Abstract
Assessment of the circumpapillary retinal nerve fiber layer (RNFL) provides crucial knowledge on the status of the optic nerve. Current circumpapillary RNFL measurements consider only thickness, but an accurate evaluation should also consider blood vessel contribution. Previous studies considered the presence of major vessels in RNFL thickness measurements from optical coherence tomography (OCT). However, such quantitative measurements do not account for smaller vessels, which could also affect circumpapillary RNFL measurements. We present an approach to automatically segregate the neuronal and vascular components in circumpapillary RNFL by combining vascular information from OCT angiography (OCTA) and structural data from OCT. Automated segmentation of the circumpapillary RNFL using a state-of-the-art deep learning network is first performed and followed by the lateral and depth-resolved localization of the vascular component by vertically projecting the vessels along the circular scan from OCTA vessels map onto the segmented RNFL. Using this proposed approach, we compare the correlations of circumpapillary RNFL thicknesses with age at different levels of vessel exclusion (exclusion of major vessels only vs both major- and micro-vessels) and also evaluate the thickness variability in 75 healthy eyes. Our results show that the ratio of major- and micro-vessels to circumpapillary RNFL achieved a stronger correlation with aging (r = 0.478, P < .001) than the ratio with only major vessels to circumpapillary RNFL (r = 0.027, P = .820). Exclusion of blood vessels from circumpapillary RNFL thickness using OCTA imaging provides a better measure of the neuronal components and could potentially improve the diagnostic performance for disease detection.
1. Introduction
The retinal nerve fiber layer (RNFL) is composed primarily of neuronal axon bundles, which are unsheathed by glial cells, and blood vessels. Structural alteration of the RNFL is mainly due to ganglion cell axonal loss from either aging or ocular diseases such as glaucoma. The loss of these neuronal cells can be quantified by measuring the thickness of the RNFL around the optic disc in vivo using Optical Coherence Tomography (OCT). Numerous works have demonstrated the use of OCT to study the thinning of RNFL in glaucoma [1–3]. Studies have shown the importance of considering the vascular component within the RNFL when performing thickness measurements, which can significantly affect the variability of RNFL thickness profiles in healthy and glaucomatous subjects [4,5]. Ye et al. also investigated the impact of the inclusion of blood vessels on RNFL measurements in severe glaucoma and showed that these measurement can be adversely affected mainly when the RNFL was thin [6].
Current approaches for detecting and segmenting blood vessels in OCT include the application of adaptive binarization on cross-sectional OCT scans [7], the creation of shadowgraphs [8] in cross-sectional OCT scans to assign the lateral vessel positions followed by an active shape model method to segment the vessels [9], and the detection of vessel axial margins in cross-sectional OCT scans by tracking the optical shadows cast onto the outer retina [6]. However, these approaches were only applied to cross-sectional OCT scans and largely relied on the presence of optical shadow artefacts for vessel localization. As such, only major vessels with salient shadows are likely to be detected while smaller vessels with less prominent shadows can be under-detected, leading to under-representation of the vascular component. Furthermore, it is important to detect the microvasculature as its reduction in density could indicate the closure of capillaries due to glaucomatous damage [10,11]. Hence, there remains a need to account for microvasculature in the retina when performing structural measurements especially for patients with ocular conditions.
OCT angiography (OCTA) is a functional extension of OCT which enables non-invasive visualization of vasculature in the retina by employing decorrelation between consecutive cross-sectional OCT scans acquired at the same retinal location. Studies have shown that the derived retinal vessel diameter measurements from OCTA has excellent consistency with adaptive optics ophthalmoscopy [12] and have demonstrated the clinical usability of this novel imaging technique in analyzing retina vasculature quantitatively in healthy and glaucomatous human eyes. Yarmohammadi et al. performed a study showing a significant association between OCTA vessel density and the severity of visual field loss and concluded that OCTA was a promising technology in glaucoma management [13]. Wu et al. evaluated the correlation between reduced macular vessel density with reductions in the RNFL and ganglion cell complex (GCC) measurements using OCTA in patients with primary open-angle glaucoma (POAG) [14]. The results showed statistically significant correlations between reduced macular vessel density and RNFL or GCC thickness in glaucomatous eyes.
In this paper, we present an approach which combines vascular information from OCTA and structural data from OCT to detect and localize the vascular component within the circumpapillary RNFL in healthy human eyes. The localization of vessels involves the automated segmentation of the circumpapillary RNFL to obtain the axial extents of vessels and the use of enface OCTA images to provide the lateral extents. We evaluated the correlations between the vascular and the neuronal components of the RNFL with age, and as well as the contribution of the vascular component to RNFL thickness variability.
2. Materials and methods
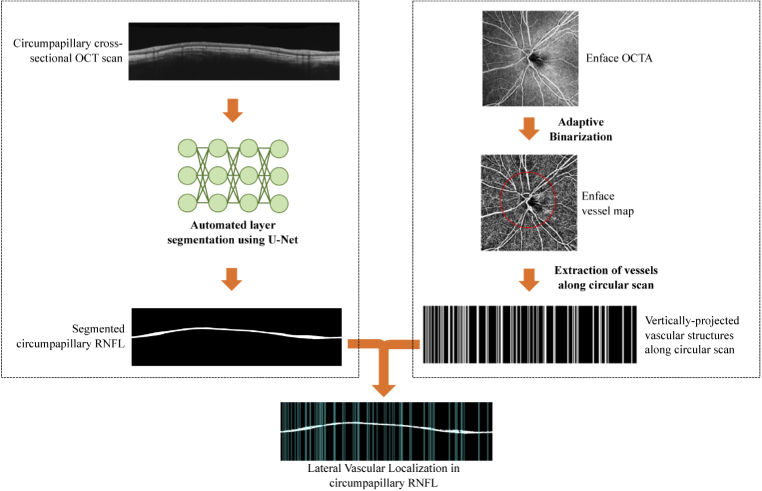
This section outlines the proposed approach for segregation of the vascular component in circumpapillary RNFL thickness measurements. We provide details on the three main processes namely, acquisition and pre-processing of OCT/OCTA images, depth-resolved vascular localisation and segregation of neuronal-vascular components. Figure 1 provides an overview of the processes in segregating vessels from the nerve fibre layer for latter analysis.
Fig. 1.

Overview of vessel-removal approach
2.1. Acquisition and pre-processing of OCT/OCTA images
The study was approved by the SingHealth Centralized Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all participants. Inclusion criteria for the healthy subjects were no evidence of ocular pathologies, including glaucoma, diabetic retinopathy, age-related macular degeneration or ocular opacity.
Both OCT and OCTA images were acquired from 43 healthy subjects using a Plex Elite 9000 (Carl Zeiss Meditec, Inc., Dublin, CA) OCT system. The demographic distribution of the dataset is summarized in Table 1.
Table 1. Subjects demographic characteristics.
| Number of subjects | 43 |
|---|---|
| Sex (male/female) | 9/34 |
| Eye (right/left) | 39/36 |
| Age (mean years ± SD) | 56.1 ± 14.2 |
| Refractive Error (mean diopters ± SD) | -1.46 ± 3.33 |
The Plex Elite 9000 uses a swept-source tunable laser with centre wavelength between 1040-1060nm. It has an acquisition rate of 100,000 A-scans/s, and the axial and lateral resolutions are 6.3µm and 20µm in tissue respectively. Each eye underwent a 6×6 mm imaging protocol centred at the optic disc. Each acquired volumetric scan consists of 500 cross-sectional images and each image consists of 500 A-scans. The acquired scans were exported to MATLAB (Mathworks Inc. Natick, MA, USA) and reconstructed as three-dimensional volumes. Enface projections of the three-dimensional OCT volumes were used to delineate the optic disc boundaries and to automatically determine the centre of the optic disc.
The circumpapillary RNFL scan is a common imaging protocol used in OCT devices for measuring the RNFL thickness to diagnose and monitor glaucoma. As shown in Fig. 2, a circular scan pattern (green circle) with diameter approximately 3.46mm distance centred at the optic nerve head, is commonly used in devices [15]. In our work, we extracted three circumpapillary cross-sectional scans at diameters of 3.44mm, 3.46mm and 3.48mm to reduce the noise and improve the visibility of RNFL boundaries. For the same angular positions along the circumference of each radius, we extracted A-scans from the volumetric scan [16,17]. We then averaged the angularly-aligned A-scans to generate a circumpapillary cross-sectional OCT scan which was rescaled to isotropic resolution. Figure 2 shows an example of a circumpapillary cross-sectional OCT scan which was extracted based on the delineated optic disc boundaries.
Fig. 2.
Generation of circumpapillary cross-sectional OCT/OCTA scan from enface OCT/OCTA. The optic disc center was automatically determined from the delineated optic disc (top left) and generated a circular cross-sectional scan (top right). A similar approach was also used to generate the corresponding circumpapillary cross-sectional OCTA scan (bottom row).
The OCTA image was generated using an optical microangiography (OMAG) technique [18] which processes the OCT data to produce three-dimensional images of blood flow within biological tissues. Its high spatial resolution allows visualization of microvasculature without the use of contrast agents. Instead, the technique uses decorrelation of optical signals backscattered by the flowing blood cells inside the vessels, to generate OCTA volumes. We generated the enface OCTA image as shown in Fig. 3, from maximum projection of the superficial capillary plexus (SCP), using a built-in segmentation software (PLEX Elite 9000 Review Software, Carl Zeiss Meditec, Dublin, CA, USA). We also extracted the circumpapillary cross-sectional OCTA scan using the same optic disc characteristics which were defined from the corresponding enface OCT image as shown in Fig. 2.
Fig. 3.
Lateral vascular localization in circumpapillary RNFL using cross-sectional OCT scan (left branch) and enface OCTA (right branch)
2.2. Lateral vascular localization
Regions of moving scatter in OCTA images which are largely attributable to red blood cells, have higher temporal decorrelation compared to static tissue. This difference in correlation values enables the generation of an enface OCTA image showing the vascular network in the retina. Figure 3 illustrates the vascular localization method using the circumpapillary cross-sectional OCT scan and a corresponding enface OCTA.
The RNFL was first automatically segmented using the U-Net [19] based convolutional neural network without applying filters or pre-processing techniques. The advantage of using a U-Net based network is that it merges a convolutional network architecture with a deconvolutional architecture to output the semantic segmentation of layer of interest, allowing extraction of vast number of features without losing the spatial information when the resolution decreases. The application of U-Net for RNFL segmentation and the details for the training and validation performance are reported in our previous work [16]. The trained model takes in an input image of a cross-sectional OCT scan which was first resized to 512×512 and generates a binarized circumpapillary RNFL segmentation which was used to mask the cross-sectional OCTA scan.
The corresponding enface OCTA image was binarized by applying adaptive thresholding [20] to extract the vascular information and generate the enface vessel map. After which, the vessels around the circular scan (diameter of 3.46mm centred at the optic disc) were extracted and vertically projected onto a cross-sectional plane. It was then combined with the segmented circumpapillary RNFL to demarcate individual blood vessels within the layer.
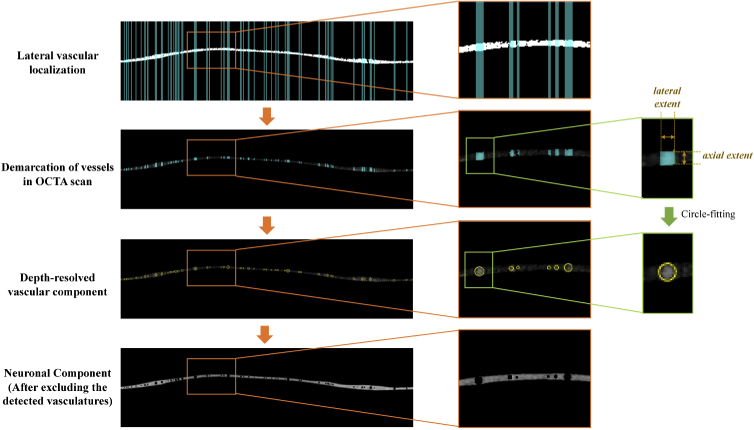
2.3. Depth-resolved vascular localization
Figure 4 illustrates the segregation of the vascular and neuronal component within the circumpapillary RNFL. As presented in the previous section, regions of interest (ROIs) indicating the presence of vascular structures from the OCTA enface vessel map were vertically projected onto the corresponding locations on the circumpapillary OCTA cross-sectional image and combined with the circumpapillary RNFL segmentation for vascular localization. Specifically, the lateral extent of the OCTA signals for each vessel in the cross-sectional OCTA scan was determined from the vertical OCTA vascular map projections, whereas the axial extent was bound by the upper and lower limits of the segmented RNFL. Using these extents, the ROIs containing each vessel were constrained laterally and axially.
Fig. 4.
The process flow from lateral vascular localization to segregation of neuronal-vascular components. The lateral extent of each vessel in the OCTA cross-sectional image was determined from the vertical projection of enface vessel map, and the axial extent was defined by the upper and lower limits of the segmented RNFL. Circle-fitting was performed for each vessel based on the defined extents.
As illustrated in Fig. 5(a), the centroid of each 8-connected vessel region was automatically determined. In our approach, we assumed that the circumpapillary scan is perpendicular to the vessels and the segmented RNFL contains the cross-section of entire vessel. Circular vessel masks V were created using the circle equation:
| (1) |
where is the ith vessel region, r is the radius of the circular vessels which is defined by half the lateral extent and, and refer to the height and width of the mesh grid generated based on the circumpapillary OCT scan. An example of the generated circular vessel masks is shown in Fig. 5(b).
Fig. 5.
(a) Illustration of single vessel mask V (red line) is generated based on half the lateral extent (r) and the determined centroid of the region (Cx, Cy). (b) Circular vessel mask was generated by fitting a circular model for each vessel ROI. (c) Binarized RNFL segmentation was obtained using the trained U-Net model. (d) Binarized RNFL with vessels excluded by masking the RNFL segmentation with the circular vessel masks.
Thereafter, these generated circular vascular masks were excluded from the neuronal layer as shown in Fig. 5(d), from which the two structural parameters, namely the RNFL thickness RT and vessel-to-thickness VT ratio were computed to compare the correlation with age. The RNFL thickness including the vascular components along the circular scan was averaged across all A-scans, to obtain an average RNFL thickness value RTave for each eye:
| (2) |
where N is the total number of columns in binarized RNFL segmentation as shown in Fig. 5(c) and refers to the thickness of the segmented RNFL at the nth A-scan. The thickness of the RNFL excluding the vascular component was also computed using (2) and denoted as RTave,nv for latter analysis. Additionally, we computed the RNFL thickness after excluding the major vessels which were only visible in OCT scans (RTave,nm) as a further comparison. The major vessels were manually selected from the detected vessel regions based on the OCT scans.
Subsequently, we calculated the proportion of vessels relative to the RNFL cross-sectional area VT ratio for all eyes. This parameter is defined as the ratio between total vessel area and the RNFL area before excluding vessels, which was computed as follows:
| (3) |
where is a circular vessel mask which were calculated using (1), I is the total number of circular vessel masks within the RNFL and Rarea is the area of RNFL before excluding vessels which was computed using (4),
| (4) |
where w is the width of the circumpapillary cross-sectional scan and is the RNFL thickness at each A-scan.
3. Results and discussion
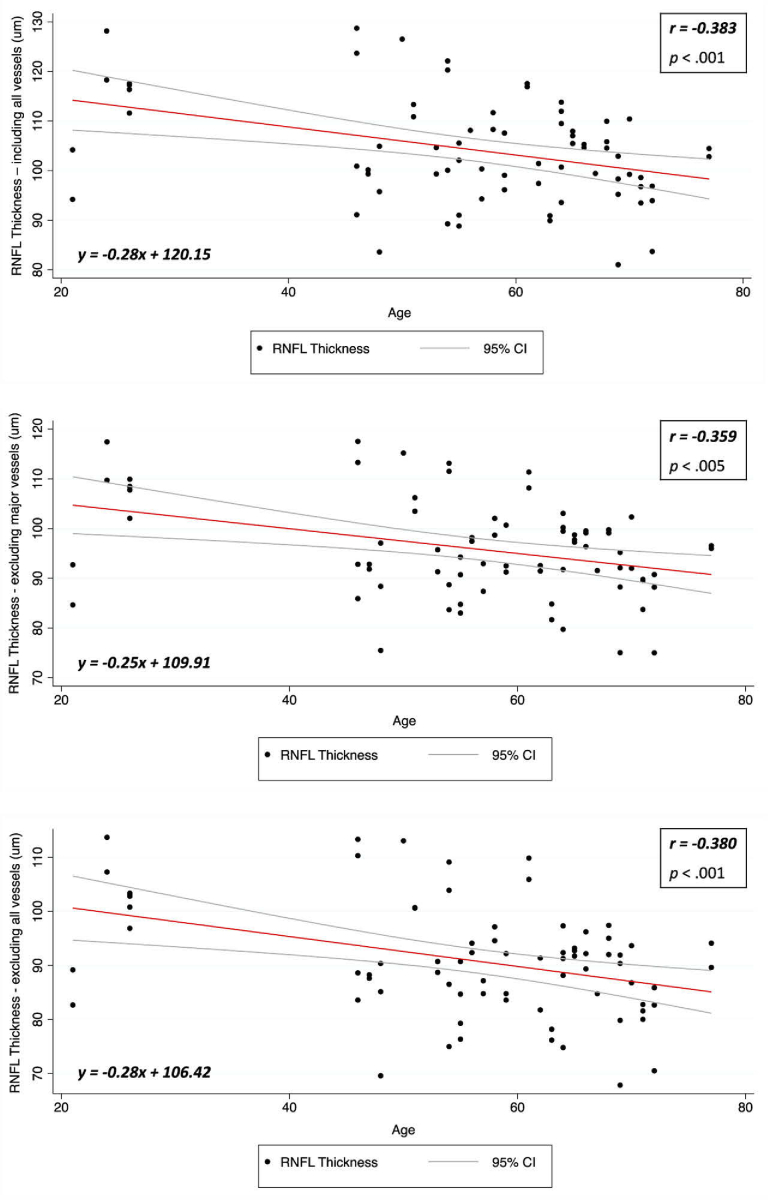
We calculated the Pearson correlation coefficient of each eye to assess the strength of correlation between RNFL thickness (including all vessels RT, after excluding major vessels RTave,nm and after excluding all vessels RTave,nv) and age. Statistical analysis was performed using a statistical software package (STATA V16.1; StataCorp). Steiger test was also used to test for the significance of differences between correlations.
The results in Table 2 show that RTave and RTave,nv have statistically significant negative correlations with age (-0.383, p < .001 and -0.380, p < .001 respectively), whereas RTave,nm has a lower negative correlation with age. The correlation plots for each parameter are presented in Fig. 6. The correlation of total vessel area with age was not statistically significant (r = 0.217, p = .061). Although RTave includes all vessels and RTave,nv excludes all vessels, the correlations with age for both were not significantly different (p = .98). While the measurement of the RNFL before vessel removal consists of neuronal cells, glial cells and vessels, by removing the vessels, we are left with the neuronal and glial components only. Since glaucoma is a disease that is defined by progressive loss of ganglion cell axonal loss, this is actually the metric which we want to measure. Therefore from our result, it shows that exclusion of the vascular component could lead to more suitable measurement of the remnant component in the RNFL which is important for a better quantification of neuronal loss. Further, the results also show that age-related thinning of the neuronal component in RNFL is not affected by the removal of all vessels.
Table 2. Statistical results of Pearson correlation coefficients for RNFL thickness and Age.
| Correlation with Age |
||
|---|---|---|
| Circumpapillary RNFL Thickness | r | p |
| Include all vessels, RTave | -0.383 | < .001 |
| After excluding major vessels, RTave,nm | -0.359 | < .005 |
| After excluding all vessels, RTave,nv | -0.380 | < .001 |
Fig. 6.
Correlation plot between RNFLT and Age (top). Correlation plot between RTave,nm and Age (bottom left). Correlation plot between RTave,nv and Age (bottom right).
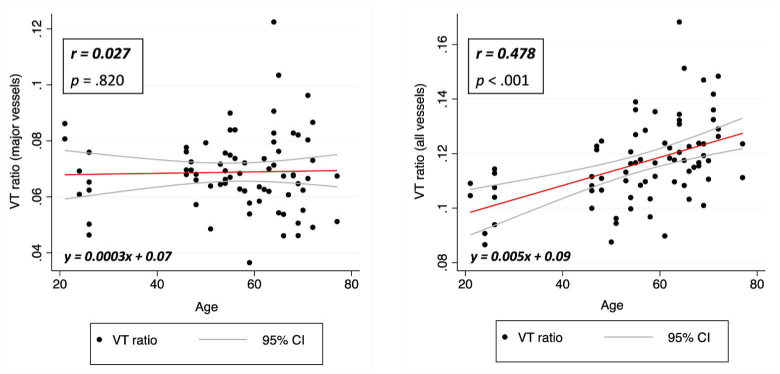
We further compared the correlation between VT ratio and age. The results are presented in Table 3 and showed that with only major vessels, the correlation between VT ratio and age was poor (r = 0.027) and was not statistically significant. However, with all vessels, a statistically significant positive correlation of 0.478 was established. The plots in Fig. 7 show the increase in the VT ratio with increasing age. This can be attributed to age-related loss of neuronal cells within the RNFL, resulting in a reduction in the RNFL thickness and consequently a higher proportion of vessels within the layer. In glaucoma, the RNFL thickness also reduces due to the loss of neuronal cells. Exclusion of only major vessels in the measurement of the RNFL could pose a challenge in identifying neuronal changes due to disease progression. It is important to also consider that the presence of microvasculature may contribute to the RNFL thickness in glaucoma [6]. Furthermore, systemic diseases such as diabetes and cardiovascular diseases could reduce vessel caliber [21] and thinning of retinal blood vessels can be mistakenly viewed as RNFL thinning. Hence, it is essential to consider the neuronal and vascular components separately for better assessment of neuronal changes within the RNFL in glaucoma [4]. The segregated vascular components could also potentially be useful for assessment of retinal vascular conditions [22,23].
Table 3. Comparison of Pearson correlation coefficients for Vessel area-to-RNFL area (VT) ratio and Age between major vessels and all vessels.
| Correlation with Age |
||
|---|---|---|
| Vessel area-to-RNFL area (VT) ratio | r | p |
| Major vessels, VTm | 0.027 | .820 |
| All vessels, VTv | 0.478 | < .001 |
Fig. 7.
Correlation plot between VTm (ratio between major vessel area and RNFL area) and Age (left). Correlation plot between VTv (ratio between total vessel area and RNFL area) and Age (right).
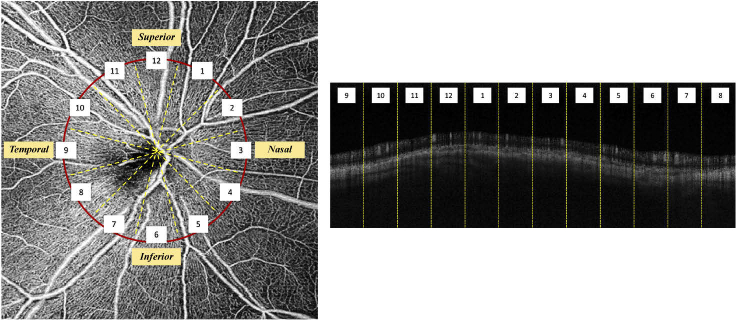
We also evaluated the variability of RNFL thickness before and after all-vessel removal along the circumpapillary profile across all eyes, as presented in Table 4, using the average thickness and standard deviation based on 12 clock-hour sectors around the optic disc. Defining the superior region at the 12 o’clock sector, the others were assigned accordingly, in the clockwise direction for the right eye and counterclockwise for the left. An example of the 12 clock-hour sectors for a right eye is illustrated in Fig. 8.
Table 4. Tabulated average RNFL thickness with and without vessels, and the average standard deviation for each clock-hour sector.
|
Average RNFL thickness (in μm)
a
|
Reduction in thickness | ||
|---|---|---|---|
| Sectors | Including vessels | Excluding vessels | |
| 1 | 107.26 ± 17.61 | 88.01 ± 16.26 | 17.95% |
| 2 | 91.81 ± 14.29 | 76.06 ± 14.31 | 17.15% |
| 3 | 76.12 ± 11.78 | 65.07 ± 10.16 | 14.52% |
| 4 | 80.68 ± 12.43 | 67.45 ± 10.48 | 16.40% |
| 5 | 104.72 ± 17.66 | 89.38 ± 15.93 | 14.65% |
| 6 | 126.89 ± 27.17 | 110.17 ± 26.08 | 13.18% |
| 7 | 145.02 ± 20.89 | 127.97 ± 21.03 | 11.78% |
| 8 | 94.71 ± 22.59 | 85.24 ± 22.21 | 10.00% |
| 9 | 77.82 ± 12.06 | 70.59 ± 13.06 | 9.29% |
| 10 | 105.15 ± 23.81 | 95.37 ± 24.00 | 9.30% |
| 11 | 133.41 ± 20.37 | 116.37 ± 19.96 | 12.78% |
| 12 | 112.61 ± 23.06 | 95.70 ± 20.38 | 15.03% |
All values are presented as mean thickness ± standard deviation.
Fig. 8.
Definition of 12 clock-hour sectors for right eye (left) and the corresponding sectors in cross-sectional OCTA scan (right).
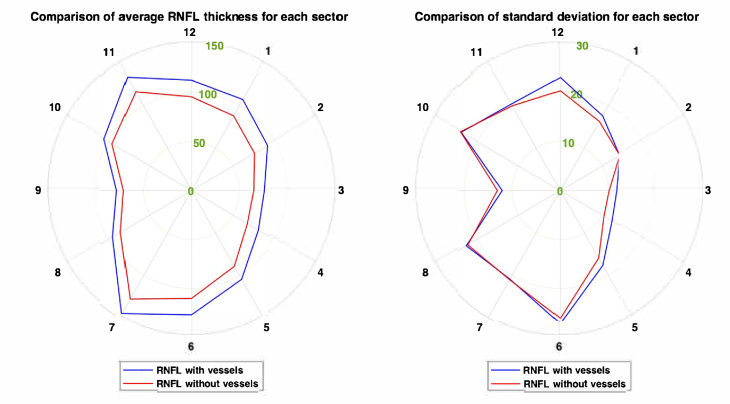
In Fig. 9, the average RNFL thickness plot is shown, and the reduction trend is supported by previous studies which have shown that the measured RNFL thicknesses in the temporal and nasal regions are generally thinner compared to the superior and inferior regions, while thicker in superior and inferior regions [3,24]. The absolute thickness variability generally reduces due to the removal of vessels. From the standard deviation plot shown in Fig. 9, it was observed that the absolute thickness variabilities in Sectors 9 and 10 were slightly higher (0.19 μm and 1 μm respectively) when all vessels are excluded. This could be due to the presence of mainly microvasculature in the temporal region where the density of microvasculature varies across all eyes. In addition, Sector 10 may consist of some major vessels which could also affect the thickness variability. The average thickness variability (range: 0.13 to 2.7 μm) after excluding all vessels was reduced in the superior (Sectors 1, 11 and 12) and inferior (Sectors 5, 6 and 7) regions. From our results, we observed that exclusion of vessels in general led to a reduction in the average RNFL thickness in all the sectors and it showed that the contribution of the vessels to the RNFL has a noticeable effect on both thickness and variability. Although there is a slight increase in percentage, the data indicates that the variability of vascular component in healthy subjects is less than that of non-vascular component. This suggests that our approach may allow us to better account for the rate of thinning of neuronal component, which is actually the defining measure of age-related disease. As such, it may potentially lead to better separation between glaucoma and healthy cases. This is particularly important in patients who have vascular disease, but no sign of ganglion cell axonal loss. Such patients may erroneously be classified as glaucomatous when they are analyzed in the conventional way, but may be considered as non-glaucomatous once the vessels are removed.
Fig. 9.
Average RNFL thickness for all eyes for 12 clock-hour sectors (blue line represents RTave; orange line represents RTave,nv) (left). Average standard deviation of RNFL thickness for all eyes for 12 clock-hour sectors (right). All values are in μm.
There are some limitations to our approach. There were more female subjects than male subjects recruited for this work. However, studies [25–28] have shown that there were no significant correlations between RNFL thickness and gender. There was a relatively greater proportion of older subjects in the current dataset. However, this is indicative of the use of RNFL thickness measurements on older individuals for detecting the presence of age-related ocular diseases and monitoring disease progression. OCTA is a technique that visualizes blood vessels by employing amplitude decorrelation between repeated consecutive OCT scans acquired at the same retinal location. Degradations in image quality caused by motion, blinking and presence of media opacity, are common in OCTA and can affect the visibility of vessels [29]. Quality checks are essential to ensure the quality of OCTA images before any processing. Next, we assumed circular cross section for all vessels in our analyses. Based on the anatomical structure of blood vessels [30], arteries generally appear more rounded cross-sectionally compared to veins due to their thicker walls with smaller lumens to withstand greater blood pressure. In contrast, veins generally appear more flattened due to the reduced venous pressure compared to arterial pressure. Nevertheless, the cross-section of retinal vessels has been assumed to be circular in most of the prior works describing the removal of blood vessels from OCT images [31]. Further work could be done to identify both retinal arteries and veins from enface OCT image, and subsequently applying a circular model for the arteries while applying an elliptical model for the identified veins. We also observed the challenge in defining the real vessel boundary due to the limitation of resolution. Nevertheless, studies have shown that OCTA is able to provide a good estimation of the vessel diameter measurement. For example, Yao et al. has reported a high correlation between the OCTA and adaptive optics ophthalmoscope measurement for both arteries and veins [12]. Matsunaga et al. also evaluated the retinal microvasculature in healthy human subjects with OCTA and showed that it is qualitatively similar to conventional fluorescein angiography [32]. Furthermore, the refractive error of each subject was not considered in our analyses as the average refractive error was -1.46 ± 3.33D and the effect on the angular distribution of RNFL bundles due to myopia is minimal. However, it is important to note that severe myopia could have potential effect on the RNFL thickness measurement [3,33,34]. Finally, we note that enface OCTA was generated from vertical maximum projection of SCP which extends below RNFL. As such, the generated enface OCTA may detect vessels which are located below RNFL and have stronger signal. This may lead to under-detection of RNFL vessels. In our work, we used the segmented RNFL boundaries to limit the axial extent and avoid detection of non-RNFL vessels.
4. Conclusion
In this work, we present a new approach to segregate retinal vascular and neuronal components within the RNFL for thickness measurement by combining structural and vascular information from OCT and OCTA images respectively. This approach uses the segmented RNFL from a cross-sectional OCT image and blood flow information from an enface OCTA image to define the lateral and axial position of major vessels and microvasculature. Evaluation of RNFL thickness and proportion of vessels to RNFL revealed a significant correlation between thickness and aging, and showed in general a lower absolute variability of average thickness profiles along circumpapillary RNFL in healthy subjects when all detected vessels were excluded. The approach could potentially increase diagnostic accuracy and aid in the detection and quantification of neuronal and vascular loss in patients with ocular disease such as diabetic retinopathy and glaucoma. Further works are needed to evaluate the diagnostic accuracy in patients with ocular disease.
Funding
National Medical Research Council10.13039/501100001349 (NMRC/CG/C010A/2017_SERI, NMRC/OFIRG/0048/2017).
Disclosures
The authors declare no conflicts of interest.
References
- 1.Leung C. K. S., Chan W. M., Hui Y. I., Yung W. H., Woo J., Tsang M. K., Tse K. K., “Analysis of retinal nerve fiber layer and optic nerve head in glaucoma with different reference plane offsets, using optical coherence tomography,” Invest. Ophthalmol. Visual Sci. 46(3), 891–899 (2005). 10.1167/iovs.04-1107 [DOI] [PubMed] [Google Scholar]
- 2.Shin J. W., Seong M., Lee J. W., Hong E. H., Uhm K. B., “Diagnostic ability of retinal nerve fiber layer thickness deviation map for localized and diffuse retinal nerve fiber layer defects,” J. Ophthalmol. 2017, 8365090 (2017). 10.1155/2017/8365090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong D., Chua J., Baskaran M., Tan B., Yao X., Chan S., Tham Y. C., Chong R., Aung T., Lamoureux E. L., Vithana E. N., Cheng C. Y., Schmetterer L., “Factors affecting the diagnostic performance of circumpapillary retinal nerve fibre layer measurement in glaucoma,” Br. J. Ophthalmol. 105(3), 397–402 (2021). 10.1136/bjophthalmol-2020-315985 [DOI] [PubMed] [Google Scholar]
- 4.Pereira I., Weber S., Holzer S., Fischer G., Vass C., Resch H., “Compensation for retinal vessel density reduces the variation of circumpapillary RNFL in healthy subjects,” PLoS One 10(3), e0120378 (2015). 10.1371/journal.pone.0120378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hood D. C., Fortune B., Arthur S. N., Xing D., Salant J. A., Ritch R., Liebmann J. M., “Blood vessel contributions to retinal nerve fiber layer thickness profiles measured with optical coherence tomography,” J Glaucoma 17(7), 519–528 (2008). 10.1097/IJG.0b013e3181629a02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye C., Yu M., Leung C. K. S., “Impact of segmentation errors and retinal blood vessels on retinal nerve fibre layer measurements using spectral-domain optical coherence tomography,” Acta Ophthalmol. 94(3), e211–e219 (2016). 10.1111/aos.12762 [DOI] [PubMed] [Google Scholar]
- 7.Stankiewicz A., Marciniak T., Dąbrowski A., Stopa M., Marciniak E., “Volumetric segmentation of human eye blood vessels based on OCT images,” in 2017 25th European Signal Processing Conference (EUSIPCO), 2017), 36–40.
- 8.Wehbe H., Ruggeri M., Jiao S., Gregori G., Puliafito C. A., Zhao W., “Automatic retinal blood flow calculation using spectral domain optical coherence tomography,” Opt. Express 15(23), 15193–15206 (2007). 10.1364/OE.15.015193 [DOI] [PubMed] [Google Scholar]
- 9.Pilch M., Wenner Y., Strohmayr E., Preising M., Friedburg C., Meyer Zu Bexten E., Lorenz B., Stieger K., “Automated segmentation of retinal blood vessels in spectral domain optical coherence tomography scans,” Biomed. Opt. Express 3(7), 1478–1491 (2012). 10.1364/BOE.3.001478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E. J., Lee K. M., Lee S. H., Kim T.-W., “OCT angiography of the peripapillary retina in primary open-angle glaucoma,” Invest. Ophthalmol. Visual Sci. 57(14), 6265–6270 (2016). 10.1167/iovs.16-20287 [DOI] [PubMed] [Google Scholar]
- 11.Chen C. L., Zhang A., Bojikian K. D., Wen J. C., Zhang Q., Xin C., Mudumbai R. C., Johnstone M. A., Chen P. P., Wang R. K., “Peripapillary retinal nerve fiber layer vascular microcirculation in glaucoma using optical coherence tomography-based microangiography,” Investigative Ophthalmology & Visual Science 57, OCT475 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao X., Ke M., Ho Y., Lin E., Wong D. W. K., Tan B., Schmetterer L., Chua J., “Comparison of retinal vessel diameter measurements from swept-source OCT angiography and adaptive optics ophthalmoscope,” British Journal of Ophthalmology, bjophthalmol 2020, 316111 (2020). 10.1136/bjophthalmol-2021-319015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarmohammadi A., Zangwill L. M., Diniz-Filho A., Suh M. H., Manalastas P. I., Fatehee N., Yousefi S., Belghith A., Saunders L. J., Medeiros F. A., Huang D., Weinreb R. N., “Optical coherence tomography angiography vessel density in healthy, glaucoma suspect, and glaucoma eyes,” Investigative Ophthalmology & Visual Science 57, OCT451 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J., Sebastian R. T., Chu C. J., McGregor F., Dick A. D., Liu L., “Reduced macular vessel density and capillary perfusion in glaucoma detected using OCT angiography,” Curr. Eye Res. 44(5), 533–540 (2019). 10.1080/02713683.2018.1563195 [DOI] [PubMed] [Google Scholar]
- 15.Heindl L. M., Adler W., El-Malahi O., Schaub F., Hermann M. M., Dietlein T. S., Cursiefen C., Enders P., “The optimal diameter for circumpapillary retinal nerve fiber layer thickness measurement by SD-OCT in glaucoma,” Journal of Glaucoma 27(12), 1086–1093 (2018). 10.1097/IJG.0000000000001027 [DOI] [PubMed] [Google Scholar]
- 16.Yow A. P., Tan B., Chua J., Aung T., Husain R., Schmetterer L., Wong D., “Automated circumpapillary retinal nerve fiber layer segmentation in high-resolution swept-source OCT,” Annual International Conference of the IEEE Engineering in Medicine and Biology Society 2020, 1832–1835 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Tan B., Chua J., Harish T., Lau A., Gan A. T. L., Tan Y. L., Wong D. W. K., Chong R. S., Ang M., Husain R., Schmetterer L., “Comparison of a commercial spectral-domain OCT and swept-source OCT based on an angiography scan for measuring circumpapillary retinal nerve fibre layer thickness,” Br. J. Ophthalmol. 104(7), 974–979 (2020). 10.1136/bjophthalmol-2019-314706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang R. K., Jacques S. L., Ma Z., Hurst S., Hanson S. R., Gruber A., “Three dimensional optical angiography,” Opt. Express 15(7), 4083–4097 (2007). 10.1364/OE.15.004083 [DOI] [PubMed] [Google Scholar]
- 19.Ronneberger O., Fischer P., Brox T., “U-Net: convolutional networks for biomedical image segmentation,” in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015 (Springer International Publishing, 2015), 234–241. [Google Scholar]
- 20.“Local adaptive thresholding,” in Encyclopedia of Biometrics, Li S. Z., Jain A., eds. (Springer, US, 2009), pp. 939. [Google Scholar]
- 21.Dumitrescu A. G., Voinea L., Badarau I. A., Paun V. A., Schowe M., Ciuluvica R., “Update on retinal vascular caliber,” Rom J Ophthalmol 61(3), 171–180 (2017). 10.22336/rjo.2017.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eladawi N., Elmogy M., Helmy O., Aboelfetouh A., Riad A. e.-d., Sandhu H., Schaal S., El-Baz A., “Automatic blood vessels segmentation based on different retinal maps from OCTA scans,” Comput. Biol. Med. 89, 150–161 (2017). 10.1016/j.compbiomed.2017.08.008 [DOI] [PubMed] [Google Scholar]
- 23.Niemeijer M., Haeker M., Ginneken B., Sonka M., Abramoff M., “Vessel segmentation in 3D spectral OCT scans of the retina,” Proc. SPIE 6914, 69141R (2008). 10.1117/12.772680 [DOI] [Google Scholar]
- 24.Patel S. B., Reddy N., Lin X., Whitson J. T., “Optical coherence tomography retinal nerve fiber layer analysis in eyes with long axial lengths,” Clin. Ophthalmol. 12, 827–832 (2018). 10.2147/OPTH.S162023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alasil T., Wang K., Keane P. A., Lee H., Baniasadi N., de Boer J. F., Chen T. C., “Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography,” Journal of Glaucoma 22(7), 532–541 (2013). 10.1097/IJG.0b013e318255bb4a [DOI] [PubMed] [Google Scholar]
- 26.Bowd C., Zangwill L. M., Blumenthal E. Z., Vasile C., Boehm A. G., Gokhale P. A., Mohammadi K., Amini P., Sankary T. M., Weinreb R. N., “Imaging of the optic disc and retinal nerve fiber layer: the effects of age, optic disc area, refractive error, and gender,” J. Opt. Soc. Am. A 19(1), 197–207 (2002). 10.1364/JOSAA.19.000197 [DOI] [PubMed] [Google Scholar]
- 27.Pakravan M., Aramesh S., Yazdani S., Yaseri M., Sedigh-Rahimabadi M., “Peripapillary retinal nerve fiber layer thickness measurement by three-dimensional optical coherence tomography in a normal population,” J. Ophthalmic Vision Res. 4, 220–227 (2009). [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S., Mendez-Hernandez C., Arribas-Pardo P., Salazar Quiñones L., Fernandez-Perez C., Garcia-Feijoo J., “Gender-related influences on superficial papillary microcirculation measured with optical coherence tomography angiography in patients with glaucoma,” Curr. Eye Res. 45(12), 1534–1542 (2020). 10.1080/02713683.2020.1755698 [DOI] [PubMed] [Google Scholar]
- 29.Enders C., Lang G. E., Dreyhaupt J., Loidl M., Lang G. K., Werner J. U., “Quantity and quality of image artifacts in optical coherence tomography angiography,” PLoS One 14(1), e0210505 (2019). 10.1371/journal.pone.0210505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Betts J. G., Young K. A., Wise J. A., Johnson E., Poe B., Kruse D. H., Korol O., Johnson J. E., Womble M., DeSaix P., Anatomy and Physiology (OpenStax, Houston, Texas, 2013). [Google Scholar]
- 31.Patel N. B., Sullivan-Mee M., Harwerth R. S., “The relationship between retinal nerve fiber layer thickness and optic nerve head neuroretinal rim tissue in glaucoma,” Invest. Ophthalmol. Visual Sci. 55(10), 6802–6816 (2014). 10.1167/iovs.14-14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsunaga D., Yi J., Puliafito C. A., Kashani A. H., “OCT angiography in healthy human subjects,” Ophthalmic Surg Lasers Imaging Retina 45(6), 510–515 (2014). 10.3928/23258160-20141118-04 [DOI] [PubMed] [Google Scholar]
- 33.Chua J., Schwarzhans F., Nguyen D. Q., Tham Y. C., Sia J. T., Lim C., Mathijia S., Cheung C., Tin A., Fischer G., Cheng C. Y., Vass C., Schmetterer L., “Compensation of retinal nerve fibre layer thickness as assessed using optical coherence tomography based on anatomical confounders,” Br. J. Ophthalmol. 104(2), 282–290 (2020). 10.1136/bjophthalmol-2019-314086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Porwal S., Nithyanandam S., Joseph M., Vasnaik A. K., “Correlation of axial length and peripapillary retinal nerve fiber layer thickness measured by Cirrus HD optical coherence tomography in myopes,” Indian J. Ophthalmol. 68(8), 1584–1586 (2020). 10.4103/ijo.IJO_1778_19 [DOI] [PMC free article] [PubMed] [Google Scholar]