Abstract
Background
BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine (3 μg or 6 μg) formulated with a toll-like receptor 7/8 agonist molecule (IMDG) adsorbed to alum (Algel). We previously reported findings from a double-blind, multicentre, randomised, controlled phase 1 trial on the safety and immunogenicity of three different formulations of BBV152 (3 μg with Algel-IMDG, 6 μg with Algel-IMDG, or 6 μg with Algel) and one Algel-only control (no antigen), with the first dose administered on day 0 and the second dose on day 14. The 3 μg and 6 μg with Algel-IMDG formulations were selected for this phase 2 study. Herein, we report interim findings of the phase 2 trial on the immunogenicity and safety of BBV152, with the first dose administered on day 0 and the second dose on day 28.
Methods
We did a double-blind, randomised, multicentre, phase 2 clinical trial to evaluate the immunogenicity and safety of BBV152 in healthy adults and adolescents (aged 12–65 years) at nine hospitals in India. Participants with positive SARS-CoV-2 nucleic acid and serology tests were excluded. Participants were randomly assigned (1:1) to receive either 3 μg with Algel-IMDG or 6 μg with Algel-IMDG. Block randomisation was done by use of an interactive web response system. Participants, investigators, study coordinators, study-related personnel, and the sponsor were masked to treatment group allocation. Two intramuscular doses of vaccine were administered on day 0 and day 28. The primary outcome was SARS-CoV-2 wild-type neutralising antibody titres and seroconversion rates (defined as a post-vaccination titre that was at least four-fold higher than the baseline titre) at 4 weeks after the second dose (day 56), measured by use of the plaque-reduction neutralisation test (PRNT50) and the microneutralisation test (MNT50). The primary outcome was assessed in all participants who had received both doses of the vaccine. Cell-mediated responses were a secondary outcome and were assessed by T-helper-1 (Th1)/Th2 profiling at 2 weeks after the second dose (day 42). Safety was assessed in all participants who received at least one dose of the vaccine. In addition, we report immunogenicity results from a follow-up blood draw collected from phase 1 trial participants at 3 months after they received the second dose (day 104). This trial is registered at ClinicalTrials.gov, NCT04471519.
Findings
Between Sept 5 and 12, 2020, 921 participants were screened, of whom 380 were enrolled and randomly assigned to the 3 μg with Algel-IMDG group (n=190) or 6 μg with Algel-IMDG group (n=190). Geometric mean titres (GMTs; PRNT50) at day 56 were significantly higher in the 6 μg with Algel-IMDG group (197·0 [95% CI 155·6–249·4]) than the 3 μg with Algel-IMDG group (100·9 [74·1–137·4]; p=0·0041). Seroconversion based on PRNT50 at day 56 was reported in 171 (92·9% [95% CI 88·2–96·2] of 184 participants in the 3 μg with Algel-IMDG group and 174 (98·3% [95·1–99·6]) of 177 participants in the 6 μg with Algel-IMDG group. GMTs (MNT50) at day 56 were 92·5 (95% CI 77·7–110·2) in the 3 μg with Algel-IMDG group and 160·1 (135·8–188·8) in the 6 μg with Algel-IMDG group. Seroconversion based on MNT50 at day 56 was reported in 162 (88·0% [95% CI 82·4–92·3]) of 184 participants in the 3 μg with Algel-IMDG group and 171 (96·6% [92·8–98·8]) of 177 participants in the 6 μg with Algel-IMDG group. The 3 μg with Algel-IMDG and 6 μg with Algel-IMDG formulations elicited T-cell responses that were biased to a Th1 phenotype at day 42. No significant difference in the proportion of participants who had a solicited local or systemic adverse reaction in the 3 μg with Algel-IMDG group (38 [20·0%; 95% CI 14·7–26·5] of 190) and the 6 μg with Algel-IMDG group (40 [21·1%; 15·5–27·5] of 190) was observed on days 0–7 and days 28–35; no serious adverse events were reported in the study. From the phase 1 trial, 3-month post-second-dose GMTs (MNT50) were 39·9 (95% CI 32·0–49·9) in the 3μg with Algel-IMDG group, 69·5 (53·7–89·9) in the 6 μg with Algel-IMDG group, 53·3 (40·1–71·0) in the 6 μg with Algel group, and 20·7 (14·5–29·5) in the Algel alone group.
Interpretation
In the phase 1 trial, BBV152 induced high neutralising antibody responses that remained elevated in all participants at 3 months after the second vaccination. In the phase 2 trial, BBV152 showed better reactogenicity and safety outcomes, and enhanced humoral and cell-mediated immune responses compared with the phase 1 trial. The 6 μg with Algel-IMDG formulation has been selected for the phase 3 efficacy trial.
Funding
Bharat Biotech International.
Translation
For the Hindi translation of the abstract see Supplementary Materials section.
Introduction
The novel human coronavirus SARS-CoV-2 has spread worldwide. To date, 194 vaccine candidates are being developed to prevent COVID-19.1 Vaccines from multiple manufacturers will be needed to address the global need for SARS-CoV-2 vaccines. Several such vaccines (inactivated, viral vector, or mRNA) have received emergency use authorisation for immunisation of health-care workers and at-risk individuals.2, 3, 4, 5 There is currently an insufficient supply of vaccines, and the mRNA-based vaccines have cold chain hurdles that countries need to overcome.
BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine formulated with a toll-like receptor (TLR) 7/8 agonist molecule adsorbed to alum (Algel-IMDG). BBV152 is stored between 2°C and 8°C, which will ease immunisation cold chain requirements.
Preclinical studies in mice, rats, and rabbits showed appropriate safety profiles and humoral and cell-mediated responses.6 Live viral challenge protective efficacy studies in hamsters and non-human primates showed rapid viral clearance in the lower and upper respiratory tracts, and the absence of lung pathology after viral challenge.7, 8
We previously reported interim findings from a double-blind, randomised, phase 1 trial on the safety and immunogenicity of three different formulations of BBV152 (3 μg with Algel-IMDG, 6 μg with Algel-IMDG, and 6 μg with Algel) and one Algel only control (without antigen).9 This phase 1 trial was done with the intention of selecting two formulations. Based on acceptable safety outcomes, and humoral and cell-mediated responses, the 3 μg with Algel-IMDG and 6 μg with Algel-IMDG formulations were selected for progression to a phase 2 trial. The decision to change the dosing schedule from a 14-day interval between the first and second doses (phase 1 trial), to a 28-day interval between the two doses (phase 2 trial) was based on ensuring commonality with other licensed COVID-19 vaccines. In the phase 1 trial, no difference in the safety and immunogenicity between the 3 μg with Algel-IMDG and 6 μg with Algel-IMDG groups was observed. In this phase 2 trial, the inclusion of a placebo group was not planned. Our objective was to increase the sample size to establish whether there are differences in immunogenicity between the 3 μg with Agel-IMDG and 6 μg with Algel-IMDG groups. Therefore, no control or Algel alone group was included in this study.
Research in context.
Evidence before this study
We searched PubMed on Jan 23, 2021, using the search terms “SARS-CoV-2”, “COVID-19”, “vaccine”, and “clinical trial”. We searched for research articles published from database inception to the date of the search, with no language restrictions. We found 12 clinical trials of COVID-19 mRNA, adenovirus vectored, protein subunit, and inactivated virus vaccines. A preferred characteristic of any COVID-19 vaccine candidate is its ability to induce T-helper-1 (Th1) responses. Whole-virion inactivated vaccines are mostly developed with alum (Algel) as the adjuvant. The response generated by alum is primarily biased to Th2. Clinical trial results from two other inactivated virus vaccines (manufactured by Sinovac and Sinopharm) reported humoral responses but minimal cell-mediated responses. Bharat Biotech developed a Vero cell-based whole-virion inactivated SARS-CoV-2 vaccine (BBV152), formulated with alum and a toll-like receptor 7/8 agonist, producing a Th1-skewed response. BBV152 showed protection in non-human primate and hamster challenge models. Data from a phase 1 study suggested adequate safety and immunogenicity findings. In January 2021, serum samples taken from 38 participants in the 6 μg with Algel-IMDG group at 4 weeks after the second dose (day 56) in the phase 2 trial were found to effectively neutralise a SARS-CoV-2 variant of concern (B.1.1.7 or 20B/501Y. V1).
Added value of this study
We report preliminary analyses for the immunogenicity and safety of BBV152 in 380 vaccinated adults and adolescents. BBV152 led to enhanced immune responses and induced T-cell responses that were biased to Th1. Due to the difference in dosing regimens between phase 1 (two doses given 2 weeks apart) and phase 2 (two doses given 4 weeks apart) trials, neutralisation responses were significantly higher in the phase 2 trial than in the phase 1 trial. Immunological differences between men and women, and across age groups were not observed. Overall, both 3 μg with Algel-IMDG and 6 μg with Algel-IMDG vaccine groups had similar safety outcomes. Follow-up data from the phase 1 trial shows that BBV152 induces durable humoral and cell-mediated immunity at 3 months after the second dose (day 104).
Implications of all the available evidence
Humoral immune responses from other inactivated SARS-CoV-2 vaccine candidates are consistent with the findings of this study. However, this is the only study of an inactivated COVID-19 vaccine candidate to report a thorough evaluation of cell-mediated responses. The 6 μg with Algel-IMDG formulation has been selected for the phase 3 efficacy trial. BBV152 (developed using a well established manufacturing platform) was safe, immunogenic (persisting for 3 months), and can be stored at 2–8°C, which is compatible with the immunisation cold chain requirements of most countries. Follow-up studies to assess efficacy and immune responses in older adults and in people with comorbidities are underway.
Herein, we report interim findings from the phase 2 trial on the immunogenicity and safety of 3 μg with Algel-IMDG and 6 μg with Algel-IMDG formulations of BBV152. Additionally, this paper reports follow-up immunological results from the phase 1 trial at 3 months after participants received the second dose (day 104).
Methods
Study design and participants
This is a randomised, multicentre, phase 2 clinical trial to evaluate the immunogenicity and safety of the whole-virion inactivated SARS-CoV-2 vaccine BBV152 in healthy male and female volunteers at nine hospitals across nine states in India. Participants were aged 12–65 years at the time of enrolment. At the screening visit, participants were tested using both SARS-CoV-2 nucleic acid and serology tests, which were done at a central laboratory (Dr Dangs Laboratory, New Delhi, India) using commercially available assays. If individuals were positive for either test, they were excluded from the trial. The median time between the screening visit and vaccination visit was 3 days (range 2–4). Individuals aged older than 65 years, pregnant or lactating women, and individuals with comorbidities were excluded. All study-related activities and the opportunity to decline or withdraw from the study were explained to participants. All participants were screened for eligibility on the basis of their health status, including their medical history, vital signs, and physical examination results, and were enrolled after providing signed and dated informed consent forms. Details of the inclusion and exclusion criteria can be found in the protocol (appendix 2 pp 50–51).
The trial was approved by the National Regulatory Authority (India) and the respective ethics committees of each participating hospital and was conducted in compliance with all International Council for Harmonization Good Clinical Practice guidelines.
Randomisation and masking
The master randomisation list was uploaded to the interactive web response system, which contained the randomisation number and intended allocation. A central depot manager uploaded the kit numbers to the respective sites. At the site-level, the system would set the randomisation number and the allotment of the kit without displaying the true group allocation; the system would allocate the same treatment group for the second visit. A block size of four was used to randomly assign (1:1) participants to either the 3 μg with Algel-IMDG group or the 6 μg with Algel-IMDG group. An unmasked contract research organisation (Sclin Soft Technologies, Hyderabad, India) generated the master randomisation code, and dispatched and labelled the vaccine vials.
Participants, investigators, study coordinators, study-related personnel, and the sponsor were masked to the treatment group allocation (excluding the unmasked contract research organisation). Participants were assigned a computer-generated randomisation code that maintained masking. A masked study nurse prepared and administered the vaccines. Each vial contained a unique code that ensured appropriate masking.
Procedures
BBV152 (manufactured by Bharat Biotech) is a whole-virion β-propiolactone-inactivated SARS-CoV-2 vaccine. The NIV-2020-770 strain was isolated from a patient with COVID-19, sequenced at the Indian Council of Medical Research-National Institute of Virology, and provided to Bharat Biotech.10 Biosafety level 3 manufacturing facilities and a well established Vero cell manufacturing platform aided the rapid development of BBV152. The NIV-2020-770 strain contains the Asp614Gly mutation, which is characterised by an aspartic acid to glycine shift at amino acid position 614 of the spike protein.10 Studies suggest that the mutation is associated with higher viral loads in patients and animal models compared with the wild-type strain11 and that NIV-2020-770 is considered to be the dominant strain in the pandemic.12
The candidates were formulated with the Algel-IMDG adjuvant, which is an imidazoquinoline class molecule (TLR 7/8 agonist) adsorbed onto Algel. After confirming their eligibility, participants were randomly assigned to the two groups. Both vaccines were stored at 2–8°C in a single-use glass vial. The appearance, colour, and viscosity of the two formulations were identical.
Vaccines were provided as a sterile liquid that was injected through an intramuscular route (deltoid muscle) at a volume of 0·5 mL per dose in a two-dose regimen on day 0 and day 28. Each glass vial contained a single dose of one of the vaccine formulations and required no additional dilution steps, therefore, no on-site dose preparation was required. No prophylactic medication (ibuprofen or acetaminophen) was prescribed either before or after vaccination. The follow-up visits were scheduled on days 42, 56, 118, and 208 after vaccination for blood collection.
Anti-IgG responses against the spike (S1) glycoprotein, receptor-binding domain (RBD), and nucleocapsid protein of SARS-CoV-2 were assessed by ELISA (Syngene, Bangalore, India), and are expressed as geometric mean titres (GMTs). Wild-type virus neutralising antibody titres in serum samples were analysed with a microneutralisation test (MNT50) and a plaque-reduction neutralisation test (PRNT50) at Bharat Biotech in a masked manner. MNT50 and PRNT50 were developed in-house. Seroconversion was defined as a post-vaccination titre at least four-fold higher than the pre-vaccination titre. To ensure the validity of our assay, an arbitrary number of serum samples (n=40) were selected at random and tested by PRNT50 at the National Institute of Virology.
Due to the absence of established SARS-CoV-2-specific correlates of protection, to compare vaccine-induced immune responses with those elicited by natural SARS-CoV-2 infections, 50 convalescent serum samples (collected 1–2 months after a nucleic acid test-based diagnosis) were tested by PRNT50 and MNT50. These serum samples were collected from self-reported symptomatic (n=35) and asymptomatic (n=15) adult (age-range not known) patients with COVID-19, and were provided by the National Institute of Virology (Pune, India). For symptomatic patients, ascertainment of severity grading and the requirement for supplemental oxygen was not available.
Cell-mediated responses were assessed in a subset of participants at three sites on day 42 and day 56. The contract research organisation generated a random code containing randomisation numbers, which was provided to the staff to identify this subset of participants. Blood (3–5 mL) was collected from participants who consented to have additional blood volume collected on day 42. Serum was used to evaluate Th1-dependent and Th2-dependent antibody isotypes, and peripheral blood mononuclear cells (PBMCs) were used to assess Th1 and Th2 cytokines. PBMCs were collected from 58 participants (29 from each group). Ten pre-vaccination samples (five from each group) collected on day 0 served as the control. Formal sample size estimations for cell-mediated responses were not done. PBMCs collected on day 42 were used to assess Th1 (interferon-γ [IFNγ], tumour necrosis factor-α [TNFα], and IL-2) and Th2 (IL-5, IL-10, and IL-13) cytokines using a Luminex multiplex assay (Luminex Corporation, Austin, TX, USA) at Indoor Biotechnologies (Bangalore, India).
PBMCs collected on day 56 were used to assess Th1 and Th2 cytokines using a cytokine bead array multiplex assay (CBA Kit, BD Biosciences, New Jersey, USA). These tests were done at Bharat Biotech.
PBMCs from a subset of randomly selected participants who consented to the additional blood volume were collected on day 104 of the phase 1 trial, and used to assess T-cell memory responses (CD4+CD45RO+ T cells and CD4+CD45RO+CD27+ T cells) at Bharat Biotech. Wild-type virus neutralisation assays (GMTs and seroconversion [MNT50] assays) were done in phase 1 participants at day 104. After antigen stimulation of these PBMCs, culture supernatants were collected on day 3 to assess cytokines and secreted SARS-CoV-2 IgG antibodies (by ELISA) on day 6. All samples were analysed in a masked manner. The details of all assay methods can be found in appendix 3 (pp 3–4).
Outcomes
The primary outcome was SARS-CoV-2 wild-type neutralising antibody titres and seroconversion rates at 4 weeks after the second dose (day 56).
A key secondary outcome was the number and proportion of participants with solicited local and systemic reactogenicity. Participants were observed for 2 h post-vaccination to assess reactogenicity. They were instructed to record local and systemic reactions within 7 days (days 0–7 and days 28–35) post-vaccination using a paper-based memory aid. The memory aid contained fields for symptom onset, severity, time to resolution, and concomitant medications, and participants were instructed to complete the form daily. The form was collected during the next visit to the site. Routine telephone calls were scheduled following the first 7 days after each vaccination. Solicited local adverse events were pain and swelling at the injection site, and systemic adverse events were fever, fatigue or malaise, myalgia, body aches, headache, nausea or vomiting, anorexia, chills, generalised rash, and diarrhoea. All unsolicited adverse events were reported by participants throughout the study. The grading scale for most adverse events was based on the US Food and Drug Administration (FDA) document for the toxicity grading scale for healthy adult and adolescent volunteers enrolled in preventive vaccine clinical trials. Adverse events for which grading was not described in the FDA guidance document were graded by use of the Common Terminology Criteria for Adverse Events. Adverse events were graded according to severity score (mild, moderate, or severe) and whether they were related or unrelated to the investigational vaccine, as detailed in the protocol (appendix 2).
Statistical analysis
We calculated that 171 participants per group were required for 90% power to detect a significant difference between GMTs in two equally sized groups, assuming the log10 (titre) is normally distributed with an SD of 0·5, the true GMT ratio is 1·5, and the groups are compared with a two-sample t test on log10 (titre) at a two-sided 5% significance level. Assuming a 10% loss of participants due to drop-out during the study, the sample size was calculated as 190 participants in each group. Sample size was calculated by use of PASS 13 software (Number Cruncher Statistical Systems, Kaysville, UT, USA).
The primary outcome was assessed in all participants who received two doses of the vaccine. Safety was assessed in all participants who received at least one dose of the vaccine. Safety endpoints are described as frequencies. GMTs with 95% CIs are presented for immunological endpoints. For continuous variables (those with <20 observations), medians and IQRs are reported. The exact binomial calculation was used for the CI estimation of proportions. Wilson's test was used to test differences in proportions. CI estimation for the GMT was based on the log10 (titre) and the assumption that the log10 (titre) was normally distributed. GMTs were compared with t tests using the means of the log10 (titre). Significance was set at p<0·05 (two-sided).
This preliminary report presents results regarding immunogenicity (days 0–56) and safety outcomes (days 0–42). Descriptive and inferential statistics were assessed using SAS, version 9.2.
The trial is registered at ClinicalTrials.gov, NCT04471519.
Role of the funding source
The funder of the study had no role in data collection, data analysis, data interpretation, or writing of this report or the statistical report, but was involved in study design. Data cleaning and analysis was done by the third party contract research organisation (Sclin Soft Technologies). Masked laboratory assessments were done at the respective laboratories, and masked datasheets were sent to the contract research organisation for decoding and analysis. The unmasked randomisation list was not shared with the study sponsor.
Results
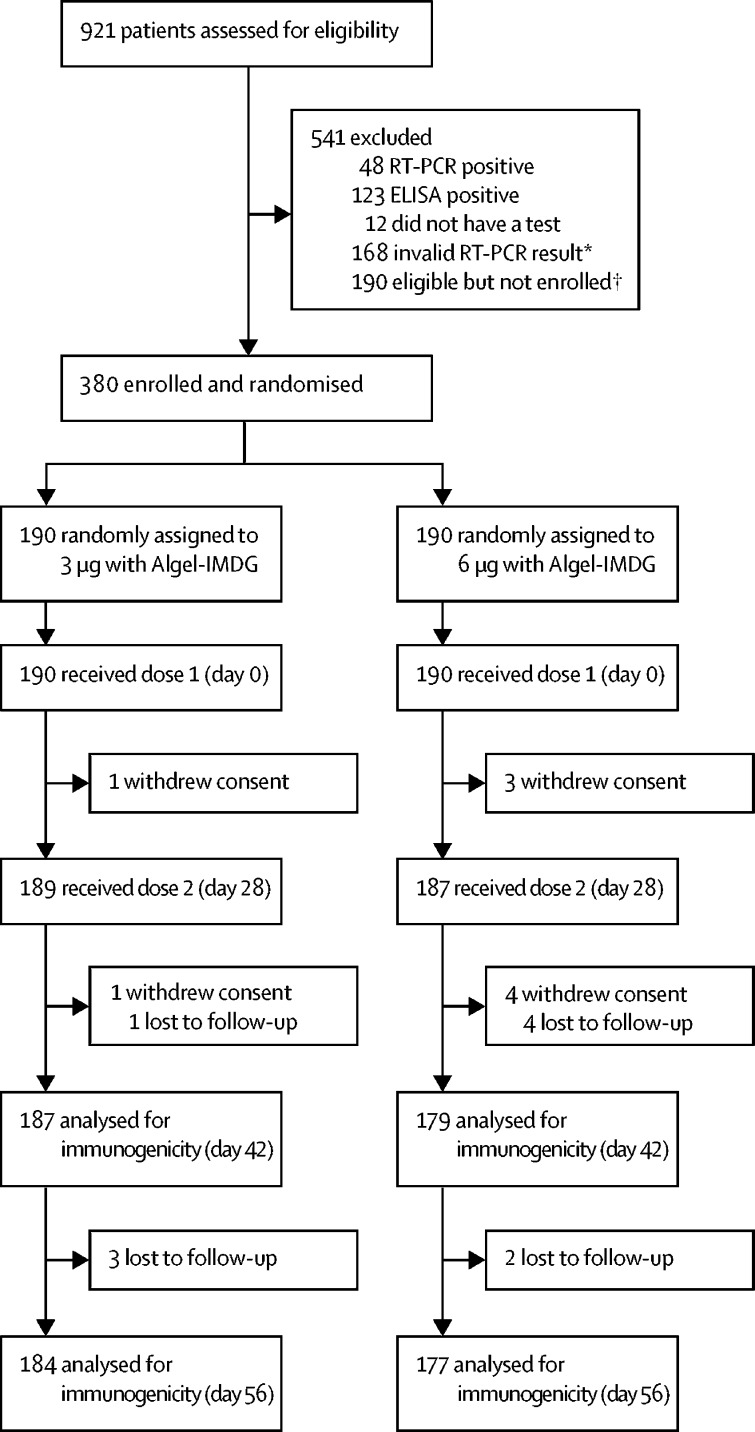
Between Sept 5 and 12, 2020, 921 potential participants were screened, 380 of whom were enrolled and randomly assigned to either the 3 μg with Algel-IMDG group (n=190) or the 6 μg with Algel-IMDG group (n=190; figure 1 ). Among the 541 individuals who were initially screened but excluded, 48 had positive nucleic acid tests and 123 had positive serology tests for SARS-CoV-2. Due to competitive recruitment, 190 individuals who were screened and found to be eligible were not enrolled. Other notable exclusions were due to inconclusive RT-PCR results (n=168). The retention rates at day 56 were 97% (184 of 190 participants) in the 3 μg with Algel-IMDG group and 93% (177 of 190 participants) in the 6 μg with Algel-IMDG group. The demographic characteristics of participants are shown in table 1 .
Figure 1.
Trial profile
*Caused by cold chain excursions during transport of the nasopharyngeal swabs from the field site to the central laboratory. †Due to competitive recruitment, all sites were screening participants individually; therefore, there was an excess of eligible participants who were not enrolled because the recruitment target was met.
Table 1.
Demographics of participants in the intention-to-treat population
| 3 μg with Algel-IMDG (n=190) | 6 μg with Algel-IMDG (n=190) | ||
|---|---|---|---|
| Age, years | |||
| Median | 34·0 (26·0–41·8) | 35·0 (27·0–44·0) | |
| ≥12 to <18 | 10 (5%) | 4 (2%) | |
| ≥18 to <55 | 173 (91%) | 176 (93%) | |
| ≥55 to ≤65 | 7 (4%) | 10 (5%) | |
| Sex | |||
| Female | 50 (26%) | 45 (24%) | |
| Male | 140 (74%) | 145 (76%) | |
| Body-mass index*, kg/m2 | 25·1 (3·4) | 24·9 (2·8) | |
Data are median (IQR), n (%), or mean (SD). The intention-to-treat population included all participants who received at least one dose.
Calculated by the participant's weight (kg) divided by the square of their height (m), measured at the time of screening.
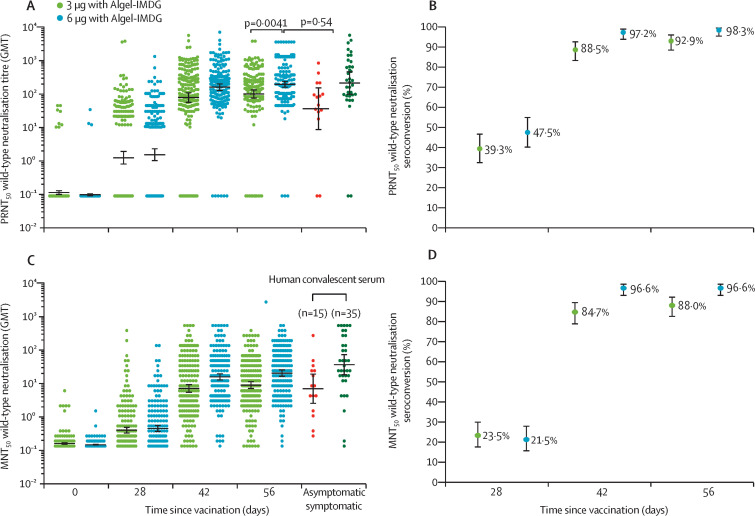
GMTs (PRNT50) at day 0 were 0·1 (95% CI 0·1–0·1) in both groups, increasing to 100·9 (74·1–137·4) in the 3 μg with Algel-IMDG group and 197·0 (155·6–249·4) in the 6 μg with Algel-IMDG group at day 56 (figure 2A ). The GMT (PRNT50) at day 56 was significantly higher in the 6 μg with Algel-IMDG group than in the 3 μg with Algel-IMDG group (p=0·0041), and was not significantly different to the GMT (PRNT50) observed in convalescent serum collected from patients who had recovered from COVID-19 (p=0·54). Seroconversion based on PRNT50 at day 56 was reported in 171 (92·9% [95% CI 88·2–96·2] of 184 participants in the 3 μg with Algel-IMDG group and 174 (98·3% [95·1–99·6]) of 177 participants in the 6 μg with Algel-IMDG group (figure 2B).
Figure 2.
SARS-CoV-2 wild-type PRNT50 GMTs (A), and seroconversion rates (B), and wild-type MNT50 GMTs (C) and seroconversion rates (D)
SARS-CoV-2 wild-type PRNT50and MNT50 GMTs at baseline (day 0), 4 weeks after the first vaccination (day 28), 2 weeks after the second vaccination (day 42), and 4 weeks after the second vaccination (day 56) in the 3 μg with Algel-IMDG (n=190) and 6 μg (n=190) with Algel-IMDG groups are shown. Seroconversion rates were defined by the proportion of post-vaccination titres that were at least four-fold higher than baseline. In A and C, the human convalescent sera panel included specimens from participants with PCR-confirmed symptomatic and asymptomatic COVID-19 obtained at least 30–60 days after diagnosis (50 samples); dots represent individual datapoints, the horizontal bars show the GMTs, and the error bars represent the 95% CIs. In B and D, the dots represent the seroconversion rates and error bars represent 95% CIs. PRNT50=plaque-reduction neutralisation test. GMT=geometric mean titre. MNT50=microneutralisation assay.
GMTs (MNT50) at day 56 were 92·5 (95% CI 77·7–110·2) in the 3 μg with Algel-IMDG group and 160·1 (135·8–188·8) in the 6 μg with Algel-IMDG group (figure 2C). Seroconversion based on MNT50 at day 56 was reported in 162 (88·0% [95% CI 82·4–92·3]) of 184 participants in the 3 μg with Algel-IMDG group and 171 (96·6% [92·8–98·8]) of 177 participants in the 6 μg with Algel-IMDG group (figure 2D; appendix 3, p 6). The PRNT50 and MNT50GMTs at day 56 were significantly higher in the 6 μg with Algel-IMDG group than the 3 μg with Algel-IMDG group. No differences in the GMTs (PRNT50) were observed in a subset of paired serum samples from both groups (20 samples from each group) analysed at the National Institute of Virology and Bharat Biotech on day 42 (2 weeks after the second vaccination; appendix 3, p 13). Seroconversion rates and GMTs across three age groups (≥12 to <18 year, ≥18 to <55 year, and ≥55 to ≤65 year groups) and between both sexes were similar, but only small numbers of participants were included in the youngest and oldest age groups (appendix 3, p 7).
IgG antibody titres (GMTs) to all epitopes (spike glycoprotein, receptor-binding domain, and nucleocapsid protein) were detected after the administration of both doses (table 2 ). Anti-spike glycoprotein IgG GMTs at day 56 were 10 413·9 (95% CI 9142·4–11 862·2) in the 3 μg with Algel-IMDG group and 9541·6 (8245·9–11 041·0) in the 6 μg with Algel-IMDG group. Both the 3 μg and 6 μg with Algel-IMDG groups showed similar anti-spike glycoprotein, anti-receptor-binding domain, and anti-nucleocapsid protein GMTs. At day 42, the anti-spike isotype mean ratios (IgG1/IgG4) were 2·4 (95% CI 1·9–2·9) in the 3 μg with Algel-IMDG group and 2·2 (1·7–2·6) in the 6 μg with Algel-IMDG group.
Table 2.
SARS-CoV-2 IgG titres against the spike glycoprotein, receptor-binding domain, and nucleocapsid protein
|
Geometric mean titre (95% CI) |
Seroconversion rate*(95% CI) |
|||
|---|---|---|---|---|
| 3 μg with Algel-IMDG | 6 μg with Algel-IMDG | 3 μg with Algel-IMDG | 6 μg with Algel-IMDG | |
| Anti-spike glycoprotein IgG | ||||
| Day 0 | 500·0 (500·0–500·0) | 500·0 (500·0–500·0) | .. | .. |
| Day 28 | 2574·2 (2228·9–2973·1) | 2240·5 (1942·4–2584·5) | 71·2% (64·1–77·6) | 65·0% (57·5–72·0) |
| Day 42 | 11528·8 (10 002·7–13 287·8) | 10040·0 (8667·0–11 630·5) | 98·4% (95·3–99·7) | 98·3% (95·1–99·7) |
| Day 56 | 10413·9 (9142·4–11 862·2) | 9541·6 (8245·9–11 041·0) | 98·4% (95·3–99·7) | 96·6% (92·8–98·8) |
| Anti-receptor binding domain IgG | ||||
| Day 0 | 500·0 (500·0–500·0) | 500·0 (500·0–500·0) | .. | .. |
| Day 28 | 1962·7 (1726·2–2231·6) | 2031·6 (1777·3–2322·3) | 58·7% (51·2–65·9) | 58·2% (50·6–65·6) |
| Day 42 | 5572·3 (4897·5, 6339·9) | 4980·8 (4366·7, 5681·3) | 94·0% (89·6, 97·0) | 93·2% (88·5, 96·5) |
| Day 56 | 5874·0 (5194·8, 6642·0) | 5558·0 (4859·9, 6356·5) | 96·2% (92·3, 98·5) | 94·4% (89·9, 97·3) |
| Anti-nucleocapsid protein IgG | ||||
| Day 0 | 500·0 (500·0–500·0) | 500·0 (500·0–500·0) | .. | .. |
| Day 28 | 2734·1 (2375·1–3147·5) | 2490·4 (2161·7–2869·2) | 72·3% (65·2–78·6) | 71·2% (63·9–77·7) |
| Day 42 | 8957·2 (7778·6–10314·3) | 9211·2 (7939·3–10 686·8) | 97·3% (93·8–99·1) | 95·5% (91·3–98·0) |
| Day 56 | 8626·0 (7528·6–9883·4) | 8754·0 (7589·4–10 097·4) | 97·3% (95·3–100·0) | 96·6% (92·8–98·8) |
ELISA results at baseline (day 0), 4 weeks after the first vaccination (day 28), 2 weeks after the second vaccination (day 42), and 4 weeks after the second vaccination (day 56) for the 3 μg with Algel-IMDG and the 6 μg with Algel-IMDG groups are shown. The number of participants in the 3 μg with Algel-IMDG group included in the immunogenicity analysis was 190 on day 0, 189 on day 28, 187 on day 42, and 184 on day 56. The number of participants in the 6 μg with Algel-IMDG group included in the immunogenicity analysis was 190 on day 0, 187 on day 28, 179 on day 42, and 177 on day 56. The cutoff for detectable antibodies was 1/500. Endpoint titre dilution for days 28, 42, and 56 sera samples were established with baseline (day 0) and interpolated from the raw optical density data of the corresponding day 0 sample. The cutoff (mean ±3 SD) for day 0 was calculated considering the absorbance of all sera dilutions (1/500 to 1/32 000) tested, except the lowest dilution (1/500).
Defined as a post-vaccination IgG titre that was at least four-fold higher than the baseline titre.
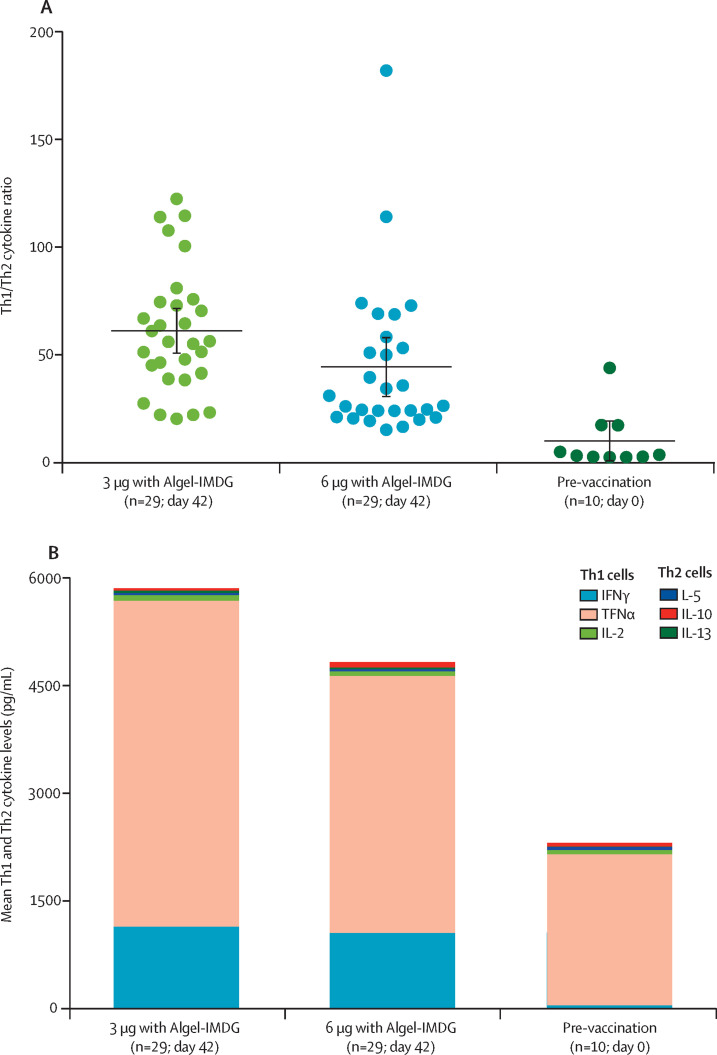
The Th1/Th2 cytokine ratio indicated bias to a Th1 cell response at day 42 (figure 3A ). Th2 responses were detected at minimal levels in both vaccine groups, as shown by IL-5, IL-10, and IL-13 levels (figure 3B). We observed a significant increase in the levels of Th1 cytokines, IFNγ, IL-2, and TNFα, on day 56 compared with day 0 (p<0·0001), as measured with the Luminex multiplex assay (appendix 3, p 12).
Figure 3.
Th1/Th2 cytokine ratios (A) and mean Th1 and Th2 cytokine levels (B) at day 42 in phase 2 participants
In A and B, cell-mediated responses in blood samples from 58 participants (29 each from the 3 μg with Algel-IMDG and 6 μg with Algel-IMDG groups), with proliferative responses to vaccination at 2 weeks after the second dose (day 42), and in ten control participants (five pre-vaccination samples from each group) are shown. In A, the Th1/Th2 ratio was calculated by the sum of IFNγ plus IL-2 cytokine levels divided by the sum of IL-5 plus IL-13 cytokine levels; horizontal bars show the mean ratios and error bars show the 95% CIs. In B, mean cytokine levels in the cell culture supernatants obtained from PBMCs stimulated with SARS-CoV-2 peptides are shown; Th1 (IFNγ, IL-2, and TNFα) and Th2 (IL-5, IL-13, and IL-10) cytokines are represented by stacked bars. Th=T-helper. IFNγ=interferon-γ. TNFα=tumour necrosis factor-α. IL=interleukin.
Solicited local adverse reactions after dose 1 (days 0–7) were reported in nine (4·7% [95% CI 2·2–8·8]) of 190 participants in the 3 μg with Algel-IMDG group and eight (4·2% [1·8–8·1]) of 190 participants in the 6 μg with Algel-IMDG group (table 3 ). Solicited systemic adverse reactions after dose 1 were reported in nine (4·7% [2·2–8·8]) participants in the 3 μg with Algel-IMDG group and 14 (7·4% [4·1–12·1]) participants in the 6 μg with Algel-IMDG group. Solicited local adverse reactions after dose 2 (days 28–35) were reported in eight (4·2% [1·8–8·1]) participants in the 3 μg with Algel-IMDG group and seven (3·7% [1·6–7·7]) participants in the 6 μg with Algel-IMDG group. Solicited systemic adverse reactions after dose 2 were reported in 12 (6·3% [3·3–10·8]) participants in the 3 μg with Algel-IMDG group and 11 (5·8% [3·0–10·1]) participants in the 6 μg with Algel-IMDG group (table 3; unsolicited adverse events are included in appendix 3, p 9).
Table 3.
Mild and moderate solicited adverse events in the safety analysis set
|
Dose 1 |
Dose 2 |
||||
|---|---|---|---|---|---|
| 3 μg with Algel-IMDG (n=190) | 6 μg with Algel-IMDG (n=190) | 3 μg with Algel-IMDG (n=190) | 6 μg with Algel-IMDG (n=190) | ||
| Local reactions | |||||
| Pain at injection site | |||||
| Mild | 5 (3%) | 6 (3%) | 7 (4%) | 4 (2%) | |
| Moderate | 1 (1%) | 0 | 0 | 1 (1%) | |
| Redness at injection site | |||||
| Mild | 1 (1%) | 1 (1%) | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | |
| Itching | |||||
| Mild | 1 (1%) | 1 (1%) | 0 | 2 (1%) | |
| Moderate | 0 | 0 | 0 | 0 | |
| Stiffness in upper arm | |||||
| Mild | 1 (1%) | 0 | 0 | 0 | |
| Moderate | 0 | 0 | 0 | 0 | |
| Weakness in injection arm | |||||
| Mild | 0 | 0 | 1 (1%) | 0 | |
| Moderate | 0 | 0 | 0 | 0 | |
| Systemic reactions | |||||
| Body ache | |||||
| Mild | 0 | 2 (1%) | 1 (1%) | 2 (1%) | |
| Moderate | 0 | 1 (1%) | 0 | 0 | |
| Fever | |||||
| Mild | 2 (1%) | 5 (3%) | 5 (3%) | 4 (2%) | |
| Moderate | 1 (1%) | 3 (2%) | 0 | 0 | |
| Headache | |||||
| Mild | 2 (1%) | 1 (1%) | 1 (1%) | 2 (1%) | |
| Moderate | 0 | 0 | 0 | 1 (1%) | |
| Malaise | |||||
| Mild | 4 (2%) | 1 (1%) | 3 (2%) | 0 | |
| Moderate | 0 | 0 | 0 | 0 | |
| Weakness | |||||
| Mild | 0 | 0 | 1 (1%) | 2 (1%) | |
| Moderate | 0 | 1 (1%) | 0 | 0 | |
| Rashes | |||||
| Mild | 0 | 0 | 1 (1%) | 0 | |
| Moderate | 0 | 0 | .. | 0 | |
Data are n (%). The safety analysis set includes all participants who received one dose of the vaccine (n=380). The number of participants who had a solicited adverse event after receiving dose 1 (days 0–7) and dose 2 (days 28–35) is shown.
No association between the dose of vaccine and the number of adverse events was observed. After both doses, the most common solicited adverse events were injection site pain, reported in five (2·6% [95% CI 0·9–6·0]) of 190 participants in the 3 μg with Algel-IMDG group and six (3·2% [1·2–6·8]) of 190 participants in the 6 μg with Algel-IMDG group. Most adverse events were mild (69 [89%] of 78 participants) and resolved within 24 h of onset. At 7 days after the second dose, solicited local and systemic adverse reactions were reported in 38 (20·0% [14·7–26·5]) of 190 participants in the 3 μg with Algel-IMDG group and 40 (21·1% [15·6–27·7]) of 190 participants in the 6 μg with Algel-IMDG group.
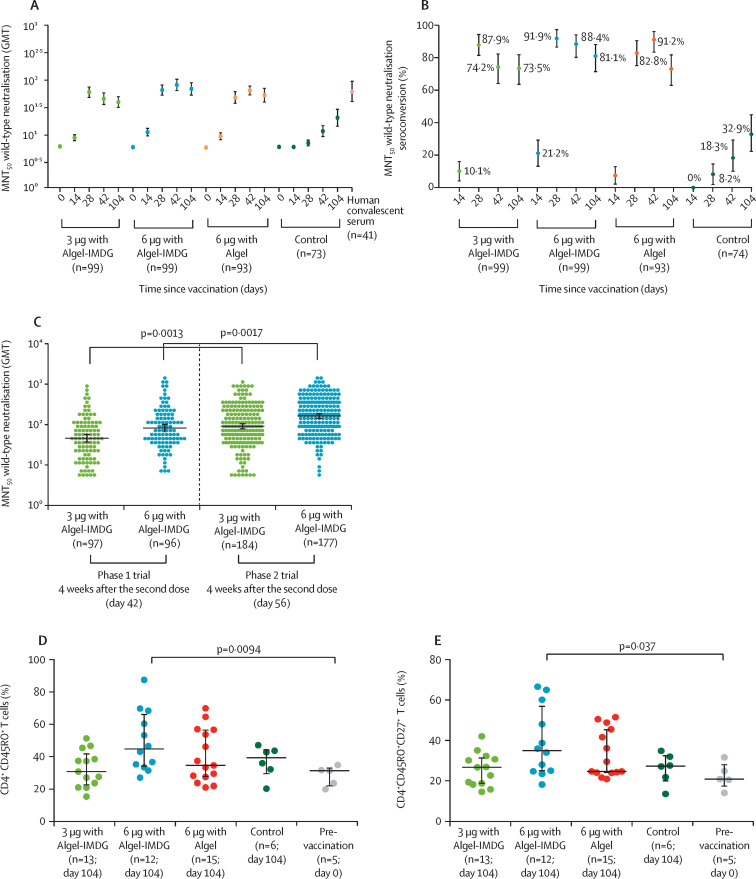
In the phase 1 trial, 97 (97%) of 100 participants in the 3 μg with Algel-IMDG group, 95 (95%) of 100 participants in the 6 μg with Algel-IMDG group, 92 (92%) of 100 participants in the 6 μg with Algel group, and 69 (92%) of 75 participants in the Algel-only control group were followed up to day 104 (3 months after the second dose). GMTs (MNT50) at day 104 were 39·9 (95% CI 32·0–49·9) in the 3 μg with Algel-IMDG group, 69·5 (53·7–89·9) in the 6 μg with Algel-IMDG group, 53·3 (40·1–71·0) in the 6 μg with Algel group, and 20·7 (14·5–29·5) in the Algel-only control group (figure 4A ). Seroconversion based on MNT50 was reported in 72 (73·5% [95% CI 63·6–81·9]) participants in the 3 μg with Algel-IMDG group, 76 (81·1% [71·4–88·1]) participants in the 6 μg with Algel-IMDG group, and 68 (73·1% [62·9–81·8]) participants in the 6 μg with Algel group (figure 4B). GMTs in the 6 μg with Algel-IMDG group were significantly higher than the 3 μg with Algel-IMDG group (appendix 3, p 7). There were no significant differences in GMTs between day 42 (2 weeks after the second dose) and 104 (3 months after the second dose) across the vaccine groups (appendix 3, p 7). Post-hoc analyses of MNT50 wild-type neutralising antibody responses in phase 1 and phase 2 participants in the 3 μg with Algel-IMDG and 6 μg with Algel-IMDG groups, showed that GMTs were significantly higher in phase 2 participants (at day 56) than in phase 1 participants (at day 42) at 4 weeks after receiving the second dose (figure 4C). At 4 weeks after the second dose of 6 μg with Algel-IMDG, the phase 1–2 GMT (MNT50) ratio was 1·9 (95% CI 1·5–2·6).
Figure 4.
SARS-CoV-2 wild-type MNT50 GMTs (A) and seroconversion rates (B) in phase 1 participants, SARS-CoV-2 wild-type MNT50 GMTs in phase 1 and phase 2 participants at 4 weeks after the second vaccination (C), and the proportion of CD4+ CD45RO+ (D) and CD4+ CD45RO+ CD27+ (E) T cells at day 104 in phase 1 participants
In the phase 1 trial, the dosing schedule was day 0 for the first dose of the vaccine and day 14 for the second dose. In the phase 2 trial, the dosing schedule was day 0 for the first dose of the vaccine and day 28 for the second dose. In A, results at baseline (day 0), 2 weeks after the second vaccination (day 28), 4 weeks after the second vaccination (day 42), and 3 months after the second vaccination (day 104) for the 3 μg and 6 μg with Algel-IMDG groups, the 6 μg with Algel group, and the Algel-only control group in the phase 1 trial are shown. The human convalescent serum panel included specimens from participants with PCR-confirmed symptomatic and asymptomatic COVID-19 obtained at least at least 30 days after diagnosis (41 samples). In B, seroconversion rates were defined by the proportion of post-vaccination titres that were at least four-fold higher than baseline. In D and E, the frequencies of antigen-specific T-cell memory responses at 3 months after the second dose (day 104) in all groups from the phase 1 trial are shown; dots are individual datapoints, and horizontal bars are medians with error bars for IQRs. GMT=geometric mean titre. MNT50=microneutralisation assay.
PBMCs from a subset of phase 1 participants at one site were collected to evaluate T-cell memory responses at day 104. Formulations with Algel-IMDG generated a T-cell memory response, as shown by an increase in the frequency of effector memory CD4+CD45RO+ T cells and CD4+CD45RO+CD27+ T cells compared with pre-vaccination (day 0) samples (figure 4D, E). Samples from Algel-alone recipients also showed a T-cell memory response. We also detected secreted IgG antibodies in the cell culture supernatant by ELISA, and the antibody titres ranged from neat (undiluted) to 1/64 (appendix 3 p 8). Further effector function of activated and differentiated T cells was shown by the levels of Th1 cytokines (appendix 3 p 8).
In phase 2 participants, nine (33%) of 27 unsolicited adverse events were reported to be related to the vaccine, as judged by a masked investigator. No significant difference in the number of unsolicited adverse events was observed between the groups (appendix 3 p 9). Severity grading scales for adverse events and the evaluation of adverse events related to the vaccine are described in appendix 3 (pp 10–11). No symptomatic SARS-CoV-2 infections were reported to the site investigators (via follow-up telephone calls or site visits) between days 0 and 118 (a scheduled visit) in phase 2 participants. However, illness visits were not scheduled and no routine SARS-CoV-2 nucleic acid testing was done. No serious adverse events were reported up to day 118 in phase 2 participants.
No new solicited or unsolicited adverse events that occurred between days 42 and 104 in phase 1 participants were considered to be related to the vaccine by the investigators. Additionally, no new serious adverse events were reported. One case of symptomatic COVID-19 was reported in the Algel-only control group. This participant received the first dose on July 17, 2020, but was considered to be lost to follow-up before the second dose was administered. The participant visited the site on Nov 27, 2020, with complaints of chronic anosmia and a history of a positive SARS-CoV-2 rapid antigen test on Aug 16, 2020.
Discussion
In this report, we present interim findings from the phase 2 clinical trial of BBV152, a whole-virion inactivated SARS-CoV-2 vaccine. The overall participant retention rates were 97% in the 3 μg with Algel-IMDG group and 93% in the 6 μg with Algel-IMDG group. Neutralising antibody titres were similar to a panel of convalescent serum samples. All elicited cytokine responses to BBV152 were biased to Th1 cells. The vaccine was well tolerated in both groups with no serious adverse events. Long-term follow-up of phase 1 trial participants showed that neutralising antibody titres persisted, and T-cell memory responses were more pronounced in the 6 μg with Algel-IMDG group compared with pre-vaccination samples.
The most common adverse event in the phase 2 trial was pain at the injection site, followed by headache, fatigue, and fever. No severe or life-threatening (ie, grade 4 and 5) solicited adverse events were reported. No significant differences in safety were observed between the two groups. However, the study was not powered to compare such differences. After either dose, the combined incidence of local and systemic adverse events in this study is lower than that of other SARS-CoV-2 vaccine platform candidates,13, 14, 15, 16 and similar to that of other inactivated SARS-CoV-2 vaccine candidates.17, 18 However, other vaccine studies have enrolled different populations and have employed varying approaches to measure adverse events.
BBV152 induced antibody binding (to spike glycoprotein and nucleocapsid protein epitopes) and neutralising antibody responses that were similar to those induced by other SARS-CoV-2 inactivated vaccine candidates.17, 18 Studies have reported the variable persistence of humoral and cell-mediated responses acquired from natural infection.19, 20 In the phase 1 trial of BBV152, we evaluated an accelerated schedule, in which the two doses were administered 2 weeks apart. At day 104 (3 months after the second dose), we observed detectable humoral and cell-mediated responses to SARS-CoV-2. Serum neutralising antibodies were detected in all phase 1 participants at day 104, and the levels of these antibodies were similar to the panel of convalescent serum samples. These findings are in accordance with those of the mRNA-1273 (Moderna) vaccine, which has received emergency use authorisation.2, 21 A sizeable T-cell memory population was also observed at this timepoint. A routine schedule, in which the two doses are administered 4 weeks apart, was evaluated in the phase 2 trial of 3 μg with Algel-IMDG and 6 μg with Algel-IMDG. We found that immune responses (MNT50) were significantly higher with the routine schedule (phase 2) than with the accelerated schedule (phase 1), which is consistent with other reports.5, 22
BBV152 is a whole-virion inactivated SARS-CoV-2 vaccine adjuvanted with Algel-IMDG. An imidazoquinoline molecule (IMDG), which is a TLR7/8 agonist, has been used to augment cell-mediated responses.23, 24 Both 3 μg with Algel-IMDG and 6 μg with Algel-IMDG formulations induced responses that were biased to a Th1 phenotype, with IgG1/IgG4 ratios greater than 1. The ratio of Th1/Th2 cytokines was clearly biased to a Th1 response, with increased IFNγ generation.
In the present study, BBV152 induced T-cell memory responses, which was shown by an increased frequency of antigen-specific CD4+ T cells expressing the memory phenotype marker CD45RO+. The increase in the CD4+CD45RO+CD27+ T-cell population also indicates the activation of the co-stimulatory marker CD27, and confirms the antigen recall memory T-cell response.25 Further, the effector function of these cells was supported by the Th1 cytokine secretion observed in ex vivo responses after stimulation of PBMCs for 3 days.26 These results further corroborate our phase 1 results showing an increased frequency of CD4+ T cells producing IFNγ in participants who received Algel-IMDG-containing formulations. Samples from participants in the Algel-only control group also showed a T-cell memory response, which corroborates a recent study published in 2020 indicating the presence of cross-reactive T-cells in individuals unexposed to SARS-CoV-2.27 Additionally, two participants in the Algel-only group showed high neutralising antibody titres and IL-6 levels at day 104 of the phase 1 study. In the phase 1 trial, the ability to secrete anti-spike glycoprotein IgG antibodies at day 104 further shows the long-lasting T-cell memory response generated by BBV152. Similar findings supporting long-term immunity were reported by Sekine and colleagues28 in convalescent patients who had previously had COVID-19. Memory B-cell responses from BBV152 are currently being evaluated. Thus far, cell-mediated responses after receipt of other SARS-CoV-2 inactivated vaccine candidates have been minimally reported.
This study was done at a time when the number of daily diagnosed COVID-19 cases was increasing rapidly. In the Algel-only control group (phase 1 trial), seroconversion was reported in six (8·2% [95% CI 1·9–14·5]) of 73 participants at day 28, 13 (18·8% [10·8–30·4]) of 69 participants at day 42, and 23 (33·3% [22·7–45·8]) of 69 participants at day 104. These results suggest that both phase 1 and 2 trials are being done during a period of high ongoing SARS-CoV-2 circulation. Since substantial SARS-CoV-2 PCR positivity was observed in the general population during the study period, in the event of natural exposure to SARS-CoV-2, it is possible that post-vaccination antibody titres in vaccinated participants could be slightly inflated. No cases of COVID-19 were reported in either group of the phase 2 trial, whereas one case of symptomatic COVID-19 was reported in the Algel-only control group of the phase 1 trial. However, illness visits were not scheduled, and routine SARS-CoV-2 nucleic acid testing was not done.
The results reported in this study do not permit efficacy assessments. The evaluation of safety outcomes requires extensive phase 3 clinical trials. We were unable to assess other immune responses (ie, binding antibody and cell-mediated responses) in convalescent serum samples due to the low quantity. Furthermore, no additional data on the age of the participant or the severity of disease from symptomatic individuals were obtained. Comparisons between phase 1 and 2 trials were not done in a randomised set of participants, and no adjustments on baseline parameters were made. Conclusions are to be considered as post-hoc analyses. Even though direct comparisons between the phase 1 and 2 trials cannot be made, the reactogenicity assessments reported in this study were substantially better in the phase 2 trial than the phase 1 trial and other trials with a placebo group.9 Additionally, the proportion of participants reporting adverse events in the phase 2 trial were lower than in the phase 1 trial. The study coordinators had verified all source documents to ensure that no data were missing or that errors had occurred. Further corroboration with phase 3 safety results is required. This study enrolled a small number of participants aged 12–18 years and 55–65 years. Follow-on studies are required to establish immunogenicity in children and in those aged 65 years and older. Withdrawals in the 6 μg with Algel-IMDG group were higher than the 3 μg with Algel-IMDG group but were not associated with adverse events. Lastly, this study population lacked ethnic, racial, and gender diversity, further underscoring the importance of evaluating BBV152 in other populations. Longitudinal follow-up of additional post-vaccination visits (at months 3, 6, and 12) is important for understanding the durability of immune responses, and is ongoing.
This study has several strengths. To ensure generalisability of the results, this study included participants from diverse geographic locations, enrolling 380 participants across nine hospitals across nine states in India. Based on follow-up data from the phase 1 trial, despite a marginal expected decline in neutralising antibody titres at day 104, BBV152 has shown the potential to provide durable humoral and cell-mediated immune responses. With several reports questioning the efficacy of SARS-CoV-2 vaccines against antigenically divergent strains, we previously reported neutralising antibody responses in homologous and heterologous strain assessments.9 Day 56 serum samples from 38 participants in the 6 μg with Algel-IMDG group of the phase 2 trial effectively neutralised a SARS-CoV-2 variant of concern (lineage B.1.1.7 or 20B/501Y. V1).29 On the basis of superior cell-mediated responses in the phase 1 trial, the 6 μg with Algel-IMDG formulation was selected for the phase 3 efficacy trial, which involves 25 800 volunteers and is currently underway (NCT04641481). BBV152 (COVAXIN) has received emergency use authorisation in India.
Data sharing
The study protocol is provided in appendix 2. De-identified, individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after the approval of a proposal, data can be shared through a secure online platform.
Acknowledgments
Acknowledgments
This work was supported and funded by Bharat Biotech International. We sincerely thank the principal and co-principal investigators, study coordinators, and health-care workers who were involved in this study. We express our gratitude to Sivasankar Baalasubramaniam from Indoor Biotechnologies (Bangalore), who assisted with cell-mediated response analyses. A special thanks to Arjun Dang and Leena Chatterjee of Dr Dangs Lab (New Delhi, India), which was the central laboratory for clinical laboratory testing. We appreciate the guidance from William Blackwelder on sample size estimation and statistical analysis planning. Shashi Kanth Muni, Sapan Kumar Behera, Jagadish Kumar, Vinay Aileni, Sandya Rani, Aparna Bathula, Amaravani Pittala of Bharat Biotech participated in protocol design and clinical trial monitoring. We thank Rakeshchandra Meka and Ramulu Chintala, and Spandana Sure of Bharat Biotech for cell-mediated assessments. This vaccine candidate could not have been developed without the efforts of Bharat Biotech's manufacturing, quality control teams. All authors would like to express their gratitude to all frontline health-care workers during this pandemic.
Contributors
All authors met the criteria for authorship set forth by the International Committee for Medical Editors. HJ, DD, DR, UP, BG, PY, and GS did the immunogenicity experiments. The contract research organisation (Sclin Soft Technologies) was responsible for analysing the data and generating the report. KMV, SRe, VS, SPr, and RE contributed to the manuscript preparation. SRe was the study coordinator and helped immensely with designing the protocol and generating the interim report. PA, SPr, NG, and BB from the Indian Council of Medical Research contributed to the writing of this paper. KE was responsible for overall supervision of the project and review of the final paper. All principal investigators (PR, SV, SKR, CS, SVR, CSG, JSK, SM, VR, and RG) were involved in the scientific review of this paper. All authors and the contract research organisation had full access to masked data in the study and all authors had final responsibility for the decision to submit for publication.
Declaration of interests
RE, HJ, BG, KMV, SRe, DD, DR, UP, SPr, and VS are employees of Bharat Biotech, with no stock options or incentives. KE is the Chairman and Managing Director of Bharat Biotech. PY, GS, PA, NG, SPa, and BB are employees of the Indian Council of Medical Research. PR, SV, SKR, CS, SVR, CSG, JSK, SM, VR, and RG were principal investigators representing the study sites.
Supplementary Materials
References
- 1.WHO Draft landscape and tracker of COVID-19 candidate vaccines. Jan 21, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines
- 2.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xia S, Zhang Y, Wang Y, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganneru B, Jogdand H, Dharam VK, et al. Evaluation of safety and immunogenicity of an adjuvanted, TH-1 skewed, whole virion inactivated SARS-CoV-2 vaccine - BBV152. bioRxiv. 2020 doi: 10.1101/2020.09.09.285445. published online Sept 12. (preprint). [DOI] [Google Scholar]
- 7.Yadav P, Ella R, Kumar S, et al. Remarkable immunogenicity and protective efficacy of BBV152, an inactivated SARS-CoV-2 vaccine in rhesus macaques. Res Square. 2020 doi: 10.21203/rs.3.rs-65715/v1. published online Sept 10. (preprint). [DOI] [Google Scholar]
- 8.Mohandas S, Yadav PD, Shete-Aich A, et al. Immunogenicity and protective efficacy of BBV152, whole virion inactivated SARS-CoV-2 vaccine candidates in the Syrian hamster model. iScience. 2021;24 doi: 10.1016/j.isci.2021.102054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(20)30942-7. published online Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarkale P, Patil S, Yadav P, et al. First isolation of SARS-CoV-2 from clinical samples in India. Indian J Med Res. 2020;151:244–250. doi: 10.4103/ijmr.IJMR_1029_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng W, Liu G, Ma H, et al. Biochemical characterization of SARS-CoV-2 nucleocapsid protein. Biochem Biophys Res Commun. 2020;527:618–623. doi: 10.1016/j.bbrc.2020.04.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Xi H, Juhas M. Biosensing detection of the SARS-CoV-2 D614G mutation. Trends Genet. 2020 doi: 10.1016/j.tig.2020.12.004. published online Dec 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulligan MJ, Lyke KE, Kitchin N, et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 14.Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh EE, Frenck RW, Falsey AR, et al. Safety and Immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y-J, Zeng G, Pan H-X, et al. Immunogenicity and safety of a SARS-CoV-2 inactivated vaccine in healthy adults aged 18–59 years: report of the randomized, double-blind, and placebo-controlled phase 2 clinical trial. medRxiv. 2020 doi: 10.1101/2020.07.31.20161216. published online Aug 10. (preprint). [DOI] [Google Scholar]
- 18.Xia S, Duan K, Zhang Y, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA. 2020;324:951–960. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for greater than six months after infection. bioRxiv. 2020 doi: 10.1101/2020.11.15.383323. published online Dec 18. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2020;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, Zeng G, Pan H, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Philbin VJ, Dowling DJ, Gallington LC, et al. Imidazoquinoline Toll-like receptor 8 agonists activate human newborn monocytes and dendritic cells through adenosine-refractory and caspase-1-dependent pathways. J Allergy Clin Immunol. 2012;130:195–204.e9. doi: 10.1016/j.jaci.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shukla NM, Salunke DB, Balakrishna R, Mutz CA, Malladi SS, David SA. Potent adjuvanticity of a pure TLR7-agonistic imidazoquinoline dendrimer. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li CK-f, Wu H, Yan H, et al. T cell responses to whole SARS coronavirus in humans. J Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mahnke YD, Brodie TM, Sallusto F, Roederer M, Lugli E. The who's who of T-cell differentiation: human memory T-cell subsets. Eur J Immunol. 2013;43:2797–2809. doi: 10.1002/eji.201343751. [DOI] [PubMed] [Google Scholar]
- 27.Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183:158. doi: 10.1016/j.cell.2020.08.017. 68.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sapkal GN, Yadav PD, Ella R, et al. Neutralization of UK-variant VUI-202012/01 with COVAXIN vaccinated human serum. bioRxiv. 2021 doi: 10.1101/2021.01.26.426986. published online Jan 26. (preprint). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study protocol is provided in appendix 2. De-identified, individual participant data will be made available when the trial is complete, upon requests directed to the corresponding author; after the approval of a proposal, data can be shared through a secure online platform.