Abstract
Obstructive sleep apnea (OSA) is associated with hypertension, cardiovascular disease, and a change in the 24 h pattern of adverse cardiovascular events and mortality. Adverse cardiovascular events occur more frequently in the middle of the night in people with OSA, earlier than the morning prevalence of these events in the general population. It is unknown if these changes are associated with a change in the underlying circadian rhythms, independent of behaviors such as sleep, physical activity, and meal intake. In this exploratory analysis, we studied the endogenous circadian rhythms of blood pressure, heart rate, melatonin and cortisol in 11 participants (48±4 years; seven with OSA) throughout a 5 day study that was originally designed to examine circadian characteristics of obstructive apnea events. After a baseline night, participants completed 10 recurring 5 h 20 min behavioral cycles divided evenly into standardized sleep and wake periods. Blood pressure and heart rate were recorded in a relaxed semirecumbent posture 15 minutes after each scheduled wake time. Salivary melatonin and cortisol concentrations were measured at 1–1.5 h intervals during wakefulness. Mixed-model cosinor analyses were performed to determine the rhythmicity of all variables with respect to external time and separately to circadian phases (aligned to the dim light melatonin onset). The circadian rhythms of blood pressure peaked much later in OSA compared to control participants (group × circadian phase, p<0.05); there was also a trend towards a slightly delayed cortisol rhythm in the OSA group. Rhythms of heart rate and melatonin did not differ between the groups. In this exploratory analysis, OSA appears to be associated with a phase change in the endogenous circadian rhythm of blood pressure during relaxed wakefulness, independent of common daily behaviors.
Keywords: sleep disordered breathing, circadian rhythms, circadian clock, blood pressure, adverse cardiovascular events, non-dipping blood pressure
Introduction
Obstructive sleep apnea (OSA) affects at least 10% of the adult US population, and depending on age, sex, and body composition can be as high as 90% in some groups (Senaratna et al. 2017). OSA is associated with hypertension, heart disease, and increased mortality (Javaheri et al. 2017), but the mechanisms that link the syndrome to its adverse health outcomes are not well understood. In OSA, the upper airway collapses repeatedly during sleep causing cycles of asphyxia, arousal and compensatory hyperventilation. Each apnea/hyperpnea cycle is associated with concomitant cycles of sleep-arousal, hypoxia-normoxia, hypercapnia-hypocapnia, bradycardia-tachycardia, ischemia-reperfusion, sympathetic activation-sympathetic withdrawal, and swings in blood pressure (BP) (Somers et al. 2008). Beyond the acute effects of each respiratory event, OSA has long been known to disrupt daily patterns of physiology1. OSA increases both daytime and nighttime BP, but the increase at night compared to day is proportionately greater, leading to more non-dipping hypertension (Noda et al. 1993; Young et al. 1997; Seif et al. 2014). Similarly, treatment of OSA with continuous positive airway pressure (CPAP) improves both daytime and nighttime BP and reduces the proportion of non-dippers (Akashiba et al. 1999; Martinez-Garcia et al. 2013; Iftikhar et al. 2014; Hu et al. 2015). Other day-night patterns that are altered by OSA include the elimination or reversal of the ocular pressure rhythm (Pepin et al. 2010), blunted and disrupted rhythms of circadian clock gene expression in white blood cells (Burioka et al. 2008a; Moreira et al. 2017; Canales et al. 2019; Yang et al. 2019), and increased nocturnal platelet activity (Barcelo et al. 2012). In the long term, OSA also changes the day-night pattern of adverse cardiovascular events and mortality. Sudden cardiac death, ventricular arrhythmias, and myocardial infarctions occur most in the morning in the general population (Muller et al. 1985; Twidale et al. 1989; Goldberg et al. 1990; Willich et al. 1992), but in OSA the peak incidence is shifted earlier into the nighttime (Gami et al. 2005; Kuniyoshi et al. 2008; Zeidan-Shwiri et al. 2011). In a study of all cardiovascular deaths, the predicted morning peak was observed in controls, but the daily pattern was flattened in those with OSA (Martins et al. 2017).
Day/night physiological and pathophysiological patterns can be driven by responses to day/night patterns in behaviors such as the daily fasting/feeding cycle, rest/activity cycle, or sleep/wake cycle, as well as the apneas and arousals that occur specifically during sleep in OSA. These day/night patterns would be considered to be evoked patterns, because they depend on daily responses to changes in behavior or the environment. But rhythms can also be driven by internal ~24 h circadian clocks in the brain and peripheral organs that coordinate endogenous rhythms of behavior, physiology, and hormonal milieu (Aschoff 1960; Mohawk et al. 2012; Thosar et al. 2018). However, studies showing that OSA can alter day/night physiological and pathophysiological patterns have usually been conducted in normal day-night conditions in which the effects of endogenous versus exogenous factors cannot be distinguished.
To study endogenous circadian rhythms, it is necessary to study the circadian modulation of physiology while controlling for the effects of common behaviors such as physical activity, meal intake, and sleep (Thosar et al. 2018). We previously conducted a forced desynchrony experiment, which standardizes behaviors evenly across a 24-h period, to measure the contribution of the endogenous circadian system to characteristics of apnea severity during sleep (Butler et al. 2015). This dataset presented an opportunity to evaluate whether internally generated circadian rhythms—not just daily rhythms—of BP and hormone secretion are altered by OSA. Therefore, we completed a retrospective study of endocrine and cardiovascular rhythms in participants with and without OSA, and focused on measures obtained during wakefulness to isolate the systemic effects of OSA on circadian rhythmicity without the acute effects of the apneas themselves (Butler et al. 2015). Rhythms were analyzed with respect to internal melatonin phase to measure the degree of internal synchrony to the central clock. Data were also analyzed with respect to the external time of day (Eastern Standard Time) to determine whether OSA shifted the rhythms with respect to the environment.
Materials and Methods
Participants.
Eleven overweight or obese participants (4 women), with untreated OSA (n=7; OSA group) or without OSA (n=4; Control group) were recruited as described previously (Butler et al. 2015). Participant details are shown in Table 1. Briefly, men and women 18–70 years old and with BMI<40 were recruited. Exclusion criteria were any cardiovascular and renal disease, diabetes, uncontrolled hypertension, other sleep disorders besides OSA, history of night shift work, and travel across time zones in the previous 3 months. Suitability for an extended in-laboratory stay was evaluated by physical exam and psychiatric interview. Assignment to the OSA and Control groups were made after the study based on the AHI during the first night in the laboratory (see Results). The study conforms to international ethical standards for the study of chronobiology (Portaluppi et al. 2010) and was approved by the Institutional Review Board of Partners Health Care; all participants provided written informed consent. Participants with BP >160/100 mmHg were excluded. One participant with OSA had medically controlled hypertension (lisinopril 20 mg/day). This participant continued to take medication during the study, and this participant’s data were removed a priori from all heart rate (HR) and BP analyses.
Table 1.
Participant characteristics. Baseline blood pressure was measured after admission to the laboratory. Habitual bed and wake time were the self-selected stable 8 h sleep periods maintained by participants for at least 1 week prior to the in-lab study. For bed times after midnight, the data were coded as bed time + 24 h. Differences tested by t-test for each OSA subset against Controls.
| Controls | OSA | OSA (No medication) | |
|---|---|---|---|
| N (male) | 4 (1) | 7 (6) | 6 (5) |
| Age (years) | 40.3 (SD 11.0) | 52.9 (SD 10.7) | 53.4 (SD 11.7) |
| BMI (kg/m2) | 36.8 (SD 6.3) | 32.8 (SD 5.2) | 33.0 (SD 5.6) |
| AHI (events/h) | 3.5 (SD 0.9) | 34.8 (SD 10.1)*** | 35.5 (SD 10.9)*** |
| Habitual bed time | 23:37 (SD 1:06) | 23:08 (SD 0:22) | 23:10 (SD 0:24) |
| Habitual wake time | 7:37 (SD 1:06) | 7:08 (SD 0:22) | 7:10 (SD 0:24) |
| DLMO (EST) | 21:34 (SD 1:44) | 20:47 (SD 0:42) | 20:35 (SD 0:33) |
| SBP (mm Hg) | 129.0 (SD 9.4) | 132.0 (SD 11.1) | 131.7 (SD 12.1) |
| DBP (mm Hg) | 75.0 (SD 2.9) | 71.8 (SD 11.6) | 69.1 (SD 10.1) |
| HR (bpm) | 76.8 (SD 19.2) | 69.1 (SD 7.3) | 70.2 (SD 7.5) |
p<0.001
To stabilize circadian rhythmicity, participants maintained a regular self-selected 8-h sleep period for at least one week prior to the laboratory study, verified by wrist actigraphy (Actiwatch, Minimitter, Bend, OR). The selected bed times ranged from 22:30 to 01:00. Participants abstained from recreational drugs, alcohol, nicotine, caffeine, and other supplements for the duration of the experiment. Comprehensive toxicology screening of urine was performed on admission to the laboratory.
Protocol.
Participants stayed in individual laboratory suites for 5 days (Fig. 1) during which they completed 10 identical cycles of a 2 h 40 min wake period in dim light (<4 lux) followed by a 2 h 40 min sleep opportunity in darkness. The rationale for this procedure, termed a forced desynchrony, is that by the end of the protocol, daily routines (identical activities, meals, BP/HR tests, and sleep opportunities) have occurred evenly spread around all circadian times (i.e., circadian phases, expressed in degrees). Therefore, this protocol design controls for daily behaviors and allows circadian rhythms to be detected in the whole dataset. Rhythms were thus estimated from 10 measurements across ~51 h, corresponding to a frequency of 9 samples per 24 h, or one sample per ~40° in the 360° circadian phase space (0° defined by dim light melatonin secretion onset; details below). This is a higher effective frequency than the 60° resolution obtained in previously published reports from longer protocols (Shea et al. 2011).
Fig. 1.
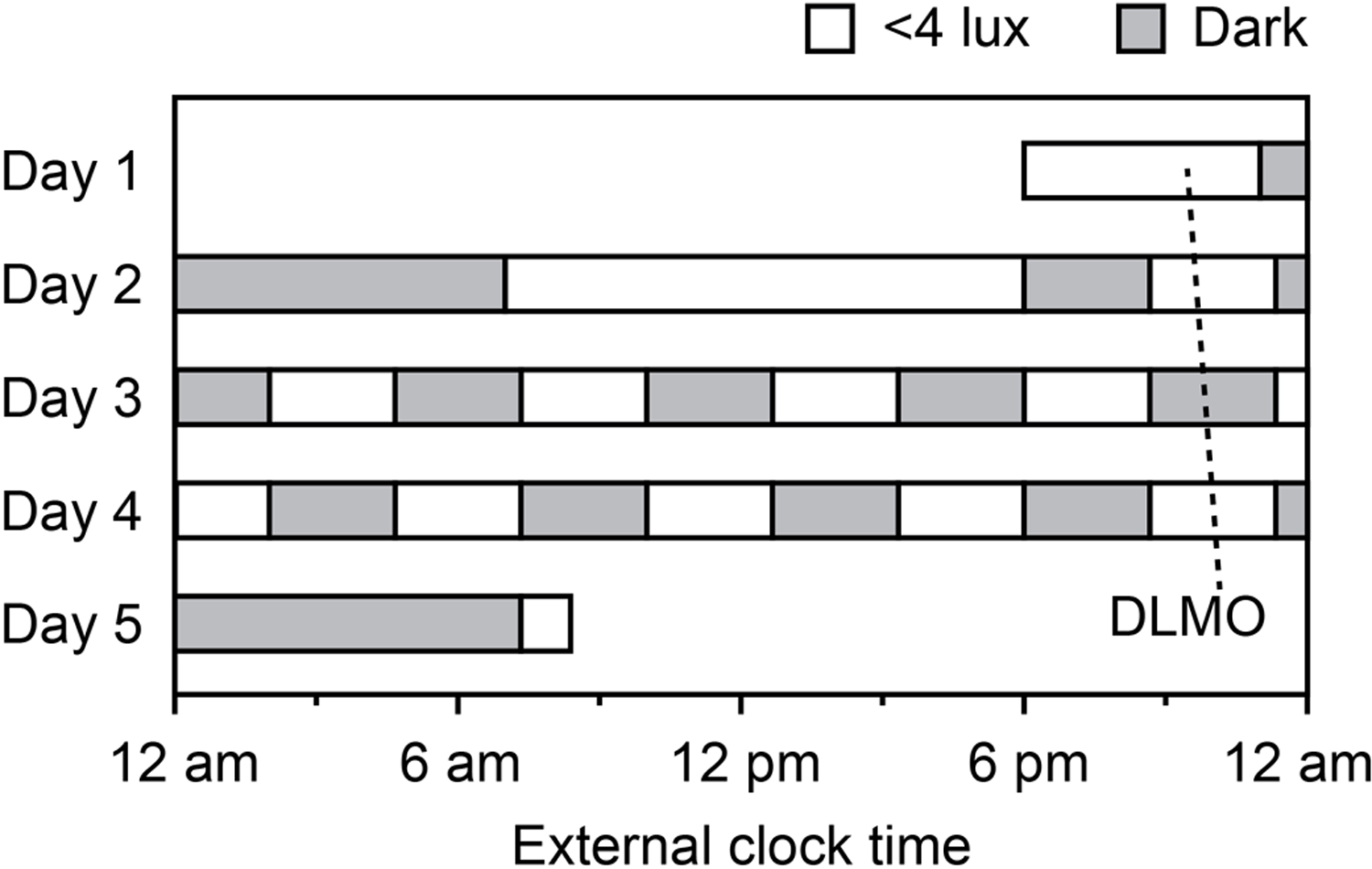
Five day in-lab protocol. Participants remained in bed during dark sleep opportunities (<0.5 lux), and were awake and out of bed during dim light wake periods (<4 lux). Blood pressure was measured 15 min after each wake time. The free-running rhythm of dim light melatonin onset (DLMO) is shown for one participant.
At the beginning of each short wake period, the head of the bed was raised to 45° and subjects remained semirecumbent for approximately 15 minutes before BP and HR were measured. BP and HR were recorded as the mean of two measures approximately 5 minutes apart, obtained on the relaxed upper arm. Trained observers used medium or large Critikon Sensacuffs, as appropriate, and an automatic oscillometric cuff sphygmomanometer (Dinamap V100, Critikon Inc., FL). Saliva samples were collected every 60–90 minutes during wakefulness, therefore allowing endogenous circadian rhythm assessment from an average of 28 samples (range: 22–31) over ~51 h. These samples were frozen at −80°C and subsequently thawed and assayed in a single batch for cortisol and melatonin by radioimmunoassay (Cortisol: Coat-a-count RIA, Siemens, Los Angeles, CA: inter- and intra-assay coefficients of variation were 4.4–7.9% and 5.4–7.7%, respectively, as reported by the manufacturer. Melatonin: Buhlmann RK-DSM2, Alpco Diagnostics, Salem, NH: inter- and intra-assay coefficients of variation at ~3.5 pg/mL were 8.9 and 4.1%, respectively, as reported by the manufacturer). Full polysomnography was recorded during the baseline night and scheduled 2h 40 min sleep periods throughout the forced desynchrony protocol (Butler et al. 2015).
Phase determination and statistical analyses.
This retrospective exploratory analysis was carried out on the data from a study that was originally designed and powered to detect differences in circadian rhythms of sleep apnea severity between people with OSA and healthy controls (Butler et al. 2015). Here, independent sample t-tests were used to compare anthropometric data between groups. Period and phase for each participant were estimated from salivary melatonin concentrations using non-orthogonal spectral analysis (NOSA) with three harmonics (Czeisler et al. 1999). Circadian phase for each individual was defined by degrees relative to dim light melatonin onset (DLMO = 0°), calculated as the interpolated time-point when melatonin levels exceeded 25% of the fitted trough-to-peak amplitude (Lewy et al. 1987; Lewy and Sack 1989). Time of day was recorded in EST. Endocrine, BP and HR data were then analyzed by time of day (without respect to habitual sleep time) and by circadian phase (relative to DLMO). These data were analyzed by mixed model cosinor analysis with factors of group (Control or OSA), time-into-protocol, circadian phase or time, and group × phase or group × time interactions. A linear parametrization of time of day or circadian phase allows the cosinor regression and prevents the discontinuity in the circularity of time variables (359° to 0° or 23:59 to 00:00) (Nelson et al. 1979; Hu et al. 2011; Cornelissen 2014). Participant was included as a random factor. Because we were interested in changes in the circadian pattern, our primary outcome of interest was the interaction effect (group × phase or group × time). Mixed model population cosinor analysis allows group-wise comparison of the waveforms by considering the inter- and intra-individual variability in a time series analysis (Van Dongen et al. 2003). For cardiovascular endpoints, a two-harmonic model (360°/180° or 24 h/12 h) was implemented as this has been extensively used to analyze daily and circadian BP rhythms (Scheer et al. 2010; Cornelissen 2014; Thosar et al. 2019). A third harmonic (120° or 8 h) was included in the regression models for endocrine variables as more samples were available and this improved the fit. In the analyses, the time-into-protocol term was never significant, thus the data could be stacked by cycle for better estimation of the waveform (Cornelissen 2014). For illustration purposes, means and standard errors for each 60° circadian bin or 4 h time bin were obtained by normalizing and binning data within each participant, and then averaging across participants (i.e., in these cases, standard errors are calculated with n=6–7 for participants with OSA or n=4 for Controls). Unless otherwise noted, data are reported as mean ± standard error of the mean. Analyses were conducted in JMP Pro 11 (SAS Institute, Cary, NC)
Results
Participant characteristics.
OSA severity was determined by AHI during the first night in the laboratory (Table 1). Thus, AHI was significantly higher in the OSA group (34.8 (SD 10.1) events/h; range 20.0–49.8) than in the Control group (3.5 (SD 0.9), range 2.7–4.3). Though not significant, participants with OSA tended to be older and were more likely to be male. Body mass index (BMI) and habitual self-selected 8 h sleep periods did not differ significantly between the groups.
Endocrine measures.
Because melatonin was the marker of central clock phases, we first tested whether OSA altered melatonin rhythms with respect to time of day. DLMO ranged from 19:13 to 23:21 EST; mean DLMO was earlier in participants with OSA by 47 min but this was not statistically significant (p>0.05; Table 1). There was also no evidence for a group effect on the rhythmic pattern of melatonin secretion with respect to time of day (Fig. 2: cosinor analysis, group × time of day interaction effect: F1,286 = 1.11, p=0.29).
Fig. 2.
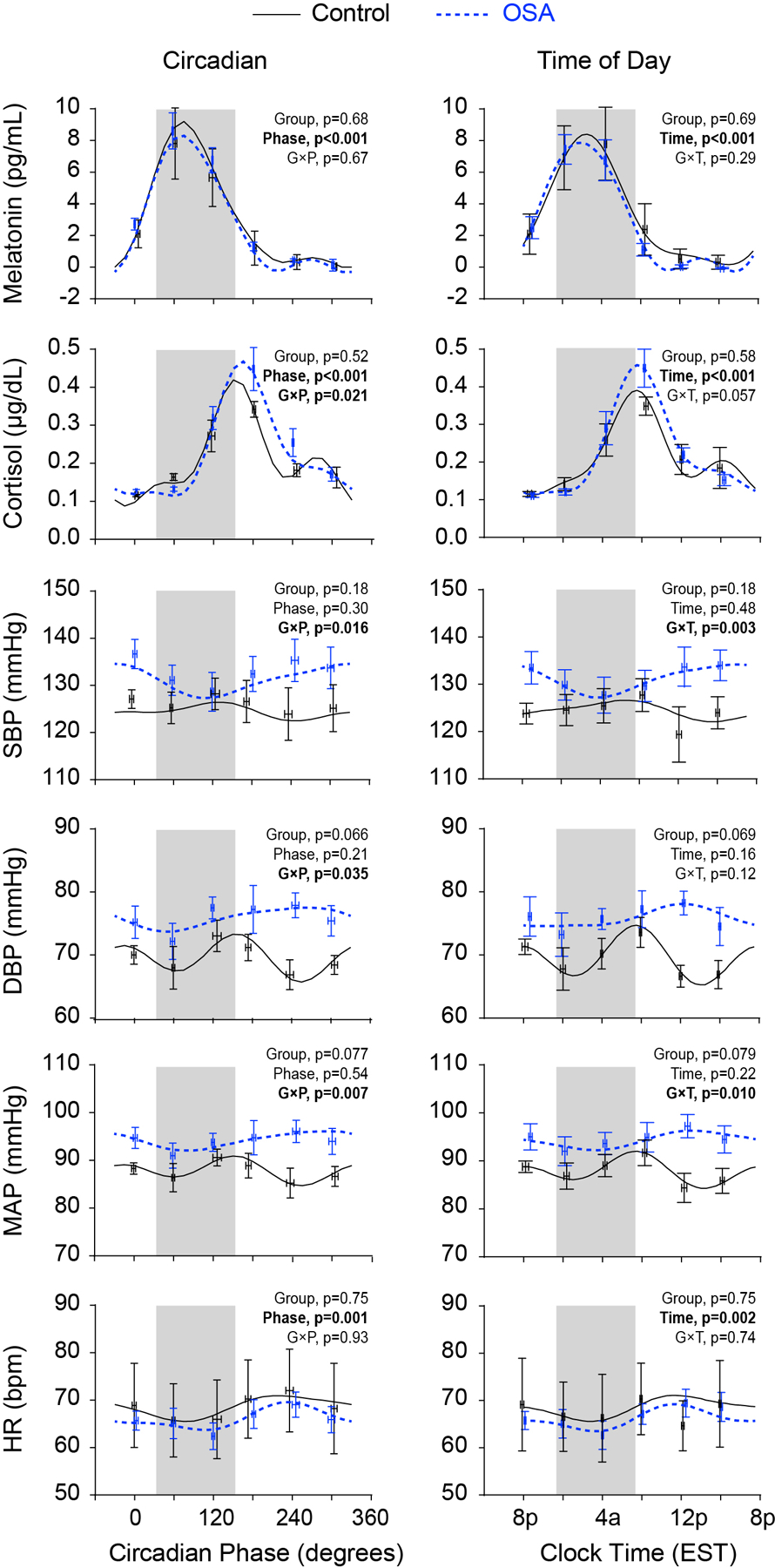
Rhythms with respect to circadian phase and external time: melatonin, cortisol, systolic blood pressure (SBP), diastolic blood pressure (DBP), mean arterial pressure (MAP) and heart rate (HR). Raw observed values are first binned by circadian phase (60° bins starting at 0°) or time-of-day (4 h bins starting at 8pm) within participant. These data are then averaged within group, and plotted as mean ± SEM (OSA, n=7 for endocrine variables, otherwise n=6; Control, n=4). Grey shading indicates the average self-selected sleep time across both groups (33°−153° on the left with 0° at DLMO, and 11:20pm-7:20am on the right). The cosinor model fits are also shown for each variable and group; p values for the fixed effects are shown in each panel.
Given heterogeneous findings with respect to cortisol patterns in OSA (Tomfohr et al. 2012), we next examined the effect of OSA on cortisol rhythms. Though there was no difference in mean level (group effect: F1,9=0.44, p=0.52), there were significant main effects of circadian phase (F1,284=257, p<0.001) and a circadian phase × group interaction (F1,284=5.4, p=0.021). The significant interaction indicates a change in phase or amplitude that depends on group: inspection of the curves revealed a higher peak concentration and a small delay in the circadian phase of cortisol in the OSA group compared to non-OSA group (Fig. 2). Similar results were obtained when analyzing by external clock time, though there was only a trend towards a significant interaction (group effect, F1,9=0.32, p=0.58; time effect, F1,284=140, p<0.001; time × group effect, F1,284=3.7, p=0.057).
Cardiovascular measures.
Repeated measures during relaxed wakefulness of systolic BP (SBP), diastolic BP (DBP), mean arterial pressure (MAP), and HR were assessed against circadian time and external time of day (EST). SBP rhythms differed between groups in both analyses (circadian phase × group interaction, F1,84=6.1, p=0.016; time × group interaction, F1,84=9.5, p=0.003; inspection of the curves showed reversed rhythms between groups, with the circadian peaks in controls almost coinciding with the circadian troughs in OSA in both the circadian and time of day plots (Figure 2). Similar results were observed in the analysis of MAP (phase and time × group interactions, both F1,84≥7.0, p≤0.01). DBP circadian rhythms also differed between groups, as revealed by the significant interaction of phase × group (F1, 84=4.6, p=0.035). This was not significant, however, in the clock time analysis (p=0.12). DBP exhibited two peaks in controls (150° and 345°), but a single peak in OSA (260°). There was a trend towards higher mean DBP in the OSA group (p<0.07). HR displayed a significant circadian and time of day rhythm (main effects of phase and time, F1,81≥10.7, p≤0.002). Nevertheless, there were no effects of group (F1,8≤0.11, p≥0.75), and no significant interaction effects (F1,81≤0.11, p≥0.74) in either analysis.
Discussion
Epidemiological evidence suggests that patients with OSA experience more adverse cardiovascular events during the night than in the general population, which has a population peak later in the morning (Willich et al. 1992; Gami et al. 2005; Kuniyoshi et al. 2008; Zeidan-Shwiri et al. 2011). A nocturnal peak in incidence of adverse cardiovascular events could reflect additional nocturnal triggers (such as the acute effects of apneas), a change in endogenous rhythms of cardiovascular risk, or a combination of these (Thosar et al. 2018). Therefore, to begin to explore how OSA alters daily rhythms of physiology, we completed a retrospective data analysis to determine whether circadian rhythms in physiology are shifted in OSA. We discovered that OSA did not appreciably affect circadian waveforms of melatonin, HR, or cortisol with respect to external time, but did alter the circadian rhythm of BP.
Changes in rhythm phase can occur in two ways. First, all internal rhythms can be shifted in synchrony relative to external time. To detect this, rhythms were analyzed with respect to external clock time. Second, different internal rhythms can be shifted to different phases, a state termed internal misalignment. To detect this, rhythms were analyzed with respect to internal circadian time as defined by melatonin secretion. We found that the time of day of DLMO did not differ appreciably between the groups, so there was little difference between the two analyses. Melatonin rhythms are directly controlled by the central circadian clock in the brain, and are generally used as a surrogate marker for central circadian phase (Klerman et al. 2002; Morris et al. 2016) in misalignment protocols. Therefore, in OSA as compared to controls, the similar times of DLMO relative to external time of day (Table 1), and the similar melatonin circadian waveforms (Figure 2) suggest no effect of OSA on the central pacemaker in the suprachiasmatic nucleus.
OSA slightly but significantly delayed the circadian rhythm of cortisol secretion, though there was no effect when analyzed against time of day (the latter nonsignificance may be a Type 2 error due to low statistical power). By inspection, there is a slight delay in the peak in circadian time but not in clock time and a small increase in peak concentration (n.s.). Nevertheless, the similarity in circadian waveforms between groups is consistent with prior work showing no effect of OSA on daily patterns (not necessarily circadian, see earlier footnote) of cortisol secretion (Entzian et al. 1996; Burioka et al. 2008a).
OSA altered the circadian rhythm of BP, as indicated by the significant phase × group and time × group interaction effects. This suggests a circadian clock contribution to the altered daily patterns of BP that are observed in people with OSA (Akashiba et al. 1999; Iftikhar et al. 2014; Seif et al. 2014; Hu et al. 2015). Though this is the first report of endogenous circadian hemodynamic rhythms in OSA, endogenous circadian BP rhythms for control participants have been reported. The peak in the BP circadian rhythm in the OSA participants here is earlier than in young (26±1 years) non-obese controls (Shea et al. 2011) but is similar to the peak time in middle-aged (51±2 years) non-obese controls without OSA (Thosar et al. 2019). Other than age, three other differences could affect comparisons across these experiments: location of study, type of circadian protocol, and body composition. In sum, the current results suggest that OSA alters hemodynamic rhythms. However, the true extent of how OSA affects cardiovascular rhythms must wait for an adequately powered study design with age, sex, and BMI matched participants.
People with OSA tend to have higher BP compared to healthy controls (Nieto et al. 2000) and both nighttime and daytime BP increase with AHI (Lavie et al. 1993). OSA increases 24-h BP values by sympathetic overactivity (Smolensky et al. 2007) and increasing the baroreflex setpoint (Brooks et al. 1999); OSA also attenuates nocturnal BP dipping through direct arousal-related BP surges and increased sympathetic tone (Somers et al. 1995). In this sample we observed this same trend in elevated mean BP in the OSA group over the circadian protocol (SBP, p=0.18; DBP, p=0.07). Nocturnal non-dipping and rising BP are risk factors for heart disease and the reason why ambulatory blood pressure monitoring is the recommended standard for assessing risk (Hermida et al. 2015). Part of OSA’s contribution to cardiovascular risk, therefore, may be in reducing dipping as this depends on the severity of OSA (Baguet et al. 2005; Mokhlesi et al. 2015). Because all of our measures were taken during relaxed wakefulness, we were not able to measure the nocturnal dipping that is generally associated with sleep (Mansoor 2002; Huang et al. 2011). The nadir of the circadian rhythms of SBP and DBP occurred during the biological night though the value of the drop would not in itself constitute dipping. In future studies, it will be of interest to determine how sleep modulates the circadian rhythms of cardiovascular function.
The circadian profiles of BP of healthy controls in our dataset (Fig. 2) differ from previously published circadian profiles of BP (Shea et al. 2011; Thosar et al. 2019). The SBP amplitude was small and DBP exhibited a striking bimodal pattern. These may be artifacts of the small sample size or may be due to experimental or demographic differences as detailed above.
Potential links between OSA and circadian timing have been reviewed recently (von Allmen et al. 2018). Respiratory disturbances elicit surges of glucocorticoids and catecholamines, two cues that are known to phase shift circadian clocks in peripheral tissues (Ueyama et al. 1999; Balsalobre et al. 2000; Cuesta et al. 2015). Some other peripheral day-night rhythms have been reported to be altered by OSA, including a reversal of the intraocular pressure rhythm in some patients (Pepin et al. 2010). Tissue necrosis factor-alpha (TNF-α), an inflammatory cytokine exhibited a blunted nocturnal profile and a daytime peak in OSA which is absent in healthy controls (Entzian et al. 1996). Day-night oscillation of interleukin-6 (IL-6) is also absent in subjects with OSA and is restored by CPAP (Burioka et al. 2008b). A direct link with the circadian clocks is suggested by the observation that OSA alters the daily expression patterns of some clock genes in white blood cells (Burioka et al. 2008a; Moreira et al. 2017; Canales et al. 2019; Yang et al. 2019). Not all circadian clocks in the body are equally sensitive to time-resetting cues which can lead to internal misalignment of peripheral and central clocks (Damiola et al. 2000; Cuesta et al. 2015; Morris et al. 2016). OSA may therefore contribute to pathology by creating a state of circadian misalignment between shifted peripheral clocks and the non-shifted central clock (e.g. as observed in the melatonin rhythm).
Strengths and Limitations.
The protocol was designed prospectively to study rhythms in the pathophysiology of obstructive events. The rigorous controlled conditions of a forced desynchrony are a strength and allow us to measure effects of the endogenous circadian clock. Additionally, through careful screening, we studied participants with OSA but without comorbid cardiometabolic disease with the exception of the one participant with controlled hypertension (whose cardiovascular data were excluded from analyses). Nevertheless, the rigor and duration of the experiment precluded recruitment of a large sample; future elaboration of OSA’s effect on central versus peripheral rhythms as well as OSA’s effect on day-night rhythms versus circadian rhythms is warranted. Additionally, there is a possibility that demographic differences in our groups could contribute to the differences in BP rhythms. Though both groups were middle-aged, the control group was relatively younger and included more women.
All analyses were conducted on measures obtained during relaxed wakefulness. This was done to eliminate any possible acute effects of the sleep-related respiratory events, and isolate the systemic long term effects that OSA may have on circadian rhythms. Any effects of OSA on circadian rhythms of BP during sleep are unknown. Future investigations which include ambulatory BP monitoring would be valuable to understand the acute effects of sleep and activity on BP within the context of a circadian protocol. We lack measures of potential mechanistic pathways, such as circulating catecholamines or aldosterone, which may be informative. If OSA is indeed responsible for disrupting hemodynamic circadian rhythms, then treatment of OSA would be predicted to normalize this.
Conclusions.
OSA has been observed to alter daily patterns of physiology, but the contributions of the nightly apneas themselves versus potential changes in endogenous rhythms had not previously been explored. Using an innovative experimental design to separate circadian rhythms from exogenously produced day/night patterns, we found a significant difference between the endogenous circadian rhythms of BP in people with OSA compared to controls. On the other hand, HR, cortisol, and melatonin secretion patterns were minimally affected by OSA. If our findings hold in studies with adequately powered sample size, it would suggest an impairment in peripherally driven physiological rhythms in OSA.
Acknowledgements
We thank the Center for Clinical Investigation staff for expert assistance, and the Harvard Catalyst Clinical and Translational Science Center for support.
Grants
This work was funded by an American Sleep Medicine Foundation Focused Project Award, T32-HL007901, and R21-HL140377 (MPB), R21-HL092407 and R01-HL125893 (SAS), F32-HL131308, KL2TR002370, and National Space Biomedical Research Institute through NCC 9–58 (SST), 1UL1-RR025758 and 8UL1-TR000170 to the Harvard Catalyst Clinical and Translational Science Center, and the Oregon Institute of Occupational Health Sciences at Oregon Health & Science University via funds from the Division of Consumer and Business Services of the State of Oregon (ORS 656.630).
Footnotes
Declaration of interests
The authors report no conflicts of interest.
In this paper, we differentiate between daily rhythms of physiological variables (those measures that are rhythmic when measured across a normal 24 hour period with typical daily patterns of eating, waking, and nightly sleep) from endogenous circadian rhythms (those measures that are rhythmic in the absence of external time cues and driven by the internal circadian system). Assuming a simple additive model, the measured daily pattern in a variable such as blood pressure would be the sum of the basal circadian rhythm in blood pressure plus the effects on blood pressure of the daily responses to environmental and behavioral changes.
References
- Akashiba T, Minemura H, Yamamoto H, Kosaka N, Saito O, Horie T. 1999. Nasal continuous positive airway pressure changes blood pressure “non-dippers” to “dippers” in patients with obstructive sleep apnea. Sleep 22(7):849–853. [DOI] [PubMed] [Google Scholar]
- Aschoff J. 1960. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol 25:11–28. [DOI] [PubMed] [Google Scholar]
- Baguet JP, Hammer L, Levy P, Pierre H, Rossini E, Mouret S, Ormezzano O, Mallion JM, Pepin JL. 2005. Night-time and diastolic hypertension are common and underestimated conditions in newly diagnosed apnoeic patients. J Hypertens 23(3):521–527. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U. 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289(5488):2344–2347. [DOI] [PubMed] [Google Scholar]
- Barcelo A, Pierola J, de la Pena M, Frontera G, Yanez A, Alonso-Fernandez A, Ayllon O, Agusti AG. 2012. Impaired circadian variation of platelet activity in patients with sleep apnea. Sleep Breath 16(2):355–360. [DOI] [PubMed] [Google Scholar]
- Brooks D, Horner RL, Floras JS, Kozar LF, Render-Teixeira CL, Phillipson EA. 1999. Baroreflex control of heart rate in a canine model of obstructive sleep apnea. Am J Respir Crit Care Med 159(4 Pt 1):1293–1297. [DOI] [PubMed] [Google Scholar]
- Burioka N, Koyanagi S, Endo M, Takata M, Fukuoka Y, Miyata M, Takeda K, Chikumi H, Ohdo S, Shimizu E. 2008a. Clock gene dysfunction in patients with obstructive sleep apnoea syndrome. Eur Respir J 32(1):105–112. [DOI] [PubMed] [Google Scholar]
- Burioka N, Miyata M, Fukuoka Y, Endo M, Shimizu E. 2008b. Day-night variations of serum interleukin-6 in patients with severe obstructive sleep apnea syndrome before and after continuous positive airway pressure (CPAP). Chronobiol Int 25(5):827–834. [DOI] [PubMed] [Google Scholar]
- Butler MP, Smales C, Wu H, Hussain MV, Mohamed YA, Morimoto M, Shea SA. 2015. The circadian system contributes to apnea lengthening across the night in obstructive sleep apnea. Sleep 38(11):1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canales MT, Holzworth M, Bozorgmehri S, Ishani A, Weiner ID, Berry RB, Beyth RJ, Gumz M. 2019. Clock gene expression is altered in veterans with sleep apnea. Physiol Genomics 51(3):77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen G. 2014. Cosinor-based rhythmometry. Theor Biol Med Model 11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Cermakian N, Boivin DB. 2015. Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J 29(4):1360–1370. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. 1999. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science 284(5423):2177–2181. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. 2000. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14(23):2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entzian P, Linnemann K, Schlaak M, Zabel P. 1996. Obstructive sleep apnea syndrome and circadian rhythms of hormones and cytokines. Am J Respir Crit Care Med 153(3):1080–1086. [DOI] [PubMed] [Google Scholar]
- Gami AS, Howard DE, Olson EJ, Somers VK. 2005. Day-night pattern of sudden death in obstructive sleep apnea. N Engl J Med 352(12):1206–1214. [DOI] [PubMed] [Google Scholar]
- Goldberg RJ, Brady P, Muller JE, Chen ZY, de Groot M, Zonneveld P, Dalen JE. 1990. Time of onset of symptoms of acute myocardial infarction. Am J Cardiol 66(2):140–144. [DOI] [PubMed] [Google Scholar]
- Hermida RC, Smolensky MH, Ayala DE, Portaluppi F. 2015. Ambulatory Blood Pressure Monitoring (ABPM) as the reference standard for diagnosis of hypertension and assessment of vascular risk in adults. Chronobiol Int 32(10):1329–1342. [DOI] [PubMed] [Google Scholar]
- Hu K, Scheer FA, Laker M, Smales C, Shea SA. 2011. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 123(9):961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Fan J, Chen S, Yin Y, Zrenner B. 2015. The role of continuous positive airway pressure in blood pressure control for patients with obstructive sleep apnea and hypertension: a meta-analysis of randomized controlled trials. J Clin Hypertens (Greenwich) 17(3):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mai W, Hu Y, Wu Y, Song Y, Qiu R, Dong Y, Kuang J. 2011. Poor sleep quality, stress status, and sympathetic nervous system activation in nondipping hypertension. Blood Press Monit 16(3):117–123. [DOI] [PubMed] [Google Scholar]
- Iftikhar IH, Valentine CW, Bittencourt LR, Cohen DL, Fedson AC, Gislason T, Penzel T, Phillips CL, Yu-sheng L, Pack AI, Magalang UJ. 2014. Effects of continuous positive airway pressure on blood pressure in patients with resistant hypertension and obstructive sleep apnea: a meta-analysis. J Hypertens 32(12):2341–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javaheri S, Barbe F, Campos-Rodriguez F, Dempsey JA, Khayat R, Malhotra A, Martinez-Garcia MA, Mehra R, Pack AI, Polotsky VY, Redline S, Somers VK. 2017. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 69(7):841–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. 2002. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms 17(2):181–193. [DOI] [PubMed] [Google Scholar]
- Kuniyoshi FH, Garcia-Touchard A, Gami AS, Romero-Corral A, van der Walt C, Pusalavidyasagar S, Kara T, Caples SM, Pressman GS, Vasquez EC, Lopez-Jimenez F, Somers VK. 2008. Day-night variation of acute myocardial infarction in obstructive sleep apnea. J Am Coll Cardiol 52(5):343–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie P, Yoffe N, Berger I, Peled R. 1993. The relationship between the severity of sleep apnea syndrome and 24-h blood pressure values in patients with obstructive sleep apnea. Chest 103(3):717–721. [DOI] [PubMed] [Google Scholar]
- Lewy AJ and Sack RL. 1989. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int 6(1):93–102. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL, Miller LS, Hoban TM. 1987. Antidepressant and circadian phase-shifting effects of light. Science 235(4786):352–354. [DOI] [PubMed] [Google Scholar]
- Mansoor GA. 2002. Sleep actigraphy in hypertensive patients with the ‘non-dipper’ blood pressure profile. J Hum Hypertens 16(4):237–242. [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia MA, Capote F, Campos-Rodriguez F, Lloberes P, Diaz de Atauri MJ, Somoza M, Masa JF, Gonzalez M, Sacristan L, Barbe F, Duran-Cantolla J, Aizpuru F, Manas E, Barreiro B, Mosteiro M, Cebrian JJ, de la Pena M, Garcia-Rio F, Maimo A, Zapater J, Hernandez C, Grau SanMarti N, Montserrat JM. 2013. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA 310(22):2407–2415. [DOI] [PubMed] [Google Scholar]
- Martins EF, Martinez D, da Silva F, Sezera L, da Rosa de Camargo R, Fiori CZ, Fuchs FD, Moraes RS. 2017. Disrupted day-night pattern of cardiovascular death in obstructive sleep apnea. Sleep Med 38:144–150. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS. 2012. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokhlesi B, Hagen EW, Finn LA, Hla KM, Carter JR, Peppard PE. 2015. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 70(11):1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira S, Rodrigues R, Barros AB, Pejanovic N, Neves-Costa A, Pedroso D, Pereira C, Fernandes D, Rodrigues JV, Barbara C, Moita LF. 2017. Changes in expression of the CLOCK gene in obstructive sleep apnea syndrome patients are not reverted by continuous positive airway pressure treatment. Front Med (Lausanne) 4:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CJ, Purvis TE, Mistretta J, Scheer FA. 2016. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J Clin Endocrinol Metab 101(3):1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T, et al. 1985. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 313(21):1315–1322. [DOI] [PubMed] [Google Scholar]
- Nelson W, Tong YL, Lee JK, Halberg F. 1979. Methods for cosinor-rhythmometry. Chronobiologia 6(4):305–323. [PubMed] [Google Scholar]
- Nieto FJ, Young TB, Lind BK, Shahar E, Samet JM, Redline S, D’Agostino RB, Newman AB, Lebowitz MD, Pickering TG. 2000. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA 283(14):1829–1836. [DOI] [PubMed] [Google Scholar]
- Noda A, Okada T, Hayashi H, Yasuma F, Yokota M. 1993. 24-hour ambulatory blood pressure variability in obstructive sleep apnea syndrome. Chest 103(5):1343–1347. [DOI] [PubMed] [Google Scholar]
- Pepin JL, Chiquet C, Tamisier R, Levy P, Almanjoumi A, Romanet JP. 2010. Frequent loss of nyctohemeral rhythm of intraocular pressure restored by nCPAP treatment in patients with severe apnea. Arch Ophthalmol 128(10):1257–1263. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. 2010. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int 27(9–10):1911–1929. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA. 2010. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci U S A 107(47):20541–20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif F, Patel SR, Walia HK, Rueschman M, Bhatt DL, Blumenthal RS, Quan SF, Gottlieb DJ, Lewis EF, Patil SP, Punjabi NM, Babineau DC, Redline S, Mehra R. 2014. Obstructive sleep apnea and diurnal nondipping hemodynamic indices in patients at increased cardiovascular risk. J Hypertens 32(2):267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senaratna CV, Perret JL, Lodge CJ, Lowe AJ, Campbell BE, Matheson MC, Hamilton GS, Dharmage SC. 2017. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med Rev 34:70–81. [DOI] [PubMed] [Google Scholar]
- Shea SA, Hilton MF, Hu K, Scheer FA. 2011. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res 108(8):980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolensky MH, Hermida RC, Castriotta RJ, Portaluppi F. 2007. Role of sleep-wake cycle on blood pressure circadian rhythms and hypertension. Sleep Med 8(6):668–680. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP, Abboud FM. 1995. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 96(4):1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, White DP, Amin R, Abraham WT, Costa F, Culebras A, Daniels S, Floras JS, Hunt CE, Olson LJ, Pickering TG, Russell R, Woo M, Young T. 2008. Sleep apnea and cardiovascular disease: an American Heart Association/American College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation 118(10):1080–1111. [DOI] [PubMed] [Google Scholar]
- Thosar SS, Berman AM, Herzig MX, McHill AW, Bowles NP, Swanson CM, Clemons NA, Butler MP, Clemons AA, Emens JS, Shea SA. 2019. Circadian rhythm of vascular function in midlife adults. Arterioscler Thromb Vasc Biol 39(6):1203–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thosar SS, Butler MP, Shea SA. 2018. Role of the circadian system in cardiovascular disease. J Clin Invest 128(6):2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomfohr LM, Edwards KM, Dimsdale JE. 2012. Is obstructive sleep apnea associated with cortisol levels? A systematic review of the research evidence. Sleep Med Rev 16(3):243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twidale N, Taylor S, Heddle WF, Ayres BF, Tonkin AM. 1989. Morning increase in the time of onset of sustained ventricular tachycardia. Am J Cardiol 64(18):1204–1206. [DOI] [PubMed] [Google Scholar]
- Ueyama T, Krout KE, Nguyen XV, Karpitskiy V, Kollert A, Mettenleiter TC, Loewy AD. 1999. Suprachiasmatic nucleus: a central autonomic clock. Nat Neurosci 2(12):1051–1053. [DOI] [PubMed] [Google Scholar]
- Van Dongen HP, Maislin G, Mullington JM, Dinges DF. 2003. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 26(2):117–126. [DOI] [PubMed] [Google Scholar]
- von Allmen DC, Francey LJ, Rogers GM, Ruben MD, Cohen AP, Wu G, Schmidt RE, Ishman SL, Amin RS, Hogenesch JB, Smith DF. 2018. Circadian dysregulation: the next frontier in obstructive sleep apnea research. Otolaryngol Head Neck Surg 159(6):948–955. [DOI] [PubMed] [Google Scholar]
- Willich SN, Goldberg RJ, Maclure M, Perriello L, Muller JE. 1992. Increased onset of sudden cardiac death in the first three hours after awakening. Am J Cardiol 70(1):65–68. [DOI] [PubMed] [Google Scholar]
- Yang MY, Lin PW, Lin HC, Lin PM, Chen IY, Friedman M, Hung CF, Salapatas AM, Lin MC, Lin SF. 2019. Alternations of circadian clock genes expression and oscillation in obstructive sleep apnea. J Clin Med 8(10):E1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. 1997. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med 157(15):1746–1752. [PubMed] [Google Scholar]
- Zeidan-Shwiri T, Aronson D, Atalla K, Blich M, Suleiman M, Marai I, Gepstein L, Lavie L, Lavie P, Boulos M. 2011. Circadian pattern of life-threatening ventricular arrhythmia in patients with sleep-disordered breathing and implantable cardioverter-defibrillators. Heart Rhythm 8(5):657–662. [DOI] [PubMed] [Google Scholar]


