Abstract
Stroke affects primarily aged and co-morbid people, aspects not properly considered to date. Since angiogenesis/vasculogenesis are key processes for stroke recovery, we purposed to determine how different co-morbidities affect the outcome and angiogenesis/vasculogenesis, using a rodent model of metabolic syndrome, and by dynamic enhanced-contrast imaging (DCE-MRI) to assess its non-invasive potential to determine these processes. Twenty/twenty-two month-old corpulent (JCR:LA-Cp/Cp), a model of metabolic syndrome and lean rats were used. After inducing the experimental ischemia by transient MCAO, angiogenesis was analyzed by histology, vasculogenesis by determination of endothelial progenitor cells in peripheral blood by flow cytometry and evaluating their pro-angiogenic properties in culture and the vascular function by DCE-MRI at 3, 7 and 28 days after tMCAO. Our results show an increased infarct volume, BBB damage and an impaired outcome in corpulent rats compared with their lean counterparts. Corpulent rats also displayed worse post-stroke angiogenesis/vasculogenesis, outcome that translated in an impaired vascular function determined by DCE-MRI. These data confirm that outcome and angiogenesis/vasculogenesis induced by stroke in old rats are negatively affected by the co-morbidities present in the corpulent genotype and also that DCE-MRI might be a technique useful for the non-invasive evaluation of vascular function and angiogenesis processes.
Keywords: Stroke, co-morbidities, angiogenesis, vasculogenesis, DCE-MRI
Introduction
Stroke represents a devastating disease, with high mortality, adult disability, socioeconomic burden and poor treatment worldwide.1,2 Stroke is associated with ageing and different co-morbidities, factors not sufficiently considered and that could explain the failure in translation to the clinic. To bridge this gap, the consideration of these co-morbidities and the use of imaging techniques have been proposed.3,4 In fact, the influence of ageing on stroke outcome and its influence on neurorepair processes such as neurogenesis has been studied experimentally and clinically, demonstrating a worse prognosis and higher mortality, being worse in the presence of other age-related co-morbidities.5–9
Angiogenesis and vasculogenesis are key processes for the initiation of brain repair. Angiogenesis, involved in the formation of neovessels in the ischemic area, is rapidly initiated after stroke by the activation of transcription factors such as HIF-1α and by the increase of growth factors (GF) such as erythropoietin and vascular endothelial growth factor (VEGF).10,11 On the other hand, vasculogenesis, by which endothelial progenitor cells (EPCs) mobilize from the bone marrow (BM) to the ischemic brain, also contributes to the formation of neovessels in the affected area. This process also starts quickly after stroke, when different molecules (cytokines and GF) are released to promote an initial mobilization of EPCs to revascularize the ischemic region,12,13 followed by a decrease in EPC counts during the chronic phase.14
Of note, impairment of angiogenesis/vasculogenesis is believed to affect negatively stroke outcome.15,16 In fact, high levels of VEGF/SDF-1α and increased EPC levels in peripheral blood translates into lower mortality and better outcome in stroke patients,12,14,17,18 hence, the importance of a non-invasive assessment of these processes. In this context, dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a technique widely used in cancer to study the vasculature/vascular function of a tumor. With this technique, vasculature can be characterized by the acquisition of serial T1-weighted images before, during and after the intravenous administration of a contrast agent (CA), which produces an increase in tissue signal intensity.19,20 To date, within the field of stroke, this technique has been used solely to assess damage to the blood-brain barrier (BBB).21–23
Co-morbidities such as ageing, obesity, atherosclerosis, insulin resistance, separately, have been reported to affect negatively angiogenesis/vasculogenesis in different experimental/clinical studies, by reducing the release of GF and the expression of their receptors in endothelial cells, by increasing the oxidative stress and by reducing the number of migrating EPCs.24,25 However, it is unknown whether these co-morbidities, all present at the same time, may worsen these neurorepair processes in old individuals, a highly growing population segment nowadays. With this background, we purposed to analyze the influence of metabolic syndrome (MS) on angiogenesis/vasculogenesis and subsequent stroke outcome, as well as and for the first time, the potential use of DCE-MRI to determine the brain perfusion and vascular function after experimental stroke in aged and co-morbid animals. To this aim we used old (20-22 month-old JCR:LA-cp (Cp) homozygous rats, which spontaneously develop MS-like phenotype.26
Materials and methods
Animals
All experiments were performed using 20-22-month-old male, lean (JCR:LA-lean (cp/-), Corpulent (Cp; JCR:LA-cp (cp/cp)), with a weight of 500 g for lean and 1100 g for Cp animals. Cp rats are homozygous for the autosomal recessive cp gene (cp/cp), and spontaneously develop MS.26 Animals were allowed free access to food and water and were maintained under temperature, humidity, and light-controlled conditions. All experimental procedures were performed in accordance with the European Parliament and of the Council Directive 2010/63/EU and Spanish legislation (Real Decreto 53/2013) and were approved by the Ethics Committee on Animal Welfare of University Complutense (PROEX No. 052/15) and are reported according to Animal Research: Reporting of In Vivo Experiments ARRIVE guidelines (EU directive 2010/63/EU)
Focal cerebral ischemia, BrdU administration and experimental groups
To study the effect of different co-morbidities on post-stroke angiogenesis and vascular function in aged rats, lean and Cp animals were randomly distributed (coin toss procedure) in naïve or ischemic groups and euthanized at 3, 7 or 28d after stroke. Taking into account our previous experience with these animals and using the statistical tool http://www.biomath.info, a total of 34 lean and 34 Cp rats were used in order to reduce the number of animals (n = 8 for ischemic lean/CP rats per time point; n = 3 for naïve lean/Cp per time point).
Focal cerebral ischemia was induced in lean and Cp rats by 90 min transient occlusion of the left middle cerebral artery by ligature (tMCAO) as described previously.27 Briefly, all the surgical procedure was conducted under anesthesia with isoflurane 5% for induction and 2% for maintenance in a mix of O2 and NO (0.2/0.8 L/min) and the temperature was maintained at 37.0 ± 0.5°C using a servo-controlled rectal probe-heating pad. Cerebral ischemia was performed by a transient ligation of the trunk of the left middle cerebral artery (MCAO) with a 9–0 suture. After 90 min of MCA occlusion, the suture was removed allowing the artery to reperfuse and 0.05 mg/kg/12h during the first 24 h of buprenorphine was subcutaneously injected as systemic analgesia. It was decided, a priori, to exclude from the study those animals that showed brain hemorrhage at any time of the surgery or with no reperfusion. None of the animals were excluded and the survival rate was 100%. In the analysis of the vascular function determined by DCE-MRI, 2 Cp at 3d, 2 at 7d and 1 at 28d were excluded from the study due to movements of the animals within the magnet during the performance of this test.
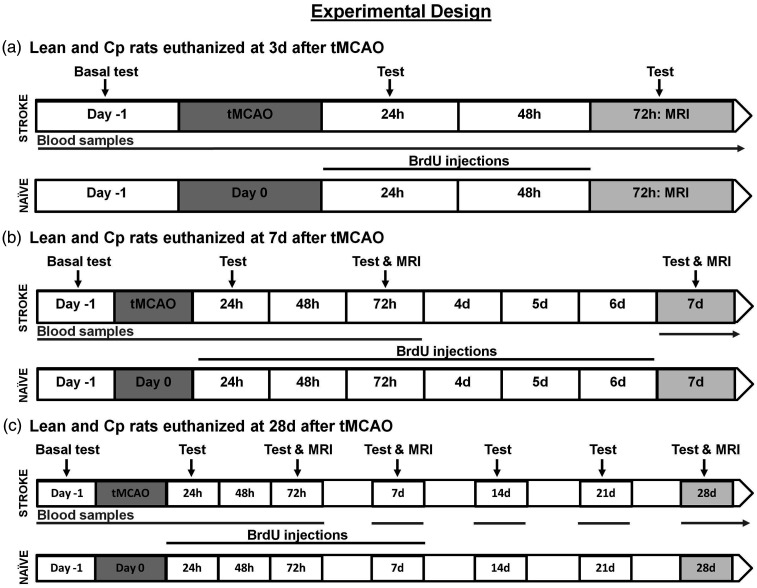
To examine the proliferation of endothelial cells and its evolution after stroke, animals were injected intraperitoneally with 50 mg/kg bromodeoxyuridine (BrdU; Sigma; UK) once a day on days 1 and 2 after tMCAO in animals euthanized at 3d, from 1 to 6 days in the groups at 7d and from 1 to 7 days in the 28d groups (Figure 1). Also, to analyze the baseline endothelial cell proliferation and brain vasculature before stroke, naïve lean and CP rats received the same BrdU injections as their corresponding stroke groups.
Figure 1.
Experimental design performed in ischemic and naïve 20–22 month-old lean/Cp rats (n = 8 and n = 3 per time point for ischemic and naïve groups respectively).
It is important to highlight that the determination of the infarct volume in T2-weighted brain images (T2-WI) was performed in all lean and Cp animals at 3d (n = 24 per experimental group) and at 7d, only those animals euthanized at this time point and at 28d (n = 16 per experimental group). Regarding the evaluation of the stroke outcome before and after cerebral ischemia, for lean and Cp animals, at basal, 24 h and 3d, all animals were analyzed (n = 24 per experimental group and time point), at 7d it was evaluated in animals euthanized at this time point and at 28d (n = 16) and for the rest of time points, only the animals sacrificed at 28d were considered (n = 8 at 14, 21 and 28d after stroke). For all the other determinations, the number of animals used per experimental group and time point appears in the corresponding section of materials and methods and in the figure legend.
Determination of infarct volume, brain perfusion and BBB damage by T2W-MRI and DCE-MRI
All the MRI studies were performed on a Bruker Biospec BMT 47/40 system (Bruker, Ettlingen, Germany) operating at 4.7 T. For the aged lean animals a 7 cm birdcage volume coil (Bruker Biospin, Ettigen, Germany) was used. For aged and MS Cp rats, due to their weight and size, a 5-cm anatomically shaped homemade surface coil was used. First, in all the animals at 3d after stroke, the reperfusion of the MCA was confirmed by magnetic resonance angiography (data not shown). The infarct volume was assessed in lean/Cp rats on T2-W brain images at 3 and 7d after stroke. At 28d after stroke, infarct volume was assessed in brain sections by Nissl staining as previously described.27 Also, in all the groups and time points, infarct volume was calculated as described by Hernandez-Jimenez in 2013.28
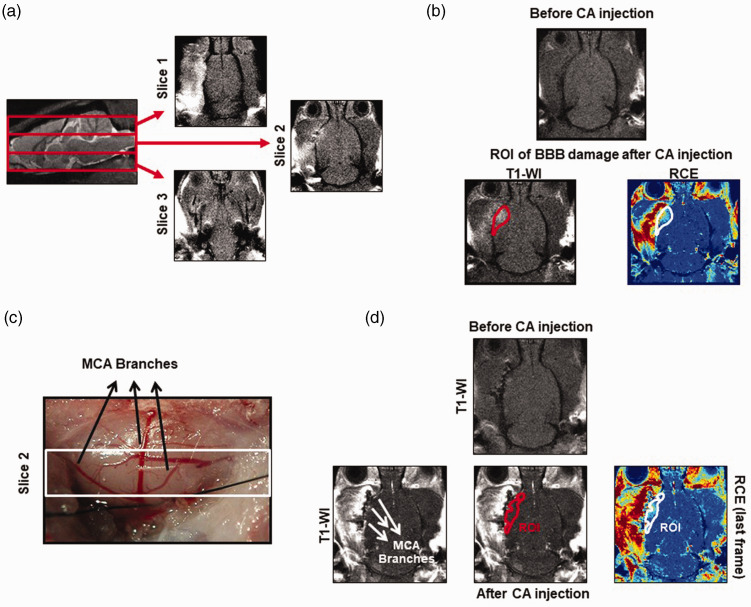
By DCE-MRI, brain perfusion, vascular function and BBB leakage were analyzed in naïve and ischemic animals at 3, 7 and 28d after stroke (Figure 1). Briefly, on anesthetized lean/cp rats, a 24 G intravenous (i.v.) cannula (Surflo® i.v. catheter; Terumo Europe) coupled to a syringe loaded with the contrast agent (CA; Gd-DTPA; Magnevist; 0.1 mmol/kg) was inserted into the tail vein. Once each animal was placed inside the magnet and the brain was located (region of interest, ROI), series of 60 repetitions of T1-weighted brain images (T1-WI), 4 before and 56 after the CA injection, were acquired with fat suppression, a repetition time of 200 ms and 9 ms of echo time; the number of averages was 2 and the matrix size 128x128. The field of view varied between 3 × 3 and 3.5 × 3.5 cm2, 3 coronal brain slices with 3 mm of thickness, covering the whole brain, were acquired and the total scan time was 52 s per repetition (Figure 2(a)). With this protocol, in the last frame of our DCE-MRI study, the BBB damage was determined by the measurement of the bright area with extravasated CA per section and, considering the 3 coronal sections of the brain, the volume of BBB damage was calculated as previously described22 (Figure 2(b)). This result was confirmed by immunohistochemical staining of endogenous rat immunoglobin G (IgG) as described previously.29 The analysis of the infarct volume and the BBB damage were performed using ImageJ software (U.S. National Institutes of Health, MD, USA). In order to analyze vascular density and function by DCE-MRI after stroke, first a region of interest (ROI) was manually drawn on each animal inside the infarct area within the territory of the MCA by the use of the IDL software (L3HARRIS™, USA). In this ROI selection, the MCA branches were excluded, to reduce the noise caused by this large vessel, and determine different parameters within the parenchyma affected by the ischemia (Figure 2(c) and (d)). Then, different semi-quantitative descriptors of brain perfusion19 were determined on the curve of relative contrast enhancement (RCE), quantified as the change in signal intensity, divided by the average signal over the frames before injection of the CA. These descriptors were the peak of the RCE (i.e., the maximum value reached on the RCE curve), the time to peak of the RCE from the injection of the CA, the clearance slope of the curve (ratio between the average signal of the first 5 frames and the last 5 frames after the injection of CA), and the area under the curve (AUC), integrating the RCE curve over the first 60 frames after injection of the CA. The RCE peak and time to peak give values about vascular density and vascular function inside the ROI, the clearance slope gives information about the vascular function and finally the AUC offers information of both function and vascular permeability.19
Figure 2.
ROI selection details to determine BBB damage and vascular parameters by DCE-MRI. (a) Images showing the 3 coronal sections with 3 mm of thickness in which the brain has been divided to analyze the vascular parameters and the BBB damage by the DCE-MRI technique. (b) T1-W and RCE images showing the ROI selected in the last frame of our DCE-MRI protocol, after CA injection to determine the BBB damage. (c) Image of the brain that correspond to slice 2 of our DCE-MRI analysis, where the transient occlusion of the MCA was performed. (d) T1-W and RCE images showing the ROI selected to determine by DCE-MRI the vascular density/function inside the ischemic brain.
Determination of stroke outcome
The stroke outcome in lean and Cp rats was analyzed by a blinded investigator, before and at different time points up to 28d after stroke (Figure 1), and by the use of a motor and a behavioral test.9 For the motor test, animals were scored as: 0 points, no deficit; 1 point, failure to extend right forepaw fully; 2 points, decreased grip of right forelimb while tail pulled; 3 points, spontaneous circling or walking to contralateral side; 4 points, walks only when stimulated with depressed level of consciousness; 5 points, unresponsive to stimulation. For the behavioural characterization of the animals, first, assessment of each animal began with observation of undisturbed behavior in a clear plastic cage: body position (completely flat, 0 to upright position, 4) and spontaneous activity (none, 0 to repeated vigorous movement, 3). Then, animals were transferred to an arena for observation of the following behaviors: transfer arousal (coma, 0 to extremely excited, 5), gait (absolute incapacity, 0 to normal, 3), touch escape (none, 0 to extremely vigorous, 3) and positional passivity (no struggle when held with a hand, 0, maximal struggle, 4). For the motor test, the higher the score the worse the neurological function, while for the behavioural test the opposite occurs, the higher the score the better the outcome.
Analysis of post-stroke angiogenesis by immunofluorescence
To determine post-stroke angiogenesis, naïve animals and ischemic lean/Cp rats were transcardially perfused at 3, 7 and 28d after stroke, and then, the brains were fixed, removed, cut (30 µm of thickness) and stained following an immunofluorescence protocol previously described27 (n = 3 for naïve and n = 6 for ischemic animals per experimental group and time point). Briefly, the cerebral vascular density and vascular proliferation were analyzed by free-floating immunofluorescence, using as primary antibodies mouse-anti rat RECA-1 (1:500; BioRad, Spain) as an endothelial cell marker, and sheep anti-BrdU (1:250; Abcam, Spain) to detect proliferation of endothelial cells. Antigens were visualized by using donkey anti-mouse Alexa Fluor 488 and anti-sheep Cy3 as secondary antibodies, to detect RECA-1 and BrdU respectively. By an investigator blinded for each experimental group, four random immunofluorescence images inside the infarct or the peri-infarct areas from five consecutive sections (with a distance of 720 µm between each section) were acquired to cover the central and widest part of the cerebral cortex affected by ischemia per animal, starting at 1.70 mm until –0.40 mm from bregma. Images were acquired as a Z-stack at 20× by laser-scanning confocal microscopy (LSM710; Zeiss, Germany). Quantification of cerebral vascular density was performed by densitometry of integrated density using Volocity 3 D image analysis software (Perkin-Elmer, USA). Quantification of the proliferation of endothelial cells was made counting RECA-1 and BrdU double positive cells using ZEN 2009 software (Zeiss). All colocalization images shown were confirmed by orthogonal projection of the z-stack files.
Study of post-stroke EPCs mobilization and SDF1-α plasma levels
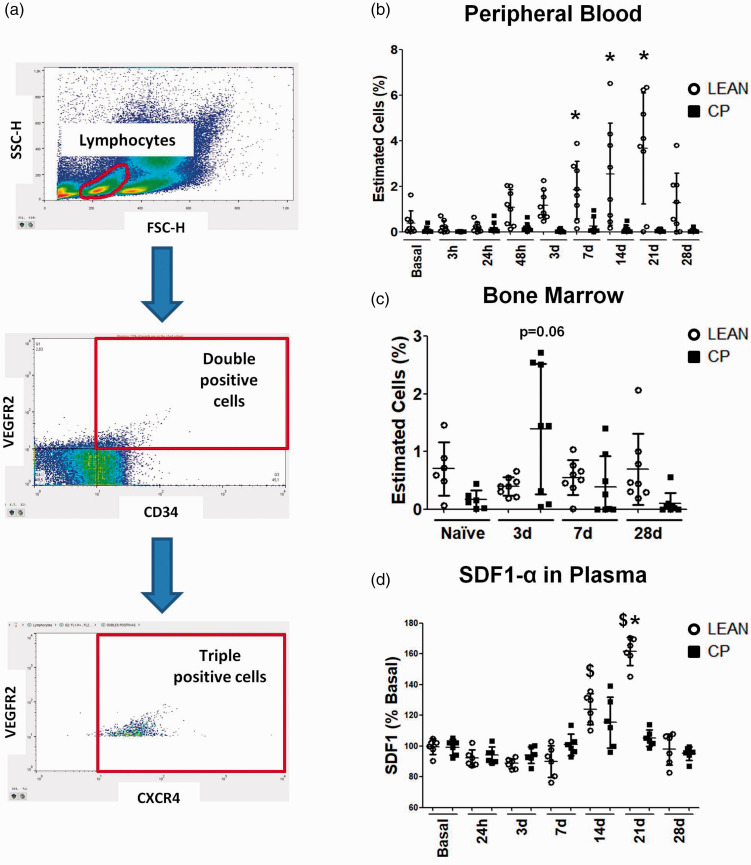
Lean and Cp blood cells were isolated before and at 3, 24, 48 and 72 h and at 7, 14, 21 and 28d after stroke (n = 8 per experimental group and time point). Bone marrow (BM) cells were also isolated from naïve (n = 6) and ischemic lean/cp rats and at 3, 7 and 28d after cerebral ischemia (n = 8 per experimental group and time point). After Fc blockage and red blood cells lysis, EPCs were stained with the following antibodies: CD309 (VEGFR2)-PE, CD184 (CXCR4)-APC (Biolegend, Spain) and CD34-FITC (Santa Cruz Biotechnologies, USA), using as negative controls the corresponding isotypic antibodies. After washing the stained cells, they were resuspended in 200 µl of FACS Flow (BD Pharmingen, USA) and 200,000 events were acquired per sample using a FACSCalibur flow cytometer with CellQuest software (BD Pharmingen, USA). Only the triple positive cells were considered as EPCs and data were obtained using FlowJo® software (FlowJo, USA).
Plasma levels of SDF1-α were determined before and after stroke (see above times) in lean and Cp rats (n = 6 per experimental group and time point) using a specific ELISA-kit (R&D Systems, Spain) following the manufacturer´s instructions.
Analysis of EPCs pro-angiogenic properties in vitro
EPCs isolated from spleen of naïve and ischemic lean/Cp rats were cultured as previously described,30 with the modification that no pools were needed due to the size of the rat spleen. Cells were in culture up to day 7, when after being identified by flow cytometry using the same markers described before, the adhesion and migration properties of these early EPCs were analyzed as previously described.31 After these protocols, EPCs were fixed with 4% paraformaldehyde (PFA) and subsequently stained with TOPRO-3 (Thermo Fisher, LifeTech, Spain). Twenty images per well were acquired as a Z-stack at 20× in a blinded manner by laser-scanning confocal microscopy, and the adhered or migrated EPCs number were counted using ImageJ software. All the experiments were performed in triplicate with a number of 6 animals per experimental group.
Statistical analysis
Results are presented as mean ± standard deviation (SD), and for the motor/behavioral data as the median and interquartile range. For the statistical analysis of the results, GraphPad Prism 5.0 software has been used. Before starting the analysis, all our data were tested with D'Agostino & Pearson omnibus normality test, to study whether they fit to gaussian distributions. Then for parametric data, Student’s t-test and two-way ANOVA followed by Bonferronís correction were used for single and multiple comparisons respectively. For non-parametric data, Mann-Whitney test was used. Differences were considered significant at p < 0.05.
Results
Stroke outcome is worsened in old MS Cp rats
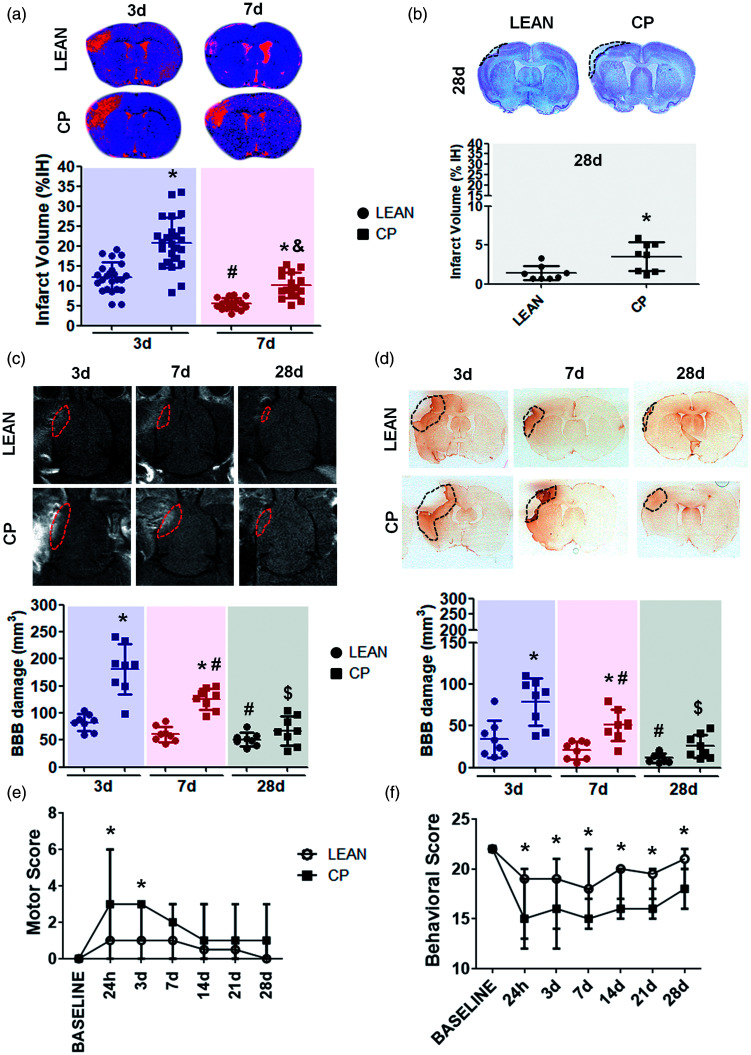
Old (20–22 months) Cp rats showed a remarkable increase in the infarct volume, determined in T2-WI at 3 and 7d after cerebral ischemia, when compared with same age lean animals (Lean-3d = 12.18 ± 3.7 vs Cp-3d = 20.82 ± 6.26, p<0.001; Lean-7d = 5.48 ± 1.44 vs Cp-7d = 10.10 ± 3.18, p<0.01; Figure 3(a)). Consistently, an increased cortex loss was also observed in Cp rats at 28d after stroke, measured by Nissl staining, when compared with lean animals at the same time point (Lean-28d = 1.39 ± 0.33 vs Cp-28d = 3.51 ± 0.65, p = 0.012; Figure 3(b)). Infarct volume, measured by MRI and histology, also showed a reduction on the lesion size along time in both Cp and lean rats, being worse for the co-morbid animals (Lean-3d vs Lean-7d and Cp-3d vs Cp-7d p<0.001; Figure 3(a) and (b)).
Figure 3.
Stroke outcome determined by MRI, histology and neurological test in lean and MS Cp old rats. (a) Infarct volume determined in lean/Cp rats at 3 and 7d after tMCAO measured in T2-WI (lean/Cp rats at 3d n = 24 respectively; lean/Cp rats at 7d n = 16 respectively). (b) Loss of cortex measured in lean/Cp animals at 28d after stroke by Nissl staining (n = 8 per experimental group). (c, d) BBB damage in lean/Cp rats at 3, 7 and 28d after tMCAO measured by DCE-MRI and IgG immunohistology (n = 8 per experimental group). (e, f) Motor and behavioral scores determined before and at 24 h, 3, 7, 14, 21 and 28d after cerebral ischemia in lean/Cp rats (lean/Cp basal, 24 h and 3d n = 24 per experimental group and time point; lean/Cp at 7d n = 16 per experimental group; lean/Cp at 14, 21 and 28d n = 8 per experimental group and time point). Data are expressed as mean±SD. *p < 0.05 vs lean, #p < 0.05 vs Lean-3d or Cp-3d, &p < 0.05 vs Cp-3d, &p < 0.05 vs Cp-7d, Student-t test and two-way ANOVA plus Bonferroni correction for single and multiple comparisons respectively. For the motor/behavioral analyses, data are expressed as the median and interquartile range, *p < 0.05 vs lean, Mann-Whitney test.
Our results on BBB damage, determined by DCE-MRI and calculated as the volume of cerebral parenchyma where extravasated Gd-DTPA was found, revealed a significant increase of this damage in obese old Cp rats at 3 and 7d after stroke, when compared with their lean counterparts (Lean-3d = 104.80 ± 85.85 vs Cp-3d = 190.21 ± 232.85, p < 0.001; Lean-7d =51.58 ± 62.29 vs Cp-7d = 136.15 ± 103.4, p < 0.01; Figure 3(c)). Despite these results, no significant differences were observed on BBB damage between Cp and lean rats at 28d. These results were confirmed by the extravasation of IgG within the brain parenchyma, which again revealed a significant increase in BBB damage in the old co-morbid animals at 3 and 7d, and a non-significant trend at 28d after stroke compared with the lean ones (Lean-3d = 23.68 ± 12.53 vs Cp-3d = 86.39 ± 42.26, p < 0.001; Lean-7d = 10.58 ± 31.6 vs Cp-7d = 70.09 ± 20.08, p < 0.001; Figure 3(d)). Similarly, to infarct volume, a resolution on BBB damage along time was observed in all the animals, although less effectively for Cp rats (Lean-3d vs Lean-28d p < 0.05, Cp-3d vs Cp-7d p < 0.01, Cp-7d vs Cp-28d p < 0.05; Figure 3(c) and (d)).
Finally, aged MS Cp rats showed greater motor and behavioral deficits than lean ones up to 28d after cerebral ischemia. As with infarct volume and BBB damage, a recovery on stroke outcome was observed, although with worse outcome for co-morbid animals (Figure 3(e) and (f)).
Angiogenesis and vasculogenesis are impaired in old MS Cp rats
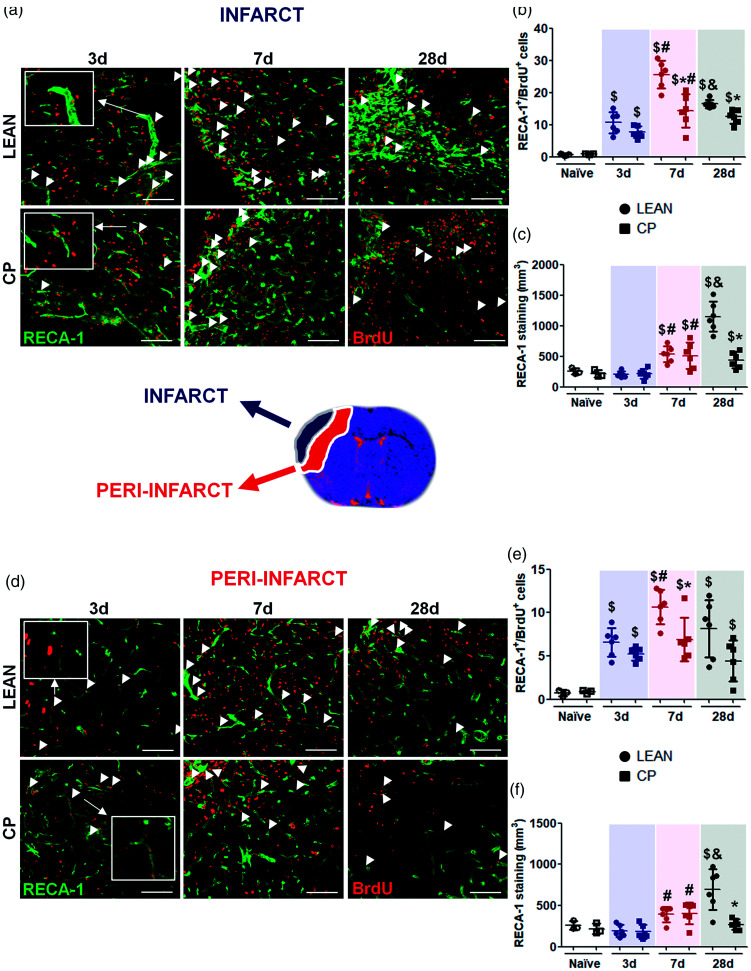
Cortex of both naïve lean and Cp old animals showed similar residual numbers of RECA-1/BrdU double positive cells and no differences in vascular density (Figure 4(b), (c), (e), (f)).
Figure 4.
Post-stroke angiogenesis in the core of the infarct and in the peri-infarct measured by immunofluorescence in lean and MS Cp old rats. (a, d) Representative images showing double positive cells for RECA-1 and BrdU in the infarct and peri-infarct in all the experimental groups (double positive cells are shown with white arrows). (b, e) Proliferation of new endothelial cells (RECA-1/BrdU double positive cells) in the cortex of naïve rats and in the infarct/peri-infarct in all the experimental groups. (c, f) Densitometry of the vascular density (expressed as µm3 of RECA-1 immunostaining) in the cortex of naïve animals and in the infarct/peri-infarct in all the experimental groups. N = 3 for naïve and n = 6 for ischemic animals per experimental group and time point. Data are expressed as mean±SD. $p < 0.05 vs Naïve; *p < 0.05 vs Lean; #p < 0.05 vs 3d; &p < 0.05 vs 7d, Mann Whitney test. Scale bar: 50 µm.
After MCAO, in the infarct core, old rats displayed an increase in endothelial cell proliferation (RECA-1+/BrdU+ cells) in both lean and Cp strains at the earliest time studied (3d after the stroke; lean-naïve = 0.72 ± 0.35 vs lean-3d = 10.63 ± 3.33, Cp-naïve = 0.86 ± 0.17 vs Cp-3d = 7.71 ± 1.79, p = 0.023), and which was maintained at day 7, although at lower extent in obese Cp rats (lean-7d = 25.59 ± 4.27 vs Cp-7d = 14.31 ± 5.16, p = 0.004), followed by a reduction at 28d mainly in lean rats, being these values again lower for Cp rats at this time point (lean-28d = 16.51 ± 1.3 vs Cp-28d = 12.48 ± 2.12, p = 0.005; Figure 4(a) and (b)). In order to study whether changes in endothelial proliferation translated to changes in vascular density, RECA-1 densitometry was determined at the same time points after MCAO. Our results show a subsequent increase in vascular density at day 7 in the infarct core of both lean and Cp old rats (lean-3d = 204.73 ± 52.76 vs lean-7d =533.85 ± 130.76 p = 0.024, Cp-3d = 218.78 ± 77.81 vs Cp-7d = 511.44 ± 214.92 p = 0.047), but only in the leans at 28d after MCAO (lean-28d = 1151.28 ± 244.62 vs Cp-28d = 439.45 ± 132.41 p = 0.002; Figure 4(a) and (c)).
In the peri-infarct area of old rats, MCAO also induced an increase in endothelial cell proliferation at day 3 after the occlusion (RECA-1+/BrdU+ cells, lean-naïve = 0.7 ± 0.3 vs lean-3d = 6.57 ± 1.67 p = 0.021, Cp-naïve = 0.79 ± 0.12 vs Cp-3d = 5.23 ± 0.76 p =0.025; Figure 4(d) and (e)), which was maintained at 7d only in the lean strain (lean-7d = 10.65 ± 1.97 vs Cp-7d = 6.91 ± 2.49 p = 0.026), again followed by a decrease at day 28 (lean-28d = 8.15 ± 3.32 vs Cp-28d = 4.44 ± 2.36 p = 0.093). On its turn, similarly to the core, an increase in vascular density was found at 7d in both lean and Cp old rats (lean-3d = 192.49 ± 71.89 vs lean-7d = 391.36 ± 91.76 p = 0.008, Cp-3d =182.06 ± 84.58 vs Cp-7d = 403.28 ± 131.86 p = 0.008), but only in the lean ones 28d after MCAO (lean-28d = 693.71 ± 247.41 vs Cp-28d = 268.07 ± 63.99 p = 0.008; Figure 4(d) and (f)).
To determine vasculogenesis, the levels of EPCs in peripheral blood and in BM of lean and Cp old rats were analyzed by flow cytometry before and at different time points after stroke. First, our results revealed no differences in basal EPC levels in peripheral blood and BM between strains. Importantly, after stroke, while lean animals showed an increase in EPC levels in peripheral blood from 48 h up to 28d, the levels of these cells in Cp animals did not change, being differences significant at 7, 14 and 21d after the surgery (i.e. lean-14d = 2.53 ± 2.25 vs Cp-14d = 0.13 ± 0.17 p < 0.001; Figure 5(b)). In contrast, when we analyzed EPC levels in BM, no differences were observed over the time after stroke, with only a trend towards increase in Cp rats at 3d compared with the non-co-morbid animals (lean-3d = 0.4 ± 0.16 vs Cp-3d = 1.39 ± 1.13 p = 0.06; Figure 5(c)).
Figure 5.
EPCs mobilization before and after cerebral ischemia in lean and MS Cp old rats. (a) Images showing the gating procedure to detect triple positive EPCs for CD34, VEGFR2 and CXCR4. (b) Percentage of estimated EPCs in peripheral blood before and at different time points after cerebral ischemia in lean/Cp rats (n = 8 per experimental group and time point). (c) Percentage of estimated EPCs in BM of naïve lean/Cp rats (n = 6) and ischemic animals 3, 7 and 28d after tMCAO (n = 8). (d) SDF1-α plasma levels, expressed as percentage of basal levels and determined by ELISA, in lean/Cp animals before and after stroke (n = 6). Data are expressed as mean±SD. $p < 0.05 vs. basal; *p < 0.05 vs Cp, (a) two-way ANOVA plus Bonferroni correction; (b, c) Mann Whitney test.
Plasma levels of SDF-1α, one of the pro-angiogenic factors involved in EPC migration, were analyzed before and after stroke by ELISA. Our data show that plasma SDF-1α levels increased 14d after MCAO with a return to baseline at 28d (Figure 5(d)). More importantly, we found a significant increase in SDF-1α levels at 21d in old lean rats which was not observed in obese counterparts (lean-21d = 161.67 ± 9.22 vs Cp-21d = 105.18 ± 5.43 p = 0.002; Figure 5(d)).
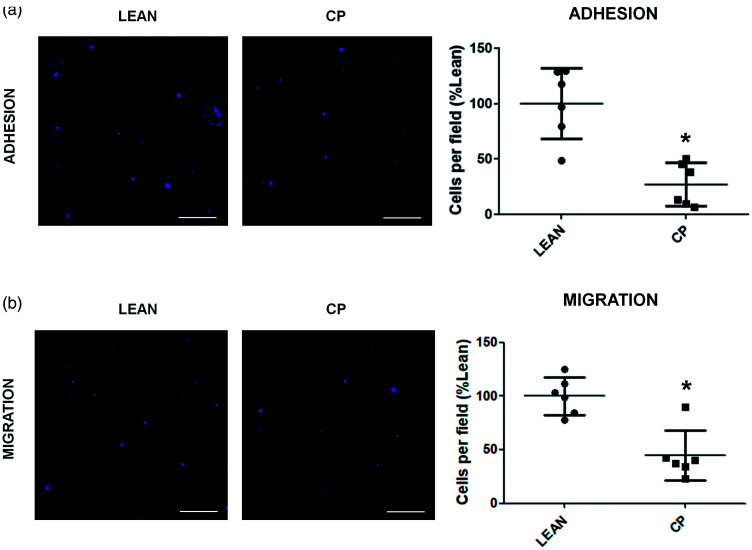
Finally, the analysis of the pro-angiogenic properties, adhesion and migration, in in vitro EPCs showed a reduction in both properties in old MS Cp rats compared to the non-co-morbid animals (lean-adhesion = 100 ± 31.86 vs Cp-adhesion = 26.97 ± 19.63 p = 0.004; lean-migration = 100 ± 17.44 vs Cp-migration = 44.45 ± 23.19 p = 0.008; Figure 6(a) and (b)).
Figure 6.
In vitro pro-angiogenic properties of EPCs from lean and MS Cp old rats. (a) Representative images and data of the adhesion of lean/Cp early EPCs at 7d of culture. (b) Representative images and results of the migration of lean/Cp early EPCs at 7d of culture. N = 6 animals per experimental group. Data are expressed as mean ± SD. *p < 0.05, Mann Whitney test. Scale bar: 25 µm.
Co-morbidities present in old Cp rats impair brain perfusion and vascular function after cerebral ischemia
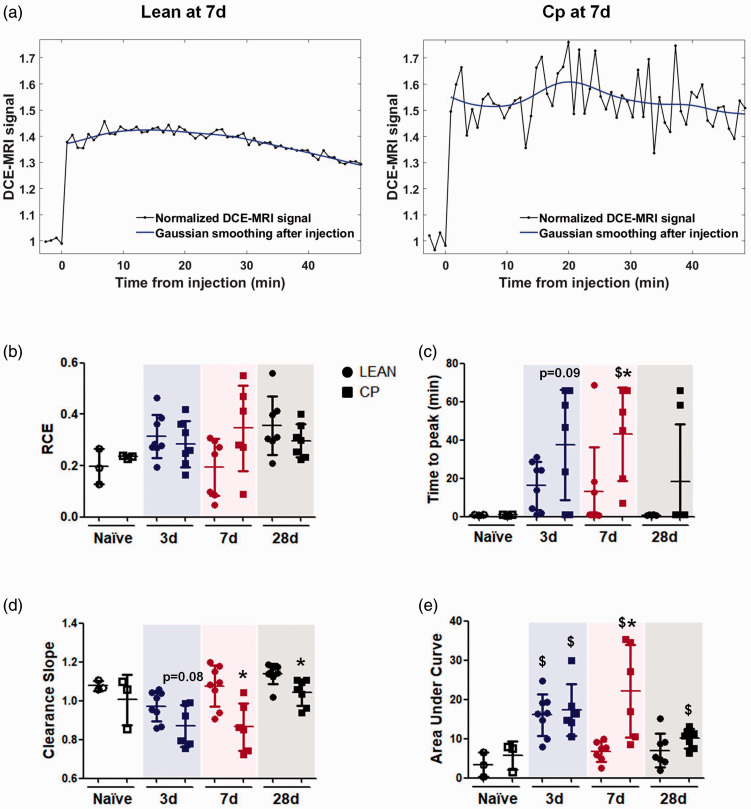
Brain perfusion and vascular function were analyzed non-invasively in both groups of old animals by the use of DCE-MRI. First, no differences in the RCE were found between lean and Cp old rats at any of the time points studied, showing that brains of both strains of old rats can reach the maximum signal of CA according to their corresponding dose, during the time of the study before and after stroke (Figure 7(b)). In contrast, the values of “time to peak”, an indicator of vascular density/functionality, was worse for obese old Cp rats at all time points, being significant at 7d (lean-7d = 12.93 ± 23.43 vs Cp-7d = 43.04 ± 24.57 p = 0.042). Other parameters of vascular function, such as CA clearance and the AUC, which show the ability of the cerebral vasculature to clear the gadolinium, revealed again a worse vascular behavior in co-morbid Cp animals, at 7d and 28d for the clearance slope (lean-7d = 1.08 ± 0.11 VS Cp-7d = 0.86 ± 0.12 p = 0.008; lean-28d = 1.14 ± 0.05 vs Cp-28d = 1.04 ± 0.07 p = 0.006) and only at 7d for the AUC after tMCAO (lean-7d = 6.80 ± 2.58 vs Cp-7d = 22.20 ± 11.82 p = 0.004), compared to lean animals, strongly supporting that co-morbidities present in old Cp rats also impair brain perfusion and vascular function after experimental stroke (Figure 7(c) to (e)). No differences were found in any of these parameters when both naïve groups were compared (Figure 7(b) to (e)).
Figure 7.
Vascular function after cerebral ischemia in lean and MS Cp old rats. (a) Representative curves of the DCE-MRI analysis of vascular function in lean/Cp rats at 7d after tMCAO. (b) Relative contrast enhancement signal (RCE; peak) in naïve and ischemic lean/Cp rats at 3, 7 and 28d after stroke. (c) Time to peak (min) of the CA in naïve and ischemic lean/Cp rats at 3, 7 and 28d after stroke. (d) Clearance slope of the CA’s curve (ratio between the average signal of the first 5 frames and the last 5 frames after the gadolinium injection) in naïve and ischemic lean/Cp rats at 3, 7 and 28d after the surgery. (e): Area under the curve during the DCE-MRI acquisition time (60 repetitions) in naïve and ischemic lean/Cp rats at 3, 7 and 28d after tMCAO. N = 3 for naïve animals and n = 6–8 for the rest of experimental groups and time point. Data are expressed as mean±SD. $p < 0.05 vs Naïve; *p < 0.05 vs Lean, Mann Whitney test.
Discussion
By 2050, it is estimated that 1 in 6 people in the world will be over the age of 65, up from 1 in 11 in 2019.32 Besides, global average life expectancy increased by 5.5 years between 2000 and 2016, the fastest increase since the 1960s, hence the need to study specific health aspects on the growing segment of the very aged (old) population. Our present findings demonstrate, for the first time, that in old rats, presenting MS display a worse stroke outcome (larger lesion size and BBB permeability, and worse neurological function), as well as a reduction in different parameters of angiogenesis and vasculogenesis, concomitantly to an impaired brain perfusion and vascular function determined by DCE-MRI.
JCR:LA-cp (Cp) homozygous rats, due to a deficient expression of the leptin receptor, develop MS characterized by obesity, atherosclerosis, insulin resistance and ventricular hypertrophy, all of them not only important risk factors26 but also conditions that may affect subsequent stroke outcome. They are, therefore, a valuable tool for the study of the impact of these common co-morbidities. In previous studies we investigated the effect of this genotype on MCAO outcome in middle-aged animals, but their impact on old animals remained unexplored.9,27 Our present data show for the first time that the stroke risk factors present in aged Cp rats not only impair BBB function up to 28d but also aggravate stroke outcome (lesion size and neurological function) when compared with aged-matched counterparts, stressing the crucial importance of the coexistence of these co-morbidities in aging. Interestingly, the repercussions of these risk factors were much more significant in old than in middle-aged animals, in which we previously found that Cp animals displayed an increased BBB damage from 24 h to 7d after stroke, but no differences in infarct volume when compared with the leans.9,27 These results confirm previous findings where the effect of ageing and different co-morbidities, considered separately, demonstrated to worsen the stroke outcome.5,7
Angiogenesis is an important neurorepair process induced after cerebral ischemia, which occurs rapidly and is important for stroke recovery,11,33 driven by the activation of HIF-1α and the release of GF (VEGF, erythropoietin, angiopoietins, etc), whose levels remain elevated during some weeks after stroke, and promote the endothelial proliferation to form new blood vessels inside the affected area.10,34,35 However, it is unknown whether this process remain in very aged animals, and whether co-morbidities may still have an impact in this setting. Our results show that MCAO is able to induce an angiogenic response in old animals, concomitant to an increased vascular density, although at a much lesser magnitude, in parallel to a worse neurological function, in rats with MS. Consistent with our data, it has been demonstrated that some of these co-morbidities, individually, have a negative effect in post-stroke angiogenesis in young rats by reducing the activation of HIF-1α, the release of GF, the number of their receptors or by increasing the oxidative stress and the inflammatory response, changes that finally produce a rarefaction and an impaired maturation of the newly formed blood vessels.24,36–38 Importantly, we have now demonstrated that old rats are still able to present an angiogenic response after experimental ischemia, demonstrated by an increase in endothelial cell proliferation and vascular density, but that is highly vulnerable to common co-morbidities such as obesity, atherosclerosis and insulin resistance.
Vasculogenesis is another related process involved in the formation of neo-vessels, which consists in the mobilization of immature endothelial cells (EPCs) from the bone marrow to the site of injury in response to different signals.39,40 Following the acute phase of stroke, it has been observed an initial increase of EPCs in peripheral blood followed by a gradual decrease to basal levels around 3 months, changes demonstrated to be involved in the formation of new blood vessels, brain repair and better outcomes.12,14,17,39,41 Our data demonstrate that old animals still present a vasculogenic response revealed by high numbers of EPCs on peripheral blood from 48 h to 21d after stroke, and which might participate in the increase of vascular density and improved outcome observed in our study. Interestingly, whereas EPC levels in BM from old lean animals show no changes along the times studied, suggesting that these cells come out into peripheral blood as they proliferate in order to increase cerebral angiogenesis after cerebral ischemia, in the old Cp rats, an increase in BM EPCs was observed 3d after stroke, with no changes in peripheral blood at any of the times studied, suggesting a poorer mobilization and subsequent migration to the injured brain, changes that may correspond with a poorer vascular density and a worse outcome when compared with the non-co-morbid animals. In this context, the CXCR4/SDF-1α axis is one of the most relevant signaling systems involved in EPCs proliferation and recruitment to the injured tissue after stroke.42 Our data show that that old lean animals also display an increase in SDF-1α at 14d, which coincides with an increased EPC mobilization in peripheral blood, similarly to the results found in stroke patients, where SDF-1α increases rapidly (7-21d) both in brain and at the peripheral level, in correlation with a high number of EPCs in peripheral blood.43 More importantly, stroke-induced plasma SDF-1α response was not observed in the co-morbid strain of old rats, in correlation with the absence of changes in blood EPC levels in blood. Our data are consistent with evidence showing a negative effect of co-morbidities in the vasculogenesis process, by reducing the proliferation, migration and increasing the mortality of EPCs after stroke due to an increase of inflammation, oxidative stress and a reduced production of attracting molecules.44–46
Finally, the use of MRI after experimental stroke has increased in the last few years, as an imaging tool that allows to improve the translation from bench to bedside, according to the STAIR recommendations.47 In this context, DCE-MRI is a technique that consists in the continuous acquisition of T1-W images, before, during and after the administration of a contrast agent. While this technique is widely used in cancer to determine the vascular density and function inside a tumor, in stroke, there are only a few number of studies, where it has been used to determine only the BBB damage.22,23,48,49 Different quantitative approaches, such as the Ketty-Tofts model, have been used to study vascular density/function and permeability. However, in that model, it is necessary to compute native T1 values of the data in order to characterize the arterial input function and to calculate the ktrans parameter.50 In our study, these determinations would extend excessively the acquisition time and would increase the mortality of our aged-Cp rats due to the co-morbidities present in them. To prevent this, we have studied the cerebral vascular density and function by using a previously described semi quantitative protocol19 and we have determined the BBB leakage by measuring the volume of brain parenchyma with extravasated CA as previously described.22 We have now demonstrated, by using DCE-MRI, BBB damage during the acute phase of stroke and a slow resolution of BBB leakage by the time, but also how co-morbidities present in Cp rats increase the BBB leakage and impair its resolution over the time. Furthermore, DCE-MRI was also useful to study vascular density and function since we demonstrated that co-morbidities worsen the time to peak (a marker of vascular density) and the clearance slope and the AUC (indicators of vascular function). These data were confirmed by histology, demonstrating that DCE-MRI is a non-invasive imaging technique that might be useful to evaluate vascular density/function and BBB damage and to predict the outcome after stroke.
In conclusion, we have demonstrated for the first time that old animals, corresponding to a very aged segment of the population, have a preserved angiogenic/vasculogenic response, important mechanisms of neurorepair, and that the concomitant presence of common co-morbidities (obesity, atherosclerosis, insulin resistance; MS) impairs these processes and worsens the outcome after stroke. In addition, our data reveal also for the first time, that DCE-MRI appears as a potential non-invasive technique to evaluate the vascular function, angiogenesis and BBB damage processes in stroke patients.
Acknowledgements
Authors wish to thank Cristina Vázquez Carballo for her help in this study during her Master Project.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from Instituto de Salud Carlos III and cofinanced by the European Development Regional Fund “A Way to Achieve Europe” (PI17/01601 and RETICS RD16/0019/0009; I.L.), from Regional Madrid Government B2017/BMD-3688 (I.L.); from Spanish Ministry of Economy and Competitiveness SAF2015-68632-R (M.A.M.); from PICATA Project (Campus of international excellence of Moncloa; J.M.P.) and from M+Vision-COFUND Project (J.M.P.) (RTI2018-098682-B-I00 (MCIU/AEI/FEDER, EU) funded by the Spanish Ministry of Science and Innovation (MJLC).
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions: J.M.P., M.A.M and I.L. designed research. J.M.P., M.H.J., V.M., M.E.F.V. performed research. S.M.A, S.D.P. designed research and supplied animals. J.M.P., M.H.J., V.M., M.E.F.V, J.O., J.M.G.S, M.J.L.C. and A.S. analyzed data. J.M.P., M.H.J., M.E.F.V., J.O., S.M.A., S.D.P., M.J.L.C., A.S., M.A.M and I.L. wrote the article. J.M.P. and M.H.J. contributed equally.
ORCID iDs: Jesús M Pradillo https://orcid.org/0000-0002-8266-7946
Juan E Ortuño https://orcid.org/0000-0001-6636-602X
Stuart M Allan https://orcid.org/0000-0001-9646-4456
References
- 1.Feigin VL, Nguyen G, Cercy K, et al. Global, regional, and Country-Specific lifetime risks of stroke, 1990 and 2016. N Engl J Med 2018; 379: 2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Catanese L, Tarsia J, Fisher M, Acute ischemic stroke therapy overview. Circ Res 2017; 120: 541–558. [DOI] [PubMed] [Google Scholar]
- 3.Albers GW, Goldstein LB, Hess DC, et al. Stroke treatment academic industry roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke 2011; 42: 2645–2650. [DOI] [PubMed] [Google Scholar]
- 4.Howells DW, Sena ES, Macleod MR.Bringing rigour to translational medicine. Nat Rev Neurol 2014; 10: 37–43. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Lin S, Chen X, et al. The effect of age, sex and strains on the performance and outcome in animal models of stroke. Neurochem Int 2019; 127: 2–11. [DOI] [PubMed] [Google Scholar]
- 6.Roy-O’Reilly M, McCullough LD.Age and sex are critical factors in ischemic stroke pathology. Endocrinology 2018; 159: 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Popa-Wagner A, Buga AM, Kokaia Z.Perturbed cellular response to brain injury during aging. Ageing Res Rev 2011; 10: 71–79. [DOI] [PubMed] [Google Scholar]
- 8.Moraga A, Pradillo JM, Garcia-Culebras A, et al. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J Neuroinflammation 2015; 12: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradillo JM, Murray KN, Coutts GA, et al. Reparative effects of interleukin-1 receptor antagonist in young and aged/co-morbid rodents after cerebral ischemia. Brain Behav Immun 2017; 61: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singh N, Sharma G, Mishra V.Hypoxia inducible factor-1: its potential role in cerebral ischemia. Cell Mol Neurobiol 2012; 32: 491–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rust R, Gantner C, Schwab ME.Pro- and antiangiogenic therapies: current status and clinical implications. Faseb J 2019; 33: 34–48. [DOI] [PubMed] [Google Scholar]
- 12.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. Mobilization, endothelial differentiation and functional capacity of endothelial progenitor cells after ischemic stroke. Microvasc Res 2010; 80: 317–323. [DOI] [PubMed] [Google Scholar]
- 13.Pias-Peleteiro J, Perez-Mato M, Lopez-Arias E, et al. Increased endothelial progenitor cell levels are associated with good outcome in intracerebral hemorrhage. Sci Rep 2016; 6: 28724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marti-Fabregas J, Crespo J, Delgado-Mederos R, et al. Endothelial progenitor cells in acute ischemic stroke. Brain Behav 2013; 3: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slevin M, Krupinski J, Slowik A, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke 2000; 31: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 16.Navarro-Sobrino M, Rosell A, Hernandez-Guillamon M, et al. A large screening of angiogenesis biomarkers and their association with neurological outcome after ischemic stroke. Atherosclerosis 2011; 216: 205–211. [DOI] [PubMed] [Google Scholar]
- 17.Sobrino T, Hurtado O, Moro MA, et al. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke 2007; 38: 2759–2764. [DOI] [PubMed] [Google Scholar]
- 18.Hermann DM, Buga AM, Popa-Wagner A.Neurovascular remodeling in the aged ischemic brain. J Neural Transm (Vienna) 2015; 122: S25–S33. [DOI] [PubMed] [Google Scholar]
- 19.Barnes SL, Whisenant JG, Loveless ME, et al. Practical dynamic contrast enhanced MRI in small animal models of cancer: data acquisition, data analysis, and interpretation. Pharmaceutics 2012; 4: 442–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ortuno JE, Ledesma-Carbayo MJ, Simoes RV, et al. DCE@urLAB: a dynamic contrast-enhanced MRI pharmacokinetic analysis tool for preclinical data. BMC Bioinformatics 2013; 14: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang WY, Wu G, Li JJ, et al. Early prediction of functional outcome using dynamic contrast enhanced magnetic resonance imaging in experimental stroke. Magn Reson Imaging 2016; 34: 1000–1007. [DOI] [PubMed] [Google Scholar]
- 22.Durukan A, Marinkovic I, Strbian D, et al. Post-ischemic blood-brain barrier leakage in rats: one-week follow-up by MRI. Brain Res 2009; 1280: 158–165. [DOI] [PubMed] [Google Scholar]
- 23.Merali Z, Huang K, Mikulis D, et al. Evolution of blood-brain-barrier permeability after acute ischemic stroke. PLoS One 2017; 12: e0171558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petcu EB, Smith RA, Miroiu RI, et al. Angiogenesis in old-aged subjects after ischemic stroke: a cautionary note for investigators. J Angiogenes Res 2010; 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai TH, Chai HT, Sun CK, et al. Obesity suppresses circulating level and function of endothelial progenitor cells and heart function. J Transl Med 2012; 10: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangat R, Su J, Scott PG, et al. Chylomicron and apoB48 metabolism in the JCR:LA corpulent rat, a model for the metabolic syndrome. Biochem Soc Trans 2007; 35: 477–481. [DOI] [PubMed] [Google Scholar]
- 27.Pradillo JM, Denes A, Greenhalgh AD, et al. Delayed administration of interleukin-1 receptor antagonist reduces ischemic brain damage and inflammation in comorbid rats. J Cereb Blood Flow Metab 2012; 32: 1810–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez-Jimenez M, Hurtado O, Cuartero MI, et al. Silent information regulator 1 protects the brain against cerebral ischemic damage. Stroke 2013; 44: 2333–2337. [DOI] [PubMed] [Google Scholar]
- 29.Greenhalgh AD, Galea J, Denes A, et al. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol 2010; 160: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosell A, Arai K, Lok J, et al. Interleukin-1beta augments angiogenic responses of murine endothelial progenitor cells in vitro. J Cereb Blood Flow Metab 2009; 29: 933–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heida NM, Muller JP, Cheng IF, et al. Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. J Am Coll Cardiol 2010; 55: 357–367. [DOI] [PubMed] [Google Scholar]
- 32.United Nations DoEaSA. Population division world population prospects2019: highlights. New York: United Nations DoEaSA , 2019.
- 33.Hatakeyama M, Ninomiya I, Kanazawa M.Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen Res 2020; 15: 16–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marti HJ, Bernaudin M, Bellail A, et al. Hypoxia-induced vascular endothelial growth factor expression precedes neovascularization after cerebral ischemia. Am J Pathol 2000; 156: 965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duran-Laforet V, Fernandez-Lopez D, Garcia-Culebras A, et al. Delayed effects of acute reperfusion on vascular remodeling and late-phase functional recovery after stroke. Front Neurosci 2019; 13: 767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prakash R, Li W, Qu Z, et al. Vascularization pattern after ischemic stroke is different in control versus diabetic rats: relevance to stroke recovery. Stroke 2013; 44: 2875–2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore SM, Zhang H, Maeda N, et al. Cardiovascular risk factors cause premature rarefaction of the collateral circulation and greater ischemic tissue injury. Angiogenesis 2015; 18: 265–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhaskar S.Impact of obesity-induced type 2 diabetes on long-term outcomes following stroke. Clin Sci (Lond) 2019; 133: 1603–1607. [DOI] [PubMed] [Google Scholar]
- 39.Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997; 275: 964–967. [DOI] [PubMed] [Google Scholar]
- 40.Liman TG, Endres M.New vessels after stroke: postischemic neovascularization and regeneration. Cerebrovasc Dis 2012; 33: 492–499. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 1999; 5: 434–438. [DOI] [PubMed] [Google Scholar]
- 42.Mao L, Huang M, Chen SC, et al. Endogenous endothelial progenitor cells participate in neovascularization via CXCR4/SDF-1 axis and improve outcome after stroke. CNS Neurosci Ther 2014; 20: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deng Y, Wang J, He G, et al. Mobilization of endothelial progenitor cell in patients with acute ischemic stroke. Neurol Sci 2018; 39: 437–443. [DOI] [PubMed] [Google Scholar]
- 44.Kushner EJ, Van Guilder GP, Maceneaney OJ, et al. Aging and endothelial progenitor cell telomere length in healthy men. Clin Chem Lab Med 2009; 47: 47–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herrera MD, Mingorance C, Rodriguez-Rodriguez R, et al. Endothelial dysfunction and aging: an update. Ageing Res Rev 2010; 9: 142–152. [DOI] [PubMed] [Google Scholar]
- 46.Chang EI, Loh SA, Ceradini DJ, et al. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation 2007; 116: 2818–2829. [DOI] [PubMed] [Google Scholar]
- 47.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin CY, Chang C, Cheung WM, et al. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: evaluation with contrast-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab 2008; 28: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 49.Israeli D, Tanne D, Daniels D, et al. The application of MRI for depiction of subtle blood brain barrier disruption in stroke. Int J Biol Sci 2010; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tofts PS.Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 1997; 7: 91–101. [DOI] [PubMed] [Google Scholar]