Abstract
Background:
Dysmenorrhea is highly prevalent; it places women at risk for other chronic pain conditions. There is a high degree of individual variability in menstrual pain severity, the number of painful sites, and co-occurring gastrointestinal symptoms. Distinct dysmenorrhea symptom-based phenotypes were previously identified, but the biological underpinnings of these phenotypes are less known. One underexplored contributor is the vaginal microbiome. The vaginal microbiota differs significantly among reproductive-age women and may modulate as well as amplify reproductive tract inflammation, which may contribute to dysmenorrhea symptoms.
Objectives:
The objective of this study was to examine associations between dysmenorrhea symptom-based phenotypes and vaginal microbiome compositions on- and off-menses.
Methods:
We conducted a prospective, longitudinal, pilot study of 20 women (aged 15–24) grouped into three dysmenorrhea symptom-based phenotypes: “mild localized pain,” “severe localized pain,” and “severe multiple pain and gastrointestinal symptoms.” Over one menstrual cycle, participants provided vaginal swabs when they were on-menses and off-menses. We assayed the vaginal microbiome using 16S rRNA gene sequencing. Permutational multivariate analysis of variance tests were used to compare microbiome compositions across phenotypes, with heat maps generated to visualize the relative abundance of bacterial taxa.
Results:
The vaginal microbiome compositions (n = 40) were different across the three phenotypes. After separating the on-menses (n = 20) and off-menses (n = 20) specimens, the statistically significant difference was seen on-menses, but not off-menses. Compared to the “mild localized pain” phenotype, participants in the “multiple severe symptoms” phenotype had a lower lactobacilli level and a higher abundance of Prevotella, Atopobium, and Gardnerella when on-menses. We also observed trends of differences across phenotypes in vaginal microbiome change from off- to on-menses.
Discussion:
The study provides proof-of-concept data to support larger studies on associations between dysmenorrhea symptom-based phenotypes and vaginal microbiome that might lead to new intervention targets and/or biomarkers for dysmenorrhea. This line of research has the potential to inform precision dysmenorrhea treatment that can improve women’s quality of life.
Keywords: dysmenorrhea, pain, phenotype, vaginal microbiome, women’s health
Between 45% to 95% of women of reproductive age experience dysmenorrhea, characterized by menstrual pain (Iacovides et al., 2015). Dysmenorrhea leads to school absences, lost work hours, impaired physical activity, poor sleep, reduced quality of life (Dawood, 2006; Iacovides et al., 2015; Rencz et al., 2017), and increases women’s susceptibility to other chronic pain syndromes (Berkley, 2013; Iacovides et al., 2015).
Individual variability in dysmenorrhea has been characterized into three phenotypes: “mild localized pain” phenotype (with mild abdominal pain), “severe localized pain” phenotype (with severe abdominal pain), and “multiple severe symptoms” phenotype (with severe pain at multiple sites and gastrointestinal symptoms; Chen et al., 2018, 2021). These phenotypes have been associated with perceived treatment ineffectiveness: Compared to the “mild localized pain” phenotype, women in the “multiple severe symptoms” phenotype were more likely to perceive common treatments as ineffective (Chen, Carpenter, LaPradd et al., 2020). According to the National Institutes of Health’s Symptom Science Model—developed by nurse scientists—integrating phenotypic and omics data is essential to understanding individual differences in symptom experiences and to developing precision-based symptom interventionsh. There is a need to study phenotype-omic associations in dysmenorrhea, so new and precision-based interventions can be developed.
The vaginal microbiota may play a role in individual differences in dysmenorrhea symptoms. A higher abundance of vaginal lactobacilli was associated with lower concentrations of pro-inflammatory immune markers in vaginal fluid (Amabebe & Anumba, 2018; Fettweis et al., 2019). Lactobacillus spp. produce lactic acid, elicit anti-inflammatory responses, and inhibit pro-inhibitory mediators (Hearps et al., 2017; Ma et al., 2012). In contrast, a diverse vaginal bacterial community promotes release of pro-inflammatory cytokines in the reproductive tract (Amabebe & Anumba, 2018; Doerflinger et al., 2014; Fettweis et al., 2019;), which may exacerbate dysmenorrhea symptoms.
To our knowledge, only one study linked dysmenorrhea to the microbiome within the reproductive tract. Among women undergoing hysteroscopy or laparoscopy (Pelzer et al., 2018), endometrial facultative anaerobes were more abundant in women with dysmenorrhea (n = 24) compared to women with menorrhagia (n = 17). However, the cross-sectional design did not sample the vaginal microbiome nor account for potential microbiome change within a menstrual cycle (Gajer et al., 2012; Hickey et al., 2013). Other limitations were a highly select clinical population, invasive sampling, and no measurement of dysmenorrhea symptom heterogeneity.
Exploring associations between the vaginal microbiome and dysmenorrhea phenotypes may elucidate mechanisms of dysmenorrhea and suggest new avenues for interventions. The purpose of the study was to examine associations between dysmenorrhea symptom-based phenotypes and vaginal microbiome compositions when women were on-menses and off-menses. We hypothesized dysmenorrhea symptom-based phenotypes would be associated with vaginal microbiome compositions on- and/or off-menses.
Methods
Study Design
In this prospective, longitudinal pilot study, 20 participants provided data at enrollment on-menses and off-menses.
Sample
The sample size was based on funding availability and funding timeline and was comparable to some previous studies involving longitudinal vaginal microbiome sample collections (Gajer et al., 2012; Hickey et al., 2013). Inclusion criteria were (a) females aged 14–24; (b) onset of menarche > 2 years prior to the study; (c) regular menstrual cycles (24–38 days) for 3 months preceding enrollment; and (d) in good general health. Exclusion criteria were (a) pregnancy or lactation; (b) diagnosis of endometriosis, uterine fibroids, polycystic ovary syndrome, or pelvic inflammatory diseases; (c) diagnosis of irritable bowel syndrome or inflammatory bowel disease; (d) use of any hormonal contraceptives within 3 months; (5) use of any intrauterine device within 30 days; (f) use of any systemic antibiotics within 3 months; and (g) use of any systemic or vaginal antifungal treatment within 3 months. Exclusion criteria were designed to reduce potential confounders of the vaginal microbiome.
Measures
Dysmenorrhea Symptom-Based Phenotypes
Based on previous research findings (Chen et al., 2018), we designed one question to identify symptom-based phenotypes. Participants were asked, “In the last 6 months, which of the following statements best describes your physical symptoms before or during menstruation?” Response options included descriptions about each phenotype: (a) “I usually had only mild abdominal cramps or mild, dull abdominal pain/discomfort, but not many other symptoms”; (b) “I usually had severe abdominal cramps, but not many other symptoms”; (c) “I usually had severe abdominal cramps, severe pain at other locations (e.g., low back, headaches, or pain all over), and severe gastrointestinal symptoms (e.g., bloating, diarrhea, and change in bowel frequencies)”; and (d) None of the above.
Dysmenorrhea symptom burden was assessed via a 14-item symptom list (Chen et al., 2015, 2018). Participants rated severity of each symptom from 0 (“not present”) to 10 (“extremely severe”). Symptoms included abdominal cramps, dull abdominal pain or discomfort, low back pain, pain in the upper thighs, headache or migraines, pain when the bladder was full, aches all over, bloating, nausea, vomiting, diarrhea (loose stools), constipation (hard stools), more bowel movements than usual, and fewer bowel movements than usual. After summing items, total scores ranged from 0 to 140 (greater symptom burden).
Demographic, Health-Related, and Behavioral Data
At enrollment, we collected self-reported demographic (age, race, ethnicity, and educational level), health-related (menstrual history, health conditions), and behavioral data (e.g., sexual behaviors, hygiene behavior, cigarette smoking, alcohol use). At both enrollment and follow-up, we collected information about participants’ recent health behaviors, including sexual behaviors and recent use of antibiotics and/or probiotics. When participants were on-menses, we asked about their use of menstrual products (e.g., tampons, pads, cups).
Vaginal Microbiome
Vaginal Swab Collection.
Participants self-collected vaginal swabs using OMNIgene-VAGINAL kits (Ottawa, Canada). Compared to provider-collected specimens, previous research suggests that self-collected specimens have the same microbial diversity and high validity (Forney et al., 2010). Vaginal swabs were collected on-menses (Days 1–3 of the menstrual cycle) and off-menses (midcycle day +/−5 days), as research suggests the vaginal microbiome profile can change during menstruation (Gajer et al., 2012; Hickey et al., 2013). This meant that for each participant the two sample collections were separated by 13 to 20 days depending on the length of their menstrual cycle. The collection kits allow for ambient temperature (−20°C to 50°C) storage for up to 30 days. Upon receipt, the specimens were aliquoted into cryovials, barcoded, and frozen at −80°C for later processing and assays.
DNA Extraction.
Nucleic acids were batch extracted from vaginal swab specimens using the Epicentre MasterPure™ Complete DNA and RNA Purification Kit. Controls were included at all steps to monitor for potential reagent contamination. DNA quality was monitored by gel electrophoresis and fluorescent dsDNA assays. Genomic DNA (gDNA) was stored at −80°C until used in creating sequencing libraries.
16S rRNA Gene Sequencing.
Multiplexed amplicon sequencing libraries were prepared using the NEXTflex 16S V4 Amplicon-Seq Library Prep Kit 2.0 (Perkin Elmer). The normalized and pooled libraries were paired-end sequenced on an Illumina MiSeq instrument (Illumina, San Diego, CA), using the 2 × 300 bp v3 chemistry. Sequences were filtered and trimmed using DADA2 (version 1.14; Callahan et al., 2016). Forward reads were trimmed at 290 bp, and reverse reads were trimmed at 260 bp. Reads with more than 10 “expected errors” were discarded. Chimeric reads were identified by the default consensus method and discarded in DATA2. A total of 5,418 operational taxonomic units (OTUs) were generated from 7,056,833 cleaned reads and annotated with BLCA software (Gao et al., 2017) using the 16S ribosomal RNA database from NCBI (dated as of September 24, 2019). As DNA was not detected in the regent controls, no control sequence was used for library construction and sequencing.
Study Procedures
The study was approved by the local institutional review board. Participants were recruited through study flyers posted on a university campus and a statewide research volunteer registry maintained at the institution. The latter was created for individuals to sign up to receive information about ongoing studies. Using the study criteria, the registry manager emailed a group of potentially eligible participants (based on age, gender, geographical location, and willingness to travel for the study visits) to inform them about the study opportunity. Interested potential participants contacted a research assistant, and the research assistant obtained their verbal permission for screening over the telephone.
An enrollment visit was scheduled for eligible and interested participants. During the visit, the research assistant explained procedures and the consent form before obtaining signed assent (aged ≤ 18) and/or signed consent (aged ≥ 18, parents of those aged ≤ 18). Participants answered self-report questionnaires through the Research Electronic Data Capture (REDCap) online platform (Harris et al., 2009). Research staff trained participants to self-collect samples using strategies described previously (Chen, Carpenter, Murphy et al., 2020). After collecting swabs at home, participants brought them to the clinical research center within 2 days.
Data Analysis
Demographic and clinical data were summarized descriptively by phenotypes using IBM SPSS Statistics for Windows, Version 26.0 (Armonk, NY: IBM Corp). Dysmenorrhea symptom burden scores were compared across phenotypes using the Kruskal–Wallis independent sample test.
Microbiome data analysis was performed in R with packages phyloseq and vegan (McMurdie & Holmes, 2013). To assess differences in bacterial community composition (i.e., beta-diversity) by phenotype, we conducted permutational multivariate analysis of variance (PERMANOVA) tests. We first conducted a PERMANOVA test when on- and off-menses specimens were combined (40 specimens), accounting for dependency between specimens from tnhe same individual. Then, we conducted PERMANOVA tests separating the 20 on-menses from the 20 off-menses specimens. Non-metric multidimensional scaling (NMDS) was used to visualize dissimilarity between phenotypes in a low-dimensional space. We compared alpha diversity between phenotypes. Specifically, we used Wilcoxon tests to compare the Shannon indices and the Simpson indices between the “mild localized pain” phenotype (n = 12) and “multiple severe symptoms” phenotype (n = 6).
We applied heatmaps to visualize the relationship between samples and their top 100 relative abundance OTUs. As the sample size for the “severe localized pain” phenotype was very small (n = 2), we compared relative OTU abundance between the “mild localized pain” phenotype (n = 12) and “multiple severe symptoms” phenotype (n = 6) during off- and on-menses stages, respectively. For these two-group comparisons, we compared OTUs with an average abundance above 0.5% in at least one phenotype group using Wilcoxon rank-sum (WRS) tests and negative binomial methods.
To compare off- to on-menses change between phenotypes, we calculated changes in bacteria abundance and used WRS tests and negative binomial (NB) methods to examine whether the amount of changes differed between phenotypes. We also used line charts to visually compare the amount of changes in abundance between the “mild localized pain” phenotype and “multiple severe symptoms” phenotype.
Results
Participant Characteristics
Participants were a mean age of 20.9 ± 3.2 years (minimum–maximum: 15–24 years). Most (n = 12, 60%) were White, while 7 (35%) were African American/Black, and one (5%) was Asian. None identified as Hispanic or Latino. Eleven (55%) had a bachelor’s degree or above. The average menstrual cycle length was 28.5 ± 2.4 days. Average age of menarche was 12.3 ± 1.7 years old.
Table 1 shows the demographic, clinical, and behavioral data by phenotypes. Participants identified with phenotypes as follows: 12 (60%) “mild localized pain” phenotype, 2 (10%) “severe localized pain” phenotype, and 6 (70%) “multiple severe symptoms” phenotype. As expected, phenotypes differed significantly in dysmenorrhea burden scores (p = 0.017).
Table 1.
Demographic, Health-related, and Behavioral Characteristics by Phenotype
| Mild Localized Pain Phenotype (n = 12) |
Severe Localized Pain Phenotype (n = 2) | Multiple Severe Symtpoms Phenotype (n = 6) | ||||
|---|---|---|---|---|---|---|
| mean (SD) | n (%) | mean (SD) | n (%) | mean (SD) | n (%) | |
| Age | 21.8 (2.6) | 19 (5.7) | 20 (3.5) | |||
| Menstrual Characteristics | ||||||
| Years since Menarche | 9.2 (3.1) | 7 (5.7) | 8.0 (2.8) | |||
| Menstrual Cycle Lenth (Days) | 27.9 (2.2) | 29.5 (2.1) | 29.2 (2.9) | |||
| Dysmenorrhea Symptom Burden Score | 29.4 (15.4) | 40.0 (9.9) | 58.2 (16.4) | |||
| Race and Ethnicity | ||||||
| White | 8 (66.7) | 2 (100) | 2 (33.3) | |||
| Black | 3 (25) | 0 (0) | 4 (66.7) | |||
| Asian | 1 (8.3) | 0 (0) | 0 (0) | |||
| Hispanic/Latino | 0 (0) | 0 (0) | 0 (0) | |||
| Medical History | ||||||
| Menorrhagia | 1 (8.3) | 0 (0) | 0 (0) | |||
| Bacterial Vaginosis | 0 (0) | 0 (0) | 0 (0) | |||
| Sexually Transmitted Infection | 1 (8.3) | 0 (0) | 0 (0) | |||
| Pelvic Inflammatory Disease | 0 (0) | 0 (0) | 0 (0) | |||
| Typical Health Behaviors | ||||||
| Sexually Active | 7 (58.3) | 1 (50) | 3 (50) | |||
| Alcohol User (1–5 times/week) | 4 (33.3) | 0 (0) | 2 (33.3) | |||
| Current Smoker | 1 (8.3) | 0 (0) | 1 (16.7) | |||
| Douching | 1 (8.3) | 0 (0) | 0 (0) | |||
| Regularly Consuming Probiotics | 1 (8.3) | 0 (0) | 0 (0) | |||
| Recent Health Behaviors | ||||||
| Took Any Antibiotics During the study | 1 (8.3) | 0 (0) | 0 (0) | |||
| Unprotected vaginal sexa | 0 (0) | 1 (50) 2 | 0 (0) | |||
| Used Tamponb | 8 (66.7) | 2 (100) | 4 (66.7) | |||
| Used Menstral Padb | 6 (50) | 1 (50) | 3 (50) | |||
| Used Menstrual Cupb | 2 (16.7) | 0 (0) | 0 (0) | |||
Note.
Within 24 hours before collecting vaginal swab.
Before on-menses vaginal swab.
Comparing Vaginal Microbiome Compositions Across Phenotypes
Based on the PERMANOVA test accounting for dependency between specimens from the same individual (n = 40), vaginal microbiome compositions were statistically different across the three phenotypes (p =.009). Figure 1 shows the dissimilarity of vaginal microbial profiles across phenotypes using the NMDS plot (a distance-based plot).
Figure 1.
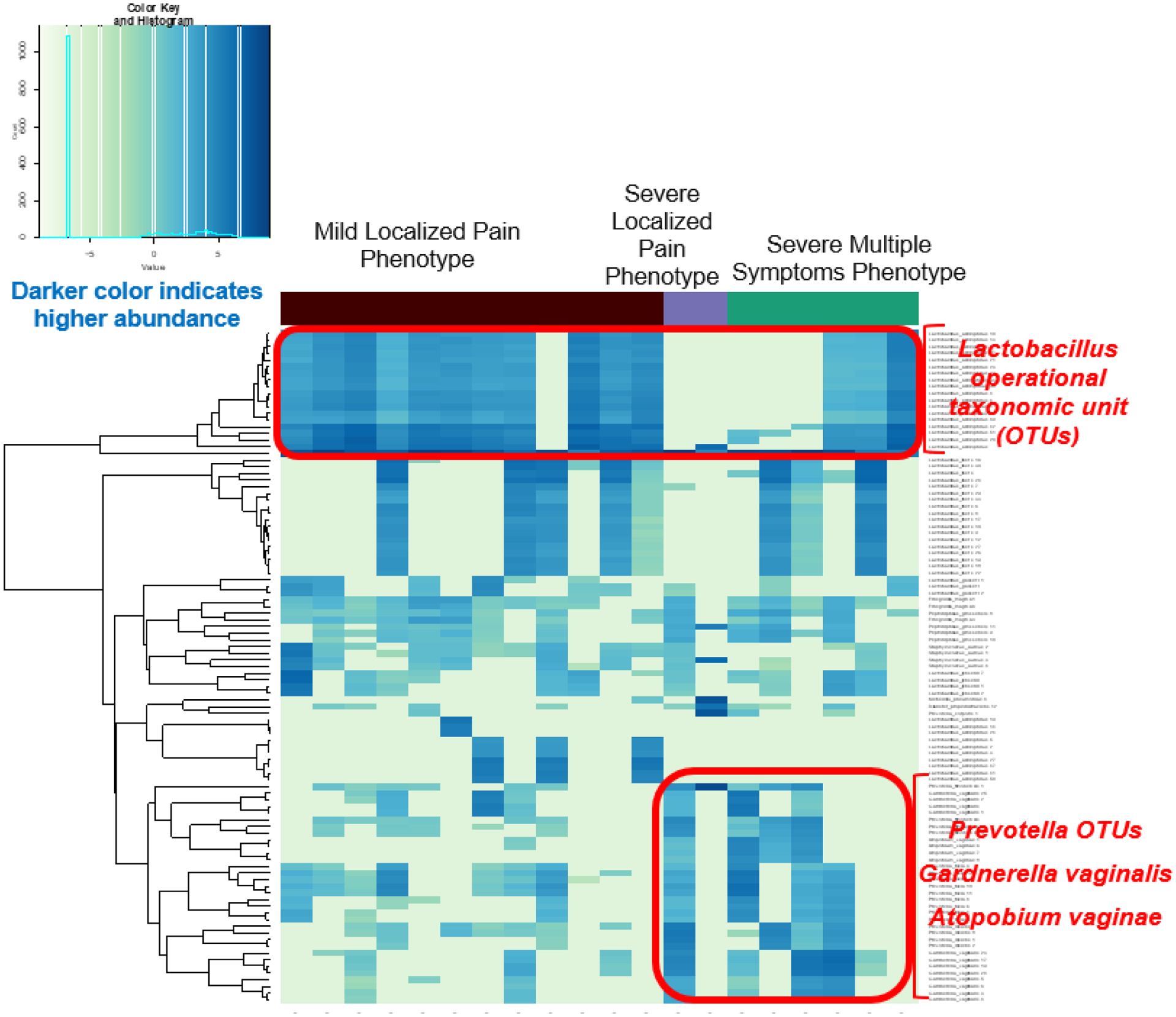
Vaginal Microbiome On-menses Differed Across Phenotypes
OTU: operational taxonomic unit. Each row represents an OTU. Each column represents an individual vaginal swab sample. The density of the color in each cell represents the relative abundance of the taxon in that sample. Darker colors indicate higher abundance. When on-menses, compared to the “mild localized pain” phenotype, participants in the “multiple severe symptoms” phenotype had a lower level of Lactobacillus crispatus and a higher abundance of Atopobium, Gardnerella, and Prevotella taxa.
For off-menses biospecimens (n = 20), no differences in bacteria composition across the three phenotypes were seen (PERMANOVA p =.394). When comparing only the “mild localized pain” phenotype and the “multiple severe symptoms” phenotype off-menses, no significant differences were seen in OTU abundance or alpha diversities (p values > .05).
For on-menses biospecimens (n = 20), significant differences in vaginal microbiota across the three phenotypes were seen (PERMANOVA p = .006). When on-menses, compared to the “mild localized pain” phenotype, participants in the “multiple severe symptoms” phenotype had a lower level of Lactobacillus crispatus and a higher abundance of Atopobium, Gardnerella, and Prevotella taxa (see Figure 1). Specifically, those in the “multiple severe symptoms” phenotype had a lower level of Lactobacillus crispatus (WRS tests: p values ≤ .038), and a higher abundance of four taxa: Atopobium vaginae (WRS tests: p values ≤ .012), Sneathia sanguinegens (WRS tests: p = 0.049, NB methods: p values ≤ .045), Gardnerella vaginalis (WRS tests: p values ≤ .079; NB methods: p values ≤ .011), and Prevotella bivia (WRS test: p = .221, NB methods: p = .036). The on-menses alpha diversities (measured by Shannon and Simpson indices) were not significantly different between the “mild localized pain” phenotype and the “multiple severe symptoms” phenotype (p values > .05). In our small sample, the p values were not significant (p values > .05) when adjusting for multiple comparisons.
Comparing Changes in Vaginal Microbiome Compositions Across Phenotypes
For changes in bacterial relative abundance from off- to on-menses, we found no statistically significant difference between the “mild localized pain” phenotype (n = 12) and “multiple severe symptom” phenotype (n = 6; p values > .05). However, trends seen in Figure 2 suggest that compared to the “mild localized pain” phenotype group, the “multiple severe symptom” phenotype group had a larger shift in lactobacilli and nonlactobacilli abundance from off- to on-menses. From off- to on-menses, women with the “multiple severe symptoms” phenotype had a larger decrease in the Lactobacillus spp. relative abundance and a larger increase in Prevotella and Gardnerella.
Figure 2.
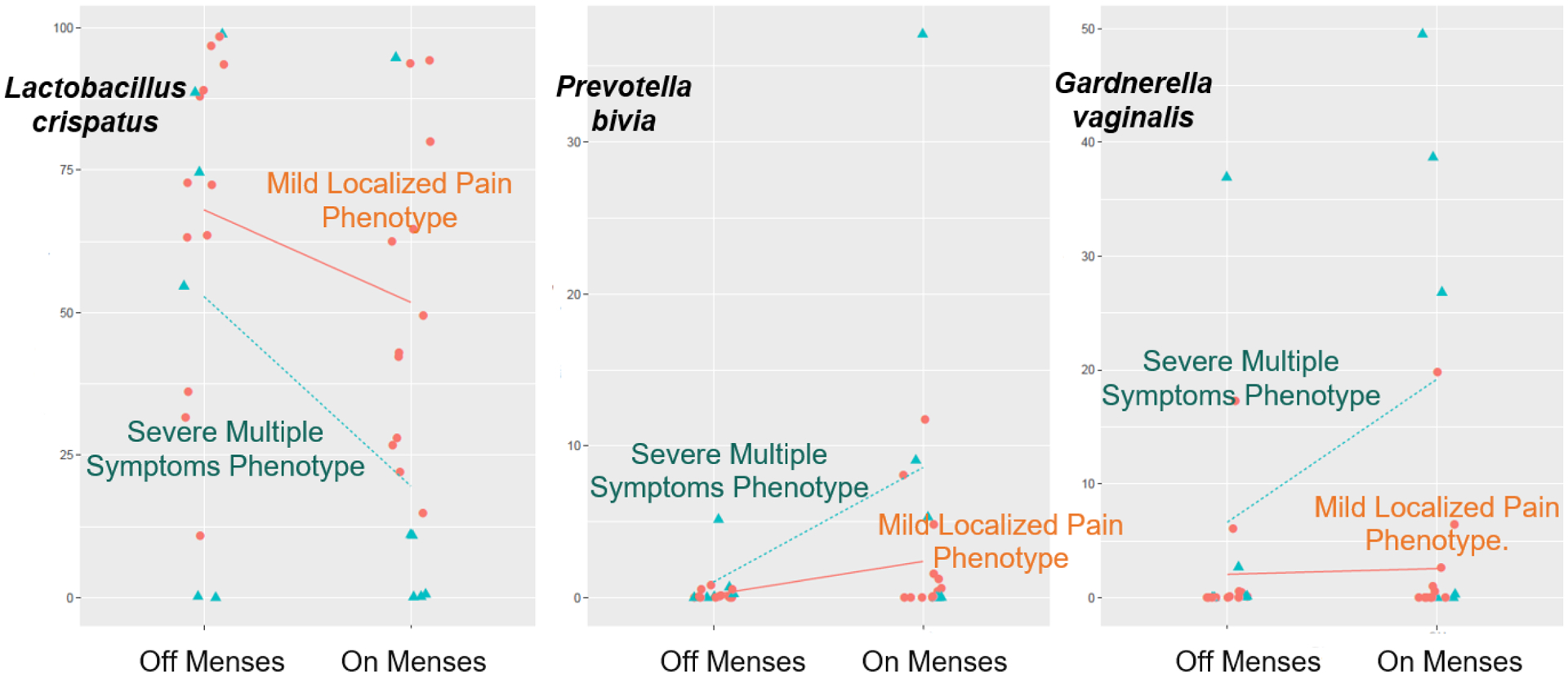
Vaginal Microbiome Change from Off-Menses to On-Menses
The lines in each plot connect the means of the relative abundance of specific bacteria from off- to on-menses. Trends seen in this figure suggest two findings. First, compared to the “mild localized pain” phenotype group, the “multiple severe symptom” phenotype group had a larger shift in lactobacilli and non-lactobacilli abundance from off- to on-menses. Second, from off- to on-menses, women with the “multiple severe symptoms” phenotype had a larger decrease in the Lactobacillus spp. relative abundance and a larger increase in Prevotella and Gardnerella.
Discussion
By analyzing the vaginal microbiomes of 20 women on- and off-menses, we found differences across three dysmenorrhea symptom-based phenotypes in on-menses vaginal microbiome compositions. We also observed differences across phenotypes in vaginal microbiome change from off- to on-menses. To our knowledge, this was the first study linking the severity of dysmenorrhea and vaginal microbiome compositions at the time of menses.
The hypothesis that dysmenorrhea symptom-based phenotypes would be associated with vaginal microbiome compositions was supported by findings showing a higher abundance of vaginal lactobacilli during menstruation in the mild versus multiple severe phenotypes. High abundance of vaginal lactobacilli was associated with lower concentrations of pro-inflammatory immune markers in vaginal fluid (Amabebe & Anumba, 2018; Fettweis et al., 2019; Ma et al., 2012). Vaginal lactic acidh produced by lactobacilli elicits anti-inflammatory responses and inhibits pro-inflammatory mediators (Fettweis et al., 2019; Hearps et al., 2017). Vaginal lactobacilli may protect some women from severe dysmenorrhea symptoms by limiting the growth of potentially pro-inflammatory bacteria and suppressing inflammation.
The hypothesis was also supported by findings showing a higher abundance of potentially pro-inflammatory bacteria (Prevotella, Atopobium, and Gardnerella) in the severe versus mild phenotype. These bacteria may promote the release of pro-inflammatory cytokines (Fettweis et al., 2019; Jean et al., 2019), alter host lipid metabolisms (Jean et al., 2019; Srinivasan et al., 2015), or even result in chronic inflammation (Wiesenfeld et al., 2012), all of which may exacerbate dysmenorrhea symptoms.
Findings suggest additional plausible mechanisms in dysmenorrhea that nurse scientists could explore. During menstruation, the breakdown of endometrial tissues releases phospholipids from cell membranes. Phospholipids are converted into arachidonic acid and then into prostaglandins (Iacovides et al., 2015). Certain vaginal bacteria such as Gardnerella may ascend to the uterus (Goldenberg et al., 2000; Swidsinski et al., 2013; Wiesenfeld et al., 2012) and secrete phospholipase A2 to act on uterine membrane phospholipids (Jean et al., 2019; Jones & Al-Mushrif, 1997), resulting in additional prostaglandin release (Bennett et al., 1990; Yang et al., 2015). Prostaglandins cause uterine muscle contractions, ischemia, hypoxia, and sensitization of nerve endings, all of which contribute to menstrual pain (Dawood, 2006). Prostaglandins may get into the circulation (Durham et al., 2010; Lundström & Green, 1978) and may contribute to gastrointestinal symptoms (e.g., nausea, vomiting, bloating, change in bowel frequency; Dawood, 2006; Heitkemper et al., 1991). In addition to promoting the release of prostaglandins, certain vaginal bacteria can stimulate the release of pro-inflammatory cytokines (Fettweis et al., 2019; Jean et al., 2019). Pro-inflammatory cytokines interact with each other and with prostaglandins (Yang et al., 2015), potentially amplifying inflammation and dysmenorrhea symptoms.
The study provides important information about the temporal stability of the vaginal microbiome in relation to dysmenorrhea. Outside of the context of dysmenorrhea, equivocal evidence exists regarding the stability of the vaginal microbiome during a menstrual cycle. In some studies, the vaginal microbiome community composition was relatively stable (Bradley et al., 2018; Chaban et al., 2014), while in others, it changed during menses (Eschenbach et al., 2000; Gajer et al., 2012). In addition to sample size and methodology differences, discrepancies in results may be due to the interindividual variability in vaginal microbiome stability. In fact, research shows that vaginal microbiome stability during menses varies considerably among individuals (Gajer et al., 2012; Hickey et al., 2013). For some women, changes in potentially pro-inflammatory bacteria (e.g., Prevotella and Gardnerella) and beneficial lactobacilli from off-menses to on-menses may be associated with a pro-inflammatory profile on-menses. Given individual differences in vaginal microbiome stability, it is important to consider vaginal microbiome dynamics in future research. Collecting samples on- and off-menses will allow researchers to account for temporal variations and further study how vaginal microbiome stability affects women’s health. Such knowledge may inform the development of personalized interventions, including those that women could use at home or those that might need to be prescribed by a nurse practitioner or other provider.
We acknowledge some study limitations. This small pilot study was limited in size, age, and ethnic diversity. Our sample size prohibited us from grouping individuals into symptom-based phenotypes using latent class analysis. We assessed health behaviors but did not control for potential confounders (e.g., race, sexual behaviors, douching, menstrual products use) given the limited sample size and variation. Some phenotypic comparisons were not significant when adjusting for multiple comparisons. Our results show the need for further research in a larger and more diverse group of women.
Conclusion
To our knowledge, this was the first study to examine relationships between dysmenorrhea symptom-based phenotypes and vaginal microbiome. Women with more severe dysmenorrhea symptom burden had a vaginal microbial profile with lower proportions of lactobacilli and higher proportions of potentially pro-inflammatory bacteria when on-menses. We also observed trends of differences across phenotypes in vaginal microbiome change from off- to on-menses. This pilot study provides important preliminary data for future research on mechanisms of dysmenorrhea, so new and individualized interventions can be developed.
Acknowledgements:
This research was supported by Grant Numbers KL2 TR002530 and UL1 TR002529 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. This research also was supported by the Center for Enhancing Quality of Life in Chronic Illness pilot grant (Chen, PI) from Indiana University School of Nursing. Biospecimens were stored in the Indiana Clinical and Translational Sciences Institute Specimen Storage Facility, which is supported, in part, by Grant NIH/NCRR RR020128. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or other funders. The authors thank Tabitha Murphy, MSN, RN, APRN, FNP-C and Patricia Brooks, BSM, CCRP for participant engagement and data collection support, All IN for Health team for recruitment support, staff at the the Indiana Clinical and Translational Sciences Institute (CTSI) Biospecimen Management Core and Xiaoli Zhang, BS for sample processing support, Leanne Hernandez and Darshay Foster at the Indiana CTSI Clinical Research Center for accommodating participants visits, Tasneem Talib, PhD, for thoughtful editorial suggestions, and research participants (and their parents for those who were younger than 18 years old) for their time and efforts.
Footnotes
Conflict(s) of Interest: Dr. Carpenter reports personal fees from RoundGlass Inc., Astellas Pharma Inc., Kappa Santé, and Sojournix; and unpaid consulting with QUE oncology. Dr. Mitchell receives research funding from Merck, and has served as a consultant for Scynexis, Inc.
Ethical Conduct of Research: This study was approved by the Institutional Review Board at the Indiana University.
References
- Amabebe E, & Anumba DOC (2018). The vaginal microenvironment: The physiologic role of lactobacilli. Frontiers in Medicine, 5, 181. 10.3389/fmed.2018.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett PR, Elder MG, & Myatt L (1990). Secretion of phospholipases by bacterial pathogens may initiate preterm labor. American Journal of Obstetrics & Gynecology, 163, 241–242. 10.1016/s0002-9378(11)90709-1 [DOI] [PubMed] [Google Scholar]
- Berkley KJ (2013). Primary dysmenorrhea: An urgent mandate. Pain, 1, 8. [Google Scholar]
- Bradley F, Birse K, Hasselrot K, Noël-Romas L, Introini A, Wefer H, Seifert M, Engstrand L, Tjernlund A, Broliden K, & Burgener AD (2018). The vaginal microbiome amplifies sex hormone-associated cyclic changes in cervicovaginal inflammation and epithelial barrier disruption. American Journal of Reproductive Immunology, 80, e12863. 10.1111/aji.12863 [DOI] [PubMed] [Google Scholar]
- Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, & Holmes SP (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nature Methods, 13, 581–583. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaban B, Links MG, Jayaprakash TP, Wagner EC, Bourque DK, Lohn Z, Albert AYK, van Schalkwyk J, Reid G, Hemmingsen SM, Hill JE, & Money DM (2014). Characterization of the vaginal microbiota of healthy Canadian women through the menstrual cycle. Microbiome, 2, 23. 10.1186/2049-2618-2-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Carpenter JS, LaPradd M, Ofner S, & Fortenberry JD (2020). Perceived ineffectiveness of pharmacological treatments for dysmenorrhea. Journal of Women’s Health. Advance online publication. 10.1089/jwh.2020.8581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Carpenter JS, Murphy T, Brooks P, Fortenberry JD (2020). Engaging adolescent and young adults in microbiome sample self-collection: Strategies for success. Biological Research in Nursing. Advance online publication. 10.1177/1099800420979606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Carpenter JS, Ofner S, LaPradd M, & Fortenberry JD (2021). Dysmenorrhea symptom-based phenotypes: A replication and extension study. Nursing Research, 70, 24–33. 10.1097/NNR.0000000000000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CX, Kwekkeboom KL, & Ward SE (2015). Self-report pain and symptom measures for primary dysmenorrhoea: A critical review. European Journal of Pain, 19, 377–391. 10.1002/ejp.556 [DOI] [PubMed] [Google Scholar]
- Chen CX, Ofner S, Bakoyannis G, Kwekkeboom KL, & Carpenter JS (2018). Symptoms-based phenotypes among women with dysmenorrhea: A latent class analysis. Western Journal of Nursing Research, 40, 1452–1468. 10.1177/0193945917731778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawood MY (2006). Primary dysmenorrhea: Advances in pathogenesis and management. Obstetrics & Gynecology, 108, 428–441. 10.1097/01.AOG.0000230214.26638.0c [DOI] [PubMed] [Google Scholar]
- Doerflinger SY, Throop AL, & Herbst-Kralovetz MM (2014). Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. Journal of Infectious Diseases, 209, 1989–1999. 10.1093/infdis/jiu004 [DOI] [PubMed] [Google Scholar]
- Durham PL, Vause CV, Derosier F, McDonald S, Cady R, & Martin V (2010). Changes in salivary prostaglandin levels during menstrual migraine with associated dysmenorrhea. Headache: The Journal of Head and Face Pain, 50, 844–851. 10.1111/j.1526-4610.2010.01657.x [DOI] [PubMed] [Google Scholar]
- Eschenbach DA, Thwin SS, Patton DL, Hooton TM, Stapleton AE, Agnew K, Winter C, Meier A, & Stamm WE (2000). Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clinical Infectious Diseases, 30, 901–907. 10.1086/313818 [DOI] [PubMed] [Google Scholar]
- Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, Xu J, Jimenez NR, Vivadelli SC, Fong SS, Sheth NU, Jean S, Lee V, Bokhari YA, Lara AM, … Buck GA (2019). The vaginal microbiome and preterm birth. Nature Medicine, 25, 1012–1021. 10.1038/s41591-019-0450-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney LJ, Gajer P, Williams CJ, Schneider GM, Koenig SSK, McCulle SL, Karlebach S, Brotman RM, Davis CC, Ault K, & Ravel J (2010). Comparison of self-collected and physician-collected vaginal swabs for microbiome analysis. Journal of Clinical Microbiology, 48, 1741–1748. 10.1128/jcm.01710-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajer P, Brotman RM, Bai G, Sakamoto J, Schütte UME, Zhong X, Koenig SSK, Fu L, Ma ZS, Zhou X, Abdo Z, Forney LJ, & Ravel J (2012). Temporal dynamics of the human vaginal microbiota. Science Translational Medicine, 4, 132ra152. 10.1126/scitranslmed.3003605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Lin H, Revanna K, & Dong Q (2017). A Bayesian taxonomic classification method for 16S rRNA gene sequences with improved species-level accuracy. BMC Bioinformatics, 18, 247. 10.1186/s12859-017-1670-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Hauth JC, & Andrews WW (2000). Intrauterine infection and preterm delivery. New England Journal of Medicine, 342, 1500–1507. 10.1056/nejm200005183422007 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics, 42, 377–381. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearps AC, Tyssen D, Srbinovski D, Bayigga L, Diaz DJD, Aldunate M, Cone RA, Gugasyan R, Anderson DJ, & Tachedjian G (2017). Vaginal lactic acid elicits an anti-inflammatory response from human cervicovaginal epithelial cells and inhibits production of pro-inflammatory mediators associated with HIV acquisition. Mucosal Immunology, 10, 1480–1490. 10.1038/mi.2017.27 [DOI] [PubMed] [Google Scholar]
- Heitkemper MM, Jarrett M, Bond EF, & Turner P (1991). GI symptoms, function, and psychophysiological arousal in dysmenorrheic women. Nursing Research, 40, 20–26. 10.1097/00006199-199101000-00005 [DOI] [PubMed] [Google Scholar]
- Hickey RJ, Abdo Z, Zhou X, Nemeth K, Hansmann M, Osborn TW III, Wang F, & Forney LJ (2013). Effects of tampons and menses on the composition and diversity of vaginal microbial communities over time. BJOG: An International Journal of Obstetrics & Gynaecology, 120, 695–706. 10.1111/1471-0528.12151 [DOI] [PubMed] [Google Scholar]
- Iacovides S, Avidon I, & Baker FC (2015). What we know about primary dysmenorrhea today: A critical review. Human Reproduction Update, 21, 762–778. 10.1093/humupd/dmv039 [DOI] [PubMed] [Google Scholar]
- Jean S, Huang B, Parikh HI, Edwards DJ, Brooks JP, Kumar NG, Sheth NU, Koparde V, Smirnova E, Huzurbazar S, Girerd PH, Wijesinghe DS, Strauss JF III, Serrano MG, Fettweis JM, Jefferson KK, & Buck GA (2019). Multi-omic microbiome profiles in the female reproductive tract in early pregnancy. Infectious Microbes & Diseases, 1, 49–60. 10.1097/IM9.0000000000000007 [DOI] [Google Scholar]
- Jones BM, & Al-Mushrif S (1997). The determination of phospholipase A2 enzyme activity in the vaginal secretions of pregnant and non-pregnant women with bacterial vaginosis-and in culture exudates of its causative organisms. Journal of Obstetrics and Gynaecology, 17, 290–292. 10.1080/01443619750113357 [DOI] [PubMed] [Google Scholar]
- Lundström V, & Green K (1978). Endogenous levels of prostaglandin F2α and its main metabolites in plasma and endometrium of normal and dysmenorrheic women. American Journal of Obstetetrics and Gynecology, 130, 640–646. 10.1016/0002-9378(78)90320-4 [DOI] [PubMed] [Google Scholar]
- Ma B, Forney LJ, & Ravel J (2012). Vaginal microbiome: Rethinking health and disease. Annual Review of Microbiology, 66, 371–389. 10.1146/annurev-micro-092611-150157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurdie PJ, & Holmes S (2013). phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE, 8, e61217. 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelzer ES, Willner D, Buttini M, & Huygens F (2018). A role for the endometrial microbiome in dysfunctional menstrual bleeding. Antonie Van Leeuwenhoek, 111, 933–943. 10.1007/s10482-017-0992-6 [DOI] [PubMed] [Google Scholar]
- Rencz F, Péntek M, Stalmeier PFM, Brodszky V, Ruzsa G, Gradvohl E, Baji P, & Gulácsi L (2017). Bleeding out the quality-adjusted life years: Evaluating the burden of primary dysmenorrhea using time trade-off and willingness-to-pay methods. Pain, 158, 2259–2267. 10.1097/j.pain.0000000000001028 [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Morgan MT, Fiedler TL, Djukovic D, Hoffman NG, Raftery D, Marrazzo JM, & Fredricks DN (2015). Metabolic signatures of bacterial vaginosis. MBio, 6. 10.1128/mBio.00204-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swidsinski A, Verstraelen H, Loening-Baucke V, Swidsinski S, Mendling W, & Halwani Z (2013). Presence of a polymicrobial endometrial biofilm in patients with bacterial vaginosis. PLoS ONE, 8, e53997. 10.1371/journal.pone.0053997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld HC, Hillier SL, Meyn LA, Amortegui AJ, & Sweet RL (2012). Subclinical pelvic inflammatory disease and infertility. Obstetrics & Gynecology, 120, 37–43. 10.1097/AOG.0b013e31825a6bc9 [DOI] [PubMed] [Google Scholar]
- Yang S, Reid G, Challis JRG, Kim SO, Gloor GB, & Bocking AD (2015). Is there a role for probiotics in the prevention of preterm birth? Frontiers in Immunology, 6, 62. 10.3389/fimmu.2015.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]


