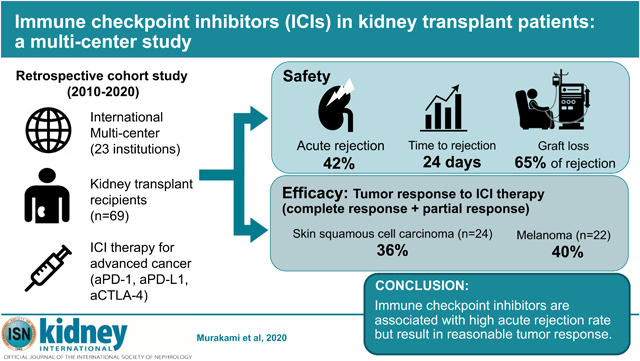
Abstract
Immune checkpoint inhibitors (ICIs) are widely used for various malignancies. However, their safety and efficacy in kidney transplant (KTx) patients have not been defined.
We conducted a multicenter retrospective cohort study of 69 KTx patients receiving ICIs between January 2010 and May 2020. For safety, we assessed the incidence, timing, and risk factors of acute graft rejection. For efficacy, objective response rate (ORR) and overall survival (OS) were assessed in cutaneous squamous cell carcinoma (cSCC) and melanoma, the most common cancers in our cohort, and compared with historical, stage-matched KTx patients with cSCC (n=23) and melanoma (n=14) not receiving ICIs.
Following ICI treatment, 29 out of 69 patients (42%) developed acute rejection, 19 of whom (65.5%) lost their allograft, compared with an acute rejection rate of 5.4% in the non-ICI historical cohort. The rejection rate was higher than the non-ICI historical cohort (5.4%). Median time from ICI initiation to rejection was 24 (IQR 20–56) days. Factors associated with a lower risk of rejection were mTOR inhibitor use (odds ratio [OR] 0.26; 95%CI, 0.09–0.72) and triple-agent immunosuppression (OR 0.67; 95%CI, 0.48–0.92). ORR was 36.4% and 40% in the cSCC and melanoma subgroup, respectively. In cSCC subgroup, OS was longer in patients treated with ICIs (median OS 19.8 months vs. 10.6 months; log rank *p=0.016), whereas in the melanoma subgroup, OS did not differ between groups (median OS 13.5 months vs. 11.4 months; log rank p=0.34).
ICIs were associated with a high risk of rejection in KTx patients but may lead to improved cancer outcomes. Prospective studies are needed to determine optimal immunosuppression strategies to improve patient outcomes.
Keywords: Immune checkpoint inhibitors, kidney transplant, rejection, onconephrology
Graphical Abstract
Introduction
Cancer is a leading cause of death in kidney transplant patients.1–3 However, the current understanding of treatment outcomes for cancer patients who are also transplant recipients is incomplete due to exclusion of these patients from most clinical trials.
Immune checkpoint inhibitors (ICIs) revolutionized the treatment of cancer and have changed the standard of care for a wide spectrum of solid and hematological malignancies.4 ICI use in solid organ transplant recipients is challenging due to two major concerns. First, immunotherapy may lead to graft rejection due to enhanced graft-directed T cell responses. Second, immunosuppressants may compromise the anti-tumor activity of immunotherapy. Small case series have provided some insight regarding ICI-associated graft rejection among solid organ transplant patients, but larger, more systematic analyses are lacking.5–9
We conducted an international, multicenter retrospective cohort study of cancer patients with kidney transplant who received ICIs for various solid malignancies between 2010 and 2020. For the clinical safety, we sought to identify the incidence, timing and risk factors for acute graft rejection following ICI therapy. For the clinical efficacy, we examined objective response rate and overall survival in cutaneous squamous cell carcinoma (cSCC) and melanoma subgroups, the two most commonly identified advanced malignancies in our cohort.
Results
Baseline characteristics of patients
Sixty-nine kidney transplant recipients were treated with ICIs (n=69, Table 1 with additional variables available in Supplemental Table 1). Patients were predominantly male (84.1%), with a median age of 65 years (IQR, 55–71). A total of 58% of the patients were living donor kidney transplant recipients. The majority (85.5%) were first-time kidney transplant recipients, without preformed donor-specific antibody (79.7%). Median baseline serum creatinine was 1.34 mg/dl (IQR, 1.1–1.72), and 56% of patients had minimal proteinuria (<0.3 g/gCr or negative dipstick) at the time of ICI initiation.
Table 1:
Patient characteristics
| Variable | ICI (n=69) | Non-ICI (n=37) |
|---|---|---|
| Age, yr | 65 (55–71) | 63 (58–68.4) |
| Female (%) | 11 (15.9) | 10 (27.0) |
| Transplant type, n (%) | ||
| LUKTx | 16 (23.2) | 6 (16.2) |
| LRKTx | 24 (34.8) | 15 (40.5) |
| DDKTx | 27 (39.1) | 12 (32.4) |
| Unknown | 2 (2.9) | 4 (10.8) |
| Baseline Cr, mg/dl | 1.34 (1.1– 1.72) | 1.2 (0.96–1.65) |
| Cancer type, n (%) | ||
| cSCC | 24 (34.8) | 23 (62.2) |
| Melanoma | 22 (31.9) | 14 (37.8) |
| NSCLC | 8 (11.6) | |
| Merkel Cell Carcinoma | 4 (5.8) | |
| RCC | 3 (4.3) | |
| Bladder | 2 (2.9) | |
| Others | 6 (8.7) | |
| Other cancer-directed therapy, n (%) | 41 (59.4) | 27 (73) |
| Transplant to ICI, yr | 9.33 (4.1–15.6) | NA |
| ICI, n (%) | NA | |
| Pembrolizumab | 29 (42.0) | |
| Nivolumab | 11 (15.9) | |
| Cemiplimab | 10 (14.5) | |
| Atezolizumab | 3 (4.3) | |
| Avelumab | 3 (4.3) | |
| Ipilimumab | 2 (2.9) | |
| PD-1/CTLA-4 combination | 11 (15.9) | |
| irAE, n (%), apart from rejection | NA | |
| None | 52 (75.4) | |
| At least one | 17 (24.6) | |
| Dermatitis | 5 (25.0) | |
| Encephalopathy | 4 (20.0) | |
| Hepatitis | 3 (15.0) | |
| Endocrinopathy | 3 (15.0) | |
| Colitis | 1 (5.0) | |
| Others | 4 (20.0) | |
| Tumor response, n (%) | ||
| CR | 5 (7.2) | 2 (5.4) |
| PR | 15 (21.7) | 2 (5.4) |
| SD | 11 (15.9) | 5 (13.5) |
| PD | 34 (49.3) | 28 (75.7) |
| Unknown | 4 (5.8) | 0 (0) |
| Follow-up, months | 12 (6.3–22.3) | 9.7 (4.2–21.4) |
Data are shown as median (IQR) and n (%). IQR; interquartile range, LUKTx; living-unrelated kidney transplant, LRKTx; living-related kidney transplant, DDKTx; deceased kidney transplant, Cr; creatinine, cSCC; cutaneous squamous cell carcinoma, NSCLC; non-small cell lung carcinoma, RCC; renal cell carcinoma, ICI; immune checkpoint inhibitor, PD-1; programmed cell death protein 1, CTLA-4; cytotoxic T-lymphocyte-associated protein 4, CR; complete response, PR; partial response, SD; stable disease, PD; progressive disease, irAE; immune-related adverse event. Other cancer-directed therapy includes chemotherapy and molecular-targeted therapy except for ICI.
Of total 69 patients, 45 patients (65.2%) underwent changes in immunosuppression regimen immediately prior to the ICI initiation, resulting in a cumulative total of 62 changes. In 43 patients (62.3%), the number of immunosuppressants remained the same before and after the ICI initiation, while 24 (34.8%) and 2 (2.9%) patients experienced a reduction and increase in the number of immunosuppressants, respectively (Supplemental Figure 1A). Of the cumulative total of 62 changes, CNI to mTORi conversion was most common (15 cases), followed by discontinuation of antimetabolite and increase of corticosteroids (14 cases each) (Supplemental Figure 1B). Most patients (49.3%) were on two-agent immunosuppressant regimen at the time of ICI initiation, and 85.5% were on corticosteroid (Supplemental Table 2). Median trough levels of tacrolimus, sirolimus and everolimus were 4.4 (IQR, 4–6.5), 7.5 (IQR, 4–10), and 4.75 (IQR, 2.9–5.7) ng/ml, respectively (Supplemental Table 3).
Advanced cSCC (n=24, 34.8%) and melanoma (n=22, 31.9%) were the most common malignancies. Overall, 59.4% of the cohort received systemic cancer-directed therapies prior to ICI. Median time from transplant to ICI initiation was 9.3 years (IQR, 4.1–15.6). The most frequently used agents were pembrolizumab (42.0%), nivolumab (15.9%), and combination therapy (15.9%, mostly concomitant ipilimumab and nivolumab, detailed in Supplemental Table 4). The objective response rate to ICI therapy was 28.9% (complete response; 7.2%, partial response; 21.7%). Extrarenal immune-related adverse events (irAE) were observed in 24.6% of patients, with dermatitis being the most common manifestation (25.0% of all irAE events). (Supplemental Table 5).
Characteristics and risk factors for acute graft rejection
A total of 29 of the 69 patients (42.0%) developed acute graft rejection, 14 of which (48.3%) were biopsy-proven (Figure 1A, Supplemental Table 6). Mixed acute T cell-mediated and antibody-mediated rejection (n=7; 50%) and pure T-cell mediated rejection (n=7; 50%) were both observed in biopsy-proven graft rejection. Rejection developed at a median of 24 days (IQR, 20–56) after ICI initiation, and 80% of all rejection episodes occurred within the first 60 days following ICI initiation (Figure 1B). Rejection was treated with intravenous high dose corticosteroids in 16 out of 29 patients (55.2%), and 3 (10.3%) patients underwent graft nephrectomy. Two patients received treatment for antibody-mediated rejection with intravenous immunoglobulin. Following rejection, 19 patients (65.5%) had allograft loss and required dialysis (Figure 1A). A higher number of immunosuppression agents used in a given patient at the time of ICI initiation (p=0.069), use of mammalian target of rapamycin (mTOR) inhibitors (p=0.021), and deceased-donor kidney transplant status (p=0.014) were associated with a lower risk of graft rejection (Table 2). Extrarenal irAE, steroid use (prednisone equivalent ³10 mg per day) or a modified protocol of mTOR inhibitor combined with steroid mini-pulse (so-called dynamic immunosuppression),10 were not associated with a lower risk of rejection. Use of mTOR inhibitors was associated with a lower risk of rejection in multivariate analyses (odds ratio 0.78; 95% CI, 0.61–0.99, Figure 2). Rejection-free graft survival and overall graft survival were both longer in mTOR inhibitor-treated patients compared to non-mTOR inhibitor-treated patients (Supplemental Figures 2A and B). Overall survival did not differ in patients who had graft rejection versus those who did not, nor was it different in patients who developed rejection and required dialysis, or patients who experienced early rejection (less than 28 days from ICI initiation), when compared to the patients without rejection (Supplemental Figure 3A–C).
Figure 1: Characteristics of graft rejection Time to graft rejection.
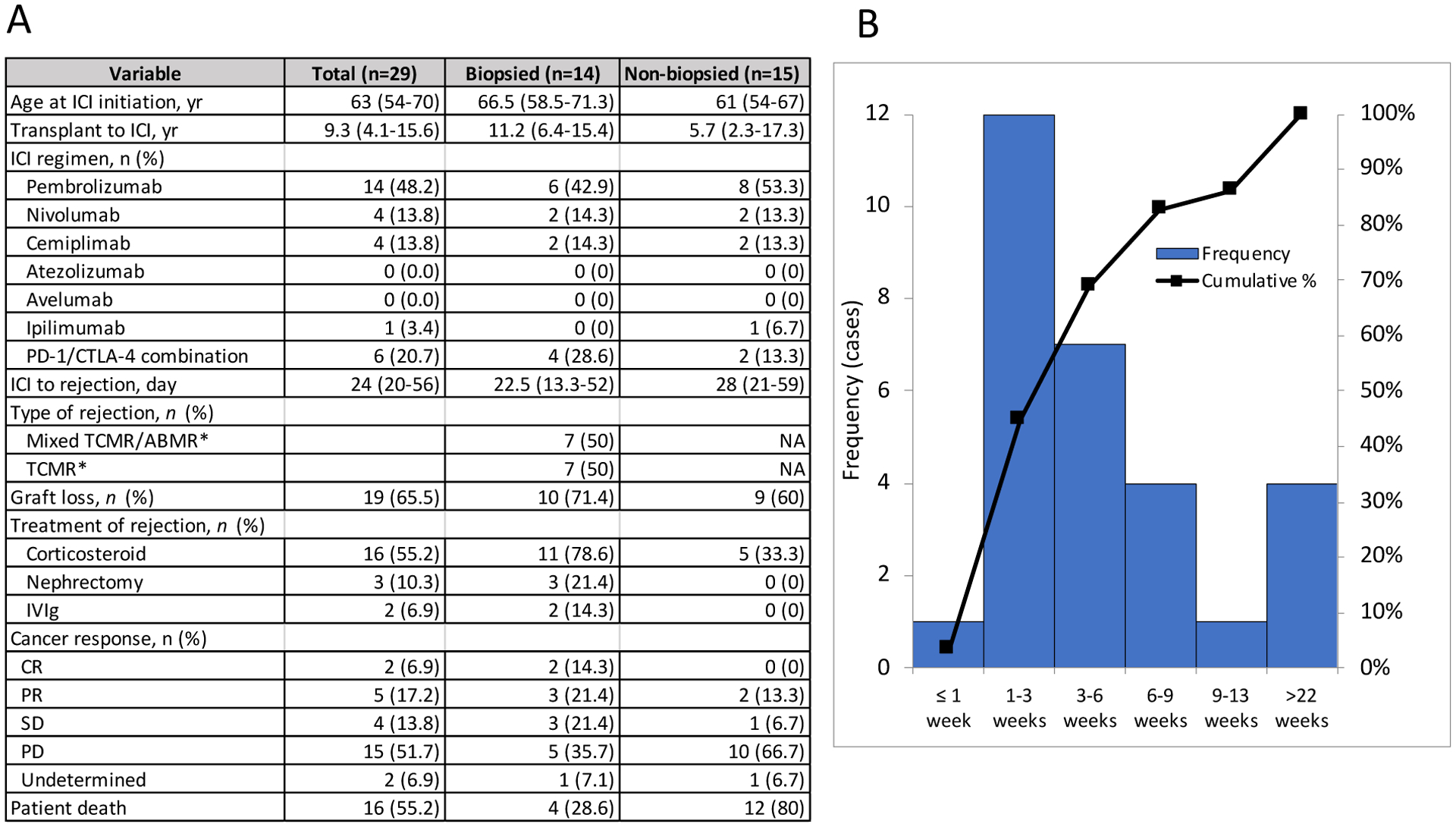
A. Characteristics of graft rejection. Data are shown as median (IQR) and n (%). *biopsy proven. ICI; immune checkpoint inhibitor, PD-1; programmed cell death protein 1, CTLA-4; cytotoxic T-lymphocyte-associated protein 4, TCMR; T-cell mediated rejection, ABMR; antibody mediated rejection, IVIg; intravenous immunoglobulin. B. Distribution of timing between ICI initiation and graft rejection, and cumulative rate of events is shown.
Table 2:
Risk factors of graft rejection
| No Rejection (n=40) | Rejection (n=29) | p | |
|---|---|---|---|
| Age at ICI initiation, yr | 65.5 (54.3–72.5) | 63 (54–70) | 0.596 |
| Female, n (%) | 8 (20.0) | 3 (10.3) | 0.336 |
| Transplant type, n (%) | 0.014 | ||
| LUKTx | 6 (15.0) | 10 (34.5) | |
| LRKTx | 13 (32.5) | 11 (37.9) | |
| DDKTx | 21 (52.5) | 6 (20.7) | |
| Unknown | 0 (0.0) | 2 (6.9) | |
| HLA antigen mismatch, n (%) | 0.425 | ||
| 0 | 5 (12.5) | 1 (3.4) | |
| 1 | 2 (5.0) | 0 (0.0) | |
| 2 | 3 (7.5) | 3 (10.3) | |
| 3 | 9 (22.5) | 7 (24.1) | |
| 4 | 3 (7.5) | 1 (3.4) | |
| 5 | 7 (17.5) | 3 (10.3) | |
| 6 | 1 (2.5) | 0 (0.0) | |
| Unknown | 10 (25.0) | 14 (48.3) | |
| DSA, n (%) | 5 (12.5) | 6 (20.7) | 0.319 |
| Cancer type, n (%) | 0.430 | ||
| cSCC | 15 (37.5) | 9 (31.0) | |
| Melanoma | 10 (25.0) | 12 (41.4) | |
| NSCLC | 4 (10.0) | 4 (13.8) | |
| Merkel Cell Carcinoma | 4 (10.0) | 0 (0.0) | |
| RCC | 2 (5.0) | 1 (3.4) | |
| Bladder | 2 (5.0) | 0 (0.0) | |
| Others | 3 (7.5) | 3 (10.3) | |
| Number of immunosuppressants, n (%) | 0.069 | ||
| 0 | 0 (0.0) | 1 (3.4) | |
| 1 | 8 (20.0) | 12 (41.4) | |
| 2 | 21 (52.5) | 13 (44.8) | |
| 3 | 11 (27.5) | 3 (10.3) | |
| Immunosuppression agents | |||
| Steroid, n (%) | 35 (87.5) | 24 (82.8) | 0.837 |
| Antimetabolite, n (%) | 12 (30.0) | 4 (13.8) | 0.199 |
| mTORi, n (%) | 22 (55.0) | 7 (24.1) | 0.021 |
| CNI, n (%) | 14 (35.0) | 12 (41.4) | 0.773 |
| Steroid ≧ 10mg*, n (%) | 5 (12.5) | 2 (6.9) | 0.69 |
| Dynamic steroid+mTORi, n (%) | 7 (17.5) | 1 (3.4) | 0.125 |
| ICI target, n (%) | 0.101 | ||
| PD-1 | 28 (70.0) | 22 (75.9) | |
| PD-L1 | 6 (15.0) | 0 (0.0) | |
| CTLA-4 | 1 (2.5) | 1 (3.4) | |
| Combination | 5 (12.5) | 6 (20.7) | |
| Nephrotoxin, n (%) | 0.306 | ||
| None | 22 (55.0) | 21 (72.4) | |
| NSAIDs | 1 (2.5) | 0 (0.0) | |
| PPI | 9 (22.5) | 6 (20.7) | |
| Antibiotics | 8 (20.0) | 2 (6.9) | |
| Tumor response, n (%) | 0.992 | ||
| CR | 3 (7.5) | 2 (6.9) | |
| PR | 9 (22.5) | 6 (20.7) | |
| SD | 7 (17.5) | 4 (13.8) | |
| PD | 19 (47.5) | 15 (51.7) | |
| Unknown | 2 (5.0) | 2 (6.9) | |
| Objective response rate [CR+PR], n (%) | 12 (30.0) | 8 (27.6) | 1 |
| Extra renal irAE, n (%) | 9 (22.5) | 8 (27.6) | 0.778 |
| Transplant to ICI, yr | 9 (4.8–14.3) | 9 (4–16) | 0.808 |
Data are shown as median (IQR) and n (%).
prednisone equivalent.
LUKTx; living-unrelated kidney transplant, LRKTx; living-related kidney transplant, DDKTx; deceased kidney transplant, DSA; donor-specific antibody at the time of transplant, cSCC; cutaneous squamous cell carcinoma, NSCLC; non-small cell lung carcinoma, RCC; renal cell carcinoma, mTORi; mammalian target of rapamycin inhibitor, CNI; calcineurin inhibitor, ICI; immune checkpoint inhibitor, PD-1; programmed cell death protein 1, PD-L1; programmed death ligand 1, CTLA-4; cytotoxic T-lymphocyte-associated protein 4, NSAIDs; nonsteroidal anti-inflammatory drugs, PPI; proton pump inhibitor, CR; complete response, PR; partial response, SD; stable disease, PD; progressive disease, irAE; immune-related adverse event
Figure 2: Risk factors of allograft rejection.
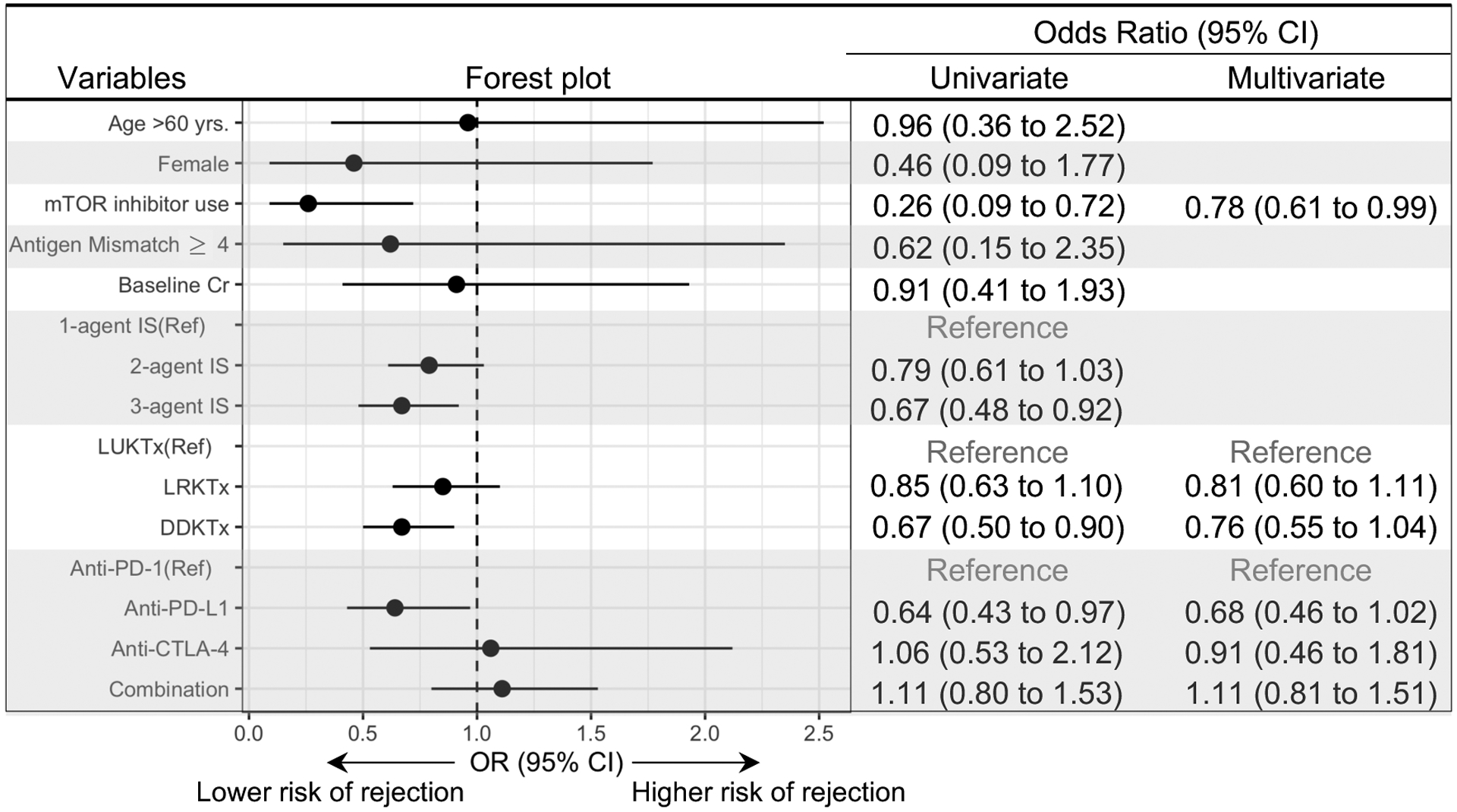
Univariate and multivariate analyses of allograft rejection. Odds ratio (OR) and 95% confidence interval (CI) are shown. Cr; serum creatinine, Ref; reference, IS; immunosuppressant, LUKT; living unrelated kidney transplant, LRKTx; living related kidney transplant; DDKTx; deceased kidney transplant.
Clinical efficacy of ICIs in patients in advanced cSCC and advanced melanoma subgroups
As cSCC and melanoma were the most common cancer types in our cohort, we sought to further investigate the clinical safety and efficacy of ICI in these two cancer types. In order to assess efficacy, historical cSCC patients with kidney transplants who did not receive ICI (non-ICI group) served as control. Baseline characteristics of ICI group (n=24) versus non-ICI group (n=23) are shown in Table 3. The number of immunosuppressants did not differ between the two groups (p=0.99). In the ICI group, 15 out of 24 (62.5%) patients had received systemic cancer-directed therapy prior to ICI, of whom 14 (93.3%) had received cetuximab, and 7 (46.7%) had received platinum-based chemotherapy. In the non-ICI group, 20 out of 23 (87%) patients had received systemic cancer-directed therapy, of whom 12 (60%) had received cetuximab and 14 (70%) had received platinum-based chemotherapy. The graft rejection rate was higher in the ICI group compared to the non-ICI group (37.5% vs. 4.3%, **p<0.01) and remained higher when stratified by the number of immunosuppressants (Figure 3A). The objective response rate to ICI was 36.4% overall, and did not differ when stratified by the number of immunosuppressants (Figure 3B and Supplemental Table 7). Both overall survival (median overall survival 19.8 vs. 10.6 months, log rank *p=0.016) and disease specific survival (median disease specific survival not reached vs 20.6 months, log rank *p=0.015) were longer in the ICI group compared to the non-ICI group (Supplemental Figure 4A and B).
Table 3:
Patient characteristics of cSCC subgroup
| ICI (n=24) | non-ICI (n=23) | ||
|---|---|---|---|
| Age, yr | 62.5 (55–71.3) | 62.7 (58–67) | |
| Female (%) | 1 (4.2) | 6 (23.1) | |
| Time from transplant to diagnosis, yr | 12 (4.7–17.4) | 11.1 (6.1–18.9) | |
| Stage at advanced diagnosis (%) | III (unresectable) | 3 (12.5) | 1 (4.3) |
| IV | 20 (83.3) | 22 (95.7) | |
| Unknown | 1 (4.2) | 0 (0) | |
| Baseline Cr, mg/dl | 1.45 (1.0–1.9) | 1.50 (1.0–1.9) | |
| Other cancer-directed therapy (%) | 15 (62.5) | 20 (87.0) | |
| ICI | |||
| Pembrolizumab (%) | 8 (33.3) | ||
| Nivolumab (%) | 2 (8.3) | ||
| Cemiplimab (%) | 10 (41.7) | ||
| Ipilimumab (%) | 1 (4.2) | ||
| Combination (%) | 3 (12.5) | ||
| Tumor response | |||
| CR (%) | 2 (8.3) | 1 (4.3) | |
| PR (%) | 6 (25) | 1 (4.3) | |
| SD (%) | 4 (16.7) | 3 (13.0) | |
| PD (%) | 10 (41.7) | *18 (78.3) | |
| Unknown (%) | 2 (8.3) | 0 (0) | |
| Number of immunosuppressants (%) | |||
| 0 | 1 (4.1) | 0 (0.0) | |
| 1 | 4 (16.7) | 6 (26.1) | |
| 2 | 15 (62.5) | 13 (56.5) | |
| 3 | 4 (16.7) | 4 (17.4) | |
| Rejection (%) | 9 (37.5) | 1 (4.3) | |
| Follow-up, months | 14.2 (10.7–26.3) | 9.7 (4.5–20.1) | |
Data are shown as median (IQR) and n (%).
cSCC; cutaneous squamous cell carcinoma, IQR; interquartile range, Cr; creatinine, ICI; immune checkpoint inhibitor, CR; complete response, PR; partial response, SD; stable disease, PD; progressive disease,
Includes 6 undetermined cases (SD vs PD). Other cancer-directed therapy includes chemotherapy and molecular-targeted therapy except for ICI.
Figure 3: Rejection rate and tumor response of cSCC subgroup.
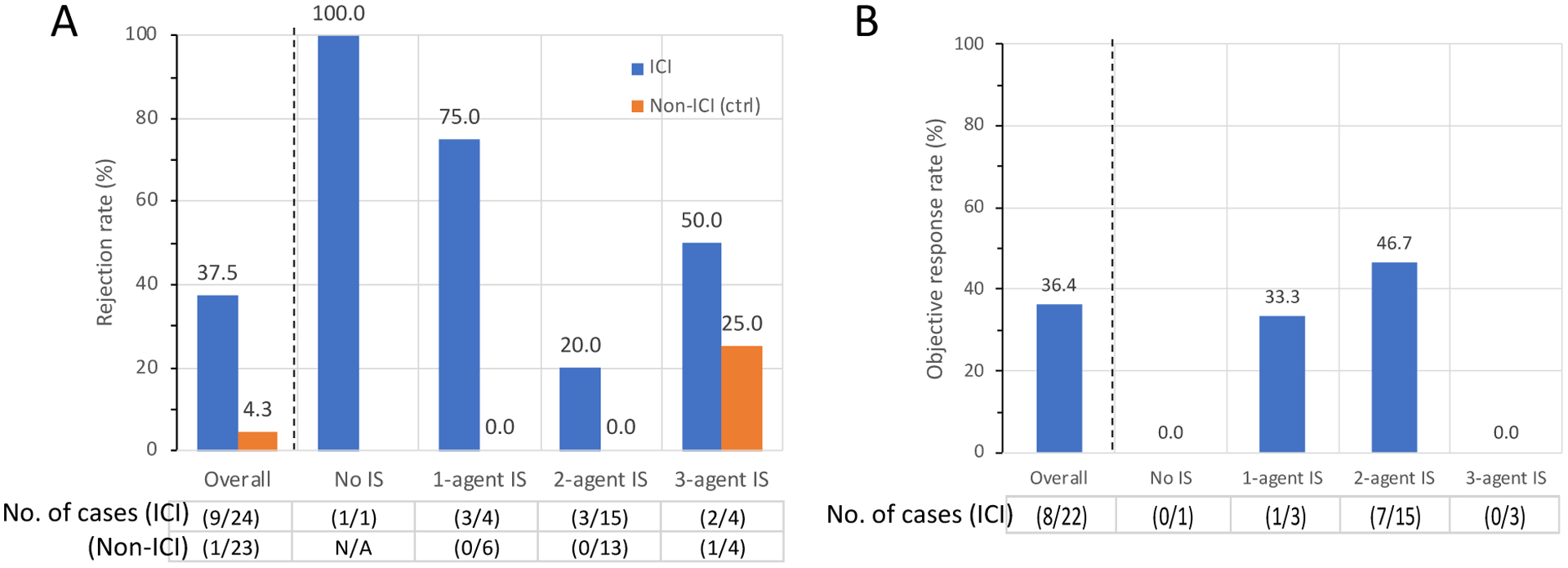
Allograft rejection rate (A) and objective response rate (ORR, B), stratified by the number of immunosuppressants. In ICI group, cancer response in 2 patients were undetermined and excluded from analysis of ORR. cSCC; cutaneous squamous cell carcinoma
In the melanoma subgroup, the historical advanced melanoma cohort not treated with ICI (non-ICI group, n=14) served as control for the clinical efficacy analysis. The number of immunosuppressants was similar between the ICI and the non-ICI-treated groups (p=0.98, Table 4). In the ICI group, 4 out of 22 (18.2%) patients had a BRAF V600 mutated melanoma, of whom 3 patients had received combination BRAF/MEK targeted therapy prior to ICI. In non-ICI group, 5 out of 14 (35.7%) patients’ melanomas were BRAF V600 mutated, of whom 4 patients received combination BRAF/MEK targeted therapy and 1 patient received BRAF inhibitor monotherapy. The graft rejection rate in the ICI group was higher than in the non-ICI group (54.5% vs. 7.1%, **p<0.01), which also remained higher when stratified by the number of immunosuppressants (Figure 4A). The objective response rate to ICIs was 40.0% overall; objective response rate specifically to anti-PD-1 monotherapy and anti-CTLA4/anti-PD-1 combination therapy was 9.1% and 87.5%, respectively. Objective response rate did not differ when stratified by the number of immunosuppressants (Figure 4B and Supplemental Table 7). Overall survival (median overall survival 13.5 vs. 11.4 months, p=0.34) and disease specific survival (median disease specific survival 29.9 vs. 21.4 months, p=0.38) did not differ between patients who received ICI and those who did not (log rank) (Supplemental Figure 4 C and D).
Table 4:
Patient characteristics of melanoma subgroup
| ICI (n=22) | non-ICI (n=14) | ||
|---|---|---|---|
| Age, yr | 68.5 (59.5–73.3) | 62 (54.8–75.6) | |
| Female (%) | 2 (9.1) | 4 (28.6) | |
| Time from transplant to diagnosis, yr | 7.1 (2.2–15.1) | 7.7 (3.5–15.3) | |
| Stage at advanced diagnosis (%) | IIIc (unresectable) | 5 (22.7) | 3 (21.4) |
| IV | 17 (77.2) | 11 (78.6) | |
| BRAF V600 mutation status | Mutation+ | 4 (18.2) | 5 (35.7) |
| Wild type | 18 (81.8) | 6 (42.9) | |
| Unknown | 0 (0.0) | 3 (21.4) | |
| Baseline Cr, mg/dl | 1.37 (1.03–1.71) | 1.05 (0.78–1.55) | |
| Other cancer-directed therapy (%) | 14 (58.3) | 7 (50.0) | |
| ICI | |||
| Pembrolizumab (%) | 11 (50.0) | ||
| Nivolumab (%) | 2 (9.1) | ||
| Ipilimumab (%) | 1 (4.5) | ||
| Combination (%) | 8 (36.3) | ||
| Tumor response | |||
| CR (%) | 1 (4.5) | 1 (7.1) | |
| PR (%) | 7 (31.8) | 1 (7.1) | |
| SD (%) | 1 (4.5) | 2 (14.3) | |
| PD (%) | 11 (50.0) | 10 (71.4) | |
| Unknown (%) | 2 (9.1) | 0 (0) | |
| Number of immunosuppressants (%) | |||
| 0 | 0 (0.0) | 3 (21.4) | |
| 1 | 8 (36.3) | 1 (7.1) | |
| 2 | 11 (50.0) | 8 (57.1) | |
| 3 | 3 (13.6) | 2 (14.3) | |
| Rejection (%) | 12 (54.5) | 1 (7.1) | |
| Follow-up, months | 9.7 (5.5–20.0) | 11.4 (3.3–22.4) | |
Data are shown as median (IQR) and n (%). ICI; immune checkpoint inhibitor, PD-1; programmed cell death protein 1, CTLA-4; cytotoxic T-lymphocyte-associated protein 4, CR; complete response, PR; partial response, SD; stable disease, PD; progressive disease. Other cancer-directed therapy includes chemotherapy and molecular-targeted therapy except for ICI.
Figure 4: Rejection rate and tumor response of melanoma subgroup.
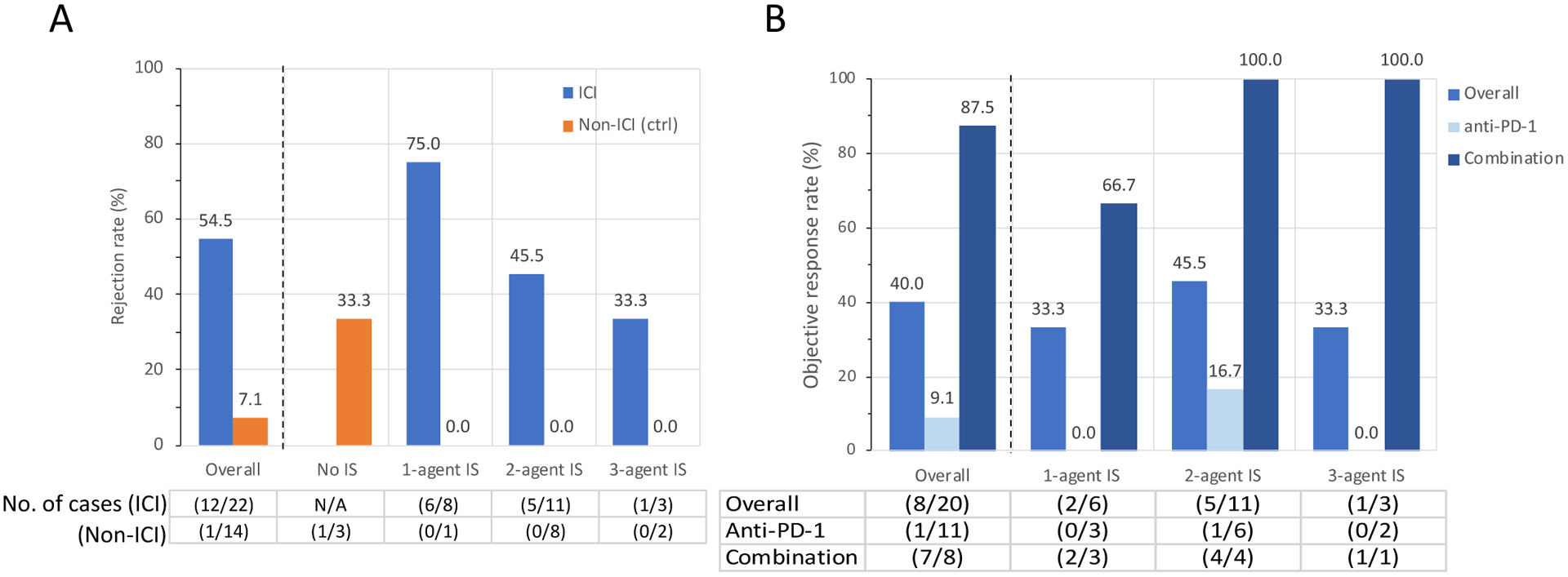
Allograft rejection rate (A) and objective response rate (ORR, B), stratified by the number of immunosuppressants. In ICI group, cancer response in 2 patients were undetermined and excluded from the analysis of ORR. In panel B, Anti-PD-1 indicates ORR of patients who received pembrolizumab or nivolumab monotherapy.
Discussion
In this multicenter retrospective cohort study, we report the incidence, timing, and risk factors of graft rejection in 69 cancer patients with kidney transplants treated with ICI. We detected a high graft rejection rate with onset of rejection within weeks following treatment, with significant graft loss after rejection episodes. In addition, our data suggests that the higher number of immunosuppressants were associated with a lower risk of acute graft rejection but that ICI may still provide a reasonable tumor response in advanced cSCC and melanoma patients with kidney transplant.
The rapid onset and severe rejection following ICI treatment in transplant recipients contrasts with the delayed onset of ICI-associated acute interstitial nephritis (AIN) seen in native kidneys.11, 12 In non-transplant patients, kidney-related irAE is characterized by AIN with an incidence of 2–3%,11 with a median onset of 14 weeks after the ICI initiation, and an 85% response rate to corticosteroid treatment to achieve partial or complete recovery.12 Our data shows that 42% of cancer patients with kidney transplant developed acute graft rejection with a median onset of less than 4 weeks, of whom 65.5% did not recover and required dialysis. It is notable that only 48% of presumed acute rejection was biopsy proven. We noted that biopsied patients were more likely to be treated, had better response to ICI and lower proportion of death (Figure 1). This may be confounded by a better overall clinical status of biopsied patients. Biopsy proven rejection included high proportion of mixed cellular and antibody-mediated rejection, and the rejections were overall very severe, with 9 out of 14 biopsied cases bearing vascular rejection component, which may explain the poor allograft outcomes. Antibody-mediated rejection is difficult to treat, and especially so in the setting of active malignancy where clinicians may be hesitant to treat it with potent immunosuppression. In addition, our findings may suggest a unique underlying mechanism of ICI-associated rejection; preexisting, graft antigen-specific memory T cells, which remained quiescent under immunosuppression, but could be activated, proliferate and elicit a rapid and robust immune response in the setting of enhanced immune response by ICI. Indeed, dynamic expansion of T cells in the early phase (<3 weeks) after ICI initiation has been observed in melanoma patients, and the expansion is seen not only in melanoma-antigen specific T cells but also in bystander T cells.13 In order to maximize tumor response to ICI therapy, while minimizing risk to the transplant allograft, it will be critical to develop a methodology to uncouple anti-cancer and anti-allograft immune response.
Modification of immunosuppression is a key therapeutic strategy in transplant patients who develop malignancies, but systematic data are lacking on the specific choice and sufficient levels of immunosuppressants. In cSCC and melanoma subgroup analyses, we demonstrated that the rejection rate was higher in ICI treated patients (37.5% and 54.5%, respectively, in cSCC and melanoma subgroups), compared to the non-ICI group (4.3% and 7.1%, respectively), despite both groups undergoing immunosuppression reduction or modification as a part of standard practice. This suggests that the higher rejection rate in ICI group cannot be solely explained by reduction of immunosuppression.
The effect of mTOR inhibitors in cancer patients with kidney transplants has been well studied. In patients with cSCC, conversion from calcineurin inhibitors to mTOR inhibitors is effective for the secondary prevention of cSCC.14, 15 For ICI-treated kidney transplant patients, a case study suggested that mTOR inhibitor therapy helped maintain graft tolerance and achieve tumor immunity simultaneously.16 Similarly, “dynamic immunosuppression” was suggested as a potentially effective way to mitigate acute rejection.10, 17 While we found that mTOR inhibitor use was associated with a lower rejection rate, this finding should be taken with a caution and needs to be validated in other studies. The actual trough levels of sirolimus (median 7.5 ng/ml) were relatively higher and closer to a full therapeutic target level than that of tacrolimus (median 4.4 ng/ml), which may confound and favor the rejection risk reduction in patients treated with mTOR inhibitors. Eight patients in our cohort received dynamic immunosuppression approach and only one patient experienced acute rejection. The effect of this regimen is the subject of an ongoing prospective study (NCT04339062).
Though the number is small, none of the 6 patients who received anti-PD-L1 (atezolizumab or avelumab) experienced rejection. In pre-clinical mouse cardiac transplant models, anti-PD-1 and anti-PD-L1 accelerated transplant rejection, suggesting PD-1 and PD-L1 are both necessary to maintain allograft tolerance.18–20 In non-transplant patients, it has been reported that anti-PD-L1 is associated with less incidence of acute kidney injury compared with anti-PD-1.21 This may be applicable to transplant population and further studies are needed to elucidate the underlying mechanisms.
Conversely, the use of immunosuppression could diminish the efficacy of immunotherapy by blunting cancer immune response. Use of corticosteroid (prednisone equivalent ³10 mg daily) has been reported to be associated with a lower efficacy of ICI in non-small cell lung cancer patients,22, 23 as well as in transplant recipients.7 In our study, the number of immunosuppressants did not appear to affect the objective response rate of cSCC or melanoma to ICIs. However, our data was not adequately powered for this endpoint, and further mechanistic and lager prospective studies are still required. Considering both rejection risk and objective response rate, it may be reasonable to maintain two-agent immunosuppression regimen while on ICI treatment, as this appears to still achieve reasonable tumor response with a lower risk of rejection (Figures 2A–B and 3A–B), as suggested in a previous meta-analysis as well.9
The prognosis of metastatic cSCC is very poor,24 and median OS is around 12 months in advanced cSCC patients with kidney transplant, without ICI therapy.25 cSCCs harbor high tumor mutational burden which is associated with response to immunotherapy.26, 27 Objective response rate of advanced cSCCs to anti-PD-1 therapies in non-transplant patients has been reported as high as 50% for cemiplimab28, and 34% for pembrolizumab (NCT03284424).29 Objective response rate in our cSCC subgroup was robust (36.4%), despite concomitant immunosuppression. Our survival estimates for advanced cSCC patients treated with ICI (19.8 months) may support the efficacy of ICI in our population. The small sample size limits our ability to provide definite conclusions, but our data can help inform clinical decision making in this challenging situation.
ICIs provide substantial survival benefit in patients with metastatic melanoma in non-transplant setting.30 The prognosis of advanced melanoma (AJCC stage III/IV) in kidney transplant patients is very poor and worse than that of non-transplant patients, with median OS less than 12 months.31 In our melanoma subgroup, median OS was 13.5 months in ICI-group, and our analysis failed to detect a difference of OS in ICI group compared to historically eligible non-ICI-treated patients with advanced melanoma. This finding may have resulted from a small sample size and the lack of statistical power, and a selection bias of our ICI-treated group to mostly previously treated patients; three out of four patients whose tumors harbored a BRAF V600 mutation had failed BRAF-targeted therapy prior to ICI. Observed objective response rate to anti-PD-1 therapy (9.1%) was lower than those reported in non-transplant cohort30, 32, but objective response rate to combination anti-CTLA-4/anti-PD-1 therapy (87.5%) was robust and comparable to that reported in non-transplant cohort.33 These results should be tested in larger prospective studies.
Although our study is to our knowledge the largest multicenter cohort of patients with advanced solid malignancies with kidney transplant who received ICI to date, there are several limitations. First, ethnicity data were not included in the analysis. Second, the exploratory, retrospective and small-sample nature of our cohort limited our ability to adjust for a number of confounders in multivariable analysis for the risk of graft rejection. Third, less than half of acute rejection were biopsy proven, which limits the accuracy of the diagnosis of rejection. Fourth, there was inherent selection bias of the non-ICI group. However, the inclusion criteria of non-ICI group were prespecified, and strictly selected to identify those patients who were eligible for ICI treatment in an effort to provide a population at historically similar risk for poor outcomes. In addition, the median year of advanced cancer diagnosis of cSCC and melanoma non-ICI subgroups were 2012 and 2013, respectively. Though these are approximately 5 years earlier than that of ICI group, they serve as reasonably contemporary control groups who received best available treatment at the time of diagnosis, knowing that ICI therapy was FDA approved in 2011 with slower adoption in transplant recipients than the general population. The comparison of outcomes using these historical cohorts suffers from the lack of power due to the small number of cases, but provides a pragmatic approach to address the risk of rejection and objective response rate. Lastly, immunosuppression modification was the providers’ choice at each institution and not standardized.
In conclusion, ICIs are a feasible option for kidney transplant recipients with advanced malignancies but should be used with caution due to a high risk of acute rejection. Close monitoring and tailoring immunosuppression are critical. Future prospective studies with sufficient patient cohorts should validate the safety and efficacy of ICIs in transplant patients.
Methods
Study overview
We conducted a multicenter retrospective cohort study of ICI use in cancer patients with kidney transplants. To identify cases, we assembled a collaborative consortium of transplant nephrologists, onconephrologists, and medical oncologists from 23 academic centers across the United States, Canada, and Europe (Supplemental Appendix 1). Protocols were approved by the Brigham and Women’s Hospital institutional review board (IRB) and by the local IRBs of participating institutions.
Patient selection and data collection
Adult (age 18 and older) kidney transplant recipients with a functioning allograft (i.e. requiring no form of renal replacement therapy), who received ICIs between January 2010 and May 2020 were retrospectively identified using clinical database queries at each participating institution. Query example: diagnostic code “kidney transplant (ICD-9-CM: V.42.0, or ICD-10-CM: Z94.0)”, AND drug administered (list of ICI agents below). Decisions regarding immunosuppressants, cancer-directed therapy, immunotherapy selection and dosing were made at the discretion of the treating institution. No patients were receiving treatment as part of a clinical trial. ICIs included: anti-cytotoxic T-lymphocyte-associated protein 4; CTLA-4 (ipilimumab, tremelimumab), anti-programmed cell death protein 1; PD-1 (nivolumab, pembrolizumab, cemiplimab), anti-programmed death-ligand 1; PD-L1 (atezolizumab, avelumab, durvalumab). Clinical data on prespecified variables were extracted for each subject from electronic medical records by investigators at each site. Missing data were reported as “unknown” for categorical values. For continuous variables, 1 baseline serum creatinine value of ICI group and 2 baseline creatinine values of non-ICI cSCC control cohort were missing and they were excluded from the analysis.
Outcomes
The primary safety outcome was acute graft rejection, which was defined by either biopsy-proven rejection, or an acute kidney injury event (defined as doubling of baseline serum creatinine) clinically suspected to be due to rejection and with no other explanation. Rejection episodes were identified via detailed chart review of laboratory results as well as all nephrology, oncology, and primary care notes. Baseline serum creatinine was defined as a stable serum creatinine value within two weeks prior to the ICI initiation. See Supplemental Appendix 2 for additional details of data collected. For the efficacy outcome, objective response rate (ORR) to ICIs was assessed. Objective response rate is defined as the proportion of the patients who achieved complete response (CR) and partial response (PR). We also assessed overall survival and disease-specific survival as exploratory outcomes. Overall survival was defined from the time of advanced cancer diagnosis qualifying for ICI use. Cancer stage at diagnosis of cSCC and melanoma was determined according to the seventh or eighth edition of the American Joint Committee on Cancer Staging System, depending on the time of diagnosis. Treatment outcome was determined using Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria in conjunction with relevant oncology notes, imaging and pathology.
Control patients (non-ICI group) with advanced cSCC or melanoma
To assess the efficacy of ICIs by overall survival and disease specific survival, a cohort of kidney transplant patients with advanced cSCC or melanoma who did not receive ICI were collected and served as comparison. The patients were identified using a database query of free text and diagnostic codes corresponding to kidney transplant and cSCC or melanoma. All subjects had a functioning allograft at the time of inclusion. Control patients with cSCC were included if they had one of the following prespecified criteria for advanced disease: distant metastatic disease (visceral metastases or nodal disease present two or more regional lymph node basins away from the primary tumor) or received systemic cancer-directed therapy for surgically unresectable disease. Control patients with melanoma were included if they had distant metastatic disease or regionally metastatic and unresectable disease receiving systemic cancer-directed therapy. The selectors were blinded from the outcomes of ICI-treated patient group. All patients who meet the above criteria were included without further selection process. Control patients were collected from Dana-Farber Cancer Institute, Massachusetts General Hospital, MD Anderson Cancer Center and Houston Methodist Cancer and Transplant Centers.
Statistical Analyses
Statistical analyses were performed using R statistics package and GraphPad Prism version 8.4.3 (GraphPad Software, San Diego, CA, USA). Descriptive statistics include frequency and percentages for categorical variables and medians and interquartile ranges (IQRs) for continuous measures. Non-parametric Mann-Whitney U tests were used for continuous variables and χ2 test or Fisher exact test (for cell size < 5) for categorical variables. Univariate and multivariable logistic regression models were used to examine risk factors for graft rejection. The following covariates were prespecified for inclusion in multivariable models based on clinical and biologic importance34: age, sex, antigen mismatch, baseline serum creatinine, mTOR inhibitor use, and the number of immunosuppressive agents. Additionally, the transplant type (living vs. deceased) and the ICI pathway targeted were included. A parsimonious multivariable risk-prediction model was constructed using only three predictors (i.e. mTOR inhibitor use, transplant type, and ICI pathway being targeted) derived from the univariable models. In survival analyses, overall survival and disease specific survival were each defined as the time from advanced cancer diagnosis which qualified for ICI-treatment to death from any cause, or to death from cancer progression, respectively. Rejection-free graft survival was defined as the time from ICI administration to rejection or graft loss (death censored). Distributions of overall survival and disease specific survival were estimated using the Kaplan-Meier method.35
Supplementary Material
Acknowledgement
The authors thank Dino Mazzarelli J.D., from Partners Healthcare Research Management, for his assistance coordinating data use agreement with each institution. NM is supported by K08DK120868 from the National Institute of Diabetes and Digestive Kidney Diseases and by an American Society of Nephrology Foundation for Kidney Research Carl W. Gottschalk Research Scholar Grant. MES is supported by K23DK117014 from the National Institute of Diabetes and Digestive Kidney Diseases. EAJ is supported by P30CA008748, R01DK114321, R01FP00008739 and DM180384. BS is a senior clinical investigator for The Research Foundation Flanders (to Fonds Wetenschappelijk Onderzoek-Vlaanderen; 1842919N). LVR is supported by R01AI143887 from the National Institutes of Allergy and Infectious Diseases, and Harold and Ellen Danser Endowed/Distinguished Chair in Transplantation at Massachusetts General Hospital. The authors thank Matthew A. Sparks M.D. for reviewing the visual abstract. A part of the study was presented as an oral abstract in the Kidney Week 2020 Reimagined.
Footnotes
Disclosures:
CDB is a consultant for Natera and CareDx. MJS serves as a consultant for NovoNordisk, Boehringer, AstraZeneca and Mundipharma.FC serves as a consultant for Natera. EAJ is chief medical officer and shareholder of Goldilocks Therapeutics, Inc. SG is a scientific coordinator for the ASCEND trial (GlaxoSmithKline). LVR had served as a consultant for CareDx and expert for Advance Medical.
Supplementary Material
Supplementary information is available on Kidney International’s web site.
References
- 1.Saran R, Robinson B, Abbott KC, et al. : US Renal Data System 2018 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 73:A7–A8, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapman JR, Webster AC, Wong G: Cancer in the transplant recipient. Cold Spring Harb Perspect Med 3, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Engels EA: Epidemiologic perspectives on immunosuppressed populations and the immunosurveillance and immunocontainment of cancer. Am J Transplant 19:3223–3232, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma P, Allison JP: The future of immune checkpoint therapy. Science 348:56–61, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Lipson EJ, Bodell MA, Kraus ES, et al. : Successful administration of ipilimumab to two kidney transplantation patients with metastatic melanoma. J Clin Oncol 32:e69–71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipson EJ, Bagnasco SM, Moore J, et al. : Tumor Regression and Allograft Rejection after Administration of Anti-PD-1. N Engl J Med 374:896–898, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdel-Wahab N, Safa H, Abudayyeh A, et al. : Checkpoint inhibitor therapy for cancer in solid organ transplantation recipients: an institutional experience and a systematic review of the literature. J Immunother Cancer 7:106, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manohar S, Thongprayoon C, Cheungpasitporn W, et al. : Systematic Review of the Safety of Immune Checkpoint Inhibitors Among Kidney Transplant Patients. Kidney Int Rep 5:149–158, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.d’Izarny-Gargas T, Durrbach A, Zaidan M: Efficacy and tolerance of immune checkpoint inhibitors in transplant patients with cancer: A systematic review. Am J Transplant , 2020 [DOI] [PubMed]
- 10.Barnett R, Barta VS, Jhaveri KD: Preserved Renal-Allograft Function and the PD-1 Pathway Inhibitor Nivolumab. N Engl J Med 376:191–192, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Seethapathy H, Zhao S, Chute DF, et al. : The Incidence, Causes, and Risk Factors of Acute Kidney Injury in Patients Receiving Immune Checkpoint Inhibitors. Clin J Am Soc Nephrol 14:1692–1700, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortazar FB, Kibbelaar ZA, Glezerman IG, et al. : Clinical Features and Outcomes of Immune Checkpoint Inhibitor-Associated AKI: A Multicenter Study. J Am Soc Nephrol 31:435–446, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fairfax BP, Taylor CA, Watson RA, et al. : Peripheral CD8+ T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med 26:193–199, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Euvrard S, Morelon E, Rostaing L, et al. : Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 367:329–339, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Dantal J, Morelon E, Rostaing L, et al. : Sirolimus for Secondary Prevention of Skin Cancer in Kidney Transplant Recipients: 5-Year Results. J Clin Oncol 36:2612–2620, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Esfahani K, Al-Aubodah T-A, Thebault P, et al. : Targeting the mTOR pathway uncouples the efficacy and toxicity of PD-1 blockade in renal transplantation. Nat Commun 10:4712, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danesh MJ, Mulvaney PM, Murakami N, et al. : Impact of corticosteroids on allograft protection in renal transplant patients receiving anti-PD-1 immunotherapy. Cancer Immunol Immunother , 2020 [DOI] [PMC free article] [PubMed]
- 18.Ito T, Ueno T, Clarkson MR, et al. : Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol 174:6648–6656, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Yang J, Popoola J, Khandwala S, et al. : Critical Role of Donor Tissue Expression of Programmed Death Ligand-1 in Regulating Cardiac Allograft Rejection and Vasculopathy. Circulation 117:660–669, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Riella LV, Watanabe T, Sage PT, et al. : Essential role of PDL1 expression on nonhematopoietic donor cells in acquired tolerance to vascularized cardiac allografts. Am J Transplant 11:832–840, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Seethapathy H, Zhao S, Strohbehn IA, et al. : Incidence and Clinical Features of Immune-Related Acute Kidney Injury in Patients Receiving Programmed Cell Death Ligand-1 Inhibitors. Kidney International Reports 5:1700–1705, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arbour KC, Mezquita L, Long N, et al. : Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol 36:2872–2878, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Ricciuti B, Dahlberg SE, Adeni A, et al. : Immune Checkpoint Inhibitor Outcomes for Patients With Non-Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J Clin Oncol 37:1927–1934, 2019 [DOI] [PubMed] [Google Scholar]
- 24.Vermorken JB, Mesia R, Rivera F, et al. : Platinum-Based Chemotherapy plus Cetuximab in Head and Neck Cancer. New England Journal of Medicine 359:1116–1127, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Lanz J, Bavinck JNB, Westhuis M, et al. : Aggressive Squamous Cell Carcinoma in Organ Transplant Recipients. JAMA Dermatol 155:66–71, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.South AP, Purdie KJ, Watt SA, et al. : NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J Invest Dermatol 134:2630–2638, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodman AM, Kato S, Bazhenova L, et al. : Tumor Mutational Burden as an Independent Predictor of Response to Immunotherapy in Diverse Cancers. Mol Cancer Ther 16:2598–2608, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migden MR, Rischin D, Schmults CD, et al. : PD-1 Blockade with Cemiplimab in Advanced Cutaneous Squamous-Cell Carcinoma. N Engl J Med 379:341–351, 2018 [DOI] [PubMed] [Google Scholar]
- 29.FDA approves pembrolizumab for cutaneous squamous cell carcinoma [Internet]. FDA , 2020[cited 2020 Jul 5] Available from: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-cutaneous-squamous-cell-carcinoma
- 30.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 381:1535–1546, 2019 [DOI] [PubMed] [Google Scholar]
- 31.Vajdic CM, Chong AH, Kelly PJ, et al. : Survival After Cutaneous Melanoma in Kidney Transplant Recipients: A Population-Based Matched Cohort Study. American Journal of Transplantation 14:1368–1375, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Robert C, Schachter J, Long GV, et al. : Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372:2521–2532, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. : Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 377:1345–1356, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nankivell BJ, Alexander SI: Rejection of the Kidney Allograft. New England Journal of Medicine 363:1451–1462, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Kaplan EL, Meier P: Nonparametric Estimation from Incomplete Observations. Journal of the American Statistical Association 53:457–481, 1958 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


