Abstract
Acute immune responses to microbial insults in the oral cavity often progress to chronic inflammatory diseases such as periodontitis and apical periodontitis. Chronic oral inflammation causes destruction of the periodontium, potentially leading to loss of the dentition. Previous investigations have demonstrated that the composition of oral immune cells, rather than the overall extent of cellular infiltration, determines the pathological development of chronic inflammation. The role of T lymphocyte populations, including Th1, Th2, Th17, and Treg cells, has been extensively described. Studies now propose pathogenic Th17 cells as a distinct subset, uniquely classifiable from traditional Th17 populations. In situ differentiation of pathogenic Th17 cells has been verified as a source of destructive inflammation, which critically drives pathogenesis in chronic inflammatory diseases such as diabetes, rheumatoid arthritis, and inflammatory bowel disease. Pathogenic Th17 cells resemble a Th1 penotype and produce not only interleukin 17 (IL-17) but also γ-interferon (IFN-γ) and granulocyte-macrophage colony-stimulating factor (GM-CSF). The proinflammatory cytokine-specific mechanisms known to induce IL-17 expression in Th17 cells are well characterized; however, differentiation mechanisms that lead to pathogenic Th17 cells are less understood. Recently, Ca2+ signaling through Ca2+ release-activated Ca2+ channels (CRAC) in T cells has been uncovered as a major signaling axis involved in the regulation of T-cell-mediated chronic inflammation. In particular, pathogenic Th17 cell–mediated immunological diseases appear to be effectively targeted via such Ca2+ signaling pathways. Pathogenic plasticity of Th17 cells has been extensively illustrated in autoimmune and chronic inflammatory diseases. Although their specific causal relationship to oral infection-induced chronic inflammatory diseases is not fully established, pathogenic Th17 cells may be involved in the underlining mechanism. This review highlights the current understanding of T-cell phenotype regulation, calcium signaling pathways in this event, and the potential role of pathogenic Th17 cells in chronic inflammatory disorders of the oral cavity.
Keywords: Th17, calcium channels, apical periodontitis, periodontitis, chronic inflammation, autoimmune diseases
Introduction: T-Cell Heterogeneity in Periodontitis and Apical Periodontitis
Inflammation of the periodontium may be considered a host defense mechanism aimed to prevent further spread of pathogens through mucosal barrier tissues or the root canal system. A significant infiltration of T cells has been demonstrated in chronic inflammation of the gingiva and periapical tissues, where T helper (Th) cells present as a heterogeneous population.
Relative ratios of T-cell populations have been measured in an effort to characterize disease phases of periodontitis and apical periodontitis. A classic study by Stashenko and Yu (1989) reported that active lesion development in rat apical periodontitis was associated with higher ratios of T helper/inducer cells over T suppressor/cytotoxic cells as compared to the chronic phase, despite similar levels of total T-cell infiltrate (Stashenko and Yu 1989). This paradigm has been repeatedly re-examined to elucidate which specific subset(s) of the T-cell population may be involved in the patterning and progression of apical periodontitis.
Within the Th cell division, Th1 cells were classically described as the primary proinflammatory effector subtype. Th1 cells are identified by their production of interferon-γ (IFN-γ), interleukin (IL) 2, and IL-12 (Jankovic et al. 2001). Th1 cells may also secrete tumor necrosis factor α (TNF-α) in response to intracellular infections by viruses and bacteria (Kidd 2003). In contrast, Th2 cells produce a different set of cytokines (IL-4, IL-5, and IL-13), which induce allergic reactions and oppose Th1 responses (Onoe et al. 2007). The migration of Th1/Th2-cell populations into target tissues occurs along specific gradients due to the expression of distinct chemokine receptors. Discordant Th1/Th2 responses were initially advocated as a determinant of lesion activity in periodontitis and apical periodontitis (see Appendix Table 1 for references).
The characterization of other effector T-cell subsets, such as T regulatory (Treg) cells and Th17 cells, and their role in inflammatory diseases has drastically improved over time. CD4+ Treg cells are defined by their expression of Foxp3 as well as CD25 and establish immune homeostasis by inhibiting inflammation (Fischer et al. 2019). In contrast, Th17 cells are largely associated with proinflammatory immune responses and the secretion of their signature cytokines—IL-17A, IL-17F, IL-21, and IL-22. It has been indicated that Th17 cells induce significant inflammatory tissue damage, including connective tissue degeneration and osteoclastic bone resorption (Okamoto et al. 2017). Therefore, interrelationships and comodulatory mechanisms between Treg and Th17 cells have been investigated to describe the disease kinetics of periodontitis and apical periodontitis (see Appendix Table 1 for references).
Tissue-resident Th17 cells play a central role in host defense and immune homeostasis. However, Th17 cells can be a pathogenic driver of chronic inflammatory disorders, referred as nonhomeostatic or infection-induced Th17 cells (Stockinger and Omenetti 2017). The development of pathologic inflammatory Th17 subpopulations is postulated as a major contributor to chronic inflammatory disorders (Appendix Table 2). This review discusses the current understanding of inflammatory or pathogenic Th17 cell differentiation. Herein, the influences of inflammatory cytokine milieus within secondary lymphoid organs as well as effector sites are summarized. Furthermore, calcium signaling in T cells has been separately identified as an important mechanism regulating the Th17-cell plasticity. While T-cell calcium signaling mechanisms have not been investigated in oral chronic inflammatory disorders, they have been intensively characterized in autoimmune diseases such as gut microbial–induced inflammatory bowel disease (Kaufmann et al. 2019). Given the clinically established link between certain systemic chronic inflammatory diseases and periodontitis as well as apical periodontitis, this review introduces T-cell calcium signaling pathways as a new avenue for mechanistic investigations.
Homeostatic Th17 Cells and Pathogenic Th17 Cells
Homeostatic Th17 cell differentiation requires IL-6 (and/or IL-1β), which facilitates the molecular association of the IL-6 receptor and gp130 phosphorylation in naive CD4+ T cells, leading to the activation of Janus tyrosine kinase (Jak). Jak then assists in dimer formation of STAT3, a transcription factor regulating the expression of RORγt—the hallmark Th17 lineage transcription factor. Activation of STAT3 and RORγt upregulates the expression of IL-17A, IL-17F, IL-21, and IL-22. In mice, IL-6 knockout mutation significantly attenuated the development of Th17 cells; however, it did not completely abolish their differentiation process. In addition to the IL-6/STAT3/RORγt pathway, an alternative differentiation mechanism was identified through the IL-21/STAT3/RORγt signaling (Korn et al. 2009) (Fig. 1).
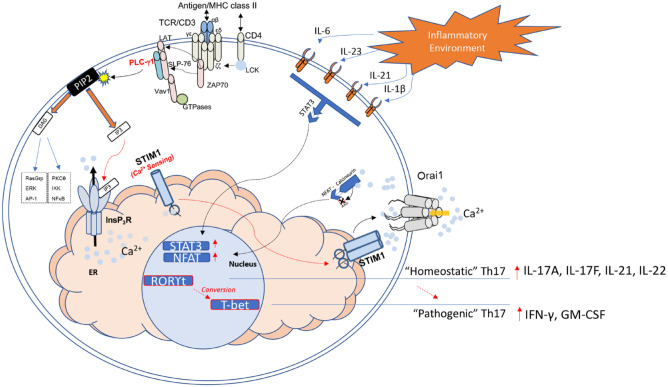
Figure 1.
Th17 pathogenesis via T-cell receptor (TCR) stimulation, cytokine signaling, and Ca2+ signaling. Antigen stimulation of the TCR complex via MHC class II interactions triggers downstream signaling pathways through activation of TCR-associated scaffolding/adaptor proteins. Phosphorylation of the TCR ζ chain by lymphocyte-specific protein tyrosine kinase (LCK) recruits ZAP70 to the TCR complex. LAT and SLP-76 are subsequently phosphorylated by ZAP70, resulting in assembly and activation of Vav1 and PLC-γ1. Phosphorylated PLC-γ1 hydrolyzes membrane-bound PIP2 into DAG and IP3 and results in the binding of IP3 to the endoplasmic reticulum (ER)–bound IP3 receptor (IP3R). Depletion of ER stores, which occurs following InsP3R-mediated ER Ca+ efflux, is detected by STIM proteins on the ER membrane. Upon sensing Ca2+ depletion, STIM oligomerizes and moves to the ER–plasma membrane (PM) junction, where it engages Orai1 to activate CRAC channels. Sustained Ca2+ entry via CRAC channels raises cytoplasmic calcium levels and allows for activation of calcineurin, which induces nuclear factor of activated T-cell (NFAT) dephosphorylation and translocation to the nucleus. At the same time, key cytokines produced within the local inflammatory environment bind to corresponding cytokine receptors along the cell membrane. Interleukin (IL) 6, IL-23, IL-21, and IL-1β signaling converge to induce STAT3-mediated transcription. Together, NFAT and STAT3 help induce expression of characteristic “homeostatic” Th17 molecular markers (RORγt, IL-17A, IL-17F, IL-21, and IL-22), which may subsequently transition to a distinct pathogenic profile involving expression of T-bet (aka T-box transcription factor TBX21), interferon-γ (INF-γ), and granulocyte-macrophage colony-stimulating factor (GM-CSF). The precise mechanism connecting SOCE, cytokine signals, and the conversion to this pathogenic lineage of Th17 cells remains to be fully elucidated. Modified from Srikanth et al. (2017).
Th17 cells are known to possess significant plasticity and can acquire a Th1-like phenotype. Inflammatory or pathogenic Th17 cells are distinguished from homeostatic Th17 cells based on their cytokine production and transcriptional signature. While homeostatic, conventional Th17 cells are identified by RORγt driven production of IL-17A, IL-17F, IL-21, and IL-22, pathogenic Th17 cells are uniquely associated with IFN-γ and GM-CSF production and predominately express the T-bet transcription factor (Fig. 1) (Lee et al. 2009; Wang et al. 2014; Krausgruber et al. 2016). Conversion of Th17 cells into this highly pathogenic phenotype has been suggested to contribute to the development of chronic inflammatory and autoimmune diseases (Ueno et al. 2018; Yang et al. 2019).
Th17 cells differentiated in vitro in the presence of IL-6, IL-1β, and IL-23 have pathogenic effects causing autoimmune encephalomyelitis (EAE) in mice following adaptive transfer (Gaublomme et al. 2015). IL-23 was identified as a key inducer of the transition to IFN-γ-producing pathogenic Th17 cells, which correlated with increased expression of T-bet (Hirota et al. 2011). Commensal microbiota and IL-23 were also found to upregulate T-bet in RORγt+ innate lymphoid cells in intestine (Klose et al. 2013). An infection-induced differentiation of pathogenic Th17 cells in the intestine was further demonstrated by segmented filamentous bacteria and Citrobacter rodentium in mice (Omenetti et al. 2019). Recently, the IL-23/IL-17 axis has become a recognized pathological mechanism of periodontitis through activation and expansion of Th17 cell populations in periodontitis (see Appendix Table 1 for references). Future studies are needed to clarify whether IFN-γ-producing pathogenic Th17 cells are involved in the oral chronic inflammatory diseases.
Takayanagi and colleagues proposed that Th17 cells in an experimental murine autoimmune arthritis model were converted from Foxp3+Treg cells (Komatsu et al. 2014). “exFoxp3” Th17 cells expressed the IL-23 receptor and activator of NF-κB ligand (RANKL), demonstrating increased osteoclastogenesis as compared to Th17 cells from the coculture model of bone marrow monocytes and synovial fibroblasts. The same group also reported the presence of exFoxp3 Th17 cells in a ligature-induced mouse periodontitis model. This cell population showed a higher expression level of IL-17 cytokines than conventional Th17 cells, suggesting a potential relevancy of Th17 plasticity to the pathogenesis of periodontitis (Appendix Table 1).
Forkhead box-O 1 (FOXO1), a member of FOXO transcription factors, has been shown to inhibit the formation of Th17 cells and suppress gene transcription of IL-17A and IL-23 receptor-target genes (Laine et al. 2015). MicroRNAs (miRNAs) target mRNA degradation at the posttranslational level and have been suggested to regulate T-cell differentiation (Muljo et al. 2005). Pathogenic Th17 cells induced in vitro in the presence of IL-6, IL-1β, and IL-23 were associated with increased expression of miRNA-183 cluster (miR-183C), which directly repressed the expression of FOXO1 (Ichiyama et al. 2016). Accordingly, the involvement of miRNA-183C has been incorporated to the mechanism of pathogenic Th17 differentiation.
Calcium Signaling as a Pathological Mechanism of Th17 Cell Differentiation
The expression of unique Ca2+ channels and regulators are examples of specialized components that contribute to the specificity of Ca2+ signaling in T cells (Srikanth et al. 2017). Oscillations of intracellular calcium concentrations in T cells are triggered by TCR stimulation and regulate the activation of transcription factors, such as nuclear factor of activated T cells (NFATs) (Figs. 1, 2). The regulation of transcription factors thereby enables control of cellular differentiation, proliferation, and cytokine production (Srikanth and Gwack 2013; Kim et al. 2014). Ca2+ release-activated Ca2+ (CRAC) channels reside on the cell membrane and mediate Ca2+ entry in response to TCR stimulation (Figs. 1, 2). These channels primarily exist as hexamers of the fundamental transmembrane pore subunit—Orai1 (Feske et al. 2006; Cai et al. 2016). Present studies demonstrate that differentiation and pathogenicity of effector Th17 populations are especially sensitive to the disruption of CRAC channel function (Kim et al. 2013; Kaufmann et al. 2019).
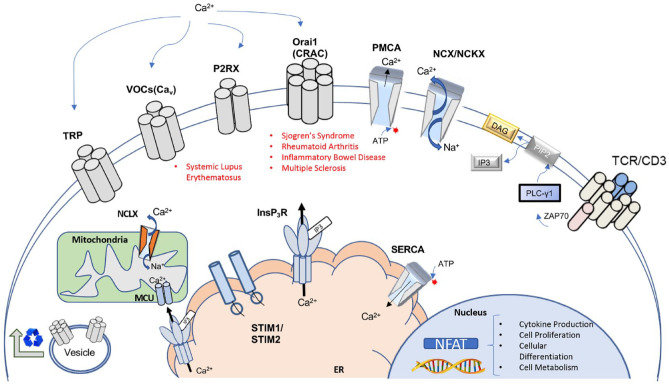
Figure 2.
Overview of major calcium channels and regulators of calcium signaling in T lymphocytes. Key components of Ca2+ homeostasis and signaling in T lymphocytes. Examples of autoimmune diseases currently associated with specific channelopathies are listed beneath respective constituents. Various channels are involved in homeostasis of Ca2+ as well as signaling events in receptor-mediated Ca2+ entry. Ca2+ influx is controlled via several main channel types: transient receptor potential (TRP) channels, voltage-gated Ca2+ channels (VOCs), purinergic ionotropic receptors (P2RXs), and calcium release-activated calcium channels (CRACs). Recycling and trafficking of channels to the plasma membrane occurs through vesicle-mediated transport from the Golgi apparatus. Transporters and ion pumps facilitate maintenance of cellular Ca2+ levels. Plasma membrane Ca2+ ATPases (PMCAs) and Na+/Ca2+ exchangers (NCKX) control efflux of cytoplasmic calcium. In the endoplasmic reticulum (ER), the sarcoplasmic/ER Ca2+ ATPase (SERCA) mediates calcium influx. T-cell receptor (TCR) stimulation generates IP3, leading to the release of ER calcium pools via InsP3 receptor (InsP3R) channels. Depletion of ER calcium stores is detected through EF-handed stromal interaction molecules 1 and 2 (STIM1, STIM2), which leads to sustained cytoplasmic Ca2+ influx via activation of Orai (CRAC channels) during T-cell activation. Contact sites between InsP3Rs and the mitochondrial Ca2+ uniporter (MCU) enable coupling of TCR stimulation to metabolic activities in the mitochondria. Mitochondrial Na+/Ca2+/Li+ exchangers (NCLXs) regulate mitochondrial Ca2+ levels. Transduction of TCR stimulation via Ca2+ signaling culminates in the activation of nuclear factor of activated T cells (NFATs). Activation of NFAT controls cellular responses associated with T-cell activation, including cytokine production, cell differentiation, and proliferation. Modified and expanded from Trebak and Kinet (2019) and Park et al. (2020).
CRAC channel activation occurs subsequent to the depletion of endoplasmic reticulum (ER) Ca2+ stores in a mechanism referred to as store-operated Ca2+ entry (SOCE). SOCE is initiated following activation of phospholipase C (PLC) within the TCR signaling cascade. Activated PLC catalyzes the release of inositol trisphosphate (IP3), which induces Ca2+ release from the ER lumen (Fig. 1) (Feske et al. 2012; Noel 2019). Stromal interaction molecules (STIM1 and STIM2) also play a vital role in SOCE and CRAC activity. STIM proteins are anchored to the ER membrane and function as Ca2+ sensors that detect ER Ca2+ depletion. After sensing ER Ca2+ depletion, STIM1 and STIM2 translocate to the ER–plasma membrane (PM) junctions defined by junctional proteins (Woo et al. 2020). Interaction between STIM1 and Orai1 at the ER-PM junction results in the activation and opening of the CRAC channels, thereupon producing a sustained increase in intracellular calcium levels (Fig. 1). Mutations of both STIM1 and Orai1, independently, have been identified in patients with severe combined immunodeficiency (SCID), which supports the critical role of CRAC channel function in the immune system (Feske et al. 2006; Picard et al. 2009; Kim et al. 2014).
Th17 cells have emerged as major contributors to numerous autoimmune and inflammatory diseases (Appendix Table 2) (Reinert-Hartwall et al. 2015; Li et al. 2017; Ueno et al. 2018; van Hamburg and Tas 2018; Schirmer et al. 2019; Yang et al. 2019). In humans, pathogenic Th17 cell infiltration or differentiation occurs at sites of tissue inflammation across various immunological and chronic inflammatory diseases, including diabetes, rheumatoid arthritis, and inflammatory bowel disease (Appendix Table 2). Evidence from corresponding animal models of these diseases is consistent with such observations in humans, demonstrating that the presence and effector functions of pathogenic Th17 cells are exceedingly correlated to the severity of immune disorders (Appendix Table 2) (Park et al. 2005; Lee et al. 2009; Ma et al. 2010; McCarl et al. 2010; Kim et al. 2013; Ip et al. 2016; Evans-Marin et al. 2018; Kaufmann et al. 2019).
Several factors determine the fate of effector T-cell differentiation. The strength of TCR-induced calcium signaling, chronicity of the antigenic stimuli, and the presence of polarizing cytokines all affect the transition of naive CD4+ precursors into Th1 versus Th2 versus Th17 phenotypes (Robert et al. 2013). Although initial differentiation of CD4+ lineages occurs in the peripheral lymphoid organs, Th17 cells transition to Th1-like, pathogenic cells after migrating to tissue-specific sites, in part through CRAC-regulated TCR signaling (Woo et al. 2018).
As reviewed, the differentiation of Th17 cells is largely controlled by TCR stimulation in conjunction with polarizing cytokines. Receptor signaling through IL-1β, IL-6, IL-21, and IL-23 induces activation of the STAT3 transcription factor and complements activation of NFAT-mediated gene expression via SOCE and TCR signaling. Deletion of Orai1, STIM1, or STIM2 in CD4+ T cells inhibits SOCE and ameliorates Th17-mediated inflammation in murine models of multiple sclerosis as well as inflammatory bowel disease (Kaufmann et al. 2016; Kaufmann et al. 2019). Evidence suggests that in addition to controlling NFAT expression, SOCE plays another unique role in Th17 pathogenicity by regulating genes involved in the electron transport chain. Deletion of STIM1 in Th17 cells impairs oxidative phosphorylation (OXPHOS) and increases mitochondrial reactive oxygen species (ROS) levels, corresponding to a nonpathogenic Th17 transcriptional signature, a loss of resistance to cell death, and protection from Th17-mediated inflammatory disease. Notably, protection from Th17-induced inflammatory disease in murine models appears independent of STAT3 activity in Stim1–/– Th17 cells. Together, these data suggest that mitochondrial function is a key determinant of Th17 pathogenicity, which is predominately regulated by Ca2+ signaling via SOCE (Kaufmann et al. 2019).
Given the sensitivity of Th17 cells to SOCE, CRAC channels and their associated components are promising candidates for therapeutic strategies in autoimmune and chronic inflammatory diseases. Defects in Ca2+ signaling and SOCE may likewise offer insight into potential mechanisms that underlie the greater susceptibility to periodontitis and apical periodontitis in patients with systemic conditions such as rheumatoid arthritis and inflammatory bowel disease.
Calcium Signaling in T-Cell–Mediated Human Diseases
Systemic autoimmune and chronic inflammatory diseases are attributed to a breakdown of homeostatic mechanisms within the immune system. Several immunological diseases are characterized by the expansion and infiltration of autoreactive T cells into normal host tissues. Chronic inflammatory diseases are associated with hypersensitive immune responses and, similar to autoimmune diseases, may arise from defects in T-cell development, differentiation, or the regulation of peripheral effector lymphocytes. Various investigations have implicated distorted Ca2+ signaling as a shared feature of these diseases (Park et al. 2020).
Diabetes is associated with an increased susceptibility to infections and impaired wound healing. Significant changes in Ca2+ homeostasis are observed in both type I and type II diabetes (Demkow et al. 2012; Wang et al. 2018). Type I diabetes is major autoimmune disease. Type I diabetes–prone strains of rats result from a functional loss of GIMPA5 (GTPase of the immune-associated nucleotide-binding protein 5), leading to the preferential survival of autoreactive T lymphocytes. The spontaneous apoptosis of most mature T lymphocytes in this model of diabetes has been linked to GIMAP5-facilitated mitochondrial Ca2+ buffering following SOCE (Chen et al. 2013). Furthermore, 2 primary consequences of diabetes, hyperglycemia and elevated oxidative stress, have been similarly related to disruption of Ca2+ metabolism. Lymphocytes in type II diabetes demonstrate amplified activity of Na+/Ca+ transporters, reduction in Ca2+-ATPase activity, and diminished Ca2+ release following TCR stimulation. A recent study also identified that SOCE is downregulated via diminished expression of Orai1 in T lymphocytes isolated from patients with type II diabetes. It was suggested that posttranscriptional modification by glycosylation of Orai1 might be responsible for these defects in Ca+ signaling (Wang et al. 2018). Hence, it is plausible that diabetes-driven defects in Ca2+ signaling may contribute to the susceptibility of diabetic patients to a range of comorbidities, including chronic oral inflammation.
Rheumatoid arthritis is characterized by chronic inflammation in the synovia of joint spaces, eventually involving the surrounding hard tissues and progressive joint destruction. In addition to autoantibodies, significant elevations of IFN-γ and IL-17 attributed to autoreactive T-cell infiltration are observed in the synovia of rheumatoid arthritis patients. Enhanced Ca2+ entry in naive T cells, overexpression of Orai1, and single-nucleotide polymorphisms (SNPs) within Orai1 coding regions have been positively correlated to rheumatoid arthritis activity (Liu et al. 2014; Yen et al. 2014). The upregulation of CRAC channels is consonant with the progression of multiple sclerosis and corresponds to amplified cytokine production at sites of disease (Park et al. 2020). Calcineurin, a protein phosphatase that activates NFAT, is a common therapeutic target in the treatment of rheumatoid arthritis. The use and effectiveness of calcineurin inhibitors in rheumatoid arthritis, as well as other immune disorders, further underscores the critical role of Ca2+-calcineurin-NFAT signaling in their pathogenesis.
Ca2+ signaling mechanisms in Th1 and Th17 cells also importantly contribute to the progression and pathogenesis of inflammatory bowel disease (Vaeth et al. 2020). Vaeth et al. (2020) demonstrated the significance of Orai1 and Orai2 heteromeric CRAC channel structures in Th1 and Th17 cells both in vitro and in vivo, where Orai1/Orai2-deficient mice are resistant to murine models of colitis (Vaeth et al. 2017). Adoptive transfer of SOCE-deficient CD4+ T cells similarly fails to induce disease in murine models due to defective Th17 cytokine production (McCarl et al. 2010; Vaeth et al. 2020). In addition to modulation of conventional Th17 cytokine production, the regulation of colitogenic Th17 cells by SOCE is mediated through other mechanisms previously described (i.e., mitochondrial function and pathogenic cell differentiation pathways; Kaufmann et al. 2019). Bioinformatic study of ulcerative colitis, a common form of inflammatory bowel disease, has also revealed significant links to gene networks associated with Ca2+ signaling, again supporting SOCE as an avenue for potential therapeutic targeting (Shi et al. 2019).
Translational Significance of T-Cell Targeting Calcium Signaling in Dentistry
The impact of Orai1 dysfunction on immune health may reveal Ca+ signaling through CRAC channels as a potential target or mechanism by which to explore effective therapeutic alternatives for chronic inflammatory conditions, including both periodontitis and refractory, nonhealing apical periodontitis. Systemic chronic inflammatory diseases directly associated with dysregulated or dysfunctional Ca2+ signaling have been correlated to increased susceptibility and/or severity of periodontitis and apical periodontitis. Aging may represent another factor altering the host response to dental infection and treatment outcomes. In fact, the severity and prevalence of periodontitis exacerbates with aging. Elderly populations display distinct reductions in memory B cells and naive T-cell populations and exhibit altered immune responses (Montecino-Rodriguez et al. 2013), culminating in a heterogenic bias toward Th17 phenotypes (Huang et al. 2008). Therefore, age-related transitions in T-cell demography may partly explain the association of advanced age to oral disease through analogous mechanisms.
The fact that principal elements of the cytokine network involved in periodontitis, such as IL-2 as well as IL-17 and IFN-γ, are affected by disruption of CRAC channel function is significant. Recent investigation has also demonstrated that Treg cells react to autoimmune neuroinflammation by suppressing Th17 cell Ca2+ signaling in the spinal cord. This finding implies that Ca2+ signaling may be an innately targeted mechanism to control inflammatory processes, making therapeutic strategies that mimic these host responses attractive (Othy et al. 2020).
The 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions revised the periodontitis diagnostic system by stages of disease severity (Tonetti et al. 2018). Moreover, the new periodontitis diagnostic system aims to clinically identify individual risk factors affecting the disease progress and treatment prognosis. For example, an association with other chronic inflammatory or immunological diseases such as diabetes, rheumatoid arthritis, and inflammatory bowel disease has led to their inclusion as risk factors. Provided that apical periodontitis is also indicated to associate with a common set of systemic diseases (Khalighinejad et al. 2016), similar considerations for endodontic diagnoses, risk factors, and treatment outcomes are warranted. Ultimately, these systemic and oral diseases may share certain underlining pathological mechanisms.
It is conceivable that T cells postulated as critical mediators of persistent infection-induced chronic inflammatory processes in periodontitis and apical periodontitis, may be influenced by manipulations of Ca2+ signaling pathways and Ca2+ channels. However, the role of T-cell Ca2+ signaling has not been investigated in periodontitis and apical periodontitis to date. Calcium channel blockers (CCBs), widely used drugs to treat hypertension, prevent Ca2+ ion influx into vascular smooth muscle cells and cardiac myocytes. Patients with the history of CCB treatment often develop drug-induced gingival enlargement or overgrowth (Livada and Shiloah 2014). With the advancing knowledge of Ca2+ signaling pathways in T-cell pathogenicity and homeostasis, oral barrier immune cells could be affected by CCBs and possibly contribute to inflammatory gingival enlargement. We propose that further investigations related to T-cell physiology may thereby offer a potential explanation for the pathogenesis of infection-induced oral chronic inflammation in periodontitis and apical periodontitis.
Author Contributions
S. Hasiakos, I. Nishimura, contributed to conception, design, data acquisition, and interpretation, drafted and critically revised the manuscript; Y. Gwack, M. Kang, contributed to data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-pdf-1-jdr-10.1177_0022034521990652 for Calcium Signaling in T Cells and Chronic Inflammatory Disorders of the Oral Cavity by S. Hasiakos, Y. Gwack, M. Kang and I. Nishimura in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
Declaration of Conflicting Interests: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: I. Nishimura is a consultant of Fujifilm Corporation and BioVinc LLC.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: S. Hasiakos is Dental Specialty and PhD Program (DSPP) Scholar supported by National Institutes of Health (NIH)/National Institute of Dental and Craniofacial Research (NIDCR) K12DE027830. The authors’ research contributing to this review was supported by NIH/National Institute of Allergy and Infectious Diseases R01AI083432, R01AI146615, R21AI149236, and R03AI147063 (Y. Gwack) and NIH/NIDCR R01DE022552 and R44DE025524 (I. Nishimura).
References
- Cai X, Zhou Y, Nwokonko RM, Loktionova NA, Wang X, Xin P, Trebak M, Wang Y, Gill DL. 2016. The orai1 store-operated calcium channel functions as a hexamer. J Biol Chem. 291(50):25764–25775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XL, Serrano D, Mayhue M, Wieden HJ, Stankova J, Boulay G, Ilangumaran S, Ramanathan S. 2013. GTPase of the immune-associated nucleotide-binding protein 5 (GIMAP5) regulates calcium influx in T-lymphocytes by promoting mitochondrial calcium accumulation. Biochem J. 449(2):353–364. [DOI] [PubMed] [Google Scholar]
- Demkow U, Winklewski P, Ciepiela O, Popko K, Lipinska A, Kucharska A, Michalska B, Wasik M. 2012. Modulatory effect of insulin on T cell receptor mediated calcium signaling is blunted in long lasting type 1 diabetes mellitus. Pharmacol Rep. 64(1):150–156. [DOI] [PubMed] [Google Scholar]
- Evans-Marin H, Rogier R, Koralov SB, Manasson J, Roeleveld D, van der Kraan PM, Scher JU, Koenders MI, Abdollahi-Roodsaz S. 2018. Microbiota-dependent involvement of Th17 cells in murine models of inflammatory arthritis. Arthritis Rheumatol. 70(12):1971–1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel SH, Tanasa B, Hogan PG, Lewis RS, Daly M, Rao A. 2006. A mutation in orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 441(7090):179–185. [DOI] [PubMed] [Google Scholar]
- Feske S, Skolnik EY, Prakriya M. 2012. Ion channels and transporters in lymphocyte function and immunity. Nat Rev Immunol. 12(7):532–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer L, Herkner C, Kitte R, Dohnke S, Riewaldt J, Kretschmer K, Garbe AI. 2019. Foxp3(+) regulatory T cells in bone and hematopoietic homeostasis. Front Endocrinol (Lausanne). 10:578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaublomme JT, Yosef N, Lee Y, Gertner RS, Yang LV, Wu C, Pandolfi PP, Mak T, Satija R, Shalek AK, et al. 2015. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell. 163(6):1400–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, et al. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 12(3):255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MC, Liao JJ, Bonasera S, Longo DL, Goetzl EJ. 2008. Nuclear factor-κB-dependent reversal of aging-induced alterations in T cell cytokines. FASEB J. 22(7):2142–2150. [DOI] [PubMed] [Google Scholar]
- Ichiyama K, Gonzalez-Martin A, Kim BS, Jin HY, Jin W, Xu W, Sabouri-Ghomi M, Xu S, Zheng P, Xiao C, et al. 2016. The microRNA-183-96-182 cluster promotes T helper 17 cell pathogenicity by negatively regulating transcription factor Foxo1 expression. Immunity. 44(6):1284–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip B, Cilfone NA, Belkina AC, DeFuria J, Jagannathan-Bogdan M, Zhu M, Kuchibhatla R, McDonnell ME, Xiao Q, Kepler TB, et al. 2016. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFα production. Obesity (Silver Spring). 24(1):102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic D, Liu Z, Gause WC. 2001. Th1- and Th2-cell commitment during infectious disease: asymmetry in divergent pathways. Trends Immunol. 22(8):450–457. [DOI] [PubMed] [Google Scholar]
- Kaufmann U, Kahlfuss S, Yang J, Ivanova E, Koralov SB, Feske S. 2019. Calcium signaling controls pathogenic Th17 cell-mediated inflammation by regulating mitochondrial function. Cell Metab. 29(5):1104–1118.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann U, Shaw PJ, Kozhaya L, Subramanian R, Gaida K, Unutmaz D, McBride HJ, Feske S. 2016. Selective orai1 inhibition ameliorates autoimmune central nervous system inflammation by suppressing effector but not regulatory T cell function. J Immunol. 196(2):573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalighinejad N, Aminoshariae MR, Aminoshariae A, Kulild JC, Mickel A, Fouad AF. 2016. Association between systemic diseases and apical periodontitis. J Endod. 42(10):1427–1434. [DOI] [PubMed] [Google Scholar]
- Kidd P. 2003. Th1/Th2 balance: the hypothesis, its limitations, and implications for health and disease. Altern Med Rev. 8(3):223–246. [PubMed] [Google Scholar]
- Kim J, Kang S, Kim J, Kwon G, Koo S. 2013. Elevated levels of T helper 17 cells are associated with disease activity in patients with rheumatoid arthritis. Ann Lab Med. 33(1):52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KD, Srikanth S, Tan YV, Yee MK, Jew M, Damoiseaux R, Jung ME, Shimizu S, An DS, Ribalet B, et al. 2014. Calcium signaling via orai1 is essential for induction of the nuclear orphan receptor pathway to drive Th17 differentiation. J Immunol. 192(1):110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose CS, Kiss EA, Schwierzeck V, Ebert K, Hoyler T, d’Hargues Y, Goppert N, Croxford AL, Waisman A, Tanriver Y, et al. 2013. A T-bet gradient controls the fate and function of CCR6-RORγt+ innate lymphoid cells. Nature. 494(7436):261–265. [DOI] [PubMed] [Google Scholar]
- Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H. 2014. Pathogenic conversion of Foxp3+ T cells into Th17 cells in autoimmune arthritis. Nat Med. 20(1):62–68. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 cells. Annu Rev Immunol. 27:485–517. [DOI] [PubMed] [Google Scholar]
- Krausgruber T, Schiering C, Adelmann K, Harrison OJ, Chomka A, Pearson C, Ahern PP, Shale M, Oukka M, Powrie F. 2016. T-bet is a key modulator of IL-23-driven pathogenic CD4(+) T cell responses in the intestine. Nat Commun. 7:11627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine A, Martin B, Luka M, Mir L, Auffray C, Lucas B, Bismuth G, Charvet C. 2015. Foxo1 is a T cell-intrinsic inhibitor of the RORγt-Th17 program.J Immunol. 195(4):1791–1803. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity. 30(1):92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Zhang SX, Ma XW, Xue YL, Gao C, Li XY. 2017. Levels of peripheral Th17 cells and serum Th17-related cytokines in patients with multiple sclerosis: a meta-analysis. Mult Scler Relat Disord. 18:20–25. [DOI] [PubMed] [Google Scholar]
- Liu S, Watanabe S, Shudou M, Kuno M, Miura H, Maeyama K. 2014. Upregulation of store-operated Ca(2+) entry in the naive CD4(+) T cells with aberrant cytokine releasing in active rheumatoid arthritis. Immunol Cell Biol. 92(9):752–760. [DOI] [PubMed] [Google Scholar]
- Livada R, Shiloah J. 2014. Calcium channel blocker-induced gingival enlargement. J Hum Hypertens. 28(1):10–14. [DOI] [PubMed] [Google Scholar]
- Ma J, McCarl CA, Khalil S, Luthy K, Feske S. 2010. T-cell-specific deletion of STIM1 and STIM2 protects mice from EAE by impairing the effector functions of Th1 and Th17 cells. Eur J Immunol. 40(11):3028–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarl CA, Khalil S, Ma J, Oh-hora M, Yamashita M, Roether J, Kawasaki T, Jairaman A, Sasaki Y, Prakriya M, et al. 2010. Store-operated Ca2+ entry through orai1 is critical for T cell-mediated autoimmunity and allograft rejection. J Immunol. 185(10):5845–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. 2013. Causes, consequences, and reversal of immune system aging. J Clin Invest. 123(3):958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muljo SA, Ansel KM, Kanellopoulou C, Livingston DM, Rao A, Rajewsky K. 2005. Aberrant T cell differentiation in the absence of dicer. J Exp Med. 202(2):261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel S. 2019. Orai1: CRACing the Th17 response in AKI. J Clin Invest. 129(11):4583–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto K, Nakashima T, Shinohara M, Negishi-Koga T, Komatsu N, Terashima A, Sawa S, Nitta T, Takayanagi H. 2017. Osteoimmunology: the conceptual framework unifying the immune and skeletal systems. Physiol Rev. 97(4):1295–1349. [DOI] [PubMed] [Google Scholar]
- Omenetti S, Bussi C, Metidji A, Iseppon A, Lee S, Tolaini M, Li Y, Kelly G, Chakravarty P, Shoaie S, et al. 2019. The intestine harbors functionally distinct homeostatic tissue-resident and inflammatory Th17 cells. Immunity. 51(1):77–89.e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoe K, Yanagawa Y, Minami K, Iijima N, Iwabuchi K. 2007. Th1 or Th2 balance regulated by interaction between dendritic cells and NKT cells. Immunol Res. 38(1–3):319–332. [DOI] [PubMed] [Google Scholar]
- Othy S, Jairaman A, Dynes JL, Dong TX, Tune C, Yeromin AV, Zavala A, Akunwafo C, Chen F, Parker I, et al. 2020. Regulatory T cells suppress Th17 cell Ca(2+) signaling in the spinal cord during murine autoimmune neuroinflammation. Proc Natl Acad Sci U S A. 117(33):20088–20099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, et al. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 6(11):1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YJ, Yoo SA, Kim M, Kim WU. 2020. The role of calcium-calcineurin-NFAT signaling pathway in health and autoimmune diseases. Front Immunol. 11:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, LeDeist F, Rieux-Laucat F, Rechavi G, Rao A, et al. 2009. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 360(19):1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert-Hartwall L, Honkanen J, Salo HM, Nieminen JK, Luopajarvi K, Harkonen T, Veijola R, Simell O, Ilonen J, Peet A, et al. 2015. Th1/Th17 plasticity is a marker of advanced beta cell autoimmunity and impaired glucose tolerance in humans. J Immunol. 194(1):68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V, Triffaux E, Savignac M, Pelletier L. 2013. Singularities of calcium signaling in effector T-lymphocytes. Biochim Biophys Acta. 1833(7):1595–1602. [DOI] [PubMed] [Google Scholar]
- Schirmer M, Garner A, Vlamakis H, Xavier RJ. 2019. Microbial genes and pathways in inflammatory bowel disease. Nat Rev Microbiol. 17(8):497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Zou R, Yang M, Mai L, Ren J, Wen J, Liu Z, Lai R. 2019. Analysis of genes involved in ulcerative colitis activity and tumorigenesis through systematic mining of gene co-expression networks. Front Physiol. 10:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Gwack Y. 2013. Orai1-NFAT signalling pathway triggered by T cell receptor stimulation. Mol Cells. 35(3):182–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth S, Woo JS, Sun Z, Gwack Y. 2017. Immunological disorders: regulation of Ca(2+) signaling in T lymphocytes. Adv Exp Med Biol. 993:397–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stashenko P, Yu SM. 1989. T helper and T suppressor cell reversal during the development of induced rat periapical lesions. J Dent Res. 68(5):830–834. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Omenetti S. 2017. The dichotomous nature of T helper 17 cells. Nat Rev Immunol. 17(9):535–544. [DOI] [PubMed] [Google Scholar]
- Tonetti MS, Greenwell H, Kornman KS. 2018. Staging and grading of periodontitis: framework and proposal of a new classification and case definition. J Periodontol. 89(Suppl 1):S159–S172. [DOI] [PubMed] [Google Scholar]
- Trebak M, Kinet JP. 2019. Calcium signaling in T cells. Nat Rev Immunol. 19(3):154–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno A, Jeffery L, Kobayashi T, Hibi T, Ghosh S, Jijon H. 2018. Th17 plasticity and its relevance to inflammatory bowel disease. J Autoimmun. 87:38–49. [DOI] [PubMed] [Google Scholar]
- Vaeth M, Kahlfuss S, Feske S. 2020. CRAC channels and calcium signaling in T cell-mediated immunity. Trends Immunol. 41(10):878–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaeth M, Yang J, Yamashita M, Zee I, Eckstein M, Knosp C, Kaufmann U, Karoly Jani P, Lacruz RS, Flockerzi V, et al. 2017. Orai2 modulates store-operated calcium entry and T cell-mediated immunity. Nat Commun. 8:14714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hamburg JP, Tas SW. 2018. Molecular mechanisms underpinning T helper 17 cell heterogeneity and functions in rheumatoid arthritis. J Autoimmun. 87:69–81. [DOI] [PubMed] [Google Scholar]
- Wang H, Wang C, Wang L, Liu T, Wang Z, You H, Zheng Y, Luo D. 2018. Orai1 downregulation impairs lymphocyte function in type 2 diabetes mellitus. Biochem Biophys Res Commun. 500(2):384–390. [DOI] [PubMed] [Google Scholar]
- Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek AB, Lee YK, Weaver CT, Yagi R, Lazarevic V. 2014. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity. 40(3):355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JS, Srikanth S, Kim KD, Elsaesser H, Lu J, Pellegrini M, Brooks DG, Sun Z, Gwack Y. 2018. CRACR2A-mediated TCR signaling promotes local effector Th1 and Th17 responses. J Immunol. 201(4):1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JS, Sun Z, Srikanth S, Gwack Y. 2020. The short isoform of extended synaptotagmin-2 controls Ca 2+ dynamics in T cells via interaction with STIM1. Sci Rep. 10(1):14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Qian FY, Zhang MF, Xu AL, Wang X, Jiang BP, Zhou LL. 2019. Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J Leukoc Biol. 106(6):1233–1240. [DOI] [PubMed] [Google Scholar]
- Yen JH, Chang CM, Hsu YW, Lee CH, Wu MS, Hwang DY, Chen BK, Liao HT, Wu MT, Chang WC. 2014. A polymorphism of ORAI1 rs7135617, is associated with susceptibility to rheumatoid arthritis. Mediators Inflamm. 2014:834831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jdr-10.1177_0022034521990652 for Calcium Signaling in T Cells and Chronic Inflammatory Disorders of the Oral Cavity by S. Hasiakos, Y. Gwack, M. Kang and I. Nishimura in Journal of Dental Research