Abstract
Early anatomical evidence suggested that the paraventricular nucleus of the thalamus (PVT) regulates arousal, as well as emotional and motivated behaviors. Here, we discuss recent studies employing modern techniques which now confirm and expand the involvement of the rodent PVT in these functions. Despite the emerging notion that the PVT is implicated in various behavioral processes, a recurrent theme here is that activity in this brain region depends on internal state information arriving from the hypothalamus and brainstem, and is influenced by prior experience. We propose that PVT’s primary function is to detect homeostatic challenges by integrating information about prior experiences, competing needs and internal state to guide adaptive behavioral responses aimed at restoring homeostasis.
Keywords: Paraventricular thalamus, internal state, valence, arousal, goal-directed behavior, homeostasis
TOWARD A NEW CONCEPTUALIZATION OF THE PVT
The rodent PVT, along with the rest of the midline and intralaminar thalamus, consists primarily of excitatory neurons thought to relay information across various cortical and subcortical regions [1,2]. However, classical anatomical studies show that, unlike neighboring thalamic structures, the PVT extends over the entire rostro-caudal length of the midline thalamus and displays unique efferent and afferent connectivity patterns with the cortex, basal forebrain, amygdala, ventral striatum, hippocampus, hypothalamus, and brainstem [3–5]. Moreover, reports of important differences in downstream connectivity between the anterior and posterior PVT (aPVT, pPVT) hint at the existence of functional segregation across the antero-posterior axis of the PVT [2, 3]. But prior technical limitations barred researchers from directly interrogating such hypotheses and gaining fundamental insights into the role of the PVT in behavior.
The rise of new technological advances has opened the door to refined mechanistic inquiries of PVT function. Recent studies using advanced approaches to monitor neuronal activity (e.g. fiber photometry and two-photon microscopy) and manipulate it (e.g. optogenetics and chemogenetics) have provided causal evidence linking the PVT to representations of valence, salience, arousal, internal state, and the regulation of emotional and motivated behaviors [6, 7]. While a holistic view of PVT function is currently lacking, these new findings place the PVT at the crossroads where interoceptive and exteroceptive signals are integrated with experience to orchestrate emotional and motivated behaviors. Here, we overview the recent literature and present an updated view of the rodent PVT and its role in behavior. Specifically, we discuss recent evidence highlighting the processing of emotional signals and internal state in the PVT (i.e. valence and arousal), and the emergence of behavioral correlates of PVT function. Most of our discussion is centered on the pPVT, since the majority of modern studies of PVT function have focused on this region. We propose that the PVT is activated by homeostatic challenges (aversive signal) and biases the selection of adaptive behaviors in order to restore homeostasis (Figure 1). Central to this discussion is the idea that experience- and state-dependent modulation of PVT circuits is critical to this process.
Figure 1. Conceptual model of the PVT’s role in homeostatic regulation.
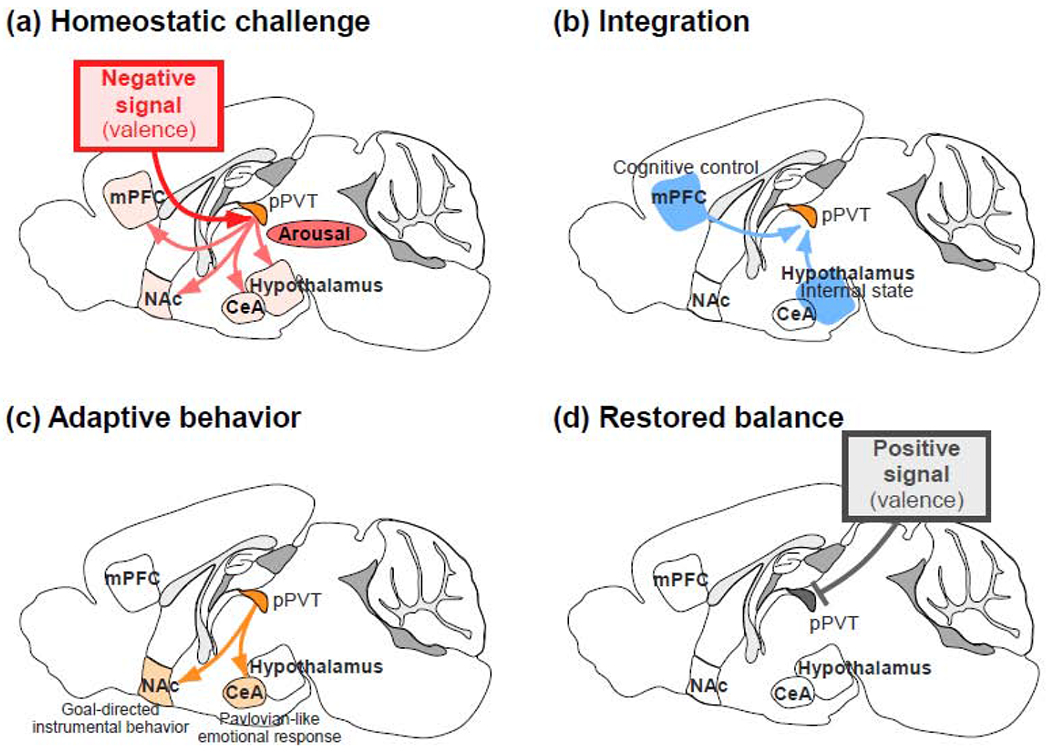
(a) Homeostatic challenges represent negative (aversive) signals that activate the pPVT, leading to the induction of aroused states via distributed downstream projections. (b) This negative signal is integrated with cognitive signals from the mPFC as well as with internal state information propagated from diverse hypothalamic and brainstem sources. (c) Following integration, the pPVT participates in the coordination of adaptive behavioral responses largely via the NAc and the CeA. (d) Reinstatement of homeostasis is associated with a positive (rewarding) signal that inhibits the pPVT. Although these four processes are depicted sequentially, they are instead likely occurring as sets of parallel processes in adaptation to an ever-changing environment.
EMOTIONAL SIGNAL PROCESSING IN THE PVT
Recent proposals suggest that emotions, such as fear or anger, can be defined as primitive central states exhibiting generalizable properties shared by different species [8]. Some of these primitives are centered on classic theories that describe emotions as a composition of two scalars: arousal (or the intensity or salience of environmental stimuli) and valence (or the hedonic value of an object or event) [9]. Based on this conceptualization, some studies on the neural basis of emotion have focused on the contribution of discrete brain regions within cortico- and mesolimbic pathways which are known to contain representations of one or both of these scalars. These include the amygdala, medial prefrontal cortex (mPFC), ventral striatum and midbrain [10]. Recently, however, this conceptual framework has been expanded to include the midline thalamus, in particular the PVT. Previously, the PVT was considered mostly as a node of the ascending arousal activating system [11], and was excluded from traditional diagrams of the emotional processing network. In this section, we first summarize evidence supporting a role for the PVT in signaling arousal states, and then discuss more recent findings demonstrating the existence of valence representations in the PVT. The following section will extend these discussions to the emerging roles of the PVT in emotional and motivated behaviors.
PVT activity is modulated by and signals arousal states
The PVT receives dense innervation from the pontine reticular formation as well as from orexinergic fibers of the hypothalamus [2, 12]. Research on the arousal-promoting nature of these pathways led to the notion that the PVT belongs to a thalamocortical arousal system. While for decades, evidence for this hypothesis was equivocal, recent studies offer strong support to this idea. First, a study centered on the dorsal medial thalamus identified calretinin-expressing (CR+) neurons within this region (mainly in the PVT) as part of a diencephalic node that controls forebrain arousal [13]. The authors demonstrated that the firing rate of CR+ neurons increases in proportion with arousal state, and that optogenetic stimulation of these neurons promotes arousal. A separate study published around the same time found that silencing PVT neurons decreases wakefulness and promotes sleep, whereas stimulation of these neurons induces wakefulness from both sleep and general anesthesia [14]. Notably, the authors linked orexinergic modulation from the hypothalamus as critical to PVT-mediated arousal [14]. Collectively, these studies offered the first evidence that neuronal activity within the PVT is causally linked to the control of arousal states. This conclusion was recently confirmed and extended by an independent study showing that dopaminergic signaling may be important to this process [15].
Various fundamental questions arise from these studies that deserve further investigation. For instance, is signaling arousal the primary function of the PVT? If not, in what circumstances is the PVT recruited to exert control over arousal? Although a systematic evaluation of these questions is currently lacking, a potential explanation to PVT’s involvement in arousal signaling may lie in the widely accepted notion that the PVT displays unique sensitivity to aversive stimuli (as we will discuss in detail below) and participates in the regulation of motivated behaviors. Within this context, the PVT may promote alertness in situations that require goal-oriented responses. This model finds some support in a recently published study that linked the activity of CR+ neurons of the PVT to starvation-induced arousal in mice [16]. Using an ethologically relevant model of need-based arousal – starvation – the authors identified CR+ projections from the PVT to the bed nucleus of the stria terminalis (BNST) as drivers of arousal in response to an apparent motivational conflict (sleep vs feeding) [16]. Collectively, these findings support the idea that the PVT’s role in arousal may be inherently linked to negative emotional states and the subsequent cognitive and motivational processes that promote homeostasis. Consistent with this view, infusion of the arousal-promoting neuropeptide orexin into the PVT, is associated with the emergence of negative emotional states [17].
It is important to note that despite the general consensus on PVT’s participation in the control of arousal, each of the described studies [13, 14, 16] implicated (for the most part) a different projection of the PVT in this process (i.e. the cortex, striatum and amygdala, respectively). These divergent conclusions regarding the downstream effectors that promote arousal may be reconciled by the profound collateralization of PVT neurons [13]. This anatomical feature could lead to the recruitment of a widely distributed neural network during aroused states, thereby coordinating cognitive, emotional and motivational processes [13]. Furthermore, while in both study [13] and [16] the activity of a majority of PVT neurons was observed to be positively correlated with arousal, the studies also reported that a large proportion of PVT neurons appear to be negatively modulated by arousal. These observations raise the possibility that while a defined class of PVT neurons drives arousal, another PVT subpopulation may antagonize this process. Indeed, a recent report demonstrated that the PVT contains a previously overlooked class of neurons that is negatively modulated by arousal and antagonizes arousal via projections to the infralimbic cortex [18]. Jointly, the studies described here support the idea that the activity of defined neuronal subpopulations of the PVT influence arousal bidirectionally and through segregated projections. Interestingly, one of these studies further showed that widespread inhibition of PVT glutamatergic neurons leads to the fragmentation of wakefulness [14]. The arguments presented above suggest that this observation may result from the modulation of distinct PVT pathways that compete over the control of wakefulness. However, future studies should aim at more systematic interrogations of the dual control of arousal exerted by divergent neuronal circuits of the PVT.
Representations of valence in the PVT
Together with recent demonstrations that the PVT is an important site for the processing of arousal, new findings have also supported the idea that the PVT contains representations of valence, with a large proportion of PVT neurons (mostly in the pPVT) selectively tuned to aversive stimuli [18–21]. Studies using in vivo calcium imaging and single-unit recordings demonstrate that aversive stimuli such as mild electrical shock, tail suspension, and air puff, are associated with acute increases in neuronal activity in the pPVT [18, 19, 22]. These observations complement early studies showing robust activation of the PVT in response to various multimodal physical and psychosocial stressors [23, 24]. They also coincide with the documented strong innervation of the PVT by nociceptive and stress-processing regions of the brainstem such as the periaqueductal gray (PAG), parabrachial nucleus (PBN), and locus coeruleus [2, 4, 19, 25]. Long lasting effects of aversive experience in the neuronal circuits of the PVT have also been reported [19–21]. For example, fear conditioning has been shown to drive persistent changes in the spontaneous firing rate of PVT neurons in rats [20]. Similarly, acute stress is associated with lasting decreases in synaptic inhibition onto D2R+ neurons of the pPVT [19]. Interestingly, pharmacological activation of D2R+ PVT neurons in mice can facilitate emergence from anesthesia [15]. This supports that the dopaminergic disinhibition of PVT neurons described in [19] contributes to both valence (aversion) and arousal. In summary, the studies discussed here demonstrate that negative experiences can drive both acute and long-term effects in the circuit activity of the PVT.
Given that the PVT is integrated into circuitry known to participate in the processing of rewarding and appetitive information, additional studies have focused on the involvement of PVT neurons in processing positive valence [26]. Using both bulk and single-cell calcium imaging approaches, studies revealed that PVT neurons are largely suppressed by both appetitive and non-appetitive rewarding experiences [18, 27, 28]. First, bulk calcium imaging using fiber photometry demonstrated that two major classes of PVT neurons are inhibited when mice transition from least-preferred temperatures to thermoneutral ones, as well as during social encounters [18, 29, 30]. Next, a two-photon calcium imaging study showed that individual PVT neurons that project to the nucleus accumbens (NAc) – a structure involved in coordinating reward-seeking and motivated behaviors – are inhibited by sucrose reward [28]. Consistent with this observation, PVT–NAc axon terminals display inhibitory responses to reward consumption [31, 32]. Collectively, these studies indicate that PVT neurons (including those that project to the NAc) are inhibited by rewarding stimuli. These inhibitory responses are distinct from neuronal activation seen in reward anticipatory stimuli [33], which are addressed later in this review. The observation that rewards are mostly associated with suppressing PVT neuronal activity further supports the notion that the PVT’s role in promoting arousal is primarily linked to the signaling of aversive states.
PVT neurons, in addition to their modulation by unconditioned aversive and rewarding stimuli, can acquire conditioned responses to cues previously associated with positive and negative outcomes [20, 21, 27, 28, 34]. Consistent with widespread valence representations in the pPVT, cues that are associated with aversive outcomes typically excite PVT neurons, whereas those associated with appetitive ones inhibit them [21, 34]. Moreover, such neuronal responses to predictive cues are sensitive to internal state changes [22]. This latter observation is consistent with the proposal discussed in more detail later in this review that PVT circuits are closely related to homeostatic regulation.
Finally, although most recent studies are consistent with the idea that the PVT (particularly the pPVT) is broadly tuned to aversive stimuli, a recent study using fiber photometry reported that the PVT is strongly activated by salient stimuli, regardless of their valence [22]. One potential explanation for the discrepancy between this study and those emphasizing PVT’s tuning to aversive stimuli (specifically) lies in the known limitations of the fiber photometry technique [35]. In this instance, the interpretation of salience encoding in the PVT was drawn at the population level and may not necessarily reflect a bona fide feature of individual PVT neurons. Growing insights into the activity of individual PVT neurons indicate that valence responses of PVT neurons are (in some cases) more heterogeneous that previously appreciated. This highlights the potential impacts that single cell imaging ang sequencing techniques may provide to broaden our classification of PVT neuronal subtypes [6].
PVT’S ROLE IN EMOTIONAL AND MOTIVATED BEHAVIORS
The PVT controls fear and avoidance behaviors
Behavioral correlates of PVT function have often been studied in the context of fear and defensive behaviors, motivated by the PVT’s distinct connectivity with brain structures implicated in fear processing—namely areas of the extended amygdala, mPFC and brainstem [3, 4, 36]. The PVT sends heavy afferents to the BNST, the central nucleus (CeA) and the basolateral amygdala (BLA)—structures recognized as major players in driving associative fear learning and fear expression [37, 38]. The PVT is also reciprocally connected with the prelimbic and infralimbic cortices, which are integral to fear retrieval and fear extinction [39], respectively, and receives visceral and nociceptive input from the PBN and PAG [4, 25, 40, 41]. Fear, it should be mentioned, is sometimes defined in relation to its conscious subjective experience. For the purpose of this review, we define fear as a central emotional state that is associated with the emergence of defensive behavior (including freezing) [8, 42].
Among brain regions innervated by the PVT, the CeA receives some of the densest innervations [43], and recent studies using optogenetic and chemogenetic tools have linked PVT–CeA connectivity to CeA-dependent processes such as the formation and retrieval of fear memory in both rats and mice [20, 21,44, 45]. In one of these studies, following auditory fear conditioning, pPVT neurons were shown to acquire auditory cue-evoked responses [20]. However, PVT’s role in fear regulation appears to be complex. First, the PVT is recruited in a time-dependent manner, whereby inactivation of the PVT impairs fear retrieval at late (>24 hr), but not early (6 hr) time points [20, 21]. Second, inactivation of PVT–CeA projections impairs fear memory retrieval and this effect persists a day later [20]. While a major conclusion from this finding was that the PVT regulates the maintenance of fear memory (e.g. reconsolidation), considering that freezing behavior was the sole metric supporting this assessment, questions remain on whether the PVT impacts fear memory maintenance as suggested. Time-dependent recruitment of the PVT is also supported by initial lesions studies showing that an intact PVT is required for neuroendocrine responses to chronic (persistent) but not acute stress [46, 47]. These findings, alongside subsequent studies highlighting a role for the pPVT in stress habituation and facilitation, suggest that the PVT may further serve to store information about aversive experiences [48].
Consistent with the forementioned conclusion, and as previously discussed, negative events are associated with the emergence of synaptic plasticity and changes in baseline firing rate in PVT neurons [19, 20]. Thus, it is plausible that these experience-dependent changes serve as “memory traces” that underlie the PVT’s influence over adaptive behavior in response to related and/or distinct future aversive episodes. However, the idea that memory-related information is stored locally in the PVT has not been tested empirically. Alternatively, the PVT may integrate information about learning and decision making from the prefrontal cortex [28, 49, 50]. Nonetheless, current findings indicate that the PVT regulates some aspects of fear memory. Future studies should aim at investigating the precise contribution of the PVT to fear-related behaviors, and the specific features of fear memory to which the PVT contributes.
The PVT drives reward seeking
While the PVT is not traditionally considered as a part of the classic mesocorticolimbic reward circuitry, it displays efferent and afferent connectivity with structures directly implicated in reward processing. In particular, the PVT densely innervates the NAc and has been shown to modulate dopaminergic signaling within this region [2, 51]. These findings indicate that the PVT may participate in regulating motivated behaviors, including reward seeking. Consistent with this proposition, pharmacological, optogenetic and chemogenetic manipulations of the PVT have been documented to impact food seeking behaviors [26–28, 33, 52–58], and fiber photometry imaging of NAc-projecting pPVT neurons demonstrate that reward seeking in a foraging task activates this pathway [32]. These findings are in agreement with previous reports showing that, unlike food rewards – which inhibit pPVT neurons–, hunger signals and reward anticipation strongly activate PVT neurons [16, 33, 59].
Importantly, metabolic information arising from hypothalamic and brainstem regions have been implicated as major modulators of PVT–NAc connectivity and subsequent food seeking related behaviors [32, 57, 58, 60–63], One study demonstrated that the PVT contains a subset of NAc-projecting neurons that is equipped with adaptations that allow for direct monitoring of glucose availability and the promotion of food seeking behavior [55]. Mounting evidence suggests that the PVT is activated by hunger signals (negative value) and subsequently drives food seeking behaviors aimed at mitigating hunger’s effects via PVT–NAc communication. Notably, a few studies centered in the aPVT have found that photoinhibition of aPVT–NAc projections promotes food seeking behavior [27, 28, 64]. These findings are consistent with the idea that the neuronal circuits of the PVT are diverse and may be organized in a way that promotes flexible and bidirectional control over behavior.
Along with its role in regulating the pursuit of natural rewards, the PVT has been documented to become activated in response to psychoactive drugs including cocaine and amphetamines [65–67]. Interestingly, these effects seem to depend on the function of D2R-like receptors in the PVT [65], which as described above, have been linked to the emergence of aversive states [19]. A recent study demonstrated that the PVT–NAc pathway is causally linked to opiate dependence [68]. Importantly, this study demonstrated that PVT neurons are not activated by drug reward per se, but instead are sensitive to and mediate the negative effects of drug withdrawal. Here, optogenetic silencing of PVT–NAc projections significantly decreased withdrawal symptoms and drug seeking behavior [68]. Another study found that cocaine withdrawal increased the probability of neurotransmitter release at PVT–NAc synapses [69]. These results are consistent with the idea that the PVT is recruited in response to aversive events. From this perspective, PVT’s involvement in drug seeking behavior can be interpreted as a means by which the negative effects of drug withdrawal are prevented (but see [70]). This notion is supported by work showing that orexin infusion in the PVT—which can cause anxiety and stress phenotypes—leads to cocaine-seeking behavior [17, 71, 72]. Furthermore, the PVT is co-targeted by the dynorphin/kappa-opioid receptor system, whose activation broadly mediates pro-addictive behavior through dysphoria, and aversive states [73–75]. Overall, while current studies provide particular support for a role of the PVT in the relapse phase of addiction (see review [26]), other studies have identified PVT’s involvement in the acquisition, reinstatement, and withdrawal phases of drug addiction [53, 68, 76, 77], Future studies should address changes in the PVT’s contribution to the progression of the phases of drug addiction as a continuum.
To summarize, we believe that the PVT’s recruitment by aversive events is a recurrent theme critical to the emergence of goal-directed behavior and subsequent reinstatement of homeostasis. Moreover, we propose that the PVT’s driving of goal-directed behavior following homeostatic challenges is achieved through negative reinforcement. As such, similar to its role in drug-seeking, PVT’s involvement in driving food seeking behavior in animals with restricted access to food or in response to a metabolic challenge may serve to prevent the aversive effects of hunger. In support of this model, in a recent study from our group interrogating circuitry mediating food seeking behavior induced by deficits in glucose availability, stimulating a projection from the brainstem drove increases in activity of PVT neurons and mimicked the aversive state of hypoglycemia in mice. Interestingly, subsequent feeding led to decreases in PVT activity [32]. Conversely, a separate study found that sustained decreased activity of the PVT driven by chronic stimulation of zona incerta inhibitory inputs led to dysregulatory weight gain and overeating [78]. These studies point toward the existence of an optimal baseline point for PVT neuronal activity, which is an important feature of homeostatic signaling [79].
THE PVT INTEGRATES EMOTIONAL AND HOMEOSTATIC SIGNALS TO GUIDE FLEXIBLE AND ADAPTIVE BEHAVIOR
Homeostasis is a dynamic process directed at achieving internal stability in response to environmental challenges. Attaining this goal dictates a system that can integrate past experiences (learning) with internally and externally generated signals to guide flexible and adaptive behavior. While substantial literature places the hypothalamus as a central region for homeostatic sensation and the regulation of energy metabolism [80, 81] we propose that the PVT is a key mediator of this integrative process. Our conclusions are bolstered by PVT’s is strong innervation from hypothalamic and brainstem nuclei known to participate in homeostatic control. In support of this view, we next discuss recent evidence suggesting that the PVT, alongside being sensitive to experience and environmental stimuli, is a site of prominent state-dependent modulation.
PVT’s role in behavior is influenced by internal state
The PVT is a site of convergence for visceroceptive and metabolic inputs from the brainstem and various hypothalamic nuclei (Figure 2), which relay energy needs [2, 30, 82]. Consistent with this anatomical connectivity, recent studies have shown that PVT neuronal responses to reward-predicting cues can be modulated by metabolic states such as thirst and hunger [16, 22]. While water and food restriction potentiate neuronal responses to reward-predictive cues, these responses are significantly attenuated in sated mice [22, 58]. This state-dependent modulation of PVT neuronal activity can be attributed, at least in part, to known neuromodulatory pathways involved in homeostatic regulation. Specifically, whereas intra-PVT blockade of orexin-2 receptors attenuated reward seeking behavior in hungry rats, infusion of orexin-A increased seeking behavior in sated subjects [58]. These findings are consistent with previous observations that the PVT is heavily innervated by orexinergic fibers from the lateral hypothalamus [12]. PVT neurons are also equipped with important adaptations for monitoring metabolic state, such as glucose levels, and promoting goal-directed behavior [55].
Figure 2. Schematic representation of major hypothalamic (pink), midbrain (blue), and hindbrain (purple) afferents to the rodent PVT (orange).
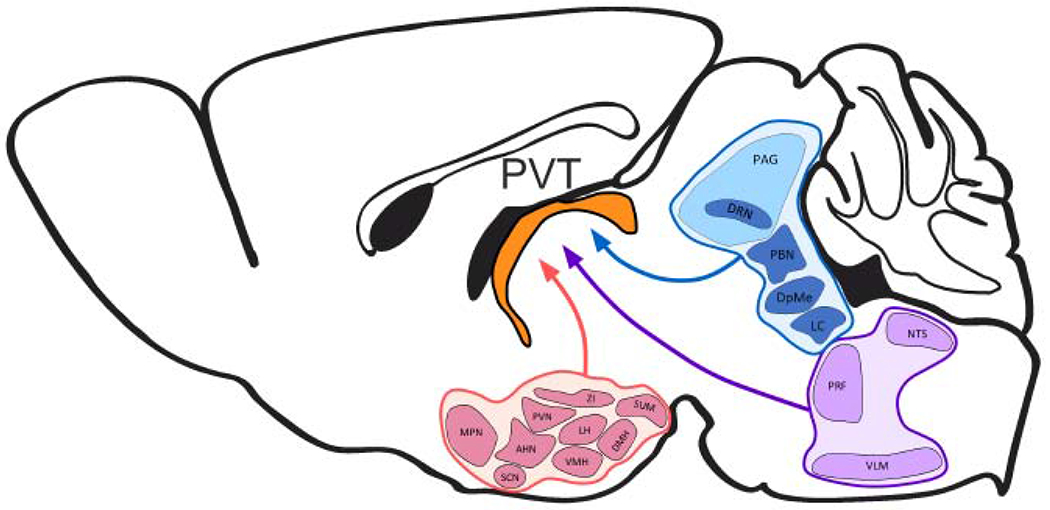
AHN, anterior hypothalamic nucleus; DMH, dorsomedial hypothalamus; DpMe, deep mesencephalic nucleus; DRN, dorsal raphe nucleus; LC, locus coeruleus; LH, lateral hypothalamus; MPN, medial preoptic nucleus; NTS, nucleus of the solitary tract; PAG, periaqueductal grey; PBN, parabrachial nucleus; PRF, pontine reticular formation; PVN, paraventricular hypothalamus; PVT, paraventricular thalamus; SCN, suprachiasmatic nucleus; SUM, supramammillary nucleus; VMH, ventromedial hypothalamus; VLM, ventrolateral medulla; ZI, zona incerta.
While the PVT’s neuronal encoding of reward-predictive cues can be potentiated by hunger signals, a recent study showed that these responses are attenuated when mice are exposed to an aversive context [22]. This report suggests that motivational conflicts can bias the responses of PVT neurons and thereby impact motivated behaviors. Similar emotional state-dependent modulation of neuronal activity has been observed in PVT-projecting neurons of the mPFC, indicating that corticothalamic neurons may contribute to state-dependent processes converging in the PVT [34, 49, 50]. Additional evidence of state dependent modulation of PVT neuronal activity and behavioral outcomes comes from a recent report demonstrating that acute stress promotes lasting disinhibition of PVT neurons via dopaminergic actions of the locus coeruleus (LC). Notably, this LC-mediated disinhibition of PVT neurons can facilitate the formation of aversive memories [19]. This finding suggests that stressed states can sensitize the PVT to aversive outcomes.
Collectively, the studies discussed here indicate that the PVT is a site of marked state-dependent modulation of neuronal activity that impact its influence over behavior. Considering that neuromodulatory systems including orexin and dopamine have been implicated in these processes, and that the PVT is densely innervated by catecholaminergic and peptidergic pathways, future studies should aim at identifying the cellular and molecular mechanisms by which neuromodulatory systems influence the neuronal circuits of the PVT and their control over behavior [1, 2, 83–85].
In the first part of this review, we summarized recent observations that highlight the representation of emotion scalars by the neuronal circuits of the PVT. Subsequently, we discussed evidence demonstrating that the PVT guides behavioral responses to both reward and aversive associations. Lastly, in the present section, we have pointed at evidence establishing a causal link between contextual and state-dependent modulation of PVT circuits, and flexible switches in motivated behavior. Together, these lines of evidence indicate that the PVT integrates information about learned associations and the environment with internally generated signals to guide behavioral outcomes. This is perhaps better illustrated in situations of motivational conflict whereby the PVT appears to arbitrate between behavioral decisions. Indeed, when mice are simultaneously presented with cues indicating both aversive and appetitive contexts, intact PVT signaling is necessary for proper execution of approach or avoidance behaviors [86, 87]. This arbitration amid motivational conflicts highlights an important feature of homeostatic systems: the balancing of internal state with environmental challenges.
The PVT orchestrates homeostatic behavioral responses through the NAc
The anatomical distribution of the efferent projections of the PVT suggests that the NAc is a major route by which the PVT guides behavior [2]. Indeed, approximately 80% of all PVT neurons innervate the NAc [88]. Here, we propose that in response to homeostatic challenges the PVT coordinates goal-directed behaviors via its projections to the NAc (Figure 1). Within this conceptual framework, innervation of PVT–NAc neurons by the hypothalamus is critical to this process. The PVT was previously hypothesized to be a central node linking hunger-related signals arising in the hypothalamus with food seeking behavior (via the NAc) [58, 62]. However, we believe that hypothalamic innervation of NAc-projecting PVT neurons is a general mechanism linking homeostatic challenges to instrumental behaviors. For example, the PVT is likely a node linking cold temperature challenges with nest building behavior [30]. Similarly, oxytocin receptors in the PVT appear to underlie maternal behaviors such as crouching over pups [89]. Importantly, oxytocin is produced by the hypothalamus and maternal behaviors have been shown to activate PVT neurons and depend on NAc function [90–92]. While evidence for PVT’s involvement in these behaviors is limited, our model is supported by broad innervation of the PVT by preoptic regions previously linked to thermoregulatory and parental behaviors [30, 93]. Additionally, despite the goal-directed nature of these responses, the aforementioned hypothalamic nuclei do not project directly to the NAc [94]. Based on this premise, we propose that the PVT is a target region by which the hypothalamus translates homeostatic signals into instrumental behaviors aimed at restoring balance. Furthermore, the PVT may leverage the balancing of competing needs by integrating information from a distributed hypothalamic network.
It is important to note that NAc-projecting PVT neurons appear to collateralize to other subcortical and cortical regions [13, 88]. Therefore, despite the NAc being its primary target, the PVT appears to influence behavior through parallel and divergent streams. For instance, the PVT may influence cognitive processes primarily via its interaction with the mPFC, and emotional responses via its projection to the amygdala [20, 21, 28, 45, 49, 95–97]. To date, however, a systematic characterization of how PVT’s distinct contribution to behavioral processes map onto discrete outputs is lacking.
An emerging concept is that the PVT may be a previously overlooked modulator of the mesostriatal pathway. Evidence from electron microscopy and microdialysis studies indicate that the PVT controls the extracellular concentration of dopamine in the NAc [51]. A separate recent study showed that the activity of PVT–NAc neurons ramps up as mice approach an anticipated reward [32], thereby resembling the approach-related dynamics in dopamine concentration in the NAc [98]. In addition to understanding what type of behaviors are regulated by the PVT and the underlying anatomical projections involved in these processes, an equally relevant task is to identify the precise cellular and circuit mechanisms by which the PVT influences motivated behavior.
CONCLUDING REMARKS AND FUTURE DIRECTIONS
In conclusion, we propose that the PVT acts as a link between circuits of homeostatic control (hypothalamus and brainstem) and those driving emotional and instrumental behaviors (amygdala and striatum). As such, the PVT may function as an integrative hub that answers to homeostatic challenges by promoting flexible adaptive behaviors (Figure 1). Moreover, while initial evidence suggests that the PVT appears to promote reward and aversive responses via segregated projections to the NAc and the CeA respectively, we hypothesize that such differential roles are likely a reflection of a divergence between circuits of instrumental and Pavlovian behavioral control and not necessarily tied to valence (see Outstanding Questions). While empirical support of the proposed model is yet to be collected, important indications from existing literature bolsters this conceptualization. For example, the NAc has been linked to instrumental responses of opposing valence (e.g. reward seeking and the avoidance of punishment), while the CeA has been associated with Pavlovian-related responses to both appetitive and aversive stimuli [99–101]. Future studies should explore the role of PVT–NAc communication in instrumental responses to negative stimuli such as avoidance, and the role of PVT–CeA communication in Pavlovian-like appetitive reactions such as incentive salience. We envision that future investigations may revise the view of the PVT as embedded in segregated pathways for reward and aversion and replace it with a conceptualization of two pathways controlling instrumental and Pavlovian responses.
OUTSTANDING QUESTIONS.
PVT projections to the NAc and the CeA are often thought to represent pathways by which the midline thalamic structure regulates positively- and negatively-valenced behaviors, respectively. Is the segregation of PVT–NAc and PVT–CeA pathways a reflection of a general role for the PVT in controlling instrumental and Pavlovian behaviors?
Evidence from in vivo electrophysiological and calcium imaging studies indicate that the neuronal circuits of the PVT are heterogenous. How do functionally distinct neuronal circuits interact locally within the PVT to control behavior?
The PVT is a site of profound convergence of neuromodulatory systems. How do these systems convey information about internal states to the neuronal ensembles of the PVT?
Studies of PVT function indicate that the PVT plays a general role in driving goal-directed behavior in response to challenge. Is this achieved through negative reinforcement?
How is information integrated in the PVT to promote arbitration in situations of conflict?
HIGHLIGHTS.
Emerging evidence indicates that the PVT plays a previously overlooked but fundamental role in the control of emotional and motivated behaviors. Heterogeneity within the PVT is thought to underlie diverse functions in behavioral control.
We propose that the PVT is an integrative node where information about prior experiences converges with interoceptive and exteroceptive signals to guide the selection of adaptive behavioral responses that promote homeostasis.
We argue that PVT’s role in homeostatic control is largely influenced by hypothalamic and hindbrain inputs that convey information about internal state.
We further argue that while PVT projections to the NAc and the CeA may appear to reflect a de facto valenced organization (e.g. reward and fear, respectively), these segregated circuits likely provide a means by which the PVT can influence both goal-directed and Pavlovian-related behavioral responses to homeostatic challenges.
PVT’s role in driving goal-directed behavior may be particularly motivated by negative reinforcement.
ACKNOWLEDGEMENTS
This work was supported by the NIMH Intramural Research Program (1ZIAMH002950, to M.A.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests to this work.
REFERENCES
- 1.Kolaj M, et al. , Intrinsic properties and neuropharmacology of midline paraventricular thalamic nucleus neurons. Front Behav Neurosci, 2014. 8: p. 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kirouac GJ, Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev, 2015. 56: p. 315–29. [DOI] [PubMed] [Google Scholar]
- 3.Moga MM, Weis RP, and Moore RY, Efferent projections of the paraventricular thalamic nucleus in the rat. J Comp Neurol, 1995. 359(2): p. 221–38. [DOI] [PubMed] [Google Scholar]
- 4.Li S and Kirouac GJ, Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct, 2012. 217(2): p. 257–73. [DOI] [PubMed] [Google Scholar]
- 5.Vertes RP and Hoover WB, Projections of the paraventricular and paratenial nuclei of the dorsal midline thalamus in the rat . J Comp Neurol, 2008. 508(2): p. 212–37. [DOI] [PubMed] [Google Scholar]
- 6.McGinty JF and Otis JM, Heterogeneity in the Paraventricular Thalamus: The Traffic Light of Motivated Behaviors. Frontiers in Behavioral Neuroscience, 2020. 14(181). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barson JR, Mack NR, and Gao W-J, The Paraventricular Nucleus of the Thalamus Is an Important Node in the Emotional Processing Network. Frontiers in Behavioral Neuroscience, 2020. 14(191). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson DJ and Adolphs R, A framework for studying emotions across species. Cell, 2014. 157(1): p. 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schachter S and Singer J, Cognitive, social, and physiological determinants of emotional state. Psychological Review, 1962. 69(5): p. 379–399. [DOI] [PubMed] [Google Scholar]
- 10.LeDoux JE, Emotion Circuits in the Brain. Annual Review of Neuroscience, 2000. 23(1): p. 155–184. [DOI] [PubMed] [Google Scholar]
- 11.Van der Werf YD, Witter MP, and Groenewegen HJ, The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev, 2002. 39(2-3): p. 107–40. [DOI] [PubMed] [Google Scholar]
- 12.Kirouac GJ, Parsons MP, and Li S, Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res, 2005. 1059(2): p. 179–88. [DOI] [PubMed] [Google Scholar]
- 13.Mátyás F, et al. , A highly collateralized thalamic cell type with arousal-predicting activity serves as a key hub for graded state transitions in the forebrain. Nat Neurosci, 2018. 21(11): p. 1551–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ren S, et al. , The paraventricular thalamus is a critical thalamic area for wakefulness. Science, 2018. 362(6413): p. 429–434. [DOI] [PubMed] [Google Scholar]
- 15.Ao Y, et al. , Application of quinpirole in the paraventricular thalamus facilitates emergence from isoflurane anesthesia in mice. Brain and Behavior, 2020. n/a(n/a): p. e01903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua R, et al. , Calretinin Neurons in the Midline Thalamus Modulate Starvation-Induced Arousal. Curr Biol, 2018. 28(24): p. 3948–3959 e4. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, et al. , Orexins in the paraventricular nucleus of the thalamus mediate anxiety-like responses in rats. Psychopharmacology (Berl), 2010. 212(2): p. 251–65. [DOI] [PubMed] [Google Scholar]
- 18.Gao C, et al. , Two genetically, anatomically and functionally distinct cell types segregate across anteroposterior axis of paraventricular thalamus. Nat Neurosci, 2020. 23(2): p. 217–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beas BS, et al. , The locus coeruleus drives disinhibition in the midline thalamus via a dopaminergic mechanism. Nat Neurosci, 2018. 21(7): p. 963–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Do-Monte FH, Quinones-Laracuente K, and Quirk GJ, A temporal shift in the circuits mediating retrieval of fear memory. Nature, 2015. 519(7544): p. 460–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penzo MA, et al. , The paraventricular thalamus controls a central amygdala fear circuit. Nature, 2015. 519(7544): p. 455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, et al. , Dynamic salience processing in paraventricular thalamus gates associative learning. Science, 2018. 362(6413): p. 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullinan WE, et al. , Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience, 1995. 64(2): p. 477–505. [DOI] [PubMed] [Google Scholar]
- 24.Chastrette N, Pfaff DW, and Gibbs RB, Effects of daytime and nighttime stress on Fos-like immunoreactivity in the paraventricular nucleus of the hypothalamus, the habenula, and the posterior paraventricular nucleus of the thalamus. Brain Research, 1991. 563(1): p. 339–344. [DOI] [PubMed] [Google Scholar]
- 25.Krout KE and Loewy AD, Parabrachial nucleus projections to midline and intralaminar thalamic nuclei of the rat. Journal of Comparative Neurology, 2000. 428(3): p. 475–494. [DOI] [PubMed] [Google Scholar]
- 26.Matzeu A, Zamora-Martinez E, and Martin-Fardon R, The paraventricular nucleus of the thalamus is recruited by both natural rewards and drugs of abuse: recent evidence of a pivotal role for orexin/hypocretin signaling in this thalamic nucleus in drug-seeking behavior. Frontiers in Behavioral Neuroscience, 2014. 8(117). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Do-Monte FH, et al. , Thalamic Regulation of Sucrose Seeking during Unexpected Reward Omission. Neuron, 2017. 94(2): p. 388–400 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otis JM, et al. , Paraventricular Thalamus Projection Neurons Integrate Cortical and Hypothalamic Signals for Cue-Reward Processing. Neuron, 2019. 103(3): p. 423–431 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ago Y, et al. , The Female Encounter Test: A Novel Method for Evaluating Reward-Seeking Behavior or Motivation in Mice. Int J Neuropsychopharmacol, 2015. 18(11): p. pyv062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan CL, et al. , Warm-Sensitive Neurons that Control Body Temperature. Cell, 2016. 167(1): p. 47–59.el5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reed SJ, et al. , Coordinated Reductions in Excitatory Input to the Nucleus Accumbens Underlie Food Consumption. Neuron, 2018. 99(6): p. 1260–1273 e4. [DOI] [PubMed] [Google Scholar]
- 32.Sofia Beas B, et al. , A ventrolateral medulla-midline thalamic circuit for hypoglycemic feeding. Nature Communications, 2020. 11(1): p. 6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haight JL, et al. , A food-predictive cue attributed with incentive salience engages subcortical afferents and efferents of the paraventricular nucleus of the thalamus. Neuroscience, 2017. 340: p. 135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otis JM, et al. , Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature, 2017. 543(7643): p. 103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y, et al. , Long-term Fiber Photometry for Neuroscience Studies. Neurosci Bull, 2019. 35(3): p. 425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Do Monte FH, et al. , Retrieving fear memories, as time goes by…. Mol Psychiatry, 2016. 21(8): p. 1027–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janak PH and Tye KM, From circuits to behaviour in the amygdala. Nature, 2015. 517(7534): p. 284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duvarci S, Bauer EP, and Paré D, The bed nucleus of the stria terminalis mediates inter-individual variations in anxiety and fear. J Neurosci, 2009. 29(33): p. 10357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Quirk GJ and Mueller D, Neural Mechanisms of Extinction Learning and Retrieval. Neuropsychopharmacology, 2008. 33(1): p. 56–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S and Su HS, Afferent connections of the thalamic paraventricular and parataenial nuclei in the rat--a retrograde tracing study with iontophoretic application of Fluoro-Gold. Brain Res, 1990. 522(1): p. 1–6. [DOI] [PubMed] [Google Scholar]
- 41.Vertes RP, A PHA-L analysis of ascending projections of the dorsal raphe nucleus in the rat. J Comp Neurol, 1991. 313(4): p. 643–68. [DOI] [PubMed] [Google Scholar]
- 42.LeDoux JE, Coming to terms with fear. Proceedings of the National Academy of Sciences, 2014. 111(8): p. 2871–2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S and Kirouac GJ, Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol, 2008. 506(2): p. 263–87. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, et al. , Lesions of the posterior paraventricular nucleus of the thalamus attenuate fear expression. Frontiers in Behavioral Neuroscience, 2014. 8(94). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen M and Bi LL, Optogenetic Long-Term Depression Induction in the PVT-CeL Circuitry Mediates Decreased Fear Memory. Mol Neurobiol, 2019. 56(7): p. 4855–4865. [DOI] [PubMed] [Google Scholar]
- 46.Bhatnagar S, et al. , A cholecystokinin-mediated pathway to the paraventricular thalamus is recruited in chronically stressed rats and regulates hypothalamic-pituitary-adrenal function. J Neurosci, 2000. 20(14): p. 5564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhatnagar S, et al. , Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol, 2002. 14(5): p. 403–10. [DOI] [PubMed] [Google Scholar]
- 48.Bhatnagar S and Dallman M, Neuroanatomical basis for facilitation of hypothalamic-pituitary-adrenal responses to a novel stressor after chronic stress. Neuroscience, 1998. 84(4): p. 1025–39. [DOI] [PubMed] [Google Scholar]
- 49.Lucantonio F, et al. , Punishment history biases corticothalamic responses to motivationally-significant stimuli. bioRxiv, 2020: p. 2020.04.06.027888. [Google Scholar]
- 50.Campus P, et al. , The paraventricular thalamus is a critical mediator of top-down control of cue-motivated behavior in rats. Elite, 2019. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parsons MP, Li S, and Kirouac GJ, Functional and anatomical connection between the paraventricular nucleus of the thalamus and dopamine fibers of the nucleus accumbens. J Comp Neurol, 2007. 500(6): p. 1050–63. [DOI] [PubMed] [Google Scholar]
- 52.Haight JL, et al. , The lateral hypothalamus and orexinergic transmission in the paraventricular thalamus promote the attribution of incentive salience to reward-associated cues. Psychopharmacology (Berl), 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hamlin AS, et al. , Paraventricular thalamus mediates context-induced reinstatement (renewal) of extinguished reward seeking. Eur J Neurosci, 2009. 29(4): p. 802–12. [DOI] [PubMed] [Google Scholar]
- 54.Matzeu A, et al. , The paraventricular nucleus of the thalamus is differentially recruited by stimuli conditioned to the availability of cocaine versus palatable food. Addict Biol, 2017. 22(1): p. 70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labouebe G, et al. , Glucose-responsive neurons of the paraventricular thalamus control sucrose-seeking behavior. Nat Neurosci, 2016. 19(8): p. 999–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheng J, et al. , Anterior Paraventricular Thalamus to Nucleus Accumbens Projection Is Involved in Feeding Behavior in a Novel Environment. Front Mol Neurosci, 2018. 11: p. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Betley JN, et al. , Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell, 2013. 155(6): p. 1337–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meffre J, et al. , Orexin in the Posterior Paraventricular Thalamus Mediates Hunger-Related Signals in the Nucleus Accumbens Core. Curr Biol, 2019. 29(19): p. 3298–3306 e4. [DOI] [PubMed] [Google Scholar]
- 59.Nakahara K, Fukui K, and Murakami N, Involvement of Thalamic Paraventricular Nucleus in the Anticipatory Reaction under Food Restriction in the Rat. Journal of Veterinary Medical Science, 2004. 66(10): p. 1297–1300. [DOI] [PubMed] [Google Scholar]
- 60.Ong ZY, et al. , Paraventricular Thalamic Control of Food Intake and Reward: Role of Glucagon-Like Peptide-1 Receptor Signaling. Neuropsychopharmacology, 2017. 42(12): p. 2387–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Livneh Y, et al. , Homeostatic circuits selectively gate food cue responses in insular cortex. Nature, 2017. 546(7660): p. 611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kelley AE, Baldo BA, and Pratt WE, A proposed hypothalamic–thalamic–striatal axis for the integration of energy balance, arousal, and food reward. Journal of Comparative Neurology, 2005. 493(1): p. 72–85. [DOI] [PubMed] [Google Scholar]
- 63.Wang C, et al. , AgRP neurons trigger long-term potentiation and facilitate food seeking. Translational Psychiatry, 2021. 11(1): p. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lafferty CK, et al. , Nucleus Accumbens Cell Type- and Input-Specific Suppression of Unproductive Reward Seeking. Cell Reports, 2020. 30(11): p. 3729–3742.e3. [DOI] [PubMed] [Google Scholar]
- 65.Deutch AY, Bubser M, and Young CD, Psychostimulant-induced Fos protein expression in the thalamic paraventricular nucleus. J Neurosci, 1998. 18(24): p. 10680–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephenson CP, et al. , The distribution of 3,4-methylenedioxymethamphetamine “Ecstasy”-induced c-fos expression in rat brain. Neuroscience, 1999. 92(3): p. 1011–23. [DOI] [PubMed] [Google Scholar]
- 67.Deutch AY, Ongür D, and Duman RS, Antipsychotic drugs induce Fos protein in the thalamic paraventricular nucleus: a novel locus of antipsychotic drug action. Neuroscience, 1995. 66(2): p. 337–46. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y, et al. , A thalamic input to the nucleus accumbens mediates opiate dependence. Nature, 2016. 530(7589): p. 219–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Neumann PA, et al. , Cocaine-Induced Synaptic Alterations in Thalamus to Nucleus Accumbens Projection. Neuropsychopharmacology, 2016. 41(9): p. 2399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Millan EZ, Ong Z, and McNally GP, Paraventricular thalamus: Gateway to feeding, appetitive motivation, and drug addiction. Prog Brain Res, 2017. 235: p. 113–137. [DOI] [PubMed] [Google Scholar]
- 71.Matzeu A, et al. , Orexin-A/Hypocretin-1 Mediates Cocaine-Seeking Behavior in the Posterior Paraventricular Nucleus of the Thalamus via Orexin/Hypocretin Receptor-2. J Pharmacol Exp Ther, 2016. 359(2): p. 273–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li Y, et al. , Changes in emotional behavior produced by orexin microinjections in the paraventricular nucleus of the thalamus. Pharmacol Biochem Behav, 2010. 95(1): p. 121–8. [DOI] [PubMed] [Google Scholar]
- 73.Knoll AT and Carlezon WA Jr., Dynorphin, stress, and depression . Brain Res, 2010. 1314: p. 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matzeu A, et al. , Dynorphin Counteracts Orexin in the Paraventricular Nucleus of the Thalamus: Cellular and Behavioral Evidence. Neuropsychopharmacology, 2018. 43(5): p. 1010–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Matzeu A and Martin-Fardon R, Drug Seeking and Relapse: New Evidence of a Role for Orexin and Dynorphin Co-transmission in the Paraventricular Nucleus of the Thalamus. Front Neurol, 2018. 9: p. 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Keyes PC, et al. , Orchestrating Opiate-Associated Memories in Thalamic Circuits . Neuron, 2020. 107(6): p. 1113–1123.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.James MH, et al. , Cocaine- and amphetamine-regulated transcript (CART) signaling within the paraventricular thalamus modulates cocaine-seeking behaviour. PLoS One, 2010. 5(9): p. e12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X and van den Pol AN, Rapid binge-like eating and body weight gain driven by zona incerta GABA neuron activation. Science, 2017. 356(6340): p. 853–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis Graeme W., Homeostatic Signaling and the Stabilization of Neural Function. Neuron, 2013. 80(3): p. 718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams G, et al. , The hypothalamus and the control of energy homeostasis: Different circuits, different purposes. Physiology & Behavior, 2001. 74(4): p. 683–701. [DOI] [PubMed] [Google Scholar]
- 81.Timper K and Brüning JC, Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis Model Mech, 2017. 10(6): p. 679–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Novak CM, et al. , Suprachiasmatic nucleus projections to the paraventricular thalamic nucleus in nocturnal rats (Rattus norvegicus) and diurnal nile grass rats (Arviacanthis niloticus). Brain Res, 2000. 874(2): p. 147–57. [DOI] [PubMed] [Google Scholar]
- 83.Lee JS, Lee EY, and Lee HS, Hypothalamic, feeding/arousal-related peptidergic projections to the paraventricular thalamic nucleus in the rat. Brain Research, 2015. 1598: p. 97–113. [DOI] [PubMed] [Google Scholar]
- 84.Kirouac GJ, Parsons MP, and Li S, Innervation of the paraventricular nucleus of the thalamus from cocaine- and amphetamine-regulated transcript (CART) containing neurons of the hypothalamus. J Comp Neurol, 2006. 497(2): p. 155–65. [DOI] [PubMed] [Google Scholar]
- 85.Otake K, Cholecystokinin and substance P immunoreactive projections to the paraventricular thalamic nucleus in the rat. Neuroscience Research, 2005. 51(4): p. 383–394. [DOI] [PubMed] [Google Scholar]
- 86.Choi EA and McNally GP, Paraventricular Thalamus Balances Danger and Reward. J Neurosci, 2017. 37(11): p. 3018–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Choi EA, et al. , Paraventricular Thalamus Controls Behavior during Motivational Conflict. J Neurosci, 2019. 39(25): p. 4945–4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dong X, Li S, and Kirouac GJ, Collateralization of projections from the paraventricular nucleus of the thalamus to the nucleus accumbens, bed nucleus of the stria terminalis, and central nucleus of the amygdala. Brain Struct Funct, 2017. 222(9): p. 3927–3943. [DOI] [PubMed] [Google Scholar]
- 89.Watarai A, et al. , The blockade of oxytocin receptors in the paraventricular thalamus reduces maternal crouching behavior over pups in lactating mice. Neurosci Lett, 2020. 720: p. 134761. [DOI] [PubMed] [Google Scholar]
- 90.Champagne FA, et al. , Variations in Nucleus Accumbens Dopamine Associated with Individual Differences in Maternal Behavior in the Rat. The Journal of Neuroscience, 2004. 24(17): p. 4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gimpl G and Fahrenholz F, The oxytocin receptor system: structure, function, and regulation. Physiol Rev, 2001. 81(2): p. 629–83. [DOI] [PubMed] [Google Scholar]
- 92.Lonstein JS, et al. , Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience, 1998. 82(1): p. 267–81. [DOI] [PubMed] [Google Scholar]
- 93.Kohl J, et al. , Functional circuit architecture underlying parental behaviour. Nature, 2018. 556(7701): p. 326–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Phillipson OT and Griffiths AC, The topographic order of inputs to nucleus accumbens in the rat. Neuroscience, 1985. 16(2): p. 275–296. [DOI] [PubMed] [Google Scholar]
- 95.Jurik A, et al. , Roles of prefrontal cortex and paraventricular thalamus in affective and mechanical components of visceral nociception. Pain, 2015. 156(12): p. 2479–91. [DOI] [PubMed] [Google Scholar]
- 96.Yamamuro K, et al. , A prefrontal-paraventricular thalamus circuit requires juvenile social experience to regulate adult sociability in mice. Nat Neurosci, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pliota P, et al. , Stress peptides sensitize fear circuitry to promote passive coping. Molecular Psychiatry, 2020. 25(2): p. 428–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Howe MW, et al. , Prolonged dopamine signalling in striatum signals proximity and value of distant rewards. Nature, 2013. 500(7464): p. 575–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Namburi P, et al. , Architectural Representation of Valence in the Limbic System. Neuropsychopharmacology, 2016. 41(7): p. 1697–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roitman MF, Wheeler RA, and Carelli RM, Nucleus Accumbens Neurons Are Innately Tuned for Rewarding and Aversive Taste Stimuli, Encode Their Predictors, and Are Linked to Motor Output. Neuron, 2005. 45(4): p. 587–597. [DOI] [PubMed] [Google Scholar]
- 101.Tye KM, Neural Circuit Motifs in Valence Processing. Neuron, 2018. 100(2): p. 436–452. [DOI] [PMC free article] [PubMed] [Google Scholar]


