Abstract
Invasive mould disease (IMD) might affect up to a third of critically ill patients with COVID-19. COVID-19-associated pulmonary aspergillosis (CAPA) is typically diagnosed on the basis of a combination of non-specific clinical, radiographical, and mycological findings, but whether most cases represent invasive disease is unresolved. We systematically reviewed autopsy series of three or more decedents with COVID-19 for evidence of IMD. We searched PubMed, Web of Science, OVID (Embase), and medRxiv for studies in English or French published from Jan 1, 2019, to Sept 26, 2020. We identified 1070 references, of which 50 studies met the criteria. These studies described autopsies from 677 decedents, with individual-level data for 443 decedents. The median age was 70·0 years (IQR 57·0–79·0). Of decedents with individual-level data, 133 (30%) had diabetes, 97 (22%) had pre-existing lung disease, and 27 (6%) had immunocompromising conditions. Of 548 decedents with such data, 320 (58%) received invasive mechanical ventilation; among 140 decedents for whom this was known, ventilation was for a median of 9·0 days (IQR 5·0–20·0). Treatment included immunomodulation in 60 decedents and antifungals in 50 decedents. Autopsy-proven IMD occurred in 11 (2%) of 677 decedents, including eight CAPA, two unspecified IMD, and one disseminated mucormycosis. Among 320 decedents who received mechanical ventilation, six (2%) had IMD. We conclude that IMD, including CAPA, is an uncommon autopsy finding in COVID-19.
Introduction
COVID-19, caused by the novel SARS-CoV-2, is responsible for a devastating pandemic causing high incidences of critical illness and death among infected persons worldwide. In addition to direct viral invasion and downstream immune dysregulation, severe disease can be complicated by secondary infections. Early autopsy studies showed several dominant pathological processes of the lungs, including diffuse alveolar damage and thromboembolism.1 Secondary infections complicating COVID-19 have been reported, but the prevalence of autopsy-proven secondary infections is poorly described.
Secondary fungal infections can complicate critical COVID-19.2, 3, 4, 5 Pulmonary aspergillosis, an invasive mould disease (IMD), occurs with increased frequency following other respiratory viral infections such as severe influenza, in which context it is associated with excess mortality.6, 7 Similarly, COVID-19-associated pulmonary aspergillosis (CAPA)8 has been reported to affect up to 39% of patients with critical COVID-19 requiring intensive care unit (ICU) care,4, 8, 9, 10, 11, 12 and has been associated with increased mortality.13, 14 Risk factors for CAPA appear to include prolonged ICU admission, lymphopenia, and immunosuppression including corticosteroid use.13, 15, 16
Diagnosing IMD in critically ill patients with COVID-19 can be challenging.17, 18 In practice, given the difficulty in obtaining tissue from critically ill patients, IMD is infrequently histologically proven. Host factors, required for meeting European Organization for Research and Treatment of Cancer and Mycosis Study Group Education and Research Consortium (EORTC/MSGERC)19 research definitions for probable IMD, are frequently absent in ICU patients with invasive pulmonary aspergillosis,20 including after severe influenza or COVID-19. Other research definitions for diagnosing CAPA in ICU patients have been proposed and include a combination of non-specific clinical, radiographical, and mycological criteria.5, 14, 17, 21 Although several ICU cohort studies have suggested high rates of probable, putative, or possible CAPA, whether these cases represent true tissue-invasive disease is unclear. If they do, then IMD would be expected to be a common finding in post-mortem examinations of decedents with fatal COVID-19.
We present a systematic review of autopsy studies done in decedents with fatal COVID-19 to establish the prevalence of autopsy-proven tissue-invasive IMD and analyse individual-level data to assess potential risk factors.
Methods
Search strategy and selection criteria
This systematic review follows PRISMA reporting guidelines and the protocol was published as part of a two-study review on PROSPERO (CRD42020204123). We identified possible studies by searching PubMed, Web of Science, OVID (Embase), and medRxiv for articles published from Jan 1, 2019, to Sept 26, 2020. We used the terms “sars cov2” OR “covid” OR “2019 ncov” OR “novel coronavirus” AND “autopsy” OR “post-mortem”. We also reviewed reference lists of eligible publications for other articles of interest in addition to a database of relevant articles maintained by the authors when new autopsy studies were published. We contacted authors of reports to obtain missing details when required or if the outcome of interest (fungal infections) was not described. We only included articles written in English or French.
Studies met inclusion criteria if they were retrospective or prospective case series of autopsy or post-mortem reports of decedents with confirmed SARS-CoV-2 infection. To minimise reporting bias from case reports (either of IMD or competing diagnoses), we only included series with at least three decedents. At minimum, the examination must have included histopathological investigation of the lungs, either through standard or minimally invasive sampling. We excluded studies if decedents were included or excluded on the basis of diagnoses other than COVID-19, or the presence or absence of complications.
All identified titles and abstracts were independently reviewed for potential inclusion criteria by two authors (BEK and ISS) and full texts were obtained for all those meeting inclusion criteria. The two same authors did a review of the full text independently, for final inclusion. A consensus was reached for all disputes. Covidence (Veritas Health Innovation, Melbourne, VIC, Australia) was used for abstract management.
In the instance of decedents being included in more than one report, we included the study with the most patient-related variables, unless the study with less individual data had a case of CAPA. If there were more decedents included in the less detailed study, we still extracted data for these remaining patients to add to the total cohort but did not extract any additional individual-level details. When a preprint study became published, we preferentially included the finalised publications.
Data analysis
The main outcome of interest was the number of decedents with autopsy-proven IMD measured by histopathology showing hyphae invading tissue. When available, we recorded the type of mould as identified in the reports. We recorded the types of autopsies done as either standard or minimally invasive. Minimally invasive included any autopsy in which multiple lung tissue samples from each lobe were obtained, either blindly or through ultrasound guidance; this practice has been applied frequently in COVID-19 to limit occupational exposures at the time of autopsy. We also recorded which country the study originated from and patient's place of death (in hospital or community). When possible, individual decedent data were also extracted. We collected age, comorbidities, length of hospital stay, duration of symptoms, days of mechanical ventilation, and immunomodulatory COVID-19-specific treatment prescribed (cytokine inhibitors or steroids). We used EORTC/MSGERC19 definitions of host factors to classify whether decedents were immunocompromised.
With regards to risk of bias assessment, we did not judge the study quality given these are small case series without a target intervention. We attempted to guard against selective reporting by contacting corresponding authors for any manuscript in which the outcome of interest (fungal infection) was not described. We also attempted to mitigate small-study effects by including only those series with three or more cases described.
Results
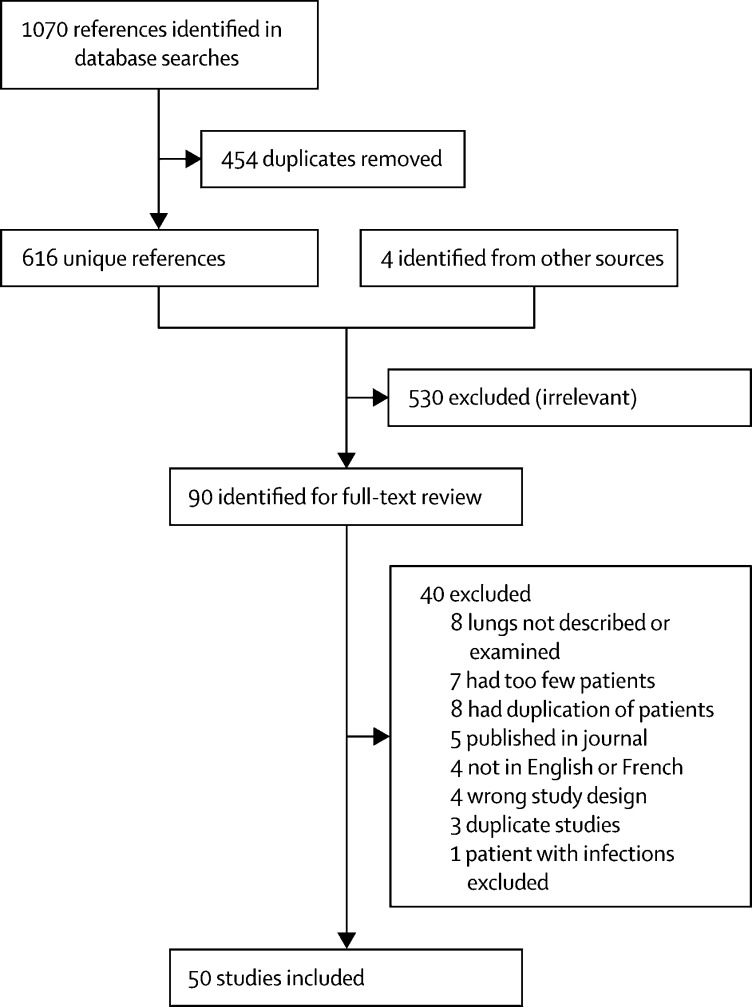
We identified 1070 references from the database searches and four from alternative sources; 454 were duplicates, 526 were excluded from title and abstract review and an additional 40 from full-text review, leaving 50 studies meeting criteria for data extraction (figure 1 ). Of these, five were preprints and the remainder were published in peer-reviewed journals. We corresponded with 39 authors. All studies were published in 2020.
Figure 1.
Flow diagram of the study selection
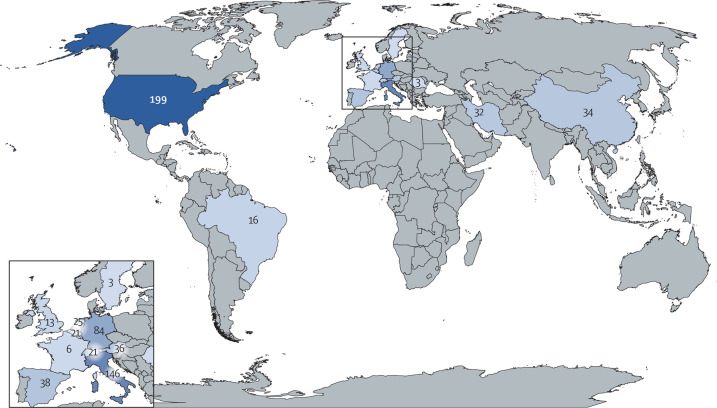
A total of 677 decedents were reported across the 50 studies (table 1 ).22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71 Information on the use of fungal stain procedures was available for 42 studies comprising 617 decedents (91%). Fungal stains were routinely used for examination of lung tissue in 17 studies (table 1) comprising 257 decedents (38%); for 25 studies, comprising 380 decedents (56%), fungal stains were added if there were findings suggestive of bronchopneumonia on haematoxylin and eosin stain. Studies were included from 15 countries, most commonly from the USA (including 199 decedents), Italy (n=146), and Germany (n=84; figure 2 ). Most autopsies were done on decedents who were admitted to hospital (n=665 [98%]) with very few from the community (n=12 [2%]). Standard autopsy was the most commonly used technique (36 [72%] studies). Minimally invasive autopsy was used in ten (20%) studies and a combination of standard and minimally invasive autopsies was used in five (10%) studies (table 1). Invasive mechanical ventilation and extracorporeal membrane oxygenation (ECMO) status was missing for 135 (20%) decedents. Of the total cohort, 320 (58%) required invasive mechanical ventilation and 15 (2%) received ECMO.
Table 1.
Summary of studies meeting criteria for data extraction
| Country | Population | Decedents included (decedents with autopsy-proven invasive mould disease) | Decedents who received invasive ventilation (decedents with autopsy-proven invasive mould disease) | Mean age, years (median) | Autopsy type | Routine fungal stains | |
|---|---|---|---|---|---|---|---|
| Beigmohammadi et al (2021)22* | Iran | Hospitalised | 7 (0)† | 7 (0) | 67·9 (72·0) | Minimally invasive | No |
| Borczuk et al (2020)23* | Italy, USA | Hospitalised | 68 (2)† | 27 (1) | (73) | Standard; 2 minimally invasive | At 1 of 3 centres involved (23 patients) |
| Böesmüeller et al (2020)24* | Germany | Hospitalised | 4 (0)† | 3 (0) | 71·8 (75·0) | Standard | Yes |
| Bradley et al (2020)25* | USA | Hospitalised | 14 (0)† | 8 (0) | 70·6 (73·5) | Standard | No |
| Brook et al (2021)26* | USA | Hospitalised | 5 (0)† | 2 (0) | (79) | Minimally invasive | No |
| Buja et al (2020)27 | USA | Hospitalised | 3 (0)† | 0 (0) | 48 (48) | Standard | No |
| Bussani et al (2020)28* | Italy | Hospitalised | 41 (0)† | 6 (0) | 79·7 | Standard | Yes |
| Carsana et al (2020)29* | Italy | Hospitalised | 38 (1)† | 1 (1), 37 ND (0) | 69 | Standard | No |
| Copin et al (2020)30* | France | Hospitalised | 6 (0) | 5 (0) | ND | Standard | No |
| De Michele et al (2020)31* | USA | Hospitalised | 40 (1)† | 23 (0) | (71·5) | Standard | No |
| Deinhardt-Emmer et al (2020)32* | Germany | Hospitalised | 11 (1)† | 7 (1) | 72·2 (78·0) | Standard | Yes |
| Dell'Aquila et al (2020)33* | Italy | Hospitalised | 12 (0) | 12 (0) | 82·3 | Standard | No |
| Desai et al (2020)34* | USA | Hospitalised | 20 (0)† | 13 (0) | 62·5 | Standard | No |
| Duarte-Neto et al (2020)35* | Brazil | Hospitalised | 10 (0) | 7 (0) | (69) | Minimally invasive | No |
| Elezkurtaj et al (2020)36* | Germany | Hospitalised | 26 (0)† | 20 (0) | 69 (69) | Standard | Yes |
| Elsoukkary et al (2021)37* | USA | Hospitalised | 9 (0)‡ | ND (0) | ND | Standard and minimally invasive | Yes |
| Falasca et al (2020)38* | Italy | Hospitalised | 22 (0)† | ND (0) | 68·0 (69·5) | Standard | No |
| Flikweert et al (2020)39 | Netherlands | Hospitalised | 7 (0)† | 7 (0) | (74) | Minimally invasive | Yes |
| Fox et al (2020)40* | USA | Hospitalised | 10 (0)† | 9 (0) | 63·0 (64·5) | Standard | No |
| Grosse et al (2020)41 | Austria | Hospitalised | 14 (0)† | 7 (0) | 80·6 (81·5) | Standard | Yes |
| Hanley et al (2020)42 | UK | Hospitalised | 10 (1)† | 4 (1) | (73) | Standard, 1 minimally invasive | ND |
| Hellman et al (2020)43* | Sweden | Hospitalised | 3 (0) | ND (0) | ND | Standard | No |
| Kimmig et al (2020)44* | USA | Hospitalised | 7 (0) | ND (0) | ND | Standard | No |
| Kommoss et al (2020)45* | Germany | Hospitalised | 13 (0)† | 9 (0) | 74·6 (78) | Standard | Yes |
| Konopka et al (2020)46* | USA | Hospitalised, community | 8 (0)† | 3 (0) | 55 (52) | Standard | No |
| Lax et al (2020)47* | Austria | Hospitalised | 11 (0)† | 2 (0) | 80·5 | Standard | Yes |
| Leppkes et al (2020)48* | Germany | Hospitalised | 8 (0) | ND (0) | ND | Standard | No |
| Li et al (2020)49 | China | Hospitalised | 30 (0)† | 26 (0) | 68·2 (68·5) | Minimally invasive | No |
| Martines et al (2020)50 | USA | Hospitalised | 8 (0)† | 6 (0) | 73·5 | Standard | ND |
| Menter et al (2020)51 | Switzerland | Hospitalised | 21 (0)† | 6 (0) | 76·4 (75·0) | Standard, some minimally invasive | No |
| Nagashima et al (2020)52* | Brazil | ND | 6 (0) | 6 (0) | ND | Minimally invasive | Yes |
| Oprinca and Muja (2020)53 | Romania | Hospitalised, community | 3 (0)† | 0 (0) | 58·7 (70·0) | Standard | No |
| Prieto-Perez et al (2020)54* | Spain | Hospitalised | 20 (0) | 9 (0) | (79) | Minimally invasive | No |
| Prilutskiy et al (2020)55* | USA | Hospitalised | 4 (0)† | 2 (0) | 74 (72) | Standard | No |
| Radermecker et al (2020)56* | Belgium | Hospitalised | 4 (0)† | 4 (0) | 60·8 (59·5) | Standard | No |
| Rapkiewicz et al (2020)57* | USA | Hospitalised | 7 (1)† | 5 (0) | 57·4 (60·0) | Standard | Yes |
| Remmelink et al (2020)58* | Belgium | Hospitalised | 17 (2)† | 11 (1) | 67·5 (68·0) | Standard | Yes |
| Roden et al (2020)59 | USA | Hospitalised | 8 (0)† | 3 (0) | (79) | Standard | Yes |
| Roncati et al (2020)60* | Italy | Hospitalised | 3 (0) | ND (0) | 53·3 (49·0) | Minimally invasive | Yes |
| Sadegh Beigee et al (2020)61 | Iran | Hospitalised | 25 (0) | ND (0) | (66) | Minimally invasive | Yes |
| Sauter et al (2020)62 | USA | Hospitalised | 1 (0)†‡ | 1 (0) | ND | Standard | ND |
| Schaefer et al (2020)63* | USA | Hospitalised | 7 (1)† | 7 (1) | 62·4 (66·0) | Standard | ND |
| Schaller et al (2020)64* | Germany | Hospitalised | 10 (0)† | 4 (0) | (79) | Standard | No |
| Schurink et al (2020)65* | Netherlands | Hospitalised | 18 (1) | 16 (ND) | ND | Standard | Yes |
| Skok et al (2020)66* | Austria | Hospitalised | 11 (0)‡ | ND (0) | ND | Standard | Yes |
| Tian et al (2020)67* | China | Hospitalised | 4 (0) | ND (0) | 73 (76) | Minimally invasive | No |
| Valdivia-Mazeyra et al (2020)68* | Spain | Hospitalised | 18 (0) | 17 (0) | 61 | Standard; 7 minimally invasive | No |
| Wichmann et al (2020)69* | Germany | Hospitalised | 12 (0)† | 5 (0) | (73) | Standard | No |
| Wu et al (2020)70* | Italy | Hospitalised | 10 (0) | 10 (0) | ND | Standard | No |
| Youd et al (2020)71 | UK | Community | 3 (0)† | 0 (0) | 82·3 (86·0) | Standard | ND |
| Total | NA | NA | 677 (11) | 320 (6) | NA | NA | NA |
Hospitalised=admitted to hospital. ND=not documented. NA=not applicable.
Author contacted.
Sufficient information for individual-level data extraction.
Some decedents included in another cohort, so patient number reduced.
Figure 2.
Geographical distribution of included decedents
Adequate information was provided for individual-level data extraction from 32 studies describing 442 decedents (table 1). Further individual-level data were added for a case of autopsy-proven IMD based on correspondence with an author; a case of autopsy-proven CAPA in a separate single-case report was the same decedent with IMD reported by Antinori and colleagues72 (and Antinori S, Università degli Studi di Milano, Milan, Italy, personal communication). In total, there were individual-level data for 443 decedents (appendix pp 1–16). The median age was 70·0 years (IQR 57·0–79·0). Most decedents had pre-existing comorbidities. Diabetes was reported for 133 (30%) decedents, pre-existing lung disease for 97 (22%), and cancer for 64 (14%). Immunocompromised status was reported for 27 decedents (6%), including solid organ transplantation in eight (2%), haematological disorders in nine (2%), and other chronic immunosuppressant use in ten (2%). The median duration of hospitalisation before death, known for 247 decedents, was 10·0 days (IQR 5·0–22·0). Data on mechanical ventilation status were available for 548 decedents (including some decedents without individual-level data): 320 (58%) decedents had received invasive mechanical ventilation. Length of ventilation, known for 140 decedents, was a median of 9·0 days (IQR 5·0–20·0). Treatment with immunomodulation was confirmed for 60 decedents: six received interleukin-6 (IL-6) inhibitors (a seventh was randomly assigned to, and received, either sarilumab or placebo), two received IL-1 inhibitors, one received both of these drug classes, 49 received steroids, and details were missing for one decedent. 50 (7%) of 677 decedents were recorded to have received antifungals.
Among the total cohort of 677 decedents, 11 (2%) had autopsy evidence of IMD; among the 320 decedents reported to receive invasive mechanical ventilation, six (2%) had IMD (table 2 ). Eight (<1%) had pulmonary aspergillosis, two (<1%) had invasive mycoses not specified, and one (<1%) had mucormycosis (table 2). Fungal diseases involved the lung only in four cases, airway only in two cases, and both airway and lung in three cases. In one case, a patient who had disseminated mucormycosis, fungal invasion was shown in the lungs, hilar lymph nodes, brain, and kidney (table 2).
Table 2.
Details of the 11 decedents with invasive mould disease
| Country | Case number in initial report | Age, years | Sex | Comorbidities | Length of illness, days | Invasive mechanical ventilation (days) | Extracorporeal membrane oxygenation | Immunotherapy | Antifungal | Mould identification | Autopsy type | Extent of fungal involvement | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borczuk et al (2020)23 | Italy | 29 | 79 | Male | Dementia, congestive heart failure, intestinal ischaemia | 9 | Yes (6) | No | None | None | Aspergillus | Standard | Airway only |
| Borczuk et al (2020)23 | Italy | 39 | 61 | Male | COPD, congestive heart failure, pharyngeal cancer | 6 | No | No | None | None | Aspergillus | Standard | Bronchopneumonia |
| Carsana et al (2020),29 Antinori et al (2020)72 | Italy | ND | 73 | Male | Diabetes, hypertension, hyperthyroidism, atrial fibrillation, obesity | ND | Yes (9) | No | None | Liposomal amphotericin B, then isavuconazole | Aspergillus fumigatus | Standard | Bronchial wall ulceration and focal necrotising pneumonia |
| De Michele et al (2020)31 | USA | ND | ND | ND | ND | ND | No | No | ND | ND | Aspergillus | Standard | Bronchopneumonia, mycetoma |
| Deinhardt-Emmer et al (2020)32 | Germany | 3 | 78 | Male | Hypertension, diabetes, chronic renal failure | 30 | Yes (7) | No | None | None | Fungus not specified | Standard | Fungal pneumonia |
| Hanley et al (2020)42 | UK | 5 | 22 | Male | Obesity | 27 | Yes (22) | No | None | Caspofungin | Mucormycete | Standard | Lungs, hilar lymph nodes, brain, kidney |
| Rapkiewicz et al (2020)57 | USA | 2 | 60 | Male | Coronary artery disease | 7 | No | No | None | None | Fungus not specified | Standard | Erosive bronchitis with hyphae; bronchopneumonia (fungal stain negative) |
| Remmelink et al (2020)58 | Belgium | 6 | 73 | Male | Hypertension, chronic renal failure | 11 | Yes (ND) | Yes | Steroids | ND | Aspergillus | Standard | Lung and trachea |
| Remmelink et al (2020)58 | Belgium | 7 | 56 | Male | None | 7 | No | No | None | ND | Aspergillus | Standard | Bilateral invasive aspergillosis (lungs) |
| Schaefer et al (2020)63 | USA | 4 | 50 | Male | Relapsed B-ALL, febrile neutropenia, invasive aspergillosis | 9 | Yes (7) | No | None | ND | Aspergillus* | Standard | Lung abscess |
| Schurink et al (2020)65 | Netherlands | ND | ND | ND | ND | ND | ND | ND | ND | ND | Aspergillus | Standard | Massive aspergillosis involving lung parenchyma |
B-ALL=b-cell acute lymphoblastic leukaemia. COPD=chronic obstructive pulmonary disease. ND=not documented.
Pre-existing diagnosis.
Decedents with and without IMD who had individual-level data available are compared in table 3 . One (11%) decedent of nine with IMD had a pre-existing immunocompromising condition, and in this patient, the diagnosis of aspergillosis pre-dated COVID-19.63 By comparison, 26 (6%) decedents of 407 without IMD were immunocompromised. One (11%) decedent of nine with IMD, for whom this was known, received immunomodulatory therapy for COVID-19 compared with 59 (14%) without IMD (table 3). Two decedents with IMD received antifungals (table 2).
Table 3.
Individual-level data for decedents with and without autopsy-proven invasive mould disease
| Invasive mould disease (n=10 [2%]) | No invasive mould disease (n=433 [98%]) | |
|---|---|---|
| Median age, years (IQR) | 60 (40–75·5) | 70 (57–79)* |
| Male | 9/9 (100%) | 260/393 (66%) |
| Pre-existing lung disease | 1/9 (11%) | 94/392 (24%) |
| Immunocompromised | 1/9 (11%) | 26/407 (6%) |
| Median duration from symptom onset to death, days (IQR) | 9 (6·8–22·5)† | 14 (9–26)† |
| Median hospital length of stay, days (IQR) | 14·0 (5·5–26·0)‡ | 10·0 (5·0–22·5)§ |
| Ventilated | 6/10 (60%) | 172/339 (51%) |
| Median ventilation time, days (IQR) | 7·0 (6·5–15·5)¶ | 9·0 (5·0–20·0)‖ |
| Host-directed therapies for COVID-19 | 1/9 (11%) | 59 (14%) |
Data missing for
60 decedents,
3 decedents,
5 decedents,
112 decedents,
1 decedent, and
37 decedents.
Discussion
In this systematic review of autopsy studies, we describe the prevalence of autopsy-proven IMD including CAPA among persons with fatal COVID-19 infections. IMD was autopsy-proven in just 2% of all decedents with COVID-19 and 2% among those reported to have received mechanical ventilation.
The rates of autopsy-proven IMD are considerably lower than rates of CAPA reported from some ICU cohort studies (1·0–39·1%).12 Given that post-mortem examinations are the most reliable way to confirm IMD, this Review provides a more robust appraisal of the true incidence of IMD complicating critical COVID-19, and suggests that the high CAPA rates published from cohort studies might be due to overdiagnosis. Previous autopsy studies have shown that pneumonias, in general, were overdiagnosed antemortem, and that discrepancy rates between clinical and necropsy findings were higher for respiratory tract infections than for other types of infection.73 Alternatively, a study done across several decades before COVID-19 found that decedents with autopsy-proven invasive pulmonary aspergillosis were frequently not diagnosed antemortem.74 Furthermore, in at least two cases in this series, the diagnosis of IMD was not made until autopsy.
CAPA might be overdiagnosed in some ICU studies because of an absence lack of specificity in the classification of probable, putative, or possible CAPA (which characterise most cases reported to date).12 There are several challenges that ICU studies face in establishing the true prevalence of CAPA. First, the clinical and radiographical signs of CAPA are difficult to distinguish from severe COVID-19 alone. Second, lower respiratory tract samples for diagnosing invasive pulmonary aspergillosis are infrequently obtained in patients with COVID-19 because bronchoscopy is considered an aerosol-generating procedure. Finally, the clinical definitions for CAPA are imperfect. Proven invasive pulmonary aspergillosis (which relies on histological evidence of tissue invasion) is rarely diagnosed and, given the tenuous clinical status of most affected patients, biopsies are rarely done. Established research criteria for the diagnosis of invasive pulmonary aspergillosis, such as the updated definitions from the EORTC/MSGERC19 are of limited utility for diagnosing invasive pulmonary aspergillosis in ICU patients because host factors are frequently absent.20 Furthermore, classic radiographical findings seen in immunocompromised hosts are uncommon in patients without host factors. Alternative diagnostic criteria have been proposed and adapted for the study of influenza-associated pulmonary aspergillosis7, 75 and now CAPA.5, 14, 17, 21 However, in critically ill patients with COVID-19, radiographical findings of IMD are non-specific and are difficult to discern from the bilateral pulmonary infiltrates due to acute respiratory distress syndrome or the thrombotic complications of COVID-19, which can result in pulmonary infarction, cavitary lesions, or halo signs. Furthermore, serum galactomannan is not sensitive, and the presence of mould in respiratory samples (detected by culture, PCR, or galactomannan) can represent airway colonisation. For example, bronchoalveolar lavage galactomannan has a high false positivity rate in patients without haematological malignancy.76 Some patients with CAPA improve without antifungals,9 which argues against true invasive disease. Furthermore, Flikweert and colleagues39 reported that histological evidence of invasive pulmonary aspergillosis was absent on post-mortem examination of six patients diagnosed with probable CAPA on the basis of bronchoalveolar lavage galactomannan (median 4·35 optical density index [IQR 3·63–4·40]) and, in two, respiratory culture growing Aspergillus fumigatus. Taken together with our findings that IMD is uncommon on autopsy studies of decedents with COVID-19, these data suggest that many cases of probable, putative, or possible CAPA do not represent true invasive disease.
Alternatively, CAPA has been associated with excess mortality.13, 77 The clinical importance of Aspergillus in the airways of patients with critical COVID-19 is unclear, but the low prevalence of autopsy-proven IMD in such patients suggests that this finding might be a marker of COVID-19 severity and not a mediator of death. Nonetheless, clinical trials are underway to assess the effect of antimould fungal prophylaxis in critically ill patients with COVID-19 (NCT04707703).
CAPA has primarily been reported among critically ill patients with COVID-19, especially those requiring mechanical ventilation, but whether this report reflects sampling bias (enhanced surveillance and access to lower respiratory samples in this patient population) or confounding (Aspergillus in the airways and critical illness or ventilation are both associated with COVID-19 severity), or whether critical illness and mechanical ventilation truly confer increased risk of CAPA is unclear. In this Review all cases of COVID-19 were severe by definition given that they resulted in death. However, only 58% of decedents (320 of 548 for whom this was known) had received mechanical ventilation, including six (60%) of ten decedents with autopsy-proven IMD for whom these data were available and 314 (58%) of 538 without IMD. These data suggest that IMD (including CAPA) is not confined to mechanically ventilated patients and that higher rates in these patients might reflect a combination of disease severity and convenience of testing.
Immune dysregulation is implicated in the pathogenesis of severe COVID-19, and immunomodulatory therapies like corticosteroids and cytokine inhibitors (IL-1 and IL-6 inhibitors) have been widely used. Corticosteroid therapy is a known risk factor for invasive pulmonary aspergillosis19 and IL-6 inhibitors could plausibly provoke IMD given impaired immune response to other pathogens.78 Only one (11%) of the nine decedents with IMD for whom treatment data were available had received these drugs, similar to 14% in the non-IMD group. Importantly, most autopsies were done before the publication of the RECOVERY trial,79 which showed dexamethasone improved mortality in patients with severe and critical COVID-19, and so prescribing of corticosteroid might have increased since then. Further studies will be needed to establish whether use of immunomodulatory therapy is associated with tissue-proven IMD.
Post-mortem examination is the most definitive means available to detect IMD and is a particular strength of this study. The presence of hyphae on histopathological investigation is simple to detect, and, therefore, most of these cases are highly likely to have been identified. We also made every effort to contact authors when histopathological findings were not specifically described to confirm that IMD had not been encountered in each case. Another strength is the inclusive nature of our Review, which included preprints, given the rapidly expanding literature on COVID-19.
There are several limitations of this Review that should be considered. First, we only included studies published in English or French, and excluded studies could possibly have contributed further data. Second, there was little clinical information from most reports. For example, the use of antifungals was uncommonly reported, and whether this reflects incompletely reported data or low rates of prescribing is unclear. Antifungal use could have reduced the sensitivity of IMD detection on autopsy. Similarly, use of immunomodulators was also infrequently reported, reducing inference about the risk of tissue-proven IMD associated with these therapies. Third, routine use of fungal stains varied by centre, and we cannot exclude the possibility that some cases of IMD were missed, particularly when the autopsy series were not specifically focused on identifying secondary infections. 38% of autopsy examinations used routine fungal staining of lung tissue, which is reassuring, and for the remainder, fungal stains were added in the presence of histopathology suggestive of bronchopneumonia to increase the sensitivity of assessing for IMD. Fourth, although our Review was exhaustive, the sample size is very small compared with the total number of COVID-19 deaths internationally; most decedents were included from just three countries (USA, Germany, and Italy) and geographical variation in incidence of IMD after COVID-19 might occur (as it does following influenza).80 Finally, although the use of minimally invasive autopsy strategies for COVID-19 has been validated,35, 81 limited sampling (ie, only smaller sections taken) during this technique might have led to underdiagnosis of IMD in some cases. However, minimally invasive autopsies comprised a small proportion of post-mortem examinations in this study.
In contrast to our findings, a study published after our systematic review was done reported alarming rates of autopsy-proven IMD in patients with COVID-19 who received care in a single German ICU.82 Of eight decedents, six had IMD, including four cases of aspergillosis and two cases of mucormycosis. Decedents had received antifungal prophylaxis with caspofungin or isavuconazole for an average for 9·5 days. All decedents had protracted ICU stays with prolonged invasive mechanical ventilation (median of 26 days [IQR 21–35]), and five received ECMO (including four with IMD). Seven of eight decedents (including five with IMD) had macrophage activation syndrome. All decedents received corticosteroids, and one additionally received tocilizumab and two additionally received anakinra. Although not suspected antemortem in any case, IMD was the autopsy-determined cause of death in four cases. In general, rates of IMD are affected by local hospital-level or ICU-level factors, such as airflow, construction, and precautions to prevent infection; this Review highlights the importance of understanding local epidemiology.
In conclusion, autopsy-proven IMD is uncommonly reported among decedents with COVID-19. However, centre-to-centre variation probably exists, and clinicians and pathologists should work together to understand local disease incidence. Given the difficulties in establishing the diagnosis of IMD antemortem, future COVID-19 autopsy studies should include pertinent clinical, radiographical, and microbiological findings and antimicrobial therapy to validate research definitions for this disease. In the interim, clinicians and pathologists alike should stay vigilant to the possibility of IMD as a complication of COVID-19.
Declaration of interests
BEK has no competing interests. CJC reports grants and personal fees from Astellas and Merck, grants from Melinta and Cidara, personal fees from The Medicines Company, Cidara, Scynexis, Shinogi, Opex, Needham & Company, and other support from Merck and T2Biosystems, outside the submitted work. MHN reports grants from the Centers for Disease Control and Prevention, Astellas, and the National Institutes of Health during the conduct of this study. ISS reports personal fees from AVIR Pharma, outside the submitted work.
Contributors
BEK searched the literature and wrote the first draft of the manuscript. BEK and ISS did the review, data extraction, and analysis, and created the figures. ISS was responsible for study design and overall supervision. All authors contributed to the data interpretation and to critical revision of the manuscript.
Supplementary Material
References
- 1.Maiese A, Manetti AC, La Russa R, et al. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2020 doi: 10.1007/s12024-020-00310-8. published online Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson GR, III, Cornely OA, Pappas PG, et al. Invasive aspergillosis as an under-recognized superinfection in COVID-19. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoenigl M. Invasive fungal disease complicating COVID-19: when it rains it pours. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1342. published online Sept 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong-James D, Youngs J, Bicanic T, et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur Respir J. 2020;56 doi: 10.1183/13993003.02554-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verweij PE, Gangneux J-P, Bassetti M, et al. Diagnosing COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e53–e55. doi: 10.1016/S2666-5247(20)30027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rijnders BJA, Schauwvlieghe AFAD, Wauters J. Influenza-associated pulmonary aspergillosis: a local or global lethal combination? Clin Infect Dis. 2020;71:1764–1767. doi: 10.1093/cid/ciaa010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schauwvlieghe AFAD, Rijnders BJA, Philips N, et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 8.Lahmer T, Kriescher S, Herner A, et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alanio A, Dellière S, Fodil S, Bretagne S, Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marr K, Platt A, Tornheim JA, et al. Aspergillosis complicating severe coronavirus disease. Emerg Infec Dis. 2021;27:18–25. doi: 10.3201/eid2701.202896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rutsaert L, Steinfort N, Van Hunsel T, et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71–74. doi: 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmanton-García J, Sprute R, Stemler J, et al. COVID-19 associated pulmonary aspergillosis, March–August 2020. Emerg Infect Dis. 2021;27:1077–1086. doi: 10.3201/eid2704.204895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bartoletti M, Pascale R, Cricca M, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. published online July 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White PL, Dhillon R, Cordey A, et al. A national strategy to diagnose coronavirus disease 2019–associated invasive fungal disease in the intensive care unit. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1298. published online Aug 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont D, Menotti J, Turc J, et al. Pulmonary aspergillosis in critically ill patients with coronavirus disease 2019 (COVID-19) Med Mycol. 2020 doi: 10.1093/mmy/myaa078. published online Sept 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koehler P, Bassetti M, Chakrabarti A, et al. Defining and managing COVID-19 associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus for research and clinical guidance. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30847-1. published online Dec 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown L-AK, Ellis J, Gorton R, De S, Stone N. Surveillance for COVID-19-associated pulmonary aspergillosis. Lancet Microbe. 2020;1:e152. doi: 10.1016/S2666-5247(20)30091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Donnelly JP, Chen SC, Kauffman CA, et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2019;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blot SI, Taccone FS, Van den Abeele, et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 21.Bassetti M, Azoulay E, Kullberg BJ, et al. EORTC/MSGERC Definitions of invasive fungal diseases: summary of activities of the Intensive Care Unit Working Group. Clin Infect Dis. 2021;72:S121–S127. doi: 10.1093/cid/ciaa1751. [DOI] [PubMed] [Google Scholar]
- 22.Beigmohammadi MT, Jahanbin B, Safaei M, et al. Pathological findings of postmortem biopsies from lung, heart, and liver of 7 deceased COVID-19 patients. Int J Surg Path. 2021;29:135–145. doi: 10.1177/1066896920935195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borczuk AC, Salvatore SP, Seshan SV, et al. COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City. Mod Path. 2020;33:2156–2168. doi: 10.1038/s41379-020-00661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Böesmüeller H, Traxler S, Bitzer M, et al. The evolution of pulmonary pathology in fatal COVID-19 disease: an autopsy study with clinical correlation. Virchows Arch. 2020;477:349–357. doi: 10.1007/s00428-020-02881-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brook OR, Piper KG, Mercado NB, et al. Feasibility and safety of ultrasound-guided minimally invasive autopsy in COVID-19 patients. Abdom Radiol (NY) 2021;46:1263–1271. doi: 10.1007/s00261-020-02753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buja LM, Wolf DA, Zhao B, et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc Pathol. 2020;48 doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bussani R, Schneider E, Zentillin L, et al. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020;61 doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carsana L, Sonzogni A, Nasr A, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copin M, Parmentier E, Duburcq T, et al. Time to consider histologic pattern of lung injury to treat critically ill patients with COVID-19 infection. Intensive Care Med. 2020;46:1124–1126. doi: 10.1007/s00134-020-06057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Michele S, Sun Y, Yilmaz MM, et al. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154:748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deinhardt-Emmer S, Wittschieber D, Sanft J, et al. Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and correlation to tissue damage. bioRxiv. 2020 doi: 10.1101/2020.07.01.182550. published online July 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dell'Aquila M, Cattani P, Fantoni M, et al. Postmortem swabs in the SARS-CoV-2 pandemic: report on 12 complete clinical autopsy cases. Arch Pathol Lab Med. 2020;144:1298–1302. doi: 10.5858/arpa.2020-0362-SA. [DOI] [PubMed] [Google Scholar]
- 34.Desai N, Neyaz A, Szabolcs A, et al. Temporal and spatial heterogeneity of host response to SARS-CoV-2 pulmonary infection. medRxiv. 2020 doi: 10.1101/2020.07.30.20165241. published online Aug 2. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duarte-Neto AN, Monteiro RAA, da Silva LFF, et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elezkurtaj S, Greuel S, Ihlow J, et al. Causes of death and comorbidities in hospitalized patients with COVID-19. Sci Rep. 2020;11 doi: 10.1038/s41598-021-82862-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsoukkary SS, Mostyka M, Dillard A, et al. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology. 2021;88:56–68. doi: 10.1159/000511325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falasca L, Nardacci R, Colombo D, et al. Post-mortem findings in Italian patients with COVID-19—a descriptive full autopsy study of cases with and without co-morbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Flikweert AW, Grootenboers MJJH, Yick DCY, et al. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fox SE, Akmatbekov A, Harbert JL, Li G, Brown JQ, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grosse C, Grosse A, Salzer HJF, Dunser MW, Motz R, Langer R. Analysis of cardiopulmonary findings in COVID-19 fatalities: high incidence of pulmonary artery thrombi and acute suppurative bronchopneumonia. Cardiovasc Pathol. 2020;49 doi: 10.1016/j.carpath.2020.107263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hanley B, Naresh KN, Roufosse C, et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hellman U, Karlsson MG, Engström-Laurent A, et al. Presence of hyaluronan in lung alveoli in severe COVID-19—an opening for new treatment options? J Biol Chem. 2020;295:15418–15422. doi: 10.1074/jbc.AC120.015967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kimmig LM, Wu D, Gold M, et al. IL6 inhibition in critically ill COVID-19 patients is associated with increased secondary infections. Front Med (Lausanne) 2020;7 doi: 10.3389/fmed.2020.583897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kommoss FKF, Schwab C, Tavernar L, et al. The pathology of severe COVID-19-related lung damage. Dtsch Arztebl Int. 2020;117:500–506. doi: 10.3238/arztebl.2020.0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konopka KE, Nguyen T, Jentzen JM, et al. Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 infection is morphologically indistinguishable from other causes of DAD. Histopathology. 2020;77:570–578. doi: 10.1111/his.14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lax SF, Skok K, Zechner P, et al. Pulmonary arterial thrombosis in COVID-19 with fatal outcome results from a prospective, single-center, clinicopathologic case series. Ann Intern Med. 2020;173:350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leppkes M, Knopl J, Naschberger E, et al. Vascular occlusion by neutrophil extracellular traps in COVID-19. EBioMedicine. 2020;58 doi: 10.1016/j.ebiom.2020.102925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Wu J, Wang S, et al. Progression to fibrosing diffuse alveolar damage in a series of 30 minimally invasive autopsies with COVID-19 pneumonia in Wuhan, China. Histopathology. 2020;78:542–555. doi: 10.1111/his.14249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martines R, Ritter J, Matkovic E, et al. Pathology and pathogenesis of SARS-CoV-2 associated with fatal coronavirus disease, United States. Emerg Infect Dis. 2020;26:2005–2015. doi: 10.3201/eid2609.202095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menter T, Haslbauer JD, Nienhold R, et al. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagashima S, Mendes MC, Camargo Martins AP, et al. Endothelial dysfunction and thrombosis in patients with COVID-19-brief report. Arterioscler Thromb Vasc Biol. 2020;40:2404–2407. doi: 10.1161/ATVBAHA.120.314860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oprinca G, Muja L. Postmortem examination of three SARS-CoV-2-positive autopsies including histopathologic and immunohistochemical analysis. Int J Legal Med. 2020;135:329–339. doi: 10.1007/s00414-020-02406-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prieto-Perez L, Fortes J, Soto C, et al. Histiocytic hyperplasia with hemophagocytosis and acute alveolar damage in COVID-19 infection. Mod Pathol. 2020;33:2139–2146. doi: 10.1038/s41379-020-0613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prilutskiy A, Kritselis M, Shevtsov A, et al. SARS-CoV-2 infection-associated hemophagocytic lymphohistiocytosis. Am J Clin Pathol. 2020;154:466–474. doi: 10.1093/ajcp/aqaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radermecker C, Detrembleur N, Guiot J, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217 doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rapkiewicz AV, Mai X, Carsons SE, et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: a case series. EClinicalMedicine. 2020;24 doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Remmelink M, De Mendonca R, D'Haene N, et al. Unspecific post-mortem findings despite multiorgan viral spread in COVID-19 patients. Crit Care. 2020;24:495. doi: 10.1186/s13054-020-03218-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roden AC, Bois MC, Johnson TF, et al. The spectrum of histopathologic findings in lungs of patients with fatal COVID-19 infection. Arch Pathol Lab Med. 2020;145:11–21. doi: 10.5858/arpa.2020-0491-SA. [DOI] [PubMed] [Google Scholar]
- 60.Roncati L, Ligabue G, Nasillo V, et al. A proof of evidence supporting abnormal immunothrombosis in severe COVID-19: naked megakaryocyte nuclei increase in the bone marrow and lungs of critically ill patients. Platelets. 2020;31:1085–1089. doi: 10.1080/09537104.2020.1810224. [DOI] [PubMed] [Google Scholar]
- 61.Sadegh Beigee F, Pourabdollah Toutkaboni M, Khalili N, et al. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol Res Pract. 2020;216 doi: 10.1016/j.prp.2020.153228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sauter JL, Baine MK, Butnor KJ, et al. Insights into pathogenesis of fatal COVID-19 pneumonia from histopathology with immunohistochemical and viral RNA studies. Histopathology. 2020;77:915–925. doi: 10.1111/his.14201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schaefer I, Padera RF, Solomon IH, et al. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod Pathol. 2020;33:2104–2114. doi: 10.1038/s41379-020-0595-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaller T, Hirschbuhl K, Burkhardt K, et al. Postmortem examination of patients with COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schurink B, Roos E, Radonic T, et al. Viral presence and immunopathology in patients with lethal COVID-19: a prospective autopsy cohort study. Lancet Microbe. 2020;1:e290–e299. doi: 10.1016/S2666-5247(20)30144-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skok K, Stelzl E, Trauner M, Kessler HH, Lax SF. Post-mortem viral dynamics and tropism in COVID-19 patients in correlation with organ damage. Virchows Arch. 2020;478:343–353. doi: 10.1007/s00428-020-02903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian S, Yong X, Huan L, et al. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Valdivia-Mazeyra M, Salas C, Nieves-Alonso J, et al. Increased number of pulmonary megakaryocytes in COVID-19 patients with diffuse alveolar damage: an autopsy study with clinical correlation and review of the literature. Virchows Arch. 2020;478:487–496. doi: 10.1007/s00428-020-02926-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wichmann D, Sperhake J, Luegehetmann M, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu MA, Fossali T, Pandolfi L, et al. COVID-19: the key role of pulmonary capillary leakage. An observational cohort study. medRxiv. 2020 doi: 10.1101/2020.05.17.20104877. published online May 21. (preprint). [DOI] [Google Scholar]
- 71.Youd E, Moore L. COVID-19 autopsy in people who died in community settings: the first series. J Clin Pathol. 2020;73:840–844. doi: 10.1136/jclinpath-2020-206710. [DOI] [PubMed] [Google Scholar]
- 72.Antinori S, Rech R, Galimberti L, et al. Invasive pulmonary aspergillosis complicating SARS-COV-2 pneumonia: a diagnostic challenge. Travel Med Infect Dis. 2020;38 doi: 10.1016/j.tmaid.2020.101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gibson TN, Shirley SE, Escoffery CT, Reid M. Discrepancies between clinical and postmortem diagnoses in Jamaica: a study from the university hospital of the West Indies. J Clin Pathol. 2004;57:980–985. doi: 10.1136/jcp.2004.016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tejerina EE, Abril E, Padilla R, et al. Invasive aspergillosis in critically ill patients: an autopsy study. Mycoses. 2019;62:673–679. doi: 10.1111/myc.12927. [DOI] [PubMed] [Google Scholar]
- 75.Verweij PE, Rijnders BJA, Brüggemann RJM, et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46:1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farmakiotis D, Le A, Weiss Z, Ismail N, Kubiak DW, Koo S. False positive bronchoalveolar lavage galactomannan: effect of host and cut-off value. Mycoses. 2019;62:204–213. doi: 10.1111/myc.12867. [DOI] [PubMed] [Google Scholar]
- 77.Permpalung N, Chiang TRP, Massie AB, et al. COVID-19 associated pulmonary aspergillosis in mechanically ventilated patients. Clin Infec Dis. 2021 doi: 10.1093/cid/ciab223. published online March 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ching CB, Gupta S, Li B, et al. Interleukin-6/Stat3 signaling has an essential role in the host antimicrobial response to urinary tract infection. Kidney Int. 2018;93:1320–1329. doi: 10.1016/j.kint.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19—preliminary report. N Engl J Med. 2020;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schwartz IS, Friedman DZP, Zapernick L, et al. High rates of influenza-associated invasive pulmonary aspergillosis may not be universal: a retrospective cohort study from Alberta, Canada. Clin Infect Dis. 2020;71:1760–1763. doi: 10.1093/cid/ciaa007. [DOI] [PubMed] [Google Scholar]
- 81.Monteiro RAA, Duarte-Neto AN, Silva LFFD, et al. Ultrasound-guided minimally invasive autopsies: a protocol for the study of pulmonary and systemic involvement of COVID-19. Clinics (Sao Paulo) 2020;75 doi: 10.6061/clinics/2020/e1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Evert K, Dienemann T, Brochhausen C, et al. Autopsy findings after long-term treatment of COVID-19 patients with microbiological correlation. Virchows Archi. 2021 doi: 10.1007/s00428-020-03014-0. published online Jan 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.