Supplemental Digital Content is available in the text
The point-prevalence of pregnancy-related pelvic girdle pain (PPGP) in a large random sample of Australian women was 44%. The identified risk factors associated with PPGP included parity, country of birth, greater duration of time spent standing, previous low back pain and/or pelvic girdle pain, and a family history of PPGP.
Keywords: associated factors, pelvic girdle pain, pregnancy, prevalence
Study Design.
Cross-sectional study conducted between December 2017 and October 2019.
Objective.
To determine the prevalence and risk factors associated with pregnancy-related pelvic girdle pain (PPGP) in Australia.
Summary of Background Data.
PPGP is a common condition worldwide yet the prevalence and associated risk factors are not known in Australia.
Methods.
A random sample of pregnant women (N = 780) of (mean [SD]) 31 (5) years of age between 14 and 38 weeks gestation attending ante-natal care in a tertiary referral hospital in Sydney, Australia was conducted. The main outcome measure was point-prevalence of PPGP as classified by recommended guidelines including a physical examination. A number of potential risk factors, including socio-demographic characteristics, country of birth, ethnicity, history of low back pain (LBP) and PPGP, family history of PPGP, occupational factors, and physical activity were investigated with logistic regression.
Results.
The point-prevalence of PPGP in a random sample of 780 Australian women was 44% with the odds of having PPGP increasing with each additional week of gestation (odds ratio [OR]) (OR 1.02). Increasing parity (P = 0.03, OR 1.15), country of birth (P = 0.03), and greater duration of time spent standing (P = 0.009, OR 1.06) were associated with PPGP. The strongest predictors of PPGP were previous LBP and/or PPGP both pregnancy (P < 0.001, OR 4.35) and not pregnancy related (P < 0.001, OR 2.24), and a family history of PPGP (P < 0.001, OR 3.76).
Conclusion.
The prevalence of PPGP in Australian women was high with almost half the sample classified with PPGP, matching data reported worldwide. The identified risk factors associated with PPGP can be included in routine ante-natal care to screen women and identify those at risk of this common and disabling condition.
Level of Evidence: 1
Pelvic girdle pain is the most common musculoskeletal disorder reported during pregnancy with pain experienced between the levels of the posterior iliac crest and the gluteal fold, as distinct from the lumbar spine.1 Women with pregnancy-related pelvic girdle pain (PPGP) usually report pain as being moderate to severe in intensity, and difficulty with physical activities, such as standing and walking.1,2 The ability to perform household and work-related duties are frequently impaired, and women with PPGP may also suffer psychosocial distress.1–4
Worldwide, the prevalence of PPGP has been reported to range from 7% to 84%.1,3,5 This large variation can be attributed to differences between studies in participant recruitment, sample size, and the method of classification, with most studies using the self-report of pain only2–6 in contrast to others which included a physical examination.7–10 Only a single study has been conducted in Australia using a small sample size of 95 women with the finding of a prevalence of 23% of PPGP determined by self-report of symptoms.6 This lack of epidemiological information limits the knowledge of how many Australian women suffer from PPGP.
There is little information about potential risk factors that lead to the development of PPGP in Australia. Previous studies from other countries have reported that PPGP is strongly associated with a history of low back pain (LBP) and prior PPGP.4–11 There are conflicting findings as to whether age, parity, exercise levels, and occupational factors are associated with PPGP.2,4,6–12 There may also be other risk factors, such as time spent in weight-bearing positions that have not been investigated despite women commonly reporting that these positions aggravate PPGP. In addition, familial history and ethnicity have also been suggested as factors associated with PPGP,6,7,12,13 however, they have not been extensively studied in the Australian population, where ethno-cultural diversity is evident with over one-third of pregnant women born overseas.14
In Australia, there is currently no routine screening for PPGP in the antenatal period. Tellingly, women report a lack of support from healthcare professionals and many do not receive any treatment.6 It is crucial that healthcare providers are aware of the factors associated with PPGP to be able to effectively identify women at risk of developing PPGP in a country with over 300,000 births annually.14 This would enable women to be offered greater support for a disorder that can benefit from timely care.6 The aims of this study were to: (1) determine the prevalence of PPGP, and (2) identify risk factors in an Australian population.
MATERIALS AND METHODS
This study was conducted as per a previously published protocol15 without changes and reported according to Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.16 Ethical approval was granted by the hospital (HREC/17/WMEAD/64) and university (RH12294) human research ethics committees.
Participants
Participants were recruited if they were aged over 18 years, between 14 and 38 weeks’ gestation and had sufficient command of English, with a health care interpreter if needed. Women were excluded if they had a medical or obstetric complication(s) affecting pregnancy, such as serious pathology of non-musculoskeletal origin including preeclampsia, serious intellectual or psychiatric impairment, systemic disease(s), or recent spinal fracture, trauma, or surgery. Data were collected from December 2017 to October 2019 at Westmead Hospital, a tertiary level facility in Sydney, Australia. A random sample of all pregnant women attending the antenatal clinic on a specific day was made using block randomization to reduce selection bias. Each participant was informed of the aims and methods of the study, and provided written and informed consent prior to data being collected during a single, face-to-face session. All data were collected by the primary investigator (D.C.).
Prevalence of PPGP
The primary outcome measure was point-prevalence of PPGP as classified according to recommended guidelines17 which included the self-report of pain in the pelvic girdle region, difficulty performing activities of daily living (such as walking, standing, or turning in bed), and positive findings on physical examination of at least two of the four tests on the same side including: the posterior pelvic pain provocation test, active straight leg raise test, palpation of long dorsal ligament test, and modified Trendelenburg test.17 The first 20 participants who reported pain in the pelvic girdle region were examined by the primary investigator (D.C.) and one other experienced physiotherapist who was not involved in the research project, to determine inter-examiner reliability of the physical examination tests.
Each participant completed the Pregnancy and Physical Activity Questionnaire (PAPQ),18 Pregnancy Mobility Index (PMI),19 and Pelvic Girdle Questionnaire (PGQ).20 Participants with PPGP rated their pain level (current and yesterday) by completing a visual analogue scale (VAS) from 0 (no pain) to 100 (worst possible pain).21
Data Collection to Determine Factors Associated With PPGP
Gestation age, parity (defined as previous deliveries >24 weeks gestation), and pregnancy type (singleton, twins, triplets) were recorded from the patient medical record and confirmed via self-report. Following measurement of height and weight, women self-reported their marital status (married/de facto or not married), education level (did not finish high school, finished high school, university completion), country of birth, and self-identified ethnicity (grouped by geographic region).22
Women were asked if they had experienced a history of non-pregnancy related previous LBP and/or pelvic girdle pain (PGP) (yes/no) and if there was a family history of PPGP with their mother and/or a sister having PPGP which was recorded as yes/no or unsure if this was not known. Participants with one or more previous pregnancies were asked if they had a history of LBP and/or PPGP (yes/no) experienced during an earlier pregnancy.
Participants self-reported information about the time they were working in an occupation in the week prior to the study (0, <20 hours, 20–40 or >40 hours). A five-point Likert Scale was used to record the type of work (from either very heavy to very light) and work satisfaction (from very bad to very good). Each participant self-reported the duration of time spent lying down, sitting, standing, and walking (hours) the day prior to the study.
Statistical Analyses
A sample size of 780 was determined a priori to allow for investigation of 18 possible risk factors with PPGP, as calculated by 10 participants per potential risk factor with division by the previously published Australian prevalence rate of 23%7 (G∗Power).23 Statistical analyses were performed using IBM SPSS for Windows Version 24.0 (IBM Corp., Armonk, NY). Inter-examiner reliability of the physical examination tests was determined by calculating the percentage agreement and Kappa coefficient (K).
The point-prevalence of PPGP was calculated by dividing the number of women classified with PPGP by the total number of women who participated in the study.
To investigate between group differences for each factor, based on normality, parametric (independent t test), and non-parametric (Pearson Chi Square test, Fishers Exact test) tests were performed to compare women with and without PPGP for continuous, categorical, and ordinal data respectively. Univariate logistic regression analyses were fitted to test individual factors for association with PPGP and multivariate logistic models were used to identify associations with more than one factor included in the models. Selection of factors to include in the multivariate models was informed by the univariate results and clinical judgement. Associations are reported as an odds ratio, 95% confidence interval (OR [95% CI]) and P values. The predictive power of the model was calculated by Nagelkerke R-square (R2) and area under receiver operating characteristic (ROC) curves (95% CI).
Secondary analyses were performed to determine whether single or multiple factors had a stronger ability to predict PPGP in subgroups of women based on clinical judgement of the important or significant factors.
RESULTS
A total of 780 women with an age (mean [SD]) of 31 (5) years and gestational age of 29 (7) weeks (Table 1) were included. The majority of women were born in Southern and Central Asia (35%) and Australia (29%), consistent with report of ethnicity (Figure 1).
TABLE 1.
Participant Profile (Mean [95% CI] or Frequency [Percentage]) and Comparison Between Women With and Without Pregnancy-Related Pelvic Girdle Pain (PPGP) (P Value)
| Predictor Variable | All Participants (N = 780) | With PPGP (N = 344) | Without PPGP (N = 436) | P Value |
| Age, yr | 31.6 (31.2, 32.0) | 31.5 (31.0, 32.0) | 31.7 (31.2, 32.2) | 0.58 |
| Gestation age, wk | 29.1 (28.5, 29.6) | 29.6 (28.9, 30.3) | 28.6 (27.9, 29.4) | 0.07 |
| Height, cm | 165.8 (165.5, 166.1) | 165.7 (165.3, 166.2) | 165.9 (165.5, 166.3) | 0.61 |
| Body mass, kg | 76.5 (75.9, 77.3) | 77.1 (76.0, 78.2) | 76.1 (75.1, 77.1) | 0.17 |
| Body mass index, kg m−2 | 27.8 (27.6, 28.1) | 28.1 (27.7, 28.4) | 27.7 (27.3, 28.0) | 0.13 |
| Parity (previous pregnancies >24 weeks gestation) | 1.1 (1.0, 1.2) | 1.2 (1.0, 1.3) | 1.0 (0.9, 1.1) | 0.03∗ |
| Pregnancy type | 0.11 | |||
| Singleton | 749 (96%) | 326 (95%) | 423 (97%) | |
| Twins | 31 (4%) | 18 (5%) | 13 (3%) | |
| LBP/PGP in previous pregnancy‡ | <0.001† | |||
| Yes | 228 (29%) | 149 (43%) | 79 (18%) | |
| No | 261 (33%) | 79 (23%) | 182 (42%) | |
| Not applicable | 291 (37%) | 116 (34%) | 175 (40%) | |
| Previous LBP/PGPP non-pregnancy related | <0.001† | |||
| Yes | 208 (27%) | 122 (35%) | 86 (20%) | |
| No | 572 (73%) | 222 (65%) | 350 (80%) | |
| Family history of PPGP | <0.001† | |||
| Yes | 201 (26%) | 130 (38%) | 71 (16%) | |
| No | 354 (45%) | 116 (34%) | 238 (55%) | |
| Unsure | 225 (29%) | 98 (28%) | 127 (29%) | |
| Marital status | 0.74 | |||
| Married/de facto | 771 (99%) | 341 (99%) | 430 (93%) | |
| Not married | 9 (1%) | 3 (<1%) | 6 (1%) | |
| Education level | 0.05 | |||
| Incomplete high school | 107 (13%) | 50 (15%) | 57 (13%) | |
| Finished high school | 443 (57%) | 208 (60%) | 235 (54%) | |
| University degree completed | 230 (29%) | 86 (25%) | 144 (33%) | |
| Work status (hours of employment previous week) | 0.28 | |||
| None | 320 (41%) | 142 (41%) | 178 (41%) | |
| 0–20 | 48 (6%) | 27 (8%) | 21 (5%) | |
| 21–40 | 126 (16%) | 57 (17%) | 69 (16%) | |
| >40 | 286 (37%) | 118 (34%) | 168 (39%) | |
| Work type§ | 0.21 | |||
| Very heavy | 18 (2%) | 11 (3%) | 7 (2%) | |
| Slightly heavy | 97 (12%) | 49 (14%) | 48 (11%) | |
| Neither heavy nor light | 123 (16%) | 50 (15%) | 73 (17%) | |
| Slightly light | 124 (16%) | 55 (16%) | 69 (16%) | |
| Very light | 98 (13%) | 37 (11%) | 61 (14%) | |
| Not applicable | 320 (41%) | 142 (41%) | 178 (41%) | |
| Work satisfaction§ | 0.07 | |||
| Very bad | 14 (2%) | 8 (2%) | 6 (1%) | |
| Somewhat bad | 41 (5%) | 24 (7%) | 17 (4%) | |
| Neither bad nor good | 95 (12%) | 46 (13%) | 49 (11%) | |
| Somewhat good | 154 (20%) | 67 (19%) | 87 (20%) | |
| Very good | 156 (20%) | 57 (17%) | 99 (23%) | |
| Not applicable | 320 (41%) | 142 (41%) | 178 (41%) | |
| Hours spent yesterday, h | ||||
| Lying down | 0.5 (0.5, 0.6) | 0.5 (0.5, 0.6) | 0.5 (0.4, 0.6) | 0.19 |
| Sitting | 8 (7.8, 8.2) | 7.7 (7.4, 8.1) | 8.2 (7.9, 8.6) | 0.05 |
| Walking | 1.5 (1.5, 1.6) | 1.5 (1.5, 1.6) | 1.5 (1.5, 1.6) | 0.93 |
| Standing | 4.6 (4.4, 4.8) | 4.9 (4.6, 5.3) | 4.3 (4.1, 4.6) | 0.02∗ |
Significant at 5% level (P < 0.05) for t test between groups.
Significant at 5% level (P < 0.05) for Chi Square test between groups.
The sample size for this variable was N = 489 representing women who reported at least one previous pregnancy >24/40.
The sample size for this variable was N = 460 representing women who reported a current work status.
LBP indicates low back pain; PGP, pelvic girdle pain.
Figure 1.
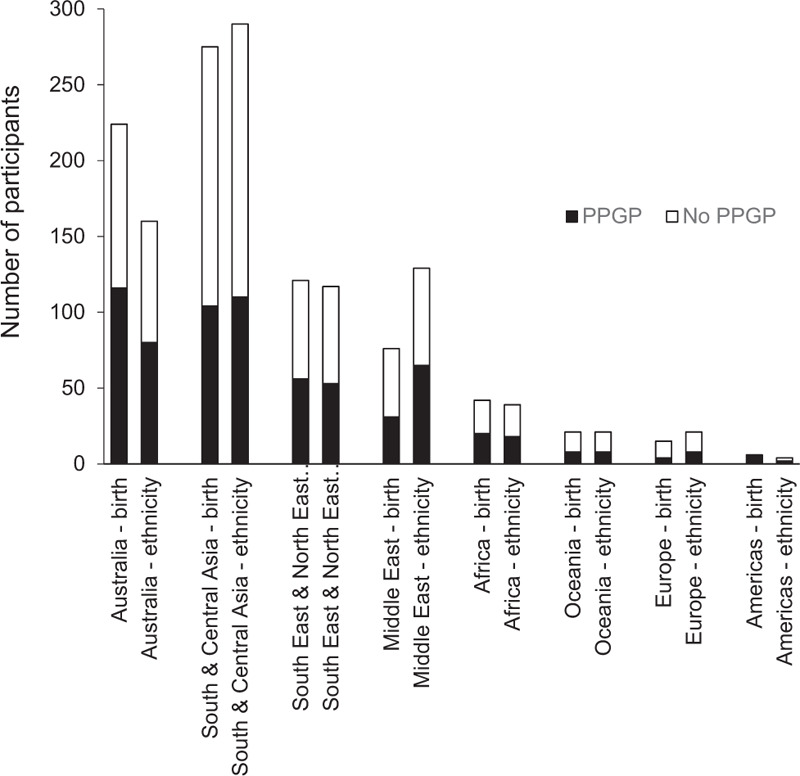
Geographic region of birth and self-identified ethnicity of women (N = 780) with and without pregnancy-related pelvic girdle pain (PPGP). Not all women born in Australia self-identified as being Australian, particularly women of Middle Eastern ethnicity.
Inter-examiner reliability of the physical examination tests was excellent with agreements of between 90% and 100% for all tests and K ranging from 0.74 to 1.00 (see Table, Supplementary Digital content 1, which details reliability findings).
Prevalence of PPGP
The point-prevalence of PPGP was 44.1% (344/780). Women with PPGP had a PGQ score (mean [95% CI]) of 42 (40–44) compared with women without PPGP who scored 7 (6–8), representing a statistically significant (P < 0.001) and clinically important difference between groups, where a higher score indicates more pain and disability.24 For women with PPGP, pain at the time of testing was (mean [SD]) 23 (28) on the VAS and pain the day prior to testing was 51 (30). Women with PPGP reported significantly more difficulty with mobility tasks on the PMI compared with women without PPGP (P < 0.001). The PAPQ scores revealed that women with PPGP performed a greater amount of weekly household and family activities compared with women without PPGP (P = 0.006).
Factors Associated With PPGP
There was statistically significantly greater (P < 0.05) parity, hours standing, frequency of a history of LBP and/or PGP (pregnancy and non-pregnancy related), and family history of PPGP in women with PPGP compared with women without PPGP (Table 1). There were statistically significant greater number of women born in Australia with PPGP compared with women without PPGP (P = 0.02) which was not evident based on women's self-identified ethnicity (P = 0.22).
The odds of having PPGP increase with every additional week of gestation from 37.6% at week 24 to 48.1% at week 38 (Table 2). There were statistically significant associations (P < 0.05) for individual factors with PPGP including parity, daily hours spent standing, a self-reported history or family history of LBP and/or PGP (pregnancy and non-pregnancy related), and a family history of PPGP. There was a statistically significant association for geographic region of birth (P = 0.03), with women born in Australia more likely to have PPGP in contrast to women born in southern and central Asia who were less likely to have PPGP (P = 0.002).
TABLE 2.
The Odds Ratio (OR [95% CI]) and P Value for Logistic Regression and the Area Under the Receiver Operator Characteristic Curve (Area ROC) (95% CI) Calculated for Each Factor Analyzed for an Association With Pregnancy-Related Pelvic Girdle Pain
| Factor | Odds Ratio (95% CI) | P Value | Area ROC (95% CI) |
| Gestation age | 1.02 (1.00, 1.04) | 0.07 | 0.53 (0.49, 0.57) |
| Parity (previous pregnancies >24 weeks gestation) | 1.15 (1.02, 1.30) | 0.03∗ | 0.55 (0.51, 0.59) |
| LBP/PGP in previous pregnancy | 4.35 (2.97, 6.35) | <0.001∗ | 0.68 (0.63, 0.72) |
| LBP/PGP non-pregnancy related | 2.24 (1.62, 3.09) | <0.001∗ | 0.58 (0.54, 0.62) |
| Family history | 3.76 (2.61, 5.41) | <0.001∗ | 0.65 (0.60, 0.70) |
| Hours standing | 1.06 (1.02, 1.11) | 0.009∗ | 0.55 (0.51, 0.59) |
| Country of birth (by region) | 0.03∗ | 0.58 (0.54, 0.62) | |
| Australia (reference level) | |||
| Oceania | 0.57 (0.23, 1.44) | 0.24 | |
| Europe | 0.34 (0.11, 1.10) | 0.07 | |
| Middle East | 0.64 (0.38, 1.09) | 0.10 | |
| Africa | 0.85 (0.44, 1.64) | 0.62 | |
| Southeast and Northeast Asia | 0.80 (0.52, 1.25) | 0.33 | |
| South and Central Asia | 0.57 (0.40, 0.81) | 0.002∗ | |
| Americas | 4.66 (0.54, 40.49) | 0.16 | |
Significant at 5% level (P < 0.05).
LBP indicates low back pain; PGP, pelvic girdle pain.
The multivariate logistic regression models investigating the predictive ability of a group of factors for all women had Nagelkerke R2 values and areas under the ROC curves which were less than acceptable for being predictive of PPGP25 (see Table, Supplementary Digital content 2, which presents logistic regression models).
Secondary analyses involved stratifying the sample of participants by clinically important and significant factors. Three logistic regression models for a subgroup of women who had more than one previous pregnancy and a known family history of PPGP (N = 358) were found to have greater ability to predict PPGP than other models (see Table, Supplementary Digital content 2, which presents logistic regression models). Nagelkerke R2 ranged from 0.25 to 0.26 (medium correlation) and areas under the ROC ranged from 0.75 to 0.76 (acceptable discrimination).24 For all models there were significant associations for a history of LBP and/or PPGP (pregnancy related) and family history of PPGP, regardless of other factors included.
DISCUSSION
Pelvic girdle pain was shown to be common in pregnancy with nearly half of Australian women reporting PPGP on a given day. This was the first large scale study conducted in Australia using recommended guidelines for classification of PPGP and powered to investigate 18 factors potentially associated with the condition. The prevalence rate is similar to that of other countries, such as Norway and Sweden,8,9 although it was greater than previously reported in Australia.6 This is likely due to the inclusion of a physical examination to confirm PPGP as distinct from LBP in these studies as well as the current study.
The finding that a history of LBP and/or PGP was associated with PPGP was consistent with previous studies.4–11 This study also demonstrated that a family history of PPGP was strongly associated with PPGP, possibly indicating a genetic or behavioral component that contributes to PPGP.7,12 This means that routinely asking women about whether they have had a past or family history of LBP or PGP may improve the ability to identify women at risk of PPGP. The knowledge that PPGP is more common with increasing parity and as gestation progresses, will allow women to be closely monitored to limit the onset or worsening of symptoms.2,4,6,8–12
A novel finding of this study was that a longer duration of standing was associated with PPGP, which aligns with the frequent report by women that activities in weight-bearing aggravate PPGP. Although axial loading of the spine and pelvis exacerbate the pain, the underlying etiology of PPGP remains unclear. The knowledge that pain may be aggravated by posture and position offers an opportunity to tailor management and reduce pain and disability, supported by the finding that women with PPGP benefited from attending physiotherapy.26
Based on the current findings, it is plausible that PPGP is not specific to ethnicity as it was not a factor that discriminated between women with and without pain. However, there may be cultural influences that increase the incidence of PPGP for women born in Australia that are not present for women born in other regions of the world. Interestingly, not all women born in Australia self-identified as being of Australian ethnicity, particularly evident in the self-report of Middle Eastern ethnicity. While the previous evidence regarding impact of ethnicity on PPGP has been conflicting,6,13 a more nuanced approach that encompasses country of birth, together with cultural and ethnic influences, is required to comprehensively investigate association with PPGP.
Strengths and Limitations
The strengths of this study include the inclusion of a large, randomly selected sample, and adherence to a published protocol using recommended guidelines. Although the setting was a major Australian metropolitan city with a culturally diverse population, it may not be representative of geographical regions such as smaller cities or towns with less cultural diversity. However, women with PPGP had a similar report of pain and disability, impaired mobility and difficulties with household activities reported in previous studies worldwide.1–15 The current pain score was lower than the pain score from previous day. This may have been influenced by the time of day the study was conducted and while women were seated. Hence, the current pain scores may not be an accurate estimate of pain experienced by women with PPGP as it is known that women generally report higher levels of pain in the evening and while moving.1,2,20 Determining a history of LBP and PPGP is influenced by recall bias and not all women could definitively report a family history of PPGP. More than a quarter of women were unsure about their family history of PPGP, therefore the association with PPGP may not be accurate. Similarly, the time spent sitting, standing, walking, and lying on the previous day was self-reported rather than using quantifiable data, such as physical activity monitors. Future studies should endeavor to investigate other variables, such as psychological and physiological factors, that may also be associated with PPGP.
CONCLUSION
This study offers new knowledge in ante-natal care by determining that the point-prevalence of PPGP in Australian women was high, with nearly half of all pregnant women reporting symptoms and associated disability. An individual and family history of LBP and/or PPGP were the strongest predictors of PPGP, while increasing parity and gestational age, being born in Australia, and a greater duration of time standing were also associated with PPGP. It is recommended that these factors should be included as part of routine clinical examination of pregnant women to readily identify women at risk of developing PPGP. Of all the factors associated with PPGP, the only modifiable factor identified was the longer duration of standing time reported by women with PPGP, which may provide an option to guide treatment. Being able to identify women at risk would allow for the provision of early education and timely management strategies which could reduce the burden of PPGP in Australia by delivering crucial healthcare to women during this critical period of their lives.
Key Points
PPGP is a common condition worldwide.
Point-prevalence of PPGP was 44.1% in Australian women and increased with gestational age.
An individual history of LBP and/or pelvic girdle pain, and a family history of PPGP were strongly associated with PPGP in Australia.
The knowledge of factors associated with this common condition will better inform healthcare providers to effectively identify women at risk of developing PPGP and provide timely management in ante-natal care.
Supplementary Material
Supplementary Material
Footnotes
The manuscript submitted does not contain information about medical device(s)/drug(s).
DC is supported by a Westmead Charitable Trust Allied Health Career Development Grant.
No relevant financial activities outside the submitted work.
Supplemental digital content is available for this article.
References
- 1.Kanakaris NK, Roberts CS, Giannoudis PV. Pregnancy-related pelvic girdle pain: an update. BMC Med 2011; 9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malmqvist S, Kjaermann I, Anderson K, et al. The association between pelvic girdle pain and sick leave during pregnancy; a retrospective study of a Norwegian population. BMC Pregnancy Childbirth 2015; 15:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van de Pol G, van Brummen HJ, Bruinse HW, et al. Pregnancy-related pelvic girdle pain in the Netherlands. Acta Obstet Gynecol Scand 2007; 86:416–422. [DOI] [PubMed] [Google Scholar]
- 4.Kovacs FM, Garcia E, Royuela A, et al. Prevalence and factors associated with low back pain and pelvic girdle pain during pregnancy. Spine (Phila Pa 1976) 2012; 37:1516–1533. [DOI] [PubMed] [Google Scholar]
- 5.Bastiaanssen JM, de Bie RA, Bastiaenen CH, et al. Etiology and prognosis of pregnancy-related pelvic girdle pain: design of a longitudinal study. BMC Public Health 2005; 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pierce H, Homer CSE, Dahlen HD, et al. Pregnancy-related lumbopelvic pain: listening to Australian women. Nurs Res Pract 2012; 2012:387428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsen EC, Wilken-Jensen C, Hansen A, et al. Symptom-giving pelvic girdle relaxation in pregnancy: prevalence and risk factors. Obstet Gynecol 1999; 78:105–110. [PubMed] [Google Scholar]
- 8.Kristiansson P, Svarsudd K, von Shoultz B. Back pain during pregnancy. Spine (Phila Pa 1976) 1996; 21:702–709. [DOI] [PubMed] [Google Scholar]
- 9.Robinson HS, Veierod MB, Mengshoel AM, et al. Pelvic girdle pain – associations between risk factors in early pregnancy and disability or pain intensity in late pregnancy: a prospective cohort study. BMC Musculoskelet Disord 2010; 11:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mousavi SJ, Parnianpour M, Vleeming A. Pregnancy related pelvic girdle pain and low back pain in an Iranian population. Spine (Phila Pa 1976) 2007; 32:E100–E104. [DOI] [PubMed] [Google Scholar]
- 11.Albert HB, Godskesen M, Korsholm L, et al. Risk factors in developing pregnancy-related pelvic girdle pain. Acta Obstet Gynecol Scand 2006; 85:539–544. [DOI] [PubMed] [Google Scholar]
- 12.Mogren IM, Pohjanen AI. Low back pain and pelvic pain during pregnancy. Spine (Phila Pa 1976) 2005; 30:983–991. [DOI] [PubMed] [Google Scholar]
- 13.Vangen S, Stoltenberg C, Stray-Pedersen B. Complaints and complications in pregnancy: a study of ethnic Norwegian and ethnic Pakistani women in Oslo. Ethn Health 1999; 4:19–28. [DOI] [PubMed] [Google Scholar]
- 14.Australian Institute of Health and Welfare 2019. Australia's mothers and babies 2017—in brief. Perinatal statistics series no. 35. Cat. no. PER 100. Canberra: AIHW. [Google Scholar]
- 15.Ceprnja D, Chipchase L, Gupta A. Prevalence of pelvic girdle pain and associated factors in Australia: a cross-sectional study protocol. BMJ Open 2017; 0:e018334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Elm E, Altman DG, Egger M, et al. STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344–349. [DOI] [PubMed] [Google Scholar]
- 17.Vleeming A, Albert HB, Östgaard HC, et al. European guidelines for the diagnosis and treatment of pelvic girdle pain. Eur Spine J 2008; 17:794–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haakstad LAH, Gundersen I, Bo K. Self-reporting compared to motion monitor in the measurement of physical activity during pregnancy. Acta Obstet Gynecol Scand 2010; 89:749–756. [DOI] [PubMed] [Google Scholar]
- 19.Van de Pol G, De Leeuw JRJ, Van Brummen HJ, et al. The pregnancy mobility index: a mobility scale during and after pregnancy. Acta Obstet Gynecol Scand 2006; 85:786–791. [DOI] [PubMed] [Google Scholar]
- 20.Stuge B, Garratt A, Krogstad Jenssen H, et al. The Pelvic Girdle Questionnaire: a condition-specific instrument for assessing activity limitations and symptoms in people with pelvic girdle pain. Phys Ther 2011; 91:1096–1108. [DOI] [PubMed] [Google Scholar]
- 21.Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health 1990; 13:227–236. [DOI] [PubMed] [Google Scholar]
- 22.Standard Australian Classification of Countries (SACC) 2nd edition. Available at: https://www.abs.gov.au/ausstats/abs@.nsf/0/C8B8914F6C683351CA25744D00818CED, accessed April 18, 2020. [Google Scholar]
- 23.Faul F, Erdfelder E, Lang A, et al. G∗Power 3: a flexible statistical power analysis program for the social, behavioural, and biomedical sciences. Behav Res Methods 2007; 39:175191. [DOI] [PubMed] [Google Scholar]
- 24.Stuge B, Jenssen HK, Grotle M. The pelvic girdle questionnaire: responsiveness and minimal important change in women with pregnancy-related pelvic girdle pain, low back pain, or both. Phys Ther 2017; 97:1103–1113. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lemeshow S, Sturdivant R. Applied Logistic Regression. 3rd ed.New York: John Wiley & Sons; 2013. [Google Scholar]
- 26.Ceprnja D, Gupta A. Does muscle energy technique have an immediate benefit for women with pregnancy-related pelvic girdle pain? Physiother Res Int 2018; 24:e1746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


