Abstract
Parkinson's disease (PD) is a chronic and progressive neurodegenerative disease caused by degeneration of dopaminergic neurons in the substantia nigra. Existing pharmaceutical treatments offer alleviation of symptoms but cannot delay disease progression and are often associated with significant side effects. Clinical studies have demonstrated that acupuncture may be beneficial for PD treatment, particularly in terms of ameliorating PD symptoms when combined with anti-PD medication, reducing the required dose of medication and associated side effects. During early stages of PD, acupuncture may even be used to replace medication. It has also been found that acupuncture can protect dopaminergic neurons from degeneration via antioxidative stress, anti-inflammatory, and antiapoptotic pathways as well as modulating the neurotransmitter balance in the basal ganglia circuit. Here, we review current studies and reflect on the potential of acupuncture as a novel and effective treatment strategy for PD. We found that particularly during the early stages, acupuncture may reduce neurodegeneration of dopaminergic neurons and regulate the balance of the dopaminergic circuit, thus delaying the progression of the disease. The benefits of acupuncture will need to be further verified through basic and clinical studies.
1. Introduction
Parkinson's disease (PD) is a chronic and progressive neurodegenerative disease typically affecting middle-aged and elderly people. The key clinical manifestation is the dysfunction of voluntary motor regulation, including bradykinesia, resting tremor, myotonia, and postural instability [1]. In addition to motor dysfunction, nonmotor symptoms may arise during progression of PD, including cognitive dysfunction, emotional disorders such as apathy, depression, anxiety, and hallucinations, and autonomic nervous dysfunction, such as constipation, hyposmia, and sleep disorders [2]. PD has a prevalence of 0.3% and is thus the second most common neurodegenerative disease after Alzheimer's disease [3]. Tragically, the physical status of PD patients declines even when treated, often deteriorating to severe disability and death. Treatment costs impose enormous financial demands on patients and their families, incurring over US$14 billion annually [4].
Pathologically, PD is characterized by the loss and degeneration of dopaminergic neurons in the substantia nigra (SN), α-synuclein (α-syn) deposition, and Lewy body formation [5]. Current treatment options for PD include medication, surgery, and exercise therapy, although medication is the first choice for PD, with the most commonly prescribed drugs being dopaminergic and anticholinergic agents [2]. These have been shown to control the initial symptoms of patients rapidly and effectively [6], but after a so-called “honeymoon period” of treatment, the effectiveness often decreases and medication may no longer be beneficial for patients with advanced PD. The fundamental reason for this phenomenon is that existing anti-PD drugs offer only symptomatic treatment but cannot delay disease progression. Moreover, anti-PD drugs can also lead to considerable side effects which can aggravate PD symptoms, and some of which still lack effective treatment [7]. Many patients with PD have therefore turned to complementary and alternative therapies which may be used in the early stages of PD or prior to the use of levodopa (L-DOPA) to relieve symptoms and delay PD progression, with the potential expectation that these therapies may also reverse or delay neuronal degeneration [8].
Acupuncture is an economical therapy in traditional Chinese medicine originating from China where it has been developed for more than 4,000 years. It can promote health or treat diseases via various different techniques such as manual acupuncture (MA), electroacupuncture (EA), moxibustion, and acupressure on specific anatomical locations (i.e., acupoints) [9]. Numerous clinical trials have shown that acupuncture exhibits significant therapeutic benefits for PD patients, reducing both motor and nonmotor symptoms. Moreover, acupuncture may help to reduce the dose and frequency of anti-PD drugs as well as alleviate their side effects [10]. The therapeutic mechanisms of acupuncture have also been shown to involve a reduction of mitochondrial dysfunction, oxidative stress, protein aggregation, impaired autophagy, and neuroinflammation [11]. In the present review, we aim to address the clinical efficacy and potential mechanisms of acupuncture in PD treatment, providing novel insights for the clinical application of acupuncture for the reversal the degeneration of dopaminergic neurons and delay of PD progression.
2. Methods
2.1. Search Strategy
We searched the PubMed database for studies published between January 2000 and December 2020 containing the keywords “Acupuncture” and “Parkinson's disease”. Only studies in English language were included. This search identified relevant 183 articles.
2.2. Study Selection
Following the search engine selection of 183 articles, a manual search was performed by screening the reference list for articles that met our inclusion criteria based on the titles and abstracts. We excluded 43 articles due to absence of an abstract, unavailability of the full text, or irrelevance to the subject, leaving 140 articles, including 43 basic research articles, 38 clinical research articles, and 59 review articles or meta-analyses. The full texts of the 43 basic research and 38 clinical research articles were obtained and assessed carefully. Out of the 38 clinical studies, 17 were excluded as they described single case reports, editorials, and uncompleted or uncontrolled trials, resulting in 21 clinical articles meeting our inclusion criteria. All 43 basic research articles met our inclusion criteria. Thus, a total of 64 articles were included in our review. A flow chart of this search process is illustrated in Figure 1.
Figure 1.
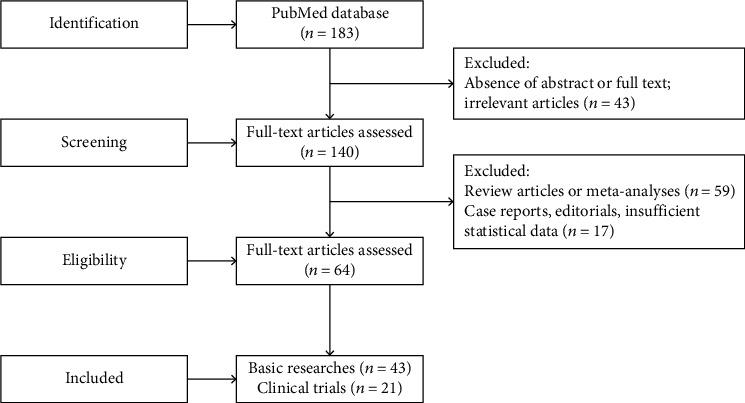
Flow chart of the review processes.
2.3. Data Extraction
Two authors independently assessed the titles and abstracts of the retrieved articles and evaluated the full-text articles. The data from finally included articles were validated and extracted according to the predefined criteria. Any disagreement was resolved by discussion between the authors.
3. Clinical Efficacy of Acupuncture in Parkinson's Disease Patients
Acupuncture is commonly and globally used as both an adjuvant therapy and monotherapy for PD treatment [8]. Comprehensive evidence for the efficacy and safety of acupuncture in PD has been provided by a total of twelve systematic reviews (SRs) or meta-analyses (MAs) assessing this topic, involving 179 randomized controlled trials (RCTs) with a total of 11,717 participants [12]. These SRs/MAs have compared the effects of acupuncture combined with medication versus medication alone, demonstrating better efficacy at symptom alleviation with the combination of acupuncture with medication compared to medication alone [13]. The Unified Parkinson's Disease Rating Scale (UPDRS) provides a comprehensive, efficient, and flexible means of monitoring PD-related disabilities and impairments as well as treatment evaluation, assessing behavior and mood, activities of daily living, motor functions, and therapeutic complications [14]. Acupuncture combined with dopaminergic drugs was superior to medication only for improving the UPDRS score [15]. Interestingly, when comparing acupuncture only with medication only, there is no significant difference in the scores. Moreover, evidence-based medicine (EBM) studies have analyzed 13 parallel RCTs and found no difference in the efficacy of acupuncture compared with medication for PD treatment [10], suggesting that acupuncture may achieve an efficacy close to that of medication. The Webster Scale is applied to assess the clinical severity of PD, rating levels of akinesia, rigidity, upper limb swing, gait, tremor, facial appearance, speech, and self-care [16]. Compared with medication only, acupuncture and medication combined have been shown to result in a greater Webster score reduction, indicating an alleviation of clinical severity [12], and compared with medication only, acupuncture alone similarly exhibited a positive effect for decreasing the Webster score [10], suggesting that acupuncture alone may be able to effectively reduce the severity of PD. EBM reviews reveal that even with an optimized oral dose of L-DOPA, there is a risk for a worsening of movement disorders, and a variety of anti-PD drugs may also induce or exacerbate nonmotor symptoms [17]. Hence, treatment with L-DOPA and other anti-PD drugs should be delayed as much as possible in patients with early-stage PD. If acupuncture achieves a similar therapeutic effect to currently available medication, it may represent a new therapeutic option for PD, especially for early stages. There is no evidence for any serious adverse reactions associated with acupuncture, suggesting that it is a relatively safe choice for PD patients. Therefore, the current evidence indicates that acupuncture may enhance the therapeutic efficacy of drugs and reduce the required dose of medication, thus also alleviating adverse effects.
Besides UPDRS and Webster scales, there are more targeted evaluation criteria corresponding to different PD symptoms [18]. The characteristics of the 15 clinical studies included in our analysis are summarized in Table 1. Using wearable sensors, Lei et al. [19] found that EA had overall beneficial impact on different temporal and spatial parameters of gait (gait speed and stride length) as well as dynamic posture control (increase of midswing angular velocity and decrease of double support). Kong et al. [20] evaluated changes in the General Fatigue score of the Multidimensional Fatigue Inventory after acupuncture treatment and found that 5-week acupuncture treatment effectively alleviated moderate and severe fatigue of PD patients. These results are similar to reports of an RCT by Kluger et al. assessing the relief of PD with fatigue by acupuncture [21]. Using functional magnetic resonance imaging (fMRI), Yu et al. [22] found enhanced connectivity in four junctions via fMRI after acupuncture. There was a significant correlation between changes in functional connectivity and the King's Parkinson's Disease Pain Scale, indicating that acupuncture relieves pain in PD patients via modulation of brain regions related to both sensory-discriminative and emotional aspects. In an unblinded trial including 20 PD patients, no adverse reactions were reported with an improvement of individual symptoms including tremor, walking, writing, bradykinesia, pain, sleep, depression, and anxiety in 85% of patients [23]. An RCT by Cristian et al. [24] showed a trend toward an improvement in the Parkinson's Disease Questionnaire, daily living activities, and UPDRS motor score following acupuncture, suggesting that acupuncture has a potential effect for alleviation of both movement and nonmotor symptoms including pain, depression, and autonomic symptoms. Using the Parkinson Disease Sleep Scale, Aroxa et al. [25] found that acupuncture was beneficial for the alleviation of sleep disorders in PD patients. Furthermore, acupuncture may be beneficial for improving oral cavity function as observed by increased mean tongue pressure and decreased average saliva swallowing reflex latency in PD patients after acupuncture [26].
Table 1.
The clinical efficacy of acupuncture in the patients with Parkinson's disease.
| Study | Clinical trial design | Clinical condition | Intervention | Comparison | Acupoints | Acupuncture parameters | Outcomes |
|---|---|---|---|---|---|---|---|
| Lei et al. (2016) [19] | RCT | PD patients | EA (n = 10) | Sham EA (n = 5) | GV20, GV14; bilateral foot motor, sensory area, balance area, LI4, ST36, GB34, BL40, SP6, KI3, LR3 | Asymmetric biphasic square wave pulse, 100 μS pulse width, “deqi”, 100 Hz or 4 Hz, three times a week, 3 weeks | Primary outcome: STHW↑, STFW↑, DTFW↑, and DTHW↑ Secondary outcome: UPRDS scores↓ |
|
| |||||||
| Kong et al. (2017) [20] | RCT | PD patients with moderately severe fatigue | Acupuncture (n = 20) | Sham acupuncture (n = 20) | CV6; bilateral PC6, ST36, LI4, SP6, KI3 | 5-30 inches, no flicking or rotation of needles after insertion, 20 minutes, twice a week, at least 3 days apart, 5 weeks, 10 sessions | Primary outcome: MFI-GF↓ Secondary outcome: MFI-total score↓, UPDRS-motor↓ |
|
| |||||||
| Kluger et al. (2016) [21] | RCT | PD patients with moderate or severe fatigue | EA (n = 47) | Sham acupuncture (n = 47) | GV20, GV24; bilateral LI10, HT7, ST36, SP6 | 0.5-1 cm, twisted three times to the right, 30 minutes, every two weeks, 6weeks | Primary outcome: MFIS (total, physical, cognitive, psychosocial)↓ Secondary outcomes: PDQ-39↓, HADS (depression)↓, ESS↓, AES↓ |
|
| |||||||
| Yu et al. (2019) [22] | Comparison trials | PD patients with pain | Acupuncture+medication (n = 9) | Medication (n = 7) | GV20; bilateral 77.18, GB34 | 5-10 mm, “deqi” for 20 s, 30 minutes, 1-3 sessions per week, separated by at least 1 day, 8 weeks | Primary outcome: KPPS↓, UPDRS (total)↓, enhanced connectivity at the four areas, a significant correlation between functional connectivity changes and KPPS |
|
| |||||||
| Shulman et al. (2002) [23] | Controlled trial | Idiopathic PD patients (stages I-III) | EA (n = 20) | Self-control | Bilateral LI4, GB34, ST36, K3, KI7, SP6, SI3, TB5 | Intensity knob: 0; electric exciter switch: 1; wave form: intermittent; twice a week, 10/16 treatments | Sleep and rest category: SIP (sleep and rest)↓ |
|
| |||||||
| Cristian et al. (2005) [24] | RCT | PD patients (stages II-III) | EA (n = 7) | Sham acupuncture group (n = 7) | GV20; bilateral K3, K10, BL60, L3, ST41, ST36, GB34, bafeng points, MH6, LI4 | 4 Hz, 20 minutes, 5 sessions, 2 weeks | NS |
|
| |||||||
| Aroxa et al. (2016) [25] | RCT | Idiopathic PD patients (stages I-III) | Acupuncture+medication (n = 11) | Medication (n = 11) | Bilateral LR3, SP6, LI4, TE5, HT7, PC6, LI11, GB20 | 30 minutes, once a week, 8 weeks | Sleep disorders evaluation: PDSS↑ |
|
| |||||||
| Fukuda et al. (2016) [26] | Controlled trial | PD patients (stages I-III) | Acupuncture (n = 13) | Self-control | Bilateral ST36, SP6, LR3, LI4, LI11, GB20, BL18, BL23 | Inserted perpendicularly, 10-20 mm, 10-15 minutes, only once | Tongue function: mean tongue pressure↑, mean swallowing reflex latency↓, saliva swallow↓ |
|
| |||||||
| Chen et al. (2015) [2] | Comparison clinical trial | PD patients | Acupuncture+medication (n = 20) | Medication (n = 20) | DU20; bilateral GB20, LI11, LI10, LI4, GB31, ST32, GB34, GB38 | MA, 5-30 mm, 15 minutes, twice a week, 18 (short-term)/36 (long-term) weeks | Short-term: UPDRS (total scores and subscores I, II, III, and IV)↓, BDI-II score↓, WHO-QOL score↓ Long-term: UPDRS (total scores and subscores I, II, III, and IV)↓, BDI-II score↓ |
|
| |||||||
| Wang et al. (2002) [27] | Controlled trial | PD patients (stages I-III) | Acupuncture (n = 29) | Blank control (n = 14) | Group 1: DU20; bilateral GB20, LI4, SP6, LR3; group 2: Extra6; bilateral LI11, SJ5, GB34, ST36, ST40, Extra21 | MA at Extra6, GB13, and GB20, then electric stimulator with continuous wave for 15 minutes; the rest acupoints: uniform reinforcing-reducing method, 40 minutes; once every other day, 3 months | The Webster's cumulative scores↓, correlation analysis of ABP indices and the cumulative scores in Webster's scale↓, the latent period of V wave and the intermittent periods of III-V peak and I-V peak↓ |
|
| |||||||
| Yeo et al. (2018) [28] | Controlled trial | Idiopathic PD patients | EA+MA (n = 10) | Self-control | Right GB34, LR3 (EA) Bilateral LR3, LI11, ST36, GB20, SP6, LI4, GB34 (MA) |
EA: 15 minutes, 120 Hz, twice a week, 8 weeks; MA: 3 to 15 mm, 15 minutes; twice a week, 8 weeks | UPDRS (total, 1, 2, 3, and4) score↓, BDI-II scores↓, neural responses (thalamus, cingulate gyrus, anterior cingulate, lingual gyrus, parahippocampal gyrus, lateral globus pallidus, mammillary body, middle temporal gyrus, cuneus, and fusiform gyrus)↑ |
|
| |||||||
| Cho et al. (2017) [29] | RCT | Idiopathic PD patients (stages I-IV) | Acupuncture+BVA (n = 29) | Sham treatment (n = 29)/conventional treatment (n = 15) | Bilateral GB20, LI11, GB34, ST36, LR3 | BVA: injected diluted bee venom 0.1 ml; EA: 1-1.5 cm, rotated at 2 Hz for 10 seconds to achieve “deqi”, 15 minutes; twice a week, 12 weeks | Primary outcome: UPDRS (part II+III) scores↓ Secondary outcomes: UPDRS (part II and III) scores↓, gait speed↓, gait number↑, PIGD↓ |
|
| |||||||
| Eng et al. (2006) [30] | Controlled trial | Idiopathic PD patients | Acupuncture+Yin Tui Na (n = 25) | Self-control | Bilateral ST42, SP3, LI11, LI15, LI20, ST7, ST36 | 7-10 minutes, 8-14 Hz, once a week, 6 months, 24 sessions | UPDRS (I and II) scores↓, UPDRS (III) scores↑, PDQ-39 total score↓, BDI↓ |
|
| |||||||
| Doo et al. (2015) [31] | Controlled trial | Idiopathic PD patients | Acupuncture and BVA+conventional treatment (n = 11) | Self-control | Bilateral GB20, LI4, GB34, ST36, LR3 | BVA: injected diluted bee venom 0.1 ml; EA: 1-1.5 cm, rotated at 2 Hz for 10 seconds to achieve “deqi”, 15 minutes; twice per week, 12 weeks | Primary outcome: UPDRS (part II+III) scores↓ Secondary outcomes: UPDRS (part II and III) scores↓, gait speed ↓, PDQL score↑ |
|
| |||||||
| Ren (2008) [32] | Controlled trial | PD patients | Acupuncture+Madopar (n = 50) | Madopar (n = 30) | From TE14 to TE2, from PC2 to PC7 (areas being tapped) Bilateral TE4, LI5, PC7, SI6, LI11, LU5, PC3, HT3, TE14, LI15, SI9, LR4, KI3, ST41, SP9, GB34, BL40, GB30, BL36 (MA) |
Tap: with a plum-blossom needle, until skin turns red; MA: uniform reinforcing and reducing method, 30 minutes; once a day, 10 sessions a course, 3-5 days between courses, 2 courses | Total effective rate↑, the minimum dose of Madopar needed↓ |
RCT: randomized controlled trial; PD: Parkinson's disease; EA: electroacupuncture; GV20: Baihui; GV14: Dazhui; LI4: Hegu; ST36: Zusanli; GB34: Yanglingquan; BL40: Weizhong; SP6: Sanyinjiao; KI3: Taixi; LR3: Taichong; STHW: single-task habitual walking; STFW: single-task fast walking; DTFW: dual-task fast walking; DTHW: dual-task habitual walking; UPDRS: Unified Parkinson's Disease Rating Scale; PC6: Neiguan; CV6: Qihai; MFI-GF: General Fatigue score of the Multidimensional Fatigue Inventory; GV24: Shenting; LI10: Shousanli; HT7: Shenmen; MFIS: Modified Fatigue Impact Scale; PDQ-39: 39-item Parkinson's Disease Questionnaire; HADS: Hospital Anxiety and Depression Scale; ESS: Epworth Sleepiness Scale; AES: Apathy Evaluation Scale; 77.18: Shenguan; KPPS: King's Parkinson's Disease Pain Scale; KI7: Fuliu; SI3: Houxi; TB5: Waiguan; SIP: Sickness Impact Profile; KI10: Yingu; BL60: Kunlun; NS: no significance; ST41: Jiexi; MH6: Yinwei; TE5: Waiguan; LI11: Quchi; GB20: Fengchi; PDSS: Parkinson's Disease Sleep Scale; BL18: Ganshu; BL23: Shenshu; DU20: Baihui; GB31: Fengshi; ST32: Futu; GB38: Juegu; MA: manual acupuncture; BDI-II: Beck Depression Inventory-Version 2; WHO-QOL: WHO quality of life; Extra6: Sishencong; SJ5: Waiguan; ST40: Fenglong; Extra21: Huatuojiaji; GB13: Benshen; ABP: auditory evoked brain stem potential; TE14: Jianliao; TE2: Yemen; PC2: Tianquan; PC7: Daling; TE4: Yangchi; LI5: Yangxi; SI6: Yanglao; LU5: Chize; PC3: Quze; HT3: Shaohai; LI15: Jianyu; SI9: Jianzhen; LR4: Zhongfeng; SP9: Yinlingquan; GB30: Huantiao; BL36: Chengfu; BVA: bee venom acupuncture; PIGD: postural instability and gait disturbance; PDQL: Parkinson's Disease Quality of Life Questionnaire; ST42: Chongyang; SP3: Taibai; LI20: Yingxiang; ST7: Xiaguan.
Taken together, the clinical studies above have concluded that acupuncture exhibits a comprehensive beneficial effect on symptoms associated with dyskinesia, psychology, behavior, emotion, and cognition of PD patients. Additionally, there are various types of acupuncture methods, such as MA, EA, and bee venom acupuncture (BVA). A combination of body and scalp acupoints is typically used in PD patients, with scalp acupoints located in the occipital, frontal, and parietal lobes as well as in motor and chorea-tremor controlled areas [33]. It has been shown that scalp acupoints cannot only directly activate and adjust functional areas of the corresponding cerebral cortex but also convey certain therapeutic advantages for movement disorders [34]: EA at scalp acupoints has been found to reduce the loss of dopaminergic neurons in the SN and delay the degeneration of dopaminergic neurons, thereby alleviating clinical symptoms and postponing the progression of PD [35]. Therefore, scalp acupoints are an indispensable part of PD acupuncture treatment. In clinical PD acupuncture trials, the acupoints LR3 (Taichong), GB34 (Yanglingquan), and ST36 (Zusanli) were the primary body acupoints used, followed by LI4 (Hegu), LI11 (Quchi), and SI3 (Houxi) [2]. Previous reports showed that GB34 significantly improves PD symptoms, in particular motor dysfunction, and attenuates dopaminergic neuronal loss in animal models of PD [36]. Other acupoints such as ST36 [37] and LR3 [38] displayed the same effects. Several preclinical randomized studies have demonstrated that acupuncture stimulation at GB34, GV20, and LR3 modulates PD-associated brain regions and results in an amelioration of locomotor function closely associated with a reduction of neuronal apoptosis in the striatum (ST) and SN [39]. Thereby, these three acupoints have been recommended as the basic setting of acupuncture in clinical treatment of PD according to the World Health Organization standards [40].
In the clinical application of acupuncture, acupoints are also selected according to different PD symptoms and complications [26, 27]. For instance, acupoints LR3, KI6 (Zhaohai), and KI7 (Fuliu) located in the lower leg are expected to relieve pain; acupoints HT3 (Shaohai), LI7 (Wenliu), and SI3 are stimulated in severe tremor; moxibustion at acupoints SP21 (Dabao) and LR14 (Qimen) or acupuncture at acupoints DU16 (Fengfu), DU14 (Dazhui), and Extra (Baliao) is used for obvious neck stiffness; acupoints DU4 (Mingmen) and UB23 (Shenshu) are stimulated for low back pain; BL15 (Xinshu), LR3, and SP6 (Sanyinjiao) points for depression and anxiety; acupoints CV23 (Lianquan) and ST4 (Dicang) for dysphagia; acupoints SP15 (Daheng), ST25 (Tianshu), CV6 (Qihai), and SJ6 (Zhigou) for constipation; acupoints CV11 (Jianli) and P6 (Neiguan) for fullness in the chest and epigastrium; and LR3, KI7, and BL18 (Ganshu), BL15, GB34, and UB13 (Feishu), UB20 (Pishu), and CV6 for hot flashes and paroxysmal sweating.
Taken together, a host of clinical studies have shown that acupuncture is beneficial for PD patients, especially for reducing the required dose of medication and related side effects [41]. However, there are several limitations in current acupuncture studies. First, outcome measures of most studies are subjective and vulnerable to potential biases, potentially reducing the credibility of the efficacy of acupuncture. Second, only few articles have defined acupoints according to different pathological stages or symptoms. Appropriate acupoints should be added according to different symptoms of patients. Third, the acupuncture protocol and stimulating parameters differ between trials. In the future, research on acupuncture treatment for PD should be carried out with higher methodological quality, optimized acupoint selection, frequency, duration, and study of long-term effects.
4. Mechanism of Acupuncture Protecting from Dopaminergic Neurodegeneration
Extensive studies have shown that genetic predisposition [42], environmental factors [43], and a variety of intracellular and extracellular pathogenic factors contribute to the development of PD, which is characterized by selective loss of dopaminergic neurons in the SN, depletion of dopamine (DA) in the ST, and the formation of Lewy bodies that are mainly composed of α-syn [44]. The reduction of striatal DA levels disrupts the neurotransmitter balance in the basal ganglia and thalamus, resulting in motor symptoms such as paralysis agitans, bradykinesia, and rigidity [45]. Loss of DA and changes in neurotransmitters such as serotonin (5-HT), norepinephrine (NE), γ-aminobutyric acid (GABA), and glutamate (Glu) in different brain regions and the peripheral nervous system lead to the occurrence of nonmotor symptoms, such as autonomic nervous dysfunction, neuropsychiatric disorders, sleep disorders, and gastrointestinal symptoms [46].
After reviewing 31 basic studies on acupuncture treatment of PD models (Table 2), we found that MA and EA were most commonly utilized, although BVA, moxibustion, acupoint injection, and laser acupuncture were also reported. It is worth mentioning that EA at high frequencies shows better efficacy than low frequencies in some animal models of PD [47, 48]. The most commonly selected acupoint was GB34 followed by LR3. Thirty studies showed that acupuncture improves motor function in PD models as assessed by the rotarod test, the cylinder test, and the pole test. Basic studies suggest that acupuncture may achieve its effectiveness for PD treatment by preventing the DA neurons from α-syn aggregation, apoptosis, oxidative stress, and modulating neuroinflammation and the basal ganglia circuit around DA neurons, which we have outlined in detail below.
Table 2.
The neuroprotective mechanisms of acupuncture in the treatment of Parkinson's disease.
| Study | PD model | Intervention | Acupoints | Acupuncture parameters | Behavioral measurements | Biochemical measurements |
|---|---|---|---|---|---|---|
| Hong et al. (2010) [49] | MPTP mice | MA | GB34 | Turned at a rate of two spins per second for 15 s | — | Grm5↓, Itpr1↓, Itpr2↓, Pde1c↓, Prkcb1↓, Plcb4↓, Atp2b3↓, Slc8a3↑, Htr5a↑, Adcy1↑, Drd5↓, Npy1r↓, Gpr156↓, Cxcl10↓, Rxfp2↑, Agtrl1↑, Inhbb↓, Cxcl5↓, IL21↓, Cox6a1↓, Uqcrq↓, Ndufc1↓, Atp5e↓, Mapt↓, Ngfr↓, Nefh↓ |
|
| ||||||
| Choi et al. (2011) [50] | MPTP mice | MA | GB34, LR3 | Turned at a rate of two spins per second for 15 s | — | TH levels in the ST and the SNpc↑, DAT↑, Ctla2a↓, EG383229↓, Ppbp↓, Ube2l6↓, EG665033↑, ENSMUSG00000055323↑, Obox6↑, Pbp2↑, Tmem150↑ |
|
| ||||||
| Yeo et al. (2013) [51] | MPTP mice | MA | GB34, LR3 | Turned at a rate of two spins per second for 15 s | — | TH in the ST, SNpc, and thalamus↑, Dusp4↑, Mafg↑, Pgm5↑, Ndph↑, Dnase1l2↑, Ucp2↓, Sp2↓, Serinc2↓ |
|
| ||||||
| Yeo et al. (2015) [39] | MPTP mice | MA | GB34, LR3 | Turned at a rate of two spins per second for 15 s | — | TH in the ST and SNpc↑, Cdh1↓, Slc6a13↓, Tph2↓, Mpzl2↓, Serping1↓, Itih2↓, Slc6a20a↓, Slc6a4↓, Ucma↓, Rdh9↓, 4921530L21Rik↑, Gm13931↑ |
|
| ||||||
| Jeon et al. (2008) [52] | MPTP mice | EA | GB34 | 2/100 Hz, 1 mA for 20 minutes | Pole test: time on the pole↓ | TH-positive cells in the SN↑, CyPA in the SN↑, MBP↑, Ube2N↑, LMP6↑, LMP2↑, lipocalin/cytosolic fatty acid-binding protein↑, eIF5A↑, CLP↑, ATPase, H1-transporting V1 subunit F↑, NSF↑ |
|
| ||||||
| Wang et al. (2013) [53] | MPTP mice | EA | ST36, SP6 | 0/100 Hz, 1-1.25-1.5 mA for 30 minutes | Open-field test: total movement time↓, movement velocity↓, total movement distance↓ | — |
|
| ||||||
| Kim et al. (2005) [54] | 6-OHDA rats | MA | ST36 | Left in acupoint for 20 minutes | Rotational behavior test: the average net number of turns/min↓ | The survival rate of TH-positive neurons↑, the density of TH-positive fibers↑ |
|
| ||||||
| Yang et al. (2011) [55] | MPTP mice | RA/EA | PC7 | Left in acupoint for 15 minutes/2 and 15 Hz, alternatively, 1 mA for 15 minutes | Pole test: descending time↓ | TH-positive neurons (RA)↑, DA in the SN↓, the intensity of radionuclide or radiopharmaceutical uptake (RA)↑, the peak time of [123I]IBZM uptake (RA)↓, HVA concentration in the SN (EA)↓ |
|
| ||||||
| Yeo and Lim (2019) [56] | MPTP mice | MA | GB34, LR3 | Turned at a rate of two spins per second for 15 s | — | TH in the ST and SNpc↑, α-syn in the SN↓, SGK1↑ |
|
| ||||||
| Yeo et al. (2020) [57] | MPTP mice | MA | GB34, LR3 | Turned at a rate of two spins per second for 15 s | — | TH in the ST and SNpc↑, α-syn in the ST and SN↓, p-α-syn 32↓, p-α-syn 16↓, p-α-syn 32/total α-syn values↑ |
|
| ||||||
| Tian et al. (2016) [58] | MPTP mice | MA | GB34 | Turned at a rate of two spins per second for 15 s, retained for 10 minutes | Rotarod test: the ORP scores↑ | α-syn in the SNpc↓, autophagosome accumulation↓, LC3-II↓, LAMP1↓, the number and function of DA neurons↑, density of TH-positive neurons in the SNpc and ST↑, DA concentration↑, synaptophysin protein↑ |
|
| ||||||
| Wang et al. (2018) [59] | Rotenone rats | Moxibustion | ST36, CV4, GV16 | Placed 2-3 cm above each acupoint, retained for 10 minutes | Behavioral scoring [60]↓ | TH-immunoreactive neurons↑, α-syn-positive aggregates↓, p-mTOR and p-p70S6K in the SNpc↓, LC3-II in the SNpc↑ |
|
| ||||||
| Song et al. (2020) [37] | MPTP mice | Acupoint injection | ST36 | Choroid plexus cells were injected into ST36, a depth of 3 mm, maintained for 1 week | Pole test: exercise capacity↑ | TH in the SNpc↑, Bax↓, cytochrome C↓, BCL-2↑, COX-2↓, iNOS↓ |
|
| ||||||
| Park et al. (2015) [61] | MPTP mice | MA | GB34 | Turned at a rate of two spins, per second for 15 s | Rotarod test: latency time↑; cylinder test: the number of wall touches↑ | TH-positive neurons in the SN↑, TH-positive fibers↑, 40 of 76 differentially expressed genes are involved in the p53 signaling network |
| MPTP+P53 knockout mice | MA | GB34 | Turned at a rate of two spins, per second for 15 s | — | TH-positive cells and fibers slightly↑ | |
|
| ||||||
| Doo et al. (2010) [62] | MPTP mice | BVA | GB34 | — | — | TH-positive neurons in the SN↑, DA fibers in the ST↑, p-c-Jun-positive cells in the SN↓ |
|
| ||||||
| Liang et al. (2002) [63] | MFB rats | EA | GV14, GV20 | 2/100 Hz, 1-2-3 mA for 30 minutes | — | TH-positive neurons on the lesion side↑, BDNF mRNA expression on the lesioned side of the VTA (100 Hz)↑, BDNF mRNA in the SNpc (100 Hz)↑ |
|
| ||||||
| Wang et al. (2013) [64] | Rotenone rats | EA | GV16, LR3 | 100 Hz, 1-1.25-1.5 mA for 30 minutes | — | BDNF mRNA expression in the SN↑, GDNF mRNA expression in the SN↑ |
|
| ||||||
| Liang et al. (2003) [48] | MFB rats | EA | GV14, GV20 | 2/100 Hz, 1-2-3 mA for 30 minutes | Rotational behavior test: day 14 the average net number of turns/min↓, day 28 the average net number of turns/min (100 Hz)↓ | GDNF mRNA-positive cells in the SNpr (100 Hz)↑, GDNF mRNA expression in the GP of the unlesioned side (2 Hz)↑, GDNF mRNA level in both the lesioned and unlesioned side of GP (100 Hz)↑ |
|
| ||||||
| Jang et al. (2020) [65] | MPTP mice | MA | GB34, ST36 | Turned at a rate of two spins per second for 30 s | Cylinder test: the number of wall touches↑; rotarod test: latency time↑; akinesia test: latency time↓; open-field test: the total distance↑, central distance↑, the ratio of central/total distance↑, the number of crossings in the central zone↑ | Dopaminergic fibers and neurons in the ST and SN↑, TH-positive neurons in the ST and SN↑, TH in the ST and SN↑, Bax↓, Bcl-2↑, GFAP↓, Iba-1↓, NF-κB↓, TNF-α↓, the Chao1 index↑, the number of observed OTUs↑, the Shannon index↑, Rikenellaceae↑, Vallitaleaceae↑, Alistipes↑, Vallitalea↑, Lachnoclostridium↑, Pseudoclostridium↑, Bacteroides xylanolyticus↑, Vallitalea pronyensis↑, Clostridium aerotolerans↑, Pseudoclostridium thermosuccinogenes↑, Roseburia faecis↑, holdemania↓, frisingicoccus↓, aestuariispira↓, sporobacter↓, rhodospirillum↓, Bifidobacterium↓, turicibacter↓, marvinbryantia↓, desulfovibrio↓, phascolarctobacterium↓, erysipelatoclostridium↓, butyricimonas↑, gracilibacter↑, phocea↑, desulfitobacterium↑, oscillibacter↑, acutalibacter↑, flintibacter↑, photosynthesis↑, glutathione metabolism↑, tetracycline biosynthesis↑, photosynthetic proteins↑, methane metabolism↓, drug metabolism↓ |
|
| ||||||
| Park et al. (2003) [66] | 6-OHDA rats | MA | Acu1: GB34, LR3; Acu2: LI4, LI11 | Turned at a rate of two spins per second for 15 s, retained for 60 s | Rotational behavior test: the average net number of turns/min (Acu1)↓ | TH-immunoreactive neurons in the ipsilateral SN (Acu1)↑, TH-immunoreactive terminals in the dorsolateral ST ipsilateral to the lesion (Acu1)↑, TrkB-positive cells in the SN (Acu1)↑ |
|
| ||||||
| Lin et al. (2017) [67] | MPTP rats | EA | GB34, LR3 | 0/50 Hz, 1 mA for 20 minutes | Rotarod test: day 9 latency time↑; rotational behavior test: the average net number of turns/min↓ | DA content in the ST↑, TH-positive neurons in the SN↑, degeneration in the ST↓, DA depletion in the ipsilateral side↓, mature BDNF, Bcl-2 and TH levels in the ipsilateral side↑, pAkt↑ |
|
| ||||||
| Zhao et al. (2019) [68] | MFB rats | EA | GV20, GV29 | 2 Hz, 1.5 mA for 10 minutes | Rotarod test: latency time↑; tail suspension test: the immobility time↓ | TH in the midbrain↑, BDNF+ cells↑, pAkt↑, pERK↑, functional TrkB FL↑, the ratio of TrkB FL/TrkB T1↑ |
| MFB rats | EA+K252a injection | GV20, GV29 | 2 Hz, 1.5 mA for 10 minutes | Rotarod test: latency time↓; tail suspension test: the immobility time↑ | TH in the midbrain↓, BDNF+ cells↓, pAkt↓, pERK1/2↓ | |
|
| ||||||
| Kim et al. (2011) [69] | MPTP mice | MA | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: latency time↑, the ORP scores↑ | pAkt↑ |
| MPTP mice | MA+LY294002 | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: latency time↓, the ORP scores↓ | pAkt↓, TH-positive dopaminergic neurons in SN↓, TH-positive dopaminergic fibers in ST↓ | |
|
| ||||||
| Hwang et al. (2019) [70] | MPTP mice | MA+Chunggan formula | GB34 | Turned at a rate of two spins per second for 30s | Rotarod test: latency time↑; cylinder test: the number of wall touches↑; pole test: descending times↓ | TH-positive dopaminergic neurons in the SNpc↑, TH protein in the SNpc and ST↑, pIκBα↓, pAkt↑, pGSK3β↑, pERK↑, pCREB↑, BDNF↑ |
|
| ||||||
| Jeon et al. (2017) [71] | MPTP mice | MA | Acu1: GB34; Acu2: HT7 | Turned at a rate of two spins per second for 30 s | Pole test: day 5 descending times (GB34)↓, day 12 descending times↓ | TH-positive cells in the SN (GB34)↑, TH immunoreactivity in the ST (GB34)↑, BrdU-positive cells in the SVZ (GB34)↑, BrdU/DCX-double stained cells in the SVZ↓, BrdU/GFAP-double stained cells in the SVZ (GB34)↑, GFAP-positive cells in the ST↑ |
|
| ||||||
| Yu et al. (2010) [38] | 6-OHDA rats | MA | GB34, LR3, ST36, SP10 | Left in acupoints for 20 minutes | Rotational behavior test: the average net number of turns/min↓ | SOD↑, GSH-Px↑, GSH↑, MDA↓ |
|
| ||||||
| Wang et al. (2011) [47] | MPTP mice | EA | ST36, SP6 | 0/100 Hz, 1-1.25-1.5 mA for 30 minutes | — | TH immunoreactivity↑, day 3 DA, DOPAC, and HVA in the ST↑, H2O2 content in the ST↓, day 3 GSH content↑, days 3 and 7 GSH-Px↓, days 7 and 14 SOD activity↑, day 7 MDA content in the ST↓ |
|
| ||||||
| Lee et al. (2018) [72] | MPTP mice | MA | Acu1: GB34; Acu2: SI3 | Turned at a rate of two spins per second for 30 s | Pole test: descending times (GB34)↓ | TH-positive neurons in the SN (GB34)↑, caspase-3 in the ST (GB34)↓, DJ-1 in the ST (GB34)↑, SOD activity (GB34)↑, CAT activity (GB34)↑ |
|
| ||||||
| Kang et al. (2007) [73] | MPTP mice | MA | GB34, LR3 | Turned at a rate of two spins per second for 15 s | — | TH-immunoreactive in the ST↑, TH-positive neurons in the SN↑, MAC-1↓, COX-2↓, iNOS↓, DA, DOPAC, and HVA in the ST↑ |
|
| ||||||
| Li et al. (2019) [74] | 6-OHDA rats | MA | CV12, ST25, CV4 | Left in acupoints for 15 minutes | Rotational behavior test: the average net number of turns/min↓ | TH-positive neurons in the SN↑, TNF-α↓, IL-1β↓, Fe levels↓, ratio of Fpn1/DMT1 mRNA↓, α-syn in the duodenum↓ |
|
| ||||||
| Yang et al. (2017) [75] | MPTP mice | MA | GB34 | Turned at 2 Hz for 15 s | — | NS |
|
| ||||||
| Kim et al. (2019) [76] | Pitx3-deficient ak/ak mice | MA+L-DOPA | GB34 | Turned at a rate of two spins per second for 15 s | Three-paw dyskinesia test, abnormal three-paw movements↓; AIM scores↓ | Pmch↑, Tac2 mRNA↑, Lcn2 mRNA↑ |
| Pitx3-deficient ak/ak mice | MA+L-DOPA+TC-MCH7c | GB34 | Turned at a rate of two spins per second for 15 s | Three-paw dyskinesia test: abnormal three-paw movements↑ | — | |
| 6-OHDA mice | MA+L-DOPA | GB34 | Turned at a rate of two spins per second for 15 s | AIM scores↓ | Pmch↑ | |
|
| ||||||
| Wattanathorn and Sutalangka (2014) [77] | 6-OHDA rats | Laser acupuncture | HT7 | Continuous blue laser beam, wavelength of 405 nm, output power 100 mW, a spot diameter of 500 μm for 10 minutes | Morris water maze: escape latency (days 7 and 14)↓, retention time (day 14)↑ | Neuron density in CA3 and dentate gyrus↑, AChE in the hippocampus↓, MAO-B activity in the hippocampus↓, GSH-Px activity in the right SN↑, MDA in the right SN↓ |
|
| ||||||
| Park et al. (2017) [78] | MPTP mice | MA | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: latency time↑; cylinder test: the number of wall touches↑ | TH-positive neurons in the SN↑, dopaminergic fibers in the ST↑, Pmch in the hypothalamus↑, double-labeled c-Fos+/MCH+ cells↑, MCH concentration in the SN↑, the expression levels of TH and synaptophysin in the SN↑, pAkt↑, and pCREB↑ |
| MPTP mice | MA+TC-MCH7c | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: latency time↓; cylinder test: the number of wall touches↓ | TH-positive neurons in the SN↓, dopaminergic fibers in the ST↓ | |
| Pitx3−/+ mice | MA | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: latency time↑ | — | |
| Pitx3−/+ mice | MA+TC-MCH7c | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: latency time↓ | — | |
| A53T Tg mice | MA | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: latency time↑; cylinder test: the number of wall touches↑ | pGSK-3β and pAMPK in the SN↑, BDNF expression in the SN↑, synuclein expression in the SN↓ | |
MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MA: manual acupuncture; GB34: Yanglingquan; Grm5: glutamate receptor metabotropic 5; Itpr1: inositol 1,4,5-triphosphate receptor 1; Itpr2: inositol 1,4,5-triphosphate receptor 2; Pde1c: phospho-diesterase 1C; Prkcb1: protein kinase beta 1; Plcb4: phospholipase C beta 4; Atp2b3: ATPase calcium-transporting plasma membrane 3; Slc8a3: solute carrier family 8, sodium/calcium exchange member 3; Htr5a: 5-hydroxytryptamine (serotonin) receptor 5A; Adcy1: adenylate cyclase 1; Drd5: dopamine receptor 5; Npy1r: neuropeptide Y receptor Y1; Gpr156: G protein-coupled receptor 156; Cxcl10: chemokine (C–X–C motif) ligand 10; Rxfp2: relaxin/insulin-like family peptide receptor 2; Agtrl1: angiotensin receptor-like 1; Inhbb: inhibin beta-B; Cxcl5: chemokine (C–X–C motif) ligand 5; IL21: interleukin 21; Cox6a1: cytochrome c oxidase, subunit VI a, polypeptide 1; Uqcrq: ubiquinol-cytochrome c reductase, complex III subunit VII; Ndufc1: NADH dehydrogenase (ubiquinone) 1, subcomplex unknown, 1; Atp5e: ATP synthase, epsilon subunit; Mapt: microtubule-associated protein tau; Ngfr: nerve growth factor receptor (TNFR superfamily, member 16); Nefh: neurofilament, heavy polypeptide; LR3: Taichong; TH: tyrosine hydroxylase; ST: striatum; SNpc: substantia nigra pars compacta; DAT: dopamine transporter; Ctla2a: cytotoxic T lymphocyte-associated protein 2 alpha; EG383229: predicted gene EG383229; Ppbp: proplatelet basic protein; Ube2l6: ubiquitin-conjugating enzyme E2L 6; EG665033: predicted gene EG665033; ENSMUSG00000055323: predicted gene ENSMUSG00000055323; Obox6: oocyte-specific homeobox 6; Pbp2: phosphatidylethanolamine-binding protein 2; Tmem150: transmembrane protein 150; Dusp4: dual specificity phosphatase 4; Mafg: v-maf musculoaponeurotic fibrosarcoma oncogene family, protein G; Pgm5: phosphoglucomutase 5; Ndph: Norrie disease homolog; Dnase1l2: deoxyribonuclease 1-like 2; Ucp2: uncoupling protein 2; Sp2: Sp2 transcription factor; Serinc2: serine incorporator 2; Cdh1: cadherin 1; Slc6a13: solute carrier family 6 (neurotransmitter transporter, GABA), member 13; Tph2: tryptophan hydroxylase 2; Mpzl2: myelin protein zero-like 2; Serping1: the serine (or cysteine) peptidase inhibitor, clade G, member 1; Itih2: interalpha trypsin inhibitor, heavy chain 2; Slc6a20a: SLC6 (neurotransmitter transporter), member 20A; Slc6a4: SLC6 (neurotransmitter transporter, serotonin), member 4; Ucma: upper zone of growth plate and cartilage matrix associated; Rdh9: retinol dehydrogenase 9; 4921530L21Rik: RIKEN cDNA 4921530L21 gene; Gm13931: predicted gene 13931; EA: electroacupuncture; CyPA: cyclophilin A; MBP: myelin basic protein; Ube2N: ubiquitin-conjugating enzyme E2N; LMP6: proteasome subunit C7-I; LMP2: 20S proteasome subunit; eIF5A: eukaryotic translation initiation factor 5A; CLP: coactosin-like protein; NSF: N-ethylmaleimidesensitive fusion protein; ST36: Zusanli; SP6: Sanyinjiao; 6-OHDA: 6-hydroxydopamine; LI4: Hegu; RA: retained acupuncture; PC7: Daling; DA: dopamine; [123I] IBZM: [123I] iodobenzamide; HVA: homovanillic acid; α-syn: α-synuclein; SGK1: serum/glucocorticoid-regulated kinase 1; p-α-syn: phosphorylated α-synuclein; ORP: overall rod performance; LC3-II: microtubule-associated protein 1 light chain 3 II; LAMP1: lysosome-associated membrane protein 1; CV4: Guanyuan; GV16: Fengfu; p-mTOR: phosphorylated mammalian target of rapamycin; p-p70S6k: phosphorylated ribosomal protein S6 kinase; BVA: bee venom acupuncture; Bax: Bcl-2-associated X protein; MDA: malondialdehyde; GSH: glutathione; TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; Bcl-2: B cell lymphoma-2; GFAP: glial fibrillary acidic protein; Iba-1: ionized calcium-binding adaptor molecule-1; NF-κB: nuclear factor kappa-B; OTUs: operational taxonomic units; COX-2: cyclooxygenase; iNOS: inducible NO synthase; p-c-Jun: phosphorylated c-Jun kinase; MFB: medial forebrain bundle; GV14: Dazhui; GV20: Baihui; BDNF: brain-derived neurotrophic factor; VTA: ventral tegmental area; GDNF: glial cell-derived neurotrophic factor; SNpr: substantia nigra pars reticulata; GP: globus pallidus; LI11: Quchi; TrkB: tyrosine kinase receptor B; GV29: Yintang; PKB/Akt: protein kinase B; p-Akt: phosphorylated Akt; pERK: phosphorylated extracellular-regulated protein kinase; TrkB FL: full-length TrkB; TrkB T1: splicing truncated isoforms TrkB; pIκBα: phosphorylated inhibitor kappa B alpha; pGSK3β: phosphorylated glycogen synthase kinase 3β; pCREB: phosphorylated cAMP-response element-binding protein; HT7: Shenmen; BrdU: 5-bromo-2′-deoxyuridine; SVZ: subventricular zone; DCX: doublecortin; SP10: Xuehai; SOD: superoxide dismutase; GSH-Px: glutathione peroxidase; DOPAC: dihydroxyphenylacetic acid; SI3: Houxi; CAT: catalase; MAC-1: macrophage antigen complex-1; CV12: Zhongwan; ST25: Tianshu; Fpn1: ferroportin1; DMT1: recombinant divalent metal transporter 1; NS: no significance; L-DOPA: levodopa; AIM: abnormal involuntary movement; Pmch: pro-melanin-concentrating hormone; Tac2: tachykinin 2; Lcn2: lipocalin 2; CA3: the CA3 region of the hippocampus; AChE: acetyl cholinesterase; MAO-B: monoamine oxidase-B; MCH: melanin-concentrating hormone; pAMPK: phosphorylated cAMP-response phosphorylated adenosine 5-monophosphate-activated protein kinase.
4.1. α-Synuclein Aggregation
During the pathogenesis of PD, soluble α-syn monomers are thought to progressively aggregate to large insoluble α-syn fibrils, called Lewy bodies [79]. Overproduction and aggregation of α-syn furthermore induces mitochondrial dysfunction, oxidative stress, and neuroinflammation, leading to damage of dopaminergic neurons in the SN and ST.
It is suggested that acupuncture may inhibit increased levels of α-syn for its neuroprotective effects. Serum/glucocorticoid-regulated kinase 1 (SGK1) is a serine threonine-specific protein kinase which may regulate α-syn. Yeo and Lim [56] found that acupuncture at GB34 and LR3 upregulated SGK1 and inhibited an α-syn increase. In SGK1 siRNA knockdown SH-SY5Y cells, the authors observed a downregulation of SGK1 in dopaminergic neurons along with an increase in α-syn expression, suggesting that acupuncture may inhibit the increase of α-syn expression by downregulation of SGK1. Other research shows that overexpression of SGK1 exerts neuroprotective functions by reducing the production of reactive oxygen species (ROS) and mitochondrial dysfunction [80]. It has been reported that in the process of α-syn aggregation, phosphorylation at serine 129 increases the neurotoxicity of α-syn. A previous study has shown that acupuncture suppressed α-syn levels in the SN and ST and inhibits increased levels of p-α-syn 32 and p-α-syn 16 at serine 129 in the nigral dopaminergic neurons, providing evidence that acupuncture may reduce neurotoxicity via inhibiting the level of α-syn and p-α-syn, thereby protecting dopaminergic neurons [57].
Acupuncture has also been demonstrated to promote the degradation of α-syn by restoring autophagy. Autophagy is essential for the removal of toxic α-syn aggregates in order to maintain intracellular homeostasis [81]. Mammalian target of rapamycin (mTOR) is a negative regulator of cellular autophagy and it has been shown that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) upregulates microtubule-associated protein 1 light chain 3 II (LC3-II) in a PD model, while downregulation lysosomal-associated membrane protein 1 (LAMP1, lysosomal structural protein) indicates that an impairment of lysosomes and the interruption of autophagosome-lysosome fusion may lead to an accumulation of autophagosomes in the substantia nigra pars compacta (SNpc) of MPTP mice [58]. Tian et al. observed that after acupuncture treatment for 4 days, LC3-II was reduced by approximately 40% and LAMP1 by approximately 20%, and more than 50% of α-syn in the SNpc was cleared, suggesting that acupuncture at GB34 restores lysosomal structures and reduces the accumulation of autophagosomes, enhancing the clearance of autophagosomes and degradation of α-syn. This group further found that acupuncture did not change levels of upstream proteins of lysosomal autophagy system (LAS), p-mTOR, p-p70s6k, and ULK1, which indicated that acupuncture activates the independent mTOR pathway to enhance the autophagic clearance of α-syn. After activation of mTOR, p70S6K and 4E-binding protein 1 (4E-BP1) are activated to form p-p70S6K and p-4E-BP1, which then inhibit autophagy. Wang et al. [59] found that moxibustion at ST36, CV4 (Guanyuan), and GV16 (Fengfu) decreased p-mTOR, p-p70s6k and α-syn levels while increasing LC3-II levels, suggesting moxibustion exerts neuroprotective effects by promoting clearance of α-syn and enhancing autophagy via the mTOR pathway. As rapamycin (mTOR antagonist) treatment results in considerable side effects in PD patients such as dyslipidemia, antiproliferative toxicity, and renal dysfunction [82], acupuncture may represent as the alternative strategy to target mTOR.
4.2. Apoptosis
It is established that nigral dopaminergic neurons in PD patients undergo apoptosis and the formation of apoptotic bodies [83]. Acupuncture may possess antiapoptosis properties via blocking apoptosis pathways of dopaminergic neurons. BVA has been found to decrease caspase-3 activity and downregulate caspase-3 and Bax gene expression, suggesting that it may inhibit the mitochondrion-dependent apoptotic pathway of dopaminergic neurons [84]. MA and acupoint injection exhibited similar effects: MA or acupoint injection at ST36 has been shown to downregulate Bax, cytochrome C, and upregulate Bcl-2 [65]. Park et al. [61] found that p53 may be involved in the neuroprotective effect of acupuncture: 40 of 76 differentially expressed genes following acupuncture were involved in the p53 signaling network, and conditionally knocking down p53 pathway genes in midbrain dopaminergic neurons attenuated the neuroprotective effect of acupuncture. The c-Jun N-terminal kinase (JNK) signaling may mediate apoptosis through phosphorylation of c-Jun [85]. Doo et al. [62] observed that BVA at GB34 can rescue dopaminergic neurons from apoptosis by inhibiting c-Jun activation in MPTP mouse, while another study suggested that MA at GB34 does not change p-c-Jun levels [69]. Therefore, the effects of different acupuncture stimulation methods on JNK need to be further clarified.
Several reports have established neurotrophic factors (NTFs) as a major player in the neuroprotective effects of acupuncture in PD treatment. Liang et al. [63] found that long-term high-frequency EA at GV14 and GV20 effectively prevented the degeneration of ventral dopaminergic neurons and upregulated the levels of brain-derived neurotrophic factor (BDNF) in the ventral subregions of the midbrain, which induced the regeneration of injured dopaminergic neurons. Subsequently, the authors also reported that EA at 2 Hz increased glial cell-derived neurotrophic factor (GDNF) mRNA in both sides of the globus pallidus, and 100 Hz EA increased GDNF mRNA in both sides of the globus pallidus and unlesioned side of SN pars reticulata, speculating that EA could regulate the retrograde transport of GDNF from ganglion to SN and restore the balance of different nuclei in the basal ganglia circuit which contributes to the behavioral improvement of medial forebrain bundle- (MFB-) lesioned rats [48]. Another study confirmed that EA upregulated BDNF and GDNF mRNA in the SN of PD models [64]. Tyrosine kinase receptor B (TrkB) is a high-affinity BDNF receptor whose activation results in the maintenance of neuronal differentiation and survival [86]. Acupuncture at GB34 and LR3 increases the TrkB expression in the damaged SN of 6-hydroxydopamine- (6-OHDA-) induced PD rats [66]. TrkB consists of different subtypes, including full-length (TrkB FL) and truncated (TrkB T1 or T2) TrkB. TrkB T1 is regarded as a dominant negative form of TrkB, which may suppress the neurotrophic activity of the BDNF/TrkB signaling pathway [87]. Hence, balancing TrkB FL and TrkB T1 is essential for neuroprotection [88]. It has been reported that the TrkB inhibitor (K252A) eliminates the neuroprotective effect of EA, and another study revealed that EA may reverse the imbalance between TrkB FL and TrkB T1 to upregulate p-Akt, p-ERK1/2, and BDNF [68]. 50 Hz EA at GB34 and LR3 has been shown to increase BDNF and downstream p-Akt levels [67], improving rotational behavior in a rat model of unilateral MPP injury, and another study revealed that inhibition of the PI3K/Akt signaling pathway blocked the protective effect of acupuncture on DA neurons [69]. Acupuncture at GB34 combined with KD5040 downregulated pIκBα and upregulated pAkt, pGSK3β, pERK, pCREB, and BDNF, which significantly improve motor function [70]. Therefore, the results presented above suggest that BDNF/TrkB and their downstream signaling pathways may mediate the neuroprotective effects of acupuncture in PD treatment. Besides, acupuncture has been found to increase the number of 5-bromo-2′-deoxyuridine- (BrdU) positive cells and to restore neurogenesis in the subventricular zone [71], which provides a new pathway for studying the molecular mechanisms of acupuncture treatment for PD.
4.3. Oxidative Stress
Aggregation of free radicals causes lipid peroxidation, which leads to excessive oxidation during the process of protein synthesis in DA neurons, destroying the structure of the cellular membrane and ultimately resulting in the death of DA neurons. The neuroprotective effects of acupuncture treatment may be mediated through the regulation of antioxidant systems, as a study reported that MA at GB34, LR3, ST36, and SP10 increases levels of superoxide dismutase (SOD), glutathione (GSH), and glutathione peroxidase (GSH-Px), and decreases levels of malondialdehyde (MDA), along with improved rotarod behavior [38], and similar results were also observed in EA and BVA treatment. For example, high-frequency EA at ST36 and SP6 increases levels of GSH and SOD and decreases striatal H2O2 and MDA levels [47]. BVA at GB34 increases GSH and paraoxonase-1 activities and decreases MDA levels [84]. In addition to increased SOD and catalase (CAT) activities, Lee et al. also observed upregulated DJ-1, which exists widely in peripheral tissues, neurons, and glial cells, playing an essential role in antioxidation via regulating the activity of SOD and CAT [72]. It is therefore speculated that the elevation of DJ-1 caused by acupuncture at GB34 may exert antioxidant effects by enhancing the activity of striatal SOD and CAT. These results indicate that acupuncture protects DA neurons from oxidative stress by restoring the balance between oxides and antioxidants.
4.4. Neuroinflammation
Microglia, the primary immune cells of the central nervous system, play a vital role in the neuroinflammation in PD [89]. Injury signaling in degenerated DA neurons can shift microglia to a pro-inflammatory “M1” phenotype, resulting in the release of ROS and cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6, and interleukin-12, which exacerbate oxidative stress and inflammation, ultimately leading to DA neuron apoptosis [90]. Inducible nitric oxide synthase (iNOS) expressed in glial cells causes nitric oxide production, which in turn activates microglia in conjunction with various proinflammatory “M1” phenotype cytokines. Acupuncture at GB34 and LR3 has been shown to attenuate the expression of macrophage antigen complex-1, a marker of microglial activation, and mitigate increases in cyclooxygenase-2 (COX-2) and iNOS expression in an MPTP-induced PD models [73]. Similarly, striatal DA levels were shown to increase from 46% to 78% within 7 days, suggesting that acupuncture exerts neuroprotective effects by attenuating MPTP-induced glial activation and neuroinflammation. Injecting choroid plexus cells at ST36 likewise decreased iNOS and COX-2 expression and enhanced exercise capacity of MPTP-induced mice [37], while BVA at GB34 has been suggested to protect dopaminergic neurons by downregulating inflammatory factors such as TNF-α and IL-1β [84].
Acupuncture can also inhibit neuroinflammation by regulating the brain-gut axis, thus alleviating movement dysfunction in PD. An altered gut microbiota has been reported to induce microglial activation and neuroinflammation, which may promote α-syn overexpression and contribute to motor dysfunction in PD models [91]. Jang et al. [65] observed that acupuncture changed the relative abundance of Butyricimonas, Holdemania, Frisingicoccus, Gracilibacter, Phocea, and Aestuariispira, which showed significant correlations with anxiety as well as motor functions. The authors proposed that acupuncture blocks inflammatory responses and apoptosis as acupuncture increased the levels of DA fibers and neurons in the SN and ST, downregulated glial fibrillary acidic protein, ionized calcium-binding adaptor molecule 1, and nuclear factor kappa B and TNF-α, and restored the conversion of Bax and Bcl-2 expression in the SN and ST. In addition, the PICRUSt-predicted functional analyses based on 16S rRNA taxonomic profiles showed that acupuncture restored physiological functions such as the glutathione and methane metabolism and other PD related pathways. Collectively, the authors postulated that modulation of the gut microbial dysbiosis and inhibition of neuroinflammation may serve as the mechanisms by which acupuncture mitigates dyskinesia and protects dopaminergic neurons in PD. Moreover, acupuncture can promote anti-inflammation by regulating inflammatory factors and the iron homeostasis in the intestine, so as to improve PD motor symptoms. Iron aggregation in the SN is an early feature of PD. Activated microglial cells regulate the import of DMT1 from microglia and downregulate the export of ferritin via FPN1, leading to accumulation of iron in microglia and ultimately damaging neurons [92]. Acupuncture at CV12 (Zhongwan), ST25, and CV4 not only reduced α-syn expression in the duodenum and inflammatory factors such as IL-1β and TNF-α in the serum and duodenum but also balanced ferritin import through DMT1 and ferritin export via FPN1 and ultimately reducing iron accumulation in the duodenum and SN [74]. It is worth mentioning that CV12, ST25, and CV4 are acupoints for the treatment of gastrointestinal symptoms, which are thought to inhibit neuroinflammation by influencing the intestinal canal to relieve movement disorders. Acupuncture has been shown to provide additional potential benefits for the brain-gut axis [93], highlighting the brain-gut-neuroinflammation-PD axis as a novel area for research.
5. Neural Circuit Alterations Underlying the Efficacy of Acupuncture Treatment for Parkinson's Disease
The measurement of neural activity provides more direct experimental evidence for the study of the mechanisms involved in the development and treatments of PD. Functional magnetic resonance imaging (fMRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) have been used to measure alterations in neural signals caused by acupuncture [94]. The pathological hallmark of PD is the loss of dopaminergic neurons in the SNpc, which leads to insufficient DA projecting to the ST and cerebral cortex and results in abnormal regulation of the neural circuitry of the basal ganglia [95]. Therefore, the functional connectivity of the SN, caudate nucleus, and thalamus, which are all part of the basal ganglia circuit, is impaired in PD patients [96]. Dopaminergic neural pathways, including the basal nuclei, thalamus, and limbic system, are also impaired in animal models of PD [97]. Consequently, Tables 3 and 4 summarize the characteristics of 6 clinical studies and 12 preclinical studies assessing the neural circuit.
Table 3.
The neural circuit mechanisms of acupuncture for Parkinson's disease in clinical researches.
| Study | Clinical trial design | Clinical condition | Intervention | Comparison | Acupoints | Acupuncture parameters | Outcomes |
|---|---|---|---|---|---|---|---|
| Chae et al. (2009) [98] | Controlled trial | PD patients (mild) | Verum acupuncture (n = 10) | Covert placebo/overt placebo (n = 10) | Left GB34 | 10 mm, manually rotated clockwise and counterclockwise once per second (1 Hz), 60 seconds, then withdrawn for 120 seconds, only once | The putamen and the primary motor cortex↑, motor function↑ (VA vs. CP) |
|
| |||||||
| Yeo et al. (2012) [99] | RCT | Idiopathic PD patients | Acupuncture (n = 12) | Acupuncture on healthy participants (n = 12) | Right GB34 | 1 cm, remained in the skin for 1 min and rotated bidirectionally for 1 min, needle remained in the skin without rotation for 1 min and then the pattern of 1 min rotation and 1 min rest was repeated, only once | Neural responses in the SN, caudate, thalamus, and putamen (impaired caused by PD)↑ |
|
| |||||||
| Yeo et al. (2014) [100] | RCT | Idiopathic PD patients | Acupuncture (n = 12) | Acupuncture on healthy participants (n = 12) | Right GB34 | 1 cm, for “stimulated” blocks: rotated bidirectionally at 1 Hz; for “not stimulated” blocks: the needle was only inserted into the skin and then left in place, only once | Neural responses in the prefrontal cortex, precentral gyrus, and putamen in PD patients↑ |
|
| |||||||
| Li et al. (2018) [101] | Controlled trial | PD patients with tremor | Acupuncture+L-DOPA (n = 14) | Sham acupuncture+L-DOPA (n = 14) | DU20, bilateral GB20, and the chorea-tremor controlled zone | 2-3 cm, reinforcing-reducing method, twirling every 10 minutes, 30 minutes, twice weekly, 12 weeks | Clinical evaluation: UPDRS (II and III) scores↓, neural responses (SN, caudate, thalamus, and putamen)↑ |
|
| |||||||
| Huang et al. (2010) [102] | RCT | PD patients | Acupuncture+L-DOPA (n = 5) | L-DOPA (n = 5) | Bilateral MS6, MS4, MS8, MS9, MS14 | Continuous wave, 50 Hz, 2-4 mA, 30 minutes, six times a week, 5 weeks | rCBF (the frontal lobe, the occipital lobe, the basal ganglion, and the cerebellum in the most affected hemisphere)↑ |
|
| |||||||
| Huang et al. (2009) [103] | RCT | PD patients | Acupuncture+Madopar (n = 5) | Madopar (n = 5) | Bilateral MS6, MS4, MS8, MS9, MS14 | Continuous wave, 50 Hz, 2–4 mA, 30 minutes, six times a week, 5 weeks | The glucose metabolisms (parietal, temporal, occipital lobes, the thalamus, and the cerebellum in the light-diseased hemisphere, and in parietal and occipital lobes of the severe-diseased hemisphere)↑ |
PD: Parkinson's disease; GB34: Yanglingquan; VA: verum acupuncture; CP: covert placebo; RCT: randomized controlled trial; SN: substantia nigra; L-DOPA: levodopa; DU20: Baihui; GB20: Fengchi; UPDRS: Unified Parkinson's Disease Rating Scale; MS6: the anterior oblique line of the vertex to temple; MS4: the lateral line III on forehead; MS8: the lateral line I of the vertex; MS9: the lateral line II of the vertex; MS14: the lower-lateral line of the occipital scalp; rCBF: regional cerebral blood flow.
Table 4.
The neural circuit mechanisms of acupuncture for Parkinson's disease in basic researches.
| Study | PD model | Intervention | Acupoints | Acupuncture parameters | Behavioral measurements | Biochemical measurements |
|---|---|---|---|---|---|---|
| Zhang et al. (2018) [104] | MPTP rhesus monkeys | EA | ST36, LI4 | 100 Hz, 3-4-5 mA for 30 minutes | The speed of movement↑, longer performance time of the affected hand↑ | The BOLD activations of the caudate nucleus, putamen, M1, cingulate gyrus, and GPe↓ |
|
| ||||||
| Lee et al. (2013) [97] | MPTP beagle dogs | RA | ST36 | Rotated clockwise and counterclockwise to generate Deqi | — | The BOLD signal intensity in the ipsilateral parietal lobe, contralateral temporal lobe, basal ganglia, pons, and cerebellum↓ |
|
| ||||||
| Khalil et al. (2015) [84] | Rotenone rats | BVA | GB34 | — | Suspension test: latency time↑; cylinder test: the number of wall touches↑ | Caspase-3 activity↓, caspase-3 genes↓, Bax genes↓, DNA damage↓, MDA↓, PON1 activity↑, GSH↑, TNF-α↓, IL-1β↓, DA↑, 5-HT↑, noradrenaline↑ |
|
| ||||||
| Kim et al. (2011) [36] | MPTP mice | MA | GB34 | Turned at a rate of two spins per second for 15 s | Rotarod test: the ORP scores↑ | TH-positive neurons in the SN↑, Nissl-positive cells in the SNpc↑, dopaminergic fibers in the ST↑, HVA in the ST↑, dopamine turnover ratios (DOPAC/DA, HVA/DA, and DOPAC+HVA/DA)↑, dopamine efflux in the ST↑, pDARPP-32 at Thr34↓, FosB in the ST↓ |
|
| ||||||
| Rui et al. (2013) [105] | 6-OHDA rats | EA | SP6, GB34, ST36 | 0/2/100 Hz, 1-3 mA for 30 minutes | Rotational behavior test: the average net number of turns/min (100 Hz)↓ | TH-positive neurons on the lesion side (100 Hz)↑, TH-positive dendritic fibers in the ST↑, DAT in the ST and SN (100 Hz)↑, D1R mRNA and protein in the ST (100 Hz)↑, D2R protein (100 Hz) in the ST↓ |
|
| ||||||
| Jia et al. (2009) [106] | MFB rats | EA | GV14, GV20 | 0/2/100 Hz, 1-2-3 mA for 30 minutes | Rotational behavior test: the average net number of turns/min (100 Hz)↓ | TH-positive neurons in the SN (100 Hz)↑, TH-positive dendritic fibers in the ipsilateral SNpr (100 Hz)↑, substance P content in the SN (100 Hz)↑, GAD67 mRNA↓ |
|
| ||||||
| Jia et al. (2010) [107] | MFB rats | EA | GV14, GV20 | 0/2/100 Hz, 1-2-3 mA for 30 minutes | Rotarod test: week 2 treadmill occupancy time (100 Hz)↑ | GABA levels in midbrain (100 Hz)↓ |
|
| ||||||
| Kim et al. (2014) [108] | 6-OHDA mice | MA+L-DOPA | GB34 | Turned at a rate of two spins per second for 15 s | Cylinder test: the number of wall touches↑; AIM scores↓ | FosB in the ST↓, GABA contents in the SNpr↓, Glu levels in the SN↓ |
|
| ||||||
| Sun et al. (2012) [109] | MFB rats | EA | GV14, GV20 | 100 Hz, 1-2-3 mA for 30 minutes | Rotarod test: treadmill occupancy time↑; rotational behavior test: the average net number of turns/min↓ | TH-positive neurons↑, ACh, Glu in the ST↓ |
PD: Parkinson's disease; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; EA: electroacupuncture; ST36: Zusanli; LI4: Hegu; M1: primary motor cortex; GPe: globus pallidus externa; RA: retained acupuncture; BVA: bee venom acupuncture; GB34: Yanglingquan; Bax: Bcl-2-associated X protein; MDA: malondialdehyde; PON1: paraoxonase-1; GSH: glutathione; TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; DA: dopamine; 5-HT: serotonin; MA: manual acupuncture; ORP: overall rod performance; SNpc: substantia nigra pars compacta; ST: striatum; HVA: homovanillic acid; DOPAC: dihydroxyphenylacetic acid; pDARPP-32: phosphorylated dopamine and adenosine 35-monophosphate-regulated phospho-protein; SP6: Sanyinjiao; DAT: dopamine transporter dopamine; D1R: D1 receptor; D2R: dopamine D2 receptor; MFB: medial forebrain bundle; GV14: Dazhui; GV20: Baihui; GAD67: glutamate decarboxylase-67; GABA: γ-aminobutyric acid; ACh: acetylcholine; Glu: glutamate.
5.1. Evidence for Neural Circuit Regulation by Acupuncture Based on Clinical Imaging Studies
Using fMRI imaging, Chae et al. [98] found that acupuncture stimulation of GB34 activated regions associated with intensified motor function as evidenced by increased BOLD signals (the index of brain activation), illustrating that acupuncture may promote the improvement of motor functions in PD patients via the basal ganglia-thalamic-cortical circuit. Furthermore, neural responses of PD patients at resting state were lower than in healthy controls in extensive brain regions including the putamen, thalamus, and supplementary motor area. This variation is secondary to DA deficiency and is related to PD severity [110]. Yeo et al. performed acupuncture stimulation in 12 PD patients and 12 healthy volunteers and compared whole-brain fMRI images of the two groups before and after acupuncture treatment. The authors found that acupuncture at GB34 increased brain activation in regions impaired by PD, such as the SN, caudate, thalamus, and putamen [99], and moreover activated the prefrontal cortex, precentral gyrus, and putamen in PD patients [100]. These results support the hypothesis that PD rehabilitation may be related to modulation of areas associated with PD by GB34 acupuncture stimulation. Besides, the dyskinesia represented by tremor may also be closely related to the activity of the compensatory circuit, in particular the cerebello-thalamo-cortical (CTC) circuit, after the basal ganglia circuit is damaged [111]. Li et al. [101] demonstrated that acupuncture alleviated PD tremor by stimulation and modulation of the cerebellum, thalamus, and motor cortex, in connection with the CTC circuit. Meanwhile, by adjusting neural activity within cognitive brain regions which connect with the CTC circuit, acupuncture may contribute to enhancing movement and improving the daily life activities. The thalamus, originally thought to be a passive transmissive structure for sensory information, plays an indispensable role in the CTC pathway along with the cerebellum and is hypothesized to be involved in movement and balance. Thalamic dysfunction may result in dyspnea with stiffness and tremor as well as nonmotor symptoms [112]. Modulation of the tremor site in the ventral thalamus may help to explain the effectiveness of acupuncture for controlling tremor symptoms [113]. Pathological changes of the basal ganglia are the basis of tremor in PD; however, the direct cause of tremor is the abnormal regulation of the CTC circuit, and the contact points of these two circuits may be located in the motor cortex or other brain locations [114]. Hence, we speculate that the tremor-related neural network may include several brain regions such as the sensorimotor cortex, cerebellum, thalamus, and basal ganglia. Within this network, the neural circuit represented by the basal ganglia and the CTC plays different roles in tremor. The restoration of function of PD patients by acupuncture may involve activation of compensatory brain regions to increase the survival rate of dopaminergic neurons or the activity of DA receptors, and to correct the network imbalance caused by the loss of the dopaminergic neurons in SN [41]. Huang et al. conducted two RCTs using PET [103] and SPECT scans [102] to compare the efficacy of EA combined with medication versus medication alone. After 5 weeks of acupuncture, PET scans of the EA+medication group showed an increased glucose metabolism in the temporal lobes, cerebellum, and thalamus of the less-affected hemisphere and in the parietal and occipital lobes of the severe-diseased hemisphere, while these changes were not observed in the medication-only group. The study highlighted that more brain regions exhibited increased metabolism in the less-affected hemisphere than in the diseased hemisphere and the survival rate of dopaminergic neurons and DA receptor activity were increased, indicating that acupuncture may also affect the number and activity of nigral cells in the less-affected hemisphere [103]. The SPECT study highlighted that regional cerebral blood flow (rCBF) of the frontal and occipital lobes, basal ganglia, and cerebellum in the more severely affected cerebral hemispheres increased, yet DAT levels in the basal ganglia remained unchanged after acupuncture treatment in PD patients, while L-DOPA treatment alone did not affect rCBF, but increased DAT activity in the basal ganglia of the most severely affected cerebral hemispheres [102].
These neuroimaging results illustrate that acupuncture enhances local brain activity and glucose metabolism and improves rCBF, which in turn reactivates both the dysfunctional motor and nonmotor neural circuits, ameliorating symptoms and improving overall quality of life. These neuroimaging quantifications rather than subjective findings helped to further objectively evaluate the effectiveness of acupuncture in PD.
5.2. Experimental Animal Model Studies Investigating the Regulation of the Basal Ganglia Circuit in Parkinson's Disease by Acupuncture
The basal ganglia circuit consists of the ST (caudate and putamen), the globus pallidus (internal and external parts), the subthalamic nucleus, and the SN, which are responsible for motor control, motor learning, emotions, and executive functions [115]. Several neurotransmitters are involved in regulating the function of the basal ganglia circuit. Once DA levels are reduced, the imbalance of DA and acetylcholine (ACh) and disorders of the neurotransmitter system contribute to the development of dyskinesia in PD [116]. GABA is the most predominant inhibitory neurotransmitter in the ST and its downstream nucleus projections are regulated by DA either via dopamine D1 receptor (D1R) or dopamine D2 receptor (D2R). SPECT imaging in PD models revealed that retained acupuncture may attenuate MPTP-induced neuronal injury [55]. Besides, fMRI studies have demonstrated that acupuncture activates the caudate nucleus, putamen, M1, cingulate gyrus, and globus pallidus externa [104], and modulates the ipsilateral parietal lobe, contralateral temporal lobe, basal ganglia, pons, and cerebellum [97], which are closely linked to the basal ganglia circuit.
Acupuncture can improve the motor function in PD models by regulation of neurotransmitters and restoration of the homeostasis of the basal ganglia circuit: first, acupuncture mediates improvements of motor function by directly affecting the dopaminergic system. Kim et al. [36] found that acupuncture improves motor function and protects dopaminergic neurons via increasing DA release, which in turn enhances the availability of DA in the synaptic cleft. Moreover, the authors observed that acupuncture reduces the expression of FosB, a marker of striatal postsynaptic dopamine depletion, and the phosphorylation of DARPP-32, which can affect postsynaptic medium spiny neurons by activating the cAMP signaling pathway. These results raised the possibility that the effects of acupuncture for the amelioration of MPTP-induced dyskinesia are mediated by upregulation of neurotransmission of postsynaptic DA and rebalanced basal ganglia circuit. EA at SP6, GB34, and ST36 with 100 Hz increased the survival of nigral DA neurons and increased the expression of striatal DAT by 253.78%, as well as elevating the levels of D1R mRNA and protein by 81.88% and 62.62%, respectively, while inhibiting increases in the D2R. These results suggested that high-frequency EA may act on both presynaptic DAT and postsynaptic DA receptors, contributing to the balance of basal ganglia output to the thalamus [105].
In terms of the regulation of other neurotransmitters, Jia et al. [106] observed that 100 Hz EA at GV14 and GV20 increased the content of nigral substance P, protected damaged DA neurons from degeneration, and reversed the abnormal elevation of GAD67 mRNA in the midbrain induced by MFB axotomy. Subsequently, the authors further found that EA at high frequency reversed increases in GABA content in the midbrain and alleviated motor coordination in MFB-lesioned rats [107]. These findings suggest that the amelioration of dyskinesia in MFB-lesioned rats may occur via restoration of the homeostasis of dopaminergic transmission and inhibition of the overoutput of GABA in the basal ganglia circuit. Sun et al. [109] reported that high-frequency EA at GV14 and GV20 reduced abnormally elevated levels of Glu and ACh on the lesioned side in the ST, improved motor coordination in MFB-lesioned rats, and protected the nigral DA neurons from degeneration. Furthermore, Glu levels were significantly correlated with the survival ratios of DA neurons in SNpc and rotational behavior, suggesting that alleviation of dyskinesia by EA may involve the downregulation of Glu and ACh in the ST. Another study found that BVA at GB34 may increase the levels of DA, NE, and 5-HT, proposing that the improvement of motor function induced by BVA may be related to increased levels of DA and NE [84].
In addition, acupuncture can also reduce L-DOPA-induced dyskinesia (LID) by regulating neurotransmitters to restore the balance of the basal ganglia circuit. As a precursor of DA, L-DOPA is used to increase DA levels and enhance synaptic DA transmission. However, after approximately 10 years of treatment with L-DOPA, most patients develop side effects, including nonmotor complications such as hallucinations, insomnia, nausea [117], and motor complications including dystonia, chorea, and athetosis, known as LID [118]. Kim et al. [108] observed that L-DOPA at half the typical dose combined with acupuncture can improve motor function. Combination therapy can also restore the levels of GABA and Glu, which are elevated by high-dose L-DOPA. Moreover, FosB expression, an early indicator of LID, is also reduced by combination therapy. These results not only suggest that acupuncture may relieve LID by balancing neurochemicals in the basal ganglia but also demonstrated a possible synergistic effect of acupuncture and L-DOPA.
In a nutshell, it is widely believed that the dysfunction of the basal ganglia circuit induced by the apoptosis of dopaminergic neurons is an essential mechanism in the development and progression of PD. The basal ganglia circuit is regulated by various neurotransmitters, and if a part in the circuit is abnormal, the function of the whole circuitry may thus be abnormal. However, since the mechanism of acupuncture for the regulation of the basal ganglia circuit which mediates improvements of PD dyskinesia has not been fully clarified so far, this paper only reviewed the published research so far. Further studies on neuropathology in this field will be beneficial to further increase the objectivity of the evaluation of the efficacy of acupuncture in PD treatment.
6. Conclusion
The effectiveness of acupuncture for PD treatment has been demonstrated by several clinical, preclinical, and basic research studies. Benefits predominantly manifested via the amelioration PD symptoms and the reversal of dopaminergic neurodegeneration in the early stages of PD. The combined application of acupuncture and anti-PD drugs may enhance the therapeutic efficacy of drugs while reducing the required dose and associated side effects. Acupuncture may protect dopaminergic neurons from degeneration via antioxidative stress, anti-inflammatory, and antiapoptotic pathways, and modulating the neurotransmitter balance in the basal ganglia circuit. As shown in Figure 2, acupuncture can exert protective effects on dopaminergic neurons through multiple pathways: (1) acupuncture reduces the abnormal aggregation of α-syn by inhibiting the production of α-syn and reinstating the autophagic clearance of α-syn; (2) acupuncture inhibits the apoptotic pathways and upregulates the expression of NTFs to activate the downstream PI3K/AKT and MEK/ERK pathways, thus promoting the survival of dopaminergic neurons; (3) acupuncture increases the level of intracellular antioxidants to reduce the oxidative stress response after PD; (4) acupuncture inhibits the activation of microglia and the release of inflammatory factors, and reverses neuroinflammation by regulating intestinal microorganisms; (5) acupuncture regulates DA and other neurotransmitters to reinstate the balance of basal ganglia circuit and exerts a synergistic effect with L-DOPA and other anti-PD drugs. Therefore, acupuncture may represent a novel and effective strategy for the treatment of PD, particularly for halting the degeneration of DA neurons at the early stages of PD to delay PD progression, although this hypothesis needs to be further verified by high-quality basic and clinical research.
Figure 2.
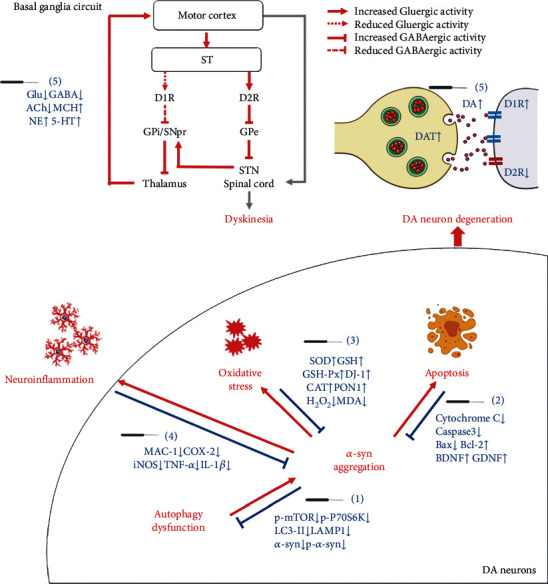
The neuroprotective mechanisms of acupuncture in the treatment of Parkinson's disease. The pathological activities associated with PD include α-syn aggregation, oxidative stress, apoptosis, neuroinflammation, and the disbalance of basal ganglia circuit (red). The key molecules (blue) in the above events are regulated by acupuncture to display neuroprotective effect in PD brain. ACh: acetylcholine; α-syn: α-synuclein; Bax: Bcl-2-associated X protein; Bcl-2: B cell lymphoma-2; BDNF: brain-derived neurotrophic factor; caspase-3: cysteinyl aspartate-specific proteinase-3; CAT: catalase; COX-2: cyclooxygenase-2; DA: dopamine; DAT: dopamine transporter; D1R: dopamine D1 receptor; D2R: dopamine D2 receptor; GABA: γ-aminobutyric acid; GDNF: glial cell-derived neurotrophic factor; Glu: glutamate; GPe: globus pallidus externus; GPi: globus pallidus internus; GSH: glutathione; GSH-Px: glutathione peroxidase; 5-HT: serotonin; IL-1β: interleukin-1β; iNOS: inducible nitric oxide synthase; LAMP1: lysosomal-associated membrane protein 1; LC3-II: microtubule-associated protein 1 light chain 3 II; MAC-1: macrophage antigen complex-1; MCH: melanin concentration hormone; MDA: malondialdehyde; NE: norepinephrine; p-α-syn: phosphorylated α-synuclein; p-mTOR: phosphorylated mammalian target of rapamycin; PON1: paraoxonase-1; p-p70S6k: phosphorylated ribosomal protein S6 kinase; SNpr: substantia nigra pars reticulate; SOD: superoxide dismutase; ST: striatum; STN: subthalamic nucleus; TNF-α: tumor necrosis factor-α.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (NSFC) (No. 82074534, No. 81804186, and No. 81904295) and the Scientific Research Projects of Tianjin Education Commission (2017KJ150).
Abbreviations
- Ach:
Acetylcholine
- AChE:
Acetylcholinesterase
- α-syn:
α-Synuclein
- Bcl-2:
B cell lymphoma-2
- BDNF:
Brain-derived neurotrophic factor
- BrdU:
5-Bromo-2′-deoxyuridine
- BVA:
Bee venom acupuncture
- Caspase:
Cysteinyl aspartate-specific proteinase
- CAT:
Catalase
- COX-2:
Cyclooxygenase-2
- CTC:
Cerebello-thalamo-cortical
- DA:
Dopamine
- DAT:
Dopamine transporter
- D1R:
Dopamine D1 receptor
- D2R:
Dopamine D2 receptor
- EA:
Electroacupuncture
- EBM:
Evidence-based medicine
- 4E-BP1:
4E-binding protein 1
- fMRI:
Functional magnetic resonance imaging
- GABA:
γ-Aminobutyric acid
- GDNF:
Glial cell-derived neurotrophic factor
- Glu:
Glutamate
- GSH:
Glutathione
- GSH-Px:
Glutathione peroxidase
- 5-HT:
Serotonin
- IL-1β:
Interleukin-1β
- iNOS:
Inducible nitric oxide synthase
- JNK:
c-Jun N-terminal kinase
- LAMP1:
Lysosomal-associated membrane protein 1
- LAS:
Lysosomal autophagy system
- LC3-II:
Microtubule-associated protein 1 light chain 3 II
- L-DOPA:
Levodopa
- LID:
L-DOPA-induced dyskinesia
- MA:
Manual acupuncture
- MAO-B:
Monoamine oxidase B
- MAs:
Meta-analyses
- MCH:
Melanin concentration hormone
- MDA:
Malondialdehyde
- MFB:
Medial forebrain bundle
- mTOR:
Mammalian target of rapamycin
- MPTP:
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NE:
Norepinephrine
- NTFs:
Neurotrophic factors
- 6-OHDA:
6-Hydroxydopamine
- PD:
Parkinson's disease
- PET:
Positron emission tomography
- rCBF:
Regional cerebral blood flow
- RCTs:
Randomized controlled trials
- ROS:
Reactive oxygen species
- SGK1:
Serum/glucocorticoid-regulated kinase 1
- SN:
Substantia nigra
- SNpc:
Substantia nigra pars compacta
- SOD:
Superoxide dismutase
- SPECT:
Single-photon emission computed tomography
- SRs:
Systematic reviews
- ST:
Striatum
- TNF-α:
Tumor necrosis factor-α
- TrkB:
Tyrosine kinase receptor B
- UPDRS:
Unified Parkinson's Disease Rating Scale.
Contributor Information
Zhifang Xu, Email: xuzhifangmsn@hotmail.com.
Meidan Zhao, Email: zhaomeidan2012@163.com.
Conflicts of Interest
The authors have no competing interests to declare.
Authors' Contributions
Yadan Zhao and Zichen Zhang contributed equally to this work.
References
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. Journal of Neurology, Neurosurgery, and Psychiatry. 2008;79(4):368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Chen F. P., Chang C. M., Shiu J. H., et al. A clinical study of integrating acupuncture and Western medicine in treating patients with Parkinson’s disease. The American Journal of Chinese Medicine. 2015;43(3):407–423. doi: 10.1142/S0192415X15500263. [DOI] [PubMed] [Google Scholar]
- 3.Cheng F. K. The use of acupuncture in patients with Parkinson’s disease. Geriatric Nursing. 2017;38(4):302–314. doi: 10.1016/j.gerinurse.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Kowal S. L., Dall T. M., Chakrabarti R., Storm M. V., Jain A. The current and projected economic burden of Parkinson’s disease in the United States. Movement Disorders: official journal of the Movement Disorder Society. 2013;28(3):311–318. doi: 10.1002/mds.25292. [DOI] [PubMed] [Google Scholar]
- 5.Simon D. K., Tanner C. M., Brundin P. Parkinson disease epidemiology, pathology, genetics, and pathophysiology. Clinics in Geriatric Medicine. 2020;36(1):1–12. doi: 10.1016/j.cger.2019.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalia L. V., Lang A. E. Parkinson’s disease. The Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 7.Catherine A. C., Fox Susan H. Clinical spectrum of levodopa-induced complications. Movement Disorders. 2015;30(1) doi: 10.1002/mds.26125. [DOI] [PubMed] [Google Scholar]
- 8.Noh H., Kwon S., Cho S. Y., et al. Effectiveness and safety of acupuncture in the treatment of Parkinson’s disease: a systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Medicine. 2017;34:86–103. doi: 10.1016/j.ctim.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Kim H. J., Jeon B. S. Is acupuncture efficacious therapy in Parkinson’s disease? Journal of the Neurological Sciences. 2014;341(1-2):1–7. doi: 10.1016/j.jns.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Huang J., Qin X., Cai X., Huang Y. Effectiveness of acupuncture in the treatment of Parkinson’s disease: an overview of systematic reviews. Frontiers in Neurology. 2020;11:p. 917. doi: 10.3389/fneur.2020.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ko J. H., Lee H., Kim S. N., Park H. J. Does acupuncture protect dopamine neurons in Parkinson’s disease rodent model?: a systematic review and meta-analysis. Frontiers in Aging Neuroscience. 2019;11:p. 102. doi: 10.3389/fnagi.2019.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao L., Li X., Li M., et al. The effectiveness of acupuncture for Parkinson’s disease: an overview of systematic reviews. Complementary Therapies in Medicine. 2020;50:p. 102383. doi: 10.1016/j.ctim.2020.102383. [DOI] [PubMed] [Google Scholar]
- 13.Qiang T., Gai C., Chai Y., et al. Combination therapy of scalp electro-acupuncture and medication for the treatment of Parkinson’s disease: a systematic review and meta-analysis. Journal of Traditional Chinese Medical Sciences. 2019;6(1):26–34. doi: 10.1016/j.jtcms.2019.01.005. [DOI] [Google Scholar]
- 14.Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Movement Disorders. 2003;18(7):738–750. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 15.Lee M. S., Shin B. C., Kong J. C., Ernst E. Effectiveness of acupuncture for Parkinson’s disease: a systematic review. Movement Disorders: official journal of the Movement Disorder Society. 2008;23(11):1505–1515. doi: 10.1002/mds.21993. [DOI] [PubMed] [Google Scholar]
- 16.Webster D. D. Critical analysis of the disability in Parkinson’s disease. Modern Treatment. 1968;5(2):257–282. [PubMed] [Google Scholar]
- 17.Seppi K., Ray Chaudhuri K., Coelho M., et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Movement Disorders: official journal of the Movement Disorder Society. 2019;34(2):180–198. doi: 10.1002/mds.27602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subramanian I. Complementary and alternative medicine and exercise in nonmotor symptoms of Parkinson’s disease. International Review of Neurobiology. 2017;134:1163–1188. doi: 10.1016/bs.irn.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 19.Lei H., Toosizadeh N., Schwenk M., et al. A pilot clinical trial to objectively assess the efficacy of electroacupuncture on gait in patients with Parkinson’s disease using body worn sensors. PloS One. 2016;11(5, article e0155613) doi: 10.1371/journal.pone.0155613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong K. H., Ng H. L., Li W., et al. Acupuncture in the treatment of fatigue in Parkinson’s disease: a pilot, randomized, controlled, study. Brain and Behavior. 2018;8(1, article e00897) doi: 10.1002/brb3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kluger B. M., Rakowski D., Christian M., et al. Randomized, controlled trial of acupuncture for fatigue in Parkinson’s disease. Movement Disorders: official journal of the Movement Disorder Society. 2016;31(7):1027–1032. doi: 10.1002/mds.26597. [DOI] [PubMed] [Google Scholar]
- 22.Yu S. W., Lin S. H., Tsai C. C., et al. Acupuncture effect and mechanism for treating pain in patients with Parkinson’s disease. Frontiers in Neurology. 2019;10:p. 1114. doi: 10.3389/fneur.2019.01114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shulman L. M., Wen X., Weiner W. J., et al. Acupuncture therapy for the symptoms of Parkinson’s disease. Movement Disorders: official journal of the Movement Disorder Society. 2002;17(4):799–802. doi: 10.1002/mds.10134. [DOI] [PubMed] [Google Scholar]
- 24.Cristian A., Katz M., Cutrone E., Walker R. H. Evaluation of acupuncture in the treatment of Parkinson’s disease: a double-blind pilot study. Movement Disorders: official journal of the Movement Disorder Society. 2005;20(9):1185–1188. doi: 10.1002/mds.20503. [DOI] [PubMed] [Google Scholar]
- 25.Aroxa F. H., Gondim I. T., Santos E. L., Coriolano M. D., Asano A. G., Asano N. M. Acupuncture as adjuvant therapy for sleep disorders in Parkinson’s disease. Journal of Acupuncture and Meridian Studies. 2017;10(1):33–38. doi: 10.1016/j.jams.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Fukuda S., Kuriyama N., Tsuru H., Egawa M. Immediate effects of acupuncture on tongue pressure including swallowing reflex latency in Parkinson’s disease. Acupuncture in Medicine: journal of the British Medical Acupuncture Society. 2016;34(1):59–61. doi: 10.1136/acupmed-2015-010811. [DOI] [PubMed] [Google Scholar]
- 27.Wang L., He C., Liu Y., Zhu L. Effect of acupuncture on the auditory evoked brain stem potential in Parkinson’s disease. Journal of Traditional Chinese Medicine. 2002;22(1):15–17. [PubMed] [Google Scholar]
- 28.Yeo S., van den Noort M., Bosch P., Lim S. A study of the effects of 8-week acupuncture treatment on patients with Parkinson’s disease. Medicine. 2018;97(50, article e13434) doi: 10.1097/MD.0000000000013434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cho S. Y., Lee Y. E., Doo K. H., et al. Efficacy of combined treatment with acupuncture and bee venom acupuncture as an adjunctive treatment for Parkinson’s disease. The Journal of Alternative and Complementary Medicine: Research on Paradigm, Practice, and Policy. 2018;24(1):25–32. doi: 10.1089/acm.2016.0250. [DOI] [PubMed] [Google Scholar]
- 30.Eng M. L., Lyons K. E., Greene M. S., Pahwa R. Open-label trial regarding the use of acupuncture and yin tui na in Parkinson’s disease outpatients: a pilot study on efficacy, tolerability, and quality of life. The Journal of Alternative and Complementary Medicine: Research on Paradigm, Practice, and Policy. 2006;12(4):395–399. doi: 10.1089/acm.2006.12.395. [DOI] [PubMed] [Google Scholar]
- 31.Doo K. H., Lee J. H., Cho S. Y., et al. A prospective open-label study of combined treatment for idiopathic Parkinson’s disease using acupuncture and bee venom acupuncture as an adjunctive treatment. The Journal of Alternative and Complementary Medicine: Research on Paradigm, Practice, and Policy. 2015;21(10):598–603. doi: 10.1089/acm.2015.0078. [DOI] [PubMed] [Google Scholar]
- 32.Ren X. M. Fifty cases of Parkinson’s disease treated by acupuncture combined with Madopar. Journal of Traditional Chinese Medicine = Chung i tsa chih ying wen pan. 2008;28(4):255–257. doi: 10.1016/s0254-6272(09)60004-2. [DOI] [PubMed] [Google Scholar]
- 33.Lee H. S., Park H. L., Lee S. J., Shin B. C., Choi J. Y., Lee M. S. Scalp acupuncture for Parkinson’s disease: a systematic review of randomized controlled trials. Chinese Journal of Integrative Medicine. 2013;19(4):297–306. doi: 10.1007/s11655-013-1431-9. [DOI] [PubMed] [Google Scholar]
- 34.Tang P. P., Xu Q., Chen D., Zhu L. L., Wu Q. H., Bao C. Effect of scalp acupuncture stimulation on cerebral cortex function and related mechanism. Zhen ci yan jiu = Acupuncture Research. 2020;45(6):504–507. doi: 10.13702/j.1000-0607.190627. [DOI] [PubMed] [Google Scholar]
- 35.Wichmann T., DeLong M. R. Pathophysiology of Parkinson’s disease: the MPTP primate model of the human disorder. Annals of the New York Academy of Sciences. 2003;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- 36.Kim S. N., Doo A. R., Park J. Y., et al. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson’s disease. PloS One. 2011;6(11, article e27566) doi: 10.1371/journal.pone.0027566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song J., Lee S. S., Lim S., Yeo S. Mechanism of the neuroprotective effect of injecting brain cells on ST36 in an animal model of Parkinson’s disease. Neuroscience Letters. 2020;717:p. 134698. doi: 10.1016/j.neulet.2019.134698. [DOI] [PubMed] [Google Scholar]
- 38.Yu Y. P., Ju W. P., Li Z. G., Wang D. Z., Wang Y. C., Xie A. M. Acupuncture inhibits oxidative stress and rotational behavior in 6-hydroxydopamine lesioned rat. Brain Research. 2010;1336:58–65. doi: 10.1016/j.brainres.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 39.Yeo S., An K. S., Hong Y. M., et al. Neuroprotective changes in degeneration-related gene expression in the substantia nigra following acupuncture in an MPTP mouse model of parkinsonism: microarray analysis. Genetics and Molecular Biology. 2015;38(1):115–127. doi: 10.1590/S1415-475738120140137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S. H., Lim S. Clinical effectiveness of acupuncture on Parkinson disease: a PRISMA-compliant systematic review and meta-analysis. Medicine. 2017;96(3, article e5836) doi: 10.1097/MD.0000000000005836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao D. Acupuncture for Parkinson’s disease: a review of clinical, animal, and functional magnetic resonance imaging studies. Journal of Traditional Chinese Medicine = Chung i tsa chih ying wen pan. 2015;35(6):709–717. doi: 10.1016/s0254-6272(15)30164-3. [DOI] [PubMed] [Google Scholar]
- 42.Zeng X. S., Geng W. S., Jia J. J., Chen L., Zhang P. P. Cellular and molecular basis of neurodegeneration in Parkinson disease. Frontiers in Aging Neuroscience. 2018;10:p. 109. doi: 10.3389/fnagi.2018.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zeng X. S., Geng W. S., Jia J. J. Neurotoxin-induced animal models of Parkinson disease: pathogenic mechanism and assessment. ASN Neuro. 2018;10 doi: 10.1177/1759091418777438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee V. M., Trojanowski J. Q. Mechanisms of Parkinson's disease linked to pathological α-synuclein: new targets for drug discovery. Neuron. 2006;52(1):33–38. doi: 10.1016/j.neuron.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Blandini F., Nappi G., Tassorelli C., Martignoni E. Functional changes of the basal ganglia circuitry in Parkinson’s disease. Progress in Neurobiology. 2000;62(1):63–88. doi: 10.1016/S0301-0082(99)00067-2. [DOI] [PubMed] [Google Scholar]
- 46.Ferrer I. Neuropathology and neurochemistry of nonmotor symptoms in Parkinson’s disease. Parkinson’s Disease. 2011;2011:13. doi: 10.4061/2011/708404.708404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H., Pan Y., Xue B., et al. The antioxidative effect of electro-acupuncture in a mouse model of Parkinson’s disease. PloS One. 2011;6(5, article e19790) doi: 10.1371/journal.pone.0019790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang X. B., Luo Y., Liu X. Y., et al. Electro-acupuncture improves behavior and upregulates GDNF mRNA in MFB transected rats. Neuroreport. 2003;14(8):1177–1181. doi: 10.1097/00001756-200306110-00015. [DOI] [PubMed] [Google Scholar]
- 49.Hong M. S., Park H. K., Yang J. S., et al. Gene expression profile of acupuncture treatment in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease model. Neurological Research. 2010;32(Supplement 1):74–78. doi: 10.1179/016164109X12537002794165. [DOI] [PubMed] [Google Scholar]
- 50.Choi Y. G., Yeo S., Hong Y. M., Kim S. H., Lim S. Changes of gene expression profiles in the cervical spinal cord by acupuncture in an MPTP-intoxicated mouse model: microarray analysis. Gene. 2011;481(1):7–16. doi: 10.1016/j.gene.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 51.Yeo S., Choi Y. G., Hong Y. M., Lim S. Neuroprotective changes of thalamic degeneration-related gene expression by acupuncture in an MPTP mouse model of parkinsonism: microarray analysis. Gene. 2013;515(2):329–338. doi: 10.1016/j.gene.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 52.Jeon S., Kim Y. J., Kim S. T., et al. Proteomic analysis of the neuroprotective mechanisms of acupuncture treatment in a Parkinson’s disease mouse model. Proteomics. 2008;8(22):4822–4832. doi: 10.1002/pmic.200700955. [DOI] [PubMed] [Google Scholar]
- 53.Wang H., Liang X., Wang X., Luo D., Jia J., Wang X. Electro-acupuncture stimulation improves spontaneous locomotor hyperactivity in MPTP intoxicated mice. PloS One. 2013;8(5, article e64403) doi: 10.1371/journal.pone.0064403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim Y. K., Lim H. H., Song Y. K., et al. Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neuroscience Letters. 2005;384(1-2):133–138. doi: 10.1016/j.neulet.2005.04.068. [DOI] [PubMed] [Google Scholar]
- 55.Yang J. L., Chen J. S., Yang Y. F., et al. Neuroprotection effects of retained acupuncture in neurotoxin-induced Parkinson's disease mice. Brain, Behavior, and Immunity. 2011;25(7):1452–1459. doi: 10.1016/j.bbi.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 56.Yeo S., Lim S. Acupuncture inhibits the increase in alpha-synuclein by modulating SGK1 in an MPTP induced parkinsonism mouse model. The American Journal of Chinese Medicine. 2019;47(3):527–539. doi: 10.1142/S0192415X19500277. [DOI] [PubMed] [Google Scholar]
- 57.Yeo S., Song J., Lim S. Acupuncture inhibits the increase in alpha-synuclein in substantia nigra in an MPTP-induced parkinsonism mouse model. Advances in Experimental Medicine and Biology. 2020;1232:401–408. doi: 10.1007/978-3-030-34461-0_51. [DOI] [PubMed] [Google Scholar]
- 58.Tian T., Sun Y., Wu H., et al. Acupuncture promotes mTOR-independent autophagic clearance of aggregation-prone proteins in mouse brain. Scientific Reports. 2016;6(1):p. 19714. doi: 10.1038/srep19714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang S. J., Wang Q., Ma J., Yu P. H., Wang Z. M., Wang B. Effect of moxibustion on mTOR-mediated autophagy in rotenone-induced Parkinson’s disease model rats. Neural Regeneration Research. 2018;13(1):112–118. doi: 10.4103/1673-5374.224380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X., Zhang N., Zhao H., et al. The protect effect of baicalin on the substantial nigra dopaminergic neuron in Parkinson’s rats induced by rotenone. Zhongfeng yu Shenjing Jibing Zazhi. 2008;2:174–177. [Google Scholar]
- 61.Park J. Y., Choi H., Baek S., et al. p53 signalling mediates acupuncture-induced neuroprotection in Parkinson’s disease. Biochemical and Biophysical Research Communications. 2015;460(3):772–779. doi: 10.1016/j.bbrc.2015.03.105. [DOI] [PubMed] [Google Scholar]
- 62.Doo A. R., Kim S. T., Kim S. N., et al. Neuroprotective effects of bee venom pharmaceutical acupuncture in acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Neurological Research. 2010;32(Supplement 1):88–91. doi: 10.1179/016164109X12537002794282. [DOI] [PubMed] [Google Scholar]
- 63.Liang X. B., Liu X. Y., Li F. Q., et al. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Research. Molecular Brain Research. 2002;108(1-2):51–59. doi: 10.1016/S0169-328X(02)00513-2. [DOI] [PubMed] [Google Scholar]
- 64.Wang S., Fang J., Ma J., et al. Electroacupuncture-regulated neurotrophic factor mRNA expression in the substantia nigra of Parkinson’s disease rats. Neural Regeneration Research. 2013;8(6):540–549. doi: 10.3969/j.issn.1673-5374.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jang J. H., Yeom M. J., Ahn S., et al. Acupuncture inhibits neuroinflammation and gut microbial dysbiosis in a mouse model of Parkinson’s disease. Brain, Behavior, and Immunity. 2020;89:641–655. doi: 10.1016/j.bbi.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 66.Park H. J., Lim S., Joo W. S., et al. Acupuncture prevents 6-hydroxydopamine-induced neuronal death in the nigrostriatal dopaminergic system in the rat Parkinson’s disease model. Experimental Neurology. 2003;180(1):93–98. doi: 10.1016/S0014-4886(02)00031-6. [DOI] [PubMed] [Google Scholar]
- 67.Lin J. G., Chen C. J., Yang H. B., Chen Y. H., Hung S. Y. Electroacupuncture promotes recovery of motor function and reduces dopaminergic neuron degeneration in rodent models of Parkinson’s disease. International Journal of Molecular Sciences. 2017;18(9):p. 1846. doi: 10.3390/ijms18091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao Y., Luo D., Ning Z., Rong J., Lao L. Electro-acupuncture ameliorated MPTP-induced parkinsonism in mice via TrkB neurotrophic signaling. Frontiers in Neuroscience. 2019;13:p. 496. doi: 10.3389/fnins.2019.00496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim S. N., Kim S. T., Doo A. R., et al. Phosphatidylinositol 3-kinase/Akt signaling pathway mediates acupuncture-induced dopaminergic neuron protection and motor function improvement in a mouse model of Parkinson’s disease. The International Journal of Neuroscience. 2011;121(10):562–569. doi: 10.3109/00207454.2011.591515. [DOI] [PubMed] [Google Scholar]
- 70.Hwang T. Y., Song M. A., Ahn S., et al. Effects of combined treatment with acupuncture and Chunggan formula in a mouse model of Parkinson’s disease. Evidence-Based Complementary and Alternative Medicine: ECAM. 2019;2019, article 3612587:14. doi: 10.1155/2019/3612587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jeon H., Ryu S., Kim D., Koo S., Ha K. T., Kim S. Acupuncture stimulation at GB34 restores MPTP-induced neurogenesis impairment in the subventricular zone of mice. Evidence-Based Complementary and Alternative Medicine: ECAM. 2017;2017, article 3971675:9. doi: 10.1155/2017/3971675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee Y., Choi G., Jeon H., et al. Acupuncture stimulation at GB34 suppresses 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced oxidative stress in the striatum of mice. The Journal of Physiological Sciences: JPS. 2018;68(4):455–462. doi: 10.1007/s12576-017-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang J. M., Park H. J., Choi Y. G., et al. Acupuncture inhibits microglial activation and inflammatory events in the MPTP-induced mouse model. Brain Research. 2007;1131(1):211–219. doi: 10.1016/j.brainres.2006.10.089. [DOI] [PubMed] [Google Scholar]
- 74.Li L., Lu J., Sun Y., Jin X. Acupuncture protects from 6-OHDA-induced neuronal damage by balancing the ratio of DMT1/Fpn1. Saudi Journal of Biological Sciences. 2019;26(8):1948–1955. doi: 10.1016/j.sjbs.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang H. J., Gao Y., Yun J. Y., et al. Acupuncture does not protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced damage of dopaminergic neurons in a preclinical mouse model of Parkinson’s disease. Neuroreport. 2017;28(1):50–55. doi: 10.1097/WNR.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 76.Kim Y. K., Lee A. R., Park H., et al. Acupuncture alleviates levodopa-induced dyskinesia via melanin-concentrating hormone in Pitx3-deficient aphakia and 6-hydroxydopamine-lesioned mice. Molecular Neurobiology. 2019;56(4):2408–2423. doi: 10.1007/s12035-018-1194-6. [DOI] [PubMed] [Google Scholar]
- 77.Wattanathorn J., Sutalangka C. Laser acupuncture at HT7 acupoint improves cognitive deficit, neuronal loss, oxidative stress, and functions of cholinergic and dopaminergic systems in animal model of Parkinson’s disease. Evidence-Based Complementary and Alternative Medicine: ECAM. 2014;2014, article 937601:8. doi: 10.1155/2014/937601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park J. Y., Kim S. N., Yoo J., et al. Novel neuroprotective effects of melanin-concentrating hormone in Parkinson’s disease. Molecular Neurobiology. 2017;54(10):7706–7721. doi: 10.1007/s12035-016-0258-8. [DOI] [PubMed] [Google Scholar]
- 79.Poewe W., Seppi K., Tanner C. M., et al. Parkinson disease. Nature Reviews. Disease Primers. 2017;3(1):p. 17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 80.Yeo S., Sung B., Hong Y. M., et al. Decreased expression of serum- and glucocorticoid-inducible kinase 1 (SGK1) promotes alpha-synuclein increase related with down-regulation of dopaminergic cell in the substantia nigra of chronic MPTP-induced parkinsonism mice and in SH-SY5Y cells. Gene. 2018;661:189–195. doi: 10.1016/j.gene.2018.03.086. [DOI] [PubMed] [Google Scholar]
- 81.Ebrahimi-Fakhari D., Cantuti-Castelvetri I., Fan Z., et al. Distinct roles in vivo for the ubiquitin-proteasome system and the autophagy-lysosomal pathway in the degradation of α-synuclein. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2011;31(41):14508–14520. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kahan B. Toxicity spectrum of inhibitors of mammalian target of rapamycin in organ transplantation: etiology, pathogenesis and treatment. Expert Opinion on Drug Safety. 2011;10(5):727–749. doi: 10.1517/14740338.2011.579898. [DOI] [PubMed] [Google Scholar]
- 83.Ruchaud S., Korfali N., Villa P., et al. Caspase-6 gene disruption reveals a requirement for lamin A cleavage in apoptotic chromatin condensation. The EMBO Journal. 2002;21(8):1967–1977. doi: 10.1093/emboj/21.8.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khalil W. K., Assaf N., ElShebiney S. A., Salem N. A. Neuroprotective effects of bee venom acupuncture therapy against rotenone-induced oxidative stress and apoptosis. Neurochemistry International. 2015;80:79–86. doi: 10.1016/j.neuint.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Willesen M. G., Gammeltoft S., Vaudano E. Activation of the c-Jun N terminal kinase pathway in an animal model of Parkinson’s disease. Annals of the New York Academy of Sciences. 2002;973(1):237–240. doi: 10.1111/j.1749-6632.2002.tb04640.x. [DOI] [PubMed] [Google Scholar]
- 86.Huang E. J., Reichardt L. F. Trk receptors: roles in neuronal signal transduction. Annual Review of Biochemistry. 2003;72(1):609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- 87.Gupta V. K., You Y., Gupta V. B., Klistorner A., Graham S. L. TrkB receptor signalling: implications in neurodegenerative, psychiatric and proliferative disorders. International Journal of Molecular Sciences. 2013;14(5):10122–10142. doi: 10.3390/ijms140510122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Andero R., Choi D. C., Ressler K. J. BDNF-TrkB receptor regulation of distributed adult neural plasticity, memory formation, and psychiatric disorders. Progress in Molecular Biology and Translational Science. 2014;122:169–192. doi: 10.1016/B978-0-12-420170-5.00006-4. [DOI] [PubMed] [Google Scholar]
- 89.Streit W. J. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–139. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 90.Blandini F. Neural and immune mechanisms in the pathogenesis of Parkinson’s disease. Journal of Neuroimmune Pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2013;8(1):189–201. doi: 10.1007/s11481-013-9435-y. [DOI] [PubMed] [Google Scholar]
- 91.Sampson T. R., Debelius J. W., Thron T., et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson's Disease. Cell. 2016;167(6):1469–1480.e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rathore K. I., Redensek A., David S. Iron homeostasis in astrocytes and microglia is differentially regulated by TNF-α and TGF-β1. Glia. 2012;60(5):738–750. doi: 10.1002/glia.22303. [DOI] [PubMed] [Google Scholar]
- 93.Li H., He T., Xu Q., et al. Acupuncture and regulation of gastrointestinal function. World Journal of Gastroenterology. 2015;21(27):8304–8313. doi: 10.3748/wjg.v21.i27.8304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dhond R. P., Kettner N., Napadow V. Neuroimaging acupuncture effects in the human brain. The Journal of Alternative and Complementary Medicine: Research on Paradigm, Practice, and Policy. 2007;13(6):603–616. doi: 10.1089/acm.2007.7040. [DOI] [PubMed] [Google Scholar]
- 95.Deuschl G., Raethjen J., Baron R., Lindemann M., Wilms H., Krack P. The pathophysiology of parkinsonian tremor: a review. Journal of Neurology. 2000;247(Supplement 5):V33–V48. doi: 10.1007/PL00007781. [DOI] [PubMed] [Google Scholar]
- 96.Wu T., Wang J., Wang C., et al. Basal ganglia circuits changes in Parkinson's disease patients. Neuroscience Letters. 2012;524(1):55–59. doi: 10.1016/j.neulet.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee S. H., Jahng G. H., Choe I. H., Choi C. B., Kim D. H., Kim H. Y. Neural pathway interference by retained acupuncture: a functional MRI study of a dog model of Parkinson’s disease. CNS Neuroscience & Therapeutics. 2013;19(8):585–595. doi: 10.1111/cns.12108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chae Y., Lee H., Kim H., et al. Parsing brain activity associated with acupuncture treatment in Parkinson’s diseases. Movement Disorders: official journal of the Movement Disorder Society. 2009;24(12):1794–1802. doi: 10.1002/mds.22673. [DOI] [PubMed] [Google Scholar]
- 99.Yeo S., Lim S., Choe I. H., et al. Acupuncture stimulation on GB34 activates neural responses associated with Parkinson’s disease. CNS Neuroscience & Therapeutics. 2012;18(9):781–790. doi: 10.1111/j.1755-5949.2012.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yeo S., Choe I. H., van den Noort M., et al. Acupuncture on GB34 activates the precentral gyrus and prefrontal cortex in Parkinson’s disease. BMC Complementary and Alternative Medicine. 2014;14(1):p. 336. doi: 10.1186/1472-6882-14-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li Z., Chen J., Cheng J., et al. Acupuncture modulates the cerebello-thalamo-cortical circuit and cognitive brain regions in patients of Parkinson’s disease with tremor. Frontiers in Aging Neuroscience. 2018;10:p. 206. doi: 10.3389/fnagi.2018.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang Y., Jiang X., Zhuo Y., Wik G. Complementary acupuncture in Parkinson’s disease: a SPECT study. The International Journal of Neuroscience. 2010;120(2):150–154. doi: 10.3109/00207450903316527. [DOI] [PubMed] [Google Scholar]
- 103.Huang Y., Jiang X., Zhuo Y., Tang A., Wik G. Complementary acupuncture treatment increases cerebral metabolism in patients with Parkinson’s disease. The International Journal of Neuroscience. 2009;119(8):1190–1197. doi: 10.1080/00207450802336774. [DOI] [PubMed] [Google Scholar]
- 104.Zhang R., Andersen A. H., Hardy P. A., et al. Objectively measuring effects of electro-acupuncture in parkinsonian rhesus monkeys. Brain Research. 2018;1678:12–19. doi: 10.1016/j.brainres.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rui G., Guangjian Z., Yong W., et al. High frequency electro-acupuncture enhances striatum DAT and D1 receptor expression, but decreases D2 receptor level in 6-OHDA lesioned rats. Behavioural Brain Research. 2013;237:263–269. doi: 10.1016/j.bbr.2012.09.047. [DOI] [PubMed] [Google Scholar]
- 106.Jia J., Sun Z., Li B., et al. Electro-acupuncture stimulation improves motor disorders in parkinsonian rats. Behavioural Brain Research. 2009;205(1):214–218. doi: 10.1016/j.bbr.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 107.Jia J., Li B., Sun Z. L., Yu F., Wang X., Wang X. M. Electro-acupuncture stimulation acts on the basal ganglia output pathway to ameliorate motor impairment in parkinsonian model rats. Behavioral Neuroscience. 2010;124(2):305–310. doi: 10.1037/a0018931. [DOI] [PubMed] [Google Scholar]
- 108.Kim S. N., Doo A. R., Park J. Y., et al. Combined treatment with acupuncture reduces effective dose and alleviates adverse effect of L-dopa by normalizing Parkinson’s disease-induced neurochemical imbalance. Brain Research. 2014;1544:33–44. doi: 10.1016/j.brainres.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sun Z., Jia J., Gong X., et al. Inhibition of glutamate and acetylcholine release in behavioral improvement induced by electroacupuncture in parkinsonian rats. Neuroscience Letters. 2012;520(1):32–37. doi: 10.1016/j.neulet.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 110.Wu T., Long X., Zang Y., et al. Regional homogeneity changes in patients with Parkinson’s disease. Human Brain Mapping. 2009;30(5):1502–1510. doi: 10.1002/hbm.20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lewis M. M., du G., Sen S., et al. Differential involvement of striato- and cerebello-thalamo-cortical pathways in tremor- and akinetic/rigid-predominant Parkinson's disease. Neuroscience. 2011;177:230–239. doi: 10.1016/j.neuroscience.2010.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bostan A. C., Dum R. P., Strick P. L. Cerebellar networks with the cerebral cortex and basal ganglia. Trends in Cognitive Sciences. 2013;17(5):241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lyons M. K. Deep brain stimulation: current and future clinical applications. Mayo Clinic Proceedings. 2011;86(7):662–672. doi: 10.4065/mcp.2011.0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bostan A. C., Strick P. L. The basal ganglia and the cerebellum: nodes in an integrated network. Nature Reviews. Neuroscience. 2018;19(6):338–350. doi: 10.1038/s41583-018-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lanciego J. L., Luquin N., Obeso J. A. Functional neuroanatomy of the basal ganglia. Cold Spring Harbor Perspectives in Medicine. 2012;2(12, article a009621) doi: 10.1101/cshperspect.a009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aosaki T., Miura M., Suzuki T., Nishimura K., Masuda M. Acetylcholine-dopamine balance hypothesis in the striatum: an update. Geriatrics & Gerontology International. 2010;10(Supplement 1):S148–S157. doi: 10.1111/j.1447-0594.2010.00588.x. [DOI] [PubMed] [Google Scholar]
- 117.Fahn S., Oakes D., Shoulson I., et al. Levodopa and the progression of Parkinson’s disease. The New England Journal of Medicine. 2004;351(24):2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 118.Manson A., Stirpe P., Schrag A. Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. Journal of Parkinson's Disease. 2012;2(3):189–198. doi: 10.3233/JPD-2012-120103. [DOI] [PubMed] [Google Scholar]


