Abstract
We recently identified epigallocatechin gallate (EGCG), a trihydroxyphenolic compound, as a dual inhibitor of lysyl oxidase-like2 (LOXL2) and TGFβ1 receptor kinase that when given orally to Idiopathic Pulmonary Fibrosis (IPF) patients reversed profibrotic biomarkers in their diagnostic biopsies. Here we extend these findings to advanced pulmonary fibrosis using cultured precision-cut lung slices (PCLS) from explants of IPF patients undergoing transplantation. During these experiments we were surprised to discover that not only did EGCG attenuate TGFβ1 signaling and new collagen accumulation but also activated MMP-dependent collagen I turnover, raising the possibility of slow fibrosis resolution with continued treatment.
Keywords: collagen, pulmonary fibrosis, collagenase
RESULTS and DISCUSSION
Trihydroxyphenolic compounds inhibit profibrotic markers in PCLS tissue and increase soluble collagen in PCLS conditioned medium
As there have been only limited attempts to analyze cultured PCLS directly from IPF explants1–3, we first established a PCLS culture system using 600 μm slices of fresh lung explants from the less fibrotic upper lobes of end-stage IPF patients (figure 1A). IPF PCLSs were apparently intact and viable at the end of the 5-day cultures as judged by H&E staining (figure 1B), PrestoBlue metabolic activity, and trypan blue viability staining (figure 1C), respectively. Next we tested two trihydroxyphenolic compounds, corilagin and EGCG, along with two approved drugs for IPF, pirfenidone and nintedanib4,5. After 5 days in serum-free medium, the slices were extracted for protein or paraffin-embedded. The profibrotic markers fibronectin, collagen I, Snail1, and pSmad3 were quantified by immunoblotting (figure1C and online supplementary figure S1). Protein extracts from IPF PCLS cultures revealed similar levels of profibrotic proteins from fresh frozen slices (D0) and slices cultured 5 days in vehicle only (DMSO) (figure 1D,E). Exposure of the cultures to corilagin or EGCG (1 μM) markedly reduced the levels of all of the profibrotic markers but had no effect on total Smad3 or α-smooth muscle actin (α-SMA). In contrast, exposure of the cultures to either pirfenidone (1 mM) or nintedanib (1 μM) had no discernible effects. Similar results were seen in cultures from each of 6 IPF patients (figure 1D,E), indicating strong inhibition of profibrotic markers in human fibrotic lungs by either corilagin or EGCG. These findings validate the important role of TGFβ1 signaling in late phase IPF. Of note, the collagen I extracted in these experiments likely represents mostly weakly cross-linked newly deposited collagen or pro-collagen solubilized by strong detergents. We attempted to measure changes in the more abundant cross-linked fibrillar collagens by quantification of second harmonic generation (SHG) signals. However, a systematic quantification of six parameters designed to determine the fraction of assembled (cross-linked) collagen of large or small fiber size among the samples showed no differences (figure 1F).
Figure 1.
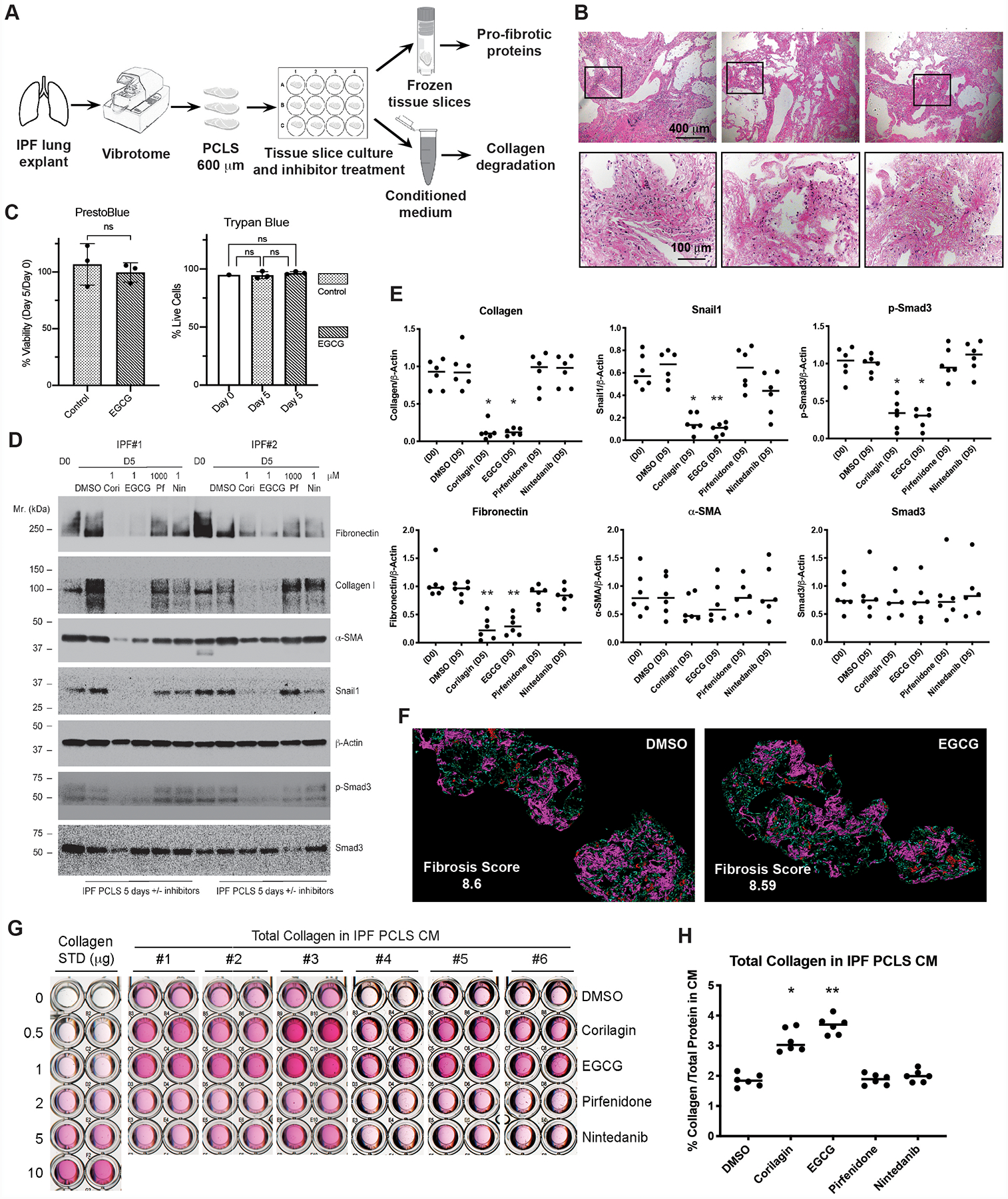
Profibrotic markers are decreased in IPF PCLS tissue while total soluble collagen in IPF PCLS conditioned medium (CM) is increased by the treatment of EGCG or corilagin, but not pirfenidone or nintedanib. (A) IPF lung slicing process and experimental setup are illustrated. (B) Hematoxylin and eosin (H&E) staining of PCLS prepared from a representative IPF donor lung and PCLS cultured with EGCG or vehicle treatment for 5 days. Scale bars: 400 μm for upper panels and 100 μm for lower panels, which show higher-magnification images for the boxed areas in upper panels. (C) PCLS metabolic activity was assessed at D0 and D5 using PrestoBlue Cell Viability Reagents both in EGCG-treated and control PCLSs. Live/Dead cell counts were assessed using Trypan Blue staining. Percent Viability: (D5 fluorescence/D0 fluorescence) X100, unpaired two-tailed t-test; Percent Live Cells: (total cells-trypan blue+ cells)/total cells X100, one-way ANOVA with post hoc Tukey test. n=3, ns: not significant. (D) PCLS lysates equalized based on total protein from Day 0 or Day 5 culture with or without inhibitors (Cori-corilagin, EGCG, Pf-pirfenidone, Nin-nintedanib) were blotted for Fibronectin, Collagen I, α-Smooth Muscle Actin (α-SMA), Snail1, β-actin, p-Smad3, and total Smad3. Data from two representative IPF patients were shown. (E) Densitometry of Western blot bands was measured using NIH ImageJ software. Density of each band normalized to β-actin from D0 and D5 cultured IPF PCLS were quantified. Differences in protein levels across the groups were tested for significance with Kruskal-Wallis distribution. Comparisons between each treated group and DMSO control group were calculated by Wilcoxon rank-sum test. Normalized band intensity is expressed as mean ± SD. N=6. *P < 0.05, **P < 0.01, no label: not significant. (F) Ten-μm paraffin-embedded tissue sections from 5-day cultured IPF PCLS treated with vehicle DMSO or EGCG were imaged using SHG microscopy (n=2 per group). Magenta/Red: larger cross-linked collagens; Blue/Green: fine cross-linked collagens. Scale bars: 400 μm. Overall fibrosis score averages are indicated in the figure. (G) Total collagen from IPF PCLS 5-day CM with or without indicated inhibitors was concentrated and measured by Sircol assay. STD, standard. (H) Calculated total collagen concentration (μg/ml) in IPF PCLS CM was normalized to total protein concentration in CM and converted to percentage of total protein in CM (mean ± SD, N=6). Differences in collagen levels across the groups were tested for significance with Kruskal-Wallis distribution. Comparisons between each treated group and DMSO control group were calculated by Wilcoxon rank-sum test. *P < 0.05, **P < 0.01, no label: not significant.
A surprising finding is the observation that the amount of collagen I extracted from the corilagin or EGCG-exposed cultures on day 5 was strikingly less than the collagen extractable from companion lung slices immediately frozen without culture (figures 1D,E). This result raised the possibility that blockade of profibrotic signaling by corilagin and EGCG also led to activation of a collagen degradation program. This possibility was confirmed by consistently increased soluble collagen as measured by Sircol assays in conditioned medium (CM) from the corilagin/EGCG but not DMSO control or pirfenidone or nintedanib-treated IPF slice cultures (figure 1G,H). One normal size PCLS (5 mm × 5 mm × 600 m) released ~1.5 μg of total collagen at 5 days.
Trihydroxyphenolic compounds increase collagen degradation in the PCLS cultures in a MMP1-dependent manner
The increased collagen signal in the CM was at least partially explained by increases in multiple sizes of soluble collagen I in each of ten IPF slice cultures treated with EGCG as compared to untreated controls (figure 2A,B). We verified that the collagen I antibody was specific by observing only the expected monomeric collagen I band in protein extracted from pepsin-treated PCLS samples (figure 2A). The appearance of high molecular weight species of variable sizes likely reflects both pro-collagen I and partially cross-linked mature collagen I. Numerous smaller (<100 kDa) fragments of collagen I in the CM suggests that the collagen degradation process is complex and may involve multiple proteinases. Some sizes lined up with MMP1-digested purified collagen I (figure 2A), suggesting that MMP1 activity might be involved. Accumulation of virtually all species indicative of solubilized cross-linked collagen I or smaller degradation fragments in IPF PCLS CM were blocked by the broad spectrum MMP inhibitor GM6001 (figure 2C–E), confirming MMPs play an important role in collagen degradation in these assays. Indeed, the MMP inhibitor TIMP1 was markedly decreased and MMP1 (also known as fibroblast or interstitial collagenase) was significantly increased in the CM of slice cultures treated with EGCG (figure 2F,G). Both increased TIMP1 expression and suppression of MMP1 are known to be effectors of ECM expansion following TGFβ1 signaling6,7, suggesting EGCG treatment triggers a collagen degradation program by inhibition of TGFβ1 signaling. Based on these findings we tested the circulating serum levels of pro-MMP1 before and after 14 days of EGCG exposure in a previously described cohort of pulmonary fibrosis patients8. Consistent with an anti-TGFβ1 signaling effect in vivo in these patients, pro-MMP1 levels were significantly higher post treatment (figure 2H).
Figure 2.
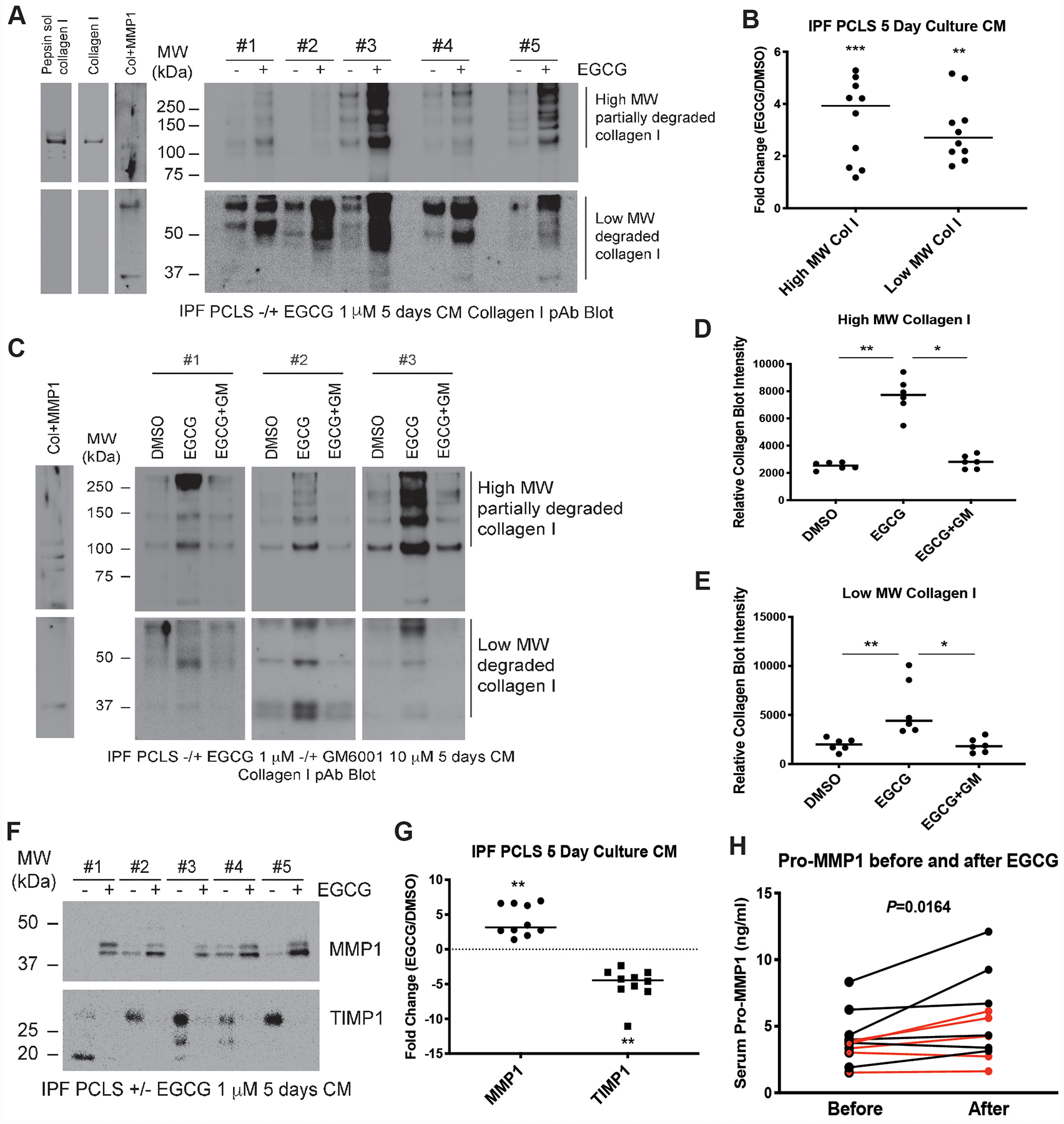
EGCG treatment induces collagen I degradation in IPF PCLS culture through inhibition of TGFβ-mediated downregulation of MMP1 and upregulation of TIMP1. (A) Concentrated CM specimens from IPF PCLS 5-day culture were blotted for Collagen I. Acid/pepsin soluble collagen extracted from IPF PCLS tissue, purified collagen I, and MMP1 digested collagen I were loaded as positive controls for collagen I blot. Representative blots from five IPF patients were shown. (B) Densitometry was performed using NIH ImageJ software. Intensity of Western blot bands were quantified and normalized to total protein and fold changes of high and low molecular weight forms of collagen I after EGCG treatment in the PCLS CM from ten IPF patients were calculated. Data were analyzed by the Wilcoxon signed-rank test (two-tailed). Data are mean ± SD. n=10. **P < 0.01, ***P < 0.001. (C) IPF PCLS 5-day CM treated with DMSO, EGCG (1 μM), or EGCG plus MMP inhibitor GM6001 (10 μM) (EGCG+GM) were blotted for Collagen I. MMP1-digested collagen I was loaded as positive control. Representative blots from three IPF patients were shown. (D and E) High molecular form (D) and low molecular form (E) collagen I Western blot bands were quantified and normalized to total protein in the PCLS CM from six IPF patients. Differences in collagen levels across the groups were tested for significance with Kruskal-Wallis distribution. Comparisons between DMSO control group and EGCG-untreated group and between EGCG-untreated group and EGCG+GM-treated group were calculated by Wilcoxon rank-sum test. Data are mean ± SD. n=6. *P < 0.05, **P < 0.01. (F) Concentrated CM specimens from IPF PCLS 5-day culture were blotted for MMP1 and TIMP1. Representative blots from five IPF patients were shown. (G) Western blot bands were quantified and normalized to total protein and fold changes of MMP1 and TIMP1 after EGCG treatment in the PCLS CM from ten IPF patients were calculated. Data were analyzed by the Wilcoxon signed-rank test (two-tailed). Data are mean ± SD. n=10. **P < 0.01. (H) Sera from a cohort of pulmonary fibrosis patients taken before and after 14 days of EGCG 600 mg orally were processed as previously described8. Serum pro-MMP1 was measured using enzyme-linked immunosorbent assay according to the manufacturers’ instructions. Data from each patient before and after EGCG treatment were compared and analyzed with the use of the Wilcoxon signed-rank test (two-tailed). Data are mean ± SD. n=11. Red dots are samples from Usual Interstitial Pneumonia (UIP, 60%) patients and black dots are samples from patients with Nonspecific Interstitial Pneumonia (NSIP, 30%) and Hypersensitivity Pneumonitis (HP, 10%).
Overall, the findings here and previously reported support a model in which EGCG firstly binds/inhibits LOXL2 and generates a TGFβ receptor I/II inhibitor that downregulates collagen I production and crosslinking, and secondly activates an alternative pathway involving MMP1 upregulation and TIMP1 downregulation that induces the degradation of collagen I9. Side-by-side comparison of EGCG and corilagin with the two FDA-approved drugs for IPF, pirfenidone and nintedanib, showed that the latter agents had no discernible effects in our assays (figure 1). One likely possibility is that because IPF lung tissues are derived from end stage patients undergoing lung transplantation, it might be that these samples had developed resistance to pirfenidone and nintedanib. Alternatively, the clear attenuation of decline in FVC among most patients taking these drugs could be due to mechanisms outside regulation of the core fibrogenic pathways attenuated by EGCG, e.g. suppression of inflammatory mediators10. If so, trihydroxyphenolic compounds may add a unique anti-fibrotic profile by directly suppressing collagen production and enhancing MMP1-mediated degradation of crosslinked and non-crosslinked collagen.
METHODS
Details are provided in the online supplementary.
Supplementary Material
Acknowledgements:
The authors thank Genevieve Montas, Darren Leong, and Elena Foster for patient serum samples and we thank the patients who participated in the clinical study.
Funding:
This work has been funded by Three Lakes Foundation (H.A.C.), NIH R01 HL142265 and R35 HL150767 (H.A.C.), and R21 AG052744 (C.J.-L.-S.).
Footnotes
Competing interests: None declared.
REFERENCES
- 1.Alsafadi HN, Uhl FE, Pineda RH, et al. Applications and Approaches for Three-Dimensional Precision-Cut Lung Slices. Disease Modeling and Drug Discovery. Am J Respir Cell Mol Biol 2020;62(6):681–91. doi: 10.1165/rcmb.2019-0276TR [published Online First: 2020/01/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kheirollahi V, Wasnick RM, Biasin V, et al. Metformin induces lipogenic differentiation in myofibroblasts to reverse lung fibrosis. Nat Commun 2019;10(1):2987. doi: 10.1038/s41467-019-10839-0 [published Online First: 2019/07/07] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milara J, Ballester B, Morell A, et al. JAK2 mediates lung fibrosis, pulmonary vascular remodelling and hypertension in idiopathic pulmonary fibrosis: an experimental study. Thorax 2018;73(6):519–29. doi: 10.1136/thoraxjnl-2017-210728 [published Online First: 2018/02/15] [DOI] [PubMed] [Google Scholar]
- 4.Maher TM, Stowasser S, Nishioka Y, et al. Biomarkers of extracellular matrix turnover in patients with idiopathic pulmonary fibrosis given nintedanib (INMARK study): a randomised, placebo-controlled study. Lancet Respir Med 2019;7(9):771–79. doi: 10.1016/S2213-2600(19)30255-3 [published Online First: 2019/07/22] [DOI] [PubMed] [Google Scholar]
- 5.Neighbors M, Cabanski CR, Ramalingam TR, et al. Prognostic and predictive biomarkers for patients with idiopathic pulmonary fibrosis treated with pirfenidone: post-hoc assessment of the CAPACITY and ASCEND trials. Lancet Respir Med 2018;6(8):615–26. doi: 10.1016/S2213-2600(18)30185-1 [published Online First: 2018/08/04] [DOI] [PubMed] [Google Scholar]
- 6.Li H, Chang HM, Shi Z, et al. ID3 mediates the TGF-beta1-induced suppression of matrix metalloproteinase-1 in human granulosa cells. FEBS J 2019;286(21):4310–27. doi: 10.1111/febs.14964 [published Online First: 2019/06/20] [DOI] [PubMed] [Google Scholar]
- 7.Todorova L, Gurcan E, Westergren-Thorsson G, et al. Budesonide/formoterol effects on metalloproteolytic balance in TGFbeta-activated human lung fibroblasts. Respir Med 2009;103(11):1755–63. doi: 10.1016/j.rmed.2009.03.018 [published Online First: 2009/04/21] [DOI] [PubMed] [Google Scholar]
- 8.Chapman HA, Wei Y, Montas G, et al. Reversal of TGFbeta1-Driven Profibrotic State in Patients with Pulmonary Fibrosis. N Engl J Med 2020;382(11):1068–70. doi: 10.1056/NEJMc1915189 [published Online First: 2020/03/12] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei Y, Kim TJ, Peng DH, et al. Fibroblast-specific inhibition of TGF-beta1 signaling attenuates lung and tumor fibrosis. J Clin Invest 2017;127(10):3675–88. doi: 10.1172/JCI94624 [published Online First: 2017/09/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y, Li H, Liu S, et al. Pirfenidone ameliorates lipopolysaccharide-induced pulmonary inflammation and fibrosis by blocking NLRP3 inflammasome activation. Mol Immunol 2018;99:134–44. doi: 10.1016/j.molimm.2018.05.003 [published Online First: 2018/05/22] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


