or sarcoidosis to suggest any other etiologies for a secondary MN. Lyme IgG and IgM were sent to investigate Lyme nephritis and were not detected, as Lyme disease has been reported to cause a diverse spectrum of glomerular disease.10–15
Given the concern for a potential drug-induced MN, lack of serologic evidence for a primary form, and its low-grade chronic nature, immunosuppressants such as cyclophosphamide or rituximab were not pursued. The patient was further diuresed with metolazone 5 mg and furosemide 80 mg IV (intravenous) twice a day with improvements to his urine output and edema. Prednisone 10 mg daily was also started for his worsening SCr, which peaked at 5.6 mg/dL about 1 month after initial presentation. When the patient came to our clinic a month later, his symptoms had drastically improved. The patient lost around 24 pounds of water weight with noticeable improvements to his edema. Upon further investigation at that time, the patient finally recalled that he had been frequently taking ibuprofen 9 months prior to symptom manifestation. He was taking 400 mg of ibuprofen three to four times a day; he had no heavy metal exposure, industrial exposure, or new vaccines in the time prior to development of anasarca. He had been using over-the-counter ibuprofen at lower doses (once a day) nearly daily for his chronic knee pain for 10 months prior to his intensification of therapy years before stopping due to inefficacy and advice of nephrologists regarding NSAID nephrotoxicity.
The findings suggesting NSAID-induced injury were tempered by finding of elevated urine copper levels. A 24-h urine panel for heavy metals showed undetectable levels of arsenic, zinc, cadmium, mercury, and lead. Copper levels were elevated at 27.1 µg/dL (upper limit of normal value 3.2 µg/dL) with a caveat that this is known to occur in high-grade proteinuria and with cirrhosis which the patient had. The patient also underwent workup for cirrhosis, including exclusion of Wilson’s disease; the patient ceruloplasmin level was within normal limits at 27 mg/dL. Eye examination did not reveal Kayser Fleischer rings as well. The liver findings were attributed by hepatology to non-alcoholic fatty liver disease (NAFLD). Unfortunately, given the patient’s financial status and insurance status, hair sampling for heavy metal toxicity was not financially viable or practical in this particular situation.
Discussion
NSAIDs have many well-described effects on renal physiology. Most of their processes of injury can be attributed to the inhibition of prostaglandin synthesis, thus altering renal hemodynamics and causing injury. NSAID-induced nephrotic syndromes are relatively uncommon, 2 and most reported cases are associated with MCD often with interstitial nephritis on renal biopsy. 16 The association between NSAID use and MN is also well known but less common. 8
A variety of NSAIDs have been implicated with nephrotic syndrome with interstitial nephritis. Of which, fenoprofen is likely the most well documented. 17 However, zomepirac, tolmetin, indomethacin, ibuprofen, naproxen, diclofenac, and sulindac have all been described. 18 Thus, NSAID-associated nephrotic syndromes (both MCD and MN) with AIN are not drug class specific nor are they dose dependent.18,19 In line with the commonly documented manifestations, our patient did not exhibit evidence of drug reaction with systemic symptoms (drug rash with eosinophilia and systemic symptoms (DRESS)) according to the RegiSCAR (Registry of Severe Cutaneous Adverse Reaction) criteria, 20 such as fever, rash, and eosinophilia.4,18 In addition, our patient’s nephrotic range proteinuria developed a few months after exposure to NSAIDs. Interestingly, our patient’s renal biopsy displayed a varied time course of the different processes. Rather than having a simultaneous process of MN with minimal change pattern and interstitial nephritis, there is evidence of a chronic stage-IV MN with concurrent separate MCD with AIN. It is unclear whether or not having an NSAID-associated MN would predispose to further injury with AIN and minimal change pattern glomerulopathy.
The diagnosis of MCD superimposed on MN on renal biopsy is difficult to make with certainty and in most cases is not possible. In the current case, the degree of podocyte foot process effacement was felt to be significantly out of proportion to the low-grade and chronic MN features on light, immunofluorescence, and electron microscopy, and thus most consistent with a superimposed MCD. The finding of elevated 24-h urine copper levels while associated with CKD (Chronic Kidney Disease) has not been associated with the glomerular findings (MCD, AIN, and secondary membranous glomerulonephritis (GN)). 21 High 24-h urine copper levels are associated with high-grade proteinuria, and this may explain these findings. 21 Thus, we favor NSAIDs as the cause of the pathological findings.
The possibility of an MN due to a separate process is also a diagnostic consideration in this case; nevertheless, the serologic workup for primary membranous antibodies and other secondary membranous etiologies was negative. However, the serologic studies for primary membranous antibodies may be negative in the chronic phase of MN. Lyme disease exposure was ruled out historically and serologically; heavy metal and industrial exposure was ruled out historically as well. Regardless, NSAIDs provide the most convincing underlying etiology that can connect all biopsy findings and clinical variables in this case. An analysis of the probability of the drug reaction by calculating a Naranjo probability score, the case scored 5 points deeming at a “probable adverse drug reaction.” 20
It is also possible that some of the manifestations discussed were due to NSAID exposure while others may have been due to another cause. Membranous glomerulonephritis, for instance, may have been due to heavy metal exposure. Primary membranous glomerulonephritis as stated prior is clinically and pathologically much less likely but is not able to be eliminated with 100% certainty. An MN with negative serological markers is always very difficult to rule out. We state this possibility, while also reaffirming our clinical suspicion of a unifying diagnosis of NSAID-induced MCD, AIN, and MN due to the clinical scenario presenting one unifying diagnosis and one exposure linked to all three. Nonetheless, this is invoking Occam’s razor to explain these events probabilistically.
As mentioned previously, NSAIDs often mediate acute renal failure through hemodynamic alterations involving prostaglandin synthesis inhibition. 22 However, in the setting of NSAID-induced nephrotic syndrome, it is more likely that an immunological mechanism is involved.1,2 The fact that these processes are not dose dependent also supports the immunological mechanism. 19 Furthermore, many stand-alone AIN or ATNs (acute tubular necrosis) can have rapid remission of AKI (acute kidney injury) within 2 months of NSAID withdrawal. The combination of nephrotic syndrome and AIN can complicate the recovery by prolonging the recovery time after stopping the NSAIDs. 14 In our case, the patient’s relative recovery may have been expedited by the addition of low-dose prednisone.
It is often difficult to obtain a complete history of NSAID use, given their widespread availability and utility. Many patients either do not recall taking NSAIDs or do not know which over-the-counter drugs are considered NSAIDs. However, as this case has shown, getting a complete medication history is vitally important in the investigation of acute renal failure etiologies. We hope to bring to light that long-term, continual ibuprofen use may be associated with the development of MN with superimposed minimal change glomerulopathy and interstitial nephritis if not intervened early enough.
Another finding that must be discussed and that could have accounted for a partial cause of the observed pathology is the 24-h urine copper level being elevated.23–25 The level was elevated at 27.1 µg/dL with a normal reference range of 3.2 µg/dL. This was cause for an extensive hepatic workup that revealed NAFLD and did not show any evidence of Wilson’s disease as evidenced by ophthalmologic examination and normal ceruloplasmin levels. There are studies linking elevated urinary copper in patients with proteinuria and cirrhosis.26,27
Conclusion
We report a complex case of secondary membranous glomerulonephropathy, with MCD, and AIN resulting most likely from NSAID exposure. Some role for heavy metal exposure cannot be completely excluded.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical permission/consent for publication: The Institutional Review Board (IRB) permission was not applied for as it is not required for individual case reports or case series with three patients or less in our institution (University of California, Los Angeles and University of California, Irvine). Consent was obtained from the patient and documented, on condition that the no identifiable data be published. Non-identifiable pathology images were documented by patient consent to be suitable for publication.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: K.K.-Z. is supported by the National Institute on Aging of the National Institutes of Health (grant R21-AG047036) and the National Institute of Diabetes, Digestive, and Kidney Disease (grants R01-DK078106, R01-DK096920, U01-DK102163, and K24-DK091419), as well as philanthropist grants from Mr. Harold Simmons and Mr. Louis Chang.
ORCID iDs: Andrew C Liu  https://orcid.org/0000-0002-4242-6873
https://orcid.org/0000-0002-4242-6873
Jonathan E Zuckerman  https://orcid.org/0000-0001-9758-2147
https://orcid.org/0000-0001-9758-2147
Lena M Ghobry  https://orcid.org/0000-0002-5744-0790
https://orcid.org/0000-0002-5744-0790
Ramy M Hanna  https://orcid.org/0000-0003-1807-8909
https://orcid.org/0000-0003-1807-8909
References
- 1. Merida E, Praga M. NSAIDs and nephrotic syndrome. Clin J Am Soc Nephrol 2019; 14(9): 1280–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zadrazil J. Nonsteroidal antiinflammatory drugs and the kidney. Vnitr Lek 2006; 52(7–8): 686–690. [PubMed] [Google Scholar]
- 3. Bach PH, Nguyen TK. Renal papillary necrosis—40 years on. Toxicol Pathol 1998; 26(1): 73–91. [DOI] [PubMed] [Google Scholar]
- 4. Whelton A. Nephrotoxicity of nonsteroidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med 1999; 106(5, Suppl. 2): 13S–24S. [DOI] [PubMed] [Google Scholar]
- 5. Glassock RJ. Secondary minimal change disease. Nephrol Dial Transp 2003; 18(Suppl. 6): vi52–vi58. [DOI] [PubMed] [Google Scholar]
- 6. Jung JH, Kang KP, Kim W, et al. Nonsteroidal antiinflammatory drug induced acute granulomatous interstitial nephritis. BMC Res Notes 2015; 8: 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rossert J. Drug-induced acute interstitial nephritis. Kidney Int 2001; 60(2): 804–817. [DOI] [PubMed] [Google Scholar]
- 8. Nawaz FA, Larsen CP, Troxell ML. Membranous nephropathy and nonsteroidal anti-inflammatory agents. Am J Kidney Dis 2013; 62(5): 1012–1017. [DOI] [PubMed] [Google Scholar]
- 9. Sethi S, Debiec H, Madden B, et al. Neural epidermal growth factor-like 1 protein (NELL-1) associated membranous nephropathy. Kidney Int 2020; 97(1): 163–174. [DOI] [PubMed] [Google Scholar]
- 10. Lyme Disease in California, http://ipm.ucanr.edu/PMG/PESTNOTES/pn7485.html#IDENTIFICATION (accessed 30 August 2020).
- 11. Florens N, Lemoine S, Guebre-Egziabher F, et al. Chronic Lyme borreliosis associated with minimal change glomerular disease: a case report. BMC Nephrol 2017; 18(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fritz CL, Kjemtrup AM, Conrad PA, et al. Seroepidemiology of emerging tickborne infectious diseases in a Northern California community. J Infect Dis 1997; 175(6): 1432–1439. [DOI] [PubMed] [Google Scholar]
- 13. Gueye S, Seck SM, Kane Y, et al. [Lyme nephritis in humans: physio-pathological bases and spectrum of kidney lesions]. Nephrol Ther 2019; 15(3): 127–135. [DOI] [PubMed] [Google Scholar]
- 14. Kwiatkowska E, Golembiewska E, Ciechanowski K, et al. Minimal-change disease secondary to Borrelia burgdorferi infection. Case Rep Nephrol 2012; 2012: 294532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stone BL, Tourand Y, Brissette CA. Brave new worlds: the expanding universe of Lyme disease. Vector Borne Zoonotic Dis 2017; 17(9): 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alper AB, Jr, Meleg-Smith S, Krane NK. Nephrotic syndrome and interstitial nephritis associated with celecoxib. Am J Kidney Dis 2002; 40(5): 1086–1090. [DOI] [PubMed] [Google Scholar]
- 17. Finkelstein A, Fraley DS, Stachura I, et al. Fenoprofen nephropathy: lipoid nephrosis and interstitial nephritis. Am J Med 1982; 72(1): 81–87. [DOI] [PubMed] [Google Scholar]
- 18. Sanchez-Borges M. Clinical management of nonsteroidal anti-inflammatory drug hypersensitivity. World Allergy Organ J 2008; 1(2): 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Radford MG, Jr, Holley KE, Grande JP, et al. Reversible membranous nephropathy associated with the use of nonsteroidal anti-inflammatory drugs. JAMA 1996; 276(6): 466–469. [PubMed] [Google Scholar]
- 20. Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int 2006; 55(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 21. Pedraza-Chaverri J, Torres-Rodriguez GA, Cruz C, et al. Copper and zinc metabolism in aminonucleoside-induced nephrotic syndrome. Nephron 1994; 66(1): 87–92. [DOI] [PubMed] [Google Scholar]
- 22. Ravnskov U. Glomerular, tubular and interstitial nephritis associated with non-steroidal antiinflammatory drugs. Br J Clin Pharmacol 1999; 47(2): 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Orr SE, Bridges CC. Chronic kidney disease and exposure to nephrotoxic metals. Int J Mol Sci 2017; 18(5): 1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lentini P, Zanoli L, Granata A, et al. Kidney and heavy metals—the role of environmental exposure. Mol Med Rep 2017; 15(5): 3413–3419. [DOI] [PubMed] [Google Scholar]
- 25. Zhang L, Liu F, Peng Y, et al. Nephrotic syndrome of minimal change disease following exposure to mercury-containing skin-lightening cream. Ann Saudi Med 2014; 34(3): 257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Frommer DJ. Urinary copper excretion and hepatic copper concentrations in liver disease. Digestion 1981; 21(4): 169–178. [DOI] [PubMed] [Google Scholar]
- 27. Stec J, Podracka L, Pavkovcekova O, et al. Zinc and copper metabolism in nephrotic syndrome. Nephron 1990; 56(2): 186–187. [DOI] [PubMed] [Google Scholar]